KEY CONCEPTS
Evidence is accumulating that maternal emotional and psychosocial experiences during pregnancy can be communicated to the fetus and influence fetal development and subsequent neurodevelopmental sequelae in offspring. It has been repeatedly documented that mothers who experience certain stressful life events during pregnancy deliver offspring with an increased risk of schizophrenia (e.g., Huttunen & Niskanen, 1978; Malaspina et al., 2008). This chapter will discuss the main research findings linking maternal stress during pregnancy with risk for schizophrenia. We also will discuss research performed in humans exploring how maternal stress during pregnancy is related to a number of obstetric events found in the histories of schizophrenia patients and is related to childhood problems that are frequently found in the premorbid period of schizophrenia. Although there are a number of preclinical studies examining stress during pregnancy and cognitive and brain abnormalities among offspring, we will focus on the primary evidence found in human studies, as these animal studies are extensively discussed in chapter 13 of this book.
Maternal Stress During Pregnancy and Risk of Schizophrenia in Offspring
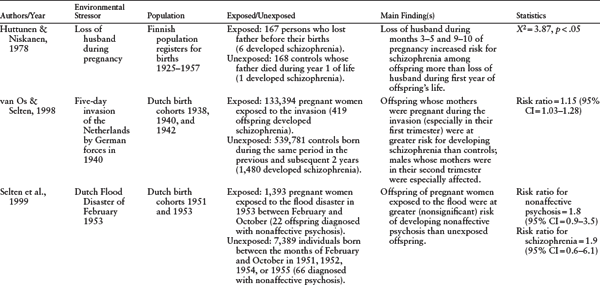
The first epidemiologic studies linking maternal stress during pregnancy to increased risk of schizophrenia in offspring were based on evidence from ecologic designs. (For a complete list of studies, see table 4.1.) Although there are some conflicting findings, many of these studies found that mothers who were exposed to a severe life event during pregnancy had offspring with an increased risk for schizophrenia. This association was first noted by Huttunen & Niskanen (1978), who found that mothers whose husbands died during months three to five or nine to ten of gestation had children who were more likely to develop schizophrenia.
Being pregnant during wartime and during natural disasters also has been associated with risk for schizophrenia in a number of studies. Specifically, children of mothers who were in their first and second trimesters of pregnancy during the May 1940 five-day invasion of the Netherlands by German forces were at a significantly increased risk for developing schizophrenia when compared to children of mothers who were pregnant two years before or after the invasion (van Os & Selten, 1998). However, the second-trimester finding appeared to be restricted to males, suggesting that male fetuses may be particularly vulnerable to maternal stress (van Os & Selten, 1998). Similarly, mothers who were in their second month of pregnancy during the 1967 Arab-Israeli War (the “Six Day War") also gave birth to offspring with a heightened risk of schizophrenia (Malaspina et al., 2008). Another study, which examined the effects of prenatal exposure to the 1953 Dutch Flood Disaster, also found an increased risk of schizophrenia in offspring; however, this finding failed to reach statistical significance, possibly because of a limited number of schizophrenia cases in the cohort (Selten et al., 1999).
Although the aforementioned studies suggest an association between maternal stress during pregnancy and increased risk for schizophrenia, ecologic studies are fraught with a number of methodologic concerns. Specifically, in all of these studies, stress was never measured in individuals but rather was assumed on the basis of life events that occurred for an entire population. Further, the aforementioned studies assessed life events that are relatively rare, severe, and likely could be associated with a range of potentially teratogenic conditions, such as malnutrition, increase in substance abuse, or other factors. The question remains as to whether severe life events, such as the ones described above, are measuring stress or other constructs such as fear, trauma, or both.
Nevertheless, three studies have prospectively measured maternal experiences that are presumed to be stressful during individual pregnancies and have linked these experiences with increased risk for schizophrenia in offspring. Khashan et al. (2008a) found that mothers who experienced a death or serious illness of one or more close relatives during the first trimester of pregnancy gave birth to offspring with a significantly increased risk of developing schizophrenia, independent of other factors associated with risk for the disorder, such as the offspring’s sex, age, family history of mental illness, place of birth, and maternal age. This is similar to the results of the population-based study by Huttunen & Niskanen (1978), except that the timing of the stressor during pregnancy differed. In a second study, Myhrman and colleagues (1996), in the Northern Finland 1966 birth cohort, found that unwantedness of a pregnancy, as rated by the mothers in the sixth or seventh month of gestation, led to a 2.5-fold increased risk of schizophrenia in offspring. In a replication attempt, Herman et al. (2006), in a birth cohort in northern California, found a similar association (unadjusted hazard ratio = 1.75, 95% CI = 0.97, 3.17) that fell slightly short of statistical significance. It is possible that the latter study failed to detect a significant result because of diminished power. Again, in these studies stress was not directly measured but rather presumed on the basis of an experience, namely unwantedness of the pregnancy, that could be associated with stress. Although it is possible that unwantedness of pregnancy is experienced as stressful to most women, it may not be the case for all women. Further, unwantedness of pregnancy also has the potential to be related to other behaviors or emotional states that could cause harm to the fetus, such as depression, substance use, lack of adequate prenatal care, or all of these.


CI = confidence interval; SSD = schizophrenia spectrum disorder.
SOURCE: Authors.
Hence, the existing studies linking stress during pregnancy to risk of psychosis in offspring are suggestive of a relationship, but lack considerable information on intermediate steps, such as maternal interpretation of life events, adequacy of coping resources, biological responses to stress, and engagement in behaviors risky to health. Despite these limitations, there is substantial evidence that fetal exposure to maternal stress is associated with a number of risk factors that have been consistently linked to schizophrenia, including obstetric complications and neurodevelopmental sequelae in offspring. In the remainder of the chapter, we will summarize these findings briefly and provide our views on how future research should proceed in investigating the role of maternal stress during pregnancy in the neurodevelopmental course of schizophrenia.
