CHARACTERISTICS OF A HORMONE
In the preceding chapters, we reviewed the electrochemical connection between neurons. This is the predominant method of communication within the brain and the focus of much attention from psychiatrists. The next four chapters describe systems that modulate the electrochemical connections. We start with hormones—molecular messengers sent through the bloodstream.
Neurons and neurotransmitters can be compared with telephone wires connecting one phone to another. Hormones are like TV signals that are broadcast across the skies and only recognized by appropriate receivers. With the advent of cell phones and cable TV, the distinction between direct and broadcast communication has blurred. This blurring has also occurred in the brain—traditional neurotransmitters sometimes function as hormones and hormones sometimes function as neurotransmitters. For example, epinephrine can be a neurotransmitter, but functions as a hormone when released from the adrenal medulla with a signal from the sympathetic division of the autonomic nervous system (ANS).
Although they can act in a similar manner, hormones differ from neurotransmitters in several key ways. Hormones tend to do the following:
1. Effect behavior and physiology in a gradual manner over days and weeks.
2. Receive reciprocal feedback.
3. Secrete in small pulsatile bursts.
4. Vary the levels on a circadian rhythm.
5. Have different effects on different organs.
The last point (no. 5) is of great interest to us and will be a large part of the focus of this chapter.
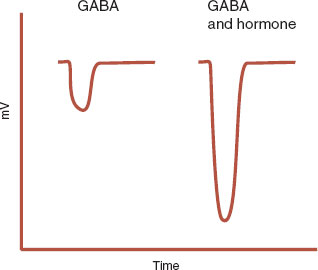
The γ-aminobutyric acid (GABA) receptor provides an example of a hormone modulating an electrochemical communication. Steroid hormones, as well as other molecules, influence the GABA receptor (see Figure 5.3). Figure 7.1 shows how a progesterone metabolite enhances the influx of Cl– ions through the GABA receptor. This will reduce the potential of the postsynaptic neuron to fire, effectively calming the neuron. This may explain the emergence of psychiatric symptoms with sex hormone fluctuations.
The difference between hormones and neurotransmitters is further blurred by the existence of neuroendocrine cells (sometimes called neurosecretory cells). Neuroendocrine cells are hybrids of neurons and endocrine cells (Figure 7.2). They receive neural signals, but secrete a hormone into the bloodstream. For example, the neuroendocrine cells of the hypothalamus receive electrical impulses from the cerebral cortex and then signal the pituitary through the bloodstream.
Classification
There are three types of hormones, which can be grouped by their chemical structure: (a) protein, (b) amine, and (c) steroid (Table 7.1). Protein hormones, such as neuropeptides, are large molecules composed of strings of amino acids. Amine hormones are small molecules derived from amino acids. Steroid hormones are composed of four interlocking rings synthesized from dietary cholesterol.
Effecting Target Cells
Hormones have two primary effects on target cells:
1. Promote differentiation and development.
2. Modulate the rate of function.
Hormones bind to specific receptors on or in the target cell in three ways. Most of the protein and amine hormones exert their effects by binding with receptors imbedded in the cell wall (as is the case with the classic neurotransmitters). The steroid hormones and thyroid hormones are lipophilic and can pass directly through the cell wall. They bind with receptors inside the cell. The steroid hormones bind with a receptor in the cytoplasm, which initiates protein synthesis when the complex couples with the DNA. The thyroid hormone receptor belongs to a family of nuclear hormone receptors. These receptors reside in the nucleus on the gene and suppress gene expression until activated by thyroid hormone (specifically T3). Figure 7.3 shows these three different receptors.

FIGURE 7.1  The Cl– current is enhanced when a progesterone metabolite is added to γ-aminobutyric acid (GABA). (Adapted from Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacologic perspectives. Trends Neurosci. 1999;22(9):410-460.)
The Cl– current is enhanced when a progesterone metabolite is added to γ-aminobutyric acid (GABA). (Adapted from Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacologic perspectives. Trends Neurosci. 1999;22(9):410-460.)
