 lung function.
lung function.Respiratory tract involvement is very common in HIV infection. Reported incidence ranged from >90% early in the epidemic to 70% in the ART era. Symptoms represent a wide disease spectrum (Box 45.1) that include conditions not directly related to HIV. Bacterial infections, e.g. sinusitis, bronchitis, otitis, and pneumonia are among the most common infectious complications, occurring with increased frequency at all CD4 counts.
Box 45.1 Spectrum of respiratory illnesses in HIV +ve patients
• Gram –ve bacilli (Pseudomonas aeruginosa, Klebsiella pneumoniae)
• Atypical mycobacteria (Mycobacterium kansasii, MAC)
• Other respiratory illnesses:
• upper respiratory tract infection (sinusitis, pharyngitis)
• Lymphocytic interstitial pneumonitis
• non-specific interstitial pneumonitis
• obstructive lung disease (asthma, chronic bronchitis)
• illicit-drug-induced lung disease
• medication-induced lung disease
• HIV itself, without pulmonary OI, leads to  lung function.
lung function.
•  Frequency of respiratory diseases with falling CD4 count and
Frequency of respiratory diseases with falling CD4 count and  duration of HIV infection.
duration of HIV infection.
• The CD4 count is a good indicator of risk of developing a specific OI:
• any CD4 count—upper respiratory tract infections, bacterial pneumonias, obstructive airway disease, TB, and bronchogenic carcinoma are more common than in the general population, and their rates increase with declining CD4 count
• <500 cells/µL: recurrent bacterial pneumonia
• <200 cells/µL:  incidence bacterial pneumonia associated with bacteraemia, M. tuberculosis, which is often extrapulmonary or disseminated, PCP and Cryptococcus neoformans pneumonia
incidence bacterial pneumonia associated with bacteraemia, M. tuberculosis, which is often extrapulmonary or disseminated, PCP and Cryptococcus neoformans pneumonia
• <100 cells/µL:  Staphylococcus aureus, Pseudomonas aeruginosa, pulmonary KS, and Toxoplasma gondii
Staphylococcus aureus, Pseudomonas aeruginosa, pulmonary KS, and Toxoplasma gondii
• <50 cells/µL: MAC, respiratory infections by endemic (e.g. Histoplasma capsulatum, Coccidioides immitis), and non-endemic fungi (e.g. Aspergillus spp.) and certain viruses (most commonly CMV). These infections are often associated with extrapulmonary or disseminated disease that dominates the clinical presentation.
• Risks of OI  by other factors, e.g. cigarette smoking (further damages lung defences).
by other factors, e.g. cigarette smoking (further damages lung defences).
• Significant  in the burden of HIV-associated respiratory disease in the ART era.
in the burden of HIV-associated respiratory disease in the ART era.
Varies with:
• Age: e.g. lymphocytic interstitial pneumonitis (LIP) occurs predominantly in children.
• Exposure (travel to or residence in endemic areas for specific pathogens): e.g. histoplasmosis.
• Specific immune defects: e.g. failure to produce antibodies against pneumococcal capsular antigen (independent of CD4 count).
• Altered/atypical clinical presentations not uncommon in the immuno-compromised
• Altered results of investigations e.g.  rate of sputum smear positivity in pulmonary TB.
rate of sputum smear positivity in pulmonary TB.
Considerable overlap of symptoms and signs, and dual infections may occur. It is rare for a particular constellation of symptoms, clinical findings, and radiological abnormalities, to be definitively diagnostic and a full investigation is usually required. However, radiological appearances may suggest different groups of conditions (See Table 45.1). As deterioration can sometimes be rapid it is important that ‘best guess’ therapy is initiated, while awaiting the result of microbiology.
