K
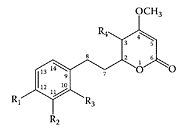
Kaempferol
Kaempferol (3,4′,5,7-tertahydroxyflavone) is a flavonol found in abundance in fruits, vegetables, and tea (Cao et al., 1997; Hertog et al., 1992). It has been shown to have anti-inflammatory and antioxidant properties in macrophages and neurons. For example, kaempferol protected rat-cortical neurons from amyloid β protein toxicity by minimizing the production of reactive-oxygen species and inhibiting caspase activity (Wang et al., 2001). It was shown previously to reduce prostaglandin E2 and nitrite production in mouse macrophages by suppressing inducible cyclooxygenase-2 and inducible nitric-oxide synthetase (Liang et al., 1999). Okamoto et al. (2002) examined the immunoregulating properties of kaempferol and found it useful for treating cellmediated immune diseases, such as acute graftversus-host disease (GVHD). In vitro studies with mice-spleen cells showed kaempferol acted directly on T cells by inhibiting Th1 cytokine production and suppressing expansion or generation of CD8+ CTLs. Subsequent treatment of C57BL/6-into-BDF 1 mice with kaempferol reduced GVHD-associated antihost CTL activity by activating Th2 cells and engraftment of the donor cells. The overall result was early recovery of body weight loss, increased survival, and reduced injury to the liver and large intestine.

Kaempferol. (From Tian et al., J. Mol. Struct., 691:197–202, 2004. With permission.)
Recent data suggest tea and vegetable consumption, such as onions, can provide protection against osteoporosis in older women (Hegarty et al., 2000; Muhlbauer and Li, 1999; New et al., 2000; Muhlbauer et al., 2002). This was attributed to the presence of flavonols, such as quercetin and kaempferol as rutin, a glycoside of quercetin. Rutin was shown previously by Horcajada-Molteni and coworkers (2000) to inhibit ovariectomy-induced osteopenia in rats. Using osteoclasts from 10-day-old rabbits, Wattel et al. (2003) showed kaempferol and quercetin both reduced bone resorption in a timeand dose-dependent manner (Figure K.57). Both flavonols induced apoptosis of mature osteoclasts in the same dose range that effectively inhibited bone resorption. Treatment of highly purified rabbit osteoclasts with 50 μM quercertin and kaempferol significantly reduced intracellular levels of reactive-oxygen species by 75 percent and 25 percent, respectively. Below this concentration, neither of these flavonols exhibited any antiradical activity, so that their antioxidant activity could not explain their inhibitory effect on bone resorption. However, they found that only kaempferol’s inhibition of bone resorption was partially reduced by addition of a pure antiestrogen. This suggested that inhibition of bone resorption by kaempferol could be partly explained by its estrogenic effect. This study demonstrated the importance of dietary sources of flavonols, such as kaempferol, as inhibitors of osteoporosis.
References
Cao, G., Sofic, E., and Prior, L.P., Antioxidant and prooxidant behaviour of flavonoids: Structureactivity relationships, Free Rad. Biol. Med., 22:749–760, 1997.
Hegarty, V.M., May, H.M., and Khaw, K.T., Tea drinking and bone mineral density in older women, Am. J. Clin. Nutr., 71:1003–1007, 2000.
Hertog, M.G.L., Hollman, P.C.H., and Katan, M.B.J., Content of potentially anticarcinogenic flavonoids in 28 vegetables and 9 fruits commonly consumed in the Netherlands, J. Agric. Food Chem., 40:2379–2383, 1992.
Horcajada-Molteni, M.N., Crespy, V., Coxam, V., Davicco, M.J., Remesy, C., and Bartlet, J.P., Rutin inhibits ovariectomy-induced osteopenia in rats, J. Bone Miner. Res., 15:2251–2258, 2000.
Liang, Y.-C., Huang, Y.-T., Tsai, S.-H., Lin-Shiau, Y.-J., Chen, C-F., and Lin, J.K., Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages, Carcinogenesis, 20:1945–1952, 1999.
