Pain and pleasure are the major driving forces of human behavior. We cover pain in this chapter but are ever so looking forward to discussing pleasure in the next chapter.
ACUTE PAIN
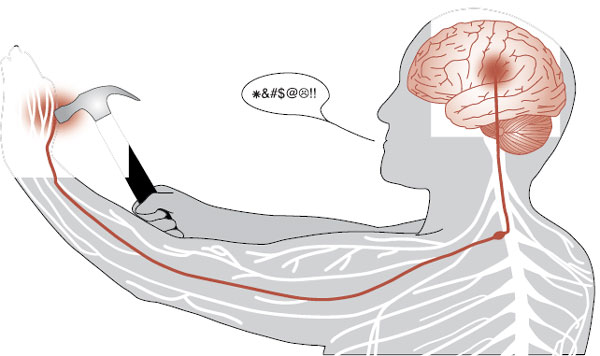
Acute pain, as we all have experienced, starts in the periphery, is relayed to the spinal cord, and then passes up to the brain where it produces a negative reaction (Figure 11.1). Pain-producing stimuli are detected by specialized afferent neurons called nociceptors. The nociceptors are free nerve endings—not an identifiable structure as we have for touch and vibration. These cells respond to a broad range of physical and chemical stimuli, but only at intensities that are capable of causing damage.
Peripheral Tissue
The nociceptors in peripheral tissue are activated by injury or tissue damage that results in the release of bradykinins, prostaglandins, and potassium (Figure 11.2). These molecules in turn cause the secretion of substance P from other branches of the axon, which stimulates the release of histamine and promotes vasodilation.
Aδ and C fibers send the signal to the dorsal horn of the spinal cord by way of the dorsal root ganglion. The Aδ fibers are myelinated axons that quickly send the first, sharp signals of pain. The C fibers are unmyelinated and send a slower, dull pain signal (Figure 11.3). It is the duller, slow pain signal of the C fibers that becomes so troublesome in chronic pain conditions (see box).
Spinal Cord
The nociceptive afferent nerve fibers synapse in the dorsal horn of the spinal cord. Information about tissue injury is passed on to the next neurons, which then cross to the contralateral side and ascend to the brain.
The signal can be modified at this point by descending fibers (discussed later) or from simultaneous activity by nonpain neurons (mechanoreceptors: Aβ fibers). The Aβ fibers can dampen the pain signal in what is called the gate theory of pain. Figure 11.4 shows how a signal from the larger mechanoreceptor activates an inhibitory interneuron in the dorsal horn, which results in a smaller signal conveyed to the brain. The gate theory explains why rubbing an injury seems to reduce the pain and is the rationale for the use of transcutaneous electrical nerve stimulation.
Ascending Pathways
There are a variety of ways to describe the different nociceptive pathways that ascend to the brain in the spinal cord. Unfortunately, there is no consensus on the proper nomenclature for these tracts and no two authors seem to use the same terms. Recently, the perception of pain—and the areas participating, in the central nervous system (CNS)—has been divided into two prominent domains: sensory-discriminative and affective-motivational. This dichotomy (summarized in Table 11.1) is a convenient way to understand the ascending pathways.
Sensory-Discriminative
The sensory-discriminative domain encompasses the traditional sensory pathway taught in the first year of medical school. The signal travels up the spinothalamic tract, synapses in the lateral thalamus, and proceeds to the somatosensory cortex. Figure 11.5 is a diagram of this pathway. This type of pain allows the subject to become aware of the location of the pain and answer the question, “where does it hurt?” However, the perception of pain is much more than just identifying the location of a noxious sensation and withdrawing the injured limb.
FIGURE 11.1  The somatosensory cortex receives the negative sensation of pain from the peripheral nerves by way of the spinal cord.
The somatosensory cortex receives the negative sensation of pain from the peripheral nerves by way of the spinal cord.
Affective-Motivational
Other ascending sensory signals communicate the intensity of a noxious stimuli. There are several tracts that transport these signals, such as the spinoreticular tract or the spinomesencephalic tract—just to name a few. The important point is that all the signals travel in the anterolateral region of the spinal cord and terminate in different locations such as the reticular formation, periaqueductal gray (PAG) matter, and the amygdala. The rest of the signals synapse in the medial thalamus before proceeding to other areas of the cerebral cortex (Figure 11.6).
The affective-motivational signals communicate the unpleasantness of the sensation and answer the question, “how much does it hurt?” In the cortex, these signals activate areas associated with emotional feelings, such as the anterior cingulate cortex (ACC), insular cortex, and prefrontal cortex—as well as the amygdala. Functional brain imaging studies over the past two decades have documented activity in these areas during pain perception—areas we typically associated with mood, attention, and fear. Activity in these regions of the brain helps us understand the concomitant depression, hyperfocus, and anxiety we see with patients in pain.
