SEXUAL DIMORPHISM
Humans are sexually dimorphic (di, “two”; morph, “type”). That is, we come in two styles. How one conceptualizes these differences depends on one’s perspective. Table 16.1 summarizes the major categories of sexual dimorphism. In this chapter, we focus on how the hormones change the morphology of the brain and how this affects the behavior and sexuality.
Pink and Blue
In general, men and women behave differently and enjoy different activities. The etiology of this difference remains a hotly debated topic. Is it nature or nurture—genetic or environmental? With humans, it is almost impossible to tease out these opposing causes. The signals a baby receives about its sexual identity start early—in the nursery. Typically, boys favor construction and transportation toys. Girls show less rough physical play and prefer toys such as dolls. Is this a product of learned gender social roles or something more innately wired in the brain?

A study with vervet monkeys suggests that the choices of toys children make to play with are more ingrained than some might think. Monkeys in large cages at the University of California, Los Angeles Primate Laboratory were allowed 5 minutes of exposure to individual toys classified as “masculine” (police car and ball) or “feminine” (doll and pot). The amount of time they were in direct contact with each of the toys was recorded. Figure 16.1 shows a male and female monkey playing with the toys and the percent time that each gender spent in contact with the toys. These results show that even nonhuman primates who are not exposed to social pressure regarding toy preference will choose gender-specific toys.
If we remember the important role of pleasure in determining behavioral preferences, we can speculate that the monkeys spend more time with the toys they enjoy. Likewise, we can speculate that the association between an object and the pleasure is hardwired in the brain. Furthermore, some of this “wiring” must have arisen early in human evolution before the emergence of our hominid ancestors.
The Boy Who Was Raised as a Girl
One of the more remarkable stories of sexually dimorphic behavior involves a tragic story of a boy raised as a girl. David was 8 months old in 1966 when his entire penis was accidentally burned beyond repair during a routine circumcision. Dr. John Money, a psychologist at Johns Hopkins Hospital with expertise in sexual reassignment, convinced the family to proceed with surgical sex change and raise the boy as a girl. Dr. Money believed that sexual identity/orientation developed after 18 months of age and children could adapt to a new sexual identity if the procedure was started early enough. David provided an ideal case study as he had an identical twin brother with a normal penis.
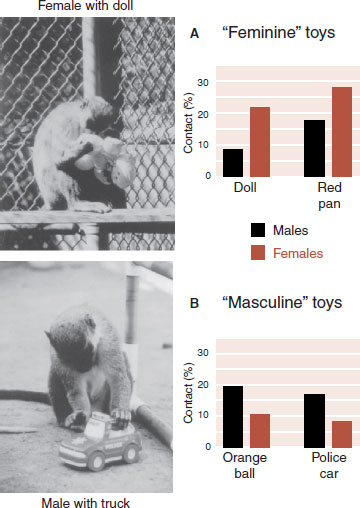
FIGURE 16.1  Male vervet monkeys spent more time in contact with “masculine” toys while females spent more time with “feminine” toys. (From Alexander GM, Hines M. Sex differences in response to children’s toys in nonhuman primates (Cercopithecus aethiops sabaeus). Evol Hum Behav. 2002;23:467-479.)
Male vervet monkeys spent more time in contact with “masculine” toys while females spent more time with “feminine” toys. (From Alexander GM, Hines M. Sex differences in response to children’s toys in nonhuman primates (Cercopithecus aethiops sabaeus). Evol Hum Behav. 2002;23:467-479.)
Amazingly, Dr. Money reported in the medical literature that the reassignment was a success, but it was in actuality a disaster. David, whose name was changed to Brenda, did not want to wear dresses, or play with dolls. She preferred to play with guns and cars. She could beat up her brother and threw a ball like a boy. Worst of all, this unusual behavior was not well received at school. She was relentlessly teased for her masculine traits. Brenda was shunned by the girls and not accepted by the boys.
By the time Brenda was 14 years old she was still unaware of the sexual reassignment and remained distressed. A local psychiatrist who was treating Brenda convinced the parents to reveal the truth. Brenda recalls her reaction, “Suddenly it all made sense why I felt the way I did. I wasn’t some sort of weirdo.”
David immediately decided to revert to his genetic sex. Within several months, he began going out in public as a boy. He stopped estrogen and started testosterone. He had bilateral mastectomies and several operations to rebuild male genitalia. He eventually married in his twenties although he could not have children. However, he battled with depression and the demons from this childhood experience. In May 2005, at the age of 38, he killed himself.
The significant point about this case is that in spite of being raised as a girl and in spite of the presence of estrogen hormones and the absence of testosterone, David continued to have male pattern psychosocial and psychosexual development. Larger case studies are consistent with David’s experience. One analysis of XY individuals assigned female roles at birth due to a severe pelvic defect (cloacal exstrophy) found that all showed masculine tendencies. Slightly more than half chose to declare themselves male when older. These studies suggest that something permanent happens in utero that determines sexual identity/orientation.
Environment
It would be naive to dismiss the significance of environment on sexually stereotypical behaviors. History is replete with examples of men and women showing varying amounts of masculine and feminine behavior that are clearly molded by shifts in social norms. Kim Wallen reviewed 30 years of research with rhesus monkeys and attempted to separate hormonal and social influences. For example, rough and tumble play is one of the most robust sexually dimorphic behaviors. Juvenile males wrestle more frequently than females in almost every rearing condition. However, if reared in a group with only males, they (males or females?) actually engage in less rough play. Likewise, mounting behavior is seen more with males than females. However, when reared in isolated, same-sex environments, males display less mounting while females display more such behavior.