Muhlbauer, R.C. and Li, F., Effect of vegetables on bone metabolism, Nature, 401:343–344, 1999.
Muhlbauer, R.C., Lozano, A., and Reinli, A., Onion and a mixture of vegetables, salads, and herbs affect bone resorption in the rat by a mechanism independent of their base excess, J. Bone Miner. Res., 17: 1230–1236, 2002.
New, S.A., Robins, S.P., Campbell, M.K., Martin, J.C., Garton, M.J., Bolton-Smith, C., Grubb, D.A., Lee, S.J., and Reid, D.M., Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am. J. Clin. Nutr., 71:142–151, 2000.
Okamoto, I., Iwaki, K., Koya-Miyata, S., Tanimoto, T., Kono, K., Ikeda, M., and Kurimoto, M., The flavonoid kaempferol suppresses the graft-versus-host reaction by inhibiting type 1 cytokine production and CD8+ T cell engraftment, Clin. Immunol., 103:132–144, 2002.
Tian, J., Liu, J., Tian, X., Hu, Z., and Chen, X., Study of the interaction of kaempferol with bovine serum albumin, J. Mol. Struct., 691:197–202, 2004.
Wang, C.-N., Chi, C.-W., Lin, Y.-L., Chen, C.-F., and Shiao, Y.-J., The neuroprotective effects of phytoestrogens on amyloid β-protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons, J. Biol. Chem., 276: 5287–5295, 2001.
Wattel, A., Kamel, S., Mentaverri, R., Lorget, F., Prouillet, C., Petit, J.-P., Fardelonne, P., and Brazier, M., Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption, Biochem. Pharmacol., 65:35–42, 2003.
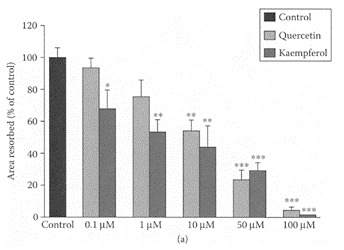
FIGURE K.57 Effect of different concentrations of quercetin and kaempferol on osteoclastic bone resorption. Osteoclasts were cultured on cortical bovine slices during 48 h in media containing either vehicle (0.1 percent DMSO—control) or flavonols, quercetin, and kaempferol (0.1–100 μM), and bone resorption assessed by measurement of total area of resorption pits. Results expressed as percent of control. Values are mean ± SEM of three independent experiments (N=5 for pit-area measurement); (*) p<0.05, (**) p<0.01, and (***) p< 0.001 compared with control group. (Wattel et al., Biochem. Pharmacol., 65:35–42, 2003. With permission.)
Kava-kava
Kava-kava (Piper methysticum), a plant native to the Pacific Islands, has been used for its soporific and narcotic effects (Bilia et al., 2002). Extracts were traditionally prepared from its macerated roots by mixing with water and coconut milk (Norton and Ruze, 1994). The active ingredients in kava-kava, known for their analgesic and anesthetic properties, are a group of lipophillic lactone derivatives with an arylethylene-α-pyrone skeleton. The major lactones are (+) kavain, (+)-methysticin, desmethoxyyangonin, yangonin, (+)-dihydrokavain, (+)-dihydromethysticin, and tetrahydroyangonin. Minor components include chalcones and essential oil. The kavalactone structures are shown in Scheme K.31.
In vitro studies showed isolated kavalactones directly affected the central nervous system and neurotransmitters by interacting with GABA-benzodiazepine receptors and by inhibiting noradrenaline uptake (Davies et al., 1992; Jussofie et al., 1994). Inhibition of noradrenaline uptake by kavalactones may explain some of their psychotropic properties (Seitz et al., 1997). The ability of kava-enriched extracts to inhibit human platelet MAO-B may also be an important mechanism for their psychotropic properties (Uebelhack et al., 1998).
