
Plate 26 HIV: oral hairy leukoplakia (42).
The gut contains the largest and the most complex lymphoid tissue of the body [gut-associated lymphoid tissue (GALT)]. The GALT contains 70–80% of the total body lymphocytes. More than 60% of CD4 cells are depleted in the first 2–4 weeks after HIV infection and are not replenished, even after immune reconstitution with ART seen in the peripheral blood in the majority of treated patients. HIV directly infects the gut mucosal cells and interaction between gp120 and certain mucosal proteins results in efficient cell-to-cell spread of HIV. Alteration in gut permeability and microbial translocation are thought to play a role in immune activation and disease progression. Similar to peripheral blood mononuclear cells, there is evidence of HIV persistence in GALT in aviraemic-treated patients. This may imply cross-infection between these 2 cellular compartments and may have a role in HIV persistence (reservoirs), despite long-term viral suppression.
In the pre-ART era opportunistic mucosal infections were a common feature of disease progression. With treatment, it is evident that patients do not demonstrate any short-term effects from the profound loss of CD4 cells of the gut.
Very common; oral lesions may alert professionals to HIV as the underlying cause. Detailed oral examination should be part of the assessment of all newly diagnosed HIV +ve individuals. Various lesions of ulcerative, raised, white, or pigmented appearance may be encountered (Boxes 42.1 and 42.2). Advice from a dentist and oral or maxillofacial surgeon should be obtained when necessary.
HSV infection is very common, with seropositivity rates approaching 80% in homosexual men living with HIV. Oral HSV, like herpes infection elsewhere, is characterized by latency (in the trigeminal ganglia). The majority are caused by HSV-1:
• 1° episodes: can occur at any age, but most common in children and young adults. May be asymptomatic to severe gingivo-stomatitis. Vesicles, typically itchy, appear on the lips, tongue, gums, buccal mucosa, or gingiva and hard palate. Unlike herpes zoster 1° HSV infection is not associated with viraemia. Lesions are initially vesicular, followed by ulcers, crusting then healing. It may take up to 3 weeks for 1° HSV lesions to heal. The duration is longer in the severely immunocompromised.
• Recurrent HSV: tends to localize to the vermilion border of the lips. Some patients experience pain or tingling sensation before the appearance of the lesions. It may take 7–10 days for oral HSV lesions to heal. Recurrent HSV infection may be more common in patients with symptomatic HIV disease, and severely immunocompromised patients tend to have more frequent and more severe attacks.
• Ocular keratitis occurs with the same frequency as in HIV –ve individuals.
• HSV oesophagitis usually with severe odynophagia occurs more frequently in patients with late HIV disease, but not necessarily associated with concomitant oral herpes,
• HSV meningitis is more likely to follow 1° genital herpes, but can occur in acute oral herpes.
• Management: labial herpes may be treated with topical acyclovir 4–6 times a day (systemic treatment; see Chapter 42, ‘Anal disease’, pp. 501–502).
Box 42.1 Differential diagnosis of white lesions in the oral cavity
• Tobacco-induced leukoplakia.
Seroprevalence rates rise with age. >90% prevalence rates have been found in homosexual men living with HIV.
• mucosal, intra-oral, typically solitary, deep, necrotic with a red margin and a white halo; difficult to differentiate from those of other aetiology
• arise when the CD4 count is <50 cells/µL
• usually occur as part of disseminated CMV disease and attempts must be made to exclude other organs involvement, e.g. the retina
• diagnosis is established by the biopsy finding of typical owl’s eye inclusions; CMV PCR on tissue sample may help
Management: For systemic management see Chapter 44, ‘Treatment’, (pp. 518–519).
Reactivation of VZV causes herpes zoster (HZ)/shingles in the immunocompromised host, including HIV infection at any stage. Rarely presents as a 1° infection in those without previous exposure to the virus. People with HIV infection are 15 times more likely to have HZ than age-matched controls. Oral HZ is latent in the trigeminal ganglion. Mandibular branch involvement presents with lesions on the lower lip and lateral border of the tongue, and maxillary branch involvement presents with lesions on the hard palate. Oral vesicles last for a few hours followed by painful ulcers, while the concomitant skin lesions may last for 2–4 weeks.
For diagnosis and management, see Chapter 47, ‘Varicella zoster virus infection’, pp. 553–554).
White adherent vertically corrugated lesion, only found in the mouth, most commonly the lateral aspect of the tongue, and can be on one side only (Plate 26). The affected area may fluctuate in size.

Plate 26 HIV: oral hairy leukoplakia (42).