Rust et al. looked at gender development in preschool children and the effect of an older sibling. They discovered that having an older brother was associated with greater masculine and less feminine behavior in boys and girls. However, boys with older sisters were more feminine but not less masculine, whereas girls with older sisters were less masculine but not more feminine.
Together, these studies suggest interplay between hormones and environment. That is, biologic factors predispose individuals to engage in specific behaviors, which can be modified by social experience.
The gonads (ovaries and testes) serve two major functions. First, they produce eggs or sperm to pass on DNA to the next generation. Second, they produce the sex hormones that not only promote the development of secondary sexual characteristics but also drive the behavior that increases the chances of an egg and sperm meeting.
HORMONES
Figure 14.8 shows the classic experiment by Berthold who was the first to establish that the testes contain a substance that controls the development of male secondary sexual characteristics. In Chapter 7, we discussed the relationship between the cortex, hypothalamus, pituitary gland, and end organ. Briefly, with input from the cortex, the hypothalamus produces gonadotropin-releasing hormone (GnRH), which in turn stimulates the anterior pituitary gland to produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH stimulate the gonads to produce the sex hormones.
Cholesterol is the precursor of all steroid hormones. Figure 7.7 shows the three major steroids synthesized from cholesterol: glucocorticoids, mineralocorticoids, and the sex steroids. The sex steroids are synthesized in the adrenals or gonads. Because the steroid hormones are lipid soluble and easily pass through the cell walls, they are released as they are synthesized.
Testosterone, as shown in Figure 16.2, is converted into 17-β-estradiol (E2) and an androgen, 5-α-dihydrotestosterone (DHT). (An androgen is a generic term for a hormone that stimulates or maintains male characteristics.) Amazingly, much of the androgen effects in the brain are actually implemented by 17-β-estradiol. For example, an injection of estrogen to a newborn rat is more masculinizing than an injection of testosterone.
Why do the maternal estrogens not masculinize all fetuses? One of the functions of α-fetoprotein during pregnancy is to bind with maternal estrogens, which are then cleared through the placenta. This protein, which does not bind testosterone effectively, prevents estrogens from reaching the brain.
We have discussed in other chapters how the sex steroids can work directly on synaptic receptors or indirectly through gene transcription (see Figures 5.3, 7.1, and 7.3). These different effects are summarized in Figure 16.3. The direct effects are fast, while the indirect effects take longer to transpire. Because the sex hormones can influence neural function, they are sometimes called neurosteroids in neuroscience literature.
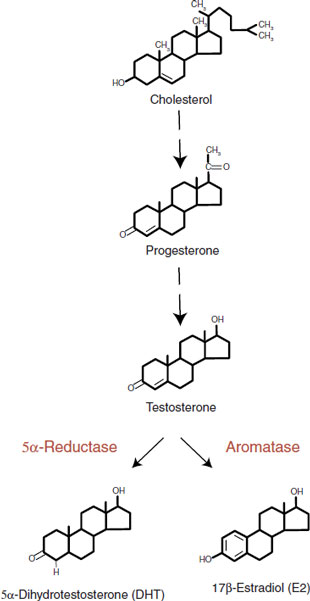
FIGURE 16.2  The sex hormones are synthesized from cholesterol. Testosterone serves as a prohormone for 17-β-estradiol.
The sex hormones are synthesized from cholesterol. Testosterone serves as a prohormone for 17-β-estradiol.
Differentiation and Activation
The development of sexual dimorphism is dependent on the sex hormones. The presence of testosterone at critical periods of time both masculinizes and defeminizes the brain. Likewise, the absence of testosterone feminizes and demasculinizes the brain. In 1959 William C. Young et al. published a classic paper that rivals Berthold’s work with roosters in helping us to understand the fundamental principles of hormones and behavior.
To understand Young’s study it is important to be aware of the different sexual postures males and females display at appropriate times. Female rodents will stand immobile and arch their backs: lordosis. Males will mount such a receptive female. Females and males rarely (although not completely) exhibit the opposite behavior. Researchers use the presence or absence of lordosis and mounting as expressions of sexual behavior. For example, castration of a male stops his mounting behavior. But this can be reinstated with injections of testosterone.
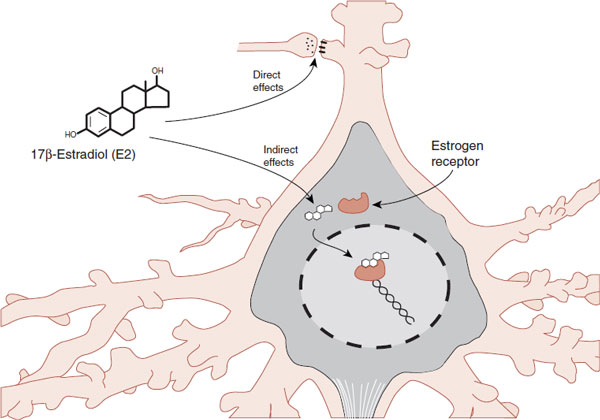
FIGURE 16.3  Steroids can directly affect the transmitter synthesis/release or postsynaptic transmitter receptors. They can also indirectly influence gene transcription. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Steroids can directly affect the transmitter synthesis/release or postsynaptic transmitter receptors. They can also indirectly influence gene transcription. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Young’s group sought to understand the effects of early and late exposure to sex hormones on sexual behavior. Their experiment, which is shown in Figure 16.4, started with injecting testosterone in a pregnant female guinea pig. Their first observation (not shown in the figure) was that female pups exposed to high doses of androgens in utero were born with masculinized external genitalia.