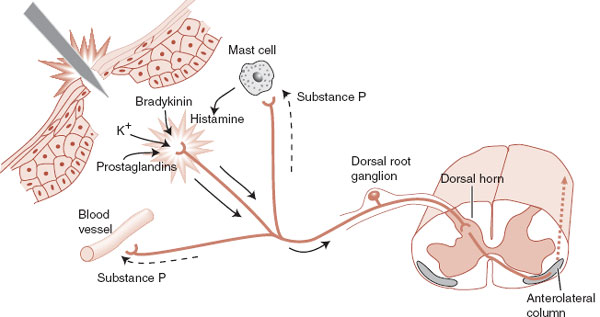
FIGURE 11.2  Peripheral nociceptive responses to acute trauma. (Adapted from Kandel ER, Schwartz JH, Jessell TM, eds. Principles of Neural Science. 4th ed. New York: McGraw-Hill; 2000.)
Peripheral nociceptive responses to acute trauma. (Adapted from Kandel ER, Schwartz JH, Jessell TM, eds. Principles of Neural Science. 4th ed. New York: McGraw-Hill; 2000.)
FIGURE 11.3  Aδ and C fibers transmit pain signals at different rates. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
Aδ and C fibers transmit pain signals at different rates. (Adapted from Bear MF, Connors BW, Paradiso MA, eds. Neuroscience: Exploring the Brain. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2007.)
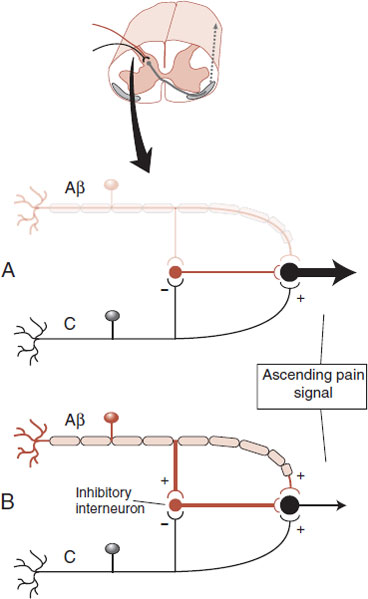
FIGURE 11.4  Gate theory of pain in the dorsal horn of the spinal cord. A. Without input from Aβ fibers a large signal is transmitted to the brain. B. A smaller signal is transmitted with input from Aβ fibers.
Gate theory of pain in the dorsal horn of the spinal cord. A. Without input from Aβ fibers a large signal is transmitted to the brain. B. A smaller signal is transmitted with input from Aβ fibers.
Figure 11.7 shows drawings of some of the areas that become active with acute pain. In this study, subjects were scanned at rest and later with a hot probe (approximately 50°C) applied to the upper right arm. Note the diverse areas that become active with acute pain—thalamus, ACC, prefrontal cortex, and insula, as well as others. The experience of pain is more than just identifying where it hurts.
It is also important to note that some of the activation is on both sides of the brain. This shows that there is more to the processing of pain signals in the CNS than implied by our simplified Figures 11.5 and 11.6.

NEUTRALIZING C FIBERS
Over 20% of American adults struggle with chronic pain. The pharmaceutical industry is actively searching for an effective analgesic devoid of narcotic side effects or addictive potential. The transient receptor potential vanilloid 1 (TRPV1) receptor is one potential target. Found primarily on small diameter sensory neurons such as C fiber neurons, the TRPV1 receptor responds to a variety of noxious chemical and physical stimuli—including capsaicin, the active component of chili peppers. Animal studies suggest that blocking this receptor reduces the aching quality of chronic pain while preserving the sensations of touch and proprioception. The National Institutes of Health and several pharmaceutical giants are studying the effect of blocking the TRPV1 receptor in humans.
One concern regarding TRPV1 is the role the sensory neurons play in modulating inflammation. Activation of the TRPV1 receptor leads to the release of pro-inflammatory cytokines. Not surprisingly, animal studies have shown that TRPV1 disruption can impair bacterial clearance, cytokine response, and ultimately mortality from sepsis—another reminder that pain and protection are intimately connected.
CONGENITAL INSENSITIVITY TO PAIN
Congenital insensitivity to pain, a term used to describe rare genetic conditions in which people lack the ability to sense pain, initially sounds like a blessing but is actually a nightmare. Individuals with this condition fail to identify or respond to noxious, injuring stimuli and suffer excessive burns, fractures, and soft tissue damage. Ultimately, the unrecognized injuries and secondary complications lead to an early death.
