H
Halichondrin B
Halichondrin B, a poly ether macrolide isolated from marine sponges and tunicates, was shown to have potent cytotoxicity properties in vitro and anticancer properties in vivo (Hirata and Uemura, 1986; Litaudon et al., 1994; Fodstad et al., 1996). The sponge Lissodendoryx n. sp. 1 was the most promising source of halichondrin B components, although it is found in four other sponges. The potential of halichondrin B as an anticancer drug (Pettit et al., 1993a) is limited by its relative scarcity. Halichondrin B disrupts mitotic spindle formation and induces mitotic arrest by inhibiting tubulin assembly and microtubule assembly (Pettit et al., 1993b). A synthetic C(1)-C(38) halichondrin subunit was reported to exhibit anticancer properties similar to that of halichondrin B (Stamos et al., 1997; Towle et al., 2001). Wang et al. (2000) synthesized simplified analogues of halichondrin B that still retained cell growth inhibitory potency in vitro. Austad and coworkers (2002) synthesized C(37)-C(54) halichondrin subunits. The National Cancer Institute selected halichondrin B for drug development.
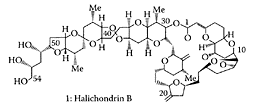
Halichondrin B. (From Seletsky et al., Bioorg. Med. Chem. Lett., 14:5547–5550, 2004. With permission.)
References
Austad, B.C., Hart, A.C., and Burke, S.D., Halichondrin B: Synthesis of the C(37)-C(54) subunit, Tetrahedron, 58:2011–2026, 2002.
Fodstad, O., Breistoel, K., Pettit, G.R., Shoemaker, R.H., and Boyd, M.P., Comparative antitumor activities of halichondrins and vineblastine against human tumor xenografts, J. Exp. Therap. Oncol., 1:119–125, 1996.
Hirata, Y. and Uemura, D., Halichondrins—antitu mor macrolides from marine sponge, Pure. Appl. Chem., 58:701–710, 1986.
Litaudon, M., Hart, J.B., Blunt, J.W., Lake, R.J., and Munro, M.H.G., Isohomohalichondrin B: A new antitumor polyether macrolide from the New Zealand deep-water sponge Lissodendoryx sp., Tetrahedron Lett., 35:9435–9438, 1994.
Pettit, G.R., Tan, R., Gao, F., Williams, M.D., Doubek, D.L., Boyd, M.R., Schmidt, J.M., Chapius, J.-C., Hamel, E., Bai, R., Hooper, J.N.A., and Tackett, L.P., Isolation and structure of halistan 1 from Eastern Indian Ocean marine sponge Phakellia carteri, J. Org. Chem., 58:2538–2541, 1993a.
Pettit, G.R., Gao, F., Doubek, D.L., Boyd, M.R., Hamel, E., Bai, R.L., Schmidt, J.M., Tackett, L.P., and Rutzler, K., Antineoplastic agents, 252, Isolation and structure of halistatin-2 from the Comoros marine sponge Axinella-carteri, Gazz. Chim. Ital., 123:371–377, 1993b.
Seletsky, B.M., Wang, Y., Hawkins, L.D., Palme, M.H., Habgood, G.J., DiPietro, L.V., Towle, M.J., Salvato, K.A., Wells, B.F., Alafs, K.K., Kishi, Y., Littlefield. B.A., and Yu, M.J., Structurally simplified macrolactone analogues of halichondrin B, Bioorg. Med. Chem. Lett., 14:5547–5550, 2004.
Stamos, D.P., Sean, S.C., and Kishi, Y., New synthetic route to the C14-C.38 segment of halichondrins, J. Org. Chem., 62:7552–7553, 1997.
Towle, M.J., Solvato, K.A., Budrow, J., Wels, B.F., Kutznetsov, G., Aalfs, K.K., Welsh, S., Zheng, W., Seletsky, B.M., Palme, M.N., Habgood, G.J., Singer, L.A., DiPietro, L.V., Wang, Y., Chen, J.J., Quincy, D.A., Davis, A., Yoshimatsu, K., Kishi, Y., Yu, M.J., and Littlefield, B.A., In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B, Cancer Res., 61:1013–1021, 2001.
Wang, Y., Habgood, G.J., Christ, W.J., Kishi, Y., Littlefield, B.A., and Yu, M.J., Structure-activity relationships of halichondrin B analogues: Modifications of C.30-C.38, Bioorg. Med. Chem. Lett., 10:1029–1032, 2000.
Hawthorn
Hawthorn (Crataegus) grows in the northern temperate regions of the world, mainly in East Asia, Europe, and North America. The bright-red berries of hawthorn fruit contain fructose, flavonoids, proanthocyanidins, triterpenes, organic acids, vitamins, and minerals (Huang, 1993). Zhang et al. (2001) reported hawthorn fruit was rich in phenolic antioxidants, particularly hyperoside, isoquercitrin, epicatechin, chlorogenic acid, quercetin, rutin, and protocatechuic acid. The ability of hawthorn fruit to lower total serum cholesterol, LDL cholesterol, and triglycerides in hyperlipidemic individuals was reported by Chen and coworkers (1995). More recent studies by Zhang et al. (2002a) showed inclusion of a 0.5 percent aqueous ethanolic extract from hawthorn-fruit powder in a semisynthetic diet containing 0.1 percent cholesterol diet to rabbits lowered serum total cholesterol and triacylglycerols by 10 percent and 13 percent, respectively (Figure H.48). A possible mechanism involved greater bile-acid excretion mediated by upregulation of hepatic cholesterol 7α-hydroxylase and inhibition of cholesterol adsorption mediated by downregulation of intestinal acyl Co A: cholesterol acyltransferase activity.
Pittler et al. (2003) examined the efficacy of hawthorn extract in treating chronic heart failure by meta-analysis of randomized trials. Their results suggested a significant benefit was derived from hawthorn extract as an adjunctive treatment for chronic heart failure. A pilot study conducted by Walker and coworkers (2002) using 36 mildly hypertensive subjects found hawthorn extract reduced the diastolic blood pressure in 10 out of the 19 subjects, with a trend in the reduction of anxiety. The small number of subjects and the low levels of hawthorn extract used in this study, however, warrants further investigation.
References
Chen, J.D., Wu, Y.Z., Tao, Z.L., Chen, Z.M., and Liu, X.P., Hawthorn (Shan Zha) drink and its lowering effect on blood lipid levels in humans and rats, World Rev. Nutr. Diet, 77:147–154, 1995.
Huang, K.C., Shan Zha: Crataegus pinnatifida, in The Pharmacology of Chinese Herbs, CRC Press, Boca Raton, Florida, 1993.