The rest of the study focused on the female pups, which were allowed to mature and were then spayed. Later, they were all given estrogen and progesterone to stimulate female sexual behavior. Each was paired with a normal male guinea pig. Sometime later, the procedure was reversed. All were injected with testosterone and paired with a receptive female. The results were striking. The females exposed to testosterone in utero failed to display lordosis when given estrogen and progesterone. However, they would mount other females when given testosterone. The control group displayed the opposite behavior.
This elegant experiment established a clear distinction between the differentiating effects of sex hormones during development and the activating effects during adulthood. The females exposed to testosterone in utero had alterations in the organization of their brains that prevented the normal activation by female sex hormones as an adult.
Human Congenital Anomalies
Occasionally, people are born with genetic alterations that give us insight into the differentiation and activation of human sexual dimorphism. One such condition is congenital adrenal hyperplasia. Children with this condition are exposed to excessive androgens due to overactive fetal adrenal glands. Paradoxically, the condition is caused by an impaired ability of the fetal adrenal gland to produce cortisol. Because the pituitary fails to receive the appropriate negative feedback, it continues to secrete adrenocorticotropic hormone (ACTH), which in turn induces hyperplasia of the androgen-producing cells of the adrenal cortex.
FIGURE 16.4  Guinea pigs exposed to testosterone in utero (A) fail to show feminine sexual behavior (B) when given female sex hormones and instead act like males (C) when given testosterone.
Guinea pigs exposed to testosterone in utero (A) fail to show feminine sexual behavior (B) when given female sex hormones and instead act like males (C) when given testosterone.
As we might predict from Young’s studies with guinea pigs, human males are unaffected by the exposure to excess adrenal androgens in utero. Females, on the other hand, are born with masculinized external genitalia. Additionally, the females tend to exhibit more rough and tumble play as children. As adults, they have an increased tendency to prefer other females as partners.
An extraordinary condition in men provides a different example of anomalous sexual development. Androgen insensitivity syndrome (AIS) is a condition in which XY (male) individuals are born as normal appearing females. The problem is caused by a mutation in the androgen receptor. These individuals produce testosterone, but the cells are unable to recognize it. Consequently, there is no activation of the genes necessary for male characteristics.
These individuals are born looking like normal little girls and are raised as such (Figure 16.5). Typically, the problem is only recognized when they fail to menstruate in adolescence. Unfortunately, they are unable to conceive as they have failed to develop uteri, fallopian tubes, and ovaries. However, their behavior is unequivocally feminine. Hines et al. examined the psychological development of 22 XY individuals with complete AIS compared with 22 XX normal controls and found no differences on any measure of psychological outcome. They concluded that these results argue against the need for ovaries and two X chromosomes in the development of traditional feminine behavior. Likewise, it reinforces the importance of the androgen receptor in masculine development. It is an interesting thought that all humans (both men and women) would develop into women unless other hormones intervene. The default model for mankind is a woman!
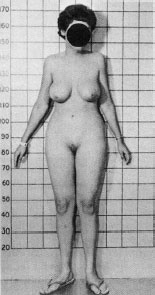
FIGURE 16.5  This person with complete androgen insensitivity syndrome has an XY genotype, but has developed unambiguous feminine characteristics. (Courtesy of John Money.)
This person with complete androgen insensitivity syndrome has an XY genotype, but has developed unambiguous feminine characteristics. (Courtesy of John Money.)
NEURONAL CIRCUITRY
Nerve Growth
Gonadal steroids grow more than just testes and breasts—they also cause selective neuronal growth. As shown in Figure 16.3, gonadal steroids stimulate gene expression through the androgen and estrogen receptors. These receptors, once bound with the hormone, also function as transcription factors, which, in turn, stimulate gene transcription and protein synthesis. Ultimately, the gonadal steroids can affect the nerve volume, dendritic length, spine density, and synaptic connectivity.
Woolley et al. demonstrated this effect by administering estradiol or placebo to ovariectomized rats and examining the structure and function of the hippocampal cells. Figure 16.6 shows two CA1 pyramidal cells and a closer examination of their dendritic spines. The estradiol-exposed neurons had 22% more spines and 30% more N-methyl-D-aspartate glutamate receptors than the controls. Furthermore, the treated neurons exhibited less electrical resistance to cellular input. So not only did the estrogen change the structure of the neuron but also the function.
TREATMENT
REVEALING THE DIAGNOSIS
In the 1950s, the standard practice was to withhold the actual diagnosis from individuals with AIS. They were told that childbearing was impossible, but not told they were genetically male. It was believed that such information would produce psychiatric disorders and possibly even thoughts of suicide. In the 1990s, the prevailing attitude shifted to full disclosure, and now it is the standard practice to reveal all the details to patients with this disorder. However, there remains a small cohort of women whose management was started in the era of less autonomy and who are still unaware of their diagnosis.
Many patients can sense when the truth is being withheld. In this age of the Internet, it is possible for curious patients to discover their own diagnosis. Some patients have avoided further medical care or even committed suicide when finally discovering their true condition. When confronted with such a patient, clinicians struggle with the appropriate manner and timing of sharing the diagnosis, particularly with a patient who has been kept in the dark for so long.