Maternal Stress During Pregnancy and Fetal Growth
Although there are some conflicting findings, patients with schizophrenia tend to have a history of lower birth weight and fetal growth than nonschizophrenia controls (see chapter 3, this book). Even though many factors can influence the growth of the fetus, it is now clear that maternal psychosocial stress during pregnancy and maternal biological responses to stress can greatly affect the growth of the fetus and result in decreased birth weight and fetal growth restriction. As discussed here, these effects appear to be dependent on the timing during pregnancy and the type of stress experienced by the mothers.
A number of studies have examined the relationship between stressful life events during pregnancy and fetal growth and low birth weight (LBW). Specifically, one study found that objective major life events in the third trimester, such as events considered to be more stressful than a wedding, were significantly associated with LBW (Newton & Hunt, 1984). It is interesting that smoking during pregnancy seemed to mediate this relationship, suggesting that behaviors risky to health may partially account for the relationship between serious life events and damage to the fetus. Similarly, Xiong et al. (2008) examined posttraumatic stress disorder (PTSD) and birth outcomes of women exposed to Hurricane Katrina. The authors found that women who were exposed to three or more severe hurricane-related events, such as feeling that one’s life was in danger and walking through flood waters, had a greater than threefold increased risk of delivering LBW infants. Neither general exposure to the hurricane nor the consequent development of PTSD was significantly associated with an increased risk of delivering an LBW infant. In a similar vein, death of a relative during pregnancy or in the six months before pregnancy has been associated with a significantly increased risk of babies weighing below the tenth percentile (Khashan et al., 2008b). Cumulatively, these findings suggest that a number of severe life events are associated with LBW and that smoking may be the link in this relationship for a portion of women. However, it remains unclear whether there are specific periods in gestation that are vulnerable to the effects of stress on LBW, given that there appears to be evidence for both early and late gestational effects of exposure to serious life events during pregnancy on fetal growth.
Another concern with the aforementioned studies is that documentation about maternal emotional responses to stressful life events was not entirely addressed. To this end, Wadhwa et al. (1993) examined the relationship between maternal appraisals of stress associated with life events and risk of LBW in a relatively healthy, nonsmoking pregnant population. It is interesting that maternal subjective reports of stress associated with life events in the first and second trimesters were significantly related to LBW; however, other measures of stress—such as chronic stress, daily hassles, psychological and physical symptom strain, and pregnancy-specific anxiety—were not related to LBW (although pregnancy-specific anxiety was significantly related to decreased gestational length). These results further support the association between maternal stressful life events during pregnancy and risk for decreased fetal growth. Moreover, these findings suggest that maternal appraisals of stress associated with the event may portend deleterious outcomes to the fetus even in the absence of behaviors that are clearly risky to health.
The question arises as to how maternal stress is translated to the fetus, ultimately resulting in disruptions in fetal development, namely fetal growth. One possibility is that fetal exposure to hormones that are associated with stress, such as glucocorticoids (e.g., cortisol), contributes to disruptions in fetal growth. Support for this idea comes from a number of studies that have found that increases in hormones associated with stress during pregnancy are related to decreased fetal growth and LBW. Specifically, one study found a significant association between the number of maternal prenatal corticosteroid administrations and decreased birth weight (French et al., 1999). Similarly, another study found that among women admitted for preterm labor and given a single prenatal treatment of glucocorticoids between 25 and 34 weeks, offspring born at term had significantly reduced length, weight, birth weight percentile, and head circumference, even when compared to offspring of mothers matched for pre-term labor admittance and gestational age and sex of infants (Davis et al., 2009). In concert with these findings, elevated levels of placental corticotropin-releasing hormone (CRH) in the thirty-third week of gestation also have been associated with increased rates of small-for-gestational-age (SGA) infants (Wadhwa et al., 2004). Further, offspring of women with high levels of CRH exhibited a 3.7-fold increased risk for fetal growth restriction over women with low CRH levels.
Lastly, it seems as if the timing of exposure to stress hormones and the sex of the fetus are key in determining the consequences to fetal growth and development. Specifically, Ellman et al. (2008) found that increases in maternal cortisol at 15 and 19 weeks gestation were significantly associated with decreases in physical and neuromuscular maturation in the newborn infant. Furthermore, these early cortisol increases were associated with a rise in placental CRH levels at 31 weeks gestation, which also led to decreases in infant physical and neuromuscular maturation, suggesting that early gestational cortisol may prime the placenta to release a surge of CRH later in gestation. In additional analyses that examined males and females separately, the above findings were significant only for males, providing support for the hypothesis that males may be more sensitive to fetal exposure to maternal stress.
Cumulatively, the extant literature suggests that stress hormones are integrally involved in fetal growth and that this involvement may be sex dependent. Altogether, studies indicate that maternal stress hormones during pregnancy may be related to a birth outcome that has been frequently found in the history of schizophrenia patients: decreased fetal growth (French et al., 1999; Wadhwa et al., 2004; Ellman et al., 2008; Davis et al., 2009).
Ethnic and Racial Differences in Responses to Stress During Pregnancy
A number of studies have found that there may be racial and ethnic disparities in schizophrenia (Fearon et al., 2006; Bresnahan et al., 2007). Specifically, individuals from African descent in the United States and the United Kingdom are at significantly increased risk for schizophrenia, even after controlling for a variety of demographic characteristics (Fearon et al., 2006; Bresnahan et al., 2007). Although the causes of this racial disparity remain elusive, there is evidence suggestive of a contribution beginning in utero.
