What creationists challenge evolutionists to show them, it seems, is a “perfect 10” transitional form, exactly halfway between, say, fish and amphibian. But no such “fishibian,” says the Institute for Creation Research (ICR), has ever been found in the fossils.
—Ronald Ecker, Dictionary of Science and Creationism
We come now to one of the classic transitions in all of evolution: How did the aquatic vertebrates crawl out on land and become four-legged (tetrapod) terrestrial animals? This subject has intrigued paleontologists and biologists for over a century, and naturally plenty of controversy and many mistakes and false leads have occurred (as in any area of science exploring a difficult topic). Creationists, of course, cannot allow themselves to admit that this transition ever occurred, so they attack it with vigor, mostly by citing out-of-date sources (even in more recent books like Gish 1995) and ignoring all the evidence that doesn’t fit their point of view. But here the creationists have been left in the dust. Dramatic new discoveries in the past 30 years have completely revolutionized what we once thought about how this transition occurred. You can take the creationist publications and wrap dead fish with them, because they have been completely debunked by what we have learned in the past decade. We don’t have every possible transitional form between fishes and tetrapods, but we now have so many steps in the sequence that to deny that this transition occurred is like the neo-Nazis denying the Holocaust—it’s a self-evident fact, and there are many fossil witnesses to bear testimony.
Before we discuss this transition in detail, a few semantic issues (some of which are exploited by creationists) need to be clarified. The old Linnaean scheme of animal classification divided the vertebrates into several obvious groups: “fishes,” “amphibians,” “reptiles,” and so on. We are all taught from a young age that amphibians are animals (such as frogs and salamanders) that live both in water and on land (amphibian literally means “living both lives” in Greek). But in the context of modern phylogenetic or cladistic classification, natural groups must include all their descendants. The lineage that leads to reptiles evolved from one group of amphibians, so amphibians must either include all vertebrates with four legs (tetrapods), or else the amphibians are a paraphyletic “grade” of evolution, halfway between reptiles and fish (fig. 10.1). To get around this problem, most modern cladistic classification schemes do not use the antiquated word “amphibian” anymore but instead use the natural monophyletic group known as tetrapods (all four-legged land animals and their relatives). If you wanted to use “amphibian” as a concept, the three living groups, or “Lissamphibia” (frogs and toads; salamanders; and the apodans, a legless group from the tropics) might be a natural monophyletic clade, and the term could be used there. But then that leaves all the various fossils that have been called amphibians out in the cold. Some extinct groups (fig. 10.1), such as the temnospondyls, may be closely related the living groups and are thus would be true amphibians.
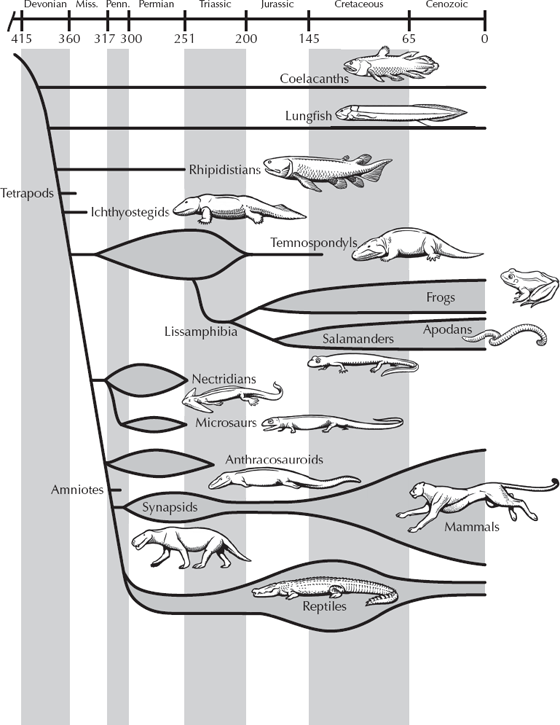
FIGURE 10.1. The relationships of the four-legged animals, or tetrapods. (Drawing by Carl Buell)
Others, such as the lepospondyls, may or may not be related to the temnospondyl-lissamphibian clade, so their inclusion is more questionable. And then there are the “anthracosaurs” (fig. 11.3), a grade of tetrapods that are the sister taxa to the reptiles (more properly, amniotes, as the next chapter will discuss). They don’t share the characters that define reptiles or amniotes, so paleontologists have traditionally left them in the amphibian wastebasket with the other tetrapods that are not amniotes. In this chapter, we will not use the term “amphibian” further, but will stick with the clumsy but accurate term “non-amniote tetrapod” when we mean frogs and salamanders and what most people call amphibians.
