L
α-Lactalbumin
α-Lactalbumin, the major protein regulator of lactose synthase in milk, has the highest content of tryptophan (Trp) and the highest Trp/ΣLarge neutral amino-acids (LNAAs) ratio among food-protein sources (Heine et al., 1996). Tryptophan is the precursor of brain serotonin (5-hydroxy-tryptamine, 5-HT), which is involved in mood disorders, such as anxiety and depression (Berk, 2000). Consequently, tryptophan was proposed as a possible treatment for depression (Meyers, 2000; Young, 2000). Because of its high level of tryptophan, Markus et al. (2000) showed that ingestion of α-lactalbumin reduced depressive feelings in stress-vulnerable human subjects compared to a casein-enriched diet. Using male Wistar rats, Orosco and coworkers (2004a) also found that ingestion of an α-lactalbumin-enriched diet induced anxiolytic and rewarding effects compared to an enriched casein diet. These effects may be related to the enhanced release of serotonin in the medial hypothalamus in rats fed 30 min meals (acutely) but disappeared after 3–6 days of diet (chronic). Based on their research findings, together with the study by Markus et al. (2003), diets enriched with α-lactalbumin appeared to be beneficial in treating stress and anxiety in the short term.
Pelligrini et al. (1999) identified antimicrobial peptides in bovine α-lactalbumin by isolating and characterizing three bacteriocidal domains. Oevermann and coworkers (2003) showed that chemical modification of the lysine residues with 3-hydroxyphthalic anhydride (3-HP) in several bovine milk protein fractions, including α-lactalbumin, yielded compounds with antiviral activity against human herpes simplex virus type 1 (HSV-1). Digestion of these modified proteins produced short peptides that had considerable potential for the treatment of herpes, as they were economical, as well as exhibited reduced antigenicity. Supplementation of an infant formula with α-lactalbumin and glycomacropeptide by Bruk et al. (2002) also benefited the human microflora by significantly reducing the presence of pathogenic bacteria.
Opioid active peptides are released from milk proteins, such as α-lactalbumin, by enzymatic hydrolysis. For example, α-lactorphin, a tetrapeptide (Tyr-Gly-Leu-Phe) released by peptic or tryptic hydrolysis of α-lactalbumin, had an amino-acid sequence corresponding to residues 50–53 in the original intact protein (Antila et al., 1991). The opioid properties associated with α-lactorphin reflected the similarity between its amino acid sequence and the N-terminal amino-acid residues of opioid peptides, such as β-endorphin, enkephalins, and dynorphin (Tyr-Gly-Gly-Phe) (Teschemacher et al., 1997). α-Lactorphin was found by Nurminen et al. (2000) to lower blood pressure in spontaneously hypertensive rats with established hypertension. Sipola and coworkers (2002) showed α-lactorphin improved vascular relaxation in the spontaneously hypertensive rats and involved nitric oxide but not vasodilatory prostanoids.
References
Antila, P., Paakkari, I., Jarvinen, A., Mattila, M.J., Laukkanen, M., Pihlanto-Leppala, A., Mantsala, P., and Hellman, J., Opioid peptides derived from in vitro proteolysis of bovine whey protein, Int. Dairy J., 1:215–229, 1991.
Berk, M., Selective serotonin reuptake inhibitors in mixed anxiety-depression, Int. Clin. Psychopharmacol., 15(Suppl. 2): S41–45, 2000.
Bruck, W.M., Graverholt, G., and Gibson, G.R., Use of batch culture and a two-stage continuous culture system to study the effect of supplemental α-lactal-bumin and glycomacropeptide on mixed populations of human gut bacteria, FEMS Microbiol. Ecol., 41: 231–237, 2002.
Heine, W., Radke, M., Wutzke, K.D., Peters, E., and Kundt, G., Alpha-lactalbumin-enriched low-protein infant formulas: A comparison to breast milk feeding, Acta Paediatr., 85:1024–1028, 1996.
Markus, C.R., Olivier, B., Panhuysen, G.E.M., Van der Gusten, J., Alles, M.S., Tuiten, A., Wastenberg, H.G.M., Fekkes, D., Koppeschaar, H.F., and de Haan, E.E.H.F., The bovine protein α-plactalbumin increases the plasma ratio of tryptophan to other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress, Am. J. Clin. Nutr., 71:1536–1544, 2000.
Meyers, S., Use of neurotransmitter precursors for treatment of depression, Altern. Med. Rev., 5:64–71, 2000.
Nurminen, M.-L., Sipola, M., Kaarto, H., PihlantoLeppala, A., Piilota, K., Korpela, R., Tossavainen, O., Korhonen, H., and Vapaatalo, H., α-Lactorphin lowers blood pressure measured by radiotelemetry in normotensive and in spontaneously hypertensive rats, Life Sci., 66:1535–1543, 2000.
Oevermann, A., Engels, M., Thomas, U., and Pellegrini, A., The antiviral activity of naturally occurring proteins and their peptide fragments after chemical modification, Antiviral Res., 59:23–33, 2003.
Orosco, M., Rouch, C., Beslot, F., Feurte, S., Regnault, A., and Dauge, V., Alpha-lactalbuminenriched diets enhance serotonin release and induce anxiolytic and rewarding effects in the rat, Behav. Brain Res., 148:1–10, 2004a.
Pellegrini, A., Thomas, U., Bramaz, N., Hunziker, P., and von Fellenberg, R., Isolation and identification of three bacteriocidal domains in the bovine α-lactalbumin molecule, Biochem. Biophys. Acta, 1426: 439–448, 1999.
Sipola, M., Finckenberg, P., Vapaatalo, H., PihlantoLeppala, A., Korhonen, H., Korpela, R., and Nurminen, M.-L., α-Lactorpin and β-lactorphin improve arterial function in spontaneously hypertensive rats, Life Sci., 71:1245–1253, 2002.
Teschemacher, H., Koch, G., and Brantl, V., Milk protein-derived opioid receptor ligands, Peptide Sci., 43:99–117, 1997.
Young, S.N., Behavioral effects of dietary neurotransmitter precursors: Basic and clinical aspects, Neurosci. Biobehav. Rev., 20:313–323, 1996.
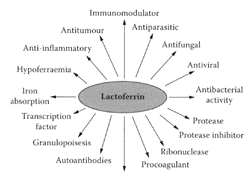
SCHEME L.33 Proposed roles for lactoferrin. (From Brock, Biochem. Cell. Biol., 80:1–6, 2002. With permission.)
Lactoferrin and Lactoferricin
see also Bovine lactoferrin Lactoferrin, an ironbinding glycoprotein present in milk, is a multifunctional protein with immunomodulation and antimicrobial activity (Vorland, 1999; Weinberg, 2001; Farnaud and Evans, 2003). These many roles for lactoferrin are summarized in Scheme L.33. In contrast, lactoferricin is a peptide released from the N-terminal part of lactoferrin by peptic digestion (Tomita et al., 1991). Compared to lactoferrin, a bilobal glycoprotein with a mass of 80 kDa, the lactoferricin-peptide structure is a loop with a cationic charge, containing 25 amino acids in the case of bovine lactoferricin (Lfcin B) and 47 aminoacid residues for human lactoferricin (Lfcin H) (Scheme L.34).
Lactoferrin is a potent inhibitor of different enveloped viruses, including herpes simplex virus (HSV) 1 and 2 (Marchetti et al., 1998), human immunodeficiency virus (HIV) (Puddu et al., 1998), human cytomegalovirus (Portelli et al., 1998), and human hepatitis C virus (Ikeda et al., 1998), as well as two naked viruses, SA11 rotavirus and poliovirus type 1 (Marchetti et al., 1999; Superti et al., 1997). Arnold and coworkers (2002) found lactoferrin was the only milk protein that inhibited adenovirus replication in a dose-dependent manner by preventing replication at an early phase of viral replication.
Bovine and human lactoferricin (Lfcin B and Lfcin H) were both shown to exhibit antiviral activity against human cytomegalovirus (HCMV) by preventing its entry into human fibroplasts (Andersen et al., 2001). A synergy between Lfcin B and the drug acyclovir (ACV) in inhibiting HSV was observed by Andersen and coworkers (2003) by a sevenfold drop in EC50 for both ACV and Lfcin B. The potency of Lfcin B increased substantially in the presence of ACV, which could reduce the amount of the drug administered, as well as the incidence of drug-resistant strains. A similar synergy was observed between bovine lactoferrin and ACV Jenssen et al. (2003) recently concluded that hydrophobicity, molecular size, special distribution of charged lipophilic amino acids, and cylic structure were features that affected the antiviral activity of Lfcin B against HSV
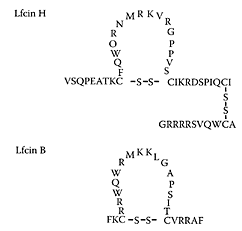
SCHEME L.34 Loop structure of human (Lfcin H) and bovine (Lfcin B) lactoferricin. Single-letter code is used to indicate the amino-acid sequence of each peptide. (From Jenssen et al., Antiviral Res., 61:101–109, 2004. With permission.)
References
Andersen, J.H., Jenssen, H., and Gutteberg, T.J., Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir, Antiviral Res., 58:209–215, 2003.
Andersen, J.H., Osbakk, S.A., Vorland, L.H., Traavik, T., and Guttenberg, Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts, Antiviral Res., 51:141–149, 2001.
Arnold, D., Di Biase, A.M., Marchetti, M., Pietrantoni, A., Valenti, P., Seganti, L., and Superti, F., Antiadenovirus activity of milk proteins: Lactoferrin prevents viral infection, Antivira Res., 53:153–158, 2002.
Brock, J.H., The physiology of lactoferrin, Biochem. Cell. Biol., 80:1–6, 2002.
Farnaud, S. and Evans, R.W., Lactoferrin—a multifunctional protein with antimicrobial properties, Mol. Immunol., 40:395–405, 2003.
Ikeda, M., Sugiyama, K., Tanaka, T., Tanaka, K., Sekihara, H., Shimotohno, K., and Kato, N., Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes, Biochem. Biophys. Res. Commun., 245:549–553, 1998.
Jenssen, H., Andersen, J.H., Uhlin-Hansen, L., Gutteberg, T.J., and Rekdal, O., Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparin sulfate, Antiviral Res., 61:101–109, 2004.
Marchetti, M., Pisani, S., Antonini, G., Valenti, P., Seganti, L., and Orsi, S., Metal complexes of bovine lactoferrin inhibit in vitro replication of herpes simplex virus type 1 and 2, Biometals, 11:89–94, 1998.
Marchetti, N.M., Superti, F., Ammendolia, M.G., Rossi, O., Valenti, P., and Seganti, L., Inhibition of poliovirus type 1 infection by iron-, manganese- and zinc-saturated lactoferrin, Med. Microbiol. Immunol., 187:199–204, 1999.
Portelli, J., Gordon, A., and May, J.T., Effect of compounds with antibacterial activities in human milk on respiratory syncytial virus and cytomegalovirus in vitro, J. Med. Microbiol., 47:1015– 1018, 1998.
Puddu, P., Borghi, P., Gessani, S., Valenti, P., Belardelli, F., and Saganti, L., Antiviral effect of bovine lactoferrin saturated with metal ions on early steps of human immunodeficiency virus type 1 infection, Int. J. Biochem. Cell Biol., 30:1055–1062, 1998.
Superti, F., Ammendolia, M.G., Valenti, P., and Seganti, L., Antirotaviral activity in milk proteins: Lactoferrin prevents rotavirus infection in the eneterocytelike cell line HT-29, Med. Microbiol. Immunol., 186: 83–91, 1997.
Tomita, M., Bellamy, W., Takase, M., Yamauchi, K., Wakabayashi, H., and Kawase, K., Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin, J. Dairy Sci., 74:4137–4142, 1991.
Vorland, L.H., Lactoferrin: A multifunctional glycoprotein, APMIS, 107:971–981, 1999.
Weinberg, E.D., Human lactoferrin: A novel therapeutic with broad spectrum potential, J. Pharm. Pharmacol., 53:1303–1310, 2001.
Lactokinin
Lactokinin is a short, biologically active peptide released by tryptic digestion of the whey milk protein β-lactoglobulin (Ala-Leu-Pro-His-Ile-Arg) (Mullally et al., 1997). It inhibits angiotensin-1-converting enzyme (ACE), an enzyme associated with the renninangiotensin system that regulates peripheral blood pressure, reducing blood pressure in hypertensive individuals. Fitzgerald and Meisel (1999) suggested that while ACE inhibitors derived from whey protein were not as potent as synthetic antihypertensive drugs, they were sufficiently active to exert an antihypertensive effect. Vermeirssen and coworkers (2002) reported that lactokinin was partly transported through a caco-2 cell monomer. Maes and coworkers (2004) showed for the first time that lactokinin modulated the release of endothelin1 (ET-1) by porcine aortic endothelial cells (Figure L.59). ET-1 is a vasoconstrictive peptide that acts via a specific receptor. Compared to 0.1 mM of the drug captopril, which decreased ET-1 release by 42 percent, lactokinin produced a 29 percent decrease under the same conditions. Thrombrin significantly stimulated the basal release of ET-1 by 66 percent.
