4 Organs of the Urinary System and their Neurovasculature
4.1 Overview of the Urinary Organs; the Kidneys in situ
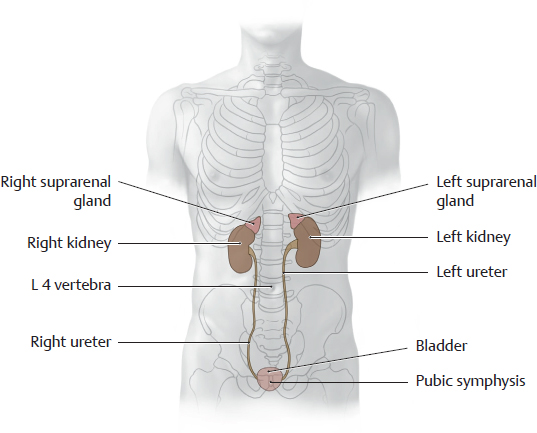
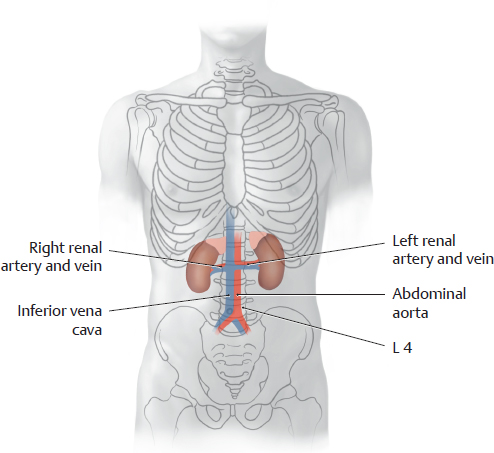
A Projection of the kidneys and other urinary organs onto the skeleton
Anterior view. The suprarenal glands are also shown to aid orientation. The kidneys are located next to the vertebral column and are high enough that they overlap the eleventh and twelfth ribs. The renal hilum is situated at the L 1/L 2 level. Usually the right kidney is somewhat lower than the left kidney due to the space occupied by the liver (see p. 382). The bladder is shown fully distended in the diagram. When empty, it is considerably smaller and is hidden behind the pubic symphysis. The ureters descend in the retroperitoneum and open into the bladder from the posterior side.
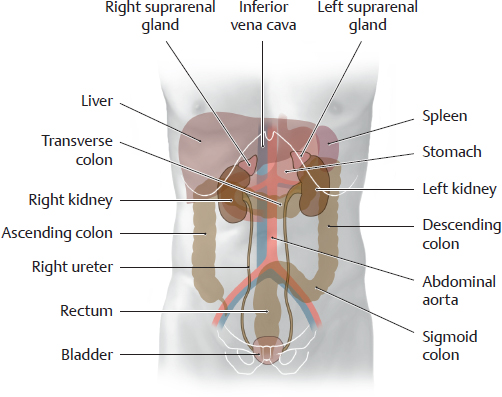
B Projection of the urinary organs onto the organs of the abdomen and pelvis
Anterior view. Owing to its large size, the liver displaces the right kidney slightly inferiorly. The bladder is shown in a fully distended state. It is anterior to the rectum in the male and anterior to the uterus (not shown here) in the female. Because of this relationship, marked distention of the rectal ampulla or enlargement of the uterus due to pregnancy exerts greater pressure on the bladder, creating an urge to urinate even when the bladder is not full. Urinary incontinence may develop due to pathological processes of longer duration, such as muscular tumors of the uterus (fibroids), or due to weakening of the bladder closure mechanism as a result of previous vaginal deliveries (descent of the muscular pelvic floor).
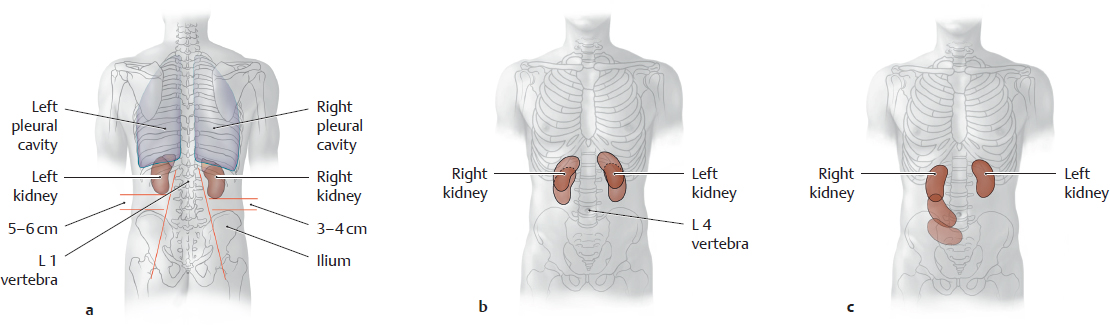
C Location of the kidneys, normal vs. pathological mobility
a Posterior view. The pleural cavities overlap the kidneys posteriorly owing to the convexity of the diaphragm.
Note that the right kidney is lower than the left kidney and is closer to the palpable iliac crest.
b, c Anterior view. The kidneys are located in the retroperitoneum just below the diaphragm. Hence they move passively with the diaphragm during respiratory excursions, moving inferiorly and slightly laterally during inspiration because of their oblique position (their inferior poles point away from the spine, see oblique red lines in a).
These passive movements may cause respiration-dependent pain in patients with renal disease. A pathological increase in renal mobility (“floating kidney,” see c) results from atrophy of the fat capsule that normally surrounds the kidneys and keeps them in a stable position. A wasting illness (e.g., metastatic tumors of varying origin) may cause such severe fatty atrophy that the kidneys descend to a lower level in the abdomen. As they are still tethered by the ureter and vascular stalk, this descent may kink the renal vessels or ureter and interfere with renal blood flow or urinary outflow.
D The urinary organs in situ
Anterior view of an opened female abdomen. The spleen and gastrointestinal organs have been removed to the sigmoid colon, and the esophagus has been pulled slightly inferiorly. The fat capsule remains partially intact on the right side, removed on the left side. The kidneys and suprarenal glands are incorporated into the retroperitoneum by the structural fat of this capsule. The moderately distended bladder is just visible above the pubic symphysis in front of the uterus. The parietal peritoneum has been removed to provide a clear view into the retroperitoneum.
Note: The ureters pass behind the ovarian vessels and in front of the iliac vessels as they descend in the retroperitoneum. These sites represent clinically important constrictions of the ureter where a stone from the renal pelvis may become lodged (see B, p. 301).
In most cases the kidneys are not oriented parallel to the coronal plane. The renal hilum, where the blood vessels and ureter enter and leave the kidneys, is directed anteromedially (see Ab, p. 292). Also, the renal superior poles are closer together than the inferior poles, so that the kidneys appear slightly “tilted” toward the midline. Thus the renal hilum also points slightly downward.
4.2 Kidneys: Location, Shape, and Structure
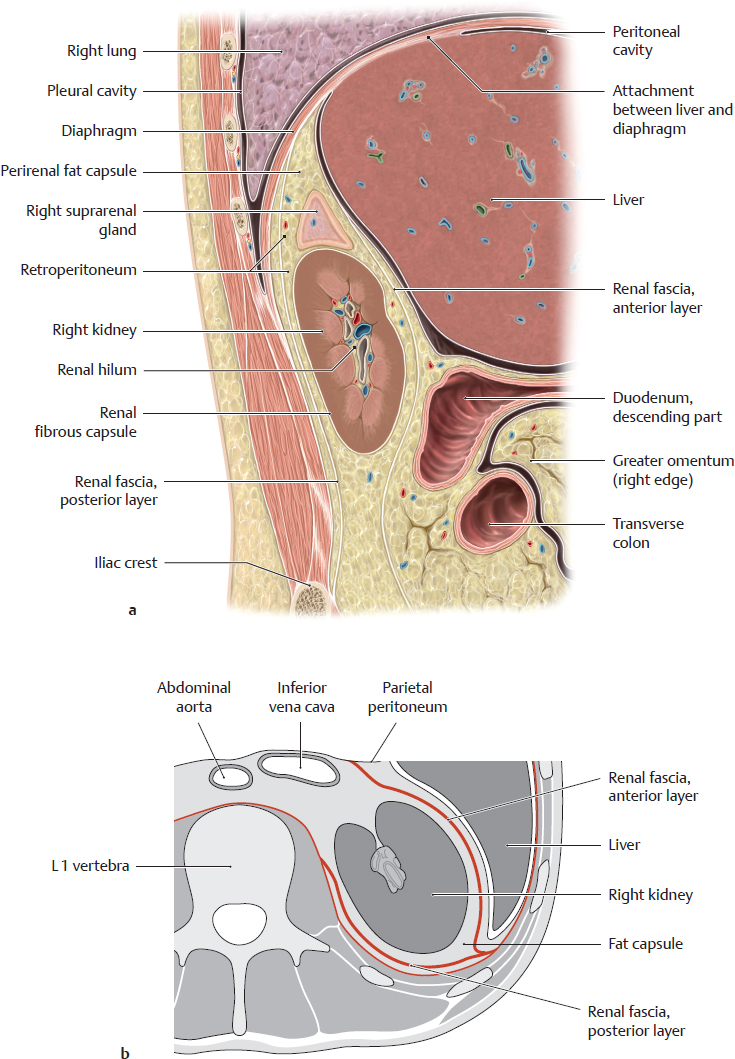
A Position of the kidneys in the renal bed
Right renal bed. a Sagittal section at approximately the level of the renal hilum, viewed from the right side. b Transverse section through the abdomen at approximately the L 1/L 2 level, viewed from above.
