16
Generalization
Perhaps—just perhaps … small populations, while teetering toward extinction and irrelevance, provide cauldrons of evolutionary novelty.
(Stern 2011, p. 218)
We can agree with Professor Popper that it does not matter for our purpose how generalisations are arrived at. The question which concerns us is what makes them acceptable.
(Ayer 2006, p. 19)
Generalizing When N = 1
WE STUDIED FINCHES ON DAPHNE: only one island! Are the evolutionary dynamics we uncovered just an interesting case history applicable only to Galápagos, or are they indicative of ubiquitous processes that are normally too subtle to be revealed in most field studies? In other words how representative are they of other island communities, in Galápagos or elsewhere, or of mainland birds, or of animals in general? Can Darwin’s finches on Daphne be considered a model system of evolution in a fluctuating environment? Obtaining answers to questions of this sort require comparative studies of other species in other places (e.g., Price 2008, Bell 2010). Some general findings are obvious, however; for example, few scientists would doubt the generality of natural selection, and Thompson (2013), with echoes of Darwin (epigraphs of chapter 4), has argued that evolution by natural selection is “relentless,” meaning pervasive and unceasing. On the other hand the importance of introgressive hybridization or interspecific competition is less well established and needs to be repeatedly assessed by measurement and experimentation (e.g., Arnold 1997, Dhondt 2012).
Daphne is small, and so are the finch populations; therefore we ask specifically to what extent can generalizations be made from studying finches with these features for a long time in one place (Grant and Grant 2010d, Billick and Price 2010)? We start with theory because it is general. Theory is explicit about genes and fitness but usually not about the intervening phenotype or the environment (fig. 4.1). Our study is explicit about the phenotype and environmentally driven fitness, but largely ignorant about the underlying genotypes. Acknowledging the difference, we use theory to interpret observations on Daphne.
THE SMALL POPULATION SYNDROME
Population genetics theory identifies several relevant genetic properties of populations that vary with population size (Crow and Kimura 1970). First, fewer mutations arise in small populations than in large ones because there are fewer genomes to mutate. Second, the probability that alleles will be lost by random genetic drift is higher in small populations. Third, as a result of both, small populations are expected to have a lower level of standing genetic variation. These three properties are mathematical consequences of small numbers of breeders (Price et al. 2010). When the reproductive success of breeders is heavily skewed, the genetically effective population size is even lower than the simple number of breeders, and the probability of genetic drift is correspondingly higher. The long-term genetically effective size is more influenced by low numbers than by high numbers, and this is important in a fluctuating environment such as Daphne. Other properties of small populations include high rates of inbreeding (Keller et al. 2006), diminished fitness, and loss of alleles; more rapid fixation of alleles, including ones with deleterious effects; reduced variation in epistatic interactions experienced by alleles (Mayr 1954); and possibly lower levels of pleiotropy (Stern 2011).
All things being equal, then, Daphne finch populations should vary less than related populations on large islands such as Santa Cruz and mainland populations. There is an additional ecological reason why this should be so. Habitats vary in plant composition along altitudinal transects on large islands such as Santa Cruz, and not on Daphne; therefore there is scope for some degree of genetic differentiation in different habitats on Santa Cruz (Kleindorfer et al. 2006, de Léon et al. 2010, Galligan et al. 2012). The expected difference in phenotypic (beak) variation of fortis, reflecting genetic variation, is in fact observed in a comparison of Daphne and Santa Cruz populations. However, several large islands support populations of fortis with lower variation than on Daphne (Grant et al. 1985). This shows that all things are not equal. The missing factor identified in the study of Daphne finches is introgression of alleles from other sympatric species.
Natural selection in small populations is sometimes described as being relatively inefficient because of the greater role of genetic drift in the loss or fixation of alleles. Selectively disadvantageous mutations are more likely to be fixed by genetic drift in small populations than in large ones. This may be happening the whole time on Daphne without us being aware of it, because we do not see the phenotypic effects of individual mutations; a single exception is described in the epilogue (chapter 17). Similarly, and for the same reason, we may fail to appreciate that stabilizing selection at the genetic level may be occurring the whole time, as typically assumed theoretically. Without denying both possibilities, we note that directional selection is occasionally strong on beak variation, and therefore not inefficient on this phenotype, and stabilizing selection on beak and body traits is apparently very rare. Moreover the Big Bird lineage did not lose any alleles at 16 microsatellite loci in three generations despite starting from a single inbred pair at the F3 generation, and inbreeding did not result in the expected loss of variation, or the extinction of either magnirostris or the Big Bird lineage.