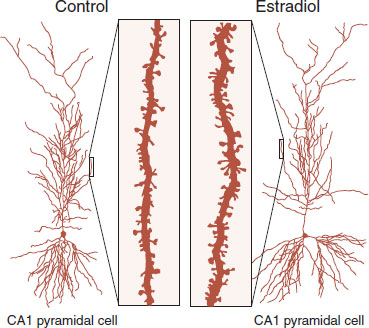
FIGURE 16.6  Ovariectomized rats treated with estradiol display greater spine formation on CA1 pyramidal cells from the hippocampus. (Adapted from Woolley CS, Weiland NG, McEwen BS, et al. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17(5):1848-1859.)
Ovariectomized rats treated with estradiol display greater spine formation on CA1 pyramidal cells from the hippocampus. (Adapted from Woolley CS, Weiland NG, McEwen BS, et al. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17(5):1848-1859.)
The astute reader might wonder about the role of growth factor proteins with sex hormones and nerve plasticity. Indeed, there is considerable evidence linking gonadal steroids with growth factors such as brain-derived neurotrophic factor (BDNF). However, it is unclear if the sex hormones stimulate the production of the growth factor protein, or work synergistically with them, or both. A study looking at the rat motor neuron sheds some light on this question.
The motor neurons projecting from the spinal cord to the skeletal muscles in rodents are generally similar for males and females. However, the male requires the additional innervation of the bulbocavernosus muscles around the penis, which are necessary for erections and copulation (Figure 16.7). Consequently, the motor neurons in the lumbar region of the spinal cord of the male rat (collectively called the spinal nucleus of the bulbocavernosus [SNB]) are approximately four times larger than in the female. These motor neurons regress in the female shortly after birth. Similar regression occurs with castrated males, although androgen treatment will preserve the motor neurons.
The dendrites of the motor neurons have extensive branchings that make connections spanning several spinal segments. Cutting the SNB motor neurons results in regression of the dendrites. Previous research has shown that testosterone or BDNF can limit the dendritic regression.
Yang et al. took this a step further. They cut the SNB motor neurons in castrated males. Then they put BDNF over the cut axons or administered testosterone, or both, in different groups of rats. A month later, the motor neurons were injected with a marker that allows visualization of the dendrites and axons after the animal is sacrificed. Figure 16.8 represents a computer-generated composite section marking the presence of the SNB motor neurons for the three groups of rats. The BDNF plus testosterone group preserved substantially more dendritic branching than seen with either alone (similar to what would be seen in a normal control). This suggests that BDNF and testosterone act synergistically to maintain the SNB motor neuron morphology.
Songbirds
The study of the sexually dimorphic brain structures of the songbirds is one of the great stories of neuroscience—one that transformed our recognition of the dynamic quality of the brain. The leader in this research is Fernando Nottebohm at the Rockefeller University in New York. He and others wanted to understand why male songbirds sing and females seldom do.
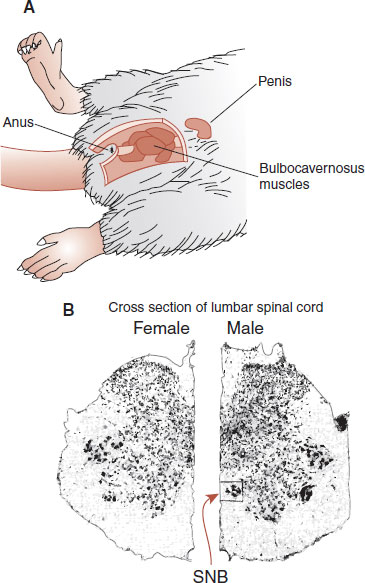
FIGURE 16.7  A. The male rat has bulbocavernosus muscles that are needed to control the penis for copulation. B. Cross sections of the female and male lumbar spinal cord show the presence of the spinal nucleus of the bulbocavernosus for the male, but not the female (A. Adapted from Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat SNB. J Neurosci. 1983;3(2):424-432.)
A. The male rat has bulbocavernosus muscles that are needed to control the penis for copulation. B. Cross sections of the female and male lumbar spinal cord show the presence of the spinal nucleus of the bulbocavernosus for the male, but not the female (A. Adapted from Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat SNB. J Neurosci. 1983;3(2):424-432.)
They initially looked at the syrinx (vocal cords) trying to find out the differences, but without success. Later, they focused on the neuronal mechanisms that control singing: the high vocal center (HVC), robust nucleus (RA), and area X. These nuclei send projections to the XII cranial nerve which controls the syrinx. Lesions of the HVC bilaterally will silence a bird.
The big discovery came when they realized that the song nuclei are approximately three times larger in males (Figure 16.9). This was the first discovery of sexual dimorphism in the brain. Furthermore, they showed that the size of the HVC correlates with the number of song syllables a male canary sings.
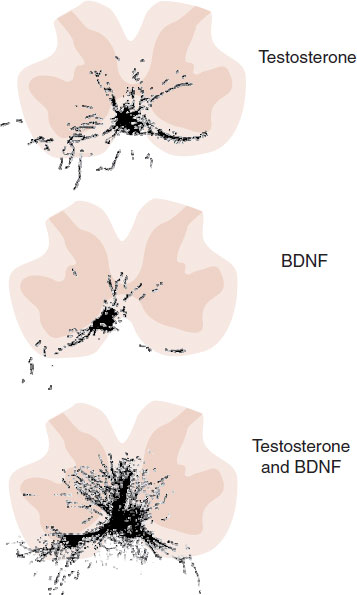
FIGURE 16.8  Composite lumbar cross sections showing the extent of spinal nucleus of the bulbocavernosus motor neurons that remain 1 month after surgical excision. Testosterone plus brain-derived neurotrophic factor preserved more of the motor neurons than either alone. (Adapted from Yang LY, Verhovshek T, Sengelaub DR. BDNF and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145(1):161-168.)