Table 45.1 Chest X-ray appearances
| Interstitial | PCP, LIP (usually in children), rarely CMV |
| Lobar | Bacterial infection |
| Nodular | KS, septic emboli, fungal infection, non-Hodgkin’s lymphoma |
| Miliary | TB |
| Pneumatocele | PCP, staphylococcal pneumonia |
| Pleural effusion | KS, TB, lymphoma (including primary effusion lymphoma) |
| Mediastinal and /or hilar lymphadenopathy | Mycobacteriosis, lymphoma, fungal infection |
Has a higher incidence than in HIV –ve people. In the pre-ART era, bacterial pneumonia was the most frequent pulmonary complication occurring in up to 42% in autopsy studies. Since the introduction of ART, the incidence of bacterial pneumonia continued to decline. An episode of bacterial pneumonia is associated with subsequent  morbidity and mortality.
morbidity and mortality.
As in the general population, S. pneumoniae and H. influenzae are the most frequently identified causes of community-acquired bacterial pneumonia. S. aureus and P. aeruginosa are more frequent than in the HIV –ve population. There are higher bacteraemia rates in pneumococcal infection and there may be  incidence of penicillin-resistant isolates. IV drug use further
incidence of penicillin-resistant isolates. IV drug use further  the risk of bacterial pneumonia. Atypical bacterial pathogens, such as Legionella pneumophila and Mycoplasma pneumoniae are infrequent causes of community-acquired bacterial pneumonia in HIV +ve individuals. Less common causes include Rhodococcus equi, which produces a cavitary pneumonia of insidious onset.
the risk of bacterial pneumonia. Atypical bacterial pathogens, such as Legionella pneumophila and Mycoplasma pneumoniae are infrequent causes of community-acquired bacterial pneumonia in HIV +ve individuals. Less common causes include Rhodococcus equi, which produces a cavitary pneumonia of insidious onset.
Most bacterial pneumonias present acutely with symptoms and radiological patterns similar to those seen in HIV –ve patients. S. pneumoniae or H. influenza typically present with consolidation. However, the radiological presentation may be indistinguishable from other OIs. H. influenzae can present with diffuse opacities, mimicking PCP. With progression of immunodeficiency pneumonias due to S. aureus and P. aeruginosa become more important and may produce cavitation.
In a patient presenting with a clinical diagnosis of bacterial pneumonia, assess and correct hypoxia, volume depletion, and hypotension, gauge severity by presence of confusion,  blood urea,
blood urea,  respiratory rate,
respiratory rate,  BP, older age group and co-morbidities. Obtain sputum and blood cultures. Consider urine testing for legionella antigen and pneumococcal antigen. Check inflammatory markers and WCC, although white cell response may be
BP, older age group and co-morbidities. Obtain sputum and blood cultures. Consider urine testing for legionella antigen and pneumococcal antigen. Check inflammatory markers and WCC, although white cell response may be  in HIV-induced marrow suppression. If severe (use CURB65 >2 or severe sepsis) commence IV antibiotic therapy with either cefuroxime 1.5 g tid plus a macrolide (e.g. clarithromycin 500 mg bd), or amoxicillin 1 g tid plus a macrolide. Consider modification to these combinations if there are features suggesting S. aureus, P. aeruginosa, or atypical organisms, such as R. equi. If recurrent and evidence of
in HIV-induced marrow suppression. If severe (use CURB65 >2 or severe sepsis) commence IV antibiotic therapy with either cefuroxime 1.5 g tid plus a macrolide (e.g. clarithromycin 500 mg bd), or amoxicillin 1 g tid plus a macrolide. Consider modification to these combinations if there are features suggesting S. aureus, P. aeruginosa, or atypical organisms, such as R. equi. If recurrent and evidence of  antibody production consider IVIg therapy or prophylactic antibiotics. For outpatient and less severe pneumonia oral amoxicillin and a macrolide may suffice.
antibody production consider IVIg therapy or prophylactic antibiotics. For outpatient and less severe pneumonia oral amoxicillin and a macrolide may suffice.
