S
Saffron
see also Crocin Saffron (Crocus sativus L.) is an important spice grown in Greece, Spain, Turkey, Iran, India, and Morocco. In folklore medicine, as well as in modern pharmacy, saffron has been reputed to be useful in treating numerous human diseases, such as cardiovascular diseases (Grisolia, 1974; Abdullaev, 1993) and neurodegenerative disorders accompanying memory impairment (Abe and Saito, 2000). It contains three main, pharmacologically active metabolites: (1) saffron-colored compounds crocins, which are unusual, water-soluble carotenoids. The digentiobiosyl ester of crocetin—α-crocin—is the major component of saffron. (2) Picrocrocin is the main substance responsible for the bitter taste in saffron. (3) Safranal is the volatile oil responsible for the characteristic saffron odor and aroma. Furthermore, saffron contains proteins, sugars, vitamins, flavonoids, amino acids, mineral matter, gums, and other chemical compounds (Rios et al., 1996; Winterhalter and Straubirger, 1971.)
Studies by Escribano and coworkers (1996) found extracts from saffron inhibited cell growth of human tumor cells. Cells treated with crocin proved very effective in inhibiting tumor growth. A growing body of research has demonstrated that the saffron extract itself and its main constituents, the carotenoids, possess chemopreventive properties against cancer. A review by Abdullaev and Espinosa-Aguirre (2004) discusses the recent literature on the anticancer activities of saffron and its main ingredients.
An earlier study by Xuan et al. (1999) on ischemic retinopathy and age-related macular degeneration found that monosaccharide analogues of crocin, because of their ability to significantly increase blood flow to the retina, could be used to alleviate this condition.
Crocetin was found to enhance oxygen diffusivity through liquids, such as the plasma. As a consequence of this property, crocetin has been observed to increase alveolar oxygen transport and to enhance pulmonary oxygenation. It improves cerebral oxygenation in hemorrhaged rats and acts positively in atherosclerosis and arthritis treatment (Giaccio, 2004).
A significant reduction in papilloma formation was found with saffron application in the preinitiation and postinitiation periods. The inhibition appeared to be partly due to the modulatory effects of saffron on some phase II detoxifying enzymes, such as glutathione S-transferase, glutathione peroxidase, catalase, and superoxide dismutase (Das et al., 2004).
In a double-blind, randomized clinical pilot trial, Noorbala et al. (2005) showed a hydroalcoholic extract from saffron was as effective as the drug fluoxetine in treating mild to moderate depression (Figure S.88). Based on their results, a larger-scale trial was strongly recommended.
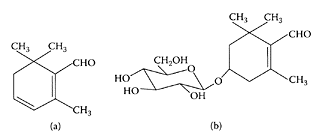
Saffranal (a) picrocrocin (b). (Adapted from Lozano et al., J. Biochem. Biophys. Methods, 43:367–378, 2000.)

FIGURE S.88 Mean ± S.E.M. scores of two groups of patients on the Hamilton Depression Rating Scale, (ns) Nonsignificant; (**) p <0.01 and (***) p <0.001. The horizontal symbols (** and ***) were used to express statistical significance vs. their respective baseline value, and ns were used for between-group comparisons. (From Noorbala et al., J. Ethnopharmacol., 97:281–284, 2005. With permission.)
References
Abdullaev, F.I., Biological effects of saffron. BioFactors, 4:83–86, 1993.
Abdullaev, F.I. and Espinosa-Aguirre, J.J., Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials, Cancer Detect. Prev., 28:426–432, 2004.
Abe, K. and Saito, H., Effects of saffron extract and its constituent crocin on learning behaviour and longterm potentiation, Phytother. Res., 14:149–152, 2000.
Das, I., Chakrabarty, R.N., and Das, S., Saffron can prevent chemically induced skin carcinogenesis in Swiss albino mice, Asian Pac. J. Cancer Prev., 5:70–76, 2004.
Escribano, J., Alonso, G.L., Coca-Prados, M., and Fernandez, J.A., Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro, Cancer Lett., 100:23–30, 1996.
Giaccio, M., Crocetin from saffron: an active component of an ancient spice, Crit. Rev. Food Sci. Nutr., 44:155–172, 2004.
Grisolia, S., Letter: hypoxia, saffron, and cardiovascular disease, Lancet, 2(78–71):41–42, 1974.
Lozano, P., Delgado, D., Gomez, D., Rubio, M., and Iborra, J.L., A non-destructive method to determine the safranal content of saffron (Crocus sativus L.) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography, J. Biochem. Biophys. Methods, 43:367–378, 2000.
Noorbala, A.A., Akhondzadeh, S., Tahmacebi-Pour, N., and Jamshidi, A.H., Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial, J. Ethnopharmacol., 97:281–284, 2005.
Rios, J.L., Recio, M.C., Giner, R.M., and Mañez, S., An update review of saffron and its active constituents, Phytother. Res., 10:189–193, 1996.
Winterhalter, P. and Straubinger, M., Saffron—renewed interest in an ancient spice, Food Rev. Int., 16:39–59, 1971.
Xuan, B., Zhou, Y.H., Min, Z.D., and Chiou, G.C., Effects of crocin analogues on ocular blood flow and retinal function, J. Ocular Pharm. Ther., 15:143–152, 1999.
Sage (Salvia officinalis)
Sage is a common aromatic and medicinal plant native to Mediterranean countries but now grown throughout Europe and North America. The odor and aromatic taste of sage are due to its volatile oil. The plant’s medicinal value resides in its crushed, dried leaves and the oil extracted from its flowers, leaves, and stems. It exhibits antibacterial qualities, inhibits viral and fungal growth (Radulescu et al., 2004), reduces perspiration and other secretions, and acts as an astringent, tightening and drying the tissues (Togel et al., 2002).
Salvia officinalis has been used in herbal medicine for many centuries. It has been suggested, on the basis of traditional medicine and its in vitro cholinergic-binding properties and modulation of mood and cognitive performance in humans, that Salvia officinalis might potentially provide a novel natural treatment for Alzheimer’s disease. A recent study demonstrated the efficacy of Salvia officinalis extract in the management of mild to moderate Alzheimer’s disease (Akhondzadeh et al., 2003).
Caffeic acid, rosmarinic acid, and oligomers of caffeic acid, with multiple catechol groups, are all constituents of Salvia officinalis, with antioxidant potential with regard to their radical-scavenging activity and the stability and structure of the intermediate radicals (Bors et al., 2004). Ursolic acid is the main component in Salvia officinalis L. leaves that is involved in sage topical anti-inflammatory activity (Baricevic et al., 2001).
Antimutagenic properties of terpenoid fractions of sage (Salvia officinalis) were demonstrated by Vujosevic and Blagojevic (2004) in mammalian system in vivo. Sage decreases the frequency of aberrant cells, induced by a potent mutagen. The acidic polysaccharide fractions from the aerial parts of sage were found to exhibit mitogenic activities, indicating that they may have adjuvant properties (Capek and Hribalova, 2004).
Lima and coworkers (2005) recently reported that drinking a water infusion (tea) of common sage (Salvia officinalis) improved the liver antioxidant status, measured as GSH content, in mice and rats. Compared to water, drinking sage tea conferred some protection in the hepatocyte cultures exposed to tert-butyl hydroperoxide (t-BHP). This was particularly evident in the presence of 1 mM tBHP (Figure S.89). These results point to the important antioxidant contribution by sage in combating oxidative stress.

FIGURE S.89 Effect of sage-tea consumption (in vivo for 14 days) on t-BHP-induced decrease in GSH content of primary hepatocyte cultures, presented as percentage of the control. Values are mean±S.E.M., n=4. *p <0.05, significantly different with Student’s t-test. (From Lima et al., J. Ethnopharm., 97:383–389, 2005. With permission.)
References
Akhondzadeh, S., Noroozian, M., Mohammadi, M., Ohadinia, S., Jamshidi, A.H., and Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomized and placebo-controlled trial, J. Clin. Pharm. Then, 28:53–59, 2003.
Baricevic, D., Sosa, S., Della Loggia, R., Tubaro, A., Simonovska, B., Krasna, A., and Zupancic, A., Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid, J. Ethnopharmacol., 75:125–132, 2001.
Bors, W., Michel, C., Stettmaier, K., Lu, Y., and Foo, L.Y., Antioxidant mechanisms of polyphenolic caffeic acid oligomers, constituents of Salvia officinalis, Biol. Res., 37:301–311, 2004.
Capek, P. and Hribalova, V., Water-soluble polysaccharides from Salvia officinalis L. possessing immunomodulatory activity, Phytochemistry, 65:1983–1992, 2004.
Lima, C.F., Andrade, P.B., Seabra, R.M., FernandesFerreira, M., and Pereira-Wilson, C., The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats, J. Ethropharm., 97:383–389, 2005.
Radulescu, V., Chiliment, S., and Oprea, E., Capillary gas chromatography-mass spectrometry of volatile and semi-volatile compounds of Salvia officinalis, J. Chromatogr. A., 1027:121–126, 2004.
Togel, B., Greve, B., and Raulin, C., Current therapeutic strategies for hyperhidrosis: a review, Eur. J. Dermatol., 12:219–223, 2002.
Vujosevic, M. and Blagojevic, J., Antimutagenic effects of extracts from sage (Salvia officinalis) in mammalian system in vivo, Acta Vet. Hung., 52:439–443, 2004.
Saponins
Saponins are a group of surface-active glycosides, produced mainly by plants and some lower marine animals and bacteria (Espada and Riguera, 1997; Yoshiki et al., 1998). They consist of a sugar moiety, such as glucose, galactose, glucuronic acid, xylose, rhamnose, or methylpentose, attached to a hydrophobic aglycone (sapogenin), which can be a triterpenoid or steroid. Triterpenoid saponins are predominant in cultivated crops, while steroid saponins are found in plants used as herbs, particularly for their health-related properties (Fenwick et al., 1991). Some saponins were found by Johnson and coworkers (1986) to increase cell permeability and facilitate the uptake of substances not previously absorbed. For example, Gee and coworkers (1993) showed the ability of quinoa saponins to increase cell permeability, which could be used to enhance drug absorption by patients. In fact, Estrada et al. (1998) found that quinoa saponins could act as adjuvants for mucosally administered vaccines. Saponins from different sources were also shown to lower serum cholesterol levels in a variety of animals and humans (Al-Habori and Raman, 1998) and to have anti-inflammatory (Wei et al., 2004) and antioxidant (Sur et al., 2001) properties. Saponin-based adjuvants stimulated the immune system, as well as enhanced antibody production at low-dose levels (Oda et al., 2000). The adjuvant activity was attributed to branched sugar chains (Bomford et al., 1992) or aldehyde groups (Kensil, 1996). Saponins from different sources were found to inhibit cancer cells in vitro (Podolak et al., 1998). Triterpenoid saponins from Acacia vitoriae were reported to selectively inhibit the growth of tumor in human breast-cancer cell lines by arresting cell cycle or by apoptosis in leukemia-cell lines (Mujoo et al., 2001). Triterpene saponins also showed a prominent IL-2-inducing activity, which may explain the mechanism involved in their immunomodulatory and anticancer effects (Yesilada et al., 2005). Francis and coworkers (2002) reviewed the biological action of saponins in animal systems. Recently, triterpenoid saponins isolated from the leaves of the Vietnamese medicinal plant Maesa balansae, showed in vitro and in vivo activity against the tropical protozoal parasite Leishmania infantum (Scheme S.54) (Germonprez et al., 2005).
References
Al-Habori, M. and Raman, A., Antidiabetic and hypocholesterolaemic effects of fenugreek, Phytother. Res., 12:233–242, 1998.
Bomford, R., Stapleton, M., Winsor, S., Beesley, J.E., Jessup, E.A., Price, K.R., and Fenwick, G.R., Adjuvanticity and IS COM formation by structurally diverse saponins, Vaccine, 10:572– 577, 1992.
Espada, A. and Riguera, R., Boussingoside E, anew triterpenoid saponin from the tubers Boussingaultia baselloides, J. Nat. Prod., 60:17–19, 1997.
Estrada, A., Li, B., and Laarveld, B., Adjuvant action of Chenopodium quinoa saponins on the induction of antibody responses to intragastric and intranasal administered antigens in mice, Comp. Immunol. Microbiol. Infect. Dis., 21:225–236, 1998.
Fenwick, G.R., Price, K.R., Tsukamoto, C., and Okubo, K. Saponins, in Saponins in Toxic Substances in Crop Plants, D’Mello, F.J.P., Duffus, C.M., and Duffus, J.H., Eds., Cambridge: The Royal Society of Chemistry, Cambridge, 1991.
Francis, G., Kerem, Z., Makkar, H.P., and Becker, K., The biological action of saponins in animal systems: a review, Br. J. Nutr., 88:587–605, 2002.
Gee, J.M., Price, K.R., Ridout, C.L., Wortley, G.M., Hurrell, R.F. and Johnson, I.T., Saponins of quinoa (Chenopodium quinoa). Effect of processing in their abundance in quinoa products and their biological effects on intestinal mucosal tissue. J. Sci. Food Agric., 63:201–209, 1993.
Germonprez, N., Maes, L., Van Puyvelde, L., Van Tri, M., Tuan, D.A., and De Kimpe, N., In vitro and in vivo anti-leishmanial activity of triterpenoid saponins isolated from Maesa balansae and some chemical derivatives, J. Med. Chem., 48:32–37, 2005.
Kensil, C.R., Saponins as vaccine adjuvants, Crit. Rev. Then Drug Carrier Sys., 13:1–55, 1996.
Leonard, S., Capote, R., Germonprez, N., Puyvelde, L.V., De Kimpe, N., Vermeersch, H., Rosier, J., Maes, L., Roets, E., and Hoeogmartens, J., Liquid chromatographic method for analysis of saponins in Maesa balansae extract active against leishmaniasis, J. Chromatogr. A, 1012:39–46, 2003.
Mujoo, K., Haridas, V., Hoffman, J.J., Wachter, G.A., Hutter, L.K., Lu, Y., Blake, M.E., Jayatilake, G.S., Bailey, D., Mills, G.B., and Gutterman, J.U., Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce apoptosis, Cancer Res., 61:5486–5490, 2001.
Oda, K., Matsuda, H., Murakami, T., Katamaya, S., Ohgitani, T., and Yoshikawa, W., Adjuvant and haemolytic activities of 47 saponins derived from medicinal and plant foods, Biol. Chem., 381:67–74, 2000.
Podolak, I., Elas, M., and Cieska, K., In vitro anti-fungal and cytotoxic activity of triterpene sapono-sides and quinoid pigments from Lysimachia vulgaris L., Phytother. Res., 12:S70–S73, 1998.
Sur, P., Chaudhuri, T., Vedasiromoni, J.R., Gomes, A., and Ganguly, D.K., Anti-inflammatory and anti-oxidant property of saponins of tea [Camellia sinen-sis (L) O. Kuntze] root extract, Phytother. Res., 15: 174–176, 2001.
Wei, F., Ma, L.Y., Jin, W.T., Ma, S.C., Han, G.Z., Khan, I.A., and Lin, R.C., Anti-inflammatory triterpenoid saponins from the seeds of Aesculus chinensis, Chem. Pharm. Bull., 52:1246–1248, 2004.
Yesilada, E., Bedir, E., Calis, I., Takaishi, Y., and Ohmoto, Y., Effects of triterpene saponins from Astragalus species on in vitro cytokine release, J. Ethnopharmacol., 96:71–77, 2005.
Yoshiki, Y., Kodov, Y.S. and Okubo, K., Relationship between chemical structures and biological activities of triterpenoid saponins from soybean., Biosci. Biotechnol Biochem., 62:2291–2299, 1998.
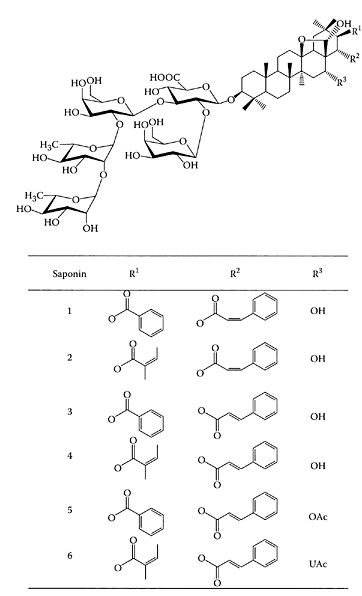
SCHEME S.54 Six triterpenoid saponins extracted from Maesa balansae. (From Leonard et al., J. Chromatogr. A, 1012:39–46, 2003. With permission.)
Sarcophytol
A Sarcophytol A (SaA) is a cembrane-type diterpene isolated from the marine soft coral Sarcophyton glaucum. It showed anti-cancer and cancer-preventive effects in two animal models: transplanted human pancreatic-cancer cells in nude mice and pancreatic carcinogenesis induced by N-nitrobis-(2-hydroxypropyl) amine in Syrian golden hamsters (Yokomatsu et al., 1994).
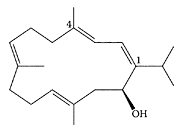
Sarcophytol A. (From Li et al., Tetrahedron Lett., 40:965–968, 1999. With permission.)
SaA also provided significant protection against the induction of genetic damage in human lung cells exposed to tobacco-specific nitrosamines (Weitberg and Corvese, 1999).
Recently, the natural cembranolide sarcophine and its lactone ring-opened analogue were oxidized to prepare hydroxylated derivatives, which were shown to have higher activity than the chemopreventive agent Sarcophytol A (Katsuyama et al., 2002).
References
Katsuyama, I., Fahmy, H., Zjawiony, J.K., Khalifa, S.I., Kilada, R.W., Konoshima, T., Takasaki, M., and Tokuda, H., Semisynthesis of new sarcophine derivatives with chemopreventive activity, J. Nat. Prod., 65:1809–1814, 2002.
Li, W.-DZ., Li, Y., and Li, Y., Concise and efficient total syntheses of (±)-sacophytols A and B, two anti-tumor cembrane diterpenoids, by an intramolecular McMurry olefination strategy, Tetrahedron Lett., 40: 965–968, 1999.
Weitberg, A.B. and Corvese, D., The effect of epigallocatechin galleate and Sarcophytol A on DNA strand breakage induced by tobacco-specific nitrosamines and stimulated human phagocytes, J. Exp. Clin. Cancer Res., 18:433–437, 1999.
Yokomatsu, H., Satake, K., Hiura, A., Tsutsumi, M., and Suganuma, M., Sarcophytol A: a new chemotherapeutic and chemopreventive agent for pancreatic cancer, Pancreas, 9:526–530, 1994.
