R
Radish (Raphanus sativus)
Unlike the West, where the radishes are small-rooted vegetables, the Far East grows them as large-rooted vegetables (Curtis, 2003). They are cultivated for their fleshy, pungent, edible roots, which are usually reddish but sometimes white or black. The leaves and roots have been used in various parts of the world to treat cancer or as antimicrobial, antifungal, and antiviral agents (Terras et al., 1993; Gutierrez and Perez, 2004). Isothiocyanates present as thioglucoside conjugates in radish were shown to inhibit the development of tumors in many experimental models investigated (Conaway et al., 2002). Radishes are recognized as a food remedy for stones, gravel, and scorbutic conditions. The juice has been used for treating gall stones (choleithiasis) and for preventing the formation of biliary caculi. Kumar (2004) showed a diet containing radishes increased excretion of calcium oxalate compared to a self-selected diet, with the crystal count significantly higher in both genders.
Glucoraphanin, the natural precursor of sulforaphane found mostly in cruciferous vegetables, but also in radishes, is known for maintaining good heath (West et al., 2004).
Using the bleomycin-Fe(III) method, the methanolic extract from radish sprouts (Raphanus sativus) was shown by Takaya et al. (2003) to be the most potent hydroxyl-radical scavenger of 11 commonly used vegetables, with close to double that of L-ascorbic acid (Figure R.86). This activity was attributed to the presence of various sinapic acid esters and flavonoids.
Matsufuji et al. (2003) attempted to isolate and characterize the reaction products of 12 acylated anthocyanins from red radish (Raphanus sativus) by reacting with 2,2′- azobis(2-amidinopropane) dihydrochloride (AAPH) to generate peroxyl radicals. A number of products were isolated, and their chemical structures determined by preparative HPLC to be p-hydroxybenzoic acid, 6-O-(E)-p-coumaroy1–2-O-β-D-glucopyranosyl- α-D-glucopyrano-side, p-coumaric acid, 6-O-(E)-feruloyl-2-O-β-D-glucopyranosyl-α-D-glucopyranoside, and ferulic acid.

FIGURE R.86 Antioxidant activity of vegetables expressed as index of activity compared to ascorbic acid (1.0). (From Takaya et al., J. Agric. Food Chem., 51:8061–8066, 2003. With permission.)]
References
Curtis, I.S., The noble radish: Past, present and future, Trends Plant Sci., 8:305–307, 2003.
Conoway, C.C., Yang, Y.M., and Chung, F.I., Isothiocyanates as cancer chemopreventive agents: Their biological activities and metabolism in rodents and humans, Curr. Drug. Metab., 3:233– 255, 2002.
Gutierrez, R.M. and Perez, R.L., Raphanus sativus (Radish): Their chemistry and biology, Curr. Drug. Metab., 3:233–255, 2004.
Kumar, A., Influence of radish consumption on urinary calcium oxalate excretion, Nepal. Med. Coll. J., 6:41–44, 2004.
Matsufuji, H., Otsuki, T., Takeda, T., Chino, M., and Takeda, M., Identification of reaction products pf acylated anthocyanins from red radish with peroxyl radicals, J. Agric. Food Chem., 51:3157–3161, 2003.
Takaya, Y., Kondo, Y., Furukawa, T., and Niwa, M., Antioxidant constituents of radish sprouts (Kaiwaredaikon), Raphanus sativus L., J. Agric. Food Chem., 51:8061–8066, 2003.
Terras, F.R.G., Torrekens, S., Van Leuven, B.P.A.F., Osborn, R.W., Vanderleyden, J., Cammue, B.P., and Broekaert, W.T., A new family of basic cysteine-rich plant antifungal proteins from Brassicaceae species, FEBS Lett., 316:233–240, 1993.
West. L.G., Meyer, K.A., Balch, B.A., Rossi, F.J., Schultz, M.R., and Haas, G.W., Glucoraphanin and 4-hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, Brussels sprouts, kale, and cabbage, J. Agric. Food Chem., 52:916–926, 2004.
Rapeseed
see also Canola Rapeseed, known scientifically as Brassica napus or Brassica rapa, is cultivated in northern climates primarily for animal feed and vegetable oil for human consumption and for biodesel. According to the USDA, rapeseed is the third leading source of vegetable oil in the world in 2000 after soy and palm. Canola, a specific variety of rapeseed bred to have a low erucic-acid content (2.0 percent) in the oil and a low glucosinolate content (<18 mmol/g) in the meal, is grown in Canada, with related varieties grown in Europe. Barrett et al. (1998) showed that cruciferous seed meals that include rapeseed exerted protective effects against tumor formation and growth. Four potent, angiotensin-converting, enzyme-inhibitory peptides were isolated by Marczak et al. (2003) from subtilisin digestion of rapeseed protein. They lowered blood pressure in spontaneously hypertensive rats, suggesting the digest may be a promising functional food for preventing and treating hypertension. Del Mar Yust et al. (2004) recently treated rapeseed protein hydrolysates with the food-grade endoprotease, alcalase, and identified two fractions rich in HIV-protease inhibitors.
Thiyam et al. (2004) recently examined the antioxidant potential of rapeseed oil by-products and found the meal contained significant amounts of phenolic compounds. Of these, the major one was sinapic acid, and in the form of its esters and glucosides. These antioxidants could make a significant contribution to the meal industry.
The genotoxin potential of rapeseed oil cooking fumes was studied by Chen et al. (1992). The cooking fumes contained mutagenic activity, suggesting Chinese women exposed to such fumes were at high risk for lung cancer. However, it should be pointed out that the rapeseed grown in China is high in glucosinolates, resulting in much higher levels of sulfur in the oil and not characteristic of canola oil. In addition, many of the homes are poorly ventilated.
References
Barret, J.E., Klopfenstein, C.F., and Leipold, H.W., Protective effects of cruciferous seed meal and hulls against colon cancer in mice, Cancer Lett., 127:83–88, 1998.
Chen, H., Yang, M., and Ye, S., A study on genotoxicity of cooking fumes from rapeseed oil, Biomed. Environ. Sci., 5:229–235, 1992.
del Mar Yust, M., Pedroche, J., Megias, C., GironCalle, J., Alaiz, M., Millan, F., and Vioique, J., Rapeseed protein hydrolysates: A source of HIV protease peptide inhibitors, Food Chem., 87:387–392, 2004.
Marczak, E.D., Usui, H., Fujita, H., Yang, Y., Yokoo, M., Lipkowski, A.W., and Yoshikawa, New antihypertensive peptides isolated from rapeseed, Peptides, 24:791–798, 2003.
Thiyam, U., Kuhlmann, A., Stockmann, H., and Schwartz, K., Prospects of rapeseed oil byproducts with respect to antioxidative potential, C.R. Chemie, 7:5611–5616, 2004.
Raspberry (Rubus idaes)
Raspberry is an aggregate fruit that is fleshy and contains seeds. It grows best in climates with cool summers and mild winters (Duel, 1996). Ethanol extracts from raspberry fruits showed in vitro anticancer activity on cervical- and breast-cancer cell lines (Wedge, 2001). Haung and coworkers (2002) proposed that the ability of black raspberries to inhibit the development of chemically induced esophageal and colon cancer in rodents and to inhibit benzo(a)pyrene-induced cell transformation in vitro may be mediated by impairing signal-transduction pathways, leading to activation of AP-1 and NF-κB, known to be involved in tumor promotion/progression.
The fruits and leaves from red raspberry (Rubus idaeus L.) and black raspberry (Rubus occidentalis L.) plants were reported to be high in phenolics, with the highest antioxidant activity found at the ripe stage. Total anthocyanin content increased with maturity, with the leaves being higher in antioxidant activity than the fruits (Wang et al., 2000). The bright color of red raspberries is due to the presence of two anthocyanins, cyanidin-3-glucoside and cyanidin-3-sophoroside (Scheme R.51) (van Elbe and Schwartz, 1996).
Juranic and coworkers (2005) correlated the antiproliferative activity of the water extracts from the seed or pulp of five raspberry cultivars on malignant human colon carcinoma LSI74 cells with ellagic acid content.
Raspberry leaves are also high in tannins and, like its relative the blackberry, may relieve acute diarrhea (Tyler, 1994). The antimicrobial properties of raspberry juice, raspberry-leaf extract, and a commercial brand of raspberryleaf tea were investigated against five human pathogenic bacteria and two fungi. Raspberry juice was found to significantly reduce the growth of several species of bacteria, including Salmonella, Shigella, and E. coli. No antimicrobial activity was detected in the leaf extract or tea (Ryan et al., 2001). Lin et al. (2005) recently demonstrated the potential of enriching wine or vodka with phenolics to inhibit H. pylori in laboratory medium. Raspberry-, cinnamon-, and peppermint-enriched wines all exhibited high antimicrobial activity, while raspberry-enriched vodka proved the most potent inhibitor of H. pylori.

SCHEME R.51 Anthocyanins of red raspberry. (From Suthanthangjai et al., 2005)
Tea made from the leaves of Rubus idaeus L. (raspberry) has been used for centuries as a uterine relaxant in folk medicine. Rojas-Vera et al. (2002) reported that methanol extracts of dried raspberry have relaxant activity on transmurally stimulated guinea-pig ileum. Many women consume the raspberry leaf herb during their pregnancy, believing that it shortens labor and makes labor “easier.” Simpson and coworkers (2001) undertook a double-blind, randomized, placebo-controlled trial with 192 low-risk women who birthed their babies between May 1999 and February 2000. Raspberry leaf, consumed in tablet (2×1.2 g per day) from 32 gestation week until labor, was found to cause no adverse effects for mother or baby, but contrary to popular belief, did not shorten the first stage of labor. The only clinically significant findings were a shortening of the second stage of labor (mean difference=9.59 minutes) and a lower rate of forceps deliveries between the treatment group and the control group (19.3 percent vs. 30.4 percent) (Wang and Lin, 2000).
References
Duel, C.L., Strawberries and raspberries, in Processing Fruits: Science and Technology; Major Processed Products, vol. 2, Somogyi, L.P., Barrett, D.M., and Hui, Y.U., Eds., Technomic Pub. Co., New York, 1996, pp. 157–177.
Huang, C., Huang, Y., Li, J., Hu, W., Aziz, R., Tang, M.S., Sun, N., Cassady, J., and Stoner, G.D., Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor κB by black raspberry extracts, Cancer Res., 62: 6857–6863, 2002.
