B
Baicalein and Baicalin
Baicalin, a flavonoid with a structure analogous to genistein, is found in Scutellaria species used widely in Chinese herbal medicine. It has a glucuronate group at the C-7 position, which is absent in its aglycone, baicalein. Baicalein and baicalin both appear to have antiviral (Kitamura et al. 1998), antioxidant (Shi et al., 1995), antitumor (Matsuzaki et al., 1996), and anti-inflammatory (Lin and Shieh, 1996) properties, as well as an ability to reduce blood pressure and relax arterial smooth-muscle cells (Chen et al., 1999). The antitumor effects of baicalin on human hepatoma cell lines was also reported by Motoo and Sabatu (1994). Po and coworkers (2002) showed baicalin, unlike genistein, suppressed 17β-estradiol-induced transactivation in MFC-7 cells expressing receptor a. Baicalin also proved to be a stronger apoptosis-inducing agent, making it a superior chemopreventive agent. Chan and coworkers (2000) reported baicalin induced apoptosis in several human prostate-cancer cell lines so that it had the potential to be a chemopreventive agent or an adjuvant for the treatment of prostate cancers.
Baicalin and baicalein were both found by Huang and coworkers (2004) to exhibit novel vascular effects by inhibiting endothelial aortic relaxation via inhibition of cyclic GMP accumulation in vascular smooth-muscle cells. If this occurred in small blood vessels, vascular permeability would be reduced, which may explain the anti-inflammatory action of these flavonoids against acute edema or by inhibiting lipoxygenase, a key enzyme involved in inflammatory response.
Shen and coworkers (2003) evaluated the mechanisms responsible for the antiinflammatory properties of baicalin and baicalein in human leukocytes. They both reduced N-formyl-methionyl-leucyl-phenylalanine (fMLP)-and phorbol-12-myristate-13-acetate (PMA)-induced reactive-oxygen intermediates in neutrophils and monocytes. The anti-inflammatory activity of baicalin and baicalein was due to the combination of baicalin scavenging the reactive-oxygen intermediates and baicalein antagonizing ligand-initiated Ca2+ influx, both of which inhibit Mac 1-dependent leukocytes.
Li et al. (2000) reported that baicalin inhibited HIV-1 infection by interfering with the interaction of HIV-1 envelope proteins with chemokine coreceptors, blocking the entry of HIV into target cells. As a result, it is viewed as a possible natural chemotherapy for HIV infection (De Clerq et al., 2000). Recent studies by Wang and coworkers (2004) showed that coupling baicalein (BA) with zinc made it a far more effective inhibitor of recombinant reverse transcriptase (RT) and HIV-1 entry into the host cells. Figure B.9 shows that inhibition of recombinant RT was far greater in the presence of lower concentrations of BA-Zn with an EC50 25.09 μM which was threefold lower than the EC50 for BA of 83.48 μM.
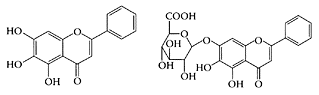
Baicalein and Baicalin. (From Zhang et al., J. Pharmaceut. Biomed. Anal., 36:637–641, 2004. With permission.)
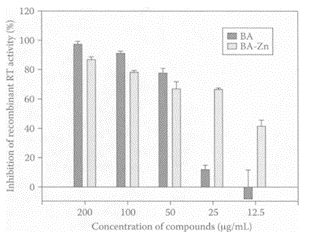
FIGURE B.9 Inhibition of recombinant HIV-1 RT activity by BA and BA-Zn. Inhibition rates were calculated according to the absorbance of the ELIZA Reader. Data expressed as means±SEM of at least three independent measurements. (From Wang et al., Biochem. Biophys. Res. Commun., 324:605–610, 2004. With permission.)
References
Chan, F.L., Choi, H.L., Chen, Z.Y., Chan, P.S.F., and Huang, C.Y., Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin, Cancer Lett., 160:219–228, 2000.
Chen, Z.Y., Su, Y.L., Lau, W.I., and Huang, Y., Endothelium-dependent contraction and direct relaxation induced by baicalin in rat mesenteric artery, Eur. J. Pharmacol., 374:41–47, 1999.
De Clerq, E., Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection, Med. Res. Rev., 20:323–349, 2000.
Huang, Y., Wong, C.M., Lau, C.-W., Yao, X., Tsang, S.Y., Su, Y.L., and Chen, Y., Inhibition of nitric oxide/cyclic GMP-mediated relaxation by purified flavonoids, baicalin and baicalein, in rat aortic rings, Biochem. Pharmacol., 67:787–794, 2004.
Kitamura, K., Honda, M., Yoshizaki, H., Yamamoto, S., Nakane, H., Fukushima, M., Ono, K., and Tokunaga, T., Baicalin, an inhibitor of HIV-1 production in vitro, Antiviral Res., 37:131–140, 1998.
Li, B.Q., Fu, T., Dongyan, Y. Mikovitz, J.A.n Ruscettim, F.W., and Wang, J.M., Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry, Biochem. Biophys. Res. Commun., 276:534– 538, 2000.
Lin, C.C. and Shieh, D.E., The anti-inflammatory activity of Scutellaria rivulas extracts and its active components, baicalin, baicalein and wogonin, Am. J. Clin. Chem., 24:31–36, 1996.
Matsuzaki, Y., Kurokawa, N., Terai, S., Matsumara, Y., Kobayashi, N., and Okita, K., Cell death induced by baicalein in human heptocellular carcinoma cell lines, Jpn. J. Cancer Res., 87:170– 177, 1996.
Motoo, Y. and Sawatu, N., Antitumor effects of saikosaponins, baicalin and baicalein on human heptoma cell lines, Cancer Lett., 86:91–95, 1994.
Po, L.S., Chen, Z.Y., Tsang, D.S.C., and Leung, L.K., Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells, Cancer Lett., 187:33–40, 2002.
Shen, Y.-C., Chiou, W.-F., Chou, Y.-C., and Chen, C.-F., Mechanisms in mediating the antiinflammatory effects of baicalin and baicalein in human leukocytes, Eur. J. Pharmacol., 465:171–181, 2003.
Shi, H., Zhao, B., and Xin, W.J., Scavenging effects of baicalin on free radicals and its protection on erythrocyte membrane from free radical injury, Mol. Bio. Int., 35:981–984, 1995.
Wang, Q., Wang, Y.-T., Pu, S.-P., and Zheng, Y.-T., Zinc coupling potentiates anti-HIV activity of baicalin, Biochem. Biophys. Res. Commun., 324:605–610, 2004.
Zhang, L., Lin, G., and Zuo, Z. High-performance liquid chromatographic method for simultaneous determination of baicalein and baicalein 7-glucuronide in rat plasma, J. Pharmaceut. Biomed. Anal., 36:637–641, 2004.
TABLE B.9
Effect of Various Fruit Extracts or Juices on NO Synthesis in Human Red-Cell Membrane
Banana
Bananas (Musa Cavendish), one of the most popular fruits worldwide, is a source of antioxidants, vitamin C, vitamin E, and β-carotene. Someya and coworkers (2002) found bananas were also high in flavonoids, with the peel being a richer source of total phenolics (907 mg/100 dry wt) compared to the pulp (232 mg/100g dry wt). This difference was reflected by the antioxidant activity of the peel extract being 2.2 times greater than the pulp. Several flavonoids were identified, including gallocatechin, catechin, and epicatechin. Of these, gallocatechin exhibited the greatest antioxidant activity and was much higher in banana peel (158 mg/100 g dry weight) compared to the pulp (29.6 mg/100 g dry weight). These researchers recommended that banana peels be considered a functional food source for combating chronic diseases and should not be discarded.
A study in India by Guha et al. (2003) showed ripe banana (Musa paradisiacal sapientum) extracts stimulated the production of nitric oxide (NO) in human erythrocyte membranes. Nitric oxide is tumoricidal, as well as induces apoptosis and differentiation in neoplastic cells (Farias-Eisner et al., 1994; Jun et al., 1996). Stimulation of nitric oxide is catalyzed by a family of isoenzymes, nitric synthase (NOS). Incubation of human red-cell membranes with different fruit extracts showed ripe banana was the most potent stimulator of NO, followed by cucumber, apple, and lemon, with pear lacking any activity (Table B.9). Inclusion of ripe bananas in the diets of mice administered Ehrlich’s ascetic carcinoma cells showed that 70 percent of those animals receiving 2 g of ripe banana (wet weight/day) died within 35 days compared to the control group which died within 5–6 days. The ability of bananas to prevent or slow down the progression of ascetic carcinoma in mice could be extended to humans.
Vitamin A deficiency and chronic diseases are a particularly serious problem in Pacific Island countries. A recent paper by Englberger and coworkers (2003) pointed out the importance of bananas as significant sources of provitamin A and β- and α-carotenes, which could alleviate this problem in Micronesia.
References
Englberger, L., Schierle, J., Marks, G.C., and Fitzgerald, M.H., Micronesian banana, taro, and other foods: Newly recognized sources of provitamin A and other carotenoids, J. Food Comp. Anal., 16:3–19, 2003.
Farias-Eisner, R., Sherman, M.P., Aeberhard, E., and Chaudhuri, G., Nitric oxide is an important mediator for tumoricidal activity in vivo, Proc. Natl. Acad. Sci. U.S.A., 91:9407–9411, 1994.
Guha, M., Basuray, S., and Sinha, A.K., Preventive effect of ripe banana in the diet on Ehrlich’s ascetic carcinoma cell induced malignant ascites in mice, Nutr. Res., 23:1081–1088, 2003.
Jun, C.-D., Lee, D.-K., and Cu, Y.-H., High-dose nitric oxide induces apoptosis in HL-60 human myeloid cells, Korean J. Exp.. Mol. Med., 28:101- 108, 1996.
Someya, S., Yoshiki, Y., and Okubo, K., Antioxidant compounds from bananas (Musa Cavendish), Food Chem., 79:351–354, 2002.
Barley
Barley is one of the major cereals grown worldwide and is particularly important in China. The major uses for barley are in malting, as well as for the feed industry. Germinated barley foodstuff (GMF) obtained from the aleurone layer and scutellum fractions of malt consists mainly of dietary fiber and glutamine-rich protein. This material was found to have a preventive and therapeutic effect in an experimental colitis model (Kanauchi et al., 1997, 1998), as well as in patients with mild to moderate ulcerative colitis (Mitsuyama et al., 1998). Bamba and coworkers (2002) fed germinated barley to patients with mild to moderate active ulcerative colitis and found significant clinical and endoscopic improvements were associated with an increase in stool butyrate levels. These results suggested GMF was a new prebiotic for the treatment of ulcerative colitis. Deguchi and coworkers (2000) produced an anthocyanintannin type pigment from barley bran-fermented broth. The purple pigment, referred to as hordeumin, scavenged superoxide radicals in a dose-dependent manner, which was attributed to the bran poly phenols, such as proanthocyanidins.
Barley is also a good source of β-glucan, the mixed-linked (1→3), (1→4)-β-D-glucan, which has been shown to have important health benefits. The β-glucan content of winter-barley cultivars grown in different locations in China were similar to those grown in Canada and Australia (Zhang et al., 2002). Because of the significant interaction between cultivars and environment on β-glucan content, they emphasized the importance of planting appropriate barley cultivars in specific areas in order to maximize β-glucan levels.
References
Bamba, T., Kanauchi, O., Andoh, A., and Fujiyama, Y., A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis, J. Gastroeneterol. Hepatol., 17:818–824, 2002.
Deguchi, T., Ohba, R., and Ueda, S., Radical scavenging activity of a purple pigment, hordeumin, from uncooked barley bran-fermented broth, J. Agric. Food Chem., 48:3198–3201, 2000. Kanauchi, O., Nakamura, T., Agate, K., Mitsuyama, K., and Iwanaga, T., Effects of germinated barley foodstuff on dextran sulfate sodium-induced colitis in rats, J. Gastroenterol., 33:179– 188, 1998.
Kanauchi, O., Iwanaga, T., and Andoh, A., The dietary fibre fraction of germinated barley foodstuff (GMF) attenuated mucosal damage and diarrhea and accelerated repair of the colonic mucosa in a rate model of experimental colitis, J. Gastroenterol. Hepatol., 16:160–168, 2001.
Mitsuyama, K., Saiki, T., and Kanauchi, O., Treatment of ulcerative colitis with germinated barley foodstuff: A pilot study, Aliment. Pharmacol. Ther., 12:1225–1230, 1998.
Zhang, G., Junmei, W., and Jinxin, C., Analysis of β-glucan content in barley cultivars from different locations of China, Food Chem., 79:251–254, 2002.
Basil (Ocimum basilicum)
Basil (Ocimum basilum L.Laminiaceae) is a common herb used for culinary and medical purposes. The essential oil obtained from basil was reported to exhibit antimicrobial activity, as well as inhibit the fungus Aspergillus ochraceus and the production of ochratoxin A (Hili et al., 1997; Basilico and Basilico, 1999). A recent study by Opalchenova and Obreshkova (2003) identified the main components in basil by gas chromatography as linalool (59.5 percent), methylchavikol (12 percent), and methylcinnamate (7.2 percent). They also examined whether basil could inhibit multidrug-resistant clinical isolates of the genera Staphyloccocus, Enterococcus, and Pseudomonas. Basil proved effective against these antibiotic-resistant tested bacteria with minimum inhibitory concentrations ranging from 0.0030–0.0007 percent.
Linalool. (From Letizia et al., Food Chem. Toxicol., 41:943–964, 2003. With permission.)
Basil oil was also reported to exhibit antiinflammatory properties against carrageenan, PGE2, leukotriene, and arachidonic acid-induced paw edema in rats (Singh, 1998, 1999 a, b, c). Courreges and Benecia (2002) further explored possible immunomodulatory effects of basil oil on mouse macrophages. Exposure of macrophages to basil oil for a 24-hour period significantly inhibited phagocytosis, with complete inhibition with dilutions of 1:2000 and 1:1000 (Table B.10).
Javanmardi et al. (2003) screened 23 Iranian basils as sources of antioxidants and phenolics. They found them to be good sources of antioxidants because of their strong radical-scavenging activities. A positive linear relationship was observed between antioxidant activity and total phenolic acids for the basil samples examined.
References
Basilico, M.Z. and Basilico, J.C., Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production, Lett. Appl. Micobiol., 29:135–141, 1999.
Courreges, M.C. and Benecia, F., In vitro antiphagocytic effect of basil oil on mouse macrophages, Fitoterapia, 73:369–374, 2002.
Hili, P., Evans, C.S., and Veness, R.G., Antimicrobial action of essential oils: The effect of dimethyl sulfoxide on the activity of cinnamon oil, Lett. Appl. Microbiol., 24:269–275, 1997.
