8
Hybridization
The extraordinary variants that crop up in a series [of museum specimens] give an impression of a process of change and experiment going on.
(Swarth 1934, p. 231)
It would be of great interest to determine the critical factors controlling the variability of each species, and to know why some species are so much more variable than others.
(Lack 1947, p. 94)
Introduction
OUR STUDY ON DAPHNE BEGAN as a small part of an archipelago-wide attempt to understand the evolutionary diversification of the finches. Since the strength of the evolutionary response to natural selection is governed by the amount of genetic variation (chapter 3), morphological averages should not be considered in isolation from the variances. Average beak size differs between species as well as between populations of the same species (fig. B.1.2), and so do phenotypic variances (Lack 1947). For example, in chapter 3 we showed that fortis vary more than scandens phenotypically and genetically. These contrasts raise the question of why some populations vary in morphological traits such as beak size much more than others and hence have greater potential for evolutionary change. Up to now we have discussed factors that are relevant to the average size and shape of beaks. In this chapter and the next we explain how addressing questions of population variation, that is, variation among members of the same population, led to a study of hybridization.
Background
Daphne is a good choice for the variation question, even if it is not ideal. The coefficient of variation for beak width of fortis males in museum collections is 6.48 (n = 31) (Grant et al. 1985). On other islands it varies from 3.63 (Fernandina, n = 14) to 10.98 (Floreana, n = 276) with an average of 7.08 for 12 populations with sample sizes greater than 10 individuals. The coefficient of variation for scandens males in museum collections is distinctly lower, and varies from 4.25 (Floreana, n = 141) to 6.44 (Marchena, n = 10) with an average of 5.78 for 10 populations. There are too few specimens from Daphne (box 2.2) for calculating the coefficient, but a large sample of live birds (both sexes) from Daphne (n = 570) has a much smaller coefficient (4.53) than the comparable one for fortis (7.76, n = 881; Grant and Grant 2000a). Thus fortis is a more variable species than scandens. Why is this?
When we started in 1973, there were two major ecological ideas to explain differences in variation between natural populations in traits that vary continuously. One hypothesized a role for diversifying selection in heterogeneous environments, and was explicitly adaptive (Van Valen 1965, Roughgarden 1972; more recently Bolnick et al. 2003, Araújo et al. 2011): the more heterogeneous the environment, the more variable the population as a result of advantages experienced by extreme individuals relative to those with average characteristics (fig. 8.1). The other suggested that gene exchange between slightly differentiated populations could elevate the variance in each one without the variation in either being adaptive; populations that exchange genes vary more than those that don’t (Soulé 1971, 1972).
Hybridization, which is one form of gene exchange, was not part of mainstream discussions of population variation at that time. Introgressive hybridization, that is, hybridization followed by backcrossing to a parental species, was considered to be generally rare and inconsequential in animals (Mayr 1963), although common in plants. Where it was observed, it gave rise to concerns about the loss of genetic identity and uniqueness of a species. This was appropriate in situations where invasive species hybridized with local residents in human-altered habitats (Cade 1983, Rhymer and Simberloff 1996). Lack (1945, 1947) looked for field evidence of hybridization in the Galápagos, failed to find it, and rejected it as a cause of the small size of fortis on Daphne (chapter 2). Although hybrid finches were produced in captivity, they died within a week of hatching (Orr 1945). Nevertheless, we paid attention to the natural breeding of finches at the start of our study because neither of the two ecological explanations seemed fully satisfactory. Our reasons were, first, Daphne appeared to be too small and homogeneous for a variable population to be maintained by diversifying selection, and second, the island appeared to be too isolated (by 8 km) from the nearest island to receive many, if any, immigrants.
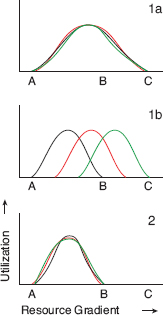
Fig. 8.1 Upper: Graphical models of the way resources are exploited by members of a species. A broad range of resources (A–C) is exploited by populations 1a and 1b; in the first, individuals are generalists; in the second, they are specialists. A narrow range of resources (A–B) is exploited by all members of the specialist species 2. Overlap of individuals in 1b is shown to be symmetrical, for simplicity. In fact overlaps are likely to be asymmetrical. From Grant et al. 1976, based on the adaptive variation arguments of Van Valen 1965 and Van Valen and Grant 1970. Alternatively specialist species might be divided into different specialist individuals (Bolnick et al. 2010).
Our first study of breeding in 1976 yielded evidence of hybridization (Grant and Price 1981, Boag and Grant 1984b). In addition to fortis and scandens there were 14 fuliginosa individuals breeding. This was a little surprising because, although very small numbers of this species had been reported on the island on previous visits (Harris 1973; box 1.3), it was not known to breed there. What was even more surprising was that eight fuliginosa females bred with fortis males. Furthermore their eggs hatched, and the young fledged. This was the first demonstration that the reproductive barrier between finch species in nature was not absolute. It raised a set of questions concerning the circumstances and consequences of interbreeding that are relevant to the potential role of hybridization in causing an elevation of morphological variances. This chapter, which focuses on the causes and fitness consequences of hybridization, leads into the topic of morphological consequences in the next chapter. Our study shows that a low level of hybridization and introgression occurs naturally in pristine, undisturbed, habitats. It gives insights into the degree to which genetic variation of a population is enhanced, particularly when species of different genetic correlations and allometries hybridize. It also gives insights into the origin and dynamics of reproductive isolation, whether partial or complete.