Saskatoon berry
The Saskatoon (Amelanchier alnifolia) is a small to large shrub, or a small tree, which belongs to the rose family. The Saskatoon, an important food source, was also used as a source wood and a medicinal plant. Today, Saskatoons are used in a wide variety of ways, from pies, jams, jellies, syrups, ice cream toppings, wine, liqueurs, and flavor concentrates to components of baked goods. The methanolic extract of Amelanchier alnifolia was found active against an enteric coronavirus, demonstrating antiviral activities at the noncytotoxic concentrations (McCutcheon et al., 1995)

References
McCutcheon, A.R., Roberts, T.E., Gibbons, E., Ellis, S.M., Babiuk, L.A., Hancock, R.E.W., and Towers, G.H.N., Antiviral screening of British Columbian medicinal plants, J. Ethnopharmacol., 49:101–110, 1995.
Savory (Satureja hortensis L.)
Savory, an annual herb of the Lamiaceae family, is used as a condiment, as well as in folk medicine, for treating infectious diseases. It contains an essential oil composed of thymol, although the main component is carvacrol, a positional isomer of thymol (30 percent to 45 percent), as well as p-cymene (max. 30 percent), γ-terpinene, α-pinene (8 percent), dipentene, borneol, 1-linalool, terpineol, and 1-carvone. Antimicrobial and antioxidant tests by Gulluce et al. (2003) showed the essential oil exhibited antimicrobial activities against all 23 bacteria and 15 fungi and yeast species tested, while linoleic-acid oxidation was inhibited by 95 percent. The evaluation of antioxidant power of glycosidically bound volatile aglycones from savory showed the antioxidative activity possessed by these compounds was comparable to that of the essential oil (Radonic and Milos, 2003). The antioxidant properties of a crude extract and its purified ethyl acetate-soluble fraction from the aerial material of savory was recently demonstrated by Dorman and Hiltunen (2004) using an Fe(III) reductive and DPPH, ABTS+, and hydroxyl free-radical-scavenging assays. Chorianopoulos et al. (2004) reported that essential oils extracted from the Satureja species represented an inexpensive source of natural antibacterial compounds for potential use in food systems to prevent the growth of foodborne bacteria and extend the shelf life of the processed food. The antibacterial activity of a number of essential oils, including savory oil, was found to be effective against vaginal microorganisms responsible for infectious gynecological diseases (Arnal-Schnebelen et al., 2004). Other species of savory plants were reported to have antinociceptive (Hajhashemi et al., 2002) and anti-inflammatory (Uslu et al., 2003) effects.
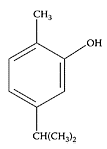
Carvacrol. (From De Vicenzi et al., Fitoterapia, 75:801–804, 2004. With permission.)
References
Arnal-Schnebelen, B., Hadji-Minaglou, F., Peroteau, J.-F., Ribeyre, F., and de Billerbeck, V.G., Essential oils in infectious gynaecological disease: a statistical study of 658 cases, Int. J. Aromather., 14:192–197, 2004.
Chorianopoulos, N., Kalpoutzakis, E., Aligiannis, N., Mitaku, S., Nychas, G-J., and Haroutounian, S.A., Essential oils of Satureja, Origanum, and Thymus species: chemical composition and antibacterial activities against foodborne pathogens, J. Agric. Food Chem., 52:8261–8267, 2004.
De Vincenzi, M., Stammati, A., De Vincenzi, A., and Silano, M., Constituents of aromatic plants: carvacrol, Fitoterapia, 75:801–804, 2004.
Dorman, H.J.D. and Hiltunen, R., Fe(III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L.) extract and subfractions, Food Chem., 88:193–199, 2004.
Gulluce, M., Sokmen, M., Daferera, D., Agar, G., Ozkan, H., Kartal, N., Polissiou, M., Sokmen, A., and Sahin, F., In vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L.., J. Agric. Food Chem., 51: 3958–3965, 2003.
Hajhashemi, V., Ghannadi, A., and Pezeshkian, S.K., Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil, J. Ethnopharmacol., 82:83–87, 2002.
Radonic, A. and Milos, M., Chemical composition and antioxidant test of free and glycosidically bound volatile compounds of savory (Satureja montana L. subsp. montana) from Croatia, Nahrung, 47:236–237, 2003.
Uslu, C., Murat Karasen, R., Sahin, F., Taysi, S., and Akcay, F., Effects of aqueous extracts of Satureja hortensis L. on rhinosinusitis treatment in rabbit, J. Ethnopharmacol., 88:225–228, 2003.
Saw palmetto
see also Palmetto berries Saw palmetto (Serenoa repens) is a North American native plant whose berries are used for medicinal purposes. A letter to the editor by Champault and coworkers in 1984 first highlighted the pharmacological benefits of saw palmetto for the treatment of benign prostatic hyperplasia. It has since become the treatment for enlarged prostate or benign prostatic hyperplasia (BPH) in Europe (Wilt et al., 1998). Using a six-month, randomized trial, Veltri et al. (2002) found that treating men with symptomatic BPH with a saw palmetto herbal blend altered DNA chromatin structure and organization in prostate epithelial cells, suggesting a possible molecular basis for its therapeutic effect. Recent studies in the United States by Gerber et al. (2000) and Gong and Gerber (2004) showed that saw palmetto improved urinary function for those suffering from BPH.
The efficacy of saw palmetto appears to be similar to medications, such as finasteride, but it is better tolerated and less expensive. There are no known drug interactions with saw palmetto, and reported side effects are minor and rare. It was also used to treat chronic prostatitis, but currently there is no evidence of its efficacy (Gordon and Shaughnessy, 2003).
References
Champault, G., Patel, J.C., and Bonnard, A.M., A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia, Br. J. Clin. Pharmacol., 18:461–462, 1984.
Gerber, G.S., Saw palmetto for the treatment of men with lower urinary tract symptoms, J. Urol., 163: 1408–1412, 2000.
Gong, E.M. and Gerber, G.S., Saw Palmetto and benign prostatic hyperplasia, Am. J. Chin. Med., 32: 331–338, 2004.
Gordon, A.E. and Shaughnessy, A.F., Saw palmetto for prostate disorders, Am. Fam. Physician, 67:1281–1283, 2003.
Veltri, R.W., Marks, L.S., Miller, M.C., Bales, W.D., Fan, J., Macairan, M.L., Epstein, J.I., and Partin, A.W., Saw palmetto alters nuclear measurements reflecting DNA content in men with symptomatic BPH: evidence for a possible molecular mechanism, Urology, 60:617–622, 2002.
Wilt, T.J., Ishani, A., Stark, G., MacDonald, R., Lau, J., and Mulrow, C., A systematic review: Saw palmetto extracts for treatment of benign prostatic hyperplasia, JAMA, 280:1604–1609, 1998.
Sea buckthorn (Hippophae rhamnoides L.)
Sea buckthorn, a temperate, hardy bush growing in Central Asia and Europe, produces nutritious and delicious berries (Roussi, 1971). The oil from the berries of sea buckthorn has been used in Chinese medicine for many centuries for treating cardiovascular disease. In fact, sea-buckthorn berries, particularly the alcohol extract of the twigs, was reported to inhibit thrombus formation or platelet aggregation (Xu and Chen, 1991). Sea buckthorn is rich in antioxidants, tocopherols (Luhua et al., 2004), carotenoids, and vitamin C, as well as phytosterols, such as sitosterol (Field et al., 1997). In the sea-buck-thorn pomace extract, the oligomeric proanthocyanidins accounted for 75 percent of the total antioxidant activity (Rosch et al., 2004). Using a supercritical extract of sea-buckthorn oil, Johansson and coworkers (2000) showed it inhibited platelet aggregation. Cheng and coworkers (2003) reported that a total flavone (TFH) extract from sea buckthorn exhibited a similar inhibitory effect to aspirin on platelet aggregation induced by collagen in mouse femoral artery (Figure S.90). This ability to prevent in vivo thrombogenesis, similar to aspirin, suggested sea buckthorn may help prevent cardiac and cerebral thrombosis.
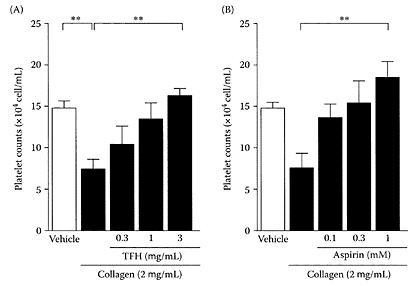
FIGURE S.90 Effects of (A) TFH and (B) aspirin on platelet aggregation induced by collagen (2 μg.mL) in whole blood from five mice. An open column indicates platelet counts in the tubes with added vehicle without collagen. Data are presented as means±S.E.M. **; p<0.01 by Bonferroni’s multiple comparison test. (From Cheng et al., Life Sci., 72:2263– 2271, 2003. With permission.)
Eccleston et al. (2002) showed sea-buck-thorn juice was rich in antioxidants and moderately decreased the susceptibility of LDL to oxidation. An earlier study by Yang and coworkers (1999) found α-linolenic acid in sea buckthorn had a beneficial effect on atopic dermatitis (AD), a condition in which the skin is dry, scaly, and itchy with eczematous inflammation and lesions. Sea buckthorn was also reported to be a hopeful drug for prevention and treatment of liver fibrosis (Gao et al., 2003).
References
Cheng, J., Kondo, K., Suzuki, Y., Ikeda, Y., Meng, X., and Umemura, K., Inhibitory effects of total flavones of Hippophae rhamnoides L. on thrombosis in mouse femoral artery and in vitro platelet aggregation, Life Sci., 72:2263–2271, 2003.
Gao, Z.L., Gu, X.H., Cheng, F.T., and Jiang, F.H., Effect of sea buckthorn on liver fibrosis: a clinical study, World J. Gastroenterol., 9:1615–7, 2003.
Eccleston, C., Baoru, Y., Tahvonen, R., Kallio, H., Rimbach, G.H., and Minihane, A.M., Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans, J. Nutr. Biochem., 13:346–354, 2002.
Johansson, A.K., Korte, H., Yang, B., Stanley, J.C., and Kallio, H.P., Sea buckthorn berry oil inhibits platelet aggregation, J. Nutr. Biochem., 11:491–495, 2000.
Luhua, Z., Ying, T., Zhengyu, Z., and Guangji, W., Determination of alpha-tocopherol in the Traditional Chinese Medicinal preparation Sea buckthorn oil capsule by non-aqueous reversed phase-HPLC, Chem. Pharm. Bull., 52:150–152, 2004.
Rosch, D., Mugge, C., Fogliano, V., and Kroh, L.W., Antioxidant oligomeric proanthocyanidins from sea buckthorn (Hippophae rhamnoides) Pomace, J. Agric. Food Chem., 52:6712–6718, 2004.
Roussi, A., The genus Hippophae L., A taxonomic study, Ann. Bot. Fennici., 8:177–227, 1971.
Xu, Q. and Chen, C., Effects of oil of Hippophae rhamnoides on the experimental thrombus formation and blood coagulation system, Res. Dev. Natu. Prod., 3:70–73, 1991.
Yang, B., Kalimo, K.O., Mattila, L.M., Kallio, S.E., Katajisto, J.K., Peltola, O.J., and Kallio, H.P., Effects of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on atopic dermatitis, J. Nutr. Biochem., 10:622–630, 1999.
Sea cucumbers
Sea cucumbers or Holothuroida, are an abundant and diverse group of worm-like, soft-bodied echinoderms. They are ubiquitous in the marine environment but particularly diverse in tropical, shallow-water coral reefs. In the Southeast Asia regions, sea cucumbers are used as food supplements and as traditional remedy for wounds (Perchenik, 1996), parasitic skin infections (Shimada, 1969), and other ailments, such as backache, joint pain, and stomach and mouth ulcers.
Sea cucumber contains a wide range of nutrients, including collagen, marine protein, essential fatty acids, and antioxidants, including vitamin E and minerals (Hawa et al., 1999).
A few species of sea cucumbers showed antibacterial activity (Ridzwan et al., 1995), and glycosphingolipids from the sea cucumber had neuritogenic activity toward the saponin biosynthesis (Kerr and Chen, 1995). Yamada (2002) isolated pheochromocytoma cell line C-type mannan-binding lectins from various species of sea cucumbers exhibiting relatively high agglutinating activity (Bulgakov et al., 2000). Recently, Kariya et al. (2004) isolated two types of fucan sulfates, types A and B, from sea cucumber (Stichopus japonicus) that were potent inhibitors of osteoclastogenesis. Type A consisted of a backbone of (163)-linked fucosyl residues substituted at C-4 with the fucosyl residues sulfated at C-2/C-4 (Scheme S.55).
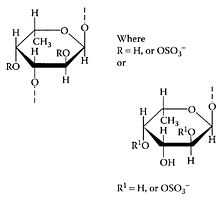
SCHEME S.55 Hypothetical structures of type A and type B fucan sulfates. Both have a backbone of (1→3)-linked fucose residues substituted with fucosyl residues at C-2 and C-4. Sulfate substitution(s) occur at C-2 and C-4 position(s). (Kariya et al., Carbohydr. Res., 339:1339–1346, 2004. With permission.)
Type B consisted of unbranched (163)-linked fucosyl residues with sulfate substitution(s) at C-2 and/or C-4. The presence of either type A or B inhibited osteoclastogenesis in an in vitro osteoclast assay by 99.8 percent and 96.3 percent, respectively, compared to the control (Figure S.91). The potent inhibition of osteoclastogenesis by fucan sulfates points to their potential for treating some of the symptoms associated with osteoporosis and rheumatoid arthritis.
References
Bulgakov, A.A., Nazarenko, E.L., Petrova, I.Y., Eliseikina, M.G., Vakhrusheva, N.M., and Zubkov, V.A., Isolation and properties of a mannan-binding lectin from the coelomic fluid of the holothurian Cucumaria japonica, Biochemistry, Moscow; 65: 933–939, 2000.
Hawa, I., Zulaikah, M., Jamaludin, M., Zainal Abidin, A.A., Kaswandi, M.A., and Ridzwan, B.H., The potential of the coelomic fluid in sea cucumber as an antioxidant, Mal. J. Nutr., 5:55–59, 1999.
Kariya, Y., Mulloy, B., Imai, K., Tominaga, A., Kaneko, T., Asari, A., Suzuki, K., Masuda, H., Kyogashima, M., and Ishii, T., Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber Stichopus japonicus and their ability to inhibit osteoclastogenesis, Carbohydr. Res., 339: 1339–1346, 2004.
Kerr, R.G. and Chen, Z., In vivo and in vitro biosynthesis of saponins in sea cucumbers, J. Nat. Prod., 58:172–176, 1995.
Perchenik, J.A., The echinoderms, in Biology of Invertebrates, 3rd ed., Dubuque, W.M.C., Ed., Brown Publisher, London, 1996, pp. 445–474.
Ridzwan, B.H., Kaswandi, M.A., Azman, Y., and Fuad, M., Screening for antibacterial agents in three species of sea cucumbers from coastal areas of Sabah, Gen. Pharmacol., 26:1539–1543, 1995.
Shimada, S., Antifungal steroid glycoside from sea cucumber, Science, 163:1462, 1969.
Yamada, K., Chemo-pharmaceutical studies on the glycosphingolipid constituents from echinoderm, sea cucumbers, as the medicinal materials, Yakugaku Zasshi., 122:1133–1143, 2002.
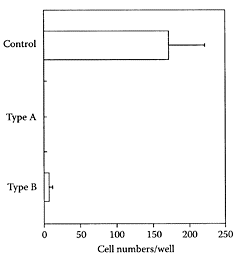
FIGURE S.91 Inhibitory effects of types A and B fucan sulfate on an in vitro osteoclast-formation assay system. Data expressed as mean±SD (n=3) taking the control as 0 percent. (From Kariya et al., Carbohydr. Res., 339:1339–1346, 2004. With permission.)
Secoisolariciresinol diglycoside
see also Lignans Secoisolariciresinol diglycoside (SDG) is a plant lignan most notably found in flaxseed (linseed). SDG is classified as a phytoestrogen with a weak estrogenic activity. The level of SDG in flaxseed typically varies between 0.6 percent and 1.8 percent. Following ingestion, SDG is converted to the aglycone Secoisolariciresinol, which is then metabolized to the mammalian lignans enterolactone and enterodiol. Most of the effects of oral SDG are mediated by enterolactone and enterodiol.
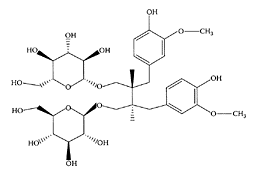
Secoisolariciresinol diglucoside. (From Coran et al., J. Chromatogr. A, 1045:217–222, 2004. With permission.)
SDG, enterolactone, and enterodiol exhibited a number of antioxidant activities (Kitts et al., 1999), including inhibition of lipid peroxidation and scavenging of hydroxy radicals. SDG inhibited mammary-tumor development (Rickard et al., 2000), as well as delayed the progression of dimethylbenz[a]anthraceneinduced mammary tumorigenesis (Rickard et al., 1999). Supplementation with SDG reduced pulmonary metastasis of mice melanoma cells and inhibited the growth of metastatic tumors formed in the lungs (Li et al., 1999). This was evident by a significant decrease in the mean tumor cross-sectional area and volume in a dose-dependent manner compared to the con-trol (Table S.61).
References
Coran, S.A., Giannellini, V., and Bambagiotti, M., High-performance thin-layer chromatographicden- sitometric determination of Secoisolariciresinol diglucoside in flaxseed, J. Chromatogr. A, 1045:217–222, 2004.
Kitts, D.D., Yuan, Y.V., Wrjewickreme, A.N., and Thompson, L.U., Antioxidant activity of the flaxseed lignan Secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone, Mol. Cell Biochem., 202:91–100, 1999.
Li, D., Yee, J.A., Thompson, L.U., and Yan, L., Dietary supplementation with secoisolariciresinol diglycoside (SDG) reduces experimental metastasis of melanoma cells in mice, Cancer Lett., 142:91–96, 1999.
Rickard, S.E., Yuan, Y.V., Chen. J., and Thompson, L.U., Dose effects of flaxseed and its lignan on N-methyl-N-nitrosourea-induced mammary tumorigenesis, Nutr. Cancer, 35:50–57, 1999.
Rickard, S.E., Yuan, Y.V., and Thompson, L.U., Plasma insulin-like growth factor I levels in rats are reduced by dietary supplementation of flaxseed or its lignan secoisolariciresinol diglycoside, Cancer Lett., 161:47–155, 2000.
Selenium
Selenium, a trace element essential in small amounts, can be toxic when taken in larger amounts. The levels in the body depend mainly on the amount of selenium in the diet, which is a function of the selenium content of the soil. Selenium is required for the functioning of more than 30 known selenoproteins, essential for normal functioning of the immune system (He et al., 2004), thyroid gland (Zagrodzki et al., 2001), and normal development, growth, metabolism, and defense of the body (Dodig and Cepelak, 2004; Kohrl et al., 2000).
A large number of studies confirmed that selenium supplementation plays a preventive and therapeutical role in diseases, such as male infertility (Foresta et al., 2002), viral infections (Broome et al., 2004), HIV (Kupka et al., 2004), cancer (recently reviewed by Patrick, 2004), and cardiovascular (Alissa et al., 2003) and autoimmune diseases (Gartner and Gasnier, 2003).