African American women are at substantially increased risk for a variety of poor birth outcomes, such as preterm delivery and LBW (Alexander et al., 2003). However, these racial differences do not seem to be related to socioeconomic status or access to prenatal care (Lu & Halfon, 2003). It has been proposed that racial differences in poor birth outcomes may be attributed, in part, to dissimilar maternal experiences across racial groups. Specifically, one study found that perceived racism significantly predicted birth weight in African Americans but not in other racial or ethnic groups (Dominguez et al., 2008). Moreover, although the number of stressful life events during pregnancy seem to be higher among African American women than among other racial or ethnic groups, their reports of subjective stress and anxiety have been found to be lower, possibly because of underreporting or denial of stressful experiences (Johnson & Crowley, 1996; Dominguez et al., 2005). Nonetheless, these findings suggest that perceived racism and increases in stressful life events may contribute to the racial disparities found in LBW among African Americans and might contribute to the increased risk for schizophrenia.
It also appears as if African Americans exhibit a different profile of stress hormone secretion during pregnancy than other racial and ethnic groups. Specifically, relatively low cortisol levels during the second trimester of pregnancy have been found among African Americans as compared to other racial and ethnic groups (Glynn et al., 2007). This endocrine profile is consistent with a pattern of hypothalamo-pituitary adrenal (HPA) axis dysregulation present in those diagnosed with PTSD. This pattern has been interpreted as being attributable to a lifetime of exposure to increased stress, adverse socioeconomic circumstances, and racial bias (Glynn et al., 2007). Although decreases in cortisol may appear to argue against a direct teratogenic role of this hormone in increasing risk for subsequent neurodevelopmental sequelae, cortisol is essential for fetal growth and development (Welberg, Seckl, & Holmes, 2001; Trainer, 2002; Murphy et al., 2006). Therefore, lower levels of cortisol at key periods of fetal development can result in deleterious birth outcomes as well. It also appears as if African American women may be more sensitive to the effects of stress hormones during pregnancy, whereby smaller increases in stress hormones are more likely to lead to other deleterious birth outcomes, such as preterm delivery (Holzman et al., 2001). Moreover, because cortisol is a potent anti-inflammatory hormone, the damaging effects to the fetus could potentially be a result of relative increases in proinflammatory cytokines among African Americans (Miller, Cohen, & Ritchey, 2002). Taken together, these results support a complex scenario whereby fetal exposure to both decreased and increased levels of cortisol can lead to deleterious effects on birth outcomes (such as LBW) and potentially to risk for schizophrenia in adulthood. Although this latter relationship has not been tested, it is clear that issues of timing of exposure to stress, fetal sex, and maternal race or ethnicity are key factors that should be explored in future investigations.
Maternal Stress During Pregnancy and Immune Functioning
As discussed in chapters 1 and 10 of this book, a number of maternal infections during pregnancy (e.g., influenza, upper respiratory infections, and toxoplasma gondii) have been repeatedly linked to increased risk of schizophrenia through a series of ecologic and, more recently, serologic studies (Brown & Derkits, 2010). Nevertheless, many infections do not appear to cross the placenta; therefore, the deleterious influences to fetal brain development seem related to maternal antiviral responses to infection, such as increases in proinflammatory cytokines (Patterson, 2009). Two studies have found that elevations in proinflammatory cytokines during pregnancy result in an increased risk of schizophrenia in offspring (Buka et al., 2001; Brown et al., 2004).
There is a preponderance of evidence that both psychological stress and neuroendocrine markers of stress affect immune functioning and susceptibility to infection, depending on the duration and severity of the stressor. Sympathetic neuronal fibers directly innervate both primary (bone marrow and thymus) and secondary (spleen and lymph nodes) lymphoid tissues, releasing hormones that bind to receptors on leukocytes, leading to multiple changes in their distribution and function (Felten & Felten, 1994; Ader, Felten, & Cohen, 2001). Similarly, multiple studies have demonstrated direct interactions between inflammatory mediators and the HPA axis. Both animal and human studies have linked multiple proinflammatory cytokines, such as interleukin-1 (IL-1), tumor necrosis factor- α (TNF-α), interleukin-8 (IL-8), and interleukin-6 (IL-6) with activation of the HPA axis, including direct activation of CRH production by the hypothalamus (Cupps & Fauci, 1982; Fontana, Weber, & Dayer, 1984; Sapolsky et al., 1987; Bernardini et al., 1990; Buckingham et al., 1994; Farina & Winkelman, 2005). Although glucocorticoids act in certain circumstances as potent anti-inflammatory agents, numerous studies suggest that exposure to stressors can lead to relative increases in inflammatory cytokines, likely due to an inflammatory rebound that occurs once the stressor has ceased (Miller, Cohen, & Ritchey, 2002). This assertion is supported by a number of studies (Segerstrom & Miller, 2004). Consistent with these findings, stress has been shown to exacerbate the consequences of influenza infections in humans and alter cytokine production in respiratory infections (Cohen, Doyle, & Skoner, 1999).
Despite consistently documented relationships between stress and immune functioning, very few studies have examined this association in pregnant populations, although the results of available studies are consistent with those from studies of nonpregnant populations. Specifically, one study found that maternal self-reported elevations in stress during pregnancy were positively correlated with higher levels of the proinflammatory cytokines IL-6 and TNF-α and with low levels of the anti-inflammatory cytokine IL-10 (Coussons-Read et al., 2005). Furthermore, increases in cortisol during weeks 19 through 21, 23 through 26, and 31 through 35 of gestation have been associated with greater prevalence of genitourinary infection (Ruiz et al., 2001).
Altogether, the aforementioned studies suggest that maternal stress during pregnancy may contribute to the previously observed relationship between maternal infection during pregnancy and risk for schizophrenia. Specifically, maternal stress during pregnancy could result in increased susceptibility to infection, more severe infections, and larger inflammatory responses to infection. Further, maternal stress during pregnancy has been found to lead to increases in inflammation in the absence of known infections (Coussons-Read et al., 2005), suggesting that the previously observed associations between increases in proinflammatory cytokines during pregnancy and risk for schizophrenia may be partially accounted for by maternal stress during pregnancy.