The renal bed is located on each side of the spine in the retroperitoneum. It contains the kidneys, which are invested by a thin organ capsule (renal fibrous capsule), and the suprarenal glands, which are surrounded by the perirenal fat capsule that also encloses the kidneys. The fat capsule is thicker posteriorly than anteriorly.
Note: Swelling of the kidney (usually due to inflammation) may cause severe pain due to stretching of the fibrous capsule.
The fat capsule is surrounded by the renal fascia, which separates it from its surroundings by two layers:
• The anterior layer behind the parietal peritoneum (to which it is fused at some sites)
• The posterior layer, which is partially attached to the transversalis fascia and muscular fasciae on the posterior trunk wall
The renal fascia, and thus the renal bed, is open inferiorly and medially to allow passage of the ureter and renal vessels. It is closed laterally and superiorly by fusion of the fascial layers. Because of this arrangement, inflammatory processes that are adjacent to the kidney but within the renal fascia tend to spread to the contralateral side or inferiorly and may spread into the pelvis.
Note: The entire renal bed moves downward during inspiratory depression of the diaphragm, indirectly causing the kidney and suprarenal gland to move as well. This differs from the liver, which is attached to the diaphragm (bare area) and is directly moved by diaphragm excursions.
B Renal bed: fasciae and capsules of the kidneys
Renal fibrous capsule |
Thin, firm connective-tissue capsule that closely invests each kidney |
Perirenal fat capsule |
Mass of fat that surrounds the kidneys and suprarenal glands and completely occupies the renal bed; it is thickest lateral and posterior to the kidneys |
Renal fascia |
Connective-tissue fascial sac that encloses the perirenal fat, portions of the abdominal aorta and inferior vena cava close to the kidney (see Ab), and the proximal ureter; subdivided into a thin anterior layer and a thick posterior layer (see Aa) |
C Structure and shape of the kidney
Anterior view (a), posterior view (b), and medial view (c) of the right kidney. The suprarenal gland is left intact in a and b, and the ureter has been cut at the level of the inferior renal pole. The fibrous capsule that directly invests the kidney is intact in a and c and has been partially opened in b to display the underlying renal parenchyma. The renal sinus (the deep space into which the hilum opens) generally contains a certain amount of structural fat, and so the vascular structures and renal pelvis are not exposed to view intraoperatively as they are in these drawings. The normal kidney measures an average of 12 x 6 x 3 cm (L x W x T) and weighs 150–180 g. It has
• two poles (superior and inferior),
• two surfaces (anterior and posterior), and
• two borders (lateral and medial).
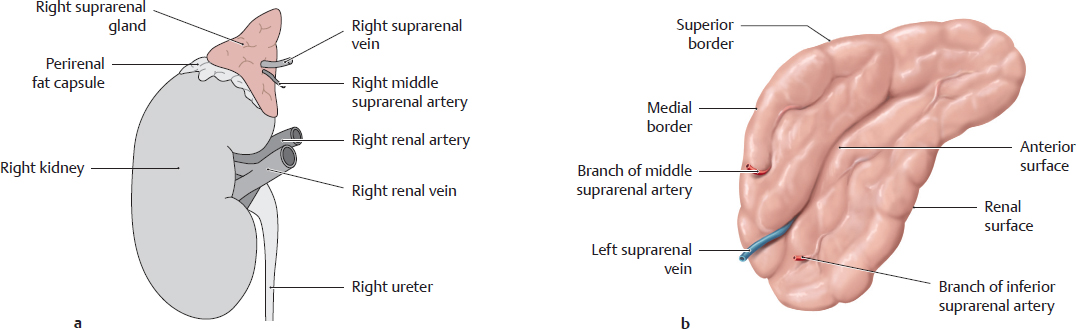
The medial border bears the renal hilum, where vascular structures and the ureter enter and leave the kidney. The shallow surface grooves result from the embryonic lobulation of the kidney. The hilar structures are usually arranged as follows from anterior to posterior (as shown in c): right renal vein, right renal artery, and right ureter.
Note: The renal artery is usually posterior to the renal vein because the right renal artery passes to the right kidney behind the inferior vena cava (where the renal veins terminate), while the left renal vein passes to the left kidney i n front of the abdominal aorta (which gives origin to the renal arteries). The left renal artery may also loop around the left renal vein from above to occupy an anterior position. The ureter leaves the renal pelvis (see p. 294) below the vessels and is usually somewhat posterior in relation to the blood vessels.
4.3 Kidneys: Architecture and Microstructure
A Macroscopic structure of the kidney
Posterior view of a right kidney with the upper half of the kidney partially removed. The renal parenchyma consists of an outer cortex and inner medulla:
• The renal cortex is a relatively thin layer that lies beneath the fibrous capsule and forms columns (renal columns) that extend between the pyramids of the medulla. The cortex and columns contain approximately 2.4 million renal corpuscles (which contain the glomeruli, see B) as well as the proximal and distal renal tubules (see C).
• The renal medulla consists of approximately 10–12 renal pyramids. The bases of the pyramids are directed toward the cortex and capsule, while their apices converge toward the renal pelvis. The medulla mainly contains the ascending and descending limbs of the renal tubules.
The renal pelvis is described on p. 296.
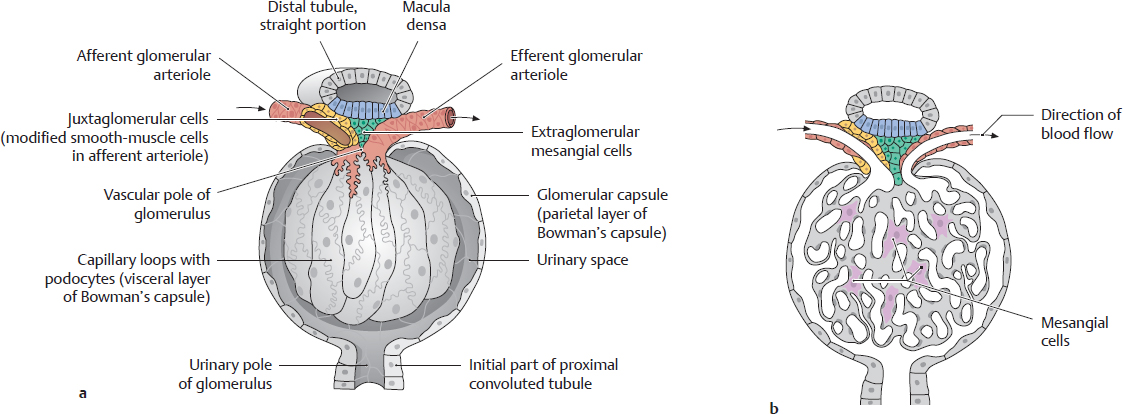
B Renal corpuscle
a With the capsule opened; b In section.
The renal corpuscle is the interface between the blood vessels and the excretory portion of the urinary tract (see C). It consists of a central convoluted vascular loop, the glomerulus, and a bulbous envelope lined by squamous epithelial cells, the glomerular capsule (Bowman’s capsule). Blood enters the glomerulus at the vascular pole of the renal corpuscle by flowing through the afferent glomerular arteriole, and it leaves the glomerulus through the efferent glomerular arteriole. The primary urine is formed within the renal corpuscle and drains through a tubular system at the urinary pole of the glomerulus. The initial portion of this tubular system that is connected to the glomerular capsule is the proximal convoluted tubule (see C)
Note: Specialized cells at the vascular pole of the renal corpuscle regulate the blood pressure that is necessary for ultrafiltration.
C Architecture of the renal vessels and intrarenal collecting system
a Renal vessels: Sectional view of a medullary pyramid with adjacent cortical areas. The intrarenal vascular and collecting systems are closely interrelated spatially and functionally. An ultrafiltrate from the blood (primary urine) drains into a microscopically small system of renal tubules. Blood flow to the kidney (a) is supplied by interlobar arteries that pass along the sides of the medullary pyramids from the renal hilum. Each interlobar artery supplies two adjacent medullary pyramids and the associated cortical zones (these branches are not shown). At the base of the pyramid, the interlobar artery gives rise to the arcuate artery, from which the interlobular arteries are distributed into the cortex as far as the fibrous renal capsule. The afferent glomerular arterioles that arise from an interlobular artery each supply one glomerulus. The efferent glomerular arterioles that emerge from the glomerulus are still carrying blood at a high oxygen tension; they supply the renal cortex or medulla.
b Intrarenal collecting system: The smallest functional unit of the kidney is the nephron, which consists of the renal corpuscle and renal tubules. Each nephron drains via a short connecting tubule into a collecting duct which collects the urine from about 10–12 nephrons. There are approximately 1 million nephrons, which process approximately 1700 liters of blood daily to form approximately 170 liters of primary urine. This primary filtrate enters the tubular system at the urinary pole of the renal corpuscle and reaches the renal papilla as the final urine, which drains into the calyceal system (approximately 1.7 liters/day). The tubular system consists of the proximal and distal tubules (each with a convoluted and straight portion) and an intermediate tubule (with descending and ascending limbs). The intermediate tubule and the adjacent straight portions of the proximal and distal tubules comprise the loop of Henle. While passing through the tubular system, substances contained in the filtrate (mainly water) are reabsorbed while other substances (e.g., ions) are secreted into the filtrate. This process yields the final urine, which passes through a collecting tubule into a collecting duct and drains through the renal papilla into the caliceal system. It is conveyed from the calyces and renal pelvis to the ureter by peristalsis.