Juranic, Z., Zizak, Z., Tasic, S., Petrovic, S., Nidzovic, S., Leposavic, A., and Stanojkovic, T., Anti-proliferative action of water extracts of seeds or pulp of five different raspberry cultivars, Food Chem., 93: 39–45, 2005.
Lin, Y.T., Dhiraj Vattem,. Labbem R.G., and Shetty, K., Enhancement of antioxidant activity and inhibition of Helicobacter pylori by phenolic phytochemical-enriched alcoholic beverages, Process Biochem., 40:2059–2065, 2005.
Rojas-Vera, J., Patel, A.V., and Dacke, C.G., Relaxant activity of raspberry (Rubus idaeus) leaf extract in guinea-pig ileum in vitro, Phytother. Res., 16:665–668, 2002.
Ryan, T., Wilkinson, J.M., and Cavanagh, H.M., Antibacterial activity of raspberry cordial in vitro, Res. Vet. Sci., 71:155–159, 2001.
Simpson, M., Parsons, M., Greenwood, J., and Wade, K., Raspberry leaf in pregnancy: Its safety and efficacy in labor, J. Midwifery Womens Health, 46:51–59, 2001.
Suthanthangjai, W., Kajda, P., and Zabetakis, I., The effect of high hydrostatic pressure on the anthocyanins of raspberry (Rubus ideaus), Food Chem., 90: 193–197, 2005.
Tyler, V.E., Herbs of Choice: The Therapeutic Use of Phytomedicinals, Pharmaceutical Products Press, Binghamton, New York, 1994, pp. 52, 139.
Von Elbe, J.H. and Schwartz, S.J., Colorants, in Food Chemistry, Fennema, O.R., Ed., Marcel Dekker, New York, 1996, pp. 611–722.
Wang, S.Y. and Lin, H-S., Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage, J. Agric. Food Chem., 48:140–146, 2000.
Wedge, D.E., Meepagala, K.M., Magee, J.B., Smith, S.H., Huang, G., and Larcom, L.L., Anticarcinogenic activity of strawberry, blueberry, and raspberry extracts to breast and cervical cancer cells, J. Med. Food, 4:49–51, 2001.
Red clover
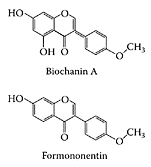
Red clover is a perennial herb that commonly grows wild in meadows throughout Europe and Asia, and has now been naturalized to grow in North America. The red flowers at the end of the branched stems are considered to be the source of its medicinal properties and are usually dried for therapeutic use. Red-clover (Trifolium pratense) extracts are becoming increasingly popular, primarily for the treatment of menopausal symptoms (Fugh-Berman and Kronenberg, 2001). Although promoted as a phytoestrogen source similar to soybeans, red clover is a medicinal herb, not a food, and traditionally has not been used for long term. Formononentin and biochanin A are the principal isoflavones of red clover, and are known to help with hot flushes, which are a common menopausal complaint. The conflicting data on randomized, controlled trials of red clover for the control of menopausal symptoms are encouraging and suggest that phytoestrogens are a treatment modality that needs pursuing (Pitkin, 2004; Barentsen, 2004).
(Adapted from Hurr and Rafii, FEMS Microbiol Lett. 192:21–25, 2000.)
Current research has focused on a red-clover extract high in isoflavones as a possible treatment for symptoms associated also with cardiovascular health. Isolated isoflavones from red clover enriched in biochanin lowered LDL-C in men (Nestel et al., 2004). Campbell and coworkers (2004) recently reported that one-month supplementation with red clover isoflavones had a positive effect on HDL cholesterol. Mean daytime systolic and diastolic blood pressures were significantly lowered during isoflavone therapy, compared to placebo, and forearm vascular endothelial function was significantly greater during isoflavone than placebo supplementation in postmenopausal, type 2 diabetic women. These data suggest that isoflavone supplementation from red clover may favorably influence blood pressure and endothelial function in postmenopausal, type 2 diabetic women (Howes et al., 2003).
Various studies also suggest that red-clover isoflavones may help prevent cancer. Jarred and coworkers (2003) found red-clover-derived isoflavones had a significant effect on prostatic growth, reducing the enlarged, nonmalignant prostate phenotype of the adult aromatase knock-out mouse, by acting as antiandrogenic agents rather than weak, estrogenic substances. Isoflavones in red clover significantly reduced the synthesis of prostaglandin E2 and thromboxane B2 (p<0.001 to p<0.05) in the murine macrophage cell line, indicating COX inhibition. Thus, it is possible that the lower rate of some cancers in populations with a high intake of dietary isoflavones may be linked to their inhibition of COX activity. In mice fed a diet supplemented with red-clover isoflavones, the prostatic epithelium displayed a significant increase in the production of estrogen-receptor beta and the adhesion protein E-cadherin, but a decrease in transforming growth factor betal. This study suggested that red-clover isoflavones represent a nontoxic dietary treatment for prostatic hyperplasia, reducing the potential for neoplastic transformation (Slater et al., 2002).
The activity of alkaline phosphatase increased following incubation of osteosarcoma cells (HOS58) with red-clover-chloroform extracts, suggesting a role for red-clover isoflavonoids in the stimulation of osteoblastic-cell activity.
References
Barentsen, R., Red clover isoflavones and menopausal health, J. Br. Menopause Soc., 1:4–7, 2004.
Campbell, M.J., Woodside, J.V., Honour, J.W., Morton, M.S., and Leathern, A.J.C., Effect of red cloverderived isoflavone supplementation on insulin-like growth factor, lipid and antioxidant status in healthy female volunteers: A pilot study, Eur. J. Clin. Nutr., 58:173–179, 2004.
Fugh-Berman, A. and Kronenberg, F., Red clover (Trifolium pratense) for menopausal women: Current state of knowledge, Menopause, 8:333–337, 2001.
Howes, J.B., Tran, D., Brillante, D., and Howes, L.G., Effects of dietary supplementation with isoflavones from red clover on ambulatory blood pressure and endothelial function in postmenopausal type 2 diabetes, Diabetes Obes. Metab., 5:25–332, 2003.
Hur, H.-G. and Rafii, F., Biotransformation of the isoflavonoids biochanin A, formononentin, and glycitein by Eubacterium limosum, FEMS Microbiol. Lett., 192:21–25, 2000.
Jarred, R.A., McPherson, S.J., Jones, M.E.E., Simpson, E.R., and Risbridger, G.P., Anti-androgenic action by red clover-derived dietary isoflavones reduces non-malignant prostate enlargement in aromatase knockout (ArKo) mice, Prostate, 56:54–64, 2003.
Lam, A.N., Demasi, M., James, M.J., Husband, A.J., and Walker, C., Effect of red clover isoflavones on COX-2 activity in murine and human monocyte/macrophage cells, Nutr. Cancer, 49:89–93, 2004.
Nestel, P., Cehun, M., Chronopoulos, A., DaSilva, L., Teede, H., and McGrath, B., A biochaninenriched isoflavone from red clover lowers LDL cholesterol in men, Eur. J. Clin. Nutr., 58:403–408, 2004.
Pitkin, J., Red clover isoflavones in practice: A clinician’s view, J. Br. Menopause Soc., 10:7–12, 2004.
Slater, M., Brown, D., and Husband, A., In the prostatic epithelium, dietary isoflavones from red clover significantly increase estrogen receptor β and E-cadherin expression but decrease transforming growth factor β1, Prostate Cancer Prostatic Dis., 5:16–21, 2002.
Wende, K., Krenn, L., Unterrieder, I., and Lindequist, U., Red clover extracts stimulate differentiation of human osteoblastic osteosarcoma HOS58 cells, Planta Med., 70:1003–1005, 2004.
Red wines
see also Wines Polyphenols, mainly flavonoids, exert protective effects on the cardiovascular system (Wollin and Jones, 2001), as well as exhibit anticancer (Bianchini and Vainio, 2003), antiviral, and antiallergic properties (Bhat et al., 2001). In coronary heart disease, the protective effects of flavonoids are antithrombic, antioxidant, antiischemic, and vasorelaxant properties (de Lorimier, 2000). It has been hypothesized that the phenomenon of a low incidence of coronary heart disease in French people may be partially related to the pharmacological properties of polyphenolic compounds included in red wine (Zenebe and Pechanova, 2002). The mechanisms underlying CHD protective benefits of red wine have not been elucidated. Recently, the polyphenol resveratrol (3,5,4′-trihydroxy-trans-stilbene), known to be abundantly present in red wine compared to white wine, beer, or spirits, has been demonstrated to elicit a broad spectrum of biological responses in in vitro and in animal studies, including effects that are compatible with the cardioprotective roles proposed for red wine. Other studies relate exposure to wine/resveratrol with reduction in myocardial damage during ischemia-reperfusion, modulation of vascular cell functions (Wu et al., 2001), inhibition of LDL oxidation, and suppression of platelet aggregation (Halpern et al., 1998; Wu et al., 2001; Wollin and Jones, 2001). Grapes contain a variety of antioxidants, including resveratrol, catechin, epicatechin, and proanthocyanidins. Of these, resveratrol is present mainly in grape skin, while proanthocyanidin is present in the seeds. Das and coworkers (1999) demonstrated that red-wine extract, as well as resveratrol and proanthocyanidins, are equally effective in reducing myocardial ischemic reperfusion injury, which suggests that these redwine polyphenolic antioxidants play a crucial role in cardioprotection.
Schafer and Bauersachs (2002) reported that red wine may beneficially affect the development of high-altitude pulmonary edema, which is the predominant cause of death due to highaltitude illness. Two cellular mechanisms have been described for the altitude-related reduction in barometric pressure: enhanced endothelin 1 production and the increased generation of reactive-oxygen species. Both were suppressed by red wine.
References
Bhat, K.P.L., Kosmeder, II, J.W., and Pezzuto, J.M., Biological effects of resveratrol, Antioxid. Redox Signal, 3:1041–1064, 2001.
Bianchini, F. and Vainio, H., Wine and resveratrol: Mechanisms of cancer prevention? Eur. J. Cancer Prev., 12:417–425, 2003.
Das, D.K., Sato, M., Ray, P.S., Maulik, G., Engelman, R.M., Bertelli, A.A., and Bertelli, A., Cardioprotection of red wine: Role of polyphenolic antioxidants, Drugs Exp. Clin. Res., 25:115–120, 1999.
de Lorimier, A.E., Alcohol, wine, and health, Am. J. Surg., 180:357–361, 2000.