Caused by Pneumocystis jirovecii, a ubiquitous fungus. Pneumocystis carinii now refers to the pneumocystis that infects rats and P. jirovecii refers to the distinct species that infects humans. The abbreviation PCP is still in use to designate pneumocystis pneumonia. PCP results from new acquisition or from reactivation of latent infection acquired earlier in life. It was an AIDS defining illness in 70–80% of patients before the widespread use of PCP prophylaxis and ART. ~90% of cases occurred in patients with CD4 counts <200 cells/µL.
Usually presents sub-acutely with symptoms  over weeks with night sweats, systemic symptoms and weight loss, dry cough, progressive dyspnoea, initially on exertion and eventually at rest, occasionally with spontaneous pneumothorax. Abnormalities on respiratory examination often minimal or absent, and significant
over weeks with night sweats, systemic symptoms and weight loss, dry cough, progressive dyspnoea, initially on exertion and eventually at rest, occasionally with spontaneous pneumothorax. Abnormalities on respiratory examination often minimal or absent, and significant  of pulmonary function may occur despite minimal chest X-ray changes.
of pulmonary function may occur despite minimal chest X-ray changes.
Empirical treatment may need to be started if unable to get definitive diagnosis through induced sputum or bronchoalveolar lavage (BAL)
If history suspicious but normal resting oxygen saturations, exercise oximetry showing  in oxygen saturation by 5% or to <90% highly suggestive of PCP.
in oxygen saturation by 5% or to <90% highly suggestive of PCP.
• High resolution CT shows ground glass appearance of interstitial pathology.
• Chest X-ray may be normal in early and mild disease, but in severe cases, the typical pattern is bi-basal perihilar interstitial infiltrates (Plate 29). Less commonly unilateral infiltrates and pneumatoceles, and rarely pneumothorax and pleural effusion.
• Atypical upper lobe involvement may be seen in patients on nebulized pentamidine prophylaxis. They may also develop extrapulmonary involvement of various organs, including eyes, spleen, and skin (due to lack of systemic effect).
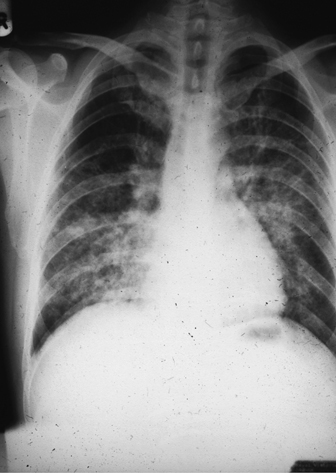
Plate 29 Chest X- ray— PCP (45).
Specific diagnosis requires demonstration of P. jirovecii cysts in respiratory secretions or lung tissue. Patients with borderline lung function may require sputum induction and bronchoscopy. Sputum/respiratory specimen is stained with immunofluorescence or histochemistry. PCR has a sensitivity of 97–98% and a specificity of 68–96%. PCR cannot differentiate between active infection and colonization:
• Induced sputum by 3% saline using an ultrasonic nebulizer. Most laboratories perform:
• fibre optic BAL-sensitivity 90–95%
• open lung biopsy sensitivity or transbronchial biopsy with histology of fixed tissue-sensitivity 95–100%. Not routinely performed as pneumothorax is common in PCP.
Prior to initiating investigations in those presenting with a history suggestive of PCP, it is essential to assess pulmonary impairment and the need for oxygen supplements by pulse oximetry and arterial blood gases.
If there is pulmonary impairment, delays in initiating PCP therapy should be kept to a minimum. ART should not be deferred beyond 1 week after acute PCP treatment, as there is an  risk of death if delayed.
risk of death if delayed.
Gold standard antibiotic therapy is co-trimoxazole 120 mg/kg in 4 divided doses daily for 3 days, if stable/improving reduce to 90 mg/kg/day for further 18 days. Haematology, LFTs, and the skin (rash seen in 1%) must be monitored carefully for evidence of toxicity or developing hypersensitivity. Folinic acid supplements may be considered if there is evidence of impaired marrow function. For non-responders or hypersensitive patients alternative regimens include clindamycin (600mg tid) plus primaquine (30 mg/day), dapsone (100 mg/day) plus trimethoprim (5 mg/kg every 6–8 hours), atovaquone (750 mg bd) and IV pentamidine (4 mg/kg).