Then I turned the page and saw the sketch, at which I stared and stared, at first in puzzlement, for I did not know of any fish of our own or indeed of any seas like that; it looked more like a lizard. And then a bomb seemed to burst in my brain, and beyond that sketch and the paper of the letter I was looking at a series of fishy creatures flashed up as on a screen, fishes no longer here, fishes that have lived in dim past ages gone, and of which often only fragmentary remains in rocks are known. I told myself sternly not to be a fool, but there was something about the sketch that seized on my imagination and told me that this was something far beyond the usual run of fishes in our seas…. I was afraid of this thing, for I could see something of what it would mean if it were true, and I also realized only too well what it would mean if I said it was what it was not.
—J. L. B. Smith, Old Fourlegs: The Story of the Coelacanth
On December 23, 1938, one of the most remarkable scientific discoveries of the past century was made in the mouth of the Chalumna River near East London off the coast of South Africa. Pulled out of a net from the trawler Nerine was a huge (almost 1.5 meters [or 5 feet] long, and weighing 58 kilograms [or 127 pounds]) shiny silvery-blue fishlike creature, the likes of which no fisherman had ever seen before (fig. 10.2). The local museum curator, Marjorie Courtenay-Latimer, was called and immediately realized it was something of great scientific importance, a new species never before caught in the waters off South Africa. As she wrote later, it was “the most beautiful fish I had ever seen, five feet long, and a pale mauve blue with iridescent silver markings.” Unfortunately, it was already dead and beginning to rot rapidly in the hot austral summer weather. She did her best to preserve it, but it was so large and rotting so fast that she eventually had to discard most of the innards and saved only the skin. She then sent a sketch of it with her letter to the foremost authority on South African fishes, James Leonard Brierly Smith, whose reaction when he opened her letter and saw her sketch is given in the quote that opens this section. He finally got to see the specimen on January 3, 1939, and as he later wrote,

FIGURE 10.2. Evolutionary transformation series of the coelacanths, from the primitive Triassic form, which looks much like other early sarcopterygians, to the highly specialized living fossil Latimeria (bottom drawing). Even though there is dramatic change in shape, these animals still retain the hallmarks of coelacanths, including the extra lobed fin in the end of the tail, the triangular opercular bone covering the gills, and the distinctive shape of the lobed pectoral, pelvic, and anal fins, but ray-finned dorsal fins. (From Clack 2002; used with permission)
Coelacanth—yes, God! Although I had come prepared, that first sight hit me like a white-hot blast and made me feel shaky and queer, my body tingled. I stood as if stricken to stone. Yes, there was not a shadow of a doubt, scale by scale, bone by bone, fin by fin, it was a true coelacanth. It could have been one of those creatures of 200 million years ago come alive again. I forgot everything else and just looked and looked, and then almost fearfully went close up and touched and stroked. (Smith 1956:73)
Smith named this astounding find Latimeria (after the discoverer) chalumnae (after where it was found), and it was the sensation of the scientific world in 1939. After 13 hard years of searching, however, Smith and the fishermen of South Africa had yet to find another and were beginning to despair. So much crucial information had been lost when its guts had been discarded! They sent out a “wanted” poster with a photograph of the fish and a £100 reward and circulated them all over the African coast. Then, in 1952, a lucky break occurred.
Another fisherman, Eric Hunt, had distributed Smith’s reward poster up and down the East African coast, and a local fisherman in the tiny Comoros Islands north of Madagascar had found another coelacanth. Soon the fish was flown back to South Africa on the orders of the prime minister himself, and it was well enough preserved that the internal organs had survived.
Since 1952, more than 100 additional specimens have been hauled out of the deep waters around the Comoros and again in South Africa, and a few years ago, another new species was discovered in Indonesia. It turns out that coelacanths live in very deep waters and only come to the surface in the dark of night, which is why they had gone undetected for so long. Unfortunately, they are now so valuable that local fishermen may be hunting them back into extinction, barely more than 75 years since this living fossil was first discovered. Coelacanths have long been known from the fossil record (fig. 10.2), but the last known fossil of a coelacanth dated back to the Cretaceous, while the dinosaurs still roamed the earth. No wonder the world was so astonished to find an animal living that had been thought to be extinct for at least 70 million years.
