W
Walnuts
Walnuts, the seeds of Juglans regia L., have been used as a folk medicine in Europe and Asia for treating coughs and stomach ache (Perry, 1980) and cancer (Duke, 1989). Hu et al. (1998) showed that frequent consumption of nuts may offer some protection from coronary heart disease. Nuts, such as walnuts, are relatively high in polyunsaturated fatty acids, particularly omega-3 fatty acids. Iwamoto and coworkers (2000) examined the effect of walnuts on the serum cholesterol of Japanese men and women. A moderate intake of walnuts of 43–57 g/d, equivalent to 12.5 percent of energy, significantly reduced LDL cholesterol levels in both Japanese men and women by 0.18 mmol/L and 0.22 mmol/L, respectively. The ratio of LDL to HDL cholesterol was also significantly lowered, as was the apolipoprotein B concentration. A subsequent study by Almario et al. (2001) confirmed the ability of walnuts to beneficially reduce LDL cholesterol in patients with combined hyperlipidemia. Another study by Iwamoto et al. (2002) confirmed that a diet containing 44–58 g/day of walnuts fed to normal Japanese men and women lowered serum cholesterol, as well as had a beneficial effect on lipoproteins. In addition to lowering cholesterol, Ros and coworkers (2004) found that substituting walnuts for monounsaturated fat in a Mediterranean diet also improved endothelium vasodilation in hypercholesterolemic subjects. This suggested that the benefits of nuts went beyond just lowering cholesterol.
In addition to the oil, a polyphenolic-rich walnut extract containing ellagic acid, gallic acid, and flavonoids was shown by Anderson et al. (2001) to be a potent antioxidant by inhibiting oxidation of human plasma and LDL in vitro. The presence of ellagic acid suggested to Fukuda et al. (2003) the possible presence of tannins, such as ellagitannins. These researchers isolated a number of tannins from the butanol extract of walnut, including three hydrolyzable tannins, glansrins A-C, together with 13 known tannins. Glansrins A-C proved to be ellagitannins, with a tergalloyl or related poly-phenolic acyl group. Fukuda et al. (2003) demonstrated the antioxidant properties of these polyphenols by their SOD-like and radical-scavenging activities. A recent study by Kearny et al. (2004) showed that a diet enriched with 64 g/day of walnuts fed to hypercholesterolemic patients over six weeks significantly reduced total cholesterol (−5 percent) and LDL cholesterol (−9 percent). An additional trend observed was a 20 percent reduction in the large VLDL particle subclass. This study further demonstrated the cardiovascular health benefits of a daily diet enriched with walnuts.
References
Almario, R.U., Vonghavaravat, V., Wong, R., and Kasim-Karakas, S.E., Effect of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia, Am. J. Clin. Nutr, 74:72–79, 2001.
Anderson, K.J., Teuber, S.S., Gobielle, A., Cremin, P., Waterhouse, A.L., and Steinberg, F.M., Walnut poly phenolic s inhibit in vitro human plasma and LDL oxidation, J. Nutr., 131:2837– 2842, 2001.
Feldman, E.B., The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease, J. Nutr,, 132:1062–1101, 2002.
Fukuda, T., Ito, H., and Yoshida, T., Antioxidative polyphenols from walnuts (Juglans regia L.), Phytochemistry, 63:795–801, 2003.
Hu, F.B., Sampfer, M.J., Manson, J.E., Colditz, G.A., Rimm, E.B., Rosner, B.A., Speizer, F.E., Hennekens, C.H., and Willett, W.C., Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study, Br. J. Med. J., 317:1341–1345, 1998.
Iwamoto, M, Imaizumi, K., Hirooka, Y., Sakai, K., Takeshit, A., and Kono, M., Serum lipid profiles in Japanese women and men during consumption of walnuts, Eur. J. Clin. Nutr., 56:629–637, 2002.
Iwamoto, M., Sato, M., Kono, M., Hirooka, Y., Sakai, K., Takeshita, A., and Imaizumi, K., Walnuts lower serum cholesterol in Japanese men and women, J. Nutr., 130:171–176, 2000.
Kearny, D., Dulaney, K., Carey, C., Capuzzi, and Morgan, J., Effects of walnut consumption as part of a heart healthy diet on atherogenic lipoprotein subclasses, J. Am. Dietet. Assoc., 104(Suppl. 2):20, 2004.
Ros, E., Nunez, I., Perez-Heras, A., Serra, M., Gilabert, R., Casals, E., and Deulofeu, R., A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation, 109:1609–1614, 2004.