Halpern, M.J., Dahlgren, A.L., Laakso, I., SeppanenLaakso, T., Dahlgren, J., and McAnulty, P.A., Redwine polyphenols and inhibition of platelet aggregation: Possible mechanisms, and potential use in health promotion and disease prevention, J. Int. Med. Res., 26:171–180, 1998.
Schafer, A. and Bauersachs, J., High-altitude pulmonary edema: Potential protection by red wine, Nutr. Metab. Cardiovasc. Dis., 12:306–310, 2002.
Wollin, S.D. and Jones, P.J., Alcohol, red wine and cardiovascular disease, J. Nutr., 131:1401– 1404, 2001.
Wu, J.M., Wang, Z.R., Hsieh, T.C., Bruder, J.L., Zou, J.G., and Huang, Y.Z., Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (review), Int. J. Mol. Med., 8:3–17, 2001.
Zenebe, W. and Pechanova, O., Effects of red wine polyphenolic compounds on the cardiovascular system, Bratisl. Lek. Listy., 103:159–165, 2002.
Rehmannia
Rehmannia refers to the root of Rehmannia glutinosa, an herb of the Scrophulariaceae family. The species name glutinosa comes from glutinous, referring to the sticky nature of the root. Another name for rehmannia is Chinese foxglove.

Rehmannia is a Chinese herb that is often combined with other herbs to treat anemia (Yuan et al., 1998; Zee-Cheng, 1992), cancer (Wei and Ru, 1997; Kamei et al., 2000), constipation, and diabetes. It has been mainly used to treat broken bones and severed sinews from falls. Oh and coworkers (2003) reported recently that Rehmannia glutinosa Libosch extracts stimulate the proliferation and activities of osteoblasts, while inhibiting the generation and resorptive activities of osteoclasts. It also shows preventive effects on osteoporotic bone loss induced by an ovariectomy. Other uses include the treatment of fatigue (Zee-Cheng, 1992) and high blood pressure (Yi et al., 1965). Rehmannia may be applied to the skin to treat eczema or psoriasis (Prieto et al., 2003) and may be beneficial in the regulation of immediate-type allergic reaction (Kim et al., 1998). It may also be used to treat cuts and wounds (Luo, 1993).
In modern times, rehmannia is especially used for treating hormonal disorders, such as menopause, thyroid imbalance, and adrenal insufficiency (Shan, 1994; Chao et al., 2003).
The main components of rehmannia are simple sugars (including glucose, galactose, fructose, sucrose, and mannitol), which make the root sticky and give it the sweet taste. About half the content of dried rehmannia is stachyose and verbascose, polysaccharides that are difficult to digest. The stachyose extract from Rehmannia glutinosa Libosch had a significant, hypoglycemic effect in glucose- and adrenaline-induced hyperglycemic and alloxan-induced diabetic rats (Zhang et al., 2004).
The major active constituents of rehmannia are iridoid glycosides. In a study of several samples of rehmannia, Luo et al. (1994) found that catalpol made up about 3–11 percent of the undried root content. The pharmacological action of catalpol and related iroids involved production of adrenal cortical hormones (HsonMou and Pui-Hay, 1986). These hormones have anti-inflammatory action (explaining the claimed benefits of rehmannia for asthma, skin diseases, and arthritis) and are also involved in the production of sex hormones (explaining the claimed benefit of treating menopause, impotence, and other signs of hormone deficiency). Recent research by Li et al. (2004) suggested catalpol were a potential neuroprotective agent, and its neuroprotective effects were achieved, at least partly, by promoting endogenous anti-oxidant enzymatic activities and reducing the formation of nitric oxide. Kim and coworkers (1999) suggested rehmannia may inhibit TNF-α secretion by inhibiting IL-1 secretion and has an anti-inflammatory activity in the central nervous system, curing some pathological-disease states.
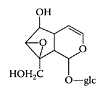
Catalpol. (Adapted from Li et al., Brain Res., 1029:179–185, 2004.)
References
Anh, N.T., Sung, T.V., Franke, K., and Wessjohann, L.A., Phytochemical studies of Rehmannia glutinosa rhizomes, Pharmazie, 58:593–595, 2003.
Chao, S.L., Huang, L.W., and Yen, H.R., Pregnancy in premature ovarian failure after therapy using Chinese herbal medicine, Chang Gung Med. J., 26:449–452, 2003.
Hson-Mou, C. and Pui-Hay, P.B., Eds., Pharmacology and Applications of Chinese Materia Medica, (2 vols.), World Scientific, Singapore, 1986.
Kamei, T., Kumano, H., Iwata, K., Nariai, Y., and Matsumoto, T., The effect of a traditional Chinese prescription for a case of lung carcinoma, J. Altern. Complement Med., 6:557–559, 2000.
Kim, H.M., An, C.S., Jung, K.Y., Choo, Y.K., Park, J.K., and Nam, S.Y., Rehmannia glutinosa inhibits tumor necrosis factor-alpha and interleukin-1 secretion from mouse astrocyte, Pharmacol. Res., 40:171–176, 1999.
Kim, H.M., Lee, E.H., Lee, S.J., Shin, T.Y., Kim, Y.J., and Kim, J.B., Effect of Rehmannia glutinosa on immediate type allergic reaction, Int. J. Immunopharmacol., 20:231–240, 1998.
Li, D.-Q., Bao, Y.-M., Zhao, J.-J., Liu, C.-P., Liu, Y., and An, L.-J., Neuroprotective properties of catalpol in transient global cerebral ischemia in gerbils: Dose-response, therapeutic timewindow and longterm efficacy, Brain Res., 1029:179–185, 2004.
Luo, Y., Determination of catalpol in rehmannia root by high performance liquid chromatography, Chinese Pharm. J., 29:8–40, 1994.
Luo, Z.H., The use of Chinese traditional medicines to improve impaired immune functions in scald mice, Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi, 9:56–58, 80, 1993.
Oh, K-O., Kim, S-W., Kim, J-Y., Ko, S-Y., Kim, H-M., Baek, J-H., Ryoo, H-M., and Kim, J-K., Effect of Rehmannia glutinosa Libosch extracts on bone metabolism, Clin. Chim. Acta, 334:185–195, 2003.
Prieto, J.M., Recio, M.C., Giner, R.M., Manez, S., Giner-Larza, E.M., and Rios, J.L., Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions, J. Pharm. Pharmacol., 55:1275–1282, 2003.
Shan, J.C., Determination of hepatocyte adrenergic alpha 1 receptor and study on actions of nourishing yin and replenishing qi drugs in experimental hyperthyroid rats, Zhongguo Zhong Xi Yi Jie He Za Zhi, 14: 96–98, 69–70, 1994.
Wei, X.L. and Ru, X.B., Effects of low-molecularweight Rehmannia glutinosa polysaccharides on p53 gene expression, Zhongguo Yao Li Xue Bao, 18:471–474, 1997.
Yi, N.Y., Chu, W., and Koang, N.K., Pharmacologic studies on liu wei di huang t’ang (decoction of Rehmannia with 6 components): its action on kidney function and blood pressure of rats with renal hypertension, Chin. Med. J., 84:433–436, 1965.
Yuan, A., Liu, C., and Huang, X., Treatment of 34 cases of chronic aplastic anemia using prepared Rehmannia polysaccharide associated with stanozolol, Zhongguo Zhong Xi Yi Jie He Za Zhi, 18:351–353, 1998.
Zee-Cheng, R.K., Shi-quan-da-bu-tang (ten significant tonic decoction), SQT, a potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs, Exp. Clin. Pharmacol., 14:725–736, 1992.
Zhang, R.X., Jia, Z.P., Kong, L.Y., Ma, H.P., Ren, J., Li, M.X., and Ge, X., Stachyose extract from Rehmannia glutinosa Libosch to lower plasma glucose in normal and diabetic rats by oral administration, Pharmazie, 59:552–556, 2004.
Resistant starch
Resistant starch is starch that resists digestion by enzymes in the small intestine. It is found naturally in many cereals and grains, as well as in some processed foods, such as extruded cereals (Brown, 2004). Resistant starch functions like a fiber in the diet, as it plays a role in gut health (Asp et al., 1996). In humans, resistant starch lowers fecal bileacid excretion (Langkilde et al., 1998). In their review, Young and Leu (2004) showed that the consumption of resistant starch dramatically affected the colonic luminal environment by facilitating apoptotic deletion of genetically damaged cells in the colon, several of which are considered to be biomarkers associated with risk for colorectal cancer. In addition, its ability to lower colonic pH is usually considered beneficial for cancer prevention, as well for mineral biovailability in the colon (Champ, 2004). Cheng and Lai (2000) showed that resistant rice starch was fermented to produce proprionic acid, which resulted in reduction in serum total cholesterol, serum LDL cholesterol, hepatic cholesterol, and hepatic triglycerides in rats. Foods in this class also have a low glycemic index and reduce postprandial-insulin levels and increase HDL cholesterol levels (Kendall et al., 2004; Park et al., 2004). Other researchers found that retrograded resistant starch was a very potent butyrate producer (Bird et al., 2000; Topping and Bird, 1999). In a recent review, Brouns and coworkers (2002) highly recommend resistant starch in relation to butyrate. Higgins et al. (2004) reported that replacement of 5.4 percent of the total dietary carbohydrate with resistant starch increased postprandial lipid oxidation significantly and therefore might decrease fat accumulation in the long term.
The protective effect of high-amylose cornstarch ingestion on trinitrobenzene sulfonic acid-induced colitis suggested to Morita et al. (2004) that it altered the colonic mucosa, possibly due to the production of cecal short-chain fatty acids.
In summary, resistant-starch intake seems to decrease postprandial glycemic and insulinemic responses, lower plasma cholesterol and triglyceride concentrations, improve whole-body insulin sensitivity, increase satiety, and reduce fat storage. These properties make resistant starch an attractive dietary target for the prevention of diseases associated with dyslipidemia and insulin resistance, as well as the development of weight-loss diets and dietary therapies for the treatment of type 2 diabetes and coronary heart disease (Higgins, 2004).
References
Asp, N.G., van Amelsvoort, J.M.M., and Hautvast, J.G.A.J., Nutritional implications of resistant starch, Nutr. Res. Rev., 9:1–31, 1996.