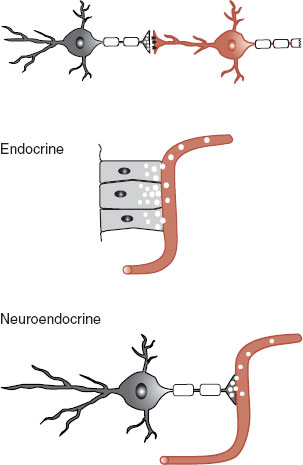
FIGURE 7.2  Chemical communication systems in humans.
Chemical communication systems in humans.

In all cases, the hormones change the function of the target cell by stimulating gene expression. This process is called a genomic effect. Some hormones effect neurons without stimulating the transcription mones effect neurons without stimulating the transcription of genes by modulating ion receptors: a nongenomic effect.
FIGURE 7.3  The three types of receptors utilized by different hormones. CREB, cyclic adenosine monophosphate response element binding; cAMP, cyclic adenosine monophosphate.
The three types of receptors utilized by different hormones. CREB, cyclic adenosine monophosphate response element binding; cAMP, cyclic adenosine monophosphate.
CENTRAL NERVOUS SYSTEM AND HORMONES
The primary endocrine glands in the brain are the hypothalamus and the pituitary gland. The hypothalamus appears to be the command center that integrates information about the state of the brain and the body by way of neuronal projections and intrinsic chemosensitive neurons. The hypothalamus coordinates the actions of the pituitary gland to maintain homeostasis in response to changes in the body and environment. Some of the essential somatic functions controlled by the hypothalamus and pituitary gland are as follows:
1. Control of blood flow (e.g., drinking, blood osmolarity, and renal clearance).
2. Regulation of energy metabolism (e.g., feeding, metabolic rate, and temperature).
3. Regulation of reproductive activity.
4. Coordination of response to threats.
5. Control of circadian rhythms.
The remarkable number of functions governed by these relatively small glands is possible due to the diversity of neuronal projections sent to the hypothalamus from the brain, as well as up the spinal cord. Additionally, the hypothalamus and pituitary gland have a multitude of intrinsic chemosensitive neurons that respond to circulating levels of various hormones. The pituitary gland, as well as some areas of the hypothalamus, is an area of the brain not protected by the blood–brain barrier. This allows for quicker feedback about the current status of target organs.
Of particular interest to behavioral neuroscientists are the direct afferent projections the hypothalamus receives from areas of the brain with well-known psychiatric functions. Four of these important projections are as follows:
1. Corticohypothalamic fibers: frontal cortex.
2. Hippocampohypothalamic fibers: hippocampus.
3. Amygdalohypothalamic fibers: amygdala.
4. Thalamohypothalamic fibers: thalamus.
These afferent fibers play a large role in the stimulation, or lack of stimulation, to the hypothalamus and the pituitary gland that results in many of the endocrine abnormalities found during different behavioral conditions.
Figure 7.4 shows the complex relationship among the cortex, hypothalamus, pituitary gland, and target organs for the neuroendocrine system. The figure illustrates the central location of the hypothalamus, the two sides of the pituitary, the variety of target organs affected, and the feedback mechanisms. Figure 7.5 shows most of the hormones associated with the anterior pituitary gland, particularly the releasing hormones excreted by the neuroendocrine cells of the hypothalamus, which stimulate the release of the tropic hormones.