The spectrum of congenital insensitivity to pain provides an example of the distinction between sensory and affective components of pain. Subjects with frank congenital insensitivity to pain are without the peripheral Aδ and C fibers. These patients lack the sensory-discriminative as well as affective components of pain and are the most at risk for harm and premature death.
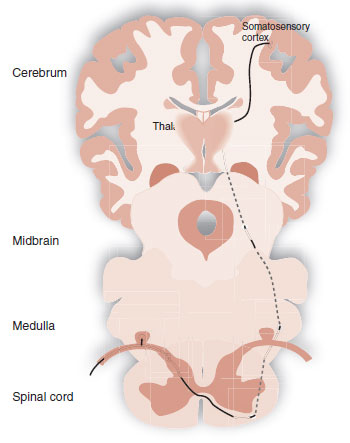
FIGURE 11.5  Spinothalamic tract transmitting the sensory-discriminative pain signal from the periphery to the somatosensory cortex.
Spinothalamic tract transmitting the sensory-discriminative pain signal from the periphery to the somatosensory cortex.
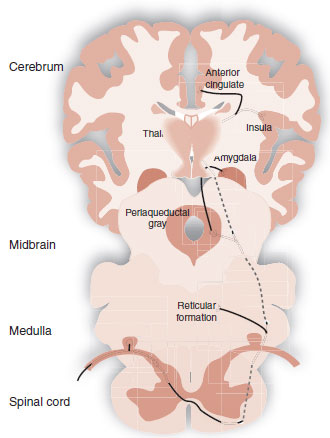
FIGURE 11.6  Affective-motivational pain tracts.
Affective-motivational pain tracts.
FIGURE 11.7  PET scans showing activity in the brain with acute pain. ACC, anterior cingulate cortex; Thal, thalamus; Cb, cerebellum; Ins, insula; PMv, ventral premotor cortex. (Adapted from Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100(14):8538-8542.)
PET scans showing activity in the brain with acute pain. ACC, anterior cingulate cortex; Thal, thalamus; Cb, cerebellum; Ins, insula; PMv, ventral premotor cortex. (Adapted from Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100(14):8538-8542.)
A milder condition, termed congenital indifference to pain, is found in individuals who can distinguish sharp and dull pain, but are indifferent to the sensation. They lack the emotional responses and normal withdrawal movements; they can feel the pain, but are not concerned. Subjects with this disorder have normal peripheral nerve fibers but seem to have an as yet unidentified central impairment of the affective-motivational component of pain.
Congenital insensitivity to pain with anhidrosis is a specific rare autosomal recessive disorder characterized by absence of pain (along with inability to sweat, unexplained episodes of fever, and mental retardation). Patients with this condition have a mutation in the gene for the Trk receptor—the receptor that binds with nerve growth factor. As we saw in Chapter 8, nerves need nerve growth factor proteins to survive. Cells lacking the receptor are unable to incorporate the growth factor and wither away (or fail to develop). The patient lacks pain fibers and therefore does not experience pain.
Pain Tolerance Spectrum
People have different abilities to tolerate pain. This has been shown in multiple psychological studies and observed by most of us in our clinical population. More recently, brain imaging studies have documented that subjects with less pain tolerance have greater activation of the cortical areas discussed earlier (ACC, insular cortex, and prefrontal cortex, along with the somatosensory cortex).
Genetic factors undoubtedly play a role in the spectrum of pain tolerance. A study reported in 2005 examined pain tolerance in 202 women and genetic variants for the gene encoding for catecholamine-O-methyltransferase (COMT), an enzyme involved in the regulation of catecholamines and enkephalins. After initially measuring pain tolerance with a noxious thermal stimuli, the researchers assessed the genetic makeup of each participant. They found that three genetic variants for COMT accounted for 11% of the variation in pain perception (a large percentage in genetic studies). Figure 11.8 shows how five different combinations of these genetic variants accounted for differing pain responsiveness. Further recent studies by the same research group examined patients in the ER after a motor vehicle accident. They found that those with the pain vulnerable genes were more likely to report neck pain, headaches, and dizziness.
One wonders if subjects who excel at physically demanding professions are more tolerant to pain than those of us with more sedentary jobs. Legend has it that Edward Villella, the famous American dancer, had his feet X-rayed in his thirties and discovered nine old fractures of which he was unaware. Ostensibly what would have crippled us was just an aching foot to Villella. Surprisingly, little has been done to study pain tolerance in professional athletes/performers. One study measured pain tolerance during limb immersion in ice water in ballet dancers. As expected, the dancers endured the ice water longer than nondancers (and men more than women). It is possible that individuals who are successful in physically demanding professions were born with, or developed, better tolerance to pain—along with being stronger and more coordinated and driven to succeed.