While incubation with 1 μM and 0.1 nM of captopril inhibited the stimulated ET-1 release by 45 percent and 62 percent compared to 32 percent and 43 percent when thrombrin was coincubated with 1 μM and 0.1 mM of lactokinin. While lactokinin was not quite as effective as captopril, nevertheless, this milk-proteinderived peptide could still be a useful treatment of hypertension. Recent data by Maes et al. (2004) showed lactokinin modulated ET-1 release by endothelial cells, which explained the antihypertensive effect of milk-protein peptides.
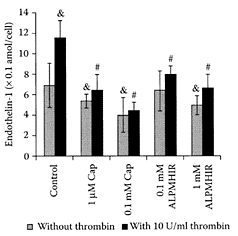
FIGURE L.59 Effect of captopril and lactokinin on basal and 10 μ/mL thrombrin-stimulated endothelial1 release by porcine aortic endothelial cells. Drugs and peptide were solubilized in medium 199 supplemented with 190 porcine serum. Cells were exposed to medium 199 with 1 percent serum, 1 μM and 0.1 mM captopril (cap), 0.1 and 1.0 mM lactokinin (ALPMHIR), with or without the addition of 10 μ/mL thrombrin (throm). & Different from ET-1 release without stimulation, p<0.01.g Different from 10 μ/mL thrombrin-stimulated ET-1 release, p< 0.01. Values are means ± 2 SD., n=10– 15. (Maes et al., Reg. Pept., 118(1–2): 105–109, 2004. With permission).
References
Fitzgerald, R.J. and Meisel, H., Lactokinins: Whey protein ACE inhibitory peptides, Nahrung, 43:165–167, 1999.
Maes, W., Van Camp, J., Vermeirssen, V., Hemeryck, M., Ketelsleghers, J.M., Schrezenmeir, J., Oostveldt, P.V., and Huyghebaert, A., Influence of the lactokinin Ala-Leu-Pro-Met-His-Ile-Arg (ALPMHIR) on the release of endothelin-1 by enodothelial cells, Reg. Pept., 118(1–2):105–109, 2004.
Mullally, M.M., Meisel, H., and Fitzgerald, R.J., Identification of a novel angiotensin-1-converting enzyme inhibitory peptide corresponding to a tryptic fraction of bovine β-lactoglobulin, FEBS Lett., 402: 99–101, 1997.
Vermeirssen, V., Deplancke, B., Tappenden, K.A., Van Camp, J., Gaskins, H.R., and Verstraete, W., Intestinal transport of the lactokinin Ala-Leu-Pro-Met-Ile-Arg through Caco-2 Bbe monolayer, J. Pept. Sci., 8:95–100, 2002.
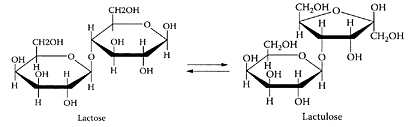
SCHEME L.35 Alkaline isomerization of lactose to lactulose. (From Montilla et al., Food Chem., 90:883–890, 2005. With permission.)
Lactulose
Lactulose (4-O-β-D-galactopyranosyl-D-fructose), an isomerized product of lactose (Scheme L.35), is a registered medicinal drug in more than 100 countries (Schumann, 2002). While it does not occur naturally in milk, it is present in very small amounts in heated milk and in UHT milk. Unlike the α 1–4 glycosidic bond in lactose, the β 1–4 glycosidic bond in lactulose cannot be broken down by the digestive enzymes, so it passes through to the intestine, where it is metabolized by the colonic bacteria. Lactulose is a prebiotic, as it is the preferred food for lactic-acid bacteria compared to the proteolytic activity of various pathogenic bacteria in the colon. It is effective in the treatment of chronic constipation and is the standard, worldwide treatment for hepatic encephalopathy (Blei and Cordoba, 2001).
Lactulose also prevents tumors by protecting DNA in human-flora associated rats exposed to dimethylhydrazine (DMH) (Rowland et al., 1996). Although very little lactulose is absorbed (0.25–2 percent), it appears to have specific beneficial immunological effects when given intravenously or in vitro (Greve et al., 1980; Liehr and Heine, 1981). Lactulose has been found to reduce urinary tract infection (UTI) and pneumonia (McCutcheon and Fulton, 1989) and stimulate calcium absorption in post-menopausal women (Van de Heuvel and Weidauer, 1999). A preparation containing lactulose was reported by Bianchi and coworkers (1994) to lower blood glucose. Lhoste et al. (2001) reported Fischer male rats innoculated with Clostridium paraputrificum fed lactuloseenriched diets increased butyrate in the caesum. The formation of butyrate has a number of beneficial effects, including preventing carcinogenesis (see butyric acid).
References
Bianchi, G.P., De Mitri, M.S., Bugianesi, E., Abbiati, R., Fabbri, A., and Marchesini, G., Lowering effects of a preparation containing fibres and lactulose on glucose and insulin levels in obesity, Ital. J. Gastroenteol. 26:174–178, 1994.
Blei, A.T. and Cordoba, J., Hepatic encephalopathy, Am. J Gastroenterol., 96:1968–1974, 2001.
Greve, J.W., Gouma, D.J., von Leeuwen, P.A.M., and Buurman, W.A., Lactulose inhibits endotoxins induced tumor necrosis factor production by monocytes. An in vitro study, Gut, 31:198–203, 1990.
Lhoste, E.F., Nugon-Baudon, L., Lory, S., Meslin, J.-C., and Andrieux, C., The fermentation of lactulose in rats inoculated with Clostridium paraputrificum influences the activities of liver and intestinal xenobiotic metabolizing enzymes, J. Sci. Food Agric., 81:1397–1404, 2001.
Liehr, H. and Heine, W.D., Treatment of endotoxemia in galactosamine hepatitis by lactulose administered intravenously, Hepato-Gastroenterol., 28: 296–298, 1981.
McCutcheon, J. and Fulton, J.D., Lowered prevalence of infection with lactulose therapy in patients in long term hospital care, J. Hosp. Infect., 13:81–86, 1989.
Montilla, A., del Castillo, M.D., Sanz, M.L., and Olano, A., Egg shell as catalyst of lactose to lactulose, Food Chem., 90:883–890, 2005.
Rowland, I.R., Bearne, C.A., Fischer, R., and PoolZobel, B.L., The effect of lactulose on DNA damage induced by DMH in the colon of human flora-associated rats, Nutr. Can., 26:37–47, 1996.
Schumann, C., Medicinal, nutritional and technological properties of lactulose. An update, Eur. J. Nutr., 41(Suppl. 1): 17–25, 2002.
Van de Heuvel, E.G. and Weidauer, T., Role of non-digestible carbohydrate lactulose in the absorption of calcium, Med. Sci. Monit., 5:1231–1237, 1999.
Lavender (Lavandula angustifolia)
Lavender oil, used in aromatherapy, is obtained from the flowering tips of the plant Lavandula angustifolia. Shellie and coworkers (2002) identified 85 components in lavender essential oil, which accounted for more than 95 percent of the oil. Of nine samples analyzed, three were closest to the ISO Standard 3515, which included acceptable ranges for linalool, 25–38 percent; linalyl acetate, 25–45 percent; lavandulyl acetate minimum, 2 percent; terpinen-4-ol, 2–6 percent; lavandulol minimum, 0.3 percent; 1,8-cineole, 0–15 percent; limonene, 0–0.5 percent; transβ-ocimene, 2–6 percent; cis-β-ocimene, 4–10 percent; 3-octanone, 0–2 percent; camphor, 0–0.5 percent; and α-terpineol, 0–1 percent. Lavender oil is a holistic relaxant thought to have carminative, antiflatulence, and anticolic properties (Tisserand, 1985). The oil was found to have a spasmolytic effect on guinea-pig ileum in vitro (Lis-Balchin et al., 1996), which is correlated with the holistic relaxant effect in man (Lis-Balchin and Hart, 1997). The spasmolytic effect of lavender and linalool were shown by Lis-Balchin and Hart (1999) to be mediated via cAMP.
The impact of aromatherapy on positive mood shifts by Knasko (1992) led to a study on the effect of lavender baths on psychological well-being by Morris (2002). Forty female university students and staff, with a mean age of 28.2 years, were randomly allocated either grapeseed oil or 80 percent grapeseed oil and 20 percent lavender oil to use in their daily bath for 14 days. Using the University of Wales Institute of Science and Technology (UWIST) Mood Adjective Checklist (Matthews et al., 1990), lavender oil was found to have a selective effect on anger-frustration in the first trial, while reducing negative responses about the future in the second trial. These results suggested lavender-oil baths may have a positive effect on psychological well-being. A recent study by Fernandez and coworkers (2004) showed differences in response to odors between newborn infants of depressed and non-depressed mothers. Only newborn infants of depressed mothers increased relative left frontal electroencephalographic (EEG) asymmetry following exposure to lavender or rosemary aroma, with no response by newborns of non-depressed mothers. The shift in right frontal EEG asymmetry is a pattern associated with a positive effect and response to positive stimuli and was related to significantly greater head turning and lip licking. A recent review of the scientific and clinical evidence for the psychological effects of lavender was prepared by Kirk-Smith (2003).
References
Fernandez, M., Hernandez-Reif, M., Field, T., Sanders, C., Diego, M., Sanders, C., and Roca, A., EEG during lavender and rosemary exposure in infants of depressed and non-depressed mothers, Infant Behav. Develop., 27:91–100, 2004.
Kirk-Smith, M., The psychological effects of lavender II: Scientific and clinical evidence, Intern. J. Aromather., 13(23):82–88, 2003.
Knasko, S.C., Ambient odour’s effect creativity, mood, and perceived health, Chem. Senses., 17:27–35, 1992.
Lis-Balchin, M. and Hart, S., Correlation of the chemical profiles of essential oil mixes with their relaxant and stimulant properties in man and smooth muscle preparations in vitro, in Proc. 27th International Symposium on Essential Oils, Franz, C.H., Mathe, A., and Buchbaer, G., Eds., Allured Pub. Corp., Carol Stream, IL, 1997, pp. 24–28.
Lis-Balchin, M. and Hart, S., Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P.Miller), Phytother. Res., 13:540–542, 1999. Lis-Balchin, M., Hart, S., Deans, S.G., and Eaglesham, E., Comparison of the pharmacological and antimicrobial action of commercial plant essential oils, J. Herbs Spices Med. Plants, 4:69–88, 1996.
Matthews, G., Jones, D., and Chamberlain, G., Refining measurement of mood: The UWIST MoodAdjective Checklist, Br. J. Psychol., 81:17–42, 1990.
Morris, N., The effects of lavender (Lavandula angustifolium) baths on psychological well-being: Two exploratory randomised control trials, Complement. Ther. Med., 10:223–228, 2002.
Shellie, R., Mondello, L., Marriott, P., and Dugo, G., Characterisation of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention and comparison with comprehensive two-dimensional gas chromatography, J. Chromatogr. A., 970:225–234, 2002.
Tisserand, R., The Art of Aromatherapy, C.W. Daniel Co. Ltd., Saffron Walden, U.K., 1985.
Lectins
Lectins are glycoproteins that combine reversibly with sugars and glycoconjugates. They cause agglutination of erythrocytes, as well as interfere with nutrient absorption by binding with glycoproteins on the epithelial surface of the small intestine (Lajolo and Genovese, 2002). They are found as phytohemagglutinins in a wide variety of plants, particularly legumes. Lectins may have possible medical uses by their ability to provoke hyperplasia of the small intestine, alter the bacterial flora, and interfere with hormone secretion, as well as enter systematic circulation (Pusztai, 1993; Pusztai and Bardocz, 1996). For example, mice fed a purified bean phytohemagglutinins had reduced tumor growth, indicating competition between the gut epithelium undergoing hyperplasia and the growing tumor (Pusztai et al., 1998). Gastman et al. (2004) reported wheatgerm agglutinin induced apoptosis by binding to surface carbohydrates (N-acetylmeuraminic or N-acetylglucosamine) of normal and malignant cells. The lectin-induced apoptosis was extremely fast and mediated via a mitochondrial pathway.
The possible use of lectins for treating obesity was shown by Pusztai and coworkers (1998) by the reduction in lipid accumulation in obese rats fed a diet containing raw kidney beans. This was attributed to a reduction in insulin levels by the bean lectins with no loss in body or muscle proteins observed.
Nishimura and coworkers (2004) recently found that bone-marrow mesenchymal stem cells, chondrocytes, and osteoblasts exposed to a lectin from the bean (Phaseolus vulgaris) increased their adhesion on plastic culture dishes or plates of hydroxy apatite, titanium, and poly-DL-lactic-co-glycolic acid (PLGA). The bean lectin, erythroagglutinin, enhanced resistance of these cells to proteases and mechanical stimuli, suggesting their potential in tissue engineering and cell therapy.
References
Gastman, B., Wang, K., Han, J., Zhu, Z., Huang, X., Wang, G.-Q., Rabinowich, H., and Gorelik, E., A novel apoptotic pathway as defined by lectin cellular initiation, Biochem. Biolphys. Res. Commun., 316: 263–271, 2004.
Lajolo, F.M. and Genovese, M.I., Nutritional significance of lectins and enzyme inhibitors from legumes, J. Agric. Food Chem., 50:6592–6598, 2002.
