CHAPTER 103
Tuberculosis and Other Mycobacterial Infections
TUBERCULOSIS
MICROBIOLOGY
Tuberculosis (TB) is caused by organisms of the Mycobacterium tuberculosis complex, which includes M. tuberculosis, the most common and important agent of human mycobacterial disease, and M. bovis, which (like several other mycobacterial species) is acquired via ingestion of unpasteurized milk. M. tuberculosis is a thin aerobic bacillus that is neutral on Gram’s staining but that, once stained, is acid-fast; i.e., it cannot be decolorized by acid alcohol because of the cell wall’s high content of mycolic acids and other lipids.
EPIDEMIOLOGY
Approximately 9.4 million new cases of TB occurred worldwide in 2009, with ~1.7 million TB-related deaths in 2008—mostly in low-income countries. Globally, TB rates are stable or falling.
• In the United States, TB primarily affects HIV-infected adults, immigrants, the elderly, and disadvantaged/marginalized populations.
• Multidrug-resistant (MDR; resistant to at least isoniazid and rifampin) and extensively drug-resistant (XDR; resistant to isoniazid, rifampin, fluoroquinolones, and either amikacin, kanamycin, or capreomycin) isolates of M. tuberculosis are increasing in frequency; ~440,000 cases of MDR-TB may have emerged in 2008, of which ~10% were probably XDR.
• Disease from a pt with pulmonary TB is spread by droplet nuclei that are aerosolized by coughing, sneezing, or speaking.
– Droplets <5–10 μm in diameter may be suspended in air for several hours.
– Transmission is determined by the intimacy and duration of contact with a pt with TB, the degree of infectiousness of the pt, and the shared environment.
– Pts with cavitary or laryngeal disease are most infectious, with as many as 105–107 acid-fast bacilli (AFB)/mL of sputum.
• Risk factors for development of active disease after M. tuberculosis infection include recent acquisition (within the preceding year), comorbidity (e.g., HIV disease, diabetes, silicosis, immunosuppression, gastrectomy), malnutrition, tobacco smoking, and presence of fibrotic lesions.
PATHOGENESIS
AFB that reach alveoli are ingested by macrophages. The bacilli impair phago-some maturation, multiply, lyse the macrophages, and spread to regional lymph nodes, from which they may disseminate throughout the body. These initial stages of infection are generally asymptomatic and induce cellular and humoral immunity.
• About 2–4 weeks after infection, a tissue-damaging response resulting from delayed-type hypersensitivity [the basis for tuberculin skin testing (TST)] destroys nonactivated macrophages that contain multiplying bacilli, and a macrophage-activating response activates cells capable of killing AFB. A granuloma forms at the site of the primary lesion and at sites of dissemination. The lesions can then either heal by fibrosis or undergo further evolution. Despite “healing,” viable bacilli can remain dormant within macrophages or in necrotic material for years.
• Cell-mediated immunity confers partial protection against TB. Cytokines secreted by alveolar macrophages contribute to disease manifestations, granuloma formation, and mycobacterial killing.
CLINICAL MANIFESTATIONS
TB is classified as pulmonary, extrapulmonary, or both.
Pulmonary TB
TB is limited to the lungs in >80% of HIV-negative pts. Primary disease may cause no or mild symptoms (fever and occasional pleuritic chest pain) in contrast to the prolonged disease course that is common in postprimary or adult-type disease.
• Primary disease is frequently located in the middle and lower lobes. The primary lesion usually heals spontaneously, and a calcified nodule (Ghon lesion) remains.
– Transient hilar and paratracheal lymphadenopathy is common.
– In immunosuppressed pts and children, primary disease may progress rapidly to significant clinical disease, with cavitation, pleural effusions, and hematogenous dissemination (miliary disease).
• Adult-type disease presents initially with nonspecific and insidious symptoms, such as diurnal fever, night sweats, weight loss, anorexia, malaise, and weakness.
– As the disease progresses, pts develop cough and purulent sputum production, often with blood streaking. Extensive cavitation may develop, with occasional massive hemoptysis following erosion of a vessel located in the wall of a cavity.
– Disease is usually localized to the apical and posterior segments of the upper lobes and the superior segments of the lower lobes.
