Of all the great transitions between major structural grades within vertebrates, the transition from basal amniotes to basal mammals is represented by the most complete and continuous fossil record, extending from the Middle Pennsylvanian to the Late Triassic and spanning some 75 to 100 million years.
—James Hopson, “Synapsid Evolution and the Radiation of Non-eutherian Mammals”
Of all the transitional series that we have examined between major groups of vertebrates, one of the best documented is the transition from primitive amniotes to mammals via the synapsids, formerly known as the “mammal-like reptiles.” As we explained previously (fig. 11.1), however, the synapsids that evolve into mammals are not reptiles and never had anything to do with the lineage that leads to reptiles. Both the earliest true reptiles (Westlothiana from the Early Carboniferous—fig. 11.4) and the earliest synapsids (Protoclepsydrops from the Early Carboniferous and Archaeothyris form the Middle Carboniferous) are equally ancient, demonstrating that their lineages diverged at the beginning of the Carboniferous. Older pre-cladistic interpretations had synapsids evolving from a paraphyletic wastebasket of primitive amniotes known as the “anaspid reptiles.” This idea is now completely discredited, and anyone who still uses the obsolete and misleading term “mammal-like reptile” clearly doesn’t know much about the current understanding of vertebrate evolution.
Focusing on the lineage of synapsids, we can put together an almost continuous series of well-preserved fossils (figs. 13.1, 13.2, 13.3, and 13.4) that span the Carboniferous and Permian and straggle into the Triassic before most lineages died out (possibly in competition from the newly emergent dinosaurs), and the remaining lineages gave rise to true mammals. Each taxon along the way shows a mosaic of mammalian characters, with some advanced features appearing early in the series, while others appeared quite late. The most primitive group is the paraphyletic wastebasket known as “pelycosaurs” (fig. 13.2A and B), which includes not only the oldest and most primitive taxa (such as Protoclepsydrops and Archaeothyris) but also the spectacular “finbacks” Dimetrodon and Edaphosaurus, which were among the largest land animals of the Early Permian (fig. 13.2B). Although the earliest forms are almost indistinguishable from the earliest true reptiles in most features, creatures such as Protoclepsydrops and Archaeothyris still show a number of unique synapsid specializations, including a hole in the side of the skull (temporal opening) beneath the postorbital and squamosal bones, the beginnings of true canine-like teeth, and a number of other subtle features in the skull and palate. Even more advanced “pelycosaurs” like Dimetrodon are still primitive in most features but clearly have the lower temporal opening in the skull and the large canines in the front of the mouth.

FIGURE 13.1. The transformation from primitive synapsids like Ophiacodon and the fin-backed Dimetrodon to the predatory gorgonopsians to the weasel-like Thrinaxodon and finally to true mammals is one of the best transitional series in the entire fossil record. (Drawing by Carl Buell)
FIGURE 13.2. Skeletons of the various transitional fossils among the synapsids. (A) The very primitive early Permian “pelycosaur” Ophiacodon. (B) The finbacked pelycosaur Dimetrodon. (C) The predatory gorgonopsian Lycaenops, with its large wolf-like skull, big canines, and more upright posture. (D) The highly mammal-like cynodont Thrinaxodon, which was the size of weasel. (Photos courtesy R. Rothman)
FIGURE 13.3. The evolution of the synapsid skulls from primitive pelycosaurs through therapsids and cynodonts to true mammals. (From Kardong 1995; reproduced by permission of the McGraw-Hill Companies)
FIGURE 13.4. The transformation of the synapsid skeleton from primitive pelycosaurs like Dimetrodon through cynodonts to true mammals. (Drawing by Carl Buell).
As we move up the cladogram through the diverse groups of synapsids (fig. 13.3), more and more mammalian characters appear in a piecemeal fashion. The next grade up from the pelycosaurs is the “therapsids,” which dominated the Late Permian landscape and evolved into a number of large wolf-sized creatures with big saber-toothed canines (fig. 13.2C), as well as lineages of huge herbivorous synapsids, some with beaks and almost no teeth, and others with thickened skulls and ugly bony knobs on their faces for display and head-to-head butting. In short, these therapsids dominated many different niches in the Late Permian terrestrial ecosystem. They were also considerably more advanced than typical pelycosaurs like Dimetrodon (fig. 13.2B). The temporal opening on the side of the skull was now much larger, presumably for expansion of jaw muscles, therefore enabling them to have a stronger bite force and even some chewing motion. If you look at the palate of the skull, there is the beginning of a secondary palate, a roof of bone that grows out from the edge of the upper jaws and encloses the original reptilian palate in a tube. This enables advanced synapsids and mammals to breathe and eat at the same time, something that reptiles (except crocodilians, which independently evolved a secondary palate) cannot do. Reptiles, with their slow metabolism, can hold their breath for a long time while they swallow a large prey item, but the presence of a secondary palate shows that synapsids must have been developing an active, “warm-blooded” metabolism and needed to process their food quickly to survive. In addition, the old single ball joint that hinged the skull to the first neck vertebra (known as the occipital condyle) is now split into a double ball joint, presumably for more flexibility in moving the head.
In therapsids, the canines are much larger, and some of the rest of the teeth are now more specialized as well, with serrated edges like steak knives. There are also striking differences in the limbs (fig. 13.4), with a much stronger more flexible shoulder girdle and an increased number of lower back vertebrae fused to the hip bones. Finally, they no longer have the sprawling limb posture seen in primitive synapsids but instead held their limbs vertically and walked fully upright. Consequently, there are many subtle changes in the shape and musculature of all the limb bones, and the finger bones are much shorter because these animals no longer sprawled and walked flat-footed with toes splayed out like a lizard’s but instead began to walk on the tips of their toes like most mammals do.