4.4 Renal Pelvis and Urinary Transport
A Structure and shape of the renal pelvis
Mid-longitudinal section through a right kidney, posterior view. The renal pelvis lies posterior to the renal vessels and is continuous inferiorly with the ureter. It may vary in shape (see B). It is usually divided into two or three indistinctly separable larger (major) calyces, which further divide into smaller (minor) calyces. They encompass the tips of the renal papillae in such a way that urine drains from the papillae into the calyx without entering the renal parenchyma. Smooth-muscle fibers in the calyces, renal pelvis and ureter (for details about wall structure, see D), enable these structures to undergo peristaltic contractions (see C). Note: Stones (see C, p. 301) that form in the calyces or renal pelvis may become so large that they more or less fill the cavity and assume its shape (caliceal stone, staghorn calculus).
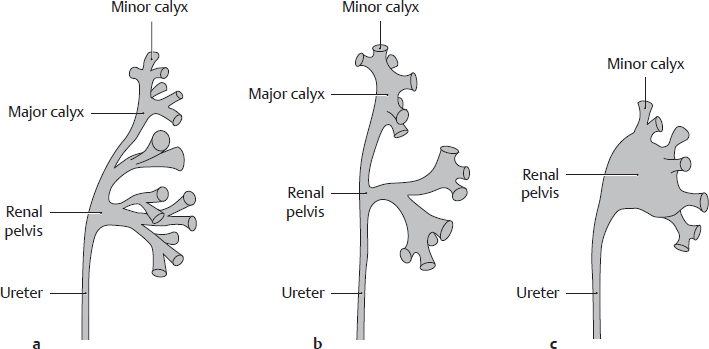
B Variations in the shape of the renal pelvis
Anterior view of the left renal pelvis. The renal pelvis and ureter develop from an outgrowth of the mesonephric duct. This “ureteric bud” grows from the bony pelvis toward the renal primordium and unites with it. Branching extensions of the renal pelvis form the major and minor calyces. The major calyces in particular vary in number and shape: neighboring major calyces may fuse and “become incorporated” into the renal pelvis. The renal pelvis is found in basic forms along with transitional forms:
• Dendritic type (with extensive branching, also called linear) (a): very fine major calyces, narrow renal pelvis;
• Transitional form (b);
• Ampullary type (c): indistinct major calyces with wide renal pelvis; minor calyces arise “directly” from the renal pelvis.
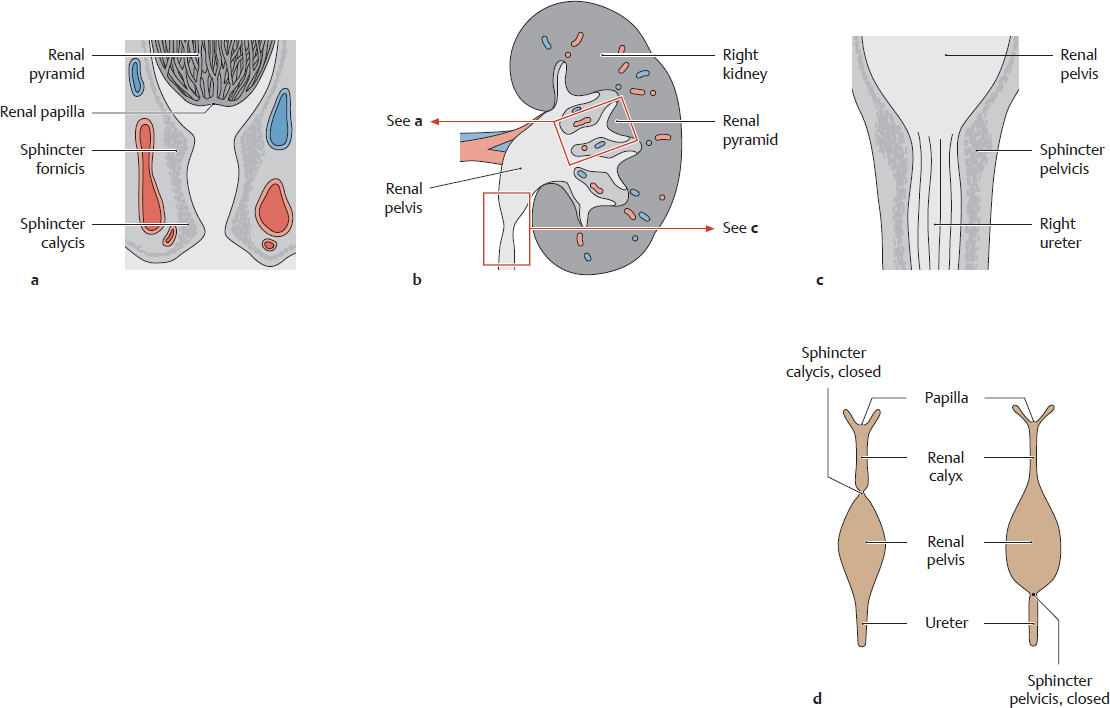
C Closure mechanisms of the renal calyces and renal pelvis; urinary transport (after Rauber and Kopsch)
Schematic representation of a kidney (b) with magnified sections of a calyx (a) and renal pelvis (c) and a dynamic functional diagram of the calyx and pelvis during urinary transport (d). Urine is transported by an active mechanism. The smooth musculature of the sphincter fornicis and calycis (a) and the sphincter pelvicis (c) (the functional sphincter system) enables the wall of the renal calyces and pelvis to contract in segments. These contractions are continuous with the peristaltic waves of the ureter, with the result that the urinary tract is never patent over its entire length but is patent in some portions and closed in others (d). This maintains a distal flow of urine from the tip of the renal papilla into the calyx, through the renal pelvis, into the ureter, and on toward the bladder while preventing the reflux of urine into the kidneys.
Note: If this active transport process is impaired (e.g., by renal stones or drugs that inhibit the ureteral muscles), urine may reflux into the kidney and incite an inflammatory process in the renal pelvis. The papillae, calyces, and pelvis are often affected jointly by disease (e.g., inflammation) because of their close proximity to one another. One of the most common diseases is suppurative bacterial pyelonephritis (pyelo-, referring to the [renal] pelvis, from Gr. pyelos—trough, tub, or vat).
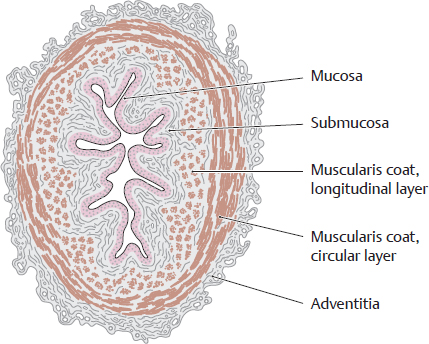
D Wall structure of the ureter
Transverse section through a ureter. A characteristic feature is the stellate lumen that appears in cross-section due to the longitudinal mucosal folds. As in the urethra and bladder, the ureteral mucosa consists of a transitional epithelium of varying height (see p. 305). The smooth muscle consists basically of a longitudinal and a circular layer. It is powerfully developed and shows a functionally spiral architecture (see E). When a renal stone enters the ureter, the smooth muscle in the ureteral wall undergoes powerful contractions in an effort to expel the stone, causing very severe pain (renal or ureteral colic). The colic may be relieved by drugs that suppress the activity of the parasympathetic nervous system, though this will also inhibit normal urinary transport to the bladder. The renal pelvis is structurally analogous to the ureter, including the stellate shape of its lumen.
E Arrangement of the ureteral musculature (after Graumann, von Keyserlingk, and Sasse)
Schematic cross-sections at various levels of the ureter. The longitudinal and circular muscle layers of the ureter wall have a slightly oblique arrangement, forming a kind of spiral that propels urine toward the bladder by peristaltic contractions. Although the ureters are richly innervated, the peristaltic contractions are instigated by spontaneously depolarizing smooth-muscle cells in the walls of the renal pelvis. Peristaltic waves of contraction (with a speed of 2–3 cm/s) are propagated through direct electrical connections (gap junctions) between adjacent smooth-muscle cells. Autonomic motor innervation and local sensory reflexes serve to modulate this intrinsic activity. This mechanism may thus have some superficial similarities to the system that controls heartbeat.
4.5 Suprarenal Glands
A Location and shape
a Location of the suprarenal gland on the right kidney. b Isolated left suprarenal gland, anterior view.
The renal surface of each suprarenal gland lies upon the superior pole of the associated kidney. A thin layer of fat separates the suprarenal gland from the renal fibrous capsule (making it easy to dissect the gland from the kidney). The perirenal fat capsule, however, encompasses both the kidney and the suprarenal gland.
Note: The entire suprarenal gland cannot be seen while in situ, and its true size is not appreciated until it has been detached from the kidney. Portions descend on the posterior surface of the kidney and are not visible in situ.
B Structure of the suprarenal gland
a Right suprarenal gland, cut open. b Histological section from a suprarenal gland.