Caused by EBV. Usually asymptomatic, but a few patients may complain of pain, and altered sensation and taste. Reported in all risk groups but more frequent in adults, men and smokers. It is not pathognomonic and occurs in other immunocompromised individuals, such as bone marrow and renal transplant recipients. Incidence and persistence increase with advancing HIV disease and declining CD4 counts. Its occurrence is associated with faster progression towards AIDS even after adjustment for CD4 count. It is not premalignant and does not need specific treatment, but regresses with improved immune function associated with ART.
Incidence dropped dramatically with the advent of ART. HPV type 44, 32, 7, 13, and 18 are the most commonly identified causes of oral warts in PLWH. Mutant strains are frequently reported. Oral warts are more common than in the general population, but there is no association with the stage of infection. HPV-16 has been associated with oral SCC in HIV-infected men. Although mostly localized to the oral cavity, laryngeal warts may occur, and may be solitary or multiple, pedunculated, or sessile with small papilliferous or cauliflower-like projections.
Surgical excision, laser, or cryotherapy.
The  incidence of gum and periodontal disease in PLWH makes dental hygiene particularly important. Periodontal disease should be managed in consultation with the dentist, and/or the maxillofacial or dental surgeon. Mild gingivitis and dental abscesses are common at all stages of HIV infection. Linear gingival erythema (LGE), necrotizing ulcerative periodontitis (NUP) and necrotizing stomatitis (NS) occur more frequently in HIV infection. They are characterized by rapid onset, increased severity, and poor response to conventional treatment.
incidence of gum and periodontal disease in PLWH makes dental hygiene particularly important. Periodontal disease should be managed in consultation with the dentist, and/or the maxillofacial or dental surgeon. Mild gingivitis and dental abscesses are common at all stages of HIV infection. Linear gingival erythema (LGE), necrotizing ulcerative periodontitis (NUP) and necrotizing stomatitis (NS) occur more frequently in HIV infection. They are characterized by rapid onset, increased severity, and poor response to conventional treatment.
• LGE: patients present with spontaneous painless gum bleeding. Examination reveals an oedematous erythematous band parallel to the free gingival margin, which may be a precursor of NUP. Commonly isolated organisms include Bacteroides gingivalis, Fusobacterium nucleatum, and Actinobacilli. Response to treatment is poor, but attention to oral hygiene and chlorhexidine mouthwashes are helpful. Systemic metronidazole may be required.
• Necrotizing periodontal diseases (NPD): acute-onset severe rapidly progressive disease, usually seen in patients with advanced HIV. Includes NUG and NS. Appears to be related to diminished resistance to bacterial infection and only differ in the severity of tissue involvement, with NUP extending into periodontal attachment resulting in more destruction. Patients complain of spontaneous bleeding, foul mouth smell and deep jaw pain. Severe gum pain precedes the appearance of signs, which include soft tissue necrosis with destruction of periodontal ligament and bone. NUP may resemble intra-oral lymphoma. Management includes oral hygiene, chlorhexidine mouthwashes and oral antibiotics e.g. metronidazole and amoxicillin together with gentle debridement.
The most common oral manifestation is irregular ulceration of the tongue or palate. Culture has low sensitivity detection rates (2–17%) so PCR should be considered.
Probably the most common opportunistic infection (OI) in HIV. >90% would have oropharyngeal candidiasis at some stage. Commonly caused by Candida albicans, but other species, such as Candida glabrata and Candida tropicalis are implicated. Although oropharyngeal candidiasis may occur at acute seroconversion, it is more frequent as the CD4 count  .
.
• Pseudomembranous candidiasis: the pseudomembranous appearance results from overgrowth of candidal hyphae, mixed with desquamated epithelium and inflammatory cells. Appears as white plaques at any site of the mouth or pharynx, and leaves an erythematous raw bleeding mucosa on scraping. Patient may complain of soreness and altered taste (Plate 25).
• Erythematous candidiasis: appears as flat, red patches of varying morphology that can be difficult to recognize and, therefore, diagnosis may be delayed. Most common sites are palate and dorsal surface of the tongue. Usually asymptomatic, but soreness and burning sensation may be reported, especially after eating salty or spicy food.
• Angular cheilitis: may appear solely or in conjunction with other forms of oropharyngeal candidiasis, resulting in redness, ulcers, and fissuring of one or both corners of the mouth.
• Hyperplastic candidiasis: the rarest form of candidiasis in PLWH. Chronic, white, and nodular, and cannot be wiped off.

Plate 25 Oral candidiasis and HIV (42).
