Emergences of Chikungunya and Zika in Africa
Diawo Diallo; Ibrahima Dia; Cheikh T. Diagne; Alioune Gaye; Mawlouth Diallo Medical Entomology Unit, Institut Pasteur de Dakar, Dakar, Senegal
Abstract
Both chikungunya virus and Zika virus were first described and isolated during studies conducted in Africa. The geographic distribution, hosts, vectors, and transmission cycles of CHIKV as well as surveillance, prevention, and methods of control in Africa are presented and reviewed in this chapter. The information is presented regionally and by country with data for specific epidemics if appropriate.
Keywords
Chikungunya virus; Zika virus; Aedes aegypti; Aedes albopictus; Africa; Epidemiology; Outbreaks; Mosquitoes
Introduction
Chikungunya (CHIK) and Zika (ZIK) are two arboviruses originated from Africa that have recently globalized, therefore becoming major public health problems worldwide. Chikungunya virus (CHIKV; family, Togaviridae; genus, Alphavirus) has been responsible for several outbreaks and sporadic cases of febrile illness in Africa since it was first identified in Tanzania in 1952 (Lumsden, 1955; Robinson, 1955; Ross, 1956). The virus was most likely present in Africa before 1952 and identified wrongly for dengue virus. The first possible record of CHIKV emergence in Africa was published in Cairo in 1779 (Carey, 1971).
The name “chikungunya” is derived from Makonde, a language spoken in Tanzania, and means “that which bends up.” It refers to the posture of patients afflicted with severe joint pain. CHIKV was formerly considered as an arbovirus of minor concern, but since 2004, it has become a major public health concern worldwide. Between 2004 and 2008, CHIKV caused large outbreaks in islands of the Indian Ocean, the Indian subcontinent, and several European countries (Charrel et al., 2007; Rezza et al., 2007). In India during this time period, more than 1 million cases of CHIKV were reported in different states (Lahariya and Pradhan, 2006; Powers and Logue, 2007). Since the first well-documented autochthonous transmission in the Americas in December 2013, there have been more than 1.6 million cases of CHIKV and at least 253 associated deaths in 44 countries and territories in the region (Weaver and Lecuit, 2015; PAHO, 2016). Further details of chikungunya in Asia, Europe, and the Americas are provided in Chapters 5–7, respectively. As described in the previous chapter, chikungunya disease in humans is mainly characterized by sudden onset of fever, severe arthralgia, rash, headache, and other symptoms including photophobia and vomiting (Powers and Logue, 2007; Weaver and Lecuit, 2015). Arthralgia can be debilitating and prolonged. The disease has an incubation period of ~ 2–10 days (usually 2–3 days) and lasts for 1–7 days (Burt et al., 2012). Chikungunya disease is generally associated with high morbidity with occasional mortality as reported during the large outbreaks since 2004 in the Indian Ocean and Americas (Renault et al., 2008; Cardona-Ospina et al., 2015).
CHIKV is primarily transmitted by mosquitoes of the genus Aedes. In Africa, there is a sylvatic transmission cycle between arboreal Aedes and monkey nonhuman primates (NHPs) (Diallo et al., 1999, 2012b) and an urban cycle between humans and the mosquitoes Aedes aegypti and Aedes albopictus (Powers, 2010).
Zika virus (ZIKV; genus Flavivirus, family Flaviviridae) was only known to a small group of arbovirogists until it reached Micronesia causing the first well-described epidemic in the Yap island in 2007 (Duffy et al., 2009). Subsequently, the virus emerged on a global scale, causing outbreaks in 2010 in Cambodia, 2013 in New Caledonia and French Polynesia, 2015 in America with occurrence of new symptoms like microcephaly and Guillain-Barré syndrome (Musso, 2015; Musso and Gubler, 2016) Chapter 3. ZIKV is endemic in Africa and Asia. The virus was first isolated in Uganda from a febrile sentinel rhesus monkey (Macaca mulatta) in 1947 and from the mosquito, Ae. africanus 1 year later (Dick et al., 1952). Human infections were first described in 1964 by a medical entomologist infected during fieldwork in Uganda (Simpson, 1964). In Africa, the disease is generally mild and associated with headaches, maculopapular rash, fever, malaise, conjunctivitis, and arthralgia (Simpson, 1964; Musso and Gubler, 2016). ZIKV is transmitted in a zoonotic cycle between arboreal Aedes spp. mosquitoes and nonhuman Primates in African and Asian forests (Diallo et al., 2014). Urban epidemics of ZIKV, involving human and Ae. aegypti or Ae. albopictus as vectors, were recently observed in Gabon in 2007, Cabo Verde in 2015–16, and in Guinea Bissau in 2016 (Grard et al., 2014). The transmission cycles of ZIKV with discussion of arthropod vectors and vertebrate hosts have been more fully described in Chapter 2.
Until these recent events, there were no specific programs dedicated to ZIKV in Africa. Therefore, data were gathered as part of surveillance efforts or outbreak investigation of other arboviruses, specifically yellow fever virus (YFV) that is considered as a major public health problem in Africa. ZIKV was just considered as an incidental and insignificant arbovirus without any public health importance.
CHIKV and ZIKV diagnostics (Chapter 9) are performed by virus isolation or detection of viral RNA by reverse transcription polymerase chain reaction (RT-PCR) during the viremic phase of disease in humans and infected mosquitoes. Anti-CHIKV and ZIKV immunoglobulin M and G antibodies are detected by capture enzyme-linked immunosorbent assay (MAC-ELISA) in patients 6 months and more after disease onsets for IgM and a much longer period for IgG. Hemagglutination inhibition (HI) antibodies generally appear within 5–7 days after disease onset (Barrett and Weaver, 2002).
As described in Chapter 8, phylogenetic analyses of both CHIKV and ZIKV strains collected in Africa and elsewhere identified three genotypes, two African and one Asian (Volk et al., 2010; Faye et al., 2014).
Despite the fact that CHIKV and ZIKV are now considered as highly significant threats to public health worldwide, there is currently no review available on their emergences in Africa. The geographic distribution, hosts, vectors, and transmission cycles of CHIKV as well as surveillance, prevention, and methods of control in Africa are presented and reviewed in this chapter.
Geographic Distribution of the Emergence of Chikungunya and Zika Viruses in Humans
Geographic Distribution of the Emergence of Chikungunya Virus in Humans
CHIKV has been detected in humans as sporadic cases, epidemics and during serological survey studies in West, Central, East, and Southern Africa (Fig. 1).
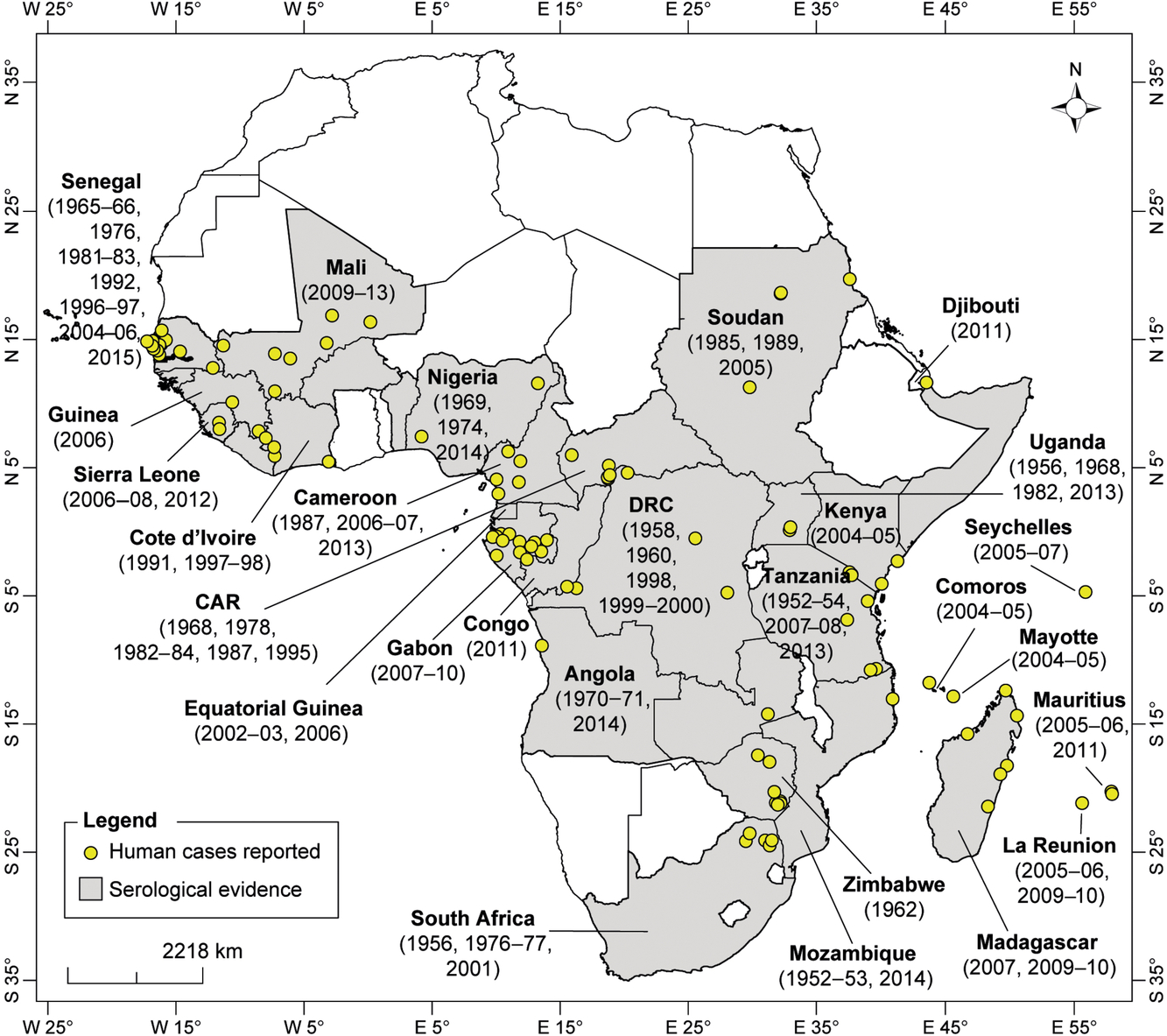
West Africa
West African countries and localities in which CHIKV strains or CHIKV antibodies have been detected in humans are presented in Fig. 2. In Senegal, most sporadic cases and CHIKV epidemics were reported in the Western region of Senegal. CHIKV was isolated from humans in Senegal in 1965–66, 1975–76, 1981–83, 1992, 1998, 2009, and 2015 (Diallo et al., 1999, 2012b; CRORA, 2013). Furthermore, CHIKV was isolated from human samples collected from Rufisque in 1966 (four strains), Touba in 1981–82 (eight strains), and Thiadiaye in 1982 (four strains). Anti-CHIKV IgM antibodies were detected in Kaffrine in 1996 and Niakhar in 1997 (Roche and Robin, 1967; Saluzzo et al., 1983; Monlun et al., 1993; Diallo et al., 1999; Thonnon et al., 1999). In 1996, 35.3% of 447 blood donors from Kaffrine were CHIKV IgM positive. In the Niakhar region, 8.5% of individuals tested in 1997 were CHIKV IgM positive (Thonnon et al., 1999).
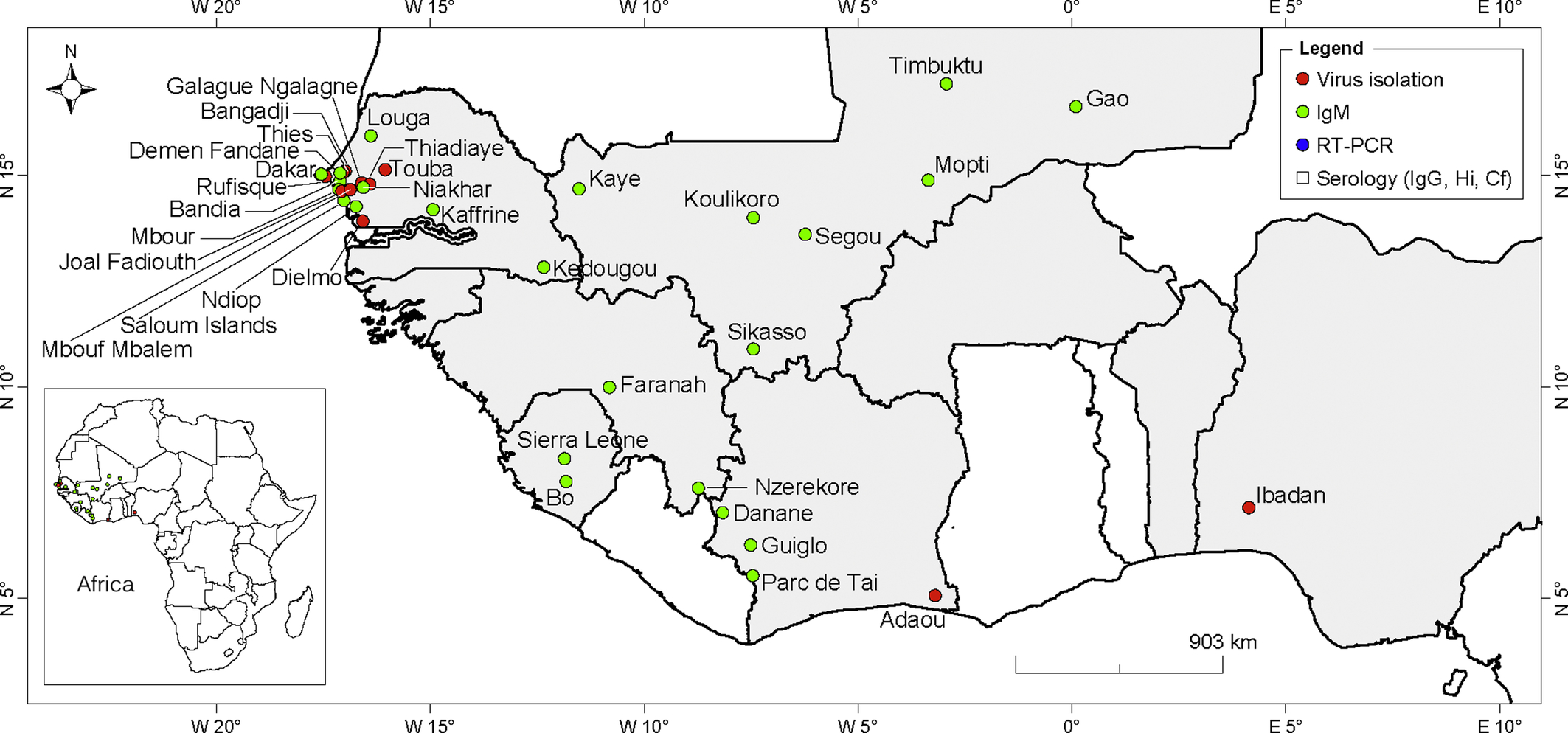
Results from serological surveys indicate that 39.8% of individuals in Niakhar and 52.6% of individuals were immune to CHIKV before the outbreaks, suggesting that the virus has been circulating in the region. Over the last 10 years, CHIKV has been reported among American Peace Corps volunteers in the Kedougou area in 2004, British soldiers in the Saloum islands in 2005, a group of patients returning from several localities in western Senegal (Thies, Louga, M’Bour, Joal-Fadiouth, and Dakar) in 2006, and the last outbreak was reported in 2015 in the Kedougou region (Pistone et al., 2009b; CRORA, 2013). In Mali, a recent serosurvey study revealed evidence of CHIKV circulation from 2009 to 2013 (Safronetz et al., 2016). The IgM prevalence ranged between 3.7% in 2010 and 7% in 2011.