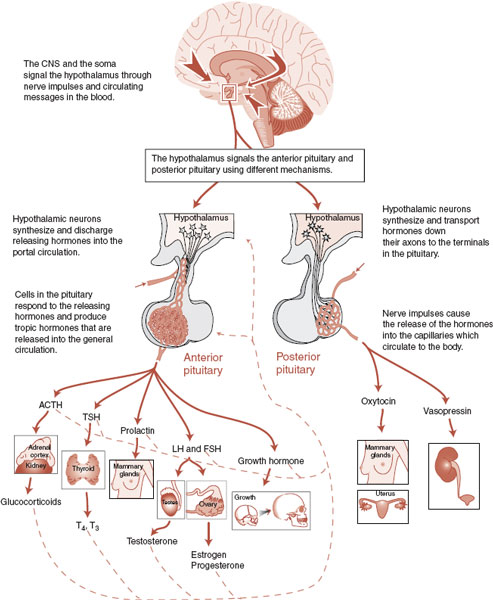
FIGURE 7.4  Information from the cerebral cortex and bloodstream converges on the hypothalamus, which instigates a cascade of actions and in turn responds to feedback from the target organs. CNS, central nervous system; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; T4, tetraiodothyronine; T3, triiodothyronine; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
Information from the cerebral cortex and bloodstream converges on the hypothalamus, which instigates a cascade of actions and in turn responds to feedback from the target organs. CNS, central nervous system; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; T4, tetraiodothyronine; T3, triiodothyronine; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
FIGURE 7.5  The anterior pituitary. Releasing hormones excreted by the hypothalamus stimulate the release of tropic hormones from the anterior pituitary. The tropic hormones then stimulate the target organ to change or release its own hormone. CRH, corticotropin-releasing hormone; TRH, thyrotropin-releasing hormone; PRH, prolactin-releasing hormone; GnRH, gonadotropin-releasing hormone; GHRH, growth hormone–releasing hormone; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; T4, tetraiodothyronine; T3, triiodothyronine.
The anterior pituitary. Releasing hormones excreted by the hypothalamus stimulate the release of tropic hormones from the anterior pituitary. The tropic hormones then stimulate the target organ to change or release its own hormone. CRH, corticotropin-releasing hormone; TRH, thyrotropin-releasing hormone; PRH, prolactin-releasing hormone; GnRH, gonadotropin-releasing hormone; GHRH, growth hormone–releasing hormone; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone; T4, tetraiodothyronine; T3, triiodothyronine.
While most of the hormones affect the brain in one way or another, we only focus on the two systems most relevant to mental illness: the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis.
THYROID: THE HYPOTHALAMIC- PITUITARY-THYROID AXIS
Thyroid hormones are involved in maintaining optimal metabolism in nearly every organ system and are integral to the temperature regulation of the body. The secretion of thyroid hormones is controlled by the HPT axis shown in Figure 7.6. The neuroendocrine cells in the hypothalamus secrete thyrotropin-releasing hormone (TRH) into the portal circulation of the pituitary. TRH binds with receptors on the thyrotroph cells of the anterior pituitary and stimulates the release of thyroidstimulating hormone (TSH). The hypothalamic
neuroendocrine cells also synthesize and release somatostatin, which inhibits the release of TSH (as well as growth hormone).
TSH stimulates the synthesis and release of two thyroid hormones from the thyroid gland: triiodothyronine (T3) and tetraiodothyronine (T4). T4 is the predominant form of thyroid released by the gland, but T3 is the more biologically potent form. T4 is converted into T3 by the target organs as well as the brain.
In humans, congenital hypothyroidism causes severe structural and functional neurologic abnormalities known as cretinism. This disorder is easily corrected, but a disaster if missed. It is one of the few medical conditions routinely screened for in the newborn nursery.
The role of thyroid hormones in the maintenance of the mature brain is less understood. The brain maintains tight control over the level of thyroid hormone in the central nervous system (CNS), and thyroid nuclear receptors are highly expressed throughout the brain, particularly within the hippocampus. Clinical features of hypothyroidism and hyperthyroidism, including significant neuropsychiatric symptoms, are described in Table 7.2.
Additionally, clinical studies have shown that the T3 hormone can augment other medications used to treat patients with treatment-resistant depression, as well as accelerate the response to antidepressants when initiating treatment for depression. However, meta-analysis of augmenting and accelerating studies found the trials to be small in number and not uniformly positive. The efficacy of liothyronine augmentation was compared with lithium augmentation as the third step for treatment-resistant depression in the STAR*D study. Both groups showed only modest remission rates (liothyronine 24.7% vs. lithium 15.9%) and were not statistically significantly different.
