Chikungunya and Zika Virus in Asia
Jamal I-Ching Sam University of Malaya, Kuala Lumpur, Malaysia
Abstract
Chikungunya virus (CHIKV) and Zika virus (ZIKV) have been present in Southeast Asia and South Asia since the 1950s. Before 2005, CHIKV in Asia was characterized by sporadic outbreaks followed by years of apparent inactivity. Since 2005, Asia has experienced widespread outbreaks, which expanded the known distribution of CHIKV to new countries and new regions, such as the Middle East and the Pacific. The epidemic East/Central/South African genotype replaced the endemic Asian genotype in many countries; however, it was the Asian strains that spread to the Americas via the Pacific, a route that ZIKV would also later take. ZIKV remained neglected until the first recorded outbreak in the Federated States of Micronesia in 2007. Clusters and sporadic cases of ZIKV are now reported throughout Southeast Asia. New and existing arboviruses continue to have major public health impact on Asia, where ideal conditions exist for transmission by the main vectors Aedes aegypti and Ae. albopictus. The history and epidemiology of these arboviruses in Asia will be reviewed in this chapter.
Keywords
Chikungunya virus; Zika virus; Aedes aegypti; Aedes albopictus; Asia; Epidemiology; Outbreaks
Acknowledgments
The author acknowledges past and present funding for chikungunya work from the University of Malaya (University Malaya Research Grant RG526-13HTM and High Impact Research grant E000013-20001), Fundamental Research Grant Scheme FP035-2015A of the Ministry of Higher Education, Malaysia, and the European Union Seventh Framework Program (Integrated Chikungunya Research, grant agreement no. 261202). The author also thanks Dr. Chong Long Chua for translating the Chinese papers.
Chikungunya Virus in Asia
Introduction
For the purposes of this chapter, the term “Asia” refers to regions commonly recognized as such (countries within East Asia, South Asia, and Southeast Asia), as well as Oceania (including the Pacific islands and Australasia) and Western Asia (or the Middle East). This would include all the countries within the WHO South-East Asia and Western Pacific Regions, and some from the Eastern Mediterranean Region. This chapter will summarize available historical and up-to-date reports of chikungunya virus (CHIKV) in various Asian countries, according to region.
The history of CHIKV in Asia can be divided into three periods:
- 1. Pre-1958: the previrology era, with historical descriptions of outbreaks of CHIKV-like illness, which were difficult to distinguish from dengue fever in the absence of modern laboratory diagnostics.
- 2. 1958–2005: after the Asian genotype of the virus was first isolated in Asia and confirmed to cause sporadic outbreaks, particularly in India and Southeast Asia, before apparently disappearing for long periods. Aedes aegypti was the main vector.
- 3. Post-2005: when epidemic Indian Ocean lineage (IOL) strains of the East/Central/South African (ECSA) genotype spread from East Africa throughout the world, leading to a massive expansion in the number of countries reporting CHIKV for the first time. Both Ae. aegypti and Ae. albopictus were implicated; in particular, the latter was involved in transmission of outbreak strains with the adaptive E1-A226V mutation.
Prior to 2005, nearly all reported CHIKV sequences in Asia were of the Asian genotype, which is considered endemic to the region (Table 1, Fig. 1). After 2005, a major epidemiological shift occurred, in that both ECSA and Asian genotypes are now present in Asia, often in the same country. After the IOL lineage arose in coastal East Africa and gave rise to two sublineages (the Indian Ocean islands sublineage and the Indian sublineage), the latter spread to India in 2005 to affect millions, and from there to other countries in South Asia, and Southeast Asia by 2008 (Volk et al., 2010). In the Southeast Asian region, it first became established in countries, for example Malaysia and Thailand, before spreading further within the region and to East Asia. In many Asian countries, for example India and Malaysia, where the Asian genotype had been historically reported and considered to be endemic, the ECSA genotype seems to have displaced the Asian genotype. There have been notable exceptions, such as the Philippines and Indonesia, where large outbreaks of Asian genotype have continued to occur despite circulation of the epidemic IOL strains. More significantly, and perhaps unexpectedly, it was Asian CHIKV that spread to the Pacific islands in 2011–13, and then seeded the subsequent massive epidemics in the Caribbean and Americas that began in late 2013 (Chen et al., 2016).
Table 1
| Country | Year | Location | Outbreak/sporadic/isolate | Culture- confirmationa | Mosquito vectorb | Reference |
|---|---|---|---|---|---|---|
| Thailand | 1958 | Bangkok | Outbreak | C | Hammon et al. (1960) | |
| 1960 | Bangkok | Outbreak | C | Hammon et al. (1960) | ||
| 1962–64 | Bangkok | Outbreak | C | Ae. aegypti | Nimmannitya et al. (1969) | |
| 1962–65 | Nakhon Ratchasima province | Outbreak | Halstead et al. (1969b) | |||
| 1975 | Surin province | Isolate | C | Volk et al. (2010) | ||
| 1978 | Bangkok | Isolate | C | Volk et al. (2010) | ||
| 1988 | Northeast Thailand | Isolate | C | Chen et al. (2016) | ||
| 1991 | Khon Kaen province | Outbreak | Thaikruea et al. (1997) | |||
| 1995 | Nakorn Si Thammarat and Nong Khai provinces | Outbreak | C | Thaikruea et al. (1997) | ||
| Bangkok | Isolates | C | Volk et al. (2010) | |||
| 1996 | Payoa province | Isolate | C | Powers et al. (2000) | ||
| Cambodia | 1961–62 | Phnom Penh | Outbreak | C | Chastel (1966) | |
| India | 1963 | Calcutta, West Bengal state | Outbreak | C | Ae. aegypti | Sarkar et al. (1964a) |
| 1964 | Madras and Vellore, Tamil Nadu state; Pondicherry (Puducherry) | Outbreak | C | Ae. aegypti | Myers et al. (1965) | |
| 1965 | Nagpur, Maharashtra state | Outbreak | C | Ae. aegypti | Rodrigues et al. (1972) | |
| 1965 | Vishakapatnam, Andhra Pradesh state | Isolate | C | Yergolkar et al. (2006) | ||
| 1971 | Chennai, Tamil Nadu state | Isolate | C | Yergolkar et al. (2006) | ||
| 1973 | Barsi, Maharashtra state | Outbreak | C | Padbidri and Gnaneswar (1979) | ||
| 2000 | Yawat, Maharashtra state | Sporadic | Cc | Ae. aegypti | Mourya et al. (2001) | |
| Ceylon (Sri Lanka) | 1965 | Colombo | Outbreak | Hermon (1967) | ||
| Vietnam | 1964–65 | Saigon, Binh Long | Outbreak | Vu Qui and Nguyen-Thi (1967) | ||
| Not stated | Sporadic | C | Deller and Russell (1967) | |||
| Philippines | 1965 | Manila | Outbreak | C | Campos et al. (1969) | |
| 1968–69 | Negros island | Outbreak | Macasaet et al. (1969) | |||
| 1985 | Bocolod, Negros | Isolate | C | Volk et al. (2010) | ||