Can be made on the clinical appearance. Candidal hyphae can be demonstrated on Gram or periodic acid Schiff staining of smears from lesions. Culture may be used, particularly in recalcitrant infection, to identify the candidal type, which may guide treatment. Sensitivities may be difficult to interpret. It does not help in making the diagnosis, as candida is a commensal of the oropharynx.
• Systemic antifungal (standard mode of therapy):
• fluconazole—50–100 mg od for 7–14 days
• itraconazole solution—100–200 mg od for 7–14 days.
• Topical antifungal (consider in mild cases):
• nystatin (as oral suspension)—100,000 units qds for 7 days and continue for 48 hours after lesions resolution
• miconazole oral gel—2.5 mL after food qds, continue for at least 7 days after lesions resolution.
Topical treatment must be retained in the mouth in contact with lesions for sufficient time.
Relapses are common and may be the result of poor compliance, rather than resistance to antifungals. Consider prophylactic therapy (fluconazole 50 mg daily) when recurrences are common, but risk of resistance.
See Chapter 54, ‘Kaposi’s sarcoma’, pp. 611–613.
Oral KS was among the first recognized features in the early AIDS epidemic. More common in MSM. Usually presents as a blue, purple, or red lesion that may be flat, papular, nodular, or rarely a large tumour (Plate 27). Large nodular lesions may ulcerate and be secondarily infected. Occasionally, the adjacent mucosa is yellow stained (due to haemosiderin). Commonest site is the hard palate, but may be seen on the gingiva, tongue, soft palate, and buccal mucosa. Local pain may be a feature in secondarily infected, ulcerative lesions. Mucosal lesions may indicate more extensive visceral involvement requiring good systemic enquiry and radiological investigation with chest X-ray and possibly computerized tomography (CT) scanning.
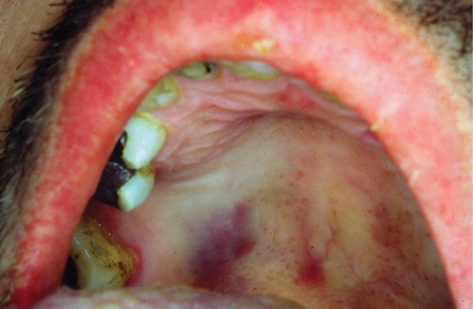
Plate 27 Oral Kaposi’s sarcoma (42).
See Chapter 54,’Kaposi’s sarcoma’, pp. 611–613.
• immune reconstitution accomplished by art may result in KS resolution, improves survival, and response to therapy
• small lesions respond to local therapy, e.g. surgical excision (may bleed excessively), laser excision, and intralesional chemotherapy
• larger lesions may be treated with radiation therapy, which may be complicated by mucositis.
EBV is implicated in the majority of cases. The vast majority are B-cell non-Hodgkin’s lymphomas. Oral lymphoma may precede the development of lymphomas at other sites. Presents as a rapidly growing tumour at any site in the mouth (Plate 28). May be diffuse or discrete nodules, or may present as ulcers of variable morphology. May mimic periodontal disease. Diagnosis by tissue biopsy.

Plate 28 Oral lymphoma and HIV (42).
Once the diagnosis is established, lymphoma must be staged by appropriate imaging. Systemic chemotherapy and local radiation are the main stay of therapy in collaboration with the maxillofacial surgeons to minimize oral side-effects.
Recurrent oral ulceration resembling aphthous ulcers is a common phenomenon in PLWH, usually well circumscribed, superficial, and varying in size:
• Herpetiform: painful clusters of 1–2 mm ulcers, usually in the mouth and soft palate.
• Minor—usually solitary, 0.5–1.0 cm in diameter, causing minimal symptoms.
• Major—2–4 cm in size and necrotic. Usually found on the tonsils and tongue base, and rarely the pharynx and nasopharynx. Typically, very painful, persisting for several weeks.
• Local analgesics: lidocaine hydrochloride 5% ointment or 10% solution as a spray; apply thinly to the ulcer using a cotton bud.
• Topical steroids: e.g. hydrocortisone muco-adhesive buccal tablets as 1 qds (allow to dissolve slowly in mouth in contact with ulcer).
• Systemic steroids may be used if associated with oesophageal ulcers 40–60 mg daily for 7–10 days. Infective aetiology must be excluded.
• Thalidomide (50–200 mg/day) produces an excellent response in resistant cases. Prescribing thalidomide needs compliance with certain requirements, especially the avoidance of pregnancy.
Salivary glands may become infiltrated with lymphocytes, predominantly CD8 cells, leading to enlargement especially the parotids.
Xerostomia may be caused by drugs such as didanosine (DDI), antihistamines, and antidepressants, but also as a manifestation of HIV infection, where the precise aetiology is unclear. Salivary amylase levels may be raised in drug-related cases.
