1
Speciation, Adaptive Radiation, and Evolution
I should like to take some one family to study thoroughly, principally with a view to the theory of the origin of species. By that means I am strongly of opinion that some definite results might be arrived at.
(Wallace 1847, letter to H. W. Bates)
Those forms which possess in some considerable degree the character of species, but which are so closely similar to some other forms, or are so closely linked to them by intermediate gradations, that naturalists do not like to rank them as distinct species, are in several respects the most important to us.
(Darwin 1859, p. 47)
Introduction
MANY OF US ARE FASCINATED by the biological world around us. We marvel at the diversity of color, pattern, form, shape, size, ferocity, tameness, speed, and ingenious things that animals and plants do to find food and mates and avoid being eaten. Some of us have peered into microscopes that have opened up a new and wonderfully diverse world. Others have had the same thrilling experience in diving off coral reefs and being dazzled by the variety of fish. Yet others have been simultaneously bewildered and stimulated by the overwhelming diversity of a tropical rain forest. All this is so enthralling that some of us not only want to know why the world is the way it is; we also want to explore, examine, and test ideas about it in order to make our own discoveries. We are evolutionary biologists.
As evolutionary biologists we ask, how do species form? If we can answer that question we have taken a large stride toward understanding the biological richness of the world. The question is old but remains unresolved because rarely is it possible to witness even a part of the process. It must generally be inferred from indirect evidence, and yet we have had the good fortune to be witnesses. In this book we describe what we have learned about speciation by tracking populations and measuring evolutionary changes across 40 years in contemporary time.
Our starting point is Darwin’s Origin of Species by Means of Natural Selection. This is a manifesto of cardinal evolutionary principles. It laid out a theory of common descent of all organisms, represented evolutionary diversification as a branching pattern, and invoked the principle of natural selection as the driving agency that caused the divergence. Darwin argued that species formed by diverging in separate locations and then, when they came together, competed with each other for food and space, and diverged yet further. By this means, repeated, complex communities built up from simpler ones. Darwin had little hope of seeing evolution occur, but he did write that young radiations of species might provide windows through which we could view the steps involved in speciation. By an indirect pathway this led us to the Galápagos Islands, to Daphne Major in particular, to the finches named after him, to a fascination with them that lasted 40 years, and even to the origin of a new species.
Adaptive Radiation of Darwin’s Finches
Finches on the Galápagos are a young radiation of ecologically diverse species that have evolved from a common ancestor (Lack 1947, Grant 1986). Other radiations of plants and animals are more spectacular in terms of both numbers of species and their diversity (e.g., Schluter 2000, Grant 2013), yet Darwin’s finches have several advantages for the study of biological diversity (box 1.1). Many populations live in pristine environments, no species has become extinct as a result of human activities, and evolution can be studied as a contemporary process.
Box 1.1. The Choice of Darwin’s Finches
When we began our Galápagos research, the best-known radiations of animals were the numerous species of cichlid fish in several of the African Great Lakes (Fryer and Iles 1972), Anolis lizards of the Caribbean (Williams 1972), Drosophila (Carson et al. 1967) and honeycreepers (Amadon 1950, Warner 1968) of Hawaii, and Darwin’s finches (Lack 1945, 1947). The major features of morphological diversity were understood as feeding adaptations caused by natural selection in spatially segregated populations, and color and pattern variation as a result of sexual selection. Deepening this understanding required two things: a better estimation of phylogenies, which only became possible much later with the development of molecular genetic markers (Wagner and Funk 1996, Givnish and Sytsma 1997), and an analysis of contemporary populations to investigate the genetic basis and ecological causes of evolutionary change. This is where Darwin’s finches had some advantages over the others. The subject was ripe for ecological analysis, and finches seemed suitable subjects because they could provide the missing focus on population biology.
Detailed population studies appeared to be feasible because some populations are small, the finches can be marked for individual recognition, and they are conspicuous and approachable, so their fates can be determined accurately. Ecological influences on their fates can be identified because the climate fluctuates strongly and the extremes are markedly different. In some years there is little or no rain (La Niña); in others there is an abundance of rain (El Niño). The change from one extreme to the other is caused by reversals in the gradient of atmospheric pressure and sea-surface temperature across the Pacific basin. It is known as the El Niño–Southern Oscillation or ENSO phenomenon. The climatic extremes occur somewhat predictably at approximately three-to seven-year intervals on average (Philander 1990, Chen et al. 2004), with multidecadal oscillations in amplitude (Schlesinger and Ramankutty 1994). Superimposed upon a normal annual cycle of hot-wet (January to April) and cool-dry (May to December) seasons, the interannual fluctuations create profound changes in both marine and terrestrial productivity. As we discovered, the swings from plenty to scarcity reveal the ecological forces that finches are subjected to, and the evolutionary consequences. These in turn help us to interpret the radiation because it is still in a natural state: no species is known to have become extinct through human agency, and several of the islands have scarcely or never been affected by human occupation or exploitation.