| 1985–86 | Mindanao, Cebu and Masbate islands | Sporadic | C | Centers for Disease Control (1986) | ||
| 1996 | Barangay Pulo, Cavite province | Outbreak | Retuya et al. (1998) | |||
| 1999 | Manila | Sporadic | Inoue et al. (2000) | |||
| 2001 | Not stated | Sporadic | Ngwe Tun et al. (2016) | |||
| Myanmar (Burma) | 1970 | Rangoon (Yangon) | Outbreak | C | Khai Ming et al. (1974) and Mathew and Thiruvengadam (1973) | |
| 1984 | Yangon | Outbreak | Thein et al. (1992) | |||
| 2004–06 | Not stated | Sporadic | Ngwe Tun et al. (2016) | |||
| Indonesia | 1973 | Kalimantan | Outbreak | Kanamitsu et al. (1979) | ||
| 1982–86 | Sumatra, Java, Kalimantan, Sulawesi, East Nusa Tenggara, Maluku, Irian Jaya | Outbreak | C | Wuryadi (1986) | ||
| 1983 | Not stated | Isolate | C | Volk et al. (2010) | ||
| 1985 | Ambon, Maluku | Isolate | C | Volk et al. (2010) | ||
| 1989 | Kupang, East Nusa Tenggara | Isolate | C | Harnett and Bucens (1990) | ||
| 1998–99 | Yogyakarta, Java | Outbreak | C | Porter et al. (2004) | ||
| 2001–03 | Java, Aceh, Sulawesi, West Nusa Tenggara | Outbreak | C | Laras et al. (2005) | ||
| 2004–05 | Not stated | Sporadic | Ngwe Tun et al. (2016) | |||
| Timor-Leste | 2004 | Dili | Sporadic | Whelan et al. (2004) | ||
| China | 1986–88 | Yunnan province | Sporadic (bats and a human) | C | Zhang et al. (1992) | |
| Malaysia | 1998–99 | Port Klang | Outbreak | C | Lam et al. (2001) |
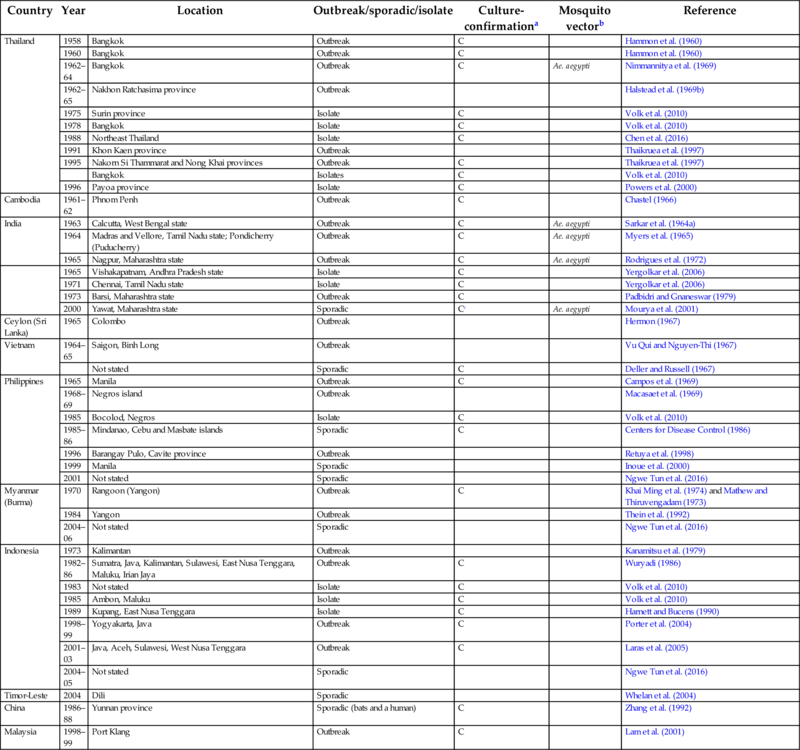
a Outbreaks listed here were at a minimum diagnosed serologically; outbreaks additionally confirmed by virus culture or PCR are indicated by C.
b The vector involved is listed here only if confirmed by culture or PCR.
c Laboratory confirmation of CHIKV was reported in Ae. aegypti, but not in humans.

The nature of endemicity of CHIKV in Asia is not yet fully understood. Several studies have detected anti-CHIKV in animals, raising the possibility of one or more sylvatic reservoirs. Neutralizing antibodies were detected in cattle, water buffalo, horses, pigs, dogs, monkeys, rabbits, and bats in Thailand (Halstead and Udomsakdi, 1966); horses, mules, and cattle in India (Bedekar and Pavri, 1969); and a chicken and a pig in Malaysia (Marchette et al., 1978). No antibodies were detected in 33 wild and domestic animals tested in Lombok in 1978 (Olson et al., 1983). Monkeys are considered the most likely animal reservoir, as reported in Africa (Chapter 4), and because they are known reservoirs for other arboviruses such as dengue and Zika viruses. However, the first reported isolation of CHIKV in animals outside Africa was from a bat (Rousettus leschenaulti) in Yunnan province, China, in 1986 (Chen and Tao, 1996). Low levels of neutralizing antibodies have been reported in two Malaysian studies of long-tailed macaques (Macaca fascicularis) at similar rates of 1.5% (6/393) (Marchette et al., 1978) and 0.7% (1/147) (Sam et al., 2015), and in northern pig-tailed macaques (M. leonina) from northern Thailand (4/38, or 11%) (Nakgoi et al., 2014). However, no seropositivity was found in other species examined. These included: 71 orangutans (Pongo pygmaeus) in East Malaysia (Wolfe et al., 2001), 115 toque macaques (M. sinica) in Sri Lanka (Peiris et al., 1993), or 12 bonnet macaques (M. radiata), 24 hanuman langurs (Presbytis entellus), and 9 rhesus monkeys (M. mulatta) in India (Bedekar and Pavri, 1969). CHIKV was only isolated for the first time in nonhuman primates in Asia from four M. fascicularis caught in Malaysia in 2007 (Apandi et al., 2009). The role of vertebrates in transmitting or maintaining CHIKV in Asia thus remains unclear. Recent CHIKV outbreaks appear to be principally caused by introduction of virus by people from affected areas.
Historical Accounts of Chikungunya-Like Epidemics, Pre-1958
After the first isolation of CHIKV during an outbreak in southern Tanganyika (now Tanzania) in 1952–53 (Ross, 1956), CHIKV disease was virologically confirmed in Asia for the first time only in Bangkok in 1958. Donald Carey, who had been working on dengue in Vellore, India, in the early 1960s, studied historical documents and made the compelling case that CHIKV disease had been present in Asia for at least 200 years (Carey, 1971). As described more fully in Chapter 3, the absence of diagnostics in the previrology era made it difficult to clinically distinguish the numerous tropical infectious diseases that had similar presentations. Physicians in many countries had been using several different terms to describe what were likely to be different diseases within a “dengue” syndrome, which included fever, headache, retro-orbital pain, arthralgia, myalgia, and rash.
Carey was influenced by the work of James Christie, a physician in Zanzibar, Tanzania, in 1870, who noted during a “dengue” outbreak of a febrile exanthema that the disease was a distinct clinical entity characterized by severe and persistent joint pain (which he himself experienced for 2 months), which was not prominent in descriptions of other “dengue” outbreaks (Christie, 1872). This disease was called “kidinga pepo” in local Swahili, meaning “cramp-like pains, produced through the agency of an evil spirit.” Christie found close clinical similarities in descriptions of certain earlier epidemics, notably an account by Bylon of an outbreak of febrile arthritic disease in Batavia (present-day Jakarta, Indonesia) in 1779, which was termed “knokkel-koorts” or “knuckle fever,” and an earlier 1823 epidemic in Zanzibar preceding epidemics in Calcutta, India (1824), and Rangoon, Burma (now Myanmar, 1824), as cited by Kuno (2015). As outbreaks of a similar disease were also reported in Calcutta and Madras, India, in 1871–72 following the Zanzibar outbreak in 1870, Carey believed that the frequent passage of ships between the East African coast and India provided a route for spread. This provides a historic parallel for the recent CHIKV epidemic caused by the East Central South African genotype from 2005 onwards, in which the two main clades (Indian subcontinent and Indian Ocean) appear to have independently emerged from coastal East Africa in 2004 or earlier (Volk et al., 2010).
Carey also suggested that “dengue” outbreaks described in Hong Kong, Burma, and Madras in 1901–02, and Calcutta in 1923 more closely resembled CHIKV disease. For example, in a description of the outbreak in Rangoon which subsequently spread northwards along a major trade route, Pridmore describes:
The sudden onset, the pain and stiffness in the fingers and other joints, with frontal headache and suffused eyes, are so characteristic that there has been little difficulty in quickly arriving at a correct diagnosis. The appearance of the rash of course drives away all doubt.
