8
Ear, Nose, & Throat Disorders
Lawrence R. Lustig, MD
Joshua S. Schindler, MD
DISEASES OF THE EAR
HEARING LOSS
ESSENTIALS OF DIAGNOSIS

 Two main types of hearing loss: conductive and sensorineural.
Two main types of hearing loss: conductive and sensorineural.
 Most commonly due to cerumen impaction, transient eustachian tube dysfunction from upper respiratory tract infection, or age-related hearing loss.
Most commonly due to cerumen impaction, transient eustachian tube dysfunction from upper respiratory tract infection, or age-related hearing loss.
 Classification & Epidemiology
Classification & Epidemiology
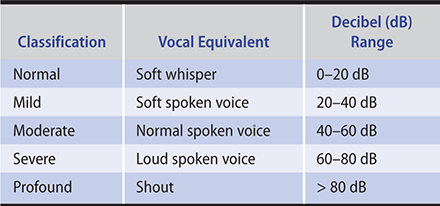
Table 8–1 categorizes hearing loss as normal, mild, moderate, severe, and profound and outlines the vocal equivalent as well as the decibel range.
Table 8–1. Hearing loss classification.
A. Conductive Hearing Loss
Conductive hearing loss results from external or middle ear dysfunction. Four mechanisms each result in impairment of the passage of sound vibrations to the inner ear: (1) obstruction (eg, cerumen impaction), (2) mass loading (eg, middle ear effusion), (3) stiffness (eg, otosclerosis), and (4) discontinuity (eg, ossicular disruption). Conductive losses in adults are most commonly due to cerumen impaction or transient eustachian tube dysfunction from upper respiratory tract infection. Persistent conductive losses usually result from chronic ear infection, trauma, or otosclerosis. Conductive hearing loss is often correctable with medical or surgical therapy, or both.
B. Sensorineural Hearing Loss
Sensory and neural causes of hearing loss are difficult to differentiate due to testing methodology and thus are often referred to as “sensorineural.” Sensorineural hearing losses are common in adults.
Sensory hearing loss results from deterioration of the cochlea, usually due to loss of hair cells from the organ of Corti. The most common form is a gradually progressive, predominantly high-frequency loss with advancing age (presbyacusis); other causes include excessive noise exposure, head trauma, and systemic diseases. Sensory hearing loss is usually not correctable with medical or surgical therapy but often may be prevented or stabilized. An exception is a sudden sensory hearing loss, which may respond to corticosteroids if delivered within several weeks of onset.
Neural hearing loss lesions involve the eighth cranial nerve, auditory nuclei, ascending tracts, or auditory cortex. Neural hearing loss is much less commonly recognized. Causes include acoustic neuroma, multiple sclerosis, and auditory neuropathy.
Cunningham LL et al. Hearing loss in adults. N Engl J Med. 2017 Dec 21;377(25):2465–73. [PMID: 29262274]
Nieman CL et al. Otolaryngology for the internist: hearing loss. Med Clin North Am. 2018 Nov;102(6):977–92. [PMID: 30342615]
 Evaluation of Hearing (Audiology)
Evaluation of Hearing (Audiology)
In a quiet room, the hearing level may be estimated by having the patient repeat aloud words presented in a soft whisper, a normal spoken voice, or a shout. A 512-Hz tuning fork is useful in differentiating conductive from sensorineural losses. In the Weber test, the tuning fork is placed on the forehead or front teeth. In conductive losses, the sound appears louder in the poorer-hearing ear, whereas in sensorineural losses it radiates to the better side. In the Rinne test, the tuning fork is placed alternately on the mastoid bone and in front of the ear canal. In conductive losses greater than 25 dB, bone conduction exceeds air conduction; in sensorineural losses, the opposite is true.
Formal audiometric studies are performed in a soundproofed room. Pure-tone thresholds in decibels (dB) are obtained over the range of 250–8000 Hz for both air and bone conduction. Conductive losses create a gap between the air and bone thresholds, whereas in sensorineural losses, both air and bone thresholds are equally diminished. Speech discrimination measures the clarity of hearing, reported as percentage correct (90–100% is normal). Auditory brainstem-evoked responses may determine whether the lesion is sensory (cochlea) or neural (central). However, MRI scanning is more sensitive and specific in detecting central lesions.
Every patient who complains of a hearing loss should be referred for audiologic evaluation unless the cause is easily remediable (eg, cerumen impaction, otitis media). Immediate audiometric referral is indicated for patients with idiopathic sudden sensorineural hearing loss because it requires treatment (corticosteroids) within a limited several-week time period. Routine audiologic screening is recommended for adults with prior exposure to potentially injurious noise levels or in adults at age 65, and every few years thereafter.
Almeyda R et al. Assessing and treating adult patients with hearing loss. Br J Hosp Med (Lond). 2018 Nov 2;79(11):628–33. [PMID: 30418825]
Musiek FE et al. Perspectives on the pure-tone audiogram. J Am Acad Audiol. 2017 Jul/Aug;28(7):655–71. [PMID: 28722648]
 Hearing Amplification
Hearing Amplification
Patients with hearing loss not correctable by medical therapy may benefit from hearing amplification. Contemporary hearing aids are comparatively free of distortion and have been miniaturized to the point where they often may be contained entirely within the ear canal or lie inconspicuously behind the ear.
For patients with conductive loss or unilateral profound sensorineural loss, bone-conducting hearing aids directly stimulate the ipsilateral cochlea (for conductive losses) or contralateral ear (profound unilateral sensorineural loss).
In most adults with severe to profound sensory hearing loss, the cochlear implant—an electronic device that is surgically implanted into the cochlea to stimulate the auditory nerve—offers socially beneficial auditory rehabilitation.
Johnson CE et al. Benefits from, satisfaction with, and self-efficacy for advanced digital hearing aids in users with mild sensorineural hearing loss. Semin Hear. 2018 May;39(2):158–71. [PMID: 29915453]
Jorgensen LE et al. Conventional amplification for children and adults with severe-to-profound hearing loss. Semin Hear. 2018 Nov;39(4):364–76. [PMID: 30374208]
McRackan TR et al. Meta-analysis of quality-of-life improvement after cochlear implantation and associations with speech recognition abilities. Laryngoscope. 2018 Apr;128(4):982–90. [PMID: 28731538]
Michaud HN et al. Aural rehabilitation for older adults with hearing loss: impacts on quality of life—a systematic review of randomized controlled trials. J Am Acad Audiol. 2017 Jul/Aug;28(7):596–609. [PMID: 28722643]
DISEASES OF THE AURICLE
Disorders of the auricle include skin cancers due to sun exposure. Traumatic auricular hematoma must be drained to prevent significant cosmetic deformity (cauliflower ear) or canal blockage resulting from dissolution of supporting cartilage. Similarly, cellulitis of the auricle must be treated promptly to prevent perichondritis and resultant deformity. Relapsing polychondritis is characterized by recurrent, frequently bilateral, painful episodes of auricular erythema and edema and sometimes progressive involvement of the cartilaginous tracheobronchial tree. Treatment with corticosteroids may help forestall cartilage dissolution. Polychondritis and perichondritis may be differentiated from cellulitis by sparing of involvement of the lobule, which does not contain cartilage.
Dalal PJ et al. Risk factors for auricular hematoma and recurrence after drainage. Laryngoscope. 2020 Mar;130(3):628–31. [PMID: 31621925]
DISEASES OF THE EAR CANAL
1. Cerumen Impaction
Cerumen is a protective secretion produced by the outer portion of the ear canal. In most persons, the ear canal is self-cleansing. Recommended hygiene consists of cleaning the external opening only with a washcloth over the index finger. Cerumen impaction is most often self-induced through ill-advised cleansing attempts by entering the canal itself. It may be relieved by the patient using detergent ear drops (eg, 3% hydrogen peroxide; 6.5% carbamide peroxide) and irrigation, or by the clinician using mechanical removal, suction, or irrigation. Irrigation is performed with water at body temperature to avoid a vestibular caloric response. The stream should be directed at the posterior ear canal wall adjacent to the cerumen plug. Irrigation should be performed only when the tympanic membrane is known to be intact.
Use of jet irrigators (eg, WaterPik) should be avoided since they may result in tympanic membrane perforations. Following irrigation, the ear canal should be thoroughly dried (eg, by the patient using a hair blow-dryer on low-power setting or by the clinician instilling isopropyl alcohol) to reduce the likelihood of external otitis. Specialty referral is indicated if impaction is frequently recurrent, if it has not responded to routine measures, or if there is tympanic membrane perforation or chronic otitis media.
Schwartz SR et al. Clinical Practice Guideline (Update): Earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017 Jan;156(1 Suppl):S1–29. [PMID: 28045591]
2. Foreign Bodies
Foreign bodies in the ear canal are more frequent in children than in adults. Firm materials may be removed with a loop or a hook, taking care not to displace the object medially toward the tympanic membrane; microscopic guidance is helpful. Aqueous irrigation should not be performed for organic foreign bodies (eg, beans, insects), because water may cause them to swell. Living insects are best immobilized before removal by filling the ear canal with lidocaine.
Karimnejad K et al. External auditory canal foreign body extraction outcomes. Ann Otol Rhinol Laryngol. 2017 Nov;126(11):755–61. [PMID: 28954532]
Shunyu NB et al. Ear, nose and throat foreign bodies removed under general anaesthesia: a retrospective study. J Clin Diagn Res. 2017 Feb;11(2):MC01–4. [PMID: 28384894]
3. External Otitis
ESSENTIALS OF DIAGNOSIS

 Painful erythema and edema of the ear canal skin.
Painful erythema and edema of the ear canal skin.
 Purulent exudate.
Purulent exudate.
 In diabetic or immunocompromised patients, osteomyelitis of the skull base (“malignant external otitis”) may occur.
In diabetic or immunocompromised patients, osteomyelitis of the skull base (“malignant external otitis”) may occur.
 General Considerations
General Considerations
External otitis presents with otalgia, frequently accompanied by pruritus and purulent discharge. There is often a history of recent water exposure (ie, swimmer’s ear) or mechanical trauma (eg, scratching, cotton applicators). External otitis is usually caused by gram-negative rods (eg, Pseudomonas, Proteus) or fungi (eg, Aspergillus), which grow in the presence of excessive moisture. In diabetic or immunocompromised patients, persistent external otitis may evolve into osteomyelitis of the skull base (so-called malignant external otitis). Usually caused by Pseudomonas aeruginosa, osteomyelitis begins in the floor of the ear canal and may extend into the middle fossa floor, the clivus, and even the contralateral skull base.
 Clinical Findings
Clinical Findings
Examination reveals erythema and edema of the ear canal skin, often with a purulent exudate (Figure 8–1). Manipulation of the auricle elicits pain. Because the lateral surface of the tympanic membrane is ear canal skin, it is often erythematous. However, in contrast to acute otitis media, it moves normally with pneumatic otoscopy. When the canal skin is very edematous, it may be impossible to visualize the tympanic membrane. Malignant external otitis typically presents with persistent foul aural discharge, granulations in the ear canal, deep otalgia, and in advanced cases, progressive palsies of cranial nerves VI, VII, IX, X, XI, or XII. Diagnosis is confirmed by the demonstration of osseous erosion on CT scanning.

Figure 8–1. Malignant external otitis in a 40-year-old woman with diabetes mellitus, with typical swelling and honey-colored crusting of the pinna. Both the external auditory canal and temporal bone were involved in the pseudomonal infection. (Used, with permission, from E.J. Mayeaux Jr, MD, in Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. The Color Atlas of Family Medicine, 2nd ed. McGraw-Hill, 2013.)
 Treatment
Treatment
Treatment of external otitis involves protection of the ear from additional moisture and avoidance of further mechanical injury by scratching. In cases of moisture in the ear (eg, swimmer’s ear), acidification with a drying agent (ie, a 50/50 mixture of isopropyl alcohol/white vinegar) is often helpful. When infected, an otic antibiotic solution or suspension of an aminoglycoside (eg, neomycin/polymyxin B) or fluoroquinolone (eg, ciprofloxacin), with or without a corticosteroid (eg, hydrocortisone), is usually effective. Purulent debris filling the ear canal should be gently removed to permit entry of the topical medication. Drops should be used abundantly (five or more drops three or four times a day) to penetrate the depths of the canal. When substantial edema of the canal wall prevents entry of drops into the ear canal, a wick is placed to facilitate their entry. In recalcitrant cases—particularly when cellulitis of the periauricular tissue has developed—oral fluoroquinolones (eg, ciprofloxacin, 500 mg twice daily for 1 week) are used because of their effectiveness against Pseudomonas. Any case of persistent otitis externa in an immunocompromised or diabetic individual must be referred for specialty evaluation.
Treatment of “malignant external otitis” requires prolonged antipseudomonal antibiotic administration, often for several months. Although intravenous therapy is often required initially (eg, ciprofloxacin 200–400 mg every 12 hours), selected patients may be graduated to oral ciprofloxacin (500–1000 mg twice daily). To avoid relapse, antibiotic therapy should be continued, even in the asymptomatic patient, until gallium scanning indicates marked reduction or resolution of the inflammation. Surgical debridement of infected bone is reserved for cases of deterioration despite medical therapy.
Mildenhall N et al. Clinician adherence to the clinical practice guideline: Acute otitis externa. Laryngoscope. 2019 Nov 15. [Epub ahead of print] [PMID: 31730729]
Peled C et al. Necrotizing otitis externa-analysis of 83 cases: clinical findings and course of disease. Otol Neurotol. 2019 Jan;40(1):56–62. [PMID: 30239427]
Wang X et al. Use of systemic antibiotics for acute otitis externa: impact of a clinical practice guideline. Otol Neurotol. 2018 Oct;39(9):1088–94. [PMID: 30124617]
4. Pruritus
Pruritus of the external auditory canal, particularly at the meatus, is common. While it may be associated with external otitis or with seborrheic dermatitis or psoriasis, most cases are self-induced from excoriation or overly zealous ear cleaning. To permit regeneration of the protective cerumen blanket, patients should be instructed to avoid use of soap and water or cotton swabs in the ear canal and avoid any scratching. Patients with excessively dry canal skin may benefit from application of mineral oil, which helps counteract dryness and repel moisture. When an inflammatory component is present, topical application of a corticosteroid (eg, 0.1% triamcinolone) may be beneficial.
5. Exostoses & Osteomas
Bony overgrowths of the ear canal are a frequent incidental finding and occasionally have clinical significance. Clinically, they present as skin-covered bony mounds in the medial ear canal obscuring the tympanic membrane to a variable degree. Solitary osteomas are of no significance as long as they do not cause obstruction or infection. Multiple exostoses, which are generally acquired from repeated exposure to cold water (eg, “surfer’s ear”), may progress and require surgical removal.
Kim SH. Exostoses of the external auditory canals. Br J Hosp Med (Lond). 2017 Mar 2;78(3):174. [PMID: 28277770]
6. Neoplasia
The most common neoplasm of the ear canal is squamous cell carcinoma. When an apparent otitis externa does not resolve on therapy, a malignancy should be suspected and biopsy performed. This disease carries a very high 5-year mortality rate because the tumor tends to invade the lymphatics of the cranial base and must be treated with wide surgical resection and radiation therapy. Adenomatous tumors, originating from the ceruminous glands, generally follow a more indolent course.
Oya R et al. Surgery with or without postoperative radiation therapy for early-stage external auditory canal squamous cell carcinoma: a meta-analysis. Otol Neurotol. 2017 Oct;38(9):1333–8. [PMID: 28796084]
Seligman KL et al. Temporal bone carcinoma: treatment patterns and survival. Laryngoscope. 2020 Jan;130(1):E11–20. [PMID: 30874314]
DISEASES OF THE EUSTACHIAN TUBE
1. Eustachian Tube Dysfunction
ESSENTIALS OF DIAGNOSIS

 Aural fullness.
Aural fullness.
 Fluctuating hearing.
Fluctuating hearing.
 Discomfort with barometric pressure change.
Discomfort with barometric pressure change.
 At risk for serous otitis media.
At risk for serous otitis media.
The tube that connects the middle ear to the nasopharynx—the eustachian tube—provides ventilation and drainage for the middle ear cleft. It is normally closed, opening only during swallowing or yawning. When eustachian tube function is compromised, air trapped within the middle ear becomes absorbed and negative pressure results. The most common causes of eustachian tube dysfunction are diseases associated with edema of the tubal lining, such as viral upper respiratory tract infections and allergy. The patient usually reports a sense of fullness in the ear and mild to moderate impairment of hearing. When the tube is only partially blocked, swallowing or yawning may elicit a popping or crackling sound. Examination may reveal retraction of the tympanic membrane and decreased mobility on pneumatic otoscopy. Following a viral illness, this disorder is usually transient, lasting days to weeks. Treatment with systemic and intranasal decongestants (eg, pseudoephedrine, 60 mg orally every 4–6 hours; oxymetazoline, 0.05% spray every 8–12 hours) combined with autoinflation by forced exhalation against closed nostrils may hasten relief. Autoinflation should not be recommended to patients with active intranasal infection, since this maneuver may precipitate middle ear infection. Allergic patients may also benefit from intranasal corticosteroids (eg, beclomethasone dipropionate, two sprays in each nostril twice daily for 2–6 weeks). Air travel, rapid altitudinal change, and underwater diving should be avoided until resolution.
Conversely, an overly patent eustachian tube (“patulous eustachian tube”) is a relatively uncommon, though quite distressing problem. Typical complaints include fullness in the ear and autophony, an exaggerated ability to hear oneself breathe and speak. A patulous eustachian tube may develop during rapid weight loss, or it may be idiopathic. In contrast to eustachian tube dysfunction, the aural pressure is often made worse by exertion and may diminish during an upper respiratory tract infection. Although physical examination is usually normal, respiratory excursions of the tympanic membrane may occasionally be detected during vigorous breathing. Treatment includes avoidance of decongestant products, insertion of a ventilating tube to reduce the outward stretch of the eardrum during phonation, and rarely, surgery on the eustachian tube itself.
Huisman JML et al. Treatment of eustachian tube dysfunction with balloon dilation: a systematic review. Laryngoscope. 2018 Jan;128(1):237–47. [PMID: 28799657]
Meyer TA et al. A randomized controlled trial of balloon dilation as a treatment for persistent Eustachian tube dysfunction with 1-year follow-up. Otol Neurotol. 2018 Aug;39(7):894–902. [PMID: 29912819]
Ward BK et al. Patulous eustachian tube dysfunction: patient demographics and comorbidities. Otol Neurotol. 2017 Oct;38(9):1362–9. [PMID: 28796094]
2. Serous Otitis Media
ESSENTIALS OF DIAGNOSIS

 Eustachian tube remains blocked for a prolonged period.
Eustachian tube remains blocked for a prolonged period.
 Resultant negative pressure results in transudation of fluid.
Resultant negative pressure results in transudation of fluid.
Prolonged eustachian tube dysfunction with resultant negative middle ear pressure may cause a transudation of fluid. In adults, serous otitis media usually occurs with an upper respiratory tract infection, with barotrauma, or with chronic allergic rhinitis, but when persistent and unilateral, nasopharyngeal carcinoma must be excluded. The tympanic membrane is dull and hypomobile, occasionally accompanied by air bubbles in the middle ear and conductive hearing loss. The treatment of serous otitis media is similar to that for eustachian tube dysfunction. When medication fails to bring relief after several months, a ventilating tube placed through the tympanic membrane may restore hearing and alleviate the sense of aural fullness. Endoscopically guided laser expansion of the nasopharyngeal orifice of the eustachian tube or balloon dilation may improve function in recalcitrant cases.
Roditi RE et al. Otitis media with effusion: our national practice. Otolaryngol Head Neck Surg. 2017 Aug;157(2):171–2. [PMID: 28535139]
Vanneste P et al. Otitis media with effusion in children: pathophysiology, diagnosis, and treatment. A review. J Otol. 2019 Jun;14(2):33–9. [PMID: 31223299]
3. Barotrauma
Persons with poor eustachian tube function (eg, congenital narrowness or acquired mucosal edema) may be unable to equalize the barometric stress exerted on the middle ear by air travel, rapid altitudinal change, or underwater diving. The problem is generally most acute during airplane descent, since the negative middle ear pressure tends to collapse and block the eustachian tube, causing pain. Several measures are useful to enhance eustachian tube function and avoid otic barotrauma. The patient should be advised to swallow, yawn, and autoinflate frequently during descent. Oral decongestants (eg, pseudoephedrine, 60–120 mg) should be taken several hours before anticipated arrival time so that they will be maximally effective during descent. Topical decongestants such as 1% phenylephrine nasal spray should be administered 1 hour before arrival.
For acute negative middle ear pressure that persists on the ground, treatment includes decongestants and attempts at autoinflation. Myringotomy (creation of a small eardrum perforation) provides immediate relief and is appropriate in the setting of severe otalgia and hearing loss. Repeated episodes of barotrauma in persons who must fly frequently may be alleviated by insertion of ventilating tubes.
Underwater diving may represent an even greater barometric stress to the ear than flying. Patients should be warned to avoid diving when they have an upper respiratory infection or episode of nasal allergy. During the descent phase of the dive, if inflation of the middle ear via the eustachian tube has not occurred, pain will develop within the first 15 feet; the dive must be aborted. In all cases, divers must descend slowly and equilibrate in stages to avoid the development of severely negative pressures in the tympanum that may result in hemorrhage (hemotympanum) or in perilymphatic fistula. In the latter, the oval or round window ruptures, resulting in sensory hearing loss and acute vertigo. During the ascent phase of a saturation dive, sensory hearing loss or vertigo may develop as the first (or only) symptom of decompression sickness. Immediate recompression will return intravascular gas bubbles to solution and restore the inner ear microcirculation.
Tympanic membrane perforation is an absolute contraindication to diving, as the patient will experience an unbalanced thermal stimulus to the semicircular canals and may experience vertigo, disorientation, and even emesis.
Rozycki SW et al. Inner ear barotrauma in divers: an evidence-based tool for evaluation and treatment. Diving Hyperb Med. 2018 Sep 30;48(3):186–93. [PMID: 30199891]
Ryan P et al. Prevention of otic barotrauma in aviation: a systematic review. Otol Neurotol. 2018 Jun;39(5):539–49. [PMID: 29595579]
DISEASES OF THE MIDDLE EAR
1. Acute Otitis Media
ESSENTIALS OF DIAGNOSIS

 Otalgia, often with an upper respiratory tract infection.
Otalgia, often with an upper respiratory tract infection.
 Erythema and hypomobility of tympanic membrane.
Erythema and hypomobility of tympanic membrane.
 General Considerations
General Considerations
Acute otitis media is a bacterial infection of the mucosally lined air-containing spaces of the temporal bone. Purulent material forms not only within the middle ear cleft but also within the pneumatized mastoid air cells and petrous apex. Acute otitis media is usually precipitated by a viral upper respiratory tract infection that causes eustachian tube obstruction. This results in accumulation of fluid and mucus, which becomes secondarily infected by bacteria. The most common pathogens are Streptococcus pneumoniae, Haemophilus influenzae, and Streptococcus pyogenes.
 Clinical Findings
Clinical Findings
Acute otitis media may occur at any age. Presenting symptoms and signs include otalgia, aural pressure, decreased hearing, and often fever. The typical physical findings are erythema and decreased mobility of the tympanic membrane (Figure 8–2). Occasionally, bullae will appear on the tympanic membrane.
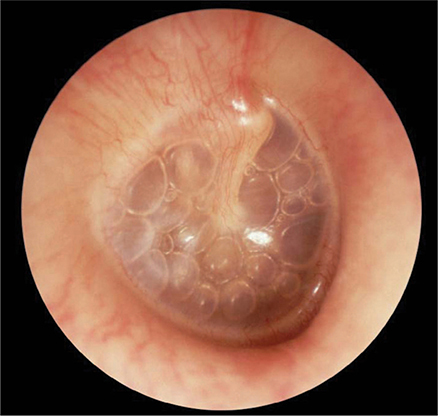
Figure 8–2. Acute otitis media with effusion of right ear, with multiple air-fluid levels visible through a translucent, slightly retracted, nonerythematous tympanic membrane. (Used, with permission, from Frank Miller, MD, in Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H. The Color Atlas of Family Medicine, 2nd ed. McGraw-Hill, 2013.)
Rarely, when middle ear empyema is severe, the tympanic membrane bulges outward. In such cases, tympanic membrane rupture is imminent. Rupture is accompanied by a sudden decrease in pain, followed by the onset of otorrhea. With appropriate therapy, spontaneous healing of the tympanic membrane occurs in most cases. When perforation persists, chronic otitis media may develop. Mastoid tenderness often accompanies acute otitis media and is due to the presence of pus within the mastoid air cells. This alone does not indicate suppurative (surgical) mastoiditis. Frank swelling over the mastoid bone or the association of cranial neuropathies or central findings indicates severe disease requiring urgent care.
 Treatment
Treatment
The treatment of acute otitis media is specific antibiotic therapy, often combined with nasal decongestants. The first-choice antibiotic is amoxicillin 1 g orally every 8 hours for 5–7 days. Alternatives (useful in resistant cases) are amoxicillin-clavulanate 875/125 mg or 2 g/125 mg ER every 12 hours for 5–10 days; or cefuroxime 500 mg or cefpodoxime 200 mg orally every 12 hours for 5–7 days.
Tympanocentesis for bacterial (aerobic and anaerobic) and fungal culture may be performed by any experienced physician. A 20-gauge spinal needle bent 90 degrees to the hub attached to a 3-mL syringe is inserted through the inferior portion of the tympanic membrane. Interposition of a pliable connecting tube between the needle and syringe permits an assistant to aspirate without inducing movement of the needle. Tympanocentesis is useful for otitis media in immunocompromised patients and when infection persists or recurs despite multiple courses of antibiotics.
Surgical drainage of the middle ear (myringotomy) is reserved for patients with severe otalgia or when complications of otitis (eg, mastoiditis, meningitis) have occurred.
Recurrent acute otitis media may be managed with long-term antibiotic prophylaxis. Single daily oral doses of sulfamethoxazole (500 mg) or amoxicillin (250 or 500 mg) are given over a period of 1–3 months. Failure of this regimen to control infection is an indication for insertion of ventilating tubes.
Hutz MJ et al. Neurological complications of acute and chronic otitis media. Curr Neurol Neurosci Rep. 2018 Feb 14;18(3):11. [PMID: 29445883]
Szmuilowicz J et al. Infections of the ear. Emerg Med Clin North Am. 2019 Feb;37(1):1–9. [PMID: 30454772]
2. Chronic Otitis Media
ESSENTIALS OF DIAGNOSIS