Frequency of Hybridization
Hybridization occurred in most years of breeding between 1976 and 1995, and several later years, but was always rare (Grant and Grant 1992b, 1996d, 1997a). The numbers of interspecific pairs across those 20 years were 45 fuliginosa × fortis and 8 scandens × fortis. These numbers contrast with numerous intraspecific pairs; 23 fuliginosa, 1,268 fortis, and 562 scandens. Thus 2.8% of fortis males and 2.3% of females paired with fuliginosa, and an additional 0.1% of males and 0.8% of females paired with scandens. The fortis × fuliginosa pairs comprise 3.4% of pairs involving fortis but 65.2% of pairs involving fuliginosa. Despite that, the proportion of fuliginosa pairs is much higher than would be expected if fortis and fuliginosa were to mate at random (Grant and Grant 1997a). Males and females of fortis and fuliginosa hybridized at approximately equal frequencies, on average, whereas scandens males hybridized consistently more frequently than scandens females.
These estimates come from observations of adult males and females attending nests. They rest on the assumptions that we identified the species correctly by morphology (box 8.1), and that we identified paternity correctly (box 3.1).
Causes of Hybridization
Why do species hybridize? Two plausible explanations for Darwin’s finches are, first, a scarcity of mates, and second, perturbation of the process of sexual imprinting, when offspring learn characteristics of parental phenotypes early in life and select mates with those characteristics. The two possibilities are considered in turn.
A SCARCITY OF CONSPECIFIC MATES
A relative scarcity of mates could explain why a rare species (fuliginosa) of either sex hybridizes with a common species (fortis). This is sometimes referred to as the Hubbs principle (Mayr 1963, Grant and Grant 1997a, Randler 2002), because it was invoked by Carl Hubbs to explain hybridization in fish species in terms of encounter rates (Hubbs 1955). Hybridization of scandens is also consistent with the principle. Even though scandens were not rare, males outnumbered females at all times, many failed to obtain mates, and male scandens (seven) hybridized more than females (one). But why did the common species, fortis, hybridize? The sex ratio of fortis was usually close to 1:1, except after the drought of 1977 (chapter 4). One possible answer is a scarcity of available mates at the end of the pairing period. As the supply of potential mates declines, the last few individuals remaining unmated might lower a critical threshold of mate acceptability and accept a heterospecific mate. Moreover the last few individuals could be young and inexperienced, with incompletely developed mate recognition systems. These plausible expectations were tested with observations on the temporal pattern of pairing, but not supported (Grant and Grant 1997a). Interspecific pairs formed throughout the pairing formation period, and therefore a scarcity of mates does not explain why fortis hybridized. Possibly female fortis chose heterospecific males holding high-quality territories, but we have no measure of territory quality to assess this. For example, territory quality confers direct benefits to hybridizing flycatchers in Europe (Wiley et al. 2007).
Box 8.1. Identifying Species
At the beginning of the study fortis and scandens clearly differed in morphology. G. scandens could be recognized unambiguously by long beaks, both absolutely and relative to beak depth or width (fig. 8.7). The males of each species could also be identified unambiguously by their songs (figs. 8.2 and 8.5). On the other hand identifying fuliginosa gave us a problem in that there were no discrete differences in morphology between fortis and fuliginosa; the frequency distributions ran into each other without a break, with the result that the combined frequency distribution had a long tail at the lower end of the axis of beak or body size measurements (fig. 9.6). Nor are the songs diagnostically different.
To identify fuliginosa individuals, we therefore adopted an operational definition by using the frequency distributions of fuliginosa and fortis measurements on the northern coast of Santa Cruz Island at Borrero Bay, where the distributions discretely differ (Grant 1993). The upper measurements of fuliginosa beak dimensions on Santa Cruz were used as markers for classifying individuals on Daphne as fuliginosa or not fuliginosa. Those individuals classified as not fuliginosa were assumed to be either fortis or fortis × fuliginosa hybrids. Pedigrees were subsequently used to assign individuals to species and hybrids. F1 hybrids and backcrosses were identified when the parents were known from observations of adults that were incubating eggs (females only) or feeding nestlings and fledglings (both social parents). All birds—F1 hybrids and backcrosses as well as nonhybrids—mate according to paternal song type (box 8.2); therefore F1 hybrids and backcrosses, including those that failed to breed, could be assigned to one population or another. We considered the 1983 populations as the baseline for studies of hybrids because we did not know if hybridizing individuals were in fact pure species, F1 hybrids, or backcrosses. In the next two chapters (9 and 10) we discuss tests of hybrid identification with genetic data, and identification of island sources of fuliginosa immigrants that sustain the population on Daphne.
IMPRINTING
Playback experiments with song and mount experiments showed that finches on Daphne can discriminate between their own and another species using either visual or acoustic cues alone (Ratcliffe and Grant 1983, 1985). Furthermore, experiments with captive finches have demonstrated that males learn their short adult song early in life from a male they interact with socially (Bowman 1983). Mates are chosen according to species-specific song and morphology, which are learned early in life though imprinting on parents and occasionally others (box 8.2). Hybridization can occur after young birds of either sex imprint on an adult of a different species and use the learned cues in choosing a heterospecific mate. Heterospecific imprinting is sometimes referred to as misimprinting (Grant and Grant 2008a). Song plays a significant role. Song is probably an important factor in the interbreeding of fortis and fuliginosa because their songs are similar. However, fuliginosa immigrate to Daphne, so we do not know the origin of their songs and the learning environment.
SONG INHERITANCE
Males, only, sing a single, structurally simple song, which is retained unaltered for life (fig. 8.2). Both sons and daughters learn songs, mainly from their fathers, according to (1) laboratory studies, (2) father-son resemblances (fig. 8.3), and (3) pairing patterns in nature (box 8.2). Learning takes place during a short sensitive period extending from day 10 to day 40 after hatching. On a coarse scale songs of fortis can be classified into four types; 72% of 263 sons sang the same type as their fathers (Grant and Grant 1996d). However, there is variation within song types, and the song types are not discretely different, so we measured eight variables to quantify temporal and frequency features of songs in order to analyze variation with principal components analysis (PCA).