With regards to sequelae:
Troublesome joint pains, with swelling and tenderness, have persisted in a small percentage of cases… In this epidemic I have neither seen or heard of any deaths. The after-effects, so troublesome in adults, in this disease are rarely seen in children.
This description does strongly suggest CHIKV. Pridmore reports that the locals called the disease “tok-kive-ana,” or “the tying or drawing together (of hands and feet) disease” (Pridmore, 1902). Like “kindinga pepo,” these historical indigenous names that eloquently describe the key disease characteristic are reminiscent of “chikungunya,” which in the Makonde language in Tanzania means “disease that bends up the joints” (Ross, 1956). A further interesting observation was that the Burmese believed that the disease visits the country every 30 years, a pattern of long interepidemic periods that were later noted for CHIKV disease in other Asian countries. This pattern is unlike the endemicity of what is currently known as dengue.
With regards to the outbreak in Hong Kong, Stedman described the sequelae thus:
…complete return to health is delayed by a recrudescence or a continuance of the pains in the limbs and joints; the pains which are not accompanied by fever occur as before … These pains are often so severe as to prevent the patient using the limbs in which they are situated … the duration of these after-pains is very variable; in some cases they disappear after a few days, in others they may persist for many weeks (Stedman, 1902)…
The isolations of dengue virus (DENV) and CHIKV in the 1940s and 1950s would finally provide the means to virologically distinguish between the two main agents causing dengue-like syndrome, and the terms “dengue” and “chikungunya” were thus assigned to the diseases as we now recognize them (Halstead, 2015). However, the etymology of “dengue” is muddied by the previous application of the term to different diseases with common symptoms and signs. Kuno cautioned that the reclassification of past “dengue” as CHIKV outbreaks mainly based on prominence of arthralgia is hampered by various factors. These include insufficient clinical and epidemiological data provided by historical medical records, the fact that joint symptoms are reported by some studies to also be prominent in dengue, and the knowledge now of many other viral causes of epidemic febrile arthritis other than CHIKV (Kuno, 2015, 2009). For example, the epidemics in Zanzibar in 1823 and 1870 could also have been due to O’nyong-nyong virus, another alphavirus that is endemic in East Africa, and cannot definitively be attributed to CHIKV without laboratory evidence.
In summary, despite their limitations, historical medical accounts strongly suggest that certain Asian epidemics (in Hong Kong, India, Indonesia, and Myanmar) in the last two centuries may have been due to CHIKV, predating the earliest viral isolation in the Asian region in 1958. This is based on clinical and epidemiological descriptions that resonate with the extensive contemporary experience with CHIKV.
Chikungunya in Southeast Asia
Thailand
Dengue hemorrhagic fever emerged as a new clinical entity in the 1950s, with severe bleeding, shock, and high fatality rates distinguishing it from classical dengue fever, as reviewed by Halstead and Cohen (2015). The first outbreak was described in Manila in 1954, and soon after, DENV was identified as the cause of “Philippine hemorrhagic fever.” In 1958 and 1960, similar outbreaks occurred in Bangkok, but both DENV and CHIKV were now isolated from cases of “Thai hemorrhagic fever” (Hammon et al., 1960; Hammon, 1961). An extensive longitudinal study of this disease was initiated in Bangkok between 1962–64, during which time 10,194 children were admitted. This revealed that while > 80% of cases of children admitted with hemorrhagic fever could be attributed to DENV, about 8% were caused by CHIKV, diagnosed serologically by a four-fold rise in HI titres in paired serum, and by culture (Nimmannitya et al., 1969). A similar proportion of hemorrhagic fever cases seen in clinics was caused by CHIKV, and an estimated 44,000–70,000 outpatient CHIKV cases were seen in 1962 alone (Halstead et al., 1969a,b). Ae. aegypti was the vector for CHIKV (Halstead et al., 1969b). Large outbreaks of hemorrhagic fever were also reported outside of Bangkok. Although CHIKV was diagnosed at a far lower rate than dengue; seroprevalence of CHIKV was found to be very high, at 19%–84% in different provinces, suggesting widespread endemicity throughout the country (Halstead et al., 1969c). It was subsequently concluded that hemorrhagic fever had a mixed etiology. While severe shock and hemorrhage were associated exclusively with DENV, both DENV and CHIKV (as a minor contributor) could cause mild hemorrhagic manifestations, sometimes limited to just a positive tourniquet test. Both nonsevere dengue and CHIKV could be very difficult to tell apart clinically, but CHIKV was far more likely to cause a maculopapular rash, conjunctival injection, and arthralgia (Nimmannitya et al., 1969).
While DENV remained the major agent of hemorrhagic fever, CHIKV continued to be identified as a minor cause in most of Thailand during surveillance between 1974 and 1976 (Gunakasem et al., 1981). Consistent with this, a retrospective study of CHIKV seroprevalence in pregnant women sampled between 1998 and 1999, mostly from Bangkok, found an overall 33.6% seroprevalence. The gradual age-associated increase suggested ongoing transmission during the 1970s (Watanaveeradej et al., 2006). By 1979–80 in Bangkok, CHIKV was no longer detected in surveillance of 1086 cases of hemorrhagic fever (including 959 sets of paired serum), or paired serum from 457 cases of undifferentiated febrile illnesses (Burke et al., 1985). This unexplained disappearance of CHIKV was also observed in India. CHIKV re-emerged in the 1990s, causing outbreaks in Khon Kaen (1991), Nakorn Si Thammarat (1995), and Nong Khai provinces (1995) in rural villages, with almost 1800 reported cases in the latter (Thaikruea et al., 1997). No hemorrhage or deaths were reported. All Thai isolates that had been sequenced up to that point, including isolates from 1958, 1962, 1975, 1985, and 1988, were from the Asian genotype (Volk et al., 2010).
Thailand experienced a nationwide outbreak starting in August 2008 in Narathiwat, the southernmost province bordering the Malaysian states of Perak and Kelantan, which were experiencing outbreaks at the time. The disease soon spread to three other southern provinces along the Thailand-Malaysia border (Yala, Pattani, and Songkhla), and then northwards to 58 other provinces throughout Thailand by December 2009 at a median rate of 7.5 km per week (Ditsuwan et al., 2011). Over 49,000 people, or 77 per 100,000, were affected (Thaikruea et al., 2011). A seroprevalence study of pregnant women in Songkhla province in November 2009, after the peak outbreak period, found CHIKV antibodies in 227/319 (71.2%), indicating high attack rates (Laoprasopwattana et al., 2016). The virus involved in this outbreak was from the ECSA genotype (E1-A226V) and closely related to those identified earlier that year in Malaysia and Singapore (Rianthavorn et al., 2010; Pongsiri et al., 2010). The southern provinces of Thailand have many plantation areas, and in Narathiwat and Songkhla, 62.9% of 421 patients surveyed worked in agriculture, almost all in rubber plantations (Laoprasopwattana et al., 2016). Overall, 86.5% of the first 23,847 cases lived in rural areas (Ditsuwan et al., 2011). CHIKV was detected in both Ae. aegypti and Ae. albopictus in Songkhla, although the infection rate was much higher in captured Ae. albopictus (53%) than in Ae. aegypti (10%) (Thavara et al., 2009), implying that Ae. albopictus may have played the main role, as expected from the implicated virus mutant A226V ECSA strain, which has higher capacity to infect Ae. albopictus than Ae. aegypti (Tsetsarkin et al., 2007) (Chapters 2 and 8). Similar viruses continued to be detected in Ratchaburi Province in central Thailand in August 2010 (Sasayama et al., 2014) and Bueng Kan Province in northern Thailand in September 2013 (Wanlapakorn et al., 2014).