Nishimura, H., Nishimura, M., Oda, R., Yamanaka, K., Matsubara, T., Ozaki, Y., Sekiya, K., Hamada, T., and Kato, Y., Lectins induce resistance to proteases and/or mechanical stimulus in all examined cells—including bone marrow mesenchymal stem cells—on various scaffolds, Exp. Cell Res., 295:119–127, 2004.
Pryme, I.F., Pusztai, A. Bardocz, S., and Ewen, S.W.B., The induction of gut hyperplasia by phytohaemagglutinins in the diet and limitation of tumor growth, Histol Histopathol., 13:575– 583, 1998.
Pusztai, A., Dietary lectins are metabolic signals for the gut and modulate immune and hormone functions, Eur. J. Clin. Nutr., 47:691–699, 1993.
Pusztai, A. and Bardocz, S., Biological effects of plant lectins on the gastrointestinal tract: Metabolic consequences and applications, Trends Glycosci. Glycotechnol., 8:149–165, 1996.
Pusztai, A., Grant, G., Buchan, W.C., Bardocz, S., de Carvalho, A.F.F.U., and Ewen, S.W.B., Lipid accumulation in obese Zucker rats is reduced by inclusion of raw kidney bean (Phaseolus vulgaris) in the diet, Br. J. Nutr., 79:213–221, 1998.
Legumes
see also Beans, Bowman-Birk protease inhibitors, Chickpea, Kidney bean, Lectins, Lentils, Peas, Resistant starch, and Soybeans Legumes play an important role in human nutrition, as they are excellent sources of proteins and complex carbohydrates. In addition to being good sources of vitamins and minerals, legumes are considered low glycemic foods, as they elicit a low blood-glucose response (Tharanathan and Mahadevamma, 2003). Legumes have long been recognized as beneficial for controlling and treating metabolic diseases, such as diabetes mellitus, coronary heart disease, and colon cancer (Simpson et al., 1981). They are consumed as whole or grains, dehusked, or as split legumes and include peas, lentils, and a variety of beans, such as red gram, black gram, cowpea, broad bean, field bean, horse bean, and kidney bean.
A number of dietary components in legumes appear to be responsible for their beneficial physiological effects, including protein and starch. Chau and coworkers (1998) incorporated 12 percent protein concentrates from three Chinese legume seeds, Phaeolus angularis, Phaseolus calcaratus, and Dolichos lablab in the diets of male Golden Syrian hamsters and found a pronounced hypocholesterolemic effect. The three legume-protein concentrates significantly (p<0.05) lowered serum triglyceride and total and LDL cholesterol levels and liver total lipids and cholesterol compared to casein. The more potent hypocholesterolemic effects were associated with P. calacaratus and D. lablab, while only the former significantly increased serum HDL cholesterol. During processing, the higher amylose content (30–40 percent) of legume starches compared to cereals (Madhusudhan and Tharanathan, 1995) results in the formation of large amounts of resistant starch. The latter is known to have important physiological benefits (Edwards, 1993).
Legumes are also rich in protease inhibitors and lectins, considered antinutritional factors (Lajolo and Genovese, 2002). However, the Bowman-Birk inhibitor was shown to have therapeutic properties that included anticarcinogenic and antiinflammatory, as well the ability to reduce ulcerative colitis in mice (Kennedy, 1998; Wan et al., 1999; Ware et al., 1999; Armstrong et al., 2000). Lectins are also recognized for their beneficial properties, including preventing gastrointestinal atrophy during total parenteral nutrition and reducing tumor growth in mice, as well as treating obesity (Pryme et al., 1998; Pusztai et al., 1998; Jordinson et al., 1999).
References
Armstrong, W.B., Kennedy, A.R., Wan, X.S., Atiba, J., McLaren, G.E., and Meyskens, F.L., Jr., Single-dose administration of Bowman-Birk inhibitor concentrate in patients with oral leukoplakia, Cancer Epidemiol. Biomarkers, 9:43–47, 2000.
Chau, C.-F., Cheung, P.C.K., and Wong, Y.-S., Hypocholesterolemic effects of protein concentrates from three Chinese indigenous legume seeds, J. Agric. Food Chem., 46:3698– 3701, 1998.
Edwards, C.A., Interactions between nutrition and intestinal microflora, Proc. Nutr., 52:375–382, 1993.
Jordinson, M., Goodlad, R.A., Brynes, A., Bliss, P., Ghatewi, M.A., Bloom, S.R., Fitzgerald, A., Grant, G., Bardocz, S., Pustzai, A., Pagnatelli, M., and Calam, J., Gastrointestinal responses to a panel of lectins in rats maintained on total parenteral nutrition, Am. J. Physiol.-Gastr. Liver Physiol., 39:G1235-G1242, 1999.
Kenndey, A.R., The Bowman-Birk inhibitor from soybeans as anticarcinogenic agent, Am. J. Clin. Nutr., 68: 1406S-1412S, 1998.
Lajolo, F.M. and Genovese, M.I., Nutritional significance of lectins and enzyme inhibitors from legumes, J. Agric. Food Chem., 50:6592–6958, 2002.
Madhushan, B. and Tharanathan, R.N., Legume and cereal starch—why differences in digestibility? Part 1, Isolation and composition of legume (green gram and Bengal gram) starches, Staerke, 47:165–171, 1995.
Pryme, I.F., Pustzai, A., Bardocz, S., and Ewen, S.W.B., The induction of gut hyperplasia by phytohemagglutinin in the diet and limitation of tumor growth, Histol. Histopathol., 13:575– 583, 1998.
Pusztai, A., Grant, G., Buchan, W.C., Bardocz, S., de Carvalho, A.F.F.U., and Erwen, S.W.B., Lipid accumulation in obese Zucker rats is reduced by inclusion of raw kidney bean (Phaseolus vulgaris) in the diet, Br. J. Nutr., 79:213–221, 1998.
Simpson, H.C., Lousley, R.S., Greekie, M., Hockaday, T.D.R., Carter, R.D., and Mann, J.I., A high carbohydrate leguminous fibre diet improves all aspects of diabetes control, Lancet, 1:1–4, 1981.
Tharanathan, R.N. and Mahadevamma, S., Grain legumes—a boon to human nutrition, Trends Food Sci. Technol., 14:507–518, 2003.
Wan, X.S., Meyskens, F.L., Jr., Armstrong, W.B., Taylor, T.H., and Kennedy, A.R., Relationship between protease activity and neu oncongene expression in patients with oral leukoplakia treated with the Bowman-Birk inhibitor, Cancer Epidemiol. Biomarkers, 8:601–608, 1999.
Ware, J.H., Wan, X.S., Newberne, P., and Kennedy, A.R., Bowman-Birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis, Digest. Dis. Sci., 44: 986–990, 1999.
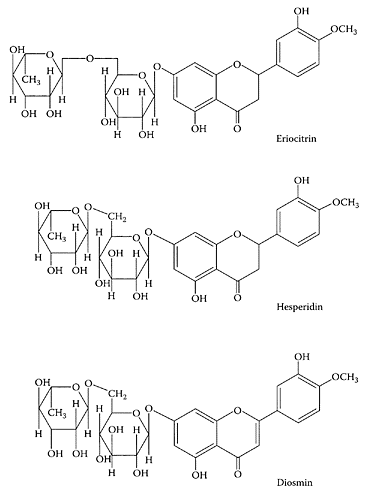
Eriocitrin, hesperidin, and diosmin. (From Del Rio et al., Food Chem., 84:457–461, 2004. With permission.)
Lemon
see also Flavonoids and Hesperidin Lemon juice is a rich source of ascorbic acid and flavonoids. The antioxidant properties of these compounds have been suggested to inhibit heart disease and certain types of cancers (Salah et al., 1995). Marin and coworkers (2002) found these nutraceuticals were higher in Fino lemon juice compared to the Vern variety. In addition, they found that different industrial-extraction systems affected the levels of these components. Miyake et al. (1997) identified the flavonoid, eriocitrin, in lemon fruit, which had considerable antioxidant activity. Ogata and coworkers (2000) showed this flavonoid induced apoptosis in HL-60 cells and may have therapeutic applications. A recent study of Citrus limon flavonoids by Del Rio et al. (2004) found that immature fruit from Lisbon and Fino-9 cultivars were excellent sources of the flavonone hesperidin, while mature fruits from Fino-9 and leaves of Eureka were good sources of the flavone diosmin and the flavonone eriocitrin. Each of these flavonoids have been shown to have pharmaceutical properties.
References
Del Rio, J.A., Fuster, M.D., Gonez, P., Porras, I., Garcia-Lidon, A., and Ortuno, A., Citrius limon: A source of flavonoids of pharmaceutical interest, Food Chem., 84:457–461, 2004.
Marin, F.R., Martinez, M., Uribesalgo, T., Casillo, S., and Frutos, M.J., Changes in nutraceutical composition of lemon juices according to different industrial extraction systems, Food Chem., 78:319–324, 2002.
Miyake, Y., Yamamoto, K., and Osawa, T., Isolation of eriocytrin (eryodictiol-7-rutinoside) from lemon fruit (Citrus limon BURM.f.) and its antioxidative activity, Food Sci. Tecnol. Int. Tokio., 3:84–89, 1997.
Ogata, S., Miyaje, Y., Yamomoto, K., Okumura, K., and Taguchi, H., Apoptosis induced by the flavonoid from lemon fruit (Citrus limon BURM. f.) and its metabolites in HL-60 cells, Biosci. Biotechnol. Biochem., 64:1975–1978, 2000.
Salah, N., Miller, N.J., Paganga, G., Tijburg, L., Bolwell, G.P., and Rice-Evans, C., Polyphenolic flavonols as scavenger of aqueous phase radicals and as chain-breaking antioxidants, Arch. Biochem. Biophys., 322:339–346, 1995.
Lemon balm (Melissa officinalis)
The leaves of lemon balm, a perennial, lemon-scented herb, are used extensively as an herbal tea in Europe for its aromatic, digestive, and antispasmodic properties in treating sleep disturbances and gastrointestinal disorders (Bisset and Wichtl, 1994). It is generally sold in combination with other herbs that elicit “calming” or sedative effects. Cerny and Schmid (1999) showed that a combination of valerian and lemon balm significantly improved the quality of sleep of healthy volunteers during 30 days of treatment with 360 mg/day and 240 mg/day of valerian and lemon balm, respectively. Acute administration of lemon balm was shown by Kennedy and coworkers (2002) to modulate the mood and cognitive performance of healthy volunteers in a dose- and time-dependent manner, as assessed using the Congitive Drug Research (CDR) computerized-test battery and two serial subtraction tasks. The calming effect and possible cholinergic modulation of lemon balm may have application in the treatment of Alzheimer’s disease. A recent double-blind, placebo-controlled study using lemon balm essential aromatherapy on 71 patients suffering from severe dementia by Ballard et al. (2004) also showed they were less agitated and socially withdrawn compared to the placebo.
Carnat and coworkers (1998) reported the presence of 0.13 percent citral (neral+geranial) and 11.8 percent total polyphenolic compounds in the essential oil of dried lemon-balm leaves. Of the latter, hydroxycinnamic compounds accounted for 11.3 percent, with rosmarinic acid 4.1 percent, and total flavonoids 0.5 percent. Herbal tea from lemon balm contained 10 mg/L essential oil, of which 74 percent was citral plus large amounts of polyphenolic compounds.
References
Ballard, C., O’Brien, J., Reichelt, K., and Perry, E., Aromatherapy as a safe and effective treatment for the management of agitation in severe dimentia: The results of a double-blind, placebo controlled trial with Melissa, J. Clin. Psychiatry, 63:553–558, 2002.
Bisset, N.G. and Wichtl, M., Herbal Drugs, Medpharm, Stuttgart, 1994.
Carnat, A.P., Carnat, A., Fraisse, D., and Lamaison, J.L., The aromatic and polyphenolic composition of lemon balm (Melissa officinalis L. subsp. Officinalis) tea, Pharmaceutica Acta Helv., 72:301–305, 1998.
Cerny, A. and Schmid, K., Tolerability and efficacy of valerian/lemon balm in healthy volunteers: A double-blind, placebo-controlled, multicentre study, Fitoterapia, 70:221–228, 1999.
Kennedy, D.O., Scholey, A.B., Tildesley, N.T.J., Perry, E.K., and Wesnes, K.A., Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm), Pharmacol. Biochem. Behav., 72:953–964, 2002.
Lemon Grass (Cymbopogon citratus)
Lemon grass, native to India, is used in Thai and Vietnamese cooking. Most commercial crops for the United States are grown in California and Florida. Using the Salmonella mutation assay, Vinitketkumnuen and coworkers (1994) showed an ethanol extract from lemon grass exhibited antimutagenic activity against a number of different mutagens. Anticancer components in lemon grass extract were found by Suaeyun et al. (1997) to inhibit azoxymethane (AOM)-initiated colon carcinogenesis in the rat. Puatanachokchai et al. (2002) showed a similar lemon-grass extract inhibited the early stages of hepatocarcinogenesis in diethyl-nitrosamine (DEN)-treated male Fischer 344 rats by reducing the number of putatively preneoplastic, glutathione S-transferase placental form-positive lesions, as well as the level of oxidative hepatocyte nuclear DNA injury, assessed by 8-hydroxyguanosine production.