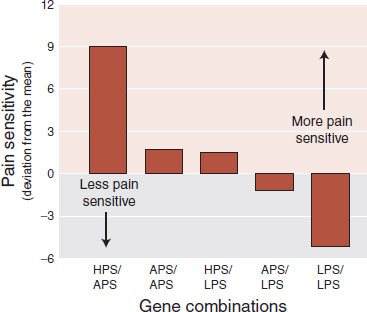
FIGURE 11.8  Pain responsiveness categorized by three major combinations of genetic variations for the catecholamine-O-methyltransferase enzyme. LPS, low pain sensitivity; APS, average pain sensitivity; HPS, high pain sensitivity. Subjects with the LPS variation were 2.3 times less likely to develop temporomandibular joint disorder. (Adapted from Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135-143.)
Pain responsiveness categorized by three major combinations of genetic variations for the catecholamine-O-methyltransferase enzyme. LPS, low pain sensitivity; APS, average pain sensitivity; HPS, high pain sensitivity. Subjects with the LPS variation were 2.3 times less likely to develop temporomandibular joint disorder. (Adapted from Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135-143.)
DESCENDING PATHWAYS AND OPIOIDS
The discovery of the opioid-mediated pain modulation circuits is one of the great stories in neuroscience. In the late 1960s, it became apparent that the brain exerts a top-down control of pain. A big break came in 1969 with the discovery that electrical stimulation of PAG, the gray matter surrounding the third ventricle and the cerebral aqueduct in the midbrain, induces analgesia. Reynolds implanted electrodes in the PAG of rats and performed abdominal surgery without problems when the electrodes were stimulated. Although the animals did not respond to the pain, they were able to move about the cage (before and after surgery) and displayed a startle response to visual or auditory stimuli even while the electrodes were active.
Subsequent work has led to a detailed knowledge of the descending pain-modulating circuits. Figure 11.9 shows a drawing of the important features of these pathways. Input from the prefrontal areas of the anterior cingulate and insular cortex as well as the hypothalamus and amygdala converges on the PAG. The PAG does not send neurons directly to the dorsal horn, but rather projects by way of intermediary nuclei such as the rostral ventral medulla (RVM). Other serotonergic and noradrenergic neurons (not shown) also project down on to the afferent pain neurons. The end result is inhibition and diminution of the pain signal that is sent up the ascending anterolateral tracts to the brain.
Opium
It is likely that opium was used as early as 4000 B.C. by the ancient Sumerians. By the 17th century, the therapeutic value of opium was well known. Morphine was first isolated in 1806 and codeine in 1832. Heroin was introduced to medicine in 1896. The uses and abuses of opium and its analogues have permeated most cultures of the world.
DISORDER
SCHIZOPHRENIA
There is a long history of noting increased pain tolerance in patients with schizophrenia, dating back to Kraepelin and Bleuler. More recently, surgeons and internists have written anecdotal reports of patients with schizophrenia who appear to experience little pain despite suffering from extremely painful physical conditions. It is not clear if the pain tolerance is a consequence of the illness, the psychotropic medications, or simply a lack of affect, that is, the patient feels the pain but fails to express it appropriately.
Hooley and Delgado sought to avoid these complicating factors by measuring pain sensitivity in the relatives of patients with schizophrenia. They found that subjects with a family history of schizophrenia showed elevated pain thresholds and tolerance. Of interest, the pain correlated with measures of self-referential thinking, magical ideation, and perceptual disturbances. The pathology of this aberrant pain sensitivity is unknown, but may be part of the genetic makeup of schizophrenia.
FIGURE 11.9  Descending pain-modulating pathways that enable the brain to inhibit the intensity of the ascending pain signals.
Descending pain-modulating pathways that enable the brain to inhibit the intensity of the ascending pain signals.
By the mid-1960s, it was known that microinjections of morphine into the PAG or the dorsal horn produced a powerful analgesia and that the opioid antagonist naloxone could block this effect. Furthermore, transection of the axons from the RVM reduces the morphine-induced analgesia. Yet, how does morphine relieve pain?
Opioid Receptors
The discovery of the opioid receptors was a major breakthrough in understanding the pain modulatory system. Three major classes of opioid receptors have been identified: μ, δ, and κ. However, most of the focus has been on the μ receptor because its activation is required for most analgesics. Indeed the affinity that a medication has for the μ receptor correlates with its potency as an analgesic. Naloxone also binds with the μ receptor and acts as the quintessential antagonist for it blocks activation and can precipitate withdrawal.