Selenium is an essential constituent of a number of enzymes, some of which have anti-oxidant functions. A deficiency of this element in animals renders them susceptible to injury by certain types of oxidative stress (Burk, 2002). In addition, selenomethionine catalyzes the reduction of peroxynitrite and low-molecular-weight organoselenium, compounds of pharmacologic interest known to catalyze the reduction of hydroperoxides or peroxynitrite with various cellular-reducing equivalents (Klotz et al., 2003). Oxidative stress plays an important role in vascular degenerative lesions observed in diabetes. Selenium is the cofactor of glutathione peroxidase, which is associated with thrombosis and cardiovascular complications of diabetes (Faure, 2003).
Analyses of pooled data from 1763 trial participants showed that statistically, individuals whose blood-selenium values were in the highest quartile (median =150 ng/mL) had significantly lower odds of developing new adenomas compared with those in the lowest. The inverse association between higher blood-selenium concentration and adenoma risk supports previous findings indicating that higher selenium status may be related to decreased risk of colorectal cancer (Jacobs et al., 2004).
Popova (2002) investigated the influence of neonatal selenium exposure on spontaneous liver-tumor formation in adult mice. Selenium was administered to pregnant CBA mice during their last week of pregnancy and for 10 days following parturition. There was a significant reduction in the incidence of spontaneous hepatomas in the adult male progeny, but not in adult females. This indicated that neonatal selenium altered hepatoma incidence in a sex-dependent manner.
References
Alissa, E.M., Bahijri, S.M., and Ferns, G.A., The controversy surrounding selenium and cardiovascular disease: a review of the evidence, Med. Sci. Monit., 9:RA9–RA18, 2003.
Broome, C.S., McArdle, F., Kyle, J.A.M., Andrews, F., Lowe, N.M., Hart, C.A., Arthur, J.R., and Jackson, M.J., An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status, Am. J. Clin. Nutr., 80: 154–162, 2004.
Burk, R.F., Selenium, an antioxidant nutrient (Review), Nutr. Clin. Care, 5:75–79, 2002.
Dodig, S. and Cepelak, I., The facts and controversies about selenium, Acta Pharm., 54:261–276, 2004.
Faure, P., Protective effects of antioxidant micronu-trients (vitamin E, zinc and selenium) in type 2 dia-betes mellitus (Review), Clin. Chem. Lab. Med., 41: 995–998, 2003.
Foresta, C., Flohe, L., Garolla, A., Roveri, A., Ursini, F., and Maiorino, M., Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase, Biol. Reprod., 67:967– 971, 2002.
Gartner, R. and Gasnier, B.C., Selenium in the treatment of autoimmune thyroiditis, Biofactors, 19:165—170, 2003.
He, S-X., Wu, B., Chang, X-M., Li, H-X., and Qiao, W., Effects of selenium on peripheral blood mononuclear cell membrane fluidity, interleukin-2 production and interleukin-2 receptor expression in patients with chronic hepatitis, World J. Gastroenterol., 10: 3531–3533, 2004.
Klotz, L-O., Kroncke, K-D., Buchczyk, D.P., and Sies, H., Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress, J. Nutr., 133:1448S- 1451S, 2003.
Kohrl, J., Brigelius-Flohe, R., Bock, A., Gartner, R., Meyer, O., and Flohe, L., Selenium in biology: facts and medical perspectives, Biol. Chem., 381:849–864, 2000.
Kupka, R., Msamanga, G.I., Spiegelman, D., Morris, S., Mugusi, F., Hunter, D.J., and Fawzi, W.W., Selenium status is associated with accelerated HIV disease progression among HIV-1- infected pregnant women in Tanzania, J. Nutr., 134:2556–2560, 2004.
Jacobs, E.T., Jiang, R., Alberts, D.S., Greenberg, E.R., Gunter, E.W., Karagas, M.R., Lanza, E., Ratnasinghe, L., Reid, M.E., Schatzkin, A., SmithWarner, S.A., Wallace, K., and Martinez, M.E., Selenium and colorectal adenoma: results of a pooled analysis, J. Natl. Cancer Inst., 96:1669–1675, 2004.
Patrick, L., Selenium biochemistry and cancer: a review of the literature, Altern. Med. Rev., 9:239– 258, 2004.
Popova, N.V., Perinatal selenium exposure decreases spontaneous liver tumorogenesis in CBA mice, Cancer Lett., 179:39–42, 2002.
Zagrodzki, P., Nicol, F., Arthur, J.R., and Slowiaczek, M., Selenoproteins in human thyroid tissues, Biofactors, 14:223–227, 2001.
Senega (Polygara senega)
This perennial herb grows in central and western North America. The name of the genus, Polygala, means “much milk,” alluding to its own profuse secretions and their effects. “Senega” is derived from the Seneca tribe of North American Indians, among whom the roots were used as a remedy for snake bites. The root contains polygalic acid, virgineic acid, pectic and tannic acids, yellow, bitter, coloring matter, cerin-fixed oil, resin, traces of volatile oil (a mixture of valeric ether and methyl salicylate), 7 percent sugar, from 2 percent to 5 percent senegin (Saitoh et al., 1994), malatesgum, woody fiber, salts, aluminum, silica, magnesium, and iron (Moes, 1966). Fresh senega root has a pleasant odor due to its content of approximately 0.1 percent methyl salicylate. The active ingredient, however, is a complex mixture in the root of triterpenoid saponins in a concentration ranging from 8 percent to 16 percent (Yoshikawa et al., 1996). The saponins act by local irritation on the lining of the stomach, causing nausea that in turn stimulates both bronchial secretion and the sweat glands.

During the early 19th century, senega root was used as an expectorant cough remedy. Today, senega root is used to treat bronchitis, tracheitis, emphysema, and inflammation of the respiratory tract (Kuribara and Tadokoro, 1989). The saponins suppress coughing, while their detergent activity breaks up phlegm (Kantee, 1973). Senega is also believed to stimulate bronchial mucous-gland secretion. Saponins in senega root may hold some potential for treatment of noninsulin-dependent diabetes (Kako et al., 1996). Senegose A, senegin II, senegin III, and senegasaponin b, hair-regrowth substances, were isolated from Polygara senega by Ishida et al. (1999).
Recently, senega saponins were reported to display immunopotentiation activity to protein and viral antigens, suggesting their potential role as vaccine adjuvants to increase specific immune responses (Estrada et al., 2000)
References
Estrada, A., Katselis, G.S., Laarveld, B., and Barl, B., Isolation and evaluation of immunological adjuvant activities of saponins from Polygala senega L., Comp. Immunol. Microbiol. Infect. Dis., 23:21–43, 2000.
Ishida, H., Inaoka, Y., Okada, M., Fukushima, M., Fukazawa, H., and Tsuji, K., Studies of the active substances in herbs used for hair treatment. III. Isolation of hair-regrowth substances from Polygara senega var. latifolia TORR. et GRAY, Biol. Pharm. Bull., 22:1249–1250, 1999.
Kako, M., Miura, T., Nishiyama, Y., Ichimaru, M., Moriyasu, M., and Kato, A., Hypoglycemic effect of the rhizomes of Polygala senega in normal and diabetic mice and its main component, the triterpenoid glycoside senegin-II, Planta Med., 62:440–443, 1996.
Kantee, H., Althaea, ipecac, senega and thyme as cough medicines, Sairaanhoitaja, 49:32, 1973.
Kuribara, H. and Tadokoro, S., Behavioral study on an antitussive and expectorant, and of its constituents. I. Effects on ambulatory activity and discrete lever-press avoidance response in mice, Arukoru Kenkyuto Yakubutsu Ison, 24:417–429, 1989.
Moes, A., A parallel study of the chemical composition of Polygala senega and of “Securidaca longepedunculata” Fres. var. parvifolia, a Congolese polygalacea, J. Pharm. Belg., 21:347–362, 1966.
Saitoh, H., Miyase, T., Ueno, A., Atarashi, K., and Saiki, Y., Senegoses J-O, oligosaccharide multiesters from the roots of Polygala senega L., Chem. Pharm. Bull., 42:641–645, 1994.
Yoshikawa, M., Murakami, T., Matsuda, H., Ueno, T., Kadoya, M., Yamahara, J., and Murakami, N., Bioactive saponins and glycosides. II. Senegae Radix. (2): Chemical structures, hypoglycemic activity, and ethanol absorption-inhibitory effect of Esenegasaponin c, Zsenegasaponin c, and Z-senegins II, III, and IV, Chem. Pharm. Bull., 44:1305–1313, 1996.
Senna
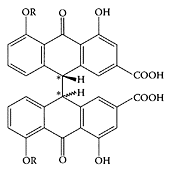
Senna, a traditional Chinese medicine, possesses multiple pharmacological activities. In particular, it can promote the motility and secretion of the gastrointestinal tract (Krumbiegel and Schulz, 1993; Valverde et al., 1999). However, its application is greatly restricted by its toxicity (Stickel et al., 2001, Adam et al., 2001). The body-weight gain (males only) of animals receiving 750 or 1500 mg/kg per day was reduced significantly and, due to the laxative properties of senna, water consumption increased, and notable changes in electrolytes in both serum and urine were observed (Mengs et al., 2004). Senna plants are small shrubs of Leguminosae, cultivated in Somalia, the Arabian peninsula, and near the Nile River. Tinnevelly senna is obtained from cultivated plants, mainly in South India and Pakistan. The senna pods (fruits) are collected during the same period as the leaves, then dried and separated into various qualities. The active principle of senna was first isolated and characterized by Stoll in 1941. The isolated glycosides were identified and attributed to the anthraquinone family (Scheme S.56). They were named sennosides A, B, C, and D. The active constituents in the pods and in the leaves are similar, but are present in larger quantities in the pods (Franz, 1993).
Much attention is being paid to senna effects on the regulation of gastrointestinal motility (Tian et al., 2000; Zhang et al., 2000). Wang et al. (2002) showed senna caused diarrhea and enhanced gastrointestinal motility through digestive-tract administration. Long-term gastric administration of senna induced inflammatory changes and cell damage in the whole gastrointestinal tract. The researchers suggested that the differential proteins screened from the colonic tissues of the model mice might mediate the enhancing effect of senna on gastrointestinal motility.
Quinquangulin and rubrofusarin are two known antimycobacterial natural products extracted from the stem and fruits of senna (Graham et al., 2004.). The piperidine alkaloid cassine is another antimicrobial compound isolated from the leaves of Senna racemosa (Sansores-Peraza et al., 2000).
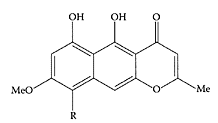
Quinquangulin (R=CH3) and ribrofusarin (R=H). (From Barbosa et al., Biochem. System. Ecol., 32:363–365, 2004. With permission.)
References
Adam, S.E., Al-Yahya, M.A., and Al-Farhan, A.H., Combined toxicity of Cassia senna and Citrullus colocynthis in rats, Vet. Hum. Toxicol., 43:70–72, 2001.
Barbosa, F.G., da Conceicao, M., de Oliveira, F., Braz-Filho, R., and Silveira, E.R., Anthaquinones and naphthopyrones from Senna rugosa, Biochem. System. Ecol., 32:363–365, 2004.
Franz, G., The senna drug and its chemistry, Pharmacology, 47:2–6, 1993.
Graham, J.G., Zhang, H., Pendland, S.L., Santarsiero, B.D., Mesecar, A.D., Cabieses, F., and Farnsworth, N.R., Antimycobacterial naphthopyrones from Senna obliqua, J. Nat. Prod., 67:225–227, 2004.
Hazra, B., Das Sarma, M.D., and Sanyal, U., Separation methods of quinonoid constituents of plants used in Oriental traditional medicines, J. Chromatogr. B, 812:259–275, 2004.
Krumbiegel, G. and Schulz, H.U., Rhein and aloeemodin kinetics from senna laxatives in man, Pharmacology, 47:120–124, 1993.
Mengs, U., Mitchell, J., McPherson, S., Gregson, R., and Tigner, J., A 13-week oral toxicity study of senna in the rat with an 8-week recovery period, Arch. Toxicol., 78:269–275, 2004.
Sansores-Peraza, P., Rosado-Vallado, M., BritoLoeza, W., Mena-Rejon, G.J., and Quijano, L., Cassine, an antimicrobial alkaloid from Senna racemosa, Fitoterapia, 71:690–692, 2000.
Stickel, F., Seitz, H.K., Hahn, E.G., and Schuppan, D., Liver toxicity of drugs of plant origin, Z. Gastroenterol., 39:225–232, 234–237, 2001.
Tian, X.L., Mourelle, M., Li, Y.L., Guarner, F., and Malagelada, J.R., The role of Chinese herbal medicines in a rat model of chronic colitis, World J. Gastroenterol., 6:40, 2000.
Valverde, A., Hay, J-M., Fingerhut, A., Boudet, M-J., Petroni, R., Pouliquen, X., Msika, S., and Flamant, Y., Senna vs polyethylene glycol for mechanical preparation the evening before elective colonic or rectal resection: a multicenter controlled trial, Arch. Surg., 134:514–519, 1999.
Wang, X., Zhong, Y.X., Lan, M., Zhang, Z.Y., Shi, Y.Q., Lu, J., Ding, J., Wu, K.C., Jin, J.P., Pan, B.R., and Fan, D.M., Screening and identification of proteins mediating senna induced gastrointestinal motility enhancement in mouse colon, World J. Gastroenterol., 8:162–167, 2002.
Zhang, H.X., Ren, P., Huang, X., and Li, Y., Regulation of the traditional Chinese medicine on gastrointestinal hormone and motility, Shijie Huaren Xiaohua Zazhi, 8:10, 2000.
Sesame
Sesame (Sesamum indicum L.), an important oilseed crop in India, Sudan, China, and Burma, has been used as a healing oil for thousands of years. Sesame seeds have a higher oil and lower protein content than soybean, with the oil content ranging from 46.4–52.0 percent, and the protein content ranging from 19.8–24.2 percent. The fatty-acid composition of sesame seeds is oleic (40.4–44.9 percent), linoleic (37.7–43.4 percent), palmitic (9.1–9.8 percent), and stearic (4.8–6.1 percent).
The mechanism by which a diet containing 24 percent sesame oil reduces levels of serum and liver cholesterol, liver LDL cholesterol, and liver lipids is not known. However, the high degree of unsaturation (85 percent) of sesame oil and the presence of linoleic acid may be important factors (Satchithanandam et al., 1996). Linoleic acid is also known to have anti-neoplastic properties. When Salerno and Smith (1991) tested lipase-digested sesame oil and undigested sesame oil, they found that both inhibited the growth of three malignant colon-cell lines. Thus, sesame contains in vitro antineoplastic properties. Lignans and lignan glycosides, such as sesamol dimer, sesamin, sesamolin, sesaminol triglucoside, and sesaminol diglucoside, isolated from sesame-methanolic extract, showed a high capacity for free-radical scavenging with the DPPH system (Suja et al., 2004). These lignans, in combination with α-tocopherol, showed a lag period in the time course of cumene hydroperoxide-mediated lipid peroxidation and a decreased rate of thiobarbituric acid reactive-product formation, suggesting recycling of α-tocopherol. Further work by Suja and coworkers (2005) showed the antioxidant activity of a crude methanol extract obtained from sesame cake was comparable to BHT at 200 ppm. In contrast, the corresponding purified extract exhibited far superior antioxidant properties to BHT at 5, 10, 50, 100, and 200 ppm levels. A typical result is shown in Figure S.92 using the thiocyanate method linoleic-acid emulsion system. Prasad et al. (2005) reported that the antioxidant properties of sesamol provided potent phytoprotection to lymphocytes against UVB radiation.

Sesamol (1) and sasamin (2). (From Chavali and Forse, Prostaglandins Leukot. Essent. Fatty Acids, 61:347–352, 1999. With permission.)
An increase in reported sesame-induced allergic reactions led Wolff et al. (2004) to identify and characterize the linear B-cell epitopes, the major allergen of sesame seed, which might provide a better understanding of the functional role the allergens play and might have implications for immunodiagnosis and probably immunotherapy. A single dose of sesame oil reduced lipid peroxidation 6 h after endotoxin intoxication. Furthermore, sesame oil given 6 h after cecal ligation and puncture significantly increased survival rate. These data suggest that sesame oil could be used as a potent antioxidant to reduce oxidative stress after the onset of sepsis in rats (Hsu and Liu, 2004).
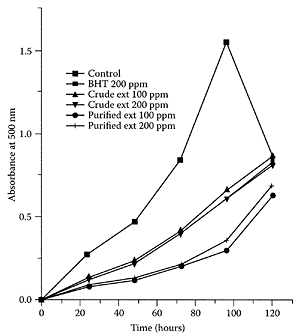
FIGURE S.92 Antioxidant activity of sesame extracts and BHT by the thiocyanate-cyanate method linoleicacid system. (From Suja et al, Food Chem., 91:213–219, 2005. With permission.)
Chen et al. (2005) recently suggested that the overall vascular fibrinolytic capacity may be enhanced by using sesamol, which regulates plasminogen activator gene expression. Sesamol is also known to reduce the synthesis of the coenzyme NADPH, which led Jacklin et al. (2003) to study the effect of oxidants on tumor and vascular endothelial cells. In preliminary studies on the effect of sesamol alone, it was clear that the compound demonstrated marked cytotoxicity.
References
Chavali, S.R. and Forse, R.A., Decreased production of interleukin-6 and prostaglandin E2 associated with inhibition of Δ-5 desaturation of ω6 fatty acids in mice fed safflower oil diets supplemented with sasamol, Prostaglandins Leukot. Essent. Fatty Acids, 61:347–352, 1999.
Chen, P-R., Lee, C-C., Chang, H., and Tsai, C.E., Sesamol regulates plasminogen activator gene expression in cultured endothelial cells: a potential effect on the fibrinolytic system, J. Nutr. Biochem., 16:59–64, 2005.
Hsu, D.Z. and Liu, M.Y., Effects of sesame oil on oxidative stress after the onset of sepsis in rats, Shock 22:582–585, 2004.
Jacklin, A., Ratledge, C., Welham, K., Bilko, D., and Newton, C.J., The sesame seed oil constituent, sesamol, induces growth arrest and apoptosis of cancer and cardiovascular cells, Ann. N.Y. Acad. Sci., 1010: 374–380, 2003.
Nakano, D., Itoh, C., Ishii, F., Kawanishi, H., Takaoka, M., Kiso, Y., Tsuruoka, N., Tanaka, T., and Matsumura, Y., Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats, Biol. Pharm. Bull., 26:1701–1705, 2003.
Prasad, N.R., Mahesh, T., Menon, V.P., Jeevanram, R.K., and Pugalendi, K.V., Photoprotective effect of sesamol on UVB-radiation induced oxidative stress in human blood lymphocytes in vitro, Environ. Toxicol. Pharmacol., 20:1–5, 2005.