• Salivary stimulants, such as sugarless sweets and chewing gum for symptomatic relief.
• Artificial saliva as necessary.
• Diffuse infiltrating lymphocytosis syndrome (DILS): due to CD8 expansion, may also involve the cervical lymph nodes and lungs. Presents with dry mouth and parotid glands enlargement.
• Benign lympho-epithelial cysts: usually painless and soft, may be single or multiple, and may enlarge gradually to involve the superficial lobes of both glands.
Imaging techniques and fine needle aspiration help to confirm diagnosis. Parotidectomy rarely needed other than for cosmetic reasons.
Box 42.2 Differential diagnosis of oral–pharyngeal ulcers
• necrotizing ulcerative periodontitis
• necrotizing ulcerative gingivitis
Dysphagia +/– odynophagia are the main symptoms. However, symptoms and signs are not discriminative enough to establish an underlying aetiology (Box 42.3). Assessment of the nutritional status and hydration is essential in managing such patients.
Box 42.3 Causes of oesophagitis
• Mycobacterium avium complex.
• Endoscopy and biopsy required to establish a definitive diagnosis.
• Double-contrast barium swallow may differentiate between infection and neoplasm, but rarely diagnostic.
• More than one pathology may co-exist and, therefore, multiple biopsies (>6) from different sites are advisable.
• Generally, culture of oesophageal specimens is not helpful as does not differentiate between colonization and actual tissue invasion, but may be useful in identifying viral and mycobacterial pathogens.
Most common cause of oesophagitis in HIV infection. Patient with oral candidiasis and odynophagia can be treated empirically for oesophageal candidiasis, and investigated if it fails to respond. Usually occurs when CD4 count <200 cells/µL and rarely asymptomatic. Endoscopic appearance classically diffuse raised plaques that can be removed from the mucosa by the endoscope. Brushing or biopsy of plaques shows candida hyphae, spores, and diffuse inflammatory infiltrates.
• fluconazole 100–200mg daily for 7–14 days.
• itraconazole 200 mg daily in 1-2 divided doses (the suspension formulation of itraconazole has a better bioavailability than the capsular form). This is especially useful in fluconazole-resistant candidiasis (rare and seen in those with very low CD4 count who have had repeated courses of fluconazole).
• liposomal amphotericin B (e.g. Amphocil®, AmbiSome®)
• voriconazole, in refractory cases and in fluconazole-resistance.
CMV causes large distal oesophageal ulcers. Biopsy required to con-firm, and exclude other or concomitant causes of oesophagitis.
CMV disease is treated with ganciclovir or foscarnet until clinical improvement. Patients may be given maintenance oral valganciclovir to prevent CMV retinitis (see Chapter 44, ‘CMV retinitis’, pp. 518–519).
Diarrhoea is an extremely common symptom in HIV infection. Can be debilitating, and require extensive and invasive investigation. In patients with CD4 >200 cells/µL, usually caused by virulent organisms and managed as in the immunocompetent. When CD4 <200 cells/µL, it is usually more severe, more likely caused by OIs, more difficult to diagnose and is less responsive to treatment. Large bowel diarrhoea is typically of small volume, may be blood-stained, and associated with lower abdominal pain and tenesmus. Small bowel diarrhoea is characteristically of large volume, offensive, and of pale colour. Diarrhoea is chronic if persists >1 month. Although infection with campylobacter and other enteric pathogens occur (see Chapter 20, ‘Proctocolitis and enteric sexually acquired infections’, pp. 259–266), particularly important organisms are cryptosporidium, microsporidium, cystoisopora, and salmonella.
Cryptosporidium spp. are protozoan parasites that primarily cause enteric illness in humans and animals. They have worldwide distribution and lack host specificity. The two species that infect humans most frequently are Cryptosporidium hominis and Cryptosporidium parvum. They are 4–6 µm intracellular protozoa and have a complete life cycle (with a sexual and an asexual stage) in the intestinal mucosa of a single host. The oocyst can survive in the environment for >3 months. Given its small size, it may bypass water filtration systems. It has an outer shell making it resistant to standard home and hospital disinfectants. It is highly infectious; the infectious dose for C. hominis 10–83 oocysts. Infection is self-limiting and is usually asymptomatic in the immunocompetent host, but can cause debilitating symptoms in patients with advanced HIV disease. Human disease is usually limited to the jejunum, but may involve all the GI and respiratory epithelium in the immunocompromised.
The incidence of cryptosporidiosis in PLWH has declined dramatically since the introduction of ART.
• Zoonotic infection: e.g. rural communities. Contact with infected lambs and calves.
