15
Themes and Issues
We add to what was learned before, raising the old questions again and again, lifting them if we can toward higher and higher ground, and ourselves with them. Why are there so many kinds of animals, and why are we among them? Probably we have been asking these questions ever since we lived in caves.
(Weiner 1994, p. 293)
Biologists do not yet fully understand how the various potential processes of evolutionary change actually integrate over time to yield the patterns in which species occupy morphospace.
(Brakefield and Jorion 2010, p. 93)
Introduction
FIGURE 15.1 SUMMARIZES THE FOUR profound morphological changes that took place in the Daphne finch community over 40 years. Two changes were additions, brought about by colonization (magnirostris) and speciation (Big Bird lineage). Two changes were morphological transformations of fortis and scandens through selection and introgressive hybridization.
From start to finish three themes dominated the study: interspecific competition for resources, enhanced variation in continuously varying traits like beak size, and the role these two play in species formation. Three themes emerged as the study progressed: natural selection as an observable, repeatable, and interpretable phenomenon with evolutionary consequences; introgressive hybridization; and the role of both in species formation. In this chapter we synthesize what we have learned about speciation before discussing some of the issues raised by our findings, including the question of how generalizable they are.
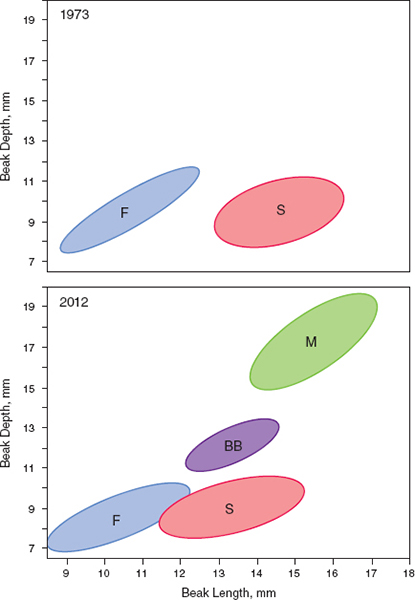
Fig. 15.1 Change in the morphological community of finches on Daphne. Between 1973 and 2012 magnirostris (M) colonized the island, a Big Bird lineage (BB) was formed, fortis (F) became smaller in beak depth, and scandens (S) became shorter in beak length through a combination of selection and hybridization. These dynamics provide a glimpse of evolution elsewhere on much longer time-scales.
Speciation, Selection, and Hybridization
A central problem in evolutionary biology is to explain how new species are formed. The world is biologically rich because it is filled with so many different kinds of species; therefore understanding speciation goes a long way toward explaining biodiversity. The Daphne study has yielded several important insights into a process that is rarely observed yet frequently discussed, debated, and necessarily inferred. In fact there is not one process but two. The first is divergence and lineage splitting: two species from one. This is part of the canonical (standard) allopatric model of speciation. The second is unequal genetic mixing of two species to form a third: three species from two—introgressive or reticulate speciation. Here we summarize and discuss our findings.
EVOLUTION
There is widespread agreement that speciation generally results from genetic differentiation of separate populations in allopatry (or parapatry), whether the populations are genetically connected by occasional gene flow or not. This is followed in many cases by the coexistence of the differentiated populations in sympatry (Mayr 1963, Coyne and Orr 2004, Price 2008). Two obvious requirements for this to happen are genetic variation and ecological opportunity. The Daphne study provides evidence of both. It shows there is abundant polygenic variation in the ecologically most important traits, beak size and shape. Moreover some of the genes involved in the development of different beak dimensions have been identified. These are expressed at the same or different stages of development but independently, and their independence helps to explain how evolutionary change takes place in beak shape as well as size (chapter 12). Thus the intrinsic potential for evolutionary change is high. Extrinsic potential, that is, ecological opportunity in the form of available resources, is very difficult to identify before it is revealed by a species actually exploiting the resources, and yet it must have been present on Daphne to have allowed two colonizations to take place, the first by magnirostris during the extreme El Niño event of 1982–83 and the second by the originator of the hybrid lineage two years earlier. Since there are many islands in the archipelago differing in ecological conditions, there is a high extrinsic potential as well as high intrinsic potential for evolutionary change in allopatry. This is a major reason why Darwin’s finch species have proliferated.
ECOLOGICAL IMPORTANCE OF FOOD: THE DAPHNE PERSPECTIVE
The four finch species on Daphne, together with related populations on other islands, illustrate the speciation problem and point to a solution. G. fortis, fuliginosa, scandens, and magnirostris each have several populations, respectively 17, 27, 15, and 12 (Grant 1986). Morphologically the 71 populations are variations on four discrete species themes (fig. B.1.2). The pattern in figure B.1.2 raises two questions. First, how is a new theme generated? Second, why are there no populations in the gaps between the means? We offer an answer to the first question here, and an answer to the second question in a section below (Size and Hybridization).