The opioid receptors are concentrated in the PAG, RVM, and dorsal horn—areas well known for pain modulation. However, the receptor can be found throughout the body, including skin, muscles, and joints, which help explain the benefits as well as typical side effects associated with opioids, for example, constipation and respiratory depression.
Endogenous Opioids
Clearly, animals did not evolve a receptor so that drug abusers could enjoy heroin. In the mid-1970s, the race was on to find the neurotransmitter that the body produces to activate the opioid receptor. The result was the discovery of the β-endorphin, enkephalins, and dynorphins: the three major classes of endogenous opioid peptides. The genes for these peptides are distributed throughout the CNS.
Figure 11.10 gives an example from the dorsal horn of the enkephalins and the μ receptors working together to decrease the pain signal sent to the brain. Note that the μ receptors are on both the presynaptic and the postsynaptic neurons.
Placebo
The use of placebos, substances with no intrinsic therapeutic value, is the oldest treatment known to man. In the modern era of medicine, the placebo response has been equated with the statement “all in your head.” Subsequently, it has been recognized that the placebo response is real—and actually in our heads. After the discovery of the endogenous opioid, it was shown that placebo anesthesia could even be reversed with naloxone.
POINT OF INTEREST
β-endorphin is derived from a larger precursor molecule called proopiomelanocortin (POMC), found primarily in the pituitary (see Figure 16.13). POMC contains other biologically active peptides, including adrenocorticotropic hormone (ACTH). Consequently, the stress response—discussed in a previous chapter—includes the release of ACTH as well as β-endorphin into the bloodstream. One wonders about the evolutionary advantage of the simultaneous synthesis and release of these two neuropeptides.
FIGURE 11.10  Descending pathways stimulate an interneuron in the dorsal horn to release enkephalins that activate the μ receptors. The effect on the presynaptic neuron is to decrease the release of glutamate and neuropeptides. With the postsynaptic neuron, stimulation of the μ receptors hyperpolarizes the membrane. These two actions result in a smaller signal coming out of the dorsal horn.
Descending pathways stimulate an interneuron in the dorsal horn to release enkephalins that activate the μ receptors. The effect on the presynaptic neuron is to decrease the release of glutamate and neuropeptides. With the postsynaptic neuron, stimulation of the μ receptors hyperpolarizes the membrane. These two actions result in a smaller signal coming out of the dorsal horn.
Brain imaging studies of subjects anticipating an effective treatment for pain but given a placebo have shown reduced activity in the pain perception areas and increased activity in the pain modulation areas—those associated with endogenous opioids (Figure 11.11). Thus, placebos work in two ways. First, by decreasing awareness in the pain-sensitive regions and second by increasing activity in regions involved with top-down suppression.
Acupuncture
Acupuncture has been used for thousands of years in China, Korea, and Japan, but only recently introduced to the West. The healing power of acupuncture is believed to work by reestablishing the proper “energy balance” in disordered organs. The treatment is conducted by inserting a needle into specific locations along meridians established through ancient clinical experience. The lack of scientific correlation or good clinical trials can be troubling to those of us who subscribe to a Western orientation of physiology and illness.
One of the difficulties in conducting good clinical trials with acupuncture is separating the specific effect of stimulating the acupoints from the placebo effect. A recent study in London sought to overcome this problem by including two placebo arms along with active acupuncture treatment. The first placebo treatment was with a blunt needle and the patients were aware they were being given an “inert” treatment. The second placebo arm intervention utilized what can best be described as a “stage needle”—when poked, the needle retracted into the handle, giving the appearance that the skin had been pierced. Surprisingly, few of the subjects were aware of the difference between the “stage needle” and real acupuncture.
While these patients—all with osteoarthritis—were being stuck, they were also being scanned with positron emission tomography (PET). The results showed that areas of the brain associated with top-down modulation of pain—dorsolateral prefrontal cortex, ACC, and midbrain—were active with “stage needles” as well as real needles. Only the ipsilateral insula was solely activated by real acupuncture. These results suggest that acupuncture works by way of a large placebo response and also a distinct, but as yet unknown, unique physiological effect.
Stress-Induced Analgesia
Perhaps the most impressive demonstration of top-down modulation of pain is the extreme analgesia shown by individuals at times of stress, for example, the athlete or performing artist who does not appreciate the pain until much later. The classic example was described by Beecher regarding men wounded in battle during the Second World War. He examined 215 men brought to a forward hospital with serious injuries: long bone fractures, penetrating wounds, etc. He found that only 25%, on being directly questioned regarding pain relief, said their pain was severe enough to want morphine. Beecher believed the relief the men experienced, when taken from the battle and brought to a safe location, blocked the pain.