Extrapulmonary TB
Any site in the body can be involved, but the most commonly affected sites are (in order of frequency) the lymph nodes, pleura, genitourinary tract, bones and joints, meninges, peritoneum, and pericardium. Up to two-thirds of HIV-infected pts with TB have extrapulmonary disease.
• Lymphadenitis occurs in 35% of extrapulmonary TB cases, especially among HIV-infected pts. Painless swelling of cervical and supraclavicular nodes (scrofula) is typical.
– Nodes are discrete early on but can develop into a matted nontender mass with a fistulous tract.
– Fine-needle aspiration or surgical-excision biopsy of the node is required for diagnosis. Cultures are positive in 70–80% of cases.
• Pleural involvement is common and results from a hypersensitivity response to mycobacterial antigens or contiguous spread of parenchymal inflammation.
– Fluid is straw-colored and exudative, with protein levels >50% of those in serum, normal to low glucose levels, a usual pH of ~7.3 (occasionally <7.2), and pleocytosis (500–6000 cells/μL). The pleural concentration of adenosine deaminase, if low, virtually excludes TB.
– Pleural biopsy is often required for diagnosis, with up to 80% of biopsy cultures positive. Direct smears and cultures of pleural fluid are less sensitive.
– Empyema is an uncommon complication of pulmonary TB and results from rupture of a cavity with many bacilli into the pleural space. In these cases, direct smears and cultures are often positive, and surgical drainage is usually required in addition to chemotherapy.
• In genitourinary disease, local symptoms predominate (e.g., frequency, dysuria, hematuria, abdominal or flank pain), and up to 75% of pts have a CXR demonstrating previous or concomitant pulmonary disease. Disease is occasionally identified only after severe destructive lesions of the kidneys have developed.
– In >90% of cases, urinalysis shows pyuria and hematuria with negative bacterial cultures.
– Mycobacterial culture of three morning urine specimens is diagnostic in 90% of cases.
• Weight-bearing joints (spine, hips, and knees) are the most common sites of skeletal disease.
– Spinal TB (Pott’s disease) often involves two or more adjacent vertebral bodies; in adults, lower thoracic/upper lumbar vertebrae are usually affected. Disease spreads to adjacent vertebral bodies, later affecting the intervertebral disk and causing collapse of vertebral bodies in advanced disease (kyphosis, gibbus). Paravertebral cold abscesses may form.
• Meningitis occurs most often in young children and HIV-seropositive pts. Disease typically evolves over 1–2 weeks and often involves paresis of cranial nerves (particularly of ocular nerves). The ultimate evolution is toward coma, with hydrocephalus and intracranial hypertension.
– CSF can have a high lymphocyte count, an elevated protein level, and a low glucose concentration. Cultures are positive in 80% of cases. PCR is ~80% sensitive but gives a false-positive result 10% of the time.
– Neurologic sequelae are seen in ~25% of treated pts; adjunctive glucocorticoids enhance survival among pts >14 years of age, but do not reduce the frequency of neurologic sequelae.
• Gastrointestinal disease can affect any portion of the GI tract (with the terminal ileum and cecum most commonly involved), causing abdominal pain, obstruction, hematochezia, and often a palpable mass. TB peritonitis can occur after spread of the organism from ruptured lymph nodes and intraabdominal organs; peritoneal biopsy is usually required for diagnosis.
• Pericarditis is characterized by an acute or subacute onset of fever, dull retrosternal pain, and sometimes a friction rub. Effusion is common. Chronic constrictive pericarditis is a potentially fatal complication, even in treated pts. Adjunctive glucocorticoids remain controversial; no conclusive data demonstrate a benefit.
• Miliary disease arises from hematogenous spread of M. tuberculosis throughout the body. Symptoms are nonspecific, and small (1- to 2-mm) granulomas may develop in many organs. Hepatomegaly, splenomegaly, lymphadenopathy, and choroidal tubercles of the eye may occur.
HIV-Associated TB
The manifestations of TB vary with the stage of HIV infection. When cell-mediated immunity is only partly compromised, pulmonary TB presents as typical upper-lobe cavitary disease. In late HIV infection, a primary TB–like pattern may be evident, with diffuse interstitial or miliary infiltrates, little or no cavitation, and intrathoracic lymphadenopathy.