Pittler, M.H., Schmidt, K., and Ernst, E., Hawthorn extract for treating chronic heart failure: Meta-analysis of randomized trials, Am. J. Med., 114:665–674, 2003.
Walker, A.F., Marakis, G., Morris, A.P., and Robinson, P.A., Promising hypotensive effect of hawthorn extract: A randomized double-blind pilot study of mild, essential hypertension, Phytother. Res., 16:48–54, 2002.
Zhang, Z., Chang, Qi, Zhu, Min., Huang, Y., Ho, W.K.K., and Chen, Z.-Y., Characterization of antioxidants present in hawthorn fruits, J. Nutr. Biochem., 12:144–152, 2001.
Zhang, Z., Ho, W.K.K., Huang, Y., and Chen, Z.-Y., Hypocholesterolemic activity of hawthorn fruit is mediated by regulation of cholesterol-7-α-hydroxylase and acyl CoA: Cholesterol acyltransferase, Food Res. Inter., 35:885–891, 2002a.
Zhang, Z., Ho, W.K.K., Huang, Y., James, A.E., Lam, L.W., and Chen, Z.Y., Hawthorn fruit is hypolipidemic in rabbits fed a high cholesterol diet, J. Nutr., 132:5–10, 2002b.
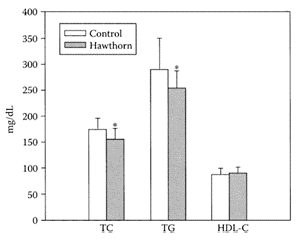
FIGURE H.48 Effects of supplementation of 0.5 percent hawthorn fruit ethanolic extract (equal to 2 percent dried-fruit powder) in diet on serum total cholesterol (TC), triacylglycerols (TG), and high-density lipoprotein cholesterol (HDL-C) in hamsters. Values are means±S.D., n=15. *Differs significantly at p<0.05 (From Zhang et al., Food Res. Inter., 35:885–891, 2002a. With permission.)
Hemp
The annual herbaceous plant hemp (Cannabis saliva L.) has been traditionally grown for its fiber and oil. Its seeds were reported to have a number of health benefits, including lowering cholesterol and high blood pressure (Jones, 1995). Hemp-seed oil is perfectly balanced with respect to the ratio (3:1) of the two essential polyunsaturated fatty acids, linoleic to linolenic acid. The presence of γ-linolenic acid in hemp oil makes it an excellent ingredient in light body oils and lipid-enriched creams (Rausch, 1995).
References
Jones, K., Nutritional and Medicinal Guide to Hemp Seed, Rainforest Botanical Laboratory, Gibsons, B.C., Canada, 1995.
Rausch, P., Verwendung von hanfsamenol in der kosmetik, in Bioresource Hemp, 2nd ed., Nova-Institute, Cologne, Germany, 1995, pp. 556–561, 1995.
Herbs
see also Individual herbs Herbs are used extensively and with increasing interest in North America in complimentary and alterna tive medicines (Eisenberg et al., 1998). Women (particularly white, middle-aged women) appear to be the major users of these nontraditional therapies (Astin, 1998; Druss and Rosenheck, 1999). Like pharmaceutical drugs, herbal medicines can be therapeutic at one dose and toxic at another (Fugh-Berman, 2001). Of particular concern, however, are the possible adverse effects of herbal-drug interactions. In a review of herbs commonly used by women, Tesch (2001) noted that some herbs, such as Ginkgo biloba, were more effective than the placebo for dementia. However, when taken with aspirin or warfarin, it inhibited the platelet-activating factor and was associated with serious bleeding. St. John’s Wort, shown to be effective for treating mild to moderate depression in the short term, suffers from many drug interactions. Ginseng may attenuate postprandial glycemia and improve psychological symptoms in perimenopausal women. A decrease in certain cancers associated with ginseng, however, is offset by its impurity and possible side effects. A review of herbal medicines and epilepsy by Spinella (2001) noted that certain herbal sedatives (kava kava, valerian, chamomile, passion flower) may potentiate the effects of antiepileptic medications, increasing their sedative and cognitive effects. However, limited evidence suggests that many of these herbal medicines, particularly those containing ephedrine and caffeine, can exacerbate seizures. A list of the clinical reports for some herb-drug interactions is summarized in Table H.37. A review of alternative medicines for treating glaucoma by Rhee et al. (2001) indicated that while Ginkgo biloba and other Chinese herbal medicines do not affect intraocular pressure, they may have a beneficial effect by improving blood flow to the optic nerve. These researchers cautioned that using some herbal medicines could have possible toxicities and side effects.
Zou et al. (2002) examined the in vitro effects of 25 purified components from commonly used herbal products on the catalytic activity of cDNA-expressed cytochrome P450 isoforms. Herbal products containing kava kava, Ginkgo biloba, garlic, or St. John’s Wort were capable of inhibiting the metabolism of coadministered medications in which the primary elimination route was via cytochrome P450. Constituents in these herbal products inhibited one or more of the cytochrome P450 isoforms at concentrations less than 10 μM. Of the three main isoforms (CYP2C9, CYP2C19, and CYP3A4) affected, CYP2C19 proved to be the most sensitive. These herbal components were capable of eliciting clinically significant drug interactions.
TABLE H.37
Clinical Reports of Selected Herb-Drug Interactions
For a more detailed discussion of the risks associated with herbal medicines, the review articles by Izzo et al. (2004) and Zhou et al. (2004) are strongly recommended.
References
Astin, J.A., Why patients use alternative medicine: Results of a national study, JAMA, 279:1548– 1553, 1998.
Druss, B.G. and Rosenheck, R.A., Association between use of unconventional therapies and conventional medical services, JAMA, 282:651–656, 1999.
Eisenberg, D.M., Davis, R.B., Ettner, S.L., Appel, S., Wilkey, S., Van Rompay, M., and Kessler, R.C., Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey, JAMA, 280:1569–1575, 1998.
Fugh-Berman, A., Herb-drug interactions, Lancet, 355:134–138, 2001.
Izzo, A.A., Di Carlo, G., Borrielli, F., and Ernst, E., 2004. Cardiovascular pharmacotherapy and herbal medicines: The risk of drug interaction, Inter. J. Cardiol., 98:1–14, 2004.
Rhee, D.J., Katz, L.J., Spaeth, G.L., and Myers, J.S., Complementary and alternative medicine for glaucoma, Surv. Ophthamol., 46:43–55, 2001.
Spinelli, M., Herbal medicines and epilepsy: The potential for benefit and adverse effects, Epilepsy Behav., 2:524–532, 2001.