A study of risk factors for CHIKV infection (as measured by seropositivity 2 years after the epidemic) was carried out in three villages in the Thung Nari subdistrict in Phatthalung Province, one of the southern provinces near the Malaysian border (Nakkhara et al., 2013). Of 507 individuals tested, 314 (61.9%) were seropositive. Independent risk factors for infection were having a garbage pile near the residence, and spending at least 8 h a day outdoors, with almost two-thirds of seropositive cases working as rubber tappers. Both would increase potential exposure to Aedes mosquito vectors.
In summary, these findings suggest that in 2008, the imported ECSA strains appear to have displaced the previously endemic Asian genotype, causing an extensive outbreak throughout the country, particularly in rural areas.
Cambodia
CHIKV and DENV were isolated in Phnom Penh, Cambodia, in 1961–62, where CHIKV activity was limited to the rainy season (Chastel, 1966). Population seroprevalence in Phnom Penh in 1962 increased with age, and as 44% of the inhabitants were < 14 years, children were believed to be the main source of spread (Chastel, 1966).
In 2011, CHIKV was identified in samples collected from a national hospital-based surveillance study during two dengue-like illness outbreaks reported in Preah Vihear province (Duong et al., 2012). At least three patients presenting with encephalitis were also diagnosed, using IgM ELISA. Phylogenetic analysis and the spatiotemporal sequence of sporadic cases and outbreaks suggested origin from Thailand into neighboring Battambang province in May 2011, and then southeastwards through a major trade route across the country to Siem Reap (June-July), Preah Viear (August and December outbreaks), Kampong Thom (July), Kampong Cham (October), and Kandal (December) provinces. The later December outbreak in Preah Viear may have been a separate reintroduction from across the Thai border.
In February 2012, a further rural outbreak was reported in Trapeang Roka village (population: 695) in Kampong Speu Province, just 30 km from Phnom Penh, with an attack rate of 44.7% over 7 weeks mainly affecting those < 46 years (Centers for Disease Control and Prevention, 2012). The virus contained the E1-A226V mutation, but Ae. aegypti and not Ae. albopictus were collected in the area. The lower incidence in older people may have been due to preexisting immunity from the last recorded circulation of CHIKV in Cambodia during the 1960s (Galatas et al., 2016). A stochastic model was developed to study the temporal dynamics of the outbreak, which estimated the reproduction number (R0) to be 6.46 (Robinson et al., 2014). This was higher than reported R0s in other CHIKV outbreaks, probably because the village is relatively isolated and has large household sizes in close proximity. R0 was likely underestimated if calculations excluded the asymptomatic cases; 5.3% in this outbreak. These subclinical infections are normally not considered in public health responses; however, this study indicates their often unrecognized potential importance in transmission during outbreaks.
Vietnam
During an outbreak of hemorrhagic fever in Saigon and Binh Long in 1964–65, a serologic study of 1196 cases revealed that most of the cases were due to DENV; however, a small percentage could be attributed to CHIKV (or a closely related group A arbovirus), especially toward the end of the outbreak (Vu Qui and Nguyen-Thi, 1967). A subsequent seroprevalence survey in Saigon revealed 148/472 (31.3%) of healthy children had anti-CHIKV antibodies at titers ≥ 1:40 (Vu Qui et al., 1967). Seropositivity by HI, complement fixation, and neutralization had been reported in Saigon and the Mekong river delta in south Vietnam (Halstead and Udomsakdi, 1966; Halstead et al., 1965). In a 1966 study of 110 American soldiers admitted with febrile illness to a military hospital in Long Binh, 10 had culture-confirmed CHIKV (Deller and Russell, 1967).
During the spread of CHIKV in Southeast Asia from 2008, no confirmed outbreaks were reported in the English literature. A retrospective study of 44 patients suspected of dengue in 2006 found that none had detectable CHIKV IgM, although 25% had neutralizing antibodies (Ngwe Tun et al., 2016). However, during a prospective study of acute febrile illness in children in My Tho city, Tien Giang Province in southern Vietnam in 2010–11, CHIKV IgM was detected in 59.4% of 150 patients, consistent with local news reports of increasing CHIKV cases (Capeding et al., 2013). Surveillance of hospitalized patients between January 2012 to February 2013 in Dong Thap, at the border with Cambodia, where CHIKV had been detected in late 2011, failed to detect CHIKV (Kim Lien et al., 2016). A larger study of 8105 participants in Southern Vietnam between 2010 and 2014 detected four CHIKV cases between August and November 2012, two of whom resided within 20 km of the Cambodian border (Quyen et al., 2017). The four CHIKV sequences were closely related to the Southeast Asian ECSA strains with the E1-A226V mutation circulating in Cambodia. These reports indicate that CHIKV was active in some parts of Vietnam during this time.
Philippines
CHIKV was first isolated in the Philippines in Manila in 1965 (Campos et al., 1969; Retuya et al., 1998). Serum collected from several sites in 1968–74 showed seropositivity rates ranging from 14% to 30%, indicating past circulation (Tesh et al., 1975).
Since the widespread lack of diagnostic capability made national surveillance difficult, Salje et al. (2016) used age-stratified serum samples, collected from Cebu City in 1973 and 2012, and tested these samples for CHIKV-neutralizing antibodies, in order to model the history of CHIKV circulation. The best-fit models to explain the temporal changes in age-stratified seroprevalence predicted outbreaks in 1968, 1986, and 1993, with probability of infection in susceptible individuals ranging between 14% and 37% in each outbreak, and with long absences of disease activity in between (Salje et al., 2016). These predictions were consistent with past reports of a serologically confirmed outbreak in Negros, a neighboring island, in 1968–69 (Macasaet et al., 1969), as cited by Salje et al. (2016), and sporadic reported cases of CHIKV in US Peace Corps volunteers stationed in Mindanao, Cebu, and Masbate islands in 1985–86 (Centers for Disease Control, 1986). Sequences of two isolates from Bacolod and Negros in 1985 were reported as being from the Asian genotype (Volk et al., 2010). There was also a serologically confirmed outbreak in 1996 in a small rural village 50 km south of Manila, on Luzon island (Retuya et al., 1998), showing the CHIKV was circulating around this time. Sporadic cases of CHIKV continued to be detected in 1999 and 2001 (Ngwe Tun et al., 2016; Inoue et al., 2000).
A nationwide outbreak of CHIKV occurred in 2011–13, starting first in Mindanao in 2011, before spreading northwards throughout the country over the next 2 years. Surprisingly, given that strains of the ECSA Indian lineage had been spreading throughout Southeast Asian since 2008, the outbreak viruses in the Philippines were of the Asian genotype, closely related to strains from Indonesia (Sy et al., 2016). Sporadic cases of ECSA CHIKV also occurred in Mindanao in 2012 and 2013, again most closely related to Indonesian strains, suggesting that the proximity of this southern island to Indonesia provides an important route of transmission. Entomological investigation of an outbreak in Laguna in June 2012 found that 79% of collected larvae were Ae. aegypti, and 21% were Ae. albopictus (Ballera et al., 2015).
A prospective community-based cohort study carried out in Cebu from 2012 to 2014 to actively evaluate circulation of CHIKV revealed valuable findings (Yoon et al., 2015; Srikiatkhachorn et al., 2016). The baseline prevalence of neutralizing antibodies was 28% and occurred almost exclusively in those > 15 years old, suggesting active transmission some time ago. Overall CHIKV infection in the cohort during the first year was 12.32/100 person years, declining to 2.84 cases/100 person years in the second year. Nobody with antibodies at the baseline was infected throughout the surveillance period, indicating the long-term protective effect of neutralizing antibodies. Most interestingly, 82% of proven infections (PCR-positive or eight-fold rise in neutralizing titres) were asymptomatic, which is much higher than other reports that suggest only 17%–28% are asymptomatic (Ayu et al., 2010). As the previous studies depended on retrospective reports of symptoms, this high rate of subclinical disease obtained by prospective and active surveillance may be more accurate. If confirmed, this would have major implications for true estimates of disease burden and transmission; for example, it would affect the assessment of blood donors, which is based on self-reported symptoms, during outbreaks.