The suprarenal gland consists of an outer cortex and an inner medulla (see a). The cortex is covered by a thin fibrous capsule and consists of three morphologically distinct zones (see b) in which adrenocortical hormones are produced and secreted into the bloodstream. These zones are, from outside to inside:
• Zona glomerulosa: mainly secretes mineralocorticoids (aldosterone)
• Zona fasciculata: mainly secretes glucocorticoids (hydrocortisone)
• Zona reticularis: sex hormones (estrogens and androgens)
Note: Loss or deficiency of both suprarenal cortices leads to Addison disease, while hyperfunction of the suprarenal cortex (or adrenocortical tumors) leads to Cushing syndrome.
The suprarenal medulla is essentially a completely different endocrine gland, of different origin, that happens to be anatomically (but also functionally) associated with the suprarenal cortex. The cortex is derived embryonically from mesoderm lining the posterior abdominal wall. The suprarenal medulla is, by contrast, a neural crest derivative, and thus has an ectodermal origin. The catecholamines epinephrine and norepinephrine are produced in the suprarenal medulla and are released into the bloodstream. From a (neuro)functional standpoint, the suprarenal medulla is less a gland than a sympathetic ganglion: presynaptic sympathetic neurons pass from the greater and lesser splanchnic nerves into the suprarenal medulla. Because the suprarenal glands are endocrine glands and sympathetic ganglia in one, they can secrete both epinephrine and glucocorticoids (cortisone) in response to stress.
C Right and left suprarenal glands in situ
Anterior view of the right (a) and left (b) kidney and suprarenal gland with the perirenal fat capsule removed. To demonstrate the vessels behind the suprarenal gland, the vena cava has been retracted medially in a and the pancreas has been retracted inferiorly in b. The principal differences between the two suprarenal glands are as follows:
• The right suprarenal gland is often somewhat smaller than the left suprarenal gland, which frequently extends inferiorly to the renal hilum.
• The right suprarenal gland is pyramid-shaped while the large left suprarenal gland is more oblong. The right suprarenal gland is normally in contact with the inferior vena cava (retracted medially here), but the left suprarenal gland is not in contact with the abdominal aorta.
• The right suprarenal vein usually opens directly into the inferior vena cava, unlike the left suprarenal vein, which opens into the left renal vein.
Note: The suprarenal glands are richly vascularized because, as endocrine organs, they release their hormones directly into the bloodstream.
4.6 Ureters in situ
A Course of the ureter in the abdomen and pelvis
Anterior view, male abdomen. All organs have been removed except the urinary organs, suprarenal glands, and a rectal stump. The esophagus has been pulled slightly downward, and the fat capsule of the right kidney has been partially preserved. A prolongation of the renal pelvis, the ureter passes inferiorly and slightly anterior in the retroperitoneum for a length of approximately 26–29 cm. It opens into the posterior aspect of the bladder. Anatomically, the ureter consists of three parts:
• Abdominal part (from the renal pelvis to the linea terminalis of the bony pelvis)
• Pelvic part (from the linea terminalis to the bladder wall)
• Intramural part (passes through the bladder wall)
The ureter is also divided into three clinical segments, based more on the presence of a free segment and two organ-bound segments than on the anatomical boundary between the abdominal and pelvic parts:
• Renal segment (connected to the kidney)
• Lumbar segment (between the kidney and bladder)
• Vesical segment (in the bladder wall, corresponds anatomically to the intramural part).
The most common congenital anomalies of the ureter are duplication anomalies and clefts. They may allow urine to back up to the kidney (e.g., a cleft may allow reflux due to deficient vesicoureteral closure), producing an infection that ascends from the bladder to the renal pelvis (bacterial pyelonephritis).
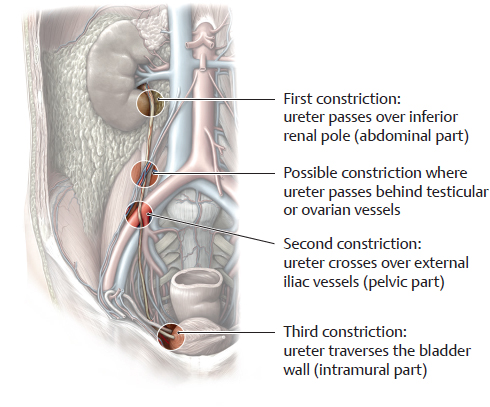
B Anatomical constrictions of the ureter
There are three normal anatomical constrictions where a stone from the renal pelvis is apt to become lodged:
• Origin of the ureter from the renal pelvis (ureteropelvic junction)
• Site where the ureter crosses over the external or common iliac vessels
• Passage of the ureter through the bladder wall (ureterovesical junction).
Occasionally a fourth constriction can be identified where the testicular or ovarian artery and vein pass in front of the ureter.
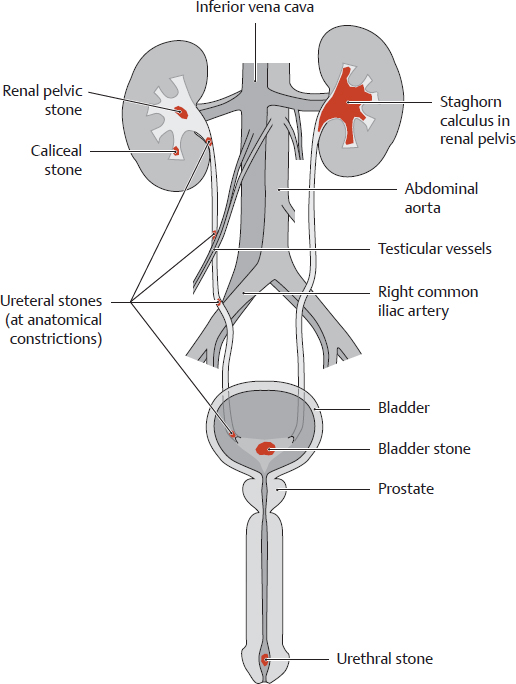
C Common sites of occurrence of urinary stones
When the solubility limit of certain compounds in the urine (e.g., uric acid) is exceeded, the compounds do not remain in solution but are precipitated to form crystallization nuclei. These calculi (“stones”) may develop anywhere in the upper urinary tract and may migrate to various sites in any of the urinary organs (renal and renal pelvic stones, ureteral stones, bladder stones, urethral stones). Larger stones are particularly apt to become lodged in the ureter, often stimulating powerful waves of muscular contractions to expel the stone and causing excruciating pain (renal colic, ureteral colic).
D Intravenous urography
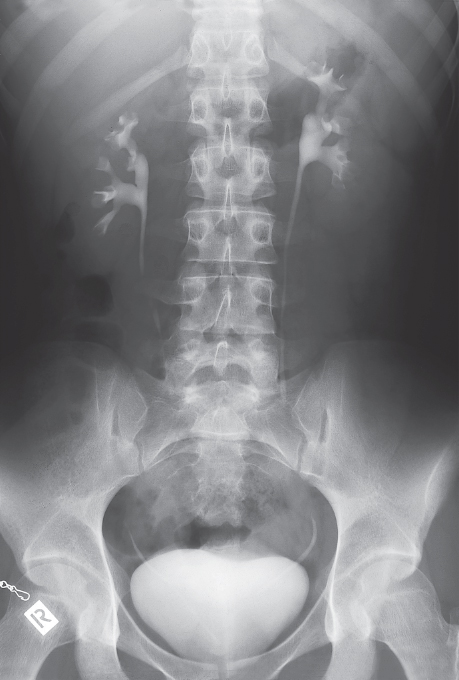
With intravenous urography, which is a radiographic examination, iodinated contrast agent is injected and excreted by the kidneys. The test provides information about renal function, and pathological findings such as anomalies, cysts, urinary obstruction, urinary stones, tumors, etc. (from: Möller, T.B., E. Reif: Taschenatlas der Roentgenanatomie, 3. Aufl. Thieme, Stuttgart 2006).
4.7 Urinary Bladder in situ
A Location and peritoneal covering of the female (a) and male (b) bladder
Midsagittal section, viewed from the left side. The bladder is shown slightly distended, raising the uterus to a slightly higher position. The peritoneum extends from the posterior surface of the anterior abdominal wall to the superior surface of the bladder and is reflected onto the organ posterior to the bladder, forming a peritoneal pouch. In the female, it forms the vesicouterine pouch; in the male, it forms the rectovesical pouch. Most of the bladder is loosely embedded in pelvic connective tissue.
Note: When the bladder is distended and thus enlarged, the upper part of the bladder, which is covered by urogenital peritoneum, is pushed so far cranially that the anterior wall of the bladder, which is embedded in the surrounding connective tissue and not covered with peritoneum, appears superior to the upper margin of the pubic symphysis (like a “sun rising on the horizon”). This provides an access route for percutaneous puncture of the distended bladder above the symphysis without having to enter the peritoneal cavity with the needle.
B Location of the bladder in the pelvis and on the pelvic floor
Superior view. The uterus is shown upright for clarity. Most of the large intestine has been removed, leaving the urogenital peritoneum intact. The transverse vesical fold, a peritoneal fold on the surface of the bladder, is effaced when the bladder is full (as shown here). In the female, the bladder lies inferior to the uterus and elevates it when distended. When the structures of the pelvic floor (levator ani and its fasciae) are weakened due to injuries sustained in a vaginal delivery, for example, they may allow the bladder to descend, resulting in incontinence.
C Comparison of the location of the bladder in the male (a and b) and female (c)
a Superior view with the bladder pulled slightly posteriorly. Unlike in B, the urogenital peritoneum has been removed; the bladder has an almost spherical shape given that it is well distended.