FIGURE 11.11  The brain imaging studies of the placebo response showing two aspects to the brain’s reaction. On the left, control minus placebo shows decreased activity in those areas that perceive pain. On the right, placebo minus control shows increased activity in areas that suppress pain from the top down. PAG, periaqueductal gray. (Adapted from Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162-1167.)
The brain imaging studies of the placebo response showing two aspects to the brain’s reaction. On the left, control minus placebo shows decreased activity in those areas that perceive pain. On the right, placebo minus control shows increased activity in areas that suppress pain from the top down. PAG, periaqueductal gray. (Adapted from Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162-1167.)
The modern belief is that stress stimulates opioid-dependent pathways that inhibit the pain signal. However, a group at the University of Georgia has revealed a role for the endocannabinoids in stress-induced analgesia independent of the opioid pathways. Several endocannabinoids rapidly accumulate in the PAG in the midbrain with stress. The group in Georgia demonstrated that stress-induced analgesia in rats could be inhibited with endocannabinoid blockers. Their results suggest that higher cortical regions such as the amygdala release endocannabinoids into the PAG during stressful times to suppress pain perception. We appreciate the brain’s wisdom in utilizing several mechanisms (endocannabinoids and opioids) to put pain on hold when other events are more important.
POINT OF INTEREST
In an almost unbelievable brain imaging study, a group in Korea has shown that stimulating a visual-related acupoint—in the foot— activated the visual cortex. The figure shows the cortical activity for visual light stimulation, real acupuncture, and sham acupuncture. These results are difficult to comprehend for those of us who believe in the Western model of medicine and suggest there may be more to this old treatment than we can explain with our current understandings of the brain.
Light and acupuncture (at the visual acupoint 1) stimulate activity in the visual cortex, whereas sham acupuncture does not.
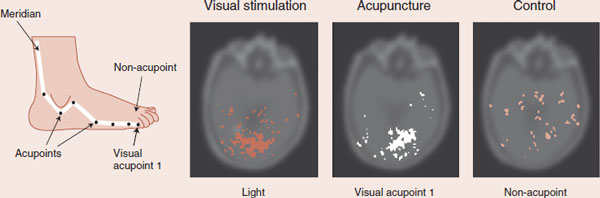
Adapted from Cho ZH, Chung SC, Jones JP, et al. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci U S A. 1998;95(5):2670-2673.
CHRONIC PAIN
Until the last half of the past century, pain was thought to be produced by a passive, direct transmission system from peripheral receptors to the cortex. This is called nociceptive pain (which we have already discussed earlier) and examples include acute trauma, arthritis, and tumor invasion. There is a veritable medical industry based on locating and correcting the source of the nociceptive pain for patients who are suffering. Unfortunately, most evaluations fail to turn up a cause that explains the pain. One reason is that the traditional model of pain fails to take into account changes in the nerves. Clearly, many patients in pain—particularly chronic, persistent pain—have developed autonomous, maladaptive pain perception independent of tissue damage.
Neuropathic pain is a heterogeneous term used to describe pain that arises from an injured nerve—either centrally or peripherally. Examples of this type of pain are postherpetic neuralgia, diabetic neuropathy, and phantom limb. The distinction between neuropathic and nociceptive pain is important when choosing proper treatment. For instance, neuropathic pain may not respond as well to nonsteroidal anti-inflammatory agents, or opioids, and is better managed with antidepressants and anticonvulsants.
Neuropathic pain is frequently persistent, does not resolve with time, and is resistant to treatment. The pain often disables patients. The pathophysiological mechanisms that underlie these neuropathic pain conditions are beginning to be teased out. One promising area of research is looking at the role of neurotrophic factors in the development of pathologic pain states.
As discussed in previous chapters, tissue injury induces an inflammatory response, which recruits immune cells that release cytokines. The cytokines are literally toxic to the sensory neurons. For example, inflammation interrupts the retrograde transport of neurotrophins from the periphery back to the cell body, where it is needed to maintain normal cell functioning. One neurotrophic factor in particular (glial cell line–derived neurotrophic factor [GDNF]) has been identified as a possible target. Several studies have shown that exogenous GDNF can prevent the development of experimental neuropathic pain.
Pain Memory
A striking example of the brain’s elaboration of pain without sensory input can be found in patients after limb amputation. Many will continue to experience pain from lesions that existed on the limb before the surgery—what some call pain memory. For example, a person with an ulcer on the foot at the time of the operation will still feel the presence of the ulcer many months after the limb has been removed.
Several clever clinicians have shown that sufficient local anesthesia of the affected limb several days prior to the amputation significantly reduces the incidence of pain memories. These results suggest that pain has an enduring effect on the brain, which can be attenuated with appropriate treatment.