The next grade up is a group called the “cynodonts,” which originated in the late Permian, survived the greatest mass extinction event in earth history at the end of the Permian, and then dominated the world of the Early Triassic. Cynodonts (the name means “dog tooth” in Greek) are very much like mammals in lots of ways (figs. 13.2D and 13.3). The temporal opening at the back of the skull is even more enlarged, allowing for several jaw muscles to develop, which in turn made it possible for cynodont jaws to chew as well as bite. The canines were large, and the postcanine teeth (premolars and molars) are now specialized and multicusped for chewing, not the simple conical piercing teeth of reptiles and primitive synapsids. The secondary palate was now almost fully developed, with the internal air passage opening not in the mouth (like in primitive synapsids) but in the back of the throat (as in mammals). Instead of sprawling, the limbs (fig. 13.4) are even more upright and specialized for rapid running, with a lightweight shoulder girdle and a reduced set of hip bones (except for the expansion of the iliac blade of the hip along the spine). In the heel, the ankle bone known as the calcaneum develops a long extension for the attachment of the Achilles tendon, a sign of much more efficient and faster running. The tail is shorter, too. The rib cage disappears from the lower back, and in some specimens, the ribs are locked together with flanges of bone. This suggests that they did not breathe by expanding the ribs in and out (like reptiles do) but instead had a solid rib cage and used a muscular wall between the lung cavity and abdominal cavity, the diaphragm, to pump air in and out of the lungs. Some advanced cynodonts, such as Thrinaxodon (fig. 13.2D) actually have small pits on their snouts that suggest the presence of whiskers. (Hair does not normally fossilize, so it is hard to know whether any given fossil had hair or not.) If so, then cynodonts probably had hair elsewhere on their bodies as well, and hair probably appeared early in synapsid evolution with the evolution of the smaller therapsids.
The most advanced cynodonts, such as the tritylodonts and the trithelodonts of the Early Triassic (fig. 13.3), were small weasel-like forms with highly specialized skulls, large temporal openings and several sets of jaw muscles, highly specialized molars and premolars, and very doglike skeletons with almost all the typical mammalian features. In these and many other features, they are so mammal-like that they have often been called mammals. Indeed, the transition from the most primitive synapsids all the way to mammals is so smooth that it is rather arbitrary where to break the continuous sequence and begin calling advanced synapsids mammals. However, most paleontologists agree that creatures like Adelobasileus, Sinoconodon, Megazostrodon (fig. 13.4), and Morganucodon, from the Late Triassic of New Mexico, China, and South Africa, are bona fide mammals, because they are dramatically smaller in body size (rat-sized or smaller), had lost the ribs from their neck vertebrae, had reduced the reptilian elements in their shoulder girdle (clavicles, interclavicles, and coracoids), and had reduced the ilium portion of the hip bones to a simple rod running along the spine. Most importantly, they had a jaw joint between the dentary bone of the jaw and the squamosal bone of the skull, the defining character of Mammalia.
Through this entire gradual transition is an even more amazing story in the jaws and ears of these animals (fig. 13.5). Early synapsids like Dimetrodon had a classic primitive amniote jaw. The front part is composed of the dentary bone (which contains the teeth) but behind it were many other bones: the coronoid bone where the jaw muscles pulled up on the jaw, the articular bone in the jaw hinge, the angular bone in the lower back part (angle) of the jaw, and several other accessory jawbones. The articular bone of the lower jaw hinged against the quadrate bone of the amniote skull, and both were abutting against the middle ear and the “stirrup” bone (the stapes), helping transmit sound from the lower jaw into the ear. But as we go through the series of more and more advanced synapsids, we see some remarkable changes in the jaw. All of the nondentary components (shaded bones in right column of figure 13.5) get smaller and smaller, until by the time we see advanced cynodonts, most of these nondentary bones are just tiny splints in the inside back of the jaw, and the dentary bones have expanded to become almost the entire jaw. The probable reason for this transformation is that a single bone (the dentary) is much stronger than a series of bones sutured together, and as synapsids became more active in chewing, they needed a jaw that could handle all the stresses. A portion of the dentary, known as the coronoid process, expanded upward and took over the attachment points of the temporalis muscles and replaced the primitive amniote coronoid bone. Likewise, the primitive articular jaw joint with the quadrate bone of the skull also became highly reduced. Meanwhile, a portion of the dentary bone expanded upward, meeting the skull in the squamosal bone region and initiating a new jaw joint. Eventually, the dentary/squamosal jaw joint will take over completely, and the primitive articular/quadrate jaw joint will be superseded.

FIGURE 13.5. The gradual transformation of the jawbones within the synapsids, as all the nondentary jawbones (shaded bones: angular, surangular, articular, coronoid, splenials, and so on) are gradually reduced to tiny splints in the inside back part of the jaw, and the dentary bone (unshaded bone) takes over as the principal jawbone. Eventually, all the nondentary jaw elements are lost in mammals, except for the articular bone, which becomes the “hammer” (malleus) bone of the middle ear. (Drawing by Carl Buell)
There is even one remarkable fossil, a trithelodont known as Diarthrognathus (fig. 13.6), which shows how this transition took place. Its name means “two jaw joint,” and indeed that’s what it has: both the old amniote articular/quadrate jaw joint still attached on both sides of the skull alongside the new dentary/squamosal jaw joint. We could not have asked for a more perfect transitional fossil, which has been caught in the act of making the transition from one set of jaw joints to the other. Eventually, the articular/quadrate jaw joint gets smaller and smaller, and no longer serves as a jaw joint, as the dentary and squamosal take over completely.
FIGURE 13.6. Diarthrognathus actually has the old articular/quadrate jaw joint of primitive synapsids in operation side by side with the mammalian dentary/squamosal jaw joint on both sides of the skull. (Drawing by McLoughlin 1980; Viking, New York; used with permission)
So what happened to the quadrate and articular? They could have vanished completely, as did most of the nondentary bones of the lower jaw. But remember the point we discussed earlier? Primitive amniotes hear with their lower jaws, and the sound is transmitted from the jaw joint into the middle ear. For example, snakes cannot hear when they rear up to face the snake charmer because their jaw is not in contact with the ground and so cannot pick up vibrations. They are responding to the body motions of the snake charmer, but the sound of the flute is just for the tourists—the snake cannot hear a thing. Sure enough, once the quadrate and articular became tiny and disconnected from the jaw joint function, they did not vanish—they are in your middle ear right now (fig. 13.7)! The quadrate bone turned into the incus or “anvil” bone, which transmits sound to the stirrup or stapes. The articular bone turned into the malleus or “hammer” bone, which transmits sound from the eardrum to the incus. So as you listen to sounds, they are being transmitted through bones that started out as part of the jaw and skull articulation. If that story seems too incredible, just think of this: when you were an embryo, your ear bones were represented by cartilage in the lower jaw and skull, and through embryonic development they shift until they reached the middle ear, retracing the path they took during evolution!