The small population paradigm of low variation may apply without qualification to populations that are essentially closed to heterospecific gene flow. The most likely examples in Galápagos are the solitary populations of fuliginosa on several arid islands that are smaller and botanically more depauperate than Daphne (Grant 1986) (fig. 15.4). These populations are presumably low in genetic variation, low in ecological opportunity, subject to stochastic fluctuations in numbers and liable to become extinct, and therefore short-lived.
THE MEDIUM POPULATION SYNDROME
Daphne is unique among islands in the Galápagos in size, degree of isolation, and vegetation. Smaller islands are arid and largely treeless, except for those close to large islands as North and South Plazas are in relation to Santa Cruz. Larger islands are generally richer floristically, except for the strongly isolated islands of Wolf and Darwin. Thus the intermediate characteristics of Daphne foster populations of intermediate characteristics. The island is large enough and floristically diverse enough to support three or more related species and not just one or two. Their populations are similar enough to allow hybridization, large enough that extinction risk is low, and large enough that effects of random genetic drift and inbreeding are minimal. All these factors point to a rich evolutionary potential that is less constrained genetically and environmentally than is usual in most small populations.
Generalizing from a unique island strains credulity. Nonetheless, from a deep temporal perspective Daphne is not alone: islands like Daphne existed in the past. Several islands acquired similar characteristics to Daphne when sea level fell and small islands became larger and higher (fig. 2.4), and new ones were formed by volcanic activity. Rises and falls in sea level occurred repeatedly, and islands separated and coalesced several times, in association with periods of glaciation. This happened as many as 10 times at 100,000 year intervals in the last million years, sufficient time for generation of the eight youngest species in the radiation and perhaps more. Their long-term survival would have been dependent upon colonization of other islands by dispersal or vicariance when coalesced islands separated (Poulakakis et al. 2012, Geist et al. MS). This broader perspective implies that favorable conditions for speciation occurred more in the past than in the present. Assuming the interpretation is relevant to speciation outside the archipelago, we suggest that the fission-fusion-replacement dynamics we see in the Galápagos occur in similar island settings with similar histories. Two examples involving hybridization are Nesospiza finches on the Tristan group of islands (Ryan et al. 1994, 2007) and Zosterops white-eyes on Reunion (Gill 1970, Warren et al. 2006). Similar dynamics may occur on continents in local, semi-isolated patches of inhomogeneous habitat, most likely at the margins of species distributions (see below). From this point of view Daphne, although singular, is a model system.
LARGE ISLANDS
If Daphne finches are unique, we do not expect hybridization on large islands. On the other hand hybridization may occur elsewhere in the archipelago but perhaps less frequently, in which case we should see signs of gene exchange between sympatric species on other islands. Those signs would be greater genetic similarity between species A and B where they occur together than where they occur on different islands. We made these comparisons between all pairs of related species, including the tree finches, on many islands, all larger than Daphne, and found overwhelming support for widespread gene exchange: species were more similar on average at microsatellite loci in sympatry than in allopatry (Grant et al. 2005). Therefore Daphne finches are not unique. Nevertheless introgression may have stronger effects on islands like Daphne because of its relatively small size.
In other respects Daphne finches are unusual. For example, they are probably subject to natural selection when food is scarce more frequently and intensely than are finches on large islands. Droughts are likely to be more severe on Daphne: the climatic evidence was discussed in the previous chapter. Furthermore Daphne lacks resident predators as well as the diversity of habitats present on large islands that may enable finches to escape the worst effects of food scarcity. On Daphne they have nowhere to go.
These comparisons are relevant to the debate on the roles of island size and isolation in evolution (MacArthur and Wilson 1967, Losos and Ricklefs 2010). Relatively small and well-isolated Galápagos islands have been considered a greater source of evolutionary novelty than large islands because they have a high proportion of endemic subspecies of finches (Lack 1945, 1947, Hamilton and Rubinoff 1967). Alternatively the high endemism might reflect long-term persistence rather than high rate of evolutionary divergence (Price et al. 2010, Rosindell and Phillimore 2011), and Cox (1990) has argued that the large central islands might have contributed more to the radiation. Evidence from Daphne and other islands (Petren et al. 2005) supports the former view.
