58 | EPIDEMIOLOGY OF SUBSTANCE USE DISORDERS
DENISE B. KANDEL, MEI-CHEN HU, AND PAMELA C. GRIESLER
The epidemiology of drug use in the general population includes two distinct streams of research. The more common stream measures consumption patterns by asking individuals whether they have ever used specific classes of drugs, and, if so, how frequently they have done so. The second stream, and one implemented more rarely, measures the extent of problematic drug use by asking individuals about behaviors and symptoms that meet criteria for a substance use disorder.
Currently, the most extensive data on substance use disorders in the US population are provided by four national surveys implemented since 2001. The ongoing National Survey on Drug Use and Health (NSDUH-Substance Abuse and Mental Health Services Administration [SAMHSA], 2011) surveys respondents 12 years of age and older annually. This survey also provides excellent data on patterns of use. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) (Compton et al., 2005; Conway et al., 2006; Grant et al., 2004a, 2004b), carried out in 2001–2002, and the National Comorbidity Survey Replication (NCS-R) (Kessler et al., 2005a, 2005b), carried out in 2001–2003, surveyed adults 18 years and older. The National Comorbidity Survey for Adolescents (NCS-A) (Kessler et al., 2012a, 2012b), carried out in 2001–2004, surveyed youths 13–18 years old. These surveys have different strengths and weaknesses and generate somewhat different estimates of rates of substance use and substance use disorders and their comorbidity with other psychiatric disorders in the population.
In this chapter, we present data on the epidemiology and phenomenology of substance use disorders from comparative and developmental perspectives. We discuss six issues:
• The definition and measurement of substance use disorders and characteristics of existing epidemiological studies
• The prevalence of substance use and substance use disorders for legal drugs (cigarettes, alcohol), illegal drugs (marijuana, cocaine), and non-medical use of prescribed psychoactive drugs in different studies among adults and adolescents
• The prevalence of substance use disorders by age, gender, and race/ethnicity
• The comorbidity of substance use disorders with other psychiatric disorders
• Developmental stages of involvement in drugs
• Adolescence as a critical exposure period
The data presented in the chapter are based on publications from these studies and secondary analyses of the data sets that we implemented to illustrate points for which the documentation was not available in published reports.
DEFINITION AND MEASUREMENT OF SUBSTANCE USE DISORDERS
The currently available data on rates of disorders are based on the diagnostic criteria for substance use disorders specified by the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV and DSM-IV-TR; American Psychiatric Association, 1994, 2000). The DSM-IV covers two maladaptive patterns of substance use: abuse, and dependence (addiction); abuse is less severe than dependence and is diagnosed only if criteria for dependence are not met. The criteria for dependence have evolved over the past 30 years with a shift of emphasis from the necessary physiological criteria of tolerance and withdrawal to behavioral criteria for compulsive use. Tolerance and withdrawal form two of the seven potential criteria (Koob et al., 2008: p. 354). Three criteria need to be experienced within a 12-month period in order for the diagnosis of a substance dependence disorder to be made. The seven criteria are: (1) tolerance; (2) withdrawal; (3) impaired control, the substance taken in larger amounts or over a longer period than intended; (4) unsuccessful quit attempts; (5) time spent obtaining, using the substance, or recovering from its effects; (6) neglect of important social, occupational, or recreational activities; (7) continued use despite persistent or recurrent physical or psychological problems caused or exacerbated by the substance. These criteria closely resemble those outlined by the International Statistical Classification of Diseases and Related Health Problems (ICD-10; World Health Organization, 1992). In both systems, the specific withdrawal symptoms vary across drugs (Koob et al., 2008: p. 354). Withdrawal from cannabis (marijuana) is not included as a criterion although a cannabis withdrawal “syndrome is now well established (Budney et al., 2003).” (Koob et al., 2008: p. 355) As noted by Hughes (2006), generic criteria are based in part on shared genotype across different drugs, common underlying neurobiological processes, as well as by common behavioral correlates, such as antisocial syndromes (Compton et al., 2005; Koob et al., 2008: p. 354; Nelson et al., 1999).
Abuse is a distinct diagnostic category that excludes individuals who meet criteria for dependence. Abuse requires recurrent substance use during a twelve-month period resulting in at least one of four harmful consequences: failure to fulfill major role obligations at work, school, or home; hazardous use, such as driving an automobile or operating a machine when impaired by substance use; legal problems, for example, arrests for substance-related disorderly conduct; and continued use despite social or interpersonal problems caused by the substance.
TABLE 58.1. Lifetime and last 12-month prevalence of nonmedical substance use among persons aged 18 and older in NSDUH 2010, NESARC 2001–2002 and NCS-R, and persons aged 12–17 in NSDUH 2010 and 13–17 in NCS-A
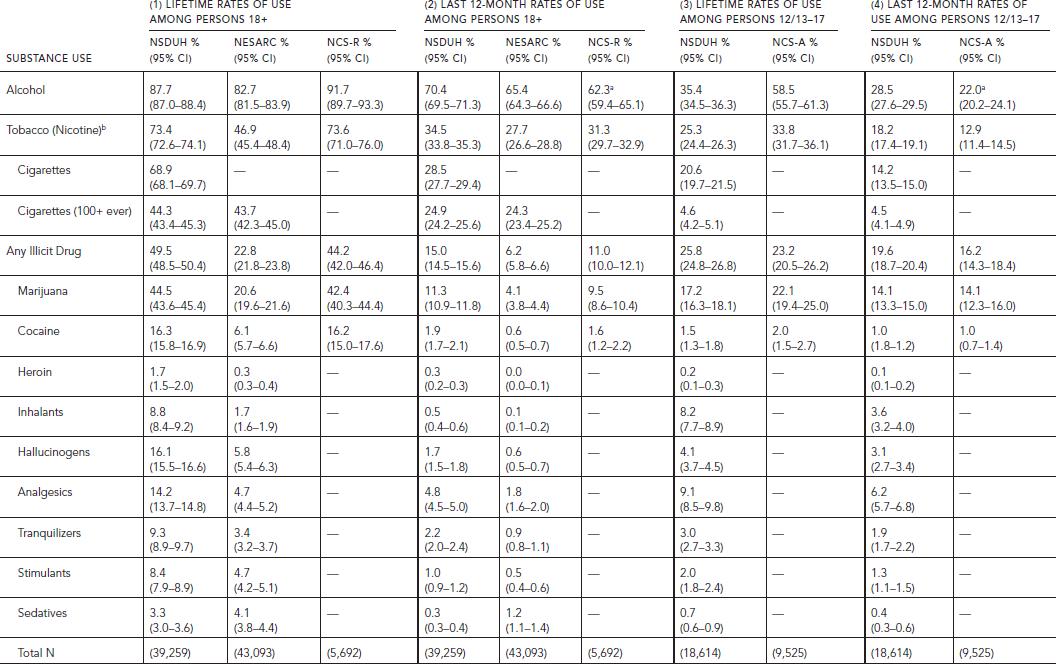
The criteria for tobacco (nicotine) dependence are the same as for alcohol and illicit drugs. Abuse does not apply to tobacco.
These definitions differ substantively from those in the fifth edition (DSM-5) of the American Psychiatric Association, which will become the standard as of 2013. In the DSM-5, the distinction between abuse and dependence will be eliminated and, for all substances including tobacco, the criteria for drug disorder will be 2 out of 11 criteria (Hasin, 2012ba, 2012b; O’Brien, 2011; Schuckit, 2012). This decision was based on secondary analysis of several large-scale epidemiological data sets. Craving was considered, in addition to the seven traditional DSM-IV criteria. The methods used to determine the relationship between abuse and dependence included factor analysis to establish unidimensionality, item response theory to assess the relationship of abuse to dependence criteria, criterion/item characteristic curves to examine the severity and discrimination of each criterion relative to each other, and total information curves to allow comparisons of two or more sets of criteria (Hasin, 2012a; Hasin et al., 2012; Saha et al., 2012). The evidence suggested that abuse and dependence formed one disorder. Craving was added as a diagnostic criterion but legal difficulties was eliminated (O’Brien, 2011), leaving a total of 11 criteria. Based on analyses of existing data sets, this definition is expected to generate rates of substance use disorders similar to those derived from combining rates of DSM-IV dependence and abuse. However, the rates will in all likelihood be higher than the rates of DSM-IV dependence discussed in this chapter, which do not include abuse.
