13
Speciation by Introgressive Hybridization
But the chief cause of our unwillingness to admit that one species has given birth to other and distinct species, is that we are always slow in admitting any great change of which we do not see the intermediates.
(Darwin 1859, p. 481)
All our knowledge has its origins in our perceptions.
(Leonardo Da Vinci; Richter 1985, p. 4)
Introduction
OVER THE PAST 20 YEARS hybridization has been seen increasingly as an important factor in speciation (reviewed in Schwenk et al. 2008, Abbott et al. 2013). An exchange of genes between related species or even genera was once thought to be a phenomenon largely restricted to plants, but is now known to be widespread and to occur from bacteria (Jain et al. 1999, Ochman et al. 2000) and corals (Veron 1995, Willis et al. 2007) to arthropods (Mallet 2005), birds (Grant and Grant 1992b, McCarthy 2006), and primates (Arnold and Meyer 2006, Schwenk et al. 2008). Even in our own evolutionary lineage there is evidence of gene transmission from Neanderthals to our direct ancestors (Reich et al. 2011). The important question is what effect does gene exchange have on the receiving populations, and in particular what is the relevance to speciation?
Hybridization can give rise to a new species through the production of fertile offspring possessing a different number of chromosomes from either parent (Coyne and Orr 2004, Mallet 2007). Polyploid species thus formed are well known in plants and in some arthropods (Bullini 1994) and cold-blooded vertebrates (Dawley and Bogert 1989), but not in birds. Hybridization of birds is more likely to cause a merging of two species as a result of introgression (chapters 9 and 10), or a transformation of them through enhanced genetic variation, than the evolution of a new species, unless the hybrids occupy a different habitat or location as happens in some plants (Huxley 1942, Rieseberg, Raymond, et al. 2003) and in a few groups of mammals (Larsen et al. 2010), salamanders (Pereira and Wake 2009), fish (Nolte and Tautz 2009, Hudson et al. 2011), flies (Schwarz et al. 2005), and butterflies (Gompertz et al. 2006, Mavárez et al. 2006, Kunte et al. 2011, Brower 2013). Two examples of bird species where this is suspected have been reported recently, and both involve a large degree of geographical isolation of the putative hybrid population. The Italian Sparrow (Passer italiae) is morphologically intermediate in plumage, and genetically intermediate, between the House Sparrow (Passer domesticus) and the Spanish Sparrow (Passer hispaniolensis), largely isolated reproductively and geographically from both of them, being the sole occupant of most of the Italian peninsula (Hermansen et al. 2011). In North America Audubon’s Warbler (Setophaga auduboni) on the western side of the Rocky Mountains is genetically and phenotypically intermediate between Myrtle’s Warbler (S. coronata) farther east and the Black-fronted Warbler (S. nigrifrons) farther south (Brelsford et al. 2011, Milá et al. 2011; reviewed in Rheindt and Edwards 2011).
In the case of Darwin’s finches hybridization could lead to the formation of a new species if hybrids from one island fly to another, ecologically different island, establish a breeding population, and undergo evolutionary divergence that culminates in reproductive isolation from the parental species (Grant et al. 2005). The colonists need not be solely introgressed hybrids for speciation to occur; they could be a mixture of F1 hybrids, backcrosses, and members of the parental species. Hybrids and parental types might interbreed, and the increased genetic variation would enhance the potential for change in a new direction (chapter 9). Alternatively the parental types might have poor post-colonization success and contribute less and less than the hybrids to future generations.
We now describe an example of incipient hybrid speciation on Daphne (Grant and Grant 2009a). Like the establishment of a breeding population of magnirostris (chapter 6) it began with immigration to the island, but unlike that event there was only a single immigrant. Its descendants formed a population that became reproductively isolated from the other finches on the island. We refer to this as introgressive speciation because backcrossing was an important part of the initial separation of the lineage from the parental species. We describe how this unlikely outcome transpired and interpret why it happened.
A Hybrid Arrives on Daphne
In 1981 Trevor Price captured a strange bird (number 5110) while doing routine netting of offspring of known parents for heritability analysis. It was young, in brown plumage, yet unusually large. In weight (29.7 g), beak length (14.1 mm), and other dimensions it was clearly beyond the range of measurements of all known resident fortis and scandens. It was more similar to fortis in beak proportions than to scandens. At the time of its arrival we, together with our assistants, had measured more than 90% of all resident fortis and scandens, and observed the breeding of the remainder, so we were confident that it had not hatched on the island. We considered it to be an immigrant fortis, and suspected it had hatched earlier in the year on neighboring Santa Cruz Island (Grant and Grant 2008c).
In 1990 we recaptured 5110, known by now to be a male and in shiny black plumage (fig. 13.1), and took a blood sample for microsatellite DNA analysis. Many years later we had assembled blood samples from several neighboring islands and were able to assign 5110 to a population of origin by using the newly developed program STRUCTURE (chapter 10; Pritchard et al. 2002, 2007). First, we confirmed it did not originate in either the fortis or scandens populations on Daphne. Second, it was assigned with more than 90% probability to the population of fortis on Santa Cruz Island. Third, in a separate analysis with the addition of Santa Cruz scandens, STRUCTURE assigned a significant fraction of the genome to scandens (0.341), the remainder being assigned to fortis (0.659). We concluded the immigrant was a hybrid, possibly a backcross to fortis of a fortis × scandens F1 hybrid (Grant and Grant 2009a). We designate this FFS as explained in figure 8.16.