BEYOND GALÁPAGOS
Where might the medium population syndrome apply to birds in continental regions? The answer is discontinuous, fragmented, or peripheral habitats. One example is glacially disturbed habitats at high latitudes (Weir and Schluter 2007). A second example is montane habitats in the Andes, especially during uplift when changes occurred at habitat margins (Burns and Naoki 2004, Fjeldså and Rahbeck 2006, Cadena et al. 2012). These include newly created patches of open habitat that witnessed rapid speciation in certain plant groups (Hughes and Eastwood 2006) and isolated cloud-forest ridges undergoing similar rapid speciation in plant species (Gentry 1989). A third example is dry and humid habitats in the neotropics that underwent cycles of expansion and contraction (Campagna et al. 2012, Jetz et al. 2012, Smith et al. 2012). A group of eleven species of capuchino seedeaters (Sporophila) in South America illustrate the role of habitat size in speciation. Their rapid diversification in the Pleistocene was associated with periodic contractions of savanna and forested habitat, so at times their populations must have been small. They occur in various sympatric associations, and like Darwin’s finches they display genetic signatures of introgressive hybridization (Campagna et al. 2012). In several respects capuchinos may be the continental equivalent of Darwin’s finches.
Our thesis, developed more extensively in a previous book (Grant and Grant 2008a), is that Darwin’s finch evolution is no different in principle from the earliest stages of evolutionary divergence elsewhere, but differs in detail. The most important difference in detail is color. Darwin’s finches are not colorful, whereas most of their continental tanager relatives are colorful and patterned, many outstandingly so (Burns 1997, Isler and Isler 1999). Sexual selection through female choice is likely to have played a much larger role in the diversification of continental species than in Darwin’s finches. Speciation, and the rate of evolution of such traits, may be governed by the rate at which new mutations arise (Lanfear et al. 2010, Price et al. 2010), except when species hybridize. Little is actually known empirically about how the mutations then spread throughout the range of a species, either from the center or the margins where hybridization with a parapatric species might occur.
A second difference is environmental, and this may affect the rate of speciation. Consider the continental tanagers again. The current number of species is about 400 (Isler and Isler 1999), many are allopatric, and they evolved in roughly 12 MY (Cracraft and Barker 2009). Assuming uniform rates of speciation and no extinction, these figures translate into a doubling of species numbers approximately every 1.5 MY. This is about one-third of the rate of accumulation of Darwin’s finch species (Grant and Grant 2008a) and is in keeping with data from other neotropical species that show a relatively slow rate of speciation (Price 2010, Weir and Price 2011). However, two groups of tanagers confound the trend with high rates of speciation. The first is a group of species in another archipelago, the Caribbean, that contain the closest relatives of Darwin’s finches (Sato et al. 2001, Burns et al. 2002). The second group comprises 49 species in the genus Tangara. Their evolution began 6.5 MYA, but most speciation (species accumulation) was concentrated in the period 3.5–5.5 MYA (Burns and Naoki 2004) at a time when the Andes were rising (Coltorti and Ollier 2000). Thus their diversification rate is more comparable to the speciation rate of Darwin’s finches, and was even higher in a concentrated period of their history. This is an important observation, since it shows that archipelagos are not necessarily the sites of fastest diversification, so their inhabitants are not necessarily exceptional.
THE SPECTER OF EXTINCTION, THE BIG UNKNOWN
We conclude the chapter with speculations about extinction. The 600 MY fossil record shows that almost all species that ever existed have become extinct (Simpson 1953). Are recent radiations exempt? Probably not. Our four decades of observations provide tantalizing hints of repeated speciation and extinction on a much shorter timescale. Thus any explanation of biological richness is incomplete without some understanding of the subtractive process of extinction and its effects on modern communities. Unfortunately almost nothing is known from direct evidence about natural extinction in recently radiating groups of organisms. The assumption we made in the preceding paragraph, that speciation rate is uniform and extinction is absent, is just that, an assumption. In the absence of fossils all we know is the minimum number of species that evolved in a group. The statistical challenge is to devise a model with extinction and speciation parameters that best fits the pattern of species accumulation through time based on molecular phylogenies (Ricklefs 2006, Glor 2010, Rabosky and Glor 2011). An example is the recent analysis of endemic vangids (Vangidae) on Madagascar. They display a standard pattern of species accumulating rapidly early in the radiation and slower later, and modeling shows the pattern is best explained by a decrease in the rate of speciation and not by an increase in the rate of extinction (Jønsson et al. 2012, Reddy et al. 2012).
Darwin’s finches are a classical example of adaptive radiation (Schluter 2000, Grant 2013), yet they lack the classical signature of an early burst of species proliferation followed by a slower rate (fig. 16.1). In this they are not alone (Harmon et al. 2010, Derryberry et al. 2011). Modeling studies have shown that the early-burst signature is erased by extinctions if they are numerous enough (Rabosky and Lovette 2008). On these grounds alone there is evidence of finch extinctions. It appears at face value that the finch radiation started slowly (fig. 16.1), and this may not be correct. Additionally, regardless of whether Darwin’s finches really exhibited an early burst or not, or whether they are still experiencing one, three lines of biological reasoning indicate extinction could have been prevalent in their history.