 Chronic otorrhea with or without otalgia.
Chronic otorrhea with or without otalgia.
 Tympanic membrane perforation with conductive hearing loss.
Tympanic membrane perforation with conductive hearing loss.
 Often amenable to surgical correction.
Often amenable to surgical correction.
 General Considerations
General Considerations
Chronic infection of the middle ear and mastoid generally develops as a consequence of recurrent acute otitis media, although it may follow other diseases and trauma. Perforation of the tympanic membrane is usually present. The bacteriology of chronic otitis media differs from that of acute otitis media. Common organisms include P aeruginosa, Proteus species, Staphylococcus aureus, and mixed anaerobic infections.
 Clinical Findings
Clinical Findings
The clinical hallmark of chronic otitis media is purulent aural discharge. Drainage may be continuous or intermittent, with increased severity during upper respiratory tract infection or following water exposure. Pain is uncommon except during acute exacerbations. Conductive hearing loss results from destruction of the tympanic membrane or ossicular chain, or both.
 Treatment
Treatment
The medical treatment of chronic otitis media includes regular removal of infected debris, use of earplugs to protect against water exposure, and topical antibiotic drops (ofloxacin 0.3% or ciprofloxacin with dexamethasone) for exacerbations. Oral ciprofloxacin, active against Pseudomonas, 500 mg twice a day for 1–6 weeks, may help dry a chronically discharging ear.
Definitive management is surgical in most cases. Successful reconstruction of the tympanic membrane may be achieved in about 90% of cases, often with elimination of infection and significant improvement in hearing. When the mastoid air cells are involved by irreversible infection, they should be exenterated at the same time through a mastoidectomy.
Emmett SD et al. Chronic ear disease. Med Clin North Am. 2018 Nov;102(6):1063–79. [PMID: 30342609]
Master A et al. Management of chronic suppurative otitis media and otosclerosis in developing countries. Otolaryngol Clin North Am. 2018 Jun;51(3):593–605. [PMID: 29525390]
 Complications of Otitis Media
Complications of Otitis Media
A. Cholesteatoma
Cholesteatoma is a special variety of chronic otitis media (Figure 8–3). The most common cause is prolonged eustachian tube dysfunction, with inward migration of the upper flaccid portion of the tympanic membrane. This creates a squamous epithelium-lined sac, which—when its neck becomes obstructed—may fill with desquamated keratin and become chronically infected. Cholesteatomas typically erode bone, with early penetration of the mastoid and destruction of the ossicular chain. Over time they may erode into the inner ear, involve the facial nerve, and on rare occasions spread intracranially. Otoscopic examination may reveal an epitympanic retraction pocket or a marginal tympanic membrane perforation that exudes keratin debris, or granulation tissue. The treatment of cholesteatoma is surgical marsupialization of the sac or its complete removal. This may require the creation of a “mastoid bowl” in which the ear canal and mastoid are joined into a large common cavity that must be periodically cleaned.

Figure 8–3. Cholesteatoma. (Used, with permission, from Vladimir Zlinsky, MD, in Roy F. Sullivan, PhD: Audiology Forum: Video Otoscopy, www.RCSullivan.com; from Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H, Tysinger J. The Color Atlas of Family Medicine. McGraw-Hill, 2009.)
Luu K et al. Updates in pediatric cholesteatoma: minimizing intervention while maximizing outcomes. Otolaryngol Clin North Am. 2019 Oct;52(5):813–23. [PMID: 31280890]
Rutkowska J et al. Cholesteatoma definition and classification: a literature review. J Int Adv Otol. 2017 Aug;13(2):266–71. [PMID: 28274903]
B. Mastoiditis
Acute suppurative mastoiditis usually evolves following several weeks of inadequately treated acute otitis media. It is characterized by postauricular pain and erythema accompanied by a spiking fever. CT scan reveals coalescence of the mastoid air cells due to destruction of their bony septa. Initial treatment consists of intravenous antibiotics (eg, cefazolin 0.5–1.5 g every 6–8 hours) directed against the most common offending organisms (S pneumoniae, H influenzae, and S pyogenes), and myringotomy for culture and drainage. Failure of medical therapy indicates the need for surgical drainage (mastoidectomy).
C. Petrous Apicitis
The medial portion of the petrous bone between the inner ear and clivus may become a site of persistent infection when the drainage of its pneumatic cell tracts becomes blocked. This may cause foul discharge, deep ear and retro-orbital pain, and sixth nerve palsy (Gradenigo syndrome); meningitis may be a complication. Treatment is with prolonged antibiotic therapy (based on culture results) and surgical drainage via petrous apicectomy.
Gadre AK et al. The changing face of petrous apicitis—a 40-year experience. Laryngoscope. 2018 Jan;128(1):195–201. [PMID: 28378370]
Ren Y et al. Acute otitis media and associated complications in United States emergency departments. Otol Neurotol. 2018 Sep;39(8):1005–11. [PMID: 30113560]
D. Facial Paralysis
Facial palsy may be associated with either acute or chronic otitis media. In the acute setting, it results from inflammation of the seventh nerve in its middle ear segment. Treatment consists of myringotomy for drainage and culture, followed by intravenous antibiotics (based on culture results). The use of corticosteroids is controversial. The prognosis is excellent, with complete recovery in most cases.
Facial palsy associated with chronic otitis media usually evolves slowly due to chronic pressure on the seventh nerve in the middle ear or mastoid by cholesteatoma. Treatment requires surgical correction of the underlying disease. The prognosis is less favorable than for facial palsy associated with acute otitis media.
Owusu JA et al. Facial nerve paralysis. Med Clin North Am. 2018 Nov;102(6):1135–43. [PMID: 30342614]
Prasad S et al. Facial nerve paralysis in acute suppurative otitis media—management. Indian J Otolaryngol Head Neck Surg. 2017 Mar;69(1):58–61. [PMID: 28239580]
Zhang W et al. The etiology of Bell’s palsy: a review. J Neurol. 2019 Mar 28. [Epub ahead of print] [PMID: 30923934]
E. Sigmoid Sinus Thrombosis
Trapped infection within the mastoid air cells adjacent to the sigmoid sinus may cause septic thrombophlebitis. This is heralded by signs of systemic sepsis (spiking fevers, chills), at times accompanied by signs of increased intracranial pressure (headache, lethargy, nausea and vomiting, papilledema). Diagnosis can be made noninvasively by magnetic resonance venography (MRV). Primary treatment is with intravenous antibiotics (based on culture results). Surgical drainage with ligation of the internal jugular vein may be indicated when embolization is suspected.
F. Central Nervous System Infection
Otogenic meningitis is by far the most common intracranial complication of ear infection. In the setting of acute suppurative otitis media, it arises from hematogenous spread of bacteria, most commonly H influenzae and S pneumoniae. In chronic otitis media, it results either from passage of infection along preformed pathways, such as the petrosquamous suture line, or from direct extension of disease through the dural plates of the petrous pyramid.
Epidural abscesses arise from direct extension of disease in the setting of chronic infection. They are usually asymptomatic but may present with deep local pain, headache, and low-grade fever. They are often discovered as an incidental finding at surgery. Brain abscess may arise in the temporal lobe or cerebellum as a result of septic thrombophlebitis adjacent to an epidural abscess. The predominant causative organisms are S aureus, S pyogenes, and S pneumoniae. Rupture into the subarachnoid space results in meningitis and often death. (See Chapter 30.)
Hutz MJ et al. Neurological complications of acute and chronic otitis media. Curr Neurol Neurosci Rep. 2018 Feb 14;18(3):11. [PMID: 29445883]
Mather M et al. Is anticoagulation beneficial in acute mastoiditis complicated by sigmoid sinus thrombosis? Laryngoscope. 2018 Nov;128(11):2435–6. [PMID: 29521448]
3. Otosclerosis
Otosclerosis is a progressive disease with a marked familial tendency that affects the bony otic capsule. Lesions involving the footplate of the stapes result in increased impedance to the passage of sound through the ossicular chain, producing conductive hearing loss. This may be treated either through the use of a hearing aid or surgical replacement of the stapes with a prosthesis (stapedectomy). When otosclerotic lesions involve the cochlea (“cochlear otosclerosis”), permanent sensory hearing loss occurs.
Gillard DM et al. Cost-effectiveness of stapedectomy vs hearing aids in the treatment of otosclerosis. JAMA Otolaryngol Head Neck Surg. 2019 Nov 7. [Epub ahead of print] [PMID: 31697352]
Yeh CF et al. Predictors of hearing outcomes after stapes surgery in otosclerosis. Acta Otolaryngol. 2019 Dec;139(12):1058–62. [PMID: 31617779]
4. Trauma to the Middle Ear
Tympanic membrane perforation may result from impact injury or explosive acoustic trauma (Figure 8–4). Spontaneous healing occurs in most cases. Persistent perforation may result from secondary infection brought on by exposure to water. During the healing period, patients should be advised to wear earplugs while swimming or bathing. Hemorrhage behind an intact tympanic membrane (hemotympanum) may follow blunt trauma or extreme barotrauma. Spontaneous resolution over several weeks is the usual course. When a conductive hearing loss greater than 30 dB persists for more than 3 months following trauma, disruption of the ossicular chain should be suspected. Middle ear exploration with reconstruction of the ossicular chain, combined with repair of the tympanic membrane when required, will usually restore hearing.

Figure 8–4. Traumatic perforation of the left tympanic membrane. (Used, with permission, from William Clark, MD, in Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H, Tysinger J. The Color Atlas of Family Medicine. McGraw-Hill, 2009.)
Sagiv D et al. Traumatic perforation of the tympanic membrane: a review of 80 cases. J Emerg Med. 2018 Feb;54(2):186–90. [PMID: 29110975]
5. Middle Ear Neoplasia
Primary middle ear tumors are rare. Glomus tumors arise either in the middle ear (glomus tympanicum) or in the jugular bulb with upward erosion into the hypotympanum (glomus jugulare). They present clinically with pulsatile tinnitus and hearing loss. A vascular mass may be visible behind an intact tympanic membrane. Large glomus jugulare tumors are often associated with multiple cranial neuropathies, especially involving nerves VII, IX, X, XI, and XII. Treatment usually requires surgery, radiotherapy, or both. Pulsatile tinnitus thus warrants magnetic resonance angiography (MRA) and MRV to rule out a vascular mass.
Killeen DE et al. Endoscopic management of middle ear paragangliomas: a case series. Otol Neurotol. 2017 Mar;38(3):408–15. [PMID: 28192382]
Marinelli JP et al. Adenomatous neuroendocrine tumors of the middle ear: a multi-institutional investigation of 32 cases and development of a staging system. Otol Neurotol. 2018 Sep;39(8):e712–21. [PMID: 30001283]
EARACHE
Earache can be caused by a variety of otologic problems, but external otitis and acute otitis media are the most common. Differentiation of the two should be apparent by pneumatic otoscopy. Pain out of proportion to the physical findings may be due to herpes zoster oticus, especially when vesicles appear in the ear canal or concha. Persistent pain and discharge from the ear suggest osteomyelitis of the skull base or cancer, and patients with these complaints should be referred for specialty evaluation.
Nonotologic causes of otalgia are numerous. The sensory innervation of the ear is derived from the trigeminal, facial, glossopharyngeal, vagal, and upper cervical nerves. Because of this rich innervation, referred otalgia is quite frequent. Temporomandibular joint dysfunction is a common cause of referred ear pain. Pain is exacerbated by chewing or psychogenic grinding of the teeth (bruxism) and may be associated with dental malocclusion. Repeated episodes of severe lancinating otalgia may occur in glossopharyngeal neuralgia. Infections and neoplasia that involve the oropharynx, hypopharynx, and larynx frequently cause otalgia. Persistent earache demands specialty referral to exclude cancer of the upper aerodigestive tract.
DISEASES OF THE INNER EAR
1. Sensory Hearing Loss
Diseases of the cochlea result in sensory hearing loss, a condition that is usually irreversible. Most cochlear diseases result in bilateral symmetric hearing loss. The presence of unilateral or asymmetric sensorineural hearing loss suggests a lesion proximal to the cochlea. The primary goals in the management of sensory hearing loss are prevention of further losses and functional improvement with amplification and auditory rehabilitation.
A. Presbyacusis
Presbyacusis, or age-related hearing loss, is the most frequent cause of sensory hearing loss and is progressive, predominantly high-frequency, and symmetrical. Various etiologic factors (eg, prior noise trauma, drug exposure, genetic predisposition) may contribute to presbyacusis. Most patients notice a loss of speech discrimination that is especially pronounced in noisy environments. About 25% of people between the ages of 65 and 75 years and almost 50% of those over 75 experience hearing difficulties.
Tawfik KO et al. Advances in understanding of presbycusis. J Neurosci Res. 2019 Apr 4. [Epub ahead of print] [PMID: 30950547]
Tu NC et al. Age-related hearing loss: unraveling the pieces. Laryngoscope Investig Otolaryngol. 2018 Feb 21;3(2):68–72. [PMID: 29721536]
Vaisbuch Y et al. Age-related hearing loss: innovations in hearing augmentation. Otolaryngol Clin North Am. 2018 Aug;51(4):705–23. [PMID: 29735277]
B. Noise Trauma
Noise trauma is the second most common cause of sensory hearing loss. Sounds exceeding 85 dB are potentially injurious to the cochlea, especially with prolonged exposures. The loss typically begins in the high frequencies (especially 4000 Hz) and, with continuing exposure, progresses to involve the speech frequencies. Among the more common sources of injurious noise are industrial machinery, weapons, and excessively loud music. Personal music devices used at excessive loudness levels may also be injurious. Monitoring noise levels in the workplace by regulatory agencies has led to preventive programs that have reduced the frequency of occupational losses. Individuals of all ages, especially those with existing hearing losses, should wear earplugs when exposed to moderately loud noises and specially designed earmuffs when exposed to explosive noises.
Bielefeld EC et al. Advances and challenges in pharmaceutical therapies to prevent and repair cochlear injuries from noise. Front Cell Neurosci. 2019 Jun 26;13:285. [PMID: 31297051]
Neitzel RL et al. Risk of noise-induced hearing loss due to recreational sound: review and recommendations. J Acoust Soc Am. 2019 Nov;146(5):3911. [PMID: 31795675]
C. Physical Trauma
Head trauma (eg, deployment of air bags during an automobile accident) has effects on the inner ear similar to those of severe acoustic trauma. Some degree of sensory hearing loss may occur following simple concussion and is frequent after skull fracture.
Mizutari K. Update on treatment options for blast-induced hearing loss. Curr Opin Otolaryngol Head Neck Surg. 2019 Oct;27(5):376–80. [PMID: 31348022]
D. Ototoxicity
Ototoxic substances may affect both the auditory and vestibular systems. The most commonly used ototoxic medications are aminoglycosides; loop diuretics; and several antineoplastic agents, notably cisplatin. These medications may cause irreversible hearing loss even when administered in therapeutic doses. When using these medications, it is important to identify high-risk patients, such as those with preexisting hearing losses or kidney disease. Patients simultaneously receiving multiple ototoxic agents are at particular risk owing to ototoxic synergy. Useful measures to reduce the risk of ototoxic injury include serial audiometry, monitoring of serum peak and trough levels, and substitution of equivalent nonototoxic drugs whenever possible.
It is possible for topical agents that enter the middle ear to be absorbed into the inner ear via the round window. When the tympanic membrane is perforated, use of potentially ototoxic ear drops (eg, neomycin, gentamicin) is best avoided.
Laurell G. Pharmacological intervention in the field of ototoxicity. HNO. 2019 Jun;67(6):434–9. [PMID: 30993373]
Rybak LP et al. Local drug delivery for prevention of hearing loss. Front Cell Neurosci. 2019 Jul 9;13:300. [PMID: 31338024]
E. Sudden Sensory Hearing Loss
Idiopathic sudden loss of hearing in one ear may occur at any age, but typically it occurs in persons over age 20 years. The cause is unknown; however, one hypothesis is that it results from a viral infection or a sudden vascular occlusion of the internal auditory artery. Prognosis is mixed, with many patients suffering permanent deafness in the involved ear, while others have complete recovery. Prompt treatment with corticosteroids has been shown to improve the odds of recovery. A common regimen is oral prednisone, 1 mg/kg/day, followed by a tapering dose over a 10-day period. Intratympanic administration of corticosteroids alone or in association with oral corticosteroids has been associated with an equal or more favorable prognosis. Because treatment appears to be most effective as close to the onset of the loss as possible, and appears not to be effective after 6 weeks, a prompt audiogram should be obtained in all patients who present with sudden hearing loss without obvious middle ear pathology.
Ahmadzai N et al. A systematic review and network meta-analysis of existing pharmacologic therapies in patients with idiopathic sudden sensorineural hearing loss. PLoS One. 2019 Sep 9;14(9):e0221713. [PMID: 31498809]
Plontke SK. Diagnostics and therapy of sudden hearing loss. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2018 Feb 19;16:Doc05. [PMID: 29503670]
F. Autoimmune Hearing Loss
Sensory hearing loss may be associated with a wide array of systemic autoimmune disorders, such as systemic lupus erythematosus, granulomatosis with polyangiitis, and Cogan syndrome (hearing loss, keratitis, aortitis). The loss is most often bilateral and progressive. The hearing level often fluctuates, with periods of deterioration alternating with partial or even complete remission. Usually, there is the gradual evolution of permanent hearing loss, which often stabilizes with some remaining auditory function but occasionally proceeds to complete deafness. Vestibular dysfunction, particularly dysequilibrium and postural instability, may accompany the auditory symptoms.
In many cases, the autoimmune pattern of audiovestibular dysfunction presents in the absence of recognized systemic autoimmune disease. Responsiveness to oral corticosteroid treatment is helpful in making the diagnosis and constitutes first-line therapy. If stabilization of hearing becomes dependent on long-term corticosteroid use, steroid-sparing immunosuppressive regimens may become necessary.
Das S et al. Demystifying autoimmune inner ear disease. Eur Arch Otorhinolaryngol. 2019 Dec;276(12):3267–74. [PMID: 31605190]
Mancini P et al. Hearing loss in autoimmune disorders: prevalence and therapeutic options. Autoimmun Rev. 2018 Jul;17(7):644–52. [PMID: 29729446]
2. Tinnitus
ESSENTIALS OF DIAGNOSIS

 Perception of abnormal ear or head noises.
Perception of abnormal ear or head noises.
 Persistent tinnitus often, though not always, indicates the presence of sensory hearing loss.
Persistent tinnitus often, though not always, indicates the presence of sensory hearing loss.
 Intermittent periods of mild, high-pitched tinnitus lasting seconds to minutes are common in normal-hearing persons.
Intermittent periods of mild, high-pitched tinnitus lasting seconds to minutes are common in normal-hearing persons.
 General Considerations
General Considerations
Tinnitus is defined as the sensation of sound in the absence of an exogenous sound source. Tinnitus can accompany any form of hearing loss, and its presence provides no diagnostic value in determining the cause of a hearing loss. Approximately 15% of the general population experiences some type of tinnitus, with prevalence beyond 20% in aging populations.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Though tinnitus is commonly associated with hearing loss, tinnitus severity correlates poorly with the degree of hearing loss. About one in seven tinnitus sufferers experiences severe annoyance, and 4% are severely disabled. When severe and persistent, tinnitus may interfere with sleep and ability to concentrate, resulting in considerable psychological distress.
Pulsatile tinnitus—often described by the patient as listening to one’s own heartbeat—should be distinguished from tonal tinnitus. Although often ascribed to conductive hearing loss, pulsatile tinnitus may be far more serious and may indicate a vascular abnormality, such as glomus tumor, venous sinus stenosis, carotid vaso-occlusive disease, arteriovenous malformation, or aneurysm. In contrast, a staccato “clicking” tinnitus may result from middle ear muscle spasm, sometimes associated with palatal myoclonus. The patient typically perceives a rapid series of popping noises, lasting seconds to a few minutes, accompanied by a fluttering feeling in the ear.
B. Diagnostic Testing
For routine, nonpulsatile tinnitus, audiometry should be ordered to rule out an associated hearing loss. For unilateral tinnitus, particularly associated with hearing loss in the absence of an obvious causative factor (ie, noise trauma), an MRI should be obtained to rule out a retrocochlear lesion, such as vestibular schwannoma. MRA and MRV and temporal bone computed tomography (CT) should be considered for patients who have pulsatile tinnitus to exclude a causative vascular lesion or sigmoid sinus abnormality.
 Treatment
Treatment
The most important treatment of tinnitus is avoidance of exposure to excessive noise, ototoxic agents, and other factors that may cause cochlear damage. Masking the tinnitus with music or through amplification of normal sounds with a hearing aid may also bring some relief. Among the numerous drugs that have been tried, oral antidepressants (eg, nortriptyline at an initial dosage of 50 mg orally at bedtime) have proved to be the most effective. In addition to masking techniques, habituation techniques, such as tinnitus retraining therapy, may prove beneficial in those with refractory symptoms.
Chari DA et al. Tinnitus. Med Clin North Am. 2018 Nov;102(6):1081–93. [PMID: 30342610]
Wu V et al. Approach to tinnitus management. Can Fam Physician. 2018 Jul;64(7):491–5. [PMID: 30002023]
Zenner HP et al. A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. Eur Arch Otorhinolaryngol. 2017 May;274(5):2079–91. [PMID: 27995315]
3. Hyperacusis
Excessive sensitivity to sound may occur in normal-hearing individuals, either in association with ear disease, following noise trauma, in patients susceptible to migraines, or for psychological reasons. Patients with cochlear dysfunction commonly experience “recruitment,” an abnormal sensitivity to loud sounds despite a reduced sensitivity to softer ones. Fitting hearing aids and other amplification devices to patients with recruitment requires use of compression circuitry to avoid uncomfortable overamplification. For normal-hearing individuals with hyperacusis, use of an earplug in noisy environments may be beneficial, though attempts should be made at habituation.
Aazh H et al. Insights from the third international conference on hyperacusis: causes, evaluation, diagnosis, and treatment. Noise Health. 2018 Jul–Aug;20(95):162–70. [PMID: 30136676]
Baguley DM et al. Hyperacusis: major research questions. HNO. 2018 May;66(5):358–63. [PMID: 29392341]
4. Vertigo
ESSENTIALS OF DIAGNOSIS