The multimodal nature of dry-season seed distributions (fig. 2.16) shows why there should be more than one granivorous finch species on large islands (Schluter and Grant 1984), but not how speciation takes place. Since the four species possess adaptations to feeding on different foods, the first question can be rephrased: how does the process of ecological divergence beyond the normal range of variation of populations of a species get started? How do finches encounter a new food type or types that potentiate speciation?
Insights from the Daphne study offer an answer by extrapolation from the observed to the unobserved. A new encounter occurs when finches arrive at an island that has a new food (fig. 1.1 and chapter 7) or when a new food unfamiliar to the finches arrives on the island. In the latter case a population of finches may go on fluctuating morphologically between more or less fixed limits that are set by the composition of the food supply and competition for it, as we observed on Daphne (chapter 11), and then starts on a new evolutionary trajectory when by chance a new food arrives on the island. Character displacement may be a key element in the directional change by providing the initial impetus in shifting the population toward a new optimum. Mutation, recombination, and additional selection would all be involved in completing the process.
Paleoclimatic evidence indicates the climate was gradually becoming drier even as it fluctuated in the last million years (Grant and Grant 2008a). The arrival in the archipelago of drought-tolerant plants, including those with medium and large seeds such as Opuntia cactus, Tribulus, and Cordia, probably triggered speciation of the cactus and granivorous ground finches (Grant and Grant 2008a). These evolutionary dynamics echo the theme in chapter 11 of approximate stasis for long periods of time followed by relatively rapid evolutionary change and speciation.
BEHAVIORAL BARRIER TO INTERBREEDING
A third requirement for the sympatric phase of speciation is a barrier to the free exchange of genes. Genetic theories of speciation stress genetic incompatibilities when nascent species interbreed (Coyne and Orr 2004, Feder et al. 2012, Abbott et al. 2013) and largely ignore the contribution of learning to a behavioral barrier that prevents interbreeding. Darwin’s finches do interbreed, on Daphne and on other islands (Grant and Grant 1989, 2008a, Grant et al. 2005), but rarely, and yet without loss of fitness when feeding conditions for hybrids and backcrosses are favorable. This shows that in the early stages of speciation, before genetic incompatibilities arise, populations are almost entirely isolated from each other by a premating behavioral barrier to interbreeding that is set up by learning through sexual imprinting; that is, the barrier is culturally inherited (chapters 6 and 8, and Grant and Grant 1996d, 1997a, 1998), and rarely breached.
An important question, therefore, is what constitutes the barrier to interbreeding, because identifying the constituents help us to understand how and why the barrier arose and how it functions. For many passerine bird species the answer would be plumage and courtship behavior (Prager and Wilson 1980, Price 2008), subject to sexual selection occurring predominantly through female choice. The answer for Darwin’s finches is song and morphology. These function in interactions between males as well as between males and females. Species cues are learned early in life as revealed by experiments (Bowman 1983, Grant and Grant 2008a). Like beak morphology, songs diverge in allopatry (fig. 15.2), probably through a combination of influences of habitat structure on song transmission (Bowman 1983), copying errors accumulating through time (Grant and Grant 1996d), and changes caused by acoustical interference with songs of other finch species (Grant and Grant 2010c) occupying somewhat different parts of the total acoustical space (Nelson and Marler 1990). These topics have been reviewed recently: by Wilkins et al. (2013) for song divergence, Grether et al. (2009) for interspecific interference, and Verzijden et al. (2012) for song learning.
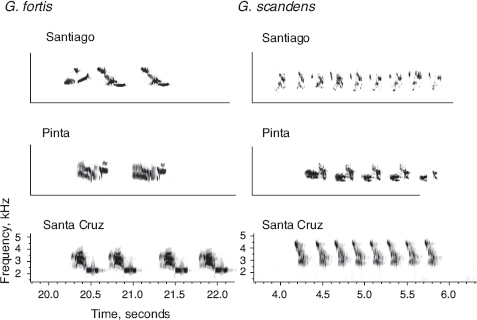
Fig. 15.2 Songs of fortis and scandens illustrate strong inter-island differences in note structure but species-specific similarity in temporal pattern. From Grant and Grant 2010c.