Composite lumbar cross sections showing the extent of spinal nucleus of the bulbocavernosus motor neurons that remain 1 month after surgical excision. Testosterone plus brain-derived neurotrophic factor preserved more of the motor neurons than either alone. (Adapted from Yang LY, Verhovshek T, Sengelaub DR. BDNF and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145(1):161-168.)
Their next discovery has fundamentally altered the way we think about the brain. Adult canaries change their songs every year, which is accomplished by adding new syllable types and discarding others. Remarkably, this occurs through the birth and death of neurons in the song nuclei. Nottebohm et al. were the first to show that working neurons develop in adult warm-blooded vertebrates—an idea that received a cool reception when first presented in 1984.
The reason for discussing this topic in this chapter is the fact that much of the differences in the male canaries’ nuclei and song production are controlled by the testosterone. Evidence to support this include the following:
1. The nuclei of the adult song system have high concentrations of testosterone.
2. Adult males with higher testosterone sing more than the adults with less testosterone.
3. Females given testosterone will sing more and show increased volume of their HVC and RA.
4. Drops in testosterone levels at the end of the breeding season correspond with the death of HVC neurons.
Hypothalamus
The hypothalamus is instrumental in regulating gonadotropin secretion. Specifically, the anterior aspect of the hypothalamus is known to control a wide variety of mating behaviors: desire, sexual behavior, and parenting. Lesions in this area can lead to alterations in sexual behavior. The preoptic area (POA) in the rat is an area where significant differences between the sexes are found (see Figure 2.8). In the male rat, the POA is five to seven times larger than in the female. The difference is so prominent that it can be accurately identified with the naked eye. This region of the POA is called the sexually dimorphic nucleus of the preoptic area (SDN-POA). Female rats given androgens will develop an SDN-POA approximately the size of that of a male. As with the SNB motor neurons, it appears that the androgens preserve the nerve cells, which otherwise waste away.
Humans
Allen et al. examined the anterior aspect of the hypothalamus in a postmortem analysis of 22 human brains: half of each sex. They focused their attention on an area that is the human equivalent of the rat SDN-POA. They identified four cell groupings within the anterior hypothalamus, which they called the interstitial nuclei of the anterior hypothalamus (INAH). They numbered the INAH from 1 to 4 and reported that INAH-2 and INAH-3 are approximately twice as large in males compared with females. Figure 16.10 shows the actual comparative micrographs through the INAH of males and females.
Sexual Orientation
Simon LeVay took this work one step further and compared the INAH for females, heterosexual males, and homosexual males. He confirmed the work by Allen et al., that is, two of the four interstitial nuclei are sexually dimorphic. However, even more interesting, he found that INAH-3 was twice as large in heterosexual men as it was in homosexual men. Although this provides compelling evidence that sexual orientation is “hardwired,” we must be cautious for there could be other explanations. For example, almost all of the homosexual men died of acquired immunodeficiency syndrome (AIDS), whereas only approximately one-third of the heterosexual men died of AIDS. Likewise, there was considerable overlap in the size of the nuclei between groups, implying that it is impossible to predict the sexual orientation of any individual based on the measurement of his INAH-3.
FIGURE 16.9  The difference in the song nuclei in canaries explains why males sing but females rarely do. HVC, high vocal center; RA, robust nucleus. (Adapted from Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628-658.)
The difference in the song nuclei in canaries explains why males sing but females rarely do. HVC, high vocal center; RA, robust nucleus. (Adapted from Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628-658.)
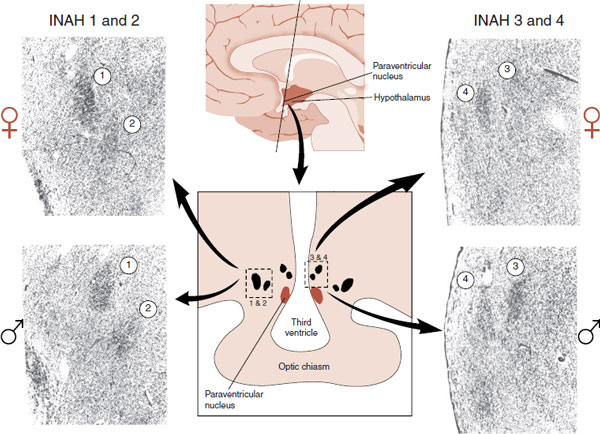
FIGURE 16.10  Representative micrographs showing the interstitial nuclei of the anterior hypothalamus for women (top) and men (bottom). Note that INAH numbers 2 and 3 are less distinct in women. (From Allen LS, Hines M, Shryne JE, et al. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9(2):497-506.)
Representative micrographs showing the interstitial nuclei of the anterior hypothalamus for women (top) and men (bottom). Note that INAH numbers 2 and 3 are less distinct in women. (From Allen LS, Hines M, Shryne JE, et al. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9(2):497-506.)
Other researchers have addressed this topic in different species. Approximately 8% of the domestic male sheep display sexual preference for other males. A group in Oregon identified a cluster of cells within the POA of the anterior hypothalamus (analogues to the INAH) that is significantly larger in rams than in ewes. They compared these nuclei for rams with different sexual preferences and found it was twice as large in heterosexual rams as it was in homosexual rams. Hence, an animal model that is consistent with the work by LeVay.
More recently, a group in Amsterdam (the capital of the sex trade industry) examined the INAH-3 nucleus from the brains of individuals with and without gonadotropin hormones compared with male-to-female transsexual individuals with completed sex reassignment surgery. The volume of the INAH-3 nucleus was larger in the males compared with females and transsexual subjects. Furthermore, they counted the neurons in the nucleus for each group and found a similar relationship as shown in Figure 16.11.