First, later-formed species may be more efficient at resource exploitation than some of the early-formed ones, and competitively replace them. Second, extinctions are to be expected because the Galápagos environment changed (Grant and Grant 2008a). The composition of the vegetation changed as the climate became progressively cooler and drier through Darwin’s finch history. Some resources and ecological opportunities would have been eliminated as others were created, resulting in both extinction and speciation. The third reason is missing morphologies. The phylogenetically basal warbler finch (Certhidea olivacea) is very different from its closest relatives in continental South America and the Caribbean on the one hand, and very different from the next-oldest Darwin’s finch species (Geospiza difficilis or Platyspiza crassirostris) on the other. The morphological transitions before and after Certhidea are so substantial that intermediate species must surely have existed and evolved into something else (pseudoextinction) or become extinct after generating one or more new species: the same reasoning from morphologically extreme species applies to the vangids mentioned above and to the Hawaiian honeycreepers (Pratt 2005). If our argument is correct, Certhidea may not be basal to the reconstructed phylogeny but a derived lineage from an ancestral species that has become extinct (Lack 1947, Petren et al. 1999b).
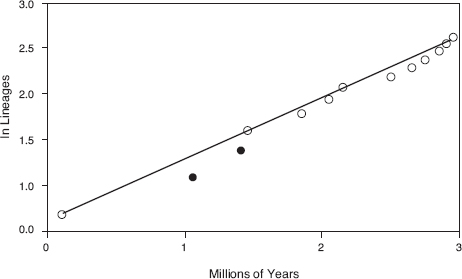
Fig. 16.1 A lineage-through-time plot of Darwin’s finches shows a regular linear increase of species on a semilog plot after an apparent lag indicated by the two solid circles (Grant and Grant 2008a). The plot is constructed by taking a backward look at number of species from present to past and using molecular data to estimate time of species separations.
In combination these considerations suggest that modern Darwin’s finch communities are the product of speciation and extinction that varied in time but not uniformly (fig. 16.2), perhaps co-occurring in pulses (Jackson 1995). Daphne has one such small community. Perhaps one day it will be possible to develop improved inferences about the past for all organisms, not just Darwin’s finches, by reading evidence of extinction as well as evolution into the genomes of extant species (Grant and Grant 2008a).
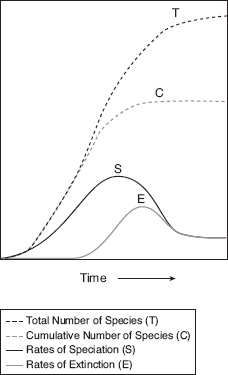
Fig. 16.2 A graphical representation of the development of the Darwin’s finch radiation. Species accumulate in a manner analogous to the MacArthur-Wilson (1967) immigration-extinction model of island biogeography (Grant 1984) and based on the same assumptions of density (diversity)-dependent processes and environmental saturation. There are three phases: (a) an early phase in which speciation, inevitably, exceeds extinction; (b) a middle phase of increasing speciation and extinction; and (c) a final phase when rates of speciation and extinction become equal and fall to a long-term equilibrial value. The logic of the last phase presupposes that a fixed, environmentally determined, long-term number of species in the archipelago has been reached. There is no evidence for the third phase in Darwin’s finches, but there is for Caribbean Anolis lizards (Rabosky and Glor 2011), and evidence of an overshoot in species accumulation in a laboratory microcosm (Meyer et al. 2011). Extinction in the second phase could be responsible for an apparent lag in speciation at the beginning of the finch radiation (fig. 16.1), and high morphological disparity in surviving species, if some of the early formed species were outcompeted and replaced by later, more efficient, and morphologically different species (e.g., Ricklefs and Cox 1972). Quantitative models differ from this scheme by continuously varying speciation and extinction uniformly through time or setting extinction to zero throughout (e.g., Ricklefs 2006, Rabosky and Lovette 2008, Rabosky and Glor 2011).
Summary
In this chapter we discuss the question of how generalizable are the results of studying finches on one island. The field observations are put in the context of theoretically expected evolution in small populations. Populations on Daphne do not conform entirely to expectation, for several reasons. Despite being small the island is floristically diverse enough to support three or more related species. Their populations are similar enough to allow hybridization, large enough that extinction risk is low, and large enough that effects of random genetic drift and inbreeding are minimal. We suggest that although Daphne is a unique island with unique biological properties, other islands of similar characteristics occurred in the archipelago in glacial periods. They may have been especially important platforms for generating new species. The same may be true in island-like situations on continents. We conclude by speculating about extinction, and suggest that future studies of avian genomes may help to reveal the signs of hitherto unknown species and extinctions. Finch populations must have frequently come and gone as new islands emerged and some were submerged.