Fig. 13.1 Two lines of descent from an immigrant. Upper left: The original immigrant hybrid fortis, 5110. Upper right: A son, 15830, produced on the A line of descent. Lower left: 19256, a member of the Big Bird lineage on the B line of descent (generation f4). Lower right: 17618, a second-generation member of the A line of descent and daughter of 15830’s brother.
Descendants
PHASE I: THE START OF A NEW LINEAGE
Bird number 5110 was not only unusual morphologically; he was unusually fit. He lived 13 years, paired with six different females, and fledged at least 18 offspring of which 5 became breeding recruits (fig. 13.2). He bred for the first time in 1983, and while many birds experienced high reproductive success in this extraordinary El Niño year (chapter 5), he only managed to acquire a mate toward the end of the prolonged breeding season. In the next El Niño year (1987) his success was much higher.
Initial choice of mates appears to have been highly selective in that the first three mates were, like him, all FFS backcrosses, but produced on Daphne; two of them were sisters. Backcross individuals were very rare in the population, as these and another sister and a brother were the only ones known to us in 1987 from pedigree analysis. The second three mates of 5110 (1991–93) were all fortis (fig. 13.3).
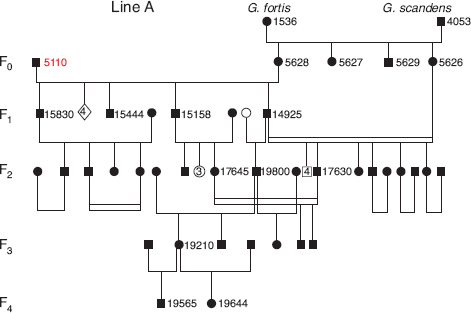
Fig. 13.2 The A line of descent from the immigrant male 5110 when paired with hybrid (backcross) females. Bird number 5110 initially bred in 1983 and 1984 with 4734, a female with a similar fortis × fortis-scandens (FFS) genetic constitution. This is omitted from the figure because only four offspring fledged from one of five nests with eggs, and they all died within a year. As shown in the figure, he then bred with the genetically similar 5628 (FFS) five times in 1987 and fledged eight young. He bred unsuccessfully with the sister 5626 in 1990. From generation 1 onward the members of the pedigree bred with each other (close inbreeding, indicated by parallel horizontal lines) or with members of the fortis population. Note: generations 3 and 4 are underestimated because the study of breeding from 1992 onward was not as comprehensive as beforehand. Male 19800 was the only known member of the pedigree to survive the 2003–4 drought. One member (19210) was alive in 2012. Symbols: squares, males; circles, females; diamonds, birds of unknown sex; filled, known by observation and/or genotype; open, unknown but inferred (see text). Multiples of nonbreeders of the same or unknown sex are shown by numbers within symbols. Birds 5110 and 15830 are illustrated in figure 13.1, and scandens 4053 is illustrated in figure 8.7.
Thus there were two lines of descent from the two groups of females, an A line from the backcrosses and a B line from fortis (fig. 13.1). They were connected at only one point. Paternity analysis with microsatellite data revealed that 5110 was cuckolded when breeding with 5821 (line B) by a son (14925) from line A. There are several instances in the pedigree of close inbreeding but no other known instances of cuckoldry in figure 13.1.
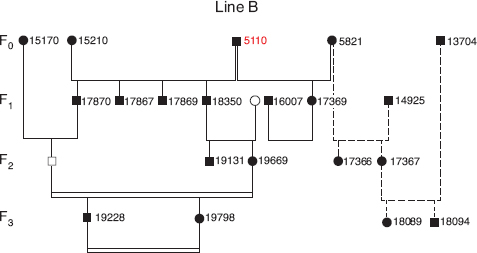
Fig. 13.3 The B line of descent up to generation 3 from the immigrant male 5110 when paired with three fortis females. In 1991 he bred with 5821. Two of three fledged young were sired by 14925, 5110’s son from the A line (fig. 13.2), and this pathway is indicated by broken lines. In 1992 he bred with 15210 and fledged at least five young. In 1993 he bred with 16594. This is not shown, because hatching and fledging success of their two eggs is unknown. Symbols as in figure 13.2. Birds 5110 and 5821 are illustrated in figure 13.10.
THE PHENOTYPIC UNIQUENESS OF 5110. Bird number 5110 was unusual in the two features identified in chapter 6 as being important in the choice of mates: morphology and song. In beak shape he resembled fortis more strongly than scandens, as expected given his genetic constitution. Large size and beak shape are likely to have been factors in the choice of 5110 by FFS females in the initial pairings, possibly also factors in the choice by fortis females. The role of song in choice of mates is not so easily assessed. His song combined a rapid down-sweep of the first note with repetitions of four to six notes (fig. 13.4). This resembles the songs of scandens to some extent but also one type of song sung by fortis, distinctly slower than scandens songs, which we refer to in the field as “chey-yey-yeh” (fig. 13.4) (Millington and Price 1985, Gibbs 1990, Grant and Grant 1996d). The origin of 5110’s song is unknown. We have never heard or recorded the song on other islands, nor did Robert Bowman (1983) in his extensive survey on many islands, and nor have Jeff Podos and Sarah Huber, who have spent much time recording ground finch songs on Santa Cruz Island (Huber and Podos 2006 and pers. comm.). The most likely explanation is that 5110 learned songs from his father and other birds of both species in his natal area, and produced a modified version of paternal song on Daphne, influenced to some degree by what he heard on Daphne during the crystallization phase of song production in his first breeding season in 1983. G. fortis males typically sing a perfect copy of their father’s song, but some learn and copy another type of song sung by other members of the population (fig. 8.3) (Grant and Grant 1996d).