Wasabi (Wasabia japonica)
Wasabi, a member of the Japanese horseradish family with a number of important, health-related compounds, is usually served as a condiment with Japanese cuisine. Sinigrin was shown by Yu et al. (2001) to be the main, sulfur-containing species in wasabi initially hydrolyzed by myrosi-nase, followed by a nonenymatic step, the Lossen rearrangement, to form isothiocyanates (Scheme W.70). Yano et al. (2000) showed isothiocyanate, 6-methylthiohexyl isothiocyanate (6MHITC), isolated from wasabi inhibited 4-(methylnitrosamino)-1-(3-pyridyl)-l-butanone (NNK)-induced lung tumorigenesis in mice, suppressing the initiation phase, that is the formation of O6-methylguanine (O6MG) (Figure W.99). O6MG is a promutagen adduct formed from activation of NNK via a-hydroxylation. 6MHIT significantly reduced the level of O6MG by 55 percent, very similar to the control containing phenylethyl isothiocyanate.
Morimitsu and coworkers (2000) isolated 6-methylsulfinylhexyl isothiocyanate from wasabi and showed it possessed antiplatelet and anticancer properties. Subsequent work by Watanabe et al. (2003) identified the active component in an ethanol extract capable of inducing apoptosis as 6-methylsulfinylhexyl isothiocyanate by its ability to inhibit the cell growth of human monoblastic leukemia U937 cells.
Shin et al. (2004) recently observed the bactericidal properties of wasabi against Helicobacter pylori. In addition to allyl isothiocyante, several other components were also responsible for the effectiveness of wasabi.
References
Morimitsu, Y., Hayashi, K., Nakagawa, Y., Fujii, H., Horio, F., Uchida, K., and Osawa, T., Antiplatelet and anticancer isothiocyanates in Japanese domestic horseradish, Wasabi, Mech. Aging Develop., 116: 125–134, 2000.
Shin, I.S., Masuda, H., and Naohide, K., Bacteriocidal activity of wasabi (Wasabia japonica) against Helicobacter pylori, Inter. J. Food Microbiol., 94: 255–261, 2004.
Watanabe, M., Ohata, M., Hayakawa, S., Isemura, M., Kumazawa, S., Nayakama, T., Furugori, M., and Kinae, N., Identification of 6-methtylsulfinylhexyl isothiocyanate as an apoptosis-inducing component in wasabi, Phytochemistry, 62:733–739, 2003.
Yano, T., Yajima, S., Virgona, N., Yano, Y., Otani, S., Kumagai, H., Sakurai, M., and Ichikawa, T., The effect of 6-methylthiohexyl isothiocyanate isolated from Wasabia japonica (wasabi) on 4- (methylnitrosamino)-1 -(3-pyridyl)-1 -(butanone-induced lung tumorigenesis in mice, Cancer Lett., 155:115–120, 2000.
Yu, E.Y., Pickering, I.J., George, G.N., and Prince, R.C., In situ observation of the generation of isothio-cyanates from sinigrin in horseradish and wasabi, Biochem. Biophys. Acta, 1527:156– 160, 2001.
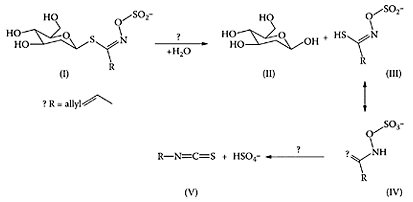
SCHEME W.70 Degradation of β-D-S-glucosides (I) by myrosinase (β-thioglucoside glucohydrolase) and related biochemistry. The initial hydrolysis of (I) yields glucose (II) and (III), which will be in equilibrium with (IV), is then thought to undergo a Lossen rearrangement to produce the isothiocyanate (V) and sulfate. (From Yu et al., Biochim. Biophys. Acta, 1527:156–160, 2001.)
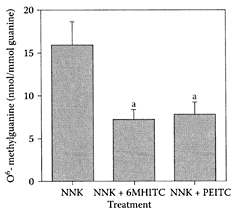
FIGURE W.99 The effect of 6MHITC treatment on pulmonary O6-methyl guanine level in mice treated with NNK. Values are expressed as the mean ± SE from five mice, (a) Significantly different from the NNK-treated group (p<0.05). (From Yano et al., Cancer Lett., 155:115–120, 2000. With permission.)
Wheat bran
Wheat bran was shown to decrease the mucosal formation of aberrant crypt foci, an important marker for determining the efficacy of colon cancer preventative agents (Earnest et al., 1999). In fact, wheat bran was found to be the most effective fiber for protecting against colon cancer. Whether this was related to fermentation of the fiber and the release of short-chain fatty acids, particularly butyric acid, a known inhibitor of tumor growth, remains unclear. Zile and coworkers (1998) also showed that inclusion of wheat bran suppressed mammary-gland tumorigenesis in experimental animals. Wheat dietary fiber comprises less than wheat bran, so that other nutrients besides dietary fiber may also protect against cancer. Such components were thought to be phenolic acids, lignans, and flavonoids (Ferguson and Harris, 1999). The effectiveness of different dietary components, wheat bran (WB), curcumin (CUR), rutin (RUT), and benzyl isothiocyanate (BIT), on the formation aberrant crypt foci (ACF), colorectal tumors, and selected gene expression in azoxymethane-treated F344 rats were compared by Wijnands et al. (2004). Of the different dietary components, only WB and CUR protected against colorectal cancer compared to RUT and BIT.