The bladder is located on the muscular sheet of the pelvic diaphragm (lying principally upon the levator ani and its fascia = superior fascia of the pelvic diaphragm). This area of contact is smaller in the male than in the female because the lesser pelvis also contains the prostate.
b and c slightly angled coronal section with the bladder and urethra opened. The portions of the bladder not covered by peritoneum are integrated into the pelvis by a connective tissue space, which has a well-developed venous plexus. This plexus and the mobile visceral peritoneum allow for considerable changes in bladder size. Like the bladder itself, the initial portion of the urethra is surrounded by connective tissue, and in the male also by the prostate, which lies upon the deep transverse perineal muscle and the levator ani of the pelvic diaphragm.
4.8 Urinary Bladder, Bladder Neck, and Urethra: Wall Structure and Function
A External morphology of the bladder and urethra
Bladder in a male (a) and a female (b), viewed from the left side.
The bladder is a hollow muscular organ that collects urine produced by the kidneys and passes it to the urethra at the right time. The maximum bladder capacity is between 500 and 700 ml (females > males). The desire to urinate is already experienced when the bladder contains about 150–200 ml, and even less in pregnant women as the growing uterus increases pressure on the bladder. A normal bladder empties fully without retaining residual urine. The bladder is subdivided into the body of the bladder, the posterior base of the bladder, and the anterior apex of the bladder, which is continuous with the median umbilical ligament (obliterated urachus) at the inner surface of the anterior trunk wall. The two ureters, which enter the bladder dorsolaterally, open into the fundus of the bladder. The urethra begins at the ventrocaudal neck of the bladder.
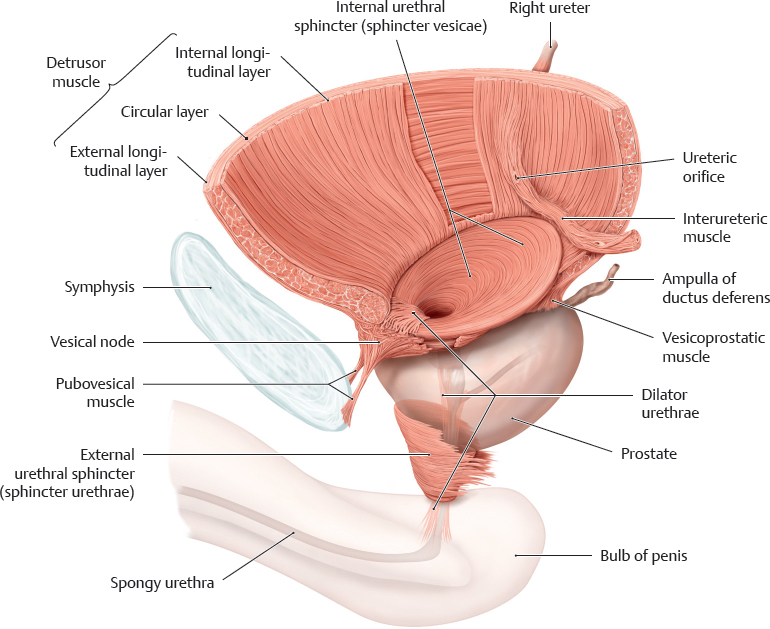
B Muscles of bladder and urethra
Bladder in the male, viewed from the left side. The main muscles of the bladder are
• Detrusor muscle (bladder emptying) and
• Internal urethral sphincter (bladder closure).
The main muscles of the urethra are
• Dilator urethrae (urethral dilation) and
• External urethral sphincter (urethral closure).
According to Dorscher et al (2001), the detrusor and internal urethral sphincter are two morphologically separate muscles (see p. 306). The detrusor consists of three layers and is responsible for firmly affixing the bladder to the pelvis in the anterior-posterior direction. Fibers of its external longitudinal muscle layer extend posteriorly to the vesicoprostatic muscle (or vesicovaginalis muscle in the female) and in the area of the vesicle node anteriorly to the pubovesical muscle, which forms an important part of the ventral suspension apparatus (see p. 307). The middle and internal layers end posteriorly above the interureteric crest (see C). The internal sphincter is elliptical in shape in the male and circular in the female. Its only function is to close the bladder. Around its posterior circumference, the internal sphincter forms the morphological basis of the bladder trigone (see C).
The dilator (see p. 306) is a fan-shaped muscle that arises from the symphysis and the tendinous arch of the pelvic fascia (see p. 307). It extends posteriorly across the urethral opening and inferiorly at the anterior surface of the urethra, where it inserts on the bulb of penis or the vestibular bulbs. The external sphincter consists of an inner layer of smooth muscle fibers and an external layer of striated muscle fibers (for more details see D, p. 307).
C Bladder neck, bladder trigone, and internal urethral orifice
Coronal section at the level of the urethral opening in the male, anterior view.
The interior of the bladder is lined with a relatively thick mucosal layer (urothelium and associated underlying connective tissue, see D). Except for at the bladder trigone, it is easily movable and is thrown into folds when the bladder is not distended. The bladder trigone is an area of smooth mucosa at the base of the bladder or at the bladder neck between the urethral opening and the two ureters, which enter the bladder on its dorsolateral aspect. In the upper border of the trigone is the interureteric crest, a ridge, produced by the interureteric muscle that extends between the orifices of the two ureters. Inferiorly, the internal urethral sphincter, shaped like an elliptical cylinder in the male and a circular cylinder in the female, surrounds the internal urethral orifice. Note the slit-like ureteral orifice and the oblique transit of the ureter through the bladder wall. This obliquity creates a normal constriction in the intramural part of the ureter (see p. 301). The oblique course, the ureteral muscle, and the bladder-wall muscle provide for functional closure of the ureteral orifice and guard against reflux.
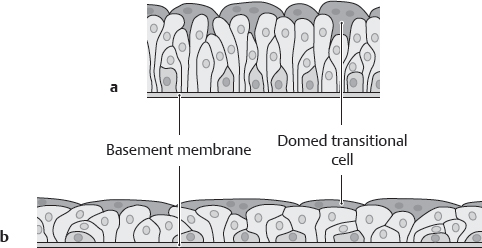
D Epithelium of the bladder mucosa
a Bladder empty: tall epithelium. b Bladder full: flattened epithelium. Like almost all portions of the urinary tract (except the distal urethra), the bladder is lined by transitional epithelium (urothelium) whose height and stratification depend on the degree of distention of the urinary tract segment. The transitional epithelium basically consists of multiple cell layers. The conspicuous cells in the surface layer are called “transitional cells” because they change shape.
Note: The total thickness of the bladder wall (muscle plus mucosa) ranges from 2 to 5 mm in the full bladder and from 8 to 15 mm in the empty bladder.
Emptying and closing the bladder: micturition and continence
Micturition is the process of emptying the bladder. The ability to hold urine with a full bladder is called continence. Coordinated interaction of muscular mechanisms for opening and closing the bladder is crucial for optimal bladder function. Involuntary (autonomic) and voluntary (pudendal nerve) control of the muscular apparatus of the bladder and the urethra play an important role in (cf. p. 316)
• Emptying the bladder completely during micturition,
• Protecting the ureteral orifice from reflux, and
• Maintaining urinary continence with a full bladder.
Emptying the bladder (micturition): activation of the sacral micturition center by a center in the brainstem (pontine micturition center); contraction of the detrusor muscle thereby raising the pressure within the bladder (supported by an increase in intraabdominal pressure, abdominal press); relaxation of the internal sphincter and contraction of the urethral dilator and pubovesical muscle, dilating the urethra (internal urethral orifice); simultaneous closure of the two ureteral orifices by the trigone muscles; relaxation of the external sphincter including both the smooth and striated muscle portions and detumescence of the submucosal venous plexus; voiding of the bladder occurs.
Closing the bladder (continence): structures responsible for maintaining continence primarily include the muscular mechanisms for closing the bladder and urethra (internal sphincter and external sphincter), the ventral suspension apparatus (see p. 307) and parts of the pelvic floor and perineal body. Optimal interaction of these distinct structures ensures continence.
Note: At rest, the urethra forms an angle between its posterior margin and the base of the bladder of 110–120 degrees (posterior vesicourethral angle) in both the male and female. An increased angle due to pelvic floor descent results in incontinence.
4.9 Functional Anatomy of Urinary Continence
A Muscles of the bladder neck and proximal urethra in the male
According to Dorschner et al (2001) and Schwalenberg et al (2010), maintaining continence occurs by the interaction of distinct functional units including the anatomically correct positions of the sphincter muscles, tone of the urethral smooth and striated musculature, and a ventral suspensory mechanism at the level of the bladder neck. Dysfunction of one of these components may cause urethral hypermobility and result in incontinence. Two muscular systems are differentiated:
• A system of sphincter muscles:
– internal urethral sphincter,
– external urethral sphincter with smooth and striated muscle portions,
– longitudinal urethral musculature with smooth ventral urethral musculature and dorsal longitudinal ejaculatory musculature.
• A musculofibrous anchoring system in the pelvic floor:
– ventral vesicourethral suspension apparatus made up of pubovesical muscle, pubourethral and puboprostatic ligaments and the tendinous arch of the pelvic fascia from which the bladder neck is suspended;
– perineal body (central tendon of the perineum) which counters and anchors the external urethral sphincter.
Note: Except for the dorsal longitudinal ejaculatory muscle, all structures are found in both males and females.