Maternal Stress During Pregnancy and Hypoxia-Associated Obstetric Complications
Some evidence suggests that maternal stress during pregnancy is associated with fetal hypoxia (reduced oxygen delivery to the fetus) and hypoxia-associated obstetric complications. Specifically, studies have investigated the relationship between stress and pregnancy-induced hypertension, a maternal health complication commonly associated with fetal hypoxia (Sharma, Norris, & Kalkunte, 2010). These studies are particularly relevant to schizophrenia research, as hypoxia-associated obstetric complications have been consistently linked to an increased risk of schizophrenia, an earlier age of onset for the disorder, and more severe brain abnormalities among patients (Cannon, 1997; McGorry et al., 2001; Cannon et al., 2002; Van Erp et al., 2002; McGorry et al., 2008; McGrath, 2008) (see chapter 3, this book). Specifically, in an examination of psychosocial work stress and pregnancy-induced hypertension, Landsbergis and Hatch (1996) found that women working outside of the home had a significantly increased incidence of pregnancy-induced hypertension than did unemployed pregnant women. However, this increased incidence was explained partly by confounding variables, and the mechanisms driving this finding were not elucidated. Similarly, Vollebregt and colleagues (2008) also discovered that working was associated with approximately twice the risk for development of preeclampsia and gestational hypertension than was not working. A more in-depth analysis revealed that this risk was explained significantly by having perceived control at work, as high or moderate work control reduced the discrepancy of these outcomes between the working and not working groups, whereas low work control increased the risks of developing preeclampsia and gestational hypertension considerably; job strain also resulted in approximately twice the risk. Notably, all of these associations were no longer significant after controlling for medical and socioeconomic covariates, again bringing the direct relationship of work stress and preeclampsia into question.
Other maternal stressors during pregnancy also have been studied in relation to hypoxia-associated obstetric complications. Specifically, in a population of black African women in Nigeria, Anorlu, Iwuala, & Odum (2005) found that a stressful home environment and stressful work during pregnancy (defined by a 5-level activity score) both were significantly associated with increased risk for developing preeclampsia. As the aforementioned studies attributed working outside the home, low work control, and high job strain to increased risk of this outcome, this study found that work per se during pregnancy was not significantly associated with preeclampsia, suggesting that specific types of stress associated with work may be involved in the development of the condition. In contrast, Sikkema and colleagues (2001) studied white women who developed preeclampsia after 33 weeks of gestation and found no significantly increased levels of cortisol, state or trait anxiety scores, or pregnancy-specific anxiety over those of normotensive control women. These apparent discrepancies may be related to racial differences in responses to stress during pregnancy, as one study found that maternal stress during pregnancy was related to elevated blood pressure in African Americans but not whites (Hilmert et al., 2008). Lastly, one study found that elevated levels of the stress hormone CRH led to restricted blood flow to the fetus, measured through ultrasonography (Harville et al., 2008).
In sum, it remains unclear whether maternal stress during pregnancy is related to increased risk for hypoxia-associated obstetric complications. There is some evidence that maternal stress during pregnancy may place individuals of African descent at increased risk for hypoxia-associated obstetric complications, namely through increases in blood pressure. In addition, there is preliminary evidence that elevations in maternal stress hormones during pregnancy lead to restricted blood flow and oxygen to the fetus. These data suggest a possible relationship between fetal hypoxia and maternal stress during pregnancy, although this question warrants further investigation.
Maternal Stress During Pregnancy and Eating Behaviors
As other chapters in this book describe in detail (chapters 2 and 12), there is growing support for a relationship between maternal nutrition and risk for schizophrenia in offspring. Specifically, both prenatal famine and increased prepregnancy body mass index (BMI) have been associated with an increased risk for schizophrenia, implicating nutritional factors in the pathogenesis of the disorder (Schaefer et al., 2000; Brown & Susser, 2008). Further, elevated homocysteine levels, which occur secondary to folate deficiency during pregnancy, also have been associated with increased risk for schizophrenia (Brown et al., 2007). It is becoming increasingly clear that stress affects eating behaviors in a variety of ways. Specifically, stress has been associated with changes in the amount of food consumed, and it influences food preference and choices. In both laboratory and naturalistic environments, studies have consistently demonstrated that people exposed to stressful situations prefer sweeter foods and foods with a higher fat content (Oliver, Wardle, & Gibson, 2000; Epel et al., 2001). Although stress also has been associated with dietary restriction, this finding may be limited to normal-weight women (Epel et al., 2001; Newman, O’Connor, & Conner, 2007; Habhab, Sheldon, & Loeb, 2009; Rutters et al., 2009a, 2009b). These studies raise the possibility that the relationship between maternal nutrition during pregnancy and risk for schizophrenia likely operates within a context of maternal experiences and, potentially, stress-related behaviors that should be considered in investigations that explore the long-term sequelae of nutrients during pregnancy.
Maternal Stress During Pregnancy and Childhood Outcomes
Maternal stress during pregnancy also has been associated with a range of childhood disturbances—including cognitive, social, and emotional difficulties—that commonly occur during the premorbid period of schizophrenia (Jones et al., 1994; Cannon et al., 1997; Cannon et al., 1999; Bearden et al., 2000; Niendam et al., 2003). Specifically, multiple types of stressors during pregnancy (e.g., life events, pregnancy anxiety, daily hassles) have been associated with a range of cognitive problems in offspring, including decreased IQ, decreased language functioning, poor attention regulation, and difficulties adapting to new situations (Buitelaar et al., 2003; Huizink et al., 2003; Slykerman et al., 2005; Laplante et al., 2008). Stress during pregnancy and elevations in stress hormones have also been linked to changes in offspring behavior, such as displaying a fearful temperament (de Weerth, van Hees, & Buitelaar, 2003; Davis et al., 2005). Moreover, in an examination of children six to nine years of age, Buss and colleagues (2010) found that maternal self-report of pregnancy anxiety at 19 weeks gestation was associated with gray matter volume reductions specifically in six distinct brain regions, including the prefrontal cortex and the medial temporal lobe, abnormalities of which have been consistently implicated in volumetric brain imaging studies in schizophrenia (Wright et al., 2000; Gur, Keshavan, & Lawrie, 2007). Cumulatively, these studies lend support to the putative role of maternal stress during pregnancy in neurodevelopmental precursors of schizophrenia; however, there still is a paucity of long-term follow-up studies to confirm these associations.