FIGURE 7.6  The hypothalamic-pituitary-thyroid axis showing the complex relationship between the brain and thyroid hormones. The important point is that tetraiodothyronine (T4) and triiodothyronine (T3) have direct effects on the brain as well as the body. CNS, central nervous system; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone.
The hypothalamic-pituitary-thyroid axis showing the complex relationship between the brain and thyroid hormones. The important point is that tetraiodothyronine (T4) and triiodothyronine (T3) have direct effects on the brain as well as the body. CNS, central nervous system; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone.
Thyroid hormones are important for the function of the adult brain, but the underlying molecular mechanism by which the HPT axis influences neuropsychiatric conditions remains unclear. Many authors in psychiatry postulate that the cognitive and emotional symptoms associated with thyroid disorders are related to changes in serotonin, norepinephrine (NE), and dopamine. Indeed, studies on rats have found increased serotonergic transmission concomitant with decreased 5-hydroxytryptamine (5-HT)1A sensitivity and increased
5-HT2A sensitivity with exogenous thyroid. With regard to NE, Gordan et al. have demonstrated anterograde transport of T3 from the cell bodies in the locus coeruleus to the nerve terminals in the hippocampus and cerebral cortex. They believe that T3 functions as a cotransmitter along with NE. This suggests that sufficient T3 is needed for proper NE activity.
POINT OF INTEREST
Thyroid hormones are the only substance produced by the body that contains iodine. Without enough iodine, the thyroid gland swells (stimulated by excessive TSH), producing goiter. The addition of small amounts of iodine to salt prevents the development of this diet-induced hypothyroidism.
An alternative explanation for the influence of thyroid hormones on the psychiatric status of the mature brain revolves around their role with nerve growth factors. Nerve growth factor genes are activated by T3 during development, although growth factors, such as brain-derived neurotropic factor (BDNF), are unaltered by thyroid hormone in the adult brain. Recently, Vaidya et al. established that 5-HT1A stimulation with chronic T3 administration altered the production of BDNF in the hippocampus, although neither did so alone. They postulate a synergistic relationship between 5-HT1A receptors and thyroid hormones in the expression of BDNF (see Chapter 21 for more on the role of BDNF in depression).
The most compelling explanation for the correlation between mood disorders and thyroid hormones appears to be related to the general effect that thyroid has on brain metabolism, much like the effect on peripheral metabolism. Positron emission tomography studies on patients with hypothyroidism show global reduction in brain activity and as much as a 23% reduction in cerebral blood flow compared with controls. In a remarkable study at the National Institutes of Mental Health, Marangell et al. examined TSH in medication-free patients with mood disorders, none of whom had overt thyroid disease. The study found an inverse relationship between TSH and global cerebral blood flow: TSH was up when cerebral blood flow was down. The areas with the greatest reduction in blood flow were the areas of the brain associated with depression: left dorsolateral prefrontal cortex (PFC) and medial PFC.
One can postulate that the hypothalamus responds to the reduced metabolism of the PFC (by way of the afferent fibers) as a result of the depression and reacts by stimulating the release of TSH. In essence, the HPT axis is seeking to correct the brain disorder.
MOOD DISORDERS
Although it is clear that thyroid disorders cause neuropsychiatric symptoms and that adding thyroid hormone can accelerate and augment the treatment of mood disorders, relatively few psychiatric patients have thyroid disease. Two analyses of clinical patients with depression found a 2% to 2.5% incidence of thyroid disease. The researchers concluded that routine screening for thyroid disease was not justified. Furthermore, although some patients with affective disorders can have mild laboratory changes, often referred to as subclinical thyroid disease, almost all anomalies resolve with effective treatment of the psychiatric disorder.
DISORDER
ANOREXIA NERVOSA
Women with anorexia nervosa, when purging and undernourished, often have symptoms that resemble hypothyroidism (cold intolerance, bradycardia, low resting metabolic rate, etc.). Thyroid studies of patients in this condition have found low normal T4, low T3, and normal TSH. Additionally, reverse T3, the metabolically inactive enantiomer of T3, is increased. This thyroid profile is called euthyroid sick syndrome. It can be produced by starvation in normal volunteers and is corrected with weight gain.