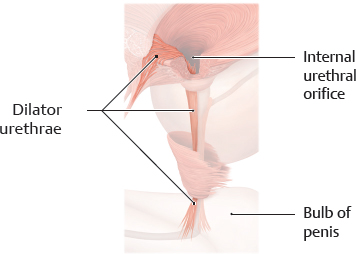
B Dilator urethrae
This is the ventral longitudinal urethral musculature. It is a fan-shaped muscle that arises from the symphysis and along the tendinous arch of the pelvic fascia (see E). It runs over the top of the ventral circumference of the internal urethral sphincter and through the internal urethral orifice. It extends inferiorly along the anterior surface of the urethra, where it inserts on the bulb of penis. The urethra is shortened and the internal urethral orifice is widened by the contraction of the longitudinal musculature. This allows initiation of micturition.
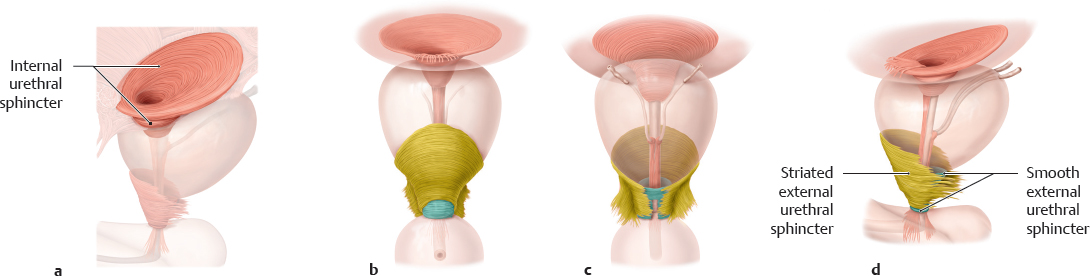
C Internal urethral sphincter and external urethral sphincter
a Internal urethral sphincter; b–d External urethral sphincter, anterior, posterior, and lateral views.
According to Dorschner et al. (2001), the internal sphincter is a distinct, independent functional sphincter. Its smooth musculature is not related to the detrusor or urethral musculature. Thus, it does not arise from the trigone or detrusor musculature. Generally, the internal sphincter is more distinct in the male than the female, in particular the urethral portion, which extends to the proximal urethra. One reason may be that in the male the internal sphincter ensures not only continence but also the effective closure of the bladder neck to prevent retrograde ejaculation (dual function). According to Dorschner et al. (see above), the external sphincter consists of
• an inner circular layer of smooth muscle, and
• an outer striated layer, which has an omega or horseshoe shape and a depression in its posterior surface.
Note: Numerous studies have shown that the striated external sphincter (like the internal sphincter) is an independent muscle and is not split off from the levator ani or the deep transverse perineal muscles.
D Integration of the external urethral sphincter into its surroundings
Anteriorly and laterally, the outer portion of the external urethral sphincter borders the distinct venous plexus (see E) and is partially interspersed with veins. According to Wallner et al. (2009) and Schwalenberg et al. (2010) the lateral fibers of the external sphincter extends into the fascia of the levator ani. Additionally, it has been discussed whether its muscle fibers are anchored to the perineal body. As a result, when the external sphincter contracts, its fibers stretch to both sides of the levator ani, where it is dynamically anchored. Whereas the circular muscle fibers of the smooth external urethral sphincter exert light but permanent pressure on the membranous urethra, the striated external urethral sphincter, which receives somatic innervation, together with the perineal body and the levator ani are able to increase the urethral closure pressure (= improved continence) during pelvic floor contraction.
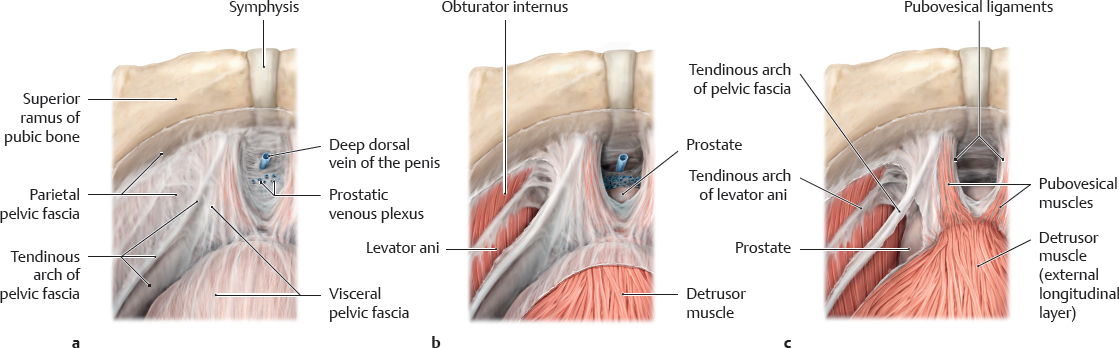
E Ventral vesicourethral suspension apparatus
Aside from the anterolateral stabilization of the vesicourethral junction, the most important function of the ventral suspension apparatus in the retropubic space is suspension of the bladder neck, which ensures continence (Schwalenberg et al. 2010). Essential components of the ventral suspension apparatus include the pubovesical muscles and the tendinous arch of the pelvic fascia, which is a thickened band of the pelvic fascia that extends from the pubic symphysis over the pelvic diaphragm to the ischial spine. There the visceral and the parietal layers (superior fascia of the pelvic diaphragm) merge. The tendinous arch of the pelvic fascia, partially due to its anterior extensions, serves as an additional aponeurotic insertion of the pubovesical muscles that extend as the continuation of the ventral outer longitudinal layer of the detrusor muscle on both sides of the symphysis to the pubic bone. The pubourethral and puboprostatic ligaments listed in the Nomina Anatomica are not ligaments in the proper sense. They are strong sheets of connective tissue of the visceral and parietal pelvic fascia and extend from the pubic symphysis to the bladder neck or prostate
Note: Protection and restoration of the structures of the ventral suspension apparatus as described above have led to a significant decrease in postoperative incontinence after surgery to remove the prostate.
4.10 Urethra
A Parts of the male urethra
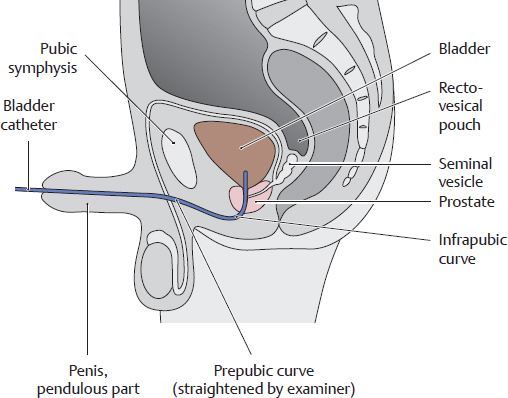
Male urogenital system in the pelvis, viewed from the right side. Unlike the female urethra, the male urethra functions as a common urinary and genital passage. It has an average length of 20 cm and consists of four parts with three constrictions and three expansions (see B). The intramural part of the urethra in the bladder wall is not shown here. While the female urethra is essentially straight (see E), the male urethra presents two curves: an infrapubic curve and a prepubic curve. These curves are important in transurethral bladder catheterization (see F).
B Wall segments, constrictions, and expansions of the male urethra (see also D)
Wall segments |
Constrictions and expansions |
Internal urethral orifice |
|
Intramural part |
First
constriction: internal urethral sphincter |
Prostatic part |
First expansion |
Membranous part |
Second constriction: external urethral sphincter |
Spongy part |
Second expansion: ampulla
Third expansion: navicular fossa |
External urethral orifice |
Third constriction |
C Location of the male urethra in the penis
Transverse section through the penile shaft. The spongy part of the urethra is contained in the corpus spongiosum of the penis. The corpus spongiosum does not become completely hard even at maximum erection, ensuring that the urethra remains patent during ejaculation. The urethral lumen often presents a flattened rather than circular shape in cross-section, with the upper and lower walls touching.
D Male urethra in longitudinal section
The whole length of the urethra has been cut open and displayed without curves, and all of the pelvic floor muscles have been removed. The four parts of the male urethra can be identified. The male urethra extends distally in the corpus spongiosum to its external orifice on the glans penis. The prostatic part of the urethra may be greatly narrowed in patients with benign prostatic enlargement (prostatic hyperplasia, see p. 338). This condition is often marked by incomplete voiding and the dribbling of urine after micturition. The residual urine left in the bladder may incite an (often bacterial in nature) inflammation of the bladder (cystitis).
E Female urethra in longitudinal section
Coronal section tilted slightly posterior, anterior view. Unlike the male urethra, the female urethra is straight and only about 3–5 cm long. Thus it is much easier to catheterize than in the male. At the same time, the short length of the female urethra increases susceptibility to urinary tract infections.
F Transurethral bladder catheterization in the male
The two curves of the male urethra (infrapubic and prepubic) and its three constrictions may pose an obstacle to transurethral catheterization. The prepubic curve can be straightened out somewhat by straightening the penile shaft.