Wheat bran has also been associated with considerable antioxidant activity due to the concentration of phenolics in the bran portion of the grain (Zhou and Yu, 2004). Bran extracts of hard wheat varieties, Akron and Trego, grown at three different locations in Colorado, were shown by Yu et al. (2004) to significantly reduce lipid peroxidation in human LDL in vitro. They also found that the bran absorbed oxygen radicals, with the potential of preventing them from attacking biological molecules. Yuan et al. (2004) showed that the water-soluble feruloyl oligosaccharides in the insoluble dietary fiber of wheat bran scavenged 2,2-diphenyl-1-picrylhydrazine (DPPH) free radicals, as well as inhibited oxidative hemolysis of rat erythrocytes induced by 2,2-azobis-2-amidinopropane dihydrochloride (AAPH) by 91.7 percent.
Thus, incorporation of wheat-bran products may provide functional foods capable of preventing atherosclerosis and related diseases, as well as a new source of natural antioxidants.
References
Earnest, D.L., Einspahr, J.G., and Alberts, D.S., Protective role of wheat bran fiber: data from marker trials, Am. J. Med., 106:32S-37S, 1999.
Ferguson, L. and Harris, P.J., Protection against cancer by wheat bran: role of dietary fiber and phytochemicals, Eur. J. Cancer Prev., 8(Suppl. 1):17–25, 1999.
Wijnands, M.V.W., van Erk, M.J., Doorbos, R.P., Krul, C.A.M., and Woutersen, R.A., Do aberrant crypt foci have predictive value for the occurrence of colorectal tumors? Potential of gene expression profiling tumors, Food Chem. Toxicol., 42:1629–1639, 2004.
Yu, L., Zhou, K., and Parry, J.W., Inhibitory effects of wheat bran extracts on human LDL oxidation and free radicals, Lebensm.-Wiss. U.-Technol., 38:463–470, 2005.
Yuan, X., Wang, J., Yao, H., and Chen, F., Free radical-scavenging capacity and inhibitory activity on rat erythrocyte hemolysis of feruloyl oligosaccharides from wheat bran insoluble dietary fiber, Lebensm.-Wiss U.-Technol., 38:877–883, 2005.
Zhou, K. and Yu, L., Effects of extraction solvent on wheat bran antioxidant activity estimation. Lebensm.-Wiss U.-Technol., 37:717–721m, 2004.
Zile, M.H., Welsch, C.W., and Welsch, M.A., Effect of wheat bran on the development of mammary tumors in female mice intact and ovariectomized treated with 7,12- dimethylbenz(a)anthracene and in mice with spontaneously developing mammary tumors, Int. J. Cancer, 75:439–443, 1998.
Wheat flour
Whole-wheat flour was reported by Adam and coworkers (2001) to lower plasma and hepatic lipids in rats. Since whole-wheat flour is normally consumed in a processed product, such as bread, Adams et al. (2003) showed breadmaking (fermentation, starch gelatinization, heating) had a hypolipidemic effect compared to native whole-wheat flour. Rats fed a semipurified diet containing 70 percent whole-wheat flour (WWF) or 70 percent desiccated whole-wheat bread (WWB) had significantly (p<0.05) lower plasma and liver cholesterol levels compared to a control fiber-free starch diet. A closer examination of the plasma lipoproteins showed the triacylglycerol fraction (TGRLP, d<1.040 kg/L) was 33 percent lower in animals fed WWF or WWB diets compared to the control, accounting for decreases of 14 percent and 24 percent, respectively. No differences were evident in the HDL fraction (Figure W.100). Total steroid excretion was significantly (p<0.01) higher in the cereal diets, but was higher with the WWB diet. This corresponded to a reduction in cholesterol absorption of 26 percent for WWB compared to 38 percent and 52 percent for the WWF and control diets.
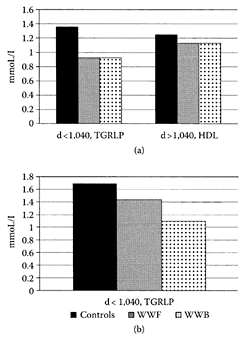
FIGURE W.100 Differences in the repartition of cholesterol (panel a) in plasma lipoprotein fractions of rats fed the control, WWF, and WWB diets. Each value is a mean of triplicate analyses of a pool of plasma. The fractions with a density of less than 1.040 kg/L corresponded to triacylglycerol-rich lipoproteins with a lower contribution of LDL. The fractions with a density higher than 1.040 kg/L corresponded essentially to HDL. (From Adam et al., Food Chem., 80:337–344, 2003. With permission.)