References
Puatanachokchai, R., Kishida, H., Denda, A., Murata, N., Konishi, Y., Vinitketkumnuen, U., and Nakae, D., Inhibitory effects of lemon grass (cymbopogon citratus) extract on the early phase of hepatocarcinogenesis after initiation with diethylnitrosamine in 344 male Fischer rats. Cancer Lett., 183:9–15, 2002.
Suaeyun, R., Kinouchi, T., Arimochi, H., Vinitketkumnuen, U., and Ohnishi, Y., Inhibitory effects of lemon grass (Cymbopogon citratus, Stapf) on formation of azoxymethane-induced DNA adducts and aberrant crypt foci in the rat colon, Carcinogenesis, 18:949–955, 1997.
Vinitketkumnuen, U., Puatanachokchai, R., Kongtawelert, P., Lertprasertsuke, N., and Matsushima, T., Antimutagenicity of lemon grass (Cymbopogon citrates, Stapf) to various known mutagens in Salmonella mutation assay, Mutat. Res., 341:71–75, 1994.
Lentils (Lens culinaris L.)
Legume seeds such as lentils provide an inexpensive source of protein for a large part of the world’s population. Like other legumes, lentils contain phytohemagglutinins and protease inhibitors, which must be destroyed by cooking before they can be utilized in the diet. Duenas and coworkers (2003) identified proanthocyanidins in the seed coat of lentils. The major monomeric flavan-3-ol identified was (+) catechin-3-glucose followed by smaller amounts of (+)-catechin and (−)-epicatechin. The latter compounds were reported to exhibit potent antioxidant and freeradical-scavenging activities and to inhibit platelet aggregation and antiulcer activity against stomach-mucosa injury (Vinson et al., 1995; Cook and Samman, 1996; Duenas et al., 2003). The large amounts of these bioactive compounds in lentil seed coats represent a potential source of nutraceuticals
References
Cook, N.C. and Samman, S., Flavonoids—chemistry, metabolism, cardiopreventive effects, and dietary source, J. Nutr. Biochem., 7:66–76, 1996.
Duenas, M., Sun, B., Hernandez, T., Estrella, I., and Spranger, M.I., Proanthocyanidin composition in the seed coat of lentils (Lens culinaris L.), J. Agric. Food Chem., 51:7999–8004, 2003.
Vinson, J.A., Dabbagh, Y.A., Serry, M.M., and Jang, J., Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease, J. Agric. Food Chem., 43:2800–2802, 1995.
Lettuce (Lactuca sativa)
Lettuce leaves are quite low in phenolic s, but Kang and Saltveit (2002) reported a fourfold increase in iceberg and romaine lettuce following heat-shock (45°C for 2.5 min. in water) treatment or wounding. This increase in phenolics was accompanied by a corresponding increase in antioxidant power (FRAP). Serafini and coworkers (2002) showed that ingestion of 260 g fresh lettuce raised the plasma-antioxidant levels in 11 healthy volunteers compared to the same lettuce stored at 5°C under modified atmosphere-packaging conditions (MAP: O2-N2, 5:95 v/v). Ingestion of the fresh lettuce resulted in significantly higher plasma total radical-trapping potential (TRAP) compared to the MAP stored lettuce. In addition, there was a significant increase in plasma quercetin, p-coumaric, caffeic acid, β-carotene, and vitamin C following consumption of fresh lettuce, which was not observed following ingestion of MAP lettuce. Thus, optimized MAP storage conditions were needed to better preserve the bioactive components of fresh-cut produce.
Nicolle and coworkers (2004) recently found male Wistar rats fed a diet containing 20 percent freeze-dried lettuce over a three-week period had increased excretion of cholesterol end products, as well as enhanced antioxidant status (Figure L.60). A slight but significant (p<0.05) decrease in cholesterol was observed in rats on the lettuce-fed diet, while triacylglycerol levels were unaffected. A decrease of −23 percent in cholesterol levels in the plasma triacylglycerolrich lipoprotein (with a minor contribution of LDL) fraction was accompanied by an increase of +18 percent in the HDL fraction and a slight decrease of −7 percent in triacylglycerol levels in the lettuce-fed rats compared to the control. Nicolle and coworkers (2004) attributed these beneficial effects to the fiber and antioxidant content of lettuce (Table L.43).
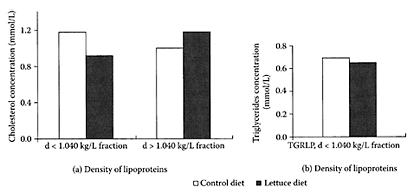
FIGURE L.60 (a) Changes in the distribution of cholesterol in the various lipoprotein fractions in rats fed control or lettuce diets. The fractions with d<1.040 kg/L correspond chiefly to triacyclyglycerol-rich lipoproteins (TGRLP), with a lower contribution of LDL. The fractions with d<1.040 kg/L correspond essentially to HDL; (b) differences in the repartition of triacylglycerols in plasma-lipoprotein fractions of rats fed control or lettuce diets. Each value is the mean of triplicate analyses of a pool of eight plasma. (Nicolle et al., Clinical Nutr., 23:605–614, 2004. With permission.)
TABLE L.43
Fiber and Antioxidant Content of Lettuce1
References
Kang, H.-M. and Saltveit, M.E., Antioxidant capacity of lettuce leaf tissue increases after wounding, J. Agric. Food Chem., 50:7536–7541, 2002.
Nicolle, C., Cardinault, N., Gueux, E., Jaffrelo, L., Rock, E., Mazur, A., Amourex, P., and Remesy, C., Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat, Clin. Nutr., 23:605–614, 2004.
Serafini, M., Bugianesi, R., Salucci, M., Azzinni, E., Ragguzzini, A., and Maiani, G., Effect of acute ingestion of fresh and stored lettuce (Lactuca saliva) on plasma total antioxidant levels in human subjects, Br. J. Nutr., 88:615–623, 2002.
Licochalcone A
Licochalcone A is an oxygenated chalcone first isolated from Chinese licorice roots, which has considerable biological activity (Nadelmann et al., 1997). Barfod and coworkers (2002) found licochalcone A and four synthetic analogues inhibited proliferation of lymphocytes, as well as the production of proinflammatory and anti-inflammatory cytokines. These results suggested that these compounds exert immunomodulatory effects, which may be useful for treating some diseases. Oral administration of licochalcone A (30 mg/kg/day) to mice with glomerular disease (Masugi-nephritis) was found by Fukai et al. (2003) to reduce the urinary-protein excretion compared to nephritic mice. Licochalcone A also exhibited weak scavenging activity against superoxide radicals.
Licochalcone A. (From Fukai et al., Fitoterapia, 74:720–724, 2003. With permission.)
References
Barfod, L., Kemp, K., Hansen, M., and Kharazmi, A., Chalcones from Chinese licorice inhibit proliferation of T cells and production of cytokines, Inter. Immunopharmacol., 2:545–555, 2002.
Fukai, T., Satoh, K., Nomura, T., and Sakagami, H., Antinephritis and radical scavenging activity of prenylflavonoids, Fitoterapia, 74:720–724, 2003.
Nadelmann, L. Tjørnelund, J., Christensen, E., and Hansen, H., High-performance liquid chromatographic determination of licochalcone A and its metabolites in biological fluids, J. Chromatogr. B., 695:389–3900, 1997.
Licorice
see also Glabridin, Glycyrrhin, and Glycyrrhizic acid Licorice root, one of the oldest and most commonly used botanicals in Chinese medicine, has been used worldwide for medicinal purposes since ancient times (Liu et al., 2000). The key components associated with its medicinal properties are triterpenes, polyphenols, polysaccharides, flavonoids, alkaloids, poly amines, and essential oils. Licorice triterpenes are nonsteroidal agents exhibiting both antioxidant and anti-inflammatory properties (Wang and Nixon, 2001). The most important triterpene in licorice, glycyrrhizin (GL), is hydrolyzed to its major metabolite, glycyrrhetinic acid (GA) (Wang et al., 1998). GA exists in two different forms, 18α-glycyrrhetinic acid (α-GA) and 18β-glycyrrhetinic acid (β-GA), both of which are antimutagens capable of inhibiting monooxygenase activity (Wang et al., 1991). Tamir and coworkers (2000) showed glabridin, an isoflavan in licorice root, acted as a phytoestrogen by inhibiting the proliferation of estrogen responsive (ER+) and estrogen nonresponsive (ER-) human breast cells at concentrations greater than 15 μm.
Rafi et al. (2001) found a novel polyphenol in licorice root, 1-(2,4-dihydroxyphenyl)-3-(4′-hydroxyphenyl) 1-propanone (β-hydroxy DHP), that was more inhibitory against Bel-2 phosphorylation in tumor cells compared to its stereoisomer α-hydroxy DHP. Bcl- 2, a protein that prevents cell death, inhibits cytochrome c from being released from mitochondria, which is essential for cell apoptosis to occur. Thus, licorice components appear to act as chemopreventive agents with potential as new pharmaceuticals for treating cancers.
Glycyrrhetinic acid (glycyrrhizic acid). (From Wang et al., J. Chromatogr. A., 811:219–224, 1998. With permission.)
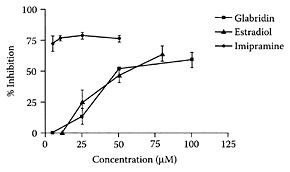
FIGURE L.61 The effect of increasing concentration of glabridin (■), estradiol (▲), and imipramine (●) on the inhibition of hSERT reuptake. 293-hSERT cells were incubated for 20 min with estradiol or ipramine at various concentrations (0–100 μM). Cells were harvested, and the incorporated radioactivity was measured with a scintillation liquid. Data are presented as percent inhibition of controls. Values are means ± SD of three experiments. (Ofir et al., J. Mol. Nurosci., 20:135– 140, 2003. With permission.)
Ofir and coworkers (2003) recently showed that licorice isoflavans and isoflavene were capable of inhibiting serotinin re-up, a known pharmacological treatment for major depression, as well as anxiety, appetite, and obsessive-compulsive disorders (Barker and Blakely, 1995). Glabridin, for example, mimicked estradiol by inhibiting serotonin reuptake in a dose-dependent manner (Figure L.61). The ability to inhibit serotonin reuptake was facilitated by the lipophilic part of the isoflavans, as well as the hydroxyl at position 2′ in the B ring. These licorice constituents appear to have considerable potential for the therapeutic treatment of mild to moderate depression in premenopausal and postmenopausal women.
Glabridin. (Adapted from Tamir et al., J. Steroid Biochem. Mol. Biol., 78:291–298, 2001.)
References
Barker, E.L. and Blakely, R.D., Norepinephrine and serotonin transporter: Molecular targets of antidepressant drugs, in Psychopharmacology: The Fourth Generation of Progress, Bloom, F.E. and Kupfer, D.J., Eds., Raven Press, New York, 1995, pp. 321–333.
Liu, H.-M., Naoki, S., Takimi, A., and Tamio, M., Constituents and their sweetness of food additive enzymatically modified licorice extract, J. Agric. Food Chem., 48:6044–6047, 2000.
Ofir, R., Tamir, S., Khatib, S., and Vaya, J., Inhibition of serotonin re-uptake by licorice constituents, J. Mol. Neurosci., 20:135–140, 2003.
Rafi, M.M., Vastano, B.C., Zhu, N., Ho, C-T., Ghai, G., Rosen, R.T., Gallo, M., and Di Paola, R.S., Novel polyphenol molecule isolated from licorice root (Glycyrrhiza glabra) induces apoptosis, G2/M cycle arrest, and Bcl-2 phosphorylation in tumor cell lines, J. Agric. Food Chem., 50:677–684, 2002.
Tamir, S., Eizenberg, M., Somjen, D., Israel, S., and Vaya, J., Estrogen-like activity of glabrene and other constituents isolated from licorice root, J. Steroid Biochem. Mol. Biol., 78:291–298, 2001.
Tamir, S., Eizenberg, M., Somjen, D., Stern, N., Shelach, R., Kaye, A., and Vaya, J., Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells, Cancer Res., 60:5704–5709, 2000.
Wang, Z., Argarwal, R., Zhou, Z.C., Bickers, D.R., and Mukhtar, H., Inhibition of mutagenicity in Salmonella typhymurium and skin tumor initiating and tumor promoting activities in SENCAR mice by glycyrrhetinic acid: Comparison of 18α- and 18β-stereoisomers, Carcinogenesis, 12:187–192, 1991.
Wang, P., Li, S.F.Y., and Lee, H.K., Determination of glycyrrhizic acid and 18-β-glycyrrhetinic acid in biological fluids by micellar electrokinetic chromatography, J. Chromatogr. A., 811:219–224, 1998.
Wang, Z. and Nixon, D., Licorice and cancer, Nutr. Cancer, 39:1–11, 2001.
