Chapter 11
Magnetic resonance angiography
One of the strengths of MRI for imaging patient tissue is its ability to acquire information regarding its function as well as its structure. The most common example is the examination of flowing blood within the vascular network using MR angiography (MRA). In Chapter 9, moving tissue was shown to produce severe image artifacts. MRA uses flowing tissue such as blood to provide the primary source of signal intensity in the image. It allows visualization of the normal, laminar flow of blood within the vascular system and its disruptions due to pathologic conditions such as stenoses or occlusions. MRA can be of particular benefit in evaluating vessel patency. MRA techniques have the advantage over conventional X-ray-based angiographic techniques in that use of a contrast agent is not always required. Consequently, multiple scans may be performed if desired (e.g., visualizing arterial and then venous flow).
The most common MRA approach is known as a “bright-blood” technique. A signal from the moving protons is accentuated relative to the stationary protons through the pulse sequence and measurement parameters. Whether the vessels are displayed as white pixels on a dark background or dark pixels on a bright background, bright-blood MRA techniques assign high pixel amplitudes to the laminar-flowing blood. Bright-blood MRA techniques can be divided into two approaches: time-of-flight and phase contrast methods. In their simplest implementation, both methods visualize arterial and venous flow simultaneously through the volume of interest. This can lead to an ambiguous identification if the vessels are in close proximity to each other. One approach to select either arterial or venous flow is to apply spatial presaturation pulses to saturate the undesired blood signal prior to its entry into the imaging volume (Figure 11.1). Presaturation pulses may also be used in “black blood” MRA techniques where the blood signal is saturated and the vessels appear dark relative to the surrounding tissue. These techniques are seldom used and will not be discussed further in this book.

Figure 11.1 Flow selection in MR angiography. (a) In the absence of a spatial presaturation pulse, flowing blood entering the imaging volume is visualized regardless of the direction of the flow. (b) A spatial presaturation pulse that saturates flowing blood prior to its entry into the imaging volume suppresses signal from that volume.
MRA techniques use gradient echo sequences as the measurement technique for data collection and may use 2D sequential slice (number of slices equal to the number of subloops) or 3D volume acquisition modes. Gradient echo sequences allow the use of short TE times (usually less than 10 ms), which minimize T2* dephasing of the blood and other tissues. The choice of a 2D versus a 3D technique is usually dictated based on the total volume of tissue to be examined and by the total scan time. Three-dimensional techniques provide the best spatial resolution in all three directions and minimize vessel misregistration artifacts. However, saturation of blood at the distal side of the excitation volume limits the volume to approximately 70 mm unless a T1 relaxation agent is used. The improved spatial resolution of 3D methods requires significantly longer scan times. The 2D method is preferable for imaging vessels with slow flow velocity, such as veins, or to limit saturation effects from the in-plane flow. Coverage of large areas of anatomy is possible in one scan since the volume of excitation is typically 3–4 mm per slice.
There are two additional problems associated with MRA. One is that the saturation pulse becomes less effective as more time elapses between the presaturation and the excitation pulses. This problem occurs both due to increased T1 relaxation of the blood as well as time-of-flight effects (see Section 11.1). This loss of effectiveness can be minimized by proper positioning of the saturation pulse near the volume of excitation. Care must be taken in positioning if there is pulsatile flow in the vessel of interest, as too close a position to the imaging volume could cause a reduction in the signal if the flow is retrograde in nature. A second problem is an exaggerated sensitivity to vessel stenosis. The stenotic region disrupts the laminar flow in the area of and distal to the stenosis, causing a loss of vessel signal to a greater extent than from the stenotic region alone. As the laminar flow is reestablished within the vessel, a bright signal can again be measured, demonstrating patency of the vessel.
11.1 Time-of-flight MRA
Time-of-flight techniques are the most time-efficient methods for obtaining MRA images. A single measurement is performed, with the stationary tissue signal suppressed relative to the flowing tissue signal for the imaging volume. A moderate excitation pulse angle and a TR much shorter than the tissue T1 values are used to accomplish this. While the stationary tissue experiences every RF excitation pulse, the flowing tissue does not. A volume of blood will be at a different location at the time of each excitation pulse due to its motion during TR (Figure 11.2). The signal from the blood volume is largest at the entry point of the slice because it has not undergone any excitation pulses. As the blood volume travels through the slice, it becomes progressively saturated as it undergoes more excitation pulses and loses signal. If the flow direction is perpendicular to the slice (through-plane flow) and the volume of excitation is small, then the volume of blood exits the slice before it is completely saturated and a significant blood signal can be measured throughout the entire slice. If the flow is contained within the excitation volume (in-plane flow), then significant saturation of the blood will occur and the blood signal will be isointense with the stationary tissue unless a T1 relaxation agent is used. The degree of blood saturation depends on the slice thickness, TR, excitation angle, and flow velocity.