References
Adam, A., Levrat-Verny, M-A., Lopez, H.W., Leuillet, M., Demigne, C., and Remesy, C., Whole wheat and triticale flours with different viscosities stimulate cecal fermentations and lower plasma and hepatic lipid in rats, J. Nutr., 131:1770–1776, 2001.
Adam, A., Lopez, H.W., Leuillet, M., Demigne, C., and Remesy, C., Whole wheat flour exerts cholesterol-lowering in rats in its native form and after use in bread-making, Food Chem., 80:337–344, 2003.
Whey
see also α-Lactalbumin and Lactoferrin The whey fraction of bovine milk is rich source of immunomodulators, including the β-lactoglobulin, α-lactalbumin, lactoferrin, lactoperoxidase, and such tissue growth factors as TGF-β (Buonous et al., 1981; Stoeck et al., 1986; Dababbi et al., 1998; Wong et al., 1997, 1998). The unique immunomodulatory properties of a whey-protein concentrate (WPC) obtained from rennet casein whey was shown by Low et al. (2001) to markedly increase the intestinal-tract antibody responses in BALB/c mice over a 12-week period. Further research by Low and coworkers (2003) showed WPC increased humoral immune response in BALB/c mice immunized with such antigens as influenza virus, diphtheria, tetanus toxoids, poliomyelitis vaccine, ovalbumin, and cholera toxin subunit. The ability of dietary WPC to boost immune response in mice may be applicable for boosting postvaccination in humans, especially in children and the elderly with suboptimal immunity.
Tong et al. (2000) examined the antioxidant activity of a high-molecular-weight (HMW) fraction from whey. Using a salmon-oil emulsion, the antioxidant activity of the HMW fraction appeared dependent on the availability of sulfhydryl groups. However, even when the sulfhydryl groups were blocked, whey proteins still exhibited significant antioxidant activity. This was attributed to the scavenging activity of other amino acids in whey proteins by the HMW fraction’s ability to scavenge peroxyl radicals generated by β-PE decay. An additional antioxidant mechanism of whey proteins was reported to be metal chelation.
References
Bounous, G., Stevenson, M.M., and Kongshavn, P.A.L., Influence of dietary α-lactalbumin hydrolysate on the immune system of mice and resistance to salmonellosis, J. Infect. Dis., 144:281, 1981.
Debabbi, H., Dubarry, M., Rautureau, M., and Tome, D., Bovine lactoferrin induces both mucosal and systemic immune responses in mice, J. Dairy Res., 65: 283–293, 1998.
Low, P.P.L., Rutherfurd, K.J., Gill, H.S., and Gross, M.L., Effect of dietary whey protein concentrate on primary and secondary antibody responses in immunized BALB/c mice, Int. Immunopharmacol., 3:393–401, 2003.
Low, P.P.L., Rutherfurd, K.J., Gross, M.L., and Gill, H.S., Enhancement of mucosal antibody responses by dietary whey protein concentrate, Food Agric. Immunol., 13:255–264, 2001.
Stoeck, M., Ruegg, V., Miescher, S., Carrel, S., Cox, D., van Fliedner, V., and Alkanis, Comparison of the immunosuppressive properties of milk growth factor and transforming growth factors β1 and β2, J. Immunol., 143:3258–3265, 1989.
Tong, L.M., Sasaki, S., McClements, D.J., and Decker, E.A., Mechanisms of the antioxidant activity of a high molecular weight fraction of whey, J. Agric. Food Chem., 48:1473–1478, 2000.
Wong, C.W., Seow, H.F., Husband, A.J., Regester, G.O., and Watson, D.L., Effects of purified bovine whey factors on cellular immune functions in ruminants, Vet. Immunol. Immunopathol., 56:85–96, 1997.
Wong, K.F., Middleton, N., Montgomery, M., Dey, M., and Carr, R.I., Immunostimulation of murine spleen cells by materials associated with bovine milk protein fractions, J. Dairy Sci., 81:1825–1835, 1998.
Wheat germ
A commercial wheat germ was enzymically hydrolyzed by Arrigoni et al. (2002) and then subjected to fermentation with fresh human feces under anaerobic conditions. The short-chain fatty acids produced were high in proprionates, suggesting wheat germ behaved as a prebiotic by its support of bifidobacteria.
References
Arrigoni, E., Jorger, F., Kolloffel, B., Roulet, I., Herenspeger, M., Meile, L., and Amado, R., In vitro fermentability of a commercial wheat germ preparation and its impact on the growth of bifidobacteria, Food Res. Intern., 35:475–481, 2002.
Whole grains
see also Wheat, Barley, Maize or Corn, Millet, Oats, Sorghum, and Triticale Epidemiological evidence supports an inverse relationship between consumption of cereal fiber or whole grains and type 2 diabetes (Meyer et al., 2000; Liu et al., 2000; Salmeron et al., 1997a, b), cardiovascular disease (Jacobs et al., 1998, 1999), and total mortality (Jacobs et al., 1998, 1999). Whole grains from cereals (wheat, rice, maize, oats, barley, triticale, sorghum, and millet) are rich in phytochemicals, such as dietary fiber, resistant starch, oligosaccharides, and phytoestrogens, some exhibiting anticarcinogenic activity (Slavin et al., 1999). Table W.63 summarizes the range of components and their health-related properties.