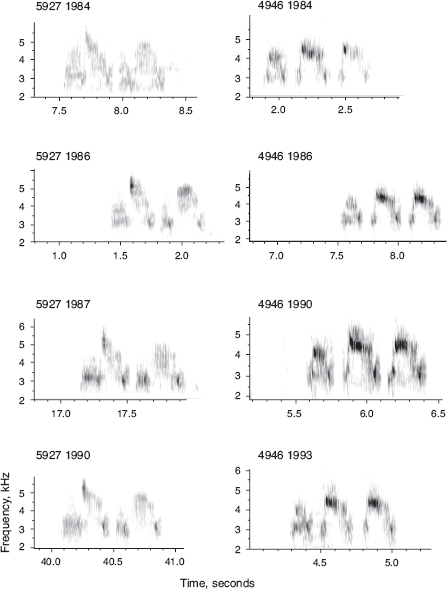
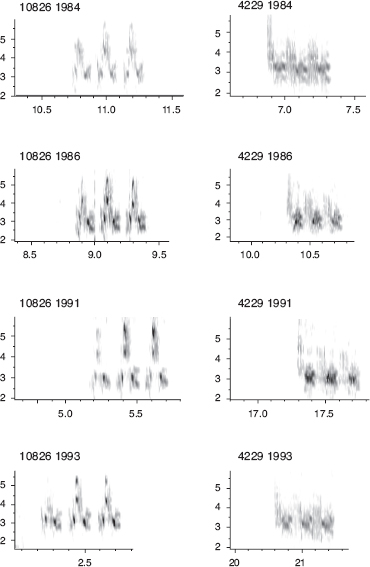
Fig. 8.2 Four song variants sung by fortis. Repeated recording across years shows that an individual’s song is retained for life. Adapted from Grant and Grant 1996d, 2008a.
Family resemblance could be due to transmission of genetic factors in addition to learning. If so we would expect the songs of sons to resemble not only the songs of their fathers but also the songs of both paternal and maternal grandfathers, whereas with learning there should be no relationship between the songs of sons and maternal grandfathers. We used PCA to discriminate between these alternatives. The resulting regressions supported the hypothesis of learned cultural transmission from father to son and gave no support to the alternative of genetic transmission (fig. 8.4). Variation in song among scandens individuals is not so striking; nevertheless the father’s song does predict the length of the first note sung by the sons (Grant and Grant 1996d) as well as trill rate (Grant and Grant 2010c). G. magnirostris sons sing the same song type as their fathers (chapter 6). We know of only a single exception.
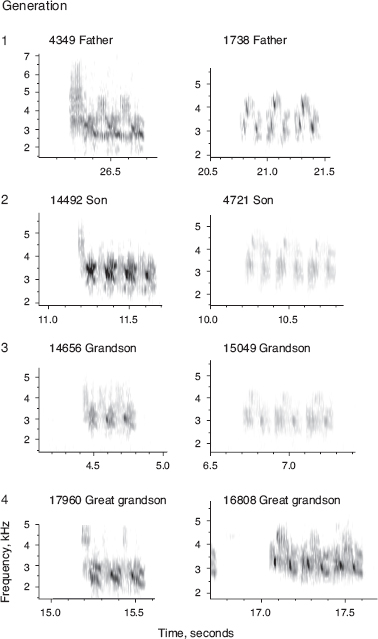
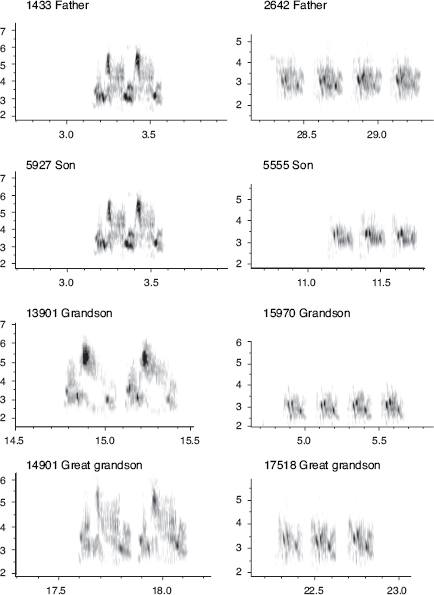
Fig. 8.3 Songs of fortis sons usually resemble the songs of their fathers. From Grant and Grant 1996d, 2008a.
Box 8.2 Cues Used in the Choice of Mates
The six species of ground finches have similar plumage, and do not differ consistently in patterns of courtship behavior, but do differ conspicuously in size and shape and in song. They have apparently identical mate recognition systems, yet pair conspecifically solely as a result of learning the specific features of their parents or similar models (Grant and Grant 1997a, 1998, Podos 2010). Experiments with stuffed museum specimens showed that individuals discriminate between their own and another species on the basis of beak size and body size in the absence of movement and acoustic cues (Ratcliffe and Grant 1985). A similar set of experiments showed that they also discriminate between species on the basis of song alone in the absence of movement and visual cues (Ratcliffe and Grant 1983).