The coupling of song and morphology could be more than a matter of learning if song features were strictly determined (constrained) by beak morphology and musculature for biomechanical reasons (Podos 1997, 2001). Song would then be analogous to an inflexible trait that is genetically correlated with beak size. This attractively simple possibility has some support from an association between beak size and trill rate and frequency bandwidth on Santa Cruz Island (Huber and Podos 2006, Podos 2010), as well as from some studies of continental species (e.g., Badyaev et al. 2008). Evidence is mixed, however, both on Santa Cruz (Huber and Podos 2006) and outside Galápagos (Slabbekoorn and Smith 2004, Seddon 2005), sometimes lacking as on Daphne (Podos 1997), or even contrary to expectation (Huber and Podos 2006).
We doubt if strict determination of song by beaks plays a role in finch speciation, notwithstanding the original correlations between beaks and songs (Podos 2001) and results of some functional and biomechanical assays (Herrel et al. 2005, 2009). There are two reasons. First, Bowman’s (1983) extensive song recordings throughout the archipelago showed that in most finch populations individuals sing fast or slow variants of song, which he labeled as basic and derived respectively. The two intraspecific variants are not associated with differences in beak size, beak shape, or body size where this has been investigated, either in fortis and scandens on Daphne (Grant and Grant 1996d, 2009a, 2010c) or conirostris (Grant and Grant 1989) and difficilis (Grant and Grant 2002b) on Genovesa. Second, songs of some Geospiza species are not constrained, as shown by the fact that species with different beak sizes and shapes sing such precise copies of each other’s songs that they elicit strong responses from the copied species (chapter 8). Moreover heterospecific singing is not restricted to Daphne, as birds are occasionally encountered elsewhere in the archipelago singing the song of another finch species with a different beak size and shape (Bowman 1983, Grant and Grant 1989).
SIZE AND HYBRIDIZATION
Occasional hybridization does more than reveal the absence of genetic incompatibilities; it shows in two ways that morphological cues are as important as song cues when they convey conflicting information about potential mates and species identity. First, singing each other’s songs can lead to interbreeding when size differences are small, as between fortis and fuliginosa or scandens, but not when size differences are large, as they are between magnirostris and fortis on Daphne. Second, introgressive hybridization leads in opposite directions, either to the reversal or collapse of speciation (chapter 10) or to the formation of a new species (chapter 13). The outcome appears to depend on the magnitude of the size differences between the interacting species. This provides an answer to the long-standing question of gaps between clusters of species (Huxley 1938, 1940, Mayr 1942). A minimum morphological difference between species is set not only by competition for food (reviewed in Grant 1986) but by the propensity of similar species to hybridize and potentially fuse into one (chapter 10).
The importance of size differences is illustrated by the fates of two lines of descent in the hybrid lineage that can be attributed to a difference in size from generation 1 onward. Members of the A line were smaller on average than members of the B line in beak and body size, and resembled fortis more closely. Since males of both lines sang the same song as the immigrant male, the key difference between them was their size. The A line became absorbed into the fortis population through interbreeding, whereas the B line prospered as a reproductively isolated population. This is the clearest example of a single factor, morphology, tipping the balance toward either fission or fusion at the secondary sympatry stage.
PHYLOGENETIC IMPLICATIONS OF HYBRIDIZATION
We began the study with a simple conception of speciation, depicted in figure 1.2 as a process of divergence. In the light of experience on Daphne we should modify it to allow for reticulation (fig. 15.3), as exemplified by the exchange of genes between fortis on the one hand and scandens and fuliginosa on the other, and convergence as shown by fortis and scandens. Furthermore, rather than just a process of fission or fusion, repeated many times to give rise to many species from one, a third possibility should be added: interbreeding of two species contributes to the formation of a third. The genetic footprint seen in sympatric populations elsewhere in the archipelago (Grant et al. 2005) suggests that introgressive hybridization may have influenced, and even produced through introgressive speciation, some of the Darwin’s finch species present today (Grant and Grant 2008a). Further tests need to be devised for detecting a genetic signature of past introgression and estimating when it occurred (e.g., Larsen et al. 2010, Rheindt and Edwards 2011).
EPHEMERALITY OF SPECIES
It is tempting to think of the Darwin’s finch radiation as a simple process of accumulation of 14 diverse species from an original species, and that all species are known. Extinction, impossible to quantify in the absence of fossils, is acknowledged as a possibility in analyses of diversity and has to be estimated by making assumptions of its frequency and temporal pattern (Ricklefs 2007, Glor 2010). Some distribution patterns of birds with a known phylogenetic history are best explained by invoking extinctions (Warren et al. 2006). The Daphne study suggests that species are likely to be arising, fusing, and collapsing more often than is generally appreciated, and unrecorded in either fossils or molecular phylogenies. For example, repeated hybridization with introgression may be thought of as repeated experimentation. Only some experiments yield results of evolutionary significance. If this view is correct, many episodes of speciation fail for every one that succeeds in reaching complete genetic isolation (Grant and Grant 2009a). Origination of ephemeral lineages will scarcely ever be observed, being rare and unpredictable in occurrence, cryptic, and local, and therefore underestimated. Long-term studies like the present one with marked individuals have the best chances of detecting and understanding them.