Once thought to be members of the passerine family Emberizidae (buntings and finches), Darwin’s finches are now classified as tanagers (Thraupinae) (Burns 1997). This Neotropical family comprises about 400 species (Isler and Isler 1999) that evolved in the last 12 million years (Cracraft and Barker 2009). The drab-colored Darwin’s finches are thus a small part of a much larger radiation of varied and often colorful birds. According to current understanding at least 13 species of finches evolved on the Galápagos in the last two to three million years, and another evolved on Cocos Island (Grant and Grant 2008a). The species are distinctive in morphology (box 1.2), especially in the size and shape of their beaks, as well as in their diets. How is all the variation to be explained?
Box 1.2. What Makes a Darwin’s Finch Species?
Lack (1945), following Swarth (1931) and earlier taxonomists, classified species by their size, proportions, and to a lesser extent plumage. For example, four species of ground finches can be recognized morphologically by their differences on any one island and the consistency of the differences across islands. The Small Ground Finch (fuliginosa), Medium Ground Finch (fortis), and Large Ground Finch (magnirostris) differ principally in average size (fig. B.1.2, appendix 1.2, fig. P.1). As size increases from one species to the next, beak size becomes both larger and more blunt. We refer to them as the granivore group because they all feed extensively on seeds. The fourth species, the Cactus Ground Finch (scandens), is about the size of the Medium Ground Finch but has a proportionately longer and narrower beak than any of the other three. As the name implies, it is a cactus (Opuntia) specialist. In other respects the species are identical. As young birds they are brown and streaked. With successive molts the males, but not females, acquire partly black then completely black plumage (Salvin 1876, Grant 1986). The remaining 10 species of Darwin’s finches, similarly recognized by morphology, have minor relevance to this book (they are briefly described in appendix 1.3). Lack confirmed the biological reality of species identified by morphology in the breeding season of 1938–39 on San Cristóbal and Santa Cruz islands. Without having the benefit of measurements of individuals, he observed members of each morphological group (aka species) pairing up and breeding with each other and not with members of another group. Later in the book (chapter 9) we discuss the additional relevance of song to the question of what constitutes a species.
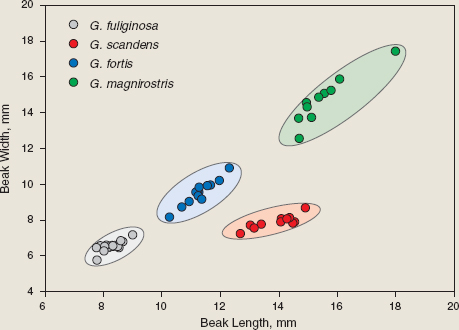
Fig. B.1.2 Morphological variation among four species of Darwin’s ground finches (males) on several islands. Data are taken from Grant et al. 1985.
Lack (1945, 1947) made the first attempt to answer this question after studying Darwin’s finches in the field. His explanation laid stress on three factors; natural selection, diversification on separate islands, and competition between species for food. Truly Darwinian! According to the Darwinian view, splitting of a species on Galápagos is initiated allopatrically when individuals disperse from one island to another and establish a new population. This is easy to visualize (fig. 1.1) because the archipelago has many islands. Colonists encounter new conditions, many die, and those surviving pass on to their offspring the heritable characteristics that contributed to their survival. In this way the population evolves by natural selection and becomes adapted to the new environment. There may be additional elements of randomness in how they evolve, if, for example, the founders are few in number or are not a representative sample of the original population and later diverge through genetic drift.
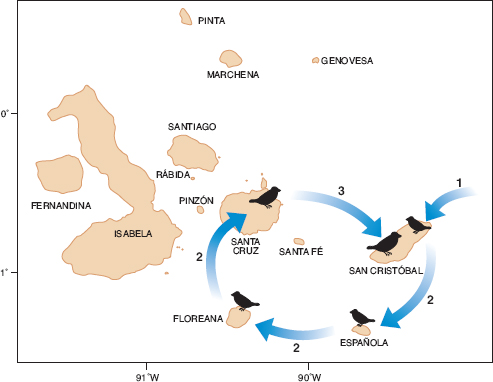
Fig. 1.1 Allopatric speciation in three stages: initial colonization (1), establishment of a second and additional populations (2), and secondary contact between two divergent populations (3). Choice of islands is arbitrary. Repetition of stages 2 and 3 in other parts of the archipelago gives rise to more species. From Grant 1981a, Grant and Grant 2008a.