Coelacanths are part of a group of vertebrates known as “lobe-finned fishes” or Sarcopterygii, which includes not only the coelacanths but also the lungfishes and a number of extinct forms known by the paraphyletic grouping “rhipidistia,” as well as their descendants, the tetrapods. Both lobe fins and ray fins share a common ancestor in the Devonian (fig. 9.1). Recent DNA sequencing has shown that coelacanths and lungfish are more closely related to each other than either is to tetrapods. While the ray-finned fish now dominate the world’s waters, lobe fins (once common in the Paleozoic and early Mesozoic) have since been reduced to a tiny remnant: the living coelacanths, three genera of lungfish, and, of course, tetrapods. Instead of fins supported by numerous bony rays (as in the fish described in the last chapter), the fins of lobe-finned fish are supported by robust bones and muscles forming a lobe, which is then surrounded by fin rays.
The first living lobe fin to be discovered and described scientifically was the South American lungfish, Lepidosiren, which although highly specialized with whiplike fins, has lungs rather than gills. In 1837, a specimen of the African lungfish, Protopterus (fig. 10.3), came to the attention of Richard Owen, England’s foremost anatomist and paleontologist. As he dissected it, he came across the undisputed evidence that this fish had lungs, although his creationist leanings refused to admit that this gave it tetrapod affinities. Its teeth consisted of large ridged biting plates, which had been known from fossils for years, and here was the source of those mysterious fossils. The clincher came when the Australian lungfish, Neoceratodus, was discovered. Not only did it have lungs, but its fins were not as highly modified as those of the African and South American species but still showed the classic lobed form. Since then, many fossil lungfish have been found, and they look much more like the earliest coelacanths (fig 10.2) and the earliest “rhipidistians,” so the peculiarities of the living lungfishes and coelacanth disappear as you go back in time.

FIGURE 10.3. Evolutionary transformation series of lungfish, from the primitive Devonian fossil Dipterus, which closely resembles the earliest coelacanths and rhipidistians, to the highly specialized living forms. (Reproduced by permission of the Royal Society of Edinburgh and P. Ahlberg; from Ahlberg and Trewin 1995)
Further study of the living lobe fins reveals another striking characteristic. Not only are their limbs constructed with robust bones and muscles (like those of a tetrapod) rather than thin bony fin rays (like most fish), but they also use them differently than most living fish. Studies of the fin motion of both coelacanths and lungfish have shown that they move their fins in a “step cycle” similar to the motion of the four limbs of tetrapods. Thus the characteristic leg motion sequence of four-legged animals was already present in lobe fins that never walked on land.
This highly specialized anatomy of the modern forms confuses creationists. They point to the peculiarities of living Latimeria or the specialized fins of some lungfish and argue that they cannot have been ancestors of tetrapods. But once again, they are thinking of ladders when we are talking about branching bushes. None of the living species are ancestral to tetrapods, and no paleontologist ever made that claim. The lungfish, coelacanths, and rhipidistians are distinct side branches, or sister taxa, to the tetrapods, and they share many unique anatomical characters that support that relationship, but they branched off back in the Devonian and have had their own unique history ever since then (fig. 9.1). We can see how close they once were when we compare Devonian fossil forms (fig. 10.3), and it is irrelevant that the peculiar modern descendants have specializations that are not found in tetrapods. Evolution is a bush, not a ladder, and organisms continue to evolve and change, long after they have branched off from their sister groups that lead to humans. It’s hard to tell whether creationists can’t get this point straight because they are clueless, misinformed, or just don’t want to face the truth.
We will leave lungfish and coelacanths aside for now and focus on the fossils that document the transformation from rhipidistians to tetrapods.
In my days it was believed that the place for a fish was in the water. A perfectly sound idea, too. If we wanted fish, for one reason or another, we knew where to find it. And not up a tree.
For many of us, fish are still associated quite definitely with water. Speaking for myself, they always will be, though certain fish seem to feel differently about it. Indeed, we hear so much these days about the climbing perch, the walking goby, and the galloping eel that a word in season appears to be needed.
Times change, of course—and I only wish I could say for the better. I know all that, but you will never convince me that a fish that is out on a limb, or strolling around in vacant lots, or hiking across the country, is getting a sane, normal view of life. I would go so far as to venture that such a fish is not a fish in his right mind.
—Will Cuppy, How to Become Extinct
As humorist Will Cuppy noted (in the preceding quote), we think of fish as belonging in the water and have trouble with the idea that they could crawl out on land. For years, evolutionary biologists and paleontologists have argued and speculated about the forces that led fishes to finally crawl out on land. The transition seems like a remarkable one. Water-living fishes needed to develop some way of breathing air, supporting themselves on land without the buoyancy of water, preventing their skins from drying out, seeing and hearing on land, and making many other physiological adjustments. Paleontologists used to speculate endlessly about why they made this apparently difficult trek. Some argued that it was to escape drying pools, and others suggested it was to escape predation in the crowded Devonian waters, or to take advantage of new food sources on land (since insects, spiders, scorpions, millipedes, and other arthropods had already colonized the land over 100 million years before vertebrates did). Unfortunately, much of this speculation was predicated on false assumptions and inadequate specimens, and most of it is irrelevant now.