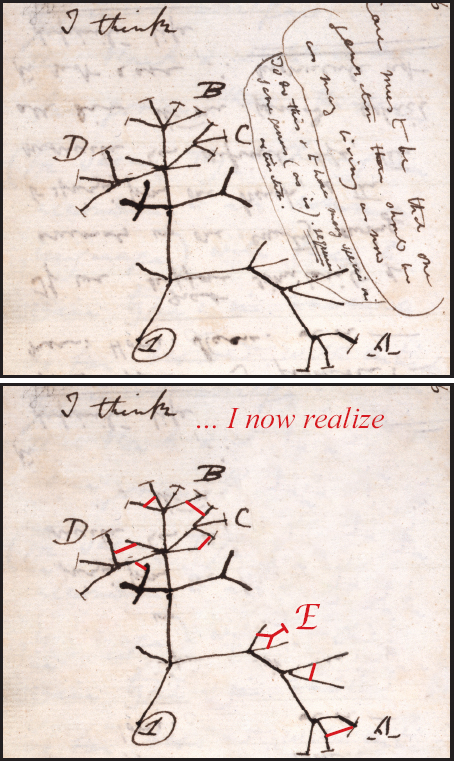
Fig. 15.3 Darwin’s first thoughts on phylogeny, from Notebook B written in 1837 (above), contrasted with how he might have depicted his thoughts with allowance for reticulation caused by introgressive hybridization (below, in red). In his famous sketch speciation is represented as a branching process. Some lines of descent become extinct: these lack terminal crossbars. In the lower panel species, and not just their genes, reticulate, and by interbreeding separate lineages may produce a new species (E). Reticulation is not a new a idea (e.g., Huxley 1942). The upper illustration is reproduced with kind permission of the Syndics of Cambridge University Library, and the lower illustration is adapted from Grant and Grant 2010a.
The 40 years spanned by our study is a short time in relation to the radiation, and yet we witnessed the origin of one incipient species by introgressive hybridization and, in the opposite direction, a process of fusion that is leading to a reduction from two species to one on Daphne. The hybrid lineage may be short-lived. We surely cannot have observed the only one in the history of the finches.
PREDICTABILITY AND EVOLVABILITY
There are predictable aspects of evolution—similar and repeated ecological, morphological, and genetic changes in similar environments (e.g., Hudson et al. 2011, Rutschmann et al. 2011, Jones et al. 2012, Zhen et al. 2012)—but also unpredictable aspects. Evolution of finches on Daphne displays both: it is genetically predictable but environmentally unpredictable.
Evolution is highly predictable when natural selection occurs because populations maintain a large amount of polygenic variation underlying body size and beak size and shape variation (chapters 3, 8, and 9). However, climatic fluctuations, which are the ultimate cause of natural selection through their effects on food supply (chapters 4 and 7), are unpredictable in their occurrence, duration, and severity, so the occurrence and direction of natural selection is unpredictable. Moreover a one-year drought may lead to strong natural selection, as in 1977 (chapter 4), whereas a two-year drought (e.g., 1988–89) may not (chapter 11). The factors governing the outcome are food types and quantity, as well as finch density at the beginning of a drought. Preceding conditions are therefore crucially important in determining whether natural selection will occur or not and, if it does, the direction it takes.
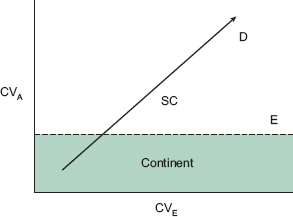
Fig. 15.4 The potential for evolution of a population is a function of both genetic (intrinsic) and environmental (extrinsic) variation. A line with arrowhead shows the direction of increasing potential. The coefficient of additive genetic variation (evolvability, chapter 3) is plotted against a similar index of environmental variation (chapter 1); both increase away from the origin. The environmental index is the long-term estimate of rainfall variance divided by the long-term mean, from figure 14.2. Expressed as a percentage of the mean, variation is five times greater on Daphne (60.9%) than on Santa Cruz (12.6%). Points on the graph refer to small (Enderby, E), medium (Daphne, D), and large (Santa Cruz, SC) islands. D and SC represent positions of two fortis populations, placed on the assumption that the unknown genetic variation is lower on Santa Cruz because introgressive hybridization is less frequent; this may not be correct (de Léon et al. 2010). The small island of Enderby has only a single population, of fuliginosa, and has been chosen because fortis does not occur alone anywhere. Its placement is hypothetical. The blue zone represents a band of conditions that might prevail in continental regions along an environmental gradient from stability (CVE ~ 0), as in lowland tropical rain forests, to instability (CVE ~ 1), as in deserts.