Tesch, B.J., Herbs commonly used by women: An evidence-based review, Clin. J. Women’s Health, 1: 89–102, 2001.
Zhou, S., Koh, H.-L., Gao, Y., Gong, Z., and Lee, E.J.D., Herbal bioactivation: The good, the bad and the ugly, Life Sci., 74:935–968, 2004.
Zou, L., Harkey, M.R., and Henderson, G.L., Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity, Life Sci., 71: 1579–1589, 2002.
Hesperidin
Hesperidin (3′,5,7-trihydroxy-4′-methoxyflavonone-7-rhamnoglucoside), is a naturally occurring bioflavonoid present in fruits and vegetables. As a component of citrusfruit peel, it was shown to lower cholesterol in rats (Bok et al., 1999; Galati et al, 1994), as well as exhibit antioxidant activity in vitro (Van Acker et al., 1996; Chan et al., 1999).
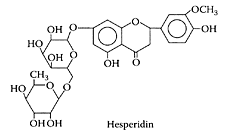
(Adapted from Kanaze et al., J. Pharm. Biomed. Anal., 36:175–181, 2004.)
A number of studies have shown dietary hesperidin, alone or in combination with diosmon, exerted anticarcinogenic effects in tongue, colon, esophageal, and urinary-bladder carcinogenic rat models (Tanaka et al., 1997a, b, 2000; Yang et al., 1997). In addition, hesperidin was also shown to have anti-inflammatory activity in mouse skin exposed to a tumor promoter (Koyuncu et al., 1999). Using the rat model for testing arthritis, Guardia and coworkers (2001) showed hesperidin inhibited both acute and chronic phases of inflammation. Sakata et al. (2003) examined the modulating effects of hesperidin on the expression and activity of COX-2 and iNOS enzymes induced by the endotoxin lipopolysaccharide (LPS). COX-2 and iNOS are inducible enzymes, which, in association with inflammatory responses, play a key role in carcinogenesis. Using mouse macrophage cells, hesperidin dramatically suppressed prostaglandin E2, nitric dioxide, and expression of iNOS protein, which could explain its anti-inflammatory and antimutagenic properties (Figure H.49).
Hesperidin and 6-methylapigenin were reported by Marder et al. (2003) as new Valeriana flavonoids with activity on the central nervous system. 2S-(−)-hesperidin was found to have sedative and sleep-enhancing properties potentiated by 6-methylapigenin. The chemistry and pharmacology of the citrus bioflavonoid hesperidin were reviewed by Garg et al. (2001).
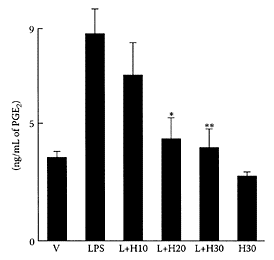
FIGURE H.49 PGE2 production in RAW 264.7 cells. PGE2 over production was induced by LPS (0.2 μg/mL medium, L) and suppressed by hesperidin (H), with various concentrations, v, cells treated with vehicle. L+ H10−L+H30, cells treated with LPS and 10, 20, and 30 μM of hesperidin. H30 cells treated with hesperidin 30 μM. Mean PGE2 concentration±SD of three separate experiments. *p<0.005 and **p<0.003 compared with L by Student’s t-test. (From Sakata et al, Cancer Lett., 199:139–145, 2003. With permission.)
References
Bok, S.H., Lee, S.H., Park, Y.B., Bae, K.H., Son, K.H., Jeong, T.S., and Choi, M.S., Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase and acyl coenzyme A: Cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus biflavonoids, J. Nutr., 128:1182–1185, 1999.
Chan, T., Galati, G., and O’Brien, P.J., Oxygen activation during peroxidase catalysed metabolism of flavones and flavonones, Chem. Biol. Interact., 122: 15–25, 1999.
Galati, E.M., Monforte, M.T., Kirjainen, S., Forestieri, A.M., Trovato, A., and Tripodo, M.M., Biological effect of hesperidin, a citrus flavonoid (note 1): Anti-inflammatory and analgesic activity, II Farmaco., 49:709–712, 1994.
Garg, A., Garg, S., Zaneveld, L.J.D., and Singla, A.K., Chemistry and pharmacology of the citrus bioflavonoid hesperidin, Phytother. Res., 15:655–669, 2001.
Guardia, T., Rotelli, A.E., Juarez, A.O., and Pelzer, L.E., Anti-inflammatory properties of plant flavonoids, effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat, Il Formaco., 56:683–687, 2001.
Kakumoto, M., Satoh, K., Horo, A., Sumida, T., Tanaka, T., and Ogawa, H., Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids diosmin and hesperidin, Carcinogenesis, 18:957–965, 1997b.
Kanaze, F.I., Kokkalou, E., Georgarakis, M., and Niopas, I., A validated solid-phase extraction HPLC method for the simultaneous determination of the citrus flavonone aglycones hesperitin and naringenin in urine, J. Pharm. Biomed. Anal., 36:175–181, 2004.
Koyuncu, H., Berkada, B., Baykut, F., Soybir, G., Alati, C., Gul, H., and Altun, M., Preventive effect of hesperidin against inflammation in CD-1 mouse skin caused by tumor promoter, Anticancer Res., 19: 3237–3241, 1999.
Marder, M., Viola, H., Wasowski, C., Fernandez, S., Medina, J.H., and Paladini, A.C., 6- Methylapigenin and hesperidin: New valeriana flavonoids with activity on the CNS, Pharmacol. Biochem. Behav., 75: 537–545, 2003.
Sakata, K., Hirose, Y., Qiao, Z., Tanaka, T., and Mori, H., Inhibition of inducible isoforms of cyclooxygase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line, Cancer Lett., 199:139–145, 2003.
Tanaka, T., Kohno, H., Murakami, M., Shimada, R., Kagami, S., Sumida, T., Azuma, Y., and Ogawa, H., Suppression of azoxymethane-induced colon carcinogenesis in male F344 rats by mandarin juices in β-cryptoxanthin and hesperidin, Int. J. Cancer, 88: 146–150, 2000.
Tanaka, T., Makita, H., Ohnishi, M., Mori, H., Satoh, K., Hara, A., Sumida, T., Fukutani, K., Tanaka, T., and Ogawa, H., Chemoprevention of 4-mitroquinoline 1-oxide induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination, Cancer Res., 57:246–252, 1997a.