Lignans
see also Flaxseed, Matairesinol, and Secoisolariciresinol Lignans are a complex group of phenolic compounds widely distributed in the plant kingdom, composed of phenylpropane dimers linked by β–β bonds with a 1,4-diarylbutane structure (Smeds and Hakala, 2003). Flaxseed is the richest source of lignans compared to other food sources, such as soybean, oat bran, and lentils, as summarized in Table L.44. The main lignan precursors in flaxseed are Secoisolariciresinol (SEC) and matairesinol (MAT) (Scheme L.36). SEC is normally present in the form of Secoisolariciresinol diglucoside (SDG), which is converted by bacteria in the gastrointestinal tract to enterodiol and enterolactone. Both enterodiol and enterolactone were shown to inhibit the growth of human colon-cancer cells at a concentration of 100 μmM (Sung et al., 1998). Supplementation of flaxseed in the diets of rats decreased the number of aberrant crypts and foci in AOM-treated rats (Serraino and Thompson, 1992). A dose-dependent reduction in metastasis and the growth of secondary tumors observed in mice fed flaxseed by Yan et al. (1998) indicated its potential for preventing metastasis.
TABLE L.44
Level of Lignans in Some Plant Food Sources
Niemeyer and Metzler (2002) examined the oxidative metabolism of lignans SEC and MAT and showed they were both excellent substrates for cytochrome P450 hydroxylation at the aliphatic and aromatic positions in the molecule. The different pathways involved are outlined in Scheme L.37. However, the genotoxic potential of these hydroxylated products, including isolariciresinol (ISL) and lariciresinol (LAR), have yet to be determined.
Owen and coworkers (2000) showed for the first time that lignans (+)-1-acetoxypinoresinol and (+)-pinoresinol were major components of the phenolic fractions in extra-virgin olive oils. They were virtually absent in the corresponding refined oils. Nurmi and coworkers (2003) reported that lignans in red wine ranged from 0.812 to 1.406 mg/L, with isolariciresinol being the main one.
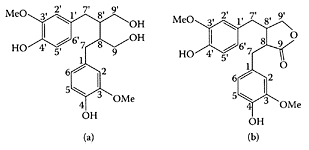
SCHEME L.36 Structure and numbering of Secoisolariciresinol (a) and matairesinol (b). (From Saarinen et al., J. Chromatogr. B., 777:311–319, 2002. With permission.)
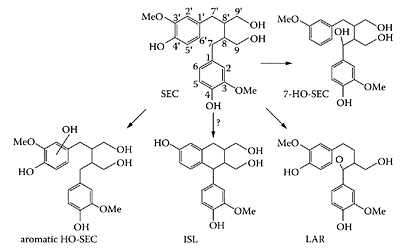
SCHEME L.37 Oxidative pathways in the metabolism of SEC. (Niemeyer and Metzler, J. Chromatogr. B., 777:321– 327, 2002. With permission.)
References
Niemeyer, H.B. and Metzler, M., Oxidative metabolites and genotoxic potential of mammalian and plant lignans in vitro, J. Chromatogr. B., 777:321–327, 2002.
Nurmi, T., Haeinonen, S., Mazur, W., Deyama, T., Nishibe, S., and Adlercreutz, H., Lignans in selected wines, Food Chem., 83:303–309, 2003.
Owen, R.W., Mier, W., Giacosa, A., Hull, W.E., Spiegelhalder, B., and Bartsch, H., Identification of lignans as major components in the phenolic fraction of olive oil, Clin. Chem., 46:976–988, 2000.
Reinli, K. and Block, G., Phytoestrogen content of foods—a codium of literature values, Nutr. Cancer, 26:123–148, 1996.
Saarinen, N.M., Smeds, A., Makela, S.I, Ammala, J., Hakala, K., Pihlava, J.-M., Ryhanen, E.-L., Sjoholm, R., and Santti, H., Structural determinants of plant lignans for the formation of enterolactone in vivo, J. Chromatogr., 777:311–319, 2002.
Serraino, M. and Thompson, L.U., The effect of flaxseed supplementation on the imitation and promotional stages of mammary tumorigenesis, Nutr. Cancer, 17:153–159, 1992.
Setchell, K.D.R. and Adlercreutz, H.A., Mammalian lignans and phytoestrogens. Recent studies on their formation, metabolism and biological role in health and disease, in Role of Gut Flora in Toxicity and Cancer, Rowland, I.R., Ed., Academic Press, New York, 1988, pp. 315–345.
Smeds, A. and Hakala, K., Liquid chromatographictandem mass spectrometric method for the plant lignan 7-hydroxymatairesinol and its potential metabolites in human plasma, J. Chromatogr. B., 793:297–308, 2003.
Sung, M.-K., Lautens, M., and Thompson, L.U., Mammalian hormones inhibit the growth of estrogen-independent human colon cells, Anti. Cancer Res., 18:1405–1408, 1998.
Taskinen, A., Eklund, P., Syoholm, R., and Hotokha, M., The molecular structure and properties of hydroxymatairesinol, an ab initio study, J. Mol. Struct., (Theochem), 677:113–124, 2004.
Yan, L., Yee, J.A., Li, D., McGuire, M.H., and Thompson, L.U., Dietary flaxseed supplementation and experiential metastasis of melanoma in mice, Cancer Lett., 124:181–186, 1998.
Limes (Citrus aurantifolia)
Limes, members of the Rutaceae family, only grow in a tropical climate. Many different varieties are cultivated in the Middle East, tropical Asia, and in Florida in the United States. Kawaii and coworkers (1999) examined the antiproliferative effects of the readily extractable fraction from 34 important citrus juices on four different human cancer cells. Of these extracts, sweet lime inhibited the proliferation of three of these lines but was much less toxic towards normal human cell lines. Gharagozloo and Ghaderi (2001) later reported a concentrated lime-juice extract exhibited immunomodulatory effects on activated cultured human mononuclear cells. The levels of extract needed to inhibit proliferation of phytohemagglutinin (PHA)-activated mononuclear cells were 250 and 500 μg/mL, while inhibition of staphyloccocal protein (SPA)-activated mononuclear cells required 500 μg/mL (Figure L.62). They suggested that it was the protein components in the lime-juice extract that appeared responsible for its immunomodulatory properties.
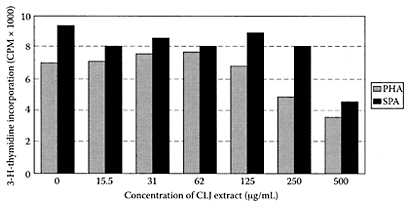
FIGURE L.62 Inhibitory effect of concentrated lime juice (CLJ) on the proliferation of PHA- and SPA-activated mononuclear cells. Proliferation of PHA-activated mononuclear cells was significantly inhibited by 250 and 500 μg/mL of CLJ extract, whereas only 500 μg/mL of the extract could induce significant inhibition in proliferation of SPA-activated mononuclear cells. Each value represents the mean ± SD of at least three independent measurements, p<0.05 was considered significant with regard to unstimulated control (0 μg/mL of CLJ extract). (From Gharagozloo and Ghaderi, J. Ethnopharmacol., 77:85–90, 2001. With permission.)
d-Limonene. (Adapted from Casuscelli et al., Appl. Cat., A: General, 274:115–122, 2004.)
References
Gharagozloo, M. and Ghaderi, A., Immunomodulatory effect of concentrated lime juice extract on activated human mononuclear cells, J. Ethnopharmacol., 77:85–90, 2001.
Kawaii, S., Tomono, Y., Katase, E., Ogawa, K., and Yano, M., Antiproliferative effects of the readily extractable fractions prepared from various citrus juices on several cancer cell lines, J. Agric. Food Chem., 47:2509–2512, 1999.
Limonene
d-Limonene is a monocyclic monoterpene in essential oils of citrus fruits, spices, and herbs. Orange peel is a particularly rich source, ranging from 90–95 percent (w/w). Limonene has been reported to exhibit chemoprotective activity against spontaneously and chemically induced tumors in the skin (Elegbede et al., 1988), liver (Dietrich and Swenberg, 1991), mammary gland (Elson et al., 1988; Maltzman et al., 1989), and lung and forestomach of rodents (Wattenberg et al., 1989; Watenberg and Coccia, 1991). Kawamori et al. (1996) showed d-limonene was effective against azoxymethane (AOM)-induced colon cancer in F344 rats. Those treated with 0.5 percent d-limonene in the drinking water had a significantly lower number of 2, 3, and 4 crypts compared to (AOM)-treated rats (Table L.45). These researchers confirmed the ability of d-limonene to inhibit formation of colonic ACF by blocking formation of (AOM)-induced ACF in the colon. Uedo and coworkers (1999) examined the mechanism involved in inhibiting gastric carcinogenesis in Wistar rats induced by N-methyl-N ′-nitrosoguanidine. Feeding 2 percent limonene significantly inhibited the induced cancer through increased apoptosis and decreased DNA synthesis.
TABLE L.45
Effect of d-Limonene on Aberrant Crypt/Focus Induced by AOM in Rat Colon1
References
Casuscelli, S.G., Crivello, M.E., Perez, C.F., Ghione, G., Herrero, E.R., Pizzio, L.R., Vasquez, P.G., Caceres, C.V., and Blanco, M.N., Effect of reaction conditions on limonene epoxidation with H2O2 catalyzed by supported Keggin heteropolycompounds, Appl. Cat., A: General, 274:115–122, 2004.
Dietrich, D.R. and Swenberg, J.A. The presence of α2u-globulin is necessary for d-limonene promotion of male rat kidney tumor, Cancer Res., 51:3512–3521, 1991.
Elegbede, J.A., Maltzman, T.H., Verma, A.K., Tanner, M.A., Elson, C.E., and Gould, M.N., Mouse skin tumor promoting activity of orange peel and d-limonene: A re-evaluation, Carcinogenesis, 7:2047–2049, 1986.
Elson, C.E., Maltzman, T.H., Boston, J.L., Tanner, M.A., and Gould, M.N., Anticarcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis, Carcinogenesis, 9:331–332, 1988.
Kawamori, T., Tanaka, T., Hirose, Y., Ohnishi, M., and Mori, H., Inhibitory effect of d-limonene on the development of colonic aberrant crypt foci induced by azoxymethane in F344 rats, Carcinogenesis, 17: 369–372, 1996.
Maltzman, T.H., Hurt, L.M., Elson, C.E., Tanner, M.A., and Gould, M.N., The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil, Carcinogenesis, 10:781–783, 1989.
Uedo, N., Tatsuta, M., Iishi, H., Baba, M., Sakai, N., Yano, H., and Otani, T., Inhibition by dlimonene of gastric carcinogenesis induced by N-methyl-N ′-nitrosoguanidine in Wistar rats, Cancer Lett., 137: 131–136, 2002.
Wattenberg, L.W., Sparnins, V.L., and Barany, G., Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur and monoterpenes, Cancer Res., 49:2689–2692, 1989.
Wattenberg, L.W. and Coccia, J.B., Inhibition of 4-(methylnitrosoamino)-1-(3-pyridyl)-1 -butanone carcinogenesis in mice by d-limonene and citrus fruit oils, Carcinogenesis, 12:115–117, 1991.
Limonin
see also Limonoids Limonin glucoside, one of the most abundant limonoids in citrus fruit, is readily available in orange-juice and citrus-juice processing byproducts (Schoch et al., 2001; Ifuku et al., 1998). Together with nomilin, they both inhibited forestomach, buccal pouch, lung, and skin carcinogenesis in rodents (Lam et al., 1989; Wada, 1996). Tanaka and coworkers (2000) clearly demonstrated inhibition of AOM-induced colonic ACF by dietary limonin or obacunone with suppression of preneoplasia to malignancy. This was evident by significantly lower incidences of multiplicities in the treated groups compared to the AOM control (Table L.46).
References
Ifuku, Y., Maseda, H., Miyake, M., Inaba, N., Ayano, S., Ozaki, Y., Maruyama, K., and Hasegawa, S., Method for manufacturing limonoid glucosides, U.S.Patent 5734046, 1998.
Kelly, C., Jewell, C., and O’Brien, N.M., The effect of dietary supplementation with the citrus limonoids, limonin and nobilin on xenobiotic-metabolizing enzymes in the liver and small intestine of the rat, Nutr. Res., 23:681–690, 2003.
Lam, L.K.T., Li, Y., and Hasegawa, S., Effects of citrus limonoids on glutathione S-transferase activity in mice, J. Agric. Food Chem., 37:878–880, 1989.
Schoch, T.K., Manners, G.D., and Hasegawa, S., Analysis of limonoid glucosides from citrus by electrospray ionization liquid chromatography-mass spectrometry, J. Agric. Food Chem., 49:1102–1108, 2001.
Tanaka, T., Maeda, M., Kohno, H., Murakami, M., Kagami, S., Miyake, M., and Wada, K., Inhibition of azoxymethane-induced colon carcinogenesis in male F344 rats by the citrus limonoids obacunone and limonin, Carcinogenesis, 22:193–198, 2001.
Wada, K., 1996. Studies on the constituents of edible and medicinal plants to affect metabolizing system in mammals, Natural Med., 50:195–203, 1996 (in Japanese).
Citrus limonoids, limonin (a) and nomilin (b). (From Kelly et al., Nutr. Res., 23:681–690, 2003. With permission.)
SCHEME L.38 Chemical structures of (a) obacunone and (b) limonin. (Tanaka et al., Carcinogenesis, 22:193–198, 2000. With permission.)