In Guinea, anti-CHIKV IgM antibodies were detected in 8 of 47 acute febrile patients analyzed by MAC-ELISA and Plaque reduction neutralization test (PRNTs) in 2006–07 (Jentes et al., 2010). The positive samples originated from N’Zérékoré and Faranah. A previous serological survey in seven prefectures of Guinea reported a mean prevalence of 51.7% and detection of immune sera in all investigated prefectures (Ivanov et al., 1992). A serological survey conducted in Sierra Leone in 1972 reported anti-CHIKV antibodies in 21.8% of serum samples tested (Robin and Mouchet, 1975). Positive cases were detected in all of the provinces investigated. The virus re-emerged in Sierra Leone in 2012 in Bo, with the identification of 400 patients with anti-CHIKV IgM antibodies (Ansumana et al., 2013). In Guinea Bissau, anti-CHIKV antibodies were found in 22% of 200 human sera analyzed between 1964 and 1965 (Pinto, 1967).
The most recent evidence of CHIKV transmission in Nigeria was in a 21-year-old male Nigerian, who was positive for CHIKV by RT-PCR. He was tested in India in September 2014 after returning from a 1-month vacation in Nigeria (Raut et al., 2015). CHIKV was previously isolated in this country in Ibadan in 1964, 1969, and 1974 and reported as so-called Igbo-Ora in 1969 (Tomori et al., 1975). Further, neutralizing antibodies against CHIKV were detected in 17.4% of 143 febrile patients tested in Borno Sate in Northern Nigeria in 2008 (Baba et al., 2013). Anti-CHIKV IgM and IgG antibodies were detected in an American who resided in Cote d’Ivoire between October and December 1997 (Pile et al., 1999). A serological survey performed from May through December 1998 in 21 villages in western Cote d’Ivoire (Danané and Guiglo districts) showed that 9.9% of febrile patients were CHIKV positive (Attoh-Touré et al., 2008). In Benin, high seroprevalence of CHIKV infection was observed in pregnant women from Cotonou in 2006–07 (Bacci et al., 2015). Anti-CHIKV IgG antibodies were detected in 36.1% of patients, suggesting that CHIKV may potentially be endemic. Furthermore, a previous study reported the detection of anti-CHIKV IgG antibodies in 5.7% of 88 German aid workers returning from Benin (Eisenhut et al., 1999). The workers had resided in Benin between 1987 and 1993.
Central Africa
Details of countries and localities where CHIKV strains or CHIKV antibodies were detected in humans are shown in Fig. 3. Epidemics of 1999 and 2000 in Kinshasha, Democratic Republic of Congo (DRC), were the biggest and first well-described CHIKV outbreaks in Central Africa (Muyembe-Tamfum et al., 2003) with more than 50,000 cases identified. CHIKV was first isolated in a rural area in the Eastern DRC in 1958 and 1960 (Osterrieth et al., 1960, 1961). In 1998, anti-CHIKV IgM antibodies were detected in 12 febrile patients during a West Nile virus outbreak in Kisangani (Nur et al., 1999).
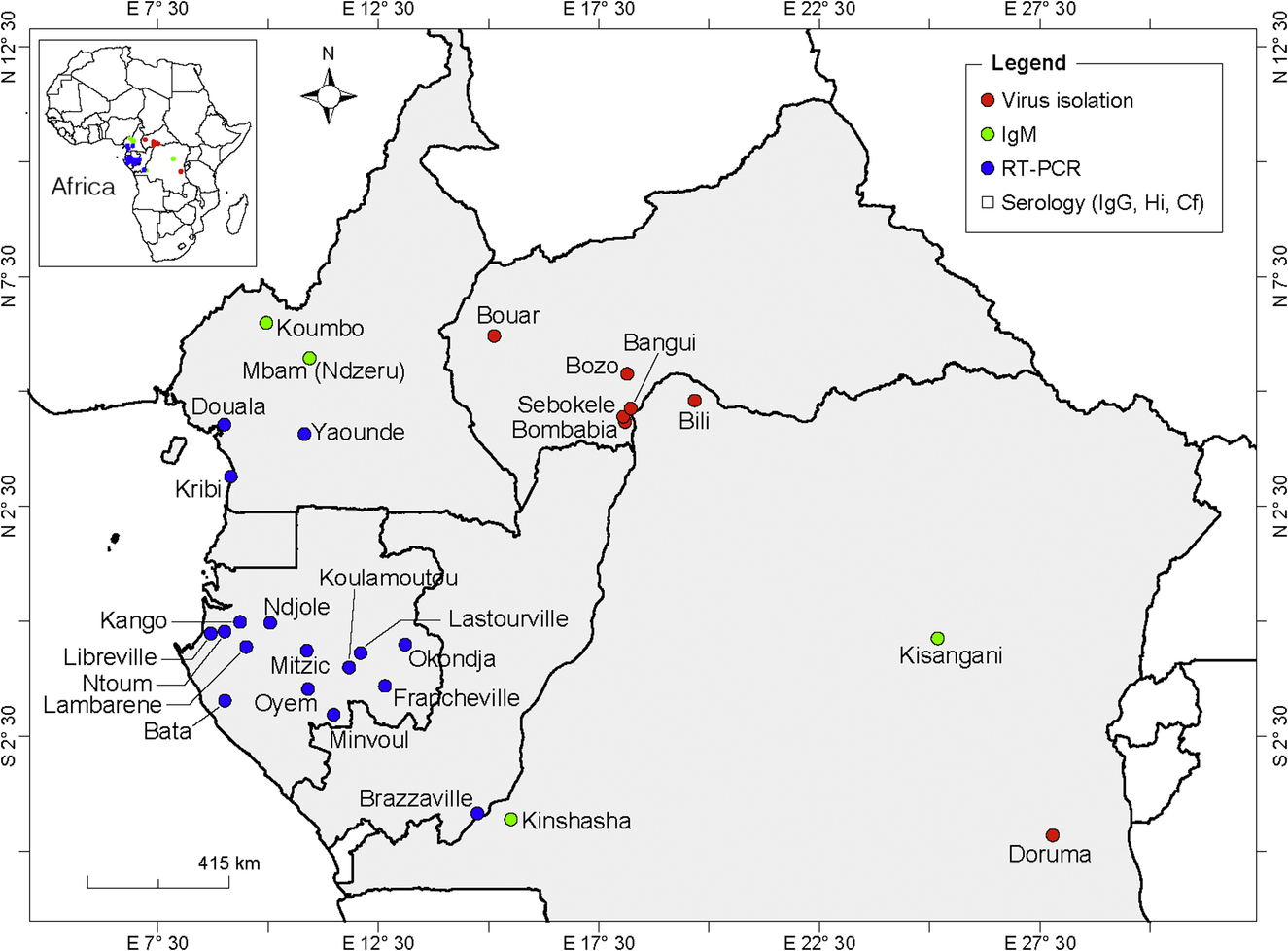
In the neighboring Republic of Congo, CHIKV genomic RNA was detected in 21 of 23 patients tested in Brazzaville in 2011 (Mombouli et al., 2013). However, a serological survey conducted before the outbreak revealed the presence of anti-CHIKV IgG antibodies in 34.4% of 517 blood donors tested, indicating prior circulation of CHIKV in this region (Moyen et al., 2014).
Serological prevalence studies showed CHIKV to be one of the most common infections in Cameroon (Kuniholm et al., 2006). The first report on an epidemic was described in 2006 in a retrospective serological survey, which showed 51.4% of the 105 individuals tested to have anti-CHIKV IgM antibodies in rural regions of Cameroon (Peyrefitte et al., 2007; Demanou et al., 2010). The most recent report on the detection of CHIKV was in June 2016 in 3 of 12 sera from febrile patients collected during a survey in the center, Southern, and littoral regions (Yaoundé, Kribi, and Douala) of Cameroon (Demanou et al., 2015).
Between 2007 and 2010, CHIKV was responsible for several outbreaks in Gabon with a total of 20,000 cases (Caron et al., 2012). In 2007, the outbreak was centered on Libreville and the Estuaire and Wole Ntem provinces, where 28.1% of patients with acute febrile illness (n = 1057) were CHIKV positive by RT-PCR. In 2008 and 2009, the virus was detected in the center and Southeastern part of the country with 158 cases. Finally, a large outbreak was centered on Franceville and the Haut Ogooue and Ogooue Lolo provinces n 2010 with 1112 cases. Antibodies against CHIKV were previously detected in human and simian sera in Libreville (8.5%) and Franceville (between 20% and 44%) in Gabon in the late 1970s (Saluzzo et al., 1982).
The CHIKV genome was amplified from 8 of 720 blood samples from febrile infants collected in Bata in Equatorial Guinea in 2002–03 (Collao et al., 2010). In 2006, CHIKV was also isolated from a Spanish traveler returning from Equatorial Guinea.
CHIKV has been isolated from human sera in several localities (Bozo, Bangui, Bonbabia, Sebokele, and Boar) in the Central African Republic in 1968, 1978, 1982–85, 1987, and 1995 (Saluzzo et al., 1980; Mathiot et al., 1988; CRORA, 2013). Antibodies against CHIKV have been detected in 17% of human sera in 1978–79 by indirect hemagglutination tests (IHA) from several parts of the country.
Southern Africa
In Southern Africa, CHIKV or antibodies against this virus were detected in residents of the countries and localities presented in Fig. 4. In the Republic of South Africa, human cases of CHIKV were detected for the first time in the rural area of the Limpopo Province in 1956. Other CHIK human cases were also reported in the same region in 1975–77 and most recently in 2001 (Jupp, 2005). In 1976, CHIKV was isolated from 8 patient sera. Furthermore, sera from 19 men were found to be CHIKV positive by serology in this rural area. Following this outbreak, anti-CHIKV antibodies were detected in 5%–46% of humans and 15%–71% of baboons from several localities of the area (Lillie, Hope, Phalaborwa, Hoedspruit, and Gravelotte) (McIntosh et al., 1977). In 1977, a group of high-school children from Pretoria and accompanied adults were infected with CHIKV after a visit to the northern Transvaal bushveld (Fourie and Morrison, 1979). NHPs were affected by CHIKV in the Ndumu area in northern Kwazulu Natal in 1964 (Jupp, 2005). No apparent human cases were recorded during this particular outbreak.
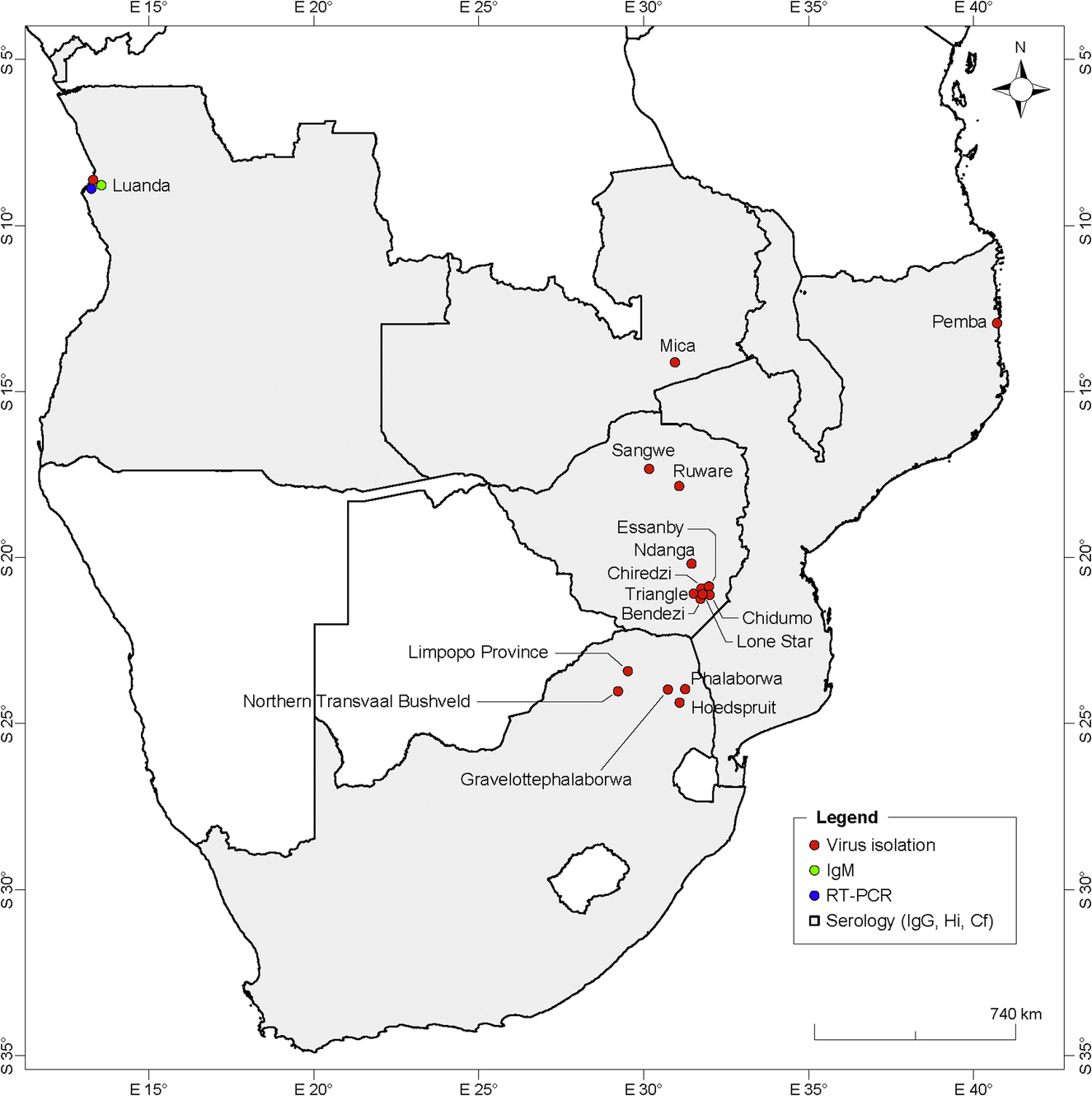
Anti-CHIKV IgM antibodies were detected in the sera of patients from Angola in 2014, indicating an ongoing outbreak (Parreira et al., 2014). CHIKV was previously detected in northern Angola in 1959 by serological analysis, with the detection of neutralizing antibodies in human sera (Kokernot et al., 1965). In 1970–71, the virus was detected in humans in Luanda by serological analysis and virus isolation (Filipe and Pinto, 1973).