• Outbreaks have been linked to drinking or swimming in contaminated water.
• Travellers to countries with poor sanitation systems.
• Human-to-human transmission, which may include sexual contact.
• Oocysts may be excreted for 2 weeks after recovery.
• PLWH with preserved immune function: similar to HIV –ve immunocompetent. Acute, self-limiting profuse, watery diarrhoea with weight loss, abdominal pain, anorexia, and fatigue. Symptoms resolve within 2–3 weeks.
• In advanced HIV disease: persistent watery diarrhoea of variable frequency and volume (1–15 L/day). Commonly associated with nausea, abdominal cramps, malabsorption, weight loss, and electrolyte disturbances. Sclerosing cholangitis, interstitial lung disease, chronic sinusitis, and otitis media are rare features.
The parasite can be easily missed on routine stool analysis, inform lab that diagnosis is considered. Special staining techniques such as auramine-phenol (fluorescent stain) are required. Intestinal mucosal biopsies may identify the organism.
Attention to fluid and nutrition is important. No proven effective agent, although paromomycin, azithromycin, and other antibiotics are used with variable degrees of success.
• Best response is by improving the immune function with ART.
• octreotide (somatostatin analogue)—effective in secretory diarrhoea.
• Raising awareness of modes of transmission.
• If CD4 <50 cells/µL: boil water for minimum 10 minutes.
• Use of fine filters to the mains water supplies.
Microsporidia are small (1–5 µm) obligate intracellular fungi of which several hundred species were identified, but only a few cause human disease. They develop in enterocytes and are excreted in faeces. Transmitted by the ingestion of contaminated food or water, and possibly through contact with infected animals. Incidence in PLWH  dramatically with the introduction of ART. The commonest microsporidia that cause disease in patients with advanced HIV infection are:
dramatically with the introduction of ART. The commonest microsporidia that cause disease in patients with advanced HIV infection are:
• Encephalitazoon intestinalis: accounts for the majority of microsporidial diarrhoea. It is associated with cholangitis, dermatitis, disseminated infection, superficial keratoconjunctivitis, and weight loss.
• Enterocytozoon bieneusi: accounts for most of remainder of microsporidial diarrhoea. It is associated with malabsorption, pulmonary disease, and cholangitis.
Identification of the organism in the stools with modified trichrome stain and immunofluorescent stains such as calcofluor stain. Molecular identification using species-specific PCR assays are also available. Various staining methods are used to visualize the organism in tissue biopsies. Transmission electron microscopy of intestinal biopsies considered as gold standard if available.
• Immune reconstitution with ART as the most effective treatment.
• Albendazole 400mg twice daily for 4 weeks produces best results for Encephalitazoon infection.
• Some relief of symptoms has been described with metronidazole,
• Co-trimoxazole, erythromycin and octreotide but all fail to eradicate the organism.
Cystoisospora belli (formerly known as Isospora belli) is a common cause of epidemic diarrhoea in tropical countries and travellers. Infection occurs by ingestion of the mature (fully sporulated) oocysts contaminating food and water. Infection is usually confined to the small intestine, but may cause acalculous cholecystitis and may disseminate in patients with advanced HIV disease.
• Crampy abdominal pain, watery diarrhoea, and weight loss similar to cryptosporidiosis.
• Steatorrhoea (involvement of the pancreatic and biliary tracts).
• Rarely involves the spleen, liver, and abdominal lymph nodes.
Oocysts (25–30 × 10–19 µm) are visualized in the stools by using special stains such as modified Ziehl–Neelson or in duodenal aspirate or biopsy.
• Co-trimoxazole 960 mg 2–4 times a day for 2–4 weeks gives good results.
• 2° prophylaxis recommended as relapses are common, e.g. co-trimoxazole as for PCP.
• ART results in significant improvement of symptoms.
Salmonellae are Gram –ve non-spore-forming rods belonging to the Enterobacteriaceae family. Non-typhoidal salmonella infections occur with  frequency in PLWH, particularly with Salmonella typhimurium. Major sources are poultry and eggs. Salmonella multiplies in the intestinal epithelium and, if not contained, it invades the mesenteric lymph nodes and disseminates. Cellular immune responses are important defence mechanisms.
frequency in PLWH, particularly with Salmonella typhimurium. Major sources are poultry and eggs. Salmonella multiplies in the intestinal epithelium and, if not contained, it invades the mesenteric lymph nodes and disseminates. Cellular immune responses are important defence mechanisms.
In HIV infection, salmonella tends to cause more systemic symptoms. A common feature is severe gastroenteritis and fever. Localized extra-intestinal focal infection may occur. Bacteraemia is common in patients with advanced HIV disease, may be recurrent and may not be accompanied by gastrointestinal symptoms.