Van Acker, S.A.B.E., Van Den Berg, D.J., Tromp, M.N.J.L., Griffioen, D.H., Van Bennekom, W.P., Van Der Vijgh, W.J.F., and Bast, A., Structural aspects of antioxidant activity of flavones or flavonones, Free Rad. Biol. Med., 20:331–342, 1996.
Yang, M., Tanaka, T., Hirose, Y., Deguchi, T., Mori, H., and Kawada, Y., Chemoprotective effects of diosmin and hesperidin on N-butyl-N-(4-hydroxybutyl)-induced urinary bladder carcinogenesis in male 1CR mice, Int. J. Cancer, 73:719–724, 1997.
High-density lipoproteins
see Lipoproteins
Honey
Honey, a complex mixture of carbohydrates, has been studied extensively (Horvath and Molnarl-Perl, 1998; Gomez Barez et al., 2000). In addition, some cyclitols or poly alcohols, such as myo-inositol and mannitol, have also been reported in edible honeys (Horvath and Molnarl-Perl, 1998). Sanz and coworkers (2004) identified quercitol, pinitol, 1-O-methylmuco-inositol, and muco-inositol for the first time in edible honey. Of 28 honeys examined, most had myo-inositol and pinitol, while only in some samples were the other cyclitols detected. The anti-inflammatory nature of (+)-pinitol, isolated from Abies pindrow leaves, was demonstrated by Singh and coworkers (2001) using the carrageenan-induced paw edema in rats. A significant reduction in edema volume was evident in the presence of pinitol with a dose of 10 mg/kg comparable to that of phenylbutazone (Table H.38).
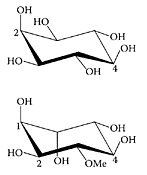
Myo-inositol and D-pinitol. (From Hart et al., Carbohydr. Res., 339:1857–1871, 2004. With permission.)
TABLE H.38
Anti-inflammatory Effect of (+)-Pinitol on Carrageenan-Induced Paw Edema in Rats1
Certain honeys derived from such floral sources as Leptospermum scoparium (Manuka) and L. polygalifolium (Meadhoney) provide additional antioxidants, antibacterial agents, and other unidentified compounds and are referred to as therapeutic honeys (Lusby et al., 2002). The ability of these honeys to prevent microbial growth in the moist-wound environment accounts, in part, for their beneficial effects in wound healing. Tonks et al. (2003) examined the wound-healing ability of three honeys (manuka, pasture, and jelly bush) on the activation state of immunoincompetent cells, using the human monocytic cell-line model MonoMac-6. All of the honeys significantly increased the release of important inflammatory cytokines TNF-α, IL-1β, and IL-6 (Figure H.50). These cytokines are both proinflammatory and anti-inflammatory. While all three honeys showed significant increases in cytokines compared to the sugar-solution control, the Australian jelly-bush honey had the greatest effect. The ability of these honeys to regulate the production of cytokines is probably due to the presence of components other than sugar. These components have yet to be identified but could involve cyclitols.
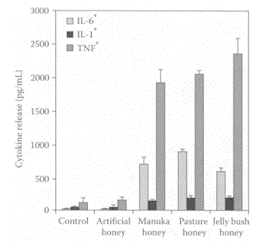
FIGURE H.50 Effect of 1 percent (w/v) honeys on TNF-α, IL-1β, and IL- 6 release from isolated human peripheral-blood monocytes. Results are expressed as mean±SD. *p<0.001 analyzed by ANOVA and Tukey’s pair-wise comparisons. (From Tonks et al., Cytokine, 21:242–247, 2003. With permission.)
References
Gomez Barez, J.A., Garcia Villanova, R.J., Elvira Garcia, S., Rivas Pala, T., Gonzalez Paramas, A.M., and Sanchez Sanchez, J., Geographical distribution of honeys through the employment of sugar patterns and common chemical quality parameters, Eur. Food Res. Technol., 210:437– 444, 2000.
Hart, J.B., Kroger, L., Falshaw, A., Falshaw, R., Farkas, E., Thiem, J., and Win, A.L., Enzymecatalysed synthesis of galactosylated 1D- and 1L-chiro-inositol, 1D-pinitol, myo-inositol and selected derivatives using the β-galactosidase from the thermophile Thermoanaerobacter sp. strain TP6-B1, Carbohydr. Res., 339:1857–1871, 2004.
Horvath, K. and Molnarl-Perl, I., Simultaneous GC-MS quantitation of o-phosphoric, aliphatic and aromatic carboxylic acids, proline, hydroxymethylfurfural and sugars as their TMS derivatives: In honeys, Chromatographia, 48:120–126, 1998.
Lusby, P.E., Coombes, A., and Wilkinson, J.M., Honey: A potent agent for wound healing? JWOCN, 29:295–300, 2002.
Sanz, M.L., Sanz, S.J., and Martinez-Castro, I., Presence of some cyclitols in honey, Food Chem., 84: 133–135, 2004.
Singh, R.K., Pandey, B.L., Tripathi, M., and Pandey, V.B., Anti-inflammatory effect of (+)-pinitol, Fitoterapia, 72:168–170, 2001.
Tonks, A.J., Cooper, R.A., Jones, K.P., Blair, S., Parton, J., and Tonks, A., Honey stimulates inflammatory cytokine production from monocytes, Cytokine, 21:242–247, 2003.
Hops (Humulus lupulus L.)
The bitter taste and aroma of beers is due to the bitter acids extracted from hops added to the sweet wort during brewing. These are composed of a- and β-acids, humulone, cohumulone, adhumulone, lupulone, colupulone, and adlupulone and their oxidation products (Scheme H.30) (Verzele and Potter, 1978). The potential of bitter acids, such as humulone, as chemotherapeutic or chemopreventive agents was evident by their ability to inhibit angiogenesis (Shimamura et al., 2001), suppress cyclooxygenase-2 transcription (Yamamoto et al., 2000), induce differentiation of myelogenous leukemia cells (Honma et al., 1998), inhibit tumor promotion by 12-O-tetradecanoyl- phorbol-13-acetate (Yasukawa et al., 1995), and induce apoptosis in human leukemia HL-60 cells (Tobe et al., 1997). Chen and Lin (2004) attempted to delineate the mechanism whereby hop bitter acids triggered apo-ptosis in human leukemia-cell lines HL-60 and U937. The hop bitter acids inhibited HL-60 cell viability in a dose-dependent manner, with an IC50 of 8.67 μg/mL (Figure H.51). The U937 cells proved much more resistant to the action of the hop bitter acids with an IC50 value of around 58.87 μg/mL. Several mechanisms were proposed for triggering apoptosis involving Fas activation and mitochondrial dysfunction.