Discussion and Future Directions
There is emerging evidence of an association between maternal stress during pregnancy and increased risk for schizophrenia. This evidence derives from multiple lines of investigation, including the relationship between severe stressful life events and schizophrenia in ecologic studies, between maternal stress during pregnancy and obstetric complications that are found in the histories of schizophrenia patients, and between maternal stress during pregnancy and childhood disturbances that occur more commonly in the premorbid period of individuals diagnosed with schizophrenia as opposed to controls.
There are, however, a number of limitations in previous studies that should be investigated in future work. Specifically, no study, to our knowledge, has prospectively examined different types of maternal stress during pregnancy, such as perceived stress and appraisals of life events, in relation to risk for the subsequent development of schizophrenia in offspring. These studies are necessary, as only rare and severe stressful life events during pregnancy (e.g., death of a spouse or exposure to war) have been examined thus far in relation to schizophrenia with no information on maternal cognitive or emotional responses to these events (Huttunen & Niskanen, 1978; Malaspina et al., 2008). Therefore, it is possible that the findings from existing studies may not generalize to most pregnant populations. Moreover, no study has determined whether maternal neuroendocrine responses to stress during pregnancy are related to the development of schizophrenia in offspring. This is necessary for us to begin to understand how maternal subjective experiences of stress during pregnancy are translated to the fetus, subsequently leading to risk for future developmental disruptions.
The role of ethnic and racial differences in relation to maternal stress during pregnancy and subsequent risk for schizophrenia also should be considered in future investigations. As reviewed earlier in this chapter (see “Ethnic and Racial Differences in Responses to Stress During Pregnancy"), there are differences in the number of life events, appraisals of life events, and endocrine responses to stress in African American populations (Dominguez et al., 2005; Glynn et al., 2007; Dominguez et al., 2008). Given these differences, it is clear that future studies would benefit by incorporating race and ethnicity into explanatory models of the relationship between prenatal stress and schizophrenia.
As discussed previously in this chapter (see “Maternal Stress During Pregnancy and Fetal Growth”), there also are findings suggesting that the timing of exposure to stress and the sex of the fetus may be important in determining the future outcomes of offspring (e.g., Ellman et al., 2008). Although this area has been relatively understudied, there is evidence for both late and early effects of stress on detrimental outcomes to offspring. Consequently, we believe that this line of research is an important next step for future work.
It also is clear that maternal behaviors during pregnancy can be affected by stress and other emotional states, some of which can have damaging influences on fetal development. In this chapter, we discussed the relationship between stress and eating (see “Maternal Stress During Pregnancy and Eating Behaviors"); however, it is clear that stress can influence rates of substance abuse and smoking, as well as cause disruptions in sleep, all of which have the potential to induce teratogenic effects (Oliver, Wardle, & Gibson, 2000; Repetti, Taylor, & Seeman, 2002; Irwin et al., 2008; Rutters et al., 2009b; Chang et al., 2010). Incorporating into future studies information on behaviors during pregnancy that are risky to maternal health may be critical in understanding important intermediate steps between prenatal stress and subsequent risk for schizophrenia.
Lastly, we discussed how maternal stress during pregnancy has been associated with a number of obstetric events that have been consistently linked to schizophrenia (see “Maternal Stress During Pregnancy and Hypoxia-Associated Obstetric Complications"). Determining whether maternal stress during pregnancy acts as an antecedent to other obstetric risk factors observed in the etiology of schizophrenia has not only the potential to elucidate important causal pathways in the development of the disorder but also the potential to influence both primary prevention strategies and potential interventions in pregnant populations.
KEY AREAS FOR FUTURE RESEARCH
Acknowledgments
Mary Iampietro was supported by a Temple University start-up award to Dr. Ellman. The authors also would like to thank Alan Brown for his helpful edits.
Selected Readings
Cohen, S., Kessler, R. C., & Gordon, L. U. (1997). Measuring Stress: A Guide for Health and Social Scientists. New York: Oxford University Press.
Dunkel Schetter, C. (2011). Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology 62: 531–558.
Ellman, L. M. & Susser, E. S. (2009). The promise of epidemiologic studies: Neuroimmune mechanisms in the etiologies of brain disorders. Neuron 64(1): 25–27.
Felten, S. Y. & Felten, D. (1994). Neural-immune interaction. Progress in Brain Research 100: 157–162.
Kinsella, M. T. & Monk, C. (2009). Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clinical Obstetrics and Gynecology 52(3): 425–440.
Miller, G. E., Cohen, S., & Ritchey, A. K. (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology 21(6): 531–541.
Segerstrom, S. C. & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin 130(4): 601–630.
Talge, N. M., Neal, C., & Glover, V. (2007). Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? Journal of Child Psychology and Psychiatry 48(3–4): 245–261.
Wadhwa, P. D., Buss, C., Entringer, S., & Swanson, J. M. (2009). Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Seminars in Reproductive Medicine 27(5): 358–368.
Weinstock, M. (2008). The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews 32(6): 1073–1086.