It turns out that crawling up on the land is no big deal. Many different teleost fishes (with flimsy ray fins, not the robust lobe fins) do it all the time. A variety of tide-pool fishes, such as gobies and sculpins, spend a lot of time out of water when the tide goes out, hunting vulnerable prey. In the southeastern United States, the walking catfish (fig. 10.4A) is a legendary pest for its ability to wriggle from one pool of water to another, using only its ray fins for propulsion. Eels, too, are capable of wriggling across the ground for some distance in search of new pools of water. The climbing perch of Africa and Southeast Asia, Anabas testudineus, travels in search of water when its ponds dry up. It walks supported by the spiny edges of the gill plates and propelled by the fins and tail and can climb low trees. The most specialized and most amphibious of all the “land fish” is the mudskipper (fig. 10.4B), which is completely adapted to living on the land-water boundary. It even has its eyes up on periscopes so it can look above water while it is swimming. The mudskipper haunts the mudflats in mangrove swamps, catching prey in the mud. It uses its ability to wriggle on land or swim in water to escape predators coming from either direction and can also climb up the exposed roots of the mangrove trees.

FIGURE 10.4. A number of ray-finned fishes have evolved the ability to live on land and crawl around, or they have modified their rayed fins into walking appendages for use in creeping along the seafloor. (A) The “walking catfish,” which wriggles along the ground between ponds when its home pond dries up or becomes too crowded. (B) The mudskipper, which spends most of its life out of water sitting on the mudflats or mangrove roots. ([A] and [B] from Romer 1959) (C) The frogfish, which has modified its ray fins into “fingers” that enable it to creep along the bottom. (From Clack 2002: fig. 4.15; used with permission)
In 2014, scientists published a remarkable study (Standen et al. 2014). They took populations of the African ray-finned fish known as the bichir, or Polypterus (distantly related to sturgeons and paddlefish), and made them cross over dry land over and over again. Within 8 months, the bichir that had had been raised on land had modified their ray fins and the muscles to control them so they were more efficient walkers; those that were kept in their normal water habitat did not change. The remarkable footage of these walking fish can be seen online by searching for videos of “walking fish Polypterus.”
None of these fishes are perfectly adapted for land, but are “jury-rigged” to live on land just long enough to accomplish certain tasks. They must inhabit humid climates and remain close to water so they don’t dry out, and they return to the water frequently to restore their water balance. They don’t have lungs but make do with their gills, swim bladders, and the moisture in the air to breathe for extended periods of time without lungs. They don’t have robust lobed fins that could become legs and feet, but do the best they can with the relatively flimsy ray fins to push themselves along on their bellies and wriggle across the ground. In fact, there are a variety of completely aquatic teleosts, such as the fingered dragonets, genus Dactylopus, and the grunt sculpin, Rhamphocottus richardsoni, that have modified their ray fins into separate “fingerlike” features that allow them to creep underwater along the sea bottom in a motion that resembles a spider or a lobster. However, these “fin fingers” are not robust or muscular or as flexible as tetrapod fingers, so they cannot manipulate objects with them. They are jury-rigged features built out of another structure (ray fins) and suboptimally modified to be “semifingers” (yet another blow to “intelligent design”). The frogfish, Antennarius, uses its fins with the fingerlike fin rays to walk along the bottom in a motion very similar to that of tetrapods (fig. 10.4C).