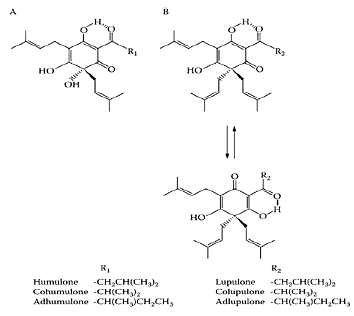
SCHEME H.30 Chemical structures of hop bitter acids: (A) α-acids; (B) β- acids. (From Chen and Lin, J. Agric. Food Chem., 52:55–64, 2004. With permission.)
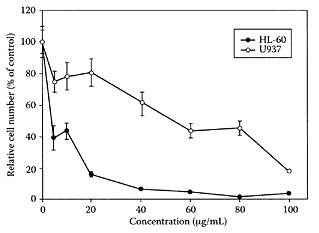
FIGURE H.51 Effect of hop bitter acids on cell viability, HL-60 and U937 cells when treated with either 5 μL/mL of DMSO as vehicle control or various concentrations of hop bitter acids for 24 h. Cell viability was determined by luminescent ATP detection assay kit, with data representing the means ± of three determinations. (From Chen and Lin, J. Agric. Food Chem., 52:55–64, 2004. With permission.)
References
Chen, W.-J. and Lin, J.-K., Mechanisms of cancer chemoprevention by hop bitter acids (beer aroma) through induction of apoptosis mediated by Fas and caspase cascades, J. Agric. Food Chem., 52:55–64, 2004.
Honma, Y., Tobe, H., Makishima, M., Yokoyama, A., and Okabe-Kado, J., Induction of differentiation of mycelogenous leukemia cells by humulone, a bitter in the hop, Leuk. Res., 22:605–610, 1998.
Shimamura, M., Hazato, T., Ashino, H., Yamamoto, Y., Iwasaki, E., Tobe, H., Yamamoto, K., and Yamamoto, S., Inhibition of angiogenesis by humulone, a bitter acid from beer hop, Biochem. Biophys. Res. Commun., 289:220–224, 2001.
Tobe, H., Kubota, M., Yamaguchi, M., Kocha, T., and Aoyagi, T., Apoptosis to HL-60 by humulone, Biosci. Biotechnol. Biochem., 61:1027–1029, 1997.
Verzele, M. and Potter, M.De, High-performance liquid chromatography of hop bitter substances, J. Chromatogr. A., 166:320–326, 1978.
Yamamoto, K., Wang, J., Yamamoto, S., and Tobe, H., Suppression of cyclooxygenase-2 gene transcription by humulon of beer hop extract studied with reference to glucocorticoid, FEBS Lett., 465:103–106, 2000.
Yasukawa, K., Takeuchi, M., and Takido, M., Humulon, a bitter in the hop, inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin, Oncology, 52:156–158, 1995.
Huperzine A and B
Huperzine A (HupA), a sesquiterpene alkaloid obtained from Chinese club moss (Huperzia serrata) or Lycopodium serrata, has been used as the folk medicine Quian Ceng Ta for centuries (Liu et al., 1986). It is a potent, highly specific, and reversible inhibitor of acetylcholinesterase, equivalent or superior to physostigmine, galanthamine, done-pezil, and tacrine, approved drugs for treating Alzheimer’s disease that are capable of crossing the blood-brain barrier (Wang et al., 1986; Wang and Tang, 1998). As a promising therapeutic agent for treating Alzheimer’s disease, Wang et al. (2000) showed that huperzine A decreased the incidence rate of vascular dementia via multiple mechanisms involving the cholinergic system, oxygen free radicals, and energy metabolism. This was evident by its ability to significantly restore choline acetyl tranferase activity in the hippocampus of rats, as well as reducing superoxide dismutase, lipid peroxide, lactate, and glucose to normal levels (Table H.39). Zhao and Tang (2002) showed huperzine A preferentially inhibited the tetrameric molecular form of acetylcholin-esterase in the cerebral cortex, hippocampus, and striatum of the rat brain, while tacrine and rivastigime preferentially inhibited the monomeric form. Physostigmine showed no form selectivity in any brain region. Donepezil varied from region to region with respect to its preferential inhibition of either the G4- and G1 selectivity. The different inhibitory preferences among acetylcholinestease inhibitors require further study in relation to their effect on Alzheimer’s disease. To understand the molecular events leading to Alzheimer’s disease, Zhang and Tang (2003) examined the effect of huperzine A on staurosporine neuronal apoptosis in primary cultured rat cortical neurons. They demonstrated for the first time that huperzine A attenuated neurotoxin staurosporine-induced apoptosis, possibly via upregula-tion of bcl-2, downregulation of bax, and reduction in immunoreactive caspase-3 proenzyme.
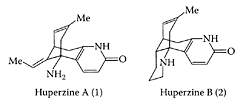
(From Lee et al., Tetrahedron Lett., 45:285–287, 2004. With permission.)
The potential of huperzine A as a therapeutic agent for Alzheimer’s disease was demonstrated by Zhang et al. (2004), who showed it increased the downregulation of secretory amyloid proteins by upregulating protein kinase C on infused rats and human embryonic kidney 293 Swedish mutant cells.
Tonduli and coworkers (2002) found huperzine A was extremely effective in preventing epileptic activity induced in rats by soman. Its ability to prevent toxicity was due to its protection of the enzyme acetylcholinesterase and reduction of hypercholinergy by soman at the electrophysiological level.
Huperzine B, a cogener of huperzine A in club moss, was reported to have a lower acetylcholinesterase inhibitory potency (Hu and Tang, 1987). Pretreatment of a rat pheochromocytoma cell line PC12 with huperzine B by Zhang and Tang (2000) showed it had similar neuroprotective effects to huperzine A against H2O2-induced injury. This neuroprotective effect could have application in the treatment of Alzheimer’s disease.
Three new Lycopodium alkaloids were recently identified by Tan et al. (2003) in Huperzia serrata as huperzine S (2β, 13β-epoxyalopecuridine), huperzine T (5α-hydroxy-6-oxodihydro-phlegmariu- rine A), and huperzine U (2,3-dihydro-12-hydroxyhuperzine B). The potential health benefits of these alkaloids still remain to be established.
References
Hu, H. and Tang, X.C., Cholinesterase inhibition by huperzine B, Acta Pharmacol. Sin., 8:18–22, 1987.
Lee, I.Y.C., Hong, J.Y., Jung, M.H., and Lee, H.W., Synthesis of huerzine B through ring closing metathesis, Tetrahedron Lett., 45:285–287, 2004.