References
Ader, R., Felten, D. L., & Cohen, N. (2001). Psychoneuroimmunology (3rd ed.). San Diego: Academic.
Alexander, G. R., Kogan, M., Bader, D., Carlo, W., Allen, M., & Mor, J. (2003). US birth weight/gestational age-specific neonatal mortality: 1995–1997 rates for whites, Hispanics, and blacks. Pediatrics 111(1): e61–e66.
Anorlu, R. I., Iwuala, N. C., & Odum, C. U. (2005). Risk factors for pre-eclampsia in Lagos, Nigeria. Australian and New Zealand Journal of Psychiatry 45(4): 278–282.
Bearden, C. E., Rosso, I. M., Hollister, J. M., Sanchez, L. E., Hadley, T., & Cannon, T. D. (2000). A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophrenia Bulletin 26(2): 395–410.
Bernardini, R., Kamilaris, T. C., Calogero, A. E., Johnson, E. O., Gomez, M. T., Gold, P. W., & Chrousos, G. P. (1990). Interactions between tumor necrosis factor-alpha, hypothalamic corticotropin-releasing hormone, and adrenocorticotropin secretion in the rat. Endocrinology 126(6): 2876–2881.
Bresnahan, M., Begg, M. D., Brown, A., Schaefer, C., Sohler, N., Insel, B., Vella, L., & Susser, E. (2007). Race and risk of schizophrenia in a US birth cohort: Another example of health disparity? International Journal of Epidemiology 36(4): 751–758.
Brown, A. S., Bottiglieri, T., Schaefer, C. A., Quesenberry, C. P., Jr., Liu, L., Bresnahan, M., & Susser, E. S. (2007). Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Archives of General Psychiatry 64(1): 31–39.
Brown, A. S. & Derkits, E. J. (2010). Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. American Journal of Psychiatry 167(3): 261–280.
Brown, A. S., Hooton, J., Schaefer, C. A., Zhang, H., Petkova, E., Babulas, V., Perrin, M., Gorman, J. M., & Susser, E. S. (2004). Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry 161(5): 889–895.
Brown, A. S. & Susser, E. S. (2008). Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophrenia Bulletin 34(6): 1054–1063.
Buckingham, J. C., Loxley, H. D., Taylor, A. D., & Flower, R. J. (1994). Cytokines, glucocorticoids and neuroendocrine function. Pharmacological Research 30(1): 35–42.
Buitelaar, J. K., Huizink, A. C., Mulder, E. J., de Medina, P. G., & Visser, G. H. (2003). Prenatal stress and cognitive development and temperament in infants. Neurobiology of Aging 24(Suppl 1): S53–S60; discussion S67–S68.
Buka, S. L., Tsuang, M. T., Torrey, E. F., Klebanoff, M. A., Wagner, R. L., & Yolken, R. H. (2001). Maternal cytokine levels during pregnancy and adult psychosis. Brain, Behavior, and Immunology 15(4): 411–420.
Buss, C., Davis, E. P., Muftuler, L. T., Head, K., & Sandman, C. A. (2010). High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 35(1): 141–153.
Cannon, M., Jones, P., Gilvarry, C., Rifkin, L., McKenzie, K., Foerster, A., & Murray, R. M. (1997). Premorbid social functioning in schizophrenia and bipolar disorder: Similarities and differences. American Journal of Psychiatry 154(11): 1544–1550.
Cannon, M., Jones, P., Huttunen, M. O., Tanskanen, A., Huttunen, T., Rabe-Hesketh, S., & Murray, R. M. (1999). School performance in Finnish children and later development of schizophrenia: A population-based longitudinal study. Archives of General Psychiatry 56(5): 457–463.
Cannon, T. D. (1997). On the nature and mechanisms of obstetric influences in schizophrenia: A review and synthesis of epidemilogic studies. International Review of Psychiatry 9: 387–397.
Cannon, T. D., van Erp, T. G., Rosso, I. M., Huttunen, M., Lonnqvist, J., Pirkola, T., Salonen, O., Valanne, L., Poutanen, V. P., & Standertskjold-Nordenstam, C. G. (2002). Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Archives of General Psychiatry 59(1): 35–41.
Chang, J. J., Pien, G. W., Duntley, S. P., & Macones, G. A. (2010). Sleep deprivation during pregnancy and maternal and fetal outcomes: Is there a relationship? Sleep Medicine Reviews 14(2): 107–114.
Cohen, S., Doyle, W. J., & Skoner, D. P. (1999). Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosomatic Medicine 61(2): 175–180.
Coussons-Read, M. E., Okun, M. L., Schmitt, M. P., & Giese, S. (2005). Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic Medicine 67(4): 625–631.
Cupps, T. R. & Fauci, A. S. (1982). Corticosteroid-mediated immunoregulation in man. Immunological Reviews 65: 133–155.
Davis, E. P., Glynn, L. M., Dunkel Schetter, C., Hobel, C., Chicz-Demet, A., & Sandman, C. A. (2005). Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Developmental Neuroscience 27(5): 299–305.
Davis, E. P., Waffarn, F., Uy, C., Hobel, C. J., Glynn, L. M., & Sandman, C. A. (2009). Effect of prenatal glucocorticoid treatment on size at birth among infants born at term gestation. Journal of Perinatology 29(11): 731–737.
de Weerth, C., van Hees, Y., & Buitelaar, J. K. (2003). Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development 74(2): 139–151.
Dominguez, T. P., Dunkel-Schetter, C., Glynn, L. M., Hobel, C., & Sandman, C. A. (2008). Racial differences in birth outcomes: The role of general, pregnancy, and racism stress. Health Psychology 27(2): 194–203.
Dominguez, T. P., Schetter, C. D., Mancuso, R., Rini, C. M., & Hobel, C. (2005). Stress in African American pregnancies: Testing the roles of various stress concepts in prediction of birth outcomes. Annals of Behavioral Medicine 29(1): 12–21.