In patients with arterial oxygen pressures <9.3 kPa or O2 saturations <92% steroid therapy is recommended, as it  risk of respiratory failure and mortality. Conventional regimen is prednisolone, or equivalent, 40 mg bd for 5 days, 40 mg od for 5 days followed by 20 mg daily for 5–10 days. There is no significant
risk of respiratory failure and mortality. Conventional regimen is prednisolone, or equivalent, 40 mg bd for 5 days, 40 mg od for 5 days followed by 20 mg daily for 5–10 days. There is no significant  in risk of other OIs, except Candida spp. and local HSV.
in risk of other OIs, except Candida spp. and local HSV.
Patients with severe PCP may progress to respiratory failure requiring ventilatory support with constant positive airways pressure (CPAP), or intubation and ventilation. Pneumothorax may require chest drain insertion. Adverse prognostic indicators include  serum lactic dehydrogenase (LDH) levels, need for high ventilatory pressures and prolonged intensive therapy unit stay.
serum lactic dehydrogenase (LDH) levels, need for high ventilatory pressures and prolonged intensive therapy unit stay.
PCP is almost completely preventable by primary prophylaxis.
• presentation with an AIDS-defining illness.
Prophylaxis with the same regimen should be continued after successful treatment of PCP.
• Co-trimoxazole [480 mg OD or 960 mg on alternate days (3 times a week)] is the drug of choice. Cutaneous hypersensitivity reactions are common, but 80% of HIV +ve patients can be desensitized by the use of gradually  doses.
doses.
• Dapsone (100 mg od) need to add pyrimethamine to protect against toxoplasmosis,
• Pentamidine isetionate (by inhalation of nebulized solution, 300 mg every 4 weeks). Does not protect against toxoplasmosis
• 1° PCP prophylaxis can be discontinued when the CD4 count has been >200 cells/µL for at least 3–6 months (rate of OIs do not  until after 2 months of ART).
until after 2 months of ART).
• Careful consideration is needed before stopping prophylaxis in patients who have had PCP or other AIDS defining illness, perhaps deferring interruption until complete viral suppression and sustained CD4 count  for 6 months.
for 6 months.
TB is the leading cause of morbidity and mortality among people living with HIV (PLWH) worldwide. Globally PLWH are 26 times more likely to develop active TB disease than the general population. Sub-Saharan Africa bears the highest TB/HIV burden and over 50% of TB patients are co-infected with HIV. In the UK, the annual incidence of TB  since 2012. In 2016, the rate of TB is ×15 higher in the non-UK born population.
since 2012. In 2016, the rate of TB is ×15 higher in the non-UK born population.
The most common route of infection is inhalation of droplets containing Mycobacterium tubercles. The immune response limits the multiplication of tubercle bacilli, but viable ones may persist for years (latent TB infection). Active TB disease develops soon after exposure to (primary disease) or after reactivation of latent infection. The risk of reactivation is much higher with untreated HIV compared with the general population. TB can occur at any CD4 count, although the risk increases with  CD4 counts.
CD4 counts.
ART results in significant  in the incidence of TB. However, even with the beneficial effects of ART, the risk and incidence of TB disease among PLWH remains higher than in the general population.
in the incidence of TB. However, even with the beneficial effects of ART, the risk and incidence of TB disease among PLWH remains higher than in the general population.
Patients diagnosed or suspected with TB should be risk-assessed and tested for HIV. PLWH presenting with a pulmonary illness should have TB excluded. Proven or suspected TB should be notified to public health authorities and contact tracing initiated.