LIMITATIONS OF MEASUREMENT: DIFFERENCES ACROSS SURVEYS
There are important differences among the surveys regarding the measurement of drug use, drug abuse, and drug dependence. Methodological features affect comparability across studies, the rates they each report, and lead to substantial variations in prevalences.
Discrepancies in estimates of prevalence and the correlates of substance use and substance use disorders between the NSDUH and NESARC were discussed in detail by Grucza et al. (2007). A discussion of NSDUH methodology compared with other surveys is also provided by Hedden et al. (2012). Two limitations of the assessments regarding alcohol and illicit drugs implemented by the National Comorbidity Studies, both the replication among adults (NCS-R) and the adolescent survey (NCS-A), have also previously been highlighted (Cottler, 2007; Grant et al., 2007). In the NCS, abuse symptoms were used as a screen so that respondents were not asked the dependence questions unless they had endorsed at least one abuse symptom. Because in the DSM-IV abuse symptoms are not required for the dependence diagnosis, this strategy results in an underestimate of dependence in the population. As illustrated by NESARC, a substantial number of individuals meet criteria for dependence although they are negative for abuse, 33.7% for those dependent on alcohol and 22.4% for those dependent on an illicit drug (Hasin and Grant, 2004; Hasin et al., 2005). The underestimate is especially pronounced among women and minorities. Second, in the NCS the assessment of abuse and dependence on marijuana and other illicit drugs was made for different illicit drugs as a group rather than for each drug separately, thereby creating a generic diagnosis of drug abuse or drug dependence, rather than one that was drug specific, as specified by the DSM-IV (Cottler, 2007). Asking about symptoms for drugs as a group may have led to underestimates of reports compared with asking about each drug separately. The principal investigators of the NCS-R and NCS-A studies acknowledged the validity of the first criticism but not the second (Kessler and Merikangas, 2007).
A third limitation of the NCS, not previously noted, pertains to the assessment of nicotine dependence. Among smokers, assessment was restricted to those who had smoked daily for a two-month period. This reduced the number of smokers eligible to report symptoms of dependence. Craving, a symptom that is not part of the DSM-IV definition, was included first in a list of eight criteria of nicotine dependence; tolerance and withdrawal were listed second and third. The logic and skip patterns of the interview schedule resulted in the inclusion of two different groups among those defined as nicotine dependent: (1) tobacco users who endorsed the first three criteria, but who were not asked the remaining five criteria, and (2) tobacco users who had endorsed up to two criteria among the first three and were asked all remaining five criteria. Finally, the definition of nicotine dependence applied to adolescents (NCS-A) did not apply the DSM-IV requirement that three symptoms be experienced within a 12-month time frame.
There are additional differences in the assessment of drug use and the eligibility of respondents for answering abuse and dependence questions across surveys. In NESARC, respondents were asked which illicit drugs they had used from a list of ten drug classes. Non-medical use of medically prescribed drugs was listed first; the last class included “any other medicines or drugs (not otherwise defined).” More detailed questions about drug-specific patterns of use were then asked for each drug reported to have been used. Symptoms of dependence were ascertained first without reference to any drugs. Respondents were then asked which “medicines or drugs did this happen with?” during the last 12 months, and before the last 12 months. The NSDUH asked only about symptoms experienced in the last 12 months but not lifetime. In NESARC, being a smoker was defined as having ever smoked at least 100 cigarettes. The nicotine dependence questions were ascertained in this restrictive group of smokers, whereas the NSDUH asked these questions of anyone who had ever smoked, even if only a puff. As noted earlier, in the NCS the last 12-month smoking and nicotine dependence questions were restricted to individuals who had smoked daily for at least two months. The alcohol abuse and dependence questions were restricted to those who had ever drunk 12 drinks in a year.
TABLE 58.2. Lifetime and last 12-month prevalence of substance abuse and dependence among persons aged 18 and older in NSDUH 2010 (N = 39,259), NESARC 2001–2002
(N = 43,093), and NCS-R (N = 5,692)
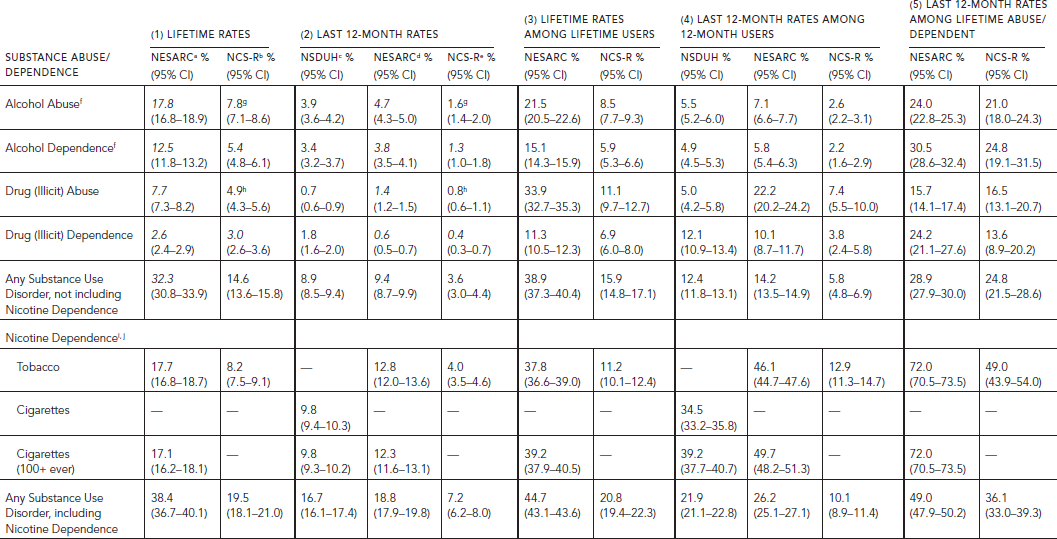
There are also differences in mode of survey administration that impact on the resulting rates of self-reported drug behavior. Of the surveys, NSDUH is the only anonymous one. In addition, the assessment of lifetime symptoms based on retrospective reports may lead to undercounts compared with repeated prospective assessments (Moffitt et al., 2010).
Thus, existing surveys have different strengths and weaknesses. NESARC has the most systematic assessment of substance use disorders and psychiatric disorders. The repeated annual NSDUH surveys provide the best data on patterns of use of legal and illegal drugs in the population. They also include detailed DSM-IV assessments of abuse and dependence on alcohol, specific illicit drugs, and the non-medical use of medically prescribed drugs for the last 12 months but not lifetime. Nicotine dependence is measured by the NDSS (Shiffman et al., 2004) rather than the DSM-IV. Anonymity in the NSDUH may have led to higher rates of self-reported drug use than in NESARC, whereas differences in instrumentation may have led to higher rates of last 12-month substance use disorder among the self-acknowledged drug users in NESARC than NSDUH. The NSDUH surveys respondents as of age 12, making possible comparisons between adolescents and adults. The nature of the NCS-R and NCS-A assessments greatly limit the quality of the data collected on substance use disorders, although they allow comparisons between adults and adolescents, because the same methodology (although flawed) was implemented in both surveys.
We implemented extensive secondary analyses of the four data sets in order to increase comparability across studies. For reasons of confidentiality, the public use NSDUH data included 84.5% of the original sample, so that in certain instances the figures in this chapter differ very slightly from published ones. To increase comparability of NCS with the other surveys, we reran NCS data on abuse excluding cases that met criteria for dependence. We also applied the DSM-IV requirement of experiencing three criteria within a 12-month period for nicotine dependence in NCS-A. We included nicotine dependence in summary measures of drug disorder for all the surveys. Finally, we excluded respondents 18 years old from the NCS-A sample to obtain a sample 13–17 years old and increase comparability with the sample of adolescents 12–17 years old in NSDUH.
Taking into account the issues discussed earlier, the data presented by any one study must be interpreted with caution. Comparisons across studies are subject to many limitations.
PREVALENCE OF SUBSTANCE USE AND DISORDERS
PREVALENCE OF SUBSTANCE USE AND DISORDERS AMONG ADULTS
The lifetime and last 12-month prevalence of use of specific substances in the NSDUH, NESARC, and NCS-R for persons aged 18 and over are presented in the first two panels of Table 58.1.