Ellman, L. M., Schetter, C. D., Hobel, C. J., Chicz-Demet, A., Glynn, L. M., & Sandman, C. A. (2008). Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology 50(3): 232–241.
Epel, E., Lapidus, R., McEwen, B., & Brownell, K. (2001). Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26(1): 37–49.
Farina, L. & Winkelman, C. (2005). A review of the role of proinflammatory cytokines in labor and noninfectious preterm labor. Biological Research for Nursing 6(3): 230–238.
Fearon, P., Kirkbride, J. B., Morgan, C., Dazzan, P., Morgan, K., Lloyd, T., Hutchinson, G., Tarrant, J., Fung, W. L., Holloway, J., et al. (2006). Incidence of schizophrenia and other psychoses in ethnic minority groups: Results from the MRC AESOP Study. Psychological Medicine 36(11): 1541–1550.
Felten, S. Y. & Felten, D. (1994). Neural-immune interaction. Progress in Brain Research 100: 157–162.
Fontana, A., Weber, E., & Dayer, J. M. (1984). Synthesis of interleukin 1/endogenous pyrogen in the brain of endotoxin-treated mice: A step in fever induction? Journal of Immunology 133(4): 1696–1698.
French, N. P., Hagan, R., Evans, S. F., Godfrey, M., & Newnham, J. P. (1999). Repeated antenatal corticosteroids: Size at birth and subsequent development. American Journal of Obstetrics and Gynecology 180(1 Pt 1): 114–121.
Glynn, L. M., Schetter, C. D., Chicz-DeMet, A., Hobel, C. J., & Sandman, C. A. (2007). Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides 28(6): 1155–1161.
Gur, R. E., Keshavan, M. S., & Lawrie, S. M. (2007). Deconstructing psychosis with human brain imaging. Schizophrenia Bulletin 33(4): 921–931.
Habhab, S., Sheldon, J. P., & Loeb, R. C. (2009). The relationship between stress, dietary restraint, and food preferences in women. Appetite 52(2): 437–444.
Harville, E. W., Savitz, D. A., Dole, N., Herring, A. H., Thorp, J. M., & Light, K. C. (2008). Stress and placental resistance measured by Doppler ultrasound in early and mid-pregnancy. Ultrasound in Obstetrics and Gynecology 32(1): 23–30.
Herman, D. B., Brown, A. S., Opler, M. G., Desai, M., Malaspina, D., Bresnahan, M., Schaefer, C. A., & Susser, E. S. (2006). Does unwantedness of pregnancy predict schizophrenia in the offspring? Findings from a prospective birth cohort study. Social Psychiatry and Psychiatric Epidemiology 41(8): 605–610.
Hilmert, C. J., Schetter, C. D., Dominguez, T. P., Abdou, C., Hobel, C. J., Glynn, L., & Sandman, C. (2008). Stress and blood pressure during pregnancy: Racial differences and associations with birthweight. Psychosomatic Medicine 70(1): 57–64.
Holzman, C., Jetton, J., Siler-Khodr, T., Fisher, R., & Rip, T. (2001). Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstetrics and Gynecology 97(5 Pt 1): 657–663.
Huizink, A. C., Robles de Medina, P. G., Mulder, E. J., Visser, G. H., & Buitelaar, J. K. (2003). Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry 44(6): 810–818.
Huttunen, M. O. & Niskanen, P. (1978). Prenatal loss of father and psychiatric disorders. Archives of General Psychiatry 35(4): 429–431.
Irwin, M. R., Wang, M., Ribeiro, D., Cho, H. J., Olmstead, R., Breen, E. C., Martinez-Maza, O., & Cole, S. (2008). Sleep loss activates cellular inflammatory signaling. Biological Psychiatry 64(6): 538–540.
Johnson, R. & Crowley, J. (1996). An analysis of stress denial. In H. Neighbors & J. Jackson (eds.), Mental Health in Black America (pp. 62–76). Thousand Oaks: Sage.
Jones, P., Rodgers, B., Murray, R., & Marmot, M. (1994). Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 344(8934): 1398–1402.
Khashan, A. S., Abel, K. M., McNamee, R., Pedersen, M. G., Webb, R. T., Baker, P. N., Kenny, L. C., & Mortensen, P. B. (2008a). Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of General Psychiatry 65(2): 146–152.
Khashan, A. S., McNamee, R., Abel, K. M., Pedersen, M. G., Webb, R. T., Kenny, L. C., Mortensen, P. B., & Baker, P. N. (2008b). Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosomatic Medicine 70(6): 688–694.
Landsbergis, P. A. & Hatch, M. C. (1996). Psychosocial work stress and pregnancy-induced hypertension. Epidemiology 7(4): 346–351.
Laplante, D. P., Brunet, A., Schmitz, N., Ciampi, A., & King, S. (2008). Project Ice Storm: Prenatal maternal stress affects cognitive and linguistic functioning in 51/2-year-old children. Journal of the American Academy of Child and Adolescent Psychiatry 47(9): 1063–1072.
Lu, M. C. & Halfon, N. (2003). Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal 7(1): 13–30.
Malaspina, D., Corcoran, C., Kleinhaus, K. R., Perrin, M. C., Fennig, S., Nahon, D., Friedlander, Y., & Harlap, S. (2008). Acute maternal stress in pregnancy and schizophrenia in offspring: A cohort prospective study. BMC Psychiatry 8(71).
McGorry, P. D., Yung, A. R., Bechdolf, A., & Amminger, P. (2008). Back to the future: Predicting and reshaping the course of psychotic disorder. Archives of General Psychiatry 65(1): 25–27.
McGorry, P. D., Yung, A. R., & Phillips, L. J. (2001). “Closing In": What features predict the onset of first-episode psychosis within an ultra-high-risk group? In C. Schulz & R. B. Zipursky (eds.), The Early Stages of Schizophrenia (pp. 3–32). Washington, DC: American Psychiatric Press.