Liu, J.S., Zhu, Y.L., Yu, C.M., Zhou, Y.-Z., Han, Y.-Y., F.-W., and Qui, B.-F., The structure of huperzine A and B, two new alkaloids exhibiting anticholinesterase activity, Can. J. Chem., 64:837–839, 1986.
Tan, C.-H., Ma, X.-Q., Chen, G.-F., and Zhu, D.-Y., Huperzines S.T., and U: New Lycopodium alkaloids from Huperzia serrata, Can. J. Chem., 81:315–318, 2003.
Tonduli, L.S., Testylier, G., Masqueliez, C., Lallement, T., and Monmaur, P., Effects of huperzine used as pre-treatment against soman-induced seizures, Neurotoxicology, 22:29–37, 2001.
Wang, L.M., Han, Y.F., and Tang, X.C., Huperzine A improves cognitive deficits caused by chronic cerebral hypoperfusion in rats, Eur. J. Pharmacol., 398:65–72, 2000.
Wang, Y.E. and Tang, X.C., Anticholesterase effects of huperzine A, E2020 and tacrine, Eur. J. Pharmacol., 349:137–142, 1998.
Wang, Y.E., Yue, D.X., and Tang, X.C., Anticholesterolase activity of huperzine A, Acta Pharmacol. Sin., 7:110–113, 1986.
Zhang, H.Y. and Tang, X.C., Huperzine B, a novel acetylcholinesterase inhibitor, attenuates hydrogen peroxide induced injury in PC 12 cells, Neurosci. Lett., 292:41–44, 2000.
Zhang, H.Y. and Tang, X.C., Huperzine A attenuates the neurotoxic effect of staurosporine in primary rat cortical neurons, Neurosci. Lett., 340:91–94, 2003.
Zhang, H.Y., Yan, H., and Tang, X.C., Huperzine A enhances the level of secretory amyloid precursor protein and protein kinase C-α in intracerebroventricular β-amyloid-(1–40) infused rats and human embryonic kidney 293 Swedish mutant rats, Neurosci. Lett., 360:21–24, 2004.
Zhao, Q. and Tang, X.C., Effects of huperzine A on acetylcholinesterase isoforms in vitro: Comparison with tacrine, donepezil, rivastigimine and physostigmine, Eur. J. Pharmacol., 455:101–107, 2002.
Hydratis canadensis
see Golden seal
Hydroxymatairesinol
Hydroxymatairesinol (HMR) found in Norway spruce (Ekman, 1976), is related to matairesinol, an abundant lignan found in rye bran. Its structure shown below consists of two guaiacol (2-methoxyphenol) units bonded to a and β positions of a γ-butyrolactone (2-oxacyclopentanone) ring via carbon atoms (Taskinen et al., 2004). Using Apcmin (adenomatous polyposis coli) mice, a genetically manipulated animal colon-cancer model, Oikarinen et al. (2000) found significantly lower numbers of adenomas in the small intestine of animals fed HMR compared to the other diets, including rye bran.
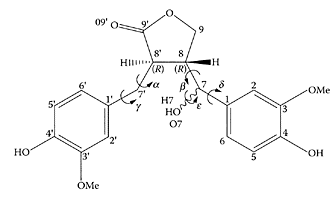
Hydroxymatairesinol. (From Taskinen et al., J. Mol. Struct., (Theochem), 677:113–124, 2004. With permission.)
References
Ekman, R., Analysis of lignans in Norway spruce by combined gas chromatography-mass spectrometry. Holzforschung, 30:79–85, 1976.
Oikarinen, S.I., Pajari, A.-M., and Mutanen, M., Chemoprotective activity of crude hydroxymatairesinol (HMR) extract on Apcmin mice, Cancer Lett., 161:253–258, 2000.
Taskinen, A., Eklund, P., Sjoholm, R., and Hotokka, The molecular structure and some properties of hydroxymatairesinol, an ab initio study, J. Mol. Struct., (Theochem), 677:113–124, 2004.
Hydroxytyrosol
see also Olive oil Hydroxytyrosol (2-(3,4-dihydroxyphenyl)ethanol, a naturally occurring phenolic compound in olive oil, is a potent antioxidant that prevents lowdensity lipoprotein from oxidation in vivo (Grignaffini et al., 1994) as well scavenges free radicals (Visioli et al., 1998). Studies showed hydroxytyrosol inhibited platelet aggregation and protected against damage by reactive-oxygen species (Petroni et al., 1995; Manna et al., 1997, 1999a, b). The ability of hydroxytyrosol to act in vivo was suggested, as it is rapidly taken up by intestinal cell lines by passive diffusion (Manna et al., 2000). Della Ragione and coworkers (2000) found hydroxytyrosol arrested cell proliferation and apoptosis in HL60 cells. They attributed hydroxytyrosol’s anti-inflammatory and chemopreventive effects to its ability to downregulate lymphocyte proliferation, which could prove beneficial in the treatment of chronic bowel pathologies, such as Chrone’s disease.
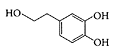
Hydroxytyrosol. (From Vogna et al., Tetrahedron Lett., 44:8289–8292, 2003. With permission.)
References
Grignaffini, P., Roma, P., Galli, C., and Catapano, A.L., Protection of low-density lipoprotein from oxidation by 3,4-dihydroxyphenylethanol, Lancet, 343: 1296–1297, 1994.
Manna, C., Galleti, P., Cucciolla, V., Moltedo, O., Leone, A., and Zappia, V., The protective effect of the olive oil polyphenol (3,4-dihydroxyphenyl)-ethanol counteracts reactive oxygen metabolite-induced cytotoxicity in Caco-2 cells, J. Nutr., 127:286–292, 1997.
Manna, C., Galleti, P., Cucciolla, V., Montedoro, G., and Zappia, V., Olive oil hydroxytyrosol protects human erythrocytes against oxidative damage, J. Nutr. Biochem., 10:159–165, 1999a.
Manna, C., Ragione, F.D., Cucciolla, V., Borriella, A., D’Angelo, S., Galletti, P., and Zappia, V., Biological effects of hydroxytyrosol, a polyphenol from olive oil endowed with antioxidant activity, Adv. Exp. Med. Biol., 472:115–130, 1999b.
Manna, C., Galletti, P., Maisto, G., Cucciolla, V., D’Angelo, S., and Zappia, V., Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco2 cells, FEBS Lett., 470:341– 344, 2000.
Petroni, A., Blasevich, M., Salami, M., Papini, N., Montedoro, G.F., and Galli, C., Inhibition of platelet aggregation and eicoisanoid production by phenolic components of olive oil, Thromb. Res., 178:151–160, 1995.