The rates of use for alcohol are very similar across the three studies, whereas those for tobacco and illicit drugs (as a group) are similar in the NSDUH and NCS-R, but much lower in NESARC (see Table 58.1, Panels 1 and 2). The discrepancy for tobacco is explained by the fact that, in NESARC, smoking at least 100 cigarettes lifetime was required to be defined as a smoker. Yet, 36% of smokers in the NSDUH never smoked 100 cigarettes. When restricted to those who smoked at least 100 cigarettes, the rate in NSDUH is identical to NESARC. It is not clear why the overall rates of lifetime or last year illicit drug use in NESARC are half those of the other two surveys. Lack of anonymity may be one explanation. The discrepancies are especially large for cocaine, with 16.3% and 16.2% in NSDUH and NCS-R, respectively, reporting lifetime use compared with only 6.1% in NESARC (see Table 58.1, Panel 1) (see Grucza et al., 2007). Despite these differences in absolute rates across studies, the relative rankings for overall prevalence of use are the same. Alcohol is the substance that is used most widely, followed by tobacco and illicit drugs. Half the adults 18 and over in the United States have used an illicit drug, including non-medical use of a prescribed drug, representing 113 million individuals in 2010. Of these drugs, marijuana is the most prevalent, followed by cocaine; almost three times as many individuals have ever used marijuana as have used cocaine (Table 58.1, Panel 1). The higher rates in NSDUH and NCS-R than NESARC increase the base of individuals eligible for being asked the questions relevant to drug abuse and dependence and the absolute number identified as meeting criteria for abuse or dependence.
Lifetime and last 12-month rates of abuse or dependence in the total adult population, lifetime rates among lifetime users of each drug, last 12-month rates among last 12-month users, and last 12-month rates among lifetime abusers or dependents are presented in Table 58.2. None of the published reports from the studies include nicotine dependence in any substance use disorder. This is a serious omission inasmuch as tobacco is one of the two most addictive substances that are used, second to heroin. Table 58.2 presents rates of abuse and dependence separately for alcohol, illicit drugs as a group, nicotine, any substance use disorder as reported in the literature (Row 5), and rates including nicotine dependence that we calculated from secondary analysis of the public use data (Row 9). The conditional rates among users specify the risk of abusing or becoming dependent on a drug among those who consumed the drug in their lifetime or the last 12 months. The conditional rates of last 12-month abuse or dependence among those who met lifetime criteria index chronicity of abuse or dependence.
Except for illicit drug dependence, the rates of lifetime and last 12-month abuse and dependence for all drug classes in the total adult population and among last 12-month users were consistently the lowest in NCS-R (Table 58.2, Panels 1, 2 and 4).
In NESARC, close to 40% of the population 18 years old and over ever met criteria for a substance disorder (abuse and/or dependence), including nicotine dependence; 17.7% met criteria for nicotine dependence, 12.5% for alcohol dependence, 2.6% for illicit drug dependence (Table 58.2, Panel 1). The 12-month rates were 80% to 28% lower than lifetime rates. Slightly less than 20% of the population met criteria for a substance use disorder within the last year in NESARC, a rate very similar to the NSDUH (Table 58.2, Panel 2). The proportion meeting criteria for abuse or substance dependence on a given drug among individuals who used the drug varied greatly across drug classes. Across all surveys, conditional upon lifetime use, nicotine emerged as the most addictive of the substances, with 37.8% of tobacco users in NESARC meeting criteria for lifetime dependence (Table 58.2, Panel 3). This compares with 28.2% among heroin users (Compton et al., 2005). Among last year users, however, heroin emerged as the most addictive substance in NSDUH, followed by tobacco, sedatives, cocaine, and analgesics. The ranking in NESARC, wherein heroin could not be ranked because of the small number of users, was tobacco, cocaine, and stimulants (data not presented). The conditional last 12-months rates among last 12-month users were the highest in NESARC for any substance use disorder with or without nicotine (Table 58.2, Panel 4), although there were differences among specific illicit substances. The rates were higher in NESARC than NSDUH for cocaine and stimulants, lower for analgesics and sedatives, and the same for alcohol, marijuana, inhalants, hallucinogens, and tranquilizers (data not presented). The proportion of individuals with an alcohol-related disorder among those who consumed alcohol in the last year was among the lowest of any of the substances. However, because so many individuals consume alcohol, this percentage translates into a large number of affected individuals. Detailed comparison across drug classes could not be made in the NCS-R.
Substance use disorders are chronic disorders. As illustrated by NESARC, close to half of those who met lifetime abuse or dependence criteria on any substance still experienced these symptoms within the last year (Table 58.2, Panel 5). These rates could not be calculated for NSDUH, which only measured last 12-months abuse and dependence. While rates of chronicity of dependence are consistently lower in NCS-R than NESARC, the patterns across drug classes are identical. Nicotine is by far the most chronic of the addictions, with 72.0% of tobacco users in NESARC meeting criteria for last 12-month dependence among those who ever met lifetime criteria. Chronicity is slightly higher for dependence on alcohol (30.5%) than illicit drugs (24.2%) (Table 58.2, Panel 5). Chronicity of dependence is also relatively high among those who consume prescribed drugs non-medically, especially analgesics (data not presented).
PREVALENCE OF SUBSTANCE USE AND DISORDERS AMONG ADOLESCENTS
The behavior of adults 18 years old and over could be compared with that of adolescents 12–17 years old in NSDUH and 13–17 years old in NCS-A. Except for inhalants, the prevalence of lifetime use of various substances is lower among adolescents than adults, with wide differences across substances (Table 58.1, Panels 3 and 4). Although the absolute rates differ between NCS-A and NSDUH, the patterns are strikingly similar in both surveys. As illustrated by NSDUH, the lifetime adolescent rates for alcohol and any tobacco use are about 40% and those for any illicit drug about 50% of those observed among adults, and only 10% for use of 100 cigarettes or more. There are great variations across specific illicit drugs in the ratio of adolescent to adult prevalence rates. Inhalants is the only drug class for which the rates are the same. With respect to last 12-month use, the age patterns reverse for illicit drugs, where adolescents have higher rates than adults, 19.6% versus 15.0% in NSDUH and 16.2% versus 11.0% in NCS.
The overall rates of any substance use disorders among lifetime users are the same among adolescents and adults, although there are differences across substances. Lifetime dependence on illicit drugs among lifetime users is higher among adolescents, whereas tobacco dependence is lower than among adults (NCS, Tables 58.2 and 58.3, Panel 3). The last 12-month rates among 12-month users are consistently higher among adolescents than adults in both surveys, except for tobacco dependence in NSDUH (Tables 58.2 and 58.3, Panel 4). The differences are greater in NCS-A than NSDUH, where three times as many adolescents (31.0%) as adults (10.1%) meet criteria for any substance use disorder among those who used those drugs within the last 12 months (NCS, Tables 58.2 and 58.3, Panel 4). Persistence is also much higher among adolescents than adults (Tables 58.2 and 58.3, Panel 5), reflecting the fact that duration since onset of drug use is much shorter for adolescents than adults.
COMORBIDITY OF USE AND DISORDERS ACROSS SUBSTANCES
Consideration of each drug class by itself underestimates the extent of drug use in the population because many individuals use more than one class of drugs. Thus, in 2010, only 8.2% of the population aged 18 and over had not experimented with any substance; 44.2% had experimented with all three major classes: alcohol, tobacco, and illicit drugs (based on NSDUH 2010).
Similarly, substance use disorders on multiple drugs tend to cooccur. In NESARC, 40.3% of individuals 18 and over who met criteria for abuse or dependence on one drug class also met criteria for abuse or dependence for at least another drug; 11.7% met criteria for two other substances. The highest comorbidity was between dependence on an illicit drug and dependence on alcohol (70%) or nicotine (69%) (secondary analysis of NESARC). Yet, only a minority of addicted individuals have been treated for their addiction (Compton et al., 2007).
PREVALENCE OF SUBSTANCE USE AND DISORDERS BY AGE, GENDER, RACE/ETHNICITY
There are important differences in the prevalence of use and substance use disorders in different subgroups in the population, but the patterns are not necessarily consistent across surveys. Age differences were examined in all four surveys, but only in NSDUH and NESARC for gender and race/ethnicity.
AGE
Differences between adolescents and adults were discussed earlier. Adulthood itself can be differentiated into periods. Four age groups (ages 12/13–17 to ages 50+) could be differentiated in NSDUH and NCS and three age groups (ages 18–25 to 50+) in NESARC. Rates of last 12-month use by age in the population are presented in Figure 58.1A–C, and rates of last 12-month dependence among last 12-month users are presented in Figure 58.1D–F. Age-related trends are presented for tobacco users in NCS, and for all cigarette users in NSDUH as well as for those who smoked at least 100 cigarettes, the NESARC definition of being a smoker, to maximize comparability between the two surveys.