Visual cues of appearance (beaks) are inherited genetically (chapter 3), whereas acoustic cues are inherited through learning, that is, culturally. Only males sing. By raising finches in soundproof chambers and playing tape-recorded conspecific or heterospecific song to them, Bowman (1983) found that song is learned during a short sensitive period early in life between day 10 and day 40 post-hatching, in other words for a few days in the nest and then for the first month as fledglings when dependent on parents for food. The song is learned mainly from the father, and once learned it is retained for life. We presume the appearance of the parents is also learned at this early time of life in association with species-specific song. This is an imprinting process that influences mating behavior later in life (the evolutionary significance is reviewed in Irwin and Price 1999, ten Cate and Vos 1999, Slabbekoorn and Smith 2002, and Price 2008). An important part of the process is learning that song and acoustic cues of similar sympatric species are different from their own species, as shown experimentally with the Blackcap (Sylvia atricapilla), a European warbler, learning the features of the Garden Warbler (S. borin) (Matyjasiak 2005).
There is some evidence of nonrandom mating within species with respect to size, but it is not strong (Grant and Grant 1997a, Grant and Grant 2008c). The evidence is correlations between the body size of parents and the mates of either their sons or daughters (Grant and Grant 2008c). The strongest associations are between fathers and the mates of their sons. In some years males change mates and the second mate is similar in size to the son’s father whereas the first is not. With regard to song, the mating rule for females is that they pair only with a male of the same or different species that sings the same species song as their father. For example, of 90 scandens females for which both the father and the mate were recorded, 86 mated with scandens males that sang scandens songs, while the remaining 4, all daughters of a scandens male that sang a fortis song, mated with fortis males. Rare exceptions to the rule, detectable only when the song of mate and father are known, amount to 2 of 482 fortis females (Grant and Grant 1996d). For non-random mating in the fortis population on Santa Cruz see Podos (2010), and for a general review of learning, sexual selection, and speciation see Verzijden et al. (2012).
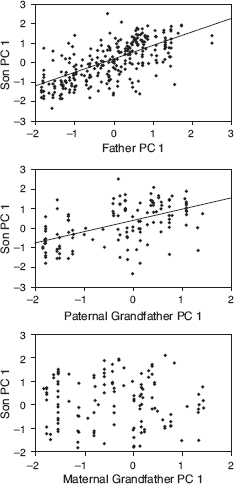
Fig. 8.4 Songs of fortis sons resemble the songs of their fathers and paternal grandfathers but not maternal grandfathers, indicating cultural but not genetic inheritance. The slope of the son-father relationship is 0.725 ± 0.047 s.e. (r = 0.671, p < 0.0001), based on 273 father-son pairs involving 133 different fathers. The slope of the son–paternal grandfather relationship is 0.540 ± 0.076 (r = 0.523, p < 0.0001), based on 136 son–paternal grandfather pairs involving 47 different paternal grandfathers. The slope of the son–maternal grandfather relationship is 0.036 ± 0.101 and not significantly different from zero (r = 0.032, p > 0.1), based on 124 son–maternal grandfather pairs involving 45 different maternal grandfathers. The slope of the paternal grandfather regression (0.540) is close to the expected value (0.525) from the square of the son-father regression (0.725). From Grant and Grant 1996d.
PERTURBATIONS OF IMPRINTING
Species differ in song characteristics (compare figs. 8.2 and 8.5), so under the normal imprinting process unambiguous song cues to species identity are learned in association with morphology and later used at the time of courtship and mate choice. Imprinting in effect sets up a premating barrier between species. However, the barrier is not impermeable; it occasionally leaks.
The normal learning process is vulnerable to perturbation if a young bird hears and learns the song of another species during its sensitive period. This we have observed happening in three ways, each rarely. First, a nest with one or more eggs is taken over by another species, and the young that hatch learn the foster father’s song. We know of one case where a fortis nest with an egg was taken over by a scandens and a young fortis was raised to fledging and sang the song of its foster father. Second, if the father dies or disappears toward the end of the nestling period, the young birds may imprint on their natal neighbor, which can be a member of another species: we know of three such cases. Third, a dominant heterospecific neighbor may repeatedly chase the father away from its nest and then sing, with the result that the young imprint on that song. We know of one case where a magnirostris influenced the subsequent song of two juvenile males out of three from two fortis nests (fig. 8.6): the third sang the same song as his father’s. Thus perturbation of the normal learning process can result in the son singing a heterospecific song, even when the father sings a normal song. However, for most cases of heterospecific singing we do not know how the imprinting occurred (box 8.3).
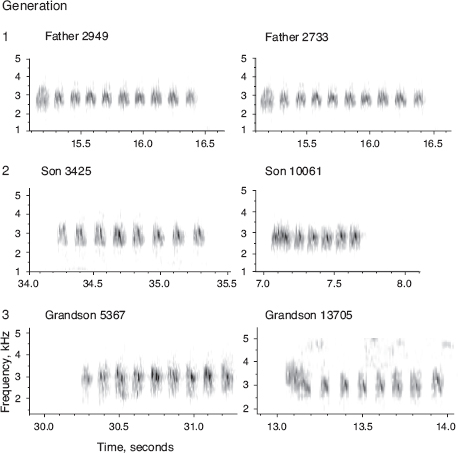
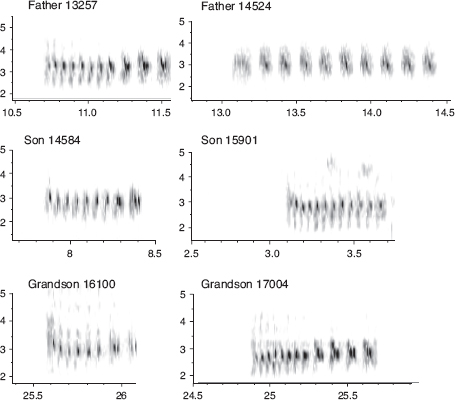
Fig. 8.5 Songs of scandens. Close copying of father’s song by sons is a rule that is occasionally broken. Songs of males 15901 and 13705 deviated from their respective fathers’ songs. From Grant and Grant 1996d.