Salerno, J.W. and Smith, D.E., The use of sesame oil and other vegetable oils in the inhibition of human colon cancer growth in vitro, Anticancer Res., 11: 209–216, 1991.
Satchithanandam, S., Chanderbhan, R., Kharroubi, A.T., Calvert, R.J., Klurfeld, D., Tepper, S.A., and Kritchevsky, D., Effect of sesame oil on serum and liver lipid profiles in the rat, Int. J. Vitam. Nutr. Res., 66:386–392, 1996.
Suja, K.P., Jayalekshmy, A., and Arumughan, C., Free radical scavenging behavior of antioxidant compounds of sesame (Sesamum indicum L.) in DPPH system, J. Agric. Food Chem., 52:912– 915, 2004.
Suja, K.P., Jayalekshmy, A., and Arumughan, C., Antioxidant activity of sesame cake extract, Food Chem., 91:213–219, 2005.
Wolff, N., Yannai, S., Karin, N., Levy, Y., Reifen, R., Dalai, I., and Cogan, U., Identification and characterization of linear B-cell epitopes of β-globulin, a major allergen of sesame seeds, J. Allergy Clin. Immunol., 114:1151–1158, 2004.
Sho-saiko-to
Sho-saiko-to (SST), introduced into Japan as an oriental classical medicine from China approximately 1,500 years ago, is currently the most representative Kampo medicine (traditional Japanese medicine). SST is used to treat chronic hepatitis and cirrhosis. Many experimental and clinical studies have demonstrated the various pharmacological effects of SST (Kusunose et al., 2002; Tajiri et al., 1991; Geerts and Rogiers, 1999). SST is a mixture drug of medicinal herbs prepared from the hot-water extraction of seven raw materials. Fifteen major, low-molecular compounds (i.e., baicalin, wogonin-7-O-glucuronide, liquiritin, their three aglycons, liquiritin apioside, glycyrrhizin, saikosaponin b1, saikosaponin b2, ginsenoside Rg1, ginsenoside Rb1, (6)-gingerol, (6)-shogaol, and arginine) that have various pharmacological actions are assumed to be responsible, at least partly, for the pharmacological effects of SST (Scheme S.57) (Ohtake et al., 2004).
In prospective studies (Oka et al., 1995), SST was found to play a chemopreventive role in the development of hepatocellular carcinoma in cirrhotic patients. Recently, the mechanisms were studied, and SST was shown to function as a potent antifibrosuppressant via the inhibition of oxidative stress in hepatocytes and hepatic stellate cells. Its active components are baicalin and baicalein. SST also exhibited anti-carcinogenic properties, as it inhibited chemical hepatocarcinogenesis in animals and suppressed the proliferation of hepatoma cells by inducing apoptosis and arresting the cell cycle (Shimizu, 2000).
SST may protect rats against lethality caused by endotoxin by its ability to regulate the heme metabolism in septic shock (Sakaguchi et al., 2005) or to inhibit TNF-α production in septic shock (Sakaguchi and Furusawa, 2004). It was also reported to reduce cholestasis in rats (Chen et al., 2004) and to suppress acute hepatic injury induced by CCl4 and to bring about an early recovery in liver function (Taira et al., 2004).
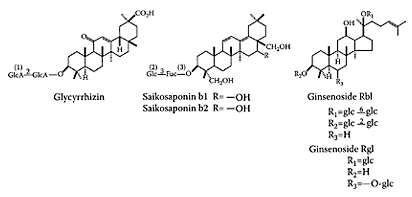
SCHEME S.57 Structures of some detected compounds in Sho-saiko-to. (1) GlcA is D-glucuronic acid; (2) Glc is D-glucopyranese; (3) Fuc is fucose. (From Ohtake et al., J. Chromatogr. B, 812:135–148, 2004. With permission.)
References
Chen, M-H., Chen, J-C., Tsai, C-C., Wang, W-C., Chang, D-C., Lin, C-C., and Hsieh, H-Y., Sho-saiko-to prevents liver fibrosis induced by bile duct ligation in rats, Am. J. Chin. Med., 32:195– 207, 2004.
Geerts, A. and Rogiers, V., Sho-saiko-to: the right blend of traditional Oriental medicine and liver cell biology, Hepatology, 29:282–284, 1999.
Kusunose, M., Qiu, B., Cui, T., Hamada, A., Yoshioka, S., Ono, M., Miyamura, M., Kyotani, S., and Nishioka, Y., Effect of Sho-saiko-to extract on hepatic inflammation and fibrosis in dimethylnitrosamine induced liver injury rats, Biol. Pharm. Bull., 25:1417–1421, 2002.
Ohtake, N., Nakai, Y., Yamamoto, M., Sakakibara, I., Takeda, S., Amagaya, S., and Aburada, M., Separation and isolation methods for analysis of the active principles of Sho-saiko-to (SST) oriental medicine, J. Chromatogr. B, 812:135–148, 2004.
Oka, H., Yamamoto, S., Kuroki, T., Harihara, S., Marumo, T., Kim, S.R., Monna, T., Kobayashi, K., and Tango, T., Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9), Cancer, 76:743–749, 1995.
Sakaguchi, S. and Furusawa, S., Preventive effects of a traditional Chinese medicine (Sho-saiko-to) on endotoxin-induced cytotoxicity and tumor necrosis factor-alpha production in J774A.1 cells, Biol. Pharm. Bull., 27:1468–1470, 2004.
Sakaguchi, S., Furusawa, S., and Iizuka, Y., Preventive effects of a traditional Chinese medicine (Shosaiko-to) on septic shock symptoms; approached from Heme metabolic disorders in endotoxemia, Biol. Pharm. Bull., 28:165–168, 2005.
Shimizu, I., Sho-saiko-to: Japanese herbal medicine for protection against hepatic fibrosis and carcinoma, J. Gastroenterol. Hepatol., 15(Suppl. 1):D84-D90, 2000.
Taira, Z., Yabe, K., Hamaguchi, Y., Hirayama, K., Kishimoto, M., Ishida, S., and Ueda, Y., Effects of Sho-saiko-to extract and its components, baicalin, baicalein, glycyrrhizin and glycyrrhetic acid, on pharmacokinetic behavior of salicylamide in carbon tetrachloride intoxicated rats, Food Chem. Toxicol., 42:803–807, 2004.
Tajiri, H., Kozaiwa, K., Ozaki, Y., Miki, K., Shimuzu, K., and Okada, S., Effect of Sho-saiko-to (Xiao-chai-hu-tang) on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease, Am. J. Chin Med., 19:121–129, 1991.
Silver birch (Betula pendula)
Betula, of the family Betulaceae, commonly known as silver birch, grows mainly in the northern hemisphere from Eastern Europe to the northern parts of China and Japan. Different parts of Betula species have various medicinal applications, which in part is due to their essential oils. More than 50 compounds were identified in the essential oil from Betula species. The main components were α-copaene (12 percent and 10 percent), germacrene D (11 percent and 18 percent), and δ-cadinene (11 percent and 15 percent) (Betül et al., 2004). Some of the caryophyllene derivatives were evaluated for antimicrobial activity (Demirci et al., 2000a, b).

The medicinal parts are the bark, leaves, buds, sap, or juice or their processed products, which are used to treat diseases, such as urinarytract disorders, skin diseases, severe infections, and inflammations. Furthermore, B. pendula flavors are used commercially as aroma and flavoring for alcoholic beverages. Other uses include applications in cosmetics.
Diverse phytochemical investigations of Betula species have shown they contain mainly phenolics, flavonoids, tannins, saponins, glycosides, sterols, and terpene derivatives (Demirci et al., 2000).
References
Demirci, B., Paper, D.H., Demirci, F., Baser, K.H.C., and Franz, G., Essential oil of Betula pendula Roth. Buds, eCAM, 1:301–303, 2004.
Demirci, B., Baser, K.H.C., Demirci, F., and Hamann, M.T., New caryophyllene derivatives from Betula litwinowii, J. Nat. Prod., 63:902–904, 2000a.
Demirci, F., Demirci, F., Ozek, T., and Baser, K.H.C., Betulenols from Betula species, Planta Med., 66: 490–493, 2000b.
Demirci, F., Demirci, B., Baser, K.H.C., and Giiven, K., The Composition and antifungal bioassay of the essential oils of different Betula species growing in Turkey, Khim Prir Soedin, 2:126–130 (cited in Chem. Nat. Comp., 36:159–165), 2000.
Sinigrin
Sinigrin, 2-propenyl glucosinolate, is a common glucosinolate in Brassica vegetables known to possess anticarcinogenic activity. Elfoul et al. (2001) showed that sinigrin can be hydrolyzed by a Bacteroides thetaiotaomicron strain of human origin to yield allyl isothiocyanate (AITC) in the large bowel of rats inoculated with this bacterium. Three other strains of Bifidobacterium sp., B. pseudocatenulatum, B. adolescentis, and B. longum, from human intestinal tract were also able to digest sinigrin (Cheng et al., 2004). This local release of isothiocyanates may explain the protective effect of cruciferous vegetables on the colon epithelium.

Singirin. (From Jen et al., J. Chromatogr. A, 912:363–368, 2001. With permission.)
Depending on target tissue and the type of compound, different mechanisms of action have been suggested to explain the anticarcinogenic actions of glucosinolates and their breakdown products, among which are the isothiocyanates (ITCs). The most frequently proposed cancerpreventive mechanisms are modulation of the activities of phase I (cytochrome P450s) and phase II (glutathione-S-transferase, UDP-glucu-ronosyl-transferase, and quinone reductase) biotransformation enzymes (Vang et al., 1999), redox regulation antiproliferation, and induction of cell-cycle arrest and by increasing the rate of apoptosis in cancer cells (Yu et al., 1998; Yang et al., 2002).
The production of allyl isothiocyanate from sinigrin was investigated in a dynamic, in vitro large-intestinal model, after inoculation with a complex microflora of human origin. Peak levels of allyl isothiocyanate were observed between 9 and 12 h after the addition of sinigrin. The conversion rate was remarkably higher if different individual human microflora were used. Between 10 percent and 30 percent (mean 19 percent) of the sinigrin was converted into allyl isothiocyanate (Krul et al., 2002)
References
Cheng, D.L., Hashimoto, K., and Uda, Y., In vitro digestion of sinigrin and glucotropaeolin by single strains of Bifidobacterium and identification of the digestive products, Food Chem. Toxicol., 42:351–357, 2004.
Elfoul, L., Rabot, S., Khelifa, N., Quinsac, A., and Rimbault, A., Formation of allyl isothiocyanate from sinigrin by Bacteroides thetaiotaomicron, FEMS Microbiol. Lett., 197:99–103, 2001.
Jen, J.-F., Lin, T.-H., Huang, J.-W., and Chung, W.-C., Direct determination of sinigrin in mustard seed without desulfatation by reversed-phase ion-pair liquid chromatography, J. Chromatogr. A, 912:363–368, 2001.
Krul, C., Humblot, C., Philippe, C., Vermeulen, M., van Nuenen, M., Havenaar, R. and Rabot, S., Metabolism of sinigrin (2-propenyl glucosinolate) by the human colonic microflora in a dynamic in vitro large-intestinal model, Carcinogenesis, 23:1009–1016, 2002.
Vang, O., Mehrota, K., Georgellis, A., and Andersen, O., Effects of dietary broccoli on rat intestinal xenobiotic metabolizing enzymes, Eur. J. Drug. Metab. Pharmacokinet., 24:353–359, 1999.
Yang, Y.M., Conaway, C.C., Chiao, J.W., Wang, C.X., Amin, S., Whysner, J., Dai, W., Reinhardt, J., and Chung, F.L., Inhibition of benzo[a]pyreneinduced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis, Cancer Res., 62:2–7, 2002.
Yu, R., Mandlekar, S., Harvey, K.J., Ucker, D.S., and Kong, A.N., Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity, Cancer Res., 58:402–408, 1998.
Sitostanol
see also Phytosterols Sitostanol, the saturated derivative of plant sterol β-sitosterol, is a natural dietary component with serum cholesterol-lowering properties (Perez-Jimenez et al., 1995; Terry et al., 1995; Jones et al., 1999). However, it is not found in significant amounts in plants but comprises up to 20 percent of the phytosterols extracted from tall oil (Ling and Joseph, 1995). The lowering of serum cholesterol by plant sterols is believed to be the result of an inhibition of cholesterol absorption in the small bowel (Normen et al., 2000), although increased bile-acid excretion has also been suggested (Becker et al., 1993). Recently, it has been reported that unesterified sitostanol is more effective in inhibiting cholesterol absorption and reducing LDL cholesterol than acetate or oleate esters (Sudhop et al., 2003).
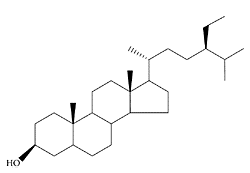
Sitostanol. (Adpated from Moreau et al., Prog. Lipid Res., 41:457–500, 2002.)
References
Becker, M., Staab, D., and von Bergman, K., Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol, J. Pediatr., 122:292–296, 1993.
Jones, P.J.H., Ntanios, F.Y., Raeini-Sarjaz, M., and Vanstone, C.A., Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men, Am. J. Clin. Nutr., 69:1144–1150, 1999.
Ling, W.H. and Jones, P.J.H., Enhanced efficacy of sitostanol-containing versus sitostanol-free phytosterol mixtures in altering lipoprotein cholesterol levels and synthesis in rats, Atherosclerosis, 118: 319–331, 1995.
Moreau, R.A., Whitaker, B.D., and Hicks, K.B., Phytosterols, phytostanols, and their conjugates in food: structural diversity, quantitative analysis, and health-promoting properties, Prog. Lipid Res., 41:457–500, 2002.
Normen, L., Dutta, P., Lia, A., and Andersson, H., Soy sterol esters and β-sitostanol ester as inhibitors of cholesterol absorption in human small bowel, Am. J. Clin. Nutr., 71:908–913, 2000.
Perez-Jimenez, F., Espino, A., Lopez-Segura, F., Blanco, J, Ruiz-Gutierrez, V., Prada, J.L., LopezMiranda, J., Jimenez-Pereperez, J. and Ordovas, J.M., Lipoprotein concentration in normolipidemic males consuming oleic acid-rich diets from two different sources: olive oil and oleic acid-rich sunflower oil, Am. J. Clin Nutr., 62:769–775, 2000.
Sudhop, T., Lutjohann, D., Agna, M., von Ameln, C., Prange, W., and von Bergmann, K., Comparison of the effects of sitostanol, sitostanol acetate, and sitostanol oleate on the inhibition of cholesterol absorption in normolipemic healthy male volunteers, a placebo controlled randomized cross-over study, Arzneimittelforschung, 53:708–713, 2003.
Terry, J.G., McGill, B.L., and Crouse, J.R., 3rd., Evaluation of the use of beta-sitostanol as a nonabsorbable marker for quantifying cholesterol absorp-tion, J. Lipid Res., 36:2267–2271, 1995.
Sitosterol
see also Phytosterols and Sitostanol Sitosterol, or β-sitosterol, belongs to dietary phytosterols. The various biological activities of phytosterols, anti-inflammatory, cholesterol-lowering, antimicrobial, antibacterial, and antifungal effects, are reviewed by Tapiero et al. (2003), Ostlund (2004), and Qullez et al. (2003). Recently, the antitumor and chemopreventive activity of sitosterols were studied by Valchakova et al. (2004). They demonstrated that sitosterol inhibited colon and breast-cancer development at various stages of tumor development, including inhibition of tumorigenesis, inhibition of tumor promotion, and induction of cell differentiation. It also effectively inhibited invasion of tumor cells and metastasis. Ju et al. (2004) also reported that dietary β-sitosterol protected against E(2)-stimulated MCF-7 tumor growth and lowered circulating E(2) levels.
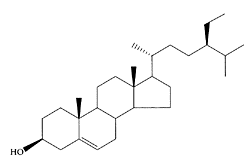
β-Sitosterol. (Adapted from Moreau et al., Prog. Lipid Res., 41:457– 500, 2002.)
Circulating levels of β-sitosterol can be affected by dietary modification. Thus, β- sitosterol can be used as a biomarker of exposure in observational studies or as a compliance indicator in dietary-intervention studies of cancer prevention (Muti et al., 2003).
References
Ju, Y.H., Clausen, L.M., Allied, K.F., Almada, A.L., and Helferich, W.G., β-sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and beta-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice, J. Nutr., 134:1145–1451, 2004.
Moreau, R.A., Whitaker, B.D., and Hicks, K.B., Phytosterols, phytostanols, and their conjugates in food: structural diversity, quantitative analysis, and health-promoting properties, Prog. Lipid Res., 41:457–500, 2002.
Muti, P., Awad, A.B., Schunemann, H., Fink, C.S., Hovey, K., Freudenheim, J.L., Wu, Y-W., Bellati, C., Pala, V., and Berrino, F., A plant food-based diet modifies the serum β-sitosterol concentration in hyperandrogenic postmenopausal women, J. Nutr., 133:4252–4255, 2003.
Ostlund, R.E., Jr., Phytosterols and cholesterol metabolism, Curr. Opin. Lipidol., 15:37–41, 2004.
Qullez, J., Garcla-Lorda, P., and Salas-Salvado, J., Potential uses and benefits of phytosterols in diet: present situation and future directions, Clin. Nutr., 22:343–351, 2003.
Tapiero, H., Townsend, D.M., and Tew, K.D., Phytosterols in the prevention of human pathologies, Biomed. Pharmacother., 57:321–325, 2003.
Vachalkova, A., Ovesna, Z. and Horvathova, K., Taraxasterol and β-sitosterol: A new natural compounds with chemoprotective/chemopreventive effects, Neoplasma, 51:407–414, 2004.
Skullcap (Scutellaria lateriflora L.)

Skullcap is a perennial member of the mint family, with 300 Scutellaria species growing worldwide. It has been traditionally used as a sedative and to treat various nervous disorders, such as anxiety (Awad et al., 2003). Chinese skullcap is also used in Traditional Chinese Medicine to treat tumors. Early laboratory studies investigating this traditional use showed possible preventive involvement in bladder, liver, and other types of cancers (Udintsev et al., 1990). The main constituents found in the plant are scutellarin, a flavonoid glycoside, together with many other flavones, catalpol, other volatile oils, bitter iridoids, and tannins (Popova et al., 1972, Popova, 1974).
In vivo animal-behavior trials, performed to test anxiolytic effects in rats orally administered skullcap extracts, demonstrated that the flavonoid baicalin, its aglycone baicalein, and the amino acids GABA and glutamine may play a role in anxiolytic activity. This is not unexpected, as baicalin and baicalein are known to bind to the benzodiazepine site of the GABA receptor and GABA is the main inhibitory neurotransmitter (Awad et al., 2003).