Gray Matter Loss
A study by a group at Northwestern University further enhances our understanding of the role of the CNS with chronic pain. Apkarian et al. scanned 26 patients with chronic back pain that had persisted for at least 1 year. They compared the results with age-matched controls and found a 5% to 11% loss of gray matter in patients with chronic back pain (Figure 11.12). Further regional analysis showed that the dorsolateral prefrontal cortex was the specific area with greatest decrease in gray matter density. These results suggest that one explanation for chronic pain may be the loss of top-down modulation of pain (e.g., the loss of the placebo response in Figure 11.11).
What causes the gray matter loss with chronic pain? Several explanations are possible. First, the patients could be genetically predisposed to have less gray matter. Small injuries result in persistent pain because the patients lack sufficient CNS modulation of the ascending pain signal. Second, in the next chapter, we will show that medications or abused substances alter the morphology of the cell (see Figure 12.13). Excessive use/abuse may have a toxic effect on neurons in the gray matter. Third, the unremitting pain signal with the associated negative affect and stress may result in an excitotoxic and inflammatory state that essentially wears out the brain circuitry.
Subsequent studies conducted to verify the Apkarian findings have confirmed gray matter loss in patients with migraines, fibromyalgia, and idiopathic facial pain. A group in Oxford demonstrated that thalamic gray matter loss in patients with persistent hip pain was reversed 9 months after successful arthroplasty. These studies demonstrate another example of the remarkable plasticity of the brain.
DEPRESSION AND ANTIDEPRESSANTS
There is a long and well-documented correlation between depression and pain. Patients with chronic pain have a high incidence of depression and patients with depression have an increased expression of painful physical symptoms. Recently, studies have shown that patients with depression and pain are less likely to achieve remission of their depression.
Antidepressants have been shown to be effective in decreasing low back pain and pain in diabetic neuropathy, postherpetic neuralgia, fibromyalgia, and migraines as well as other pains. Traditionally, the tricyclics were used, but with the development of cleaner medications, other agents were tried. Of interest, the selective serotonin reuptake inhibitors (SSRIs) have been disappointing—often not separating from placebo in controlled trials. It appears that only agents that inhibit the reuptake of norepinephrine as well as serotonin are effective for pain reduction. Recently, the newer agents that tout a dual mechanism of action (venlafaxine and duloxetine) have been shown to reduce pain due to diabetic neuropathy. Duloxetine even has an Food and Drug Administration (FDA) indication for peripheral diabetic neuropathy.
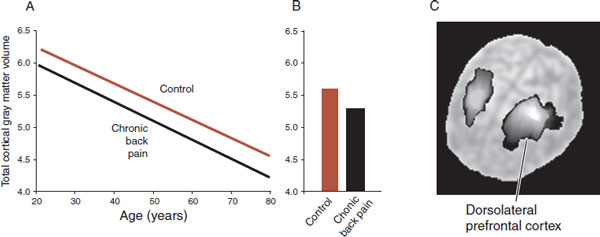
FIGURE 11.12  Gray matter loss with chronic back pain. A. Decrease in total gray matter volume for patients with chronic back pain compared with controls. B. Average for both groups. C. The dorsolateral prefrontal cortex is the specific region with decreased gray matter density. (Adapted from Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410-10415.)
Gray matter loss with chronic back pain. A. Decrease in total gray matter volume for patients with chronic back pain compared with controls. B. Average for both groups. C. The dorsolateral prefrontal cortex is the specific region with decreased gray matter density. (Adapted from Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410-10415.)
TMS, PFC, AND POSTOPERATIVE PAIN
Transcranial magnetic stimulation (TMS) is a noninvasive procedure that can stimulate the cerebral cortex. The prefrontal cortex (PFC) has been implicated as a region that modulates pain tolerance. A cleaver collaboration between anesthesiology and psychiatry shows that activation of the PFC with TMS can decrease pain perception.
Patients undergoing gastric bypass surgery were randomized to receive 20 minutes of either active or sham TMS immediately after surgery. The total morphine administered by patient-controlled analgesia pumps was tracked as an indirect measurement of pain. The figure shows that patients who received active TMS used approximately 40% less morphine in the 48-hours after the operation.
One 20-minute session of TMS applied to the left PFC postoperatively greatly reduced the total morphine administered to the patients.
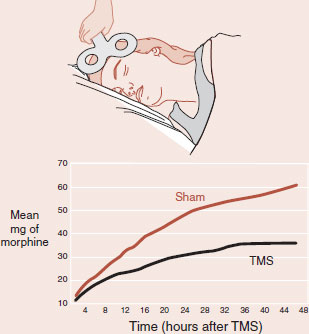
Adapted from Borckardt JJ, Weinstein M, Reeves ST, et al. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology. 2006;105(3):557-562.