Myanmar (Previously Burma)
A serological survey of 2060 children with hemorrhagic fever admitted in Rangoon between 1970 and 1972 showed multiple etiologies, with different major causes in each year: chikungunya (51%) in 1970, influenza (39%) in 1971, and dengue (29%) in 1972 (Khai Ming et al., 1974). CHIKV was isolated from serum of a patient repatriated to India in October 1970 (Mathew and Thiruvengadam, 1973). A subsequent seroprevalence study found widely distributed anti-CHIKV HI antibodies in Ae. aegypti-endemic areas throughout the country, with the highest rates in Rangoon (Thaung et al., 1975). CHIKV was also the predominant cause of a further outbreak of hemorrhagic fever in Yangon (formerly Rangoon) in 1984 (Thein et al., 1992). Retrospective CHIKV IgM testing performed on samples collected in 2004-6 from patients clinically suspected of dengue in Myanmar found 4/225 (1.8%) positive (Ngwe Tun et al., 2016). Increases in CHIKV cases were reported in 2010 from Ayeyarwady Division, Yangon Division, Rakhine State and Shan State, representing large areas in the south and east regions of the country (ProMED-mail, 2010). During an outbreak of dengue in July-October 2010 in Mandalay, IgM against CHIKV only was seen in 5% of patients (Tun et al., 2014). The four CHIKV isolates obtained were most closely related to ECSA genotype strains from neighboring Thailand, which had experienced extensive outbreaks in 2008–09.
Indonesia
Serum samples collected from lifelong residents of 10 sites in Indonesia revealed widespread CHIKV seropositivity (Kanamitsu et al., 1979). Most of these showed increasing CHIKV seropositivity rates only after 30 years of age, suggesting that CHIKV had been inactive during the intervening period. The exceptions were Balikpapan and Samarinda (in East Kalimantan, Borneo), which had high seropositivity rates of 36%–42% across all age groups, following a recent outbreak there of serologically confirmed CHIKV.
An extensive series of CHIKV epidemics (confirmed by culture) occurred between 1982 and 1986, starting in South Sumatra before spreading to many other regions, including Java, Kalimantan, Sulawesi, East Nusa Tenggara, the Mollucas, and Irian Jaya (Wuryadi, 1986). The outbreaks had high attack rates of 40%–85%, affected all ages, and lasted 3 months at each site, suggesting extremely intense transmission. No severe hemorrhage, shock, or deaths were reported. A hiatus of 12 years was followed by a virologically confirmed outbreak in Yojyakarta, Java, in December 1998 to February 1999 (Porter et al., 2004). Another large, multi-island epidemic then occurred between 2001 and 2003, with 24 reported outbreaks and over 5000 suspected cases mainly throughout Java, and in Aceh, Sulawesi, and West Nusa Tenggara (Laras et al., 2005). Bleeding was reported in 5%–8% of cases in the Java outbreaks.
Since 2003, the Indonesian Ministry of Health has reported up to several thousand cases a year, with a notable surge to 83,756 (11 provinces) and 52,703 (20 provinces), in 2009 and 2010 respectively (Ministry of Health, Indonesia, 2011, 2016). This included Bali, a popular tourist destination that also attracts large numbers of local migrant workers from other Indonesian islands as well as over two million international visitors a year (Yoshikawa and Kusriastuti, 2013). Sequencing of 28 PCR-confirmed cases from five districts in East and West Java, Bali, West Nusa Tenggara, and West Kalimantan showed that all were of the Asian genotype, except the two sequences from West Kalimantan, which were of the ECSA genotype and clustered with isolates from neighboring Malaysia, which had a large outbreak between 2008 and 2010 (Maha et al., 2015). CHIKV sequences from Surabaya, East Java, collected in 2010 and 2011 were also shown to be from the Asian genotype, with the responsible vector being identified as Ae. aegypti (Mulyatno et al., 2012).
A prospective cohort study was carried out among 4380 subjects working in three factories in Bandung, West Java, between 2000 and 2004 and between 2006 and 2008 (Kosasih et al., 2013). The data showed that CHIKV cases had occurred every month during the study periods, accounting for 7.1% of acute febrile episodes and an incidence rate of 10.1/1000 person years. Only the Asian genotype was identified, and this important study is one of the first to show endemic background infection at a given site. These recent studies suggest that the Asian genotype of CHIKV continues to cause endemic disease and outbreaks in much of Indonesia. The occasional reports of the Southeast Asian CHIKV (ECSA genotype), such as the two sequences from West Kalimantan and six cases in Taiwan in 2009–10 with recent travel to Indonesia (the precise locations are not stated) (Yang et al., 2016), show that the ECSA genotype is circulating, but has not become as well established as it has in other nations in the region. The reasons for this are unknown.
Timor-Leste
Timor-Leste shares a border with East Nusa Tenggara, Indonesia, and appears to be often involved when CHIKV spreads through the Indonesian islands. It is also an important source of imported cases into Australia, with a serologically diagnosed case of CHIKV imported from Dili in Australia in 2004 (Whelan et al., 2004), and another case in 2010, which was of the Asian genotype (ProMED-mail, 2013).
Malaysia
A seroprevalence study showed that 64/184 (35%) of adults from rural and urban areas in northern Malaysia in 1973 were seropositive for CHIKV, with minimal reactivity against other alphaviruses including Ross River, Getah, Bebaru, and Sindbis viruses (Tesh et al., 1975). Low levels of CHIKV antibody (10.5%) were reported in healthy people throughout Peninsular (West) Malaysia in the 1960s, more commonly in the northern states bordering Thailand, and more frequently in rural and forest-dwelling than urban residents (Marchette et al., 1980). In a rural village in Sarawak (East Malaysia), the overall seroprevalence of CHIKV antibodies was similarly low at 27/449 (6.1%), but most of those were individuals aged > 45 years (prevalence of 37.7%) (Bowen et al., 1975). As urban centers of west-central states had little population immunity and abundant Ae. aegypti, Marchette et al. (1980) predicted they would be highly vulnerable to future outbreaks (Marchette et al., 1980).
This prediction was fulfilled when the first laboratory-confirmed outbreak of CHIKV due to the Asian genotype occurred in Port Klang, affecting 51 people between December 1998 and February 1999 (Lam et al., 2001). The second outbreak occurred in Bagan Panchor, a small fishing village in Perak state, in March 2006. Interestingly, despite the ongoing epidemics of CHIKV of the ECSA genotype in India and the Indian Ocean, this outbreak was caused by an Asian strain similar to that involved in the Klang outbreak (AbuBakar et al., 2007). Many economic migrants from the region live in these outbreak sites, raising the possibility of introduction from other countries. A limited seroprevalence study in Bagan Panchor indicated an attack rate of 55.6%, and an asymptomatic rate of 17.5% (Ayu et al., 2010).
In late 2007/early 2008, CHIKV was isolated from long-tailed macaques (M. fascicularis) in Kuala Lipis, Pahang (Apandi et al., 2009), over 300 km away by road from Bagan Panchor. This raised the possibility of endemic sylvatic circulation of Asian CHIKV (believed to occur in Africa) rather than introduction of the virus from migrants. In a later study of 147 long-tailed macaques captured in 20 human-populated sites in 5 states in late 2009 and 2010, just after a nationwide outbreak of CHIKV in 2008–09, only one was found to be seropositive for CHIKV, and none were PCR-positive (Sam et al., 2015). A similarly low seroprevalence of 1.5% in 393 long-tailed macaques was reported in the past (Marchette et al., 1978). Although nonhuman primates may play a minor role in maintaining a sylvatic reservoir of CHIKV, the involvement of other domestic and wild vertebrates, which have previously shown sporadic seropositivity, has not been conclusively excluded.