Phytoestrogens appear to play a role in reducing the risk of cardiovascular disease, diabetes, and some cancers. The regular consumption of whole grains significantly reduces the risk of coronary heart disease (Rimm et al., 1996; Jacobs et al., 1998; Liu et al., 1999). Jacobs and coworkers (2002) reported that whole-grain elevated serum enterolactone in hyperinsulinemic women. Previous studies showed that the risk of incidence of heart disease was reduced in Finnish men when serum enterolactone was in the upper quartile (Vanharanta et al., 1999). McKeown et al. (2002) reported that increased intake of whole grains had a favorable effect on the metabolic risk factors associated with cardiovascular disease and type 2 diabetes. Pereira and coworkers (2002) confirmed the improvement of insulin insensitivity in hyperinsulinemic and obese adults following the consumption of whole grains. A prospective study in men by Fung et al. (2002) also found that diets high in whole grains reduced the risk of type 2 diabetes and should replace refined grains.
TABLE W.63
Selected Components in Whole Grains and Their Postulated Mechanisms
References
Fung, T.T., Hu, F.B., Pereira, M.A., Liu, S., Stampfer, M.J., Colditz, G.A., and Willett, W.C., Whole-grain intake and the risk of type 2 diabetes: a prospective study in men, Am. J. Clin. Nutr., 76:535–540, 2002.
Jacobs, D.R., Jr., Meyer, K.A., Kushi, L.H., and Folsom, A.R., Whole-grain intake may reduce risk of ischemic heart disease death in postmenopausal women: Iowa Women’s Health Study, Am. J. Clin. Nutr., 68:248–257, 1998.
Jacobs, D.R., Pereira, M.A., Stumpf, K., Pins, J.J., and Adlecreutz, H., Whole grain food intake elevates serum enterolactone, Br. J. Nutr., 88:111–116, 2002.
Kohlmeier, L., Simonsen, N., and Mohus, K., Dietary modifiers of carcinogenesis, Environ. Health Perspect., 103(Suppl): 177–184, 1995.
Liu, S., Stampfer, M.J., Hu, F.B., Giovannucci, E., Rimm, E., Manson, J.E., Hennekens, C.H., and Willett, W.C., Whole-grain consumption and risk of coronary heart disease: results from Nurses’ Health Study, Am. J. Clin. Nutr., 70:412–419, 1999.
Liu, S., Stampfer, M.J., and Hu, F.B., A prospective study of whole grain intake and risk of type 2 diabetes in women, Am. J. Pub. Hlth., 90:1409–1415, 2000.
McKeown, N.M., Meigs, J.B., Liu, S., Wilson, P.W.F., and Jacques, P.F., Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham offspring study, Am. J. Clin. Nutr., 76: 390–398, 2002.
Meyer, K.A., Kushi, L.H., Jacobs, Jr., D.R., Slavin, J.S., Sellers, T.A., and Folsom, A.R., Carbohydrates, dietary fiber and incident of 2 diabetes in older women, Am. J. Clin. Nutr., 71:921–930, 2000.
Pereira, M.A., Jacobs, D.A., Pins, J.J., Raatz, S.K., Gross, M.D., Slavin, J.L., and Seaquist, E.R., Effect of whole grains on insulin sensitivity in overweight, hyperinsulinemic adults, Am. J. Clin. Nutr., 75:848–855, 2002.
Rimm, E.B., Ascherio, A., Giovannucci, E., Spiegelman, D., Stampfer, M.J., and Willett, W.C., Vegetable, fruit, and cereal intake and risk of coronary heart disease among men, JAMA, 275:447–451, 1996.
Salmeron, J., Ascherio, A., Rimm, E.B., Colditz, G.A., Spiegelman, D., Jenkins, D.J., Stampfer, M.J., Wing, A.L., and Willett, W.C., Dietary fiber, glycemic load, and risk of NIDDM in men, Diabet. Care, 20:545–550, 1997.
Salmeron, J., Manson, J.E., Stampfer, M.J., Colditz, G.A., Wing, A.L., and Willett, W.C., Dietary fiber, glycemic load and risk of non-insulin-dependent diabetes mellitus in women, JAMA, 277:472–477, 1997.
Slavin, J.L., Jacobs, D., and Marquart, L., Wholegrain consumption and chronic disease: protective mechanisms, Nutr. Cancer, 27:14–21, 1997.
Slavin, J.L., Martini, M.C., Jacobs, D.R., Jr., and Marquart, L., Plausible mechanisms for the protectiveness of whole grains, Am. J. Clin. Nutr., 70:459S– 463S, 1999.