This decrease in active T3 thyroid profile seems like a physiologic adaptation to malnutrition, with the goal of preserving calories and limiting the expenditure of energy. Some patients with eating disorders will surreptitiously take exogenous thyroid to stimulate their metabolism and try to lose weight.
HYPOTHALAMIC-PITUITARY-ADRENAL AXIS AND STRESS
The HPA axis controls the synthesis and release of the corticosteroids. The corticosteroids are derived from dietary cholesterol in the adrenal cortex and include the mineralocorticoids, sex hormones, and glucocorticoids (Figure 7.7). The mineralocorticoid aldosterone assists in the maintenance of the proper ionic balance by stimulating the kidney to conserve sodium and excrete potassium. The sex hormones are secreted in negligible amounts, but have physiologic significance, and are covered in more detail in Chapter 16. The hormone of greatest interest to the mental health community is the glucocorticoid cortisol.
Cortisol is of interest because it mobilizes energy (by promoting catabolic activity) and increases cardiovascular tone. At the same time, cortisol suppresses anabolic activity, such as reproduction, growth, digestion, and immunity. The release of cortisol varies throughout the day, with maximal secretion in the early morning hours to effectively prepare the brain and body for the rigors of the day. Cortisol also plays a large part in acute and chronic stress, which we discuss subsequently.
The secretion of the adrenal cortex is controlled by the hypothalamus (Figure 7.8). The hypothalamus, with input from the cortex and feedback through the blood, synthesizes and releases corticotropin-releasing hormone (CRH) into the portal circulation of the pituitary, which in turn stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary. The input to the hypothalamus from the cortex includes inhibitory signals from the hippocampus and activating signals from the amygdala. In other words, a healthy hippocampus turns down the HPA axis, while an active amygdala turns it up. This is important in understanding the endocrine role in depression and anxiety.
POINT OF INTEREST
CRH is an example of a hormone that has multiple functions. The primary role of CRH is the stimulation of ACTH, but CRH receptors can be found throughout the brain, not just on the anterior pituitary, suggesting other, as yet unknown, effects for this hormone.
FIGURE 7.7  The corticosteroids are built from the sterol backbone of cholesterol by the addition or removal of side groups.
The corticosteroids are built from the sterol backbone of cholesterol by the addition or removal of side groups.
ACTH is released into the systemic circulation from the anterior pituitary. ACTH starts as a large propeptide precursor that is cleaved into smaller segments, some of which (such as ACTH) are biologically active (see Figure 16.13). ACTH stimulates the release of cortisol from the adrenal cortex. This has a variety of physiologic effects, including changes in the CNS. The high incidence of psychiatric symptoms in patients with primary endocrine disorders, such as Cushing’s disease and Addison’s disease, is supportive evidence of the direct effects of cortisol on the brain.
Stress
In his book Why Zebras Don’t Get Ulcers, Sapolsky uses a clever example to illustrate the difference between the acute physical stress that a zebra experiences when running from a lion and the chronic psychological stress that many people experience in modern industrial societies. The acute response is the biologic equivalent of mobilizing troops to handle a perceived threat. The sympathetic activation by the ANS and the liberation of cortisol bring the body and brain to an alert, fight-or-flight orientation. Cortisol has the effect of mobilizing energy, increasing cerebral glucose, and turning down the nonessential functions (i.e., erections and digestions). Ultimately, the brain becomes more focused and vigilant.
The body and brain pay a price for maintaining a heightened state of alertness when the stress persists and the person cannot adapt. As seen with Addison’s and Cushing’s diseases, too little or too much cortisol is pathologic. The benefits of cortisol can be graphed as an upside-down “U.” Moderation is best.
FIGURE 7.8  The hypothalamic-pituitary-adrenal axis. Note that adrenocorticotropic hormone (ACTH) releases an array of hormones with a diurnal variation. As with all hormones, there are direct effects on the cerebral cortex. CNS, central nervous system; CRH, corticotropin-releasing hormone.