 Either a sensation of motion when there is no motion or an exaggerated sense of motion in response to movement.
Either a sensation of motion when there is no motion or an exaggerated sense of motion in response to movement.
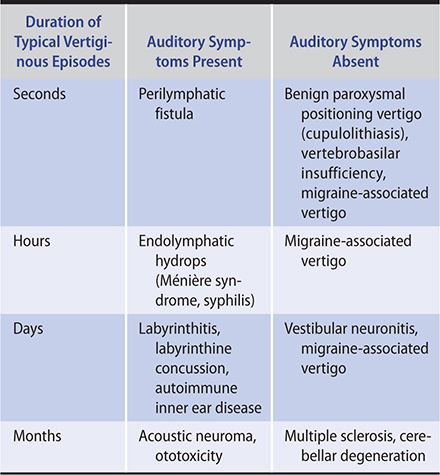
 Duration of vertigo episodes and association with hearing loss are the keys to diagnosis.
Duration of vertigo episodes and association with hearing loss are the keys to diagnosis.
 Must differentiate peripheral from central etiologies of vestibular dysfunction.
Must differentiate peripheral from central etiologies of vestibular dysfunction.
 Peripheral: Onset is sudden; often associated with tinnitus and hearing loss; horizontal nystagmus may be present.
Peripheral: Onset is sudden; often associated with tinnitus and hearing loss; horizontal nystagmus may be present.
 Central: Onset is gradual; no associated auditory symptoms.
Central: Onset is gradual; no associated auditory symptoms.
 Evaluation includes audiogram and electronystagmography (ENG) or videonystagmography (VNG) and head MRI.
Evaluation includes audiogram and electronystagmography (ENG) or videonystagmography (VNG) and head MRI.
 General Considerations
General Considerations
Vertigo can be caused by either a peripheral or central etiology, or both (Table 8–2).
Peripheral causes
Vestibular neuritis/labyrinthitis
Ménière disease
Benign paroxysmal positioning vertigo
Ethanol intoxication
Inner ear barotraumas
Semicircular canal dehiscence
Central causes
Seizure
Multiple sclerosis
Wernicke encephalopathy
Chiari malformation
Cerebellar ataxia syndromes
Mixed central and peripheral causes
Migraine
Stroke and vascular insufficiency
Posterior inferior cerebellar artery stroke
Anterior inferior cerebellar artery stroke
Vertebral artery insufficiency
Vasculitides
Cogan syndrome
Susac syndrome
Granulomatosis with polyangiitis (formerly Wegener granulomatosis)
Behçet disease
Cerebellopontine angle tumors
Vestibular schwannoma
Meningioma
Infections
Lyme disease
Syphilis
Vascular compression
Hyperviscosity syndromes
Waldenström macroglobulinemia
Endocrinopathies
Hypothyroidism
Pendred syndrome
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Vertigo is the cardinal symptom of vestibular disease. Vertigo is typically experienced as a distinct “spinning” sensation or a sense of tumbling or of falling forward or backward. It should be distinguished from imbalance, light-headedness, and syncope, all of which are nonvestibular in origin (Table 8–3).
Table 8–3. Common vestibular disorders: differential diagnosis based on classic presentations.
1. Peripheral vestibular disease—Peripheral vestibulopathy usually causes vertigo of sudden onset, may be so severe that the patient is unable to walk or stand, and is frequently accompanied by nausea and vomiting. Tinnitus and hearing loss may be associated and provide strong support for a peripheral (ie, otologic) origin.
Critical elements of the history include the duration of the discrete vertiginous episodes (seconds, minutes to hours, or days), and associated symptoms (hearing loss). Triggers should be sought, including diet (eg, high salt in the case of Ménière disease), stress, fatigue, and bright lights (eg, migraine-associated dizziness).
The physical examination of the patient with vertigo includes evaluation of the ears, observation of eye motion and nystagmus in response to head turning, cranial nerve examination, and Romberg testing. In acute peripheral lesions, nystagmus is usually horizontal with a rotatory component; the fast phase usually beats away from the diseased side. Visual fixation tends to inhibit nystagmus except in very acute peripheral lesions or with CNS disease. In benign paroxysmal positioning vertigo, Dix-Hallpike testing (quickly lowering the patient to the supine position with the head extending over the edge and placed 30 degrees lower than the body, turned either to the left or right) will elicit a delayed-onset (~10 sec) fatiguable nystagmus. Nonfatigable nystagmus in this position indicates CNS disease.
Since visual fixation often suppresses observed nystagmus, many of these maneuvers are performed with Frenzel goggles, which prevent visual fixation, and often bring out subtle forms of nystagmus. The Fukuda test can demonstrate vestibular asymmetry when the patient steps in place with eyes closed and consistently rotates in one direction.
2. Central disease—In contrast, vertigo arising from CNS disease (Table 8–2) tends to develop gradually and then becomes progressively more severe and debilitating. Nystagmus is not always present but can occur in any direction, may be dissociated in the two eyes, and is often nonfatigable, vertical rather than horizontal in orientation, without latency, and unsuppressed by visual fixation. ENG is useful in documenting these characteristics. Evaluation of central audiovestibular dysfunction requires MRI of the brain.
Episodic vertigo can occur in patients with diplopia from external ophthalmoplegia and is maximal when the patient looks in the direction where the separation of images is greatest. Cerebral lesions involving the temporal cortex may also produce vertigo; it is sometimes the initial symptom of a seizure. Finally, vertigo may be a feature of a number of systemic disorders and can occur as a side effect of certain anticonvulsant, antibiotic, hypnotic, analgesic, and tranquilizer medications or of alcohol.
Welgampola MS et al. Dizziness demystified. Pract Neurol. 2019 Dec;19(6):492–501. [PMID: 31326945]
B. Laboratory Findings
Laboratory investigations, such as audiologic evaluation, caloric stimulation, ENG, VNG, vestibular-evoked myogenic potentials (VEMPs), and MRI, are indicated in patients with persistent vertigo or when CNS disease is suspected. These studies help distinguish between central and peripheral lesions and identify causes requiring specific therapy. ENG consists of objective recording of the nystagmus induced by head and body movements, gaze, and caloric stimulation. It is helpful in quantifying the degree of vestibular hypofunction.
Sorathia S et al. Dizziness and the otolaryngology point of view. Med Clin North Am. 2018 Nov;102(6):1001–12. [PMID: 30342604]
Whitman GT. Dizziness. Am J Med. 2018 Dec;131(12):1431–7. [PMID: 29859806]
 Vertigo Syndromes Due to Peripheral Lesions
Vertigo Syndromes Due to Peripheral Lesions
A. Endolymphatic Hydrops (Ménière Syndrome)
The cause of Ménière syndrome is unknown. The classic syndrome consists of episodic vertigo, with discrete vertigo spells lasting 20 minutes to several hours in association with fluctuating low-frequency sensorineural hearing loss, tinnitus (usually low-tone and “blowing” in quality), and a sensation of unilateral aural pressure (Table 8–3). These symptoms in the absence of hearing fluctuations suggest migraine-associated dizziness. Symptoms wax and wane as the endolymphatic pressure rises and falls. Caloric testing commonly reveals loss or impairment of thermally induced nystagmus on the involved side. Primary treatment involves a low-salt diet and diuretics (eg, acetazolamide). For symptomatic relief of acute vertigo attacks, oral meclizine (25 mg) or diazepam (2–5 mg) can be used. In refractory cases, patients may undergo intratympanic corticosteroid injections, endolymphatic sac decompression, or vestibular ablation, either through transtympanic gentamicin, vestibular nerve section, or surgical labyrinthectomy.
Gibson WPR. Meniere’s disease. Adv Otorhinolaryngol. 2019;82:77–86. [PMID: 30947172]
B. Labyrinthitis
Patients with labyrinthitis suffer from acute onset of continuous, usually severe vertigo lasting several days to a week, accompanied by hearing loss and tinnitus. During a recovery period that lasts for several weeks, the vertigo gradually improves. Hearing may return to normal or remain permanently impaired in the involved ear. The cause of labyrinthitis is unknown. Treatment consists of antibiotics, if the patient is febrile or has symptoms of a bacterial infection, and supportive care. Vestibular suppressants are useful during the acute phase of the attack (eg, diazepam or meclizine) but should be discontinued as soon as feasible to avoid long-term dysequilibrium from inadequate compensation.
Welgampola MS et al. Dizziness demystified. Pract Neurol. 2019 Dec;19(6):492–501. [PMID: 31326945]
C. Benign Paroxysmal Positioning Vertigo
Patients suffering from recurrent spells of vertigo, lasting a few minutes per spell, associated with changes in head position (often provoked by rolling over in bed), usually have benign paroxysmal positioning vertigo (BPPV). The term “positioning vertigo” is more accurate than “positional vertigo” because it is provoked by changes in head position rather than by the maintenance of a particular posture.
The typical symptoms of BPPV occur in clusters that persist for several days. There is a brief (10–15 sec) latency period following a head movement before symptoms develop, and the acute vertigo subsides within 10–60 seconds, though the patient may remain imbalanced for several hours. Constant repetition of the positional change leads to habituation. Since some CNS disorders can mimic BPPV (eg, vertebrobasilar insufficiency), recurrent cases warrant head MRI/MRA. In central lesions, there is no latent period, fatigability, or habituation of the symptoms and signs. Treatment of BPPV involves physical therapy protocols (eg, the Epley maneuver or Brandt-Daroff exercises), based on the theory that it results from cupulolithiasis (free-floating statoconia, also known as otoconia) within a semicircular canal.
Argaet EC et al. Benign positional vertigo, its diagnosis, treatment and mimics. Clin Neurophysiol Pract. 2019 Apr 6;4:97–111. [PMID: 31193795]
Instrum RS et al. Benign paroxysmal positional vertigo. Adv Otorhinolaryngol. 2019;82:67–76. [PMID: 30947198]
D. Vestibular Neuronitis
In vestibular neuronitis, a paroxysmal, usually single attack of vertigo occurs without accompanying impairment of auditory function and will persist for several days to a week before gradually abating. During the acute phase, examination reveals nystagmus and absent responses to caloric stimulation on one or both sides. The cause of the disorder is unclear though presumed to be viral. Treatment consists of supportive care, including oral diazepam, 2–5 mg every 6–12 hours, or meclizine, 25–100 mg divided two to three times daily, during the acute phases of the vertigo only, followed by vestibular therapy if the patient does not completely compensate.
Bronstein AM et al. Long-term clinical outcome in vestibular neuritis. Curr Opin Neurol. 2019 Feb;32(1):174–80. [PMID: 30566414]
van Esch BF et al. Clinical characteristics of benign recurrent vestibulopathy: clearly distinctive from vestibular migraine and Menière’s disease? Otol Neurotol. 2017 Oct;38(9):e357–63. [PMID: 28834943]
E. Traumatic Vertigo
Labyrinthine concussion is the most common cause of vertigo following head injury. Symptoms generally diminish within several days but may linger for a month or more. Basilar skull fractures that traverse the inner ear usually result in severe vertigo lasting several days to a week and deafness in the involved ear. Chronic posttraumatic vertigo may result from cupulolithiasis. This occurs when traumatically detached statoconia (otoconia) settle on the ampulla of the posterior semicircular canal and cause an excessive degree of cupular deflection in response to head motion. Clinically, this presents as episodic positioning vertigo. Treatment consists of supportive care and vestibular suppressant medication (diazepam or meclizine) during the acute phase of the attack, and vestibular therapy.
Marcus HJ et al. Vestibular dysfunction in acute traumatic brain injury. J Neurol. 2019 Oct;266(10):2430–3. [PMID: 31201499]
F. Perilymphatic Fistula
Leakage of perilymphatic fluid from the inner ear into the tympanic cavity via the round or oval window is a rare cause of vertigo and sensory hearing loss. Most cases result from either physical injury (eg, blunt head trauma, hand slap to ear); extreme barotrauma during airflight, scuba diving, etc; or vigorous Valsalva maneuvers (eg, during weight lifting). Treatment may require middle ear exploration and window sealing with a tissue graft.
Deveze A et al. Diagnosis and treatment of perilymphatic fistula. Adv Otorhinolaryngol. 2018;81:133–45. [PMID: 29794455]
G. Cervical Vertigo
Position receptors located in the facets of the cervical spine are important physiologically in the coordination of head and eye movements. Cervical proprioceptive dysfunction is a common cause of vertigo triggered by neck movements. This disturbance often commences after neck injury, particularly hyperextension; it is also associated with degenerative cervical spine disease. Although symptoms vary, vertigo may be triggered by assuming a particular head position as opposed to moving to a new head position (the latter typical of labyrinthine dysfunction). Cervical vertigo may often be confused with migraine-associated vertigo, which is also associated with head movement. Management consists of neck movement exercises to the extent permitted by orthopedic considerations.
Devaraja K. Approach to cervicogenic dizziness: a comprehensive review of its aetiopathology and management. Eur Arch Otorhinolaryngol. 2018 Oct;275(10):2421–33. [PMID: 30094486]
Ranalli P. An overview of central vertigo disorders. Adv Otorhinolaryngol. 2019;82:127–33. [PMID: 30947212]
H. Migrainous Vertigo
Episodic vertigo is frequently associated with migraine headache. Head trauma may also be a precipitating feature. The vertigo may be temporally related to the headache and last up to several hours, or it may also occur in the absence of any headache. Migrainous vertigo may resemble Ménière disease but without associated hearing loss or tinnitus. Accompanying symptoms may include head pressure; visual, motion, or auditory sensitivity; and photosensitivity. Symptoms typically worsen with lack of sleep and anxiety or stress. Food triggers include caffeine, chocolate, and alcohol, among others. There is often a history of motion intolerance (easily carsick as a child). Migrainous vertigo may be familial. Treatment includes dietary and lifestyle changes (improved sleep pattern, avoidance of stress) and antimigraine prophylactic medication.
Hain T et al. Migraine associated vertigo. Adv Otorhinolaryngol. 2019;82:119–26. [PMID: 30947176]
I. Superior Semicircular Canal Dehiscence
Deficiency in the bony covering of the superior semicircular canal may be associated with vertigo triggered by loud noise exposure, straining, and an apparent conductive hearing loss. Autophony is also a common feature. Diagnosis is with coronal high-resolution CT scan and VEMPs. Surgically resurfacing or plugging the dehiscent canal can improve symptoms.
Ahmed W et al. Systematic review of round window operations for the treatment of superior semicircular canal dehiscence. J Int Adv Otol. 2019 Aug;15(2):209–14. [PMID: 31418721]
Naert L et al. Aggregating the symptoms of superior semicircular canal dehiscence syndrome. Laryngoscope. 2018 Aug;128(8):1932–8. [PMID: 29280497]
 Vertigo Syndromes Due to Central Lesions
Vertigo Syndromes Due to Central Lesions
CNS causes of vertigo include brainstem vascular disease, arteriovenous malformations, tumors of the brainstem and cerebellum, multiple sclerosis, and vertebrobasilar migraine (Table 8–2). Vertigo of central origin often becomes unremitting and disabling. The associated nystagmus is often nonfatigable, vertical rather than horizontal in orientation, without latency, and unsuppressed by visual fixation. ENG is useful in documenting these characteristics. There are commonly other signs of brainstem dysfunction (eg, cranial nerve palsies; motor, sensory, or cerebellar deficits in the limbs) or of increased intracranial pressure. Auditory function is generally spared. The underlying cause should be treated.
Choi JY et al. Central vertigo. Curr Opin Neurol. 2018 Feb;31(1):81–9. [PMID: 29084063]
Ranalli P. An overview of central vertigo disorders. Adv Otorhinolaryngol. 2019;82:127–33. [PMID: 30947212]
DISEASES OF THE CENTRAL AUDITORY & VESTIBULAR SYSTEMS
Lesions of the eighth cranial nerve and central audiovestibular pathways produce neural hearing loss and vertigo (Table 8–3). One characteristic of neural hearing loss is deterioration of speech discrimination out of proportion to the decrease in pure tone thresholds. Another is auditory adaptation, wherein a steady tone appears to the listener to decay and eventually disappear. Auditory evoked responses are useful in distinguishing cochlear from neural losses and may give insight into the site of lesion within the central pathways.
The evaluation of central audiovestibular disorders usually requires imaging of the internal auditory canal, cerebellopontine angle, and brain with enhanced MRI.
1. Vestibular Schwannoma (Acoustic Neuroma)
Eighth cranial nerve schwannomas are among the most common intracranial tumors. Most are unilateral, but about 5% are associated with the hereditary syndrome neurofibromatosis type 2, in which bilateral eighth nerve tumors may be accompanied by meningiomas and other intracranial and spinal tumors. These benign lesions arise within the internal auditory canal and gradually grow to involve the cerebellopontine angle, eventually compressing the pons and resulting in hydrocephalus. Their typical auditory symptoms are unilateral hearing loss with a deterioration of speech discrimination exceeding that predicted by the degree of pure tone loss. Nonclassic presentations, such as sudden unilateral hearing loss, are fairly common. Any individual with a unilateral or asymmetric sensorineural hearing loss should be evaluated for an intracranial mass lesion. Vestibular dysfunction more often takes the form of continuous dysequilibrium than episodic vertigo. Diagnosis is made by enhanced MRI. Treatment consists of observation, microsurgical excision, or stereotactic radiotherapy, depending on such factors as patient age, underlying health, and size of the tumor. Bevacizumab (vascular endothelial growth factor blocker) has shown promise for treatment of tumors in neurofibromatosis type 2.
Kalogeridi MA et al. Stereotactic radiosurgery and radiotherapy for acoustic neuromas. Neurosurg Rev. 2019 Apr 13. [Epub ahead of print] [PMID: 30982152]
Leon J et al. Observation or stereotactic radiosurgery for newly diagnosed vestibular schwannomas: a systematic review and meta-analysis. J Radiosurg SBRT. 2019;6(2):91–100. [PMID: 31641546]
2. Vascular Compromise
Vertebrobasilar insufficiency is a common cause of vertigo in the elderly. It is often triggered by changes in posture or extension of the neck. Reduced flow in the vertebrobasilar system may be demonstrated noninvasively through MRA. Empiric treatment is with vasodilators and aspirin.
Cornelius JF et al. Compression syndromes of the vertebral artery at the craniocervical junction. Acta Neurochir Suppl. 2019;125:151–8. [PMID: 30610316]
3. Multiple Sclerosis
Patients with multiple sclerosis may suffer from episodic vertigo and chronic imbalance. Hearing loss in this disease is most commonly unilateral and of rapid onset. Spontaneous recovery may occur.
Kattah JC et al. Eye movements in demyelinating, autoimmune and metabolic disorders. Curr Opin Neurol. 2020 Feb;33(1):111–6. [PMID: 31770124]
OTOLOGIC MANIFESTATIONS OF AIDS
The otologic manifestations of AIDS are protean. The pinna and external auditory canal may be affected by Kaposi sarcoma and by persistent and potentially invasive fungal infections (particularly Aspergillus fumigatus). Serous otitis media due to eustachian tube dysfunction may arise from adenoidal hypertrophy (HIV lymphadenopathy), recurrent mucosal viral infections, or an obstructing nasopharyngeal tumor (eg, lymphoma). Unfortunately, ventilating tubes are seldom helpful and may trigger profuse watery otorrhea. Acute otitis media is usually caused by typical bacterial organisms, including Proteus, Staphylococcus, and Pseudomonas, and rarely, by Pneumocystis jirovecii. Sensorineural hearing loss is common and, in some cases, results from viral CNS infection. In cases of progressive hearing loss, cryptococcal meningitis and syphilis must be excluded. Acute facial paralysis due to herpes zoster infection (Ramsay Hunt syndrome) occurs commonly and follows a clinical course similar to that in nonimmunocompromised patients. Treatment is with high-dose acyclovir (see Chapter 32). Corticosteroids may also be effective as an adjunct.
Bao S et al. Otorhinolaryngological profile and surgical intervention in patients with HIV/AIDS. Sci Rep. 2018 Aug 13;8(1):12045. [PMID: 30104657]
Matas CG et al. Audiological and electrophysiological alterations in HIV-infected individuals subjected or not to antiretroviral therapy. Braz J Otorhinolaryngol. 2018 Sep–Oct;84(5):574–82. [PMID: 28823692]
DISEASES OF THE NOSE & PARANASAL SINUSES
INFECTIONS OF THE NOSE & PARANASAL SINUSES
1. Acute Viral Rhinosinusitis (Common Cold)
ESSENTIALS OF DIAGNOSIS

 Nasal congestion, clear rhinorrhea, and hyposmia.
Nasal congestion, clear rhinorrhea, and hyposmia.
 Associated malaise, headache, and cough.
Associated malaise, headache, and cough.
 Erythematous, engorged nasal mucosa without intranasal purulence.
Erythematous, engorged nasal mucosa without intranasal purulence.
 Symptoms are self-limited, lasting less than 4 weeks and typically less than 10 days.
Symptoms are self-limited, lasting less than 4 weeks and typically less than 10 days.
 Clinical Findings
Clinical Findings
Because there are numerous serologic types of rhinoviruses, adenoviruses, and other viruses, patients remain susceptible to the common cold throughout life. These infections, while generally quite benign and self-limited, have been implicated in the development or exacerbation of more serious conditions, such as acute bacterial sinusitis and acute otitis media, asthma, cystic fibrosis, and bronchitis. Nasal congestion, decreased sense of smell, watery rhinorrhea, and sneezing, accompanied by general malaise, throat discomfort and, occasionally, headache, are typical in viral infections. Nasal examination usually shows erythematous, edematous mucosa and a watery discharge. The presence of purulent nasal discharge suggests bacterial rhinosinusitis.
 Treatment
Treatment
There are no effective antiviral therapies for either the prevention or treatment of most viral rhinitis despite a common misperception among patients that antibiotics are helpful. Prevention of influenza virus infection by boosting the immune system using the annually created vaccine may be the most effective management strategy. Oseltamivir is the first neuramidase inhibitor approved for the treatment and prevention of influenza virus infection, but its use is generally limited to those patients considered high risk. These high-risk patients include young children, pregnant women, and adults older than 65 years of age. Oseltamivir is hard to use because it must be started within 48 hours for optimal effect. Buffered hypertonic saline (3–5%) nasal irrigation has been shown to improve symptoms and reduce the need for nonsteroidal anti-inflammatory drugs (NSAIDs). Other supportive measures, such as oral decongestants (pseudoephedrine, 30–60 mg every 4–6 hours or 120 mg twice daily), may provide some relief of rhinorrhea and nasal obstruction. Nasal sprays, such as oxymetazoline or phenylephrine, are rapidly effective but should not be used for more than a few days to prevent rebound congestion. Withdrawal of the drug after prolonged use leads to rhinitis medicamentosa, an almost addictive need for continuous usage. Treatment of rhinitis medicamentosa requires mandatory cessation of the sprays, and this is often extremely frustrating for patients. Topical intranasal corticosteroids (eg, flunisolide, 2 sprays in each nostril twice daily), intranasal anticholinergic (ipratropium 0.06% nasal spray, 2–3 sprays every 8 hours as needed), or a short tapering course of oral prednisone may help during the withdrawal process.
 Complications
Complications
Other than mild eustachian tube dysfunction or transient middle ear effusion, complications of viral rhinitis are unusual. Secondary acute bacterial rhinosinusitis is a well-accepted complication of acute viral rhinitis and is suggested by persistence of symptoms beyond 10 days with purulent green or yellow nasal secretions and unilateral facial or tooth pain.
Bergmark RW et al. Diagnosis and first-line treatment of chronic sinusitis. JAMA. 2017 Dec 19;318(23):2344–5. [PMID: 29260210]
Tan KS et al. Impact of respiratory virus infections in exacerbation of acute and chronic rhinosinusitis. Curr Allergy Asthma Rep. 2017 Apr;17(4):24. [PMID: 28389843]
2. Acute Bacterial Rhinosinusitis (Sinusitis)
ESSENTIALS OF DIAGNOSIS