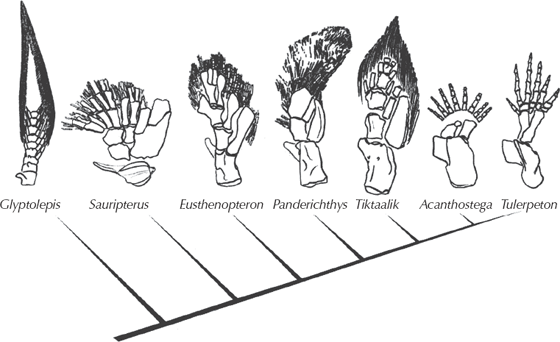
When it comes to deciphering how lobe fins crawled out on land, we have some remarkable fossils that demonstrate some of the steps—although preservation of soft features like the nature of their skin and gills or lungs cannot be demonstrated from the bony skeleton. The paraphyletic rhipidistians of the Devonian include a variety of peculiar fish with robust lobed fins such as Eusthenopteron (fig. 10.5). Although rhipidistians are fishlike with an aquatic body and multiple fins, the lobe fins have key elements that are homologous, bone for bone, with those in the tetrapod limb. For example, in the pectoral fin, the robust element closest to the body looks very much like the upper arm bone, or humerus, of primitive tetrapods. At the far end of this bone are a pair of bones, which are homologous (and look much like) the radius and ulna of the lower arm in tetrapods. Beyond those bones are a series of smaller rodlike bones that are homologous with the wrist and finger elements. The fin is then surrounded by a series of rays that support the fin membrane itself. If you look at the pelvic fin, the homologies with the thigh bone (femur), shinbones (tibia and fibula), and ankle and foot bones (tarsals and metatarsals) are equally apparent.

FIGURE 10.5. The evolutionary modifications of the skull, limbs, and the rest of the skeleton from a rhipidistian like Eusthenopteron to a tetrapod like Ichythyostega. (Drawing by Carl Buell).
The similarities don’t stop there. The detailed bone-for-bone structure of the spine of Eusthenopteron is a dead ringer for that in primitive tetrapods and totally unlike that in any other group of fish. The detailed patterns of the bones in the skull are also bone-for-bone identical with those in primitive tetrapods; only the relative proportions change in tetrapods, which greatly reduces the bones covering the gills and expands the bones in the front of the snout (fig. 10.5). The lungs or gills themselves don’t fossilize, of course, but it’s reasonable to assume that Eusthenopteron already had lungs because its primitive sister group (the lungfish) and advanced sister taxa (the tetrapods) do. In short, you could not ask for a better fishibian than Eusthenopteron. If you study it in detail with the eyes of anatomist (instead of reading superficially about it, as creationists do), you can see all the elements of the tetrapods already in place. Given how easily many modern fish now walk on land, it’s not hard to imagine Eusthenopteron doing so as well.
The next step is a series of remarkable fossils that demonstrate the step-by-step transition to becoming a tetrapod (figs. 10.5–10.8). These include a variety of more tetrapod-like fish (Panderichthys, Elginerpeton, Ventastega, and Metaxygnathus) known from only partial specimens from the Late Devonian. Panderichthys (figs. 10.6, 10.7, and 10.10) was a very tetrapod-like lobe-finned fish. Unlike Eusthenopteron, these creatures had flattened bodies and upward-facing eyes, and frontal bones, like tetrapods, and a straight tails with a well-developed tail fin. The braincase of Panderichthys was originally classified as belonging to a tetrapod, not a fish, until the rest of the body was found. The teeth have the characteristic enfolding of the enamel (“labyrinthodont” teeth) that characterizes the teeth of later tetrapods. Panderichthys had both gills and well-developed lungs with nostrils, so it could breathe either way. Most importantly, like tetrapods, Panderichthys has lost the dorsal fins and anal fins, leaving only the remarkably footlike lobed pectoral and pelvic fins. This is a classic fishibian: tetrapod-like skull and body and braincase and lungs, all the rest of the fishy fins lost, but still retaining true fins that become the hands and feet.