FIGURE 13.7. The ear region also undergoes a dramatic transformation, as the articular bone of the lower jaw hinge and the quadrate bone of the jaw hinge in the skull shift to the middle ear and become the incus and the malleus (“anvil” and “hammer”). This same transformation can be seen not only in fossils but also during the embryology of a mammal. When you were an embryo, your middle ear bones started out in your jaw.
The most amazing clinching fossil, however, is Yanoconodon, described by my friend Luo Zhexi and his colleagues (Luo et al. 2007) from the Lower Cretaceous Yixian Formation of Hebei Province. It is equivalent in age to the beds in the Liaoning Province that produced all the birds described in the last chapter. It is a beautiful complete specimen (fig. 13.8) with all the bones articulated in a death pose as it died and was preserved in the delicate lake sediments. The most amazing thing about the specimen is that the middle ear bones are still connected to the lower jaw! This animal could hear with its quadrate/articular (incus/malleus) like any other mammal, yet the bones had still not migrated to the middle ear!

FIGURE 13.8. The primitive triconodont mammal Yanoconodon allini from the Lower Cretaceous of China, which still retains its ear bones attached to its lower jaw. (A) Photograph of the original articulated specimen. (B and C) Sketch of the skeleton, labeling the bones, and a reconstruction of the skeleton. (D) Detailed diagrams of the lower jaw, showing the ring of ear bones still attached to the lower jaw while functioning for hearing. Specimen (a) is the jaw of the primitive Triassic mammal Morganucodon; (b) is Yanoconodon, with a detail of the ear bones shown in (c) Diagram (d) is the lower jaw of the triconodont Repenomamus. For further details, see Luo et al. (2007:288–293). (Courtesy Zhexi Luo/Carnegie Museum of Natural History)
All of this amazing evidence, of course, is hard for creationists to stomach. Some, such as Duane Gish, tried to ridicule the whole idea by joking about these creatures “chewing and hearing while rearticulating their jaws.” He never explained to his audience how most reptiles indeed hear with their lower jaw, or that we have fossils like Diarthrognathus with both jaw articulations operating simultaneously, or that human ear bones were originally in their jaws during their early embryonic stages. Gish (1995:147–173) attempted to discredit this beautiful evolutionary sequence of synapsids by his usual method: quoting people out of context or quoting outdated sources that do not reflect what we know now. He mines Tom Kemp’s (1982) 35-year-old book for quotes that seem to say there was no transitional sequence in synapsids—but if you read the quotes closely, what Kemp is saying is that we don’t have many gradual transformations between each of the synapsid genera (most of which are known only from a few fossils). But that does not mean that we can’t line up the genera (as we did here) and produce a beautiful evolutionary sequence among the genera (fig. 13.3). In other cases, Gish claimed that there is a black hole of missing fossils in the Mesozoic—but that gap has long ago been filled by some amazing fossils. Most of the rest of Gish’s criticisms reveal that he had absolutely no firsthand knowledge of these fossils or their anatomy but simply mines other people’s work for quotes that seem to support his biases and then pulls them out of context so they seem to say something that the author never intended. Once again, this is dishonest and unscientific. If Gish had been really interested in the truth, he would have done his homework, learned some anatomy and paleontology, and studied the fossils for himself—and he would have found one of the best macroevolutionary transitions between two major groups of animals ever documented.
How do the intelligent design creationists handle this extraordinary transitional series? Wells (2000) doesn’t address it, nor does Sarfati (1999, 2002). Davis and Kenyon (2004:100–101) quote a few evolutionists out of context and even concede, “Without a doubt, the Therapsids are highly suggestive of a Darwinian lineage.” But then they betray their complete lack of understanding of evolution and try to discount the entire example by arguing that it is not a single ancestral lineage but many different lineages. That is exactly how most evolutionary transitions work in a bushy, branching system—not as “missing links” on a nonexistent “chain of being” (the common creationist misunderstanding) but as multiple, closely related lineages that each show progressively more mammalian characteristics.
With malleus Aforethought
Mammals
Got an earful
Of their ancestors’ Jaws
—John Burns, Biograffiti
When some people think of life during the Mesozoic, or “age of dinosaurs,” they assume that the dinosaurs dominated the planet, and mammals had not yet evolved. In fact, the earliest mammals evolved from cynodonts in the Late Triassic at exactly the same time as the early dinosaurs (which may have outcompeted the last of the larger synapsids that once ruled the Triassic). But while dinosaurs soon came to dominate the globe for the next 130 million years, mammals remained small and inconspicuous, seldom getting much larger than the size of a house cat. Most mammals apparently lived in the nooks and crannies of the world of the “terrible lizards,” hiding in the vegetation, and probably coming out mainly at night. In fact, the first two-thirds of mammalian history was the story of these tiny Mesozoic mammals. Only after the nonavian dinosaurs vanished at the end of the Cretaceous did the world open up for mammals so that they could dominate the planet.
For over a century, very little was known about Mesozoic mammals. Because they were so tiny and delicate, the best we could find were tiny pinhead-sized teeth and partial jaws from animals the size of shrews. None were known from almost any other part of the skeleton. When I first began to work on Jurassic mammals for my master’s thesis in 1977, this state of affairs was still true after a century. I had access to a new collection of jaws and teeth from the famous Upper Jurassic Morrison Formation dinosaur quarries at Como Bluff, Wyoming. This allowed me to survey all the early Mesozoic mammals known at the time and conduct the first cladistic analysis of all these poorly understood species. I did several different papers on that research and published them. One result was that I was able to show that the long-abused paraphyletic group “Pantotheria” was a hopeless wastebasket and needed to be abandoned. Indeed, most paleontologists since then have stopped using this obsolete concept. Around the campfire in the long field season of 1977, my graduate advisor Malcolm McKenna, my fellow graduate students, and I daydreamed about what it would be like if we had skulls or even partial skeletons of these critters, instead of tiny, frustratingly incomplete teeth and jaws. For a century before us, everyone else who worked on Mesozoic mammals must have felt the same way. But they did the best they could with what they had.