Figure 11.2 Time-of-flight effect. During data collection, the imaging volume experiences multiple RF pulses. Flowing blood (colored box) experiences the first RF pulse (upper left). During the first TR period, the excited blood volume moves (upper right) and only a portion experiences the second RF pulse. During the second TR time period, the initial volume of blood continues to move (lower left). By the end of a third TR time period, the initial volume of blood is entirely outside the volume of excitation and does not contribute to the detected signal (lower right).
Time-of-flight techniques suffer from two limitations. One is that it provides only a qualitative assessment of flow velocity. The second problem is that there is incomplete suppression of the background tissue due to the faster T1 relaxation times of the stationary tissues relative to the blood, particularly fat. Magnetization transfer (MT) pulses are often incorporated for additional suppression of the stationary tissue water to enhance the ability for small vessel detection at the expense of increased TR and relative fat signal (Figure 11.3). Three-dimensional time-of-flight techniques produce increased saturation of the blood due to the large number of excitation pulses of the imaging volume. For this reason, they are suitable only for visualizing vessels with fast flow velocities, such as moderate-sized arteries.

Figure 11.3 Time-of-flight MRA showing effects of magnetization transfer pulse. Other measurement parameters are: pulse sequence, three-dimensional refocused gradient echo, post excitation; TR, 42 ms; TE, 7 ms; excitation angle, 25°; acquisition matrix, 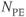 , 192 and
, 192 and 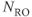 , 512 with twofold readout oversampling; FOV,
, 512 with twofold readout oversampling; FOV,  ;
; 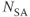 , 1; effective slice thickness, 0.78 mm. (a) Source image, one from data set acquired without MT pulse; (b) transverse post processed image of (a); (c) source image, one from data set acquired with MT pulse; (d) transverse post processed image of (c). Note reduction of background gray and white matter in (c) compared to (a) and improved visualization of vessels in (d) compared to (b) (arrow).
, 1; effective slice thickness, 0.78 mm. (a) Source image, one from data set acquired without MT pulse; (b) transverse post processed image of (a); (c) source image, one from data set acquired with MT pulse; (d) transverse post processed image of (c). Note reduction of background gray and white matter in (c) compared to (a) and improved visualization of vessels in (d) compared to (b) (arrow).
Two approaches have been developed to overcome this saturation. For 3D examinations with through-plane flow, the excitation pulse can be modified to produce a ramped or nonuniform range of excitation angles across the imaging volume (Figure 11.4). The effective excitation ramp is oriented so that increased energy is applied to protons located at the distal side of the imaging volume. This induces more transverse magnetization and more signal from the more saturated protons (Figure 11.5). A second approach is analogous to traditional X-ray angiography and is used for examinations with in-plane flow such as the visualization of pulmonary or abdominal arteries. T1 contrast agents can be administered as a bolus to shorten the T1 relaxation time of blood and obtain more blood signal (Figure 11.6). Measurement times can be reduced to a few seconds through the use of interpolation in the slice selection. These short times will enable serial scan volumes coupled with suspension of respiration to be acquired during the measurement. Subtraction of pre- and postcontrast volumes eliminates the residual signal from stationary tissue. Use of contrast agents for abdominal MRA has enabled rapid, reliable, and high-quality studies to be obtained with minimal contamination from patient motion. Studies of peripheral vessels by MRA can also be performed following contrast administration (Figure 11.7). However, caution must be used in the use of contrast media in patients with compromised renal function (see Chapter 15).