McGrath, J. (2008). Hypotheses desert us, while data defend us. Schizophrenia Research 102(1–3): 27–28.
Miller, G. E., Cohen, S., & Ritchey, A. K. (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology 21(6): 531–541.
Murphy, V. E., Smith, R., Giles, W. B., & Clifton, V. L. (2006). Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocrine Reviews 27(2): 141–169.
Myhrman, A., Rantakallio, P., Isohanni, M., Jones, P., & Partanen, U. (1996). Unwantedness of a pregnancy and schizophrenia in the child. British Journal of Psychiatry 169(5): 637–640.
Newman, E., O’Connor, D. B., & Conner, M. (2007). Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology 32(2): 125–132.
Newton, R. W. & Hunt, L. P. (1984). Psychosocial stress in pregnancy and its relation to low birth weight. British Medical Journal (Clinical Research Ed) 288(6425): 1191–1194.
Niendam, T. A., Bearden, C. E., Rosso, I. M., Sanchez, L. E., Hadley, T., Nuechterlein, K. H., & Cannon, T. D. (2003). A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. American Journal of Psychiatry 160(11): 2060–2062.
Oliver, G., Wardle, J., & Gibson, E. L. (2000). Stress and food choice: A laboratory study. Psychosomatic Medicine 62(6): 853–865.
Patterson, P. H. (2009). Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behavioural Brain Research 204(2): 313–321.
Repetti, R. L., Taylor, S. E., & Seeman, T. E. (2002). Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin 128(2): 330–366.
Ruiz, R. J., Fullerton, J., Brown, C. E., & Schoolfield, J. (2001). Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biological Research for Nursing 3(1): 39–48.
Rutters, F., Nieuwenhuizen, A. G., Lemmens, S. G., Born, J. M., & Westerterp-Plantenga, M. S. (2009a). Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring) 17(1): 72–77.
Rutters, F., Nieuwenhuizen, A. G., Lemmens, S. G., Born, J. M., & Westerterp-Plantenga, M. S. (2009b). Hyperactivity of the HPA axis is related to dietary restraint in normal weight women. Physiology and Behavior 96(2): 315–319.
Sapolsky, R., Rivier, C., Yamamoto, G., Plotsky, P., & Vale, W. (1987). Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238(4826): 522–524.
Schaefer, C. A., Brown, A. S., Wyatt, R. J., Kline, J., Begg, M. D., Bresnahan, M. A., & Susser, E. S. (2000). Maternal prepregnant body mass and risk of schizophrenia in adult offspring. Schizophrenia Bulletin 26(2): 275–286.
Segerstrom, S. C. & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin 130(4): 601–630.
Selten, J. P., van der Graaf, Y., van Duursen, R., Gispen-de Wied, C. C., & Kahn, R. S. (1999). Psychotic illness after prenatal exposure to the 1953 Dutch Flood Disaster. Schizophrenia Research 35(3): 243–245.
Sharma, S., Norris, W. E., & Kalkunte, S. (2010). Beyond the threshold: An etiological bridge between hypoxia and immunity in preeclampsia. Journal of Reproductive Immunology 85: 112–116.
Sikkema, J. M., Robles de Medina, P. G., Schaad, R. R., Mulder, E. J., Bruinse, H. W., Buitelaar, J. K., Visser, G. H., & Franx, A. (2001). Salivary cortisol levels and anxiety are not increased in women destined to develop preeclampsia. Journal of Psychosomatic Research 50(1): 45–49.
Slykerman, R. F., Thompson, J. M., Pryor, J. E., Becroft, D. M., Robinson, E., Clark, P. M., Wild, C. J., & Mitchell, E. A. (2005). Maternal stress, social support and preschool children’s intelligence. Early Human Development 81(10): 815–821.
Trainer, P. J. (2002). Corticosteroids and pregnancy. Seminars in Reproductive Medicine 20(4): 375–380.
Van Erp, T. G., Saleh, P. A., Rosso, I. M., Huttunen, M., Lonnqvist, J., Pirkola, T., Salonen, O., Valanne, L., Poutanen, V. P., Standertskjold-Nordenstam, C. G., et al. (2002). Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. American Journal of Psychiatry 159(9): 1514–1520.
van Os, J. & Selten, J. P. (1998). Prenatal exposure to maternal stress and subsequent schizophrenia: The May 1940 invasion of The Netherlands. British Journal of Psychiatry 172: 324–326.
Vollebregt, K. C., van der Wal, M. F., Wolf, H., Vrijkotte, T. G., Boer, K., & Bonsel, G. J. (2008). Is psychosocial stress in first ongoing pregnancies associated with pre-eclampsia and gestational hypertension? Bjog 115(5): 607–615.
Wadhwa, P. D., Garite, T. J., Porto, M., Glynn, L., Chicz-DeMet, A., Dunkel-Schetter, C., & Sandman, C. A. (2004). Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. American Journal of Obstetrics and Gynecology 191(4): 1063–1069.
Wadhwa, P. D., Sandman, C. A., Porto, M., Dunkel-Schetter, C., & Garite, T. J. (1993). The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. American Journal of Obstetrics and Gynecology 169(4): 858–865.
Welberg, L. A., Seckl, J. R., & Holmes, M. C. (2001). Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: Possible implications for behaviour. Neuroscience 104(1): 71–79.
Wright, I. C., Rabe-Hesketh, S., Woodruff, P. W., David, A. S., Murray, R. M., & Bullmore, E. T. (2000). Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry 157(1): 16–25.
Xiong, X., Harville, E. W., Mattison, D. R., Elkind-Hirsch, K., Pridjian, G., & Buekens, P. (2008). Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. American Journal of Medical Sciences 336(2): 111–115.