Javanmardi, J., Stushnoff, C., Locke, E., and Vivanco, J.M., Antioxidant activity and total phenol content of Iranian Ocimum accessions, Food Chem., 83: 547–550, 2003.
Letizia, C.S., Cocchiara, J., Lalko, J., and Api, A.M., Fragrance material review of linalool, Food Chem. Toxicol., 41:943–964, 2003.
Opalchenova, G. and Obreshkova, D., Comparative studies on the activity of basil—an essential oil from Ocimum basilicum L., J. Micobiol. Methods, 54:105–110, 2003.
Singh, S., Comparative evaluation of anti-inflammatory potential of fixed oil of different species of Ocimum and its possible mechanism of action, Indian J. Exp. Biol., 36:1028–1031, 1998.
Singh, S., Mechanism of action of anti-inflammatory effect of fixed oil of Ocimum basilicum Linn, Indian J. Exp. Biol., 37:248–252, 1999a.
Singh, S., Evaluation of gastric antiulcer activity of fixed oil of Ocumum basilicum Linn and its possible mechanism of action, Indian J. Exp. Biol., 37:253–257, 1999b.
Singh, S., Effect of Ocimum sanctum fixed oil on vascular permeability and leukocytes migration, Indian J. Exp. Biol., 37:1136–1138, 1999c.
Beans
Beans are an important part of our diet and represent a good source of protein and nutrients. The consumption of beans, particularly in Mexico, has a long history and was estimated at 19.5 kg/annum per capita (Gonzalez de Mejia, 1990). The importance of phenolic compounds in plant foods, including beans, is related to their effect on nutritional and esthetic properties. In addition to their antioxidant and chelating properties, they are able to scavenge reactive-oxygen species and electrophiles, as well as modulate cellular-enzyme activities (Huang and Ferraro, 1992). The antimutagenic properties of the phenolic compounds from common beans (Phaseolus vulgaris) were reported by Gonzalez de Maija et al. (1999). The majority of poly phenols were located in the seed coat with negligible amounts in the cotyledons. The key antimutagenic compounds in beans, easily extracted with methanol, were phenols, while low-molecular-weight hydrolyzable phenols were present in the aqueous extract. The phenolic compounds specifically identified were catechin, tannic acid, and ellagic acid. These compounds were effective against the mutagenic activities of 1-nitropyrene (1-NP) and benzo[α]pyrene using the Salmonella typhimurium tester strain YG1024 in the plateincorporation test. Dose-dependent inhibition was observed for all the samples tested. Doses of 500 μg equivalent catechin/plate resulted in 63%, 81%, and 83% inhibition for water, water/methanol, and methanol extracts, respectively. The greatest inhibition was evident for the methanol extract at lower doses of 50 μg equivalent catechin/plate. These results were consistent with earlier findings by Mandal and coworkers (1987) regarding the antimutagenic effects of ellagic acid.
References
Gonzalez de Mejia, E., Caracterizacion fiscoquimica e implicaciones nutricias de las lectinas de frijol tepari y sus hibridos, Ph.D dissertation, CIN-VESTAV-Unidad Irapuato, Irapuato, Gto, Mexico, 1990.
Gonzalez de Mejia, E., Castano-Tostado, E., and Loarca-Pina, G., Antimutagenic effects of natural phenolic compounds in beans, Met. Res. Gen. Toxicol. Environ. Mutageneis., 441:1–9, 1999.
Huang, M. and Ferraro, T., Phenolic compounds in food and cancer prevention, in Phenolic Compounds in Food and their Effects on Health. II. Analysis, Occurrence and Chemistry, C.Ho, C., Lee, C.Y., and Huang, M., Eds., American Chemical Society, Washington, D.C., 1992, pp. 8–35.
Mandal, S., Ahuja, A., Shivapurkar, N.M., Sheng, S.J., Groopman, J.D., and Stoner, G.D., Inhibition of aflatoxin B in Salmonella typhimurium and DNA damage in cultured rat and human tracheobronchial tissues by ellagic acid, Carcinogenesis, 8:1651–1656, 1987.
Bearberry
see also Uva-ursi Bearberry (Arctostaphylos uva-ursi L.) is a small shrub that grows in the northern latitudes and high mountains of Europe, Asia, and America. Its astringent leaves have medicinal properties and are used as a disinfectant in the treatment of lower urinary-tract infections. One of the principal components of bearberry-leaf extracts is arbutin (hydroquinone-1-O-β-D-glucoside), which forms urinary metabolites that are conjugates with glucuronic and sulfuric acids (Paper et al., 1993; Siegers et al., 1997). These metabolites appear to be precursors of hydroquinone, which is released in the lower urinary tract where it kills or inhibits bacteria. Pegg and coworkers (2001) reported the presence of a natural antioxidant in the ethanol extracts of bearberry leaves, which proved to be very effective in nitrite-free processed meats. Bacterial surface hydrophobicity appears to be related to the ability of certain pathogens to cause infection. Thus, an increase in hydrophobicity is strongly correlated with enhanced pathogenic potential (Absolom, 1988; Andersson et al., 1998). Altering surface hydrophobicity could provide an effective way of decreasing the viability of pathogenic bacteria in food or in the gastrointestinal tract. Annuk et al. (1999) compared aqueous extracts of four medicinal plants, including bearberry, on the cell surface hydrophobicity of the Gram-negative pathogen Hylobacter pylori. Bearberry extract proved to be the richest source of tannic acid and was attributed for the decrease in cell surface hydrophobicity and its antibacterial activity against Hylobacter pylori. Recent work by Dykes and coworkers (2003 a) examined the effect of an antioxidant ethanolic extract from bearberry leaves on the surface hydrophobicity of 25 food-related bacteria. The bearberry extract significantly decreased the hydrophobicity of only four bacteria, while significantly increasing the hydrophobicity of 14. These researchers cautioned against marketing a particular extract, such as bearberry, based on a single claim, as there may be detrimental effects on food-related bacteria associated with such nutraceuticals. For example, increased antibiotic resistance in bacteria was recently associated with their exposure to certain neutraceutical extracts (Ward et al., 2002). However, Dykes et al. (2003b) also studied the effect of an ethanolic extract from bearberry, alone or in combination with nisin, on 25 food-related bacteria. Although bearberry did not exhibit any antimicrobial activity, it enhanced the antibacterial efficacy of nisin against Gram-positive bacteria, particularly Brochothrix thermosphacta.
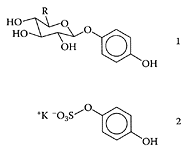
Arbutin (1, R=CH2OH) and hydroquinone glucuronide (1, R=COOH) and hydroquinone sulfate potassium salt (2). (From Glockle et al., J. Chromatogr. B, 761:261–266, 2001. With permission.)
References
Absolom, D.R., The role of bacterial hydrophobicity in infection: Bacterial adhesion and phagocyte ingestion, Can. J. Microbiol., 34:287–298, 1988.
Andersson, A., Granum, P.E., and Ronner, U., The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism, Internal. J. Food Microbiol., 39:93–99, 1998.
Annuk, H., Hirmo, S., Turi, E., Mikelsaar, M., Arak, E., and Wadstrom, T., Effect on cell surface hydrophobicity and susceptibility of Helicobacter pylori, FEMS Microbiol. Lett., 172:41–45, 1999.
Dykes, G.A., Amarowicz, R., and Pegg, R.B., An antioxidant bearberry (Arctostaphylos uva-ursi) extract modulates surface hydrophobicity of a wide range of food-related bacteria: Implications for functional food safety, Food Control, 14:515–518, 2003a.
Dykes, G.A., Amarowicz, R., and Pegg, R.B., Enhancement of nisin antibacterial activity by a bearberry (Arctostaphylos uva-ursi) leaf extract, Food Microbiol., 20:211–216, 2003b.
Glockl, I., Blaschke, G., and Veit, M., Validated methods for direct determination of hydroquinone glucuronide and sulfate in human urine after oral intake of bearberry leaf extract by capillary zone electrophoresis, J. Chromatogr., B. 761:261–266, 2001.
Paper, D.H., Kohler, J., and Franz, G., 1993. Bioavailability of drug preparations containing a leaf extract of Arctostaphylos uva-ursi (L.), Pharmaceut. Pharmacol. Lett., 3:63–66, 1993.
Pegg, R.B., Amarowicz, R., and Barl, B., Applications of plant phenolics in model and meat systems, in Proceedings of the 47th International Congress of Meat Science. Technology, Krakow, Poland, Vol. II, 234–235, 2001.
Siegers, C.P., Siegers, J.P., Pentz, C., Bodinet, C., and Freudenstein, J., Metabolism of arbutin from uva ursi-extracts in humans, Pharm. Pharmacol. Lett., 7: 90–92, 1997.
Ward, P., Fasitsas, S., and Katz, S.E., Inhibition, resistance development, and increased antibiotic and antimicrobial resistance caused by nutraceuticals, J. Food Prot., 65:528–533, 2002.
Beer
Epidemiological studies showed an inverse relationship between moderate ethanol consumption and risk of coronary heart disease (Gronback et al. 1995; Kannel and Ellison, 1996). Reduced mortality was associated with the consumption of beer and wine but not with spirits. An increase in plasma antioxidant levels was reported by Ghiselli and coworkers (1999) in healthy, fasting nonsmokers consuming 500 mL beer in the morning. The antioxidants present in beer were phenolic acids, of which syringic and sinapic acids were the most significant. Wei et al. (2001) examined the antioxidant properties of volatiles extracted from stout beer, particularly phenylethyl alcohol, maltol, and 2-furanmethanol. Measuring antioxidant activity, as the reduction in the oxidation of hexanal to hexanoic acid, 2-furanmethanol and maltol were far more effective in preventing hexanal oxidation compared to phenylethyl alcohol (Figure B.10).
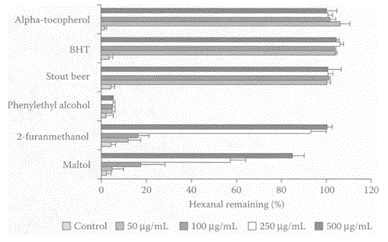
FIGURE B.10 Relative amounts of hexanal remaining in hexane samples containing volatiles, (a) phenylethyl alcohol; (b) 2-furanmethanol; (c) maltol. (From Wei et al., J. Agric. Food Chem., 49:4097–4101, 2001. With permission.)
In addition to polyphenols, Gorinstein et al. (2002) reported the presence of protein and amino acids in beer. To assess whether these proteins were biologically active, these researchers examined the effect of lyophilized polyphenol-free beer and lyophilized polyphenol-free wine on rat-plasma lipids over four weeks. Only the group fed the diet supplemented with beer significantly lowered total cholesterol, LDL cholesterol, and triacylglycerols, pointing to the potential contribution by the proteins and essential amino acids present in beer. Thus, moderate consumption of beer appears to be as beneficial as moderate consumption of wine.
Arimoto-Koyabashi and coworkers (1999) found native and freeze-dried Japanese-beer samples inhibited the genotoxicity of several heterocyclic amines and N-methyl-N ′- nitro-N-nitrosoguani-dine (MNNG). Kimura et al. (1999) isolated an antimutagen in beer, glycine betaine, but it had no effect on MNNG. However, the first nucleoside with antimutagenic properties against MNNG was reported by Yoshikawa et al. (2002) who identified a pseudouridine compound in one of six antimutagenic fractions isolated from freeze-dried beer. Pseudouridine was present at around 0.4 mg/100 mL beer but only accounted for 3 percent of the beer’s total antimutagenicity. The major compounds responsible for the bioactive properties of the beer still remained to be identified.
Nozawa et al. (2004) reported the inhibitory effects of four commercial beers, two pilsner-types, a black beer, and a stout beer, against five heterocyclic amine carcinogens. They all exhibited antimutagenic properties by inhibiting the genotoxic effects of these carcinogens, as well as significantly reducing the number of ACF in rats fed a diet containing these carcinogens.
Bamforth (2002), in reviewing the nutritional properties of beer, noted its contribution to certain B vitamins, minerals, antioxidants, and possibly fiber.
References
Arimoto-Kobayashi, S., Sugiyama, C., Harada, N., Takeuchi, M., Takemura, M., and Hayatsu, H., Inhibitory effects of beer and other alcoholic beverages on mutagenesis and DNA adduct formation induced by several carcinogens, J. Agric. Food Chem., 47: 221–230, 1999.
Bamforth, C.W., Nutritional aspects of beer—a review, Nutr. Res., 22:227–237, 2001.
Ghiselli, A., Natella, F., Guidi, A., Montanari, L., Fantozzi, P., and Scaccini, C., Beer increases plasma antioxidant capacity in humans, J. Nutr. Biochem., 11:76–80, 2000.
Gorinstein, S., Leontowicz, H., Lojek, A., Leontowicz, M., Eiz, M., Stager, M.A.G., Montes. J.M.B., Toledo, F., Arancibia-Avila, P., and Trakhtenberg, S., Hypolipidemic effect of beer proteins in experiment on rats, Lebensm. Wiss u-Technol., 35:265–271, 2002.
Gronback, A.M., Deis, A., Sorensen, T.I.A., Becker, U., Schnohr, P., and Jensen, G., Mortality associated with moderate intakes of wine, beer, or spirits, BMJ, 310:1165–1169, 1995.
Kannel, W.B. and Ellison, R.C., Alcohol and coronary disease: The evidence for a protective effect, Clin. Chim. Acta, 246:59–76, 1996.
Kimura, S., Hayatsu, H., and Arimoto-Kobayashi, S., Glycine betaine in beer as an antimutagenic substance against 2-chloro-4-methylthiobutanoic acid, the samma fish mutagen, Mutat. Res., 439:267–276, 1999.
Nozawa, H., Tazumi, K., Sato, K., Yoshida, A., Takata, J., Arimoto-Kobayashi, S., and Kondo, K., Inhibitory effects of beer on heterocyclic amine-induced mutagenesis and PhIP-induced aberrant crypt foci in rat colon, Mutat. Res., 559:177–187, 2004.
Wei, A., Mura, K., and Shibamoto, T., Antioxidative activity of volatile chemicals extracted from beer, J. Agric. Food Chem., 49:4097–4101, 2001.
Yoshikawa, T., Kimura, S., Hatano, T., Okamoto, K., Hayatsu, H., and Arimoto-Kobayashi, S., Pseudouridine, an antimutagenic substance in beer towards N-methyl 1-N ′-nitro-Nnitrosoguanidine (MNNG), Food Chem. Toxicol., 40:1165–1170, 2002.
