Chapter 3
Relaxation
As mentioned in Chapter 2, the MR measurement can be analyzed in terms of energy transfer. The process by which the protons release the energy that they absorbed from the RF pulse is known as relaxation. Relaxation is a fundamental aspect of MR, as essential as energy absorption, and provides the primary mechanism for image contrast as discussed in Chapter 6. In resonance absorption, RF energy is absorbed by the protons only when it is broadcast at the correct frequency. The additional energy disturbs the equilibrium arrangement of spins parallel and antiparallel to 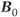 . Following excitation, relaxation occurs in which the protons release this added energy and return to their original configuration through naturally occurring processes. It is a time-dependent process and is characterized by a rate constant known as the relaxation time. Although it is individual protons that absorb the energy, relaxation times are measured for an entire sample of spins and are statistical or average quantities. Relaxation times are measured for gray matter or cerebrospinal fluid as bulk samples rather than for the individual water or fat molecules within the organs. Two relaxation times can be measured, known as T1 and T2. While both times measure the spontaneous energy transfer by an excited proton, they differ in the final disposition of the energy.
. Following excitation, relaxation occurs in which the protons release this added energy and return to their original configuration through naturally occurring processes. It is a time-dependent process and is characterized by a rate constant known as the relaxation time. Although it is individual protons that absorb the energy, relaxation times are measured for an entire sample of spins and are statistical or average quantities. Relaxation times are measured for gray matter or cerebrospinal fluid as bulk samples rather than for the individual water or fat molecules within the organs. Two relaxation times can be measured, known as T1 and T2. While both times measure the spontaneous energy transfer by an excited proton, they differ in the final disposition of the energy.
3.1 T1 relaxation and saturation
In a modern MR experiment, pulsed RF energy is applied to the protons repeatedly with a delay time between the pulses. This time between pulses allows the excited protons to give up the absorbed energy (T1 relaxation). As the protons give up this energy to their surroundings, the population difference (spin up versus spin down) is reestablished so that net absorption can reoccur after the next pulse. In the macroscopic picture, 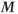 returns toward its initial value
returns toward its initial value 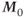 as more energy is dissipated. Since
as more energy is dissipated. Since 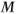 is the ultimate source of the MR signal, the more energy dissipated, the more signal is generated following the next RF pulse.
is the ultimate source of the MR signal, the more energy dissipated, the more signal is generated following the next RF pulse.
For practical reasons, the time between successive RF pulses is usually insufficient for complete T1 relaxation so that 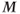 will not be completely restored to
will not be completely restored to 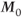 . Application of a second RF pulse prior to complete relaxation will rotate
. Application of a second RF pulse prior to complete relaxation will rotate 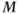 into the transverse plane, but with a smaller magnitude than following the first RF pulse. The following experiment describes the situation (Figure 3.2):
into the transverse plane, but with a smaller magnitude than following the first RF pulse. The following experiment describes the situation (Figure 3.2):
- A 90° RF pulse is applied.
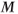 is rotated into the transverse plane.
is rotated into the transverse plane. - A time
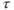 elapses, insufficient for complete T1 relaxation. The longitudinal magnetization at the end of
elapses, insufficient for complete T1 relaxation. The longitudinal magnetization at the end of 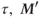 , is less than in step 1.
, is less than in step 1. - A second 90° RF pulse is applied.
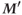 is rotated into the transverse plane.
is rotated into the transverse plane. - After a second time
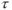 elapses,
elapses, 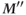 is produced. It is smaller in magnitude than
is produced. It is smaller in magnitude than 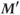 , but the difference is less than the difference between
, but the difference is less than the difference between 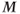 and
and 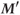 .
.
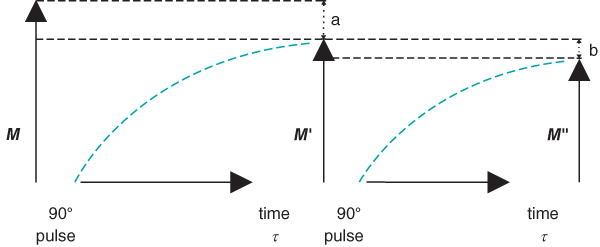
Figure 3.2 Following a 90° RF pulse, longitudinal magnetization is regenerated through T1 relaxation. If the time between successive RF pulses  is insufficient for complete recovery of M, only
is insufficient for complete recovery of M, only  will be present at the time of the next RF pulse (a). If time
will be present at the time of the next RF pulse (a). If time  elapses again, only
elapses again, only 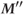 will be present (b).
will be present (b). 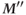 will be smaller than
will be smaller than 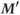 , but the difference will be less than the difference between M and
, but the difference will be less than the difference between M and 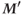 .
.