Limonoids
see also Limonin Limonoids are a group of highly oxygenated triterpenoids found in members of the Rutaceae (citrus fruits) and Maliaceae (neem) families. Citrus fruits are particularly rich sources of limonoids, with the most prevalent being obacunone and limonin (Scheme L.38). They impart bitterness to citrus juices, but as the fruit matures, they form glycosides, which are tasteless and water soluble. The anticancer properties of limonoids are attributed to their induction of the phase II enzyme, glutathione S-transferase (Lam et al., 1989; Kelly et al., 2003). Administration of high doses of citrus limonoids to four groups of healthy male and female subjects were shown by Manners and coworkers (2003) to be readily bioavailable and nontoxic.
References
Kelly, C., Jewell, C., and O’Brien, N.M., The effect dietary supplementation with the citrus limonoids, limonin and nomilin on xenobiotic-metabolizing enzymes in the liver and small intestine of the rat, Nutr. Res., 23:681–690, 2003.
Lam, L.K.Y., Li, Y., and Hasegawa, S., Effect of citrus limonoids on glutathione S-transferase in mice, J. Agric. Food Chem., 37:878–880, 1989.
Manners, G.D., Jacob, R.A., Breksa, A.P., III, Schoch, T.K., and Hasegawa, S., Bioavailability of citrus limonoids in humans, J. Agric. Food Chem., 51:4156–4161, 2003.
α-Lipoic acid
α-Lipoic acid (thioctic acid), a short-chain fatty acid with two sulfur atoms, is a naturally occurring coenzyme of pyruvate and α-ketoglutarate dehydrogenases. It can be reduced to dihydrolipoic acid, with the two sulfur atoms converted to sulfhydryl groups. Lipoic acid is found mainly in meat and liver and could not be detected in vegetables (Hiroyuki, 1998). It appears to be a useful, therapeutic agent for neurological and liver disorders (Packer et al., 1995; Bustamante et al., 1998). A recent study by Obrosova and coworkers (2003) confirmed the effectiveness of DL-α-lipoic acid as an antioxidant by reducing oxidative stress in rat renal cortex during early diabetes. Preclinical trials were recommended to assess the efficacy of lipoic acid for treating diabetic complications, such as diabetic nephropathy. Gibson et al. (2003) also confirmed the major role of oxidative stress in diabetic autonomic and somatic neuropathy, by the ability of α-lipoic acid to protect autonomic nerves of gastric fundus and the vascular supply of these nerves and neuronal-cell bodies from damage by reactive-oxygen species (ROS). As seen in Figure L.63, α-lipoic acid corrected the defective, relaxation-impaired gastric fundus nonadrenergic, noncholinergic (NANC) nerves caused by diabetes by providing 82.8 percent protection for maximum relaxation (16 Hz, p<0.001).
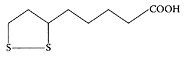
α-Lipoic acid. (From Sitton et al., J. Biochem. Biophys. Methods, 61:119–124, 2004. With permission.)
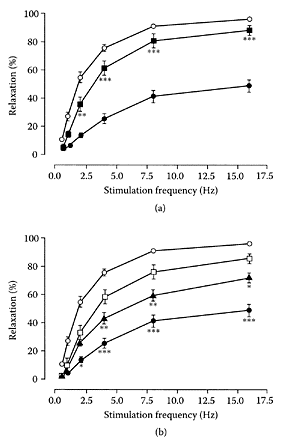
FIGURE L.63 (a,b) Effect of diabetes and chronic α-lipoic-acid treatment on NANC-mediated frequencyresponse curves in 5-hydroxytryptamine (5-HT) precontracted rat gastric fundus longtitudinal muscle strips, (a) Prevention study: nondiabetic control group, n=18. Eight-week diabetic control group, n=20;, α-lipoicacid prevention treatment diabetic group, n=9. Data are mean ± SEM. **p<0.01, ***p< 0.001 compared to eight-week diabetic control group, (b) Intervention study: nondiabetic ( ) and 8 week diabetic ( ) control groups as in (a), plotted for comparison; 4 week diabetic control group, n=17; α-lipoic acid intervention group treated for 4 weeks following 4 weeks of untreated diabetes, n=8. Data are mean ±. *p<0.05, **p<0.01, ***p<0.001 vs. α-lipoic acid intervention treatment group (Gibson et al., Free Rad. Biol. Med., 35:160–168, 2003. With permission.)
Lapenna et al. (2003) recently showed that it was the reduced form of lipoic acid, dihydrolipoic acid, not lipoic acid, that inhibited 15-lipoxygenase-dependent lipid peroxidation, suggesting possible antioxidant and antiathrogenic properties.
References
Bustamante, J., Lodge, J.K., Marcocci, L., Trischler, H.J., Packer, L., and Rihn, B.H., Alpha-lipoic acid in liver metabolism and disease, Free Rad. Biol. Med., 24:1023–1039, 1998.
Gibson, T.M., Cotter, M.A., and Cameron, N.E., Effects of alpha-lipoic acid on impaired gastric fundus innervation in diabetic rats, Free Rad. Biol. Med., 35:160–168, 2003.
Hiroyuki, K., Chromatographic analysis of lipoic acid and related compounds, J. Chromatogr., B717: 247–262, 1998.
Lapenna, D., Ciofani, G., Pierdomenico, S.D., Giamberardino, M.A., and Cuccurullo, F., Dihydrolic acid inhibits 15-lipoxygenase-dependent lipid peroxidation, Free Rad. Biol. Med., 35:1203–1209, 2003.
Obrosova, I.G., Fathallah, L., Liu, E., and NouroozZadeh, J., Early oxidative stress in the diabetic kidney: Effect of DL-α-lipoic acid, Free Rad. Biol. Med., 34:186–195, 2003.
Packer, L., Witt, E.H., and Tritschler, H.J., Alphalipoic acid as a biological antioxidant, Free Rad. Biol. Med., 19:227–250, 1995.
Sitton, A., Schmid, M.G., Gubitz, G., and AboulEnein, H.Y., Determination of lipoic acid in dietary supplement preparations by capillary electrophoresis, Biochem. Biophys. Methods, 61:119–124, 2004.
Lipoproteins
Lipoproteins are conjugated proteins in which simple proteins are combined with lipid components, such as cholesterol or triacylglycerols. They are classified according to their density as low-density (LDL) or highdensity (HDL) lipoproteins. LDL and HDL both transport lipids in the watery fluids of the body; however, HDL transports cholesterol from the peripheral tissues to the liver for oxidation. High LDL and low HDL levels are associated with a high risk of ischemic heart disease (Gordon and Rifkind, 1989). However, a high level of HDL cholesterol appears to protect the arterial wall from the formation of atherosclerotic lesions by removing lipids (Yancey et al., 2003).
The protective effect afforded by HDL against ischemic heart disease appears to be mediated via a reduced cardiac tumor necrosis factor-α (TNF-α) content and enhanced cardiac prostaglandin release (Calabresi et al., 2003). Apoliprotein-specific synthetic HDLs made by combining phosphatidylcholine and apolipoprotein A-1 were proposed by Sirtori et al. (1999) as novel, therapeutic tools for treating cardiovascular diseases. They reported that it was possible to produce synthetic HDLs on a large scale and to safely administer high doses to humans. Subsequent research showed the effectiveness of these synthetic HDLs in animal models of atherosclerosis, arterial thrombosis, and hemorrhagic and septic shock (Sha et al., 2001; Cockerill et al., 2001; Chiesa et al., 2002). Rossoni et al. (2004) demonstrated the cardioprotective effects of administering synthetic HDLs to isolated rat hearts 10 min prior to ischemia by the rapid and dose-dependent improvement in postischemic cardiac function. The left ventricular developed pressure recovered to 71±3.2 compared to 40.5±3.8 mm Hg for the saline-treated hearts, while cardiac perfusion pressure increased to 100.3±6.2 compared to 132.0±9.0 mm Hg.
LDL cholesterol, because of its detrimental relationship to cardiovascular disease, cannot be considered a nutraceutical. Nevertheless, the oxidized form of this low-density lipoprotein (OxLDLs) appears to function as a specific delivery system for photosensitizers to the scavenger receptors expressed on the macrophages in atherosclerotic lesions, enhancing the benefits of photodynamic therapy (De Vries et al., 1999). Photodynamic therapy, a promising new therapy for cardiovascular pathologies, such as atherosclerosis and retetenosis, involves the specific delivery of a photosensitizer, such as aluminum phthalcyanine chloride (AIPc), to the atherosclerotic plaque, where it is activated by light of a specific wavelength, reducing the narrowing of the artery (Nyamekye et al., 1996).
References
Calabresi, L., Rossoni, G., Gomaraschi, M., Sisto, F., Berti, F., and Franceschini, G., High-density lipoprotein protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-α content and enhancing prostoglandin release, Circ. Res., 92:330–337, 2003.
Chiesa, G., Monteggia, E., Marchesi, M., Lorenzon, P., Laurello, M., Lorusso, V., Di Mario, C., Karvouni, E., Newton, R.S., and Bisgaier, C.L., Franceshini, G., and Sirtoni, C.R., Recombinant apolipoprotein A-1Milano infusion into rabbit carotid artery rapidly removes lipid from fatty streaks, Circ. Res., 90:974–980, 2002.
Cockerill, G.W., McDonald, M.C., Mota-Filipe, H., Cuzzocrea, S., Miller. N.E., and Thiemermann, C., High density lipoproteins reduce organ injury and organ dysfunction in a rat model of hemorrhagic shock, FASEB J., 15:1941–1952, 2001.
De Vries, H.E., Moor, A.C.E., Dubbelman, T.M.A.R., Van Berkel, T.J.C., and Kuiper, J., Oxidized low-density lipoprotein delivery system for photosensitizers: Implications for photodynamic therapy of atherosclerosis, J. Pharmacol. Exp. Then, 289:528–534, 1999.
Gordon, D.J. and Rifkind, B.M., High density lipoprotein: The clinical implications of recent studies, N. Engl. J. Med., 321:1311–1316, 1989.
Nyamekye, I., Buonaccorsi, G., McEwan, J., MacRobert, A., Bown, S., and Bishop, C., Inhibition of intimal hyperplasia in balloon injured arteries with adjunctive phthalocyanine sensitized photodynamic therapy, Eur. J. Endovasc. Surg., 11:19–28, 1996.
Rossoni, G., Gomaraschi, M., Berti, F., Sirtori, C.R., Franceschini, G., and Calabresi, L., Synthetic highdensity lipoprotein exert cardioprotective effects in myocardial ischemia/reperfusion injury, J. Pharmacol. Exp. Ther., 308:79–84, 2004.
Sha, P.K., Yano, J., Reyes, O., Chyu, R.Y., Kaul, S., Bisgaier, C.L., Drake, S., and Cercek, B., High-dose recombinant apolipoprotein A-1Milano mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E-deficient mice: Potential implications for acute plaque stabilization, Circulation, 103:3047–3050, 2001.
Sirtori, C.R., Calabresi, L., and Franceschini, G., Recombinant apolipoproteins for the treatment of vascular diseases, Atherosclerosis, 142:29–40, 1999.
Yancey, P.G., Bortnick, A.E., Kelmer-Weibel, G., Llera-Moya, M., Phillips, M.C., and Rothblat, G.H., Importance of different pathways of cellular cholesterol efflux, Arterioscler. Thromb. Vasc. Biol., 23: 712–719, 2003.
Lobeline
Lobeline, an alkaloid constituent of Indian tobacco (Lobelia inflata), is a nicotine antagonist and may be useful as a smoking cessation agent. A clinical trial by Schneider and Olsson (1996) found that treatment with 7.5 mg lobeline resulted in a sustained abstinence from tobacco over the last four weeks of the study in 10 out of 34 treated subjects compared to 8 out of 47 subjects on the placebo. A multicenter study of sublingual lobeline tablet use and cessation of smoking with 750 subjects by Glover and coworkers (1998) found no statistical differences between lobeline and the placebo. In one of three sites, however, there was significant efficacy so that lobeline as a smokingcessation agent still remains controversial. The ability of lobeline to inhibit amphetamineinduced release of dopamine in vitro and amphetamine-induced hyperactivity suggested to Dwoskin and Crooks (2002) that lobeline and its analogues could act as therapeutic agents for treating methamphetamine abuse. The potential of lobeline as a novel pharmacotherapy for treating psychostimulant abuse was further confirmed by Miller and coworkers (2003), who showed lobeline attenuated locomotor stimulation in rats induced by the repeated nicotine administration.
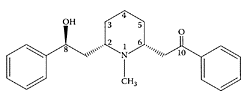
Lobeline. (From Dwoskin and Crooks, Biochem. Pharmacol., 63:89– 98, 2002. With permission.)
References
Dwoskin, L.P. and Crooks, P.A., A novel mechanism of action and potential use of lobeline as a treatment for psychostimulant abuse, Biochem. Pharmacol., 63:89–98, 2002.
Glover, E.D., Leischow, S.J., Rennard, S.I., Glover, P.N., Daughton, D., Quirin, J.N., Schneider, F.H., and Mione, P.J., A smoking cessation trial with lobeline sulfate: A pilot study, Am. J. Health Behav., 22:62–74, 1998.
Miller, D.K., Harrod, S.B., Green, T.A., Wong, M.Y., Bardo, M.T., and Dwoskin, L.P., Lobeline attenuates locomotor stimulation induced by repeated nicotine administration in rats, Pharmacol. Biochem. Behav., 74:279–286, 2003.
Schneider, F.H. and Olsson, T.A., Clinical-experience with lobeline as a smoking cessation agent, Med. Chem. Res., 6:562–570, 1996.