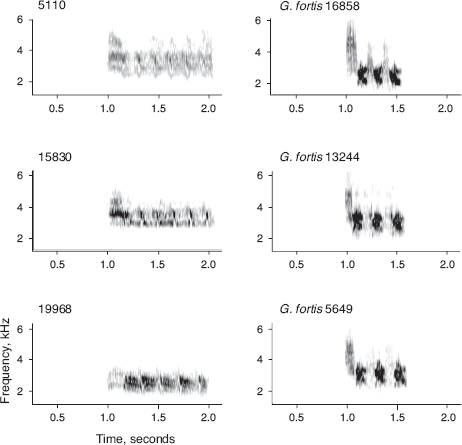
Fig. 13.4 Songs of the hybrid lineage and the most similar type of song sung by fortis, type III (“chey-yeh-yeh”; Gibbs 1990). A few songs of scandens resemble the hybrid lineage songs, perhaps as a result of copying, as they were not present on the island until 1993. From Grant and Grant 2009a.
PHASE II: GENERATIONS 1 AND 2
Not all members of the next two generations (F1 and F2) were genotyped; nevertheless it appears from our reconstruction that only the B line contributed to the lineage of large birds (fig. 13.3). This is surprising because 5110 was genetically more similar to members of the A line, which initially were a mixture of fortis and scandens genes, than to members of the B line. Our conclusions regarding lines of descent in these generations and later ones are based on observations of breeding pairs and genetic inferences (box 13.1 and appendix 13.1). The genetic inferences make use of the fortunate fact that 5110 was homozygous for a rare allele (183) at microsatellite locus Gf.11. The rarity of the genotype can be appreciated by comparison with other species: only 2 of 2,302 fortis, 10 of 956 scandens, and no fuliginosa (n = 43) or magnirostris (n = 590) were homozygous for the 183 allele at this locus.
In the B line of descent two of four sons (F1 generation) produced by 5110 × 15210 survived long enough to breed and produce the F2 generation. One of them (18350) bred with an unidentified female that may have been a fortis. The other (17870) bred with an identified fortis female (fig. 13.3). There was no known or suspected interbreeding with scandens.
Box 13.1. Pedigree
Here we explain the procedure for identifying missing members of the pedigree. When the genotypes of both parents are known but possible offspring have not been recorded at a nest, the procedure is straightforward. A search is made among the genotypes of all unknown birds for an individual with the combination of alleles that is consistent with inheritance from the known parents. The procedure is different when one parent and an offspring are known but the other parent is not known. All the alleles contributed by the known parent to its offspring are subtracted from the offspring’s genotype. The unknown parent must have contributed the remaining alleles. A search is then made among the genotypes of all known potential parents for these remaining alleles. Potential parents are excluded on the basis of mismatches of more than two base pairs at one or more loci. For example, by this subtraction-exclusion procedure we identified 19669 (generation 2) as the mother of 19228 and 19798 (generation 3; fig. 13.3). Importantly, no other bird’s genotype matched both of the genotypes of the two generation 3 birds except for 19131, a male that was not known to be alive in the breeding season of 2002. The father of 19669 was identified as 18350, but the mother could not be found among the genotyped birds. All known females of the A lineage were excluded. As explained in the text, song and size provide additional information for identifying positions of males in the pedigree. Appendix 13.1 provides more detail on the identification of breeders. Previous reconstructions of the pedigree have been revised as more connections have been established (Grant and Grant 2008c, 2009a).

Fig. 13.5 Generation 3 and a Big Bird family. Upper left: Father, male 19228. Upper right: Mother, female 19798. Lower left: Male 19228 cracking Tribulus cistoides fruits using the same technique as magnirostris (fig. 6.10). Lower right: Male 19228 feeding fledgling son 9807.
PHASE III: ENDOGAMY AND REPRODUCTIVE ISOLATION
The fates of the two lines of descent differed, and the A line will be discussed later. All members of the B line died in the devastating drought of 2003–4 except for two, a male (19228) and a female (19798) (fig. 13.5). Their genotypes matched the genotype of 19669. For this reason, and because they were homozygous (183/183) at locus Gf.11, we consider them to be siblings, members of the F3 generation.
The pedigree of generations F3 to F6 is shown in figure 13.6. All individuals in these generations were homozygous (183/183) at locus Gf.11. All those we were able to capture as adults and measure were large in body and beak measurements (figs. 13.7 and 13.8). All adult males sang the song sung by 5110 (figs. 13.4 and 13.9), and those that reached a mature black plumage were shiny black like 5110. The consistency in all four traits—genetics, morphology, song, and plumage—indicates that members of the lineage bred with each other (endogamy) and were thus reproductively isolated from fortis. In view of their large size in all dimensions we refer to them as the Big Bird lineage (fig. 13.10).