For many MRI experiments such as standard spin echo and gradient echo imaging, a steady state of 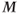 is present because multiple RF pulses are applied and the repetition time TR between the pulses is nearly always less than sufficient for complete relaxation. To produce this steady state prior to data collection, additional RF pulses are applied to the tissue immediately prior to the main imaging pulses. These extra RF pulses are known as preparatory pulses or dummy pulses because the generated signals are usually ignored. These preparatory pulses ensure that
is present because multiple RF pulses are applied and the repetition time TR between the pulses is nearly always less than sufficient for complete relaxation. To produce this steady state prior to data collection, additional RF pulses are applied to the tissue immediately prior to the main imaging pulses. These extra RF pulses are known as preparatory pulses or dummy pulses because the generated signals are usually ignored. These preparatory pulses ensure that 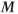 has the same magnitude prior to every measurement during the scan.
has the same magnitude prior to every measurement during the scan.
The rate of RF pulse application and the efficiency of energy transfer must have the proper balance. Suppose the RF energy is applied faster than T1 relaxation can occur. A comparison of the microscopic and macroscopic pictures is useful at this point. In the microscopic picture, the protons in the lower energy level absorb the RF energy and the protons in the upper energy level are stimulated to emit their energy. As more energy is transmitted, the proton populations of the two levels will gradually equalize. When this equalization occurs, no further net absorption of energy is possible, a condition known as saturation (Figure 3.3). In the macroscopic picture, 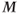 will rotate continuously but gradually get smaller in magnitude until it disappears as the net population difference approaches zero. Since there is no net magnetization, there will be no coherence of proton motion in the transverse plane and thus no signal is produced. This condition is known as saturation. There is a limited amount of energy that a collection of protons can absorb before they become saturated. In a conventional MR measurement, each tissue will experience different amounts of saturation, due to their different T1 relaxation times. As a result, the contribution of a tissue to
will rotate continuously but gradually get smaller in magnitude until it disappears as the net population difference approaches zero. Since there is no net magnetization, there will be no coherence of proton motion in the transverse plane and thus no signal is produced. This condition is known as saturation. There is a limited amount of energy that a collection of protons can absorb before they become saturated. In a conventional MR measurement, each tissue will experience different amounts of saturation, due to their different T1 relaxation times. As a result, the contribution of a tissue to 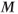 will differ, as will its maximum potential signal.
will differ, as will its maximum potential signal.
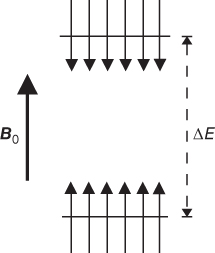
Figure 3.3 Saturation. If RF pulses are applied faster than the T1 relaxation processes can dissipate the energy, the spin populations equalize between the two energy levels. As a result, there is no difference in the number of spins and no net magnetization.
As mentioned earlier, spin-lattice relaxation measures the rate of energy transfer from an excited proton to its surroundings. The key to this energy transfer is the presence of some type of molecular motion (e.g., vibration, rotation) of the lattice in the vicinity of the excited proton with an intrinsic frequency, 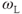 , that matches the resonant frequency,
, that matches the resonant frequency, 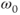 . The closer
. The closer 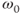 is to
is to 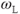 , the more readily the motion absorbs the energy and the more frequently this energy transfer occurs, allowing the collection of protons to return to its equilibrium configuration sooner. In tissues, the nature of the protein molecular structure and any metal ions that may be present have a pronounced effect on the particular
, the more readily the motion absorbs the energy and the more frequently this energy transfer occurs, allowing the collection of protons to return to its equilibrium configuration sooner. In tissues, the nature of the protein molecular structure and any metal ions that may be present have a pronounced effect on the particular  . Metals ions such as iron or manganese can have significant magnetic moments that may influence the local environment. While the particular protein structures are different for many tissues, the molecular rotation or tumbling of most proteins typically have
. Metals ions such as iron or manganese can have significant magnetic moments that may influence the local environment. While the particular protein structures are different for many tissues, the molecular rotation or tumbling of most proteins typically have 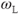 of approximately 1 MHz. Therefore, at lower resonant frequencies (lower
of approximately 1 MHz. Therefore, at lower resonant frequencies (lower 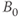 ), there is a better match between
), there is a better match between 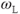 and
and 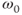 . This enables a more efficient energy transfer to occur and thus T1 is shorter. This is the basis for the frequency dependence of T1; namely, T1 decreases with decreasing strength of the magnetic field. This is also the reason that a larger
. This enables a more efficient energy transfer to occur and thus T1 is shorter. This is the basis for the frequency dependence of T1; namely, T1 decreases with decreasing strength of the magnetic field. This is also the reason that a larger 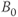 does not necessarily translate to a greater signal, as saturation is more prevalent due to the longer T1 times.
does not necessarily translate to a greater signal, as saturation is more prevalent due to the longer T1 times.