Anti-CHIKV antibodies were detected in Zambia in 1959 (Rodger, 1961), Malawi in 1987–89 (van den Bosch and Lloyd, 2000), and Southern Zimbabwe in 1961–62 and 1971 (McIntosh et al., 1963b; Swanepoel and Cruickshank, 1974).
In Mozambique, the most recent emergence of CHIKV was in a biologically confirmed positive patient from Pemba in northern Mozambique in 2014 (Aly, 2015). In 2013, anti-CHIKV IgG antibodies were found in 24.6% of 208 acute febrile patients in Maputo. Antibody titers were observed to increase in 4.3% of the positive patients, suggesting recent infection (Gudo et al., 2015). The emergence of CHIKV was previously reported in northern Mozambique with the occurrence of small outbreaks and sporadic cases in 1952–53 (Lumsden, 1955). Furthermore, anti-CHIKV antibodies were detected in 21.9% of 870 sera collected in several areas throughout the country in 1957 (Kokernot et al., 1960).
East Africa and the Indian Ocean
The geographical distribution of CHIKV and anti-CHIKV antibodies detected in East Africa and the Indian Ocean is shown in Fig. 5. In Somalia, samples analyzed for CHIKV during surveillance program between 1985 and 1987 were all negative. However, IgG antibodies to CHIKV were detected in 4% of patients with febrile symptom in Barbera in 1989 (Hibbs et al., 1993). The most recent manifestation of CHIKV in Somalia was the detection of IgM and IgG antibodies against the virus in two travelers returning from Mogadishu in 2016 (Zammarchi et al., 2016).
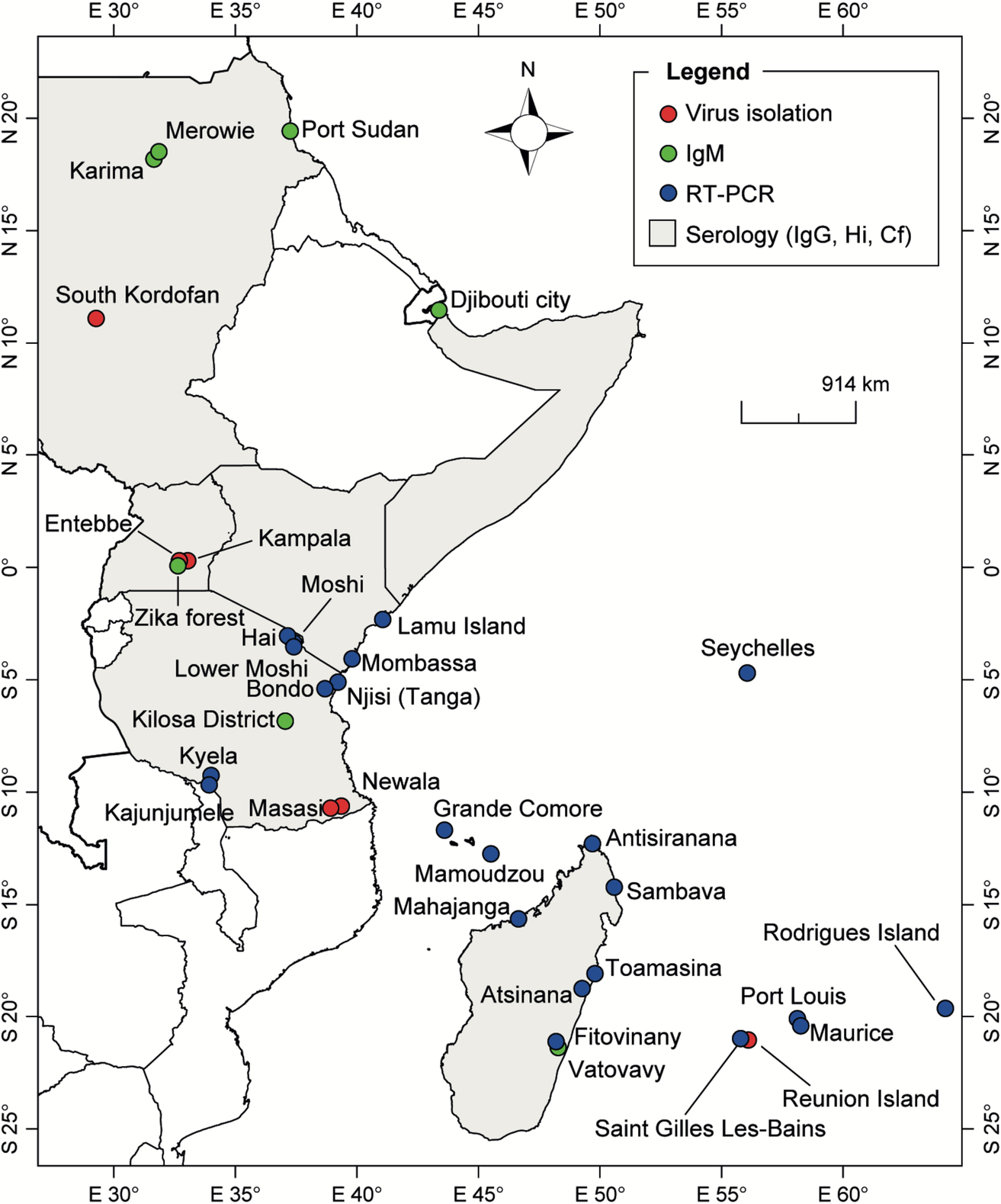
In Djibouti, the unique human seroepidemiological survey conducted in 1987 revealed little threat to the inhabitants of Djibouti for nine hemorrhagic fever viruses tested including CHIKV. Only one seropositive subject from Ethiopia had antibodies to CHIKV, most likely from a previous infection contracted in Ethiopia (Salah et al., 1988). A serological survey in 2010 has shown that only 2.6% of the population had antibodies against CHIKV. However, a CHIKV outbreak was reported in this country in 2011 (Andayi et al., 2014).
In Sudan, CHIKV was responsible for 605 cases of acute febrile illness during an outbreak in 2005 in South Kordofan. Indeed, the virus was isolated from febrile patients during the outbreak. Serologic evidences of recent CHIKV infection were also observed among the patients with acute febrile illness and asymptomatic cases (Gould et al., 2008). Serological evidence of CHIKV infection was previously detected among soldiers in Port Sudan and during serosurveys conducted in the Sennar district and in Northern Sudan (Salim and Porterfield, 1973; Watts et al., 1994). During the 1960 Yellow Fever (YF) epidemic in Ethiopia, antibodies against members of the Alphavirus family, represented by CHIKV, were analyzed. Relatively low levels were detected in all territories (Serie et al., 1964).
CHIKV was first isolated and described in Tanzania during an outbreak of dengue-like febrile illness in 1952–53, which affected the Newala and Masasi districts (Ross, 1956). In 2007–08, 7.9% of a cohort of 700 pediatric and adult hospitalized febrile patients in Moshi, in northern Tanzania, were CHIKV positive by RT-PCR suggesting active circulation of CHIKV during this period (Hertz et al., 2012; Crump et al., 2013). A serological study of febrile children in the Kilosa district in 2013 showed that 4.7% (n = 367) of children were CHIKV IgM positive, indicating recent circulation (Chipwaza et al., 2014). A cross-sectional study in three localities of north-eastern Tanzania (Bondo, Hai, and Lower Moshi) in 2013 and 2014 showed that 12.9% (49/381) and 13.8% (86/622) of febrile and afebrile participants, respectively, were CHIKV IgM positive (Kajeguka et al., 2016). Among the febrile patients, 11 were PCR positive.
CHIKV was first isolated from human serum in 1956 in Uganda. Only sporadic cases were reported and no large outbreak was documented. This may be due to the apparent nonanthropophilic nature of the main vector Ae. aegypti, which is not well adapted to the human environment in this country. Five human cases of CHIKV were detected in the Entebbe area, during an epizootic of CHIKV in the Mukono district in 1982 (Kalunda et al., 1985). During this outbreak, CHIKV was isolated from seven human sera. A serological survey in 1984 showed that CHIKV was the most prevalent arbovirus circulating in villages of the Karamojat district (Rodhain et al., 1989).
In Kenya, CHIK was first reported during a serological survey conducted in 1968–69 in Central Nyanza, the Kitui and the Malindi districts (Geser et al., 1970). In this study, 1500 individuals were randomly selected and CHIKV antibodies were found in 54.6% of subjects from Nyanza and 50.8% from Malindi. CHIKV antibodies were not detected in individuals from the Kitui district. After evidence of the circulation of CHIKV from the previous study, an outbreak of CHIK was reported on Lamu Island in 2004, with at least 1300 potential cases (Sergon et al., 2008). During this outbreak, IgM antibodies against CHIKV were detected in 60 of 130 human sera tested. Furthermore, CHIKV was detected by isolation or RT-PCR in an additional 22 sera. An outbreak of CHIKV also occurred in Mombasa in 2004 (Sang et al., 2008).
Interestingly, several retrospective serological surveys in Kenya covering different periods reported trace levels of human infection. In 1994–95, retrospective analysis of samples collected in a healthcare center from patients with a misdiagnosed condition revealed five samples positive for CHIKV (three in 1994 and two in 1995) (Sanders et al., 1999). In a study performed on serum samples collected in 2000–03 from the Msambweni district and in 2004 from inland children, it was observed that samples collected from the lowlands were more likely to be seropositive for CHIKV than in children in the highlands (42% vs 0%). A retrospective serological survey in 2007 using HIV-negative blood samples confirmed IgG antibodies against CHIKV in 4.5% of 1091 samples tested (Ochieng et al., 2015). In a recent serological survey conducted using PRNT, 11.5% of febrile children recruited in a district hospital of Busia, Kenya, in 2010 had antibodies against CHIKV (Mwongula et al., 2013).
The first major outbreak of CHIKV occurred in the Indian Ocean between 2004 and 2007 and started in coastal Kenya (Pialoux et al., 2007). The outbreak spread to the Comoros Islands in 2004–05, Mayotte, Reunion Island, Mauritius, Seychelles, and Madagascar (Renault et al., 2007; Beesoon et al., 2008; Ratsitorahina et al., 2008). In 2005, antibodies against CHIKV were detected in 63% of samples analyzed from Grande Comoros Island (Sergon et al., 2008).
In Mauritius, the first CHIKV outbreak occurred in 2005 with an initial transmission focused on the capital Port Louis, extending thereafter to other localities as well as to Rodrigues Island in 2006. At the end of 2006, < 1% of the inhabitants of Rodrigues Island were affected. No other cases of CHIKV were officially reported in Mauritius between 2006 and January 2011, with Mauritius authorities reporting an indigenous case (Beesoon et al., 2008; Renault et al., 2012).
In the Seychelles archipelago, evidence of human CHIKV infection was reported in 1979 (Calisher et al., 1981). No clinical cases of CHIKV were diagnosed during the 20th century. The first outbreak was reported between July 2005 and late 2007 and affected ~ 12% of the population (Renault et al., 2012). Cases were reported in all districts of the three main islands of the archipelago (Mahe, Praslin, and La Digue).
In 2005–06, a CHIKV epidemic of unprecedented magnitude was reported in Reunion Island. More than one-third of the population (40%) was affected. A mutation facilitating the adaptation of the virus to Ae. albopictus was suspected of being responsible and also for the particularly intense spread of the virus (Tsetsarkin et al., 2007, 2009; Charrel et al., 2007; Pialoux et al., 2007; Renault et al., 2007, 2012). Two other CHIKV outbreaks were reported in 2009 and 2010 on the western coast of Reunion Island (with more than 100 cases) and in travelers returning from this island (D'Ortenzio et al., 2009, 2011).
CHIKV was first detected in Mayotte in 2005 at the end of the rainy season and was followed by a large-scale epidemic in 2006, which affected between 26% and 37.2% of the population (Sissoko et al., 2008a,b, 2010; Renault et al., 2012)
Many imported cases from Madagascar were reported in France and Reunion Island in visitors coming from Sambava in Northeast Madagascar in 2007, but also from Toamasina in 2009 and 2010 (Pistone et al., 2009a; Renault et al., 2012). Several transmission foci were detected by RT-PCR and/or IgM in 2007 in the coastal areas of Antisiranana (north coast) and Mahajanga (West coast), in 2009 in Toamasina, and in 2010 in the southeastern coastal provinces of Vatovavy Fitovinany and Atsinana. In October 2009, the CHIKV IgM prevalence was 27.5% (of the samples from Mananjary) and 5.2% (of the samples from Manakara) in pregnant women in the eastern coast 2–4 months after the peak of an outbreak of febrile disease (Schwarz et al., 2012).
Geographic Distribution of ZIKV Emergence in Humans
In Africa, evidences of ZIKV emergence in human have been shown by epidemics, sporadic cases, and serological studies in humans (Fig. 6).