Taken together, the above studies suggest a possible explanation for the continuum of human sexual behavior. Small differences in nuclei in the anterior hypothalamus produce significant differences in sexual identity and behavior. Early exposure to sexual hormones during critical periods or other environmental factors or genetics may alter the development of nuclei that steer one’s sexual orientation and preferences.
PSYCHIATRIC DISORDERS
Cognitive Decline
There is considerable evidence that estrogens (and presumably testosterone) are neuroprotective. Figure 16.6 shows the robust increase in spine formation that can be induced by estrogen in hippocampal neurons. Presumably, such an arborization of the neurons enhances neural connections. Research with rodents and nonhuman primates demonstrates beneficial effects of estrogen on cognition. Multiple observational studies in humans have found that hormone replacement protects against the development of Alzheimer’s disease, although there are other risks of continued hormone replacement.
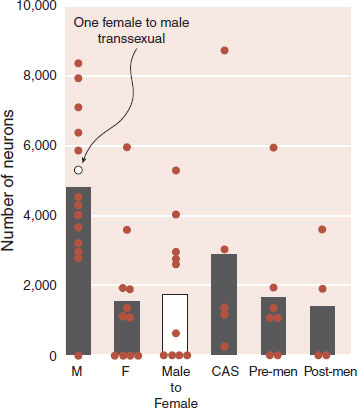
FIGURE 16.11  Postmortem analysis of the number of neurons in the INAH-3 nucleus for males (M), females (F), male-to-female transsexual, and castrated males due to prostate cancer (CAS). All the women are separated by menopausal status in the last two bars. One female to male transsexual is included with the males. (Adapted from Garcia-Falgueras A, Swaab DF. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain. 2008;131:3132-3146.)
Postmortem analysis of the number of neurons in the INAH-3 nucleus for males (M), females (F), male-to-female transsexual, and castrated males due to prostate cancer (CAS). All the women are separated by menopausal status in the last two bars. One female to male transsexual is included with the males. (Adapted from Garcia-Falgueras A, Swaab DF. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain. 2008;131:3132-3146.)
Figure 16.12 is an example of one such observational study with humans. This was a study of more than 3,000 elderly people from one county in Utah. The objective was to test for the development of Alzheimer’s disease and see if a history of hormone replacement therapy (HRT) was protective. Note that the men (who presumably maintain adequate levels of sex hormones) fare better than the women in terms of developing Alzheimer’s disease. Likewise, the women who took HRT displayed a dose–response effect. That is, the longer a woman took HRT the less likely she was to develop Alzheimer’s disease.
TREATMENT
HOMOSEXUALITY
A few mental health professionals across the country offer treatment for individuals who want to be heterosexual rather than homosexual. Hardly any studies exist that establish the effectiveness of such treatment. However, Robert Spitzer has published a survey of 200 individuals who claim to have changed their sexual orientation. Many reported changing from predominantly or exclusively homosexual to predominantly heterosexual. Few reported complete changes. Is it possible that some motivated individuals were able to change their brain or were they not actually homosexual at the start? Only prospective studies will answer this controversial question.
FIGURE 16.12  A prediction of the incidence of Alzheimer’s disease calculated from data collected about men and women over 3 years shows the beneficial effects of sex hormones. HRT, hormone replacement therapy. (Adapted from Zandi PP, Carlson MC, Plassman BL, et al. HRT and incidence of Alzheimer’s disease in older women: the Cache County Study. JAMA. 2002;288(17):2123-2129.)
A prediction of the incidence of Alzheimer’s disease calculated from data collected about men and women over 3 years shows the beneficial effects of sex hormones. HRT, hormone replacement therapy. (Adapted from Zandi PP, Carlson MC, Plassman BL, et al. HRT and incidence of Alzheimer’s disease in older women: the Cache County Study. JAMA. 2002;288(17):2123-2129.)
Clinical trials of HRT and cognition have not been as uniformly positive as we would hope. A review of the studies suggests that unopposed estrogen improves the verbal memory in women younger than 65 years. For older women, the results are neutral. Janicki and Schupf, in a recent review of hormonal influences on cognition and Alzheimer’s progression, concluded that “overall data from epidemiologic studies, observational studies and clinical trials of HRT, studies of endogenous hormones, and evaluation of genetic variants involved in estrogen biosynthesis and receptor activity indicate that estrogen plays an important role in the pathogenesis of cognitive decline and risk for Alzheimer’s Disease in both men and women.” So while estrogen may not be the panacea for preventing Alzheimer’s disease, it does appear to have some neuroprotective benefits.
Sexual Dysfunction
A survey of adults between the ages of 18 and 59 in the United States found a high prevalence of sexual dysfunction: 31% for men and 43% for women. The most common complaint for men was premature ejaculation, whereas for women it was lack of interest. There are pharmacologic interventions available for premature ejaculation. For example, the selective serotonin reuptake inhibitors make it harder to have an orgasm, although they are not U.S. Food and Drug Administration (FDA) approved for this indication.
POINT OF INTEREST
Does pregnancy change the maternal brain? Studies with rodents have shown that the hormones of pregnancy trigger changes in the POA and hippocampus of the maternal brain. These changes may explain why mother rats perform better on tests of memory (navigating a maze) than virgin rats of similar age. Has nature developed a mechanism to make mothers smarter caregivers?