Vanharanta, M., Voutilainen, S., Lakka, T.A., van der Lee, M., Adlecruetz, H., and Salonen, J.T., Risk of acute coronary events according to serum concentrations of enterolactone: a prospective population-based case-control study, Lancet, 354:2112–2115, 1999.
Wine
see also Red wines and Resveratrol The relationship between wine and cardiovascular disease arose from the fact that in certain parts of France, where people consumed greater amounts of animal fats, the incidence of cardiovascular disease was remarkably low compared to North America. This phenomenon, referred to as the “French Paradox,” was subsequently attributed to the greater consumption of red wine. Frankel and coworkers (1993) showed it was the phenolics in wine that inhibited oxidation of human LDL cholesterol and, hence, the progression of atherosclerosis. This observation was confirmed by other researchers, including Tedesco et al. (2000), who demonstrated the beneficial effects of the nonalcoholic wine components, mainly polyphenols, in protecting red blood cells against oxidative stress. FernandezPachon et al. (2004) assessed the antioxidant activity of different wine samples and found it was higher in red wine compared to either white or sherry wines. Auger et al. (2001) showed a phenolic extract from red wine reduced atherosclerosis in hypercholesterolemic Golden Syrian hamsters. One of the major polyphenols found in red wine is resveratrol (3,5,4′- trihydroxystilbene) present as trans and cis isomers, depending on the type of wine (Scheme W.71). Most studies, however, have focused on the trans isomer and its antioxidant and antiproliferative activities in vitro (Briviba et al., 2002; Olas and Wachowicz, 2002; Zoberi et al., 2002). Resveratrol also inhibits angiogenesis, the process of new blood-cell growth associated with tumor growth and metastasis (Cao et al., 2002).
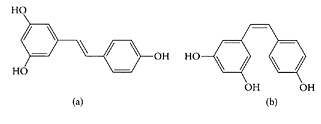
SCHEME W.71 Structure of the trans- (a) and cis- (b) isomers of resveratrol. (From Kolouchova-Hanzlikova et al., Food Chem., 87:151–158, 2004. With permission.)
Among the proposed mechanisms for the protective action of red wine is its effect on HDL metabolism (Gaziano et al., 1993; Lavy et al., 1994). HDL not only reverses cholesterol transport but also inhibits accumulation of lipid peroxides on LDL (Aviram et al., 1998a). Paraoxonase (PON), an enzyme on HDL particles, is responsible for the antioxidant properties of HDL by inhibiting or preventing oxidation of LDL and HDL. In addition, PON was thought to stimulate cholesterol efflux, the first step in reversing cholesterol transport (Aviram et al., 1998a, b; Mackness et al., 1993). Sarandol et al. (2004) examined the effect of wine consumption on the serum paraoxonase/ary-lesterase activities of PON, as well as lipoprotein oxidizability in 14 healthy males between the ages of 25 and 38 years. While there was no change in paraoxonase activity, there was a 22 percent decrease in arylesterase activity following wine consumption. However, the ability of red wine to protect lipoproteins from oxidation was clearly evident by the significant (29 percent) decrease in oxidation of apolipoprotein B-containing lipoproteins. Gomez-Cordoves et al. (2001) also showed wine phenolics could decrease melanogenic activity, suggesting their potential as therapeutic agents in the treatment of human melanoma.
References
Auger, C., Caporiccio, B., Landrault, N., Teissedre, P.L., Laurent, C., Cros, G., Besancon, P., and Rouanet, J.-M., Red wine phenolic compounds reduce plasma lipids and apolipoprotein B and prevent early aortic atherosclerosis in hypercholesterolemic Golden Syrian hamsters (Mesocricetus auratus), J. Nutr., 132:1207–1213, 2002.
Aviram, M., Billecke, S., Sorenson, R., Bisgaier, C., Newton, R., Rosenblat, M., Erogul, J., Hsu, C., Dunlop, C., and La Du, B., Paraoxonase active site required for the protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R, Arterioscler. Thromb. Vasc. Biol., 18:1617–1624, 1998a.
Aviram, M., Rosenblat, M., Bisgaier, C.L., Newton, R.S., Primo-Parmo, S.L., and La Du, B.N., Paraoxonase inhibits high density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase, J. Clin. Invest, 101:1581–1590, 1998b.
Briviba, K., Pan, L., and Rechkemmer, G., Red wine polyphenols inhibit the growth of colon carcinoma cells and modulate the activation pattern of mitogen activated protein kinases, J. Nutr., 132:2814–2828, 2002.
Cao, Y., Cao, R., and Brakenhielm, E., Antiangiogenic mechanisms of diet-derived polyphenols, J. Nutr. Biochem., 13:380–390, 2002.
Fernandez-Pachon, M.S., Villano, D., Garcia-Parrilla, M.C., and Troncoso, A.M., Antioxidant activity of wines and relation with their polyphenolic composition, Anal. Chim. Acta, 513:113– 118, 2004.