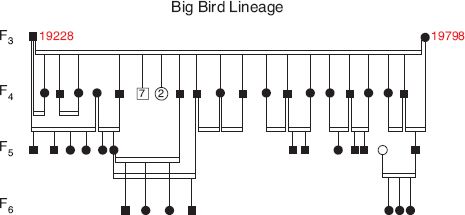
Fig. 13.6 The Big Bird lineage, an extension of the B line of descent from the original immigrant 5110 (fig. 13.3). Symbols as in figure 13.3. One pair has been omitted for simplicity: 19352 × 19742. They did not produce offspring. Note the original male 19228 bred with two daughters. In each case they were paired with brothers, so the offspring were extra-pair young.
Direct observations of breeding and behavior verify the conclusion of reproductive isolation. All pairs observed breeding in the years 2006 to 2012 were made up of members of this lineage: we observed no mixed pairs. As early as 1993, when the A line of descent was at its most numerous, most males established territories adjacent to each other (fig. 13.11). After 2005 the same pattern of contiguous territories was evident among males of the Big Bird lineage (fig. 13.12), with the majority of males in acoustic contact with at least one other. Playback of tape-recorded song and observations showed them to be aggressive to other members of the lineage that entered their territories but not to members of the other three species, which they ignored unless they approached to within a few meters of an active nest or a favored song perch. In these respects their behavior resembled the intraspecific aggressive behavior of fortis, scandens, and magnirostris.
All evidence thus points to reproductive isolation of the Big Bird lineage. Nevertheless males may have engaged in cryptic extra-pair mating with females of the other species (exogamy). If so we would expect offspring that carried the 183 allele to match their fathers at every one of the other 15 microsatellite loci. To test this we examined several hundred individuals of known genotype of fortis (n = 665), scandens (n = 432), and magnirostris (n = 308) captured in 2006–12. The frequency of the 183 allele was low in all three species: 0.04 in magnirostris, 0.08 in fortis, and 0.17 in scandens. None of them with the 183 allele matched any member of the lineage at the remaining loci. Moreover none of the fortis or scandens were large. We conclude that members of the Big Bird lineage bred with each other and not with members of the other species. Significantly, all known and suspected extra-pair fertilizations were within lineage (fig. 13.6). If this is not simply a reflection of the tendency to mate with extra-pair neighbors (appendix 5.1), the pattern is strong evidence for within-group mate choice. Close inbreeding prevailed, and average heterozygosity declined (fig. 13.13), but without loss of any of the 28 alleles present in the combined genotypes of the brother (19228) and sister (19798) of generation F3.
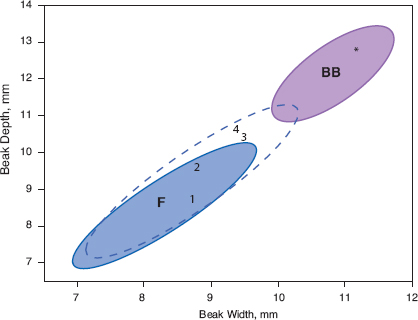
Fig. 13.7 Beak sizes of the Big Bird lineage in comparison with fortis present in the years 2006–12. Symbols: Big Birds in purple; fortis before the 2004 drought shown with a broken line (95% density ellipse) and after the drought in blue; asterisk 5110. Numbers refer to four female parents: 1 = 5628, mother of the A lineage (fig. 13.2); 2 = 15210, mother of the B lineage (fig. 13.3); 3 = 5821, another mate of 5110 whose offspring did not survive; 4 = 15170, mate of 5110’s son 17870 and grandmother of the Big Bird lineage (figs. 13.3 and 13.6).

Fig. 13.8 Big Bird in comparison with a Medium Ground Finch. Left: Big Bird 9807, distinctly larger than fortis and with a large, blunt beak despite being an immature only one to two months old, as indicated by the multicolored lower mandible. Figure 13.5 shows it being fed by its father. Right: fortis 19181, of typical size and proportions.
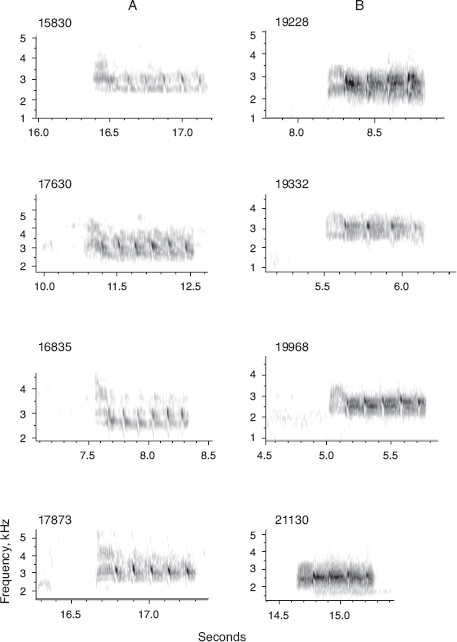
Fig. 13.9 Songs of several members of the two lines of descent (A and B) from the original immigrant male 5110, and the song of one scandens (21130) that sang a B lineage song in 2011. On the A line 15830 was a son of 5110, 16835 was his son, and 17630 was another grandson of 5110. Male 17873 was a grandson raised by 5110 but fathered by 5110’s son 14925 (see figs. 13.2 and 13.3). On the B line 19228 was the male in generation 3, and 19332 and 19668 were his sons. In addition 3 of 10 recorded A line males sang a fortis type 1 song (see fig. 8.2), whereas all 17 B line males (some not recorded) sang the same song as 5110. Note the subtle difference in tempo and pitch between the A and B line songs. The difference probably arose in generation 3 because there were no other B lineage males present when 19228 first sang.