 Purulent yellow-green nasal discharge or expectoration.
Purulent yellow-green nasal discharge or expectoration.
 Facial pain or pressure over the affected sinus or sinuses.
Facial pain or pressure over the affected sinus or sinuses.
 Nasal obstruction.
Nasal obstruction.
 Acute onset of symptoms (between 1 and 4 weeks’ duration).
Acute onset of symptoms (between 1 and 4 weeks’ duration).
 Associated cough, malaise, fever, and headache.
Associated cough, malaise, fever, and headache.
 General Considerations
General Considerations
Compared with viral rhinitis, acute bacterial rhinosinusitis infections are uncommon, but they still affect nearly 20 million Americans annually and account for over 2 billion dollars in health care expenditures.
Acute bacterial rhinosinusitis is believed to be the result of impaired mucociliary clearance, inflammation of the nasal cavity mucosa, and obstruction of the ostiomeatal complex, or sinus “pore.” Edematous mucosa causes obstruction of the complex, resulting in the accumulation of mucus in the sinus cavity that becomes secondarily infected by bacteria. The largest of these ostiomeatal complexes is deep to the middle turbinate in the middle meatus. This complex is actually a confluence of complexes draining the maxillary, ethmoid, and frontal sinuses. The sphenoid drains from a separate complex between the septum and superior turbinate.
The typical pathogens of bacterial rhinosinusitis are S pneumoniae, other streptococci, H influenzae, and less commonly, S aureus and Moraxella catarrhalis. Pathogens vary regionally in both prevalence and drug resistance; about 25% of healthy asymptomatic individuals may, if sinus aspirates are cultured, harbor such bacteria as well.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
There are no agreed-upon criteria for the diagnosis of acute bacterial rhinosinusitis in adults. Major symptoms include purulent nasal drainage, nasal obstruction or congestion, facial pain/pressure, altered smell, cough, and fever. Minor symptoms include headache, otalgia, halitosis, dental pain, and fatigue. Many of the more specific symptoms and signs relate to the affected sinus(es). Bacterial rhinosinusitis can be distinguished from viral rhinitis by persistence of symptoms for more than 10 days after onset or worsening of symptoms within 10 days after initial improvement. Acute rhinosinusitis is defined as lasting less than 4 weeks, and subacute rhinosinusitis, as lasting 4–12 weeks.
Acute maxillary sinusitis is the most common form of acute bacterial rhinosinusitis because the maxillary is the largest sinus with a single drainage pathway that is easily obstructed. Unilateral facial fullness, pressure, and tenderness over the cheek are common symptoms, but may not always be present. Pain may refer to the upper incisor and canine teeth via branches of the trigeminal nerve, which traverse the floor of the sinus. Purulent nasal drainage should be noted with nasal airway obstruction or facial pain (pressure). Maxillary sinusitis may result from dental infection, and teeth that are tender should be carefully examined for signs of abscess. Drainage of the periapical abscess or removal of the diseased tooth typically resolves the sinus infection.
Acute ethmoiditis in adults is often accompanied by maxillary sinusitis, and symptoms are similar to those described above. Localized ethmoid sinusitis may present with pain and pressure over the high lateral wall of the nose between the eyes that may radiate to the orbit.
Sphenoid sinusitis is usually seen in the setting of pansinusitis or infection of all the paranasal sinuses on at least one side. The patient may complain of a headache “in the middle of the head” and often points to the vertex.
Acute frontal sinusitis may cause pain and tenderness of the forehead. This is most easily elicited by palpation of the orbital roof just below the medial end of the eyebrow.
Hospital-associated sinusitis is a form of acute bacterial rhinosinusitis that may present without the usual symptoms. Instead, it may be a cause of fever in critically ill patients. It is often associated with prolonged presence of a nasogastric or, rarely, nasotracheal tube causing nasal mucosal inflammation and ostiomeatal complex obstruction. Pansinusitis on the side of the tube is common on imaging studies.
B. Imaging
The diagnosis of acute bacterial rhinosinusitis can usually be made on clinical grounds alone. Although more sensitive than clinical examination, routine radiographs are not cost-effective and are not recommended by the Agency for Health Care Policy and Research or American Association of Otolaryngology Guidelines. Consensus guidelines recommend imaging when clinical criteria are difficult to evaluate, when the patient does not respond to appropriate therapy or has been treated repeatedly with antibiotics, when intracranial involvement or cerebrospinal fluid rhinorrhea is suspected, when complicated dental infection is suspected, or when symptoms of more serious infection are noted.
When necessary, noncontrast screening coronal CT scans are more cost-effective and provide more information than conventional sinus films. CT provides a rapid and effective means to assess all of the paranasal sinuses, identify areas of greater concern (such as bony dehiscence, periosteal elevation, or maxillary tooth root exposure within the sinus), and speed appropriate therapy.
CT scans are reasonably sensitive but are not specific. Swollen soft tissue and fluid may be difficult to distinguish when opacification of the sinus is due to other conditions, such as chronic rhinosinusitis, nasal polyposis, or mucus retention cysts. Sinus abnormalities can be seen in most patients with an upper respiratory infection, while bacterial rhinosinusitis develops in only 2%.
If malignancy, intracranial extension, or opportunistic infection is suspected, MRI with gadolinium should be ordered instead of, or in addition to, CT. MRI will distinguish tumor from fluid, inflammation, and inspissated mucus far better than CT, and will better delineate tumor extent (eg, involvement of adjacent structures, such as the orbit, skull base, and palate). Bone destruction can be demonstrated as well by MRI as by CT.
 Treatment
Treatment
All patients with acute bacterial rhinosinusitis should have careful evaluation of pain. The European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2012 recommends NSAIDs, saline nasal sprays, and nasal decongestants (pseudoephedrine, 30–60 mg every 6 hours, up to 240 mg/day; nasal oxymetazoline, 0.05% or oxymetazoline, 0.05–0.1%, one or two sprays in each nostril every 6–8 hours for up to 3 days) for symptom reduction in viral rhinitis and bacterial rhinosinusitis without complication. In cases of suspected bacterial rhinosinusitis, intranasal corticosteroids (eg, high-dose mometasone furoate 200 mcg each nostril twice daily for 21 days) have demonstrated efficacy in reducing nasal symptoms and are recommended. Other medications, such as mucolytics, vitamin C, probiotics, and antihistamines, have not demonstrated efficacy in the management of acute rhinosinusitis.
Antibiotic therapy should be reserved for complicated or protracted acute bacterial rhinosinusitis. Between 40% and 69% of patients with acute bacterial rhinosinusitis improve symptomatically within 2 weeks without antibiotic therapy. Antibiotic treatment is controversial in uncomplicated cases of clinically diagnosed acute bacterial rhinosinusitis because only 5% of patients will note a shorter duration of illness with treatment, and antibiotic treatment is associated with nearly twice the number of adverse events compared with placebo. Antibiotics may be considered when symptoms last more than 10 days or when symptoms (including fever, facial pain, and swelling of the face) are severe or when cases are complicated (such as immunodeficiency). In these patients, administration of antibiotics does reduce the incidence of clinical failure by 50% and represents the most cost-effective treatment strategy.
Selection of antibiotics is usually empiric and based on a number of factors, including regional patterns of antibiotic resistance, antibiotic allergy, cost, and patient tolerance. For adults younger than 65 years with mild to moderate acute bacterial rhinosinusitis, the recommended first-line therapy is amoxicillin-clavulanate (500 mg/125 mg orally three times daily or 875 mg/125 mg orally twice daily for 5–7 days), or in those with severe sinusitis, high-dose amoxicillin-clavulanate (2000 mg/125 mg extended-release orally twice daily for 7–10 days). In patients with a high risk for penicillin-resistant S pneumoniae (age over 65 years, hospitalization in the prior 5 days, antibiotic use in the prior month, immunocompromised status, multiple comorbidities, or severe sinus infection), the recommended first-line therapy is the high-dose amoxicillin-clavulanate option (2000 mg/125 mg extended-release orally twice daily for 7–10 days). For those with penicillin allergy or hepatic impairment, then doxycycline (100 mg orally twice daily or 200 mg orally once daily for 5–7 days), or clindamycin (150–300 mg every 6 hours) plus a cephalosporin (cefixime 400 mg orally once daily or cefpodoxime proxetil 200 mg orally twice daily) for 10 days are options. Macrolides, trimethoprim-sulfamethoxazole, and second- or third-generation cephalosporins are not recommended for empiric therapy.
Hospital-associated infections in critically ill patients are treated differently from community-acquired infections. Removal of a nasogastric tube and improved nasal hygiene (nasal saline sprays, humidification of supplemental nasal oxygen, and nasal decongestants) are critical interventions and often curative in mild cases without aggressive antibiotic use. Endoscopic or transantral cultures may help direct medical therapy in complicated cases. In addition, broad-spectrum antibiotic coverage directed at P aeruginosa, S aureus (including methicillin-resistant strains), and anaerobes may be required.
 Complications
Complications
Local complications of acute bacterial rhinosinusitis include orbital cellulitis and abscess, osteomyelitis, cavernous sinus thrombosis, and intracranial extension.
Orbital complications typically occur by extension of ethmoid sinusitis through the lamina papyracea, a thin layer of bone that comprises the medial orbital wall. Any change in the ocular examination necessitates immediate CT imaging. Extension in this area may cause orbital cellulitis leading to proptosis, gaze restriction, and orbital pain. Select cases are responsive to intravenous antibiotics, with or without corticosteroids, and should be managed in close conjunction with an ophthalmologist or otolaryngologist, or both. Extension through the lamina papyracea can also lead to subperiosteal abscess formation (orbital abscess). Such abscesses cause marked proptosis, ophthalmoplegia, and pain with medial gaze. While some cases respond to antibiotics, such findings should prompt an immediate referral to a specialist for consideration of decompression and evacuation. Failure to intervene quickly may lead to permanent visual impairment and a “frozen globe.”
Osteomyelitis requires prolonged antibiotics as well as removal of necrotic bone. The frontal sinus is most commonly affected, with bone involvement suggested by a tender swelling of the forehead (Pott puffy tumor). Following treatment, secondary cosmetic reconstructive procedures may be necessary.
Intracranial complications of sinusitis can occur either through hematogenous spread, as in cavernous sinus thrombosis and meningitis, or by direct extension, as in epidural and intraparenchymal brain abscesses. Fortunately, they are rare today. Cavernous sinus thrombosis is heralded by ophthalmoplegia, chemosis, and visual loss; the diagnosis is most commonly confirmed by MRI. When identified early, cavernous sinus thrombosis typically responds to intravenous antibiotics. Frontal epidural and intracranial abscesses are often clinically silent, but may present with altered mental status, persistent fever, or severe headache.
 When to Refer
When to Refer
Failure of acute bacterial rhinosinusitis to resolve after an adequate course of oral antibiotics necessitates referral to an otolaryngologist for evaluation. Endoscopic cultures may direct further treatment choices. Nasal endoscopy and CT scan are indicated when symptoms persist longer than 4–12 weeks. Any patients with suspected extension of disease outside the sinuses should be evaluated urgently by an otolaryngologist and imaging should be obtained.
 When to Admit
When to Admit
• Facial swelling and erythema indicative of facial cellulitis.
• Proptosis.
• Vision change or gaze abnormality indicative of orbital cellulitis.
• Abscess or cavernous sinus involvement.
• Mental status changes suggestive of intracranial extension.
• Immunocompromised status.
• Failure to respond to appropriate first-line treatment or symptoms persisting longer than 4 weeks.
Ebell MH et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019 Mar;17(2):164–72. [PMID: 30858261]
Lemiengre MB et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018 Sep 10;9:CD006089. [PMID: 30198548]
Snidvongs K et al. Update on intranasal medications in rhinosinusitis. Curr Allergy Asthma Rep. 2017 Jul;17(7):47. [PMID: 28602009]
3. Nasal Vestibulitis & S aureus Nasal Colonization
Inflammation of the nasal vestibule may result from folliculitis of the hairs that line this orifice and is usually the result of nasal manipulation or hair trimming. Systemic antibiotics effective against S aureus (such as dicloxacillin, 250 mg orally four times daily for 7–10 days) are indicated. Topical mupirocin 2% nasal ointment (applied two or three times daily) may be a helpful addition and may prevent future occurrences. If recurrent, the addition of rifampin (10 mg/kg orally twice daily for the last 4 days of dicloxacillin treatment) may eliminate the S aureus carrier state. If a furuncle exists, it should be incised and drained, preferably intranasally. Adequate treatment of these infections is important to prevent retrograde spread of infection through valveless veins into the cavernous sinus and intracranial structures.
S aureus is the leading nosocomial pathogen, and nasal carriage is a well-defined risk factor in the development and spread of nosocomial infections. Nasal and extranasal methicillin-resistant S aureus (MRSA) colonization are associated with a 30% risk of developing an invasive MRSA infection during hospital stays. While the vast majority have no vestibulitis symptoms, screening by nasal swabs and polymerase chain reaction (PCR) based assays has demonstrated a 30% rate of S aureus colonization in hospital patients and an 11% rate of MRSA colonization in intensive care unit patients. Elimination of the carrier state is challenging, but studies of mupirocin 2% nasal ointment application with chlorhexidine facial washing (40 mg/mL) twice daily for 5 days have demonstrated decolonization in 39% of patients.
Lipschitz N et al. Nasal vestibulitis: etiology, risk factors, and clinical characteristics: a retrospective study of 118 cases. Diagn Microbiol Infect Dis. 2017 Oct;89(2):131–4. [PMID: 28780999]
Septimus EJ. Nasal decolonization: what antimicrobials are most effective prior to surgery? Am J Infect Control. 2019 Jun;47S:A53–7. [PMID: 31146851]
4. Invasive Fungal Sinusitis
Invasive fungal sinusitis is rare and includes both rhinocerebral mucormycosis (Mucor, Absidia, and Rhizopus sp.) and other invasive fungal infections, such as Aspergillus. The fungus spreads rapidly through vascular channels and may be lethal if not detected early. Patients with mucormycosis almost invariably have some degree of immunocompromise, such as diabetes mellitus, long-term corticosteroid therapy, neutropenia associated with chemotherapy for hematologic malignancy, or end-stage renal disease. Occasional cases of sinonasal infection with Aspergillus sp. have been reported in patients with untreated HIV/AIDS. The initial symptoms may be similar to those of acute bacterial rhinosinusitis, although facial pain is often more severe. Nasal drainage is typically clear or straw-colored, rather than purulent, and visual symptoms may be noted at presentation in the absence of significant nasal findings. On examination, the classic finding of mucormycosis is a black eschar on the middle turbinate, but this finding is not universal and may not be apparent if the infection is deep or high within the nasal bones. Often the mucosa appears normal or simply pale and dry. This may be noted on the hard palate as well. Early diagnosis requires suspicion of the disease and nasal biopsy with silver stains, revealing broad nonseptate hyphae within tissues and necrosis with vascular occlusion. Because CT or MRI may initially show only soft tissue changes, biopsy and ultimate debridement should be based on the clinical setting rather than radiographic demonstration of bony destruction or intracranial changes.
Invasive fungal sinusitis represents a medical and surgical emergency. Once recognized, voriconazole may be started by intravenous infusion, and prompt wide surgical debridement is indicated for patients with reversible immune deficiency (eg, poorly controlled hyperglycemia in diabetes). Other antifungals, including amphotericin or the less nephrotoxic lipid-based amphotericin B (Ambisome) and caspofungin, are alternatives to voriconazole and may be added to voriconazole depending on the fungus. Surgical management, while necessary for any possibility of cure, often results in tremendous disfigurement and functional deficits (eg, often resulting in the loss of at least one eye). Even with early diagnosis and immediate appropriate intervention, the prognosis is guarded. In persons with diabetes, the mortality rate is about 20%. If kidney disease is present or develops, mortality is over 50%; in the setting of AIDS or hematologic malignancy with neutropenia, mortality approaches 100%. Whether to undertake aggressive surgical management should be considered carefully because many patients are gravely ill at the time of diagnosis, and overall disease-specific survival is only about 57%.
Craig JR. Updates in management of acute invasive fungal rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2019 Feb;27(1):29–36. [PMID: 30585877]
Ergun O et al. Acute invasive fungal rhinosinusitis: presentation of 19 cases, review of the literature, and a new classification system. J Oral Maxillofac Surg. 2017 Apr;75(4):767.e1–9. [PMID: 27918884]
Kashkouli MB et al. Outcomes and factors affecting them in patients with rhino-orbito-cerebral mucormycosis. Br J Ophthalmol. 2019 Oct;103(10):1460–5. [PMID: 30514712]
ALLERGIC RHINITIS
ESSENTIALS OF DIAGNOSIS

 Clear rhinorrhea, sneezing, tearing, eye irritation, and pruritus.
Clear rhinorrhea, sneezing, tearing, eye irritation, and pruritus.
 Associated symptoms include cough, bronchospasm, and eczematous dermatitis.
Associated symptoms include cough, bronchospasm, and eczematous dermatitis.
 Environmental allergen exposure in the presence of allergen-specific IgE.
Environmental allergen exposure in the presence of allergen-specific IgE.
 General Considerations
General Considerations
Allergic rhinitis is very common in the United States with population studies reporting a prevalence of 20–30% of adults and up to 40% of children. Allergic rhinitis adversely affects school and work performance, costing about $6 billion annually in the United States through direct costs of therapy as well as the indirect costs of sleep deprivation, fatigue and reduced productivity, or absenteeism. Seasonal allergic rhinitis is most commonly caused by pollens and spores. Flowering shrub and tree pollens are most common in the spring, flowering plants and grasses in the summer, and ragweed and molds in the fall. Interestingly, climate change may have an impact on the occurrence of allergic rhinitis since increased temperature and carbon dioxide exposure cause increased pollen production in ragweed plants and since the extended duration of summer correlates with longer periods of pollen production in these and other flowering weeds. Dust, household mites, air pollution, and pet dander may produce year-round symptoms, termed “perennial rhinitis.”
 Clinical Findings
Clinical Findings
The symptoms of “hay fever” are similar to those of viral rhinitis but are usually persistent and may show seasonal variation. Nasal symptoms are often accompanied by eye irritation, pruritus, conjunctival erythema, and excessive tearing. Many patients have a strong family history of atopy or allergy.
The clinician should be careful to distinguish allergic rhinitis from other types of nonallergic rhinitis. Vasomotor rhinitis (sometimes called senile rhinitis) is caused by increased sensitivity of the vidian nerve and is a common cause of clear rhinorrhea in elderly persons. Often patients will report that they have troubling rhinorrhea in response to numerous nasal stimuli, including warm or cold air, odors or scents, light, or particulate matter. Other types of rhinitis, including gustatory, atrophic, and drug-induced rhinorrhea, have also been described.
On physical examination, the mucosa of the turbinates is usually pale or violaceous because of venous engorgement. This is in contrast to the erythema of viral rhinitis. Nasal polyps, which are yellowish boggy masses of hypertrophic mucosa, are associated with long-standing allergic rhinitis.
 Treatment
Treatment
A. Intranasal Corticosteroids
Intranasal corticosteroid sprays remain the mainstay of treatment of allergic rhinitis. They are more effective—and frequently less expensive—than nonsedating antihistamines, though patients should be reminded that there may be a delay in onset of relief of 2 or more weeks. Corticosteroid sprays may also shrink hypertrophic nasal mucosa and nasal polyps, thereby providing an improved nasal airway and ostiomeatal complex drainage. Because of this effect, intranasal corticosteroids are critical in treating allergy in patients prone to recurrent acute bacterial rhinosinusitis or chronic rhinosinusitis. Available preparations include beclomethasone (42 mcg/spray twice daily per nostril), flunisolide (25 mcg/spray twice daily per nostril), mometasone furoate (200 mcg once daily per nostril), budesonide (100 mcg twice daily per nostril), and fluticasone propionate (200 mcg once daily per nostril). All are considered equally effective. Probably the most critical factors are compliance with regular use and proper introduction into the nasal cavity. In order to deliver medication to the region of the middle meatus, proper application involves holding the bottle straight up with the head tilted forward and pointing the bottle toward the ipsilateral ear when spraying. Side effects are limited, the most annoying being epistaxis (perhaps related to incorrect delivery of the drug toward the nasal septum).
B. Antihistamines
Antihistamines offer temporary, but immediate, control of many of the most troubling symptoms of allergic rhinitis. Effective oral antihistamines include nonsedating loratadine (10 mg once daily), desloratadine (5 mg once daily), and fexofenadine (60 mg twice daily or 120 mg once daily), and minimally sedating cetirizine (10 mg once daily). Brompheniramine or chlorpheniramine (4 mg orally every 6–8 hours, or 8–12 mg orally every 8–12 hours as a sustained-release tablet) and clemastine (1.34–2.68 mg orally twice daily) may be less expensive but are usually associated with some drowsiness. The safety and efficacy of the newer, less-sedating antihistamines is so compelling that one of them, the H1-receptor antagonist nasal spray azelastine (1–2 sprays per nostril daily), is now included in the treatment guidelines of many consensus statements; however, some patients object to its bitter taste. Other side effects of oral antihistamines besides sedation include xerostomia and antihistamine tolerance (with eventual return of allergy symptoms despite initial benefit after several months of use). In such patients, typically those with perennial allergy, alternating effective antihistamines periodically can control symptoms over the long term.
C. Adjunctive Treatment Measures
Antileukotriene medications, such as montelukast (10 mg/day orally), alone or with cetirizine (10 mg/day orally) or loratadine (10 mg/day orally), may improve nasal rhinorrhea, sneezing, and congestion. Cromolyn sodium and sodium nedocromil may be useful adjunct agents for allergic rhinitis. They work by stabilizing mast cells and preventing proinflammatory mediator release. As topical agents, they have very few side effects, but they must be initiated well before allergen exposure (up to 4 weeks before). The most useful form of cromolyn is probably the ophthalmologic preparation placed dropwise into the nasal cavity. Intranasal cromolyn is cleared rapidly and must be administered four times daily for continued symptom relief. In practice, it is not nearly as effective as inhaled corticosteroid.
Intranasal anticholinergic agents, such as ipratropium bromide 0.03% or 0.06% sprays (42–84 mcg per nostril three times daily), may be helpful adjuncts when rhinorrhea is a major symptom. They are not as effective for treating allergic rhinitis but are more useful for treating vasomotor rhinitis.
Avoiding or reducing exposure to airborne allergens is the most effective means of alleviating symptoms of allergic rhinitis. Depending on the allergen, this can be extremely difficult. Maintaining an allergen-free environment by covering pillows and mattresses with plastic covers, substituting synthetic materials (foam mattress, acrylics) for animal products (wool, horsehair), and removing dust-collecting household fixtures (carpets, drapes, bedspreads, wicker) is worth the attempt to help more troubled patients. Air purifiers and dust filters may also aid in maintaining an allergen-free environment. Nasal saline irrigations are a useful adjunct in the treatment of allergic rhinitis to mechanically flush the allergens from the nasal cavity. When symptoms are extremely bothersome, a search for offending allergens may prove helpful. This can either be done by serum radioallergosorbent test (RAST) testing or skin testing by an allergist.
In some cases, allergic rhinitis symptoms are inadequately relieved by medication and avoidance measures. Often, such patients have a strong family history of atopy and may also have lower respiratory manifestations, such as allergic asthma. Referral to an allergist for immunotherapy may be appropriate. Such treatment involves proper identification of offending allergens, progressively increasing doses of allergen(s), and eventual maintenance dose administration over a period of 3–5 years. Immunotherapy has been proven to reduce circulating IgE levels in patients with allergic rhinitis and reduce the need for allergy medications. Both subcutaneous and topical immunotherapy have been shown to be effective in the long-term treatment of refractory allergic rhinitis. Emerging evidence demonstrating the safety and efficacy of sublingual and intranasal immunotherapy may allow these outpatient treatment options to replace more traditional parenteral allergen desensitization for allergic rhinitis in the near future.
Fein MN et al. CSACI position statement: Newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy Asthma Clin Immunol. 2019 Oct 1;15:61. [PMID: 3158299]
Meng Y et al. Recent developments and highlights in allergic rhinitis. Allergy. 2019 Dec;74(12):2320–8. [PMID: 31571226]
Reitsma S et al. Recent developments and highlights in rhinitis and allergen immunotherapy. Allergy. 2018 Dec;73(12):2306–13. [PMID: 30260494]
Singh AK et al. Fungal rhinosinusitis: microbiological and histopathological perspective. J Clin Diagn Res. 2017 Jul;11(7):DC10–12. [PMID: 28892889]
Small P et al. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018 Sep 12;14(Suppl 2):51. [PMID: 30263033]
OLFACTORY DYSFUNCTION
ESSENTIALS OF DIAGNOSIS

 Subjective diminished smell or taste sensation.
Subjective diminished smell or taste sensation.
 Lack of objective nasal obstruction.
Lack of objective nasal obstruction.
 Objective decrease in olfaction demonstrated by testing.
Objective decrease in olfaction demonstrated by testing.
 General Considerations
General Considerations
Anatomic blockage of the nasal cavity with subsequent airflow disruption is the most common cause of olfactory dysfunction (hyposmia or anosmia). Polyps, septal deformities, and nasal tumors may be the cause. Transient olfactory dysfunction often accompanies the common cold, nasal allergies, and perennial rhinitis through changes in the nasal and olfactory epithelium. About 20% of olfactory dysfunction is idiopathic, although it often follows a viral illness. Central nervous system neoplasms, especially those that involve the olfactory groove or temporal lobe, may affect olfaction and must be considered in patients with no other explanation for their hyposmia or for other neurologic signs. Head trauma is a rare but severe cause of olfactory dysfunction. Shearing of the olfactory neurites is more commonly associated with anosmia. Absent, diminished, or distorted smell or taste has been reported in a wide variety of endocrine, nutritional, and nervous disorders (eg, Parkinson disease and Alzheimer disease).
 Clinical Findings
Clinical Findings
Evaluation of olfactory dysfunction should include a thorough history of systemic illnesses and medication use as well as a physical examination focusing on the nose and nervous system. Nasal obstruction (from polyps, trauma, foreign bodies, or nasal masses) can cause functional hyposmia. Most clinical offices are not set up to test olfaction, but such tests may at times be worthwhile if only to assess whether a patient possesses any sense of smell at all. The University of Pennsylvania Smell Identification Test (UPSIT) is available commercially and is a simple, self-administered “scratch-and-sniff” test that is useful in differentiating hyposmia, anosmia, and malingering.
 Treatment
Treatment
Hyposmia secondary to nasal polyposis, obstruction, and chronic rhinosinusitis may respond to endoscopic sinus surgery. Unfortunately, there is no specific treatment for primary disruption of olfaction; some disturbances spontaneously resolve. The degree of hyposmia is the greatest predictor of recovery, with less severe hyposmia recovering at a much higher rate. In permanent hyposmia, counseling should be offered about seasoning foods (such as using pepper that stimulates the trigeminal as well as olfactory chemoreceptors, rather than table salt) and safety issues (such as installing home smoke alarms and using electric rather than gas appliances).
Doty RL. Age-related deficits in taste and smell. Otolaryngol Clin North Am. 2018 Aug;51(4):815–25. [PMID: 30001793]
Howell J et al. Head trauma and olfactory function. World J Otorhinolaryngol Head Neck Surg. 2018 Mar 14;4(1):39–45. [PMID: 30035260]
Werner S et al. Olfactory dysfunction revisited: a reappraisal of work-related olfactory dysfunction caused by chemicals. J Occup Med Toxicol. 2018 Sep 4;13:28. [PMID: 30202422]
EPISTAXIS
ESSENTIALS OF DIAGNOSIS

