
IN CONTEXT
Physics
1913 Niels Bohr uses the concept of quantized light to explain the specific energy levels associated with electrons inside atoms.
1924 Louis de Broglie proposes that just as light can exhibit particle-like properties so, on the smallest scale, particles might sometimes show wave-like behaviour.
1927 Heisenberg and Bohr put forward the highly influential Copenhagen interpretation of the way that quantum-level events affect the large-scale (macroscopic) world.
1929 Heisenberg and Wolfgang Pauli work on the development of quantum field theory, whose foundations have been laid by Paul Dirac.
Following Louis de Broglie’s suggestion in 1924 that on the smallest scales of matter, subatomic particles could display wave-like properties, a number of physicists turned their attention to the question of understanding how the complex properties of an atom could arise from the interaction of “matter waves” associated with its constituent particles. In 1925, German scientists Werner Heisenberg, Max Born, and Pascual Jordan used “matrix mechanics” to model the hydrogen atom’s development over time. This approach was later supplanted by Erwin Schrödinger’s wavefunction.
Working with Danish physicist Niels Bohr, Heisenberg built on Schrödinger’s work to develop the “Copenhagen interpretation” of the way that quantum systems, governed by the laws of probability, interact with the large-scale world. One key element of this is the “uncertainty principle”, which limits the accuracy to which we can determine properties in quantum systems.
The uncertainty principle arose as a mathematical consequence of matrix mechanics. Heisenberg realized that his mathematical method would not allow certain pairs of properties to be determined simultaneously with absolute precision. For example, the more accurately one measures a particle’s position, the less accurately one can determine its momentum, and vice versa. Heisenberg found that for these two properties in particular, the relationship could be written as: ΔxΔp ≥ ħ/2 where Δx is the uncertainty of position, Δp the uncertainty of momentum, and h is a modified version of Planck’s constant.
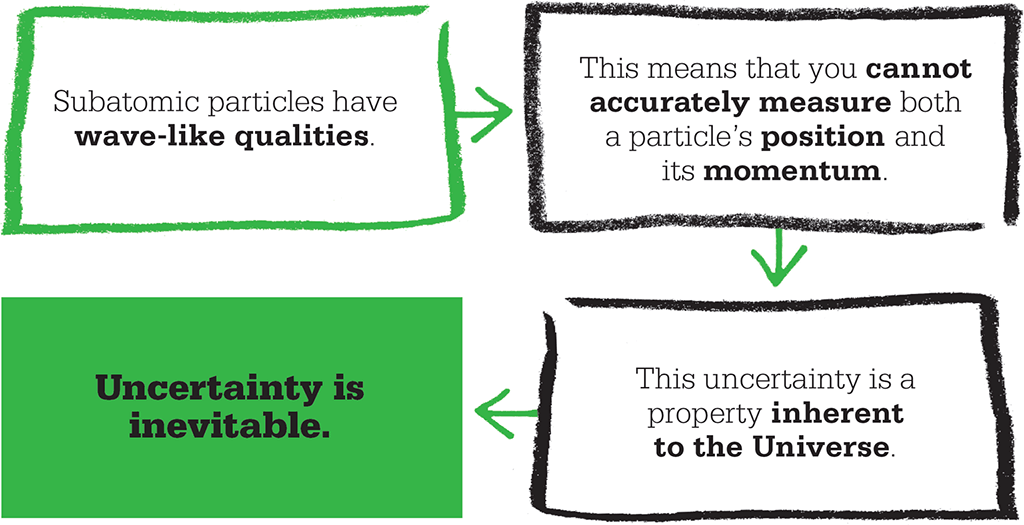
An uncertain Universe
The uncertainty principle is often described as a consequence of quantum-scale measurements – for instance, it is sometimes said that determining a subatomic particle’s position involves the application of a force of some sort that means its kinetic energy and momentum are less well defined. This explanation, put forward at first by Heisenberg himself, led various scientists including Einstein to spend time devising thought-experiments that might obtain a simultaneous and accurate measurement of position and momentum by some form of “trickery”. However, the truth is far stranger – it turns out that uncertainty is an inherent feature of quantum systems.
A helpful way of thinking about the issue is to consider the matter waves associated with the particles: in this situation, the particle’s momentum affects its overall energy and therefore its wavelength – but the more tightly we pin down the particle’s position, the less information we have about its wavefunction, and therefore about its wavelength. Conversely, accurately measuring the wavelength requires us to consider a broader region of space, and therefore sacrifices information about the particle’s precise location. Such ideas might seem strangely at odds with those we experience in the large-scale world, but they have nevertheless been proved real by many experiments, and form an important foundation of modern physics. The uncertainty principle explains seemingly strange real-life phenomena such as quantum tunnelling, in which a particle can “tunnel” through a barrier even if its energy suggests that it should not be able to.
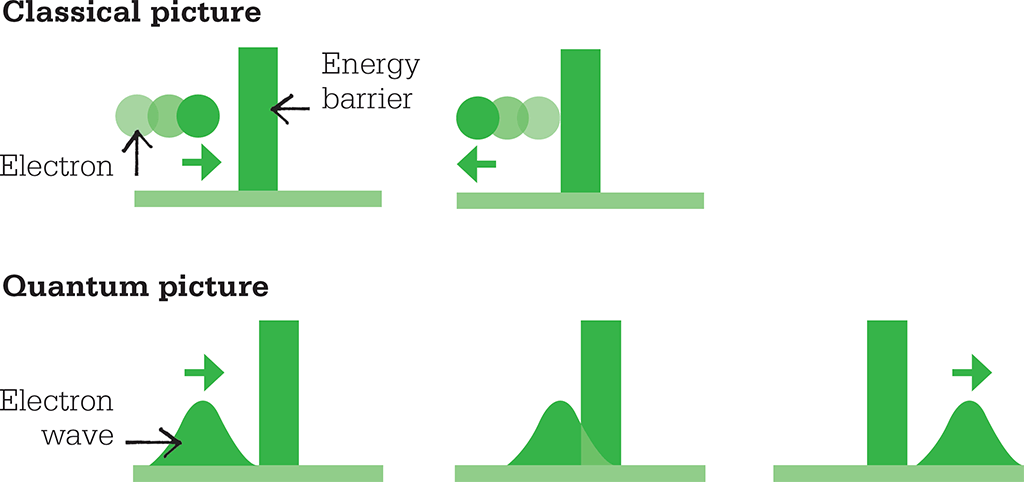
Quantum tunelling is explained by Heisenberg’s principle. There is a non-zero chance that an electron can pass through a barrier even if it appears to have too little energy to do so.
WERNER HEISENBERG

Born in the southern German town of Würzburg in 1901, Werner Heisenberg studied mathematics and physics at the universities of Munich and Göttingen, where he studied under Max Born and met his future collaborator Niels Bohr for the first time.
He is best known for his work on the Copenhagen interpretation and the uncertainty principle, but Heisenberg also made important contributions to quantum field theory and developed his own theory of antimatter. Awarded the Nobel Prize in Physics in 1932, he became one of its youngest recipients, and his stature enabled him to speak out against the Nazis after they seized power the following year. However, he chose to stay in Germany and led the country’s nuclear energy programme during World War II.
Key works
1927 Quantum Theoretical Re-interpretation of Kinematic and Mechanical Relations
1930 The Physical Principles of the Quantum Theory
1958 Physics and Philosophy
See also: Albert Einstein • Erwin Schrödinger • Paul Dirac • Richard Feynman • Hugh Everett III