Fig. 13.10 Big Bird lineage: males on the left, females on the right. Upper left: 5110, the original immigrant male. Upper right: 5821, one of 5110’s fortis mates, although not the mother of the lineage (photo not available)—it is included to indicate size and shape of the beak. Upper middle left: 19256 (generation f4). Upper middle right: 19566 (generation f4), long-term mate of 19256. Lower middle left: 19410 (generation f4). Lower middle right: 19321 (generation f5: offspring of 19256 × 19566 f4). Lower left: 19721 (generation f4). Lower right: 19364 (generation f4).
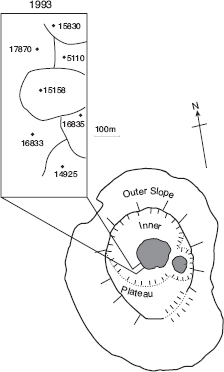
Fig. 13.11 A local community of the hybrid lineage in 1993. The original immigrant, 5110, established a territory here in 1983 and held it until 1994. Two of his sons that hatched in 1987 established territories in adjacent locations in 1991: 15158 and 15830. A third son, 14925, established a territory next to his brother 15158. Two of 14925’s sons that hatched in 1991, 16833 and 16835, established adjacent territories in 1993, and one of them, 16833, bred with a sister, 16834, from the same natal nest. Two other sons of 14925 established territories respectively ~50 m to the north (17630) and ~100 m to the northeast (17948) on the inner slope near the crater. All individuals were members of the A line of descent. One member of the B line, 17870, hatched in 1991 and established a territory next to his father 5110, while a brother (18350) hatched in 1992 and established a territory on the outer slope in sector 11 (fig. A.3.1). All males of the two lines sang the song of 5110. The pattern of aggregated and contiguous territories of relatives is highly unusual. In contrast only 4 of 251 fortis offspring (2 males and 2 females) that hatched in 1987 (1.6%) bred on territories adjacent to their parents’ territories. For scandens the numbers are 2 (both daughters) out of a total of 50 (4.0%). There is only one record in the sample of 80 fortis and 8 scandens grand-offspring of grandsons (2) establishing territories adjacent to parents and grandparents. The grandfather was a fortis × fuliginosa F1 hybrid. One of the grandsons bred with a granddaughter of 5110, thereby combining the genes of fortis, scandens, and fuliginosa in the offspring (Grant and Grant 2010b). Filled circles indicate nest locations. From Grant and Grant 2009a.
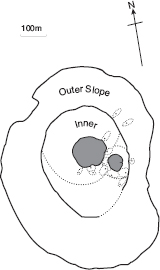
Fig. 13.12 Contiguous territories of Big Birds (line B) in 2010. Only one male of the A line (not shown) was known to be present at this time; it sang the type 1 song of fortis and held a territory on the outer slope in acoustic isolation from males of the Big Bird lineage. The distribution illustrates the fact that territories are held on the inner and outer slopes of the crater but not on the plateau (fig. 3.11), where the three other species breed.
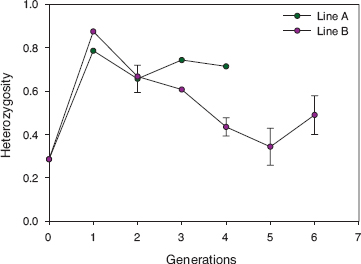
Fig. 13.13 Average multilocus heterozygosity of birds hatched in successive generations in the A line and the B line (Big Bird lineage), beginning with 5110 at generation 0. For each generation the fractions of 14 autosomal loci that are heterozygous have been averaged across individuals. A strong decrease in average heterozygosity in the B line followed a population bottleneck of a single breeding pair at generation 3 (figs. 13.3 and 13.6). Sample sizes for the following generations are 4, 19, 5, and 2 for the A line and 4, 6, 2, 27, 11, and 7 for the B line. Even though sample sizes are generally low, the difference between lines is very large when the largest samples are compared: generation 2 of the A line (n = 19) has twice the average heterozygosity of generation 5 of the B line (n = 27). Ninety-five-percent confidence limits are shown for samples larger than 6 individuals. For comparison, the long-term average heterozygosity of fortis and scandens is approximately 0.67 (fig. 10.12). Averages for each of the last three generations of the B line are significantly lower than the average at generation 2 for the A line.