• Extrapulmonary disease occurs frequently; common forms include lymphadenitis, meningitis, pleuritis, pericarditis, mycobacteremia, and disseminated disease.
• Immune reconstitution inflammatory syndrome (IRIS), which may occur 1–3 months after initiation of antiretroviral therapy, may exacerbate the signs and symptoms of TB.
DIAGNOSIS
The key to diagnosis is a high index of suspicion.
• AFB microscopy of diagnostic specimens—i.e., light microscopy of specimens stained with Ziehl-Neelsen basic fuchsin dyes or fluorescence microscopy of samples stained with auramine-rhodamine—can provide a presumptive diagnosis. In suspected pulmonary TB, 2 or 3 sputum samples should be examined.
• Definitive diagnosis requires growth of M. tuberculosis in culture or identification of the organism’s DNA in clinical samples.
– Liquid media and speciation by molecular methods have decreased the time required for diagnostic confirmation to 2–3 weeks (from 4–8 weeks).
– Nucleic acid amplification is useful not only for rapid confirmation of TB in AFB-positive specimens but also for diagnosis of AFB-negative pulmonary and extrapulmonary TB.
• Drug susceptibility can be assessed via indirect testing on solid media (which takes ≥8 weeks), direct testing in liquid media (which takes ~3 weeks), or PCR (which can provide results within hours).
• TST is of limited value in active disease because of low sensitivity and specificity but is the most widely used screening test for latent TB infection.
• Interferon γ release assays (IGRAs) measure the release of interferon γ by T cells after stimulation with TB-specific antigens and are more specific for M. tuberculosis than is TST.
– In low-incidence settings, IGRAs may be more sensitive than TST.
– In high TB- and/or HIV-burden settings, the performance of IGRAs has varied greatly.
DRUGS
First-Line Agents
• Rifampin: Rifampin is the most important and potent antituberculous agent. The standard dosage in adults is 600 mg/d.
– The drug distributes well throughout body tissues, including inflamed meninges. It turns body fluids (e.g., urine, saliva, tears) red-orange and is excreted through bile and the enterohepatic circulation.
– Rifampin is usually well tolerated; adverse events are infrequent and generally mild.
– Of note, rifampin is a potent inducer of the hepatic cytochrome P450 system and decreases the half-life of many other drugs.
• Isoniazid: Isoniazid is a critical drug for active and latent TB disease. The usual adult dosage is 300 mg/d or 900 mg twice per week.
– Isoniazid is distributed well throughout the body and infected tissues, including CSF and caseous granulomas.
– The most important toxicities are hepatotoxicity and peripheral neuropathy.
• Isoniazid-associated hepatitis is idiosyncratic and increases with age and alcohol use and in the postpartum period.
• Because peripheral neuropathy can result from interference with pyridoxine metabolism, pyridoxine (25–50 mg/d) should be given to pts with other risk factors for neuropathy, such as diabetes, alcohol abuse, or malnutrition.
• Ethambutol: The least potent first-line agent, ethambutol is synergistic with the other drugs in the standard first-line regimen. Ethambutol is usually given at a dosage of 15 mg/kg daily.
– The drug is distributed throughout the body but reaches only low levels in CSF.
– This agent can cause dose-dependent optic neuritis, producing central scotoma and impairing both visual acuity and the ability to see green.
• Pyrazinamide: The usual dosage is 15–30 mg/kg daily (maximum, 2 g/d). The drug distributes well throughout the body, including the CSF.
• Hyperuricemia that can be managed conservatively is common.
• Clinical gout is rare.
Other Effective Agents
• Streptomycin: The usual adult dose is 0.5–1.0 g IM daily or 5 times per week. Streptomycin causes ototoxicity (primarily vestibulotoxicity) but is less nephrotoxic than other aminoglycosides.
• Rifabutin: Rifabutin has fewer drug interactions than rifampin and is active in vitro against some rifampin-resistant strains of M. tuberculosis. Rifabutin reaches tissue concentrations 5 to 10 times higher than those in plasma and has a much longer half-life than rifampin. The drug is well tolerated, and its adverse effects are dose related.