Classic presentation of pulmonary TB is night sweats/fever, cough, pleuritic chest pain, haemoptysis, and weight loss. Less typical presentations occur as CD4 count  . Lobar distribution of pulmonary infection may be atypical and mimic community-acquired pneumonia. Disseminated and extrapulmonary disease are more common with
. Lobar distribution of pulmonary infection may be atypical and mimic community-acquired pneumonia. Disseminated and extrapulmonary disease are more common with  CD4 count. Although most cases are caused by reactivation of latent infection, 1° infection with rapid progression to active TB can occur when CD4 count <100 cells/µL. TB has an additional immunosuppressive effect in HIV infection.
CD4 count. Although most cases are caused by reactivation of latent infection, 1° infection with rapid progression to active TB can occur when CD4 count <100 cells/µL. TB has an additional immunosuppressive effect in HIV infection.
• Microscopy for acid fast bacilli (AFB) on respiratory samples (expectorated sputum, induced sputum, or BAL), followed by molecular testing in conjunction with culture and drug sensitivity testing. Examination of at least 3 sputum samples is the standard practice. Careful control of infection precautions are required to avoid nosocomial transmission of TB during such procedures. Smear positivity  if advanced immunodeficiency.
if advanced immunodeficiency.
• TB pleural effusion: obtain cultures on pulmonary samples even in the absence of obvious parenchymal involvement, as the yield of sputum culture in induced samples approaches 55%. Diagnosis is established by detection of M. tuberculosis in pleural fluid or pleural biopsy by finding caseating granulomas, together with AFB.
• Radiology: in patients with preserved immune function typical upper lobe cavitary changes occur. In those with  CD4 counts appearances may be more extensive mimicking other infections.
CD4 counts appearances may be more extensive mimicking other infections.
• If extra-pulmonary findings (e.g. lymphadenopathy, bone marrow abnormalities) tissue histology and cultures may be diagnostic.
• Latent TB: interferon-gamma release assay (IGRA) is recommended for all PLWH at high risk of TB (those with recent exposure and those from high and medium TB-incidence countries) regardless of their CD4 count and ART.
• Diagnosis of multi-drug resistant TB: molecular techniques (rifampicin resistance gene probe), in addition to phenotypic drug susceptibilities should be performed to achieve rapid detection of drug/s resistant strains.
Drug therapy of TB-co-infected PLWH is complex due  drug toxicities, drug–drug interactions, and paradoxical reactions. If TB is strongly suspected, empirical therapy, after appropriate cultures taken, with 4 drugs should be initiated immediately. Even if cultures are negative, a full course may be required if there is clinical response. Treatment of active TB may lead to
drug toxicities, drug–drug interactions, and paradoxical reactions. If TB is strongly suspected, empirical therapy, after appropriate cultures taken, with 4 drugs should be initiated immediately. Even if cultures are negative, a full course may be required if there is clinical response. Treatment of active TB may lead to  in CD4 counts.
in CD4 counts.
For fully sensitive organisms, a 6-month course of TB therapy is sufficient for respiratory and most extrapulmonary TB. Initial phase with quadruple therapy of isoniazid (300 mg daily), rifampicin (600 mg daily), pyrazinamide (1.5–2 g daily), and ethambutol (15 mg/kg/day) with monitoring precautions until mycobacterial drug sensitivities are known. Intensive phase of 2 months with quadruple therapy should be followed by 4 months continuation phase of rifampicin and isoniazid, if fully sensitive. If MDRTB suspected seek expert advice on initial regimen and further management
Monitor for visual symptoms and LFTs regularly.
▶ Check visual acuity prior to starting ethambutol.
▶ There are very important drug–drug interactions between anti-TB and ART drugs because of their varying enzyme inducing and enzyme inhibiting effects ( Chapter 55, ‘Antiretroviral drug–drug interactions’, p. 643).
Chapter 55, ‘Antiretroviral drug–drug interactions’, p. 643).
• If not on therapy, ART should be started as soon as practical and within 8 weeks of TB diagnosis.