Origin of Reproductive Isolation
Bird number 5110 was distinctive in morphology and song. These characteristics were passed on to successive generations through genetic and cultural transmission respectively. Viewed from an interspecific perspective, the differences between the Big Bird lineage and other species are twin components of a barrier to interbreeding. Tracing back the lineage from generation F5, we can ask when did the barrier to interbreeding arise? The answer cannot be the F1 generation, because sons had no surviving sisters, as far as we know, and therefore they had to breed with females of the fortis population. The barrier arose at generation 2 when 17870’s son bred with 18350’s daughter (19669).This was the first generation to have a choice between a relative and members of the fortis population. Reproductive isolation of the B lineage from fortis continued from then on. Intragroup mate recognition and endogamy in generations F3 to F5 may have been facilitated by the scarcity of potential fortis mates of appropriate size as a result of selection in 2003–4 (chapter 7).
Reproductively isolated from the other species, the B line individuals are behaving as a separate species. Regardless of whether they should be considered a separate species or not (box 13.2), they provide a valuable example and insight into one way in which a new species forms. The lineage originated allopatrically and became reproductively isolated sympatrically (box 13.3) at the F2 generation. Both members of the breeding pair were third-generation backcrosses, assuming correct identification of 5110 as a first-generation backcross. Subsequent generations of the lineage retained that genetic composition by breeding endogamously and forming an inbreeding swarm.
This is speciation of a special sort: introgressive speciation.
Fate of the A Line of Descent
Further insight into how the reproductive barrier arose is gained by comparing the two lines of descent. From generation 2 onward there was no interbreeding between members of the A and B lines; they were reproductively isolated from each other. Members of the A line, in contrast to the B line, bred with fortis and became integrated into the fortis population. Size may have played a role: members of the A line had a smaller (hybrid) mother than members of the B line (fig. 13.7), and from generation 1 onward were smaller on average than members of the B line (fig. 13.14) as a result of the repeated breeding with fortis (fig. 13.1). In contrast to the morphological difference between the two lines, males of both lines sang 5110’s song (fig. 13.9). Thus it appears that beak and body size were more important than song in choice of mates and hence as components of the barrier to interbreeding. A similar barrier occurs between fortis and the much larger magnirostris (chapter 8).
Box 13.2 A Question of Species
Discretely different from fortis in song, and almost so in beak size, the Big Bird lineage has functioned as a biological species through being reproductively isolated from fortis as well as the other species. Therefore the question arises as to whether it is appropriate to refer it as a new species. Several concepts of species, including the biological species concept, share the characteristic of a lineage that maintains continuity and integrity through successive generations. They have been unified and referred to as the lineage concept of species (de Queiroz 1998, 2011). The Big Bird lineage conforms to this concept of a species, and therefore it would be justified to give it a scientific name, such as Geospiza strenuirostris. The only problem with doing so is the short life of the lineage as a reproductively isolated entity. How many generations of exclusively within-group mating are needed before the group is recognized as a separate species? The answer has to be arbitrary. If, say, the number 10 is chosen, then after 10 generations the conclusion will be reached that the lineage has achieved the status of a separate species, and it actually did so at generation 2! Faced with this conundrum, we maintain only that the Big Bird lineage is functioning as a reproductively isolated species, a species “in the making,” and has done so for five generations. Of greater interest and importance than its taxonomy and nomenclature are the insights it provides into how a new species arises and either persists or becomes extinct.
Initially members of the A line were partly isolated reproductively from fortis and apparently heading in the same direction of complete isolation taken by the B line. The tendency for the A line male territories to be contiguous, even clustered, in 1993 (fig. 13.11) suggests that the males recognized each other as distinct from the other species. Song may have played a major role in this recognition. Females possessed the same recognition as the males, as suggested by four pairings of close relatives. These two features of territory contiguity and close inbreeding also characterized the B line. However, isolation of the A line from fortis weakened and eventually disappeared; they became absorbed into the population of fortis. Only one member of line A, 19800 (a male), survived the 2003–4 drought, and he died in 2008. He and his known descendants continued the trend of breeding only with fortis up to our last study of breeding in 2012. Thus lines A and B had different fates, and we suggest the reason for this was their difference in size (fig. 13.14).
Box 13.3 A Question of Speciation
The reproductive and ecological isolation of the Big Bird lineage on Daphne has allopatric and sympatric elements. It does not fall neatly and discretely into categories of allopatric or sympatric species, because the hybrid lineage initiated by the interbreeding of fortis and scandens on one island became reproductively isolated from the two parental species on another island. The isolation owes its origin to introgressive hybridization on Santa Cruz Island. The hybrid immigrant possessed the traits, passed on to subsequent generations with little alteration, that conferred success on the lineage. This conforms to the classical allopatric model of speciation, in which evolution in allopatry, specifically adaptive divergence, facilitates coexistence in sympatry at the secondary contact stage of a speciation cycle (e.g., Sobel et al. 2010, Harrison 2012), with or without continuing gene flow or further divergence in sympatry (chapter 1). In other respects speciation was essentially sympatric, because the assortative mating and the barrier to interbreeding arose only in sympatry (e.g., see Dieckmann and Doebeli 1999, Ryan et al. 2007, Huber et al. 2007, Ito and Dieckmann 2007, Grant and Grant 2009b, Hendry et al. 2009, van Doorn et al. 2009). The traits that constitute the barrier, distinctive morphology and song, were possessed by the immigrant. The barrier was not sufficient to prevent interbreeding at generation 0, for had it been so the immigrant would have died without reproducing. Instead the barrier became effective only after a potential mate (daughter) had been produced at generation 1 by outbreeding. In other words the reproductive barrier was latent. There was no evolution of morphology or song from generation 1 onward, therefore no evolution of a reproductive barrier in sympatry. The development of reproductive isolation of the Big Bird lineage was not typical of allopatric, sympatric, or ecological speciation, although it comprised elements of all of them.