In August 2006, an ECSA genotype was isolated in Malaysia for the first time, in a traveler who had returned to Batu Gajah, Perak, from Chennai, in Tamil Nadu state, India, where there was an ongoing outbreak (Soon et al., 2007). No associated cases arose from this imported case. The viral sequences from this outbreak are not available, but contemporaneous sequences from India possess the E1-226A residue. In December 2006, an outbreak affected over 50 people in Ipoh, also in Perak state; again, the index case had recently returned from Tamil Nadu (Noridah et al., 2007). The outbreak was centered around a group of scrap metal yards that employed mainly Indian migrants, and where the metal scraps were found to contain stagnant water. CHIKV was isolated from Ae. albopictus.
Starting in April 2008, CHIKV outbreaks in the southernmost state of Johor spread northwards and eastwards (including across the South China Sea to East Malaysia) to affect all 15 states and federal territories by 2010, making this the largest outbreak ever to affect Malaysia (Sam et al., 2009; Chua, 2010). Over 13,000 cases were reported by the end of 2009, with at least two associated deaths (Sam et al., 2010; Chua et al., 2010). The causative strains were ECSA genotype with E1-226V, were closely related to Indian strains from Kerala, India, from 2007, and formed a cluster with later strains from neighboring Singapore, Thailand, and China, suggesting Malaysia as the source (Sam et al., 2009, 2012; Apandi et al., 2010).
Epidemiological studies in five states indicated that rural residence, particularly in rubber or palm oil plantations, was associated with a higher risk of CHIKV infection (Azami et al., 2013; Yusoff et al., 2013). In laboratory mosquito studies, the ECSA strain was found to infect midguts and disseminate to salivary glands at higher rates in Malaysian Ae. albopictus compared to Ae. aegypti, while the Asian strain infected Ae. aegypti at higher rates (Sam et al., 2012). Ae. albopictus was strongly suspected to be the responsible vector for this outbreak; for example, mosquito collection in four affected districts in Kelantan state in 2009 showed that Ae. albopictus was the most common species, while Ae. aegypti was not found (Rozilawati et al., 2011). However, as PCR was negative for CHIKV, the role of Ae. albopictus in this major outbreak has not been confirmed definitively. Ae. albopictus is generally the predominant mosquito species present in plantations (Chang et al., 1997; Afizah et al., 2015). Based on the observations, it is therefore speculated that in Malaysia, the imported outbreak strain, with the E1-A226V mutation known to increase adaptation to Ae. albopictus (Tsetsarkin et al., 2007), was able to spread widely across the country, particularly in rural areas where this vector predominates over Ae. aegypti.
Singapore
In November 2006, Taiwan reported an imported case of CHIKV in a student from Singapore (Shu et al., 2008). Singapore reported a few imported CHIKV cases in late 2006–07, mainly from India and Sri Lanka (Ng et al., 2009). January 2008 saw the first confirmed local outbreak with 13 cases, caused by ECSA viruses with parental wild-type E1-226A, occurring in the urban Little India district, so-named because the population is predominantly Indian (Leo et al., 2009). Ae. aegypti was strongly implicated as it was the only vector found in the affected, highly urbanized area, and the epidemiological link to India was supported by phylogenetic analysis (Ng et al., 2009).
A second outbreak occurred later that year from July 2008, which affected 1072 people, and with 83% of the clusters in rural or suburban areas (Ho et al., 2011). This was due to the introduction of a different ECSA virus carrying the mutant E1-A226V, most likely from neighboring Malaysia where phylogenetically similar viruses were causing an ongoing nationwide outbreak that started in Johor state in April (Sam et al., 2009). The daily traffic across the causeway linking Johor and Singapore is estimated to be up to 100,000 vehicles (National Library Board, Singapore, 2016) and of the first 231 cases, 108 (47%) were imported, and 92% of these had traveled to Malaysia (Ng et al., 2009). CHIKV was subsequently isolated from Ae. albopictus, the predominant species caught in the field (no Ae. aegypti was found). The early identification of a different vector to the January outbreak led to modification of the vector control strategy, targeting the outdoor Ae. albopictus with more outdoor fogging, residual spraying of external walls, and removal of overgrown vegetation (Tan et al., 2011).
Re-emergence of CHIKV was reported in rural areas in early 2013, with over 1000 cases; again, the causative ECSA virus carried E1-A226V, and Ae. albopictus was confirmed as the main vector (Oon and Ng, 2014).
In summary, Singapore, as an important regional travel hub and place of employment for regional workers, has had multiple introductions of CHIKV. This led to an interesting real-life manifestation in 2008 of the laboratory findings that the E1-A226V mutation increases transmission in Ae. albopictus. The early January outbreak involved the parental E1-226A viruses transmitted by Ae. aegypti in urban areas, while the later outbreaks were caused by the mutated E1-A226V viruses efficiently transmitted by Ae. albopictus where it dominates in rural areas. A highly integrated approach between laboratory and field to identify this switch enabled early changes to vector control, and subsequently outbreak control.
An important and valuable finding was that commercial CHIKV IgM assays performed with considerably different sensitivities when used in the two outbreaks in 2008, probably because of differences in antibody binding to the envelope proteins used as antigens (Yap et al., 2010). This emphasizes the critical value of field evaluation of new assays, rather than depending on reported performances based on carefully selected panels of serum.
Laos
Seropositivity rates of up to 30% was reported in Vientiane, Laos, in the 1960s and 1970s (Halstead and Udomsakdi, 1966; Kanamitsu et al., 1979). Between July and September 2012, CHIKV was detected for the first time in outbreaks affecting almost 200 people in 10 villages in Champassak Province in southern Laos (Soulaphy et al., 2013). Champassak shares a border with Preah Viear province in Cambodia, which had reported an outbreak in December 2011. In 2013, a dengue outbreak affecting 50,000 people occurred in Laos. A study in Champasak Hospital during this dengue outbreak showed that CHIKV was still circulating, and strains had genetic similarity to ECSA (E1-A226V) strains from Cambodia (Phommanivong et al., 2016).
Brunei
Despite being surrounded by Sabah and Sarawak states of Malaysia, which experienced extensive outbreaks in 2009–10, Brunei only reported its first case of imported CHIKV from Java, Indonesia in 2011, followed by a few cases of apparently locally acquired CHIKV (Liew and Yung, 2012). Diagnosis was by IgM detection, and no sequences are available.
Chikungunya in South Asia
India
Between July and December 1963, a city-wide outbreak of hemorrhagic fever occurred in Calcutta, affecting an estimated 25,000 people with 200 deaths (Sarkar et al., 1964a). Epidemiological and virological analysis revealed two overlapping outbreaks, with the earlier curve peaking in September, and characterized by circulatory failure with or without bleeding and high mortality in children; a single DENV was isolated out of 90 sera, while seroconversion to a group B (flavivirus) arbovirus (likely DENV) was shown in several patients (Sarkar et al., 1964b). A second type of dengue-like illness with few hemorrhagic manifestations and low mortality had its peak in November; CHIKV (35/90 samples) was isolated from this group of patients. This was an early example of the proven concurrent circulation of both CHIKV and DENV, although the overlap in prominent hemorrhagic disease is unusual for CHIKV. A retrospective serological analysis of samples collected in Calcutta in 1955 and 1960 showed that 14/313 (4.5%) had neutralizing antibodies to CHIKV, none of whom were aged < 26 years (Pavri, 1964).