Recently, Baikal-skullcap extract was reported to potentiate the anti-metastatic effect of cyclophosphamide in mice with Lewis lung carcinoma. It modulated cytotoxic activity of natural-killer cells and peritoneal macrophages during tumor growth (Kaplya et al., 2004).
References
Awad, R., Arnason, J.T., Trudeau, V., Bergeron, C., Budzinski, J.W., Foster, B.C., and Merali, Z., Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): a medicinal plant with anxiolytic properties, Phytomedicine, 10:640–649, 2003.
Kaplya, O.A., Sherstoboev, E.Y., Zueva, E.P., Razina, T.G., Amosova, E.N., and Krylova, S.G., Effect of baikal skullcap extract administered alone or in combination with cyclophosphamide on natural cytotoxicity system in mice with Lewis lung carcinoma, Bull. Exp. Biol. Med., 137:471–474, 2004.
Popova, T.P., Litvinenko, V.I., Gella, E.V., and Ammosov, O.S., Chemical composition and medicinal properties of common skullcap, Farm Zh., 27: 58–61, 1972.
Popova, T.P., Flavone glycosides in the roots of the Baikal skullcap, Farm Zh., 29:91–92, 1974.
Udintsev, S.N., Razina, T.G., and Iaremenko, K.V., The antitumor effect of Baikal skullcap, Vopr Onkol., 36:602–607, 1990.
Sorghum (sorghum vulgare)
Sorghum is the major food crop in the semiarid regions of Africa and Asia. It provides a component to the diets of many people in the form of unleavened breads, boiled porridge or gruel, malted beverages, and specialty foods, such as popped grain and beer (Anglani, 1998). A syrup is produced from sweet sorghum. The crop is also used for building material, fencing, fodder for animals, and for brooms. In the United States, sorghum grain is used primarily for livestock feed, and the stems and foliage for green chop, hay, silage, and pasture.

A comparison of the nutritional and chemical parameters of 10 varieties of sorghum showed components to range from lipids (2.70–3.75 percent), raw fiber (60.0–64.7 percent), protein (9.01–11.43 percent), no nitrogen extract (77.65–83.07 percent), starch (60.5–64.20 percent), tannin (2.50–10.16 mg/g), and total calories (380–4000 kcal). Ash content, with values of 1.17–1.91 percent, protein digestibility (23.8–38.8 percent), and in situ starch (54.4–66.6 percent) were not statistically different (Torres Cepeda et al., 1996)
Sorghum is a rich source of various phytochemicals, including tannins, phenolic acids, anthocyanins, phytosterols, and policosanols (Awika and Rooney, 2004). These phytochemicals are known to impact human health. Sorghum fractions possess high antioxidant activity in vitro, relative to other cereals or fruits. Epidemiological studies suggest that, in comparison to other cereals, sorghum consumption reduces the risk of certain types of cancer in humans. Kamath et al. (2004), using the DPPH model system for assessing antiradical properties, recently identified various extracts from sorghum flour that exhibited significant, greater antioxidant activity than BHT (Figure S.93).
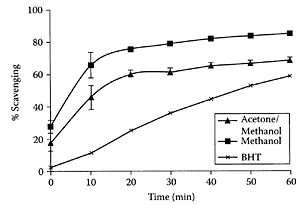
FIGURE S.93 Effect of subfractions of methanol extracts on quenching DPPH radicals-time related effect. 50 μL (0.2 mg) of either extract of sorghum was employed for quenching. An equal volume of respective solvents was used in control. 50 μL (10 mM) BHT was used. Each value represents mean ± standard error (n— 6). (From Kamath et al., J. Cereal Sci., 40:283–288, 2004. With permission.)
Even though they were unable to correlate anti-oxidant activity and phenolic content, diets rich in sorghum could still be helpful in combating chronic diseases involving free radicals. This explains why sorghum phytochemicals promote cardiovascular health and are involved in cancer prevention (Awika and Rooney, 2004).
References
Anglani, C., Sorghum for human food—a review, Plant Foods Hum. Nutr., 52:85–95, 1998.
Awika, J.M. and Rooney, L.W., Sorghum phytochemicals and their potential impact on human health, Phytochemistry, 65:1199–1221, 2004.
Kamath, V.G., Chandrashekar, A., and Ranjini, P.S., Antiradical properties of sorghum (Sorghum bicolor L. Moench) flour extracts, J. Cereal Sci., 40:283–288, 2004.
Torres Cepeda, T.E., Alanis Guzman, M.G., and Maiti, R., Relationship between nutritional composition and anatomical parameters in sorghum (Sorghum bicolor L. Moench), Arch. Latinoam. Nutr., 46: 253–259, 1996.
Southernwood (Artemisia abrotanum)
There are two different cultivated strains of southernwood. The traditional type has a vague, lemon-like smell, while the more recently bred type has an even more intense and dominant smell. Despite their significant bitterness, the leaves of both types are well-suited for culinary usage. Various sources reported different compositions for the essential oil (0.2 percent), with some claiming absinthol as the main component, while others report the heterocyclic sesquiterpenes davanol and davanone plus carlinene and 1,8-cineol. Among the nonvolatile constituents reported are the alkaloid abrotin and coumarins. Although southernwood contains significant amounts of bitter sesquiterpene lactones (absinthin) and the glycoside rutin, it is still less bitter than its close relative, wormwood.

A nasal-spray formulation containing an extract of Artemisia abrotanum L. was developed for therapeutic use in patients with allergic rhinitis and other upper-airway disorders. The extract used contains a mixture of essential oils (4 mg/mL) and flavonols (2.5 microg/mL), of which some components have been shown to possess anti-inflammatory, expectorant, and spasmolytic, as well as antiseptic and antimicrobial, activities. The most important constituents in the essential-oil fraction are 1,8-cineole, linalool, and davanone, while the flavonol fraction contains centauredin, casticin, and quercetin dimethyl-ethers (Remberg, 2004).
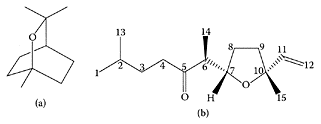
Structures of 1,8-cineole (a) and davanone (b) (Adapted from Tisevec et al., Biochem. Systems Ecol., 32:525–527, 2004; Silvester et al. Ind. Crops Prod., 12:53–56, 2000)
Lactones and sesquiterpenes, isolated from the methanol extract of the aerial parts of Artemisia sylvatica, displayed inhibitory activity on the LPS-induced NF-κB activation, NO production, and TNF-α production (Jin et al., 2004). Artemisia iwayomogi extract also inhibited mast-cell-derived, immediate-type allergic reactions and involvement of intracellular Ca(2+), proinflammatory cytokines, p38 MAPK, and NF-κB (Kim et al., 2005). These results support the pharmacological use of Artemisia sylvatica and Artemisia iwayomogi, which have been employed as herbal medicines for inflammation treatment.
References
Jin, H.Z., Lee, J.H., Lee, D., Hong, Y.S., Kim, Y.H., and Lee, J.J., Inhibitors of the LPS-induced NF-κB activation from Artemisia sylvatica, Phytochemistry, 65:2247–2253, 2004.
Kim, S.H., Choi, C.H., Kim, S.Y., Eun, J.S., and Shin, T.Y., Anti-allergic effects of Artemisia iwayomogi on mast cell-mediated allergy model, Exp. Biol. Med., 230:82–88, 2005.
Remberg, P., Bjork, L., Hedner, T., and Sterner, O., Characteristics, clinical effect profile and tolerability of a nasal spray preparation of Artemisia abrotanum L. for allergic rhinitis, Phytomedicine, 11:36–42, 2004.
Silvestre, A.J.D., Valega, M. and Cavaliero, J.A.S., Chemical transformation of 1,8-cineole: Synthesis of seudenone, an insect pheronome, Ind Crops Prod., 12:53–56, 2000.
Tisevec, V., Milosavljevie, S., Vajs, V., Janackovic, P., Jovic, D.L.J., Tetrahydroguran-type sesquiterpenes from Artemisia lobelii All Var. biasolettiana (Vis.) K. Malay., Biochem. Systems Ecol., 32:527–532, 2004.
Soybeans
see also Daidzein and Genistein A large number of components contribute to the diverse biological activities of soybeans: hormonal, immunological, bacteriological, and digestive effects. These components include isoflavones (genistein, daidzein, biochanin), saponins, Kunitz inhibitor, Bowman-Birk inhibitor, soyacystatin, phytoestrogens, Maillard products, soybean hydrophobic protein, soy allergens, lecithins, allergens, raffinose, stachyose, and 2-pentyl pyridine (Csaky and Fekete, 2004).
Soy isoflavones (genistein, daidzein, biochanin) are known to protect against different cancers (Sarkar and Li, 2003), cardiovascular disease (Hasler, 2002), and bone loss (Harkness, 2004). Many studies have demonstrated the effect of soy isoflavones on specific target molecules and signaling pathways, cell proliferation and differentiation, cell-cycle regulation, apoptosis, angiogenesis, cell adhesion and migration, metastasis, and activity of different enzymes. Isoflavones are also classified as phytoestrogens with weak estrogenic properties (Valachovicova et al., 2004). Interleukin-6 is a pleiotropic cytokine that plays a crucial role in immune physiology and is tightly controlled by hormonal-feedback mechanisms. Isoflavones modulate IL-6 gene-expression levels and may have therapeutical benefit in preventing cancer progression, aging discomforts, and restoring immune homeostasis (Dijsselbloem et al., 2004).
A systematic review of randomized clinical trials performed to evaluate the benefit of soy for the treatment of perimenopausal symptoms provides some evidence for the efficacy of soy preparations for perimenopausal symptoms, but the heterogeneity of the studies performed makes it difficult to achieve a definitive statement (Huntley and Ernst, 2004).
The ability of soybean extracts to inhibit mouse mammary adenocarcinoma tumor growth was not only due to the presence of genistein but to other constituents present (Hewitt and Singletary, 2003). Recent studies demonstrated a direct effect of soy saponins on cancer cells, which further leads to elucidating the nature of soy constituents involved in cancer protection (Kerwin, 2004).
The Bowman-Birk inhibitor, a serine protease inhibitor derived from soybeans, is presently being evaluated in clinical trials for its ability to serve as a cancer preventive or anti-inflammatory agent (Kennedy et al., 2002). Kunitz inhibitor was also found to inhibit cell invasiveness through suppression of urokinasetype plasminogen activator signaling cascade (Kobayashi et al., 2004).
Soy infant formulas are widely used, but only a few studies have evaluated their long-term safety or specific forms of toxicity, such as the effects of genistein and daidzein in soy infant formula, on the endocrine or immune systems. In addition, there is inconsistency in the existing data, which point to the need for more clinical and epidemiological studies (Chen and Rogan, 2004).
Soybean oil is the world’s most widely used, edible oil. In the United States, soybean oil accounts for nearly 80 percent of edible-oil consumption. It contains 61 percent polyunsaturated fat and 24 percent monounsaturated fat. It is one of the few vegetable oils to contain linolenic acid, an omega-3 fatty acid (7.2 percent C18:3n-3) known to prevent cardiovascular diseases. Soybean oil also contains 54 percent C18:2n-6 linoleic acid, which is required for normal immune response (Meydani et al., 1991).
References
Csaky, I. and Fekete, S., Soybean: feed quality and safety. Part 1. Biologically active components, a review, Acta Vet. Hung., 52:299–313, 2004.
Chen, A. and Rogan, W.J., Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants, Annu. Rev. Nutr., 24:33–54, 2004.
Dijsselbloem, N., Vanden Berghe, W., De Naeyer, A., and Haegeman, G., Soy isoflavone phyto-pharmaceuticals in interleukin-6 affections, Multi-purpose nutraceuticals at the crossroad of hormone replacement, anti-cancer and anti-inflammatory therapy, Biochem. Pharmacol., 68:1171–1185, 2004.
Harkness, L., Soy and bone, where do we stand? Orthop. Nurs., 23:12–17, 2004.
Hasler, C.M., The cardiovascular effects of soy products, J. Cardiovasc. Nurs., 16:50–63, 2002.
Hewitt, A.L. and Singletary, K.W., Soy extract inhibits mammary adenocarcinoma growth in a syngeneic mouse model, Cancer Lett., 192:133–143, 2003.
Huntley, A.L. and Ernst, E., Soy for the treatment of perimenopausal symptoms—a systematic review, Maturitas, 47:1–9, 2004.
Kennedy, A.R., Billings, P.C., Wan, X.S., and Newberne, P.M., Effects of Bowman-Birk inhibitor on rat colon carcinogenesis, Nutr. Cancer, 43:174–186, 2002.
Kerwin, S.M., Soy saponins and the anticancer effects of soybeans and soy-based foods, Curr. Med. Chem. Anti-Canc. Agents, 4:263–272, 2004.
Kobayashi, H., Suzuki, M., Kanayama, N., and Terao, T., A soybean Kunitz trypsin inhibitor suppresses ovarian cancer cell invasion by blocking urokinase upregulation, Clin. Exp. Metastasis, 21: 159–166, 2004.
Meydani, S.N., Lichtenstein, A.H., White, P.J., Goodnight, S.H., Elson, C.E., Woods, M., Gorbach, S.L., and Schaefer, E.J., Food use and health effects of soybean and sunflower oils, J. Am. Coll. Nutr., 10: 406–428, 1991.
Sarkar, F.H. and Li, Y., Soy isoflavones and cancer prevention, Cancer Invest., 21:744–757, 2003.
Valachovicova, T., Slivova, V., and Sliva, D., Cellular and physiological effects of soy flavonoids, Mini Rev. Med. Chem., 4:881–887, 2004.
Soy fibers
Due to its neutral taste and light color, soy fiber can be incorporated into a variety of high-fiber and reduced-calorie products, such as baked goods, cereal, and beverages. Clinical studies showed soy fiber provided all the benefits associated with both soluble and insoluble fiber. A soy-fiber-rich diet (6 percent) significantly lowered serum total cholesterol (TC), LDL-C, and atherosclerotic index, increased the ratio of HDL-L/TC, lowered serum fibrinogen (FB), platelet aggregation, and prolonged clotting time in rats (Wang et al., 1996). Lo et al. (1987) suggested a complementary role for soy fibers and soy protein in preventing atherosclerosis in rabbits. Bile acids were markedly lower in bran-soy treated females with cholelithiasis (Belonovskaia and Kliashtornaia, 1992).
References
Belonovskaia, L.K. and Kliashtornaia, O.S., The effect of soy bran on the bile acid spectrum of patients with cholelithiasis, Vopr Pitan., (4): 15–17, 1992.
Lo, G.S., Evans, R.H., Phillips, K.S., Dahlgren, R.R., and Steinke, F.H., Effect of soy fiber and soy protein on cholesterol metabolism and atherosclerosis in rabbits, Atherosclerosis, 64:47–54, 1987.
Wang, C., Zhao, L., and Chen, Y., Hypolipidemic action of soy fiber and its effects on platelet aggregation and coagulation time in rats, Zhonghua Yu Fang Yi Xue Za Zhi, 30:205–208, 1996.
Soy protein
The U.S. Food and Drug Administration approved (1999) the association of soy proteins with coronary prevention. This claim was based on studies demonstrating that soyprotein components were primarily responsible for reducing cholesterolemia (Greaves et al., 1999; Sirtori et al., 1998; Nestel, 2002). Proteins appear to elicit the hypocholesterolemic response, mainly by activating liver LDL receptors (Baum et al., 1998), a mechanism tentatively attributed to specific protein components, i.e., the 7S globulin and its α-α′ subunits (Lovati et al., 2000).
Plant-derived proteins, such as soy protein, were shown by Damasceno and coworkers (2000) to have a beneficial effect on atherosclerosis. Using a soy-protein isolate, they found a reduction in the level of oxidized LDL, as well as in the production of oxidized LDL antibodies, in rabbits.
Proteomic comparison of soy proteins used for clinical studies on hypercholesterolemia, particularly in Europe and the United States, indicate differences in the protein composition. These results may explain the variability found in experimental and clinical studies (Gianazza et al., 2003).
References
Baum, J.A., Teng, H., Erdman, J.W., Jr., Weigel, R.M., Klein, B.P., Persky, V.W., Freels, S., Surya, P., Bakhit, R.M., Ramos, E., Shay, N.F., and Potter, S.M., Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women, Am. J. Clin. Nutr., 68:545–551, 1998.
Damasceno, N.R.T., Goto, H., Rodrigues, F.M.D., Dias, C.T.S., Okawabata, F.S., Abdalla, D.S.P., and Gidlund, M., Soy protein isolate reduces the oxidizability of LDL and the generation of oxidized LDL autoantibodies in rabbits with diet-induced atherosclerosis, J. Nutr., 130:2641– 2647, 2000.
Food and Drug Administration, Food labeling health claims: soy protein and coronary heart disease, Final rule, Fed. Regist., 64:57699–57733, 1999.
Gianazza, E., Eberini, I., Arnoldi, A., Wait, R., and Sirtori, C.R., A proteomic investigation of isolated soy proteins with variable effects in experimental and clinical studies, J. Nutr., 133:9– 14, 2003.
Greaves, K.A., Parks, J.S., Williams, J.K., and Wagner, J.D., Intact dietary soy protein, but not adding an isoflavone-rich soy extract to casein, improves plasma lipids in ovariectomized cynomolgus monkeys, J. Nutr., 129:1585–1592, 1999.
Lovati, M.R., Manzoni, C., Gianazza, E., Arnoldi, A., Kurowska, E., Carroll, K.K., and Sirtori, C.R., Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells, J. Nutr., 130:2543– 2549, 2000.
Nestel, P., Role of soy protein in cholesterol-lowering: how good is it? Arterioscler. Thromb. Vasc. Biol., 22:1743–1744, 2002.
Sirtori, C.R., Lovati, M.R., Manzini, C., Gianazza, E., Bondioli, A., Staels, B., and Auwerx, J., Reduction of serum cholesterol by soy proteins: clinical experience and potential molecular mechanisms, Nutr. Metab. Cardiovasc. Dis., 8:334–340, 1999.
Spearmint
Like peppermint, spearmint (Mentha spicata) is a popular food-flavoring agent and a valuable medicinal herb. The essential oil of spearmint was reported to have anti-bacterial (Imai et al., 2001) and antifungal (Soliman and Badeaa, 2002) activities, as well as anti-inflammatory activities, possibly through the suppression of neutrophil recruitment into the peritoneal cavity, as demonstrated in mice (Abe et al., 2004).
A water extract from spearmint inhibited the mutagenic activity of the parent compound, 2-amino-3-methyl-3H-imidazo[4,5-f]quinoline (IQ), in the presence of rat liver. These findings suggest that spearmint extract protects against IQ, and possibly other heterocyclic amines, through inhibition of carcinogen activation and via direct effects on the activated metabolite (Yu et al., 2004). Spearmint was also found as an effective chemopreventive agent that may suppress benzoyl peroxide-induced cutaneous oxidative stress, toxicity, and hyperproliferative effects in the skin of mice (Saleem et al., 2000).