Imprinting on another species can lead to hybridization (fig. 8.7) but does not always do so. Heterospecifically imprinted males transmit ambiguous signals, in having the song of one species and the morphology of the other. As a result not all hybridize: some mate conspecifically. Over a period of more than 20 years we noted 11 instances of fortis and scandens males singing each other’s (heterospecific) song, and three of them paired heterospecifically (Grant and Grant 1996d, 1998). Apparently some females are more influenced by morphology than by song when the two sets of cues are in conflict, and for others the reverse is true. An example is given in figure 8.8. Two fortis sisters paired with different heterospecific mates even though the father sang a normal fortis song. We presume the normal imprinting process was perturbed after fledging. In both cases the morphology of the mates was just beyond the normal intraspecific range, and in one case the song was clearly beyond the normal conspecific range.
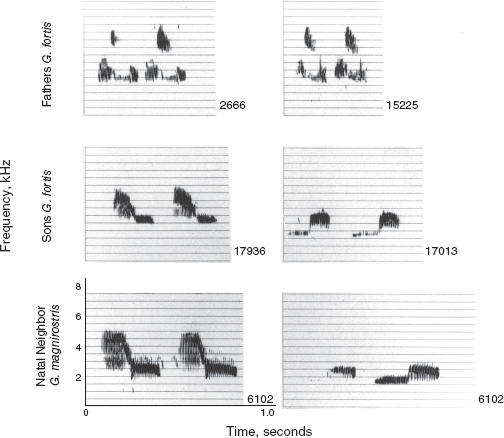
Fig. 8.6 Heterospecific copying of song. Two fortis (17936 and 17013) copied the two songs sung by a neighboring magnirostris male (6102) and not the songs sung by their respective fathers (2666 and 15225). Their fathers were adjacent neighbors, and the magnirostris territory overlapped both fortis territories. Male 15225 was the son of 2666— a rare case of a son occupying a territory next to that of his father. The magnirostris is also a rare case of an individual singing two songs. In neither case did the imprinting lead to hybridization. In contrast to 17013, a brother (17011) raised in the same nest was not imprinted on the magnirostris song. He sang the same song as his father and paired with a fortis female. From Grant and Grant 1996d.
Such variation in the pattern of mating gives no indication of genetically based mate preferences. Controlled experiments would be needed to expose them if they exist. Experimental studies of imprinting on other species by cross-fostering have given similar, nonuniform, results in the pattern of mating (Clayton 1990, Slagsvold et al. 2002).
Box 8.3 Heterospecific Imprinting as a Cause of Hybridization
The general impression is one of heterogeneity, and at best only partial predictability, in the pattern of hybridization. This could be a consequence of the fact that heterospecifically imprinted birds, like F1 hybrids themselves, have a broader morphological range of potential mates from which to choose (Gee 2003) as a result of early experience in or out of the nest. Two examples illustrate this possibility. First, two sisters from the same nest paired with males of different species (fig. 8.8). Second, finches that pair heterospecifically may change mates and pair conspecifically. For example, a female fortis paired first with a scandens male, then a fortis male, returned to the first scandens male, and following his death paired with a new fortis male (Grant and Grant 1997a). Experience after leaving the nest, or possibly even off the natal territory, may occasionally modify the canalizing influence of early experience. For example, sexual and aggressive interactions may influence choice of a mate (Bischoff and Clayton 1991).
G. MAGNIROSTRIS
G. magnirostris did not hybridize with the other species at any time throughout the study, in spite of nine fortis learning and singing a magnirostris song (fig. 8.6). Female magnirostris, being twice their size, may not have recognized them as potential mates, but often they were not given a chance to explore courtship possibilities, because whenever a fortis sang a magnirostris song it was immediately and repeatedly chased and harassed by magnirostris males. The only one of these fortis males that acquired a mate did so after reducing song output and singing at low volume and low in the bushes: the mate was a fortis. A single scandens male imprinted on a magnirostris song (fig. 8.9) received the same harassment, responded in the same quiet and cryptic way, and also acquired a mate, but in this case it was a fortis! Her history was not known. Song copying must have been precise to elicit such strong responses from magnirostris males; usually each species ignores songs of the others.
Importantly the mating patterns show that acoustic cues and visual cues complement each other (Baker and Baker 1990). When morphological differences between species are small, as between fortis on the one hand and fuliginosa or scandens on the other, learning the song of another species can lead to hybridization, but when the size difference between species is large, the barrier to interbreeding is robust, for then even learning the song of another species does not lead to hybridization.

Fig. 8.7 Birds imprinted on another species and a hybrid. Upper: scandens 2054 sang a fortis song. Middle: fortis 18459 sang a scandens song. Lower: scandens 4053 sang a fortis song. The top two birds have measurements typical of their respective species and are therefore not likely to have been hybrids. In contrast the lower bird’s measurements were close to those of fortis, and therefore the bird is suspected of being an F1 hybrid.
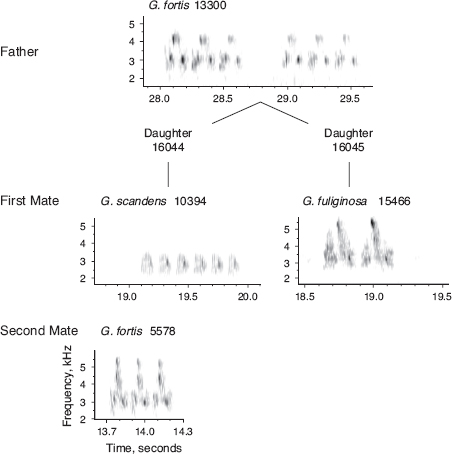
Fig. 8.8 A unique example of heterogeneity of pairing within a family. One daughter (16044) of a fortis male (13300) and female (13687) paired with a scandens male (10394), and later with a fortis male (5578). Another daughter (16045) paired with a fuliginosa male (15466). From Grant and Grant (1997a). Later genetic analysis with microsatellite DNA markers (box 10.1) confirmed the fortis identities of 13300 and 13687 but also revealed that 5578 was a fortis × fuliginosa F1 and 15466 was a rare fortis × fuliginosa F2. Although 10394 was genotyped as scandens, its small size suggests the possibility of it being a fortis × scandens F1.