• If CD4 count ≤ 100 cells/µL: start ART as soon as practical and within 2 weeks.
• Efavirenz, raltegravir or dolutegravir may be used with tenofovir/emtricitabine or abacavir/lamivudine.
• Rifabutin (150 mg od or 350 mg ×3/week) instead of rifampicin, where ART regimen includes a ritonavir-boosted protease inhibitor.
• Do not use fixed-dose combinations containing tenofovir alafenamide (TAF) when co-administered with rifampicin or rifabutin.
• Avoid nevirapine in ART-naïve individuals, when TB regimen includes rifampicin.
▶ If already established on ART with fully suppressed VL:
• ART should not be interrupted.
• Start rifampicin-based TB regimen if ART consists of efavirenz, nevirapine, dolutegravir, or raltegravir, plus two NRTIs.
• Rifabutin instead of rifampicin where established ART necessitate use of ritonavir.
Where there is no suspicion of resistant organism the patient would be regarded as no longer an infection risk after 2 weeks of therapy.
IGRA is recommended for all PLWH. If +ve, consider treatment of latent infection in individuals at high risk of TB (those with recent exposure, and those from high and medium TB-incidence countries) and no evidence of active tuberculosis (asymptomatic, normal CXR) regardless of their CD4 count and ART.
• Isoniazid od for 6–9 months, or isoniazid and rifampicin for 3 months
See Chapter 55, ‘Immune reconstitution’, p. 642.
Characterized by the worsening of existing or the development of new symptoms, signs, or radiological abnormalities after the initiation of ART. These are not explained by another disease or TB treatment failure. IRIS is, therefore, a diagnosis of exclusion. It is thought to result from unmasking of an occult OI, not clinically apparent before ART. It is more common with low CD4 count, after a prompt  of CD4 count and rapid
of CD4 count and rapid  of HIV VL. It is usually short-lived, but may last for several months. May require steroids or interruption of ART until TB is better controlled.
of HIV VL. It is usually short-lived, but may last for several months. May require steroids or interruption of ART until TB is better controlled.
MAC consists of M. avium, M. intracellulare, and M. chimaera, and are ubiquitous in the environment. MAC is the most common cause of non-tuberculous mycobacterial infection in patients with AIDS, typically when the CD4 counts <50 cells/µL. The incidence is 20–40% in the absence of effective ART. The incidence among PLWH has fallen dramatically with ART.
Characteristically produce generalized bacteraemic disease, often in patients with wasting syndrome. MAC infection in the ART era is almost always localized and related to IRIS. During immune reconstitution as a result of ART, previously subclinical infection in the lungs may become apparent as an inflammatory response leading to pulmonary inflammation and X-ray changes.
The incidence of cryptococcosis has declined substantially with ART. Most new infections are recognized in newly diagnosed HIV with CD4 counts typically <100 cells/µL. Pulmonary disease is less frequent than meningitis, although the lung is the most likely portal of entry. Pulmonary involvement can be asymptomatic, but precedes the onset of disseminated disease in the majority of patients.
Cough (productive and unproductive), fever, malaise, shortness of breath, and pleuritic chest pain.
• Chest X-ray may be normal, or show focal or diffuse infiltrates that mimic PCP, solitary nodules, consolidation, hilar and mediastinal lymphadenopathy, and pleural effusions.
• High resolution CT scan must be performed if pulmonary involvement is suspected. May show diffuse, small lesions similar to TB or infiltrates similar to bronchopneumonia. Cavitation and bronchiectasis may also be present.
• Organism are more likely isolated from BAL, rather than sputum.
• Cryptococcal antigen can be detected in blood, BAL, or pleural fluid and biopsy specimens.
Early treatment of pulmonary lesions can prevent disseminated disease. Treatment consists of liposomal amphotericin B, combined with flucytosine as induction therapy, followed by fluconazole or itraconazole (see Chapter 46, ‘HIV: neurological disorders’, p. 544).