Extensive clinical studies, using a number of rating scales (Hamilton Anxiety Scale and Clinical Global Impressions Scale), all showed the efficacy of kavalactones as an anxiolytic drug. For example, Lehmann et al. (1996) demonstrated the effectiveness of kava-kava extract for treating anxiety disorders, while Voltz and Kieser (1997) showed the same extract significantly improved patients suffering from anxiety of nonpsychotic origin. Overall, studies showed good tolerance and low incidence of adverse effects associated with kava-kava treatment, including a systematic review and meta-analysis (Pittler and Ernst, 2000). Cagnacci and coworkers (2003) recently found an improvement in the mood of perimenopausal women, particular in anxiety, following administration of kava-kava. Lehrl (2004) reported that sleep disturbances associated with nonpsychotic disorders were effectively and safely treated with a kava extract WS®1490.
A number of adverse cases, however, were reported in Germany, where kava-kava was associated with dopamine antagonism (Schelosky et al., 1995), while seven cases of hepatitis were attributed directly to kava-kava intake (Strahl et al., 1998; Escher et al., 2001; Russmann et al., 2001). Stickel and coworkers (2003) pointed to the potential hepatoxicity of kava in Germany, which led to hepatic necrosis or cholestatic hepatitis in patients given alcoholic and acetonic kava extracts. High doses of kava lactones were recently shown by Gow et al. (2003) to have serious hepatoxic side effects. Such lactones are normally metabolized by the cytochrome P450 system in the liver (Schmidt et al., 1999) and by lactone hydrolases in the serum (Bargota et al., 2003). A recent study by Whitton et al. (2003) attributed the toxicity of kava-kava lactones to the particular extraction method, as they were 30 times higher in the standardized preparations compared to the traditional aqueous-extraction method. The various solvents used to extract kava lactones resulted in markedly different yields in the dried extract, ranging from 100 percent for the standardized methods involving acetone or 96 percent alcohol to 15 percent and 2.97 percent using the more traditional extractants of 25 percent ethanol or water, respectively (Table K.40). The higher levels in the more standardized extracts would saturate the detoxification pathways leading to hepato side effects. Since glutathione plays a crucial role in the phase II conversion of lactones to excretable waste products, its depletion could explain the increased side effects observed for kavalactones. Schmidt and coworkers (2001) reported that sesquiterpene lactones bind to glutathione, allowing faster clearance by lactone hydrolases in the hepatocytes. Whitton and coworkers (2003) showed that supplementation with glutathione rendered the kava lactone nontoxic to eukaryotic cells by a similar mechanism in which the lactone ring was opened up via the Michael reaction (Scheme K.32), bypassing the cytochrome P50 pathway. The adverse hepatotoxic effects were found with the tablets and capsules made using standardized kava extracts in which glutathione was either absent or present at very low levels. Traditional preparations, on the other hand, contained high levels of glutathione, which probably explains their safe use for many years. To avoid these adverse effects, supplementation with glutathione appears to be essential. The recent banning of kava in the U.K. and Europe is presently under review.
TABLE K.40
Extraction of Kava Lactones from Roots of P. Methysticum with Different Solvents1

SCHEME K.32 The Michael reaction between kawain and glutathione. (From Whitton et al., Phytochemistry, 64:673–679, 2003. With permission.)
References
Bargota, R.S., Akhtar, M., Biggadike, K., Gani, D., and Allemann, R.K., Structure-activity relationship on human serum paraxonona (PONI) using substrate analogues and inhibitors, Bioorg. Med. Chem. Lett., 13:1623–1626, 2003.
Bilia, A.R., Gallori, S., and Vincieri, F.F., Kava-kava and anxiety: Growing knowledge about the efficacy and safety, Life Sci., 70:2581–2597, 2002.
Bilia, A.R., Scalise, L., Bergonzi, M.C., and Vincieri, F.F., Analysis of kava lactones from Piper methyticum (Kava-Kava), J. Chromatogr., B., 812:203–214, 2004.