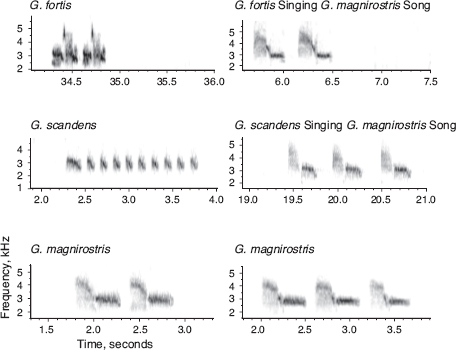
Fig. 8.9 Copying of magnirostris song by scandens 19936 in 2010 and fortis 17936 a decade earlier. Both bred with fortis females. Typical fortis and scandens songs are shown on the left, and typical magnirostris songs of two individuals are shown at the bottom.
Fitness Consequences of Hybridization
VIABILITY
Hybridization is important in the context of speciation because it provides an opportunity to test the degree of genetic compatibility between species. The first question to be answered is this: are the hybrids viable? In the first eight years none survived long enough to breed. G. scandens × fortis hybrids were too rare for analysis (2 pairs). G. fuliginosa × fortis hybrids were more numerous (12 pairs), and their survival was significantly weaker than age-matched fortis (fig. 8.10). One possible reason for this was an intrinsic physiological weakness due to their particular heterospecific combination of genes. An alternative reason was extrinsic: food conditions were not suitable for hybrids. During this time, large and hard seeds dominated the dry-season seed supply. G. fortis × fuliginosa hybrids are unable to crack large and hard seeds with beaks that are intermediate in size between the beaks of the parental species, and the small seeds they might have exploited were scarce. G. fortis × scandens hybrids with similarly intermediate beak sizes are inefficient at cracking Opuntia seeds, a scandens food, and unable to crack Tribulus fruits, a fortis food (fig. 8.11; Grant and Grant 1996b).
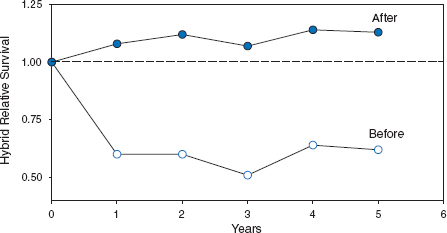
Fig. 8.10 Relative fitness of fuliginosa × fortis hybrids was lower before 1983 (open circles) than after 1983 (solid circles). Relative fitness is depicted as survival of fuliginosa × fortis F1 hybrids in relation to fortis survival for two groups of cohorts, the 1976–81 cohorts and the 1983–87 cohorts. The broken line indicates equal fitness of fortis and the F1 hybrids. The fitness of the 1976–81 hybrids is significantly lower than the fitness of fortis over the first age interval, and remained low thereafter. There are no significant differences in fitness for the 1983–87 cohorts. Sample sizes are 957 fortis and 32 hybrids hatched in 1976–81, and 2,376 fortis and 68 hybrids hatched in 1983–87. These constitute an estimated 80% (1976–81) and 97% (1983–87) of the total fortis and hybrid fledglings produced on the island in those years. From Grant and Grant 1993.
Subsequent observations showed the extrinsic reason to be correct. From 1983 onward, when small and soft seeds dominated the food supply, hybrids survived well, in agreement with the extrinsic hypothesis. Hybrids survived surprisingly well relative to the parental species, in fact as well as if not slightly better than them (fig. 8.12). This clearly demonstrates they are not physiologically weak, and there is no evidence here of genetic incompatibility between the hybridizing species (Grant and Grant 2008b).
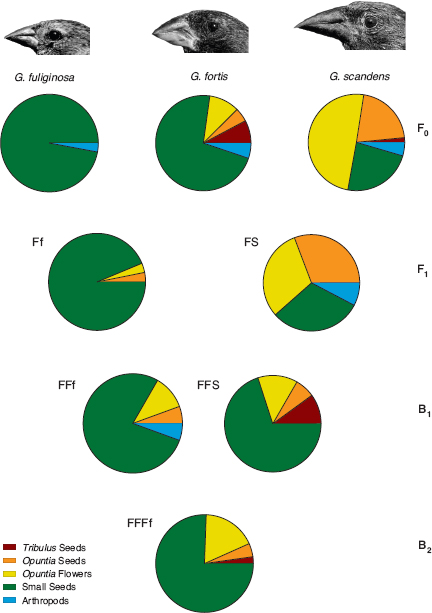
Fig. 8.11 Diets of hybrids and backcrosses. From Grant and Grant 1996b.