TABLE 58.3. Lifetime and last 12-month prevalence of substance abuse and dependence among persons aged 12–17 in NSDUH 2010 (N = 18,614) and aged 13–17 in NCS-A (N = 9,525)
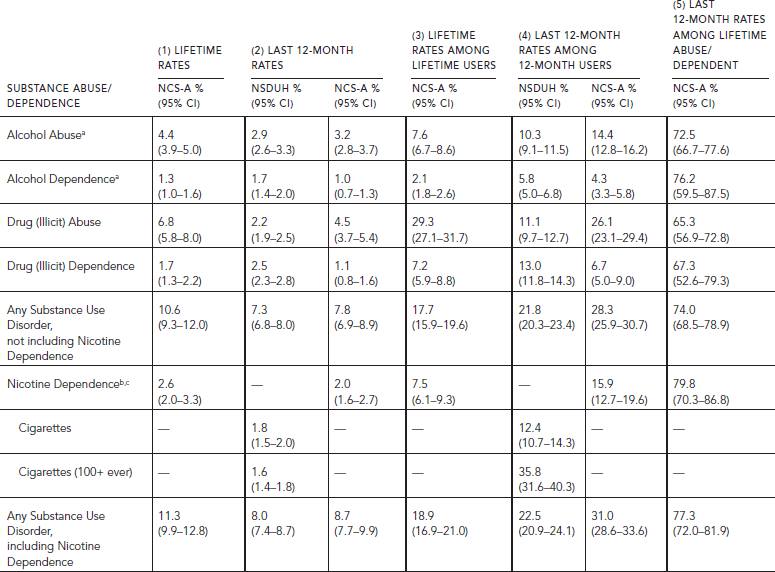
Age-related patterns are very similar across the surveys, although the absolute rates of use or dependence may differ. The prevalence of substance use varies greatly by age. With the exception of tobacco (cigarettes), age-related differences are stronger for use in the population than for dependence among those who used a particular substance.
Rates of use of all substances increase sharply throughout adolescence, and decline also sharply from ages 18–25 for illicit drugs (see Fig. 58.1A–C). Use of cigarettes declines more slowly; the decline for those who smoked at least 100 cigarettes occurs at older ages. In NSDUH and NESARC, the prevalence of drinking alcohol remains at fairly stable levels from ages 18 to 49, when rates start to decline. In NCS-R, rates decline as of ages 26–34.
Among last 12-month users, age-related patterns for dependence differ from those for use itself (see Fig. 58.1D–F). For all cigarette users (NSDUH), conditional rates of nicotine dependence rise sharply from adolescence to the mid-thirties and then more slowly thereafter (see Fig. 58.1D). Rates of nicotine dependence (for those who smoked 100 cigarettes or more or used tobacco daily) change little with age in NSDUH and NCS but decline as of age 35–49 in NESARC (see Fig. 58.1D–F). The rates of alcohol dependence decline gradually as of ages 18–25, except for a sharp drop from ages 18–25 to 26–34 in NESARC. In the three surveys, the conditional rates of dependence on illicit drugs decline slowly over time as of age 18–25 (see Fig. 58.1D–F).
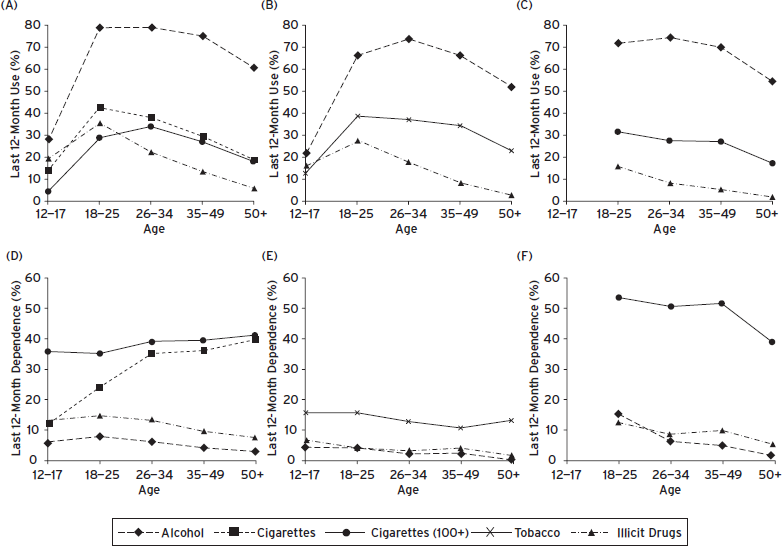
Figure 58.1 Prevalence of last 12-month use by age of alcohol and illicit drugs in (A) NSDUH, (B) NCS-R, NCS-A, (C) NESARC; of any cigarettes (NSDUH); of any cigarettes 100+ lifetime (NSDUH, NESARC); or of tobacco (NCS-R, NCS-A); and prevalence of last 12-month dependence by age among last 12-month users in (D) NSDUH, (E) NCS-R, NCS-A, (F) NESARC.
GENDER
Gender patterns for substance use and substance use disorders are similar in NSDUH and NESARC (Table 58.4). For all substances, including nonmedical use of psychotherapeutics, males have consistently higher rates of use than females, with the rates higher by 10–20% for alcohol, and 50–60% higher for tobacco and all illicit drugs combined. Gender differences are especially pronounced for marijuana and cocaine.
Among drug users, the rates of abuse and dependence on alcohol and illicit drugs are also consistently higher for males than females, except for nicotine, for which the rates of dependence are the same for males and females in NSDUH but significantly higher for females than males in NESARC (see Table 58.4). Combining all substances, the conditional rates of overall substance use disorders, including nicotine, are 39% higher in NSDUH and 28% higher in NESARC among males than females.
RACE/ETHNICITY
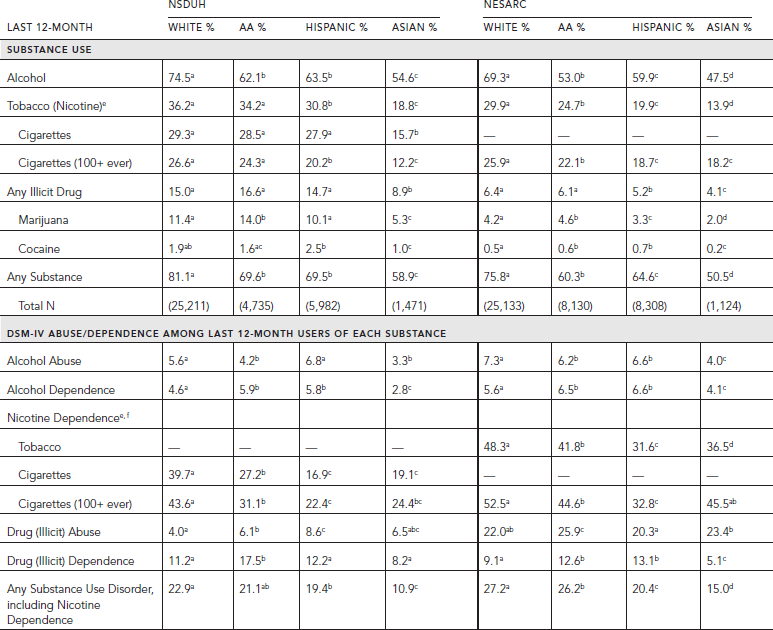
Racial/ethnic differences in patterns of substance use and disorders in the population are similar in NESARC and NSDUH (Table 58.5). In both surveys, Asians have the lowest rates of use and disorder for every drug class, except for tobacco dependence and illicit drug abuse in NESARC. In NESARC, whites have the highest rates of any group for alcohol abuse and nicotine dependence; African-Americans and Hispanics have the highest rates of illicit drug dependence. In the NSDUH, ethnic patterns are less consistent. Whites have much higher rates of nicotine dependence among last 12-month smokers than any other racial/ethnic group. African-Americans tend to have higher rates of dependence on illicit drugs than any other group. The most striking differences are the higher rates of alcohol use and nicotine dependence among whites than any other group, and the higher rate of dependence on illicit drugs among African-Americans (see Table 58.5).