Aspergillus spp. colonize the lungs of individuals with underlying lung pathology. Invasive aspergillosis occurs when lung parenchymal infection disseminates to other organs. This can occur in PLWH, but is rare in the absence of other risk factors, such as neutropenia, transplantation, or steroid use.
Symptoms include fever, dyspnoea, cough, chest pain, and haemoptysis. 20% have unilateral or bilateral diffuse or nodular infiltrates.
•  30% have thick-walled upper lobe cavities.
30% have thick-walled upper lobe cavities.
•  20% have unilateral or bilateral diffuse or nodular infiltrates.
20% have unilateral or bilateral diffuse or nodular infiltrates.
• CT chest may show ‘Halo’ sign.
• Sputum, BAL, blood, bone marrow, and/or tissue biopsies should be examined by microscopy and cultured for fungi. Only 10–30% have positive findings on sputum culture. BAL has a higher yield.
• Culture is required as can be difficult to distinguish aspergillus from other fungal species by microscopy alone.
• Serum PCR and/or galactomannan ELISA may be helpful, but need careful interpretation
• Liposomal amphotericin: test dose 1 mg (give over 10 minutes) then 3–5 mg/kg od.
• Voriconazole 6 mg/kg bd 24 hours, as a loading dose, followed by 4 mg/kg 7 days. 200 mg bd (often used as first line). Isavuconazole or posaconazole may be alternatives.
• Caspofungin 70 mg stat then 50 mg/day
In patients with severe neutropenia granulocyte macrophage colony stimulating factor may have an adjunctive role. Interactions must be reviewed (see Chapter 55, ‘Antiretroviral drug–drug interactions’, p. 643).
Patients with PCP often have CMV isolated from bronchial washings or lung biopsy. In most cases, this represents viral replication without pneumonitis and the patient responds to PCP therapy alone. Active pneumonitis occurs much less frequently than in patients taking immunosuppressive therapy after transplant. Hypoxia is usual.
• Chest X-ray shows diffuse interstitial infiltrates.
• CMV PCR is not specific and often +ve, representing high levels in blood, rather than lung disease; definitive diagnosis is through histology and identification of CMV in lung tissue.
Patients with interstitial pneumonia and positive CMV identification in lung tissue should be treated with anti-CMV therapy using ganciclovir or foscarnet (see Chapter 46, ‘Distal symmetric polyneuropathy’, p. 548).
Patients with HIV-related persistent generalized lymphadenopathy have sub-diaphragmatic lymphadenopathy, but do not have significant hilar or mediastinal node enlargement. Hilar or mediastinal adenopathy implies significant pathology. The differential diagnosis includes:
May be seen in active PCP, but other radiological abnormalities usually co-exist. A careful search for peripheral lymphadenopathy, skin lesions, and other abnormalities that could be subjected to histological and microbiological examinations should be sought. If necessary, mediastinoscopy and biopsy can be performed.
1° Pulmonary hypertension can occur as a consequence of HIV infection (0.5% in PLWH vs 0.0015% if HIV –ve). IV drug users may develop pulmonary small vessel obstruction due to injection of particulate material or may suffer recurrent pulmonary emboli.
Intense fatigue, breathlessness, and faintness on exertion are features of severe pulmonary hypertension. Clinical features include accentuated pulmonary component of the second heart sound, third heart sound, left lower parasternal heave, pan-systolic murmur indicative of tricuspid valve regurgitation, jugular venous distension, hepatojugular reflux, ascites, and peripheral oedema. Should be considered in unexplained shortness of breath
• ECG: RV strain, RBBB, right axis deviation.
• Ultrasonography of the deep veins of the leg.
• Right heart catheterization is the gold standard diagnostic test.
• ART may improve mortality and morbidity.
• Specific drug therapy in collaboration with specialist (anticoagulants, calcium channel blockers, endothelin receptor antagonists and phosphodiesterase type-5 inhibitors).