• Rifapentine: Rifapentine is similar to rifampin but can be given once or twice weekly. This drug is not approved for the treatment of HIV-infected pts because of elevated rates of relapse.
Second-Line Agents
• Fluoroquinolones: Levofloxacin, gatifloxacin (no longer marketed in the United States because of its severe toxicity), and moxifloxacin have solid, broad antimycobacterial activity. Ciprofloxacin is no longer recommended for treatment of TB because of poor efficacy.
• Other agents are used uncommonly but may be needed in disease caused by resistant strains of M. tuberculosis.
REGIMENS
See Table 103-1.
TABLE 103-1 RECOMMENDED REGIMENS FOR TREATMENT tOF TB
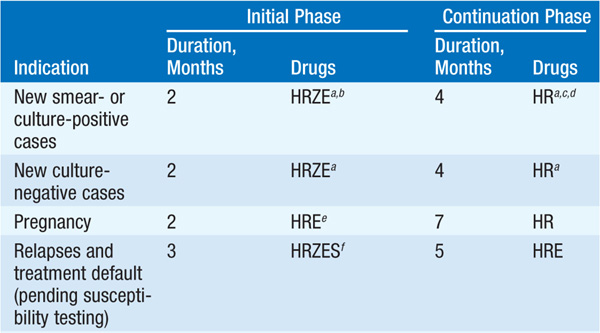
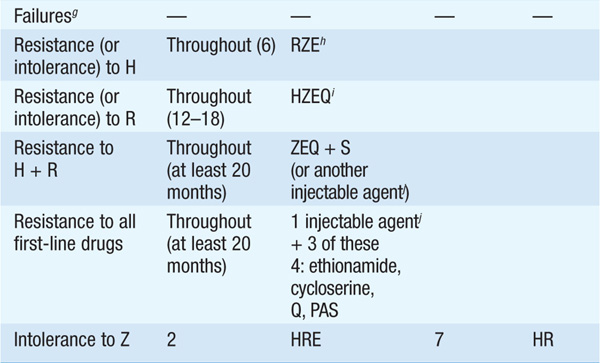


• During the initial phase, the majority of tubercle bacilli are killed, symptoms resolve, and usually the pt becomes noninfectious. The continuation phase is required to eliminate persisting mycobacteria and prevent relapse.
• Nonadherence to the regimen is the most important impediment to cure. Directly observed treatment (especially during the initial 2 months) and fixed drug-combination products should be used if possible.
• Bacteriologic evaluation is the preferred method of monitoring response to treatment.
– Virtually all pts should have negative sputum cultures after 3 months of treatment. If the culture remains positive, treatment failure and drug resistance should be suspected.
– With extrapulmonary TB, bacteriologic monitoring may not be feasible. In these cases, the response to treatment must be assessed clinically and radiographically.
• Drug resistance may be either primary (i.e., present in a strain prior to therapy) or acquired (i.e., arising during treatment because of an inadequate regimen or noncompliance).
• Close monitoring for drug toxicity should take place during treatment and should include baseline LFTs and monthly questioning about possible hepatitis symptoms. High-risk pts (e.g., older pts, pts who use alcohol daily) should have LFT values monitored during treatment.
For pts with symptomatic hepatitis and those with marked (five- to sixfold) elevations in serum levels of aspartate aminotransferase, treatment should be stopped and drugs reintroduced one at a time after liver function has returned to normal.
• Three important considerations are relevant to TB treatment in HIV-infected pts: an increased frequency of paradoxical reactions, interactions between antiretroviral agents and rifamycins, and development of rifampin monoresistance with widely spaced intermittent treatments.
PREVENTION
• Vaccination: An attenuated strain of M. bovis, bacille Calmette-Guérin (BCG), protects infants and young children from serious forms of TB (e.g., meningitis and miliary disease) and is recommended for routine use in countries with high TB prevalence.
• Treatment of latent infection: Candidates for chemoprophylaxis are identified by TST or IGRA. Positive skin tests are determined by reaction size and risk group (Table 103-2). Drug treatment should be considered for pts with evidence of latent infection (Table 103-3). Isoniazid should not be given to persons with active liver disease.