Frankel, E.N., Kinsella, J.E., German, J.B., Parks, E., and Kinsella, J.E., Inhibition of oxidation of human low density lipoprotein by phenolic substances in red wine, Lancet, 341:454–457, 1993.
Gaziano, J.M., Buring, J.E., Breslow, J.L., Goldhaber, S.Z., Rosner, B., VanDenburgh, M., Willett, W., and Hennekens, C.H., Moderate alcohol consumption intake, increased levels of high-density lipoprotein and its subfractions and decreased risk of myocardial infarction, N. Engl. J. Med., 329:1829–1834, 1993.
Gomez-Cordoves, C., Bartolome, B., Vieira, W., and Virador, V.M., Effects of wine phenolics and sorghum tannins on tyrosinase activity and growth of melanoma cells, J. Agric. Food Chem., 49:1620–1624, 2001.
Kolouchova-Hanzlikova, I., Melzoch, K., Filip, V., and Smidrkal, J., Rapid method for resveratrol by HPLC with electrochemical and UV detections in wines, Food Chem., 87:151–158, 2004.
Lavy, A., Fuhrman, N.B., Markel, A., Danker, G., Ben-Amotz, A., Presser, D., and Aviram, M., Effect of dietary supplementation of red or white wine on human blood chemistry, hematology and coagulation: favorable effect of red wine on plasma high-density lipoprotein, Ann. Nutr. Metab., 38:287–294, 1994.
Mackness, M.I., Arrol, S., Abbott, X., and Durrington, P.N., Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase, Atherscler., 104: 129–135, 1993.
Olas, B. and Wachowicz, B., Resveratrol and vitamin C as antioxidants in blood platelets, Thromb. Res., 106:143–148, 2002.
Sarandol, E., Serdar, Z., Dirican, M., and Safak, O., Effects of red wine consumption on serum paraoxonase/arylesterase activities and lipoprotein oxidizability in healthy-men, J. Nutr. Biochem., 14:507–512, 2003.
Tedesco, I., Russo, M., Russo, P., Iacomino, G., Russo, G.L., Carraturo, A., Faruolo, C., Moio, L., and Palumbo, R., Antioxidant effect of red wine polyphenols on red blood cells, J. Nutr. Biochem., 11:114–119,2000.
Zoberi, I., Bradbury, C.M., Curry, H.A., Bisht, K.S., Goswami, P.C., Roti, J.L., and Gius, D., Radiosensitizing and antiproliferative effects of resveratrol in two human cervical tumor lines, Cancer Lett., 175: 165–173, 2002.
Winter savory
Winter savory (Satureja montana L.), an aromatic and medicinal herb used as a folk remedy for many diseases, grows along the Adriatic coast and parts of Croatia. It contains a number of biologically active components including triterpenes, flavonoids, and rosmarinic acid (Escudero et al., 1985; ThomasBarberan et al., 1987; Reschke, 1983). Madsen et al. (1996) found extracts from a number of plants, including winter savory, were high in antioxidants. The broad-spectrum antimicrobial activity of the essential oil and ethanol extracts from winter savory was demonstrated in several studies, including its ability to control potential pathogenic and spoilage yeasts (Pepeljnak et al., 1999; Ciani et al., 2000).
The value of savory oil was attributed by Lawrence (1979) to its high carvacrol content and fresh, spicy phenolic notes reminiscent of oregano and thyme. Carvacrol was approved as a flavoring agent by the FDA and the Council of Europe at a level of 2 ppm in beverages, 5 ppm in fold, and 25 ppm in candy (De Vincent et al., 2004). These researchers pointed out the need for long-term toxicological studies to establish its safety. Mastelic and Kerkovic (2003) found a moderate correlation between the chemical composition of savory oil and its volatile aglycones. The compounds identified were thymol, p-cymene-9-ol, geraniol, 1-octen3-ol, carvacrol, and nerol.
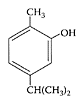
Carvacrol. (From De Vicenzi et al., Fitoterapia, 75:801–804, 2004. With permission.)
References
Ciani, M., Menghini, L., Mariani, F., Pagiotti, R., Menghini, A., and Fatichenti, F., Antimicrobial properties of essential of Satureja montana L. on pathogenic and spoilage yeasts, Biotechnol. Lett., 22: 1007–1010, 2000.
De Vincenzi, M., Stammati, A., De Vencenzi, A., and Silano, M., Constituents of aromatic plants carvacol, Fitoterapia, 75:801–804, 2004.
Escudero, J., Lopez, J.C., Rabanal, R.M., and Valverde, S., Secondary metabolites from Satureja species. New triterpenoid from Satureja-Acionos, J. Nat. Prod., 48:128–132, 1985.
Lawrence, B.M., Essential Oils, Allured Publishing, Wheaton, IL, 1979.