Thus the evolutionary potential of a population, as well as its predictability, is not just a function of genetic variation, or of environmental variation (including the social environment), but a product of the interaction between the two. A simple graph depicts the concept (fig. 15.4) and shows that the evolutionary potential varies among locations. Responses to environmental variation depend on the nature of the variation. The climatic environment may fluctuate as we have seen on Daphne and result in evolutionary change that fluctuates about a long-term mean within limits. The environment may also change to a new state, gradually through a change in those limits, or abruptly with the introduction of a novel food item, or new species.
A possible example of abrupt change on Daphne is the arrival of caltrop (Tribulus). T. cistoides is believed to be an adventive to the New World (Svenson 1946, Porter 1967). A dozen relatives occur in the Old World, in Europe and Africa: one is food for camels in the Arabian desert (Thesiger 1959)! If caltrop is indeed an adventive, it arrived in Galápagos no earlier than 1535 on Fray de Berlanga’s vessel, and on Daphne after that, perhaps attached to the feet of boobies (Sula) or gulls (Larus). Tribulus is part of the food niche of fortis, and a critical part of the niches of magnirostris and the hybrid lineage. A parallel example without any known or supposed human influence is the inter-island dispersal of seeds of Opuntia species and Cordia lutea, which are critical for the survival of magnirostris and conirostris on other islands (Grant and Grant 1982).
Overview
The value of Darwin’s finches lies in what they reveal about the early stages of speciation in a young adaptive radiation, when ecological divergence has proceeded dramatically without the kind of genetic divergence that impairs fertility and viability (Grant and Grant 1997a). Daphne finches have shown us that speciation occurs by hesitant, wavering steps rather than by a large stride. It is pushed in one direction and then in another by selection, and pulled by hybridization in both: more a journey along a winding rural lane than an expressway. Experimental. Barriers to interbreeding are imperfect. Introgressive hybridization may cause them to decay and yet, paradoxically, it also provides the genetic variation for a new directional change. Recognizing the primary role of natural selection in their divergence, we could consider Darwin’s finches to be an exemplar of ecological (Van Valen 1976, Schluter 1996, 2009, Nosil 2012) or ecogenetic speciation. Recognizing the primary role of learning in reproductive isolation, they could be considered an exemplar of ethological speciation (Dobzhansky 1941, Mayr 1942). In fact they are both.
In conclusion, speciation of Darwin’s finches is ecologically driven by natural selection with reproductive isolation as an incidental effect (Fisher 1930, Dobzhansky 1940) or by-product of morphological divergence in allopatry (Grant 1986, Schluter 1996, 2000, Grant et al. 2000, Grant and Grant 2008a). It is also influenced by song, which constitutes an important element of the barrier to interbreeding by being culturally inherited and coupled with morphology through associative learning and not because it is determined by beak size. This may be a common form of speciation in birds because many species of birds learn their songs from conspecifics at an early age (Irwin and Price 1999, ten Cate and Vos 1999). We think of speciation as an eco-behavioral-genetic process of evolutionary divergence (Grant and Grant 2008a). Genetic incompatibilities arise much later through mutation, and eventually the door to gene exchange closes forever. However, in this young radiation of Darwin’s finches all species, apparently, still have their doors open (Grant 1986).
Summary
In this chapter we discuss the major themes of the study and some of the issues raised by our findings. The major themes are variation, natural selection, speciation, interspecific competition, and hybridization. The major issues are the causes of divergence of populations to the point of little or no gene exchange, the existence of morphological gaps between species, and the roles of song and beak size in speciation. We consider phylogenetic implications of hybridization, and the implication from the hybrid lineage that species may be arising and becoming extinct more frequently than is generally appreciated. Daphne finches show that speciation occurs by hesitant, wavering steps. Their evolution is genetically predictable but environmentally unpredictable, and so the evolutionary potential of a finch population, as well as its predictability, is a function of genetic variation, environmental variation, and their interaction. We end the chapter with an overview of speciation. Our main conclusion is that speciation of Darwin’s finches is ecologically driven by natural selection, with reproductive isolation as an incidental effect or by-product of morphological and song divergence in allopatry.