Beets (Beta vulgaris)
see also Betalains Red beets are very popular vegetables used for the production of such ethnic foods as borscht. Kapadia and coworkers (1996) showed a root extract from red beet exhibited the strongest in vitro inhibitory effect on Epstein-Barr virus early antigen (ENV-EA) induction using Raji cells compared to capsanthin, cranberry, red onion skin, and short and long red bell peppers. The root extract also significantly inhibited tumors in mice skin and lungs. Bobek et al. (2000) fed diets supplemented with 15 percent fiber isolated from red beet to Wistar rats with hypercholesterolemia and chemically induced colon cancer. The red-beet diet significantly reduced serum cholesterol (-30 percent) and triacylglycerol (-40 percent) levels while significantly increasing HDL cholesterol. In addition, the number of animals bearing tumors was reduced by 30 percent, although it did not significantly affect the incidence of colon tumors.
Kanner and coworkers (2001) identified a new class of dietary cationized antioxidants in red beets, the betalains. The major one was betanin, a betanidin 5-O-β-glucoside. A relatively low concentration of betanin was found to inhibit lipid peroxidation of membranes or linoleate emulsion by the “free iron” redox cycle, H2O2-activated metmyoglobin, lipoxygenase. The bioavailability of betanin was demonstrated by the presence of betacyanin in the urine of four volunteers 2–4 h following the consumption of 300 mL of red beet juice containing 120 mg of the antioxidant.
References
Bobek, P., Galbavy, S., and Mariassyova, M., The effect of red beet (Beta vulgaris var. rubra) fiber on alimentary hypercholesterolemia and chemically induced colon carcinogenesis in rats, Nahrung, 44: 184–187, 2000.
Kanner, J., Harel, S., and Granit, R., Betalains—A new class of dietary cationized antioxidants, J. Agric. Food Chem., 49:5178–5185, 2001.
Kapadia, G.J., Tokuda, H., Konoshima, T., and Nishino, H., Chemoprevention of lung and skin cancer by Beta vulgaris (beet) root extract, Cancer Lett., 100:211–214, 1996.
Bell pepper (Capsicum annuum)
see also Chili Peppers and Peppers Bell pepper iss used extensively in North Africa as a spice to enhance the flavor of food. Its juice was shown to inhibit N-methylnitrosourea-induced colon carcinogenesis in rats (Narisawa et al., 2000). Maoka and coworkers (2001) found that the carotenoids obtained from the fruits of bell peppers exhibited potent antitumor properties both in vivo and in vitro. Carotenoids from bell pepper were previously shown to inhibit the mutagenicity of 1-nitropyrene, 1,6-dinitropyrene, and 1,8-dinitropyrene by 87 percent, 79 percent, and 73 percent, respectively (Gonzalez de Meija et al., 1998). Capsaicin, a major component in bell pepper, was shown to inhibit mutagens and carcinogens by modulating cytochrome P450 monooxygenases (Miller et al., 1993). El-Hamss et al. (2003) recently showed bell pepper had strong chemopreventive properties by its antimutagenic effect against promutagen ethyl carbamate (EC) and against the alkylating agent methyl methanesulfonate (MMS) in larvae of Drosophila melanogaster, using the wing Somatic Mutation and Recombination Test (SMART). The 2-day-old larvae were pretreated with bell and black peppers 24 h prior to treatment with EC and MMS. In the presence of 5 percent bell pepper, there was a significant reduction (p<0.05) in mutational events by 39 percent from the original 1.02 spots/wing in the presence of EC (10 mM). This was somewhat greater than the 20 percent reduction of wing/spots in the presence of 5 percent bell pepper from the original 4.60 spots/wing in the presence of 0.005 percent MMS (Figure B.11). Black pepper was only effective in reducing mutations induced by EC. The inhibitory effect was attributed to such compounds as β-carotene, together with small amounts of capsaicin.
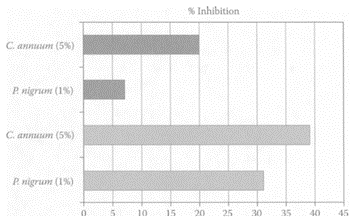
FIGURE B.11 Pre-treatment effect with bell (C.capsicum) and black (P.nigrum) peppers on the mutagenicity of 10 mM EC (shaded) and 0.005 percent MMS (black). (Adapted from E1 Hamss et al., Food Chem. Toxicol., 41:41–47, 2003. With permission.)
References
E1 Hamss, R., Idaomar, M., and Alonoso-Moraga, A., and Munoz-Serrano, A., Antimutagenic properties of bell and black peppers, Food Chem. Toxicol., 41:41–47, 2003.
Gonzalez de Mejia, E., Quintanar-Hernandez, A., Loarca-Pina, G., and Wurgler, F.A., Antimutagenic activity of carotenoids in green peppers against some nitroarenes, Mutat Res., 416:11–19, 1998.
Maoka, T., Mochida, K., Kozuka, M., Ito, Y., Fujiwara, Y., Hashimoto, K., Enjo, F., Ogata, M., Nabakuni, Y., Tokuda, H., and Nishino, H., Cancer chemopreventive activity of carotenoids in the fruit of red paprika Capsicum anuum L., Cancer Lett., 172:103–109, 2001.
Miller, C.H., Zhang, Z., Hamilton, S.M., and Teele, R.W., Effects of capsaicin on liver microsomal metabolism of the tobacco-specific nitrosamine NNK, Cancer Lett., 75:45–52, 1993.
Narisawa, T., Fukaura, Y., Hasebe, M., Nomura, S., Oshima, S., and Inakuma, Prevention of Nmethylnitrosoreau- induced colon carinogenesis in rats by oxygenated carotenoid capsanthin and capsanthinrich paprika juice, Proc. Soc. Expt. Biol. Med., 224:116–122, 2000.
Berries
see also Individual berries Berries are rich sources of phenolic pigments and anthocyanins. They are relatively high in phenolic antioxidants, which correlates with their anthocyanin and phenolic compounds (Heinonen et al., 1998; Prior et al., 1998). Berries, such as strawberries and black raspberries, have been shown to have chemopreventive properties against cancers. Ellagic acid, a major component in these fruits, was found to inhibit carcinogenesis in in vitro and in vivo studies. Xue et al. (2001) reported that in addition to ellagic acid, a methanolic extract of strawberries and black raspberries also displayed chemopreventive properties. Miranda-Rottmann and coworkers (2002) showed Chilean berry [Aristotelia chilensis (ach)] was much higher in phenolics than blackberry, cranberry, and strawberry, with much higher scores for total radical-trapping potential (TRAP) and other in vitro antioxidant capacity tests. The anthocyanins present in ach juice prevented copper-induced LDL oxidation, indicating its possible antiantherogenic properties. Netzel et al. (2002) demonstrated the ability of a composite antioxidant-rich juice (30 percent grape, 25 percent black currant, 15 percent elderberry, 10 percent sour cherry, 10 percent blackberry, and 10 percent aronia) to significantly increase the plasma antioxidant activity (30 percent after 2 h) and significantly reduce plasma MDA (18 percent after 4 h) in six healthy volunteers (Figure B.12 a, b).
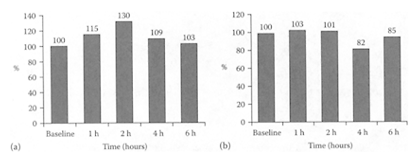
FIGURE B.12 (a) Relative changes in plasma antioxidant after juice consumption, (b) Relative changes in plasma MDA after juice consumption. (From Netzel et al., Food Res. Intern., 35:213–216, 2002. With permission.)
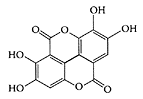
Ellagic acid. (Adapted from Mertens-Talcott and Percival, Cancer Lett., 218:141–151, 2005.)
References
Heinonen, I.M., Meyer, A.S., and Frankel, E.N., Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation, J. Agric. Food Chem., 46:4107–4112, 1998.
Mertens-Talcott, S.U. and Percival, S.S., Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells, Cancer Lett., 218:141–151, 2005.
Miranda-Rottmann, S., Aspillaga, A.A., Pereez, D.D., Vasquez, L., Martinez, L., and Leighton, F., Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress, J. Agric. Food Chem., 50:7542–7547, 2002.
Netzel, M., Strass, G., Kaul, C., Bitsch, I., Dietrich, H., and Bitsch, R., In vivo antioxidative capacity of a composite berry juice, Food Res. Intern., 35:213–216, 2002.
Prior, R.L., Cao, G., Marton, A., Sofic, E., McEwen, J.J., O’Brien, C., Lischner, N.Y., Ehlenfeldt, M., Kalt, W., Krewer, G., and Mainland, C.M., Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species, J. Agric. Food Chem., 46:2686–2693, 1998.
Xue, H., Aziz, R.M., Sun, N., Cassady, J.M., Kamen dulis, L.M., Xu, Y., Stoner, G.D., and Klaunig, J.E., Inhibition of cellular transformation by berry extracts, Carcinogenesis, 22:831–833, 2001.
All trans-β-carotene. (Adapted from Keijer et al, Biochem. Biophys. Acta, 1740:139–146, 2005)
β-Carotene
β-Carotene is an important antioxidant present in fruits and vegetables. As a carotenoid it is a potent quencher of singlet oxygen and would be expected to exert a protective effect against sunlight-induced erythema in human skin (Gollnick et al. 1996; Biesalski and Obermiller-Jevic, 2001) and photoimmunosuppression (Fuller et al., 1992; Herraiz et al., 1992). Trekli and coworkers (2003) examined the effect of β-carotene on UVA-induced HO-1 gene expression in a cultured human fibroblast line FEK4. Activation of the HO-1 gene has become a sensitive marker for oxidative stress. These researchers showed β-carotene inhibited activation of the HO-1 gene, probably through scavenging singlet oxygen.
Considerable controversy surrounds β-carotene, as three randomized clinical trials showed that it alone, or in combination with vitamins A and E, increased lung-cancer incidence and mortality in heavy smokers and in asbestos workers (The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group, 1994; Stram et al., 2002; Omenn et al., 1996a, b). Paolini et al. (2003) reviewed the data on β-carotene and suggested it was harmful when given as the sole supplement to smokers or individuals exposed to environmental carcinogens. The high levels of cytochrome P450 isoforms induced by β-carotene under these conditions could predispose individuals to higher cancer risk as a result of bioctivation of procarcinogens or by increased production of reactive-oxygen species. Thus, β-carotene may act as a cocarcinogen, particularly in individuals exposed to tobacco smoke or industrial settings. However, under normal circumstances, β-carotene exhibits both antioxidant and anticancer properties.
References
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group, The effect of vitamin E and β-carotene on the incidence of lung cancer and other cancers in male smokers, N. Engl. J. Med., 330: 1029–1035, 1994.
Biesalski, H.K. and Obermueller-Jevic, U.C., UV light, β-carotene, and human skin: Beneficial and potentially harmful effects, Arch. Biochem. Biophys., 389:1–6, 2001.
Fuller, C.J., Faulkner, H., Bendich, A., Parker, R.S., and Roe, D.A., Effect of β-carotene supplementation on photosuppression of delayed-type hypersensitivity in normal young men, Am. J. Clin. Nutr., 56:684–690, 1992.
Gollnick, H.P.M., Hopfenmueller, W., Hemmes, C., Chun, S.C., Schmid, C., Sundameier, K., and Biesalski, H.K., Systemic β-carotene plus topical UV sunscreen are an optimal protection against harmful effects of natural UV sunlight, Eur. J. Dermatol., 6: 200–295, 1996.
Herraiz, L.A., Hsieh, W.C., Bendich, A., Parker, R.S., and Swanson, J.E., Effect of UV exposure and β-carotene supplementation on delayed-type sensitivity response in healthy older males, J. Am. Coll. Nutr., 176:617–624, 1998.
Keijer, J., Bunschoten, A., Palou, A., and Franssenvan Hal, N.L.W., Beta-carotene and the application of transcriptomics in risk-benefit evaluation of natural dietary components, Biochem. Biophys. Acta, 1740:139–146, 2005.
Omenn, G.S., Goodman, G.E., Thornquist, M.D., Balmes, J., Cullen, M.R., Glass, A., Keogh, J.P., Meyskens, F.L., Jr., Valanis, B., Williams, J.H., Jr., Barnhartm S., Cherniack, M.G., Brodkin, C.A., and Hammer, S., Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and efficacy trial. J. Ntl. Cancer Inst., 88:1550–1559, 1996a.
Omenn, G.S., Goodman, G.E., Thornquist, M.D., Balmes, J., Cullen, M.R., Glass, A., Keogh, J.P., Meyskens, F.L., Jr., Valanis, B., Williams, J.H., Jr., Barnhart, S., and Hammar, S., Effects of a combination of β-carotene and vitamin A on lung cancer and cardiovascular disease, N. Engl. J. Med., 334:1150–1155, 1996b.
Paolini, M., Abdel-Rahman, S.Z., Sapone, A., Pedulli, G.F., Perocco, P., Cantelli-Forti, G., and Legator, M.S., ß-Carotene: A cancer chemopreventive agent or a co-carcinogen, Mut. Res., 543:195–200, 2003.
Stram, D.O., Huberman, M., and Wu, A., Is residual confounding a reasonable explanation for the apparent protective effects of β-carotene found in epidemiologic studies of lung cancers and smokers? Am. J. Epidemiol., 155:622–628, 2002.
Trekli, M.C., Riss, G., Goralczyk, R., and Tyrrell, R.M., Beta-carotene suppresses UVA-induced HO-1 gene expression in cultured FEK4, Free Rad. Biol. Med., 34:456–464, 2003.
Betalains
Betalains are water-soluble pigments containing nitrogen and include red-violet betacyanins and yellow betaxanthins. Betacyanins are a group of compounds exhibiting antioxidant and radical-scavenging activities (Escribano et al., 1998; Pedrano and Escribano, 2000). Betalains are a class of cationic antioxidants found in red beets. One of the major ones, betanin or betanidin 5-O-β-glucoside, was shown by Kanner and coworkers (2001) to inhibit linoleate peroxidation with an IC50 value of 0.4 μM compared to 1.2 and 5.0 for catechin and α-tocopherol, respectively. Betanin (IC50< 2.5 μM) inhibited lipid peroxidation of membranes or linoleate emulsions catalyzed by the “free iron” redox cycle, H2O2-activated metmyoglobin or lipoxygenase and was more effective than catechin. The bioavailability of betanin was demonstrated in four volunteers with 0.5–0.9 percent detected in the urine. An excellent review of betalain was published by Strack and coworkers (2002).