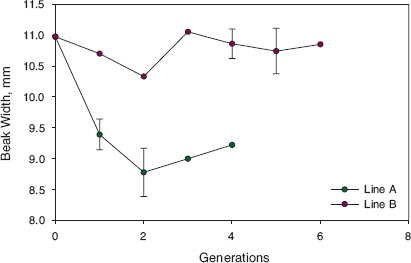
Fig. 13.14 Average beak width of birds hatched in successive generations in the A line and the B line (Big Bird lineage). The founder, 5110, is the sole member of generation 0 for both lines. Large average size persisted across generations in the B line, in part due to the high frequency of inbreeding, whereas average beak width of the A line fell in generation 1, owing to 5110 breeding with a small backcross female (BW = 8.2 mm), and remained at approximately that size for the following generations. The difference in generation 1 is pronounced. Measurements of 8 line-A birds (maximum 9.8 mm) all fall well below the measurement of the single line B bird (10.7 mm). Ninety-five-percent confidence limits on estimates of the means are shown with vertical lines for only those based on samples larger than 5 individuals. Sample sizes are 8, 10, 3, and 2 for the four generations of the A line and 1, 2, 2, 25, 8, and 2 for the B line. Sex is known for most but not all birds. Corrections for sexual dimorphism (Price 1984b) have not been applied; they are too small to account for the differences between lines.
Success of the Lineage So Far
The Big Bird lineage has persisted for more than 30 years since the arrival of its founder in 1981 (fig. 13.15). What determined its success? Intrinsic factors associated with the hybrid nature of the genome, conferring hybrid vigor, could be part of the answer. Extrinsic factors affecting survival are a second part.
INTRINSIC FACTORS
We have no direct measure of an intrinsic factor affecting vigor or robustness. If longevity can be considered a reflection of an intrinsic property of vigor, as opposed to a response to extrinsic ecological opportunity (chapter 6), then Big Birds showed signs of vigor, starting with 5110 who lived for 13 years. Finches rarely live this long. Only 0.62% of fortis that hatched in 1976–98 and who survived their first year (n = 1,445) lived for 13 years or more. Moreover the Big Birds appear to have survived the severe drought of 2003–4 relatively well, even though this amounted to only two birds out of a known eight (25%) in the B line. Survival of banded birds of the other species was 18.5% fortis (n = 173), 25.4% scandens (n = 110), and 3.9% magnirostris (n = 122). The two drought-surviving Big Birds, 19798 and 19228, lived for 8–9 and 9–10 years respectively.
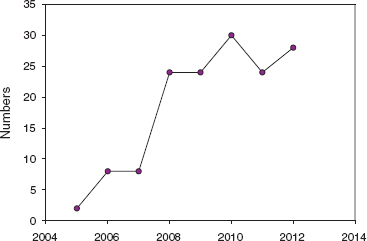
Fig. 13.15 Numbers of adult Big Birds after the 2003–4 drought and censused in January and February each year. The sex ratio was approximately equal among banded birds: 24 males and 21 females, summed across years. Included are an estimated 12–15 additional birds that were not banded but recognized individually by subtle plumage and beak-color features. Adult males outnumbered females throughout the growth of the population, not through differential production but through differential survival: the sex ratio of genotyped nestlings and fledglings was, if anything, female-biased (4 males : 8 females). In 2011 the number of breeding females was reduced to 6, but there were more in 2012 at our last census early in the breeding season.
Genetic composition provides another reflection of vigor. The genome of 5110 is expected to be unusually heterozygous owing to its hybrid origin. In fact, surprisingly, it was unusually homozygous: 10 of the 14 autosomal microsatellite loci were homozygous. To put this in perspective only 0.66% of the total fortis sample (n = 2,123) were homozygous at 10 or more of the 14 loci. Low heterozygosity at the microsatellite loci does not necessarily contradict the notion of hybrid vigor. It may reflect genome-wide transgressive segregation, in which beneficial alleles are combined in homozygous form in the hybrid genome (Rieseberg, Widmer et al. 2003, Stelkens and Seehausen 2009). The result is a genotype and phenotype beyond the range of both parents and potentially with high fitness.
EXTRINSIC FACTORS
Success of the lineage after 2005 was enhanced by the heavy mortality experienced by the other species in the drought of 2003–4. More niche space for the Big Birds opened up with the morphological and ecological divergence of fortis and magnirostris (character displacement) in 2004–5 (chapter 7). As a result the Big Birds occupy ecological and morphological space in a zone between the other species (fig. 13.16). For example, in average beak width they are approximately equidistant from fortis (27.7% smaller), scandens (25.7% smaller), and magnirostris (37.2% larger). Lineage members have a distinctive generalist diet. They crack open Tribulus fruits (fig. 13.5), like magnirostris (fig. 6.10), feed on a variety of small seeds picked up from the ground, and feed on the seeds, nectar, and pollen of Opuntia (fig. 13.17). They differ from magnirostris in feeding on Opuntia flowers, they differ from scandens and most fortis in feeding on Tribulus seeds, and they differ from large fortis in efficiency when opening Tribulus fruits.