The process of colonization and dispersal is repeated from one island to another until the two diverging lineages eventually come together on an island. As discussed in several chapters in this book, what happens at the point of secondary contact is crucially important in the speciation process. If members of the resident population and the immigrants do not interbreed, but the immigrants breed among themselves, two species will have been formed. Alternatively residents and immigrants might interbreed to some degree if prior divergence had not proceeded far. A tension might then exist between opposing tendencies: between fusion into a single population through interbreeding, and fission through divergent selection. The tendency to diverge would be expected if individuals produced by interbreeding had lower fitness than members of the parental populations, as would be the case if they suffered from an ecological disadvantage in competition for food or were physiologically weak. The end point of the divergence of the salient characters—character displacement—is reduced competition for food, a strengthened barrier to interbreeding, and enhanced prospects of long-term coexistence of these species (Grant 1981a, 1986).
Species and Speciation
Species differ (box 1.2). If they interbreed, they do so rarely yet remain distinct. They are said to be reproductively isolated from each other by behavioral barriers that prevent or inhibit interbreeding, or, if they do interbreed, by genetic barriers that prevent the formation of fertile offspring. To be more precise, it is individuals that interbreed, not species, and a species is a collection of individuals in one or more populations that are capable of interbreeding with little or no loss of offspring fitness. This is the biological species concept, with the essence of species being their complete or near-complete separation from each other.
Speciation is the evolutionary process that gives rise to the differences. It occurs when one species splits into two noninterbreeding populations or sets of populations (fig. 1.2). The challenge we face is to explain the product, species and their attributes, when the process by which they are produced is scarcely ever seen. To that end we need to answer the how, why, when, and where questions of speciation (Grant and Grant 2008a). What are the important factors that cause populations to diverge, how do they operate, and what are the circumstances? What prevents them from interbreeding and fusing into a single population? To answer these questions we chose to study finches on a small island: Daphne Major.
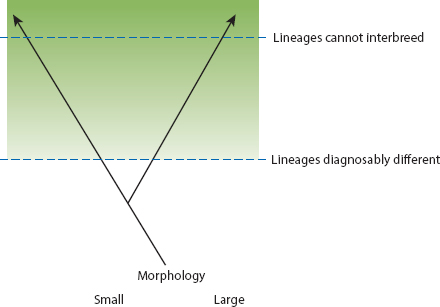
Fig. 1.2 Y diagram of speciation. The process is represented as a splitting and divergence of lineages. Opinions vary on when the lineages merit recognition as two species because divergence is gradual (discussed in Grant and Grant 2008a).
Daphne
Daphne (box 1.3, figs. P.2 and P.3, figs. 1.3 and 1.4) is centrally located in the main part of the archipelago about 8 km from the much larger islands of Santa Cruz to the south and Seymour to the east (fig. P.3). It is a pyroclastic or tuff cone that was formed explosively by underwater volcanic activity. Approximately three-quarters of a kilometer long and 120 m high, it has never had a human settlement. The center is a crater floor that is periodically occupied by breeding Blue-footed Boobies (Sula nebouxii). Topographically there are three vegetated habitats: an inner slope, an outer slope, and an area on the southern side with a more gentle slope of about 15 degrees that we refer to euphemistically as the plateau (figs. 1.4 and 1.5). All three habitats have shallow soils, seasonally deciduous annual and perennial plants, and clusters of Opuntia (prickly pear cactus) bushes where finches nest. The two main finch species are the Medium Ground Finch (Geospiza fortis) and the Cactus Finch (G. scandens).


Fig. 1.3 Daphne Major landing. Left: The wave-cut, barnacle-covered platform used for landing and departing at low tide; arrows indicate “steps” (M. Wikelski). Right: Exit, leaving when the sea is calm.
Associated with its small size (0.34 km2), the most important feature of Daphne is its ecological simplicity. It has a community of fewer than 60 plant species, most of which are rare (appendix 1.1), and a similarly low diversity of insects and spiders. Breeding populations of finches rarely exceed 150 pairs. Small numbers make it relatively easy to determine how environmental factors affect morphological traits of finches, and how change in the environment brings about change in morphology. Their tameness makes them easy to observe. Of paramount importance for this study, they can be uniquely marked so that each individual can be identified by observation.