COMORBIDITY OF SUBSTANCE USE DISORDERS WITH PSYCHIATRIC DISORDERS
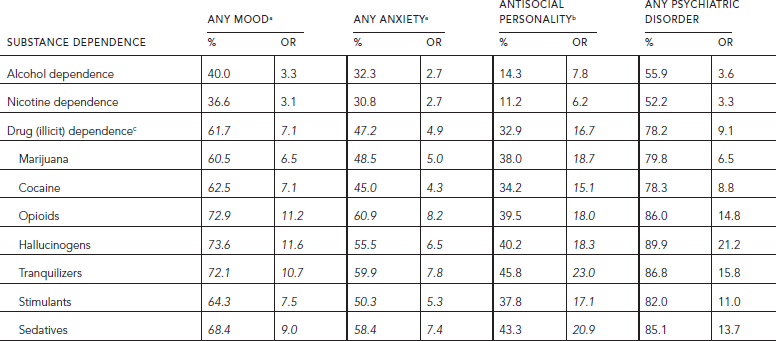
“There is extensive comorbidity between addiction and mental illness, as documented by NESARC. Among individuals with a diagnosis of abuse or dependence on illicit drugs, 40.9% met criteria for mood disorder, 29.9% for anxiety disorder (Conway et al., 2006), 18.3% for antisocial personality disorder, and 57.5% for any psychiatric disorder (unpublished analysis). The rates were at least 50% higher among those who met criteria specifically for illicit drug dependence (i.e., 61.7% for mood, 47.2% for anxiety, 32.9% for antisocial personality, and 78.2% for any psychiatric disorder) (Koob et al., 2008: p. 362) (Table 58.6). The rates among those dependent on alcohol or nicotine were similar and lower than those for the seven classes of illicit drugs that were considered. For alcohol dependence, the rates were 40.0% for mood, 32.3% for anxiety, 14.3% for antisocial personality, and 55.9% for any disorder. Comorbidity rates among those dependent on nicotine were similar to those dependent on alcohol, namely 36.6%, 30.8%, 11.2%, and 52.2%, respectively. The associations between psychiatric disorders and substance dependence are highly statistically significant for all substances and all classes of psychiatric disorders. The associations are somewhat higher with mood than anxiety disorders, and much higher—by a factor of two or three—with antisocial personality than mood or anxiety disorders” (Koob et al., 2008: p. 362) with odds ratios ranging from 15 to 23 for illicit drugs (Table 58.6). Among the mood disorders, mania has the highest association with dependence for every illicit drug, as does panic with agoraphobia among anxiety disorders (Conway et al., 2006, see Tables 4 and 5).
TABLE 58.4. Last 12-month prevalence of nonmedical substance use and last 12-month DSM-IV abuse and dependence among last 12-month users aged 18 and older by gender in NSDUH 2010 and NESARC 2001–2002

For all three broad classes of psychiatric disorders, the lower the overall prevalence of dependence on a specific substance in the population, the greater its comorbidity with psychiatric disorders (Compton et al., 2005; Conway et al., 2006).
“A particularly strong comorbidity, observed in clinical samples but not in the general population, because of the small numbers of affected individuals, is that of schizophrenia with smoking and nicotine dependence. Rates of smoking are three to four times higher among schizophrenics than in the general population and higher than among individuals diagnosed with other psychiatric disorders (Kumari and Postma, 2005; Volkow, 2009; Wing et al., 2012). Smoking may represent self-medication for the cognitive and negative symptoms prominent in schizophrenia, given the interaction of nicotine with dopaminergic and glutamatergic transmitter systems (de de Leon and Diaz, 2005; Kumari and Postma, 2005).
As suggested by Compton et al. (2005), the strong association with antisocial personality disorder across various substances may reflect an underlying comorbidity factor rather than substance specific links. This has important implications regarding the potential commonality of selected mechanisms and genetic factors underlying substance use disorders and psychiatric disorders, especially antisocial personality disorder.” (Koob et al., 2008: p. 362)
To the extent that gender differences appear, comorbidity of psychiatric disorders with substance use disorders is consistently higher among females than males. Comorbidity is higher for any mood disorder with alcohol and nicotine dependence, and for antisocial personality with alcohol, nicotine, and any illicit drug dependence (Compton et al., 2005; Grant et al., 2004a; Unpublished analysis of NESARC; data not presented). There are several gender differences in the association of specific anxiety and mood disorders with specific illicit drug disorders (see Conway et al., 2006). “Although rates of psychiatric disorders vary substantially across racial/ethnic groups, the association between alcohol dependence and illicit drug dependence with mood and anxiety disorders is similar among whites, African-Americans, Hispanics, and Asians (Smith et al., 2006).” (Koob et al., 2008: p. 362) Hispanics have the highest rate of mood disorder comorbid with nicotine dependence, while African-Americans have the lowest, and Asians the highest, rates of antisocial personality disorder comorbid with nicotine and illicit drug dependence (unpublished analysis of NESARC).
TABLE 58.5. Last 12-month prevalence of substance use and last 12-month DSM-IV abuse and dependence among last 12-month users aged 18 and older by race/ethnicity in NSDUH 2010 and NESARC 2001–2002
DEVELOPMENT OF PSYCHIATRIC COMORBIDITY WITH DRUG DEPENDENCE
“These cross-sectional associations do not inform on developmental processes underlying comorbidity, whether mental illness follows and causes drug dependence or whether drug dependence follows and causes mental illness. For some cases, mental illness and addiction may cooccur independently (Grant et al., 2004b); for others, there might be a sequential relationship. The direction of causality between psychiatric disorders and dependence is ambiguous because both pathways of influence have been documented. For instance, among adolescents and young adults, when drug use and psychiatric disorders have their onset, depression, social anxiety, and disruptive disorders predict the onset of smoking and nicotine dependence (Breslau et al., 2004; Karp et al., 2006; Kessler, 2004), but prior smoking and nicotine dependence also predict depression, disruptive disorders, panic attacks and disorder, and agoraphobia (Boden et al., 2010; Breslau et al., 2004; Isensee et al., 2003; Klungsøyr et al., 2006).” (Koob et al., 2008: p. 362) Analysis that we conducted in a longitudinal sample of adolescent smokers (mean age 16.7) indicated that DSM-IV psychiatric disorders preceded and, for the most part, predicted the onset of nicotine dependence, while nicotine dependence rarely predicted psychiatric disorder (Griesler et al., 2008, 2011). Anxiety, mood, and disruptive disorders had their onset at least 2.5 years before nicotine dependence. Psychiatric disorders started, on average, between ages 10.6 and 11.7, tobacco use at age 13, the first symptom of nicotine dependence at age 14.3, and full nicotine dependence at age 14.7. Panic disorder, attention-deficit hyperactivity disorder, and oppositional defiant disorder predicted the onset of nicotine dependence; nicotine dependence predicted the onset of oppositional defiant disorder. Depression and nicotine dependence did not predict each other. In adults, psychiatric disorders predicted persistent course of alcohol, nicotine, and illicit drug dependence but substance use disorders did not predict persistence of mood or anxiety disorders (Hasin and Kilcoyne, 2012).
TABLE 58.6. Lifetime comorbidity of dependence (percentages and unadjusted odds ratios) on alcohol, nicotine, and illicit drugs with three classes of psychiatric disorders among persons aged 18 and older in NESARC 2001–2002 (N = 43,093)
The mechanisms underlying the comorbidity between substance use disorders and psychiatric disorders are not properly understood. Both classes of disorder may be caused by shared genetic or environmental factors. “It is likely that different neurobiological factors are involved in comorbidity depending on its development. When mental illness is followed by dependence on some types of drugs, comorbidity might reflect self-medication. However, when drug dependence is followed by mental illness, chronic drug exposure itself may lead to changes in the brain that would increase the risk for mental illness, particularly in persons with genetic vulnerability. For example, the high prevalence of smoking after individuals become depressed could reflect the antidepressant effects of nicotine and of monoamine oxidase A (MAOA) and B (MAOB) inhibition by cigarette smoking (Fowler et al., 2003).” (Koob et al., 2008: p. 362)
DEVELOPMENTAL STAGES OF INVOLVEMENT IN DRUGS
“Not only are there regular patterns of cooccurrence of use and dependence across drugs, but there are regular sequences of progression from the use of one drug class to another. The existence of a developmental sequence of involvement in drugs is one of the best replicated findings in the epidemiology of drug use (Kandel, 2002). In the United States and other Western societies, a regular sequence of progression has consistently been found. The use of cigarettes or alcohol precedes the use of marijuana and, in turn, the use of marijuana precedes the use of other illicit drugs (Fig. 58.2). Very few individuals among those who have tried cocaine have not previously used marijuana (2%), cigarettes (3.6%), or alcohol (2.2%) (based on NSDUH 2010). The majority (66.8%) of individuals have used alcohol or cigarettes prior to marijuana use or at the same age. Such behavioral regularities have given rise to the gateway hypothesis” (Koob et al., 2008: p. 360) to emphasize that certain drugs serve as gateways for the use of other substances. The drugs used earlier increase the risk of using other drugs. Even now, when rates of marijuana use among young people have greatly increased and even surpass slightly those of cigarette use, the majority of those who have experimented with marijuana or cocaine had first experimented with cigarettes or alcohol. Thus, among high school seniors in the 2010 Monitoring the Future (MTF) Survey, 42.2% ever smoked cigarettes, 71.0% ever drank alcohol, 43.8% ever used marijuana or hashish, and 5.5% ever used cocaine (Johnston et al., 2010). Yet, 54.8% had started smoking cigarettes or using alcohol prior to using marijuana and 33.7% had started using these drugs in the same grade; only 8.6% of marijuana users had started to use marijuana before smoking or drinking alcohol; 2.9% had only used marijuana. Parallel percentages for cocaine are 73.3% who smoked or drank alcohol prior to starting using cocaine; 19.9% started in the same grade, and 6.8% used cocaine prior to cigarettes or alcohol (unpublished analysis of MTF).