Madsen, H.L., Nielsen, B.R., Bertelsen, G., and Skibsted, L.H., Screening of antioxidative activity of spices. A comparison between assays based on ESR spin trapping and electrochemical measurement of oxygen consumption, Food Chem., 57:331–337, 1996.
Mastelic, J. and Jerkovic, I., Gas chromatographymass spectrometry analysis of free and glycoconjugated aroma compounds of seasonally collected Satureja montana L., Food Chem., 80:135–140, 2003.
Pepeljnjak, S., Stanic, G., and Potocki, P., Antimicrobial activity of the ethanol extract of Satureja montana sp. montana, Acta Pharm., 49:65–69, 1999.
Reschke, A., Capillary gas chromatographic determination of rosmarinic acid in leafy spices, Z. Lebensm. Unters Forsch., 176:116–119, 1983.
Thomas-Barberan, F.A., Husain, S.Z., and Gil, M.J., The distribution of methylated flavones in the Lamiaceae, Biochem. Sys. Ecol., 16:43–46, 1987.
Witch hazel
Witch hazel (Hamamelis virginiana L.), a deciduous shrub native to Eastern North America and Canada, has been used in skin-care products and for treating sunburn, irritated skin, and atopic eczema (Korting et al., 1995). One of the main components in the bark extract from witch hazel is hamamelitannin, a compound composed of two gallate moieties and the sugar hamamelose (Hartisch and Kolodzie, 1996). Hamamelitannin was reported to protect cells from ultraviolet B radiation and to scavenge superoxide and hydroxyl radicals (Masaki et al., 1995a, b). In vitro studies by Habtermariam (2002) found hamamelitannin inhibited the activity of the tumor necrosis factor (α-TNF), preventing its cytotoxic induction of endothelial cell death. An important prerequisite of endothelial cell death is DNA fragmentation. Hamamelitannin prevented DNA fragmentation by α-TNF in a concentration-dependent manner, as shown in Figure W.101. However, it had no effect on TNF-induced upregulation of endothelial adhesiveness. In addition to tannins, Dauer et al. (2003) reported that catechin present in the bark of Hamamelis virginiana also protected human hepatoma cells (Hep G2) from benz(a)pyrene (BP) and (±)-anti-benz( a)pyrene-7,8-dihydrodiol-9,10-epoxide (BPDE)-induced DNA damage by inactivation of the mutagen. These compounds may prevent genetic damage caused by genotoxins present in the diet or environment.
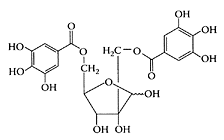
Structure of hamamelitannin (2,5′-di-O-galloyl hamamelose). (Adapted from Dauer et al., Phytochemistry, 63:199–207, 2003.)
References
Dauer, A., Hensel, A., Lhoste, E., Knasmuller, S., and Mersch-Sundermann, V., Genotoxic and antigenotoxic effects of catechin and tannins from the bark of Hamamelis virginiana L. in metabolically competent human hepatoma cells (Hep G2) using single cell electrophoresis, Phytochemistry, 63:199–207, 2003.
Habtemariam, S., Hamamelitannin from Hamamelis virginiana inhibits the tumor necrosis factor-α- TNF-induced endothelial cell death in vitro, Toxicon., 40: 83–88, 2002.
Hartisch, C. and Kolodziej, H., Galloylhamameloses and proanthocyanidins from Hamamelis virginiana, Phytochemistry, 42:191–198, 1996.
Korting, H.C., Schafer-Korting, M., Kloverlorn, W., Kloverlorn, G., Martin, C., and Laux, P., Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema, Eur. J. Clin. Pharmacol., 48:461–465, 1995.
Masaki, H., Atsumi, T., and Sakurai, H., Protective activity of hamamelitannin on cell damage of murine skin fibroblasts induced by UVB irradiation, J. Dermatol. Sci., 10:25–34, 1995a.
Masaki, H., Atsumi, T., and Sakurai, H., Peroxyl radical scavenging activities in hamamelitannin in chemical and biological systems, Free Rad. Res., 22: 419–430, 1995b.
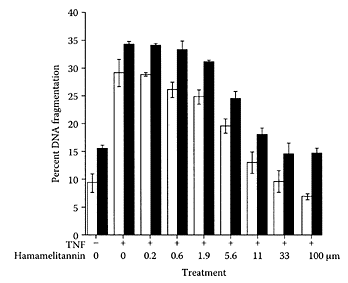
FIGURE W.101 Inhibition of TNF-mediated DNA fragmentation by hamamelitannin. Endothelial cells were treated with TNF and actinomycin D in the presence or absence of hamamelitannin. DNA leakage was assessed to the medium (open bars), and DNA fragmentation is quantified from cell lysates (solid bars). Results are mean values ± SEM from four separate experiments. (From Habtemariam, Toxicon., 40:83–88, 2003. With permission.)