References
Escribano, J., Pedreno, M.A., Garcia-Carmona, F., and Munoz, R., Characterization of the antiradical activity of betalains from Beta vulgaris roots, Phytochem. Anal., 9:124–127, 1998.
Kanner, J., Harel, S., and Granit, R., Betalains—A new class of dietary cationized antioxidants, J. Agric. Food Chem., 49:5178–5185, 2001.
Pedreno, M.A. and Escribano, J., Studying the oxidation and antiradical activity of betalain from beetroot, J. Biol., 35:49–59, 2000.
Strack, D., Vogt, T., and Schliemann, W., Recent advances in betalain research, Phytochemistry, 62: 247–269, 2002.
Bilberry
Bilberry, a low-growing shrub, grows mainly in the U.K., northern Europe, and Asia. Its dark-blue berries are used mainly for tarts and preserves. The main phenolic constituents in bilberry (Vaccinium myrtillus L.) are anthocyanins. Madhavi et al. (1998) identified a major flavonoid fraction containing proanthocyanidins and anthocyanin pigments together with a hexane extract containing chlorophylls, carotenoids, sterols, and lipids in tissues extracted from bilberry fruits and callus cultures. Many of these constituents not only protected against initiation of carcinogenesis but prevented proliferation of the cancer cell lines. This was evident by the ability of the hexane extract to induce the phase II xenobiotic detoxification enzyme, quinone reductase (QR), in murine hepatoma cells.
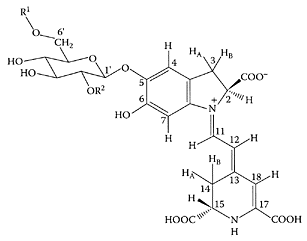
Betanin (R1=R2=H). (From Strack et al., Phytochemistry, 62:247–269, 2002. With permission.)
A study by Logan and Wong (2001) showed bilberry may be beneficial in the treatment of chronic-fatigue syndrome through its control of oxidative stress. Head (2001) suggested a number of botanicals, including bilberries, could also prevent cataracts, particularly by inhibition of aldolase reductase activity. Katsube et al. (2003) recently reported that the phenolic and anthocyanin content, as well as DPPH radical-scavenging activity, was highest in the bilberry extract (Table B.11). Those anthocyanins eluted with 20–40 percent and 40 percent metanol were the more potent inhibitors of the growth of human promelocytic leukemia HL60 and HCT116 cancer cells, inducing apoptosis in the HL60 cells. The anticancer effects exhibited by bilberries make them a potential functional food.
References
Head, K.A., Natural therapies for ocular disorders, part two: Cataracts and glaucoma, Altern. Med. Rev., 6:141–166, 2001.
Katsube, N., Iwashita, K., Tsushida, T., Yamaki, K., and Kobori, M., Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins, J. Agric. Food Chem., 51:68–75, 2003.
Logan, A.C. and Wong, C., Chronic fatigue syndrome: Oxidative stress and dietary modifications, Altern. Med. Rev., 6:450–459, 2001.
Madhavi, D.L., Bomser, J., Smith, M.A.L., and Singletary, K., Isolation of bioactive constituents from Vaccinium myrtillus (bilberry) fruits and cell cultures, Plant Sci., 131:95–103, 1998.
Bitter tea (Ligustrum pedunculare)
Bitter tea is a popular beverage in China. In contrast to green and oolong teas, which are prepared from the leaves of Camellia sinensis, bitter tea is brewed from the leaves of 10 species in five different families (He et al., 1992). Wong and coworkers (2001) characterized the antioxidants present in one of these species, Ligustrum purpuracens, as two phenylethanoid glycosides, acteoside and ligpurposide A. Both proved to be effective antioxidants comparable to green tea catechins. Further work by Chen and coworkers (2002) characterized the antioxidants in Ligustrum pedunculare, another species used for brewing bitter tea in Suchuan Province of China. He et al. (1994) previously isolated eight phenylethanoid or monoterpene glycosides in this species. Chen and coworkers (2002) showed the crude glycoside fraction prevented the oxidation of human low-density lipoprotein (Scheme B.8). Four out of the eight monoterpene glycosides (lipedosides B-V, B-VI, A-I and A-II) protected LDL from Cu2+-mediated oxidation, as well as exhibited free-radical scavenging activity on DPPH equivalent to that of α-tocopherol (Figure B.13).
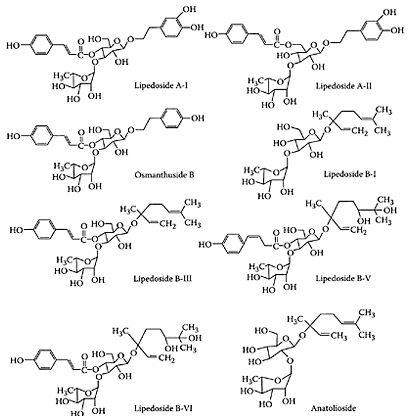
SCHEME B.8 Structures of phenylethanoid and monoterpene glycosides in bitter-tea beverage derived from the plant L.pedunculare. (From Chen et al., J. Agric. Food Chem., 50:7530–7535, 2002. With permission.)
References
Chen, Z.Y., Wong, I.Y., Leung, M.W., He, Z.D., and Huang, Y., Characterization of antioxidants present in bitter tea (Ligustrum pedunculare), J. Agric. Food Chem., 50:7530–7535, 2002.
He, Z.D., Liu, Y.Q., and Yang, C.R., Glycosides from Ligustrum purpurascen, Acta Bot. Yunnanica., 14:328–336, 1992.
He, Z.D., Ueda, S., Akaji, M., Fujita, T., Inoue, K., and Yang, C., Monoterpenoid and phenylethanoid glycosides from Ligustrum pedunculare, Phytochemistry, 36:709–716, 1994.
Wong, I.Y., He, Z.D., Huang, Y., and Chen, Z.Y., Antioxidative activities of phenylethanoid glycosides from Ligustrum purpurascens, J. Agric. Food Chem., 49:3113–3119, 2001.
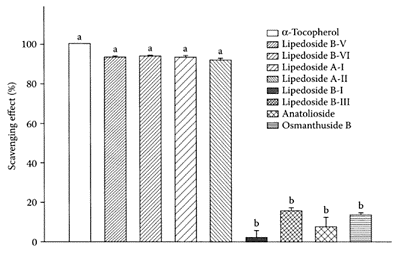
FIGURE B.13 Free-radicalscavenging effects of eight phenylethanoid or monoterpene glycosides (10 μM) isolated from L.pedunculare. 2,2-Diphenyl-1-picrylhydrazyl DPPH was used as a stable free radical, α-Tocopherol was used as a reference antioxidant. Means with different letters (a, b) differ significantly at p< 0.05. (From Chen et al., J. Agric. Food Chem., 50:7530– 7535, 2002. With permission.)
Black beans
Carmona et al. (1996) showed that condensed tannins isolated from black beans strongly inhibited α-amylase, maltase, sucrase, and lactase enzyme activities in vitro. The tannins also affected in vitro glucose uptake by rat-everted intestinal sleeves, which could explain the reduction in carbohydrate bioavailability found in animals fed high tannin diets. Chao and coworkers (1998) showed that water and organic-sol vent extracts of black bean exhibited much higher antioxidant capacity compared to equivalent extracts from soybeans. The ability to inhibit LDL oxidation in plasma of patients suffering from cardiovascular disease was positively correlated with GSH, genistein, anthocyanin, and TAS in the water extract, and vitamin E, genistein, and anthocyanin in the organic extracts for all treatments.
The ability of black beans to bind bile acids in vitro was examined by Kahlon and Woodruff (2002). The cholesterol-lowering properties of food components can be predicted from their ability to bind bile acids, which in turn lowers the risk of cardiovascular disease. These researchers found that both black beans and pinto beans had much higher bile-acid-binding properties compared to soybean protein, suggesting black beans have important health-promoting properties. Recent research by Bawadi et al. (2004) showed that the water-soluble condensed tannins isolated from black beans inhibited the growth of Caco-2 colon, MCF-7 and Hs578T breast, and DV145 prostatic cancer cells without affecting normal human fibroblast lung cells.
References
Bawadi, H.A., Bansode, R.R., Trappey, A., Truax, R.E., and Losso, J.N., Inhibition of Caco-2 colon, MCF-7 and Hs578T breast, and DU145 prostatic cancer cell proliferation by watersoluble black bean condensed tannins, Cancer Lett., 218:153–162, 2005.
Carmona. A., Borgudd, L., Borges, G., and LevyBenshimol, A., Effect of black bean tannins on in vitro carbohydrate digestion and absorption, J. Nutr. Biochem., 7:445–450, 1996.
Chao, P.-Y., Tai, W.-C., Wang, S.-P., and Hu, S.-P., The antioxidative capacity of black bean extract, Atherosclerosis, 136(1), P, S37-S61, 1998.
Kahlon, T.S. and Woodruff, C.L., In vitro binding of bile acids by soy protein, pinto beans, black beans and wheat gluten, Food Chem., 79:425–429, 2002.
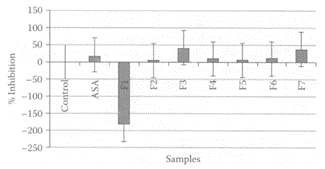
FIGURE B.14 Histogram of inhibition of hyaluronidase by crude extract (F1) and fractions (F2-F7) from chromatographic separation of blackberry fruit. (From Marquina et al., Fitoterapia, 73:727–729, 2002. With permission.)
Blackberry (Rubus fructicosus B.)
Blackberry is a small tree with red-blackish berries rich in polyphenols (Otaiza, 1997). It was reported that blackberry fruit exhibited antiinflammatory activity (Thinquino, 1993). Marquina et al. (2002) assessed the anti-inflammatory activity of a number of different aqueous extract fractions from blackberry based on their ability to inhibit hyaluronidase activity. Several of these fractions were stronger inhibitors than aspirin (Figure B.14).
References
Marquina, M.A., Corao, C.M., Araujo, L., Buitrago, D., and Sosa, M., Hyaluronidase inhibitory activity from polyphenols in the fruit of blackberry (Rubus fructicosus B.), Fitoterapia, 73:727– 729, 2002.
Otaiza, R., Plantas usuales en la medicina popular de Merida, Etidad por el consejo de desarrollo cientific, y tecnologia (C.D.C.H.T.) ULA, Medria, P, 177, 1997.
Thinquino, B., Terapias naturales. Publicaciones Latinoamericana Rayos de Luiz, Colombia, P, 330, 1993.
Black cohosh
Black cohosh (Actea racemosa and Cimicifuga racemosa) is related to the buttercup family, a perennial plant native to North America. Its botanical name was recently changed from Cimicifuga racemosa (L.) Nutt. to Actea racemosa (Ranunculaceae) (Compton et al., 1998a, b). Extracts prepared from its roots and rhizomes are standardized to 26-deoxyactein content, a member of a group of compounds known as saponins (Chen et al., 2001). It is used as an alternative to hormone-replacement therapy to treat hot flashes and other menopausal symptoms (Lieberman, 1998; Hardy, 2000). A randomized, double-blind, placebo-controlled study of 80 menopausal women treated with a black-cohosh extract was compared to a control of conjugated estrogens over a 12-week period (Stoll, 1987). A marked decrease in hot flashes was observed for women on black-cohosh extract (4.9 to 0.7 hot flashes/day) compared to either the control (5.1 to 3.1 hot flashes/day) or estrogen group (5.2 to 3.2 hot flashes/day). A later study also found a similar decrease in hot flashes was experienced by breast-cancer survivors treated with black cohosh or an antiestrogen (Jacobson et al., 2002). The American College of Obstetricians and Gynecologists (ACOG) suggest black cohosh may be helpful in the short term for women with vasomotor symptoms of menopause (ACOG, 2001). Burdette and coworkers (2002) showed methyl caffeate, ferulic acid, and caffeic acid were the primary antioxidants present in methanol extracts of black cohosh that scavenged oxygen radicals and prevented menadione-induced DNA damage.
Li and coworkers (2003) attempted to analyze caffeic-acid derivatives in black cohosh using liquid chromatography/tandem mass spectrometry. A number of derivatives were detected, with six identified as caffeic acid, ferulic acid, isoferulic acid, fukinolic acid, cimicifugic acid A, and cimicifugic acid. Novel compounds with dehydrofukiic acid groups were also reported for the first time in black cohosh. The bioactive properties of these compounds require extensive investigations.
References
American College of Obstetricians and Gynecologists, Use of botanicals for management of meno-pausal symptoms, ACOG Practice Bulletin, 28:1–11, 2001.
Burdette, J.E., Chen, S.-N., Lu, Z.-Z., Xu, H., White, B.E., Fabricant, D.S., Liu, J., Fong, H.H., Farns worth, N.R., Constantinou, A.I., van Breeman, R.B., Pezzuto, J.M., and Bolton, J.L., Black cohosh (Cimicifua racemosa L.) protects against menadioneinduced DNA damage through scavenging reactive oxygen species: Bioassay-directed isolation and characterization of active principles, J. Agric. Food Chem., 50:7022–7028, 2002.
Chen, S.-N., Li, W., Fabricant, D.S., and Santasiero, B.D., Isolation, structure and elucidation and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein, J. Natural Prod., 65:601–605, 2001.
Compton, J.A., Culham, A., Gibbings, J.G., and Jury, S.L., Phylogeny of Actaea including Cimicifuga (Ranunculaceae) inferred from nrDNA ITS sequence variation, Biochem. System. Ecol., 26:185–197, 1998a.
Compton, J.A., Culham, A., and Jury, S.L., Reclassification of Actaea to include Cimicifuga and Souliea (Ranunculaceae); phylogeny inferred from morphology, nrDNA ITS, and cpDNA trnL-F sequence variation, Taxon, 47:593–634, 1998b.
Hardy, M.L., Herbs of special interest to women, J. Am. Pharm. Assoc., 40:234–242, 2000.
identified for the first timeJacobson, J.S., Troxel, A.B., and Evans, J., Klaus, L., Vahdat, L., Kinne, D., Lo, M.S., Moore, A., Rosenmann, P.J., Kaufman, E.L., Neuget, A.I., and Grann, V.R., Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast disease, J. Clin. Oncol., 19:2739–2745, 2001.
Li, W.L., Sun, Y., Liang, W., Fitzloff, J.F., and van Breeman, R.B., Identification of caffeic acid derivatives in Actea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/tandem mass spectrometry, Rapid Commun. Mass Spectrom., 17: 978–982, 2003.
Lieberman, S., A review of effectiveness of Cimicifuga racemosa (black cohosh) for the symptoms of menopause, J. Women’s Health, 7:525–529, 1998.