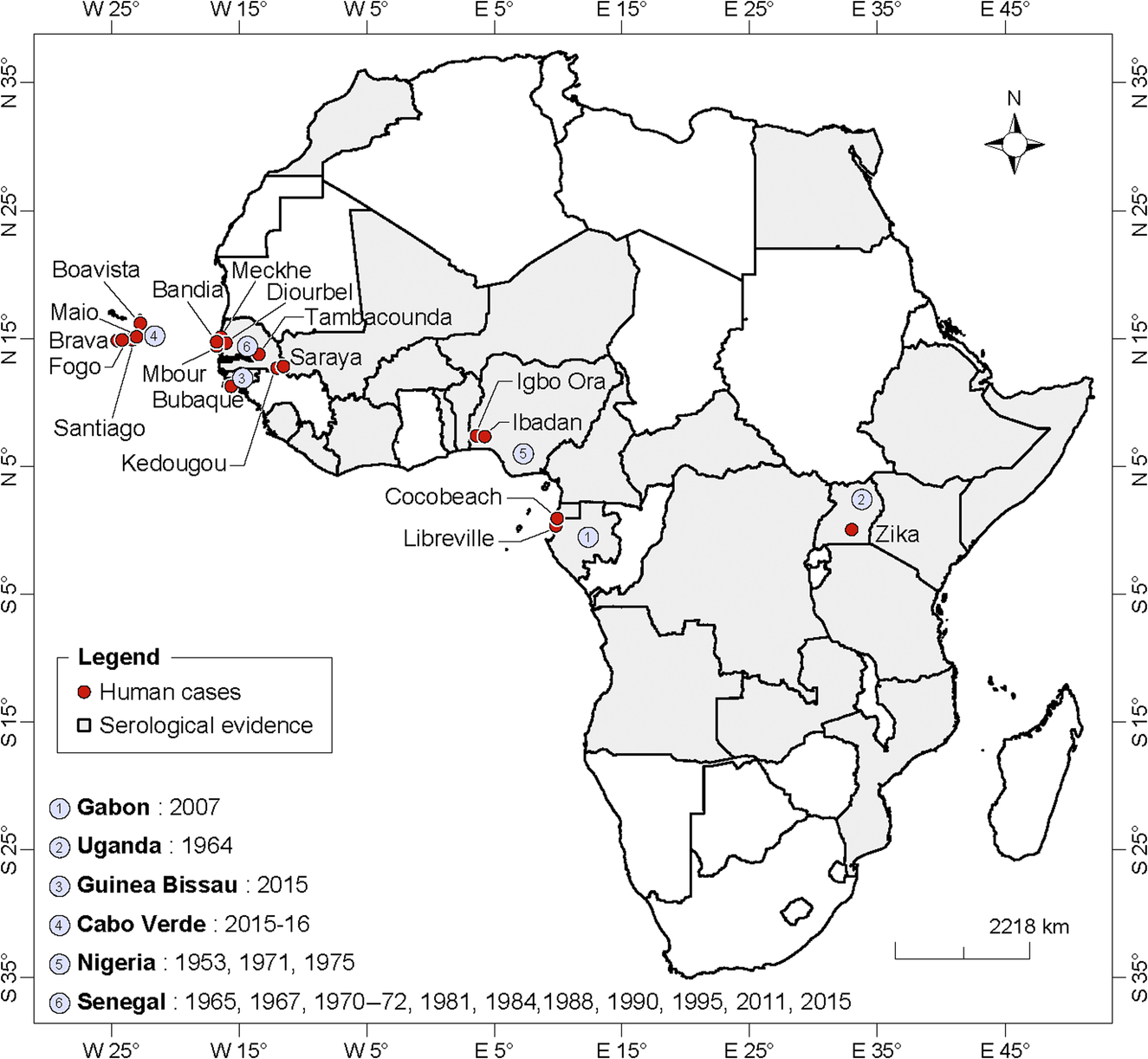
West Africa
The first evidence of ZIKV presence in Senegal was the detection of IH antibodies in 33% of the 440 human sera collected in several localities of the country (Dakar, Casamance, Ferlo, and Fleuve) in 1962 (Bres et al., 1963). In the western part of this country, ZIKV infection has been recorded from children under 10 years in 1965 in Diourbel. Serological studies detected antibodies against ZIKV from children between 0 and 4 years in Bandia in 1967, 1970–72. Between 1972 and 1975, a serosurvey revealed that 58.3% of the populations of Diourbel, Casamance, Sine Saloum, and Senegal Oriental were IgM positive for ZIKV (Renaudet et al., 1978). The population of Mekhé (Panthiou-Sine and Niakhène) also tested positive for IgM/IgG in 1981 (Dakar, 1981).
A serosurvey conducted in southeastern Senegal identified IgM antibodies to ZIKV in human samples collected in the departments of Saraya (12.5%) and Kedougou including the city of Kedougou (11.9%), and the villages of Dindefello (5.7%), Fongolimbi (13.6%), Salemata (10.1%), and Khossanto (6.2%) in 1988; and Saraya (2.2%), Dindefello (5.6%), Tomboronkoto (4.4%), Salemata (2.7%), Khossanto (4.0%), and Bandafassi (4.8%) in 1990 (Monlun et al., 1993). These results suggest that ZIKV outbreaks occurred in human in 1988 and 1990 in southeastern Senegal. ZIKV was isolated from a scientist infected in Kedougou in 1990 (Monlun et al., 1993). In Kedougou, other studies indicated child as well as expatriate infection in 1984 and 1995. The most recent outbreaks were reported in 2011 (Diallo et al., 2014) and 2015 following an amplification of the sylvatic cycle in Kedougou. Human cases have been reported during both outbreaks with 14 and 17 positives with IgM antibodies to ZIKV in 2011 and 2015, respectively. In addition, Mbour and Tambacouda reported ZIK cases as part surveillance of febrile syndromes in 2015.
In Guinea Bissau, anti-ZIKV antibodies were detected in 11% of the 921 human sera collected in 1964–65 by Pinto (Pinto, 1967). The prevalence was 2%, 8%, and 14% in coast, inland, and nonresident persons, respectively. More recently, the country reported for the first time an outbreak of ZIK in 2016. Human cases were detected by PCR from 4 patients among 21 collected in the Island of Bubaque in the region of Bijagos.
ZIKV antibodies have been detected in 52% of the human sera collected during serosurveys conducted in the Malian localities of Nioro du Sahel in 1964 and Yanfolila in 1967 (Bres, 1970). A human serosurvey conducted in five localities of Burkina faso (Dori, Banfora, Bobodioulasso, Diébougou, and Zignaré) in 1963–64 showed 53% of ZIKV antibodies (Bres, 1970).
The most recent serosurvey in Côte d’Ivoire conducted in 1999 in the Comoe National Park area detected IgG antibodies against ZIKV in 48% of the 42 sera collected during a YF outbreak investigation (Akoua-Koffi et al., 2001, 2004). The only other known serosurvey conducted between 1963 and 1965 in six localities (Bouaké, Tiassalé, Korhogo, Man, and Daloa Sassandra) showed that 20% of the sera were positive for antibodies to ZIKV (Bres 1970). ZIKV was considered as responsible for infection of part of the central nervous system observed in 45.3% of 64 patients tested from March 1997 to March 1998 in Abidjan (Akoua-Koffi et al., 2004).
In Nigeria, the first ZIKV strain was isolated from a young girl in 1953 (Macnamara, 1954). Neutralizing antibodies were detected in 44% of 97 sera collected in children under 16 years from Ilaro in 1951 (Macnamara et al., 1959). Theiler detected the presence of ZIKV IH antibodies in 55.1% of the 207 human sera collected in another locality of Nigeria (Ilobi) in 1955 (Theiler, 1961). During a serosurvey conducted in 1966–67, percentages of human sera positive for ZIKV antibodies were 51.1%, 46.3%, and 12.2% in the Forest (Imosan), Savanna (Fugar, Iressa, and Ado), and swamp (Ase, Orhua) areas, respectively (Robin, in Bres, 1970). Human occurrence of ZIK in Nigeria was also reported by Moore and collaborators, who isolated the virus from three patients in Ibadan in 1975 (Moore et al., 1974). Two isolations from human were also obtained during a retrospective analysis of samples collected from 1971 to 1975 in Igbo Ora-Oyo state (Fagbami, 1977, 1979). Serological survey conducted in several Nigerian communities including urban and rural populations identified antibodies to ZIKV commonly in the sera tested (Macnamara et al., 1959; Boorman and Draper, 1968; Monath et al., 1974; Fagbami, 1979).
An early serosurvey in Sierra Leone revealed that among the antibodies against the group B detected, ZIK was the most common (Robin and Mouchet, 1975). By using IH test, prevalence recorded were 7 in samples from the western zone including Freetown; 5.3% in Walihun, Sembehun, Sahn Iionia, Pujohun; 14% in samples from Malcari and Magburalca; and 5.9% in the south of the forest (Lalehun Labour Camp, Raoma Kangama, Kayima) and North of the sananah (Bafodia, Koinadugu).
Serological surveys for ZIKV were conducted in several other countries in West Africa (Bres, 1970) and the percentage of positive sera was 44% Benin (between Djougou and Savalou in 1967), 31% in Togo (Trevis, Sokodé, Pagouda, Niamtougou, Dapango in 1964–66), and 18% in Niger (Tera in 1965).
The first huge urban outbreak of ZIKV in Africa was reported in Cabo Verde in 2015–16. In 2015, cases of skin rash and prurit, associated with fevers, were reported notably in adults and women. In May 2016, 7557 suspected cases were reported mainly in the island of Santiago, Fogo, and Maio (http://www.who.int/mediacentre/news/releases/2016/zika-cabo-verde/en/). Out of 64 first blood samples processed by Institut Pasteur de Dakar, IgM antibodies against ZIKV were detected in 15 patients including 2 positives by RT-PCR.
Central Africa
Human cases of ZIKV infection were first reported in Gabon in 2007, concomitantly to a dengue and CHIK outbreak (Grard et al., 2014). ZIKV RNA was detected in four human samples collected in Libreville (in the suburbs of Diba-Diba, Nzeng-Ayong, PK8, and PK9) and Coccobeach. Before this outbreak, evidence of human ZIKV infections was limited to serological surveys performed in Libreville in 1967 (with 7% of tested samples positive for antibodies to ZIKV), 1975 and 1979–80 (14.7% of positive samples) (Bres, 1970; Jan et al., 1978; Saluzzo et al., 1982).
All serological studies carried out in the CAR in 1961–62 (samples collected in Botambi, Obo, Bouar, Bangassou, and Kem-Gribingui), 1963–64 (Bangui, Obo, Lobaye), and 1979 (Lobaye) showed the presence and high prevalence of antibodies to ZIKV in the population (Chippaux-Hyppolite and Chippaux, 1966; Bres, 1970), (Gonzalez et al., 1979). The percentage of positive sera was 48.8% in 1961–62, 6.9% in 1963–64, and 26.3%–27.4% in 1979.
In Cameroon, the last seroprevalence studies indicated the evidence of ZIKV in human infection with 11.4% of the tested sera positive to ZIKV antibodies (Fokam et al., 2010). A previous study conducted in 1964–66 in the whole country showed a prevalence of 17% (Salaün and Brottes, 1967).
East Africa
In Uganda, a study reported the first evidence of neutralizing antibodies in human sera collected in 1952 (Smithburn, 1952) and the first isolation of ZIKV from a human in 1964 (Simpson, 1964). This isolation is the unique human case reported in Uganda where 12.8% of the 261 human sera collected in 4 districts (Bwamba, Toro, Center, and Molambi-Gambo) in 1945, 1947–48 were positive for ZIKV antibodies (Smithburn, 1952). The percentage of positive sera varied between zero (Molambi-Gambo) and 29.5% (adults from Bwamba). Antibodies to ZIKV were found in human sera collected in the Karamoja district in this country in 1967–69 and in 1984 (Henderson et al., 1968; Rodhain et al., 1989). Neutralizing antibodies to ZIKV were detected in 6 out of 12 human sera tested in the locality of Tanga, a Tanzania port in the Indian Ocean by Smithburn in 1952.
In Ethiopia, the hemagglutination tests performed on sera collected during a YF epidemic in 1960 revealed the circulation of ZIKV in the affected area. Seroprevalences ranged from 40% to 60% in the Chouchouma valley (Gora, Manera, Tchabera, Goya villages), between 3% and 40% in the Omo basin [Boreda Kocha (40%), Wallamo Kaffa (~ 3%), Chouchouma Tchabera (~ 8%), Opa (~ 13%)] and ~ 17% in Didessa valley. A large serosurvey conducted between 1961 and 1964 in Ethiopia detected IH ZIKV antibodies in 6%–12% of the sera collected (Serie et al., 1968b). Two persons developed antibodies against ZIKV during a longitudinal serosurvey in the locality of Manera (Serie et al., 1968b).
In Kenya, a low seroprevalence of ZIKV was reported in Nyanza (3.3%), Kitui (1.3%) but a high prevalence was reported in Malindi (52%). The second study in northern Kenya resulted in seroprevalence rates of 0%–13% (Geser et al., 1970).
In 1966, three human sera were found positive for ZIKV by IH test near Mogadishu in Somalia (Henderson et al., 1968).
Southern Africa
In the southern part of Africa, a recent risk assessment conducted in 2013 in Zambia found a prevalence of 6% to ZIKV IgG-IgM antibodies in this country (Babaniyi et al., 2015).
Evidence of ZIKV emergence in Angola was detected by a serosurvey conducted in 14 localities across the country during 1960 (Kokernot et al., 1965). ZIKV IH antibodies were detected in 27% (n = 492) of the sera tested, with 48% of the IH-positive samples having neutralizing antibodies. Neutralizing antibodies to ZIKV were detected in 4% (n = 249) of the human sera collected during a serological survey in Mozambique in 1957 (Kokernot et al., 1960).
North Africa: Egypt and Morocco
Neutralizing ZIKV antibodies were detected in 1 out of 180 human sera tested in the 1950s in Egypt (Smithburn et al., 1954). Antibodies to ZIKV were also found in a low percentage of sera tested in Morocco in 1968–69 (Bres, 1970).
Coinfection
Because the emergence of CHIKV and ZIKV in Africa occurred in malaria and other arbovirus endemic areas, coinfection has been frequently reported in acute febrile patients. Further, arboviruses coinfections were also reported in mosquitoes. Malaria and CHIKV coinfection was found in 13.6% of CHIKV-positive patients in Cote d’Ivoire in 1998, in four of eight CHIKV-positive samples from Equatorial Guinea in 2002–03, in five of nine CHIKV-positive samples during the 2000 outbreak in Kinshasa (DRC), 0.6% of CHIKV-positive febrile children in Tanzania in 2013, and 23% of CHIKV-positive cases during the 2012 CHIKV outbreak in Sierra Leone (Pastorino et al., 2004; Attoh-Touré et al., 2008; Collao et al., 2010; Ansumana et al., 2013; Chipwaza et al., 2014).
Dengue virus (DENV) and CHIKV coinfection have also been frequently documented during simultaneous emergence in Africa. Dengue 2 (DENV-2) and CHIKV coinfection was found in 32 febrile patients and in one Ae. albopictus specimen in Gabon during outbreaks between 2007 and 2010 and in 1.0% of febrile children in Tanzania during a serological survey in 2013 (Caron et al., 2012; Chipwaza et al., 2014). Dengue 1 (DENV-1) and CHIKV coinfection was reported in 10 of 55 patients tested during an outbreak in Madagascar in 2006 (Ratsitorahina et al., 2008). In 2014, dengue 4 (DENV-4) and CHIKV coinfection was detected by RT-PCR in a woman returning to Portugal from Luanda (Angola) (Parreira et al., 2014).
In Guinea, Jentes et al. (2010) found evidence of coinfection with CHIKV and an additional arbovirus (West Nile, Tahyna, and another untypeable Bunyavirus) in three cases. During the 2005 outbreak of CHIKV in south Kordofan, two individuals were coinfected with both YFV and CHIKV and one individual with both WNV and CHIKV (Gould et al., 2008).
For the first time, CHIKV and HIV coinfection was found in febrile patients during 2007–08 in northern Tanzania (Hertz et al., 2012). CHIKV and HIV coinfection was also reported during the 2012 CHIKV outbreak in Sierra Leone, with 9.3% of CHIKV-positive cases coinfected with HIV (Ansumana et al., 2013).
Coinfections were common during the 2012 CHIKV outbreak in Sierra Leone. From individuals coinfected with malaria and HIV, 33 (8.3%) of CHIKV cases were coinfected with hepatitis B virus, and a smaller number of individuals with hepatitis A, hepatitis C, tuberculosis, typhoid, and syphilis (Ansumana et al., 2013).