Lack of interest in sex, on the other hand, is difficult to treat. Certainly, the qualities of the relationship, secondary medical conditions, and the presence of other psychiatric disorders have strong influences on the joy of sex. However, some women find sex lacking. As many as one in three women never or infrequently achieve orgasm during intercourse—the number is still only one in five with masturbation. Studies with twins show a genetic component to orgasmic ability. Estimated heritability for difficulty achieving orgasm during intercourse is 34%—on par with anxiety and depressive disorders (see Table 6.1).
Dopaminergic mechanisms may play a large role in modulating sexual drive and orgasmic quality. As discussed in Chapter 12, the dopamine pathways to the nucleus accumbens are active in motivated behavior. Dopamine agonists such as the stimulant medications and cocaine are anecdotally reported to enhance human sexual behavior. More recently, genetic studies have shown that different genotypes of the dopamine D4 receptor correlate more or less closely with sexual desire and arousal. This suggests that biology as well as environmental signals (in close proximity to Mr or Miss Right) interact to determine the sexual experience.
The prospect of giving women an approved medication to enhance sexual desire, although of interest to the pharmaceutical industry and feared by domineering fathers everywhere, is currently not an option. The phosphodiesterase-5 inhibitors (e.g., Viagra) have been tested in large trials for female sexual dysfunction and failed to enhance the interest any more than placebo. The testosterone patch, although effective for some patients, failed to win the FDA approval owing to safety concerns.
Recent research suggests that α-melanocyte–stimulating hormone (α-MSH) may be, what some call, a genuine aphrodisiac. We mentioned in Chapter 11 that a large precursor neuropeptide in the pituitary gland, proopiomelanocortin (POMC), is cleaved to form active neuropeptides, which include ACTH, β-endorphin, and α-MSH (see Figure 16.13). α-MSH is the peptide that also causes the skin to darken in patients with Addison’s disease and also suppresses appetite (see Chapter 13). The story is told that a company was testing a melanocortin product as a possible tanning agent that did not require sun exposure. During early testing the medication triggered erections in most of the men. Eureka! Subsequently, a peptide analog of α-MSH called bremelanotide (a melanocortin receptor agonist) was developed for further study.
Research with animals has shown that bremelanotide enhances female sexual solicitation in rats. The female rats became overtly flirtatious in a rodent sort of way—even climbing through little holes in the walls to get to the males. A study with married women in Iran (of all places) using an intranasal spray to administer the medication reported greater intercourse satisfaction in those receiving the active medication. Unfortunately, testing has been halted due to increased blood pressure in a few subjects. The company is considering subcutaneous administration as a means to eliminate the hypertensive adverse effect.
It is not clear why the pituitary neuropeptide α-MSH enhances sexual interest. Studies in which the medication was injected directly into a female rat’s lateral ventricles found increased solicitations, establishing that its effects are mediated centrally. Additionally, α-MSH activated the Fos proteins (markers of gene expression) in the POA of the hypothalamus as well as the nucleus accumbens—areas that would be consistent with sexual pleasure.
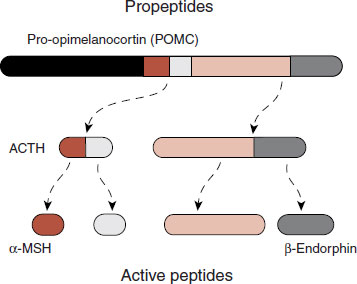
FIGURE 16.13  The propeptide POMC is produced in the pituitary gland, where it is cleaved to smaller active peptides such as ACTH, α-MSH, and β-endorphin.
The propeptide POMC is produced in the pituitary gland, where it is cleaved to smaller active peptides such as ACTH, α-MSH, and β-endorphin.
Mood Disorders
The lifetime prevalence of mood disorders in women is approximately twice that of men. Although the cause of this difference remains undetermined, one possible explanation is the sex hormones—or more specifically, the fluctuation in sex hormones. Figure 16.14 shows the alterations in estrogen levels for a hypothetical woman across her life span. The times of greatest risk for mood disturbances are during times of fluctuating estrogen levels: menarche, premenstrual syndrome (PMS), postpartum, and perimenopausal.
The correlation between dropping sex hormones and depressive symptoms is not limited to women. The difference is the fluctuations. Men typically have stable testosterone levels. However, when testosterone drops, psychiatric symptoms become more prevalent. In one veterans administration (VA) study, the researchers followed up the testosterone level and emergence of depressive illness in men older than 45 for 2 years. They found that 22% of the hypogonadal men (total testosterone <200 ng/dL) experienced depression, whereas only 7% of the eugonadal men did. Another group looked at the emergence of psychiatric symptoms in men treated for prostate cancer with GnRH agonists (see sidebar), which causes testosterone to plummet. They reported significant increases in anxiety and depression while the testosterone level was low.
TREATMENT
GnRH AGONIST
Clinicians can override the pulsatile nature of a releasing hormone to effectively shut down the production of the sex hormones and treat problems such as endometriosis, prostate cancer, and early-onset puberty.
This treatment has also been used with sex offenders as a form of “chemical castration.” In this process, a long-acting GnRH agonist, leuprolide (Lupron Depot), inhibits the release of LH and FSH, although it stimulates the receptor. This effect is achieved because the continuous stimulation—in contrast to the usual intermittent stimulation—causes the desensitization of receptors in the pituitary. As a result, LH and FSH are not produced, which subsequently reduces the production of sex hormones.
FIGURE 16.14  Estrogen levels fluctuate for women from puberty to menopause. The times of greatest vulnerability for depression occur when estrogen levels are changing. (Adapted from Stahl SM. Essential Psychopharmacology. Neuroscientific Basis of Practical Applications. 2nd ed. New York, NY: Cambridge University Press; 2000.)