I moved on from Mesozoic mammals shortly thereafter because there were no more new specimens to study at the time. I also preferred working on larger and more easily studied mammals like camels, horses, and rhinos, which do not require a microscope to see or photograph. Since then, there has been a veritable explosion of new Mesozoic mammal fossils. Not only do we have many more new species based on jaws and teeth, but there have been some extraordinary finds that have decent skulls (fig. 13.9), and in a few cases, even articulated skeletons for many of the different groups. What these specimens show is that most Mesozoic mammals were small, insectivorous creatures, living much like the modern shrew in most of their habits. A few were slightly larger in body size, and one specimen from the Cretaceous of China, known as Repenomamus, was over a meter long and actually has a baby psittacosaur dinosaur in its stomach. Generally speaking, however, it appears that mammals avoided the dinosaurs and were not in any position to compete with them, let alone eat them.

FIGURE 13.9. The skulls of some of the better known Mesozoic mammals. (A) Early Jurassic Sinoconodon. (B) Early Jurassic Morganucodon. (C) Early Cretaceous Vincelestes. (D) The Paleocene multituberculate Ptilodus, representative of the long-lived Mesozoic radiation of multituberculates. Abbreviations: sq-den jt, squamosal-dentary joint; ref lam, reflected lamina; art, articular bone; m1, first lower molar; p4, fourth lower premolar. (From Hopson 1994: fig. 9; courtesy J. Hopson)
The most amazing specimens of Mesozoic mammals come from the same Lower Cretaceous Liaoning lake beds that yielded the many feathered dinosaurs and early birds discussed in the previous chapter. These include a complete specimen of the oldest known marsupial, Sinodelphys szalayi (fig. 13.10), which preserves not only the bones in articulation but even the impressions of the fur and soft tissues. This fossil shows that marsupials (the pouched mammals, which today include opossums, kangaroos, and koalas) had already split off from the main mammalian stem at 120 million years ago, and opossum-like marsupial teeth are known from most of the Cretaceous. The Liaoning beds also yield one of the oldest known placental mammals, Eomaia scansoria, and the fossil also preserves the hair and soft tissues (fig. 13.11). An even earlier placental fossil is Juramaia, from the Jurassic of China. Thus the split between marsupials and placentals (the mammals that give live birth to their young, including most living mammals) occurred much earlier in the Cretaceous than we thought just a few years ago. By the Late Cretaceous, both marsupials and placentals were evolving very fast, and most of the archaic groups of Mesozoic mammals were gone.


FIGURE 13.10. The beautifully preserved complete fossil of the oldest known marsupial, Sinodelphys szalayi, from the Lower Cretaceous of China. (A) The complete skeleton of the type specimen. (B) Artistic reconstruction by Carl Buell of the appearance of Sinodelphys. See Luo et al. (2003: 1934–1940). (Courtesy Zhexi Luo/Carnegie Museum of Natural History)
FIGURE 13.11. The beautifully preserved fossil (complete with hair) of one of the oldest known placental mammals, Eomaia scansoria, from the Lower Cretaceous of China. (A) Photograph of the type specimen. (B) Sketch of the skeleton and reconstruction of the skeleton as it appeared in life. See Ji et al. (2002:816–822). (Courtesy Zhexi Luo/Carnegie Museum of Natural History)
The placental or eutherian mammals comprise about twenty living orders and several extinct ones. The morphological and adaptive range of this group is extraordinary; diversification has produced lineages as varied as humans and their primate relatives, flying bats, swimming whales, ant-eating anteaters, pangolins, and aardvarks, a baroque extravagance of horned, antlered and trunk-nosed herbivores (ungulates), as well as the supremely diverse rats, mice, beaver and porcupines of the order Rodentia. Such adaptive diversity, and the emergence of thousands of living and fossil species, apparently resulted from a radiation beginning in the late Mesozoic between 65 and 80 million years ago. This explosive radiation is one of the more intriguing chapters in vertebrate history.
—Michael J. Novacek, “The Radiation of Placental Mammals”
At the end of the Cretaceous 66 million years ago, the nonavian dinosaurs vanished from the planet. One group of scientists argue that the impact of a big rock from space did them in, while another group points out that there were too many survivors who could not have outlived such an extreme catastrophe. Instead, they suggest that the extinction was due to more gradual changes, and this is supported by evidence of the fossil record (for a review of the topic, see Prothero 2016). Whatever the cause, by the early Paleocene, the terrestrial realm was devoid of large animals, and there were plenty of vacant ecological niches for any opportunistic creature to occupy. Within a million years after the end of the Cretaceous, mammals began an explosive evolutionary radiation, with many new groups appearing for the first time in the fossil record, and the mostly shrew-sized Mesozoic mammals evolving into much larger dog-sized and even cow-sized animals. By the middle Eocene, only 15 million years after the nonavian dinosaurs had vanished, almost all the living orders of mammals (rodents, rabbits, bats, whales, carnivores, primates, and so on) had appeared, although they were very primitive members of those families that look nothing like their living descendants. Paleontologists frequently point to the evolutionary radiation of Cenozoic mammals as a classic example of what life can do when competition is suddenly removed and there are many new ecological resources and adaptive zones left vacant.
Deciphering this evolutionary explosion has been one of the major challenges for paleontologists for over a century. The major problem is that for a very long time the fossil record of mammals was very poor in Cretaceous and Paleocene rocks, so paleontologists had only fragmentary teeth and jaws to work with, and only from a few places such as North America and Europe. Complete skeletons were extremely rare, skulls were scarce and often badly distorted or damaged because deposits this old are usually crushed under the weight of millions of tons of rocks that were deposited on top of them and often deformed by later mountain-building events as well. Paleontologists did the best they could by matching the patterns of teeth from the Paleocene with those from the Cretaceous and attempting to construct ancestor-descendant sequences. Because the hard enamel on teeth is often the most durable part of the skeleton, the teeth and jaws are usually the only parts to survive the beating caused by scavengers and river currents and trampling. Basing our understanding of mammals largely on their teeth may seem inadequate, but fortunately teeth are the most diagnostic part of most mammals, even when we do have the luxury of fossilization of the rest of the skeleton. Teeth not only preserve patterns of their ancestry in the intricate details of their cusps and crests, but they also reflect (to varying degrees) the diet of the animal as well. Thus, if we had to choose only one part of the animal skeleton to be preserved, we are lucky that the most useful part happens to be what fossilizes. Some vertebrate paleontologists joked (after seeing one talk after another on the protocones, paracones, metacones, and other cusps on mammalian teeth) that we “protoconologists” seem to think that one tooth gave rise to another tooth ad infinitum. But this is not by choice, but by necessity. By contrast, a fish or reptile paleontologist usually cannot do much with teeth or other fragments and typically only works with nearly complete specimens. This is one of the reasons that the mammalian fossil record is the most complete and detailed and densely fossiliferous of all vertebrate groups. We can do many things with fossil mammals that cannot be done with any other group of vertebrates.