About 18.7% of CHIV and 88.9% of ZIKV infected patients were found to be coinfected with Plasmodium in Kedougou between 2009 and 2013 (Sow et al., 2016). The first human ZIKV case detected in Nigeria was also infected by malaria (Macnamara, 1954). CHIKV and ZIKV have been found coinfecting two pools of Ae. albopictus in Libreville in 2007 (Grard et al., 2014). Since the analysis was performed on pooled mosquitoes, this does not necessarily suggest coinfection of individual mosquitoes.
Mosquito and Other Vectors
Field Detection and Bioecology
Countries and localities where CHIKV has emerged in mosquitoes are presented in Fig. 7. Different mosquito species are vectors of CHIKV in Africa (Jupp and McIntosh, 1988; Diallo et al., 1999, 2012b; CRORA, 2013). The main vectors of CHIKV are the Aedes mosquitoes of the subgenera Diceromyia, Stegomyia, and Aedimorphus (Table 1).
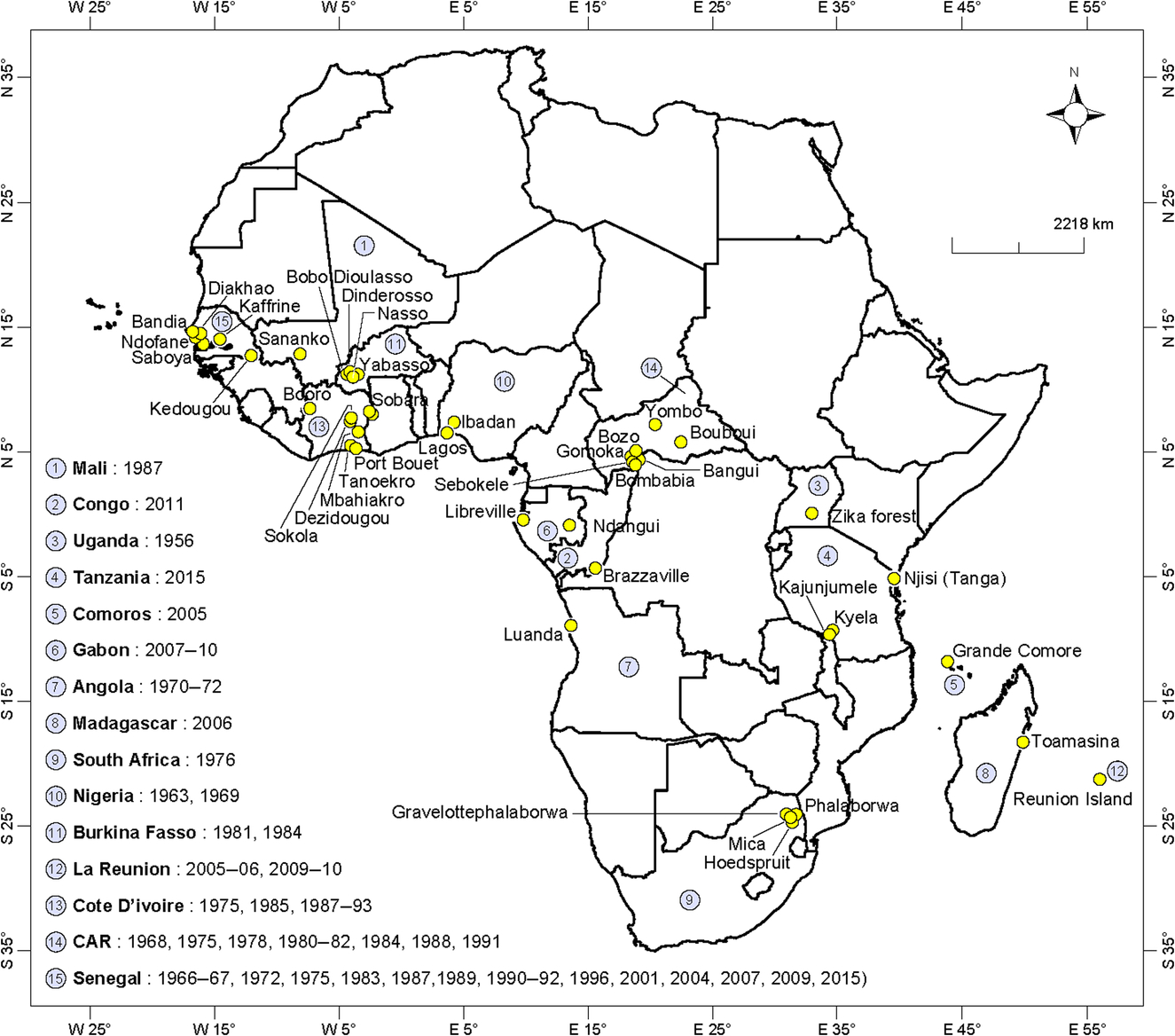
Table 1
| Country of origin | Species | References |
|---|---|---|
| Senegal | Aedes furcifer, Aedes furcifer male, Aedes aegypti, Aedes africanus, Aedes centropunctatus, Aedes dalzieli, Aedes hirsutus, Aedes luteocephalus, Aedes metallicus, Aedes neoafricanus, Aedes taylori, Aedes argentopunctatus, Aedes vittatus, Culex poicilipes, Culex ethiopicus, Mansonia uniformis, Anopheles coustani, Anopheles funestus, Anopheles domicola, Anopheles rufipes | Diallo et al. (1999, 2012b) and CRORA (2013) |
| Cote d’Ivoire | Aedes gr abnormalis, Aedes argenteopunctatus, Aedes cumminsii, Aedes gr mutilus, Aedes vittatus, Aedes cordellieri, Aedes furcifer, Aedes furcifer male, Aedes ingrami, Aedes graham, Aedes jamoti, Aedes gr palpalis, Aedes taeniorostris, Aedes aegypti, Aedes africanus, Aedes luteocephalus, Aedes opok, Aedes usambara, Eretmapodites gr inornatus, Culex gr decens, Culex guiarti, Culex quinquefasciatus, Culex weschei, Culex cinereus, Coquillettidia maculipennis, Mansonia africana, Mansonia uniformis | CRORA (2013) |
| Burkina Faso | Aedes furcifer, Aedes africanus, Aedes luteocephalus | Robert et al. (1993) and CRORA (2013) |
| Nigeria | Aedes aegypti | Moore et al. (1974) |
| Mali | Aedes furcifer | CRORA (2013) |
| Republic of Central Africa | Aedes africanus, Aedes gr africanus, Aedes opok, Aedes vittatus, Mansonia africana, Anopheles funestus | Geoffroy (1982) and CRORA (2013) |
| Gabon | Aedes aegypti, Aedes albopictus | Pages et al. (2009) and Caron et al. (2012) |
| Republic of Congo | Aedes aegypti, Aedes albopictus | Mombouli et al. (2013) |
| South Africa | Aedes furcifer | Jupp (2005) |
| Uganda | Aedes africanus, Mansonia africana, coquillettidia fuscopennata | McCrae et al. (1971) |
| Tanzania | Aedes aegypti, Aedes africanus | Lumsden (1955) and Bisimwa et al. (2016) |
| Angola | Aedes aegypti | Filipe and Pinto (1973) |
| Reunion | Aedes albopictus, Culex quinquefasciatus | Bessaud et al. (2006) |
| Mauritius | Aedes albopictus | Beesoon et al. (2008) |
| Comoros | Aedes aegypti | Sang et al. (2008) |
| Madagascar | Aedes albopictus | Ratsitorahina et al. (2008) |
Amplifications of sylvatic CHIKV have been detected in mosquito pools (mainly Ae. furcifer, Ae. luteocephalus, Ae. dalzieli, Ae. taylori) in 1975, 1979, 1983, 1992, 2009, and 2015 in the Kedougou region in southeastern Senegal (Diallo et al., 1999, 2012b; CRORA, 2013). CHIKV was previously isolated from several mosquito species (Ae. irritans, Ae. luteocephalus, Ae. aegypti, and Anopheles gambiae) in 1966–67 and Ornithodoros erraticus sonrai in 1967 in Western Senegal. Furthermore, CHIKV was isolated from Ixodidae ticks in Guinea (Konstantinov, 1990; Butenko, 1996). In Mali, the sole CHIKV isolation was from Ae. furcifer in 1987 (CRORA, 2013). In Burkina Faso, the virus was only detected in mosquito pools from Ae. africanus in 1981 and Ae. furcifer and Ae. luteocephalus at Dinderosso and Yabosso in 1984 (Robert et al., 1993; CRORA, 2013). CHIKV was isolated in more than 28 mosquito species in 1975, 1985, 1987–91, 1993, and 1997 in Cote d’Ivoire (CRORA, 2013). In Nigeria, CHIKV was isolated from different mosquito species, mostly from Mansonia africana in Lagos in 1963 and Ae. aegypti, which was collected in Ibadan during the 1969 outbreak (Boorman and Draper, 1968; Moore et al., 1974). The detection of CHIKV from a male Ae. furcifer in Senegal (Kedougou region) and Cote d’Ivoire may suggest vertical transmission of CHIKV virus (Diallo et al., 2012b). In the Republic of Congo, CHIKV was first detected in 13 pools of Ae. aegypti and Ae. albopictus collected in six locations in Brazzaville in 2011 (Mombouli et al., 2013). CHIKV was isolated from pools of six mosquito species collected throughout the Central African Republic (Gomoka, Bozo, Bouboui, Yombo, and Sebokele) in 1968, 1975, 1978, 1980–81, 1984, 1988, and 1991 (Saluzzo et al., 1980; CRORA, 2013). In South Africa, CHIKV was isolated from 16 pools of the Ae. furcifer/taylori group (mainly Ae. furcifer) collected from two farms located approximately 40 km from Phalaborwa (Eastern Transvaal) in April 1976 (McIntosh et al., 1977). In 1970–71, only one CHIKV strain was isolated in Angola from a pool of 33 Ae. aegypti specimens collected in Luanda (Filipe and Pinto, 1973). In Uganda, CHIKV was isolated from Ae. africanus, Ma. africana, and Coquillettidia fuscopennata, which was collected in the Zika forest in 1956. An entomological study conducted in the Kyala district of Tanzania in 2015 (Bisimwa et al., 2016) reported on the detection of CHIKV by RT-PCR from mosquito pools collected in the localities of Kyela (Ae. africanus and Ae. aegypti) and Kajunjumele and Njisi (Ae. aegypti). On the Reunion island, Ae. albopictus and Culex quinquefasciatus were found to be naturally infected by CHIKV (Bessaud et al., 2006). During the 2006 outbreak, CHIKV was detected in 24 pools of Ae. albopictus collected in the region of transmission. In Madagascar, only Ae. albopictus was found to be naturally infected by CHIKV (Ratsitorahina et al., 2008).
ZIKV was isolated for the first time in a pool of Ae. africanus in Uganda in 1948 (Dick et al., 1952). Later, the virus has been isolated in Uganda mainly from the same species in 1956, 1962–64, and 1969–70 (Weinbren and Williams, 1958; Haddow et al., 1964; Simpson, 1964; McCrae and Kirya, 1982). Up to now, ZIKV has been detected in Africa from several countries and localities (Fig. 8) from 26 mosquito species (Table 2) belonging mainly to the genus Aedes, subgenera Diceromyia, Stegomyia, and Aedimorphus (Diallo et al., 2014). In West Africa, ZIKV was isolated or detected by RT-PCR from several mosquito pools belonging to 25 species. In Senegal, several sylvatic amplifications of the virus have been reported in 1968–69, 1972, 1974, 77, 1980–81, 1985–89, 1991–92, 1994, 1997–99, 2001–03, 2011, and 2015. In south eastern Senegal, ZIKV is the most frequently isolated arbovirus. Indeed, it emerged 22 times in 40 years of surveillance and is the only virus that has been continuously detected in mosquitoes during 8 consecutive years. More than 400 ZIKV strains have been isolated from mosquitoes in this area. It is important to note that isolation from Ae. aegypti was very rare. An entomological study conducted in 2011 showed that ZIKV was transmitted in five land cover classes including forests, savannas, barrens, agricultures, and villages in a wide area of southeastern Senegal. The virus was detected in Ae. vittatus and Ae. furcifer within villages. During years of amplification, the virus is detected as soon as the beginning of the rainy season corresponding to the beginning of the mosquito population activity in the region. Among the 17 species found associated to ZIKV during these amplifications Ae. luteocephalus, Ae. africanus, Ae. furcifer, Ae. taylori, and Ae. dalzieli are the most common. In Cote d’ivoire, 12 species were found infected (mainly Ae. furcifer, Ae. africanus, and Ae. luteocaphalus) in 1973, 1975, 1979, 1980–82, and 1999. The infected mosquitoes were collected from Dabakala, Kong, Touba, Odiemé, and Taï. In Burkina Faso, five species were identified (mainly Ae. luteocephalus and Ae. furcifer) in 1978 and 1983. In Burkina Faso, an important amplification of the sylvatic cycle of ZIKV has been observed in forest gallery of Dinderesso near Bobo dioulasso in 1978. The virus was isolated from mosquitoes collected in Fada Ngourm (five strains) region and in the forests of Dinderosso (three strains) and Yabasso (one strain) (Robert et al., 1993). In Nigeria, ZIKV was isolated only from Ae. luteocephalus collected in the forest environment in 1969 (Lee and Moore, 1972). The virus was isolated from Ae. africanus and Ae. opok in CAR in 1968, 1976, and 1979 and from Ae. albopictus in Gabon in 2007 (Grard et al., 2014).