Stoll, W., Phytotherapy influences atrophic vaginal epithelium: Double-blind study of Cimicifuga vs estrogenic substances, Therapeutikon, 1:23–37, 1987.
Black currant (Ribes nigrum)
see also Gamma Linolenic Acid (GLA) Black currant berries have a very dark coloration due to their content of anthocyanin pigments. They also contain large amounts of flavonoids, phenolic acids and proanthocyanidins. The seeds are also recognized for their content of γ-linolenic acid (GLA), an important polyunsaturated fatty acid, which has important health-related properties. GLA remains stable in the seed due to the presence of large amounts of antioxidants. The berries are used in beverages due to their high content of these antioxidants (Constantino et al., 1993). Lu and Foo (2002) confirmed the presence of four major anthocyanins based on rutinosides and glucosides of delphinidin and cyanidin. They also identified for the first time aureisidin and 1-cinnamyl-β-D-glucoside in black currant. The importance of these anthocyanins is related to their antioxidant and pharmaceutical properties (Andersen et al., 1998; Constantino et al., 1992, 1993).
References
Andersen, O.M., Helland, D.E., and Andersen, K.J., Anthocyanidin and nthocyanidin derivatives, and their isolation, for treatment of cancer, diseases caused by lesions in connective tissues, and diseases caused by viruses, PCT Int. Appl., WO 97 41, 137 (Cl. C07H17/065), 6 November 1997, NO appl. 96/5,418 17 December 1996 (Chemical Abstracts 128, 10325k), 1997.
Constantino, L., Albasini, A., Rastelli, G., and Benvenuti, S., Activity of crude polyphenolic extracts as scavengers of superoxide radicals and inhibitors of xanthine oxidase, Planta Med., 58:342–344, 1992.
Constantino, L., Rastelli, G., Rossi, T., Bertoldi, M., and Albasini, A., Antilipoperoxidant activity of polyphenolic extracts of Ribes nigrum L. Plant Medicinales et Phytotherapie., 26:207–214, 1993.
Lu, Y. and Foo, L.Y., Polyphenolic constituents of blackcurrant seed residue, Food Chem., 80:71– 76, 2003.
Black pepper (Piper nigrum)
see also Piperine Black pepper is a common spice used in foods. It is rich in the alkaloid piperine, which is a member of a group of compounds based on the structure of vanillin, referred to as “vanilloids.” It has been shown previously to enhance the serum levels of drugs and nutrients in animals and humans (Bano et al., 1991; Majeed et al., 1996). Badmaev and coworkers (2000) showed that piperine significantly increased the plasma levels of coenzyme Q10 by almost a third compared to the placebo. This effect could be attributed to an earlier study on its thermonutrient activity (Badmaev et al., 1999).
References
Badmaev, V., Majeed, M., and Norkus, E., Piperine, an alkaloid derived from black pepper increases serum response of beta-carotene during 14-days of oral beta-carotene supplementation, Nutr. Res., 19: 381–388, 1999.
Badmaev, V., Majeed, M., and Prakash, L., Piperine derived from black pepper increases the plasma levels of coenzyme Q10 following oral supplementation, J. Nutr. Biochem., 11:109–113, 2000.
Bano, G., Raina, R.K., Zutshi, U., Johri, R.K., and Atal, K., The effect of piperine on pharmacokinetics of phenytoin in healthy volunteers, Planta Med., 53: 568–569, 1987.
Majeed, M., Badmaev, V., and Rajendran, R., Use of piperine to increase the bioavailability of nutritional compounds, US Patent No. 5,536,506 and No. 5,744,161, 1998.
Black tea
Black tea is the fermented tea, which contains a group of yellow- to darkbrown-colored polyphenolic compounds formed during fermentation (Xiao et al., 1998). Theaflavins (TF) and thearubigins (TR) are the two major classes of polyphenols responsible for its color and taste. During fermentation, these pigments form a mixture of catechin dimers, trimers, or multipolymers, referred to as tea pigments (Nursten, 1997). Animal and clinical studies have demonstrated the ability of tea pigments to treat hypertension, decrease blood sugar, and prevent atherosclerosis and cancer (Morse et al., 1997: Ye, 1997). Tea pigments were also shown to increase superoxide dismutase (SOD) activity and decrease lipid-peroxidation levels in experimental animals (Li et al., 1998; Ren et al., 1998). Cadneri et al. (2000) showed that polyphenolic extracts from black tea was similar to wine extracts in their ability to protect rats against AOM-induced colon carcinogenesis (Table B.12). Black-tea extracts were far more effective than green-tea extracts in increasing apoptosis of the tumors. The anticarcinogenic properties of black-tea extracts were demonstrated by Shukla and Taneja (2002), who reported significant decreases in the number of diethylnitrosamine (DEN)-induced pulmonary tumors in Swiss albino mice fed 2 percent and 4 percent blacktea extracts. Yaping and coworkers (2003) recently showed that tea pigments had similar free-radical-scavenging abilities to tea polyphenols, which further supports their role in disease prevention.
A melanin-like pigment was isolated by Sava et al. (2001) from black tea leaves by alkaline extraction, acid hydrolysis, and precipitation. The isolated pigment had immunostimulating activity, suggesting possible health benefits. Significant antimutagenic effects were also reported by Gupta et al. (2002) for black tea and its polyphenols using the Ames Salmonella assays. Recent work by Besra et al. (2003) also demonstrated the antidiarrheal properties of a hot-water extract of black tea.
References
Besra, S.E., Gomes, A., Ganguly, D.K., and Vedasiromoni, J.R., Antidiarrhoeal activity of hot water extract of black tea (Camellia sinensis), Phytother. Res., 17:380–384, 2003.
Caderni, G., De Filippo, C., Luceri, C., Salvadori, M., Giannini, A., Biggeri, A., Remy, S., Cheynier, V., and Dolara, P., Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats, Carcinogenesis, 21: 1965–1969, 2000.
Gupta, S., Chaudhuri, T., Seth, P., Ganguly, D.K., and Giri, A.K., Antimutagenic effects of black tea (World Blend) and its two active polyphenols theafla-vins and thearubigins in Salmonella assays, Phytother. Res., 16:655–661, 2002.
Li, N., Han, H., and Wang, Z., Protective effect of tea pigments on oxidative damage by free radical in guinea pig, Zhong Guo Zhong Yi Yao Technology, 29:23–24, 1998 (in Chinese).
Morse, M.A., Kresty, L.A., Steele, V.E., Kelloff, G.J., Boone, C.W., Balentine, D.A., Harbowy, M.E., and Stoner, G.D, Effects of theaflavins on N-nitrosomethylbenzylamine-induced esophageal tumorigenesis, Nutr. Cancer Int. J., 29:7–12, 1997.
Nursten, H.E., 1997. Chemistry of tea infusions, in Chemical and Biological Properties of Tea Infusions, Schubert, R. and Spiro, M., Eds., German Medical Information Services, Frankfurt, 1997, pp. 10–83.
Ren, M., Zheng, Y., and Xu, S., 1998. The inhibitory effect of tea pigments on lipid peroxidation in mice, Jiang Xi Med. Acta, 38:49–51, 1998 (in Chinese).
Sava, V.M., Galkin, B.N., Hong, M.Y., Yang, P.C., and Huang, G.S., A novel melanin-like pigment derived from black tea leaves with immuno-stimulating activity, Food Res. Inter., 34:337–343, 2001.
Shukla, Y. and Taneja, P., Anticarcinogenic effect of black tea on pulmonary tumors in Swiss albino mice, Cancer Lett., 176:137–141, 2002.
Yaping, Z., Wenli, Y., Dapu, W., Xiaofeng, L., and Tianxi, H., Chemiluminescence determination of free radical scavenging abilities of “tea pigments” and comparison with “tea polyphenols,” Food Chem., 80:115–118, 2003.
Ye, W., The study and application of tea pigment, China Food Add., 4:23–24, 1997 (in Chinese).
Xiao, W., Zhong, J., Xiao, H, and Li, D., The mechanism of formation of tea pigments during the industrial processing of tea known as “fermentation,” Fujian Tea, 3:8–12, 1998.
Blueberries
Blueberries are rich sources of procyanidins and anthocyanins. Catechin and epicatechin were reported as monomers with (epi)catechin oligomer units exclusively singly linked (B-type) (Prior et al., 2001). Blueberries were among those fruit that strongly reduced the genotoxicity of 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) in a dose-dependent manner in metabolically competent Chinese hamster-lung fibroblast V9 cells (Edenharder et al., 2002). Mazza and coworkers (2002) reported that 19 out of 25 anthocyanins, both intact glycosylated and possibly acylated forms, were absorbed by human subjects who consumed a high-fat diet together with a freezedried blueberry powder. The increase in serum anthocyanin levels correlated with an increase in serum antioxidant activity (ORAC) (Figure B.15).
Kay and Holub (2002) reported that consumption by healthy human subjects of a freeze-dried powder from wild blueberries
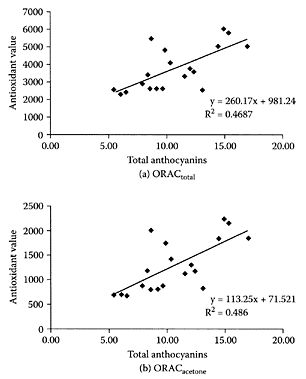
FIGURE B.15 Correlation between serum antioxidant capacity and concentration of serum total anthocyanins. Antioxidant value expressed as micromoles of Trolox equivalents per liter, and anthocyanins expressed (nanograms per milliliter of serum) as cyanidin 3-glucoside chloride. (From Mazza et al., J. Agric. Food Chem., 50:7731–7737, 2002. With permission.)
(Vaccinium angustifolium), together with a high-fat meal, significantly increased serumantioxidant status (determined by ORAC and TAS assays) compared to control diets. This increase in blood-antioxidant status has been associated with decreased risk in atherosclerosis (Durak et al., 2001) and cancer (Ching et al., 2002).
A number of proanthocyanidin fractions were recently separated from wild-blueberry extracts by Schmidt et al. (2004). Of these, only the high-molecular-weight proanthocyanidin oligomers exhibited antiproliferation and antiadhesion properties. For example, two fractions composed predominantly of four to eight linked oligomeric proanthocyanidins with average degrees of polymerization of 3.25 and 5.65 prevented adhesion of the organism responsible for urinary infections, Escherichia coli. However, only the latter fraction exhibited significant antiproliferation activity against human prostate and mouse liver-cancer cells.
References
Ching, S., Ingram, D., Hahnel, R., Beilby, J., and Rossi, E., Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study, J. Nutr., 132: 303–306, 2002.
Durak, I.I., Kacmaz, M., Cimen, M.Y.B., Buyukkocak, U., and Ozturk, H.S., Blood oxidant/antioxidant status of atherosclerotic patients, Int. J. Cardiol., 77:293–297, 2001.
Edenharder, R., Sager, J.W., Glatt, H., Muckel, E., and Platt, K.L., Protection by beverages, fruits, vegetables, herbs, and flavonoids against genotoxicity of 2-acetylaminofluorene and 2-amino-1 -methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in metabolically competent V9 cells, Mutat. Res., 521:57–62, 2002.
Kay, C.D. and Holub, B.J., The effect of wild blueberry (Vaccinium angustifolium) consumption on postprandial serum antioxidant status in human subjects, Br. J. Nutr., 88:389–397, 2002.
Mazza, G., Kay, C.D., Cottrell, T., and Holub, B.J., Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects, J. Agric. Food Chem., 50:7731–7737, 2002.
Prior, R.L., Lazarus, S.A., Cao, G., Muccitelli, H., and Hammerstone, J.F., Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using highperformance liquid chromatography/mass spectrometry, J. Agric. Food Chem., 49:1270–1276, 2001.
Schmidt, B.M., Howell, A.B., McEniry, B., Knight, C.T., Seigler, D., Erdman J.W., Jr., and Lila, M.A., Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium Ait.) fruits, J. Agric. Food Chem., 52:6433–6442, 2004.
Borage oil
Borage oil is a high gamma-linolenic acid (GLA; C18:3n-6) oil extracted from borage seeds (Borago officinalis L.). The composition of the oil is shown in Table B.13. GLA accounts for almost 25 percent of the total fatty acids in borage oil. Mounting evidence points to GLA as a potent blood-pressure-lowering nutrient, making it a potential dietary intervention for hypertension (Das, 1995; Narce and Poisson, 1995; Engler et al., 1992). Engler and Engler (1998) found GLA-rich oils, such as borage oil, increased the composition of GLA and dihomogamma-linolenic acid in the plasma, hepatic, and vascular tissue of spontaneously hypertensive rats. The changes in fatty-acid profiles brought about by GLA-enriched oils were attributed to its favorable blood-pressure-lowering effect.
TABLE B.13
Typical Fatty-Acid Composition of Borage Oil
Another role for GLA is its ability to attenuate body-fat accumulation in rats. Obese Zucker rats fed black currant oil containing 70 percent GLA were found to have lower bodyfat content compared with those animals fed soybean oil (Phinney et al., 1993). Takahashi and coworkers (2000) showed that GLA-rich borage oil reduced white adipose tissue weight compared to safflower oil by increasing gene expressions of the uncoupling protein 1 in brown adipose tissue.
Consumption of borage oil was also shown by Brosche and Platt (2000) to significantly and statistically improve skin function in the elderly. They reported a 34 percent reduction in itching, as well as in dry skin from 42 to 14 percent. Another benefit of borage oil is in the treatment of rheumatoid arthritis, due to its abil-ity to decrease the tumor necrosis factor (TNF-α), a central tissue destructive mediator in rheumatoid arthritis (Belch and Hill, 2000). Kast (2001) reviewed double-blind studies suggesting borage oil was beneficial for treating rheumatoid arthritis. He proposed a mechanism whereby GLA in borage oil raised protaglandin E levels, which in turn increased cAMP levels that suppressed TNF-α. He further cautioned against the use of nonsteroidal anti-inflammatory drugs, which would undermine the effects of borage oil.
Gadek and coworkers (1999) showed that enteral nutrition with special diets containing either EPA or GLA reduced the number of neutrophils in brochoalveolar lavage fluid, as well as reducing pulmonary inflammation. This resulted in improved clinical outcomes in patients suffering from acute respiratory distress syndrome (ARDS). To explain the anti-inflammatory effects of EPA and GLA, Gillis and coworkers (2002) hypothesized this was due to induction of neutrophil apoptosis. Their studies showed that EPA and GLA, alone or in combination, triggered the induction of apoptosis and secondary necrosis in human promy elocytic HL-60 cells. Thus, inclusion of GLA and EPA could improve clinical outcomes in ARDS.