Ragione, F.D., Cucciolla, V., Borriello, A., Della Petra, V., Pontoni, G., Racioppi, L., Manna, C., Galleti, P., and Zappia, V., Hydroxytyrosol, a natural molecule occurring in olive oil induces cytochrome c-dependent apoptosis, Biochem. Biophys. Res. Commun., 278:733–739, 2000.
Visioli, F., Bellomo, G., and Galli, C., Free radical scavenging properties of olive oil polyphenols, Biochem. Biophys. Res. Commun., 247:60–64, 1998.
Vogna, D., Pezzella, A., Panzella, L., Napolitano, A., and d’Ischia, M., Oxidative chemistry of hydroxytyrosol: Isolation and characterization of novel methanooxocinobenzodioxinone derivatives, Tetrahedron Lett., 44:8289–8292, 2003.
Hyperforin
see also St. John’s Wort The major lipophilic constituent in the herb St. John’s Wort (Hypericum perforatum) is the acylphloroglucinol derivative, hyperforin (Laakmann et al., 1998; Singer et al., 1999). In addition to its antibacterial activity (Gurevich et al., 1971), hyperforin was also shown to inhibit the growth of autologous MT-450 breast carcinoma in immunocompetent Wistar rats in a similar manner to that of the cytotoxic drug paclitaxel (Schempp et al., 2002). The antiproliferative and apoptosis-inducing properties of hyperforin were recently demonstrated by Hostanska et al. (2003) in several leukemia cell lines (Figure H.52). Hyperforin treatment of leukemic cells resulted in inhibition of their growth in a dose-dependent manner. Apoptosis was induced by hyperforin in leukemia U937 and K562 cells by enhancement of caspase-9 and caspase-3, and caspase-8 and caspase-3, respectively. They also reported synergism between hyperforin and hypericin, a naphthodianthrone in St. John’s Wort, on tumor-growth inhibition.
(From Hostanka, K. et al., Eur. J. Pharmaceutics Biopharmaceutics, 56:121–132, 2003. With permission.)
St. John’s Wort (Hypericum perforatum) extracts have traditionally been used to treat mild depression, although not all clinical trials have confirmed this property (Whiskey et al., 2001; Kasper, 2001; Bilia et al., 2002). The antidepressant properties of hyperforin were attributed to its ability to inhibit monoamine reuptake (Muller et al., 1997; Chatterjee et al., 1998). These workers showed that in contrast to other antidepressants, hyperforin potently inhibited synaptosomal uptake of the aminoacid transmitters GAB A and L-glutamate. Roz and coworkers (2002) reported hyperforin inhibited the uptake of monoamines by the rat-brain synaptic vesicles in a dose-dependent manner. To explain this phenomenon, Roz and Rehavi (2003) found hyperforin acted like a protonophore, reducing the pH across the synaptic-vesicle membrane, which interfered with monoamine storage.
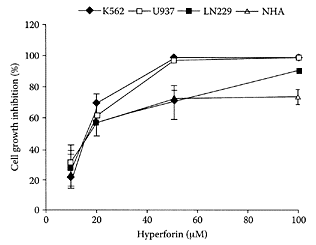
FIGURE H.52 Effect of exposure to hyperforin on cell growth of leukemia K562, U937, LN229, and NHA cells. Cells (5×103/well) were grown for 48 h in the presence of various concentrations of hyperforin and cell growth assessed by the tatrazolium salt, WST-1. (From Hostanska et al., Eur. J. Pharmaceutics Biopharmaceutics., 56:121–132, 2003. With permission.)
References
Bilia, A.R., Gallori, S., and Vincieri, F.F., St. John’s wort and depression: Efficacy, safety and tolerability—an update, Life Sci., 70:3077–3096, 2002.
Chatterjee, S.S., Bhattacharya, S.K., Wounemann, M., Singer, A., and Muller, W.E., Hyperforin as a possible antidepressant component of hypericum extracts, Life Sci., 63:499–510, 1998.
Gurevich, A.I., Dobrynin, V.N., Kolosov, M.N., Popravko, S.A., Riabova, I.D., Chernov, B.K., Derbentseva, N.A., Aisenman, B.E., and Gargulya, A.D., Hyperforin an antibiotic from Hypericum perforatum, Antibiotiki, 16:510–513, 1971.
Hostanska, K., Reichling, J., Bommer, S., Weber, M., and Sailer, R., Hyperforin a constituent of St. John’s wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cells lines, Eur. J. Pharmaceutics Biopharmaceutics., 56:121–132, 2003.
Kasper, S., Hypericum perforatum—a review of clinical studies, Pharmacopsychiatry, 34(Suppl. 1): 51–55, 2001.
Laakmann, G., Schule, C., Baghai, T., and Kieser, M., St. John’s wort in mild to moderate depression: The relevance of hyperforin for the clinical efficacy, Pharmacopsychiatry, 31:54– 59, 1998.
Muller, W.E., Rolli, M., Schafer, C., and Hafner, U., Effects of hypericum extract (LI 160) in the biochemical models of antidepressant activity, Pharmacopsychiatry, 30 (Suppl. 2): 102–107, 1997.
Roz, N., Mazur, Y., Hirshfeld, A., and Rehavi, M., Inhibition of vesicular uptake of monoamines by hyperforin, Life Sci., 71:2227–2237, 2002.
Roz, N. and Rehavi, M., Hyperforin inhibits vesicular uptake of monoamines by dissipating pH gradient across synaptic vesicle membrane, Life Sci., 73:461–470, 2003.
Schempp, C.M., Kirkin, V., Simon-Haarhaus, B., Kersten, A., Kiss, J., Termeer, C.C., Gilb, B., Kaufman, T., Borner, C., Sleeman, J.P., and Simon, J.C., Inhibition of tumor cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis, Oncogene., 21:1242–1250, 2002.
Singer, A., Wonnemann, M., and Muller, W.E., Hyperforin, a major antidepressant constituent of St. John’s wort, inhibits serotonin uptake by elevating free intracellular Na11, J. Pharmacol. Exp. Ther., 290: 1363–1368, 1999.
Whiskey, E., Wernecke, U., and Taylor, D., A systematic review of meta-analysis of Hypericum perforatum in depression: A comprehensive clinical review, Int. Clin. Psychopharmacol., 16(5):239–252, 2001.