 Bleeding from a unilateral anterior nasal cavity most common.
Bleeding from a unilateral anterior nasal cavity most common.
 Most cases may be successfully treated by direct pressure on the bleeding site for 15 minutes. When this is inadequate, topical sympathomimetics and various nasal tamponade methods are usually effective.
Most cases may be successfully treated by direct pressure on the bleeding site for 15 minutes. When this is inadequate, topical sympathomimetics and various nasal tamponade methods are usually effective.
 Posterior, bilateral, or large-volume epistaxis should be triaged immediately to a specialist in a critical care setting.
Posterior, bilateral, or large-volume epistaxis should be triaged immediately to a specialist in a critical care setting.
 General Considerations
General Considerations
Epistaxis is an extremely common problem in the primary care setting. Bleeding is most common in the anterior septum where a confluence of veins creates a superficial venous plexus (Kiesselbach plexus). Predisposing factors include nasal trauma (nose picking, foreign bodies, forceful nose blowing), rhinitis, nasal mucosal drying from low humidity or supplemental nasal oxygen, deviation of the nasal septum, atherosclerotic disease, hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome), inhaled nasal cocaine (or other illicit drug), and alcohol abuse. Poorly controlled hypertension is associated with epistaxis. Anticoagulation or antiplatelet medications may be associated with a higher incidence, more frequent recurrence, and greater difficulty in control of epistaxis, but they do not cause it.
 Clinical Findings
Clinical Findings
Laboratory assessment of bleeding parameters may be indicated, especially in recurrent epistaxis. Once the acute episode has passed, careful examination of the nose and paranasal sinuses is indicated to rule out neoplasia and hereditary hemorrhagic telangiectasia.
Repeated evaluation for diagnosis and treatment of clinically significant hypertension should be performed following control of epistaxis and removal of any packing.
 Treatment
Treatment
Most cases of anterior epistaxis may be successfully treated by direct pressure on the site by compression of the nares continuously for 15 minutes. Venous pressure is reduced in the sitting position, and slight leaning forward lessens the swallowing of blood. Short-acting topical nasal decongestants (eg, phenylephrine, 0.125–1% solution, one or two sprays), which act as vasoconstrictors, may also help. When the bleeding does not readily subside, the nose should be examined, using good illumination and suction, in an attempt to locate the bleeding site. Topical 4% cocaine applied either as a spray or on a cotton strip serves both as an anesthetic and a vasoconstrictor. If cocaine is unavailable, a topical decongestant (eg, oxymetazoline) and a topical anesthetic (eg, tetracaine or lidocaine) provide similar results. When visible, the bleeding site may be cauterized with silver nitrate, diathermy, or electrocautery. A supplemental patch of Surgicel or Gelfoam may be helpful with a moisture barrier, such as petroleum-based ointment, to prevent drying and crusting. Warfarin may be continued in the setting of controlled epistaxis, although resorbable packing may be preferable in these patients.
Occasionally, a site of bleeding may be inaccessible to direct control, or attempts at direct control may be unsuccessful. In such cases, there are a number of alternatives. When the site of bleeding is anterior, a hemostatic sealant, pneumatic or other nasal tamponade, or anterior packing may suffice as the latter may be accomplished with several feet of lubricated iodoform packing systematically placed in the floor of the nose and then the vault of the nose.
About 5% of nasal bleeding originates in the posterior nasal cavity, commonly associated with atherosclerotic disease and hypertension. In such cases, it may be necessary to consult an otolaryngologist for a pack to occlude the choana before placing a pack anteriorly. In emergency settings, double balloon packs (Epistat) may facilitate rapid control of bleeding with little or no mucosal trauma. Because such packing is uncomfortable, bleeding may persist, and vasovagal syncope is possible, hospitalization for monitoring and stabilization is indicated. Posterior nasal packing is quite uncomfortable and may require an opioid analgesic for pain control.
Surgical management of epistaxis, through ligation of the nasal arterial supply (internal maxillary artery and ethmoid arteries) is indicated when direct pressure and nasal packing fail. The most common approach to surgical treatment is endoscopic sphenopalatine artery ligation. This method has a reported efficacy of 73–100% in studies; however, it may miss bleeds caused by the ethmoid arterial supply. Alternatively, endovascular epistaxis control is highly effective (75–92%) and can address all sources of intranasal bleeding except those from the anterior ethmoid artery. Its use may be reserved for when a surgical approach fails because it is associated with a 1.1–1.5% risk of stroke.
After control of epistaxis, the patient is advised to avoid straining and vigorous exercise for several days. Nasal saline should be applied to the packing frequently to keep the packing moist. Avoidance of hot or spicy foods and tobacco is also advisable, since these may cause nasal vasodilation. Avoiding nasal trauma, including nose picking, is an obvious necessity. Lubrication with petroleum jelly or bacitracin ointment and increased home humidity may also be useful ancillary measures. Finally, antistaphylococcal antibiotics (eg, cephalexin, 500 mg orally four times daily, or clindamycin, 150 mg orally four times daily) are indicated to reduce the risk of toxic shock syndrome developing while the packing remains in place (at least 5 days).
 When to Refer
When to Refer
• Patients with recurrent epistaxis, large-volume epistaxis, and episodic epistaxis with associated nasal obstruction should be referred to an otolaryngologist for endoscopic evaluation and possible imaging.
• Those with ongoing bleeding beyond 15 minutes should be taken to a local emergency department if the clinician is not prepared to manage acute epistaxis.
Khan M et al. Initial assessment in the management of adult epistaxis: systematic review. J Laryngol Otol. 2017 Dec;131(12):1035–55. [PMID: 29280694]
Krulewitz NA et al. Epistaxis. Emerg Med Clin North Am. 2019 Feb;37(1):29–39. [PMID: 30454778]
Swords C et al. Surgical and interventional radiological management of adult epistaxis: systematic review. J Laryngol Otol. 2017 Dec;131(12):1108–30. [PMID: 29280696]
Williams A et al. Haematological factors in the management of adult epistaxis: systematic review. J Laryngol Otol. 2017 Dec;131(12):1093–107. [PMID: 29280698]
NASAL TRAUMA
The nasal pyramid is the most frequently fractured bone in the body. Fracture is suggested by crepitance or palpably mobile bony segments. Epistaxis and pain are common, as are soft-tissue hematomas (“black eye”). It is important to make certain that there is no palpable step-off of the infraorbital rim, which would indicate the presence of a zygomatic complex fracture. Radiologic confirmation may at times be helpful but is not necessary in uncomplicated nasal fractures. It is also important to assess for possible concomitant additional facial, spine, pulmonary, or intracranial injuries when the circumstances of injury are suggestive, as in the case of automobile and motorcycle accidents.
Treatment is aimed at maintaining long-term nasal airway patency and cosmesis. Closed reduction can be performed under local or general anesthesia; closed reduction under general anesthesia appears to afford better patient satisfaction and decreased need for subsequent revision septoplasty or rhinoplasty.
Intranasal examination should be performed in all cases to rule out septal hematoma, which appears as a widening of the anterior septum, visible just posterior to the columella. The septal cartilage receives its only nutrition from its closely adherent mucoperichondrium. An untreated subperichondrial hematoma will result in loss of the nasal cartilage with resultant saddle nose deformity. Septal hematomas may become infected, with S aureus most commonly, and should be drained with an incision in the inferior mucoperichondrium on both sides. The drained fluid should be sent for culture.
Packing for 2–5 days is often helpful to help prevent re-formation of the hematoma. Antibiotics with antistaphylococcal efficacy (eg, cephalexin, 500 mg four times daily, or clindamycin, 150 mg four times daily) should be given for 3–5 days or the duration of the packing to reduce the risk of toxic shock syndrome.
TUMORS & GRANULOMATOUS DISEASE
1. Benign Nasal Tumors
A. Nasal Polyps
Nasal polyps are pale, edematous, mucosally covered masses commonly seen in patients with allergic rhinitis. They may result in chronic nasal obstruction and a diminished sense of smell. In patients with nasal polyps and a history of asthma, aspirin should be avoided as it may precipitate a severe episode of bronchospasm, known as triad asthma (Samter triad). Such patients may have an immunologic salicylate sensitivity.
Use of topical intranasal corticosteroids improves the quality of life in patients with nasal polyposis and chronic rhinosinusitis. Initial treatment with topical nasal corticosteroids (see Allergic Rhinitis section for specific drugs) for 1–3 months is usually successful for small polyps and may reduce the need for operation. A short course of oral corticosteroids (eg, prednisone, 6-day course using 21 [5-mg] tablets: 6 tablets [30 mg] on day 1 and tapering by 1 tablet [5 mg] each day) may also be of benefit, but when polyps are massive or medical management is unsuccessful obstructing polyps may be removed surgically. In healthy persons, this is a minor outpatient procedure. In recurrent polyposis, it may be necessary to remove polyps from the ethmoid, sphenoid, and maxillary sinuses to provide longer-lasting relief and open the affected sinuses. Intranasal corticosteroids should be continued following polyp removal to prevent recurrence, and the clinician should consider allergen testing to determine the offending allergen and avoidance measures.
Brescia G. Role of blood inflammatory cells in chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 2019 Jan;139(1):48–51. [PMID: 30686139]
Song WJ et al. Chronic rhinosinusitis with nasal polyps in older adults: clinical presentation, pathophysiology, and comorbidity. Curr Allergy Asthma Rep. 2019 Sep 5;19(10):46. [PMID: 31486905]
B. Inverted Papillomas
Inverted papillomas are benign tumors caused by human papillomavirus (HPV) that usually arise on the lateral nasal wall. They present with unilateral nasal obstruction and occasionally hemorrhage. They are often easily seen on anterior rhinoscopy as cauliflower-like growths in or around the middle meatus. Because squamous cell carcinoma is seen in about 10% of inverted or schneiderian papillomas, complete excision is strongly recommended. This usually requires an endoscopic medial maxillectomy. While rare, very extensive disease may require an open inferior or total maxillectomy for complete removal. Because recurrence rates for inverted papillomas are reported to be as high as 20%, subsequent clinical and radiologic follow-up is imperative. All excised tissue (not just a portion) should be carefully reviewed by the pathologist to be sure no carcinoma is present.
Gamrot-Wrzoł M et al. Risk factors of recurrence and malignant transformation of sinonasal inverted papilloma. Biomed Res Int. 2017;2017:9195163. [PMID: 29250552]
Peng R et al. Outcomes of sinonasal inverted papilloma resection by surgical approach: an updated systematic review and meta-analysis. Int Forum Allergy Rhinol. 2019 Jun;9(6):573–81. [PMID: 30748098]
2. Malignant Nasopharyngeal & Paranasal Sinus Tumors
Though rare, malignant tumors of the nose, nasopharynx, and paranasal sinuses are quite problematic because they tend to remain asymptomatic until late in their course. Squamous cell carcinoma is the most common cancer found in the sinuses and nasopharynx. It is especially common in the nasopharynx, where it obstructs the eustachian tube and results in serous otitis media. Nasopharyngeal carcinoma (nonkeratinizing squamous cell carcinoma or lymphoepithelioma) is usually associated with elevated IgA antibody to the viral capsid antigen of the Epstein-Barr virus (EBV). It is particularly common in patients of southern Chinese descent and has a weaker association with tobacco exposure than other head and neck squamous cell carcinomas. Adenocarcinomas, mucosal melanomas, sarcomas, and non-Hodgkin lymphomas are less commonly encountered neoplasms of this area.
Early symptoms are nonspecific, mimicking those of rhinitis or sinusitis. Unilateral nasal obstruction, otitis media, and discharge are common, with pain and recurrent hemorrhage often clues to the diagnosis of cancer. Any adult with persistent unilateral nasal symptoms or new otitis media should be thoroughly evaluated with nasal endoscopy and nasopharyngoscopy. A high index of suspicion remains a key to early diagnosis of these tumors. Patients often present with advanced symptoms, such as proptosis, expansion of a cheek, or ill-fitting maxillary dentures. Malar hypesthesia, due to involvement of the infraorbital nerve, is common in maxillary sinus tumors. Biopsy is necessary for definitive diagnosis, and MRI is the best imaging study to delineate the extent of disease and plan appropriate surgery and radiation.
Treatment depends on the tumor type and the extent of disease. Very early stage disease may be treated with megavoltage radiation therapy alone, but advanced nasopharyngeal carcinoma is best treated with concurrent radiation and cisplatin followed by adjuvant chemotherapy with cisplatin and fluorouracil. This chemoradiation therapy protocol significantly decreases local, nodal, and distant failures and increases progression-free and overall survival in advanced stage disease. Locally recurrent nasopharyngeal carcinoma may in selected cases be treated with repeat irradiation protocols or surgery with moderate success and a high degree of concern about local wound healing. Other squamous cell carcinomas are best treated—when resectable—with a combination of surgery and irradiation. Cranial base surgery, which can be done endoscopically using image navigation, appears to be an effective modality in improving the overall prognosis in paranasal sinus malignancies eroding the ethmoid roof. Although the prognosis is poor for advanced tumors, the results of treating resectable tumors of paranasal sinus origin have improved with the wider use of skull base resections and intensity-modulated radiation therapy. Cure rates are often 45–60%.
El-Sharkawy A et al. Epstein-Barr virus-associated malignancies: roles of viral oncoproteins in carcinogenesis. Front Oncol. 2018 Aug 2;8:265. [PMID: 30116721]
Lam WKJ et al. Recent advances in the management of nasopharyngeal carcinoma. F1000Res. 2018 Nov 21;7:1829. [PMID: 30519454]
Vartanian JG et al. Orbital exenteration for sinonasal malignancies: indications, rehabilitation and oncologic outcomes. Curr Opin Otolaryngol Head Neck Surg. 2018 Apr;26(2):122–6. [PMID: 29465436]
3. Sinonasal Inflammatory Disease (Granulomatosis with Polyangiitis & Sarcoidosis)
The nose and paranasal sinuses are involved in over 90% of cases of granulomatosis with polyangiitis. It is often not realized that involvement at these sites is more common than involvement of lungs or kidneys. Examination shows bloodstained crusts and friable mucosa. Biopsy, when positive, shows necrotizing granulomas and vasculitis. Other recognized sites of granulomatosis with polyangiitis in the head and neck include the subglottis and the middle ear. For treatment of granulomatosis with polyangiitis, see Chapter 20.
Sarcoidosis commonly involves the paranasal sinuses and is clinically similar to other chronic sinonasal inflammatory processes. Sinonasal symptoms, including rhinorrhea, nasal obstruction, and hyposmia or anosmia, may precede diagnosis of sarcoidosis in other organ systems. Clinically, the turbinates appear engorged with small white granulomas. Biopsy shows classic noncaseating granulomas. Notably, patients with sinonasal involvement generally have more trouble managing sarcoidosis in other organ systems.
Polymorphic reticulosis (midline malignant reticulosis, idiopathic midline destructive disease, lethal midline granuloma)—as the multitude of apt descriptive terms suggests—is not well understood but appears to be a nasal T-cell or NK-cell lymphoma. In contrast to granulomatosis with polyangiitis, involvement is limited to the mid-face, and there may be extensive bone destruction. Many destructive lesions of the mucosa and nasal structures labeled as polymorphic reticulosis are in fact non-Hodgkin lymphoma of either NK-cell or T-cell origin. Immunophenotyping, especially for CD56 expression, is essential in the histologic evaluation. Even when apparently localized, these lymphomas have a poor prognosis, with progression and death within a year the rule.
Chapman MN et al. Sarcoidosis in the head and neck: an illustrative review of clinical presentations and imaging findings. AJR Am J Roentgenol. 2017 Jan;208(1):66–75. [PMID: 27657552]
D’Anza B et al. Sinonasal imaging findings in granulomatosis with polyangiitis (Wegener granulomatosis): a systematic review. Am J Rhinol Allergy. 2017 Jan 1;31(1):16–21. [PMID: 28234146]
Edriss H et al. Sinonasal and laryngeal sarcoidosis—an uncommon presentation and management challenge. Am J Med Sci. 2019 Feb;357(2):93–102. [PMID: 30665498]
Felicetti M et al. Ear, nose and throat involvement in granulomatosis with polyangiitis: how it presents and how it determines disease severity and long-term outcomes. Clin Rheumatol. 2018 Apr;37(4):1075–83. [PMID: 29460094]
Kühn D et al. Manifestation of granulomatosis with polyangiitis in head and neck. Clin Exp Rheumatol. 2018 Mar–Apr;36 Suppl 111(2):78–84. [PMID: 29799391]
DISEASES OF THE ORAL CAVITY & PHARYNX
LEUKOPLAKIA, ERYTHROPLAKIA, ORAL LICHEN PLANUS, & ORAL CANCER
ESSENTIALS OF DIAGNOSIS

 Leukoplakia: A white lesion that cannot be removed by rubbing the mucosal surface.
Leukoplakia: A white lesion that cannot be removed by rubbing the mucosal surface.
 Erythroplakia: Similar to leukoplakia except that it has a definite erythematous component.
Erythroplakia: Similar to leukoplakia except that it has a definite erythematous component.
 Oral Lichen Planus: Most commonly presents as lacy leukoplakia but may be erosive; definitive diagnosis requires biopsy.
Oral Lichen Planus: Most commonly presents as lacy leukoplakia but may be erosive; definitive diagnosis requires biopsy.
 Oral Cancer: Early lesions appear as leukoplakia or erythroplakia; more advanced lesions will be larger, with invasion into the tongue such that a mass lesion is palpable. Ulceration may be present.
Oral Cancer: Early lesions appear as leukoplakia or erythroplakia; more advanced lesions will be larger, with invasion into the tongue such that a mass lesion is palpable. Ulceration may be present.
 Oropharynx Cancer: Unilateral throat masses, typically presenting with painful swallowing and weight loss.
Oropharynx Cancer: Unilateral throat masses, typically presenting with painful swallowing and weight loss.
Leukoplakic regions range from small to several centimeters in diameter (Figure 8–5). Histologically, they are often hyperkeratoses occurring in response to chronic irritation (eg, from dentures, tobacco, lichen planus); about 2–6%, however, represent either dysplasia or early invasive squamous cell carcinoma. Distinguishing between leukoplakia and erythroplakia is important because about 90% of cases of erythroplakia are either dysplasia or carcinoma. Squamous cell carcinoma accounts for 90% of oral cancer. Alcohol and tobacco use are the major epidemiologic risk factors.
Figure 8–5. Leukoplakia with moderate dysplasia on the lateral border of the tongue. (Used, with permission, from Ellen Eisenberg, DMD, in Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H, Tysinger J. The Color Atlas of Family Medicine. McGraw-Hill, 2009.)
The differential diagnosis may include oral candidiasis, necrotizing sialometaplasia, pseudoepitheliomatous hyperplasia, median rhomboid glossitis, and vesiculoerosive inflammatory disease, such as erosive lichen planus. This should not be confused with the brown-black gingival melanin pigmentation—diffuse or speckled—common in nonwhites, blue-black embedded fragments of dental amalgam, or other systemic disorders associated with general pigmentation (neurofibromatosis, familial polyposis, Addison disease). Intraoral melanoma is extremely rare and carries a dismal prognosis.
Any area of erythroplakia, enlarging area of leukoplakia, or a lesion that has submucosal depth on palpation should have an incisional biopsy or an exfoliative cytologic examination. Ulcerative lesions are particularly suspicious and worrisome. Specialty referral should be sought early both for diagnosis and treatment. A systematic intraoral examination—including the lateral tongue, floor of the mouth, gingiva, buccal area, palate, and tonsillar fossae—and palpation of the neck for enlarged lymph nodes should be part of any general physical examination, especially in patients over the age of 45 who smoke tobacco or drink immoderately. Indirect or fiberoptic examination of the nasopharynx, oropharynx, hypopharynx, and larynx by an otolaryngologist, head and neck surgeon, or radiation oncologist should also be considered for such patients when there is unexplained or persistent throat or ear pain, oral or nasal bleeding, or oral erythroplakia. Fine-needle aspiration (FNA) biopsy may expedite the diagnosis if an enlarged lymph node is found.
To date, there remain no approved therapies for reversing or stabilizing leukoplakia or erythroplakia. Clinical trials have suggested a role for beta-carotene, celecoxib, vitamin E, and retinoids in producing regression of leukoplakia and reducing the incidence of recurrent squamous cell carcinomas. None have demonstrated benefit in large studies and these agents are not in general use today. The mainstays of management are surveillance following elimination of carcinogenic irritants (eg, smoking tobacco, chewing tobacco or betel nut, drinking alcohol) along with serial biopsies and excisions.
Oral lichen planus is a relatively common (0.5–2% of the population) chronic inflammatory autoimmune disease that may be difficult to diagnose clinically because of its numerous distinct phenotypic subtypes. For example, the reticular pattern may mimic candidiasis or hyperkeratosis, while the erosive pattern may mimic squamous cell carcinoma. Management begins with distinguishing it from other oral lesions. Exfoliative cytology or a small incisional or excisional biopsy is indicated, especially if squamous cell carcinoma is suspected. Therapy of lichen planus is aimed at managing pain and discomfort. Daily topical corticosteroid remains the most effective treatment for symptomatic lichen planus, but cyclosporines, retinoids, and tacrolimus have also been used. Many experts think there is a low rate (1%) of squamous cell carcinoma arising within lichen planus (in addition to the possibility of clinical misdiagnosis) and prevention of malignant transformation remains a goal of treatment. Photodynamic therapy is being studied as an approach to both treatment of symptomatic lichen planus as well as prevention of malignant transformation, but there is no compelling evidence to argue for widespread application of this technique at this time.
Hairy leukoplakia occurs on the lateral border of the tongue and is a common early finding in HIV infection (see Chapter 31). It often develops quickly and appears as slightly raised leukoplakic areas with a corrugated or “hairy” surface (Figure 8–6). While much more prevalent in HIV-positive patients, hairy leukoplakia can occur following solid organ transplantation and is associated with Epstein-Barr virus infection and long-term systemic corticosteroid use. Hairy leukoplakia waxes and wanes over time with generally modest irritative symptoms. Acyclovir, valacyclovir, and famciclovir have all been used for treatment but produce only temporary resolution of the condition. It does not appear to predispose to malignant transformation.
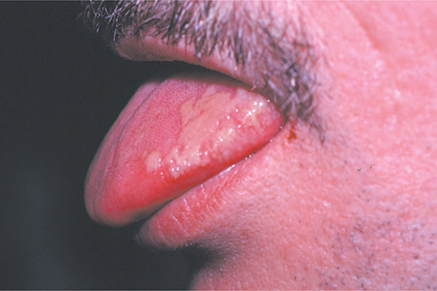
Figure 8–6. Oral hairy leukoplakia on the side of the tongue in AIDS. (Used, with permission, from Richard P. Usatine, MD, in Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H, Tysinger J. The Color Atlas of Family Medicine. McGraw-Hill, 2009.)
Oral cavity squamous cell carcinoma can be hard to distinguish from other oral lesions, but early detection is the key to successful management (Figure 8–7). Raised, firm, white lesions with ulcers at the base are highly suspicious and generally quite painful on even gentle palpation. Lesions less than 4 mm in depth have a low propensity to metastasize. Most patients in whom the tumor is detected before it is 2 cm in diameter are cured by local resection. Radiation is reserved for patients with positive margins or metastatic disease. Large tumors are usually treated with a combination of resection, neck dissection, and external beam radiation. Reconstruction, if required, is done at the time of resection and can involve the use of myocutaneous flaps or vascularized free flaps with or without bone.
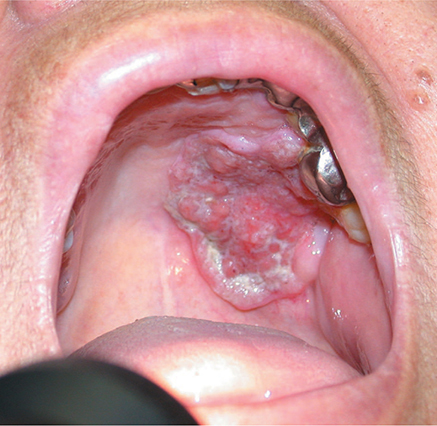
Figure 8–7. Squamous cell carcinoma of the palate. (Used, with permission, from Frank Miller, MD, in Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H, Tysinger J. The Color Atlas of Family Medicine. McGraw-Hill, 2009.)
Oropharyngeal squamous cell carcinoma generally presents later than oral cavity squamous cell carcinoma. The lesions tend to be larger and are often buried within the lymphoid tissue of the palatine or lingual tonsils. Most patients note only unilateral odynophagia and weight loss, but ipsilateral cervical lymphadenopathy is often identified by the careful clinician. While these tumors are typically associated with known carcinogens such as tobacco and alcohol, their epidemiology has changed dramatically over the past 20 years. Despite demonstrated reductions in tobacco and alcohol use within developed nations, the incidence of oropharyngeal squamous cell carcinoma has not declined over this period. Known as a possible cause of head and neck cancer since 1983, the human papillomavirus (HPV)—most commonly, type 16—is now believed to be the cause of up to 70% of all oropharyngeal squamous cell carcinoma. HPV-positive tumors are readily distinguished by immunostaining of primary tumor or fine-needle aspiration biopsy specimens for the p16 protein, a tumor suppressor protein that is highly correlated with the presence of HPV. These tumors often present in advanced stages of the disease with regional cervical lymph node metastases (stages III and IV), but have a better prognosis than similarly staged lesions in tobacco and alcohol users. This difference in disease control is so apparent in multicenter studies that, based on the presence or absence of the p16 protein, two distinct staging systems for oropharyngeal squamous cell carcinoma were introduced in 2018. Ongoing clinical trials are trying to determine if a reduction in treatment intensity is warranted for HPV-associated cancers.
Awadallah M et al. Management update of potentially premalignant oral epithelial lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018 Jun;125(6):628–36. [PMID: 29656948]
Chera BS et al. Current status and future directions of treatment deintensification in human papilloma virus–associated oropharyngeal squamous cell carcinoma. Semin Radiat Oncol. 2018 Jan;28(1):27–34. [PMID: 29173753]
Gupta S et al. Interventions for the management of oral lichen planus: a review of the conventional and novel therapies. Oral Dis. 2017 Nov;23(8):1029–42. [PMID: 28055124]
Huang SH et al. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017 Jul;18(7):40. [PMID: 28555375]
Mello FW et al. Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med. 2018 Aug;47(7):633–40. [PMID: 29738071]
ORAL CANDIDIASIS
ESSENTIALS OF DIAGNOSIS