The hypothalamic-pituitary-adrenal axis. Note that adrenocorticotropic hormone (ACTH) releases an array of hormones with a diurnal variation. As with all hormones, there are direct effects on the cerebral cortex. CNS, central nervous system; CRH, corticotropin-releasing hormone.
ADDISON’S AND CUSHING’S DISEASES
Addison’s disease, first described by Thomas Addison in 1855, results from a loss of cortisol and aldosterone secretion due to the near total or total destruction of both the adrenal glands. Classic symptoms include anorexia, nausea, and hypotension, along with neuropsychiatric symptoms of apathy, fatigue, irritability, and cognitive impairment.
Cushing’s disease is named after neurosurgeon Harvey Cushing, who in 1932 linked adrenal hyperplasia and hypercortisolemia with a pituitary adenoma. The somatic effects of excessive cortisol include easy bruising, truncal obesity, muscle atrophy, osteoporosis, and impaired immune response. The psychiatric symptoms are similar to endogenous depression: irritability, depressed mood, insomnia, and trouble with memory/concentration. Patients can also experience euphoria and even hypomania, as well as psychosis. However, these symptoms are more common in patients taking glucocorticoid medications. With both Addison’s and Cushing’s diseases, most psychiatric symptoms remit with the resumption of normal endocrine status.
Pathologic consequences of heightened sympathetic activity and HPA activation are hypertension, formation of atherosclerotic plaque, diabetes, ulcers, and impaired immune function. In the brain, the most dramatic negative effect involves the hippocampus, a structure with ample glucocorticoid receptors and afferent fibers to the hypothalamus. The hippocampus is well known for its role in memory, and excess glucocorticoids have indeed been shown to have the following effects:
1. Impair memory performance.
2. Disrupt long-term potentiation (LTP).
3. Induce atrophy of hippocampal dendrites.
4. Shrink the hippocampus.
5. Decrease neurogenesis (next chapter).
These cognitive changes do not develop without an active amygdala. The amygdala, well known for its role in anxiety, is stimulated by glucocorticoids, which in turn potentiates the hippocampus (see Figure 7.9). Chronic stress increases the dendritic arborization of neurons in the basolateral amygdala, which may be a reason people have trouble forgetting traumatic events.
Cushing’s Disease
Excessive glucocorticoid levels, for any reason, have been shown to cause impairments in declarative memory as well as hippocampal atrophy. In a remarkable analysis of patients with Cushing’s disease who underwent neurosurgical resection, Starkman et al. showed that decreasing urinary cortisol correlated with increase in the hippocampal volume. In a follow-up study, they showed that a greater improvement in memory (word list learning) was associated with greater increase in the hippocampal volume.
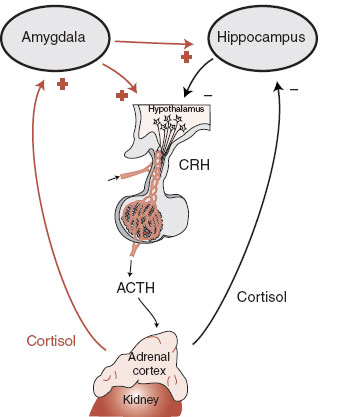
FIGURE 7.9  A schematic representation of the connections and feedback between the hypothalamic-pituitary-adrenal axis and the amygdala and hippocampus. CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone.
A schematic representation of the connections and feedback between the hypothalamic-pituitary-adrenal axis and the amygdala and hippocampus. CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone.
Aging
There is evidence that glucocorticoids may contribute to age-related neuronal atrophy and cognitive decline. An essential aspect of a healthy, adaptive stress response is the ability to shut off the system when the threat has passed. Studies on rats have shown that the HPA axis is slower to turn off as the animal ages. With age, it is exposed to more continuous high levels of glucocorticoids. In humans, the ability to turn off the HPA axis and the sympathetic nervous system in tranquil times is a variable trait. Some people are able to do this more effectively than others. Possibly this is a feature of being more resilient.