• isolation of the organism is necessary for a definitive diagnosis
• salmonella may be isolated in the blood before stools become +ve
• diagnosis should be considered in all PLWH with fever +/– diarrhoea, and blood and stool cultures taken.
Salmonella requires prompt treatment in PLWH because of severity, high relapse rates, and higher risk of dissemination:
• fluid and electrolyte replacement
• oral ciprofloxacin 750 mg bd (the drug of choice)
• amoxicillin, but bacterial resistance is  .
.
• Avoid eating undercooked food with particular care during travel.
• Long-term suppressive antibiotic therapy may be required if recurrent episodes. It may be possible to stop such therapy if there is significant immune reconstitution with ART. Ciprofloxacin is the drug of choice though co-trimoxazole has some effect.
• AIDS patients have  rates of carriage, which has implications in those working in the food industry.
rates of carriage, which has implications in those working in the food industry.
Shigellae are Gram –ve bacteria that can colonize and invade the intestinal cells, causing disease ranging from mild gastroenteritis to severe acute watery diarrhoea, which may have blood or mucus. The genus Shigella belongs to the family Enterobacteriacae and consists of four species; Shigella dysenteriae, S. flexneri, S. boydii, and S. sonnei. Historically, in the UK, most cases of shigellosis were associated with travel. Recently, there is a sustained outbreak of S. flexneri amongst MSM. Sexual practices such as rimming (oro-anal contact), sharing of sex toys, and multiple partners play a role. These practices are often linked to parties and chemsex (the use of certain substances that lead to disinhibition and engagement in high risk sexual practices).
By stools culture or PCR
Attention to hygiene and fluid intake needed. Usually self-limiting and does not require antibiotics. Antibiotic treatment, if needed, better guided by sensitivity testing, as Shigella often resistant to multiple antibiotics. Ciprofloxacin can be commenced pending lab results.
An idiopathic form of diarrhoea that can occur during the acute phase of HIV infection through advanced disease. Histologically, villous blunting, crypt hyperplasia, inflammatory infiltrates of the lamina propria, immune activation, impaired mucosal repair and regeneration. Absorption and secretory functions are also affected. The pathogenic mechanism/s remains unclear, but may involve effects of HIV itself on the GI tract and the GALT. Viral proteins secreted or shed from infected cells may have toxic effects on cells of the GI tract. HIV can affect the local humoral immunity and gut motility as a result of autonomic dysfunction. This leads to rapid cell turnover and functional immaturity of intestinal epithelium, resulting in lower enzyme production, which leads to impaired absorption. Evidence of malabsorption, such as abnormal d-xylose test, low vitamin B12 levels, and impaired triolein breath tests are described in patients with advanced HIV. These abnormalities may occur in the absence of intestinal disease. HIV enteropathy is a diagnosis of exclusion (Fig 42.1). It improves with ART, but it may also occur in patients receiving ART.

Fig 42.1 Evaluation of chronic diarrhoea in PLWH
Every attempt should be made to find an underling cause, especially infection (Box 42.4). Address factors contributing to diarrhoea, such as alcoholism and drugs. Principles of therapy are attention to nutrition and symptomatic control of diarrhoea. Improves with ART.
Box 42.4 Common causes of diarrhoea in HIV
• Salmonella, Shigella, Campylobacter, E. coli, C. difficile
• Giardia, Cryptosporidium, Microsporidum, Cystoisospora & Entamoeba
• CD4 < 50 cells/µl—Cryptosporidium, Microsporidium.
• CD4 < 50 cells/µl— CMV, HSV.
Anal complaints and anal lesions are frequent presentations in those with HIV infection, especially MSM. Anal pain, pruritus, discharge, and bleeding should be assessed by enquiring about sexual practices, followed by anal and rectal examination by proctoscopy. Gonorrhoea, chlamydia, syphilis, and anogenital warts are common and must be excluded.
Although hsv2 causes the majority of infection, hsv1 is  . Patients with advanced hiv disease tend to have more recurrences and prolonged episodes that are less likely to resolve spontaneously.
. Patients with advanced hiv disease tend to have more recurrences and prolonged episodes that are less likely to resolve spontaneously.
Typical painful vesicles and ulcers occur in the anal and peri-anal region. Severe disease may result in diffuse peri-anal ulceration. The distal rectum may be involved, resulting in anal discharge, painful defaecation, rectal bleeding, and tenesmus. Fever and painful inguinal adenopathy occur more commonly in primary attacks.
See Chapter 22, ‘Anogenital herpes’, pp. 277–289.