 Fluctuating throat or mouth discomfort.
Fluctuating throat or mouth discomfort.
 Systemic or local immunosuppression, such as recent corticosteroid, chemotherapy, or antibiotic use.
Systemic or local immunosuppression, such as recent corticosteroid, chemotherapy, or antibiotic use.
 Erythema of the oral cavity or oropharynx with creamy-white, curd-like patches.
Erythema of the oral cavity or oropharynx with creamy-white, curd-like patches.
 Rapid resolution of symptoms with appropriate treatment.
Rapid resolution of symptoms with appropriate treatment.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Oral candidiasis (thrush) is usually painful and looks like creamy-white curd-like patches overlying erythematous mucosa (see Figure 6–22). Because these white areas are easily rubbed off (eg, by a tongue depressor)—unlike leukoplakia or lichen planus—only the underlying irregular erythema may be seen. Oral candidiasis is commonly associated with the following risk factors: (1) use of dentures, (2) debilitated state with poor oral hygiene, (3) diabetes mellitus, (4) anemia, (5) chemotherapy or local irradiation, (6) corticosteroid use (oral or systemic), or (7) broad-spectrum antibiotics. Another manifestation of candidiasis is angular cheilitis (also seen in nutritional deficiencies) (Figure 8–8).
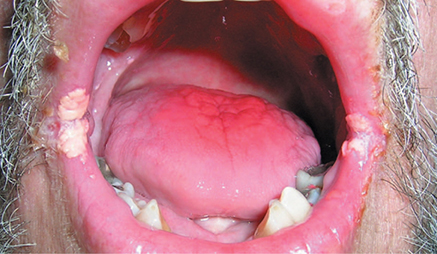
Figure 8–8. Severe angular cheilitis in HIV-positive man with oral thrush. (Used, with permission, from Richard P. Usatine, MD, in Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H, Tysinger J. The Color Atlas of Family Medicine. McGraw-Hill, 2009.)
B. Diagnostic Studies
The diagnosis is made clinically. A wet preparation using potassium hydroxide will reveal spores and may show nonseptate mycelia. Biopsy will show intraepithelial pseudomycelia of Candida albicans.
Candidiasis is often the first manifestation of HIV infection, and HIV testing should be considered in patients with no known predisposing cause for Candida overgrowth (see also Chapter 31). The US Department of Health Services Clinical Practice Guideline for Evaluation and Management of Early HIV Infection recommends examination of the oral mucosa with each clinician visit as well as at a dental examination every 6 months for individuals infected with HIV.
 Treatment
Treatment
Effective antifungal therapy may be achieved with any of the following: fluconazole (100 mg orally daily for 7 days), ketoconazole (200–400 mg orally with breakfast [requires acidic gastric environment for absorption] for 7–14 days), clotrimazole troches (10 mg dissolved orally five times daily), or nystatin mouth rinses (500,000 units [5 mL of 100,000 units/mL] held in the mouth before swallowing three times daily). In patients with HIV infection, however, longer courses of therapy with fluconazole may be needed, and oral itraconazole (200 mg/day) may be indicated in fluconazole-refractory cases. Many of the Candida species in these patients are resistant to first-line azoles and may require newer drugs, such as voriconazole. In addition, 0.12% chlorhexidine or half-strength hydrogen peroxide mouth rinses may provide local relief. Nystatin powder (100,000 units/g) applied to dentures three or four times daily and rinsed off for several weeks may help denture wearers.
Lewis MAO et al. Diagnosis and management of oral candidosis. Br Dent J. 2017 Nov 10;223(9):675–81. [PMID: 29123282]
GLOSSITIS, GLOSSODYNIA, DYSGEUSIA, & BURNING MOUTH SYNDROME
Inflammation of the tongue with loss of filiform papillae leads to a red, smooth-surfaced tongue (glossitis). Rarely painful, it may be secondary to nutritional deficiencies (eg, niacin, riboflavin, iron, or vitamin E), drug reactions, dehydration, irritants, or foods and liquids, and possibly to autoimmune reactions or psoriasis. If the primary cause cannot be identified and corrected, empiric nutritional replacement therapy may be of value.
Glossodynia is burning and pain of the tongue, which may occur with or without glossitis. In the absence of any clinical findings, it has been termed “burning mouth syndrome.” Glossodynia with glossitis has been associated with diabetes mellitus, drugs (eg, diuretics), tobacco, xerostomia, and candidiasis as well as the listed causes of glossitis. The burning mouth syndrome typically has no identifiable associated risk factors and seems to be most common in postmenopausal women. Treating possible underlying causes, changing long-term medications to alternative ones, and smoking cessation may resolve symptoms of glossitis. Effective treatments for the burning mouth syndrome include alpha-lipoic acid and clonazepam. Clonazepam is most effective as a rapid-dissolving tablet placed on the tongue in doses from 0.25 mg to 0.5 mg every 8–12 hours. Both glossodynia and the burning mouth syndrome are benign, and reassurance that there is no infection or tumor is likely to be appreciated. Unilateral symptoms, symptoms that cannot be related to a specific medication, and symptoms and signs involving regions supplied by other cranial nerves all may suggest neuropathology, and imaging of the brain, brainstem, and skull base with MRI should be considered.
de Campos WG et al. Treatment of symptomatic benign migratory glossitis: a systematic review. Clin Oral Investig. 2018 Sep;22(7):2487–93. [PMID: 29982968]
Liu YF et al. Burning mouth syndrome: a systematic review of treatments. Oral Dis. 2018 Apr;24(3):325–34. [PMID: 28247977]
INTRAORAL ULCERATIVE LESIONS
1. Necrotizing Ulcerative Gingivitis (Trench Mouth, Vincent Angina)
Necrotizing ulcerative gingivitis, often caused by an infection with both spirochetes and fusiform bacilli, is common in young adults under stress (classically in students at examination time). Underlying systemic diseases may also predispose to this disorder. Clinically, there is painful acute gingival inflammation and necrosis, often with bleeding, halitosis, fever, and cervical lymphadenopathy. Warm half-strength peroxide rinses and oral penicillin (250 mg three times daily for 10 days) may help. Dental gingival curettage may prove necessary.
Dufty J et al. Necrotising ulcerative gingivitis: a literature review. Oral Health Prev Dent. 2017;15(4):321–7. [PMID: 28761942]
2. Aphthous Ulcer (Canker Sore, Ulcerative Stomatitis)
Aphthous ulcers are very common and easy to recognize. Their cause remains uncertain, although an association with human herpesvirus 6 has been suggested. Found on freely moving, nonkeratinized mucosa (eg, buccal and labial mucosa and not attached gingiva or palate), they may be single or multiple, are usually recurrent, and appear as painful small round ulcerations with yellow-gray fibrinoid centers surrounded by red halos. Minor aphthous ulcers are less than 1 cm in diameter and generally heal in 10–14 days. Major aphthous ulcers are greater than 1 cm in diameter and can be disabling due to the degree of associated oral pain. Stress seems to be a major predisposing factor to the eruptions of aphthous ulcers. A study found that the frequency of viral rhinitis and bedtime after 11 pm were independent predictors of aphthous ulcer frequency and severity in college students.
Treatment is challenging because no single systemic treatment has proven effective. Topical corticosteroids (triamcinolone acetonide, 0.1%, or fluocinonide ointment, 0.05%) in an adhesive base (Orabase Plain) do appear to provide symptomatic relief in many patients. Other topical therapies shown to be effective in controlled studies include diclofenac 3% in hyaluronan 2.5%, doxymycine-cyanoacrylate, mouthwashes containing the enzymes amyloglucosidase and glucose oxidase, and amlexanox 5% oral paste. A 1-week tapering course of prednisone (40–60 mg/day) has also been used successfully. Cimetidine maintenance therapy may be useful in patients with recurrent aphthous ulcers. Thalidomide has been used selectively in recurrent aphthous ulcerations in HIV-positive patients.
Large or persistent areas of ulcerative stomatitis may be secondary to erythema multiforme or drug allergies, acute herpes simplex, pemphigus, pemphigoid, epidermolysis bullosa acquisita, bullous lichen planus, Behçet disease, or inflammatory bowel disease. Squamous cell carcinoma may occasionally present in this fashion. When the diagnosis is not clear, incisional biopsy is indicated.
Fitzpatrick SG et al. Ulcerated lesions of the oral mucosa: clinical and histologic review. Head Neck Pathol. 2019 Mar;13(1):91–102. [PMID: 30701449]
Saikaly SK et al. Recurrent aphthous ulceration: a review of potential causes and novel treatments. J Dermatolog Treat. 2018 Sep;29(6):542–52. [PMID: 29278022]
3. Herpes Stomatitis
Herpes gingivostomatitis is common, mild, and short-lived and requires no intervention in most adults. In immunocompromised persons, however, reactivation of herpes simplex virus infection is frequent and may be severe. Clinically, there is initial burning, followed by typical small vesicles that rupture and form scabs. Lesions are most commonly found on the attached gingiva and mucocutaneous junction of the lip, but lesions can also form on the tongue, buccal mucosa, and soft palate. Acyclovir (200–800 mg orally five times daily for 7–10 days) or valacyclovir (1000 mg orally twice daily for 7–10 days) may shorten the course and reduce postherpetic pain. These treatments may be effective only when started within 24–48 hours of the onset of initial symptoms (pain, itching, burning) and are not effective once vesicles have erupted. Differential diagnosis includes aphthous stomatitis, erythema multiforme, syphilitic chancre, and carcinoma.
Mazzarello V et al. Do sunscreen prevent recurrent herpes labialis in summer? J Dermatolog Treat. 2019 Mar;30(2):179–82. [PMID: 29804485]
PHARYNGITIS & TONSILLITIS
ESSENTIALS OF DIAGNOSIS

 Sore throat.
Sore throat.
 Fever.
Fever.
 Anterior cervical adenopathy.
Anterior cervical adenopathy.
 Tonsillar exudate.
Tonsillar exudate.
 Focus is to treat group A beta-hemolytic Streptococcus infection to prevent rheumatic sequelae.
Focus is to treat group A beta-hemolytic Streptococcus infection to prevent rheumatic sequelae.
 General Considerations
General Considerations
Pharyngitis and tonsillitis account for over 10% of all office visits to primary care clinicians and 50% of outpatient antibiotic use. The main concern is determining who is likely to have a group A beta-hemolytic streptococcal (GABHS) infection, since this can lead to subsequent complications, such as rheumatic fever and glomerulonephritis. A second public health policy concern is reducing the extraordinary cost (both in dollars and in the development of antibiotic-resistant S pneumoniae) in the United States associated with unnecessary antibiotic use. Questions being asked include: Have the rapid antigen tests supplanted the need to culture a throat under most circumstances? Are clinical criteria alone a sufficient basis for decisions about which patients should be given antibiotics? Should any patient receive any antibiotic other than penicillin (or erythromycin if penicillin-allergic)? For how long should treatment be continued? Numerous well-done studies and experience with rapid laboratory tests for detection of streptococci (eliminating the delay caused by culturing) informed a consensus experience.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The clinical features most suggestive of GABHS pharyngitis include fever over 38°C, tender anterior cervical adenopathy, lack of a cough, and pharyngotonsillar exudate (Figure 8–9). These four features (the Centor criteria), when present, strongly suggest GABHS. When two or three of the four are present, there is an intermediate likelihood of GABHS. When only one criterion is present, GABHS is unlikely. Sore throat may be severe, with odynophagia, tender adenopathy, and a scarlatiniform rash. An elevated white count and left shift are also possible. Hoarseness, cough, and coryza are not suggestive of this disease.
Figure 8–9. Streptococcal pharyngitis showing tonsillar exudate and erythema. (From Michael Nguyen, MD; reproduced, with permission, from Usatine RP, Smith MA, Mayeaux EJ Jr, Chumley H, Tysinger J. The Color Atlas of Family Medicine. McGraw-Hill, 2009.)
Marked lymphadenopathy and a shaggy, white-purple tonsillar exudate, often extending into the nasopharynx, suggest mononucleosis, especially if present in a young adult. With about 90% sensitivity, lymphocyte-to-white-blood-cell ratios of greater than 35% suggest EBV infection and not tonsillitis. Hepatosplenomegaly and a positive heterophile agglutination test or elevated anti-EBV titer are corroborative. However, about one-third of patients with infectious mononucleosis have secondary streptococcal tonsillitis, requiring treatment. Ampicillin should routinely be avoided if mononucleosis is suspected because it induces a rash that might be misinterpreted by the patient as a penicillin allergy. Diphtheria (extremely rare but described in the alcoholic population) presents with low-grade fever and an ill patient with a gray tonsillar pseudomembrane.
The most common pathogens other than GABHS in the differential diagnosis of “sore throat” are viruses, Neisseria gonorrhoeae, Mycoplasma, and Chlamydia trachomatis. Rhinorrhea and lack of exudate would suggest a virus, but in practice it is not possible to confidently distinguish viral upper respiratory infection from GABHS on clinical grounds alone. Infections with Corynebacterium diphtheria, anaerobic streptococci, and Corynebacterium haemolyticum (which responds better to erythromycin than penicillin) may also mimic pharyngitis due to GABHS.
B. Laboratory Findings
A single-swab throat culture is 90–95% sensitive and the rapid antigen detection testing (RADT) is 90–99% sensitive for GABHS. Results from the RADT are available in about 15 minutes.
 Treatment
Treatment
The Infectious Diseases Society of America recommends laboratory confirmation of the clinical diagnosis by means of either throat culture or RADT of the throat swab. The American College of Physicians–American Society of Internal Medicine (ACP-ASIM), in collaboration with the Centers for Disease Control and Prevention, advocates use of a clinical algorithm alone—in lieu of microbiologic testing—for confirmation of the diagnosis in adults for whom the suspicion of streptococcal infection is high. Others examine the assumptions of the ACP-ASIM guideline for using a clinical algorithm alone and question whether those recommendations will achieve the stated objective of dramatically decreasing excess antibiotic use. A reasonable strategy to follow is that patients with zero or one Centor criteria are at very low risk for GABHS and therefore do not need throat cultures or RADT of the throat swab and should not receive antibiotics. Patients with two or three Centor criteria need throat cultures or RADT of the throat swab, since positive results would warrant antibiotic treatment. Patients who have four Centor criteria are likely to have GABHS and can receive empiric therapy without throat culture or RADT.
A single intramuscular injection of benzathine penicillin or procaine penicillin, 1.2 million units is an effective antibiotic treatment, but the injection is painful. It is now used for patients if compliance with an oral regimen is an issue. Currently, oral treatment is effective and preferred. Penicillin V potassium (250 mg orally three times daily or 500 mg twice daily for 10 days) or cefuroxime axetil (250 mg orally twice daily for 5–10 days) are both effective. The efficacy of a 5-day regimen of penicillin V potassium appears to be similar to that of a 10-day course, with a 94% clinical response rate and an 84% streptococcal eradication rate. Erythromycin (also active against Mycoplasma and Chlamydia) is a reasonable alternative to penicillin in allergic patients. Cephalosporins are somewhat more effective than penicillin in producing bacteriologic cures; 5-day courses of cefpodoxime and cefuroxime have been successful. The macrolide antibiotics have also been reported to be successful in shorter-duration regimens. Azithromycin (500 mg once daily), because of its long half-life, needs to be taken for only 3 days.
Adequate antibiotic treatment usually avoids the streptococcal complications of scarlet fever, rheumatic myocarditis, glomerulonephritis, and local abscess formation.
Antibiotics for treatment failures are also somewhat controversial. Surprisingly, penicillin-tolerant strains are not isolated more frequently in those who fail treatment than in those treated successfully with penicillin. The reasons for failure appear to be complex, and a second course of treatment with the same drug is reasonable. Alternatives to penicillin include cefuroxime and other cephalosporins, dicloxacillin (which is beta-lactamase–resistant), and amoxicillin with clavulanate. When there is a history of penicillin allergy, alternatives should be used, such as erythromycin. Erythromycin resistance—with failure rates of about 25%—is an increasing problem in many areas. In cases of severe penicillin allergy, cephalosporins should be avoided since cross-reaction occurs in greater than 8% of cases.
Ancillary treatment of pharyngitis includes analgesics and anti-inflammatory agents, such as aspirin, acetaminophen, and corticosteroids. In meta-analysis, corticosteroids increased the likelihood of complete pain resolution at 24 hours by threefold without an increase in recurrence or adverse events. Some patients find that salt water gargling is soothing. In severe cases, anesthetic gargles and lozenges (eg, benzocaine) may provide additional symptomatic relief. Occasionally, odynophagia is so intense that hospitalization for intravenous hydration and antibiotics is necessary. (See Chapter 33.)
Patients who have had rheumatic fever should be treated with a continuous course of antimicrobial prophylaxis (penicillin G, 500 mg once daily orally, or erythromycin, 250 mg twice daily orally) for at least 5 years.
Berkley J. Management of pharyngitis. Circulation. 2018 Oct 30;138(18):1920–2. [PMID: 30372134]
Brook I. Treatment challenges of group A beta-hemolytic streptococcal pharyngo-tonsillitis. Int Arch Otorhinolaryngol. 2017 Jul;21(3):286–96. [PMID: 28680500]
Klein MR. Infections of the oropharynx. Emerg Med Clin North Am. 2019 Feb;37(1):69–80. [PMID: 30454781]
Munck H et al. Antibiotics for recurrent acute pharyngo-tonsillitis: systematic review. Eur J Clin Microbiol Infect Dis. 2018 Jul;37(7):1221–30. [PMID: 29651614]
PERITONSILLAR ABSCESS & CELLULITIS
When infection penetrates the tonsillar capsule and involves the surrounding tissues, peritonsillar cellulitis results. Peritonsillar abscess (quinsy) and cellulitis present with severe sore throat, odynophagia, trismus, medial deviation of the soft palate and peritonsillar fold, and an abnormal muffled (“hot potato”) voice. Following therapy, peritonsillar cellulitis usually either resolves over several days or evolves into peritonsillar abscess. Ultrasound may be a useful adjunct to clinical suspicion, but imaging is not required for the diagnosis. The existence of an abscess may be confirmed by aspirating pus from the peritonsillar fold just superior and medial to the upper pole of the tonsil. A 19-gauge or 21-gauge needle should be passed medial to the molar and no deeper than 1 cm, because the internal carotid artery may lie more medially than its usual location and pass posterior and deep to the tonsillar fossa. Most commonly, patients with peritonsillar abscess present to the emergency department and receive a dose of parenteral amoxicillin (1 g), amoxicillin-sulbactam (3 g), or clindamycin (600–900 mg). Less severe cases and patients who are able to tolerate oral intake may be treated for 7–10 days with oral antibiotics, including amoxicillin, 500 mg three times a day; amoxicillin-clavulanate, 875 mg twice a day; or clindamycin, 300 mg four times daily. Although antibiotic treatment is generally undisputed, there is controversy regarding the surgical management of peritonsillar abscess. Methods include needle aspiration, incision and drainage, and tonsillectomy. Some clinicians incise and drain the area and continue with parenteral antibiotics, whereas others aspirate only and monitor as an outpatient. To drain the abscess and avoid recurrence, it may be appropriate to consider immediate tonsillectomy (quinsy tonsillectomy). About 10% of patients with peritonsillar abscess exhibit relative indications for tonsillectomy. All three approaches are effective. Regardless of the method used, one must be sure the abscess is adequately treated, since complications such as extension to the retropharyngeal, deep neck, and posterior mediastinal spaces are possible. Bacteria may also be aspirated into the lungs, resulting in pneumonia. There is controversy about whether a single abscess is a sufficient indication for tonsillectomy; about 30% of patients aged 17–30 who do not undergo early planned tonsillectomy following peritonsillar abscess ultimately undergo surgery, and only about 13% of those over 30 have their tonsils removed.
Klein MR. Infections of the oropharynx. Emerg Med Clin North Am. 2019 Feb;37(1):69–80. [PMID: 30454781]
Luo MS et al. Needle aspiration versus incision and drainage under local anaesthesia for the treatment of peritonsillar abscess. Eur Arch Otorhinolaryngol. 2020 Feb;277(2):645–6. [PMID: 31555918]
DEEP NECK INFECTIONS
ESSENTIALS OF DIAGNOSIS

 Marked acute neck pain and swelling.
Marked acute neck pain and swelling.
 Abscesses are emergencies because rapid airway compromise may occur.
Abscesses are emergencies because rapid airway compromise may occur.
 May spread to the mediastinum or cause sepsis.
May spread to the mediastinum or cause sepsis.
 General Considerations
General Considerations
Ludwig angina is the most commonly encountered neck space infection. It is a cellulitis of the sublingual and submaxillary spaces, often arising from infection of the mandibular dentition. Deep neck abscesses most commonly originate from odontogenic infections. Other causes include suppurative lymphadenitis, direct spread of pharyngeal infection, penetrating trauma, pharyngoesophageal foreign bodies, cervical osteomyelitis, and intravenous injection of the internal jugular vein, especially in drug abusers. Recurrent deep neck infection may suggest an underlying congenital lesion, such as a branchial cleft cyst. Suppurative lymphadenopathy in middle-aged persons who smoke and drink alcohol regularly should be considered a manifestation of malignancy (typically metastatic squamous cell carcinoma) until proven otherwise.
 Clinical Findings
Clinical Findings
Patients with Ludwig angina have edema and erythema of the upper neck under the chin and often of the floor of the mouth. The tongue may be displaced upward and backward by the posterior spread of cellulitis, and coalescence of pus is often present in the floor of mouth. This may lead to occlusion of the airway. Microbiologic isolates include streptococci, staphylococci, Bacteroides, and Fusobacterium. Patients with diabetes may have different flora, including Klebsiella, and a more aggressive clinical course.
Patients with deep neck abscesses usually present with marked neck pain and swelling. Fever is common but not always present. Deep neck abscesses are emergencies because they may rapidly compromise the airway. Untreated or inadequately treated, they may spread to the mediastinum or cause sepsis.
Contrast-enhanced CT usually augments the clinical examination in defining the extent of the infection. It often will distinguish inflammation and phlegmon (requiring antibiotics) from abscess (requiring drainage) and define for the surgeon the extent of an abscess. CT with MRI may also identify thrombophlebitis of the internal jugular vein secondary to oropharyngeal inflammation. This condition, known as Lemierre syndrome, is rare and usually associated with severe headache. The presence of pulmonary infiltrates consistent with septic emboli in the setting of a neck abscess should lead one to suspect Lemierre syndrome or injection drug use, or both.
 Treatment
Treatment
Usual doses of penicillin plus metronidazole, ampicillin-sulbactam, clindamycin, or selective cephalosporins are good initial choices for treatment of Ludwig angina. Culture and sensitivity data are then used to refine the choice. Dental consultation is advisable to address the offending tooth or teeth. External drainage via bilateral submental incisions is required if the airway is threatened or when medical therapy has not reversed the process.
Treatment of deep neck abscesses includes securing the airway, intravenous antibiotics, and incision and drainage. When the infection involves the floor of the mouth, base of the tongue, or the supraglottic or paraglottic space, the airway may be secured either by intubation or tracheotomy. Tracheotomy is preferable in the patients with substantial pharyngeal edema, since attempts at intubation may precipitate acute airway obstruction. Bleeding in association with a deep neck abscess is very rare but suggests carotid artery or internal jugular vein involvement and requires prompt neck exploration both for drainage of pus and for vascular control.
Patients with Lemierre syndrome require prompt institution of antibiotics appropriate for Fusobacterium necrophorum as well as the more usual upper airway pathogens. The use of anticoagulation in treatment is of no proven benefit.
Brito TP et al. Deep neck abscesses: study of 101 cases. Braz J Otorhinolaryngol. 2017 May–Jun;83(3):341–8. [PMID: 27236632]
Li RM et al. Infections of the neck. Emerg Med Clin North Am. 2019 Feb;37(1):95–107. [PMID: 30454783]
Mejzlik J et al. Univariate and multivariate models for the prediction of life-threatening complications in 586 cases of deep neck space infections: retrospective multi-institutional study. J Laryngol Otol. 2017 Sep;131(9):779–84. [PMID: 28578716]
SNORING
ESSENTIALS OF DIAGNOSIS