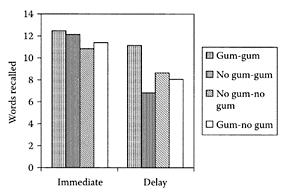
FIGURE S.94 Mean number of words recalled (maximum 15) after no retention interval (“Immediate”) or after 24 h (“Delay”). Participants in two of the four groups chewed gum at initial learning (gum-gum, gum-no gum), and two of the groups chewed gum during both of the recall tests (gum-gum, no gum-gum). (From Baker et al., Appetite, 43:207–210, 2004. With permission.)
The constituents of anti-inflammatory and hemostatic active sites of Mentha spicata includes: ursane, 3-methoxy-4-methylbenzaldehyde, veratric acid, 5-hydroxy-3′,4′,6,7-tetramethoxyflavone, diosmetin, thymonin, and daucosterol (Zheng et al., 2002). Two monoterpenoid glycosides, spicatoside A and spicato-side B, were also isolated from whole herbs of Mentha spicata L. and found to have anti-inflammatory and hemostatic activities (Zheng et al., 2003). Recently, Akdogan et al. (2004) reported on lipid peroxidation and hepatic damage that occurs after Mentha spicata administration in rat liver and on nephrotoxic changes (Akdogan et al., 2003).
One of the major uses of spearmint is in chewing gum. A recent study by Baker et al. (2004) showed that chewing spearmint gum not only promoted initial learning but also led to context-dependent effects upon memory (Figure S.94). The same effects were also observed with sucking the gum.
References
Abe, S., Maruyama, N., Hayama, K., Intuye, S., Oshima, H., and Yamaguchi, H., Suppression of neutrophil recruitment in mice by geranium essential oil, Mediators Inflamm., 13:21–24, 2004.
Akdogan, M., Ozguner, M., Aydin, G., and Gokalp, O., Investigation of biochemical and histopathological effects of Mentha piperita Labiatae and Mentha spicata Labiatae on liver tissue in rats, Hum. Exp. Toxicol., 23:21–28, 2004.
Akdogan, M., Kilinc, I., Oncu, M., Karaoz, E., and Delibas, N., Investigation of biochemical and histopathological effects of Mentha piperita L. and Mentha spicata L. on kidney tissue in rats, Hum. Exp. Toxicol., 22:213–219, 2003.
Baker, J.R., Bezance, J.B., Zellaby, E., and Aggleton, J.P., Chewing gum can produce context-dependent effects upon memory, Appetite, 43:207–210, 2004.
Imai, H., Osawa, K., Yasuda, H., Hamashima, H., Arai, T., and Sasatsu, M., Inhibition by the essential oils of peppermint and spearmint of the growth of pathogenic bacteria, Microbios, 106:3l-39, 2001.
Saleem, M., Alam, A., and Sultana, S., Attenuation of benzoyl peroxide-mediated cutaneous oxidative stress and hyperproliferative response by the prophylactic treatment of mice with spearmint (Mentha spicata), Food Chem. Toxicol., 38:939–948, 2000.
Soliman, K.M. and Badeaa, R.I., Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi, Food Chem. Toxicol., 40:1669–1675, 2002.
Yu, T-W., Xu, M., and Dashwood, R.H., Antimutagenic activity of spearmint, Environ. Mol. Mutagen., 44:387–393, 2004.
Zheng, J., Zhao, D.S., Wu, B., and Wu, L.J., A study on chemical constituents in the herb of Mentha spicata, Zhongguo Zhong Yao Za Zhi, 27:749–751, 2002.
Zheng, J., Wu, L.J., Zheng, L., Wu, B., and Song, A.H., Two new monoterpenoid glycosides from Mentha spicata L., J. Asian Nat. Prod. Res., 5:69–73, 2003.
Spices
see also Individual spices Spices have been used for generations by humans as food and to treat ailments. Commonly used spices, such as garlic, black cumin, cloves, cinnamon, thyme, allspices, bay leaves, mustard, and rosemary, possess antimicrobial properties that, in some cases, can be used therapeutically. Other spices, such as saffron—a food colorant, turmeric—a yellow-colored spice, tea—either green or black—and flaxseed do contain potent phytochemicals, including carotenoids, curcumins, catechins, and lignan, respectively, which provide significant protection against cancer (Lai and Roy, 2004).
The improvement of food flavors was facilitated by the addition of different spices, such as garlic, red chili, and cloves. Subsequent research showed that it was the antioxidant activity of the spice extracts, due to the presence of high levels of antioxidants (Madsen and Bertelsen, 1995).
Spices have long been recognized for their digestive stimulant action. This action seems to be mediated by stimulating the liver to secrete bile rich in bile acids, components that are vital for fat digestion and absorption, and by stimulating enzymes that are responsible for digestion (reviewed by Platel and Srinivasan, 2004).
References
Madsen, H.L. and Bertelsen, G., Spices as antioxidants, Trends Food Sci. Technol., 6:271–277, 1995.
Lai, P.K. and Roy, J., Antimicrobial and chemopreventive properties of herbs and spices, Curr. Med. Chem., 11:1451–1460, 2004.
Platel, K. and Srinivasan, K., Digestive stimulant action of spices: a myth or reality? Indian J. Med. Res., 119:167–179, 2004.
Spinach
Spinach leaves, another good source of flavonoids (Goldbohm et al., 1998), contain other active components that exhibit antioxidative (Kuti and Konuru, 2004), antiproliferative (Sani et al., 2004), and anti-inflammatory (Lomnitski et al., 2000) activities. Spinach extracts have numerous beneficial effects, including chemo and central nervous system protection and anticancer and antiaging functions (Galli et al., 2002; Lomnitski et al., 2003).
A water-soluble antioxidant mixture isolated from spinach leaves contained both flavonoids and p-coumaric-acid derivatives (Bergman et al., 2001). It was found to be nonmutagenic and to show promising anticarcinogenic effects in experimental models, such as skin and prostate cancer (Lomnitski et al., 2003). Spinach is relatively rich in nitrogenous substances, hydrocarbons, and iron sesqui-oxide, which account for 3.3 percent of the total ash.
References
Bergman, M., Varshavsky, L., Gottlieb, H.E., and Grossman, S., The antioxidant activity of aqueous spinach extract: chemical identification of active fractions, Phytochemistry, 58:143– 152, 2001.
Galli, R.L., Shukitt-Hale, B., Youdim, K.A., and Joseph, J.A., Fruit polyphenolics and brain aging: nutritional interventions targeting age-related neuronal and behavioral deficits, Ann. N.Y. Acad. Sci., 959:128–132, 2002.
Goldbohm, R.A., Hertog, M.G.L., Brants, H.A.M., van Poppel, G., and ven den Brandt, P.A., Intake of flavonoids and cancer risk: a prospective cohort study, in Polyphenols in Foods, EUR18169-COST916-Bioactive Plant Cell Wall Components in Nutrition and Health, Amado, R., Andersson, H., Bardocz, S., and Serra, F., Eds., European Commission, Luxembourg, 1998, pp. 159–166.
Kuti, J.O. and Konuru, H.B., Antioxidant capacity and phenolic content in leaf extracts of tree spinach (Cnidoscolus spp.), J. Agric. Food Chem., 52:117–121, 2004.
Lomnitski, L., Bergman, M., Nyska, A., Ben-Shaul, V., and Grossman, S., Composition, efficacy, and safety of spinach extracts, Nutr. Cancer., 46:222–231, 2003.
Lomnitski, L., Carbonatto, M., Ben-Shaul, V., Peano, S., Conz, A., Corradin, L., Maronpot, R.R., Grossman, S., and Nyska, A., The prophylactic effects of natural water-soluble antioxidant from spinach and apocynin in a rabbit model of lipopolysaccharideinduced endotoxemia, Toxicol. Pathol., 28:588–600, 2000.
Sani, H.A., Rahmat, A., Ismail, M., Rosli, R., and Endrini, S., Potential anticancer effect of red spinach (Amaranthus gangeticus) extract, Asia Pac. J. Clin. Nutr., 13:396–400, 2004.

Squalene. (From Auwarter et al., Forensic Sci. Int., 145:149–159, 2004. With permission.)
Squalene
Squalene, a unique hydrocarbon, was first discovered in shark-liver oil in 1906 and named after the Latin root “squalus” (shark). It is a very potent antioxidant because of its six double bonds. Desai and coworkers (1996) showed squalene and a squalene-containing compound, Roidex, both partially prevented the development of chemical-induced cancer and to cause the regression of some of the existing tumors in a mouse-skin model. Dietary squalene was also found to lower plasma cholesterol because of its ability to downregulate HMG-CoA reductase, a key enzyme in cholesterol synthesis. Chan et al. (1996) reported that squalene enhanced the effect of pravastatin (a cholesterol-lowering drug) in patients over 20 weeks. The anticancer properties of squalene, particularly its ability to scavenge free radicals and oxygen-reactive species, was also demonstrated in skin subjected to radiation (Morliere et al., 1995). A study by O’Sullivan and coworkers (2002) showed it was squalene, not ω-3 fatty acids eicosapentaenoic (EPA) and docosapentahexaenoic (DHA) acids, that protected Chinese hamster V79 fibroblast cells from H2O2-induced DNA damage.
The effectiveness of squalene as an adjuvant was recently demonstrated by Suli et al. (2004), who showed it increased the immunogenic activity of nonpotentiated rabies vaccine by approximately 1.8-fold.
References
Auwarter, V., Kiebling, B., and Pragst, F., Squalene in hair—a natural reference substance for the improved interpretation of fatty acid ethyl ester concentrations with respect to alcohol misuse, Forensic Sci. Int., 145:149–159, 2004.
Chan, P., Tomlinson, B., Lee, C.B., and Lee, Y.S., Effectiveness and safety of low dose pravastin and squalene, alone and in combination, in elderly patients with hypercholesterolemia, J. Clin. Pharmacol., 36:422–427, 1996.
Desai, K.N., Wei, H., and Lamartiniere, C.A., The preventative and therapeutic potential of the squalene-containing compound, Roidex, on tumor promotion and regression, Cancer Lett., 101:93–96, 1996.
Morliere, P., Moysan, A., and Tirache, I., Action spectrum for UV-induced lipid peroxidation in cultured human skin fibroblast, Free Rad. Biol. Meet., 19:365–371, 1995.
O’Sullivan, L., Woods, J.A., and O’Brien, N.M., Squalene but not n-3 fatty acids protect against hydrogen peroxide-induced sister chromatid exchanges in Chinese hamster V79 cells, Nutr. Res., 22:847–857, 2002.
Suli, J., Benisek, Z., Elias, D., Svrcek, S., Ondrejkova, A., Ondrejka, R., and Bajova, V., Experimental squalene adjuvant. I. Preparation and testing of its effectiveness, Vaccine, 22:3464–3469, 2004.
St. John’s wort (Hypericum perforation)
see also Hyperforin and Hypericin Hypericin is a naturally occurring substance found in the common St. John’s wort that can also be synthesized from the anthraquinone derivative emodin. It has been used traditionally throughout the history of folk medicine. In the last three decades, St. John’s wort has also become the subject of intensive biochemical research and is proving to be a multifunctional agent in drug and medicinal applications. Recent studies suggest it has antidepressive (Hirano et al., 2004; Zanoli, 2004; Muller et al., 2000), antineoplastic (Dona et al., 2004), antitumor (Gartner et al., 2004), and antiviral (human immunodeficiency and hepatitis C virus) properties (Jacobson et al., 2001; Kubin et al., 2005).

Hyperforins, a family of antimicrobial acylphloroglucinols, is thought to be a primary bioactive ingredient for antidepressive effects in the herb; and hypericins, a family of phototoxic anthraquinones, exhibits antimicrobial, antiviral, and antiherbivore properties in vitro, are two different classes of secondary metabolites produced by Hypericum perforatum L (Sirvent et al., 2003; Kirakosyan et al., 2004).
St. John’s wort preparation has been used in large quantities in Germany for treating mild to moderate depression (Muller et al., 2000). Its efficacy has been demonstrated in several double-blind depression trials and some open-label studies with anxiety disorders. There is pharmacokinetic evidence for the serotonergic, domaminergic, and GABA minergic activity of hypericum, all of which are implicated in social anxiety disorder (Hirano et al., 2004; Zanoli, 2004).
References
Dona, M., Dell’Aica, I., Pezzato, E., Sartor, L., Calíbrese, F., Della Barbera, M., Donella-Deana, A., Appendino, G., Borsarini, A., Caniato, R., and Garbosa, S., Hyperforin inhibits cancer invasion and metastasis, Cancer Res., 64:6225–6232, 2004.
Gartner, M., Muller, T., Simon, J.C., Giannis, A., and Sleeman, J.P., Aristoforin, a novel stable derivative of hyperforin, is a potent anticancer agent, Chembiochem, 6(1):171–177, 2004.
Hirano, K., Kato, Y., Uchida, S., Sugimoto, Y., Yamada, J., Umegaki, K., and Yamada, S., Effects of oral administration of extracts of Hypericum perforatum (St John’s wort) on brain serotonin transporter, serotonin uptake and behaviour in mice, J. Pharm. Pharmacol., 56:1589–1595, 2004.
Jacobson, J.M., Feinman, L., Liebes, L., Ostrow, N., Koslowski, V., Tobia, A., Cabana, B.E., Lee, D., Spritzler, J., and Prince, A.M., Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John’s wort plant, in patients with chronic hepatitis C virus infection, Antimicrob. Agents Chemother., 45:517–524, 2001.
Kirakosyan, A., Sirvent, T.M., Gibson, D.M., and Kaufman, P.B., The production of hypericins and hyperforin by in vitro cultures of St. John’s wort (Hypericum perforatum), Biotechnol. Appl. Biochem., 39:71–81, 2004.
Kubin, A., Wierrani, F., Burner, U., Alth, G., and Grunberger, W., Hypericin—the facts about a controversial agent, Curr. Pharm. Des., 11:233–253, 2005.
Muller, W.E., Singer, A., and Wonnemann, M., Mechanism of action of St. John’s wort extract, Schweiz Rundsch. Med. Prax., 89:2111–2121, 2000.
Sirvent, T.M., Krasnoff, S.B., and Gibson, D.M., Induction of hypericins and hyperforins in Hypericum perforatum in response to damage by herbivores, J. Chem. Ecol., 29:2667–2681, 2003.
Zanoli, P., Role of hyperforin in the pharmacological activities of St. John’s Wort, CNS Drug Rev., 10:203–218, 2004.
Stilbenes
see also Resveratrol Stilbenes are nonflavonoid phenolics present primarily in grapes, berries, and wine products. Stilbenes are induced following stress, such as pathogenic attack and UV-C irradiation. One of the most extensively studied Stilbenes is trans-resveratrol (3,5,4′-trihydroxystilbene), whose health benefits (Granados-Soto, 2003) include antioxidant (Caruso et al., 2004), antimutagenic (Orsini and Verotta, 1999), antiinflammatory (Donnelly et al., 2004), antiestrogenic (PozoGuisado et al., 2004), antiarrhthymic, and cardioprotective (Dong and Ren, 2004), as well as anticancer agent (Aggarwal et al., 2004; Kundu and Surh, 2004) properties.
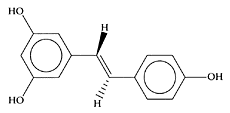
Resveratrol. (From Li et al., Free Rad. Biol. Med., 38:243–257, 2005. With permission.)
Cantos and coworkers (2002) used UV-C irradiation pulses to enhance the production of stilbenes in four grape varieties. Using this procedure, the total resveratrol content increased from 3.4-fold in Flame to 2315-fold in Red Globe. Using this method, the UVC-irradiated grapes were considered a new functional food because of its enrichment with stilbenes.
Recently, several active stilbenes (piceatannol, 3,5,4′-trimethyrpiceatannol, resveratrol, trimethylresveratrol) were reported to exhibit antiallergic activities. They inhibited ionomycin-induced β-hexosaminidase release, suggesting that inhibition of Ca(2+) influx or degranulation mechanisms after Ca(2+) influx is important for their activities. Piceatannol, 3,5,4′-trimethylpiceatannol, resveratrol, and trimethylresveratrol also inhibited in vitro antigen-induced release of TNF-α and IL-4 significantly (Matsuda et al., 2004).
References
Aggarwal, B.B., Bhardwaj, A., Aggarwal, R.S., Seeram, N.P., Shishodia, S., and Takada, Y., Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies, Anticancer Res., 24: 2783–2841, 2004.
Cantos, E., Epsin, J.C., and Tomas-Barberan, F.A., Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses, J. Agric. Food Chem., 50:6322–6329, 2002.
Caruso, F., Tanski, J., Villegas-Estrada, A., and Rossi, M., Structural basis for antioxidant activity of transresveratrol: ab initio calculations and crystal and molecular structure, J. Agric. Food Chem., 52:7279–7285, 2004.
Dong, H.H. and Ren, H.L., New progression in the study of protective properties of resveratrol in anti-cardiovascular disease, Bratisl Lek Listy., 105:225–229, 2004.
Donnelly, L.E., Newton, R., Kennedy, G.E., Fenwick, P.S., Leung, R.H.K., Ito, K., Russell, R.E.F., and Barnes, P.J., Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms, Am. J. Physiol. Lung Cell Mol. Physiol., 287:L774-L783, 2004.
Granados-Soto, V., Pleiotropic effects of resveratrol, Drug News Perspect., 16:299–307, 2003.
Kundu, J.K. and Surh, Y-J., Molecular basis of chemoprevention by resveratrol: NF-κB and AP-1 as potential targets, Mutat. Res., 555:65–80, 2004.
Li, H.-L., Wang, A.-B., Huang, Y., Liu, D.-P., Wei, C., Williams, G.M., Zhang, C.-N., Liu, G., Liu, Y.-Q., Hao, D.-L., Hui, R.-T., Lin, M., and Liang, C.-C., Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways, Free Rad. Biol. Med., 38: 243–257, 2005.
Matsuda, H., Tewtrakul, S., Morikawa, T., and Yoshikawa, M., Anti-allergic activity of stilbenes from Korean rhubarb (Rheum undulatum L.): structure requirements for inhibition of antigen-induced degranulation and their effects on the release of TNF-α and IL-4 in RBL-2H3 cells, Bioorg. Med. Chem., 12:4871–4876, 2004.
Orsini, F. and Verotta, L., Stilbenes and bibenzyls with potential anticancer or chemopreventive activity, Adv. Exp. Med. Biol., 472:169–186, 1999.
Pozo-Guisado, E., Lorenzo-Benayas, M.J., and Fernandez-Salguero, P.M., Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor α-dependent mechanism: Relevance in cell proliferation, Int. J. Cancer, 109:167–173, 2004.