Cagnacci, A., Arangino, S., Renzi, A., Zanni, A.L., Malmusi, S., and Volpe, A., Kava-kava administration reduces anxiety in perimenopausal women, Maturitas, 44:103–109, 2003.
Davies, L., Drew, C.A., Duffield, P., Johnston, G.A.P., and Jamieson, D.D., Kava pyrones and resin studies on GABAA, GABAB and benzodiazepine binding sites in rodent brain, Pharmacol. Toxicol., 71:120–126, 1992.
Escher, M., Desmeules, J., Giostra, E., and Mentha, G., Drug points: Hepatitis associated with kava, a herbal remedy for anxiety, Br. Med. J., 322:139, 2001.
Gow, P.J., Connelly, N.J., Hill, R.L., Crowley, P., and Augus, P.W., Fatal fulminant hepatic failure induced by a natural therapy containing kava, Med. J. Australia, 178:442–443, 2003.
Jussofie, B., Schmiz, A., and Hiemke, C., Kavapyrone enriched extract from Piper methysticum as a modulator of GABA binding site in different regions of the rat brain, Psychopharmacol., 116:469–474, 1994.
Lehmann, E., Kinzler, E., and Friedemann, J., Efficacy of a special Kava extract in patients with studies of anxiety, tension and restlessness of nonmental origin. A double-blind placebocontrolled study of four weeks treatment, Phytomedicine, 3:113–119, 1996.
Lehrl, S., Clinical efficacy of kawa extract WS® 1490 in sleep disturbances associated with anxiety disorders: Results of a multicenter, randomized, placebocontrolled double-blind clinical trial, J. Affective Disorders, 78:101–110, 2004.
Norton, S.A. and Ruze, P., Kava dermopathy, J. Am. Acad. Dermatol., 31:89–97, 1994.
Pittler, M.H. and Ernst, E., Efficacy of kava for treating anxiety: Systematic and meta-analysis, J. Clin. Psychopharmacol., 1:84–89, 2000.
Russman, S., Lauterburg, B.H., and Helbling, A., Kava hepatotoxicity, Ann. Intern. Med., 135:68– 69, 2001.
Schelosky, L., Raffauf, C., Jendroska, K., and Poewe, W., Kava and dopamine antagonism, J. Nenrol. Neurosurg. Psych., 58:639–640, 1995.
Schmidt, T.L. Ly-Pahl, H.L., and Merfort, I., Helananolide type sesquiterpene lactones, Part 5: The role of glutathione addition under physiological conditions, Bioorg. Med. Chem., 7:2849–2855, 1999.
Schmidt, T.L., Lyss, G., Pahl, H.L., and Merfort, I., Helananolide type sesquiterpene lactones, Part 7: The role of glutathione addition under physiological conditions, Biorg. Med. Chem., 9:2189– 2194, 2001.
Seitz, U., Schule, A., and Gleitz, J., [3H]-Monoamine uptake, inhibition properties of kavapyrones, Planta Med., 63:548–549, 1997.
Stickel, F., Baumuller, H.-M., Seitz, K., Vasilakis, D., Seitz, G., Seitz, H.K., and Schuppan, D., Hepatitis induced by kava (Piper methysticum rhizoma), J. Hepatol., 39:62–67, 2003.
Strahl, S., Ehret, V., Dahm, H.H., and Maier, K.P., Necrotizing hepatitis after taking herbal medication (extracts of kava or of a common or lesser celandine), Dtsch. Med. Wochenschr., 123:1410–1414, 1998 (in German).
Uebelhack, R., Franke, L., and Schewe, H.-J., Inhibition of platelet MAO-B by kavapyroneenriched extract from kava-kava, Pharmacopsychiatry, 31: 187–192, 1998.
Volz, H.P. and Kieser, M., Kava-kava extract WS1490 versus placebo in anxiety disorders—a randomized placebo-controlled 25-week outpatient trial, Pharmacopsychiatry, 30:1–5, 1997.