Figure 11.4 TONE RF pulse. (a) Standard excitation pulses provide uniform energy deposition across the slice, which gradually increases saturation of, and reduces the transverse magnetization from, blood located at the exit side of the slice, causing a loss of signal. (b) Nonuniform excitation pulses known as ramped or tilted optimized nonuniform excitation (TONE) RF pulses increase the excitation across the slice. Although the amount of saturation increases, the resulting transverse magnetization remains constant, so that the blood signal remains uniform throughout the imaging volume.

Figure 11.5 Time-of-flight MRA showing effects of TONE RF pulse. Measurement parameters are: pulse sequence, three-dimensional refocused gradient echo, post excitation; TR, 42 ms; TE, 7 ms; excitation angle, 25°; acquisition matrix, 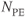 , 192 and
, 192 and 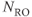 , 512 with twofold readout oversampling; FOV,
, 512 with twofold readout oversampling; FOV, 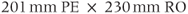 ;
; 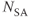 , 1; effective slice thickness, 0.78 mm. (a) Sagittal projection of volume using normal uniform excitation pulse; (b) sagittal projection of volume using TONE excitation pulse. Note improved signal from vessels (arrow).
, 1; effective slice thickness, 0.78 mm. (a) Sagittal projection of volume using normal uniform excitation pulse; (b) sagittal projection of volume using TONE excitation pulse. Note improved signal from vessels (arrow).

Figure 11.6 MR angiography of aorta and renal arteries following bolus administration of gadolinium–chelate contrast agent. Measurement parameters are: pulse sequence, three-dimensional refocused gradient echo, postexcitation; TR, 3 ms; TE, 1.1 ms; excitation angle, 20°; acquisition matrix, 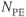 , 128 and
, 128 and 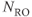 , 256; FOV,
, 256; FOV, 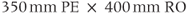 ;
; 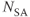 , 1; effective slice thickness, 2.0 mm. (a) Source image, one from data set. Image was obtained following subtraction of unenhanced scan from enhanced scan. (b) Coronal maximum intensity projection image of (a).
, 1; effective slice thickness, 2.0 mm. (a) Source image, one from data set. Image was obtained following subtraction of unenhanced scan from enhanced scan. (b) Coronal maximum intensity projection image of (a).