When I was an undergraduate, my final project in my vertebrate paleontology class was to identify a collection of early Eocene mammal teeth from the Bighorn Basin of Wyoming and then try to research their origins in the scientific literature. I did my best on the project using what was published at the time. But when I came to the American Museum of Natural History in 1976 to begin graduate school at Columbia University, I was in for a shock. Here were the very best minds in the business working with the best fossils of every group of mammals. They were all feverishly studying specimens and drawing cladograms, using this new approach to decipher the century-long puzzle of the relationships of the major groups of fossil and living mammals. I gave a copy of my humble undergraduate thesis to my friend, Earl Manning, the collections manager and a former graduate student, and he tore it to shreds because the new cladistic approach (plus his better knowledge of the actual specimens) gave him a perspective that I could never have obtained as an undergrad reading older published literature. (This is a lesson for creationists: you can’t do research by reading other people’s work in the literature. Until you do the research with the real specimens yourself, you have no right to talk about these things). Soon, I was discovering for myself the groundbreaking new thinking about mammalian relationships, and the century-old problem of the relationships of the orders of mammals was soon to be solved.
Just a year before I arrived in New York, my graduate advisor Malcolm McKenna published the first cladistic analysis of fossil mammals. At the time he did it, it caused howls of shock and outrage. Cladistics was already second nature to entomologists and ichthyologists by then, but vertebrate paleontologists and mammalogists tended to be more conservative. Although many had heard of cladistics, and some had tried it out on their own group of organisms, none had ever used it to decipher the higher-order relationships of mammals before. But it was just the tool that was needed to address this complex problem that had eluded solution for over a century. Instead of trying to find more primitive ancestral teeth in earlier beds, paleontologists could now use cladistics to take advantage of the anatomy of the entire organism, not just the teeth. This worked especially well for living groups that had little or no fossil record but had all this anatomical data in the soft tissues and skeletons of the living members of the group. In many cases, these living groups with limited fossil records had not entered into the analysis, because paleontologists were only concerned with “connecting the dots” between the teeth that were actually preserved in their local basin.
By the late 1970s and early 1980s, mammalian paleontologists had been studying specimens and collecting reams of anatomical data on every system in living mammals, as well as the important information about the skeletons of fossil mammals. Malcolm McKenna, Mike Novacek, and Andy Wyss published some of the first cladograms that covered all the placental mammals, while I collaborated with Earl Manning on the first cladogram (originally prepared by him in 1977) that covered all the hoofed mammals, or ungulates. All of these cladograms were published in the mid-1980s (Novacek and Wyss 1986; Novacek et al. 1988; Novacek 1992, 1994; Prothero et al. 1986, 1988), especially in the landmark volume on amniote relationships from a 1986 London symposium (Benton 1988a, 1988b), and in a later volume based on a 1990 American Museum symposium on mammalian interrelationships (Szalay et al. 1993).
By the mid-1990s, almost all these cladograms on mammalian relationships seemed to converge on a common topology, with some minor unresolved differences of opinion (fig. 13.12). Some things, however, were very clear and were based on multiple well-supported, well-corroborated analyses. As McKenna had predicted in 1975, the most primitive group of placentals is not the insectivorous mammals (as paleontologists had long thought), but the xenarthrans (sloths, armadillos, anteaters, and their relatives). The rodents and rabbits are closely related after all (most paleontologists thought they were convergent) and formed a natural group called the Glires (pronounced “GLY-reez”). The closest relatives of primates were the tree shrews (no surprise to anthropologists here) and also the colugos (a bizarre Asian species incorrectly referred to as “flying lemurs,” even though they are not lemurs and they glide but do not fly). These groups together were called the Archonta. Carnivorous mammals formed their own clade, as did the true insectivores (shrews, moles, and hedgehogs). Finally, the hoofed mammals (subject of the next chapter) also formed a distinct well-supported clade, the Ungulata. Together, these groups seemed to cut the Gordian knot of mammalian interrelationships that had puzzled the great minds of paleontology for decades.

FIGURE 13.12. The evolutionary radiation of placental mammals, modified from Novacek (1994). (Drawing by Carl Buell)
But science is never this simple. Once we seemed to have achieved consensus, another source of information had to be considered: molecular data. In the 1980s and early 1990s, all we had were a few protein sequences for a few examples of each order, and most of the data seemed to be consistent with the topology that the anatomical cladograms were producing. But by the late 1990s, molecular biologists were sequencing the mitochondrial and nuclear DNA of mammals directly, and some of their results are still not consistent with what the anatomy and fossil record seems to indicate (Springer and Kirsch 1993; Stanhope et al. 1993, 1996; Madsen et al. 2001; Springer et al. 2004, 2005; Murphy et al. 2001a, 2001b). For example, tenrecs don’t seem to be related to other insectivores, elephant shrews don’t cluster with Glires nor do elephants, aardvarks, and hyraxes cluster with Ungulata. Instead, they are united (along with some other insectivorous African groups) in a molecular clade known as Afrotheria, and their branch point is even more ancient than that of the xenarthrans. Ungulates (excluding elephants and their relatives) still clustered together, but with carnivores as their sister group, followed by the bats, and then the insectivores, forming a clade the molecular biologists call Laurasiatheria. Rodents and rabbits still cluster as the Glires, with the Archonta (primates, colugos, and tree shrews, but not bats) as their sister group, forming a group known as the Euarchontoglires. Thus nearly all the topology of the anatomical tree is retained, a strong corroboration from these two completely independent sources of data that we must be getting close to the true pattern of relationships of placental mammals. Eventually, these inconsistencies between anatomical and molecular data sets will be ironed out, but for now we have at least most of the “big picture.”