However, since publication of the original observation in 1975 (Kandel, 1975; Kandel and Faust, 1975), “surprisingly little progress has been made in addressing fundamental questions that derive from the finding that the use of one class of drug is followed by the use of another class. We still do not know (1) whether the use of a class of drugs used first, such as tobacco, is a cause of the use of the second class, such as cocaine, or whether the sequence is determined exclusively by availability of the drug or other social factors; and (2) what biological mechanisms underlie this progression in drug use. Although epidemiological studies have established the sequence between substances and specified their association, epidemiological studies cannot establish a causal progression nor can they identify the underlying cellular and molecular mechanisms that could contribute to the gateway sequence of drug use.” (Koob et al., 2008: p. 360)
To obtain biological insights into the transition from nicotine to cocaine in the development of drug abuse and address how gateway drugs exert their effects, Kandel and colleagues at Columbia University have bridged the epidemiology of drug abuse and molecular biology by developing a mouse model of this epidemiological sequence so as to explore the behavioral, physiological, and molecular genetic mechanisms underlying the gateway sequence. Animal models can provide a rigorous test of drug use progression in which drug-taking behavior can be observed in relation to well-defined prior experiences with specific drugs, independently of any social or legal constraints regulating and defining drug use. In mice, one can readily control the order in which drugs are taken so that the order is the only determinant of outcome. Alternate specifications of the sequential order of drug presentation can help resolve the possibility that the ordered use between any two drugs is only determined by social factors related to availability of different substances.
Most drugs of abuse exert their addictive effects through effects on the striatum (Thomas et al., 2001). The nucleus accumbens (NAc) in the ventral striatum is critical for reward and addiction and is a site of convergence and integration of rewarding input from dopaminergic neurons in the ventral tegmental area (VTA) and glutamatergic input from the amygdala and the prefrontal cortex. The core of the NAc is made up primarily of GABAergic inhibitory spiny neurons. The NAc sends inhibitory feedback to the dopaminergic cells of the VTA. Reduction of excitatory input to the NAc is thought to decrease inhibitory output from the NAc to the VTA and thereby contribute, through disinhibition, to the increased reward and enhanced locomotion activation observed after cocaine administration (Kauer and Malenka, 2007).
We examined in mice how sequential administration of nicotine and cocaine alters locomotor sensitization and conditioned place preference, two addiction-related behaviors that are modulated by drugs of abuse, and three physiological and molecular markers in the nucleus accumbens of the striatum: synaptic plasticity; transcription of FosB, an immediate response gene implicated in addiction to many drugs of abuse and in the response to other rewarding stimuli; and histone acetylation in chromatin (Levine et al., 2011).
Pretreatment of mice with nicotine for seven days increased the response to cocaine. Locomotor sensitization was increased by 98%, conditioned place preference by 78%, and cocaine-induced reduction in long-term potentiation (LTP) by 24%. Responses to cocaine were altered only when nicotine was administered first, and nicotine and cocaine were then administered concurrently. Reversing the order of drug administration was ineffective. Cocaine had no effect on nicotine-induced behaviors and synaptic plasticity. We found that nicotine by itself induced only a small increase in FosB expression in the striatum. However, nicotine also inhibited histone deacetylase (HDAC) activity, leading to a more widespread acetylation of histones at a larger number of genome locations in the striatum than did cocaine alone, thereby creating an environment that was primed for the induction of a number of genes. This ability of nicotine to hyperacetylate chromatin widely by inhibiting HDACs was not shared by cocaine, which caused a more local and transient acetylation. When a second drug of abuse, in this case cocaine, was given to animals after nicotine exposure, the higher histone acetylation levels led to greater activation of FosB and, likely, other genes.
Further, we found that a histone deacetylase inhibitor simulated the actions of nicotine by priming the response to cocaine and enhancing FosB gene expression and LTP depression in the nucleus accumbens. Conversely, in a genetic mouse model characterized by reduced histone acetylation, the effects of cocaine on LTP were diminished. We achieved a similar effect by infusing a low dose of theophylline, an activator of histone deacetylase, into the nucleus accumbens.
Cocaine must be administered to the animals while they are actively exposed to nicotine. These results from mice prompted an analysis of epidemiological data, which indicated that most cocaine users initiate cocaine use after the onset of smoking and while actively still smoking, and that initiating cocaine use after smoking increases the risk of becoming dependent on cocaine, consistent with our data from mice.
HDAC activators may be of potential clinical utility in the treatment of addiction because they could decrease FosB expression in response to cocaine. Modifying HDAC activators so that they target the striatum specifically would be particularly desirable because systemic treatments with HDAC activators or histone acetyltransferase inhibitors are likely to have cognitive and other deleterious effects.
DEVELOPMENTAL FACTORS: ADOLESCENCE AS A CRITICAL EXPOSURE PERIOD
“Normal developmental processes may result in higher risk for drug use at certain stages of the life cycle than others and experimentation with drugs at a particular stage of the life cycle may have lifelong consequences for subsequent extensiveness and chronicity of use. Epidemiological data on patterns of drug use in human populations as well as animal studies support the notion of critical developmental periods for drug behavior (Purves et al., 2001). Experimentation most often starts in adolescence, as does addiction (Wagner and Anthony, 2002), a period when the brain is still undergoing significant developmental changes (Dahl and Spear, 2004).” (Koob et al., 2008: p. 363) The rates of drug use increase dramatically during adolescence. Rates of use of different substances double from early to late adolescence, as illustrated by Monitoring the Future, a national study of drug use among high school students. In 2011, lifetime rates of use in the 8th, 10th, and 12th grades for alcohol were 33.1%, 56.0%, and 70.0%, respectively; for cigarettes, 18.4%, 30.4%, and 40.0%; and for any illicit substances, 20.1%, 37.7%, and 49.9% (Johnston et al., 2011).
“Although the effects of drugs of abuse during this stage of development have not been adequately investigated, initial exposure in adolescence is associated with more chronic and intensive use, and greater risk of developing a substance use disorder (e.g., nicotine dependence, alcoholism, or dependence on illicit drugs) when cigarette smoking, drinking, or the use of illicit drugs starts early during adolescence compared with initiation at older ages (Hingson et al., 2006; Kandel, 2003; Kandel and Chen, 2000; Volkow, 2006).” (Koob et al., 2008: p. 363) In NESARC, 47% of those who reported having started drinking before age 14 became dependent on alcohol compared with 9% of those who started drinking at age 21 or older (Hingson et al., 2006). In NSDUH, 12.8% of those who had first started using marijuana before age 15 met criteria for last year dependence on an illicit drug within the last year compared with 2.6% of those who first started using marijuana at age 18 or older (SAMHSA, 2011: p. 72). As we described in the preceding, the rates of drug abuse or dependence among drug users are higher among adolescents than adults. “Normal adolescent-specific behaviors (such as risk-taking, novelty-seeking, response to peer pressure) increase the propensity of experimenting with legal and illegal drugs (Spear, 2000), which might reflect incomplete development of brain regions (e.g., myelination of frontal lobe regions) involved in the processes of executive control and motivation (Sowell et al., 2003).