Whitton, P.A., Lau, A., Salisbury, A., Whitehouse, J., and Evans, C.S., Kava lactones and the kavakava controversy, Phytochemistry, 64:673–679, 2003.
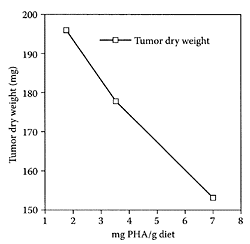
FIGURE K.58 Tumor growth expressed as dry tumor mass related to body dry weight in mice fed diets containing increasing amounts of phytohemagglutinins (PHA). (Pryme et al., Cancer Lett., 146:87–91, 1999. With permission.)
Kidney Bean
see also Beans and Lectins Kidney beans (Phaseolus vulgaris) are a variety of beans with a dark, red skin. Like most legumes, kidney beans contain a toxic lectin component that is normally inactivated by boiling to prevent gastric upset. Lectins or phytohemagglutinins, however, have been shown to exert beneficial health benefits. A number of studies reported that phytohemagglutinin in raw kidney bean diminished the growth of Krebs II non-Hodgkin lymphoma tumors in NMRI mice (Pryme et al., 1994a, b, 1996). Pryme and coworkers (1999) found that phytohemagglutinins curtailed the growth of established nonHodgkin lymphoma tumors (five days after tumor development was initiated) by as much as 30–40 percent in female NMRI mice fed diets containing increasing levels of phytohemagglutinins in a dose-dependent manner, as shown in Figure K.58. These results further confirm the importance of red kidney bean as a functional food due, in part, to the presence of bioactive lectins.
References
Pryme, I.F., Bardocz, S., Grant, G., Duguid, T.J., Brown, D.S., and Pusztai, A., The plant lectin PHA as a tool for reducing the progression of tumor growth, in, COST 98, Effects of Antinutients on the Nutritional Value of Legume Diets, Vol. 5, Bardocz, S. and Pusztai, A., Eds., EC Publications, Luxembourg, 1996, pp. 24–29.
Pryme, I.F., Bardocz, S., and Pusztai, A., A diet containing the lectin phytohaemagglutinin (PHA) slows down the proliferation of Krebs II cell tumors in mice, Cancer Lett., 76:133–137, 1994a.
Pryme, I.F., Bardocz, S., Pusztai, A., and Ewen, S.W.B., The growth of an established murine nonHodgkin lymphoma tumor is limited by switching to a phytohaemagglutinin-containing diet, Cancer Lett., 146:87–91, 1999.
Pryme, I.F., Pustzai, A., and Bardocz, S., The initial growth of Krebs II tumor cells, effect of phytohemagglutinin in the diet, Int. J. Oncol., 5:1105–1107, 1994b.
Kiwifruit
Kiwifruit has become extremely popular over the past decade. Two varieties are grown, one with green flesh and the other with yellow flesh. In addition to being a rich source of vitamin C, those grown in Asia have been used in Chinese traditional medicine for the treatment of different cancers (Zhi, 1980). Sheng (1984) reported a 30–40 percent inhibition of sarcoma in mice fed kiwifruit, while Song (1984a, b) showed kiwifruit juice inhibited cancer-cell growth. Using the Ames’ test, Liu and Peng (1994) found that some kiwi-fruit extracts exhibited a 95 percent inhibition of cancer. Motohashi and coworkers (2002) recently reported valuable bioactive compounds in kiwi gold fruit extracts. For example, hexane and acetone extracts proved selectively cytotoxic against human oral cell lines, while the more hydrophyllic 70 percent methanol fractions had higher anti-HIV, radical-generating, and O2−-scavenging activities.