Using a double-blind, monocentric trial with parallel groups of healthy male volunteers between the ages of 18 and 30, Duriez et al. (1997) showed it was possible to provide oral supplements of borage oil (3 g/day) over six weeks without having any adverse effects on platelet aggregation.
References
Belch, J.J. and Hill, A., Evening primrose oil and borage oil in rheumatic conditions, Am. J. Clin. Nutr., 71:352–356S, 2000.
Brosche, T. and Platt, D., Effect of borage oil consumption on fatty acid metabolism, transdermal water loss and skin parameters in elderly people, Arch. Gerontol. Geratr., 30:139–150, 2000.
Das, U.N., Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease, Prostaglandins Leukotr. Essen. Fatty Acids, 52:387–391, 1995.
Duriez, P., Luc, G., Jude, B., Bordet, J.C., Lacroix, B., Bonte, J.P., Parra, H.J., and Bard, J.M., A therapeutic dosage (3 g/day) of borage oil supplementation has no effect on platelet aggregation in healthy volunteers, Atherosclerosis, 134:189, 1997.
Engler, M.M. and Engler, M.B., The effects of dietary evening primrose, blackcurrant, borage and fungal oils on plasma, hepatic and vascular tissue fatty acid composition in the spontaneously hypertensive rats, Nutr. Res., 18:1533–1544, 1998.
Gadek, J.E., DeMichele, S.J., Karlstad, M.D., Pacht, E.R., Donahoe, M., Alberston, T.E., Van Hoozen, C., Wennberg, A.K., Nelson, J.L., and Noursalehi, M., Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome, Crit. Care Med., 27:1409–1420, 1999.
Gillis, R.C., Daley, B.J., Enderson, B.L., and Karlstad, M.D., Eicosapentanoic acid and γ-linolenic acid induce apoptosis in HL-60 cells, J. Surgical Res., 107:145–153, 2002.
Kast, R.E., Borage oil reduction of rheumatoid arthritis activity may be mediated by increased cAMP that suppresses tumor necrosis factor-alpha, Int. Immunopharmacol, 1:2197–2199, 2001.
Narce, M. and Poisson, J-P., Age-related depletion of linoleic acid desaturation in liver microsomes from young spontaneously hypertensive rats, Prostaglandins Leukotr. Essen. Fatty Acids, 53:59–63, 1995.
Phinney, S.D., Tang, A.B., Thurmond, A.C., Nakamura, M.T., and Stern, J.S., Abnormal polyunsaturated-lipid metabolism in the obese Zucker rat with partial metabolic correction by γ-linolenic acid administration, Metabolism, 42:1127–1140, 1993.
Takahashi, Y., Ide, T., and Fujita, H., Dietary gammalinolenic acid in the form of borage oil causes less body fat accumulation accompanying an increase in uncoupling protein 1 mRNA level in brown adipose tissue, Comp. Biochem. Physiol., Part B, 127:213–222, 2000.
Bovine lactoferrin
see also Lactoferrin Lactoferrin, an iron-binding protein found in milk, also possesses bacteriocidal activity. A 25-residue peptide released from the aminoterminal region of bovine lactoferrin catalyzed at acidic pH by pepsin was shown to have potent bacteriocidal activity (Bellamy et al., 1992). Such a reaction can occur in the stomach, in which a stable lactoferricin B is released into the intestine (Kuwata et al., 1998a, b). The intact peptide is extremely basic, with five arginine and three lysine residues. Lactoferrin appears to regulate immune and inflammatory responses by regulating the production of some cytokines, including interleukins and tumor necrosis factor-α (TNF-α) (Brock et al., 2000; Choe and Lee, 1999). Strom and coworkers (2001) examined the effects of charge and lipophilicity on the antibacterial activity of an undacapeptide (FKCRRWQWRMK) derived from bovine lactoferricin. All undecapeptides had Tryp residues in positions 6 and 8 and Arg in positions 5 and 9 and were more effective against Gram-positive bacteria, such as Staphyloccus aureus, with a higher bacteriocidal effect against Escherichia coli than Pseudomonas aeruginosa.
References
Bellamy, W., Takase, M., Yamauchi, K., Wakabayashi, H., Kawasa, T., and Tomita, M., Antibacterial spectrum of lactoferricin B, a potent bacteriocidal peptide derived from the N-terminal region of bovine lactoferrin, J. Appl. Bacteriol., 73:472–479, 1992.
Brock, J.H., Guillen, G., and Thompson, C., Anti-inflammatory and immune-regulatory properties of lactoferrin, in Lactoferrin, Structure, Function and Applications, Shimazaka, K., Ed., Elsevier Science, New York, 2000, pp. 119–128.
Choe, Y.H. and Lee, S.W., Effect of lactoferrin on the production of tumor necrosis factor-α and nitric oxide, J. Cell Biochem., 76:30–36, 1999.
Kuwata, H., Yip, T.T., Tomita, M., and Hutchens, T.W., Direct evidence of the generation in human stomach of an antimicrobial peptide domain (Lactoferrin) from ingested lactoferrin, Biochem. Biophys. Acta, 1429:129–141, 1998a.
Kuwata, H., Yip, T.T., Yamauchi, K., Teraguchi, S., Hayasawa, H., Tomita, M., and Hutchens, T.W., The survival of ingested lactoferrin in the gastrointestinal tract of adult mice, Biochem. J., 334:321–323, 1998b.
Strom, M.B., Rekdal, O., and Svendsen, J.S., The effects of charge and lipophilicity on the antibacterial activity of undecapeptides derived from bovine lactoferricin, J. Peptide Sci., 8:36– 43, 2001.
Bovine plasma
Only a small portion of blood taken from an animal after slaughter is used as emulsifiers, stabilizers, clarifiers, and as nutrients for foods. Interest in identification of bioactive peptides led to isolation of bovine blood plasma hydrolysates, including opioid peptides (Zhao et al., 1997), bradykinin-potentiating peptides (Piot et al., 1992), and several angiotensin 1-converting enzymes (ACE) (Hyun and Shin, 2000; Suetsuna, 1995). Janitha et al. (2002) reported the production of a number of bioactive peptides, following hydrolysis of defribinated plasma (DBP), a by-product of meat-processing plants, with a microbial protease. Examination of the different protein hydrolysates, following various degrees of hydrolysis (DH), showed an increase in ACE inhibition accompanied an increase in DH. The highest inhibitory activity was found for the 42 percent DH hydrolysate. Peptides were separated by size-exclusion chromatography, and the fraction with the greatest inhibitory activity contained peptides with GYP, HL(1), HPY, HPGH, L(1)F, SPY, and YPH sequences. Park and Hyun (2002) reported the production of bioactive peptides with antigenotoxic activity following enzymatic hydrolysis of bovine plasma proteins with several different proteases.
References
Hyun, C-K. and Shin, H-K., Utilization of bovine blood plasma proteins for the production of angiotensin 1 converting inhibitory peptides, Process Biochem., 36:65–71, 2000.
Janitha, P.K., Wanasundara, P.D., Ross, A.R.S., Amarowicz, R., Ambrose, S.J., Pegg, R.B., and Shand, P.J., Peptides with angiotensin 1-converting enzyme (ACE) inhibitory activity from defibrinated, hydrolyzed bovine plasma, J. Agric. Food Chem., 50: 6981–6988, 2002.
Park, K-J. and Hyun, C-K., Angenotoxin effects of the peptides derived from bovine blood plasma proteins, Enz. Microb. Technol., 30:633–638, 2002.
Piot, J.M., Zhao, Q., Guillochon, D., Ricart, G., and Thomas, D., Isolation and characterization of a bradykinin-potentiating peotide from a bovine peptic hemoglobin hydrolysate, FEBS Lett., 299:75–79, 1992.
Suetsuna, Y., Novel tripeptides and angiotensin converting enzyme inhibitors, Japanese Patent 07–188183, 1995.
Zhao, Q., Coeur, C.L., and Piot, J.M., Analysis of peptides from bovine hemoglobin and tuna myoglobin enzymatic hydrolysate: Use of HPLC with on-line second order derivative spectroscopy for the characterization of biologically active peptides, Anal. Chim. Acta, 352:201–220, 1997.
Bowman-Birk protease inhibitor
The Bowman-Birk protease inhibitor (BBI), is a family of different forms and isoforms of natural polypeptide serine protease inhibitors of trypsin and chymotrypsin found in legume seeds, such as soybeans, chickpeas, and peanuts, and, to a lesser extent, in cereals, such as barley. Preclinical studies showed BBIs were effective suppressors of carcinogenesis both in vivo and in vitro (Kennedy, 1998). While the specific target(s) affected by BBIs have yet to be identified, indirect targets appear to be a modulation of superoxide anion radical production, oncongene levels, DNA repair, immune effects, and arachidonic-acid metabolism (Lippmann and Matrisian, 2000).
TABLE B.14
Clinical Response to BBI Concentrate with Respect to Dose Administered
Early work by von Hofe et al. (1991) noted soybean BBI effectively inhibited esophageal carcinogenesis induced by N-nitrosomethyl-benzylamine (NMBzA) in male Sprague-Dawley rats. A reduction of 45 percent in the frequency of papillomas and carcinomas was observed in rats receiving BBI in three tablets a week. The ability of BBI to prevent the development of malignancies has been demonstrated in a number of animal models (Kennedy, 1993). A phase I clinical trial conducted by Armstrong et al. (2000a) showed an oral dose of a BBI concentrate given to 24 patients suffering from oral leukoplakia was nontoxic. This was followed by a phase IIa clinical trial by Armstrong et al. (2000b) in which the same BBI concentrate was administered to 32 patients with oral leukoplakia. A 31 percent clinical response was observed, including two complete and eight partial, determined by pretreatment and posttreatment for individual and total lesion areas and analysis (Table B.14). The mean total lesion area decreased significantly (p<0.004) from 614 to 435 mm2 following treatment with BBI concentrate with a linear dose-response relationship observed. The absence of toxicity combined with a dose-dependent decrease in oral leukoplakia area will require further randomized clinical trials to determine the efficacy of BBI concentrate in treating this condition.
References
Armstrong, W.B., Kennedy, A.R., Wan, X.S., Atiba, J., McLaren, C.E., and Meyskens, F.L., Jr., Singledose administration of Bowman-Birk inhibitor concentrate in patients with oral leukoplakia, Cancer Epidemiol. Biomark. Prev., 9:43–47, 2000a.
Armstrong, W.B., Kennedy, A.R., Wan, X.S., Taylor, T.H., Nguyen, Q.A., Jensen, J., Thompson, W., Lagerberg, W., and Meyskens, F.L., Jr., Clinical modulation of oral leukoplakia and protease activity by Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention trial, Clin. Cancer. Res., 6:4684–4691, 2000b.
Kennedy, A.R., Overview: Anticarcinogenic activity of protease inhibitors, in Protease Inhibitors as Cancer Chemopreventive Agents, Troll, W. and Kennedy, A.R., Eds., Plenum Press, New York, 1993, pp. 9–64.
Kennedy, A.R., Chemopreventative agents: Protease inhibitor, Pharmacol. Ther., 78:167–209, 1998.
Lippmann, S.M. and Matrisian, L.M., Protease inhibitors in oral carcinogenesis and chemoprevention, Clin. Cancer Res., 6:4599–4603, 2000.
Von Hofe, E., Newberne, P.M., and Kennedy, A.R., Inhibition of N-nitrosomethylbenzylamineinduced esophageal neoplasms by the Bowman Birk protease inhibitor, Carcinogenesis, 12:2147–2150, 1991.
Boxwood (Buxus sempervirens)
Boxwood is a popular woody, ornamental plant grown throughout Europe and North America. In folk medicine, extracts from Buxus are used to cure different diseases, including the treatment of HIV infections (Valmet, 1983; Durrant et al., 1996, 1998). It is a rich source of steroidal alkaloids, with four new alkaloids extracted from its leaves by Loru and coworkers (2000). Some of these alkaloids may be responsible for some of the health-related properties attributed to boxwood (Scheme B.9).
SCHEME B.9 Structure of four new alkaloids isolated from Buxus sempervirens. (From Loru et al., Phytochemistry, 54:951–957, 2000. With permission).
References
Durant, J., Chantre, P., Gonzalez, G., Vandermander, J., Half on, P., and Rousse, B., Efficacy and safety of Buxus sempervirens L. preparations (SPV30) in HIV-infected asymptomatic patients: A multicentre, randomized, double-blind, placebo-controlled trial, Phytochemistry, 5:1–10, 1998.
Durant, J., Vandermander, J., Chanre, P., and Dellamonica, P., in Communication at conference on AIDS, Vancouver, Canada (Abstr. LB6040), 1996.
Loru, F., Duval, D., Aumelas, A., Akeb, F., Guedon, D., and Guedj, R., Four steroidal alkaloids from the leaves of Buxus sempervirens, Phytochemistry, 54:951–957, 2000.
Valmet, J., Phytotherapie traitement des maladies par les plantes, Maloine, Paris, 1983.
Brassica vegetables
see also Crucifera Brassica vegetables are among the most frequently consumed vegetables around the world (Lange et al., 1992a, b). They include white cabbage, red cabbage, broccoli, cauliflower, Brussels sprouts, and Savoy cabbage, as well as rape and mustard. They all contain glucosinolates, which undergo degradation to isothiocyanates, indoles, and nitriles (Scheme B.10). The chemopreventive properties of these vegetables are related to the ability of their bioactive components to inhibit phase I enzymes and to activate phase II enzymes, such as glutathione S-transferase (GST).
Brassica vegetables, such as broccoli, cauliflower, Brussels sprouts, and kale, have been reported to exhibit strong anticancer properties. A diet rich in Brussels sprouts decreased urinary excretion of 8-oxidG, indicative of DNA damage (Verhagen et al., 1997), while a low risk of lung cancer in Chinese men was associated with a high urinary excretion of isothiocyanates (London et al., 2000). One explanation for the decreased cancer risk associated with vegetable intake is related to induction or inhibition of biotransformation enzymes. Lampe and coworkers (2000) found Brassica vegetables increased while apiceous vegetables decreased cytochrome P450 1A2 in human subjects. Steinkellner et al. (2001) showed that it was the degradation compounds of glucosinolates in Brassica vegetables that were responsible for the protective effect against carcinogens. For example, indoles and isothiocyanates attenuated the carcinogenic effects of poly cyclic aromatic hydrocarbons (PAHs), as well as against heterocyclic amines.
References
Lampe, J.W., King, I.B., Li, S., Grate, M.T., Barale, K.V., Chen, C., Feng, Z., and Potter, J.D., Brassica vegetables increase apiceous vegetables decrease cytochrome P450 1A2 activity in humans: Changes in caffeine metabolite ratios in response to controlled vegetable diets, Carcinogenesis, 21:1157– 1162, 2000.