Stinging nettle
The nettle tribe, Urticaceae, is spread throughout the world, with about 500 species, mainly tropical, though several, like the British species of stinging nettle, grows in temperate climates. The British species, belonging to the genus Urtica (the name derived from the Latin, uro, to burn), are known for their well-armed leaves with stinging hairs with the burning properties fluid.
Water extract of stinging nettle has powerful antioxidant activity, evaluated using different antioxidant tests, including reducing power, free-radical scavenging, superoxide-anion-radical scavenging, hydrogen-peroxide scavenging, and metal-chelating activities. It also showed antimicrobial activity against nine microorganisms, antiulcer activity against ethanol-induced ulcerogenesis, and analgesic effect on acetic acid-induced stretching (Gulcin et al., 2004).
The long-term use of the stinging nettle leaf extract, an adjuvant remedy in rheumatic diseases dependent on a cytokine suppressive effect, was found effective in the prevention of chronic murine colitis. This effect seems to be due to a decrease in the Th1 response and may be a new therapeutic option for prolonging remission in inflammatory-bowel disease (Konrad et al., 2005). The clinical efficacy of stinging-nettle-leaf extracts in treatment of rheumatoid arthritis is explained by the ability of 13-hydroxyoctadecatrienic acid, one of the more active anti-inflammatory substances in stinging-nettle-leaf extracts, to suppress the expression of matrix metalloproteinases, which are known to have a role in inflammatory joint diseases (Schulze-Tanzil et al., 2002).
The antiproliferative effect of a methanolic extract of stinging-nettle roots on human prostatic epithelial and stromal cells was observed both in in vivo and in in vitro systems (Konrad et al., 2000). In animal models, the induced growth of prostatic lobe could be reduced by the polysaccharide fraction of the methanolic extract of stinging-nettle roots by 33.8 percent (Lichius et al., 1999). In many European countries, phytopharmaceuticals are commonly used for managing benign prostatic hyperplasia and associated lower urinary-tract symptoms, with these products representing up to 80 percent of all drugs prescribed for this disorder. Extracts from the fruits of saw palmetto and the roots of stinging nettle are particularly popular.
Although extracts from the stinging nettle may provide therapeutic value for some inflammatory medical conditions, it can also cause a wide range of cutaneous reactions. Contact with the hairs or spines on the stems and leaves of the stinging nettle causes the release of several biologically active substances that cause itching, dermatitis, and urticaria within moments of contact (Anderson et al., 2003).
References
Anderson, B.E., Miller, C.J., and Adams, D.R., Stinging nettle dermatitis, Am. J. Contact Dermat., 14:44–46, 2003.
Caliskaner, Z., Karaayvaz, M. and Ozturk, S., Misuse of a herb: stinging nettle (Urtica urens) induced severe tongue oedema, Complementary Ther. Med., 12:57–58, 2004.
Gulcin, I., Kufrevioglu, O.I., Oktay, M., and Buyukokuroglu, M.E., Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.), J. Ethnopharmacol., 90(2–3):205– 15, 2004.
Koch, E., Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms, Planta Med., 67:489–500, 2001.
Konrad, A., Mahler, M., Arni, S., Flogerzi, B., Klingelhofer, S., and Seibold, F., Ameliorative effect of IDS 30, a stinging nettle leaf extract, on chronic colitis, Int. J. Colorectal Dis., 20:9– 17, 2005.
Konrad, L., Muller, H.H., Lenz, C., Laubinger, H., Aumuller, G., and Lichius, J.J., Antiproliferative effect on human prostate cancer cells by a stinging nettle root (Urtica dioica) extract, Planta Med., 66: 44–47, 2000.
Lichius, J.J., Renneberg, H., Blaschek, W., Aumuller, G., and Muth, C., The inhibiting effects of components of stinging nettle roots on experimentally induced prostatic hyperplasia in mice, Planta Med., 65:666–668, 1999.
Schulze-Tanzil, G., de, Souza P., Behnke, B., Klingelhoefer, S., Scheid, A., and Shakibaei M., Effects of the antirheumatic remedy Hox alpha—a new stinging nettle leaf extract—on matrix metalloproteinases in human chondrocytes in vitro, Histol. Histopathol., 17:477–485, 2002.
Strawberries
Strawberries are rich in ascorbic acid and a wide range of phenolic compounds. The significant inhibition by strawberry juice of the endogenous formation of N-nitrosoamino acids in humans could not be solely attributed to ascorbic acid (Hesler et al., 1992). Subsequent research showed that strawberries were high in antioxidant activity and capable of enhancing antioxidant capacity in humans (Wang et al., 1996; Cao et al., 1998). Strawberry supplementation in the diet of N-nitroso-methyl-benzylamine-treated rats reduced the multiplicity of esophageal tumors (Stoner et al., 1997). Ellagic acid was subsequently identified as one of the chemopreventive components present in strawberries, responsible for inhibiting cancers. It appears to prevent the binding of the reactive-carcinogenic components with DNA, as well as stimulate detoxification enzymes (Teel, 1986; Ahn et al., 1996). Treating Syrian hamster embryo (SHE) cells with the carcinogen benzo[a]pyrene for seven days, Xue and coworkers (2001) found that a methanolic extract from freeze-dried strawberries (Fragara ananassa) appeared to display chemopreventive activity by interfering with the uptake, activation, and detoxification of the carcinogen or by intervention of DNA binding and repair.
In strawberries, the most abundant bioactive compounds are ellagic acid and certain flavonoids: anthocyanin, catechin, quercetin, and kaempferol. These compounds in strawberries have potent antioxidant power, which helps to lower risk of cardiovascular events. Furthermore, strawberry extracts have been shown to inhibit COX enzymes in vitro, which would modulate the inflammatory process. Individual compounds in strawberries have demonstrated anticancer activity in several different experimental systems. Preliminary animal studies have indicated that diets rich in strawberries may also have the potential to provide benefits to the aging brain (Hannum, 2004).
References
Ann, D., Putt, D., Kresty, L., Stoner, G.D., Fromm, D., and Hollenberg, P.F., The effects of dietary ellagic acid on rat hepatic and esophageal mucosal cytochromes P450 and phase II enzymes, Carcinogenesis, 17:821–828, 1996.
Cao, G., Russell, R.M., Lischner, N., and Prior, R.L., Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or Vitamin C in elderly women, J. Nutr., 128:2383–2390, 1998.
Hannum, S.M., Potential impact of strawberries on human health: a review of the science, Crit. Rev. Food Sci. Nutr., 44:1–17, 2004.
Hesler, M.A., Hotchkiss, J.H., and Rose, D.A., Influence of fruit and vegetable juices on the endogenous formation of N-nitrosoproline and N-nitrosothiazolidine-4-carboxylic acid in humans on controlled diets, Carcinogenesis, 13:2277–2280, 1992.
Stoner, G.D., Kresty, L.A., Lu, J., Porter, C., Siglin, J.C., and Morse, M.A., Inhibitory effect of strawberries on esophageal tumorigenesis and O6-methylguanine levels in the F344 rat, Proc. Annu. Meet. Am. Assoc. Cancer Res., 38:A2462, 1997.
Teel, R.W., Dixit, R., and Stoner, G.D., The effect of ellagic acid on the uptake, persistence, metabolism and DNA-binding of benzo[a]pyrene in cultured explants of strain A/J mouse lung, Carcinogenesis, 6:391–395, 1985.
Wang, H., Cao, G., and Prior, R.L., Total antioxidant capacity of fruits, J. Agric. Food Chem., 44:701–705, 1996.
Xue, H., Aziz, R.M., Sun, N., Cassady, J.M., Kamendulis, L.M., Xu, Y., Stoner, G.D., and Klaunig, J.E., Inhibition of cellular transformation of berry extracts, Carcinogenesis, 22:351–356, 2001.
Sugar-beet fiber
Sugar-beet fiber, like wheat bran and flaxseed, is useful in the treatment of constipation, colon diverticulosis, and adiposity (Trepel, 2004). Rations containing sugar-beet pulp, given to broiler breeder females, were associated with higher water contents in the gastrointestinal tract, and it was proposed that this improved satiety and welfare (Hocking et al., 2004).
Mataumoto and coworkers (2001) showed that sugar-beet fiber reduced the ovariectomyinduced elevation in plasma cholesterol in 6-week-old ovariectomized female rats. Plasma total and non-HDL cholesterol were lowered significantly by 29 percent and 47 percent in rats fed sugar-beet fiber in comparison to the control diet.
References
Hocking, P.M., Zaczek, V., Jones, E.K., and Macleod, M.G., Different concentrations and sources of dietary fibre may improve the welfare of female broiler breeders, Br. Poult. Sci., 45:9–19, 2004.
Mataumoto, J., Kishida, T., and Ebihara, K., Sugar beet fiber suppresses ovarian hormone deficiency-induced hypercholesterolemia in rats, Nutr. Res., 21: 1519–1527, 2001.
Trepel, F., Dietary fibre: more than a matter of dietetics. II. Preventative and therapeutic uses, Wien Klin. Wochenschr., 116:511–522, 2004.
Sulforaphane
Sulforaphane [1-isothiocyanato- (4R)-(methylsulfinyl) butane], an isothiocyanate present naturally in widely consumed vegetables, has a particularly high concentration in broccoli. It was isolated from SAGA broccoli as the major phase II enzyme inducer, present in organic solvent extracts of this vegetable (Zhang et al., 1994). Sulforaphane was found to block chemical-initiated tumor formation in rats (Faulkner et al., 1998). The effectiveness of sulforaphane is based on induction of hepatic detoxifying enzymes. Sulforaphane is a very potent inducer of phase II enzymes, UDP-glucuronosyltransferase 1A1, and glutathione S-transferase A1 (Bacon et al., 2003). It was shown to inhibit cytochrome P-450 (CYP2E1) involved in the activation of a variety of carcinogens (Barcelo et al., 1996; Faulkner et al., 1998).
Sulforaphane. (Adapted from Wu et al., Mutat. Res., 589:81–102, 2005.)
Recent studies report that induction of thioredoxin reductase by sulforaphane is mediated by putative antioxidant response elements found in the promoter similar to the upregulating mechanism of other antioxidant enzymes (Hintze et al., 2003). In highly proliferative HT29 cells, sulforaphane induces a cell-cycle arrest, followed by cell death caused by a proapoptotic protein bax-dependent pathway. These results suggest that in addition to the activation of detoxifying enzyme activities, specific mechanisms, such as apoptosis, are also involved in the sulforaphane-associated chemoprevention of cancer (Gamet-Payrastre et al., 2000; Misiewicz et al., 2004). Similar results were found in leukemic cells (Fimognari et al., 2002) and human melanoma cells (Misiewicz et al., 2003) with recent reports suggesting sulforaphane can halt human breast-cancer cells (Johnston, 2004). Animal studies demonstrated that sulforaphane significantly reduces the formation of total and multicrypt foci in azoxymethaneinduced colonic aberrant crypt foci F344 rats, during both the initiation phase and the postinitiation treatment, indicative of its potential to prevent colon cancer (Chung et al., 2000). Recent studies demonstrated the potential of sulforaphane for treating pancreatic cancer (Pham et al., 2004). Jackson and Singletary (2004) reported sulforaphane was an effective inhibitor of human mcf-7 mammary-cancer cells.
A potent decrease in lipopolysaccharide-induced secretion of proinflammatory and procarcinogenic signaling factors (i.e., NO, prostaglandin E2, and tumor-necrosis factor) in cultured Raw 264.7 macrophages after sulforaphane treatment suggested that anti-inflammatory mechanisms contribute to sulforaphane-mediated cancer chemoprevention (Heiss et al., 2001).
Sulforaphane was also shown to be a potent bactericidal agent against both extracellular and intracellular H. pylori in vitro. Haristoy et al. (2003) investigated the efficacy of sulforaphane in vivo against H. pylori by using a recently developed model, which uses human gastric xenografts in nude mice. They observed that H. pylori can be eradicated from human gastric xenografts after a short-term administration of sulforaphane at a dose that can be achieved in the human diet. Thus, sulforaphane delivered in the diet could be beneficial for the treatment of H. pylori-associated gastric diseases.
References
Bacon, J.R., Williamson, G., Garner, R.C., Lappin, G., Langouet, S., and Bao, Y., Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes, Carcinogenesis, 24:1903–1911, 2003.
Barcelo, S., Gardiner, J.M., Gesher, A., and Chipman, J.K., CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane, Carcinogenesis, 17:277–282, 1996.
Chung, F-L., Conaway, C.C., Rao, C.V., and Reddy, B.S., Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate, Carcinogenesis, 21:2287–2291, 2000.
Faulkner, K., Mithen, R., and Williamson, G., Selective increase of the potential anticarcinogen 4-methylsulphinylbutyl glucosinolate in broccoli, Carcinogenesis, 19:605–609, 1998.
Fimognari, C., Nusse, M., Cesari, R., Iori, R., Cantelli-Forti, G., and Hrelia, P., Growth inhibition, cellcycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane, Carcinogenesis, 23:581–586, 2002.
Garnet-Payrastre, L., Li, P., Lumeau, S., Cassar, G., Dupont, M-A., Chevolleau, S., Gasc, N., Tulliez, J., and Terce, F., Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells, Cancer Res., 60:1426–1433, 2000.
Haristoy, X., Angioi-Duprez, K., Duprez, A., and Lozniewski, A., Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice, Antimicrob. Agents Chemother., 47:3982–3984, 2003.
Heiss, E., Herhaus, C., Klimo, K., Bartsch, H., and Gerhauser, C., Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms, J. Biol. Chem., 276:32008– 32015, 2001.
Hintze, K.J., Wald, K.A., Zeng, H., Jeffery, E.H., and Finley, J.W., Thioredoxin reductase in human hepatoma cells is transcriptionally regulated by sulforaphane and other electrophiles via an antioxidant response element, J. Nutr., 133:2721–2727, 2003.
Jackson, S.J.T. and Singletary, K.W., Sulforaphane inhibits human mcf-7 mammary cancer cell mitotic progression and tubulin polymerization, J. Nutr., 134:2229–2236, 2004.
Johnston, N., Sulforaphane halts breast cancer cell growth, Drug Discov. Today, 9:908, 2004.
Misiewicz, I., Skupinska, K., and Kasprzycka-Guttman, T., Sulforaphane and 2-oxohexyl isothiocyanate induce cell growth arrest and apoptosis in L-1210 leukemia and ME-18 melanoma cells, Oncol. Rep., 10:2045–2050, 2003.
Misiewicz, I., Skupinska, K., Kowalska, E., Lubinski, J., and Kasprzycka-Guttman, T., Sulforaphane-mediated induction of a phase 2 detoxifying enzyme NAD(P)H:quinone reductase and apoptosis in human lymphoblastoid cells, Acta Biochem. Pol., 51: 711–721, 2004.
Pham, N.A., Jacobberger, J.W., Schimmer, A.D., Cao, P., Gronda, M., and Hedley, D.W., The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice, Mol. Cancer Ther., 3:1239–1248, 2004.
Wu, X., Kassie, F., and Mersch-Sundermann, V., Induction of apoptosis in tumor cells by naturally occurring sulfur-containing compounds, Mutat. Res., 589:81–102, 2005.
Zhang, Y., Kensler, T.W., Cho, C.G., Posner, G.H., and Talalay, P., Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates, Proc. Natl. Acad. Sci. U.S.A., 91:3147–3150, 1994.
Summer savory (Satureja hortensis)
Satureja hortensis L. (Lamiaceae) is an annual herb, traditionally used in the Eastern Anatolia region of Turkey as folk medicine for treatment of different infectious diseases and disorders. In Iranian folk medicine, it was used as muscleand bone-pain reliever.
Uslu and coworkers (2003) showed that the activity of nitric-oxide synthase enzyme, and concentration of nitric-oxide metabolites were both significantly reduced by topical administration of S. hortensis extract. Histological examination demonstrated reduced inflammation. Thus, their data suggest that S. hortensis extract may have the potential as an antiinflammation agent in the treatment of rhinosinusitis diseases. Antimicrobial test results showed that the essential oil of S. hortensis had great potential in antimicrobial activities against different bacteria, fungi, and yeast species. Thymol (29.0 percent), carvacrol (26.5 percent), γ-terpinene (22.6 percent), and p-cymene (9.3 percent) were the main components in essential oil of S. hortensis (Gulluce et al., 2003).
The essential oil of S. hortensis was also found as a relaxant of rat-isolated ileum. In addition to antispasmodic activity in vitro, it inhibited castor oil-induced diarrhea in mice. Thus, S. hortensis essential oil may have clinical benefits for treatment of some gastrointestinal disorders (Hajhashemi et al., 2000).
The polyphenolic fraction and the essential oil of S. hortensis L. were both shown to have antinociceptive and anti-inflammatory effects (Hajhashemi et al., 2002). The antioxidant capacities and total phenol content were demonstrated in the ethanol and acetone extracts of the dried material from a number of sources, including summer savory (Exarchou et al., 2002).
References
Exarchou, V., Nenadis, N., Tsimidou, M., Gerothanassis, I.P., Troganis, A., and Boskou, D., Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory, J. Agric. Food Chem., 50:5294–5299, 2002.
Gulluce, M., Sokmen, M., Daferera, D., Agar, G., Ozkan, H., Kartal, N., Polissiou, M., Sokmen, A., and Sahin, F., In vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L., J. Agric. Food Chem., 51: 3958–3965, 2003.
Hajhashemi, V., Ghannadi, A., and Pezeshkian, S.K., Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil, J. Ethnopharmacol., 82:83–87, 2002.
Hajhashemi, V., Sadraei, H., Ghannadi, A.R., and Mohseni, M., Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil, J. Ethnopharmacol., 71:187–192, 2000.
Uslu, C., Murat Karasen, R., Sahin, F., Taysi, S., and Akcay, F., Effects of aqueous extracts of Satureja hortensis L. on rhinosinusitis treatment in rabbit, J. Ethnopharmacol., 88:225–228, 2003.
Sunflower
see Sunflower oil and Sunflower seed protein
Sunflower oil
Alpaslan and Gunduz (2000) showed that growing conditions significantly affected the fatty-acid compositions of sunflower varieties. In the 1991 and 1992 crop years, they ranged for oil content 44.2–51.2 percent (on dry weight basis) and 43.0–51.5 percent (on dry weight basis); oleic acid 14.8–18.5 percent and 32.9–40.1 percent; linoleic acid 69.5– 74.5 percent and 49.7–55.7 percent; and tocopherol content (as α-tocopherol) 648–860 mg/kg and 524–880 mg/kg, respectively.
A significantly higher LDL susceptibility to oxidation was observed after sunfloweroil intake in comparison with virgin olive oil, in spite of an increase in LDL α-tocopherol concentration in the sunflower-oil group. These results provide further evidence that sunfloweroil-enriched diets do not protect LDL against oxidation, as virgin olive oil does, in patients with peripheral vascular disease (Aguilera et al., 2004).