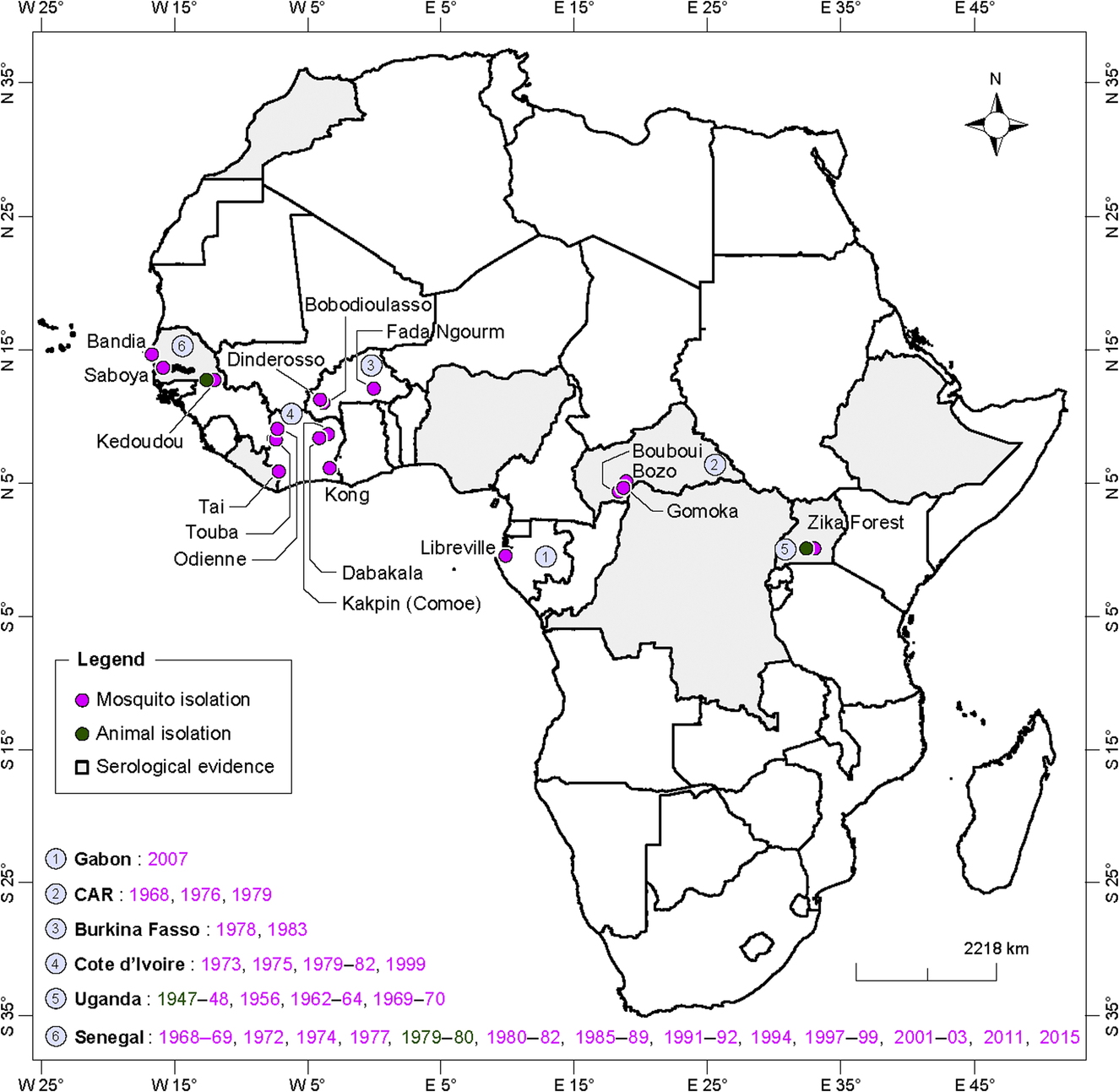
Table 2
| Country of origin | Species | References |
|---|---|---|
| Senegal | Ae. aegypti, Ae. africanus, An. coustani, An. gambiae, Cx. perfuscus, Ma. uniformis, Ae. dalzieli, Ae. fowleri, Ae. furcifer, Ae. hirsutus, Ae. luteocephalus, Ae. metallicus, Ae. neoafricanus, Ae. taylori, Ae. unilineatus, Ae. vittatus, An. coustani, An. gambiae, Cx. perfuscus, Ma. uniformis | Cornet et al. (1979) and Diallo et al. (2014)) |
| Côte d’Ivoire | Ae. aegypti, Ae. africanus, Ae. flavicollis, Ae. furcifer, Ae. grahami, Ae. luteocephalus, Ae. opok, Ae. taeniarostris, Ae. vittatus, Er. Quinquevittatus, Er. Inornatus | Akoua-Koffi et al. (2001) and CRORA (2013) |
| Burkina Faso | Ae. aegypti, Ae. furcifer, Ae. jamoti, Ae. luteocephalus, Ae. opok | Hervy and Legros (1980) and Robert et al. (1993) |
| CAR | Ae. africanus, Ae. opok | Geoffroy (1982) |
| Uganda | Ae. africanus, Ae. apicoargenteus | Dick et al. (1952), Weinbren and Williams (1958), Haddow et al. (1964), Simpson (1964), and McCrae and Kirya (1982) |
| Nigeria | Ae. luteocephalus | Lee and Moore (1972) |
| Gabon | Ae. albopictus | Grard et al. (2014) |
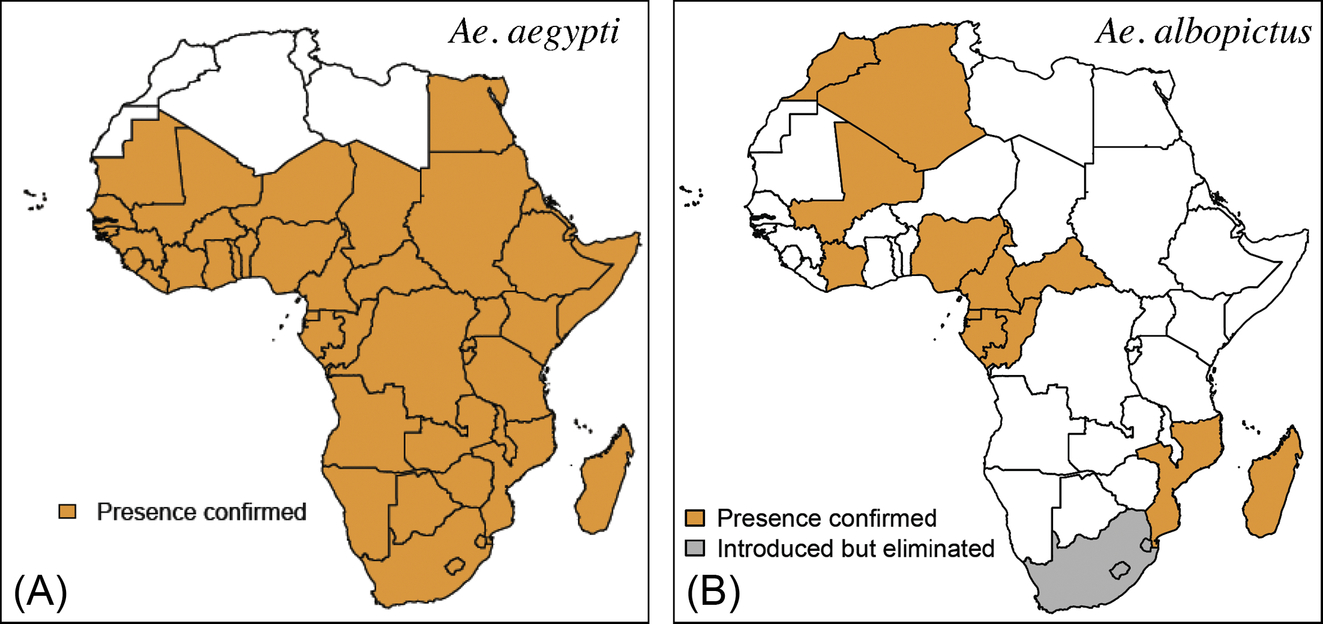
While numerous mosquito species have been observed associated with both CHIKV and ZIKV in nature, Ae. aegypti and Ae. albopictus are the two main epidemic vectors (Fig. 9). Indeed, in the urban cycle, Ae. aegypti was proven responsible for CHIK epidemics in western and central Senegal, Tanzania, Angola, Mozambique, Kenya, and Comoros while Ae. albopictus was the vector in Reunion Island, Seychelles, Mauritius, Madagascar, Gabon, and Cameroon. Concerning ZIKV, Ae. aegypti was the unique vector found in Cabo Verde while Ae. albopictus was incriminated in Gabon.
Ae. aegypti primarily breeds in artificial containers in Africa; however, some populations of the sylvatic Ae. aegypti formosus can be found breeding in tree holes and several other natural containers (Diallo et al., 2012a). Artificial breeding sites are man-made containers including water storage and discarded containers. Ae. aegypti females mainly feed on humans, indoors, and during the daytime (Lounibos, 2002).
There are two subspecies of Ae. aegypti that can be distinguished by ecological and behavioral characteristics: Ae. aegypti aegypti, the domestic and peridomestic forms in urban areas of the tropics, considered as an evolved form of Ae. aegypti formosus, the ancestral African tree hole form. Previous studies show that only Ae. aegypti aegypti exists in Asia and the New World, while both subspecies exist in Africa with limited distribution of Ae. aegypti aegypti on the east coast of Africa (Tabachnick and Powell, 1979; Powell et al., 1980). The presence of Ae. aegypti aegypti in West and Central Africa remains debatable mainly because of the lack of reliable methods to distinguish the different subspecies, while recent studies assume that both forms exist in West Africa (Sylla et al., 2009). Based on morphological aspects (white or dark color, presence of white scales on the first abdominal tergum), it would be possible to classify some domestic African populations as Ae. aegypti aegypti. However, these morphological keys are not accurate enough to distinguish Ae. aegypti aegypti from Ae. aegypti formosus or intermediary forms. Further investigations are required to better characterize these populations. The discordance between morphological identification and molecular classification sustains this statement. Even differences are often observed using morphological and bioecological parameters, with Ae. aegypti populations not displaying significant genetic differentiation (Brown et al., 2011). Whatever be the taxonomic considerations, we can assume that there are at least two different populations in Africa based on vector bionomics, which are:
- • A domestic subpopulation that is highly anthropophilic and primarily endophilic. They are indoor, daytime, and crepuscular biters, which use artificial water containers (e.g., water storage containers, old tires, and discarded containers) as breeding sites and display features similar to Ae. aegypti aegypti. Because of the artificial nature of these breeding sites, this subpopulation is present in all urbanized settings and throughout the whole year (Lounibos, 2002; Reiter, 2010).
- • A wild subpopulation exhibiting a zoophilic tendency and breeding in natural habitats (e.g., rock holes, tree holes, and fruit husks).
In recent studies performed in Mozambique, Ae. aegypti formosus (usually considered to be the wild-type population) was prevalent in the capital city, Maputo (Higa et al., 2015). This finding confirms how speculative considerations are concerning Ae. aegypti in Africa.
In Africa, Ae. aegypti has been previously reported in North Africa in Algeria, Egypt, Libya, Morocco, and Tunisia (Holstein, 1967). However, the most recent data limited its current distribution in sub-Saharan Africa (Fig. 9). The absence of Ae. aegypti in the Maghreb requires consideration in light of ecological modifications that may be induced by climate changes. Furthermore, the recent report of Ae. aegypti in Mauritania (Mint Lekweiry et al., 2015), which had been previously declared free for at least up to 30 years, and in southern Egypt (Shoukry et al., 2012) warrants further investigation.
Ae. albopictus is a mosquito originating from Asia, which expanded its distribution in several Indian Ocean and African countries several years ago (Fig. 9). It is an opportunistic daytime and outdoor feeder. However, it generally prefers humans and can be found feeding and resting indoors. Like Ae. aegypti, this species breeds in storage and discarded containers, albeit outdoors. The major role of Ae. albopictus in CHIKV transmission was highlighted during the outbreak in Reunion Island, where the species was suspected to be responsible for a single amino acid change from an alanine (A) to valine (V) at E1 envelope glycoprotein amino acid 226 of the ECSA genotype, which enhanced its vector competence (Vazeille et al., 2007; Tsetsarkin et al., 2009; Higgs and Vanlandingham, 2015).
These considerations, which are associated with recent changes in its geographic distribution, expose Africa to being at a great risk of CHIKV emergence. In continental Africa, Ae. albopictus was first reported in South Africa in 1989, but was eradicated at an early stage. In 1991, Ae. albopictus was reported in Nigeria. In the last decade, Ae. albopictus has spread mainly into Central Africa, with reports of infestation in Equatorial Guinea in 2003, Cameroon in 2005, Gabon in 2006, Central African Republic in 2008, in the Republic of Congo in 2009, Cote d’Ivoire in 2009, and Mali in 2010. The most recent countries reporting of Ae. albopictus infestation are Mozambique, Morocco, and Algeria in 2015 (Fontenille and Toto, 2001; Paupy et al., 2009; Bennouna et al., 2017; Kampango and Abilio, 2016; Muller et al., 2016).
In the sylvatic and rural environment, the main vectors of CHIKV are Ae. furcifer, Ae. luteocephalus, and Ae. taylori in West Africa, Ae. furcifer in South Africa, and Ae. africanus in Eastern Africa (Table 1). For ZIKV, the main vectors are Ae. furcifer, Ae. luteocephalus, Ae. africanus, Ae. vittatus, Ae. dalzieli, and Ae. taylori in West Africa and Ae. africanus in Central and Eastern Africa.
These main vectors in the sylvatic environment breed primarily in tree holes and fruit husks in forest galleries and savanna land covers. They are mainly primatophilic, crepuscular, and outdoor feeders, but can be found feeding on humans. CHIKV-infected Ae. furcifer specimens were collected feeding on humans within villages, even indoors, which suggests that this species is the main vector of CHIKV in humans in a sylvatic environment, especially in West and South Africa (Diallo et al., 2012a,b). Ae. africanus most probably played an identical role during rural CHIKV outbreaks in Uganda and Cameroon (McCrae et al., 1971; Demanou et al., 2010).
CHIKV and ZIKV have also been occasionally isolated in a wide range of mosquito species. These Mosquito species are members of the genera Aedes (Ae. vittatus, Ae. neoafricanus, Ae. hirsutus, Ae. fulgens, Ae. argenteopunctatus, Ae. dalzieli, Ae. vigilax, and Ae. camptorhynchites), Culex (Cx. poicilipes, Cx. ethiopicus, and Cx. quinquefasciatus), Mansonia (Ma. africana and Ma. uniformis), and Anopheles (An. coustani, An. funestus, An. rufipes, and An. domicola) for CHIKV (Jupp et al., 1981; Jupp and McIntosh, 1990; Diallo et al., 1999, 2012b; Bessaud et al., 2006). For ZIKV, the mosquitoes occasionally found associated with the virus are members of the genera Aedes (Ae. albopictus, Ae. apicoargenteus, Ae. flavicollis, Ae. fowleri, Ae. grahami, Ae. hirsutus, Ae. jamoti, Ae. metallicus, Ae. neoafricanus, Ae. opok, and Ae. taeniarostris), Anopheles (An. coustani and An. gambiae), Culex (Cx. perfuscus), Eretmapodites (Er. inornatus and Er. quinquevittatus), and Mansonia (Ma. uniformis).
Vector Competence Studies
Most laboratory studies on the vector competence of mosquitoes from Africa for CHIKV were performed with populations of Ae. aegypti from Senegal (Dakar and Kedougou), Nigeria (Lagos), Cameroon, Cabo Verde, and Mayotte, and for Ae. albopictus from Mauritius, Mayotte, Reunion Island, Madagascar, and Cameroon (Gilotra and Shah, 1967; Tesh et al., 1976; Vazeille et al., 2007; Martin et al., 2010; Paupy et al., 2010; Raharimalala et al., 2012; Diagne et al., 2014). These studies showed a high level of global vector competences of the different populations of Ae. aegypti and Ae. albopictus (Table 3). The population of Ae. aegypti from Dakar was unable to transmit CHIKV.