 Noise produced on inspiration during sleep.
Noise produced on inspiration during sleep.
 Snoring is associated with obstructive sleep apnea (OSA) but has no disruption of sleep on clinical sleep evaluation.
Snoring is associated with obstructive sleep apnea (OSA) but has no disruption of sleep on clinical sleep evaluation.
 General Considerations
General Considerations
Ventilation disorders during sleep are extremely common. While OSA occurs in 5–10% of Americans, clinically relevant snoring may occur in as many as 59%. In general, sleep-disordered breathing problems are attributed to narrowing of the upper aerodigestive tract during sleep due to changes in position, muscle tone, and soft tissue hypertrophy or laxity. The most common sites of obstruction are the oropharynx and the base of the tongue. The spectrum of the problem ranges from simple snoring without cessation of airflow to OSA with long periods of apnea and life-threatening physiologic sequelae. OSA is discussed in Chapter 9. In contrast to OSA, snoring is almost exclusively a social problem, and despite its prevalence and association with OSA, there is comparatively little known about the management of this problem.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
All patients who complain of snoring should be evaluated for OSA as discussed in Chapter 9. Symptoms of OSA (including snoring, excessive daytime somnolence, daytime headaches, and weight gain) may be present in as many as 30% of patients without demonstrable apnea or hypopnea on formal testing. Clinical examination should include examination of the nasal cavity, nasopharynx, oropharynx, and larynx to help exclude other causes of dynamic airway obstruction. In many cases of isolated snoring, the palate and uvula appear enlarged and elongated with excessive mucosa hanging below the muscular portion of the soft palate.
B. Imaging and Diagnostic Testing
Sleep examination with polysomnography is strongly advised in the evaluation of a patient with complaints of snoring. Radiographic imaging of the head or neck is generally not necessary.
 Treatment
Treatment
Expeditious and inexpensive management solutions of snoring are sought, often with little or no benefit. Diet modification and physical exercise can lead to improvement in snoring through the weight loss and improvement in pharyngeal tone that accompanies overall physical conditioning. Position change during sleep can be effective, and time-honored treatments, such as placing a golf or tennis ball into a pocket sewn on the back of the pajama top worn during sleep, may satisfactorily eliminate symptoms by ensuring recumbency on one side. Although numerous pharmacologic therapies have been endorsed, none demonstrate any significant utility when scrutinized.
Anatomic management of snoring can be challenging. As with OSA, snoring can come from a number of sites in the upper aerodigestive tract. While medical or surgical correction of nasal obstruction may help alleviate snoring problems, most interventions aim to improve airflow through the nasopharynx and oropharynx. Nonsurgical options include mandibular advancement appliances designed to pull the base of the tongue forward and continuous positive airway pressure via face or nasal mask. Compliance with both of these treatment options is problematic because snorers without OSA do not notice the physiologic benefits of these devices noted by patients with sleep apnea.
Surgical correction of snoring is most commonly directed at the soft palate. Historical approaches involved resection of redundant mucosa and the uvula similar to uvulopalatopharyngoplasty that is used for OSA. Regardless of how limited the procedure or what technique was used, the postoperative pain, expense of general anesthesia, and high recurrence rates limit the utility of these procedures. Office-based approaches are more widely used because of these limitations. Most of these procedures aim to stiffen the palate to prevent vibration rather than remove it. A series of procedures, including injection snoreplasty, radiofrequency thermal fibrosis, and an implantable palatal device, have been used with variable success and patient tolerance. The techniques can be technically challenging. Persistent symptoms may occur following initial treatment necessitating costly (and sometimes painful) repeat procedures. The durability of these procedures in alleviating symptoms is also poorly understood, and late failures can lead to patient and clinician frustration.
De Meyer MMD et al. Systematic review of the different aspects of primary snoring. Sleep Med Rev. 2019 Jun;45:88–94. [PMID: 30978609]
Demko BG. The evolution of oral appliance therapy for snoring and sleep apnea: where did we come from, where are we, and where are we going? Sleep Med Clin. 2018 Dec;13(4):467–87. [PMID: 30396442]
DISEASES OF THE SALIVARY GLANDS
ACUTE INFLAMMATORY SALIVARY GLAND DISORDERS
1. Sialadenitis
Acute bacterial sialadenitis most commonly affects either the parotid or submandibular gland. It typically presents with acute swelling of the gland, increased pain and swelling with meals, and tenderness and erythema of the duct opening. Pus often can be massaged from the duct. Sialadenitis often occurs in the setting of dehydration or in association with chronic illness. Underlying Sjögren syndrome and chronic periodontitis may contribute. Ductal obstruction, often by an inspissated mucous plug, is followed by salivary stasis and secondary infection. The most common organism recovered from purulent draining saliva is S aureus. Treatment consists of intravenous antibiotics, such as nafcillin (1 g intravenously every 4–6 hours), and measures to increase salivary flow, including hydration, warm compresses, sialagogues (eg, lemon drops), and massage of the gland. Treatment can usually then be switched to an oral agent based on clinical improvement and microbiologic results to complete a 10-day treatment course. Less severe cases can often be treated with oral antibiotics with similar spectrum. Complete resolution of parotid swelling and pain can take 2–3 weeks. Failure of the process to improve and ultimately resolve on this regimen suggests abscess formation, ductal stricture, stone, or tumor causing obstruction. Ultrasound or CT scan may be helpful in establishing the diagnosis. In the setting of acute illness, a severe and potentially life-threatening form of sialadenitis, sometimes called suppurative sialadenitis, may develop. The causative organism is usually S aureus, but often no pus will drain from Stensen papilla. These patients often do not respond to rehydration and intravenous antibiotics and thus may require operative incision and drainage to resolve the infection.
2. Sialolithiasis
Calculus formation is more common in the Wharton duct (draining the submandibular glands) than in the Stensen duct (draining the parotid glands). Clinically, a patient may note postprandial pain and local swelling, often with a history of recurrent acute sialadenitis. Stones in the Wharton duct are usually large and radiopaque, whereas those in the Stensen duct are usually radiolucent and smaller. Those very close to the orifice of the Wharton duct may be palpated manually in the anterior floor of the mouth and removed intraorally by dilating or incising the distal duct. Those more than 1.5–2 cm from the duct are too close to the lingual nerve to be removed safely in this manner. Similarly, dilation of the Stensen duct, located on the buccal surface opposite the second maxillary molar, may relieve distal stricture or allow a small stone to pass. Sialoendoscopy for the management of chronic sialolithiasis is superior to extracorporeal shock-wave lithotripsy and fluoroscopically guided basket retrieval. Repeated episodes of sialadenitis are usually associated with stricture and chronic infection. If the obstruction cannot be safely removed or dilated, excision of the gland may be necessary to relieve recurrent symptoms.
Delagnes EA et al. Sialadenitis without sialolithiasis: prospective outcomes after sialendoscopy-assisted salivary duct surgery. Laryngoscope. 2017 May;127(5):1073–9. [PMID: 27701754]
Kessler AT et al. Review of the major and minor salivary glands, Part 1: anatomy, infectious, and inflammatory processes. J Clin Imaging Sci. 2018 Nov 15;8:47. [PMID: 30546931]
Xiao JQ et al. Evaluation of sialendoscopy-assisted treatment of submandibular gland stones. J Oral Maxillofac Surg. 2017 Feb;75(2):309–16. [PMID: 27663537]
CHRONIC INFLAMMATORY & INFILTRATIVE DISORDERS OF THE SALIVARY GLANDS
Numerous infiltrative disorders may cause unilateral or bilateral parotid gland enlargement. Sjögren syndrome and sarcoidosis are examples of lymphoepithelial and granulomatous diseases that may affect the salivary glands. Metabolic disorders, including alcoholism, diabetes mellitus, and vitamin deficiencies, may also cause diffuse enlargement. Several drugs have been associated with parotid enlargement, including thioureas, iodine, and drugs with cholinergic effects (eg, phenothiazines), which stimulate salivary flow and cause more viscous saliva.
SALIVARY GLAND TUMORS
Approximately 80% of salivary gland tumors occur in the parotid gland. In adults, about 80% of these are benign. In the submandibular triangle, it is sometimes difficult to distinguish a primary submandibular gland tumor from a metastatic submandibular space node. Only 50–60% of primary submandibular tumors are benign. Tumors of the minor salivary glands are most likely to be malignant, with adenoid cystic carcinoma predominating, and may be found throughout the oral cavity or oropharynx.
Most parotid tumors present as an asymptomatic mass in the superficial part of the gland. Their presence may have been noted by the patient for months or years. Facial nerve involvement correlates strongly with malignancy. Tumors may extend deep to the plane of the facial nerve or may originate in the parapharyngeal space. In such cases, medial deviation of the soft palate is visible on intraoral examination. MRI and CT scans have largely replaced sialography in defining the extent of tumor. At least one new study demonstrates the potential benefit of enhanced MRI imaging in distinguishing among Warthin tumors and pleomorphic adenomas and malignant salivary gland tumors.
When the clinician encounters a patient with an otherwise asymptomatic salivary gland mass where tumor is the most likely diagnosis, the choice is whether to simply excise the mass via a parotidectomy with facial nerve dissection or submandibular gland excision or to first obtain an FNA biopsy. Although the accuracy of FNA biopsy for malignancy has been reported to be quite high, results vary among institutions. If a negative FNA biopsy would lead to a decision not to proceed to surgery, then it should be considered. Poor overall health of the patient and the possibility of inflammatory disease as the cause of the mass are situations where FNA biopsy might be helpful. In otherwise straightforward nonrecurrent cases, excision is indicated. In benign and small, low-grade malignant tumors, no additional treatment is needed. Postoperative irradiation is indicated for larger and high-grade cancers.
D’heygere E et al. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018 Apr;26(2):142–51. [PMID: 29373327]
Ogawa T et al. Clinical utility of dynamic-enhanced MRI in salivary gland tumors: retrospective study and literature review. Eur Arch Otorhinolaryngol. 2018 Jun;275(6):1613–21. [PMID: 29623392]
DISEASES OF THE LARYNX
DYSPHONIA, HOARSENESS, & STRIDOR
The primary symptoms of laryngeal disease are hoarseness and stridor. Hoarseness is caused by an abnormal vibration of the vocal folds. The voice is breathy when too much air passes incompletely apposed vocal folds, as in unilateral vocal fold paralysis or vocal fold mass. The voice is harsh when the vocal folds are stiff and vibrate irregularly, as is the case in laryngitis or malignancy. Heavy, edematous vocal folds produce a rough, low-pitched vocal quality. Stridor (a high-pitched, typically inspiratory, sound) is the result of turbulent airflow from a narrowed upper airway. Airway narrowing at or above the vocal folds produces inspiratory stridor. Airway narrowing below the vocal fold level produces either expiratory or biphasic stridor. The timing and rapidity of onset of stridor are critically important in determining the seriousness of the airway problem. All cases of stridor should be evaluated by a specialist and rapid-onset stridor should be evaluated emergently.
Evaluation of an abnormal voice begins with obtaining a history of the circumstances preceding its onset and an examination of the airway. Any patient with hoarseness that has persisted beyond 2 weeks should be evaluated by an otolaryngologist with laryngoscopy. Especially when the patient has a history of tobacco use, laryngeal cancer or lung cancer (leading to paralysis of a recurrent laryngeal nerve) must be strongly considered. In addition to structural causes of dysphonia, laryngoscopy can help identify functional problems with the voice, including vocal fold paralysis, muscle tension dysphonia, and spasmodic dysphonia.
Andreassen ML et al. Emerging techniques in assessment and treatment of muscle tension dysphonia. Curr Opin Otolaryngol Head Neck Surg. 2017 Dec;25(6):447–52. [PMID: 28984699]
Stachler RJ et al. Clinical Practice Guideline: Hoarseness (dysphonia) (update). Otolaryngol Head Neck Surg. 2018 Mar;158(1 Suppl):S1–42. [PMID: 29494321]
COMMON LARYNGEAL DISORDERS
1. Acute Laryngitis
Acute laryngitis is probably the most common cause of hoarseness, which may persist for a week or so after other symptoms of an upper respiratory infection have cleared. The patient should be warned to avoid vigorous use of the voice (singing, shouting) until their voice returns to normal, since persistent use may lead to the formation of traumatic vocal fold hemorrhage, polyps, and cysts. Although thought to be usually viral in origin, both M catarrhalis and H influenzae may be isolated from the nasopharynx at higher than expected frequencies. Despite this finding, a meta-analysis has failed to demonstrate any convincing evidence that antibiotics significantly alter the natural resolution of acute laryngitis. Erythromycin may speed improvement of hoarseness at 1 week and cough at 2 weeks when measured subjectively. Oral or intramuscular corticosteroids may be used in highly selected cases of professional vocalists to speed recovery and allow scheduled performances. Examination of the vocal folds and assessment of vocal technique are mandatory prior to corticosteroid initiation, since inflamed vocal folds are at greater risk for hemorrhage and the subsequent development of traumatic vocal fold pathology.
Jaworek AJ et al. Acute infectious laryngitis: a case series. Ear Nose Throat J. 2018 Sep;97(9):306–13. [PMID: 30273430]
2. Laryngopharyngeal Reflux
ESSENTIALS OF DIAGNOSIS

 Commonly associated with hoarseness, throat irritation, and chronic cough.
Commonly associated with hoarseness, throat irritation, and chronic cough.
 Symptoms typically occur when upright, and half of patients do not experience heartburn.
Symptoms typically occur when upright, and half of patients do not experience heartburn.
 Laryngoscopy is critical to exclude other causes of hoarseness.
Laryngoscopy is critical to exclude other causes of hoarseness.
 Diagnosis is made based on response to proton-pump inhibitor therapy.
Diagnosis is made based on response to proton-pump inhibitor therapy.
 Treatment failure with proton-pump inhibitors is common and suggests other etiologies.
Treatment failure with proton-pump inhibitors is common and suggests other etiologies.
Gastroesophageal reflux into the larynx (laryngopharyngeal reflux) is considered a cause of chronic hoarseness when other causes of abnormal vocal fold vibration (such as tumor or nodules) have been excluded by laryngoscopy. Gastroesophageal reflux disease (GERD) has also been suggested as a contributing factor to other symptoms, such as throat clearing, throat discomfort, chronic cough, a sensation of postnasal drip, esophageal spasm, and some cases of asthma. Since less than half of patients with laryngeal acid exposure have typical symptoms of heartburn and regurgitation, the lack of such symptoms should not be construed as eliminating this cause. Indeed, most patients with symptomatic laryngopharyngeal reflux, as it is now called, do not meet criteria for GERD by pH probe testing and these entities must be considered separately. The prevalence of this condition is hotly debated in the literature, and laryngopharyngeal reflux may not be as common as once thought.
Evaluation should initially exclude other causes of dysphonia through laryngoscopy; consultation with an otolaryngologist is advisable. Many clinicians opt for an empiric trial of a proton-pump inhibitor since no gold standard exists for diagnosing this condition. Such an empiric trial should not precede visualization of the vocal folds to exclude other causes of hoarseness. When used, the American Academy of Otolaryngology—Head and Neck Surgery recommends twice-daily therapy with full-strength proton-pump inhibitor (eg, omeprazole 40 mg orally twice daily, or equivalent) for a minimum of 3 months. Patients may note improvement in symptoms after 3 months, but the changes in the larynx often take 6 months to resolve. If symptoms improve and cessation of therapy leads to symptoms again, then a proton-pump inhibitor is resumed at the lowest dose effective for remission, usually daily but at times on a demand basis. Although H2-receptor antagonists are an alternative to proton-pump inhibitors, they are generally both less clinically effective and less cost-effective. Nonresponders should undergo pH testing and manometry. Twenty-four-hour pH monitoring of the pharynx should best document laryngopharyngeal reflux and is advocated by some as the initial management step, but it is costly, more difficult, and less available than lower esophageal monitoring alone. Double pH probe (proximal and distal esophageal probes) testing is the best option for evaluation, since lower esophageal pH monitoring alone does not correlate well with laryngopharyngeal reflux symptoms. Oropharyngeal pH probe testing is available, but its ability to predict response to reflux treatment in patients with laryngopharyngeal reflux is not known.
Lechien JR et al. Voice outcomes of laryngopharyngeal reflux treatment: a systematic review of 1483 patients. Eur Arch Otorhinolaryngol. 2017 Jan;274(1):1–23. [PMID: 27007132]
Lechien JR et al. Laryngopharyngeal reflux disease: clinical presentation, diagnosis and therapeutic challenges in 2018. Curr Opin Otolaryngol Head Neck Surg. 2018 Dec;26(6):392–402. [PMID: 30234664]
Lechien JR et al. Association between laryngopharyngeal reflux and benign vocal folds lesions: a systematic review. Laryngoscope. 2019 Sep;129(9):E329–41. [PMID: 30892725]
3. Recurrent Respiratory Papillomatosis
Papillomas are common lesions of the larynx and other sites where ciliated and squamous epithelia meet. Unlike oral papillomas, recurrent respiratory papillomatosis typically becomes symptomatic, with hoarseness that occasionally progresses over weeks to months. These papillomas are almost always due to HPV types 6 and 11. Repeated laser vaporizations or cold knife resections via operative laryngoscopy are the mainstay of treatment. Severe cases can cause airway compromise in adults and may require treatment as often as every 6 weeks to maintain airway patency. Extension can occur into the trachea and lungs. Tracheotomy should be avoided, if possible, since it introduces an additional squamociliary junction for which papillomas appear to have an affinity. Interferon treatment has been under investigation for many years but is only indicated in severe cases with pulmonary involvement. Rarely, cases of malignant transformation have been reported (often in smokers), but recurrent respiratory papillomatosis should generally be thought of as a benign condition. Cidofovir (a cytosine nucleotide analog in use to treat cytomegalovirus retinitis) has been used with success as intralesional therapy for recurrent respiratory papillomatosis. Because cidofovir causes adenocarcinomas in laboratory animals, its potential for carcinogenesis is being monitored. The quadrivalent and new 9 serotype recombinant human HPV vaccines (Gardasil and Gardasil 9) offer hope for the eventual prevention of this benign, but terribly morbid, disease.
Fortes HR et al. Recurrent respiratory papillomatosis: a state-of-the-art review. Respir Med. 2017 May;126:116–21. [PMID: 28427542]
Ivancic R et al. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2018 Jan 14;3(1):22–34. [PMID: 29492465]
4. Epiglottitis
Epiglottitis (or, more correctly, supraglottitis) should be suspected when a patient presents with a rapidly developing sore throat or when odynophagia (pain on swallowing) is out of proportion to apparently minimal oropharyngeal findings on examination. It is more common in diabetic patients and may be viral or bacterial in origin. Rarely in the era of H influenzae type b vaccine is this bacterium isolated in adults. Unlike in children, indirect laryngoscopy is generally safe and may demonstrate a swollen, erythematous epiglottis. Lateral plain radiographs may demonstrate an enlarged epiglottis (the epiglottis “thumb sign”). Initial treatment is hospitalization for intravenous antibiotics—eg, ceftizoxime, 1–2 g intravenously every 8–12 hours; or cefuroxime, 750–1500 mg intravenously every 8 hours; and dexamethasone, usually 4–10 mg as initial bolus, then 4 mg intravenously every 6 hours—and observation of the airway. Corticosteroids may be tapered as symptoms and signs resolve. Similarly, substitution of oral antibiotics may be appropriate to complete a 10-day course. Less than 10% of adults require intubation. Indications for intubation are dyspnea, rapid pace of sore throat (where progression to airway compromise may occur before the effects of corticosteroids and antibiotics), and endolaryngeal abscess noted on CT imaging. If the patient is not intubated, prudence suggests monitoring oxygen saturation with continuous pulse oximetry and initial admission to a monitored unit.
Baiu I et al. Epiglottitis. JAMA. 2019 May 21;321(19):1946. [PMID: 31112260]
Chen C et al. Acute epiglottitis in the immunocompromised host: case report and review of the literature. Open Forum Infect Dis. 2018 Feb 17;5(3):ofy038. [PMID: 29564363]
MASSES OF THE LARYNX
1. Traumatic Lesions of the Vocal Folds
Vocal fold nodules are smooth, paired lesions that form at the junction of the anterior one-third and posterior two-thirds of the vocal folds. They are a common cause of hoarseness resulting from vocal abuse. In adults, they are referred to as “singer’s nodules” and in children as “screamer’s nodules.” Treatment requires modification of voice habits, and referral to a speech therapist is indicated. While nearly all true nodules will resolve with behavior modification, recalcitrant nodules may require surgical excision. Often, additional pathology, such as a polyp or cyst, may be encountered.
Vocal fold polyps are unilateral masses that form within the superficial lamina propria of the vocal fold. They are related to vocal trauma and seem to follow resolution of vocal fold hemorrhage. Small, sessile polyps may resolve with conservative measures, such as voice rest and corticosteroids, but larger polyps are often irreversible and require operative removal to restore normal voice.
Vocal fold cysts are also considered traumatic lesions of the vocal folds and are either true cysts with an epithelial lining or pseudocysts. They typically form from mucus-secreting glands on the inferior aspect of the vocal folds. Cysts may fluctuate in size from week to week and cause a variable degree of hoarseness. They rarely, if ever, resolve completely and may leave behind a sulcus, or vocal fold scar, if they decompress or are marsupialized. Such scarring can be a frustrating cause of permanent dysphonia.
Polypoid corditis is different from vocal fold polyps and may form from loss of elastin fibers and loosening of the intracellular junctions within the lamina propria. This loss allows swelling of the gelatinous matrix of the superficial lamina propria (called Reinke edema). These changes in the vocal folds are strongly associated with smoking, but also with vocal abuse, chemical industrial irritants, and hypothyroidism. While this problem is common in both male and female smokers, women seem more troubled by the characteristic decline in modal pitch caused by the increased mass of the vocal folds. If the patient stops smoking or the lesions cause stridor and airway obstruction, surgical resection of the hyperplastic vocal fold mucosa may be indicated to improve the voice or airway, or both.
A common but often unrecognized cause of hoarseness and odynophonia are contact ulcers or their close relatives, granulomas. Both lesions form on the vocal processes of the arytenoid cartilages, and patients often can correctly inform the clinician which side is affected. The cause of these ulcers and granulomas is disputed, but they are clearly related to trauma and may be related to exposure of the underlying perichondrium. They are common following intubation and generally resolve quite quickly. Chronic ulceration or granuloma formation has been associated with gastroesophageal reflux but is also common in patients with muscle tension dysphonia. Treatment is often multimodal, and an inhaled corticosteroid (eg, fluticasone 440 mcg twice daily) may be the most effective pharmacologic therapy. Adjunctive treatment measures include proton-pump inhibitor therapy (omeprazole 40 mg orally twice daily, or equivalent) and voice therapy with special attention to vocal hygiene. Rare cases can be quite stubborn and persistent without efficacious therapy. Surgical removal is rarely, if ever, required for nonobstructive lesions.
Ogawa M et al. Is voice therapy effective for the treatment of dysphonic patients with benign vocal fold lesions? Auris Nasus Larynx. 2018 Aug;45(4):661–6. [PMID: 28844607]
White A. Management of benign vocal fold lesions: current perspectives on the role for voice therapy. Curr Opin Otolaryngol Head Neck Surg. 2019 Jun;27(3):185–90. [PMID: 30893134]
2. Laryngeal Leukoplakia
Leukoplakia of the vocal folds is commonly found in association with hoarseness in smokers. Direct laryngoscopy with biopsy is advised in almost all cases. Histologic examination usually demonstrates mild, moderate, or severe dysplasia. In some cases, invasive squamous cell carcinoma is present in the initial biopsy specimen. Cessation of smoking may reverse or stabilize mild or moderate dysplasia. Some patients—estimated to be less than 5% of those with mild dysplasia and about 35–60% of those with severe dysplasia—will subsequently develop squamous cell carcinoma. Treatment options include proton-pump inhibitor therapy, close follow-up with laryngovideostroboscopy, serial resection, and external beam radiation therapy.
Parker NP. Vocal fold leukoplakia: incidence, management, and prevention. Curr Opin Otolaryngol Head Neck Surg. 2017 Dec;25(6):464–8. [PMID: 28857841]
Sezen Goktas S et al. A new approach to vocal cord leukoplakia and evaluation of proton pump inhibitor treatment. Eur Arch Otorhinolaryngol. 2019 Feb;276(2):467–71. [PMID: 30607560]
3. Squamous Cell Carcinoma of the Larynx
ESSENTIALS OF DIAGNOSIS