Prompt treatment with specific antiviral drugs reduces the duration of symptoms and viral shedding. Chronic episodes (>4 weeks) occur more frequently in patients with advanced HIV disease. Acyclovir-resistant hsv has been reported in association with hiv infection. Valaciclovir and famciclovir have better bioavailability.
Recommended initial treatment in the immunocompromised:
• aciclovir 400 mg 5 times a day for 10 days
• famciclovir 500 mg bd for 10 days (cost may limit use)
• valaciclovir 1000 mg bd for 10 days.
Suppressive treatment (interrupted and reviewed every 6–12 months):
• aciclovir 200 mg qds (or 400 mg bd)
• famciclovir 500 mg bd (cost may limit use).
Anal warts common in PLWH, especially MSM. HPV6 and 11 are the most frequent types, but others including 16, 18, 31, and 33 have been detected. Patients with advanced HIV may have exuberant plaques in the peri-anal region, as well as flexural areas. Extensive anal and peri-anal warts may present with rectal bleeding and constipation.
 Chapter 23, ‘Anogenital warts’, pp. 291–304.
Chapter 23, ‘Anogenital warts’, pp. 291–304.
No treatment ensures HPV eradication and is similar to the HIV –ve, although persistence and recurrences are more common, but may improve with immune reconstitution produced by ART.
In the UK, an HPV vaccination scheme is offered to MSM up to and including the age of 45 years using the quadrivalent vaccine (HPV types 6, 11, 16 and 18).
The epithelium of the transitional zone between the anus and colonic mucosa is susceptible to intra-epithelial neoplasia and invasive cancer, similar to those seen in the female cervix. AIN1–3 precedes the development of anal cancer, similar to cervical cancer. HPV (type 16 and 18) infection, receptive anal intercourse, and HIV infection are co-factors for the development of these changes. The incidence of AIN and anal carcinoma, especially in MSM, is  as life expectancy rises with availability of ART, which does not appear to be protective.
as life expectancy rises with availability of ART, which does not appear to be protective.
Anal carcinoma may be prevented by the early detection and treatment of AIN3. Anal cytology can be used to detect dyskaryosis, although this is not routine clinical practice. In the absence of anal cytology annual digital rectal examination is recommended to detect early lesions.
Low grade AIN lesions can be treated conservatively by regular monitoring and high grade lesions (AIN3) by ablative surgical techniques.
Pancreatic involvement in HIV infection is common, but is usually asymptomatic. Drugs, alcohol, and OIs are the usual causes. Pancreatic disease tends to occur in conjunction with hepatic and hepatobiliary disease. Presentation may be with acute or chronic pancreatitis. Elevation of serum amylase or lipase may predate symptoms development.
Patients usually severely ill, in shock, with acute abdominal pain, nausea, and vomiting. Causes include:
• CMV—most common infective cause
• cryptococcus and toxoplasmosis
• Drugs such as didanosine, co-trimoxazole, and pentamidine.
• Very high levels of triglycerides associated with protease inhibitors.
The serum amylase is elevated and CT scan demonstrates an oedematous, enlarged pancreas with surrounding fluid. Admission to a high dependency unit for cardiovascular and electrolyte support is usually required.
Typically presents with pancreatic exocrine deficiency. Chronic diarrhoea and malabsorption are the usual presenting features. As with HIV –ve patients, the discontinuation of any offending drugs and alcohol may result in symptomatic improvement. HIV-related cholangiopathy is a recognized underlying cause.
Chronic abdominal pain (especially if history of acute pancreatitis) is suggestive of chronic pancreatitis. This may be accompanied by pancreatic insufficiency symptoms, e.g. steatorrhoea, weight loss, and other evidence of malabsorption. Screen by measuring faecal elastase, or chromotrypsin and triolein breath tests (serum amylase and lipase are usually normal). The best diagnostic test is endoscopic retrograde cholangiopancreatography (ERCP). Magnetic resonance cholangiopancreatography and endoscopic ultrasonography can be helpful.
• pancreatic enzyme supplement.
HIV itself has been found in Kupffer cells, hepatic macrophages, and hepatocytes. Prior to ART, the most common causes of liver dysfunction were OIs (including CMV and mycobacterium infections) and AIDS-related neoplasms, such as lymphoma and KS. In the ART era the spectrum of liver disease has shifted to concomitant infection with chronic HCV and HBV, medication-related hepatotoxicity, alcohol abuse, and non-alcoholic fatty liver disease. In ART era, liver disease is the most common non-AIDS-related cause of death among PLWH, accounting for 14–18% of all deaths. In some series, ~ half of deaths among hospitalized PLWH in the ART era have been attributed to liver disease.