In comparison to other commercially available antimicrobial agents, ozonized sunflower oil demonstrated significant antimicrobial activity and anti-inflammatory and wound-healing properties (Rodrigues et al., 2004). Treatment of preterm infants <34 weeks in Egypt with sunflower-seed oil resulted in a significant improvement in skin condition and a highly significant reduction in the incidence of nosocomial infections compared with infants not receiving topical prophylaxis. This study suggests the potential of topical therapy to reduce infections and save newborn lives in developing countries (Darmstadt et al., 2004).
Sesame oil and sunflower oil offered 20 percent and 40 percent protection, respectively, in mouse-skin tumor model. The antioxidant capabilities of these compounds could not solely explain the observed anticancer characteristics. Thus, the observed chemopreventive effects warrant more attention, since they already exist in the population with no known adverse effects (Kapadia et al., 2002).
References
Aguilera, C.M., Mesa, M.D., Ramirez-Tortosa, M.C., Nestares, M.T., Ros, E., and Gil, A., Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease, Clin. Nutr., 23:673–681, 2004.
Alpaslan, M. and Gunduz, H., The effects of growing conditions on oil content, fatty acid composition and tocopherol content of some sunflower varieties produced in Turkey, Nahrung, 44:434–437, 2000.
Darmstadt, G.L., Badrawi, N., Law, P.A., Ahmed, S., Bashir, M., Iskander, I., Al Said, D., El Kholy, A., Husein, M.H., Alam, A., Winch, P.J., Gipson, R., and Santosham, M., Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial, Pediatr. Infect. Dis. J., 23:719–725, 2004.
Kapadia, G.J., Azuine, M.A., Tokuda, H., Takasaki, M., Mukainaka, T., Konoshima, T., and Nishino, H., Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis, Pharmacol. Res., 45:499–505, 2002.
Rodrigues, K.L., Cardoso, C.C., Caputo, L.R., Carvalho, J.C.T., Fiorini, J.E., and Schneedorf, J.M., Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds, Inflammopharmacology, 12:261–270, 2004.
Sunflower-seed protein
In addition to oil, sunflower also produces a protein. Over the past few decades, evidence has accrued that ingestion of plant proteins lowers plasma cholesterol in animals (Carroll, 1995). One mechanism suggests that plant proteins suppress the intestinal absorption or reabsorption of neutral lipids, reducing the cholesterol pool within the body (Van Deer Meer and Beynen, 1987). Sen and Bhattacharyya (2000) enzymatically extracted dehulled sunflower seeds with pectinase to produce a protein-rich fraction low in fiber and chlorogenic acid. The protein-rich fraction significantly reduced plasma cholesterol (p<0.02) and triglyceride (p<0.02) in rats fed the sunflower-seed protein fraction compared to casein (Table S.62). While the HDL-cholesterol levels were significantly reduced by the sunflower-seed protein fraction, the total cholesterol/HDL cholesterol and LDL cholesterol/HDL cholesterol ratios were not significantly different. Thus, the sunflower-seed protein fraction exhibited similar hypolipidemic effects to casein.
References
Carrol, K.K. and Kurowska, E.M., Soy consumption and cholesterol reduction, review of animal and human studies, J. Nutr., 125:594S–597S, 1995.
Sen, M. and Bhattacharyya, D.K., Hypolipidemic effect of enzymatically extracted sunflower seed protein fraction, J. Sci. Food Agric., 81:347–352, 2000.
Vandermeer, R. and Beynen, A.C., Species dependent responsiveness of serum cholesterol to dietary proteins, J. Am. Oil Chem. Soc., 64:1172–1177, 1987.
Sweet basil
Basil or sweet basil (Ocimum basilicum) is cultivated throughout India. It is rich in essential oils that have been the subject of numerous chemical studies (Grayer et al., 1996). Sweet basil was grown by local people as a medicinal plant, culinary herb, and antimicrobial agent. Some 30 monoterpenoids, sesquiterpenoids, and phenylpropanoids identified in basil oil as the major components (more than 20 percent of the total essential-oil composition) were linalool, methyl chavicol, eugenol, methyl eugenol, and geraniol (Grayer et al., 1996).
Basil essential oils and their principal constituents were found to exhibit antimicrobial activity against a wide range of Gram-negative and Gram-positive bacteria, yeast, and mold (Suppakul et al., 2003). Linalool alone showed a moderate antifungal activity, while eugenol showed no activity at all. Mixing the two components in a ratio similar to their concentrations in the original oil was found to enhance the antifungal properties of basil oil, indicating a synergistic effect (Edris and Farrag, 2003).
The major antioxidant compound in the methanolic extract of sweet basil was confirmed as rosmarinic acid. The results showed that one rosmarinic acid can capture 1.52 radicals, and, furthermore, the existence of a synergistic effect between α-tocopherol and rosmarinic acid was revealed (Jayasinghe et al., 2003).
The effects of an alcoholic extract of the fresh leaves of sweet basil on Swiss albino mice were increasing the activity of xenobiotic metabolizing phase I and phase II enzymes, elevating antioxidant-enzyme response by increasing significantly the hepatic glutathione reductase, superoxide dismutase, and catalase activities, increasing glutathione content, and decreasing lipid peroxidation and lactate dehydrogenase activity in the liver after eight to nine weeks. Furthermore, chemopreventive response was evident from the reduced tumor, as well as from the reduced percentage of tumor-bearing animals (Dasgupta et al., 2004).
Recently Fang and coworkers (2004) reported that essential oil from sweet basil can act as skin-permeation enhancers to promote the percutaneous absorption of drugs. This might be mainly due to improvement in the partitioning of the drugs to the stratum corneum.
References
Dasgupta, T., Rao, A.R., and Yadava, P.K., Chemomodulatory efficacy of basil leaf (Ocimum basilicum) on drug metabolizing and antioxidant enzymes, and on carcinogen-induced skin and forestomach papillomagenesis, Phytomedicine, 11:139–151, 2004.
Edris, A.E. and Farrag, E.S., Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase, Nahrung, 47:117–121, 2003.
Fang, J.Y., Leu, Y.L., Hwang, T.L., and Cheng, H.C., Essential oils from sweet basil (Ocimum basilicum) as novel enhancers to accelerate transdermal drug delivery, Biol. Pharm. Bull., 27:1819–1825, 2004.
Grayer, R.J., Kite, G.C., Goldstone, F.J., Bryan, S.E., Paton, A., and Putievsky, E., Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum, Phytochemistry, 43:1033–1039, 1996.
Jayasinghe, C., Gotoh, N., Aoki, T., and Wada, S., Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.), J. Agric. Food Chem., 51(15):4442–9, 2003.
Suppakul, P., Miltz, J., Sonneveld, K., and Bigger, S.W., Antimicrobial properties of basil and its possible application in food packaging, J. Agric. Food Chem., 51:3197–3207, 2003.
Sweet flag (Acorus calamus)
Sweet flag (Acorus calamus L.) is a perennial shrub that grows in damp, marshy places in India, North America, and Europe. The rhizomes of Acorus calamus are empirically used for treating a wide variety of human diseases. It is recognized as an Indian drug with antibacterial, anticonvulsant, antiveratrinic, and antiarrhythmic action (Madan et al., 1960), as well as a tranquilizing agent (Menon and Dandiya, 1967).
The ethanol extract of Acorus calamus rhizomes exhibited a large number of actions on the CNS similar to α-asarone (an active principle of A. calamus) but differed from the latter in several other respects. These differences could be due to the effects of other chemicals in the plant extract (Vohora et al., 1990). The alcoholic extract also antagonized spontaneous motor activity and also amphetamine-induced hyperactivity in mice. It was less potent than chloropromazine, but still exerted sedative and tranquilizing action (Panchal et al., 1989).
Neurobehavioral changes produced by acrylamide could be prevented following treatment with Acorus calamus rhizomes (Shukla et al., 2002). Administration of a 50 percent ethanolic extract (100 and 200 mg/kg), as well as saponins (10 mg/kg) isolated from the extract showed significant hypolipidemic activity (Parab and Mengi, 2002). Recently, Mehrotra and coworkers (2003) demonstrated the anti-proliferative and immunosuppressive potential of an ethanolic extract from Acorus calamus in vitro.
References
Madan, B.R., Arora, B.B., and Kapila, K., Anticonvulsant, antiveratrinic and antiarrhythmic actions of Acorus calamus Linn.—an Indian indigenous drug, Arch. Int. Pharmacodyn. Ther., 124:201– 211, 1960.
Menon, M.K. and Dandiya, P.C., The mechanism of the tranquilizing action of asarone from Acorus calamus Linn., J. Pharm. Pharmacol., 19:170–175, 1967.
Mehrotra, S., Mishra, K.P., Maurya, R., Srimal, R.C., Yadav, V.S., Pandey, R., and Singh, V.K., Anticellular and immunosuppressive properties of ethanolic extract of Acorus calamus rhizome, Int. Immunopharmacol., 3:53–561, 2003.
Panchal, G.M., Venkatakrishna-Bhatt, H., Doctor, R.B., and Vajpayee, S., Pharmacology of Acorus calamus L., Indian J. Exp. Biol., 27:561–567, 1989.
Parab, R.S. and Mengi, S.A., Hypolipidemic activity of Acorus calamus L. in rats, Fitoterapia, 73:451–455, 2002.
Shukla, P.K., Khanna, V.K., Ali, M.M., Maurya, R.R., Handa, S.S., and Srimal, R.C., Protective effect of Acorus calamus against acrylamide induced neurotoxicity, Phytother. Res., 16:256– 260, 2002.
Vohora, S.B., Shah, S.A., and Dandiya, P.C., Central nervous system studies on an ethanol extract of Acorus calamus rhizomes, J. Ethnopharmacol., 28: 53–62, 1990.
Sweet potatoes
Sweet potatoes, a crop native to South America, are easy to grow and have become a staple food in many African countries. The orange-fleshed sweet-potato variety is rich in β-carotene, which offers hope to the population who are currently beyond the reach of a vitamin A supplement. The effect of 60 days of daily supplementation with 750 mg retinol equivalents (RE) of pureed sweet potatoes were tested by Haskell et al. (2004). The overall geometric mean of initial vitamin A stores was 0.108 +/− 0.067 mmol. Relative to the low vitamin A control group, the estimated mean changes in vitamin A stores were 0.029 mmol. Vitamin A equivalency factors (β-carotene :retinol, wt:wt) were estimated as approximately 13:1 for sweet potato. Thus, daily consumption of pureed sweet potatoes has a positive effect on vitamin A stores in populations at risk of vitamin A deficiency (Haskell et al., 2004).
The major phenolic components contained in the 70 percent methanol extract of sweet potatoes with strong antioxidative activity were identified as chlorogenic acid and isochlorogenic acid-1, -2, and -3. The other minor free phenolics were identified as caffeic acid and 4-O-caffeoylquinic acid. Chlorogenic acid and isochlorogenic acids, however, had only a slight antioxidative activity. Thus, the effective antioxidant activity of the sweet-potato extract was proposed to be mainly based on the synergistic effect of phenolic compounds with amino acids (Hayase and Kato, 1984). The dietary fiber content of sweet potatoes was found to range from 9–12 percent for cured roots. Soluble and insoluble dietary fiber averaged 5–30 percent and 543 percent, respectively (Mullin et al., 1994).
Anthocyanins are the chemical components that give the intense color to sweet potatoes, as in many other fruits and vegetables. Epidemiological investigations have indicated that moderate consumption of anthocyanin products is associated with a lower risk of cardiovascular disease and improvement of visual functions (Hou, 2003). Recently, the leaves of sweet potatoes were found to be rich in nutritive and functional components. They were found to contain a large amount of protein, showing high aminoacid score, soluble dietary fibers and minerals, particularly iron, and vitamins, such as carotene, vitamin B2, vitamin C, and vitamin E. Further-more, polyphenol content in the leaves was comparatively high. These results suggest that the whole parts of sweet potatoes should be utilized as valuable foodstuffs (Ishida et al., 2000).
References
Haskell, M.J., Jamil, K.M., Hassan, F., Peerson, J.M., Hossain, M.I., Fuchs, G.J., and Brown, K.H., Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men, Am. J. Clin. Nutr., 80:705–714, 2004.
Hayase, F. and Kato, H., Antioxidative components of sweet potatoes, J. Nutr. Sci. Vitaminol., 30:37–46, 1984.
Hou, D.X., Potential mechanisms of cancer. Dietary fibre in sweet potatoes, Food Res. Int., 27:563–565, 1994.
Ishida, H., Suzuno, H., Sugiyama, N., Innami, S., Tadokoro, T. and Maekawa, A., Nutritive evaluation of chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir), Food Chem., 68:359–367, 2000.
Mullin, J., Rosa, N. and Reynolds, L.B., Dietary fibre in sweet potatoes, Food Res. Int., 27:563– 565, 1994.

Swinholide A (1): n=1, R1=R2=Me
Swinholide B (2): n=1, R1=H, R2=Me
Swinholide C (3): n=1,R1=Me, R2=H
Swinholides A, B, and C. (From Hayakawa and Miyashita, Tetrahedron Lett., 41:707–711, 2000. With permission.)
Swinholide
The marine natural products swinholides A (1), B (2), and C (3), 44-membered dimeric macrolides isolated from the Okinawan marine sponge Theonella swinhoei (Carmely and Cashman, 1985), have been shown to exhibit potent cytotoxicity against a variety of human carcinoma-cell lines, as well as a broad spectrum of antifungal activity (Tanaka et al., 1990; Doi et al., 1991). Their structures are characterized by the C2-symmetrical dimeric macrolides in which two polypropionate-derived chains take axial orientation on a tetrahydropyran ring. Their unique structures and potent anticancer activities have elicited much attention from synthetic organic chemists toward the synthesis of polypropionate-derived bioactive compounds (Paterson et al., 1994; Hiroyuki and Masaaki, 2000).
References
Cannery, S. and Kashman, Y., Structure of swinholidea, a new macrolide from the marine sponge Theonella swinhoei, Tetrahedron Lett., 26:511–514, 1985.
Doi, M., Ishida, T., Koybayashi, M. and Kitagawa, I. Molecular conformation of swinholide A, a potent cytotoxic dimeric macrolide from the Okinwan marine sponge Theonella swinhoei: X-ray crystal structure of its diketone, J. Org. Chem., 56:3629–3643, 1991.
Hayakawa, H. and Miyashita, M., An efficient and stereoselective construction of the C(9)-C(17) dihydropyran segment of swinholides A-C via a novel reductive cleavage of an epoxy aldehyde, Tetrahedron Lett., 41:707–711, 2000.
Tanaka, J., Higa, T., Kobayashi, M., and Kitagawa, I., Marine natural products. XXIV. The absolute stereostructure of misakinolide A, a potent cytotoxic dimeric macrolide from an Okinawan marine sponge Theonella sp., Chem. Pharm. Bull., 38:967–2970, 1990.
Paterson, I., Yeung, K.-S., Ward, R.A., Cumming, J.G., and Smith, J.D., Total synthesis of swinholide A and hemiswinholide A, J. Am. Chem. Soc., 116: 9391–9392, 1994.
Symbiotic
see also Prebiotics and Probiotics Prebiotics, usually polysaccharides, exhibit strong bioactivity, and the ingestion of prebiotics has been shown to reduce the rate of infection and restore health in sick and postoperative patients. Probiotics are bacteria that reduce or eliminate potentially pathogenic microorganisms, as well as various toxins, mutagens, and carcinogens. They modulate the innate and adaptive immune defense mechanisms, promote apoptosis, and release many nutrients, antioxidants, growth, coagulation, and other factors necessary for recovery. A combination of prebiotics and probiotics is referred to as “synbiotics” if it has a stronger effect on intestinal diseases than probiotics or prebiotics alone (Holmes, 2003; Tuohy et al., 2003).
Synbiotics treatment was also found to contribute in the treatment of severe acute pancreatitis, chronic hepatitis, and liver transplantation (Bengmark, 2003). It was also reported as an alternative to lactulose for the management of minimal hepatic encephalopathy in patients with cirrhosis (Liu et al., 2004).
Roller and coworkers (2004) demonstrated that synbiotic supplementation in carcinogentreated rats primarily modulated immune functions in the Peyer’s patches, with a reduction in the number of colon tumors. Other evidence for the cancer-preventing properties of probiotics and prebiotics is derived from studies on fecalenzyme activities in animals and humans, inhibition of genotoxicity of known carcinogens in vitro and in vivo, suppression of carcinogeninduced preneoplastic lesions and tumors in laboratory animals. Some of these studies indicate that combinations of probiotics and prebiotics are more effective than either one of these treatments (Burns and Rowland, 2000).
Synbiotic treatment to short-bowel patients with refractory enterocolitis was very promising. It improved the intestinal bacterial flora, increased short-chain fatty acids in the feces, and accelerated their body-weight gain (Kanamori et al., 2004). Oral supplements with synbiotic also increased energy intake and promoted weight gain in acutely ill children receiving antibiotics (Schrezenmeir et al., 2004).
References
Bengmark, S., Use of some pre-, pro- and synbiotics in critically ill patients, Best Pract. Res. Clin. Gastroenterol., 17:833–848, 2003.
Burns, A.J. and Rowland. I.R., Anti-carcinogenicity of probiotics and prebiotics, Curr. Issues Infest. Microbiol., 1:13–24, 2000.
Holmes, S., Are probiotics and other functional foods the medicines of the future? Prof. Nurse, 18:627–630, 2003.
Kanamori, Y., Sugiyama, M., Hashizume, K., Yuki, N., Morotomi, M., and Tanaka, R., Experience of long-term synbiotic therapy in seven short bowel patients with refractory enterocolitis, J. Pediatr. Surg., 39:1686–1692, 2004.
Kanamori, Y., Hashizume, K., Sugiyama, M., Mortomi, M., Yuki, N., and Tanaka, R., A novel synbiotic therapy dramatically improved the intestinal function of a pediatric patient with laryngotracheo-esophageal cleft (LTEC) in the intensive care unit, Clin. Nutr., 21:527–530, 2002.
Liu, Q., Duan, Z.P., Ha da, K., Bengmark, S., Kurtovic, J., and Riordan, S.M., Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis, Hepatology, 39:1441–1449, 2004.
Roller, M., Pietro Femia, A., Caderni, G., Rechkemmer, G., and Watzl, B., Intestinal immunity of rats with colon cancer is modulated by oligofructoseenriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis, Br. J. Nutr., 92: 931–938, 2004.
Schrezenmeir, J., Heller, K., McCue, M., Llamas, C., Lam, W., Burow, H., Kindling-Rohracker, M., Fischer, W., Sengespeik, H.C., Comer, G.M., and Alarcon, P., Benefits of oral supplementation with and without synbiotics in young children with acute bacterial infections, Clin. Pediatr., 43:239–249, 2004.
Tuohy, K.M., Probert, H.M., Smejkal, C.W., and Gibson, G.R., Using probiotics and prebiotics to improve gut health, Drug Discov. Today, 8:692–700, 2003.