 New and persistent (more than 2 weeks’ duration) hoarseness in a smoker.
New and persistent (more than 2 weeks’ duration) hoarseness in a smoker.
 Persistent throat or ear pain, especially with swallowing.
Persistent throat or ear pain, especially with swallowing.
 Neck mass.
Neck mass.
 Hemoptysis.
Hemoptysis.
 Stridor or other symptoms of a compromised airway.
Stridor or other symptoms of a compromised airway.
 General Considerations
General Considerations
Squamous cell carcinoma of the larynx, the most common malignancy of the larynx, occurs almost exclusively in patients with a history of significant tobacco use. Squamous cell carcinoma is usually seen in men aged 50–70 years; an estimated 13,150 new cases in both sexes (10,490 in men) will be seen in the United States in 2018. There may be an association between laryngeal cancer and HPV type 16 or 18 infection, but this association is much less strong than that between HPV 16 or 18 and oropharyngeal cancer. In both cancer types, the association with HPV seems to be strongest in nonsmokers. Laryngeal cancer is very treatable and early detection is the key to maximizing posttreatment voice, swallowing, and breathing function.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
A change in voice quality is most often the presenting complaint, although throat or ear pain, hemoptysis, dysphagia, weight loss, and airway compromise may occur. Because of their early impact on vocal quality, glottic cancers are among the smallest detectable human malignancies and treatment success is very high with early lesions. Neck metastases are not common in early glottic (true vocal fold) cancer in which the vocal folds are mobile, but a third of patients in whom there is impaired fold mobility will also have involved lymph nodes at neck dissection. Supraglottic carcinoma (false vocal folds, aryepiglottic folds, epiglottis), on the other hand, often metastasizes to both sides of the neck early in the disease. Complete head and neck examination, including laryngoscopy, by an experienced clinician is mandated for any person with the concerning symptoms listed under Essentials of Diagnosis.
B. Imaging and Laboratory Studies
Radiologic evaluation by CT or MRI is helpful in assessing tumor extent. Imaging evaluates neck nodes, tumor volume, and cartilage sclerosis or destruction. A chest CT scan is indicated if there are level VI enlarged nodes (around the trachea and the thyroid gland) or level IV enlarged nodes (inferior to the cricoid cartilage along the internal jugular vein), or if a chest film is concerning for a second primary lesion or metastases. Laboratory evaluation includes complete blood count and liver biochemical tests. Formal cardiopulmonary evaluation may be indicated, especially if partial laryngeal surgery is being considered. All partial laryngectomy candidates should have good to excellent lung function and exercise tolerance because chronic microaspiration may be expected following the procedure. A positron emission tomography (PET) scan or CT-PET scan may be indicated to assess for distant metastases when there appears to be advanced local or regional disease.
C. Biopsy
Diagnosis is made by biopsy at the time of laryngoscopy when true fold mobility and arytenoid fixation, as well as surface tumor extent, can be evaluated. Most otolaryngologists recommend esophagoscopy and bronchoscopy at the same time to exclude synchronous primary tumor. Although an FNA biopsy of an enlarged neck node may have already been done, it is generally acceptable to assume radiographically enlarged neck nodes (greater than 1–1.5 cm) or nodes with necrotic centers are neck metastases. Open biopsies of nodal metastases should be discouraged because they may lead to higher rates of tumor treatment failure.
D. Tumor Staging
The American Joint Committee on Cancer (AJCC) staging of laryngeal cancers uses the TNM system to describe tumor extent and can be used for prognosis. Early laryngeal cancers, T1 and T2 (stage I and II) lesions, involve 1–2 laryngeal subsites locally and have no nodal metastases or profound functional abnormalities. T3 and T4 lesions may involve multiple laryngeal subsites with limitation of laryngeal mobility. These locally advanced lesions are stage III or IV cancers, and any size tumor with regional nodal metastases is at least a stage III tumor. Stage I and II lesions are generally treated with single-modality therapy (surgery or radiation), while multimodality therapy, usually including chemotherapy with radiation therapy, is reserved for more advanced stage III and IV lesions.
 Treatment
Treatment
Treatment of laryngeal carcinoma has four goals: cure, preservation of safe and effective swallowing, preservation of useful voice, and avoidance of a permanent tracheostoma. For early glottic and supraglottic cancers, radiation therapy is the standard of care since cure rates are greater than 95% and 80%, respectively. That said, radiation therapy carries substantial morbidity, and many early tumors (T1 and T2 lesions, without involved nodes) and selected advanced tumors (T3 and T4) may be treated with partial laryngectomy if at least one cricoarytenoid unit can be preserved. Five-year locoregional cure rates exceed 80–90% with surgery, and patient-reported satisfaction is excellent. In supraglottic tumors, even when clinically N0, elective limited neck dissection is indicated following surgical resection because of the high risk of neck node involvement.
Advanced stage III and IV tumors represent a challenging and ever-changing treatment dilemma. Twenty-five years ago, total laryngectomy was often recommended for such patients. However, the 1994 VA study (with induction cisplatin and 5-fluorouracil followed by irradiation alone in responders) demonstrated that two-thirds of patients could preserve their larynx. Subsequent studies have further defined multimodal therapy. Cisplatin-based chemotherapy concomitant with radiation therapy has been shown to be superior to either irradiation alone or induction chemotherapy followed by radiation. The same benefits have been demonstrated with the epidermal growth factor receptor blocker cetuximab with lower overall systemic toxicity and better patient tolerance. However, chemoradiation using either cetuximab or cisplatin is associated with prolonged gastrostomy-dependent dysphagia.
The high rate of dysphagia and morbidity associated with severe laryngeal stenosis following chemoradiation has prompted a reevaluation of the role of extended, but less-than-total, laryngeal resection for selected advanced laryngeal carcinoma in which at least one cricoarytenoid unit is intact (organ preservation surgery). In addition to the late complications, clinicians have noted that the overall success in the treatment of larynx cancer has declined in parallel with the increase in organ preservation chemoradiation therapy over the past 20 years. Some experts have proposed that this decline is the direct result of the shift in management of advanced laryngeal cancer away from surgery. Organ preservation surgery should be considered and discussed as an alternative to chemoradiation but may require referral to an appropriate regional center where such techniques are offered. After thorough evaluation of candidacy and discussion of the treatment options, patient choice plays a critical role in the ultimate decision to pursue surgery or chemoradiation as a definitive treatment modality. The patient and treating clinicians must carefully consider different early and late side effects and complications associated with different treatment modalities.
The presence of malignant adenopathy in the neck affects the prognosis greatly. Supraglottic tumors metastasize early and bilaterally to the neck, and this must be included in the treatment plans even when the neck is apparently uninvolved. Glottic tumors in which the true vocal folds are mobile (T1 or T2) have less than a 5% rate of nodal involvement; when a fold is immobile, the rate of ipsilateral nodal involvement climbs to about 30%. An involved neck is treated by surgery or chemoradiation, or both. This decision will depend on the treatment chosen for the larynx and the extent of neck involvement.
Total laryngectomy is largely reserved for patients with advanced resectable tumors with extralaryngeal spread or cartilage involvement, for those with persistent tumor following chemoradiation, and for patients with recurrent or second primary tumor following previous radiation therapy. Voice rehabilitation via a primary (or at times secondary) tracheoesophageal puncture produces intelligible and serviceable speech in about 75–85% of patients. Indwelling prostheses that are changed every 3–6 months are a common alternative to patient-inserted prostheses, which need changing more frequently.
Long-term follow-up is critical in head and neck cancer patients. In addition to the 3–4% annual rate of second tumors and monitoring for recurrence, psychosocial aspects of treatment are common. Dysphagia, impaired communication, and altered appearance may result in patient difficulties adapting to the workplace and to social interactions. In addition, smoking cessation and alcohol abatement are common challenges. Nevertheless, about 65% of patients with larynx cancer are cured, most have useful speech, and many resume their prior livelihoods with adaptations.
 When to Refer
When to Refer
• Specialty referral should be sought early for diagnosis and treatment.
• Indirect or fiberoptic examination of the nasopharynx, oropharynx, hypopharynx, and larynx by a otolaryngologist–head and neck surgeon should be considered for patients with oral erythroplakia, unexplained throat or ear pain, unexplained oral or nasal bleeding, firm neck mass, or visible oral cavity or oropharyngeal mass.
 When to Admit
When to Admit
• Airway compromise, hemorrhage, dehydration.
• To determine an effective pain management regimen for severe pain.
Moskovitz J et al. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep. 2018 Mar 3;20(2):22. [PMID: 29502288]
Patel KB et al. Treatment of early stage supraglottic squamous cell carcinoma: meta-analysis comparing primary surgery versus primary radiotherapy. J Otolaryngol Head Neck Surg. 2018 Mar 5;47(1):19. [PMID: 29506564]
VOCAL FOLD PARALYSIS
Vocal fold paralysis can result from a lesion or damage to either the vagus or recurrent laryngeal nerve and usually results in breathy dysphonia and effortful voicing. Common causes of unilateral recurrent laryngeal nerve involvement include thyroid surgery (and occasionally thyroid cancer), other neck surgery (anterior discectomy and carotid endarterectomy), and mediastinal or apical involvement by lung cancer. Skull base tumors often involve or abut upon lower cranial nerves and may affect the vagus nerve directly, or the vagus nerve may be damaged during surgical management of the lesion. While iatrogenic injury is the most common cause of unilateral vocal fold paralysis, the second most common cause is idiopathic. However, before deciding whether the paralysis is due to iatrogenic injury or is idiopathic, the clinician must exclude other causes, such as malignancy. In the absence of other cranial neuropathies, a CT scan with contrast from the skull base to the aorto-pulmonary window (the span of the recurrent laryngeal nerve) should be performed. If other cranial nerve deficits or high vagal weakness with palate paralysis is noted, an MRI scan of the brain and brainstem is warranted.
Unlike unilateral fold paralysis, bilateral fold paralysis usually causes inspiratory stridor with deep inspiration. If the onset of bilateral fold paralysis is insidious, it may be asymptomatic at rest, and the patient may have a normal voice. However, the acute onset of bilateral vocal fold paralysis with inspiratory stridor at rest should be managed by a specialist immediately in a critical care environment. Causes of bilateral fold paralysis include thyroid surgery, esophageal cancer, and ventricular shunt malfunction. Unilateral or bilateral fold immobility may also be seen in cricoarytenoid arthritis secondary to advanced rheumatoid arthritis, intubation injuries, glottic and subglottic stenosis, and, of course, laryngeal cancer. The goal of intervention is the creation of a safe airway with minimal reduction in voice quality and airway protection from aspiration. A number of fold lateralization procedures for bilateral paralysis have been advocated as a means of removing the tracheotomy tube.
Unilateral vocal fold paralysis is occasionally temporary and may take over a year to resolve spontaneously. Surgical management of persistent or irrecoverable symptomatic unilateral vocal fold paralysis has evolved over the last several decades. The primary goal is medialization of the paralyzed fold in order to create a stable platform for vocal fold vibration. Additional goals include advancing diet and improving pulmonary toilet by facilitating cough. Success has been reported for years with injection laryngoplasty using Teflon, Gelfoam, fat, and collagen. Teflon is the only permanent injectable material, but its use is discouraged because of granuloma formation within the vocal folds of some patients. Temporary injectable materials, such as collagen or fat, provide excellent temporary restoration of voice and can be placed under local or general anesthesia. Once the paralysis is determined to be permanent, formal medialization thyroplasty may be performed by creating a small window in the thyroid cartilage and placing an implant between the thyroarytenoid muscle and inner table of the thyroid cartilage. This procedure moves the vocal fold medially and creates a stable platform for bilateral, symmetric mucosal vibration.
Desuter G et al. Voice outcome indicators for unilateral vocal fold paralysis surgery: a review of the literature. Eur Arch Otorhinolaryngol. 2018 Feb;275(2):459–68. [PMID: 29264655]
TRACHEOSTOMY & CRICOTHYROTOMY
There are two primary indications for tracheotomy: airway obstruction at or above the level of the larynx and respiratory failure requiring prolonged mechanical ventilation. In an acute emergency, cricothyrotomy secures an airway more rapidly than tracheotomy, with fewer potential immediate complications, such as pneumothorax and hemorrhage. Percutaneous dilatational tracheotomy as an elective bedside (or intensive care unit) procedure has undergone scrutiny in recent years as an alternative to tracheotomy. In experienced hands, the various methods of percutaneous tracheotomy have been documented to be safe in carefully selected patients. Simultaneous videobronchoscopy can reduce the incidence of major complications. The major cost reduction comes from avoiding the operating room. Bedside tracheotomy (in the intensive care unit) achieves similar cost reduction and is advocated by some experts as slightly less costly than the percutaneous procedures.
The most common indication for elective tracheotomy is the need for prolonged mechanical ventilation. There is no firm rule about how many days a patient must be intubated before conversion to tracheotomy should be advised. The incidence of serious complications, such as subglottic stenosis, increases with extended endotracheal intubation. As soon as it is apparent that the patient will require protracted ventilatory support, tracheotomy should replace the endotracheal tube. Less frequent indications for tracheostomy are life-threatening aspiration pneumonia, the need to improve pulmonary toilet to correct problems related to insufficient clearing of tracheobronchial secretions, and sleep apnea.
Posttracheotomy care requires humidified air to prevent secretions from crusting and occluding the inner cannula of the tracheotomy tube. The tracheotomy tube should be cleaned several times daily. The most frequent early complication of tracheotomy is dislodgment of the tracheotomy tube. Surgical creation of an inferiorly based tracheal flap sutured to the inferior neck skin may make reinsertion of a dislodged tube easier. It should be recalled that the act of swallowing requires elevation of the larynx, which is limited by tracheotomy. Therefore, frequent tracheal and bronchial suctioning is often required to clear the aspirated saliva as well as the increased tracheobronchial secretions. Care of the skin around the stoma is important to prevent maceration and secondary infection.
Adly A et al. Timing of tracheostomy in patients with prolonged endotracheal intubation: a systematic review. Eur Arch Otorhinolaryngol. 2018 Mar;275(3):679–90. [PMID: 29255970]
Bontempo LJ. Tracheostomy emergencies. Emerg Med Clin North Am. 2019 Feb;37(1):109–19. [PMID: 30454773]
Cooper JD. Tracheal injuries complicating prolonged intubation and tracheostomy. Thorac Surg Clin. 2018 May;28(2):139–44. [PMID: 29627046]
Yildirim E. Principles of urgent management of acute airway obstruction. Thorac Surg Clin. 2018 Aug;28(3):415–28. [PMID: 30054079]
FOREIGN BODIES IN THE UPPER AERODIGESTIVE TRACT
FOREIGN BODIES IN THE TRACHEA & BRONCHI
Aspiration of foreign bodies occurs much less frequently in adults than in children. Older adults and denture wearers appear to be at greatest risk. Wider familiarity with the Heimlich maneuver has reduced deaths. If the maneuver is unsuccessful, cricothyrotomy may be necessary. Plain chest radiographs may reveal a radiopaque foreign body. Detection of radiolucent foreign bodies may be aided by inspiration-expiration films that demonstrate air trapping distal to the obstructed segment. Atelectasis and pneumonia may occur later.
Tracheal and bronchial foreign bodies should be removed under general anesthesia with rigid bronchoscopy by a skilled endoscopist working with an experienced anesthesiologist.
FOREIGN BODIES IN THE ESOPHAGUS
Foreign bodies in the esophagus create urgent but not life-threatening situations as long as the airway is not compromised. There is probably time to consult an experienced clinician for management. It is a useful diagnostic sign of complete obstruction if the patient is drooling or cannot handle secretions. Patients may often point to the exact level of the obstruction. Indirect laryngoscopy often shows pooling of saliva at the esophageal inlet. Plain films may detect radiopaque foreign bodies, such as chicken bones. Coins tend to align in the coronal plane in the esophagus and sagittally in the trachea. If a foreign body is suspected, a barium swallow may help make the diagnosis.
The treatment of an esophageal foreign body depends very much on identification of its nature. In children, swallowed nonfood objects are common. In adults, however, food foreign bodies are more common, and there is the greater possibility of underlying esophageal pathology. Endoscopic removal and examination are usually best via flexible esophagoscopy or rigid laryngoscopy and esophagoscopy. If there is nothing sharp, such as a bone, some clinicians advocate a hospitalized 24-hour observation period prior to esophagoscopy, noting that spontaneous passage of the foreign body will occur in 50% of adult patients. In the management of meat obstruction, the use of papain (meat tenderizer) should be discouraged because it can damage the esophageal mucosa and lead to stenosis or perforation.
Chirica M et al. Esophageal emergencies: WSES guidelines. World J Emerg Surg. 2019 May 31;14:26. [PMID: 31164915]
Zhong Q et al. Esophageal foreign body ingestion in adults on weekdays and holidays: a retrospective study of 1058 patients. Medicine (Baltimore). 2017 Oct;96(43):e8409. [PMID: 29069038]
DISEASES PRESENTING AS NECK MASSES
The differential diagnosis of neck masses is heavily dependent on the location in the neck, the age of the patient, and the presence of associated disease processes. Rapid growth and tenderness suggest an inflammatory process, while firm, painless, and slowly enlarging masses are often neoplastic. In young adults, most neck masses are benign (branchial cleft cyst, thyroglossal duct cyst, reactive lymphadenitis), although malignancy should always be considered (lymphoma, metastatic thyroid carcinoma). Lymphadenopathy is common in HIV-positive persons, but a growing or dominant mass may well represent lymphoma. In adults over age 40, cancer is the most common cause of persistent neck mass. A metastasis from squamous cell carcinoma arising within the mouth, pharynx, larynx, or upper esophagus should be suspected, especially if there is a history of tobacco or significant alcohol use. Especially among patients younger than 30 or older than 70, lymphoma should be considered. In any case, a comprehensive otolaryngologic examination is needed. Cytologic evaluation of the neck mass via FNA biopsy is likely to be the next step if a primary tumor is not obvious on physical examination.
CONGENITAL LESIONS PRESENTING AS NECK MASSES IN ADULTS
1. Branchial Cleft Cysts
Branchial cleft cysts usually present as a soft cystic mass along the anterior border of the sternocleidomastoid muscle. These lesions are usually recognized in the second or third decades of life, often when they suddenly swell or become infected. To prevent recurrent infection and possible carcinoma, they should be completely excised, along with their fistulous tracts.
First branchial cleft cysts present high in the neck, sometimes just below the ear. A fistulous connection with the floor of the external auditory canal may be present. Second branchial cleft cysts, which are far more common, may communicate with the tonsillar fossa. Third branchial cleft cysts, which may communicate with the piriform sinus, are rare and present low in the neck.
Ha EJ et al. Efficacy and safety of ethanol ablation for branchial cleft cysts. AJNR Am J Neuroradiol. 2017 Dec;38(12):2351–6. [PMID: 28970243]
2. Thyroglossal Duct Cysts
Thyroglossal duct cysts occur along the embryologic course of the thyroid’s descent from the tuberculum impar of the tongue base to its usual position in the low neck. Although they may occur at any age, they are most common before age 20. They present as a midline neck mass, often just below the hyoid bone, which moves with swallowing. Surgical excision is recommended to prevent recurrent infection. This requires removal of the entire fistulous tract along with the middle portion of the hyoid bone through which many of the fistulas pass. Preoperative evaluation should include a thyroid ultrasound to confirm anatomic position of the thyroid.
Ross J et al. Thyroglossal duct cyst surgery: a ten-year single institution experience. Int J Pediatr Otorhinolaryngol. 2017 Oct;101:132–6. [PMID: 28964283]
INFECTIOUS & INFLAMMATORY NECK MASSES
1. Reactive Cervical Lymphadenopathy
Normal lymph nodes in the neck are usually less than 1 cm in length. Infections involving the pharynx, salivary glands, and scalp often cause tender enlargement of neck nodes. Enlarged nodes are common in HIV-infected persons. Except for the occasional node that suppurates and requires incision and drainage, treatment is directed against the underlying infection. An enlarged node (larger than 1.5 cm) or node with a necrotic center that is not associated with an obvious infection should be further evaluated, especially if the patient has a history of smoking, alcohol use, or prior cancer. Other common indications for FNA biopsy of a node include its persistence or continued enlargement. Common causes of cervical adenopathy include tumor (squamous cell carcinoma, lymphoma, occasional metastases from non-head and neck sites) and infection (eg, reactive nodes, mycobacteria, and cat-scratch disease). Rare causes of adenopathy include Kikuchi disease (histiocytic necrotizing lymphadenitis) and autoimmune adenopathy.
2. Tuberculous & Nontuberculous Mycobacterial Lymphadenitis
Granulomatous neck masses are not uncommon. The differential diagnosis includes mycobacterial adenitis, sarcoidosis, and cat-scratch disease due to Bartonella henselae. The incidence of mycobacterial lymphadenitis is on the rise both in immunocompromised and immunocompetent individuals. The usual presentation of granulomatous disease in the neck is simply single or matted nodes. Although mycobacterial adenitis can extend to the skin and drain externally (as described for atypical mycobacteria and referred to as scrofula), this late presentation is no longer common.
FNA biopsy is usually the best initial diagnostic approach: cytology, smear for acid-fast bacilli, mycobacterial culture, and a sensitivity test can all be done. PCR from FNA (or from excised tissue) is the most sensitive test and is particularly useful when conventional methods have not been diagnostic but clinical impression remains consistent for tuberculous infection. While FNA has a high sensitivity (about 88%), its specificity is low (49%); thus, an excisional biopsy is often required to confirm the diagnosis.
See Table 9–15 for current recommended treatment of tuberculosis infection, which includes infection of the lymph nodes (tuberculous lymphadenopathy). For atypical (nontuberculous) infection of the lymph nodes, treatment depends on the sensitivity results of culture, but antibiotics likely to be useful include 6 months of isoniazid and rifampin and, for at least the first 2 months, ethambutol—all in standard dosages. Some would totally excise the involved nodes prior to chemotherapy, depending on location and other factors, but this can lead to chronic draining fistulas.
Białek EJ et al. Mistakes in ultrasound diagnosis of superficial lymph nodes. J Ultrason. 2017 Mar;17(68):59–65. [PMID: 28439430]
Pang P et al. Clinical study of tuberculosis in the head and neck region—11 years’ experience and a review of the literature. Emerg Microbes Infect. 2018 Jan 10;7(1):4. [PMID: 29323108]
3. Lyme Disease
Lyme disease, caused by the spirochete Borrelia burgdorferi and transmitted by ticks of the Ixodes genus, may have protean manifestations, but over 75% of patients have symptoms involving the head and neck. Facial paralysis, dysesthesias, dysgeusia, or other cranial neuropathies are most common. Headache, pain, and cervical lymphadenopathy may occur. See Chapter 34 for a more detailed discussion.
Bush LM et al. Tick-borne illness–Lyme disease. Dis Mon. 2018 May;64(5):195–212. [PMID: 29402399]
Cruickshank M et al; Guideline Committee. Lyme disease: summary of NICE guidance. BMJ. 2018 Apr 12;361:k1261. [PMID: 29650513]
Shapiro ED et al. Lyme disease in 2018: what is new (and what is not). JAMA. 2018 Aug 21;320(7):635–36. [PMID: 30073279]
TUMOR METASTASES
In older adults, 80% of firm, persistent, and enlarging neck masses are metastatic in origin. The great majority of these arise from squamous cell carcinoma of the upper aerodigestive tract. A complete head and neck examination may reveal the tumor of origin, but examination under anesthesia with direct laryngoscopy, esophagoscopy, and bronchoscopy is usually required to fully evaluate the tumor and exclude second primaries.
It is often helpful to obtain a cytologic diagnosis if initial head and neck examination fails to reveal the primary tumor. An open biopsy should be done only when neither physical examination by an experienced clinician specializing in head and neck cancer nor FNA biopsy performed by an experienced cytopathologist yields a diagnosis. In such a setting, one should strongly consider obtaining an MRI or PET scan prior to open biopsy, as these methods may yield valuable information about a possible presumed primary site or another site for FNA.
With the exception of papillary thyroid carcinoma, non–squamous cell metastases to the neck are infrequent. While tumors that are not primary in the head or neck seldom metastasize to the cervical lymph nodes, the supraclavicular lymph nodes are quite often involved by lung, gastroesophageal, and breast tumors. Infradiaphragmatic tumors, with the exception of renal cell carcinoma and testicular cancer, rarely metastasize to the neck.
Bochtler T et al. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2018 May;35(3):199–206. [PMID: 29203116]
Rahimi-Nedjat RK et al. Sentinel lymph node biopsy in malignant melanoma of the head and neck. J Craniomaxillofac Surg. 2018 Jun;46(6):1027–31. [PMID: 29735384]
LYMPHOMA
About 10% of lymphomas present in the head and neck. Multiple rubbery nodes, especially in young adults or in patients who have AIDS, are suggestive of lymphoma. A thorough physical examination may demonstrate other sites of nodal or organ involvement. FNA biopsy may be diagnostic, but open biopsy is often required to determine architecture and an appropriate treatment course.
Cabeçadas J et al. Lymphomas of the head and neck region: an update. Virchows Arch. 2019 Jun;474(6):649–65. [PMID: 30778677]
Oishi N et al. Head and neck lymphomas in HIV patients: a clinical perspective. Int Arch Otorhinolaryngol. 2017 Oct;21(4):399–407. [PMID: 29018505]