Estrogen levels fluctuate for women from puberty to menopause. The times of greatest vulnerability for depression occur when estrogen levels are changing. (Adapted from Stahl SM. Essential Psychopharmacology. Neuroscientific Basis of Practical Applications. 2nd ed. New York, NY: Cambridge University Press; 2000.)
Some of the most convincing data regarding the effects of sex hormones on mood have been treatment studies. With both men and women, positive results have been shown for improving mood when sex hormones were administered. Figure 16.15 shows two representative studies. These studies have many differences, but they both reveal less depression with sex hormones.
The study on the left is with perimenopausal women who have mild or moderate depressive symptoms. One group was given placebo and the other estradiol. No antidepressants were used. The graph on the right was a study with men. All the men had failed antidepressant therapy and had low or borderline testosterone levels. Everyone remained on his antidepressant while half the group also received supplemental testosterone gel.
These are both small studies and need to be repeated with larger numbers. Likewise, it is important to remember that hormone replacement is not without significant risks. However, the studies demonstrate the powerful effects sex hormones can have on mood.
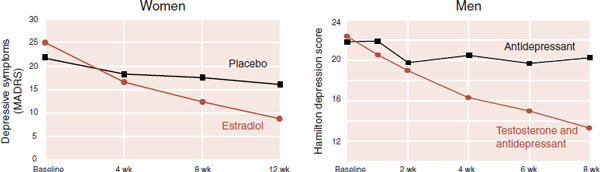
FIGURE 16.15  Two studies highlighting the mood-altering effects of sex hormones for women and men with sluggish gonads. (Left: Adapted from Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529-534. Right: Adapted from Pope HG Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160(1):105-111.)
Two studies highlighting the mood-altering effects of sex hormones for women and men with sluggish gonads. (Left: Adapted from Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529-534. Right: Adapted from Pope HG Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160(1):105-111.)
HOT FLASHES AND ANTIDEPRESSANTS
Some women want treatment for the hot flashes associated with menopause, but do not want HRT. The newer antidepressants have been shown to have some benefit. In the best study, the researchers felt that venlafaxine was clearly superior to placebo, but not as effective as estrogen. Their study stands as further evidence that mood and gonadal steroids are linked in ways that we are only beginning to understand.
Traumatic Brain Injury
Traumatic brain injury (TBI) is increasingly recognized as more than just short-term confusion. Soldiers returning from the current wars and football players are reporting enduring problems from the cortical bruising and microscopic tears the brain sustains when it is rattled around inside the hard skull. Memory, cognition, and impulse control in many cases never return to baseline. Although there is currently no medical treatment for TBI, progesterone, often thought of as the “pregnancy hormone,” is generating considerable interest. Studies with animals and a few humans suggest that progesterone, administered shortly after the head injury, may have nurturing properties that enable the brain to repair more effectively. A large phase III trial called ProTECT III is underway in the United States with an expected completion date in the middle of 2015.
Pregnancy and Depression
One can get the impression that sex hormones keep people happy. However, it is not that simple. For example, pregnancy, a time of very high estrogen and progesterone levels, has been conceptualized as a period of emotional well-being—even protective against psychiatric disorders. However, a recent study was conducted on women with a history of depression who became pregnant. Some women continued their antidepressants while others stopped them during the pregnancy. The relapse rates for depression during the pregnancy were 26% for those still on their medication and 68% for those who discontinued their antidepressants.
Clearly, pregnancy is not protective against depression for women with a history of depression. Some think the high levels of progesterone during pregnancy may have negative effects on mood. Others speculated about the negative effects of low BDNF during pregnancy. More research is needed to understand this paradoxical finding.
Summary
The sex hormones are not just for fostering the procreation of the species. One focus of this chapter has been on the nourishing capacity the neurosteroids have on the brain cells and subsequently on behavior and emotions. European physicians are more familiar with this body of research and are more comfortable utilizing small doses of hormone replacement to optimize the treatment of mental problems—a perspective we may embrace as the gonads of the baby boomers languish.
QUESTIONS
1. Female gonadal steroids include all of the following, except
a. Testosterone.
b. Estradiol.
c. Progesterone.
d. GnRH.
2. Many of the androgen effects in the brain are triggered by
a. 17-β-Estradiol.
b. 5-α-DHT.
c. Aromatase.
d. α-Fetoprotein.
3. Which rat will display lordosis when primed with estrogens and progesterones and paired with a sexually active male?
a. Males of normal pregnancies.
b. Males exposed to androgens in utero.
c. Females of normal pregnancies.
d. Females exposed to androgens in utero.
4. Known effects of female sex hormones include all of the following, except
a. Increased spine formation.
b. Decreased risk of stroke.
c. Increase γ-aminobutyric acid inhibition.
d. Gene transcription.
5. Evidence of sexual dimorphism in the vertebrate central nervous system include all of the following, except
a. SNB.
b. The robust nucleus.
c. SDN-POA.
d. Paraventricular nucleus.
6. As suggested by some research, which area is smaller in homosexual men compared with heterosexual men?
a. INAH-1.
b. INAH-2.
c. INAH-3.
d. INAH-4.
7. The propeptide POMC is cleaved into all the following active peptides, except
a. Δ-Fos.
b. ACTH.
c. α-MSH.
d. β-Endorphin.
8. Evidence of the importance of sex hormones and mood includes all of the following except
a. PMS.
b. Athletes on anabolic steroids.
c. Chemical castration for prostate cancer.
d. Randomized controlled trials.
See Answers section at the end of the book.