Lange, R., Baumgrass, R., Diedrich, M., Henschel, K.-P., and Kujawa, M., Glucosinolate in der Ernahrung-Pro und Contra einer Naturstoffklasse. Teil I: Ausgangs situation, Problem stellung, Analytik, Verzehr, Ernahr, Umsch, 39:252–257, 1992a.
Lange, L., Baumgrass, R., Diedrich, M., Henschel, K.-P., and Kujawa, M., Glucosinolate in der Ernahrung-Pro und Contra einer Naturastoff. Teil II: Abbau und Stoffwechsel Ernahr, Umsch, 39:252–257, 1992b.
London, S.J., Yuan, J.M., Chung, F.L., Gao, Y.T., Coetzee, G.A., Ross, R.K., and Yu, M.C., Isothiocyanates, glutathione C-transferase M1 and T1 polymorphisms, and lung-cancer risk: A prospective study of men in Shangai, China, Lancet, 356:724–729, 2000.
Pessina, A., Thomas, R.M., Palmieri, S., and Lussi, P.L., An improved method for the purification of myrosinase and its physicochemical characterization, Arch. Biochem. Biophys., 280:383–389, 1990.
Steinkellner, H., Rabot, S., Fretwald, C., Nobis, E., Scharf, G., Chabicovsky, M., Knasmuller, S., and Kassie, F., Effects of cruciferous vegetables and their constituents on drug metabolizing enzymes involved in the bioactivation of DNA-reactive dietary carcinogens, Mutat. Res., 480– 481:285–297, 2001.
Verhagen, H., de Vries, A., Nijhoff, N.A., Schouten, A., van Poppel, G., Peters, W.H., and van den Berg, H., Effects of Brussels sprouts on oxidative DNA-damage in man, Cancer Lett., 114:127– 130, 1997.
SCHEME B.10 Chemical structures of glucosinolates and their breakdown products following enzymatic hydrolysis by myrosinase. (Adapted from Pessina et al. (1990) by Steinkellner et al., Mutat. Res., 480– 481:285–297, 2001. With permission.)
Broccoli
Consumption of Brassica vegetables, such as broccoli, leads to excretion of isothiocyanates (ITCs) in the urine. These compounds are produced by enzymic hydrolysis of intact thioglucoside conjugates or glucosinolates and may have a role as cancer chemopreventative agents (Conaway et al., 2002). The major glucosinolate in broccoli, glucoraphanin, is hydrolyzed by myrosinase to sulforaphane or sulforaphane nitrile (Scheme B.11). Matusheski and Jeffery (2001) compared the biactivity of these metabolites in mouse hepatoma cells.
Sulforaphane proved to be the most potent in inducing phase II detoxification enzymes and had much greater potential as a chemoprotective agent than the corresponding nitrile. Keck et al. (2003) showed intact broccoli glucosinolates (Broccoli-GS) enhanced quinone reductase (QR) in the liver and colon of Fischer 344 rats far more than when fed hydrolyzed broccoli (Broccoli-HP) (Table B.15). There were no significant differences in the colonic or hepatic QR activity of rats fed a purified sulforaphane (SF) diet or the intact Broccoli-GS diet. They suggested that urinary sulforaphane conjugate of mercapturic acid was a useful biomarker for assessing the effects of dietary broccoli on QR induction in the liver and colon and could be extrapolated to measure the relative cancer prevention effects of broccoli. Based on the oxygen-radical absorbance capacity (ORAC) assay, broccoli was shown to be seventh in antioxidant capacity after kale, Brussels sprouts, alfalfa sprout, beets, and spinach broccoli (Cao et al., 1996). However, using linoleic-acid emulsions and phospholipid bilayers, Azuma and coworkers (1999) found broccoli (Brassica oleraceae var italica) exhibited the greatest antioxidant activity compared to 25 vegetable extracts (Wallig et al., 1999). The many antioxidants present in broccoli include carotenoids, tocopherols, acorbic acid, and flavonoids (Kurilich et al., 1999; Plumb et al., 1997). Using the ORAC assay, Kurilich et al. (2002) showed considerable variability in the antioxidant capacity of eight broccoli genotypes. They were unable to explain the variability based on ascorbic acid and flavonoid content of the hydrophyllic extracts sug-gesting the presence of other antioxidants or synergism. The carotenoids in the lipophylic extracts correlated with antioxidant capacity and accounted for the majority of the variability in this fraction.
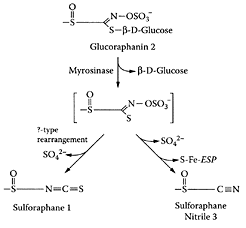
SCHEME B.11 Hydrolysis of glucoraphanin to sulforaphanes. (From Matusheski and Jeffery, J. Agric. Food Chem., 49:5743–5749, 2001. With permission.)
References
Azuma, K., Ippoushi, K., Ito, H., Higashio, H., and Terao, J., Evaluation of the antioxidant activity of vegetable extracts in linoleic emulsion and phospholipid bilayers, J. Sci. Food Agric., 79:2010–2016, 1999.
Cao, G., Sofic, E., and Prior, R., Antioxidant activity of tea and common vegetables. J. Agric. Food Chem., 44:3426–3431, 1996.
Conaway, C.C., Yang, Y., and Chung, F.-L., Isothio cyantes as cancer chemopreventive agents: Their biological activities and metabolism in rodents and humans, Curr. Drug Metab., 3:233– 255, 2002.
Keck, A.-S., Qiao, Q., and Jeffery, E.H., Food matrix effects on bioactivity of broccoli-derived sulforaphane in liver and colon of F344 rats, J. Agric. Food Chem., 51:3320–3327, 2003.
Kurilich, A.C., Tsau, G.J., Brown, A., Howard, L., Klein, B.P., Jeffery, E.H., Kushad, M., Wallig, MA., and Juvik, J.A., Carotene, tocopherol, and ascorbate contents in subspecies of Brassica oleracea, J. Agric. Food Chem., 47:1576–1581, 1999.
Kurilich, A.C., Jeffery, E.H., Juvik, J.A., Wallig, M.A., and Klein, B.P., Antioxidant capacity of different broccoli (Brassica oleracea) genotypes using the oxygen radical absorbance capacity (ORAC) assay, J. Agric. Food Chem., 50:5053–5057, 2002.
Matusheski, N.V. and Jeffery, E.H., Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile, J. Agric. Food Chem., 49:5743–5749, 2001.
Plumb, G.W., Price, K.R., Rhodes, M.J.C., and Williamson, G., Antioxidant properties of the major polyphenolic compounds in broccoli, Free Radical Res., 27:429–435, 1997.
Wallig, M.A., Azuma, K., Ippoushi, K., Ito, H., Higashio, H., and Terao, J., Evaluation of the antioxidant activity of vegetable extracts in linoleic emulsion and phospholipid bilayers, J. Sci. Food Agric., 79:2010–2016, 1999.
Broccoli sprouts
Research conducted at Johns Hopkins University School of Medicine showed that broccoli sprouts contain from 20 to 50 times higher levels of sulforaphane glucosinolates than adult cooked broccoli and could provide better anticancer protection (Nestle, 1998). Previous studies by Fahey et al. (1997) showed that broccoli sprouts were rich in enzyme inducers that protect against carcinogenesis. For example, broccoli contains large amounts of isothiocyanates, sulforaphane, or 4methyl-sulfinyl-butyl isothiocyanate, that are potent inducers of phase II enzymes. Isothiocyanates occur naturally as thioglucoside conjugates and appear to inhibit the development of cancerous tumors. Chung et al. (2000) confirmed the ability of sulforaphane and phenylethyl isothiocyanate to inhibit the development of colonic aberrant crypt foci during the initiation period in experimental male rats treated with azoxymethane (AOM), an initiator of colon cancer. Based on their observations, Fahey and Talalay (2001) patented their discovery for the development of cancer chemoprotective food products based on broccoli sprouts.

Sulforaphane. (From Konwinski et al., Toxicol. Lett., 153:343–355, 2004. With permission.)
References
Chung, F.L., Conaway, C.C., Rao, C.V., and Reddy, B.S., Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenylethyl isothiocyanate, Carcinogenesis, 21:2287–2291, 2000.
Fahey, J.W. and Talalay, P., Cancer Chemoprotective Food Products, U.S. Patent 6,177,122 B1, 2001.
Fahey, J.W., Zhang, Y., and Talalay, P., Broccoli sprouts; An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens, Proc. Natl. Acad. Sci., 94:10367–10367, 1997.
Konwinski, R.R., Haddad, R., Chun, J.A., Klenow, S., Larson, S.C., Haab, B.B., and Furge, L.L., Oltipraz, 3H-1,2-thione and sulforaphane induce overlapping and protective antioxidant responses in murine microglial cells, Toxicol. Lett., 153:343–355, 2004.
Nestle, M., Broccoli sprouts in cancer prevention, Nutr. Rev., 56:127–130, 1998.
Brussels sprouts
Brussels sprouts (Brassica oleracea var. gemmifera) are particularly rich in the glucosinolate sinigrin. Sinigrin is hydrolyzed by myrosinase to allyl isothiocyanate (AITC), which was shown to induce glutathione S-transferase activity in the liver and small intestine of rats (Bogaards et al., 1990). Musk and Johnson (1993) found that AITC selectively induced cell death in the undifferentiated phenotype of the HT29 human cell tumor cell line. Smith et al. (1998) reported that ingestion of sinigrin inhibited dimethylhydrazine-induced aberrant crypt foci, as well as induced apoptosis in the rat colon. The ability of AITC to act as a suppressor of colorectal carcinogenesis was further investigated by Smith and coworkers (2003). Freeze-dried raw and microwave-cooked Brussels sprouts containing high levels of glucosinolates significantly enhanced apoptosis and reduced mitosis in 1,2-dimethylhydrazine (DMH)-induced colonic mucosal crypts. The absence of any effect in blanched-sprout tissue was attributed to the inactivation of myrosinase and the presence of only intact glucosinolates. This study confirmed the importance of glucosinolate degradation products in affecting cell proliferation and apoptosis.
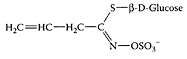
Sinigrin structure. (From Jen et al., J. Chromatogr., A., 912:363–368, 2001. With permission.)
References
Bogaards, J.J., vav Ommen, B., Falke, H.E., Willems, M.I., and van Bladeren, P.J., Glutathione Stransferase subunit induction patterns of Brussels sprouts, allyl isothiocyanate and goitrin in rat liver and small intestinal mucosa: A new approach for the identification of inducing xenobiotics, Food Chem. Toxicol., 28:81–88, 1990.
Jen, J.-F., Lin, T.-H., Huang, J.-W., and Chung, W.-C., Direct determination of sinigrin in mustard seed without desulatation by reversed phase ion-pair liquid chromatography, J. Chromatogr., A., 912:363–368, 2001.
Musk, S.R. and Johnson, I.T., Allyl isothiocyanate is selectively toxic to transformed cells of the human colorectal tumor line HT29, Carcinogenesis, 14: 2079–2083, 1993.
Smith, T.K., Lund, E.K., and Johnson, I.T., Inhibition of dimethylhydrazine-induced aberrant crypt foci and induction of apoptosis in rat colon following oral administration of the glucosinolate sinigrin, Carcinogenesis, 19:267–273, 1998.
Smith, T.K., Mithen, R., and Johnson, I.T., Effects of Brassica vegetable juice on the induction of apoptosis and aberrant crypt foci in rat colonic mucosal crypts, Carcinogenesis, 24:491–495, 2003.
Bryonolic acid
Bryonolic acid is a multiflorane compound found in saffron as the p-aminobenzoate derivative. In his discussion of medicinal plants, Thatte et al. (2000) suggested compounds, such as bryonolic acid, induced programmed cell death arresting the proliferation of cancerous cell lines. Two novel multiflorane p-aminobenzoates were detected by Appendino and coworkers (2000) in zucchini seeds, while bryonolic acid was the sole multiforane constituent found in zucchini sprouts.
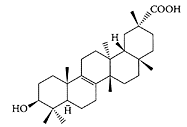
Bryonolic acid. (Adapted from from Appendino et al., Fitoterapia, 71:258–263, 2000.)
References
Appendino, G., Japukovic, J., Belloro, E., and Marchesini, A., Triterpenoid p-aminobenzoates from seeds of zucchini, Fitoterapia, 71:258–263, 2000.
Thatte, U., Bagadey, S., and Dahanukar, S., Modulation of programmed cell death by medicinal plants, Cell. Mol. Biol., 46:199–214, 2000.
Bryostatins
Bryostatins, a group of marine macrocyclic lactones with a unique polyacetate backbone, have considerable potential as chemopreventive agents (Petite, 1996). Their low toxicity combined with their antineoplastic activity has made bryostatins ideal for treating cancer. Bryostatin 1, first isolated and characterized in 1982, is recognized for its immune stimulation, growth inhibition, induction of differentiation, and enhancement of cytotoxicity of other drugs directed at target cells (Watters and Parsons, 1999).
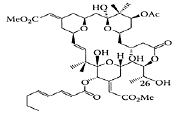
Bryostatin 1. (Baryza et al., Chem. Biol., 11:1261–1267, 2004. With permission.)
Studies on bryostatins have focused on their interaction with enzymes and cell lines or on how these enzyme activities or cellular events affect apoptosis. For example, the effect of bryostatin 1 on protein kinase C isoenzymes has been studied extensively. This family of 12 isoenzymes plays a central role in cell signaling and other processes and is activated by bryostatin 1, phorbol esters (a group of tumor promoters), and diacylglycerol. Hennings et al. (1987) showed bryostatin 1 inhibited tumor promotion by phorbol esters in SENCAR mouse skin.
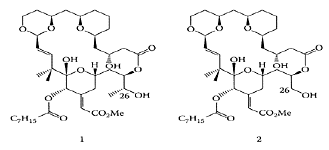
SCHEME B.12 Structures of analogs 1 and 2. (From Baryza et al., Chem. Biol., 11:1261–1267, 2004. With permission.)
Preclinical trials to investigate bryostatin 1 as an anticancer drug showed it inhibited the growth of rabbit papillomas in a dose-dependent manner but did not provide a cure (Bodily et al., 1999). Other studies showed that bryostatin 1, in combination with anticancer drugs, proved more effective. For example, a cure was reported for WSUCLL-bearing SCID mice (5/5) using a combination of auristatin PE followed by bryostatin every second day over a six-day period (Mohammed et al., 1998). A phase II trial by Nezhat et al. (2004) found a combination of bryostatin 1 and the drug cisplatin ineffective in patients with advanced-stage or recurrent cervical cancer. However, several analogs (analogs 1 and 2) of bryostatin 1 were later shown by Baryza and coworkers (2004) to be 50 times more potent than bryostatin at inducing translocation of PKCδ-GFF from the cytosol of rat basophilic leukemia (RBL) cells, suggesting great potential in cancer therapy (Scheme B.12). A review on bryostatins by Mutter and Wills (2000) is strongly recommended.