The Darwin’s finch radiation is the macrocosm; Daphne finches are the microcosm. To throw light on the macrocosm, we studied the microcosm for 40 years, and witnessed evolution.

Fig. 1.4 Three habitats. Upper: Plateau, 1995. The fallen tree (near right) was present throughout the 40 years. Middle: Inner slope and plateau, 1983. Lower: Outer slope, 2012.

Fig. 1.5 Change in vegetation over 73 years. Upper: View across the crater floor in 1939 (L.S.V. Venables). Middle: 1973. Blue-footed boobies (Sula ne bouxii) nesting on the crater floor. Lower: 2012. The pattern of vegetation on the crater floor, principally Croton scouleri, reflects drainage of rainwater to the lowest level. Notice in the left part of the figures that the extent of dark green cactus bushes increased over the years. Finches roost, nest, and in some years (e.g., 1992) die in large numbers in the bushes fringing the crater floor.
Evolution Observed
Figure 1.6 shows an intriuing pattern of change through time. The average beak size of the fortis population did not remain constant for 40 years but increased in 1978 and decreased in 2005. Beak size evolved. A host of compelling questions arise when we confront a pattern of change like this. First, why did it occur? The strong and rapid transitions in average beak size at these two times implicate natural selection. How strong was selection, and what caused it? What is the source of beak-size variation, and what maintains it when selection occurs? To what extent is it genetically based? What is the relevance to speciation?
Box 1.3. Recent History of Daphne
There is not one Daphne but two: Daphne Major and Daphne Minor (Chica) (figs. P.2 and 2.4). The Daphnes were named after the British naval vessel H.M.S. Daphne that visited Galápagos in 1846, possibly by Midshipman G.W.F. Edwardes, who was the first to show them on a map (K. T. Grant, pers. comm.). Beebe (1924) coined the terms Major and Minor (Woram 2013). The Daphnes differ in two respects. Whereas it is difficult to climb onto Daphne Major with camping supplies, it is impossible to do so on Daphne Minor without ropes. Daphne Minor has been climbed once. Also Daphne Minor has a crater lined with blocks of lava (Grant et al. 1980), indicating volcanic activity above the sea, whereas the Daphne Major crater lacks lava.
Daphne Major, hereafter Daphne, was put on the map ornithologically by Rollo Beck. He and companions collected specimens of finches in 1901 and 1905–6 for Walter Rothschild’s museum in England and the California Academy of Sciences respectively (Gifford 1919). William Beebe (1924) reached a wide audience with an engaging description of a day on Daphne in his popular book Galápagos: World’s End. David Lack never visited the island, but his assistant L.S.V. Venables did for one day in January 1939 (fig. 1.5), and reported that fortis (identified as fuliginosa!) were common all over the island whereas there were very few scandens in cactus clumps adjacent to the crater floor. He saw one magnirostris. Lack believed the Medium Ground Finch (fortis) was almost the sole occupant on the basis of these observations, those of the collectors (Gifford 1919), and 42 specimens in museums. In addition three Small (fuliginosa) and four Cactus (scandens) Ground Finches had been collected (Lack 1945), and Beebe (1924) had observed the breeding of a single pair of Large Ground Finches (magnirostris) in 1923. In the 1960s Daphne was a place for seabird research by David Snow and Michael Harris. Incidental observations on the finches (Harris 1973, 1974) provided the most up-to-date information on their status when we made our first visit to the island in April 1973. Providentially The Flora of Galápagos (Wiggins and Porter 1971), an invaluable field guide to the plants of Daphne, had been published just before.
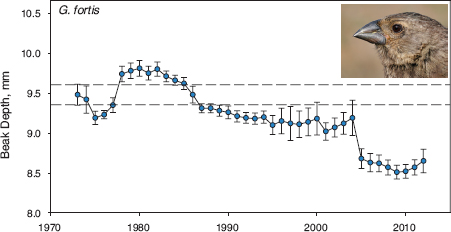
Fig. 1.6 Evolutionary trajectory of fortis beak size over 40 years. Means and 95% confidence limits are shown for all birds alive in each year. Parallel horizontal lines mark the upper and lower 95% confidence limits on the first estimate of a mean based on a large sample size (n = 221) in 1973.