The importance of adolescence as a critical developmental risk period for drug involvement and substance dependence is supported by work on rodents, which documents the greater vulnerability of adolescent than adult animals to various drugs. For example, in rats, exposure to nicotine during adolescence is associated with greater nicotine self-administration than first exposure in adulthood, a difference that persists when the adolescent rats reach adulthood (Levin et al., 2003). Adolescent rats appear to be more sensitive than adults to the rewarding actions of nicotine (Belluzzi et al., 2004).” (Koob et al., 2008: p. 363) Similarly, nicotine pretreatment leads to enhanced cocaine self-administration and locomotor activity in response to cocaine in adolescent but not adult rats (McQuown et al., 2007, 2009). Drug exposure during adolescence might result in different neuroadaptations from those during adulthood. In rodents, exposure to nicotine during the period corresponding to adolescence, but not adulthood, leads to alterations in cholinergic systems in the cerebral cortex, midbrain, and hippocampus (Abreu-Villaca et al., 2003), including changes in nicotine receptors and enhancement of the reinforcing responses to nicotine (Adriani et al., 2003). “In addition, enhanced expression of plasticity-related genes following injection of nicotine in adolescent compared with adult rats has been observed throughout the brain, especially the forebrain (Schochet et al., 2005). Similar vulnerabilities in adolescence compared with adulthood have been reported for alcohol (Rezvani and Levin, 2004; Ristuccia and Spear, 2005)” (Koob et al., 2008: p. 363), amphetamines (McPherson and Lawrence, 2006), and morphine (White and Holtzman, 2005). Future research should allow clarification of whether neurobiological changes are the reason adolescents appear to become addicted to nicotine with lower nicotine exposure than adults (Kandel and Chen, 2000). Similarly, future research will allow us to determine if the increased neuroadaptations to alcohol during adolescence compared with adulthood (Slawecki and Roth, 2004) explains the greater vulnerability to alcoholism in individuals who start using alcohol early in life (Grant et al., 2001).
CONCLUSIONS
Data currently available to assess the extent of substance use disorders in the United States have many shortcomings and make comparison and replication across studies difficult. To overcome some of the limitations, we implemented secondary analyses of data from four surveys: NSDUH, NESARC, NCS-R, and NCS-A. The use of legal and illegal drugs in the United States is pervasive. In 2010, more than 90% of the adult population had ever used at least one substance and 45% of adolescents had done so (NSDUH). In 2001–2002, close to 40% of adult lifetime substance users met criteria for any lifetime substance use disorder, whether abuse or dependence on alcohol, nicotine, or an illicit drug. The highest lifetime rates of dependence were observed for nicotine dependence (17.7%), followed by alcohol (12.5%) and illicit drugs (2.6%) (NESARC). Adolescence appears to be a period of high risk for substance use disorders among those who have experimented with legal and illegal substances. Although the lifetime and last 12-month prevalence of use is lower among adolescents than adults, except for last 12-month use of illicit drugs, the rates of lifetime disorders among lifetime users are similar among adolescents and adults (NCS). The rates are even higher for last 12-month disorders among last 12-month adolescent users than adult users both in NCS and NSDUH, with the exception of dependence on nicotine among all cigarette users in NSDUH. Ages 18–25 represent the period of highest drug use prevalence for most substances. Whereas use of cigarettes and illicit drugs peaks in early adulthood at ages 18–25, the use of alcohol stabilizes through the late forties, when it starts its decline. The rates of dependence among users tend to remain flat for cigarette smokers and, as of age 18–25, to decline but slowly for users of other substances throughout adulthood.
Nicotine represents the most serious public health problem of the drugs that are used because not only does it affect almost a fifth of the population but it is also the most chronic of the addictions, with more than 70% of adults who ever experienced symptoms of nicotine dependence reporting such symptoms within the last year. Furthermore, individuals who experiment with tobacco are more likely subsequently to experiment with illicit drugs, such as marijuana and cocaine. Recent work carried out using mice models identified molecular effects of nicotine on the brain that could explain its chronicity and its effects on other substances. Nicotine primes the response to cocaine by inhibiting histone deacetylase activity, causing global histone acetylation in the striatum, thereby inducing transcriptional activation of the FosB gene implicated in addiction. Understanding the pathways involved in this process may lead to the development of drugs that would impact in the addictive process.
Substance abuse and dependence are highly comorbid with other psychiatric disorders, especially among those who meet criteria for dependence on an illicit drug. In that group, close to 80% also meet criteria for a mood, anxiety, or antisocial personality disorder. The comorbidity between substance use disorders and psychiatric disorders is especially strong for antisocial personality disorder. Common biological and environment factors may underlie the observed associations between these two classes of disorder. Although much work remains to be done to understand the direction of causality between psychiatric and substance use disorders, it would appear that psychiatric disorders are more likely a cause of substance use disorders than substance use disorders are a cause of psychiatric disorders. Research on adolescents suggests that psychiatric disorders are more likely to precede and lead to substance use disorders than the reverse. Similarly, among adults, psychiatric disorders, in particular mood and anxiety disorders, predict persistence of substance use disorders but not the reverse.
Early onset of drug use during adolescence leads to more extensive and chronic use than later onset postadolescence, highlighting the importance of implementing prevention and educational efforts in that developmental period.
The use of animal models, imaging, and longitudinal epidemiological studies, in which individuals can be followed at relatively closely spaced intervals and for a long period of time, would help resolve some of the questions that are raised by the abuse of drugs in the population.
DISCLOSURES
No conflicts of interest to disclose for any of the authors. Support was provided by grant K05-DA0081 from the National Institute on Drug Abuse to Denise Kandel.
ACKNOWLEDGMENTS
Sections of this chapter were previously published in Koob, G.F., Volkow, N.D., et al. (2008). Pathophysiology of addiction. In Tasman, A., Kay, J., Lieberman, J.A., First, M.B., and Maj, M., eds., Psychiatry, 3rd ed., vol. 1. West Sussex, England: Wiley, pp. 354–378.
REFERENCES
Abreu-Villaca, Y., Seidler, F.J., et al. (2003). Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology 28(11):1935–1949.
Adriani, W., Spijker, S., et al. (2003). Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J. Neurosci. 23(11):4712–4716.
American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Washington, DC: Author.
American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, Text Revision, 4th Edition. Washington, DC: Author.
Belluzzi, J.D., Lee, A.G., et al. (2004). Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology 174(3):389–395.
Boden, J.M., Fergusson, D.M., et al. (2010). Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br. J. Psychiatry 196(6):440–446.
Breslau, N., Novak, S.P., et al. (2004). Psychiatric disorders and stages of smoking. Biol. Psychiatry 55(1):69–76.
Budney, A.J., Moore, B.A., et al. (2003). The time course and significance of cannabis withdrawal. J. Abnorm. Psychol. 112(3):393–402.
Compton, W.M., Conway, K.P., et al. (2005). Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 66(6):677–685.
Compton, W.M., Thomas, Y.F., et al. (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 64(5):566–576.
Conway, K.P., Compton, W., et al. (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 67(2):247–257.
Cottler, L.B. (2007). Drug use disorders in the National Comorbidity Survey: have we come a long way? Arch. Gen. Psychiatry 64(3):380–381.
Dahl, R.E., and Spear, L.P. (2004). Adolescent Brain Development: Vulnerability and Opportunities. New York: New York Academy of Sciences.
de Leon, J., and Diaz, F.J. (2005). A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 76(2–3):135–157.
Fowler, J.S., Logan, J., et al. (2003). Monoamine oxidase and cigarette smoking. Neurotoxicology 24(1):75–82.
Grant, B.F., Compton, W.M., et al. (2007). Errors in assessing DSM-IV substance use disorders. Arch. Gen. Psychiatry 64(3):379–380.
Grant, B.F., Hasin, D.S., et al. (2004a). Nicotine dependence and psychiatric disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 61(11):1107–1115.
Grant, B.F., Stinson, F.S., et al. (2004b). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 61(8):807–816.
Grant, B.F., Stinson, F.S., et al. (2001). Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J. Subst. Abuse 13(4):493–504.
Griesler, P.C., Hu, M.C., et al. (2008). Comorbidity of psychiatric disorders and nicotine dependence among adolescents: findings from a prospective, longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 47(11):1340–1350.
Griesler, P.C., Hu, M.C., et al. (2011). Comorbid psychiatric disorders and nicotine dependence in adolescence. Addiction 106(5):1010–1020.
Grucza, R.A., Abbacchi, A.M., et al. (2007). Discrepancies in estimates of prevalence and correlates of substance use and disorders between two national surveys. Addiction 102(4):623–629.