Figure 11.7 Peripheral MRA, postprocessed images. Images were acquired at three different scan table positions. Measurement parameters: pulse sequence, two-dimensional spoiled gradient echo; TR, 300 ms: TE, 3.6 ms; excitation angle, 50°; acquisition matrix, 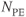 , 144 and
, 144 and 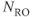 , 256; FOV,
, 256; FOV, 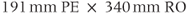 ;
; 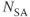 , 1; slice thickness, 4.0 mm; pulse triggered. (Reproduced with permission of H. Cecil Charles, Duke University.)
, 1; slice thickness, 4.0 mm; pulse triggered. (Reproduced with permission of H. Cecil Charles, Duke University.)
While the use of contrast media aids in MRA examinations of anatomy that can be acquired in a single-scan volume, its use for scanning larger anatomical regions such as peripheral vessels in the lower extremities or for dynamic examinations is problematic. The timing when contrast media is in the anatomical area of interest can be short for arterial vessels. To achieve images with good spatial resolution as well as good temporal resolution, techniques have been developed that do not scan  -space uniformly in time; in other words, those lines of
-space uniformly in time; in other words, those lines of  -space that primarily determine contrast (low amplitude
-space that primarily determine contrast (low amplitude  ) are acquired more frequently than those that primarily affect spatial resolution (high amplitude
) are acquired more frequently than those that primarily affect spatial resolution (high amplitude  ). The signals from the high amplitude
). The signals from the high amplitude  lines are used in multiple data sets to create images with less inter-set time variation. These techniques are known as TWIST (Time-Resolved Angiography with Interleaved Stochastic Trajectories), TRICKS (Time-Resolved Imaging of Contrast Kinetics), or 4D-TRAK (4D Time-Resolved MR Angiography with Keyhole).
lines are used in multiple data sets to create images with less inter-set time variation. These techniques are known as TWIST (Time-Resolved Angiography with Interleaved Stochastic Trajectories), TRICKS (Time-Resolved Imaging of Contrast Kinetics), or 4D-TRAK (4D Time-Resolved MR Angiography with Keyhole).
11.2 Phase contrast MRA
Phase contrast MRA is a technique in which the background tissue signal is subtracted from a flow-enhanced image to produce flow-only images. The acquisition sequence may produce a one-dimensional profile, or standard 2D or 3D images. A minimum of two images is measured at each slice position. One image, known as the reference image, is acquired with flow compensation (see Chapter 10). Subsequent images are acquired following application of additional gradient pulses that induce a phase shift in blood moving with a particular direction and flow velocity. The moving protons in the chosen direction are rephased at the echo time TE and the resultant complex or phase image is subtracted from the reference complex or phase image to produce images of only the moving protons. In the complex difference image, the signal amplitude of the blood will depend on its velocity, with a maximum signal obtained from flowing blood with an operator-specified velocity known as the velocity encoding value  . In the phase difference image the pixel values will be proportional to the blood velocity, within a maximum range determined by the
. In the phase difference image the pixel values will be proportional to the blood velocity, within a maximum range determined by the  . Additional scans may be performed to sensitize flow in other directions or at other velocities. The resultant difference images may be combined to produce images sensitive to flow in any direction (Figure 11.8).
. Additional scans may be performed to sensitize flow in other directions or at other velocities. The resultant difference images may be combined to produce images sensitive to flow in any direction (Figure 11.8).