Table 3
| Species | Origin of the mosquito | References |
|---|---|---|
| Aedes furcifer | South Africa | Paterson and McIntosh (1964) and Jupp et al. (1981) |
| Aedes aegypti | South Africa, Cameroom, Senegal, Cabo Verde | Jupp et al. (1981), Jupp and McIntosh (1990), Paupy et al. (2010), Vazeille et al. (2013), and Diagne et al. (2014) |
| Aedes fulgens | South Africa | Jupp et al. (1981) and Jupp and McIntosh (1990) |
| Aedes vittatus | Senegal, South Africa | Jupp and McIntosh (1990) and Diagne et al. (2014) |
| Aedes albopictus | Cameroon, Madagascar | Tesh et al. (1976) and Paupy et al. (2010) |
| Mansonia africana | South Africa | Jupp et al. (1981) |
| Aedes calceatus | South Africa | McIntosh et al. (1977) |
| Culex horridus | South Africa | Jupp et al. (1981) |
| Eretmapodites chrysogaster | Liberia | Gilotra and Shah (1967) |
| Aedes metallicus | South Africa | Jupp and McIntosh (1990) |
| Ae. ledgeri | South Africa | Jupp and McIntosh (1990) |
| Ae. circumluteolus | South Africa | McIntosh and Jupp (1970) |
| Ae. simpsoni | South Africa | McIntosh and Jupp (1970) |
In addition to Ae. aegypti and Ae. albopictus, the vector competence of other species readily feeding on men and was found to be abundant in CHIKV-emerging areas in Africa, including Ae. furcifer, Ae. vittatus, Ae. fulgens, Cx. quinquefasciatus, Cx. horridus, Er. chrysogaster, and Ma. africana have been successfully assessed by few studies (McIntosh et al., 1965; Gilotra and Shah, 1967; Mangiafico, 1971; Jupp et al., 1981; Diagne et al., 2014). Cx. quinquefasciatus was infected by CHIKV after feeding on a viremic animal (Jupp et al., 1981).
Experimental studies showed that populations of Ae. aegypti (from Dakar and Kedougou), Ae. unilineatus, Ae. vittatus, and Ae. luteocephalus (from Kedougou), orally infected, were susceptible to ZIKV infection (Table 4), but only a small proportion of the population of Ae. vittatus and Ae. luteocephalus was able to transmit the virus (Diagne et al., 2015). However, a population of Ae. aegypti from Kebemer (140 km of Dakar), intrathoracically infected, was highly competent to ZIKV with a transmission rate of 88% only 7 days postinfection (Cornet et al., 1979). Boorman and Porterfield (1956)) were the first to demonstrate successfully the vector competence of Nigerian population of Ae. aegypti for ZIKV. Indeed, they were able to show the infection of mouse blood through a mouse skin membrane and a rhesus monkey by infected mosquitoes.
Table 4
| Species | Origin of the mosquito | References |
|---|---|---|
| Aedes aegypti | Senegal, Nigeria | Boorman and Porterfield (1956) and Diagne et al. (2015) |
| Aedes vittatus | Senegal | Diagne et al. (2015) |
| Aedes luteocephalus | Senegal | Diagne et al. (2015) |
| Aedes unilineatus | Senegal | Diagne et al. (2015) |
Other Vertebrate Hosts
CHIKV or anti-CHIKV antibodies were detected in animals in several countries and localities in Africa (Fig. 10). In southeastern Senegal, CHIKV was isolated from three monkey species (Cercopithecus aethiops in 1972, Papio papio in 1975, and Erythrocebus patas in 1983). The virus was isolated beforehand from several wild animal species including bats (captured at Gagnick, Gossas, and Rao in 1962–63), palm squirrels [(Xerus erythropus) captured at Bandia in 1966], a monkey [(Cercopithecus aethiops) captured at Saboya in 1966], and bushbabies [(Galago senegalensis) captured at Saboya in 1967] in western Senegal (Bres et al., 1969; Diallo et al., 1999). CHIKV has been isolated in several nonhuman primates (including bushbabies, vervet monkeys, and baboons) and the golden sparrow (Auripasser luteus) in Nigeria (Moore et al., 1974).
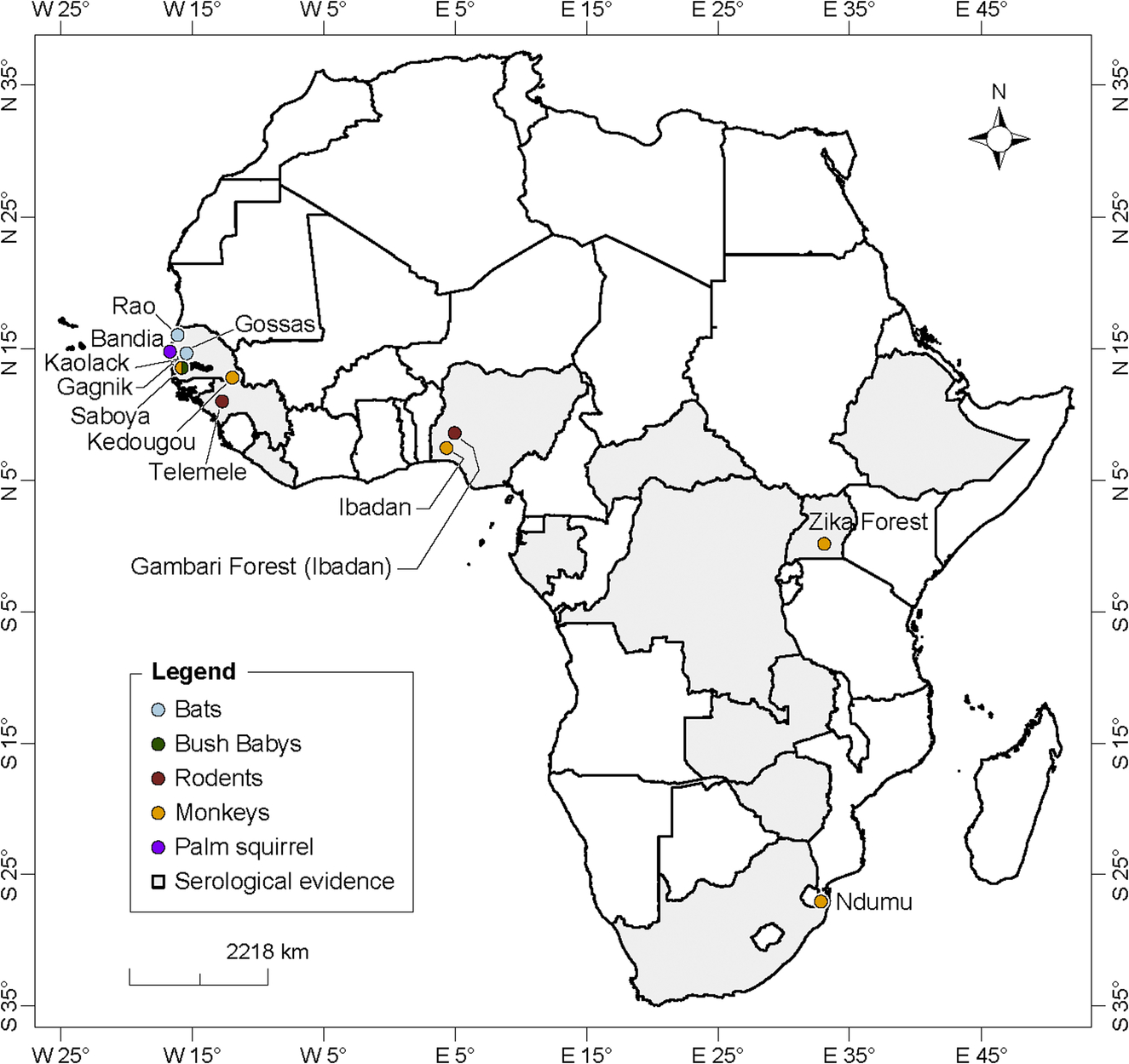
Anti-CHIKV antibodies have been detected in 11 birds and three reptiles species in 1966 in Senegal, in several others species including wild chimpanzees in DRC, 13 nonhuman primates [vervets (Ceropithecus aethiops pygerythrus) and baboons (Papio ursius)] and 17 birds species in Zimbabwe, in colobus monkeys (Colobus a. abyssinicus) and a baboon (Papio dogueri) in Ethiopia, monkeys in different habitats in Uganda, wild migratory birds in Senegal, and domestic animals including horses in Nigeria, and bovines in Guinea and South Africa (Osterrieth et al., 1961; McIntosh et al., 1964; Dickinson et al., 1965; Andral et al., 1968; Cornet et al., 1968; Henderson et al., 1969; Renaudet et al., 1978; Olaleye et al., 1989; Konstantinov, 1990; Adesina and Odelola, 1991). Specific neutralizing antibodies against CHIKV have been found in elephants from Zambia (2/3, 67%) and the DRC (2/21, 10%), in 1 of 10 forest buffalos from the Garamba National Park of DRC, and in 20% of 25 mandrills tested from the Lope National Park of Gabon (Kading et al., 2013). An epizootic of CHIKV was observed in NHPs in northern Kwazulu Natal in 1964 (Jupp, 2005). No human case was noted during this outbreak.
Mastomys, Arvicanthis, and Aethomys rodents experimentally infected with CHIKV showed low-level viremia for 2 days while high-level viremia was observed for 5 days in Mystromys rodents (McIntosh, 1961). All animals seroconverted. Three monkeys (Macaca radiata, Cercopithecus aethiops, and Papio ursinus) demonstrated viremia for 3–4 days viremia and antibodies 1 month post-CHKV inoculation (McIntosh et al., 1963a; Paul and Singh, 1968). Two African bats (Tadarida aegyptiaca and Pipistrellus nanus) were found to be susceptible to infection and showed 3 days of viremia (Jupp and McIntosh, 1988).
ZIKV isolation and detection from wild vertebrates are presented in Fig. 8. ZIKV was isolated for the first time from wild vertebrate in a rhesus monkey (Macacca mulatta) used as a sentinel in the Zika Forest in Uganda (Dick et al., 1952). The virus was also isolated in 2 NHPs (Cercopithecus aethiops, Erythrocebus patas) in Senegal. A serosurvey done in 1967 and 1968 in Senegal detected IH antibodies against ZIKV in 24% of the 41 wild mammals tested (Chunickin, in Bres, 1970). Serosurveys studies reported evidences of several ZIKV epizootics in NHPs in Uganda (in Entebbe in 1947, 1948, 1956, 1962, 1963, 1969, and 1970), southeastern Senegal (1973 and 1976) and CAR (1976) (McCrae and Kirya, 1982; Kirya and Okia, 1977; Renaudet et al., 1978; Cornet et al., 1979; Geoffroy, 1982). Anti-ZIKV antibodies were detected in NHPs in Nigeria in 1969 (83.3%; n = 24), Ethiopia (Colobus guereza, Papio cynocephalus) in 1962 (50%; n = 35) and 1964 (25%; n = 28), in CAR (C. neglectus, C. nictitans, C. aethiops, Cercocebus sp., Galago demidovi, Pan troglodytes) DRC (Pan troglodytes), Gabon in 1979–80 and Liberia (Pan troglodytes) (Andral et al., 1968; Serie et al., 1968a; Carey, 1971; Geoffroy, 1982). Antibodies to ZIKV were also identified in bats in Ethiopia and CAR (Tadaridea and Molossidae), birds in Morocco and rodents (Praomys sp, Lophuromys sikapusi, Anomalurus sp.) in CAR (Serie et al., 1968a; Geoffroy, 1982).
Phylogeny
Phylogenetic studies done in CHIKV strains isolated from Africa indicated the existence of a Western African (WA) and East-Central-South African (ECSA) lineages (Volk et al., 2010). The WA lineage was recently separated in two groups: West Africa I (WAF I) and West Africa II (WAF II). The WAF I is specific to West Africa including strains collected during 1966–2005 in Senegal and Cote d’Ivoire and WAF II is closely related to the ECSA lineage including strains collected from Senegal, Cote d’Ivoire, and the Central African Republic (Diagne et al., 2014).
CHIKV strains isolated from Gabon, Cameroon, DRC, and Equatorial Guinea belong to the ECSA phylogroup. Strains from the 2000 outbreak in DRC showed a close genetic relationship with a strain isolated in 1996 from the Central African Republic and Uganda (de Lamballerie et al., 2008; Collao et al., 2010; Caron et al., 2012; Demanou et al., 2015).
Phylogenetic studies on ZIKV strains showed that the virus has three distinct clades, the West African, East African, and Asian clade (Faye et al., 2014). Strains originated from two West African countries (Senegal and Cote d’Ivoire) were found in both African clades. The Gabonese strain of ZIKV is a member of the African lineage and the West African phylogenetic group (Grard et al., 2014).