During the second half of 1964, outbreaks of a febrile illness with severe joint pains and rash and “practically nil” mortality were reported in Vellore, Madras, and Pondicherry (Rao, 1966). Extensive laboratory investigation isolated 272 CHIKV isolates from 509 human serum samples (and only 2 DENV isolates), and 110 CHIKV isolates from 141 Ae. aegypti pools (Myers et al., 1965). A small number of cases were shown to be due to DENV and Chandipura viruses. A similar CHIKV-predominant outbreak occurred in Nagpur, Maharashtra state, in April-June 1965, with an estimated > 50% case incidence in highly populated areas; in virus studies, 23/60 human sera and 5/34 pools of Ae. aegypti yielded CHIKV (Rodrigues et al., 1972). In Madras, a random survey of 2769 (1.9% of total) houses and 38,861 (2.1%) inhabitants found that 21% had been affected by the febrile illness in 99/100 divisions, giving a total projected 378,871 cases in the city, with the highest attack rates in those aged from 10 to 29 years (Sharma et al., 1965). In support of this high attack rate, a retrospective survey of serum collected 8 years earlier in Madras state (but not the city) found 30/277 (10.8%) positive for CHIKV antibodies, with higher seropositivity of 27.1% in those aged > 40 years (Banerjee, 1965). A survey of afebrile hospital patients in Madras city at the end of the outbreak found 319/831 (38.4%) were seropositive (Dandawate et al., 1965). In Vellore, in a small set of 67 preoutbreak serum samples, 15/24 (62.5%) of individuals aged > 30 years were seropositive for CHIKV, but none younger than 30 years; postoutbreak, 28% of 90 samples in people aged < 30 years were seropositive (Carey et al., 1969). Together with the seroprevalence findings from Calcutta (Pavri, 1964), these data indicate that CHIKV had previously circulated in these regions (perhaps 30–40 years ago, leaving some immunity in older adults), and that a high level of population susceptibility preceded the explosive outbreaks in 1963–64. An illuminating comment from a health officer in Madras was that “the epidemic appeared to be very explosive like a common vehicle epidemic instead of an arbodisease” (Rao, 1965). Similarly, high attack rates would be reported in the CHIKV epidemics after 2005.
A localized outbreak occurred in Barsi, Maharashtra state, in 1973, which affected 37.5% of the town (Padbidri and Gnaneswar, 1979). In 2000, an unspecified number of febrile cases (some of whom had hemorrhagic manifestations) occurred in Yawat, a small rural town in Maharashtra state, but DENV serological tests were negative (Mourya et al., 2001). Both CHIKV and DENV were isolated from Ae. aegypti collected from homes in the town, although the authors reported that the CHIKV strain was noticeably less virulent in mice and replicated at lower rates than their control strain from the Calcutta outbreak in 1963. Later, the Yawat isolate IND-00-MH4 was sequenced and initially reported to be of Asian genotype (Yadav et al., 2003), but was subsequently resequenced and reported to be within the Central African lineage of the ECSA genotype (Yergolkar et al., 2006; Arankalle et al., 2007). However, subsequent comprehensive phylogenetic analyses of full genomes of CHIKV considered the IND-00-MH4 sequence to be a likely result of contamination, as the sequence was almost identical (99.4%) to the UgAg4155 strain isolated in 1982; this was not compatible with the estimated substitution rate over the 18 years between isolation dates (Volk et al., 2010; Chen et al., 2016). Of interest, the strain 655873, isolated in Vishakapatnam, Andhra Pradesh, in 1965, was reported in the same paper by Yadav et al. (2003) to be from the Central African lineage (Yadav et al., 2003), but this has not been reconfirmed in subsequent studies. Therefore, apart from strains 655873 and IND-00-MH4, all other pre-2005 CHIKV sequences reported to date from Asia have been from the Asian genotype (Chen et al., 2016; Yergolkar et al., 2006). The reports of these two strains, however, provide the tantalizing possibility for even earlier importation of the ECSA genotype, which did not appear to result in establishment or significant outbreaks. This possibility warrants further study.
After the large outbreaks from 1963 to 1965, and the limited outbreaks in Barsi (1973) and Yawat (2000), CHIKV would not be reported in significant numbers in India until 2005. Carey et al. (1969) had found it surprising that “CHIKV appeared so suddenly in the area and then vanished” in the 4 years after the 1964 outbreak (Carey et al., 1969), considering the continued presence of DENV and Ae. aegypti. Similarly, no CHIKV was reported in Calcutta in the 20 years following the 1963 outbreak, and seropositivity fell to 4.4% (17/389 samples) and was absent in children and young adults (Neogi et al., 1995). Pavri (1986) also subsequently noted that the unexplained disappearance of CHIKV “for 7–8 years or even 2–3 decades” had been seen in India, Burma, and Sri Lanka (Pavri, 1986).
The next outbreak in India would be of unprecedented magnitude. It started in October 2005 when cases of suspected fever were reported in Andhra Pradesh and Karnataka, and it was the first time that the ECSA genotype was confirmed to have caused an outbreak in Asia (Yergolkar et al., 2006). High densities of Ae. aegypti (with negligible or absent Ae. albopictus) were found in most affected areas, which were mainly rural. In certain areas, for example, the Lakshadweep islands, Ae. albopictus was predominant and was suspected to be the main vector instead (Samuel et al., 2009). CHIKV from five different states were very closely related, and all carried E1-226A, despite the predominant presence of the E1-226V strain in the Indian Ocean (Arankalle et al., 2007). However, a later study retrospectively reported that CHIKV strains isolated in West Bengal as early as October 2006 carried the E1A-226V mutation, which may be the first description of this adaptive mutation in India (Taraphdar and Chatterjee, 2015).
Up to December 2006, an estimated 1.38 million people in 15 states had been affected. Most cases occurred in the southern, southwest and southeastern states (Tamil Nadu, Kerala, Karnataka, Andhra Pradesh), but the disease also spread northwards to central states (Orissa, Madhya Pradesh, Gujarat, Jharkhand, West Bengal) and northern central states (Uttar Pradesh, Delhi, Rajasthan), but spared the extreme northern and northeastern states. From a high of 1582 per million, the incidence dropped to 67, 105, 80, 28, and 15 from 2007 to 2011 (Muniaraj, 2014); however, annual cases numbering in the tens of thousands continued to be reported up to 2016 (National Vector Borne Disease Control Programme-India, 2016).
Several studies have looked at the socioeconomic impact of the outbreaks in India. An overall burden of 45 disability adjusted life years per million was estimated during 2006, mainly due to chronic arthralgia; furthermore, an estimated 7.4 million working days was lost due to acute CHIKV alone (Krishnamoorthy et al., 2009). Based on interviews with 242 individuals in an affected village with an average daily wage of USD 1.05, the total of all direct and indirect costs was extrapolated for Andhra Pradesh state and estimated at USD 12 million in 2006 alone, of which out-of-pocket direct medical costs made up 68% (Seyler et al., 2010). A similar study of 857 households in Kerala found that catastrophic health expenditure (as defined as out-of-pocket health expenditure exceeding monthly per-capita income or the international poverty line of USD 32.40 per month) was incurred by about a third (Vijayakumar et al., 2013). In a study carried out in Tamil Nadu to score health-related quality of life with the SF-36 questionnaire, 403 CHIKV patients were followed up at 5 months. The proportion who had not recovered and still suffered joint pains was 24%, and had significantly lower scores in all physical and mental domains, especially physical functioning, physical role functioning, body pain, social functioning (Ramachandran et al., 2012). These studies all show the profound impact of the disease particularly in rural areas.
Such was the extent and impact of the epidemic in 2006 that Kalantri wrote:
The socio-economic impact was tremendous: school attendance dropped, productivity at work declined sharply and farmers could not tend to their crops. Festivals passed by almost unnoticed and marriages were postponed. Sufferers lost their wages, sold household items and were forced to borrow money at high interest rates.
The quoted case figures are nevertheless underestimates as CHIKV was not universally surveyed, diagnosed, or notified, especially by private doctors or alternative medicine practitioners, to whom patients often turned.
In Ahmedabad city (population 3.8 million), Gujarat state, the epidemic peak in August-November 2008 was associated with 2944 excess all-cause deaths compared to the preceding 4 years (Mavalankar et al., 2008). A similar finding was made in Port Blair (population: 136,000), the capital of Andaman and Nicobar Islands, where there were 78 excess deaths in 2006, and most occurred during the peak of the CHIKV outbreak in August-October 2008 (Manimunda et al., 2011). A series of 65 severe laboratory-confirmed CHIKV cases from the same city showed that most of the 18 deaths were in males aged > 60 years, and had atypical manifestations including renal and hepatic dysfunction, and encephalitis (Tandale et al., 2009). This supported previous reports from Reunion Island and Mauritius that the CHIKV epidemics were associated with increased deaths (Renault et al., 2008).