Hypericin
see also St. John’s Wort Hypericin, a naphthodianthrone present in St. John’s wort (Hypericum perforatum L.), has received increasing attention because of its antiviral, an tire tro viral, and photodynamic properties (Gulick et al., 1999; Kamuhabwa et al, 2000; Lavie et al., 1995). In the presence of oxygen and light stimulation, hypericin is one of the most powerful photosensitizers in nature by generating reactive-oxygen species (ROS) capable of destroying tumors (Agostinis et al., 2002). The phototherapeutic properties of hypericin are important in the new therapeutic approach to the treatment of superficial neoplastic lesion, known as photodynamic therapy (PDT). Chen and de Witte (2000) found hypericin was a potent and effective tumor photosensitizer as a PDT tool using a mouse P388 lymphoma-tumor model. Treatment with hypericin (2, 5, and 20 mg/kg, i.p.) 2 h prior to light irradiation significantly (p<0.01) prolonged the life span of the mice (Figure H.53). The efficacy of PDT treatment with hypericin was highest after 2 h compared to 24- and 48-h intervals. Ali and coworkers (2001) showed that photoactivated hypericin induced apoptosis in human mucosal carcinoma cells by activating caspase proteases, particularly caspase-3.
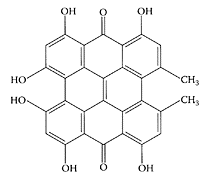
Hypericin. (From Hostanska et al., Eur. J. Pharmaceutics Biopharmaceutics., 56:121–132, 2003. With permission.)
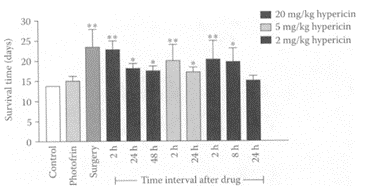
FIGURE H.53 Survival time of DBA/2 mice bearing subcutaneously transplanted P388 lymphoma cells after PDT and surgical excision. Tumor was exposed to 595 nm light 2–48 h after a 2, 5, or 20 mg/kg dose (i.p.) of hypericin and to 630 nm light 24 h after a 5 mg/kg dose (i.p.) of photofrin. For both cases, the light dose was 120 J/cm2, delivered at the intensity of 1 mW/cm2. The control group shown was the data of tumor-bearing mice without treatment. Each column represents the mean±SD (bars) for at least four animals. **p<0.01, *p<0.05, compared with the control. (From Chen and de Witte, Cancer Lett., 150:111–117, 2000. With permission.)
As an exogenous fluorophore, hypericin showed excellent sensitivity of above 90 percent in the fluorescent diagnosis of bladder cancer, suggesting its use in the early detection of this disease in situ (D’Hallewin et al, 2002). Using isolated crayfish neuron, Uzdensky et al. (2003) showed the potential of hypericin and its water-soluble derivative, developed using polyvinylpyrrolidone as carrier, for the visualization and selective photodynamic treatment of malignant gliomas.
The efficacy of St. John’s wort for the treatment of mild and moderate depression still remains controversial. Nevertheless, more than 30 clinical trials have found similar efficacy between St. John’s wort and low doses of tricyclic antidepressants, without their attendant side effects (Greeson et al., 2001; Schultz, 2002). Simmen and coworkers (2003) examined the effect of three St. John’s wort constituents, hyperforin, hypericin, and pseudohypericin. The latter is the hydroxylated derivative of hypericin found in substantially larger amounts compared to hypericin, although no pharmacological effects have yet been ascribed to it. For the first time, these researchers showed the functional antagonism of corticotrophin-releasing factor (CRF1) receptor by all three compounds, providing evidence for their role in the antidepressant efficacy of St. John’s wort. This is because selective CRF1 receptor agonists represent a new class of anxiolytics/antidepressants. Hypericin and hyperforin affected both CRF and calcitonin, while pseudohypericin selectively antagonized CRF and was considered the only real CRF1 antagonist.
References
Agostinis, P., Vantieghem, A., Merlevede, W., and de Witte, P.A.M., Hypericin in cancer treatment: More light on the way, Inter. J. Biochem. Cell Biol., 34: 221–241, 2002.
Ali, S.M., Chee, S.K., Yuen, O.K., and Olivo, M., Hypericin and hypercrellin induced apoptosis in human mucosal carcinoma cells, J. Photochem. Photobiol., 65:59–73, 2001.
Chen, B. and de Witte, P.A., Photodynamic therapy efficacy and tissue distribution of hypericin in a mouse P388 lymphoma tumor model, Cancer Lett., 150:111–117, 2000.
D’Hallewin, M.-A., Bezdetnaya, L., and Guillemin, F., Fluorescence detection of bladder cancer: A review, Eur. Urol., 42:417–425, 2002.
Greeson, J.M., Sanford, B., and Monti, D.A., St. John’s wort (Hypercum perforatum): A review of the current pharmacological, toxicological, and classical literature, Psychophramacology, 153:402–414, 2001.
Gulick, R.M., McAuliffe, V., Holden-Wiltse, J., Crumpacker, C., Liebes, L., Stein, D.S., Meehan, P., Hussey, J., and Forscht, F.T., Phase 1 studies of hypericin, the active compound in St. John’s wort, as an antiretroviral agent in HIV-infected adults, AIDS Clinical Trials Group Protocols 150 and 258, Ann. Intern. Med., 130:510–514, 1999.
Hostanska, K., Reichling, J., Bommer, S., Weber, M., and Sailer, R., Hyperforin a constituent of St. John’s wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cells lines, Eur. J. Pharmaceutics Biopharmaceutics., 56:121–132, 2003.
Kamuhabwa, A.R., Agostinis, P.A., D’Hallewin, M.A., Kasran, A., and de Witte, P.A.M., Photodynamic activity of hypericin in human urinary bladder carcinoma cells, Anticancer Res., 20:2579–2584, 2000.
Lavie, G., Mazur, D., Lavie, D., and Meruelo, D., The chemical and biological properties of hypericin: A compound with a broad spectrum of biological activities, Med. Res. Rev., 15:111– 119, 1995.
Schultz, V., Clinical trials with Hypericum extracts in patients with depression—results, comparisons, conclusions for therapy with antidepressant drugs, Phytomedicine, 9:468–474, 2002.
Simmen, U., Bobirnac, I., Ullmer, C., Lubbert, H., Buter, K.B., Schaffner, W., and Schoeffter, P., Antagonist effect of pseudohyerick at CRF, receptor, Eur. J. Pharmacol., 458:251–256, 2003.
Uzdensky, A.B., Bragin, D.E., Kolosov, M.S., Kubin, A., Loew, H.G., and Moan, J., Photodynamic effect of hypericin and a water-soluble derivative on isolated crayfish neuron and surrounding glial cells. J. Phtochem. Photobiol., 72:27–33, 2003.