Transmission Cycle
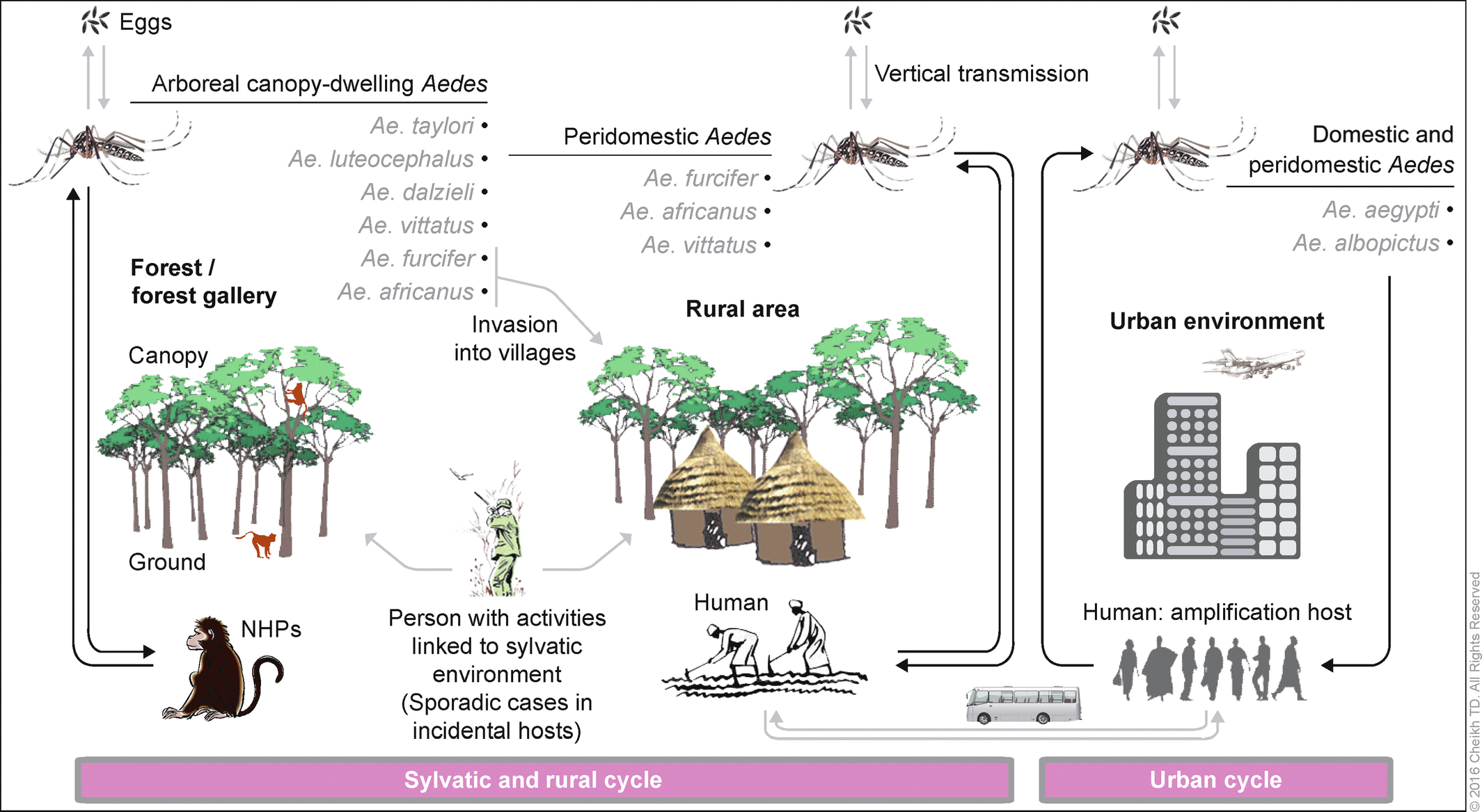
In Africa, CHIKV and ZIKV circulates in two distinct sylvatic and urban transmission cycles (Fig. 11). In the sylvatic cycle, these viruses are transmitted between arboreal forest canopy-dwelling Aedes vectors and nonhuman primates. During sylvatic amplification, humans are considered as incidental hosts and are infected when they enter the forest or by infected vectors (Ae. furcifer in West and South Africa, and Ae. africanus in East and Central Africa) invading villages from the forest (Jupp and McIntosh, 1988; Diallo et al., 1999, 2012b, 2014). Infected mosquitoes were collected as they landed on men in other land cover classes during the 2009 (CHIKV) and (ZIKV) amplifications in southeastern Senegal. Only sporadic cases and small rural outbreaks are observed in this sylvatic cycle (Powers and Logue, 2007; Diallo et al., 2014). Other vertebrates have been associated with both viruses, but their implications in the transmission cycle remain speculative. Until 2004, the urban CHIKV cycle in Africa involved only humans as a vertebrate host and Ae. aegypti as a vector (Diallo et al., 1999; Weaver and Lecuit, 2015). Between 2005 and 2010, the CHIKV E1-226V variant was mainly transmitted by Ae. albopictus in the Indian Ocean islands and Central Africa and was responsible for massive outbreaks with hundreds of thousands of cases (Pages et al., 2009; Renault et al., 2012). Ae. albopictus has also been the only vector of the urban cycle of ZIKV in Gabon in 2007 (Grard et al., 2014). No viral strain was detected during the ZIKV outbreak in Cabo Verde in 2015–16, but Ae. aegypti was the only potential vector found in the urban area where the outbreak occurred. A rural cycle, in which the virus is introduced within villages by Ae. furcifer or Ae. africanus and transmitted between humans by domestic Ae. aegypti, should not be excluded in some rural areas of Africa. The detection of CHIKV and ZIKV from a male Ae. furcifer in the Kedougou area, southeastern Senegal, and CHIKV from a male of the same species in Cote d’Ivoire may suggest vertical transmission from infected females to its progeny. Natural vertical transmission of CHIKV was also recently reported in Ae. albopictus from Reunion Island and in Madagascar during CHIKV outbreaks (Delatte et al., 2008; Ratsitorahina et al., 2008). During adverse conditions and interepidemic periods, the viruses are conserved in vertebrate reservoirs and in desiccant-resistant Aedes eggs. Moreover, venereal transmission of CHIKV by Ae. aegypti has also been demonstrated experimentally. In humans, vertical transmission of CHIKV was reported during the 2005–06 outbreak in Reunion Island (Fritel et al., 2010).
Surveillance
An epidemiological, entomological, and animal surveillance system specific to CHIKV or ZIKV was not implemented in Africa before the 2005–07 Indian Ocean outbreak. Indeed, emergences of CHIKV and ZIKV have been detected during outbreak investigations of febrile illness or from males that become ill during human-landing collection of mosquitoes (Weinbren, 1958; McCrae et al., 1971; Monlun et al., 1993; Diallo et al., 1999; Thonnon et al., 1999). During and after the Indian Ocean CHIKV outbreak, surveillance was based on a network of sentinel physicians and medical biology laboratories monitoring and reporting all fever cases that met CHIKV case definition. An active surveillance was conducted around confirmed cases to detect all potential cases during the low incidence period of the outbreaks (Renault et al., 2012). During the peak of the outbreak, only passive surveillance, which only included the identification and reporting of cases by the network of sentinel physicians, was possible. A surveillance of deaths and burial places was also implemented. In southeastern Senegal, a focus of sylvatic CHIKV and ZIKV amplification, an epidemiological surveillance system based on a network of the main health centers located in the area, was initiated in 2009. A blood sample is collected from each febrile patient frequenting these health centers and sent to a field laboratory of the Institut Pasteur de Dakar located in Kedougou. All samples are tested by ELISA and RT-PCR for CHIKV and ZIKV infection. In the event of a positive sample, an active surveillance is organized in and around the dwelling of the positive cases to detect all other potential cases. This system allowed for the detection and investigation of CHIKV emergence (in 2009 and 2015) and ZIKV (in 2011 and 2015) in the area.
Entomological surveillance of CHIKV and ZIKV in Africa has focused on both aquatic and adult stages (Diallo et al., 2012a,b, 2014). Adult surveillance comprised the collection and identification of host seeking female mosquitoes in forest galleries, villages, and other habitats where contact between vectors and vertebrate hosts may occur. This surveillance was also performed in houses and in the surroundings of confirmed positive cases during epidemics. Mosquito collection is followed by an attempt to detect the virus by isolation or RT-PCR. Human-landing catch is the only currently effective method to collect host-seeking female mosquito vectors (Achee et al., 2015; Higgs, 2015). Surveillance focusing on adult mosquitoes detected emergences of CHIKV and ZIKV in many countries, sometimes in the absence of any detectable human manifestation (Monlun et al., 1993; CRORA, 2013). It also helped to identify vectors involved in the emergence of these viruses and understand their mechanisms. Monitoring of aquatic stages was usually performed in the context of the risk assessment of emergence or during outbreak investigations to follow the vector abundance pattern and describe breeding sites (Diallo et al., 2012a). All wet containers in selected houses or forests in the area were inspected to determine the presence or absence of immature population of Aedes vectors. This surveillance allowed for the estimation of entomological risk indices and identification of potential vectors in a given area. Surveillance of bats, monkeys, and rodents not specific to CHIKV or ZIKV has resulted in the detection of these viruses in Senegal and other African countries, indicating that animal surveillance could be efficient in CHIKV and ZIKV monitoring (Geoffroy, 1982; McCrae and Kirya, 1982; Diallo et al., 1999)
In previous emergences, epidemiological and entomological surveillance has assisted in identifying affected areas or those potentially at risk. It has also helped in determining the initiation of prevention and control measures and assessment of the impact of measures taken.
Prevention and Control
Because of the lack of commercial vaccines or specific medical therapy, vector control is the only currently known effective method for the prevention and control of CHIKV and ZIKV emergences in Africa. Efforts to prevent and control the emergence of CHIKV and ZIKV in Africa involve source reduction, adulticiding, protection from mosquito bites, and social communication (Renault et al., 2012). Source reduction consisted of the mechanical destruction or removal of breeding sites in domestic environments, spraying larvicides, and/or the introduction of larvivorous fish in containers that cannot be destroyed. Adulticiding was carried out by ground and aerial spraying of insecticide in high-risk villages and where clusters of confirmed cases were observed. The population was also advised to be protecting themselves during emergence by implementing measures such as wearing long clothes, using repellents, and securing doors and windows. Social sensitization was conducted using different media such as TV, radio, and posters to educate the population on the disease and vectors, and to sensitize them on the need for participation in control measures. These different strategies have been used, but are dependent on a country’s wealth, transmission cycle, and vectors species involved. During the 2006 outbreak in Madagascar, the vector control strategies implemented were the removal of tires from rooftops, information campaigns, and community education (Ratsitorahina et al., 2008).
In Reunion Island and Mayotte, vector control was based on social awareness campaigns promoting the removal, destruction, or alteration of immature breeding sources and peridomestic spraying of chemical insecticides to kill adult mosquitoes (Sissoko et al., 2008b). During the 2005 outbreak in South Kordofan in Sudan, vector control strategies included ground and aerial space spraying of insecticides, indoor fumigation using synthetic pyrethroids, larviciding using Abate (Temephos), and educating the population on water storage management (Gould et al., 2008). During the 2015 CHIKV and ZIKV outbreaks in southeastern Senegal, vector control activities were implemented for the first time and included source reduction (removal of miscellaneous breeding containers and larviciding in other breeding sites), adulticiding within and around villages with confirmed cases by ground spraying, and social sensitization (using mass media and local leaders) to educate the local population in miscellaneous breeding site removal and personal protection measures. The same measures were used during the ZIKV outbreak in Cabo Verde in 2015–16. During the 2007 CHIKV outbreak in Gabon, the destruction or elimination of unwanted natural and artificial water containers was an effective Aedes vector control strategy in the French military camp of Libreville (Pages et al., 2009).
Perspectives
CHIKV and ZIKV will probably continue to emerge in Africa and outbreaks will be exacerbated by virus evolution, changing pattern in vector and host ecology, and urbanization. For an effective and timely response to future CHIKV and ZIKV emergences, health systems will require improved surveillance networks supported by specialized and well-equipped laboratories.
The data analysis of virus-vector associations and the vector competence for ZIKV and CHIKV has shown a great diversity of species that may be involved in the transmission. However, the role of most of them remain virtual and our current appreciation may be the unique reflect of the tools used, the level of expertise or the quality and type of studies conducted in the different countries. One of the major gaps in CHIKV and ZIKV vectors study and surveillance is the lack of efficient sampling tools for both epidemic and sylvatic vectors. Human-landing collections (HLCs) are performed to collect host-seeking mosquitoes and estimate the EIR. This is the only effective method for estimating host-vector interactions and for collecting both domestic and sylvatic host-seeking vectors. So far, HLCs are not recommended usually, especially during epidemics. Due to ethical considerations, its relevance is still debated and questioned (Achee et al., 2015; Higgs, 2015). Alternative methods have been used to collect CHIKV vectors. The BG Sentinel trap (BGS), which is baited with lures (e.g., CO2, octenol, and BG-Lure), is effective in capturing all population types (unfed, engorged, and gravid) of Ae. aegypti adult females and Ae. albopictus. BGS uses a combination of attractive visual cues and convection currents that mimic those created by the human body, thus closely resembling HLCs. It also has the advantage of being collapsible, light, and operable all day long. However, data are lacking about the effectiveness of BGS traps in collecting sylvatic vector species. Therefore, alternative methods need to be further explored since arbovirus studies or surveillance depends on high quality of entomological sampling.
Vector competence studies should be performed on all species found infected with CHIKV or ZIKV in natural habitats or found abundant in CHIKV and ZIKV endemic areas. Appropriate field and experimental studies are required to determine the relative role of this highly diverse mosquito species present in Africa in CHIKV transmission. Generally, surveillance programs for CHIKV routinely collect and analyze Aedes spp., but systematically exclude other genera from testing such as Anopheles, which can in turn lead to bias in the observed results. For example, An. gambiae remains the main vector of malaria in Africa, but it is also a vector of the O’nyong-nyong virus, which is closely related to CHIKV. Furthermore, malaria often coexists with arboviruses such as CHIKV in several regions of Africa; therefore, it is probable that An. gambiae can ingest blood from a person infected with this arbovirus.
Hence, considering variations related to, at least, interactions with the high complexity of natural mosquito populations in Africa, can we really restrict vector competence for CHIKV to the Aedes species? To date, no study has clearly demonstrated that An. gambiae does not have the capacity to also transmit CHIKV. The low transmission rates of ZIKV by several Aedes species observed by Diagne et al. (2015) may be due to several factors including virus titers and strains. Only the African lineage was tested. Further studies using Asian and American strains (responsible for all urban epidemics and complications observed) are required. Furthermore, specific attention should be paid to Cx. quinquefasciatuis regarding the conflicting data that showed its highest vector competence compared to Ae. aegypti by two studies (Guedes et al., 2017; Guo et al., 2016) but a total refractoriness to ZIKV by others (Fernandes et al., 2016; Amraoui et al., 2016; Huang et al., 2016; Boccolini et al., 2016; Lourenço-de-Oliveira and Failloux, 2017). Cx. quinquefasciatus is ubiquitous in Africa and present in domestic environments all year round because of its association to artificial breeding sites. It is abundant and anthropophagic, and is thus, a good candidate for CHIKV and ZIKV transmission in urban settings.
Further studies are required on potential vertebrate reservoirs and insecticide susceptibility, and resistance of vectors. Animal and human serological studies in all sub-Saharan countries are also required for a better understanding of the ecology of CHIKV and ZIKV and to define areas of risk in Africa. CHIKV and ZIKV control strategies should be prepared and regularly updated and adapted to each epidemiological context.
Most of the studies reported in this review date back to the 1960s and 1980s. Many of the associated research programs ended thereafter because of scientific policy changes within the institutions that supported such activities. We can assume that the efforts to study, prevent, and control arbovirus diseases were widely better 30 years ago than now. Currently, the main actions are responses to epidemics. Moreover, most of the knowledge on CHIKV and ZIKV was obtained as part of yellow fever studies that focused on enzootic cycles based on early epidemiological concepts. Therefore, if significant progress has been observed concerning arbovirus vectors in the forest ecotype, the urban environment was not seriously investigated for entomological risk, although within the two biotopes, the mechanisms of transmission are quite different.
In most of the countries analyzed, few research activities focused on arbovirus vectors while the vector biology and ecology are key elements for understanding the epidemiology of arboviral diseases as well as the development of effective and economical methods for their prevention and control. For instance, Ae. aegypti is certainly one of the most studied mosquitoes in the world but mainly from the Asian and American continent and as part of dengue project. The biology and ecology of this species is still very imperfectly known in Africa where malaria, the main public health threat, mobilized the energies for a long time. Parameters like the habitats for development, the resting sites, the life expectancy, the adult dispersal capabilities, and the interactions with human and vertebrate reservoirs are essential elements for the development of control strategies and require an accurate estimation