Bird, A.R., Brown, I.L., and Topping, D.L., Starches, resistant starch, the gut microflora and human health, Curr. Issues Intest. Microbiol., 1:25–27, 2000.
Brouns, F., Kettlitz, B., and Arrigoni, E., Resistant starch and “the butyrate revolution,” Trends Food Sci. Technol., 13:251–261, 2002.
Brown, I.L., Applications and uses of resistant starch, J. AOAC Int., 87:727–732, 2004.
Champ, M-J., Physiological aspects of resistant starch and in vivo measurements, J. AOAC Int., 87: 749–755, 2004.
Cheng, H.-H. and Lai, M.-H., Fermentation of resistant rice starch produces proprionate reducing serum and hepatic cholesterol in rats, J. Nutr., 130:1991–1995, 2000.
Higgins, J.A., Higbee, D.R., Donahoo, W.T., Brown, I.L., Bell, M.L., and Bessesen, D.H., Resistant starch consumption promotes lipid oxidation, Nutr. Metab., 1:8, 2004.
Higgins, J.A., Resistant starch: metabolic effects and potential health benefits, J. AOAC Int., 87:761–768, 2004.
Kendall, C.W.C., Emam, A., Augustin, L.S.A., and Jenkins, D.J.A., Resistant starches and health, J. AOAC Int., 87:769–774, 2004.
Langkilde, A.M., Ekwall, H., Bjork, I., Asp, N.-G., and Andersson, H., Retrograded high-amylose corn starch reduces cholic acid excretion from the small bowel in ileostomy subjects, Eur. J. Clin. Nutr., 52: 790–795, 1998.
Morita, T., Tanabe, H., Sugiyama, K., Kasaoka, S., and Kiriyama, S., Dietary resistant starch alters the characteristics of colonic mucosa and exerts a protective effect on trinitrobenzene sulfonic acid-induced colitis in rats, Biosci. Biotechnol. Biochem., 68:2155–2164, 2004.
Park, O.J., Kang, N.E., Chang, M.J., and Kim, W.K., Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects, J. Nutr. Sci. Vitaminol., 50:93–99, 2004.
Topping, D.L. and Bird, A.R., Foods, nutrients and digestive health, Aust. J. Nutr. Dietet., 56(Suppl. 3): 522–534, 1999.
Young, G.P. and Le Leu, R.K., Resistant starch and colorectal neoplasia, J. AOAC Int., 87:775– 786, 2004.
Resveratrol
Resveratrol is a trihydroxystilbene in the skins of grapes and in wine. It is a powerful phytoestrogen with a wide range of pharmacological and therapeutic health benefits. The beneficial effects of wine on cardiovascular health include prevention of oxidative damage, vasodilation, and prevention of platelet aggregation. Laden and Porter (2001) showed it was resveratrol that inhibited purified human squalene monooxygenase, a rate-limiting enzyme in cholesterol biosynthesis. Thus, protection by resveratrol is related to inhibition of cholesterol synthesis. Other mechanisms for the protection of the cardiovascular system by resveratrol include defense against ischemic-reperfusion injury, promotion of vasorelaxation, protection and maintenance of intact endothelium, antiatherosclerotic properties, inhibition of low-density lipoprotein oxidation, suppression of platelet aggregation, and estrogen-like actions (Hao and He, 2004). Gusman and coworkers (2001) reappraised the chemopreventive and chemotherapeutic properties of resveratrol. The literature confirmed the ability of resveratrol to inhibit activation of carcinogenic compounds, stimulate detoxification, prevent interaction with DNA, and, finally, to suppress tumor progression (Teel and Huynh, 1998). Bhat and Pezzuto (2001) reported that resveratrol exerted antiproliferative effects in cultured human endometrial adenocarcinoma (Ishikawa) cells involving either both estrogen-dependent and estrogen-independent mechanisms. Resveratrol has been shown to significantly alter the cellular physiology of tumor cells, as well as block initial and progression of the tumors. Zoberi et al. (2002) showed that resveratrol altered both cell-cycle progression and cytotoxic response to ionizing radiation in two cervical tumor cell lines. Resveratrol was also shown by Niles and coworkers (2003) to inhibit growth and induce apoptosis in two human melanoma-cell lines. Thus, resveratrol could be effective as a therapeutic or chemopreventive agent against melanoma.

Resveratrol. (From Li et al., Free Rad. Biol. Med., 38:243–257, 2005. With permission.)
TABLE R.60
Mechanisms of Resveratrol in Cells In Vitro Related to Cancer Chemoprevention
Pharmacokinetic studies revealed that the target organs of resveratrol are liver and kidney, where it is concentrated after absorption, and is mainly converted to a sulfated form and a glucuronide conjugate. In vivo, resveratrol blocks the multistep process of carcinogenesis at various stages: it blocks carcinogen activation by inhibiting aryl hydrocarbon-induced CYP1A1 expression and activity, and suppresses tumor initiation, promotion, and progression. Besides chemopreventive effects, resveratrol appears to exhibit therapeutic effects against cancer (Aggarwal et al., 2004). Kundu and Surh (2004) reviewed the molecular mechanisms underlying chemoprevention by resveratrol, with special focus on its effect on cellular-signaling cascades mediated by NF-κB and AP-1. The various mechanisms associated with cancer prevention by resveratrol are listed in Table R.60.
Mertens-Talcott and Percival (2005) recently reported that ellagic acid and quercetin both interacted synergistically with resveratrol, inducing apoptosis and causing transient cell-cycle arrest in human leukemia cells (MOLT-4).
Liu and Liu (2004) showed both resveratrol and its analogue, isorhapontigenin, inhibited oxidation of LDL and the generation of reactive-oxygen species. Li and coworkers (2005) recently reported that isorhapontigenin prevented cardiac hypertrophy, a major cause of morbidity and mortality worldwide. As an antioxidant, the mechanism involved inhibition of intracellular signaling transduction pathways.

Isorhapontigenin. (From Li et al., Free Rad. Biol. Med., 38:243–257, 2005. With permission.)
The ability of resveratrol to protect against age-related macular degeneration (AMD) was recently demonstrated by King et al. (2005), who showed resveratrol significantly reduced cell proliferation of a human retinal epithelium cell line (ARPE-19). At a concentration of 100 μmol/L, resveratrol inhibited H2O2-induced intracellular oxidation and protected retinal pigment epithelium from H2O2-induced cell death.
References
Aggarwal, B.B., Bhardwa, A., Aggarwal, R.S., Seeram, N.P., Shishodia, S., and Takada, Y., Role of resveratrol in prevention and therapy of cancer: pre-clinical and clinical studies, Anticancer Res., 24: 2783–2840, 2004.
Bhat, K.P.L. and Pezzuto, J.M., Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishkawa) cells, Cancer Res., 61:6137–6144, 2001.
Gescher, A.J. and Steward, W.P., Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy of resveratrol: a conundrum, Cancer Epidemiol. Biomarkers Prev., 12:953–957, 2003.
Gusman, J., Malonne, H., and Atassi, G., A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol, Carcinogenesis, 22:1111–1117, 2001.
Hao, H.D. and He, L.R., Mechanisms of cardiovascular protection by resveratrol, J. Med. Food, 7:290–298, 2004.
King, R.E., Kent, K.D., and Bomser, J.A., Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition, Chemico-Biol. Interact., 151:142–149, 2005.
Kundu, J.K. and Surh, Y-J., Molecular basis of chemoprevention by resveratrol: NF-κB and AP-1 as potential targets, Mutat. Res., 555:65–80, 2004.
Laden, B.P. and Porter, T.D., Resveratrol inhibits human squalene monooxygenase, Nutr. Res., 21: 747–753, 2001.
Li, H.-L., Wang, A.-B., Huang, Y., Liu, D.-P., Wei, C., Williams, G.M., Zhang, C.-N., Liu, G., Liu, Y.-Q., Hao, D.-L., Hui, R.-T., Lin, M., and Liang, C.-C., Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways, Free Rad. Biol. Med., 38: 243–247, 2005.
Liu, Y. And Liu, G., Isorhapontigenin and resveratrol suppress oxLDL-induced proliferaton and activation of ERK1/2 mitogen-activated protein kinase of bovine aortic smooth muscle cells, Biochem. Pharmacol., 67:777–785, 2004.
Mertens-Talcott, S.U. and Percival, S.S., Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells, Cancer Lett., 218:141–151, 2005.
Niles, R.M., McFarland, M., Weimer, M.B., Redkar, A., Fu, Y.-M., and Meadows, G.G., Resveratrol is a potent inducer of apoptosis in human melanoma cells, Cancer Lett., 190:157– 163, 2003.
Pervaiz, S., Resveratrol: from grapevines to mammalian biology, FASEB J., 17:1975–1985, 2003.
Teel, R.W. and Huynh, H., Modulation by phytochemicals of cytochrome P450-linked enzyme activity, Cancer Lett., 133:135–141, 1998.
Zoberi, I., Bradbury, C.M., Curry, H.A., Bisht, K.S., Goswami, P.C., Roti Roti, J.L., and Gius, D., Radio sensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines, Cancer Lett., 175:165–173, 2002.
Retinoic acid
see also Retinol and Vitamin A Retinoic acid (RA), a transcriptionally active metabolite of vitamin A (retinol), activates two families of nuclear-retinoid receptors that have the potential to regulate the expression of a large number of genes (Soprano et al., 2004). Retinoids are essential for normal embryo development and epithelial differentiation (Klug et al., 1989; Gajovic et al., 1998). These compounds are also involved in chemoprevention and differentiation therapy of some cancers (Hayashi et al., 2000; Yang et al., 2002), with particularly impressive results in the management of acute promyelocytic leukemia (Otsuki et al., 2004; Avvisati and Tallman, 2003). RA is derived from retinol by oxidation through retinol and retinal dehydrogenases, and several cytochrome P450S. The mechanisms that serve to adjust the metabolism of vitamin A to maintain retinoid homeostasis and prevent retinoid excess are not well understood, but the diet has some effects (Ross, 2003).
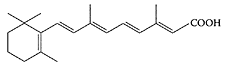
All-trans-retinoic acid (RA). (From Seo et al., Eur. J. Pharm. Biopharm., 58:681–687, 2004. With permission.)
Maden and Hind (2004) have shown that RA is also required during alveologenesis and throughout life for the maintenance of lung alveoli. When rats are deprived of dietary retinol they lose alveoli and show the features of emphysema.