>80% of those with HIV infection are estimated to have abnormal LFTs at some time during the course of their life. This does not necessarily indicate the presence of liver disease. Important causes of abnormal LFTs and liver disease are (Box 42.5):
• Viral co-infections: HCV, HBV, HDV, CMV, EBV.
• Antiretrovirals—all may cause raised ALT, more common in hepatitis co-infection. Abnormal LFTs were seen in 14–20% of patients starting ART. The overall frequency of grade 3/4 ALT elevation occurs in 1–18% patients starting any ART. Atazanavir causes clinically insignificant raised bilirubin due to uridine-glucuronosyltransferase inhibition.
• Many of the drugs used in prophylaxis and treatment of OI lead to liver toxicity.
• Nucleotide reverse transcriptase inhibitors (NRTIs) particularly DDI and zidovudine (AZT) may cause mitochondrial toxicity, leading to hepatic steatosis.
• Bacterial, fungal, and protozoal infections, and neoplasms occur much less frequently. The pattern of LFTs and the clinical stage of HIV infection may help to narrow the diagnostic options.
• IRIS, a paradoxical worsening of pre-existing infection due to rapid immune restoration in the setting of successful HIV RNA suppression
• ‘Chemsex’ is an emerging phenomenon, where substances such as mephedrone, crystal meth, and gamma hydroxybutyrate (GHB)/gamma-butyrolactone (GB) are used in parties where participants are disinhibited and are engaged in high-risk condomless sex with multiple partners. In these parties, drugs are commonly injected (slamming), resulting in increase in blood-borne virus infections, including hepatitis B, hepatitis C, and HIV, and  rates of STIs.
rates of STIs.
Box 42.5 Main causes of liver disease in HIV patients
Liver damage may lead to:
• Hepatic steatosis is common in HIV, due to NRTI-induced mitochondrial toxicity, or part of metabolic syndrome associated with protease inhibitor (PI), and HCV co-infection. May lead to steatohepatitis and cirrhosis.
• Cirrhosis is a common cause of morbidity and mortality. Generally associated with viral hepatitis, but 10% may be related to drugs.
• Nodular regenerative hyperplasia is a cause of portal hypertension and variceal bleed in the absence of fibrosis and may be caused by HIV +/–anti-retrovirals, e.g. DDI
Prior infection with viral hepatitis, alcohol intake, prescribed and recreational drug use, anti-retroviral history, and recent travel.
Fever, hypotension, signs of chronic liver disease, and nutritional status (obesity and malnutrition).
Mixed hepatic and obstructive liver enzyme abnormalities are common, but rarely diagnostic in isolation.
• Predominant  of serum transaminases usually indicate hepatitis, but lacks sensitivity and specificity. A new hepatitis viral infection or reactivation of hepatitis B and/or C infection with immune reconstitution should be excluded. Consider drug hepatotoxicity and investigate for other infections.
of serum transaminases usually indicate hepatitis, but lacks sensitivity and specificity. A new hepatitis viral infection or reactivation of hepatitis B and/or C infection with immune reconstitution should be excluded. Consider drug hepatotoxicity and investigate for other infections.
• Predominant  of serum alkaline phosphatase usually indicates biliary obstruction. Initial assessment is by ultrasound scan, or CT of the liver and abdomen. Lesions identified can be targeted by image-guided biopsy. ERCP must be considered if imaging techniques reveal intrahepatic and/or extrahepatic duct dilatation.
of serum alkaline phosphatase usually indicates biliary obstruction. Initial assessment is by ultrasound scan, or CT of the liver and abdomen. Lesions identified can be targeted by image-guided biopsy. ERCP must be considered if imaging techniques reveal intrahepatic and/or extrahepatic duct dilatation.
Presents with right upper quadrant abdominal pain, weight loss. Investigations to exclude common biliary conditions, such as cholelithiasis should proceed before attributing to HIV infection.
Similar to sclerosing cholangitis, presenting with right upper abdominal pain associated with nausea, vomiting, fever, and marked elevation of serum alkaline phosphatase. Ultrasound shows dilated intra- and/or extrahepatic bile ducts. ERCP provides further structural delineation. Some cases are associated with cryptosporidial and CMV infections.
Presents with similar symptoms to HIV-associated cholangiopathy. Ultrasound of the abdomen normally shows thickened dilated gall bladder, but may be normal. Can be complicated by recurrent cholangitis. Associated with CMV, Cryptosporidium parvum, microsporidia, and MAC infection, but no cause in >50%.
Diagnosis by ultrasound or technetium scintigraphy.
Treatment is cholecystectomy.