References
Baryza, J.L., Brenner, S.E., Craske, M.L., Meyer, T., and Wender, P.A., Simplified analogs of bryostatin with anticancer activity display greater potency for translocation of PKCδ-GFB, Chem. Biol., 11:1261–1267, 2004.
Bodily, J.M., Hoopes, D.J., Roeder, B.L., Gilbert, S.G., Pettit, G.R., Herald, C.L., Rollins, D.N., and Robinson, R.A., The inhibitory effects of bryostatin 1 administration on the growth of rabbit papillomas, Cancer Lett., 136:67–74, 1999.
Hennings, H., Blumberg, P.M., Pettit, G.R., Herald, C.L., Shores, R., and Yuspa, S.H., Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in Sencar mouse skin, Carcinogenesis, 8:1343–1346, 1987.
Mohammed, R.M., Varterasian, M.L., Almatchy, V.P., Hannoudi, G.N., Pettit, G.R., and Al-Katib, A., Successful treatment of human chronic lymphatic leukemia xenografts with combination biological agents auristatin PE and bryostatin 1, Clin. Cancer Res., 4:1337–1343, 1998.
Mutter, R. and Wills, M., Chemistry and clinical biology of the bryostatins, Bioorg. Med. Chem., 8:1841–1860, 2000.
Nezhat, F., Wadler, S.W., Muggia, F., Mandeli, J., Goldberg, G., Rahaman, J., Runowicz, C., Murgo, A.J,. and Gardner, G.J., Phase II trial of the combination of bryostatin-1 and cisplatin in advanced or recurrent carcinoma of the cervix: A New York Gynecologic Oncology Group Study, Gynecol. Oncol., 93: 144–148, 2004.
Petite, G.R., Progress in the discovery of biosynthetic anticancer drugs, Nat. Prod., 59:812–821, 1996.
Watters, D.J. and Parsons, P.G., Critical targets of protein kinase C in differentiation of tumor cells, Biochem. Pharmacol., 58:383–388, 1999.
Buckwheat
Buckwheat is a pseudocereal grown in North America, including Western Canada. The protein in buckwheat was shown by Kayashita and coworkers (1997) to lower plasma cholesterol and raise fecal neutral sterols in cholesterol-fed rats because of its low digestibility. In addition, buckwheat protein was found to retard the ability of 7,12-dimethylbenzyl [a] anthracene-induced mammary carcinogenesis in rats by lowering serum estradiol. The ability of buckwheat protein to suppress plasma cholesterol in rats fed a cholesterol-free diet was shown by Tomotake et al. (2001) to be stronger than a soybean protein isolate. The effect was attributed to the enhanced excretion of fecal neutral and acidic steroids. Yokozawa et al. (2001) reported that an aqueous extract from buckwheat ameliorated renal injury in rats induced by ischemia-reperfusion. The buckwheat extract also protected cultured proximal tubule cells subjected to hypoxia-reoxygenation, which was attributed to preventing oxygen free radicals from attacking the cell membranes. Earlier work by Lee et al. (1998) reported antioxidant and free-radical- scavenging activities among buckwheat-seed components. Holasova et al. (2002) showed the antioxidant activity of buckwheat seeds was higher than those of oats, barley, and buckwheat straws and hulls. The antioxidant activity resided primarily with the methanol-soluble components.
The hypoglycemic effects of consuming buckwheat flour or biscuits containing buckwheat flour in patients with diabetes was first reported in 1992 by several researchers (Lu et al., 1992; Wang et al., 1992). Kawa and coworkers (2003) recently showed that a single, oral dose of buckwheat concentrate significantly lowered elevated serum-glucose concentrations in streptozotocin-diabetic rats by 12–19 percent at 90 and 120 minutes after administration (Figure B.16). The active component in buckwheat responsible for the glucose-lowering effect appeared to be d-chiro-inositol, which is present at 0.2 percent in the concentrate. Fonteles et al. (2000) reported that a singe dose of intragastric d-chiro-inositol (10 mg/kg) fed to streptozotocin-treated rats resulted in a 30–40 percent decrease in plasma-glucose concentrations. Buckwheat concentrate, a good source of d-chiro-inositol, could be beneficial for the treatment of diabetes.
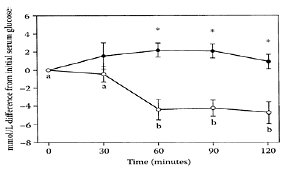
FIGURE B.16 Effect of low-dose buckwheat concentrate (10 mg of D-chiro-inositol/kg of body weight) or placebo given to STZ rats in the fed state on serum-glucose concentration. Data are expressed as the mmol/L difference from initial serum glucose concentrations (28.4±0.95 mmol/L) for the placebo low-dose (, n=9) and the low-dose buckwheat concentrate (, n=8) groups. Asterisks (*) indicate differences (p<0.001) between placebo-treated and buckwheat-treated rats. Data points with different letters indicate differences (p<0.01) within a group, determined by Duncan’s multiple range. (From Kawa et al., J. Agric. Food Chem., 51:7287–7291, 2003. With permission.)
A statistically significant correlation was observed between total phenolics and rutin and antioxidant activity of buckwheat. Steadman et al. (2000, 2001a) reported buckwheat bran was a good source of protein, lipid and dietary fiber, fagopyritols, d-chiro-mositol, and other soluble carbohydrates. Steadman and coworkers (2001b) cautioned against the use of buckwheat bran for medicinal purposes because of the high levels of phytate and tannin present. Li and coworkers (2002) reported the production of peptides from buckwheat protein that inhibited angiotensin 1-converting enzymes, which lowered the systolic pressure in hypertensive rats. While the intact protein had some ACE inhibitory activity, it was enhanced substantially by hydrolysis with chymotrypsin and trypsin. This effect was not exhibited by rutin. A recent study by Prestamo et al. (2003) showed buckwheat acted as a prebiotic by increasing lactic-acid bacteria while decreasing mesophilic bacteria in the intestine of rats.
References
Fonteles, M.C., Almeida, M.Q., and Larner J., Antihyperglycemic effects of 3-O-methyl-D-Chiroinositol and D-chiro-inositol associated with manganese in Sreptozotocin diabetic rats, Horm. Metab. Res., 32:129–132, 2000.
Holasova, M., Fiedlerova, V., Smrinova, H., Orsak, M., Lachman, J., and Vavreinova, S., Buckwheat—the source of antioxidant activity in functional foods, Food Res. Inter., 35:207– 211, 2002.
Kawa, J., Przybylski, R., and Taylor, C., Buckwheat concentrate reduces serum glucose in streptozotocindiabetic rats, J. Agric. Food Chem., 51:7287–7291, 2003.
Kayashita, J., Shimoaka, I., Yamazaki, M., and Kato, N., Consumption of buckwheat protein lowers plasmas cholesterol and raises fecal neutral sterols in cholesterol-fed rats because of its low digestibility, J. Nutr., 127:13 95–1400, 1997.
Kayashita, J., Shimaoka, I., Nakajoh, M., Kishida, N., and Kato, N., Consumption of a buckwheat protein extract retards 7,12-dimethylbenz[alpha] anthracene-induced mammary carcinogenesis in rats, Biosci. Biotechnol. Biochem., 63:1837–1839, 1999.
Lee, Y.C., Przbylski, R., and Eskin, N.A.M., Antioxidant and radical scavenging activities of buckwheat seed components, J. Am. Oil Chem. Soc., 75:1595–1601, 1998.
Li, C.H., Matsui, T., Matsumoto, K., Yamasaki, R., and Kawasaki, T., Latent production of angiotensin 1-converting enzyme inhibitors from buckwheat protein, J. Peptide Sci,. 8:267–274, 2002.
Lu, C., Zu, J., Zho, P., Ma, H., Tong, H., Jin, Y., and Li, S., Clinical application and therapeutic effect of composite tartary buckwheat flour on hyperglycemia and hyperlipidemia, in Proceedings of the 5th International Symposium on Buckwheat, Lin, R., Zhou, M., Tao, Y., Li, J., and Zhang, Z., Eds., Agriculture Publishing House, Beijing, China, 1992, pp. 458–462.
Prestamo, G., Pedrazuela, A., Penas, E., Lasuncion, M.A., and Arroyo, G., Role of buckwheat diets on rats as prebiotic and healthy food, Nutr. Res., 23:803–814, 2003.
Lee, Y.C., Przybylkski, R., and Eskin, N.A.M., Antioxidant and radical-scavenging activities of buckwheat seed components, J. Am. Oil Chem. Soc., 75: 1595–1601, 1998.
Steadman, K.J., Burgoon, M.S., Schuster, R.L., Lewis, B.A., Edwardson, S.E., and Obendorf, R.L., Fagopyritols, D-chiro-inositol, and other soluble carbohydrates in buckwheat seed milling fractions, J. Agric. Food Chem., 48:2843–2847, 2000.
Steadman, K.J., Burgoon, M.S., Lewis, B.A., Edwardson, S.E., and Obendorf, R.L., Buckwheat seed milling fractions: Description, macronutrient composition, and dietary fiber, J. Cereal Sci., 33: 271–278, 2001a.
Steadman, K.J., Burgoon, M.S., Lewis, B.A., Edwardson, S.E., and Obendorf, R.L., Minerals, phytic acid, tannin and rutin in buckwheat seed, milling fractions, J. Sci. Food Agric., 81:1094– 1100, 2001b.
Tomotake, H., Shimaoka, I., Kayashita, J., Yokoyama, F., Nakajoh, M., and Kato, N., Stronger suppression of plasma cholesterol and enhancement of the fecal excretion of steroids by a buckwheat protein product than by a soy protein isolate in rats fed on a cholesterol-free diet, Biosci. Biotechnol. Biochem., 65:1412–1414, 2001.
Wang, J., Liu, Z., Fu, X., and Run, M., A clinical observation on the hypoglycemic effect of Xinjiang buckwheat, in Proceedings of the 5th International Symposium on Buckwheat, Lin, R., Zhou, M., Tao, Y., Li, J., and Zhang, Z., Eds., Agriculture Publishing House, Beijing, China, 1992, pp. 465–467.
Yokozawa, T., Fujii, H., Kosuna, K., and Nonaka, G., Effects of buckwheat in a renal ischemiareperfusion model, Biosci. Biotechnol. Biochem., 65:396–400, 2002.
Butyric acid
Butyric acid, a short-chain fatty acid, is a by-product of bacterial fermentation of dietary fiber. In addition to making the fecal pH more acid, short-chain fatty acids, such as butyric acid, decrease the activity of bacterial
CH3-CH2-CH2-CH2-COOH
7α-dehydroxylase, which converts bile acid from primary to secondary (Hill, 1975), a cancer promoter. Butyric acid appears to be responsible for the beneficial effect of fiber on bowel cancer (Riggs and coworkers, 1977). In vivo and in vitro studies with rats showed butyric acid acts as a potent anti-inflammatory agent (Andoh et al., 1999) while another study showed it induced apoptosis in myeloid leukemia (HL-60) cell lines (Celabresse et al., 1993). Abrahamse and coworkers (1999) found butyrate reduced DNA damage induced by hydrogen peroxide in rat colon cells, pointing to butyrate having anticarcinogenic effects via its antioxidant properties. Rosignoli et al. (2001) confirmed butyrate’s ability to reduce H2O2-induced DNA damage in colon cells, although the mechanism of action still remains unknown. Sodium butyrate was also shown by Sasahara and coworkers (2002) to inhibit the growth of colon cancer by suppressing expression of inducible nitric-oxide synthase (iNOS) involving mechanisms independent from histone acetylation. An oral butyrate derivative, tributyrin, was reported by Clarke et al. (2001) to be a potent inhibitor of colorectal cancer by inducing apoptosis through activation of caspase-3 activity. Chethankumar and coworkers (2002) reported that butyric acid that supplemented high-fiber diets fed to streptozoto cin-induced diabetic rats slowed down the diabetic process by inhibiting intestinal and renal disaccharidases, slowing down the release of glucose and its absorption.
References
Abrahamse, S.L., Pool-Zobel, B.L., and Rechkemmer, G., Potential of short chain fatty acids to modulate the induction of DNA damage and changes in the intracellular calcium concentration by oxidative stress in isolated rat distal colon cells, Carcinogenesis, 20:629–634, 1999.
Andoh, A., Kimura, T., Fukuda, M., Araki, Y., Fuyiyama, Y., and Bamba, T., Rapid intestinal ischemia reperfusion injury is suppressed in genetically mast cell deficient Ws/Ws rats, Clin. Exp. Immunol., 116: 90–93, 1999.
Celabresse, C., Venturini, L., Ronco, G., Villa, P., Chomienne, C., and Belpomme, D., Butyric acid and its monosaccharide ester induce apoptosis in the HL-60 cell line. Biochem. Biophys. Res. Commun., 195:31–38, 1993.
Chethankumar, M., Salimath, P.V., and Sambiah, K., Butyric acid modulates activities of intestinal and renal disaccharidases in experimentally induced diabetic rats, Nahrung, 46:345–348, 2002.
Clarke, K.O., Feinman, R., and Harrison, E., Tributyrin, an oral butyrate analogue, induces apoptosis through activation of caspase-3, Cancer Lett., 171: 57–65, 2001.
Hill, M.J., The role of colon anaerobes in the metabolism of bile acids and steroids, and its relation to colon cancer, Cancer, 36(6):2387–2400, 1975.
Riggs, M.G., Whittaker, R.G., Neumann, J.R., and Ingram, V.M., Butyrate causes histone modification in HeLa and Friend erytholeukemia cells, Nature, 268:462–464, 1977.
Rosignoli, P., Fabiani, R., De Bartolomeo, A., Spinozzi, F., Agea, E., Pelli, M.A., and Morozzi, G., Protective activity of butyrate on hydrogen peroxide-induced DNA damage in isolated human colonocytes and HT29 tumor cells, Carcinogenesis, 22:1675–1680, 2001.
Sasahara, Y., Mutoh, M., Takahashi, M., Fukuda, K., Tanaka, N., Sugimura, T., and Wakabayashi, K., Suppression of promoter-dependent transcriptional activity of inducible nitric oxide synthase by sodium butyrate in colon cancer cells, Cancer Lett., 177: 155–161, 2002.