Hasin, D.S. (2012a, April 17). DSM-V Substance Use Disorders. Paper presented at the Drugs and Society Seminar, Columbia University, New York.
Hasin, D.S. (2012b). Combining abuse and dependence in DSM-5. J. Stud. Alcohol Drugs 73(4):702–704.
Hasin, D.S., Fenton, M.C., et al. (2012). Analyses related to the development of DSM-5 criteria for substance use related disorders: 2. proposed DSM-5 criteria for alcohol, cannabis, cocaine and heroin disorders in 663 substance abuse patients. Drug Alcohol Depen. 122(1–2):28–37.
Hasin, D.S., and Grant, B.F. (2004). The co-occurrence of DSM-IV alcohol abuse in DSM-IV alcohol dependence: results of the National Epidemiologic Survey on Alcohol and Related Conditions on heterogeneity that differ by population subgroup. Arch. Gen. Psychiatry 61(9):891–896.
Hasin, D.S., Hatzenbueler, M., et al. (2005). Co-occurring DSM-IV drug abuse in DSM-IV drug dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depen. 80(1):117–123.
Hasin, D.S., and Kilcoyne, B. (2012). Comorbidity of psychiatric and substance use disorders in the United States: current issues and findings from the NESARC. Curr. Opin. Psychiatry 25(3):165–171.
Hedden, S., Gfroerer, J., et al. (2012). The NSDUH Data Review: Comparison of NSDUH Mental Health Data and Methods with Other Data Sources. Rockville, MD: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality.
Hingson, R.W., Heeren, T., et al. (2006). Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch. Pediat. Adol. Med. 160(7):739–746.
Hughes, J.R. (2006). Should criteria for drug dependence differ across drugs? Addiction 101(Suppl 1):134–141.
Isensee, B., Wittchen, H.U., et al. (2003). Smoking increases the risk of panic: findings from a prospective community study. Arch. Gen. Psychiatry 60(7):692–700.
Johnston, L.D., O’Malley, P.M., et al. (2010, December 14). National press release: Marijuana use is rising; ecstasy use is beginning to rise; and alcohol use is declining among U.S. teens. Ann Arbor: University of Michigan News Service.
Johnston, L.D., O’Malley, P.M., et al. (2011, December 14). National press release: Marijuana use continues to rise among U.S. teens, while alcohol use hits historic lows. Ann Arbor: University of Michigan News Service.
Kandel, D.B. (1975). Stages in adolescent involvement in drug use. Science 190(4217):912–914.
Kandel, D.B. (2002). Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. Cambridge, UK: Cambridge University Press.
Kandel, D.B. (2003). The Natural History of Smoking and Nicotine Dependence. Paper presented at the Proceedings of the Royal Society of Canada 2002 Symposium on Addictions: Impact on Canada, Ottawa, Ontario, Canada.
Kandel, D.B., and Chen, K. (2000). Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob. Res. 2(3):263–274.
Kandel, D.B., and Faust, R. (1975). Sequence and stages in patterns of adolescent drug use. Arch. Gen. Psychiatry 32(7):923–932.
Karp, I., O’Loughlin, J., et al. (2006). Risk factors for tobacco dependence in adolescent smokers. Tob. Control 15(3):199–204.
Kauer, J.A., and Malenka, R.C. (2007). Synaptic plasticity and addiction. Nat. Rev. Neurosci. 8(11):844–858.
Kessler, R.C. (2004). The epidemiology of dual diagnosis. Biol. Psychiatry 56(10):730–737.
Kessler, R.C., Avenevoli, S., et al. (2012a). Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch. Gen. Psychiatry 69(4):372–380.
Kessler, R.C., Avenevoli, S., et al. (2012b). Lifetime co-morbidity of DSM-IV disorders in the U.S. National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Psychol. Med. 42(9):1–14.
Kessler, R.C., Berglund, P., et al. (2005a). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62(6):593–602.
Kessler, R.C., Chiu, W.T., et al. (2005b). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62(6):617–627.
Kessler, R.C., and Merikangas, K.R. (2007). Drug use disorders in the National Comorbidity Survey: have we come a long way? In reply. Arch. Gen. Psychiatry 64(3):381–382.
Klungsøyr, O., Nygard, J.F., et al. (2006). Cigarette smoking and incidence of first depressive episode: an 11-year, population-based follow-up study. Am. J. Epidemiol. 163(5):421–432.
Koob, G.F., Volkow, N.D., et al. (2008). Pathophysiology of addiction. In: Tasman, A., Kay, J., Lieberman, J.A., First, M.B., and Maj, M., eds. Psychiatry, 3rd Edition, Volume 1. West Sussex, UK: Wiley, pp. 354–378.
Kumari, V., and Postma, P. (2005). Nicotine use in schizophrenia: the self-medication hypotheses. Neurosci. Biobehav. Rev. 29(6):1021–1034.
Levin, E.D., Rezvani, A.H., et al. (2003). Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology 169(2):141–149.
Levine, A., Huang, Y., et al. (2011). Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci. Transl. Med. 3(107):107ra109.
McPherson, C.S., and Lawrence, A.J. (2006). Exposure to amphetamine in rats during periadolescence establishes behavioural and extrastriatal neural sensitization in adulthood. Int. J. Neuropsychopharmacol. 9(4):377–392.
McQuown, S.C., Belluzzi, J.D., et al. (2007). Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol. Teratol. 29(1):66–73.
McQuown, S.C., Dao, J.M., et al. (2009). Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats. Psychopharmacology 207(1):143–152.
Moffitt, T.E., Caspi, A., et al. (2010). How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol. Med. 40(6):899–909.
Nelson, C.B., Rehm, J., et al. (1999). Factor structures for DSM-IV substance disorder criteria endorsed by alcohol, cannabis, cocaine and opiate users: results from the WHO reliability and validity study. Addiction 94(6):843–855.
O’Brien, C. (2011). Addiction and dependence in DSM-V. Addiction 106(5):866–867.
Purves, D., Augustine, G.J., et al. (2001). Neuroscience, 2nd Edition. Sunderland, MA: Sinauer.
Rezvani, A.H., and Levin, E.D. (2004). Adolescent and adult rats respond differently to nicotine and alcohol: motor activity and body temperature. Int. J. Dev. Neurosci. 22(5–6):349–354.
Ristuccia, R.C., and Spear, L.P. (2005). Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol Clin. Exp. Res. 29(10):1809–1820.
Saha, T.D., Compton, W.M., et al. (2012). Analyses related to the development of DSM-5 criteria for substance use related disorders: 1. toward amphetamine, cocaine and prescription drug use disorder continua using Item Response Theory. Drug Alcohol Depen. 122(1–2):38–46.
Schochet, T.L., Kelley, A.E., et al. (2005). Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience 135(1):285–297.
Schuckit, M.A. (2012). Editor’s corner: editorial in reply to the comments of Griffith Edwards. J. Stud. Alcohol Drugs 73(4):521–522.
Shiffman, S., Waters, A., et al. (2004). The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 6(2):327–348.
Slawecki, C.J., and Roth, J. (2004). Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcohol Clin. Exp. Res. 28(4):598–607.
Smith, S.M., Stinson, F.S., et al. (2006). Race/ethnic differences in the prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol. Med. 36(7):987–998.
Sowell, E.R., Peterson, B.S., et al. (2003). Mapping cortical change across the human life span. Nat. Neurosci. 6(3):309–315.
Spear, L.P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24(4):417–463.
Substance Abuse and Mental Health Services Administration [SAMHSA]. (2011). Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings (NSDUH Series H-41, HHS Publication No. [SMA] 11-4658). Rockville, MD: Author.
Thomas, M.J., Beurrier, C., et al. (2001). Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 4(12):1217–1223.
Volkow, N.D. (2006). Altered pathways: drug abuse and age of onset. Add. Prof. 4(3):26, 29.
Volkow, N.D. (2009). Substance use disorders in schizophrenia: clinical implications of comorbidity. Schizophr. Bull. 35(3):469–472.
Wagner, F.A., and Anthony, J.C. (2002). From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 26(4):479–488.
White, D.A., and Holtzman, S.G. (2005). Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur. J. Pharmacol. 528(1–3):119–123.
Wing, V.C., Wass, C.E., et al. (2012). A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Ann. NY Acad. Sci. 1248:89–106.
World Health Organization. (1992). International Statistical Classification of Diseases and Related Health Problems, 10th revision. Geneva: Author.