How do the antidepressants decrease pain? One possibility could be the beneficial effect of correcting the mood. Yet, SSRIs give disappointing results. Likewise, patients without depression will show some pain reduction. Another possibility is the enhancement of the descending pathways. Some of the fibers projecting from the brain stem down onto the dorsal horn of the spinal cord are serotonergic and noradrenergic. More action from these neurons would further dampen the signals from the periphery.
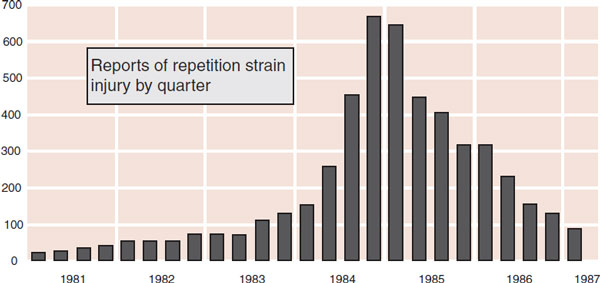
FIGURE 11.13  A psychosomatic pain epidemic. The graph shows reports of repetition strain injury at Telecom in Australia by quarter. (Adapted from Hocking B. Epidemiological aspects of “repetition strain injury” in Telecom Australia. Med J Aust. 1987;147(5):218-222.)
A psychosomatic pain epidemic. The graph shows reports of repetition strain injury at Telecom in Australia by quarter. (Adapted from Hocking B. Epidemiological aspects of “repetition strain injury” in Telecom Australia. Med J Aust. 1987;147(5):218-222.)
A final possibility for understanding the effectiveness of antidepressants for pain is a cortical mechanism. PET scans of both depressed patients and patients with pain show decreased activity in the prefrontal cortex and increased activity in the insula and ACC. It is possible that antidepressants moderate the pain perception by correcting the cortical imbalance associated with depression and pain.
Epidemic of Unexplained Pain
Let us not forget that pain is highly influenced by one’s psychosocial expectations. A good example is the epidemic of vague upper limb pain, from Australia, called repetitive strain injury (RSI). This condition was attributed to repetitive keyboard movements by telegraphists, but failed to follow the usual medical model. Reports in the medical literature and sensational media coverage as well as union and legal advocacy produced a dramatic increase in reports of RSI (Figure 11.13). The complaints peaked in 1984 and then declined, as the condition came to be perceived as psychosomatic and the courts went against the litigants. Similar epidemics have occurred in the United Kingdom and Japan.
Another classic study analyzed the development of late whiplash syndrome in Lithuania—a country where few drivers have car insurance or seek legal compensation after an accident. The authors questioned the subjects involved in known rear-end car collisions, 1 to 3 years after the accident, regarding neck pain and headaches, and compared their symptoms with matched control subjects. There was no significant difference between the groups. The authors concluded, “expectation of disability, a family history, and attribution of preexisting symptoms to the trauma may be more important determinants for the evolution of the late whiplash syndrome.”
The important point is that the perception of pain is a product of the brain’s abstraction and elaboration of sensory input. Many factors affect that process—not just nociceptive signals from the periphery.
QUESTIONS
1. Slow aching pain signals
a. Aδ fibers.
b. Aβ fibers.
c. C fibers.
d. D fibers.
2. Simultaneous input from these nerve fibers explains the inhibitory effect of the gate theory of pain.
a. Aδ fibers.
b. Aβ fibers.
c. C fibers.
d. D fibers.
3. Not associated with the affective-motivational pathways of pain
a. Somatosensory cortex.
b. “How much does it hurt?”
c. Medial thalamus.
d. Anterior cingulate and insular cortex.
4. Condition associated with increased pain tolerance
a. Depression.
b. Anxiety.
c. Fibromyalgia.
d. Schizophrenia.
5. Not part of the descending pathways of pain
a. Periaqueductal gray.
b. Dorsal horn.
c. Spinothalamic tract.
d. Rostral ventral medulla.
6. The primary opioid receptor
a. β receptor.
b. δ receptor.
c. κ receptor.
d. μ receptor.
7. Unlikely explanation for chronic persistent pain
a. Genetic predisposition.
b. Diminished placebo response.
c. Changes in the gray matter.
d. Damaged nerves.
8. Lacks significant analgesic effects
a. SSRIs.
b. Opioids.
c. Nonsteroidal anti-inflammatory drugs.
d. Anticonvulsants.
See Answers section at the end of the book.