Figure 11.8 Phase-contrast MRA. Venc, 30 cm s−1 in all directions. Other measurement parameters: pulse sequence, two-dimensional spoiled gradient echo; TR, 211 ms; TE, 8.4 ms; excitation angle, 15°; acquisition matrix, 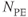 , 192 and
, 192 and  , 256; FOV, 240 mm PE × 240 mm RO;
, 256; FOV, 240 mm PE × 240 mm RO; 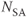 , 1; slice thickness, 40.0 mm. (a) Flow image, flow direction A–P; (b) flow image, flow direction R–L; (c) flow image, flow direction S–I; (d) magnitude sum of images (a) to (c); (e) phase image, flow direction A–P; (f) phase image, flow direction R–L; (g) phase image, flow direction S–I.
, 1; slice thickness, 40.0 mm. (a) Flow image, flow direction A–P; (b) flow image, flow direction R–L; (c) flow image, flow direction S–I; (d) magnitude sum of images (a) to (c); (e) phase image, flow direction A–P; (f) phase image, flow direction R–L; (g) phase image, flow direction S–I.
Phase contrast MRA has several advantages over time-of-flight techniques. Being subtraction rather than saturation techniques, they have better background suppression than time-of-flight methods. Through their directional sensitization, phase contrast methods also enable flow in each primary direction to be separately assessed. Finally, quantitation of the flow velocity in each direction is possible. However, phase contrast methods suffer from two problems. One is a significantly longer scan time. Four separate acquisitions are required to measure flow in all three directions. The scan time is four times longer than for a time-of-flight method with similar acquisition parameters. In addition, prior knowledge of the maximum velocity is necessary to ensure that the proper  is used. The maximum signal in the complex difference image is achieved for blood flowing at the chosen
is used. The maximum signal in the complex difference image is achieved for blood flowing at the chosen  . Flow velocities exceeding this velocity are misregistered in the image and appear as slower flow, a situation known as flow aliasing (for instance, a
. Flow velocities exceeding this velocity are misregistered in the image and appear as slower flow, a situation known as flow aliasing (for instance, a  of
of  will have equal signal amplitudes for blood flowing at 25 and
will have equal signal amplitudes for blood flowing at 25 and  ). Flow aliasing is analogous to the high-frequency aliasing problem observed in phase encoding (see Chapter 5). The blood signal is reduced at these higher velocities until a complete loss of the flow signal occurs at flow velocities that are even multiples of the
). Flow aliasing is analogous to the high-frequency aliasing problem observed in phase encoding (see Chapter 5). The blood signal is reduced at these higher velocities until a complete loss of the flow signal occurs at flow velocities that are even multiples of the  . At the other extreme, a
. At the other extreme, a  too large will minimize contrast between velocity changes within a vessel (for example, at 30 or
too large will minimize contrast between velocity changes within a vessel (for example, at 30 or  flow for a
flow for a  of
of  ). Proper choice of the
). Proper choice of the  allows adequate visualization of all flow with good sensitivity to velocity variations within a vessel. This choice can be facilitated by the use of velocity measurements measured using Doppler ultrasound techniques or by scanning the volume using 2D techniques with multiple
allows adequate visualization of all flow with good sensitivity to velocity variations within a vessel. This choice can be facilitated by the use of velocity measurements measured using Doppler ultrasound techniques or by scanning the volume using 2D techniques with multiple  values to see which one provides the best results.
values to see which one provides the best results.
11.3 Maximum intensity projection
Regardless of the choice of acquisition method, examination of the individual images from an MRA scan is recommended as they can provide details regarding flow variations within a vessel. However, a vessel is seldom located within a single slice but usually extends through several slices at an arbitrary angle, making the vessel tortuosity and the spatial relationship between vessels difficult to assess, particularly if the vessels are oriented perpendicular to the imaging volume. Bright-blood MRA images may be analyzed using a postprocessing technique known as maximum intensity projection (MIP) to better visualize the three-dimensional vessel topography.
The MIP process generates images frequently from the entire set of MRA images. A view direction is chosen and the entire set of images is projected along that direction on to a perpendicular plane using a “ray tracing” approach. The pixel of maximum intensity along the ray is chosen as the pixel for the MIP image, regardless of the input slice where the pixel is located (Figure 11.9). Because the bright-blood MRA technique accentuates the blood signal over the stationary tissue signal, the MIP process preferentially selects blood vessels whenever encountered, enabling the entire vessel to be examined no matter where it is located within the imaging volume. Multiple images may be obtained from the same data set through a change of the view direction (rotation of the projection angle). Vessels that are superimposed in one projection may be clearly resolved in another one. It is also possible to perform the MIP process on a subset of the data, a so-called “targeted” approach. This method is useful for isolating the left and right carotid arteries, for elimination of suborbital or subcutaneous fat in cerebral MRA studies, or for tailored reconstruction of the renal arteries. Care must be taken in the definition of the targeted area that the vessel of interest does not leave the area. The MIP process causes the vessel to be cut off at the edge of the defined region, simulating a vessel occlusion (Figure 11.10). Careful examination of the source images and/or MIP images in multiple orientations is necessary to ensure the inclusion of the entire vessel within the reconstructed area.

Figure 11.9 Maximum intensity projection (MIP). The MR images are acquired so that moving blood has pixels of maximal intensity. The MIP process maps the pixels of maximum intensity into a single projection or view, regardless of which slice the pixel was located in. Changing the direction of projection provides a different perspective of the vessels.

Figure 11.10 Maximum intensity projection. Note lack of vessel in (a) (arrow), which is caused by improper exclusion (b). Processing of the complete data set shows the entire vessel (c).
Another post-processing approach is known as segmentation. It uses an operator-selected region known as a “seed” region to identify the vessel. The seed region is expanded to include high-intensity pixels in contiguous regions. Since the MRA scan techniques accentuates the blood signal, the vessels will be preferentially chosen. This approach depends on adequate contrast between the blood and the tissue near the vessel.