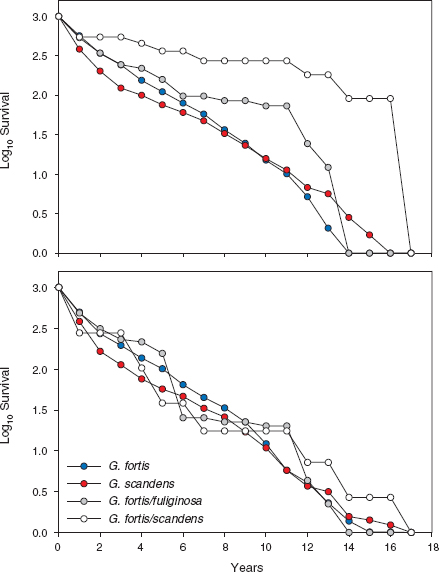
Fig. 8.12 Composite survival curves of seven cohorts of finches, hatched in the years 1978, 1981, 1983, 1984, 1987, 1991, and 1998. Upper: Numbers were summed across years, standardized to 1,000 at fledging, and converted to logs. Lower: Numbers in each cohort were first standardized to 1,000 at fledging and converted to logarithms, then summed across years and averaged. The hybrid groups comprise F1 hybrids and first generation backcrosses. From Grant and Grant 2010b. Higher survival of the fortis × scandens hybrids in the upper panel is a consistent feature of the three cohorts with largest sample sizes: 1983, 1987, and 1991 (Grant and Grant 2008b).

Fig. 8.13 Three species, hybrids, and backcrosses. Top row: Representatives of the three hybridizing species, fuliginosa (left), fortis (center), and scandens (right). Middle row: F1 hybrids, fuliginosa × fortis (left) and fortis × scandens (center and right). Bottom row: First-generation backcrosses, fuliginosa × fortis backcrossed to fortis (left) and fortis × scandens backcrossed to fortis (center) and to scandens (right). Modified from Grant and Grant 2008a.
FERTILITY
The second question is, are the hybrids fertile? The answer is yes. They bred in six of the nine years in the period 1983–91, when all breeding was monitored. They had no difficulty in acquiring mates and produced as many eggs, nestlings, fledglings, and recruits as the parental species. Hybrids and backcrosses (fig. 8.13) with scandens genes had apparently lower hatching success than those with fuliginosa genes; nevertheless in terms of fledgling success they all did about as well as the parental species and sometimes distinctly better. In short we could find no systematic difference in any of these reproductive categories between hybrids and parental species (Grant and Grant 1992b).
OVERALL FITNESS
Fitnesses of hybrids and nonhybrids produced in 1987 are compared in figure 8.14. The 1987 cohorts are chosen because they are the largest for making such comparisons. They were living at a time when small and soft seeds were abundant. An integrated measure of fitness is shown over the first four years as the product of survival of fledglings, recruitment to the breeding population, and production of fledglings in the breeding seasons of 1990 and 1991. Hybrids replaced themselves during this period, whereas the three parental hybridizing species did not. G. fortis and scandens reached replacement levels one year later, in 1992, whereas fuliginosa failed altogether to replace themselves. The fitness differences for this cohort arose largely from higher breeding success of hybrids and backcrosses.
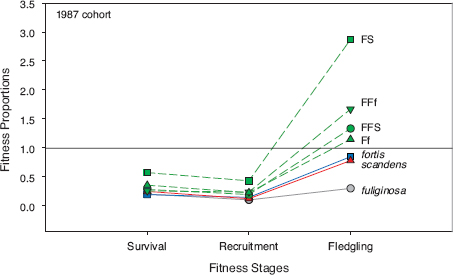
Fig. 8.14 Fitnesses of the 1987 cohorts over the first four years, expressed as survival to adulthood at stage 1, the product of survival and recruitment to the breeding population at stage 2, and production of survival, recruitment and fledglings produced in the breeding seasons of 1990 and 1991 at stage 3. A value of 1.0 for a class of finches indicates numerical replacement; the starting number of fledglings hatched in 1987 has been replaced by an equal number of fledglings hatched in 1990 and 1991. The fitness values for hybrids and backcrosses exceed 1.0 (replacement), whereas the three species all have fitness values less than 1.0. G. fortis and scandens reached replacement levels in 1992, whereas fuliginosa failed to replace themselves. Symbols: FS (fortis × scandens), Ff (fortis × fuliginosa), FFf (fortis × Ff), and FFS (fortis × FS). Adapted from Grant and Grant 1992b.
Genetic factors, over and above those affecting beak or body size, may also contribute because the hybridizing parental populations show some deleterious effects of inbreeding (Gibbs and Grant 1989, Keller et al. 2002, Grant et al. 2003, Markert et al. 2004). Hybridization can enhance fitness to different degrees by counteracting the effects of inbreeding depression through the masking of exposed deleterious alleles in heterozygotes. Alternatively deleterious alleles at one locus in one parental genome might be masked or compensated for by alleles at a different locus in the other parent’s (complementary epistasis). A small sample of hybrids and inbred birds, matched with nonhybrids and noninbreds as controls, shows the expected relationships (fig. 8.15). Relative fitness increased with mean expected heterozygosity across the total spectrum of scandens from inbred to outbred. The trends for fortis are the same but quantitatively and statistically weaker, although at the same rate as in scandens. Purging of deleterious alleles in scandens may be retarded by recurrent introgression of alleles from fortis, not all of which will be advantageous in a scandens background (Keller et al. 2002).

Fig. 8.15 Relative fitness of inbred birds and hybrid backcrosses of the 1991 cohort. Upper: Theoretical expectation of fitness of the two groups relative to the fitness of noninbred birds and nonhybrids. As genetic incompatibilities between species increase in time, the relative fitness of hybrids is expected to decline, as shown by the broken line with arrow through alternatives a, b, and c. Middle: The expected pattern is realized in scandens (middle), with relative fitness measured as the difference in longevity; the data conform most closely to alternative a above. The same pattern is realized in fortis (lower), though weaker and conforming to a or b but not c. Hybrid backcross (scandens × fortis) birds were matched with nonhybrid birds of the same sex as controls in time and space; they bred at the same time, usually on adjacent territories. The same was done for inbred and noninbred pairs. The horizontal axis is expected heterozygosity for 14 microsatellite loci. Arrowheads mark the extremes of close inbreeding (f = 0.25) and F1 hybrids. Mean expected heterozygosities of outbred birds (f=0) are indicated by broken vertical lines. The scandens slope is significantly different from 0, whereas the fortis slope is not, and the two slopes are statistically indistinguishable. However, in the large sample of fortis (n = 211) from this cohort mean heterozygosity increased and inbreeding coefficient decreased with age in the first two years owing to differential mortality (Markert et al. 2004). From Grant et al. 2003.