Ping and coworkers (2005) recently examined the effect of all-trans-retinoic acid on p62, a tumor-associated autoantigen identified with autoantibodies from patients with hepatocellular carcinoma (Zhang et al., 1999). RA induced apoptosis in a human gastric cancer-cell line BGC-823 by downregulation and translocation of p62.
References
Avvisati, G. and Tallman, M.S., All-trans retinoic acid in acute promyelocytic leukaemia, Best Pract. Res. Clin. Haematol., 16:419–432, 2003.
Gajovic, S., Chowdhury, K., and Gruss, P., Genes expressed after retinoic acid-mediated differentiation of embryoid bodies are likely to be expressed during embryo development, Exp. Cell Res., 242:138–143, 1998.
Hayashi, K., Yokozaki, H., Naka, K., Yasui, W., Yajin, K., Lotan, R., and Tahara, E., Effect of 9- cis-retinoic acid on oral squamous cell carcinoma cell lines, Cancer Lett., 151:199–208, 2000.
Klug, S., Creech-Kraft, J., Wildi, E., Merker, H.J., Persaud, T.V., Nau, H., and Neubert, D., Influence of 13-cis and all-trans retinoic acid on rat embryonic development in vitro: correlation with isomerisation and drug transfer to the embryo, Arch. Toxicol., 63: 185–192, 1989.
Maden, M. and Hind, M., Retinoic acid in alveolar development, maintenance and regeneration, Philos. Trans. R. Soc. Lond. B. Biol. Sci., 359:799–808, 2004.
Otsuki, T., Sakaguchi, H., Hatayama, T., Wu, P., Takata, A., and Hyodoh, F., Effects of all-trans retinoic acid (ATRA) on human myeloma cells, Leuk. Lymphoma, 44:1651–1656, 2003.
Ping, S., Wang, S., Zhang, J., and Peng, X., Effect of all-trans-retinoic acid on mRNA binding protein p62 in human gastric cancer cells, Int. J. Biochem. Cell Biol., 37:616–627, 2005.
Ross, A.C., Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic acid oxidation, J. Nutr., 133:291–296, 2003.
Seo, S.J., Kim, S.H., Sasagawa, T., Choi, Y.J., Akaike, T., and Cho, C.S., Delivery of all trans retinoic acid (RA) to hepatocyte cell line from RA/galactosyl α-cyclodextrin inclusion compound, Eur. J. Pharm. Biopharm., 58:681–687, 2004.
Soprano, D.R., Qin, P., and Soprano, K.J., Retinoic acid receptors and cancers, Annu. Rev. Nutr., 24:201–221, 2004.
Yang, Q., Sakurai, T., and Kakudo, K., Retinoid, retinoic acid receptor beta and breast cancer, Breast Cancer Res. Treat., 76:167–173, 2002.
Zhang, J.Y., Chan, E.K.L., Peng, X.X., and Tan, E.M., A novel cytoplasmic protein with RNAbinding motifs is an autoantigen in human hepatocellular carcinoma, J. Exp. Med., 189:1101– 1110, 1999.
Retinol
see also Retinoic acid, Vitamin A Retinol, the alcohol form of vitamin A, is stored as retinyl esters and delivered from liver stores into the bloodstream as retinol bound to a retinol-binding protein. In situations of high vitamin A demand (e.g., inflammation, diseases, prenatal period), this supply can be insufficient because of delayed production of retinol-binding protein, leading to local deficiencies and impairment of structure and function in the respective tissues. This delay may be overcome by cellular-retinyl esters stores that can be enriched by topically applied retinyl esters (Biesalski and Nohr, 2004).
The metabolism of vitamin A (retinol) to retinyl esters by lecithin:retinol acyl-transferase has been found to be substantially reduced in human carcinoma cell lines. Recently, Boorjian and coworkers (2004) tested normal and malignant bladder-tissue specimens from human patients and found a significant reduction in lecithin: retinol acyl-transf erase expression in bladder cancer with an inverse correlation between lecithin:retinol acyl-transferase mRNA and protein expression with increasing tumor stage. These data suggest that loss of lecithin:retinol acyl-transferase expression is associated with invasive bladder cancer.
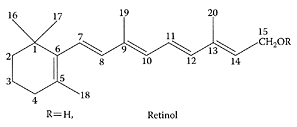
Retinol structure. (From Choi et al., Anal. Chim. Acta, 512:141–147, 2004. With permission.)
The all trans form of retinol is a naturally occurring form, which can be converted to a corresponding geometrical isomer, 9-cis-retinol form. In fact, 9-cis-retinol in combination with cis-retinol dehydrogenase was found to inhibit breast-cancer cell proliferation by producing retinol metabolites other than 9-cis-retinoic acid (Paik et al., 2005). An epidemiological study on postmenopausal women in Sweden revealed that chronic excess of retinol intake (> 1.5 mg/day) decreased bone-mineral density and increased hip-fracture risk (Whiting and Lemke, 1999). Skeletal effects of toxic amounts of vitamin A are known from acute toxic exposure to chronic high-dose intake of vitamin A. Such effects have led experts to speculate that long-term consumption of diets high in vitamin A (retinol) that stimulate bone resorption and inhibit bone formation may contribute to osteoporosis and hip fractures (Genaro Pde and Martini, 2004; Boucher et al., 2003).
References
Biesalski, H.K. and Nohr, D., New aspects in vitamin a metabolism: the role of retinyl esters as systemic and local sources for retinol in mucous epithelia, J. Nutr., 134:3453S–3457S, 2004.
Boucher, B.J., Chandra, R.K., Melhus, H., and Michaelssohn, K., Serum retinol levels and fracture risk, N. Engl. J. Med., 348:1927–1928, 2003.
Boorjian, S., Tickoo, S.K., Mongan, N.P., Yu, H., Bok, D., Rando, R.R., Nanus, D.M., Scherr, D.S., and Gudas, L.J., Reduced lecithin: retinol acyltransferase expression correlates with increased pathologic tumor stage in bladder cancer, Clin. Cancer Res., 10:3429–3437, 2004.
Choi, Y.H., Kim, H.K., Wilson, E.G., Erkelens, C., Trijzelaar, B., and Verpoorte, R., Quantitative analysis of retinol and retinol palmitate in vitamin tablets using 1H-nuclear magnetic resonance spectroscopy, Anal. Chim. Acta, 512:141–147, 2004.
de Souza Genaro, P.S. and Martin, L.A., Vitamin A supplementation and risk of skeletal fracture, Nutr. Rev., 62:65–67, 2004.
Holick, C.N., Michaud, D.S., Stolzenberg-Solomon, R., Mayne, S.T., Pietinen, P., Taylor, P.R., Virtamo, J., and Albanes, D., Dietary carotenoids, serum β-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study, Am. J. Epidemiol., 156:536–547, 2002.
Paik, J., Blaner, W.S., and Swisshelm, K., Cis-retinol dehydrogenase: 9-cis-retinol metabolism and its effect on proliferation of human MCF7 breast cancer cells, Exp. Cell Res., 303:183–196, 2005.
Schuurman, A.G., Goldbohm, R.A., Brants, H.A., and van den Brandt, P.A., A prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands), Cancer Causes Con., 13:573–582, 2002.
Whiting, S.J. and Lemke, B., Excess retinol intake may explain the high incidence of osteoporosis in northern Europe, Nutr. Rev., 57:192–195, 1999.
Rhubarb
The rhubarb plant originated in Tibet or Mongolia, and from the 16th to 18th centuries was used medicinally in Europe and Asia. It served as a laxative, antiphlogistic, and homeostatic in the treatment of constipation, diarrhea, jaundice, gastrointestinal hemorrhage, menstrual disorders, conjunctivitis, traumatic injuries, superficial supportive supperative sores, and ulcers (Peigen et al., 1984; Gu et al., 2000). Chunsheng et al. (2000) suggested rhubarb can ameliorate acute lung injury by inhibiting intercellular adhesion molecule mRNA expression. It can also be applied externally for thermal burns. Chen et al. (2001) reported that rhubarb reduced intestinal juice IgA content in mice caused by burn, which suggested an important mechanism of rhubarb was involved in protecting the muco-membranous barrier.

Red rhubarb stalks.
The edible stalk, about an inch wide, is often more than a foot long and is composed of 95 percent water. It is a fair source of potassium, contributing minor amounts of vitamins, and is low in sodium. Rhubarb’s crisp, sour stalks are rich in vitamin C, dietary fiber, and calcium, although the calcium is combined with oxalic acid. Oxalic acid can lead to an increase in urinary-oxalate excretion, which is a risk factor for kidney-stone formation. Rhubarb is somewhat acidic (pH 3.1–3.2), with one cup of diced rhubarb containing about 26 calories.
Stilbenes were isolated from Korean rhubarb by Matsuda et al. (2004) and their antiallergic activities studied in vitro. Their results revealed that 3,5,4′- trimethylpiceatannol exhibited the most potent inhibition against β-hexosaminidase release as a marker of degranulation, followed by trimethylresveratrol (Scheme R.52). Piceatannol, 3,5,4′-trimethylpiceatannol, resveratrol, and trimethylresveratrol all significantly inhibited antigen-induced release of TNF-α and IL-4.
Iizuka et al. (2004) attempted to estimate the antioxidative activity of rhubarb components on low-density lipoprotein (LDL). They reported a significant, multiple correlation coefficient for antioxidative activities on LDL (R=0.914, p<0.01) involving five components: aloe-emodin, chrysophanol, emodin 1-O-β-D-glucoside, lindleyin, and 6-hydroxymusizin 8-O-β-D-glucoside.
Rhubarb was also reported to exert protective effects on severe acute pancreatitis, probably by inhibiting inflammation of the pancreas, improving pancreatic microcirculation, and altering exocrine secretion (Zhao et al., 2004). Emodin and rhein isolated from rhubarb were found to be major iNOS inhibitors and may possibly serve as bioactive substances for anti-inflammation effects (Wang et al., 2002).
References
Chen, X.L., Huang, X.L., and Wu, H., Effect of rhubarb on intestinal immune associated secretion in healthy mice and in burn mice, Zhongguo Zhong Xi Yi Jie He Za Zhi, 21:754–756, 2001.
Chunsheng, L., Peichun, G., and Xinhua, H., Expression of intercellular adhesion molecule in lung tissues of experimental acute lung injury and the effect of Rhubarb on it, Chin. Med. Sci. J., 15:93–97, 2000.