What both the anatomical and molecular phylogenies of mammals show consistently is that the great radiation was already underway before the nonavian dinosaurs vanished at the end of the Cretaceous. We knew that the most primitive fossils of placentals and marsupials dated back to the Late Jurassic, about 140 million years ago, but the branching history of all the major orders of placentals was difficult to decipher with just teeth alone. There were teeth in the Late Cretaceous–earliest Paleocene that were clearly ungulates (the zhelestids and Protungulatum), primates (Purgatorius), and carnivore relatives (Cimolestes), but most of the placental teeth of the Late Cretaceous were insectivorous mammals, both true Insectivora (related to living moles, shrews, and hedgehogs) or members of unrelated groups that just happened to have the same diet. Now that the new molecular phylogenies are out, they suggest that nearly all the major placental orders differentiated in the Cretaceous, and a bit earlier than the traditional phylogeny suggests. The discrepancy is still being resolved, but the data point in the same direction: the explosive radiation was already underway with the primitive members of the lineages before the nonavian dinosaurs disappeared. Only after the landscape was cleared of large animals did these lineages then diverge in ecology and body size and begin to occupy the newly available niches, ultimately specializing into things as different as bats and whales.
Creationists, of course, don’t keep up with or understand any of this, and they misinterpret most of what they do bother to read. Once again, Gish (1995:184) decried the supposed lack of transitional forms among the orders and supported his case with grossly out-of-date quotations. Clearly, he has no idea how much progress we have made in the past 20 years. In some cases, he is simply wrong. He claims (p. 188) that there are no transitional forms for rodents and quotes Romer’s 60-year-old textbook as his source. If he had bothered to read anything more recent (published in the 1970s and 1980s, long before his 1995 edition; most recently summarized by Meng et al. [2003] and Meng and Wyss [2005]), he would have learned about the amazing discoveries of the anagalids (fig. 13.13) and mimotonids from the Paleocene beds of China. These fossils are now hailed as a classic primitive linking taxon that apparently gave rise to both rodents and rabbits in Asia during the Paleocene. In addition to the transitional anagalids, the most primitive true rodents and rabbits also appeared in Asia before they spread to other continents in the early Eocene—and the differences between them are so subtle that only specialists can tell them apart. What Gish doesn’t dare mention, of course, is that once rodents appear in the record in the Eocene of the rest of the northern continents, they have an extraordinary fossil record, with hundreds of specimens spanning every meter of section in places such as the Big Badlands of South Dakota. As Martin (2004) points out, in many parts of the Cenozoic, you can get hundreds of distinctive rodent teeth in every meter of sediment and use them to date the rocks very precisely. And if Gish had read any recent works on rodent paleontology, there are many, many transitional forms between the major groups of rodents. Not only that, but the molecular phylogenies of rodents are now closely reflecting the traditional anatomical phylogenies, so we have a very robust database of their evolution.

FIGURE 13.13. The Paleocene and Eocene fossils of China include anagalids and eurymylids, which are the transitional form linking rabbits and rodents with other mammals. This is the skull of Rhombomylus, which has rodent-like and rabbit-like features such as the chisel-shaped incisors, diastema, and cheek teeth, yet it is transitional between both groups. (Courtesy Meng Jin)
In other cases, creationist accusations are simply misleading and unreasonable. Gish (1995) and Davis and Kenyon (2004:102) argue that the fossil record is so good that even bats should fossilize easily, and these creationists harp on the fact that the earliest bats from the Eocene seem to look a lot like modern bats. If they knew anything at all about bats and fossilization processes, they would realize that bats have very delicate skeletons and tiny hollow bones, so they are very rarely fossilized (Simmons and Geisler 1998; Simmons 2005). We have just a handful of bat fossils over the entire Cenozoic, and most of them are just jaws and teeth. There are just a few extraordinarily complete bat specimens with wing membranes from amazing localities like Messel in Germany and the Green River Shale in Wyoming, all stagnant lake deposits that happened to preserve beautiful fossils, but the creationist emphasis on these one or two extraordinary specimens gives the false impression that we should be finding these lucky accidents all the time. If we were fortunate enough to have a similar extraordinary deposit like Liaoning or Messel for the Paleocene, we might find better transitional bat fossils, but more than a century of looking still hasn’t produced such a locality. More importantly, the creationists are wrong when they claim that these Eocene bats look just like modern bats. That may be true for an untrained, unobservant amateur, but anyone who knows bat anatomy can tell how primitive these fossils are. For one thing, the earliest bats did not yet have the ear structure necessary for the modern system of echolocation that bats use to catch insects on the wing. Their large skulls and eyes show that they probably hunted by day using sight, not at night using echolocation. In addition, Eocene bats have many other primitive features of the skull, hands, and feet that are not found in any other living bat. They may have had wings, but to someone who actually knows their fossils and mammals, a bat is not just a bat!
A lion’s work hours are only when he’s hungry; once he’s satisfied, the predator and prey live peacefully together.
—Chuck Jones, Animator
Bats and rats usually don’t interest too many people (except as pests) nor do most of the other orders of mammals, such as xenarthrans (which have an excellent fossil record with many transitional fossils of ground sloths and huge armored armadillos) or the insectivores (which have an amazing fossil record going back to the Cretaceous). So we will not dwell on these examples further for space reasons, but we will focus on two large mammal groups that do excite people: the hoofed mammals (or ungulates), subject of the next chapter, and the carnivorous mammals.