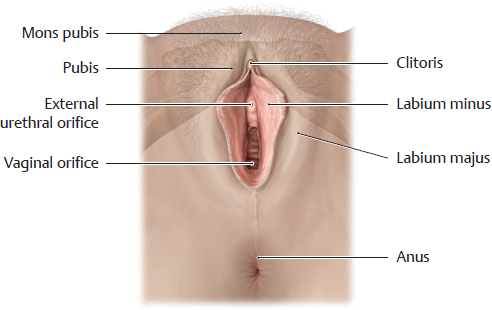
G External orifice of the female urethra
Viewed from below. The pubic bone (pubis) is shown in shadow to aid orientation. The external urethral orifice is located between the labia minora, anterior to the vagina. Despite its proximity to the female external genitalia, the female urethra functions exclusively as a urinary passage. The close topographical relationship of the urethra and external genitalia is important during embryonic development, however: both the urethra and the vagina initially have a common opening at the urogenital sinus and become separated only after further development. Failure of this separation results in an abnormal fistulous connection between the vagina and urethra, a urethrovaginal fistula. Even with normal embryonic development, the proximity of the urethra (which is physiologically germ-free) to the vagina (which is not a sterile zone) predisposes to bacterial inflammation of the urethra (urethritis). Given the short length of the female urethra, the infection can easily ascend to the bladder (cystitis).
4.11 Arteries and Veins of the Kidneys and Suprarenal Glands: Overview
A Overview of the arteries and veins of the kidneys and suprarenal glands
Anterior view. The esophagus has been pulled slightly inferiorly, and the right kidney and suprarenal gland have been pulled away from the inferior vena cava to show the vascular anatomy of the suprarenal gland. The other abdominal organs have been removed.
Renal artery: The renal arteries branch from the sides of the abdominal aorta at the level of the L 1/L 2 vertebrae (see C). The right renal artery runs posterior to the inferior vena cava (shown transparent in the drawing), and the left renal artery runs posterior to the left renal vein. Each renal artery divides into an anterior and posterior branch. The renal arteries give off inferior suprarenal arteries to the suprarenal gland, capsular (perirenal) branches to tissue surrounding the kidney and to the renal capsule (fibrous capsule and perirenal fat capsule, removed here for clarity), and ureteral branches to the upper portion of the ureter and the distal renal pelvis. Possible variants are illustrated in E, p. 313. Suprarenal arteries: Superior, middle, and inferior suprarenal arteries (from the inferior phrenic artery, abdominal aorta, and renal artery, see above).
Renal vein: The renal vein on each side is generally formed by the union of two or three venous branches (variants are shown in F, p. 313). While the left renal vein receives the left suprarenal vein and left testicular or ovarian vein, the right renal vein opens directly into the inferior vena cava without receiving these tributaries (see also D). The renal vein also receives capsular branches from the renal fibrous capsule in addition to small branches from the renal pelvis and proximal ureter (not shown here).
Suprarenal veins:
Note: The three main arteries of the suprarenal glands (see above) are generally accompanied by only one vein (rarely two), the suprarenal vein. While the left suprarenal vein opens into the left renal vein, frequently anastomosing with the left inferior phrenic vein (as shown here), the right suprarenal vein empties directly into the inferior vena cava (see also D).
B Arteries and veins of the kidneys and suprarenal glands
Anterior view. The right kidney and suprarenal gland have been slightly retracted from the inferior vena cava to display their blood vessels more clearly. It is evident in this diagram and in A that the suprarenal glands have a more complex vascular anatomy than the kidneys: More than 50 small branches may pass from the arterial trunks of the suprarenal glands (superior, middle, and inferior suprarenal arteries) into the glands.
Note that the three main arteries of the suprarenal glands are generally accompanied by only one vein, the suprarenal vein. This vessel opens directly into the inferior vena cava on the right side and into the renal vein on the left side (see D).
C Projection of the renal arteries and veins onto the vertebral column
The renal artery arises from the abdominal aorta at the L 1/L 2 level. Note: The renal veins lie anterior to the arteries.
D Tributaries of the left renal vein
The renal vein has more tributaries on the left side than on the right. The left renal vein receives the left suprarenal vein (often anastomosing with the left inferior phrenic vein, see A) and the left testicular/ovarian vein, whereas the corresponding veins on the right side open directly into the inferior vena cava. Because of this arrangement, varicose dilations of the veins in the spermatic cord (varicoceles) are more common on the left side than on the right.
4.12 Arteries and Veins of the Kidneys and Suprarenal Glands: Variants
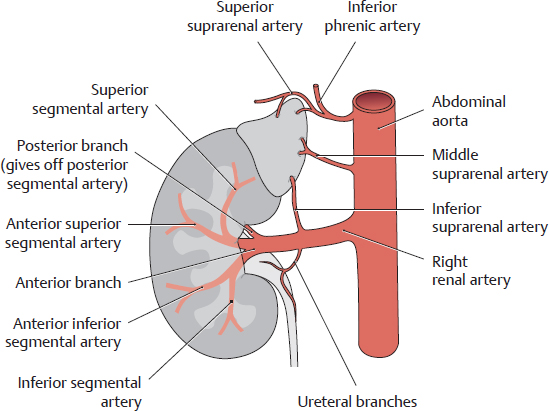
A Division of the renal artery into segmental arteries
Anterior view of the left kidney.
The main trunk of the renal artery divides into an anterior and posterior branch. The anterior branch divides further into four segmental arteries:
• Superior segmental artery
• Anterior superior segmental artery
• Anterior inferior segmental artery
• Inferior segmental artery
The posterior branch gives rise to only one segmental vessel, the posterior segmental artery.
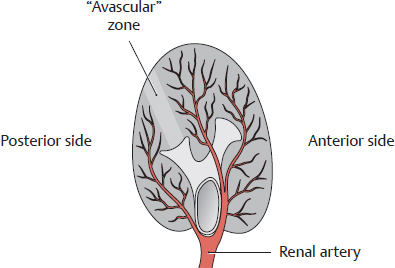
B “Avascular” zone in the kidney
Inferior view of the right kidney.
Between the posterior segment and anterior segments is a relatively avascular zone of the kidney, which otherwise is very heavily vascularized. This zone provides an important line of access for intrarenal surgery.
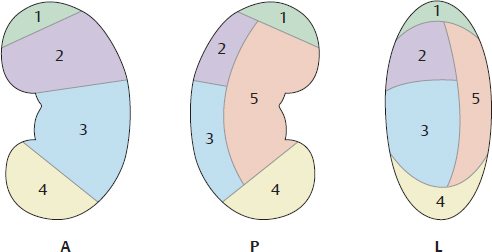
C Vascular segmentation of the kidney
Left kidney viewed from the anterior (A), posterior (P), and lateral (L) sides.
The renal artery and its branches divide the kidney into five segments:
1 Superior segment
2 Anterior superior segment
3 Anterior inferior segment
4 Inferior segment
5 Posterior segment
D Relationship of the renal arterial branches to the renal segments
Anterior view of the right kidney, demonstrating the origins of the renal artery, middle suprarenal artery, and inferior phrenic artery from the abdominal aorta.
Note the division of the renal artery into an anterior branch (anterior segments, superior and inferior segments) and a posterior branch (for the posterior segment, see also A). The upper part of the ureter is supplied by ureteral branches from the renal artery.
E Variants of the renal arteries
Anterior view of the right kidney.
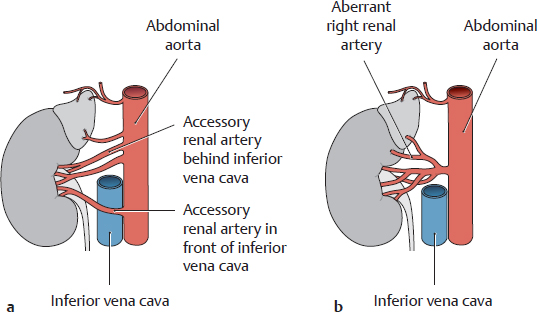
a Two accessory renal arteries (one crossing in front of the inferior vena cava): Accessory renal arteries are extra arteries that pass from the abdominal aorta to the renal hilum. As a common variant with accessory renal arteries, the inferior suprarenal artery does not arise from the renal artery.
b An aberrant renal artery is one that does not enter the kidney at the renal hilum.
F Variants of the renal veins
Anterior view.
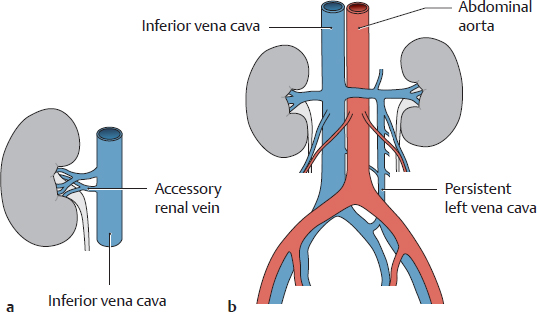
a Accessory (supernumerary) renal veins
b A left vena cava (persistent lower part of the supracardinal vein) ascends to the level of the left renal vein and opens into it.
4.13 Lymphatic Drainage of the Kidneys, Suprarenal Glands, Ureter, and Urinary Bladder
A Lymphatic drainage of the kidney, suprarenal gland, and ureter (abdominal part; the pelvic part is shown in C)
Anterior view. The following lymphatic pathways are important in this region (see also p. 221):
• Right kidney and suprarenal gland: drain to the right lumbar lymph nodes (lateral caval, precaval, and retrocaval lymph nodes, see B), then to the right lumbar trunk.
• Left kidney and suprarenal gland: drain to the left lumbar lymph nodes (lateral aortic, preaortic, and retroaortic lymph nodes, see B), then to the left lumbar trunk.
• Ureter (abdominal part): follows the pathway for the right and left kidneys and suprarenal glands (see also C).
The lumbar lymph nodes additionally function as collecting lymph nodes for the common iliac nodes.