Gu, J., Zhang, X., Fei, Z., Wen, A., Qin, S., Yi, S., Chen, Y., and Li, X., Rhubarb extracts in treating complications of severe cerebral injury, Chin. Med. J., 113:29–531, 2000.
Iizuka, A., Iijima, O.T., Kondo, K., Itakura, H., Yoshie, F., Miyamoto, H., Kubo, M., Higuchi, M., Takeda, H., and Matsumiya, T., Evaluation of rhubarb using antioxidative activity as an index of pharmacological usefulness, J. Ethnopharmacol., 91:89–94, 2004.
Matsuda, H., Tewtrakul, S., Morikawa, T., and Yoshikawa, M., Anti-allergic activity of stilbenes from Korean rhubarb (Rheum undulatum L.): structure requirements for inhibition of antigeninduced degranulation and their effects on the release of TNF-α and IL-4 in RBL-2H3 cells, Bioorg. Med. Chem., 12:871–4876, 2004.
Peigen, X., Liyi, H., and Liwei, W., Ethnopharmacologic study of Chinese rhubarb, J. Ethnopharmacol., 10:275–293, 1984.
Wang, C.C., Huang, Y.J., Chen, L.G., Lee, L.T., and Yang, L.L., Inducible nitric oxide synthase inhibitors of Chinese herbs III, Rheum palmatum, Planta Med., 68:869–874, 2002.
Yamagishi, T., Nishizawa, M., Ikura, M., Hikichi, K., Nonaka, G., and Nishioka, I., New laxative constituents of rhubarb, isolation and characterization of rheinosides A, B, C and D, Chem. Pharm. Bull., 35: 3132–3138, 1987.
Zhao, Y-Q., Liu, X-H., Ito, T., and Qian, J.M., Protective effects of rhubarb on experimental severe acute pancreatitis, World J. Gastroenterol., 10:1005–1009, 2004.
Rice
Rice (Oryzae sativum) is the principal food crop in Asia, where the incidence of breast and colon cancer is markedly below that found in the Western world. Hudson et al. (2000) investigated the potential colon and breast tumor-suppressive properties of rice. Their results suggested that brown rice and bran contain compounds had putative cancer chemopreventive properties. The phenols exhibiting this activity were present in brown-rice bran, such as tricin (Cai et al., 2004). However, they are present at much lower levels in white compared to brown rice. Thus, the consumption of rice bran or brown rice instead of milled white rice may be advantageous with respect to cancer prevention.
While tricin was a potent inhibitor of breast tumor-cell growth in vitro, Cai et al. (2004) found it had little effect on nude mice bearing human-derived malignant MDA-MB-468 breast-tumor cells. However, the high levels of tricin in the gastrointestinal tract after dietary intake may prove beneficial in preventing colorectal cancer.

Cancertricin structure. (4′,5,7-trihydroxy-3′,5′-dimethoxy-flavone). (From Cai et al., Br. J. Cancer, 91:1364–1371, 2004. With permission.)
Other investigations of potential beneficial effects of specific rice constituents in terms of prevention or amelioration of malignant disease have been published. These reports suggest that rice constituents counteract chemical-induced mutagenicity (Kang et al., 1996; Nam and Kang, 1997), tumor promotion (Yasukawa et al., 1998), carcinogenicity (Aoe et al., 1993), and established neoplastic growth in rodents (Hayashi et al., 1998; Koide et al., 1996). However, relatively little is known about which specific molecules may be responsible for these activities. Some of the evidence concerning the chemopreventive and antitumor properties of rice suggests that it is predominantly the bran portion of the grain that contains biologically active substances. The preventive potential of rice bran extract against the oxygen radical-related chronic diseases, such as cardiovascular diseases and cancer, antioxidative and antigenotoxic activities of the rice-bran extracts was demonstrated recently by Higashi-Okai et al. (2004).
Rice-bran oil is tenaciously believed to be a healthy vegetable oil in Asian countries. It exerts hypocholesterolemic activity in relation to more commonly used vegetable oils and is characterized by a relatively high content of nonfatty-acid components, some of which are known to have beneficial health effects, such as gamma-oryzanol and tocotrienols that could participate in its hypocholesterolemic effects (Sugano et al., 1999).
References
Aoe, S., Oda, T., Tojima, T., Tanaka, M., Tatsumi, K., and Mizutani, T., Effects of rice bran hemicellulose on 1,2-dimethylhydrazine-induced intestinal carcinogenesis in Fischer 344 rats, Nutr. Cancer, 20: 41–49, 1993.
Cai, H., Hudson, E.A., Mann, P., Verschoyle, R.D., Greaves, P., Manson, M.M., Steward, W.P., and Gescher, A.J., Growth-inhibitory and cell cycle-arresting properties of the rice bran constituent tricin in human-derived breast cancer cells in vitro and in nude mice in vivo, Br. J. Cancer, 91:1364–1371, 2004.
Hayashi, Y., Nishikawa, Y., Mori, H., Tamura, H., Matsushita, Y.-I., and Matsui, T., Antitumor activity of (10E,12Z)-9-hydroxy-10,12-octadecadienoic acid from rice bran, J. Ferment. Bioengineer., 86:149–153, 1998.
Higashi-Okai, K., Kanbara, K., Amano, K., Hagiwara, A., Sugita, C., Matsumoto, N., and Okay, Y., Potent antioxidative and antigenotoxic activity in aqueous extract of Japanese rice bran— association with peroxidase activity, Phytother. Res., 18:628–633, 2004.
Hudson, E.A., Dinh, P.A., Kokubun, T., Simmonds, M.S.J., and Gescher, A., Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells, Cancer Epidemiol. Biomarkers Prev., 9:1163–1170, 2000.
Kang, M.Y., Choi, Y.H., and Nam, S.H., Inhibitory mechanism of colored rice bran extract against mutagenicity induced by chemical mutagen mitomycin C, Agric. Chem. Biotech., 39:424–429, 1996.
Koide, T., Kamei, H., Hashimoto, Y., Kojima, T., and Hasegawa, M., Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice, Cancer Biother. Radiopharm., 11:73– 277, 1996.
Nam, S.H. and Kang, M.Y., In vitro inhibitory effect of colored rice bran extracts on carcinogenicity, Agric. Chem. Biotech., 40:307–312, 1997.
Sugano, M., Koba, K., and Tsuji, E., Health benefits of rice bran oil, Anticancer Res., 19:3651– 3657, 1999.
Yasukawa, K., Akihisa, T., Kimura, Y., Tamura, T., and Takido, M., Inhibitory effect of cycloartenol ferulate, a component of rice bran, on tumor promotion in two-stage carcinogenesis in mouse skin, Biol. Pharm. Bull., 21:1072–1076, 1998.
Rice starch
Rice starch cannot be completely digested by enzymes in the small intestine. Cheng and Lai (2000) demonstrated that resistant rice starch is fermented to produce propionic acid, which reduced serum total cholesterol, serum LDL cholesterol, hepatic cholesterol, and hepatic triglyceride in rats. Kim et al. (2003) reported that resistant starch from rice could also shorten the intestinal transit time and could lower plasma total lipid and cholesterol concentrations compared to diabetic control.
Rice-starch-based oral rehydration solution (ORS) has been shown to be a suitable alternative to glucose-based ORS in the treatment of both choleragenic and noncholeragenic dehydration in older infants and in children and also in the rehydration of acute diarrheal dehydration in infants below 6 months of age (Iyngkaran and Yadav, 1998).
Rice starch added to bath water was found to have beneficial effects on impaired barrier function, as evaluated by trans-epidermal water-loss measurements. Rice starch in powder or formulated in a bath product is therefore recommended by de Paepe et al. (2002) as a skin-repair bathing additive for barrier-damaged skin, particularly in the case of atopicdermatitis patients.
References
Cheng, H.H. and Lai, M.H., Fermentation of resistant rice starch produces propionate reducing serum and hepatic cholesterol in rats, J. Nutr., 30:1991–1995, 2000.
de Paepe, K., Hachem, J-P., Vanpee, E., Roseeuw, D., and Rogiers, V., Effect of rice starch as a bath additive on the barrier function of healthy but SLS-damaged skin and skin of atopic patients, Acta Derm. Venereol., 82:184–186,2002.
Iyngkaran, N. and Yadav, M., Rice-starch oral rehydration therapy in neonates and young infants, J. Trop. Pediatr., 44:199–203, 1998.
Kim, W.K., Chung, M.K., Kang, N.E., Kim, M.H., and Park, O.J., Effect of resistant starch from corn or rice on glucose control, colonic events, and blood lipid concentrations in streptozotocin-induced diabetic rats, J. Nutr. Biochem., 14:166–172, 2003.
Rooibos tea
Rooibos tea is an herbal tea produced from the leaves and fine stems of the South African leguminous shrub Aspalathus linearis, also known as Rooibos. The herbal tea is considered a health drink due to the presence of beneficial phenolic antioxidants. The antioxidant properties of Rooibos tea were found to be similar to green, oolong, and black tea (von Gadow et al., 1997a). Rooibos tea, however, contains a unique compound, aspalathin, that mimics superoxide dismutase (SOD) (Yoshikawa et al., 1990; Ito et al., 1991). Compared to BHA, BHT, and α-tocopherol, aspalathin exhibited the highest radical-scavenging activity (von Gadow et al., 1997b). In vitro and in vivo studies found rooibos tea exhibited anti-mutagenic properties against aflatoxin B1 and 2-acetylamino fluorine-induced mutagenesis (Marnewick et al., 2000; Marnewick et al., 2004a). In addition, aqueous extracts of rooibos tea enhanced phase II detoxifying enzymes, glutathione-S transferase, and UDP-glucuronyl transferase in rat liver, stabilizing glutathione (GSH) (Marnewick et al., 2003). Ethanol/ acetone (E/A)-soluble fractions prepared from methanolic extracts of processed and unprocessed South African herbal teas, rooibos, and honeybush compared to green tea were recently shown by Marnewick and coworkers (2004b) to inhibit tumor promotion in mouse skin. Using the two-stage mouse-skin carcinogenesis assay with the tumor promoter 12-O-tetra decanoylphorbol-13-acetate (TPA) on ICR mouse skin initiated with 7,12-dimethyl benz[a]anthracene (DMBA), they found herbaltea fractions significantly (p<0.001) decreased tumor volume, as well as delayed their development (Figure R.87). Compared to the control, tumors did not appear in the DMBA/TPA-treated mice at 4 and 12 weeks when maintained on processed and unprocessed rooibos, respectively. Green tea exhibited 100 percent inhibition compared to 90 percent and 84.2 percent inhibition for unprocessed and processed honeybush. While processed and unprocessed rooibos proved to be the least effective, they nevertheless accounted for an impressive 75 percent and 60 percent inhibition of tumor promotion, respectively. The variability in tumor inhibition exhibited by these herbal teas was attributed to differences in their flavonol/proanthocyanidin and flavonol/flavone composition and nonpolyphenolic components.