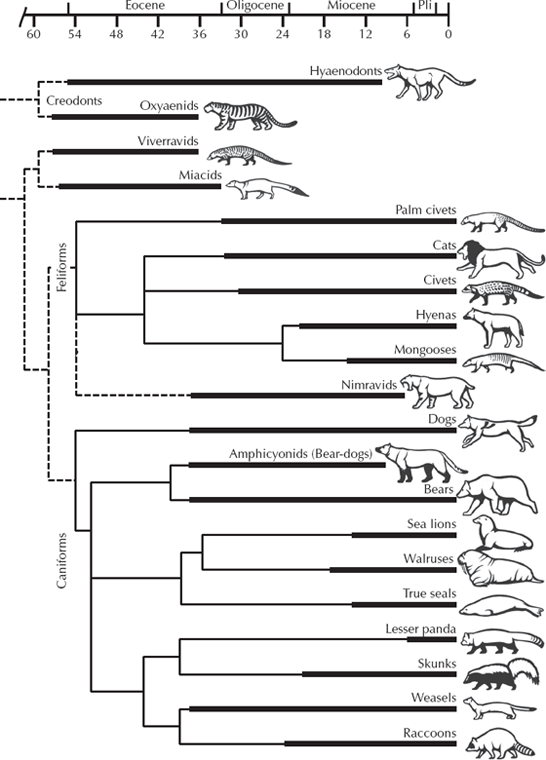
Lots of people love cats and dogs, and many are pet owners, so carnivores are near and dear to many hearts. Those science documentaries on cable TV and the children’s books about prehistoric mammals love to show saber-toothed cats, dire wolves, and cave bears, but there is far more to carnivore evolution than just these glamorous creatures. Carnivorous mammals are usually not as commonly fossilized as their herbivorous prey. Because it usually takes many prey animals to support one predator, the population numbers of carnivores are always small, and their chances of fossilization are further diminished. Nevertheless, they have an excellent fossil record going back through the entire Cenozoic, with far more diversity of forms than can be seen today with modern cats, dogs, bears, and their relatives. We can trace the living order Carnivora back to the most primitive groups, an assemblage of highly primitive carnivorans known as miacids known from the Paleocene of the northern continents (fig. 13.14). All of these creatures were small, archaic weasel-sized creatures that look nothing like their descendant families but had all the hallmarks of the Carnivora. In the next few million years of the Eocene and Oligocene, we soon see the divergence of a number of modern families (fig. 13.15). For example, the dogs appear in the middle Eocene and soon have an incredible evolutionary radiation of dozens of genera and hundreds of species. They evolved into a wide spectrum of forms, from some that were smaller than weasels (Hesperocyon) to the huge borophagine dogs, hyena-like forms with crushing teeth for breaking bones—far more diversity than dogs show today (fig. 13.16).

FIGURE 13.14. The most primitive true carnivorans are known as miacids, which were shaped roughly like weasels or raccoons, but were much more primitive. They were probably ancestral to nearly all living carnivorans. (Drawing by Carl Buell)
FIGURE 13.15. The evolutionary history of the carnivorous mammals. (Drawing by Carl Buell)
FIGURE 13.16. The family tree of the dogs, showing the variation from weasel-like Hesperocyon to the huge hyena-like bone-crushing borophagine dogs. (Based on information supplied by Xiaoming Wang; drawing by Carl Buell)
True cats appear in the early Oligocene with Pseudailurus, a creature that looked more like a weasel than a cat (fig. 13.17). But by the Miocene, they began to take on their catlike characteristics and evolved a variety of forms, including several different acquisitions of saber-like canines. In addition, there was an earlier family of catlike forms known as nimravids, which parallel cat evolution in many ways (including the evolution of several “saber-toothed” forms) but are unrelated to cats.
FIGURE 13.17. The family trees of the Felidae, or cat family, and of the Nimravidae, or “false cats,” which parallel cats in many ways but are not closely related. (Drawing by Carl Buell)
Bears have a history going back to the early Oligocene as well, but nearly all the early bears were small badger-like forms that then developed into fast-running dog-shaped forms like Hemicyon. Only late in their evolution did bears become large and develop teeth for an omnivorous diet. The fossil record of the mustelids (weasels, skunks, otters, badgers, wolverines, and their kin) and of raccoons and their relatives are also quite good (see Baskin 1998a, 1998b), although all the early members of these families are very primitive and would look nothing like their living descendants if you saw them today. Nevertheless, the details of their teeth, skulls, and skeletons are highly distinctive and unmistakable. Considering their rarity, the fossil record of most carnivorans is remarkably good, and there is no shortage of diversity of fossils or transitional forms between nearly every fossil group known.
Finally, one of the most amazing of all transitions in mammals is the origin of pinnipeds (seals, sea lions, and walruses). At one time, paleontologists thought that seals were related to weasels and that sea lions and walruses were related to bears, but recent cladistic and molecular phylogenetic analyses (Wyss 1987, 1988) have shown conclusively that all pinnipeds are monophyletic and closely related only to bears (fig. 13.15). And there are beautiful transitional fossils that link them. Oligocene deposits of Europe yield bears known as amphicynodontids, which were terrestrial animals yet had many features that link them to pinnipeds. Lower Miocene beds of California and Oregon yield the enaliarctines, which are the first truly marine relatives of seals and sea lions (Mitchell and Tedford 1973; Barnes 1989; Berta et al. 1989; Berta and Ray 1990). Although they retained many primitive skull features seen in the bearlike amphicynodontids, they also have some specializations of seals and sea lions, including enlarged eyes, an enlarged nasal cavity for regulating the temperature of the blood as they swim, and larger openings for the muscles that control their lips and whiskers. They also have reduced their olfactory lobes of the brain (since the sense of smell is not very important to aquatic mammalian predators) and improved the drainage of blood to their brains as an aid to diving. Their bodies (fig. 13.18) also had rudimentary flippers and streamlined shapes, so they would definitely remind us of the living seals, although they were very primitive looking and their flippers were clearly not as advanced as seen in modern pinnipeds. However, their bodies were still not as fully aquatic as the later seals and sea lions. Instead, they may have had lifestyles not too different from that of the sea otter.

FIGURE 13.18. The earliest known relative of the seals, Enaliarctos, which was seal-like in many features but did not have the fully specialized skull, nasal region, or ear region of modern seals and sea lions. Their webbed feet and hands were more like the condition in otters and not fully modified into flippers. (Courtesy A. Berta)
Not long after the enaliarctines, we find the first members of all the living pinniped groups in the late early to middle Miocene, including the first true seals (Pontophoca, Praepusa, and Cryptophoca in the middle Miocene of Europe and Leptophoca in the middle Miocene of North America), the first sea lions (Pithanotaria in the middle Miocene of the Pacific Northwest of North America), and the first walruses (the desmatophocines of the early Miocene of North America and Prototaria of the early Miocene of Japan).