TABLE 103-2 TUBERCULIN REACTION SIZE AND TREATMENT OF LATENT TB INFECTION
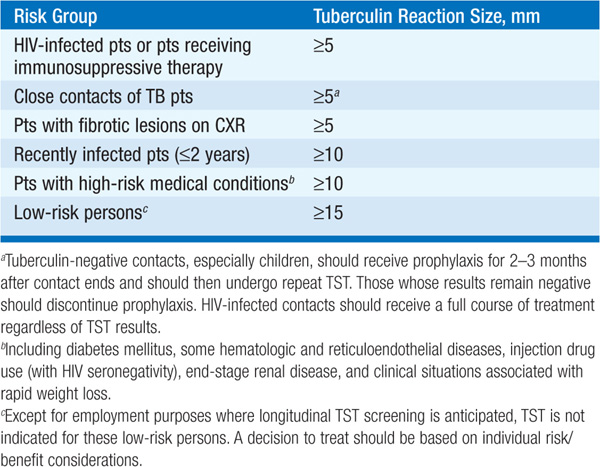
TABLE 103-3 REVISED DRUG REGIMENS FOR TREATMENT OF LATENT TUBERCULOSIS INFECTION (LTBI) IN ADULTS



LEPROSY
MICROBIOLOGY AND EPIDEMIOLOGY
Leprosy is a nonfatal chronic infectious disease caused by M. leprae, an obligate intracellular bacterial species indistinguishable microscopically from other mycobacteria. The organism is confined to humans, armadillos (in some locales), and sphagnum moss.
• M. leprae cannot yet be cultured in vitro. The organism has a doubling time in mice of 2 weeks (compared with 20 min for Escherichia coli and 1 day for M. tuberculosis).
• Leprosy, which is associated with poverty and rural residence, is a disease of the developing world; its global prevalence is difficult to assess and is variously estimated at 0.6–8 million.
– More than 80% of the world’s cases occur in a few countries: India, China, Myanmar, Indonesia, Nepal, Brazil, Nigeria, and Madagascar.
– In the U.S., ~4000 people have leprosy and 100–200 new cases are reported annually.
• The route of transmission is uncertain but may be via nasal droplets, contact with infected soil, or insect vectors.
CLINICAL MANIFESTATIONS
The spectrum from polar tuberculoid (TT) to polar lepromatous (LL) disease is associated with an evolution from asymmetric localized macules and plaques to nodular and indurated symmetric generalized skin manifestations, an increasing bacterial load, and loss of M. leprae–specific cellular immunity. Prognosis, complications, and intensity of antimicrobial therapy depend on where a pt presents on the clinical spectrum. The incubation period ranges from 2 to 40 years but is usually 5–7 years.
Tuberculoid Leprosy (TL)
At the less severe end of the disease spectrum, TL results in symptoms confined to the skin and peripheral nerves.
• One or several hypopigmented macules or plaques with sharp margins that are hypesthetic and have lost sweat glands and hair follicles are present. AFB are few or absent.
• There is asymmetric enlargement of one or several peripheral nerves—most often the ulnar, posterior auricular, peroneal, and posterior tibial nerves—associated with hypesthesia and myopathy.
Lepromatous Leprosy (LL)
Pts develop symmetrically distributed skin nodules, raised plaques, and diffuse dermal infiltration that can cause leonine facies, loss of eyebrows and lashes, pendulous earlobes, and dry scaling.
• Numerous bacilli are present in skin (up to 109/g), nerves, and all organs except the lungs and CNS.
• Nerve enlargement and damage are usually symmetric and are due to bacillary invasion.
COMPLICATIONS
• Reactional states: These common, immunologically mediated inflammatory states cause considerable morbidity. Erythema nodosum leprosum—characterized by painful erythematous papules that resolve spontaneously in ~1 week—occurs in ~50% of pts near the LL end of the disease spectrum within 2 years of initiation of therapy.
• Extremities: Neuropathy results in insensitivity and affects fine touch, pain, and heat receptors. The loss of distal digits in leprosy is a consequence of insensitivity, trauma, secondary infection, and—in lepromatous pts—a poorly understood and sometimes profound osteolytic process.
• Eyes: Owing to cranial nerve palsies, lagophthalmos and corneal insensitivity may complicate leprosy, resulting in trauma, secondary infection, and (without treatment) corneal ulcerations and opacities. Leprosy is a major cause of blindness in low-income countries.