FIGURE 10.6. Phylogeny of the transitional series from rhipidistians through primitive tetrapods. (Drawing by Carl Buell)
FIGURE 10.7. The transformation of the pectoral fin of lobe fins into the hand and forelimb of primitive tetrapods. Each bone of the lobed fin is homologous with one of the limb bones of the tetrapod, and the main difference is modifications in shape and robustness and the loss of the fin rays, which are replaced by fingers. (From Shubin et al. 2006; fig. 4; by permission of the Nature Publishing Group)
FIGURE 10.8. Sketch of the skeletons of Acanthostega (top) and Ichthyostega (bottom), showing the mixture of fishlike features (tail fins, lateral line systems, gill slits) and tetrapod features (robust limbs and shoulder and hip bones, reduced back of skull, expansion of snout). (Drawing courtesy M. Coates)
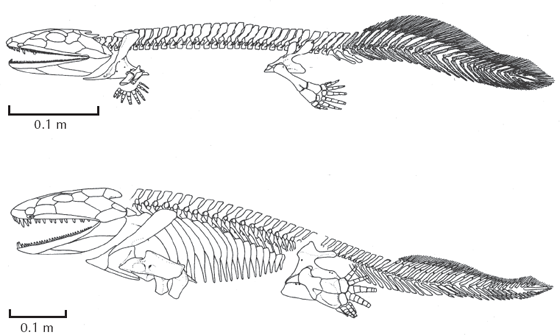
Another important recent discovery is several nearly complete skeletons of Acanthostega (figs. 10.6–10.8) by Jenny Clack, Michael Coates, Per Ahlberg, and others. Acanthostega still had a well-developed fin on the tail, large gill openings, and even gills preserved on the inside of the skeleton! It had an ear region adapted for hearing in water, not on land. Yet it also had modified the lobed fin into a tetrapod limb with as many as seven or eight fingers, although the hand and foot were not well adapted for walking, but much better suited for swimming or creeping along the bottom. This is also shown by the proportions of its limbs, which are more like those of Eusthenopteron than of tetrapods, indicating that its limbs were not very good for land locomotion. Acanthostega also had the robust vertebrae in the spine that could support its weight out of water. It’s a perfect fishibian: gills, fins, ears like a fish, but spine and limbs like a tetrapod. Clack and Coates have convincingly shown that it probably spent most of its time in the water, even though it has the limbs capable of some land locomotion. Like the teleost fish mentioned earlier, it only needed to crawl up on land once in a while, but most of its life was probably aquatic. For that matter, most living salamanders and newts are almost entirely aquatic and use their limbs primarily to swim and push along through the vegetation underwater.
The clinching piece of evidence was announced just over a decade ago (Daeschler et al. 2006; Shubin et al. 2006). Nicknamed the “Fishapod” but formally named Tiktaalik, this Late Devonian fossil from Ellesmere Island in the Canadian Arctic (fig. 10.9) was more fishlike than Ichthyostega or Acanthostega, yet its limbs show the perfect transition between fins and feet (fig. 10.7). It had fishlike scales, lower jaw, fin rays, and palate, but unlike any fish, it had a shortened skull roof and mobile neck (for whipping the head sideways to catch prey), an ear region capable of hearing in both land and water, and a wrist joint that anticipates the condition seen in terrestrial tetrapods. Thanks to this discovery, we now have a beautiful transitional sequence from fully aquatic lobe-finned fish like Eusthenopteron to more amphibian-like forms such as Panderichthys and Tiktaalik to fully four-legged forms like Acanthostega and Ichthyostega (which still retain fishlike gills, tail fins, and lateral line systems on the face). This sequence is now so smoothly gradational that it’s hard to tell where the fishes end and the amphibians begin—yet is it clear even to a creationist that Eusthenopteron is a fish and Ichthyostega is an amphibian.

FIGURE 10.9. The newly discovered “fishibian” Tiktaalik. (A) Photograph of the nearly complete articulated skeleton. (B) Reconstruction of what the animal looked like in life next to the specimen. (Photos courtesy N. Shubin, University of Chicago, and T. Daeschler, Academy of Natural Sciences, Philadelphia)
The discovery that early tetrapods had seven or eight fingers came as shock at first. We are so accustomed to the fact that all living tetrapods have only five fingers and toes (or fewer) that we assumed that they always had that number. Early reconstructions of the poorly known hand of Ichthyostega often drew in a five-fingered hand, even though there was no evidence one way or another. But in other ways, this large number of toes is not so mysterious. My fellow Columbia student, good friend, and coauthor Neil Shubin at the University of Chicago worked on this problem for his dissertation while he was a graduate student at Harvard. People had always assumed that fingers formed from the branching of the fin rays in lobe fins, symmetrically down the central axis. But Neil studied the embryological development of salamander limbs (Shubin and Alberch 1986) and found that fingers bud off in an arch, from one side of the hand to the other (not symmetrically down the middle axis). This beautifully matches the hand in Acanthostega because it apparently had extra fingers (just like extra fin rays) that budded off from this embryonic development pattern. To get the modern pattern of five fingers, the development has to shut down just a bit earlier. Since this discovery, the Hox genes that control this developmental sequence have been identified, so it is clear that changing a limb from seven or eight fingers to just five is not a big problem. It just requires a small adjustment in the developmental timing of limb budding.