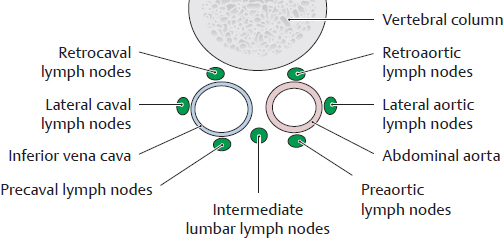
B Classification of the lumbar lymph nodes
Transverse section, viewed from above. The lumbar lymph nodes are distributed around the abdominal aorta and inferior vena cava. They are divided into three groups based on their relationship to these vessels:
• Left lumbar lymph nodes (around the aorta)
• Intermediate lumbar lymph nodes (between the aorta and inferior vena cava)
• Right lumbar lymph nodes (around the inferior vena cava)
These groups are further divided into subgroups (see legend of A).
C Lymph nodes of the ureter
Anterior view of the right ureter.
The lymphatic drainage of the ureter is roughly divided into two levels:
• Abdominal part of the ureter: lumbar lymph nodes
– Right: lateral caval lymph nodes (right lumbar lymph nodes)
– Left: lateral aortic lymph nodes (left lumbar lymph nodes)
• Pelvic part of the ureter: external and internal iliac lymph nodes.
Both pathways empty into the lumbar trunks.
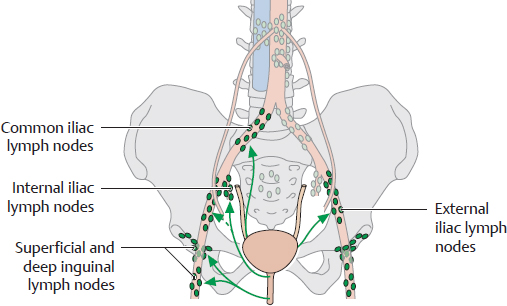
D Overview of the pelvic lymph nodes and the lymphatic drainage of the bladder
Anterior view of an opened female abdomen and pelvis. All organs have been removed except for the bladder and a small rectal stump, and the peritoneum has been removed. The bladder is distended, making it visible above the pubic symphysis. This drawing clearly shows the numerous parietal lymph nodes that are distributed around the iliac vessels in the pelvis (see E). Lymph from the bladder usually drains first to groups of visceral lymph nodes: the lateral vesical lymph nodes and the pre- and retrovesical lymph nodes (known collectively as the paravesical nodes). These nodes are embedded in the pelvic connective tissue surrounding the bladder and lie so deep within the pelvis that they are not visible here. Lymph from these visceral nodes drains directly or indirectly along two major pathways, reaching lymph nodes lateral to the abdominal aorta and inferior vena cava (lumbar lymph nodes) and finally entering the lumbar trunks. These pathways are illustrated in F.
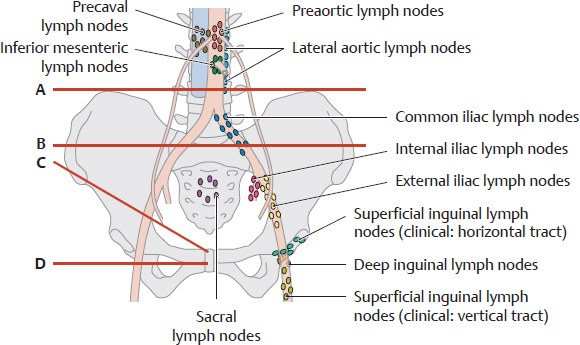
E Overview of the pelvic lymph nodes
The pelvic lymph nodes are distributed along major blood vessels and in front of the sacrum. The blood vessels are not visualized by lymphography (contrast radiography of the lymph nodes), however, and so the location of the pelvic lymph nodes must be determined by other means. One method is to use four reference lines based on skeletal landmarks:
A Iliolumbar line: horizontal line tangent to the superior borders of the iliac crests
B Iliosacral line: horizontal line through the center of the sacroiliac joint
C Inguinal line: line along the inguinal ligament
D Obturator line: horizontal line through the center of the obturator foramen
F Lymphatic drainage of the bladder and urethra
The bladder is drained by two principal pathways:
• Cranially along the visceral iliac vessels (see D)
• To the internal and external iliac lymph nodes (mainly at the base of the bladder)
Portions of the bladder near the internal urethral orifice are drained by the superficial and deep inguinal lymph nodes. The urethra drains chiefly to the deep and superficial inguinal lymph nodes (the latter mainly draining areas near the external urethral orifice). The proximal portions of the urethra are drained by the iliac lymph nodes, particularly the internal iliac nodes.
Note: The penis, like the urethra, is drained by the superficial and deep inguinal lymph nodes.
4.14 Autonomic Innervation of the Urinary Organs and Suprarenal Glands
A Overview of the autonomic innervation of the urinary organs and suprarenal glands
Anterior view into an opened male abdomen and pelvis. The stomach has been largely removed and pulled slightly inferior with the esophagus for better exposure. The right kidney has been displaced slightly laterally, and the bladder has been straightened and retracted to the left. The pelvis has been sectioned in a coronal plane passing approximately through the center of the acetabula. The autonomic innervation of the urinary organs and suprarenal glands varies according to the location of the specific organ:
• The kidneys in the retroperitoneum and portions of the upper urinary tract (proximal ureters) receive sympathetic fibers initially from the lesser, least, and lumbar splanchnic nerves (see B), which synapse with the postsynaptic neuron in the aorticorenal or renal ganglia. The parasympathetic fibers originate from the posterior vagal trunk and partly from the pelvic splanchnic nerves (the plexuses are described in B).
• The suprarenal cortex and medulla in the retroperitoneum receive sympathetic fibers from the greater and lesser splanchnic nerves. They receive parasympathetic fibers from the posterior vagal trunk, which pass with the renal plexus to the suprarenal glands as the suprarenal plexus. The sympathetic autonomic innervation of the suprarenal medulla is exceptional in that the suprarenal medulla is supplied only by presynaptic sympathetic fibers from the suprarenal plexus. These axons directly innervate the suprarenal medullary cells. At present, there is no convincing evidence that the suprarenal medulla receives parasympathetic innervation.
• The bladder, most of the abdominal part and pelvic part of the ureter (and the urethra, not shown here) in the pelvis (see D) receive sympathetic fibers from the lumbar and sacral splanchnic nerves, and they receive parasympathetic fibers from the pelvic splanchnic nerves (S2–S4). The plexuses are shown in D.
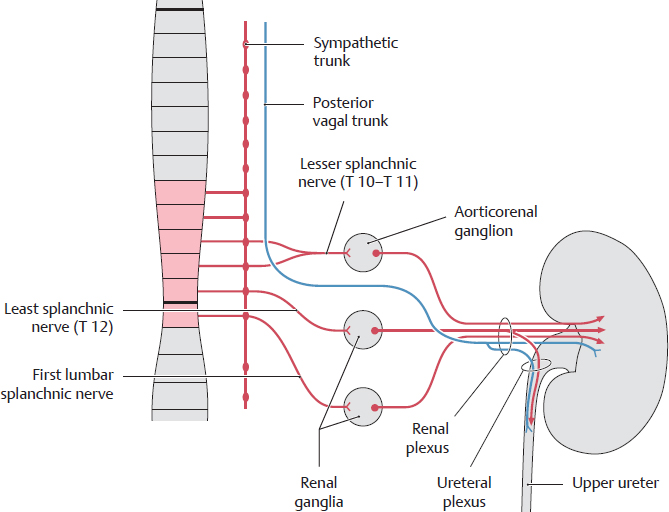
B Autonomic innervation of the kidney and upper ureter
The sympathetic fibers from the aorticorenal ganglia and renal ganglia combine with the parasympathetic fibers from the posterior vagal trunk to form the renal plexus, which passes to the kidney. Branches from that plexus form the ureteral plexus, which supplies the abdominal (upper) part of the ureter. The presynaptic sympathetic fibers come primarily from the thoracic splanchnic nerves.
Note: Due to their topographic proximity, the aorticorenal ganglia often merge with the celiac ganglia. Illustrations providing an overview, such as on page 70, often show the kidneys as receiving their innervation from the celiac ganglia. Functionally, however, the aorticorenal ganglia are separate and are thus separately mentioned in the schematic diagram.
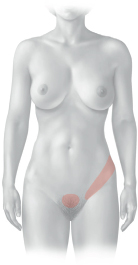
C Referred pain from the left kidney and bladder
Pain associated with diseases of the kidney and bladder (inflammation, calculi) may be perceived in these skin areas. Occasionally the pain radiates into the groin (“loin to groin pain”).
D Autonomic innervation of the bladder and the abdominal and pelvic parts of the ureter
Sympathetic fibers from the lumbar and sacral splanchnic nerves pass with the parasympathetic fibers from the pelvic splanchnic nerves to the inferior hypogastric plexus. Branches from that plexus are distributed to form additional plexuses, including the vesical and ureteral plexuses that supply the bladder and ureter (its abdominal and pelvic parts). For the parasympathetic fibers, the synapse with the postsynaptic neuron is located entirely in the inferior hypogastric plexus (or organ wall).
The sympathetic fibers synapse partly in the inferior mesenteric ganglion and partly in the inferior hypogastric plexus (see the fibers that continue from the superior hypogastric plexus to the inferior hypogastric plexus). Note: With a complete transection of the spinal cord, the effect of higher CNS centers on the central parasympathetic neurons of S2–S4 (pelvic splanchnic nerves) is abolished. Because the pelvic splanchnic nerves initiate and control micturition, a complete cord lesion also causes problems of bladder control.