An antifungal, thaumatin-like protein composed of a single-chain 21 kDa was isolated by Wang and Ng (2002) from the green-flesh kiwifruit variety. The N-terminal sequences of thaumatin-like proteins (TLP) from mono- and dicotyledons exhibited 65–80 percent identity with TLP from kiwifruit (Table K.41). Of particular note was the presence of the fifth residue (F) not present in any of the other TLPs. The kiwi protein exerted antifungal activity against Botyris cinerea and suppressed Mycosphaerella arachidicola and Coprinus comatus. Wang and Ng (2002) also found that kiwi TLP inhibited HIV-1 reverse transcriptase, similar to that reported for French bean TLP (Ye et al., 1999).
TABLE K.41
Comparison of N-Terminal Sequences of Kiwifruit TLP with Other TLPSs1
References
Liu, C. and Peng, M., Big Dictionary of Anticancer Plants, Hubei Science and Technology Publisher, Hubei, 1994, pp. 959–961.
Motohashi, N., Shirataki, Y., Kawase, M., Tani, S., Sakagami, H., Satoh, K., Kurihara, T., Nakashima, H., Mucsi, I., Varga, A., and Molnar, J., Cancer prevention and therapy with kiwifruit in Chinese folklore medicine: A study of kiwifruit extracts, J. Ethnopharmacol., 81:357–364, 2002.
Sheng, Z., Handbook of Chinese Cancer Treatment, Chongquing Publishers, Chongquing, Sichuan Province, 1994, pp. 661–662.
Song, P., Healthy application by kiwifruit juice, Nutr. Res., 6:35–40, 1984a.
Song, P., Anticancer activity of Chinese kiwifruit, Nutr. Res., 6:109–114, 1984b.
Wang, H. and Ng, T.B., Isolation of an antifungal thaumatin-like protein from kiwi fruits, Phytochemistry, 61:1–6, 2002.
Ye, X.Y., Wang, H.X., and Ng, T.B., First chromatographic isolation of an antifungal thaumatinlike protein from French bean legumes and demonstration of its antifungal activity, Biophys. Res. Commun., 263:1002–1013, 1999.
Zhi, C.-J., Chinese Anti-Cancer Agents, Hua-lian Publishers, Taipei, 1980, pp. 1, 74–75.
Kurosu
Kurosu is one of the traditional vinegars in Japan produced from unpolished rice by fermentation. It has been reported to have medicinal properties, such as improving blood fluidity and preventing hypertension (Nishikawa et al., 2001). Studies by Nishidai and coworkers (2000) showed an ethyl-acetate extract from Kurosu exhibited both antioxidant activity, as well as antitumor properties in mice. Shimoji et al. (2002) first identified dihydroferulic acid and dihydrosinapic acid as the major phenolics in Kurosu responsible for its radicalscavenging activity. These compounds were present at much higher levels in Kurosu compared to common rice vinegar (polished-rice vinegar), as shown in Table K.42. The higher content of antioxidant compounds in Kurosu, particularly dihydroferulic and dihydrosinapic acids, probably explains the almost twofold greater scavenging activity by Kurosu compared to rice vinegar.
References
Nishidai, S., Nakamura, Y., Torikai, K., Yamamoto, M., Ishihara, N., Mori, H., and Ohigashi, H., Kurosu, a traditional vinegar produced from unpolished rice, suppresses lipid peroxidation in vitro and in mouse skin, Biosci. Biotechnol. Biochem., 64:1909–1914, 2000.
Nishikawa, Y., Takata, Y., Nagai, Y., ori, T., Kawada, T., and Ishihara, N., Antihypertensive effect of Kurosu extract, a traditional vinegar produced from unpolished rice, in the SHR rats, Nippon Syokuhin Kagaku Kogaku Kaishi, 48:73–75, 2001 (in Japanese).
Shimoji, Y., Tamura, Y., Nakamura, Y., and Nanda, K., Shoho, N., Nishakawa, Y., Ishihara, N., Uenakai, K., and Ohigashi, H., Isolation and identification of DPPH radical scavenging activity compounds in Kurosu (Japanese unpolished rice vinegar), J. Agric. Food Chem., 50:6501–6503, 2002.