• Nerve abscesses: Pts with leprosy can develop abscesses of nerves (most commonly the ulnar) and require urgent surgical decompression to prevent irreversible sequelae.
DIAGNOSIS
In TL, the advancing edge of a skin lesion should be biopsied. In LL, biopsy of even normal-appearing skin often yields positive results. Serology, skin testing, and PCR of the skin offer little diagnostic assistance.
DRUGS
• Rifampin (600 mg daily or monthly) is the only agent bactericidal against M. leprae. See the preceding section on M. tuberculosis for more details on rifampin.
• Monotherapy with dapsone (50–100 mg/d) results in only a 2.5% resistance-related relapse rate.
– A decrease in hemoglobin levels of ~1 g/dL is a common adverse effect; the sulfone syndrome (high fever, anemia, exfoliative dermatitis, and a mononucleosis-type blood picture) occurs rarely.
– G6PD deficiency must be ruled out before therapy to avoid hemolytic anemia.
• Clofazimine (50–100 mg/d, 100 mg 3 times per week, or 300 mg monthly) is a phenazine iminoquinone dye that is weakly active against M. leprae. Adverse effects include red-black skin discoloration.
REGIMENS
Given the unreliability of skin smears and the lack of accessibility to histopathology in many countries in which leprosy is endemic, treatment regimens are based on the number of lesions present.
• Paucibacillary disease in adults (<6 skin lesions) is treated with dapsone (100 mg/d) and rifampin (600 mg monthly, supervised) for 6 months or with dapsone (100 mg/d) for 5 years. For a single lesion, a single dose of rifampin (600 mg), ofloxacin (400 mg), and minocycline (100 mg) is recommended.
• Multibacillary disease in adults (≥6 skin lesions) is treated with dapsone (100 mg/d) plus clofazimine (50 mg/d)—unsupervised—in addition to rifampin (600 mg monthly) plus clofazimine (300 mg monthly)—supervised—for 1–2 years.
– Some experts prefer rifampin (600 mg/d) for 3 years and dapsone (100 mg/d) for life.
– Relapse can occur years later; prolonged follow-up is needed.
• Reactional states
– Lesions at risk for ulceration or in cosmetically important areas can be treated with glucocorticoids (40–60 mg/d for at least 3 months).
– If erythema nodosum leprosum is present and persists despite two short courses of steroids (40–60 mg/d for 1–2 weeks), thalidomide (100–300 mg nightly) should be given. Because of thalidomide’s teratogenicity, its use is strictly regulated.
INFECTIONS WITH NONTUBERCULOUS MYCOBACTERIA (NTM)
Mycobacteria other than those of the M. tuberculosis complex and M. leprae are referred to as nontuberculous or atypical mycobacteria and are ubiquitous in soil and water.
MICROBIOLOGY
NTM are broadly differentiated into rapidly and slowly growing forms (<7 days and ≥7 days, respectively). M. abscessus, M. fortuitum, and M. chelonae are examples of rapid growers; species such as M. avium and M. intracellulare (the M. avium complex, or MAC), M. kansasii, M. ulcerans, and M. marinum are slow growers.
EPIDEMIOLOGY
Most NTM cause disease in humans only rarely unless some aspect of host defense is impaired (as in bronchiectasis) or breached (as by inoculation—e.g., during liposuction or trauma). The bulk of nontuberculous mycobacterial disease in North America is due to M. kansasii, MAC organisms, and M. abscessus.
CLINICAL MANIFESTATIONS
Although there are many NTM species, the clinical presentations they cause can be broadly categorized by the organ system(s) affected.
• Disseminated disease is now quite rare; even pts with advanced HIV infection do not often develop disseminated NTM infection, given improved treatment of HIV infection and effective antimycobacterial prophylaxis.
– Organisms typically spread from the bowel to the bone marrow and bloodstream, but disease is indolent, and it can take weeks or months for the pt to present for medical attention with malaise, fever, weight loss, organomegaly, and lymphadenopathy.
– A child with involvement of ≥2 organ systems and no iatrogenic cause should be evaluated for defects in the interferon γ/interleukin-12 pathway.