The most remarkable feature of the trajectory is the fact that fortis are no longer the same as they were 40 years ago. Change is not inevitable, however, as figure 1.7 shows. The trajectory of scandens is flat except for minor excursions; beak depth has remained the same for 40 years. Why did one species (fortis) change and the other (scandens) did not? Was scandens subject to natural selection but lacked the genetic variation to respond evolutionarily? Did scandens change in other traits? Did the two species interbreed, and if so with what result? Did they compete for food?
Among the several unexpected things that happened in the 40 years two events that are highly relevant to these questions stand out. One was the arrival of a hybrid from neighboring Santa Cruz Island. Years later the descendants were breeding among themselves: they were behaving as a new species! How could that happen in such a short time? Why did it happen? Why did they not breed with fortis or scandens?
The second event was the establishment of a breeding population of the Large Ground Finch (G. magnirostris) at the end of 1982. Thirty years later there were 50 pairs on the island. How could another species fit into the community? Did it compete with the residents for food, and if so what were the consequences, both evolutionary and ecological? How might the arrival of a new species throw light on speciation?
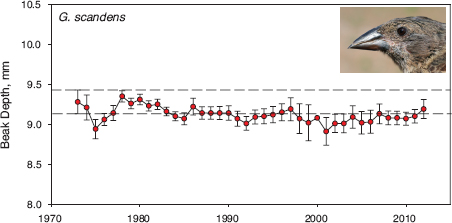
Fig. 1.7 Evolutionary trajectory of scandens beak size over 40 years. Means and 95% confidence limits are shown for all birds alive in each year. Parallel horizontal lines mark the upper and lower 95% confidence limits on the estimate of the mean in 1973 (n = 71).
All these questions are interdependent. By unraveling the dependencies we are able to reveal causes and complexities of evolution in contemporary time.
Chapters of the Book
We address these questions and describe the events that gave rise to them in the following chapters. The sequence is partly dictated by the nature of the study. In the first half we attempted to find every nest on the island and to mark every nestling uniquely. After we stopped doing this, our information on relatedness and biological success of individuals was reduced. On the other hand genetic data on relatedness became available in the second half. G. magnirostris became an important factor only in the second half. Thus the two halves of the study differ, and the organization of the chapters reflects this.
We start with an observation and a historical question in chapter 2. The Medium Ground Finch is exceptionally small on Daphne. What caused its evolution? This apparently simple problem was the magnet that drew us to Daphne in the first place. In contrast to the initial historical perspective all subsequent chapters are concerned with evolution as a contemporary process. Chapters 3 and 4 discuss the genetic basis of morphological variation and the evolutionary responses to natural selection in the first half of the study (fig. 1.6). Chapter 5 considers how the breeding component of fitness might affect the evolutionary trajectory. The arrival of magnirostris and its subsequent fate is described in chapter 6: how a population became established and why it prospered. It was a new factor, a competitive influence on fortis, and a cause of evolutionary change in the fortis trajectory (chapter 7). Chapters 8 to 10 discuss rare but persistent introgressive hybridization through backcrossing between fortis on the one hand and scandens and immigrant fuliginosa on the other, and the genetic and fitness effects of introgression on the trajectory depicted in figures 1.6 and 1.7. Chapter 11 surveys morphological evolution across the 40 years in both fortis and scandens, and in traits other than beak size, contrasting the relative influence of selection and introgressive hybridization on each of the two species. Chapter 12 quantifies the role of selection during the morphological transformation of one species into another, drawing upon knowledge of genes expressed during development as well as upon adult morphology. Chapter 13 discusses the events leading up to the formation of a new, reproductively isolated, lineage of finches. Chapter 14 speculates about two futures: the future of the finches if the anticipated environmental change occurs in Galápagos, and the future of phylogenetic understanding from genomic studies. Chapter 15 is a synthesis of the main evolutionary findings, and chapter 16 extends the discussion with some generalizations, and implications. An epilogue (chapter 17) completes the book. It stresses the value of continuous long-term study of ecology and evolution.
Summary
Darwin’s finches on the Galápagos islands are a model system for the study of speciation and adaptive radiation, that is, the rapid evolution of morphologically and ecologically diverse species from an ancestor. Core ingredients of a theory to explain how and why the radiation occurred are natural selection, allopatric divergence, reproductive isolation, and interspecific competition. Our task is to determine how these ingredients occur, and how they are connected. In this book we describe what we learned about evolution by studying four species of ground finches on the single island of Daphne Major over a period of 40 years.