Future Prospects
It is unlikely that the drama we have witnessed will result in a long-lasting species. The small population may become extinct through the deleterious effects of inbreeding (e.g., Nieminen et al. 2001), such as those shown by magnirostris in the first few generations (chapter 6), and demographic stochasticity. Nevertheless it need not be doomed; rare interbreeding with fortis or scandens or even magnirostris could provide an escape from those effects. Two examples involving males that were apparently imprinted on another species hint at how this could happen. One male fortis (18570) sang the typical song of the lineage in 1993 and paired with a granddaughter of 5110 on the A line. Breeding success was unknown. One male scandens (21131) was recorded singing the song of the lineage in 2011 and bred with a female that looked like a scandens × fortis hybrid but was not captured. Genetic rescue, combined with exploitation of a unique, generalized niche, may enable the Big Bird lineage to flourish for quite some time. Future prospects are not necessarily bleak. They are discussed further in the next chapter.
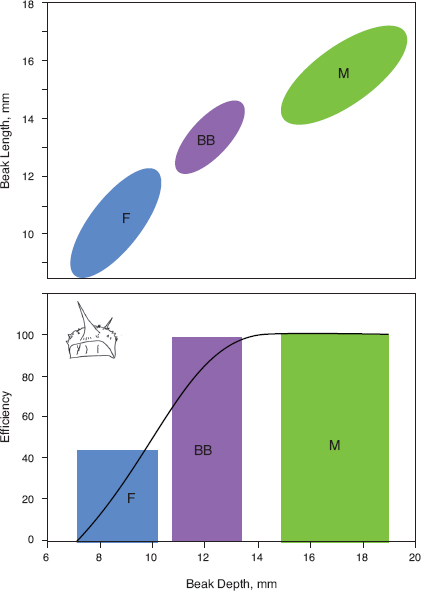
Fig. 13.16 Morphology and diet. Upper: Morphological separation of the hybrid (Big Bird) lineage (BB) from fortis (F) and magnirostris (M), illustrated with 95% density ellipses. Lower: A composite curve of feeding efficiency on the woody fruits of caltrop, Tribulus cistoides, in relation to beak depth of fortis, magnirostris, and the BB lineage, averaged from numerous timed observations. G. scandens, with different jaw musculature (Bowman 1961), does not crack caltrop fruits. Efficiency is defined as 1/CT: CT = time to crack the fruit in seconds (Grant 1981b). A minimum beak depth is needed to crack a fruit, and several fortis have smaller beaks than the minimum. G. magnirostris apparently do not vary in their efficiency in relation to beak depth or other dimensions. Their efficiency has been scaled to 100.
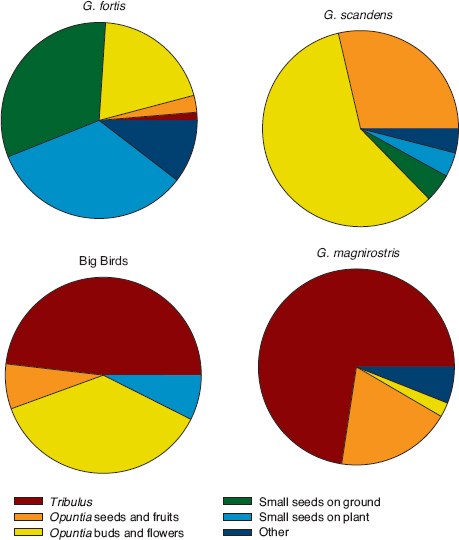
Fig. 13.17 Diets based on feeding observations of the following birds, one observation per bird, 2006–11: 190 fortis, 27 Big Birds, 140 scandens, and 84 magnirostris (see also fig. 6.11).
Summary
The chapter discusses the possibility of speciation occurring through hybridization and the development of reproductive isolation of a hybrid lineage from the two species that gave rise to it. In 1981 a fortis × scandens hybrid male, probably a backcross to fortis, immigrated from the nearby large island of Santa Cruz. It was exceptionally large and sang a unique song. We followed its fate and the fate of most descendants for the next 30 years by making observations of breeding pairs, banding their offspring, and inferring relatedness by using a genetic marker. The immigrant initially bred with a hybrid female, giving rise to one line of descent in subsequent generations (line A). He later bred with fortis females, and one of them gave rise to a second line of descent (line B). The two lines experienced different fates, probably owing to differences in average body size. The A line (smaller birds) became reproductively absorbed into the fortis population, whereas the B line became reproductively isolated from the other species. Members of the B line were reduced to a single brother-sister pair by a severe drought and heavy mortality in 2003–4. From 2005 onward they and their offspring bred endogamously, and never with fortis or scandens. Ecological success and reproductive isolation of the lineage were enhanced by heavy mortality in the 2003–4 drought of their principal competitors, magnirostris and large-beaked members of the fortis population. These observations provide insight into speciation and hence into the origin of a new species. They show how a single diploid immigrant can start the process by breeding with a resident species; tolerance of the effects of inbreeding is needed to sustain and complete the process. The barrier to interbreeding arose behaviorally and without genetic change in sympatry. This is a rare example of speciation by introgressive hybridization: introgressive speciation.
