Finally, we come to Ichthyostega (figs. 10.6, 10.7, and 10.8), the classic transitional form, found in Upper Devonian rocks of Greenland in the 1930s by Danish and Swedish expeditions. It was described briefly by Säve-Söderbergh in 1932, but not fully monographed in detail until 1996 by Erik Jarvik. This creature is very similar to Acanthostega, only not as completely preserved. It is also slightly more advanced than Acanthostega in the direction of tetrapods, with a smaller tail fin and slightly longer more tetrapod-like limbs. However, it still retains a lot of fishy characteristics, such as the tail fin, the bones covering the gill slits (opercular bones), and especially the series of canals on the face (lateral line canals) for sensing movement and electrical currents under water. This lateral line system is a feature found in most sharks, teleosts, and many other aquatic animals. Once again, we have a classic fishibian: limbs and spine like a tetrapod, but tail fin, gills, and lateral line canals like a fish.
Creationists, of course, cannot admit that this animal is a true transitional form, so they stoop to all sorts of dishonest arguments to deny its fishibian features. Gish (1978; he learned nothing new in his 1995 edition) is typical of the bunch. Ichthyostega had limbs and feet, so it must be an amphibian, and then Gish goes on to quote outdated ideas about it. Gish (1978, 1995) keeps claiming that there are no fossils that document the transition from fins to feet, but now with Panderichthys, Tiktaalik, and Acanthostega, we do have fossils that had seven or eight fingers or toes on their limbs, which are clearly still used as fins, not as hands or feet. He never mentions the gill covers, the lateral line system, or the tail fin, aquatic features of Ichthyostega that are not found in most tetrapods. This is a clear case of selective citation of evidence and deliberately misleading argumentation. Either Gish can’t read the description of the fossil well enough to understand that it has features of both fish and tetrapods, or he is deliberately trying to fool the reader by denying the obvious and well-documented fishlike features of these fossils. Either way, it is extremely poor science and very dishonest.
The creationist arguments usually stop right there: mislead the reader into thinking that Ichthyostega is “just” an amphibian and don’t mention all its fishy features, and then move on to another topic. Well, that argument is finished for good. The amazing array of new transitional fossils (fig. 10.6) documents the transition in such detail that the creationists can’t dodge behind trying to just discredit Ichthyostega anymore. The discovery of Acanthostega and Tiktaalik vastly improves our knowledge of the origin of tetrapods and shows that the earliest tetrapods used their legs not for walking on land but primarily for walking under the water! All of the old arguments about tetrapods needing robust limbs to crawl out to another pool or chase new prey are completely obsolete now that we know that walking underwater was the primary function of the limbs of Ichthyostega and Acanthostega. And all of their fishy features, such as the ear region, lateral line canals, and tail fins now make sense if we think of them as comparable to modern newts and salamanders that only rarely crawl out on land. As we saw from all the walking teleost fish, crawling out of the water is not such a feat after all, especially if you spend most of your time in the water. For that matter, most amphibians spend most if not all their time in the water, so they haven’t made as great an evolutionary leap as the old dogmas once suggested.
How do the ID creationists deal with this extraordinary evidence? They cloud the issue by denying these fossils exist, or by distortion and misstatements. Davis and Kenyon (2004:103, figs. 4–8) show a 55-year-old sketch of Ichthyostega and Eusthenopteron but make no mention of all the other transitional fossils that were well documented before their book was published in 2004. They show (figs. 4–9, p. 104) the fin and limb bones of each of these creatures but ignore all the beautiful transitional fossils that have been documented in the past 50 years. They make a big deal about how dramatic this transition was, yet falsely claim that “no such transitional species have been recovered.” Thanks to Panderichthys and Acanthostega, and now Tiktaalik, that falsehood can be safely laid to rest—but I have no expectation that creationist books will ever acknowledge the existence of these fossils. I’m sure they will simply replay their discredited and outdated arguments.
From the primitive forms like Ichthyostega and Acanthostega, the tetrapods began a great radiation of more advanced terrestrial forms in the Carboniferous. Some, like Greererpeton (figs. 10.6 and 10.10) had long fishy bodies not much different from those of Acanthostega. Their limbs and shoulder bones were considerably more advanced and terrestrial than those of Acanthostega, yet they still had fishlike features, such as the lateral line canals. By the mid-Carboniferous, we find that tetrapods have branched into many different lineages, including the big flat-bodied flat-skulled temnospondyls (fig. 10.1), the more delicate lepospondyls (some of which became legless and converged on snakes and apodans), and the anthracosaur lineage that leads to amniotes. And, by the middle Carboniferous, we find the first true amniotes as well. We will discuss them in the next chapter.