Lupien et al. followed up 51 healthy volunteers over 6 years and annually measured 24-hour plasma cortisol. They assessed memory and hippocampal volume in subgroups of those with high levels of cortisol and compared those findings with the groups with lower levels of cortisol. They found greater impairments in memory and a 14% reduction in the volume of the hippocampus in the group with high cortisol levels. This suggests that prolonged exposure to glucocorticoids either reduces the ability of neurons to resist insults or directly damages the neurons.
Depression and Anxiety
There is a long history of correlating alterations in the HPA axis with anxiety and depression. A few recent studies demonstrate the application of new research to this correlation. One research group in the Netherlands has analyzed glucocorticoid receptor number before military deployment and then assessed psychiatric symptoms upon return from combat. Glucocorticoid receptors were indirectly measured from the dexamethasone binding capacity of peripheral leukocytes. In separate studies they found greater numbers of glucocorticoid receptors, predeployment, in those individuals who developed depression and PTSD. With PTSD, they found that with an increase of every 1,000 glucocorticoid receptors, the risk of developing the disorder increased 7.5-fold.
Another group at Emory University analyzed polymorphisms of the CRH receptor, history of child abuse, and depression as an adult (Figure 7.10). A greater incidence of child abuse predicted higher adult depression scores. However, genetic differences of the CRH receptor influence the subsequent depressed mood. In this case, and there were other genetic changes with similar results, having the two base pairs AA at this location protected the abused person from developing depression. The CRH receptor, child abuse, depression connection is an example of the current understanding of mental illness. That is, disorders develop in genetically vulnerable people who are exposed to detrimental environmental events.
Although the HPA axis seems to play an important role in psychiatric disorders, attempts to alter the HPA axis, for example, CRH blockers, have not yet proven to be effective treatments.
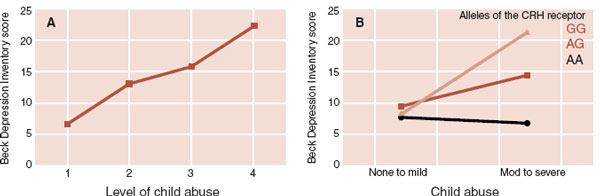
FIGURE 7.10  A. More incidence of abuse as a child led to increased depressive symptoms as an adult. B. Single nucleotide polymorphism of the corticotropin-releasing hormone (CRH) receptor alters the propensity of an abused individual to develop depression.
A. More incidence of abuse as a child led to increased depressive symptoms as an adult. B. Single nucleotide polymorphism of the corticotropin-releasing hormone (CRH) receptor alters the propensity of an abused individual to develop depression.
1. The primary mechanism by which the estrogen molecule stimulates the cell is?
a. Binding to a protein receptor imbedded in the cell wall.
b. Activating a G-protein-coupled receptor.
c. Binding with a receptor in the cytoplasm.
d. Coupling with a receptor in the nucleus.
2. Which of the following is true?
a. Thyroid hormone can enhance the GABA current.
b. Protein hormones bind a receptor on the DNA.
c. Hormones are used for fast signals.
d. Neuroendocrine cells excrete directly into the circulation.
3. Which of the following is correct?
a. GnRH → LH → estrogen.
b. GHRH → LH → testosterone.
c. TRH → TSH → prolactin.
d. CRH → TSH → cortisol.
4. Possible explanations for the role of thyroid hormone in mood disorders include all of the following except
a. Changes in brain metabolism.
b. Effects on growth hormones.
c. Changes in glucocorticoid receptors.
d. Effects on 5-HT receptors.
5. Women in the acute phase of anorexia nervosa exhibit a thyroid profile called “euthyroid sick syndrome,” which includes all of the following except
a. Low T3.
b. Low reverse T3.
c. Low to normal T4.
d. Normal TSH.
6. The triad of hippocampal atrophy, hypercortisolemia, and cognitive impairment has been found with all the following except
a. Cushing’s disease.
b. Addison’s disease.
c. Alzheimer’s disease.
d. Major depression.
7. All of the following go together except
a. Amygdala atrophy.
b. Hippocampal atrophy.
See Answers section at the end of the book.