The best documented of these transitions is the evolution of walruses. The most primitive walruses, such as the middle Miocene Proneotherium and Neotherium (fig. 13.19A), are barely different from Enaliarctos, except that they are larger and more robust, and already begin to show the size and canine tusk differences between males and females that is so characteristic of walruses and not enaliarctines. Slightly later in the middle Miocene, we find Imagotaria, which was about the size of the living walrus, with ear bones already suited to underwater hearing. Imagotaria was beginning to develop the tusk-like canines and simplified peg-like molars and premolars in its cheek teeth that all walruses have. By the late Miocene and early Pliocene, there were at least eight different kinds of walruses along the Pacific Rim, most of which looked like unusually large sea lions with short to medium-sized tusks. They included Pontolis, Gomphotaria (fig. 13.19C), and Dusignathus, with completely peg-like simplified cheek teeth, large lower tusks, and small upper canine tusks, and Aivukus (fig. 13.19B), with slightly larger upper tusks, broad cheek teeth, and a deep lower jaw with small canines. Aivukus apparently lived in shallow water as a bottom feeder and crushed its food like modern walruses do. Then, in the latest Miocene and early Pliocene, the walruses spread through the Central American seaway (there was no Panamanian land bridge until the middle Pliocene), up the Atlantic Coast, and on to Europe and the Mediterranean. In the early Pliocene of Europe and the African coast of the Mediterranean, we find fossils of Alachtherium, which looked slightly more like a modern walrus, with large tusks, reduced peg-like molars, a deep lower jaw with no canines, and only a few other cheek teeth. In almost every respect, it is an ideal intermediate between the primitive walruses and the modern groups. Finally, the late Miocene and Pliocene also yield an even better transitional form, Valenictus (fig. 13.19D), which has long tusks (but still considerably shorter than the tusks of the living walrus, Odobenus, fig. 13.19E), and almost no teeth in the lower jaw. These walruses also had the characteristic arching of the palate that we see in living walruses. This arched palate, combined with the action of the tongue, allows living walruses to suck their prey (mostly mollusks) into their mouths, where they then crush the shells and suck out the contents.

FIGURE 13.19. The evolution of walruses from primitive forms that resembled sea lions. (A) Proneotherium from the early Miocene, which has short canines and relatively primitive teeth but several distinctive features found only in walruses. (B) Aivukus from the late Miocene with larger canine tusks and simpler peg-like cheek teeth. (C) The dusignathine walrus Gomphotaria, with its big upper and lower tusks. (D) The more advanced walrus Valenictus, with upper tusks almost as large as those of the living species, a highly arched palate, and greatly reduced cheek teeth. (E) The living walrus, Odobenus rosmarus. (Photo [C] courtesy L. Barnes; [B] after Repenning and Tedford 1975, courtesy U.S. Geological Survey; all others courtesy T. Deméré)
If a creationist saw the end-members of this transitional series, they would not guess the connection, until we put all the transitional fossils in between them and demonstrated this dramatic example of land animals adapting to marine life. The walruses, in particular, give us an amazing array of transitional forms from sea lion–like early forms to those with intermediate conditions of the tusks and cheek teeth. Ironically, some of the best specimens of these “transitional” walruses are on display at the San Diego Natural History Museum, only a short drive from the original ICR headquarters. Gazing at them in the display case is one of the most powerful proofs that transitional forms are real. We will see an even better transitional series when we examine whales in chapter 14.
For Further Reading
Benton, M. J., ed. 1988. The Phylogeny and Classification of the Tetrapods. Vol. 2, Mammals. Oxford, U.K.: Clarendon.
Benton, M. J. 2014. Vertebrate Palaeontology. 4th ed. New York: Wiley-Blackwell.
Carroll, R. L. 1988. Vertebrate Paleontology and Evolution. New York: Freeman.
Gittleman, J., ed. 1996. Carnivore Biology, Behavior, and Evolution. Ithaca, N.Y.: Cornell University Press.
Hopson, J. A. 1994. Synapsid evolution and the radiation of non-eutherian mammals. In Major Features of Vertebrate Evolution, ed. D. R. Prothero and R. M. Schoch. Paleontological Society Short Course 7:190–219.
Janis, C., K. M. Scott, and L. L. Jacobs, eds. 1998. Evolution of Tertiary Mammals of North America. Vol. 1, Terrestrial Carnivores, Ungulates and Ungulate-Like Mammals. New York: Cambridge University Press
Janis, C., G. F. Gunnell, and M. D. Uhen, eds. 2008. Evolution of Tertiary Mammals of North America. Vol. 2, Small Mammals, Xenarthrans, and Marine Mammals. New York: Cambridge University Press.
Kielan-Jaworowska, Z., R. L. Cifelli, and Z.-X. Luo. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. New York: Columbia University Press.
Li, C. K., R. W. Wilson, and M. R. Dawson. 1987. The origin of rodents and lagomorphs. Current Mammalogy 1:97–108.
Luckett, W. P., and J.-L. Hartenberger, eds. 1985. Evolutionary Relationships Among Rodents. New York: Plenum.
McKenna, M. C., and S. K. Bell. 1997. Classification of Mammals. New York: Columbia University Press.
McLoughlin, J. C. 1980. Synapsida: A New Look into the Origin of Mammals. New York: Viking.
Novacek, M. J. 1992. Mammalian phylogeny: shaking the tree. Nature 356:121–125.
Novacek, M. J. 1994. The radiation of placental mammals. In Major Features of Vertebrate Evolution, ed. D. R. Prothero and R. M. Schoch. Paleontological Society Short Course 7:220–237.
Novacek, M. J., and A. R. Wyss. 1986. Higher-level relationships of Recent eutherian orders: morphological evidence. Cladistics 2:257–287.
Peters, D. 1991. From the Beginning: The Story of Human Evolution. New York: Morrow.
Prothero, D. R. 1994. Mammalian evolution. In Major Features of Vertebrate Evolution. ed. D. R. Prothero and R. M. Schoch. Paleontological Society Short Course 7:238–270.
Prothero, D. R. 2006. After the Dinosaurs: The Age of Mammals. Bloomington: Indiana University Press.
Prothero, D. R. 2013. Bringing Fossils to Life: An Introduction to Paleobiology. 3rd ed. New York: Columbia University Press.
Prothero, D. R. 2016. The Princeton Field Guide to Prehistoric Mammals. Princeton, N.J.: Princeton University Press.
Rose, K. D., and J. D. Archibald, eds. 2005. The Rise of Placental Mammals. Baltimore, Md.: Johns Hopkins University Press.
Savage, R. J. G., and M. R. Long. 1986. Mammal Evolution: An Illustrated Guide. New York: Facts-on-File.
Szalay, F. S., M. J. Novacek, and M. C. McKenna, eds. 1993. Mammal Phylogeny. New York: Springer-Verlag.
Turner, A., and M. Anton. 2004. National Geographic Prehistoric Mammals. Washington, D.C.: National Geographic Society.