Lovage (Levisticum officinale Koch.)
Lovage, a member of the Apiaceae family, is an aromatic and perennial medicinal herb grown extensively in Europe. The name is derived from its reputation as a love charm or aphrodisiac (Stuart, 1989). The essential oil from its leaves, seeds, and roots is used in food, beverages, and perfumery (Cu et al., 1990). Of 191 compounds identified in the oil, Toulemonde and Noleau (1988) showed that β-phellandrene accounted for 63 percent of the seed oil, while n-butylidene-4,5-dihydrophthalide was the major constituent in root oil (67 percent). Bylaite and coworkers (1998) showed that lovage seeds and flowers were the richest sources of oil with α-terpinyl acetate, the major constituent in the leaves and stems (up to 70 percent), while β-phellandrene accounted for 61.5 percent and 40.8 percent of the seed and flower oils, respectively. They also identified Z-ligustilide as a major phthalide in lovage leaves and stem oils, ranging from 4.4 percent-11.7 percent and 4.8–13.8 percent, respectively, depending on the harvesting time. Later work by Bylaite and coworkers (2000), using dynamic headspacegas chromatography and olfactometry analysis, showed that while β-phellandrene was the dominant constituent in lovage oil, its impact on aroma was not the most significant.
The medicinal properties of lovage can be traced back to the Benedictine monks who recommended chewing the seed to aid digestion and relieve flatulence (Stuart, 1989). Lovage roots were also known for centuries to possess carminative and spasmolytic activity (Segebrecht and Schilcher, 1989). Its use as a folk medicine in Europe is related to its calming effect on the stomach, as well as in the treatment of congestion, rheumatism, and migraine headaches. It was approved in Germany for inflamed urinary tract and preventing kidney stones (Hogg et al, 2001). Excessive dosages of lovage should be avoided by pregnant women, as it promotes the onset of menstruation, while its irritant action can cause kidney damage (Maybe et al., 1988; Stuart, 1989)
References
Bylaite, E., Roozen, J.P., Legger, A., Venskutonis, R.P., and Posthumus, M.A., Dynamic headspace-gas chromatography-olfactometry analysis of different anatomical parts of lovage (Levisticum officinale Koch.) at eight growing stages, J. Agric. Food Chem., 48:6183–6190, 2000.
Bylaite, E., Venskutonis, R.P., and Roozen, J.P., Influence of harvesting time on the composition of volatile components in different anatomical parts of lovage (Levisticum officinale Koch.), J. Agric. Food Chem., 46:3735–3740, 1998.
Cu, J.-Q., Pu, F., Shi, Y., Perineu, F., Delmas, M., and Gaset, A., The chemical composition of lovage headspace and essential oil produced by solvent extraction with various solvents, J. Essent. Oil Res., 2:53–59, 1990.
Hogg, C.L., Svoboda, K.P., Hampson, J.B., and Brocklehurst, S., Investigation into the composition and bioactivity of essential oil from lovage (Levisticum officinale W.D.J. Koch), Inter. J. Aromather., 11: 144–151, 2001.
Matbe, R., McIntyre, M., Michael, P., Duff, G., and Stevens, J., The Complete New Herbal, Hamilton, London, 1988.
Segebrecht, S. and Schilcher, H., Ligustilide: Guiding component for preparation of Levisticum officinale roots, Planta Med., 55:572–573, 1989.
Stuart, M., The Encyclopedia of Herbs and Herbalism, Macdonald and Co. Ltd., 1989
Toulemonde, B. and Noleau, I., Volatile constituents of lovage (Levisticum officinale Koch.), in Flavors and Fragrances, A World Perspective, Lawrence, B.M., Mookherjee, B.D., and Willis, B.J., Eds., Elsevier Science Publishers, Netherlands, 1988, pp. 641–657.
Low-density lipoprotein
see Lipoproteins
Lunasin
Lunasin, a soybean peptide consisting of 43 amino acids, contains at the carboxyl end none Asp(D) residues, an Arg-Gly-Arg (RGD) cell adhesion motif, and a predicted helix with structural homology to a conserved region of chromatin-binding proteins. Galvez and de Lumen (1999) reported that transfection of mammalian cells with the lunasin gene arrested mitosis, resulting in cell death. Galvez et al. (2001) further confirmed the chemopreventive properties of soybean lunasin by its ability to induce apoptosis in the SENCAR mouse-skin cancer model. Application of lunasin (250 μg/week) reduced skin-tumor incidence/mouse by 70 percent, as well as delayed the appearance of tumors by two weeks compared to the control. The antitumor activity of lunasin action resulted from its ability to prevent histone acetylation by binding preferentially to deacylated histone H4 in vitro. Jeong and coworkers (2003) showed the feasibility of large-scale production of soybean lunasin capable of suppressing the formation of mammalian cells by an oncongene.
Using Western blot analysis, Jeong et al. (2002) isolated and purified a lunasin peptide from barley. Different barley lunasin fractions were shown to inhibit colony formation in isopropyl-β-D-thiogalactoside (IPTG)-induced, ras-stably infected mouse-fibroblast cells as effectively as a chemically synthesized lunasin at a concentration of 10 μM (Figure L.64). These fractions also inhibited histone acetylation, attributed previously to the antitumor properties of soybean lunasin. Identification of lunasin in barley suggests it could be present in other plant seeds.
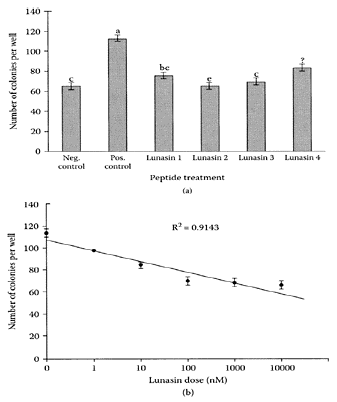
FIGURE L.64 Purified lunasin inhibits colony formation in IPTG-induced ras stably transformed 2–12 cells, (a) At a concentration of 10 μM, barley lunasin purified using different methods was as effective in inhibiting colony formation as synthetic lunasin. Negative control was not treated with IPTG, while positive control was treated with IPTG without lunasin. Lunasin 1 is the crude extract of barley lunasin; lunasin 2 is lunasin 1 purified by ion-exchange chromatography by elution at 0.7 M NaCl and not dialyzed before bioassay; lunasin 3 is lunasin 2 purified by immunoaffinity chromatography; and lunasin 4 is synthetic lunasin. Treatment means (± standard errors) with similar letters are not significantly different from each other, as analyzed by a OneWay ANOVA followed by Duncan’s Multiple Range Test, (b) Dose response of immuno-purified barley lunasin fraction in suppression of colony formation. Each lunasin dose represents the means (± standard error) of triplicate experiments. (Jeong et al., J. Agric. Food Chem., 50:5903–5908, 2002. With permission)
References
Galvez, A.F., Chen, N., Macbieh, J., and de Lumen, B.O., Chemopreventive property of a soybean peptide (lunasin) that binds to deacylated histones and inhibits acetylation, Cancer Res., 61:7473–7478, 2001.
Galvez, A.F. and de Lumen, B.O., A soybean cDNA encoding a chromatin-binding peptide inhibits mitosis of mammalian cells, Nat. Biotech., 17:495–500, 1999.
Jeong, H.J., Lam, Y., and de Lumen, B.O., Barley lunasin suppresses ras-induced colony formation and inhibits core histone acetylation in mammalian cells, J. Agric. Food Chem., 50:5903–5908, 2002.
Jeong, H.J., Park, J.H., Lam, Y., and de Lumen, B.O., Characterization of lunasin isolated from soybean, J. Agric. Food Chem., 51:7901–7906, 2003.
Lutein
Lutein is a 40-carbon hydroxylated carotenoid or xanthophyll yellow pigment in dark-green vegetables, as well as in egg yolk, corn, orange juice, melon, and orange peppers masked by chlorophyll (Pfander, 1992; Sommerburg et al., 1998). It is found, together with zeaxanthin, in large concentrations in the area of the human retina involved in central vision, known as macula lutea.
Both of these pigments are implicated in the pathogenesis of macular degeneration, a condition leading to loss of vision in the elderly (Seddon et al., 1994).
Epidemiological evidence pointed to an association between macular degeneration and carotenoid intake. The concentration of lutein and zeaxanthin were significantly lower in eyes suffering from macular degeneration compared to healthy controls (Beatty et al., 2001). Researchers at the University of Pennsylvania showed that supplementation of lutein by patients suffering from choroideremia, a genetically linked retinal disease, significantly increased the macular optical density (Duncan et al., 2002). A 50 percent increase in macular pigment optical density was also reported by Richer and coworkers (2002), which further established the role of lutein in improving visual function in patients suffering from age-related macular degeneration. Semba and Dagnelie (2003) concluded that lutein and zeaxan thin acted as antioxidants reducing photo-oxidative stress in the retina by deactivating highly reactive singlet oxygen 1O2. A two-year, double-blind, placebo-controlled study by Olmedilla and coworkers (2003) showed that only supplementation with lutein improved visual acuity of patients with age-related cataracts compared to α-tocopherol and the placebo (Figure L.65). These results suggested that long-term supplementation with lutein could be beneficial to individuals suffering from agerelated cataracts.
References
Alves, Rodrigues, A., and Shao, A., The science behind lutein, Toxicol. Lett., 150:57–83, 2004.
Beatty, S., Murray, I.J., Henson, D.B., Garden, D., Koh, H.H., and Boulton, M.E., Macular pigment and risk for age-related macular degeneration in subjects from a northern European population, Invest. Ophthamol. Vis. Sci., 40:439–446, 2001.
Duncan, J.L., Aleman, T.S., Gardner, L.M., De Castro, E., Marks, Bennett, J.D.A., Emmons, J.M., Bieber, M.L., Steinberg, J.D., Stone, E.M., MacDonald, I.M., Cideciyan, A.V., Maguire, M.G., and Jacobson, S.G., Macular pigment and lutein supplementation in choroideremia, Exp. Eye Res., 74:371–381, 2002.
Olmedilla, B., Pharm, D., Granado, F., Blanco, I., and Vaquero, M., Lutein, but not α-tocopherol, supplementation improves visual function in patients with age-related cataracts: A 2 year double-blind, placebo-controlled pilot study, Nutrition, 19:21–24, 2003.
Pfander, H., Carotenoids: An overview, Methods Enzymol., 213:3–13, 1992.
Richer, S., Stiles, W., Statkute, L., Pei, K.Y., Frankowski, J., Nyland, J., Pulido, J., and Rudy, Y., The lutein antioxidant trial (LAST)(Abstract), ARVO, B539, 2002.
Seddon, J., Ajani, U.A., Sperduto, R.D., Hiller, R., Blair, N., Burton, T.C., Farber, M.D., Grogouda, E.S., Haller, J., and Miller, D.T., Dietary carotenoids, vitamins A, C, and E and advanced age-related macular degeneration, Eye disease case-control study group, J. Am. Med. Assoc., 272:1413–1420, 1994.
Semba, R.D. and Dagnelie, G., Are lutein and zeaxanthin conditionally essential nutrients for eye health? Med. Hypoth., 61:465–472, 2003.
Sommerburg, O., Kuenen, J.E.E., Bird, A.C., and van Kiujik, F.J.G.M., Fruits and vegetables that are sources of lutein and zeaxanthin: Macular pigments in human eyes, Br. J. Ophthamol., 82:907–910, 1998.
Lutein. (Adapted from Alves-Rodrigues and Shao, Toxicol. Lett., 150:57–83, 2004.)
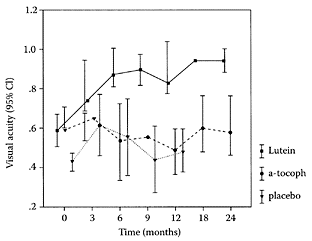
FIGURE L.65 Changes in visual acuity of patients with cataracts during supplementation study (eyes were assessed individually). Lutein group (n=9), α-tocopherol (n=10), and placebo group (n=7), CI, confidence interval. (Olmedilla et al., Nutrition, 19:21–24, 2003. With permission.)
Luteolin
Luteolin, a 3′, 4′, 5,7-tetrahydroxyflavone, is found in the glycosylated form in celery, green pepper, perilla leaf, and chamomile tea (Shimoi et al., 1998). It has been shown to exhibit antimutagenic, antitumorigenic, antioxidant, and anti-inflammatory properties (Samejima et al., 1995; Kim et al., 1999; Casagrande and Darbon, 2001; Xagorari et al., 2001). Casagrande and Darbon (2003) found luteolin was a potent inhibitor of lipopolysaccharide (LPS)-stimulated nuclear factor-kappa B (NF-κB) transcriptional activity in Rat-1 fibroplasts by modulating the transcription complex assembly in the fibroplasts. Miagkov et al. (1998) showed NF-κB played a critical role in chronic inflammation.
Luteolin. (From Li et al., J. Pharm. Biomed. Anal., 37:615–620, 2005. With permission.)
References
Casagrande, F. and Darbon, J-M., Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclindependent kinases CDK2 and CDK1, Biochem. Pharmacol., 61:1205–1215, 2001.