• Pulmonary disease represents the most common NTM infection in industrialized countries. MAC organisms are most commonly involved in North America. Pts present with months or years of throat clearing, nagging cough, and slowly progressive fatigue. M. kansasii can cause a TB-like syndrome, with hemoptysis, chest pain, and cavitary lung disease.
• Isolated cervical lymphadenopathy is the most common NTM infection among young children in North America and is most frequently caused by MAC organisms. The nodes are typically firm and painless and develop in the absence of systemic symptoms.
• Skin and soft tissue disease usually requires a break in the skin for introduction of the organism. Different NTM species are associated with specific exposures.
– M. fortuitum is linked to pedicure bath–associated infections, particularly if skin abrasion (e.g., during leg shaving) has immediately preceded the pedicure.
– Rapidly growing NTM are associated with outbreaks of infection acquired via skin contamination from surgical instruments (especially in cosmetic surgery), injections, and other procedures. These infections are typically accompanied by painful, erythematous, draining subcutaneous nodules, usually without associated fever or systemic symptoms.
– M. marinum can be acquired from fish tanks, swimming pools, barnacles, and fish scales. Pts typically develop papules or ulcers (“fish-tank granuloma”) that can progress to tendonitis and tender nodules on the arm in a pattern similar to that caused by Sporothrix schenckii. Lesions appear days or weeks after acquisition of the organism.
– M. ulcerans is a waterborne organism found primarily in tropical areas, especially in Africa. Skin lesions are typically painless, clean ulcers that slough and can cause osteomyelitis.
DIAGNOSIS
Similar to M. tuberculosis, NTM can be detected on acid-fast or fluoro-chrome smears of clinical samples and can be cultured on mycobacterial medium. Isolation of NTM from a clinical specimen may reflect colonization and requires an assessment of the organism’s clinical significance.
• Isolation of NTM from blood specimens is clear evidence of disease; many NTM species require special media and will not grow in standard blood culture medium.
• The American Thoracic Society has published guidelines for the diagnosis of pulmonary NTM disease that require the growth of NTM from 2 of 3 sputum samples, a positive bronchoalveolar lavage sample, or a pulmonary parenchyma biopsy sample with granulomatous inflammation or mycobacteria found on section and NTM in culture. Although these guidelines are specific to MAC, M. abscessus, and M. kansasii, they probably apply to other NTM as well.
• The only antibiotic susceptibility assessment indicated is testing of MAC organisms for susceptibility to clarithromycin and of M. kansasii for susceptibility to rifampin.
TREATMENT Infections with NTM
Since NTM disease evolves over a long period, it is rarely necessary to begin treatment on an emergency basis before identifying the infecting species.
• MAC infection requires multidrug therapy with a macrolide (clarithromycin or azithromycin), ethambutol, and a rifamycin (rifampin or rifabutin). Therapy is prolonged, generally continuing for 12 months after culture conversion; typically, a course lasts for at least 18 months.
• M. kansasii lung disease is similar to TB in many ways and is also effectively treated with isoniazid (300 mg/d), rifampin (600 mg/d), and ethambutol (15 mg/kg per day).
• Extrapulmonary disease due to rapidly growing NTM is often treated successfully with a macrolide and another drug (with the choice based on in vitro susceptibility). Pulmonary disease due to M. abscessus is difficult to cure and often requires repeated courses that include a macrolide along with an IV-administered agent such as amikacin, a carbapenem, cefoxitin, or tigecycline.
• M. marinum infection is effectively treated with any combination of a macrolide, ethambutol, and a rifamycin for 1–2 months after clinical resolution of isolated soft-tissue disease; tendon and bone involvement may require longer courses in light of clinical evolution.
• Treatment of infections caused by other NTM is less well defined, but macrolides and aminoglycosides are usually effective, with other agents added as indicated.

For a more detailed discussion, see Raviglione MC, O’Brien RJ: Tuberculosis, Chap. 165, p. 1340; Gelber RH: Leprosy, Chap. 166, p. 1359; Holland SM: Nontuberculous Mycobacterial Infections, Chap. 167, p. 1367; and O’Donnell MR, Saukkonen JJ: Antimycobacterial Agents, Chap. 168, p. 1371, in HPIM-18.