FIGURE R.87 Inhibitory effect of topical application of various E/A polyphenolic fractions on TPA-induced tumor promotion. The percentage of mice with tumors is plotted as a function of the treatment period (weeks). The fractions include Rp, processed rooibos; Rg, unprocessed rooibos; Hp, processed honeybush; Hg, unprocessed honeybush; and Gr, green tea. The number of animals per group=15–20. (From Marnewick et al., Cancer Lett., 224:193–202, 2005.)
References
Ito, A., Shinohara, K., and Kator, K., Protective action of Rooibos tea (Aspalathis linearis) extract against inactivation of L5178Y cells by H2O2, in Proceedings of the International Symposium on Tea Science, Shizuoka, Japan, 1991, pp. 381–384.
Jaganyi, D. and Wheeler, P.J., Rooibos tea: equilibrium and extraction kinetics of aspalathin, Food Chem., 83:121–126, 2003.
Marnewick, J.L., Batenburg, W., Wart, P., Joubert, E., Swanevelder, S., and Gelderblom, W.C.A., Ex vivo modulation of chemical-induced mutagenesis by sub cellular liver fraction of rats treated with rooibos (Aspalathus linearis) tea, honeybush (Cyclopia intermedia) tea, as well as green and black (Camellia sinensis) teas, Mutat. Res., 558:145–154, 2004a.
Marnewick, J.L., Gelderblom, W.C.A., and Joubert, E., An investigation of the antimutagenic properties of South African herbal teas, Mutat. Res., 471:157–166, 2000.
Marnewick, J., Joubert, E., Joseph, S., Swanevelder, S., Swart, P., and Gelderblom, W., Inhibition of tumor promotion in mouse skin by extracts of rooibos (Aspalathis linearis) and honeybush (Cyclopedia intermedia), unique South African herbal teas, Cancer Lett., 224:193–202, 2005.
Marnewick, J.L., Joubert, E., Swart, P., Joubert, E., van der Westhuizen, F., and Gelderblom, W.C.A., Modulation of hepatic drug metabolizing enzymes and oxidative status of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia) green and black (Camellia sinensis) teas in rats, J. Agric. Food Chem., 51:8113–8119, 2003.
von Gadow, A., Joubert, E., and Hansmann, C.F., Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, Oolong and black tea, Food Chem., 60:73–77, 1997a.
von Gadow, A., Joubert, E., and Hansmann, C.F., Comparison of the antioxidant activity of aspalathin with that of other plant phenols of Rooibos tea (Aspalathus linearis), alphatocopherol, BHT, and BHA, J. Agric. Food Chem., 45:632–638, 1997b.
Yoshikawa, T., Naito, Y., Oyamada, H., Ueda, S., Tanigawa, S., Takemura, T., Sugino, S., and Kondo, M., Scavenging effect of Aspalathus linearis (Rooibos tea) on active oxygen species, in Antioxidants in Therapy and Preventative Medicine, Emerit, I., Packer, L., and Auclair, C., Eds., Plenum Press, New York. 1990, pp. 171–174.

Aspalathin. (From Jaganyi and Wheeler, Food Chem., 83:121–126, 2003. With permission.)
Rosemary
see also Rosmarinic acid Rosemary (Rosmarinus officinalis Linn.) is a common household plant. It is used as food flavoring and a beverage drink, as well as in cosmetics. In folk medicine, it is used as an antispasmodic in renal colic and dysmenorrhoea, to relieve respiratory disorders, to stimulate growth of hair, and as a mild analgesic and antimicrobial agent (Newall, 1996). Extract of rosemary relaxes smooth muscles of trachea and intestine, and has choleretic, hepatoprotective, and antitumerogenic activity (Al-Sereiti et al., 1999). The leaves of rosemary contain valuable essential oils rich in mono- and sesquiterpenes, including borneol, camphor, carophyllene, cineol, humulene, linalool, and thujone “salviol.” The strong, antioxidant activity associated with rosemary leaves is associated with these phenolic diterpenes.
The most important constituents of rosemary are caffeic acid and its derivatives, such as rosmarinic acid. These compounds and other phenolic diterpenes, flavonoids, and phenolic acids (Ho et al., 2000) have antioxidant effects. Slamenova et al. (2002) also reported that rosemary extract exhibits a protective effect against oxidative damage to DNA as a consequence of scavenging of both OH radicals and singlet oxygen.
Rosmarinic acid, a caffeic-acid derivative, is well-absorbed from the gastrointestinal tract and from the skin. It increases the production of prostaglandin E2 and reduces the production of leukotriene B4 in human polymorphonuclear leucocytes, and inhibits the complement system (Al-Sereiti et al., 1999). It also showed therapeutic potential in treatment or prevention of bronchial asthma, spasmogenic disorders, peptic ulcer, inflammatory diseases, hepatotoxicity, atherosclerosis, ischaemic heart disease, cataract, cancer, and poor sperm motility (Rampart et al., 1986; Al-Sereiti et al., 1999).
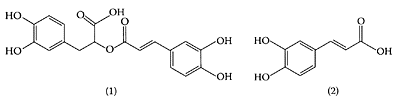
Rosmarinic acid (1) and caffeic acid (2). (From Wang et al., Food Chem., 87:307–311, 2004.)
Among the antioxidant compounds in rosemary leaves, ~90 percent of the antioxidant activity can be attributed to carnosol and carnosic acid. Topical application of rosemary extract, carnosol, or ursolic acid to mouse skin inhibited the covalent binding of benzo[a]pyrene to epidermal DNA, tumor initiation by 7,12-dimethylbenz[a]anthracene (DMBA), TPA-induced tumor promotion, ornithine decarboxylase activity, and inflammation (Huang et al., 1994).
Additional studies revealed that carnosic acid and carnosol strongly inhibited phase I enzyme CYP 450 activities and induced the expression of the phase II enzyme, glutathione S-transferase (GST) (Mace et al., 1998). These results give insight into different mechanisms involved in the chemopreventive actions of rosemary.
Recently Lo et al. (2002) demonstrated that carnosol can suppress the NO production and iNOS gene expression by inhibiting NF-κB activation, and provide possible mechanisms for its anti-inflammatory and chemopreventive action.
References
Al-Sereiti, M.R., Abu-Amer, K.M., and Sen, P., Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials, Indian J. Exp. Biol., 37:124–130, 1999.
Ho, C.T., Wang, M., Wei, G.J., Huang, T.C., and Huang, M.T., Chemistry and antioxidative factors in rosemary and sage, Biofactors, 13:161–166, 2000.
Huang, M.T., Ho, C.T., Wang, Z.Y., Ferraro, T., Lou, Y.R., Stauber, K., Ma, W., Georgiadis, C., Laskin, J.D., and Conney, A.H., Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid, Cancer Res., 54:701–708, 1994.
Lo, A-H., Liang, Y-C., Lin-Shiau, S-Y., Ho, C-T., and Lin, J-K., Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages, Carcinogenesis, 23:983–991, 2002.
Mace, K., Offord, E.A., Harris, C.C., and Pfeifer, A.M., Development of in vitro models for cellular and molecular studies in toxicology and chemoprevention, Arch. Toxicol. Suppl., 20:227–236, 1998.
Newall, C.A., Herbal Medicines—A Guide For Health Care Professionals, The Pharmaceutical Press, London, 1996.
Rampart, M., Beetens, J.R., Bult, H., Herman, A.G., Parnham, M.J., and Winkelmann, J., Complement-dependent stimulation of prostacyclin biosynthesis: inhibition by rosmarinic acid, Biochem. Pharmacol., 35:1397–1400, 1986.
Slamenova, D., Kuboskova, K., Horvathova, E., and Robichova, S., Rosemary-stimulated reduction of DNA strand breaks and FPG-sensitive sites in mammalian cells treated with H2O2 or visible light-excited Methylene Blue, Cancer Lett., 177:145–153, 2002.
Wang, H., Provan, G.J., and Helliwell, P.K., Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC, Food Chem., 87:307–311, 2004.
Rosmarinic acid
see also Rosemary Rosmarinic acid is a phenolic compound widely distributed in Labiatae herbs, such as rosemary, sweet basil, and perilla (Scarpati and Oriente, 1958; Makino et al., 1998). This naturally occurring polyphenol exhibits antioxidant (Tada et al., 1996) and anti-inflammatory effects, such as inhibitory effects on a complement-dependent inflammatory process (Peake et al., 1991), 5-lipoxygenase (Yamamoto et al., 1998), and histamine release from mast cells (Rimando et al., 1987). Sanbong et al. (2003) showed that it inhibited diesel-exhaust particles (DEP)-induced lung injury by reducing the expression of the macrophage inflammatory protein-1α.
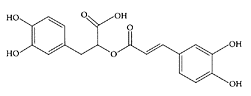
Rosmarinic acid. (From Wang et al., Food Chem., 87:307–311, 2004. With permission.)
Toshiaki et al. (2000) reported rosmarinic acid inhibited cytokine-induced mesangial-cell proliferation and suppressed platelet-derived growth factor (PDGF) and c-myc mRNA expression in PDGF-stimulated mesangial cells, all of which suggest that it might be a promising agent to prevent mesangial-cell proliferation.
References
Makino, T., Ono, T., Muso, E., and Honda, G., Inhibitory effect of Perilla frutescens and its phenolic constituents on cultured murine mesangial cell proliferation, Planta Med., 64:541–545, 1998.
Peake, P.W., Pussell, B.A., Martyn, P., Timmermans, V., and Charles worth, J.A., The inhibitory effect of rosmarinic acid on complement involves the C5 convertase, Int. J. Immunopharmacol., 13:853–857, 1991.
Rimando, A.M., Inoshiri, S., Otsuka, H. et al., Screening for mast cell histamine release inhibitory activity of Philippine medicinal plants. Active constituent of Ehretia microphyll, Shoyakugaku Zasshi, 41:242–247, 1987.