The Mating Pattern of Hybrids
Hybrid (F1) sons have intermediate morphology between their heterospecific parents, and sing the same songs as their fathers. Therefore they transmit ambiguous signals about their identity, like heterospecifically imprinted parental species but to a lesser degree. The importance of song in mate choice is shown by the fact that the mating rule which applies to species—females pair with males that sing the same species song as their own fathers (box 8.2)—applies to F1 hybrids as well, despite some morphological ambiguity (Grant and Grant 1997b). Hybrids are generally too rare to breed with each other and produce an F2 generation (see fig. 8.8 for one of only two known cases involving fuliginosa × fortis F1s); instead they backcross to one of the parental species. Hybrids formed by the interbreeding of fortis and scandens backcrossed to the two parental species equally, the direction depending on paternal song, whereas those formed by the interbreeding of fortis and fuliginosa backcrossed entirely to fortis, due to the rarity of fuliginosa. Moreover male hybrids sang a fortis song, as did the fathers of the female hybrids.
Consistent with the pattern of breeding according to father’s song type, all backcrosses bred with the parental species to which they were most related or with other hybrids and not with the parental species to which they were least related. For example, all 8 backcrosses to scandens (four males and four females) paired with scandens, and all 10 backcrosses to fortis (eight males and two females) paired with either fortis, backcrosses with a predominantly fortis constitution, or, in one case, a fortis × scandens F1 female.
Pair formation along lines of paternal song continued in the next generation. We noticed an apparent tendency for backcrosses to pair at a relatively high frequency with other hybrid classes, that is, F1 hybrids and backcrosses. Four of 16 first-generation fuliginosa × fortis backcrosses (0.25) paired with another hybrid class derived from fuliginosa and fortis, and 3 out of 10 scandens × fortis backcrosses (0.30) paired with hybrid classes derived from the same species. These are much higher than mean expectations for those years (1987–93) based on random sampling, which are 0.07 and 0.16 respectively. We interpret this pattern to be the result of a broad range of parental stimuli experienced by hybrids and backcrosses early in life that is translated into a broad range of acceptable mates later in life.
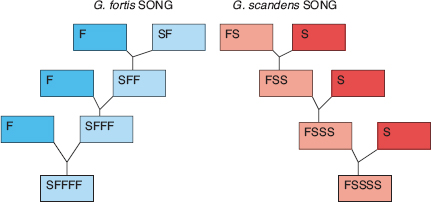
Fig. 8.16 The mating pattern of interbreeding fortis (F) and scandens (S), and their F1 hybrids and backcrosses. Both sons and daughters imprint on their father’s song and mate according to species song type. Hybrids do not sing intermediate or combined songs. As a result of introgressive hybridization genes flow from one species to another, but the two populations are kept apart by song differences and mating assortment based on song. FS refers to a F1 hybrid, the product of a fortis father imprinted on a scandens song mated to a scandens female. FSS refers to a first-generation backcross, FSSS refers to a second-generation backcross, and the same system is used for scandens backcrosses to fortis. From Grant and Grant 2008a.
The chief implication of these mating patterns is that with rare exceptions the direction of gene transfer in the first generation of interbreeding is not reversed in the next two to four generations of backcrossing (fig. 8.16).
Conclusions
The poor survival and lack of breeding of hybrids prior to 1983 stand in marked contrast to the superior fitness of hybrids afterward, especially the 1987 cohort. The contrast illustrates the dependence of hybrid fitness on the environment. In a fluctuating environment fitnesses are not constant but fluctuate in response to changing ecological (food) conditions as well as social conditions (fig. 4.1). This gives rise to opposing tendencies of population fusion caused by introgressive hybridization and fission caused by directional selection (Grant and Grant 1983, 2008b). As discussed in the next chapter fusion refuels the genetic variation, enhances morphological variation, and helps to explain why genetic variation is not eroded but is maintained at the observed high level (chapter 3) or even increased.
Summary
This chapter and the next one address the question of why some populations vary in morphological traits much more than others. The present chapter focuses on the causes and fitness consequences of hybridization, and leads into the topic of morphological consequences of introgressive hybridization in the next chapter. The two main findings are, first, the normal barrier to gene exchange is behavioral and not one of genetic incompatibility, and second, mate choice is determined by learning; there is no indication of genetically based mating preferences.
G. fortis hybridizes with fuliginosa and scandens, rarely but repeatedly. A relative scarcity of mates can explain why a rare species (fuliginosa) hybridizes with a common species (fortis). Hybridization of scandens, even though it is a common species, can be explained similarly because males outnumbered females at all times and many failed to obtain mates. Hybridization of fortis is understood in some cases. Mates are chosen according to species-specific song and morphology, which are learned early in life through imprinting on parents and occasionally others. Perturbation of the normal learning process can result in imprinting on another species, and this can lead to hybridization but does not do so always. With regard to consequences of hybridization, survival of hybrids depends on dry-season feeding conditions. Survival was poor before the 1982–83 El Niño year of substantial food production. In later, relatively wet, years it was as good as, if not slightly better than, survival of the parental species. Not only did the hybrids survive well; they bred successfully and backcrossed to each of the parental species, the direction in each case depending on paternal song. As a result of episodic introgression the fortis and scandens populations oscillated between fusion caused by introgressive hybridization and fission caused by directional selection.


