Kim, H.K., Cheon, B.S., Kim, Y.-H., Kim, S.Y., and Kim, H.P., Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships, Biochem. Pharmacol., 58:759–765, 1999.
Kim, S.-H., Shin, K.-J., Kim, Y.-H., Han, M.S., Lee, T.G., Kim, E., Ryu, S.H., and Suh, P.-G., Luteolin inhibits the nuclear factor κB transcriptional activity of rat-1 fibroblasts, Biochem. Pharmacol., 66:955–963, 2003.
Li, L., Jiang, H., Wu, H., and Zeng, S., Simultaneous determination of luteolin and apigenin in dog plasma by RP-HPLC, J. Pharm. Biomed. Anal., 37:615–620, 2005.
Miagkov, A.V., Kovalenko, D.V., Brown, C.E., Didsbury, J.R., Cogswell, J.P., Stimpson, S.A., Baldwin, A.S., and Makarov, S.S., NF-κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint, Proc. Natl. Acad. Sci., 95:13859–13864, 1998.
Samejima. K., Kanazawa, K., Ashida, H., and Danno, G., Luteolin: A strong antimutagen against dietary carcinogen, Tr-P-2, in peppermint, sage, and thyme, J. Agric. Food Chem., 43:410–414, 1995.
Shimoi, K., Okada, H., Furugori, M., Goda, T., Takase, S., Suzuki, M., Hara, Y., and Kinae, N., Intestinal absorption of luteolin and luteolin 7-O-betaglucoside in rats and humans, FEBS Lett., 438:220–224, 1998.
Xagorari, A., Papapetropolous, A., Mauromatis, A., Economou, M., Fotsis, T., and Roussos, C., Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages, J. Pharmacol. Exp. Ther., 296:181–187, 2001.
Lycopene
Lycopene is an acyclic isomer of the carotenoid β-carotene without any vitamin A activity (Stahl and Sies, 1996). It is found in abundance in fresh, ripe tomatoes and to a lesser extent in watermelon, papaya, guava, and grapefruit. Structurally, lycopene is a highly unsaturated, straight-chain hydrocarbon in which 11 of its 13 double bonds are conjugated (Argawal and Rao, 2000). It occurs predominantly in the trans isomer but undergoes isomerization to the cis isomer during heat processing. In fact, the increased levels of the cis isomer accounts for the much greater absorption of lycopene in processed tomato products (Stahl and Sies, 1992). Lycopene is a potent antioxidant with singlet oxygen-quenching ability twice that of β-carotene and 10 times that of α-tocopherol and is also able to inactivate hydrogen peroxide and nitrogen dioxide (Bohm et al, 2001; Heber and Lu, 2002).
Epidemiological studies showed that diets supplemented with lycopene reduced the risk of many chronic diseases, such as cancer and heart disease (Edward, 1999). The benefits associated with lycopene are related to its potent antioxidant properties through its ability to scavenge free radicals (Mortensen et al., 1997). Porrini and Riso (2000) showed that a daily consumption of 25 grams of tomato paste by healthy young women significantly increased plasma and lymphocyte lycopene concentrations after 14 days. Exposure of collected blood lymphocyte samples to free radicals using the Comet Test showed a significant reduction of 50 percent DNA damage compared to the control samples. A further study by Chen and associates (2001) showed that dietary lycopene fed to men with prostate cancer signifi-cantly reduced hydroxylated guanosine (8-OHdG), a by-product and useful biomarker of oxidative DNA damage in cell nuclei, by 21.3 percent. This indicated that lycopene exerted a protective effect on white blood cells by reducing oxidative damage in cancerous prostate tissue. Bowen and coworkers (2002) showed that consumption of tomato-sauce dishes containing 30 mg of lycopene per day for three weeks by 22 patients with localized prostate adenocarcinoma significantly reduced serum-prostate-specific antigen (PSA) levels and DNA oxidation. A recent examination of patients undergoing colonoscopy for colorectal adenomas by Erhardt et al. (2003) showed plasma lycopene concentrations were inversely related to adenoma risk, further supporting the protective role of lycopene against colorectal cancer.
Nara and coworkers (2001) reported that only when HL-60 human promyelocytic leukemia cells were exposed to an autoxidized mixture of lycopene (6 μM) for five days did they undergo apoptosis. Zhang et al. (2003) subsequently identified an oxidized cleavage product of lycopene, (E,E,E)-4-methyl-8-oxo-2,4,6-nonatrienal (MON), which they found induced DNA fragmentation and apoptosis in a time-and dose-dependent manner. Cell viability was reduced to 71.6 percent of the control in the presence of 10 μM MON (Figure L.66).
(E,E,E)-4-methyl-8-oxo-2,4,6-nonatrienal (MON). (From Zhang et al., Free Rad. Biol. Med., 35:1653–1663, 2003. With permission.)
Lycopene. (Adapted from Alves-Rodrigues, A. and Shao, A., Toxicol. Lett., 150:57–83, 2004.)
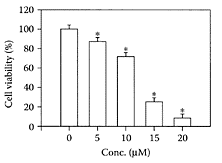
FIGURE L.66 Effect of (E,E,E)-4-methyl-8-oxo2,4,6-nonatrienal on the viability of HL-60 cells. The cell viability was evaluated by the MTT method and is expressed as the percentage of the value of the control culture treated with the vehicle (THF) alone. Values represent means ± SD of eight wells. The asterisk indicates a value significantly different from the vehicle value (p<0.01). Statistical comparisons were made by the Scheff’s F-test. (Zhang et al., Free Rad. Biol. Med., 35:1653–1663, 2003.)
Lycopene can also prevent cardiovascular disease. Supplementing the diet of six healthy human subjects with 60 mg/day of lycopene for three months significantly reduced their plasma LDL cholesterol levels by 14 percent (Furhman et al., 1997). Treatment of hypertensive patients with 15 mg per day of lycopene in the form of a capsule was found by Paran and Engelhard (2001) to reduce systolic blood pressure by almost 100 mm Hg over an eight-week period. Mohanty et al. (2002) also found that lycopene protected the human lens from oxidative damage and had potential as an anticataract agent.
Lycopene’s potent antioxidant property could provide a treatment for neurodegenerative diseases. A recent study by Suganuma and coworkers (2003) found dietary lycopene attenuated the age-related learning in senescenceaccelerated mice (SAMPS) by ameliorating the memory deficits in these animals. A recent review of lycopene and human health by Rao and Rao (2003) is recommended.
References
Alves, Rodrigues, A. and Shao, A., The science behind lutein, Toxicol. Lett., 150:57–83, 2004.
Argawal, S. and Rao, A.V., Carotenoids and chronic diseases, Drug Metab. Drug Interact., 17:189–210, 2000.
Bohm, F., Edge, R., Burke, M., and Truscott, T.G., Dietary uptake of lycopene protects human cells from singlet oxygen and nitrogen dioxide-ROS components from cigarette smoke, J. Photochem. Photobiol. B. Biol., 64:176–178, 2001.
Bowen, P., Chen, L., Stacewicz-Sapuntzakis, M., Duncan, C., Sharifi, R., Ghosh, L., Kim, H.S., Christov-Tzelkov, K., and van Breemen, R., Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis, Exp. Biol. Med., 227:886–893, 2002.
Chen, L., Stacewicz-Sapuntzakis, M., Duncan, C., Sharafi, R., Ghosh, L., Van Breeman, R., Ashton, D., and Bowen, P.E., Oxidative damage in prostate cancer patients consuming tomato sauce-based entrees as a whole food intervention, J. Natl. Cancer Inst., 93:1872–1879, 2001.
Erhardt, J.G., Meisner, C., Bode, J.C., and Bode, C., Lycopene, β-carotene and colorectal adenomas, Am. J. Clin. Nutr., 78:1219–1224, 2003.
Furhman, B., Elis, A., and Aviram, M., Hypocholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophage, Biochem. Biophys. Res. Commun., 233:58–662, 1997.
Edward, G., Tomato, tomato-based products, lycopene, and cancer: Review of epidemiological literature, J. Natl. Cancer Inst., 91:317–331, 1999.
Giovannucci, E., Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature, J. Natl. Cancer Inst., 91:317–331, 1999.
Giovannucci, E., Ascherio, A., Rimm, E.B., Stampfer, M.J., Colditz, G.A., and Willett, W.C., Intake of carotenoids and retinol in relation to risk of prostate cancer, J. Natl. Cancer Inst., 87:1767–1776, 1995.
Heber, D. and Lu, Q.-Y., Overview of mechanisms of action of lycopene, Exp. Biol. Med., 227:920–923, 2002.
Hurham, B., Elis, A., and Aviram, M., Hypocholesterolemic effect of lycopene and β-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophage, Biochem. Biophys. Res. Commun., 233:658–662, 1997.
Mohanty, I., Joshi, S., Trivedi, D., Srivastava, S., and Gupta, S.K., Lycopene prevents sugarinduced morphological changes and modulates antioxidant status of human lens epithelial cells, Br. J. Nutr., 88:347–354, 2002.
Mortensen, A., Skibsted, L.H., Sampson, J., RiceEvans, C., and Everett, S.A., Comparative mechanisms and rates of free scavenging by carotenoid antioxidants, FEBS Lett., 418(1–2):91– 97, 1997.
Nara, E., Hayashi, H., Kotake, M., Miyashita, K., and Nagao, A., Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leucemia cells, Nutr. Cancer, 39:273–283, 2001.
Paran, E. and Engelhard, Y., Effect of Lyc-O-Mato, standardized tomato extract on blood pressure, serum lipoproteins, plasma homocysteine and oxidative stress markers in grade 1 hypertensive patients, Proceedings of the 16th Annual Scientific Meeting of the Society of Hypertension, San Francisco, 2001.
Porrini, M. and Riso, P., Lymphocyte lycopene concentration and DNA protection from oxidative damage is increased in women after a short period of tomato consumption, J. Nutr., 130:189– 192, 2000.
Rao, A.V. and Rao, L.G., Lycopene and human health, Nutr. Genom. Funct. Foods, 1:35–44, 2003.
Stahl, W. and Sies, H., Uptake of lycopene and its geometrical isomers is greater from heatprocessed than from unprocessed tomato juice in humans, J. Nutr., 122:2161–2166, 1992.
Stahl, W. and Sies, H., Lycopene: A biologically important carotenoid in humans? Arch. Biochem. Biophys., 336:1–9, 1996.
Suganuma, H., Hiran, T., Kaburagi, S., Hayakawa, K., and Inakuma, T., Ameliorative effects of dietary carotenoids on memory deficits in senescence-accelerated mice (SAMPS), Int. Congress Series, 1260: 129–135, 2003.
Zhang, H., Kotake-Nara, E., Ono, H., and Nagao, A., A novel cleavage product formed by autoxidation of lycopene induces apoptosis in HL-60 cells, Free Rad. Biol. Med., 35:1653– 1663, 2003.
Lysine
The essential amino acid, L-lysine, has been used for many years as its monohydrochloride salt (LMH) to improve the diets of many Third World countries (Flodin, 1993). Research in the 1970s suggested that oral supplements of lysine monohydrochloride suppressed recurrent herpes simplex infections (Griffith et al., 1978). As a result, pharmaceutical-grade LMH supplements are readily available in 500-mg tablets. An excellent review of the pharmacology and toxicology of lysine was published by Flodin (1997). Sarubin (2003) suggested that daily supplements of up to 3 grams of lysine appear to be safe. However, Marcason (2003) cautioned that diets high in lysine or with high lysine:arginine ratios were found to be hypercholesterolemic in some animal studies.
Poly-L-lysine has also been shown to inhibit the herpes simplex virus type 1 (HSV) by possibly preventing its adsorption (Langeland et al., 1988; WuDunn and Spear, 1989). Egal and coworkers (1999) found that magainins, a class of cationic peptides rich in lysine and octanoyl groups originally isolated from the skin of the African clawed frog (Xeenopus laevis), had a direct antiviral effect on HSV.
References
Egal, M., Conrad, M., MacDonald, D.L., Maloy, W.L., Motley, M., and Genco, C.A., Antiviral effects of synthetic membrane-active peptides on herpes simplex virus type 1, Int. J. Antimicrob. Agents, 13: 57–60, 1999.
Flodin, N.W., Lysine supplementation of cereal foods: A retrospective, J. Am. Coll. Nutr., 12:486– 500, 1993.
Flodin, N.W., The metabolic roles, pharmacology, and toxicology of lysine, J. Am. Coll. Nutr., 16:7–21, 1997.
Griffith, R.S., Norins, A.L., and Kagan, C., A multicentered study of lysine therapy in herpes simplex infection, Dermatologica, 156:257–267, 1978.
Langeland, N., Moore, L.J., Holmsen, H., and Haar, L., Interaction of poly lysine with the cellular receptor for herpes simplex virus type 1, J. Gen. Virol., 69:1137–1145, 1988.
Marcason, W., Will taking the amino acid supplement lysine prevent or treat herpes simplex virus? J. Am. Diet. Assoc., 103:351, 2003.
Sarubin, F., Lysine, The Health Professional’s Guide to Popular Dietary Supplements, 2nd ed., American Dietetic Association, Chicago, 2003, pp. 269–273.
WuDunn, D. and Spear, P.G., Initial interaction of herpes simplex virus with cells is binding to heparan sulfate, J. Virol., 63:52–58, 1989.