Sanbongi, C., Takano, H., Osakabe, N., Sasa, N., Natsume, M., Yanagisawa, R., Inoue, K., Kato, Y., Osawa, T., and Yoshikawa, T., Rosmarinic acid inhibits lung injury induced by diesel exhaust particles, Free Rad. Biol. Med., 34:1060–1609, 2003.
Scarpati, M.L. and Oriente, G., Isolation and constitution of rosmarinic acid from Rosmarinus officinalis, Ricerca Sci., 28:2329–2333, 1958.
Tada, M., Matsumoto, R., Yamaguchi, H., and Chiba, K., Novel antioxidants isolated from Perilla frutescens Britton var. crispa (Thunb.), Biosci. Biotech. Biochem., 60:1093–1095, 1996.
Toshiaki, M., Ono, T., Muso, E., Yosida, H., Honda, G., and Sasayray, S., Inhibitory effects of rosmarinic acid on the proliferation of cultured murine mesangial cells, Nephrol. Dial. Transplant, 15:1140–1145, 2000.
Wang, H., Provan, G.J., and Helliwell, P.K., Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC, Food Chem., 87:307–311, 2004.
Yamamoto, H., Sakakibara, J., Nagatsu, A., and Sekiya, K., Inhibitors of arachidonate lipoxygenase from defatted perilla seed, J. Agri. Food Chem., 46: 862–865, 1998.
Rosy or Madagascar periwinkle (Other names: Cape Periwinkle, Catharanthus roseus, Church Flower, Red Periwinkle)

Rosy periwinkle (Catharanthus roseus), a medicinal plant found on the island of Madagascar, was used in traditional medicines for the treatment of cancer, Hodgkin’s disease, and leukemia in children (Cardinali, 1973). Synthetic vincristine, used to treat leukemia, is only 20 percent as effective as the natural product derived from Catharanthus roseus. It was also found to have some potent blood-sugar-lowering activity (Chattopadhyay, 1999). Wang et al. (2004) reported that aqueous extracts of Catharanthus roseus significantly inhibited proliferation of cultured bovine aortic endothelial cells at a concentration of 1 g dry herb/mL, suggesting its role as a potential antiangiogenic agent. However, the use of Catharanthus roseus is not recommended due to the risk of severe side effects (Carod-Artal, 2003). Chemicals derived from it are used in prescription-only anticancer drugs (Ram and Kumari, 2001). Catharanthus roseus and the drugs derived from it have been associated with causing birth defects, neurotoxicity, bone-marrow suppression, and sensitivity to sunlight (Mathur and Chaudan, 1985). In addition, it may also cause gastrointestinal complaints, headache, and muscle weakness.
References
Cardinali, G., Place of Vinca rosea alkaloids (Catharanthus roseus) in the treatment of Hodgkin’s disease, Haematologica, 53:51–64, 1973.
Carod-Artal, F.J., Neurological syndromes linked with the intake of plants and fungi containing a toxic component (I), neurotoxic syndromes caused by the ingestion of plants, seeds and fruits, Rev. Neurol., 36: 860–871, 2003.
Chattopadhyay, R.R., A comparative evaluation of some blood sugar lowering agents of plant origin, J. Ethnopharmacol., 67:367–372, 1999.
Mathur, R. and Chaudan, S., Antifertility efficacy of Catharanthus roseus Linn: a biochemical and histological study, Acta Eur. Fertil., 16:203–205, 1985.
Ram, V.J. and Kumari, S., Natural products of plant origin as anticancer agents, Drug News Perspect., 14: 465–482, 2001.
Wang, S., Zheng, Z., Weng, Y., Yu, Y., Zhang, D., Fan, W., Dai, R., and Hu, Z., Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts, Life Sci., 74:2467–2478, 2004.
Rye
see also Ferulic acid Rye bran contains, in addition to a high-content dietary fiber, plant lignans and other bioactive compounds, such as alkylresorcinols (AR) (Ross et al., 2004). These are phenolic lipids present in large amounts in the bran fraction of rye (Scheme R.53). They are amphiphilic 1,3-dihydroxybenzene derivatives with an odd-numbered alkyl chain at position 5 in the benzene ring (Kozubek and Tyman, 1999). Early research reported serious growth inhibition and other pathological symptoms in several animal species (Sedlet et al., 1984). Other reports, however, suggest ARs have anti-bacterial and antifungal properties, as well as antiparasitic, antitumor, and antioxidant properties (Kozubek and Tyman, 1999). Gasiorowski and coworkers (1996) showed AR markedly decreased the mutagenic effects of a number of mutagens using the Ames test. Kamil-Eldin and coworkers (2001) showed AR exhibited antioxidant activity in vitro, but it was still poor relative to α-tocopherol.
At present, evidence from studies in human subjects does not warrant the conclusion that rye, whole grains, or phytoestrogens protect against cancer. Some studies, however, have pointed in that direction, especially in relation to cancers of the upper digestive tract and of the colon (Grasten et al., 2000). Rye foods also improved bowel health, as assessed by relevant markers (McIntyre et al., 1993; McIntosh et al., 2003). In comparison to wheat, rye is a slightly better source of total dietary fiber and is more commonly used in whole-grain food forms, which, together with cellulose, contributes more mixed linked 1→3,1→4 β-glucan and arabinoxylan (Aman et al., 1997). The latter fiber types are of particular interest, because they are present in soluble and insoluble forms, and arabinoxylan is considered to be an optimal substrate for fermentative generation of short-chain fatty acids in particular, and of butyrate in the colon. High concentration of butyrate in the colon is hypothesized to improve bowel health and lower cancer risk by several possible mechanisms (Bach et al., 1997). Ferulic acid, the major phenolic compound in rye bran and an antioxidant in vitro, however, did not produce a measurable antioxidative effect on human LDL (Harder et al., 2004).

SCHEME R.53 Skeletal structures of alkylresorcinol-related analogs in rye. (From Suzuki et al., Phytochemistry, 52:281–289, 1999. With permission.)

Ferulic acid. (Adapted from Hynes and O’Coinceanainn, J. Inorg. Biochem., 98:1457–1464, 2004.)
A number of prospective epidemiological studies have clearly shown a protective effect by whole-grain cereals against myocardial infarctions (Pietinen et al., 1996). A corresponding protective effect against diabetes (Leinonen et al., 1999) and ischemic stroke (brain infarct) have also been demonstrated (Hallmans et al., 2003). A high-fiber rye diet decreased insulin secretion, measured as decreased excretion of C-peptide in the urine and decreased plasma insulin peaks at the end of the day, during nibbling regimen (Lundin et al., 2004).
References
Aman, P., Nilsson, M., and Anderson, R., Positive health effects of rye, Cereal Foods World, 42:684–688, 1997.
Bach Knudsen, K.E., Johansen, H.N., and Glitso, L., Rye dietary fiber and fermentation in the colon, Cereal Foods World, 42:690–694, 1997.
Gasiorowski, K., Szyba, K., Brokos, B., and Kozubek, A., Antimutagenic activity of alkylresorcinols from cereal grains, Cancer Lett., 106:109–115, 1996.
Grasten, S.M., Juntunen, K.S., Poutanen, K.S., Gylling, H.K., Miettinen, T.A., and Mykkanen, H.M., Rye bread improves bowel function and decreases the concentrations of some compounds that are putative colon cancer risk markers in middle-aged women and men, J. Nutr., 130:2215– 2221, 2000.
Hallmans, G., Zhang, J.X., Lundin, E., Stattin, P., Johansson, A., Johansson, I., Hulten, K., Winkvist, A., Aman, P., Lenner, P., and Adlercreutz, H., Rye, lignans and human health, Proc. Nutr. Soc., 62:193–199, 2003.
Harder, H., Tetens, I., Let, M.B., and Meyer, A.S., Rye bran bread intake elevates urinary excretion of ferulic acid in humans, but does not affect the susceptibility of LDL to oxidation ex vivo, Eur. J. Nutr., 43:230–236, 2004.
Hynes, M.J. and O’Coinceanainn, M., The kinetics and mechanisms of reactions of iron (III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin, J. Inorg. Biochem., 98:1457–1464, 2004.
Kamil-Eldin, A., Pouru, A., Eliasson, C., and Aman, P., Alkylresorcinols as antioxidants: hydrogen donation and peroxyl scavenging effects, J. Sci. Food Agric., 81:353–356, 2001.
Kozubek, A. and Tyman, J.H.P., Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity, Chem. Rev., 99:1–26, 1999.
Leinonen, K., Liukkonen, K., Poutanen, K., Uusitupa, M., and Mykkanen, H., Rye bread decreases postprandial insulin response but does not alter glucose response in healthy Finnish subjects, Eur. J. Clin. Nutr., 53:262–267, 1999.
Lundin, E.A., Zhang, J.X., Lairon, D., Tidehag, P., Aman, P., Adlercreutz, H., and Hallmans, G., Effects of meal frequency and high-fibre rye-bread diet on glucose and lipid metabolism and ileal excretion of energy and sterols in ileostomy subjects, Eur. J. Clin. Nutr, 58:410–1419, 2004.
McIntyre, A., Gibson, P.R., and Young, G.P., Butyrate production from dietary fiber and protection against large bowel cancer in a rat model, Gut, 34: 386–391, 1993.
McIntosh, G.H., Noakes, M., Royle, P.J., and Foster, P.R., Whole-grain rye and wheat foods and markers of bowel health in overweight middle-aged men, Am. J. Clin. Nutr., 77:967–974, 2003.
Pietinen, P., Rimm, E.B., Korhonen, P., Hartman, A.M., Willett, W.C., Albanes, D., and Virtamo, J., Intake of dietry fibre and risk of coronary heart disease in a cohort of Finnish men, The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, Circulation, 94:2720–2727, 1996.
Ross, A.B., Kamal-Eldin, A., and Aman, P., Dietary alkylresorcinols: absorption, bioactivities, and possible use as biomarkers of whole-grain wheat- and rye-rich foods. Nutr. Rev., 62:81–95, 2004.
Sedlet, K., Mathias, M., and Lorenz, K., Growthdepressing effects of 5-n-pentadecylresorcinol: a model for cereal alkylresorcinols, Cereal Chem., 61: 239–241, 1984.
Suzuki, Y., Esumi, Y., and Yamaguchi, I., Structures of 5-alkylresorcinol-related analogues in rye, Phytochemistry, 52:281–289, 1999.