3.2 T2 relaxation, T2* relaxation, and spin echoes
There are several potential causes for a loss of transverse coherence to 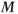 . One is the molecular motions of the adjacent spins due to vibrations or rotations. This irreversible movement is responsible for spin–spin relaxation or the true T2. Another cause arises from the fact that a proton never experiences a magnetic field that is 100% uniform or homogeneous in value. As the proton precesses, it experiences a fluctuating local magnetic field, causing a change in
. One is the molecular motions of the adjacent spins due to vibrations or rotations. This irreversible movement is responsible for spin–spin relaxation or the true T2. Another cause arises from the fact that a proton never experiences a magnetic field that is 100% uniform or homogeneous in value. As the proton precesses, it experiences a fluctuating local magnetic field, causing a change in 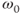 and a loss in transverse phase coherence. This nonuniformity in
and a loss in transverse phase coherence. This nonuniformity in 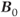 comes from three sources:
comes from three sources:
- Main field inhomogeneity. There is always some degree of nonuniformity to
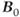 due to imperfections in magnet manufacturing, interactions with nearby building walls, or other sources of metal. This field distortion is constant during the measurement time.
due to imperfections in magnet manufacturing, interactions with nearby building walls, or other sources of metal. This field distortion is constant during the measurement time. - Sample-induced inhomogeneity. Differences in the magnetic susceptibility or degree of magnetic polarization of adjacent tissues (e.g., bone, air) will distort the local magnetic field near the interface between the different tissues. Provided there is no motion of the sample, this inhomogeneity is also of constant magnitude and is present as long as the patient is present within the magnet.
- Imaging gradients. As discussed in Chapter 4, the technique used for spatial localization generates a magnetic field inhomogeneity that induces proton dephasing. This inhomogeneity is transient during the measurement.
Some sources of proton dephasing can be reversed by the application of a 180° RF pulse, which is described by the following sequence of events (Figure 3.6):
- A 90° RF pulse.
- A short delay of time
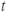 .
. - A 180° RF pulse.
- A second time delay
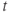 .
.
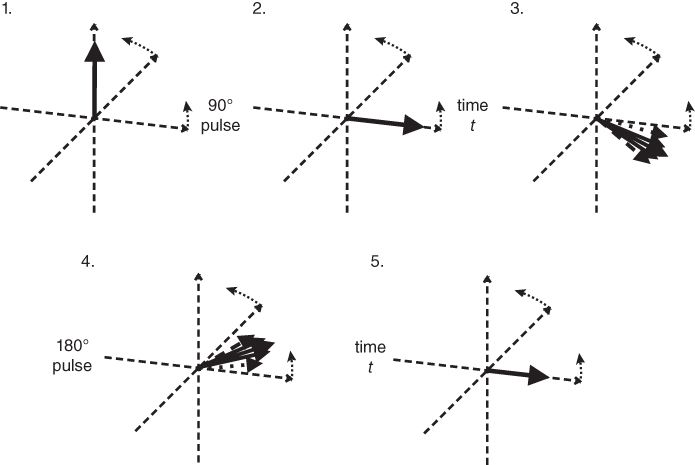
Figure 3.6 A rotation frame slower than 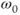 is assumed for this figure. Net magnetization M (arrow) is oriented parallel to
is assumed for this figure. Net magnetization M (arrow) is oriented parallel to 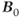 (not shown) prior to an RF pulse (1). Application of a 90° RF pulse rotates M into the transverse plane (2). Due to the
(not shown) prior to an RF pulse (1). Application of a 90° RF pulse rotates M into the transverse plane (2). Due to the 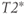 relaxation processes, the protons become asynchronous with each other during time t (3). Application of a 180° RF pulse causes the protons to reverse their phase relative to the transmitter phase. The protons that precessed most rapidly are farthest behind (dashed arrow), while the slowest protons are in front (dotted arrow) (4). Allowing time t to elapse again allows the protons to regain their phase coherence in the transverse plane (5), generating a signal in the receiver coil known as a spin echo. The loss in magnitude of the reformed coherence relative to the original coherence (2) is due to irreversible processes (i.e., true spin–spin or T2 relaxation). Equation (3.3) describes the decay of
relaxation processes, the protons become asynchronous with each other during time t (3). Application of a 180° RF pulse causes the protons to reverse their phase relative to the transmitter phase. The protons that precessed most rapidly are farthest behind (dashed arrow), while the slowest protons are in front (dotted arrow) (4). Allowing time t to elapse again allows the protons to regain their phase coherence in the transverse plane (5), generating a signal in the receiver coil known as a spin echo. The loss in magnitude of the reformed coherence relative to the original coherence (2) is due to irreversible processes (i.e., true spin–spin or T2 relaxation). Equation (3.3) describes the decay of 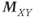 if T2 is used instead of
if T2 is used instead of 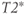 .
.
Following the echo formation, the protons continue to precess and dephase a second time as the sources of dephasing continue to affect them. Application of a second 180° RF pulse again reverses the proton phases and generates another coherence to the protons, producing another spin echo. This second echo differs from the first echo by the increased amount of T2 relaxation contributing to the signal loss. This process of spin echo formation by 180° RF pulses can be repeated as many times as desired, until T2 relaxation completely dephases the protons. The use of multiple 180° pulses maintains phase coherence to the protons longer than the use of a single 180° RF pulse because of the significant dephasing that the field inhomogeneity induces over very short time periods.