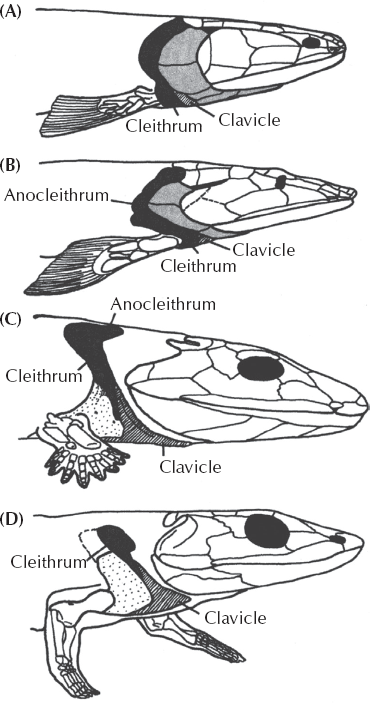
FIGURE 10.10. Drawing of the evolutionary transition of the bones from the skull, shoulder girdle, and forelimb, from fully aquatic rhipidistians like (A) Eusthenopteron to the slightly more tetrapod-like (B) Panderichthys to the more advanced (C) Acanthostega and concluding with a fully terrestrial tetrapod, (D) Greererpeton. The fishlike elements of the shoulder girdle, such as the cleithrum and anocleithrum, are gradually reduced, while the clavicles (our “collarbones”) become the largest bone in the shoulder girdle. Meanwhile, the major bones covering the gill slits (shaded here in gray) are reduced and then lost, the eyes shift backward as the snout expands, and the cheek regions are reduced. (From Clack 2002: fig. 6.4; used with permission)
Creationists frequently taunt scientists by pointing to an image of something as specialized as a frog and saying that there is no way they could imagine a transitional fossil between frogs and other amphibians. But in 2008, a fossil was announced that put this question to rest (Anderson et al. 2008). Formally named Gerobatrachus hottoni, it was dubbed “frogamander” by the press because it had features of both frogs and salamanders (fig. 10.11). It had a long tail and salamander-like body, but its head is short with a rounded snout like that of a frog. It also had the large eyes and large eardrum found in frogs and not salamanders. Most importantly, its teeth are attached to the jaw on tiny pedestals with a distinct base, a feature that defines the modern frogs and amphibians as a natural group.
FIGURE 10.11. (A) The only specimen of Gerobatrachus hottoni. (Courtesy Diane Scott and Jason Anderson) (B) A reconstruction of it in life. (Courtesy Nobumichi Tamura)
There are also fossil frogs from the Triassic such as Triadobatrachus. They looked a bit more like living frogs but do not yet have the shortened trunk, reduced number of vertebrae, long hipbones and extremely long jumping hind legs that living frogs do. In short, the transition from a primitive amphibian to a modern frog has now been completely filled by transitional fossils.
So much new information has now been discovered that I highly recommend reading the books by Zimmer (1998) and Clack (2002) to get the full story. Other useful references are also mentioned (not all of which were published before the new discoveries).
Anderson, J. S., R. R. Reisz, D. Scott, N. B. Fröbisch, and S. S. Sumida. 2008. A stem batrachian from the Early Permian of Texas and the origin of frogs and salamanders. Nature 453:515–518.
Benton, M. J. 2014. Vertebrate Palaeontology. 4th ed. New York: Wiley-Blackwell.
Carroll, R. L. 1988. Vertebrate Paleontology and Evolution. New York: Freeman.
Clack, J. A. 2002. Gaining Ground: The Origin and Early Evolution of Tetrapods. Bloomington: Indiana University Press.
Daeschler, E. B., N. H. Shubin, and F. A. Jenkins Jr. 2006. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440:757–773.
Long, J. A. 2010. The Rise of Fishes, 2nd ed. Baltimore, Md.: Johns Hopkins University Press.
Maisey, J. G. 1996. Discovering Fossil Fishes. New York: Holt.
Moy-Thomas, J., and R. S. Miles. 1971. Palaeozoic Fishes. Philadelphia: Saunders.
Prothero, D. R. 2013. Bringing Fossils to Life: An Introduction to Paleobiology, 3rd ed. New York: Columbia University Press.
Shubin, N. H., E. B. Daeschler, and F. A. Jenkins Jr. 2006. The pectoral fin of Tiktaalik roseae and the origins of the tetrapod limb. Nature 440:764–771.
Standen, E. M., T. Y. Du, and H. C. E. Larsson. 2014. Developmental plasticity and the origin of tetrapods. Nature 513: 54–58.
Thomson, K. S. 1991. Living Fossil. New York: Norton.
Weinberg, S. 2000. A Fish Caught in Time: The Search for the Coelacanth. New York: HarperCollins.
Zimmer, C. 1998. At the Water’s Edge: Macroevolution and the Transformation of Life. New York: Free Press.