While the 2005–06 epidemic appeared to have been caused by CHIKV (E1-226A) related to East African strains, there was an important shift from 2007 onwards in that two sublineages of ECSA virus (both E1-226A and E1-A226V) began to cocirculate in India. This situation appears to be unique in Asia, and it is suggested that the delineation of circulating CHIKV is dictated by the relative proportions of Ae. aegypti and Ae. albopictus across the vast spectrum of urban to rural settings found in India (Sumathy and Ella, 2012). Entomological studies in outbreak areas have been patchy, with many reporting Aedes densities only (some without speciation), and very few attempting detection of CHIKV from mosquitoes or larvae collected in the field, which would more definitively implicate a species as the vector.
Strains with E1-226A (related to the 2006 Indian viruses, and probably mainly transmitted by Ae. aegypti) continued to cause outbreaks in Tamil Nadu (2009–10) (Sumathy and Ella, 2012), Jharkhand (July 2011) (Gurav et al., 2012), Delhi (September 2011) (Afreen et al., 2014), and Andhra Pradesh (2007–09, September 2013) (Naresh Kumar et al., 2016; Parashar et al., 2015).
In some regions, notably Kerala and Karnataka states, strains with the E1-A226V mutation emerged and became dominant. Kerala was the hardest-hit state in 2007 by a resurgence of disease, reporting 56% of the country's cases. This time, sequenced viruses were shown to carry the E1-A226V mutation (Kumar et al., 2008). Since they clustered with the 2006 Indian (E1-226A) strains rather than the Indian Ocean (E1-226V) strains, it is most likely that the E1-A226V mutation arose independently in India and was not a further introduction from the Indian Ocean epidemics (Santhosh et al., 2008). Together with other instances of independent acquisition of this Ae. albopictus-adaptive mutation in Reunion and Cameroon/Gabon, this was an example of “evolutionary convergence” (de Lamballerie et al., 2008).
Regarding mosquitoes, Ae. albopictus was found in high densities in outbreak areas in Kerala and constituted from 85% to 92% of total larvae and eggs collected (compared to zero to 4% of Ae. aegypti), particularly in the worst-affected Kottayam and Pathanamthitta districts, which have the largest area of rubber plantations in the state (Kumar et al., 2008). Kerala produces 85% of the national rubber output, and when filled with rainwater, the cups which are left attached to trees to collect latex are highly likely to become mosquito-breeding sites, especially for Ae. albopictus (Sumodan, 2012). Ae. albopictus larvae were also extensively found in the leaf axils (angle between a leaf stalk and the stem) of banana and pineapple plants, which are also mass cultivated in Kerala (Eapen et al., 2010). The confirmed detection of CHIKV in Ae. albopictus in affected areas was reported in 2008–10 (Niyas et al., 2010; Kumar et al., 2012). Two novel mutations described in Kerala strains, E2-K252Q and E2-L210Q, were later shown to act as second-step mutations that further increased adaptation to Ae. albopictus (Tsetsarkin et al., 2014). In 2008, as seen in adjoining Kerala, the state of Karnataka (which also has large numbers of plantations) reported a sharp rise in cases, accounting for over 70% of the country's total cases (Santhosh et al., 2009); there was again a shift in virus, with the sequences closely clustering with the Kerala strains containing E1-A226V, and CHIKV was also identified in Ae. albopictus (Kumar et al., 2012).
In other areas, both E1-226 variants appeared to cocirculate. Detection of both E1-226A (from Ae. aegypti) and E1-A226V (from Ae. albopictus) viruses was described in Lucknow, Uttar Pradesh, in north India, in 2010–11 (Nyari et al., 2016). Although the minimum infection rate was higher overall for Ae. aegypti, suggesting that it may be the primary vector, mixed populations of Ae. aegypti and Ae. albopictus were seen particularly in indoor collection sites in urban areas. In Orissa (Odisha), the two ECSA variants circulated in different years, with E1-A226V detected in 2010 (Das et al., 2012) and E1-226A detected in 2013 (Saswat et al., 2015). During the 2010 study in Orissa, mosquito collections from outbreak sites included 56% Ae. albopictus (from which CHIKV RNA was detected) and 28% Ae. aegypti, showing the abundant presence of both species (Das et al., 2012). In West Bengal, there was an interesting clear geographical distinction, as strains from rural areas between 2006 and 2012 had the E1-A226V residue, while strains from urban Kolkata (where Ae. aegypti predominates) in 2011–12 had E1-226A (Taraphdar and Chatterjee, 2015).
The origins and initial spread of the Indian sublineage within Southeast Asia were strongly inferred based on evolutionary network analysis of sequences collected between 2006–08 in Sri Lanka and Singapore, which formed five separate subclades (Hapuarachchi et al., 2010). Initial introductions from India likely occurred in Sri Lanka in 2007 and Singapore in January 2008, with CHIKV strains bearing E1-226A. In early 2008, there were at least two further introductions into Sri Lanka, likely from India, carrying the E1-A226V mutation. An ECSA strain carrying E1-A226V closely related to Kerala strains was then introduced into Malaysia in April 2008, which would eventually spread to the whole country (Sam et al., 2009). Phylogenetic analysis allows the inference that imported strains from Malaysia were responsible for subsequent outbreaks that year in neighboring Singapore (commencing in May) (Ng et al., 2009) and Thailand (commencing August) (Rianthavorn et al., 2010), and from then on to other countries in East and Southeast Asia. Hence the Southeast Asian strains, which form a single monophyletic clade (Chen et al., 2016), have become established in the region, likely originated from India.
Sri Lanka (Previously Ceylon)
An outbreak of CHIKV was reported in Colombo, Ceylon (now Sri Lanka) in 1965, although this was diagnosed serologically and also indicated cocirculation of group B arboviruses (Hermon, 1967; Rao, 1971). Preoutbreak seroprevalence was 8% in 11- to 40-year-olds and 45% in those over 40 years, suggesting that CHIKV or a similar virus had been active decades earlier. Anti-CHIKV antibodies were detected in 74/611 (12.1%) of serum samples collected from around the country, with the lowest rates in the central hilly areas and the highest in Trincomalee (37.0%), a busy port on the northeast coast (Vesenjak-Hirjan et al., 1969).
In a sample of 206 febrile patients tested in Kandy in 2004–05, with a median age of 31 years (range: 13–76 years), none had serological evidence of past CHIKV infection (Panning et al., 2009). This supported the absence of reports of CHIKV since the 1960s (albeit CHIKV testing was not available), and showed high population susceptibility that underlay the subsequent large outbreaks of 2006–08. A major CHIKV outbreak that coincided with circulation of DENV started in October 2006 and continued into 2007, and caused over 37,000 suspected cases (Kularatne et al., 2009; Reller et al., 2013). This outbreak was caused by CHIKV (E1-226A), which clustered with sequences from India (Reller et al., 2013), and occurred mainly in urban and coastal areas (Hapuarachchi et al., 2010). The outbreak also involved Jaffna district in northern Sri Lanka, which had land routes closed off from the rest of the country due to the ongoing civil war, leading to shortages of everyday items including mosquito coils (Surendran et al., 2007). No CHIKV diagnostics were available in this region, and over 10,000 suspected outpatient cases were seen between November and December 2006.
A further outbreak affecting a similar number of people occurred in 2008, mainly in rural areas with abundant banana and rubber plantations where Ae. albopictus predominates (Hapuarachchi et al., 2010). This time, the viruses carried the E1-A226V mutation and formed at least two clear clusters separated from sequences from the region, suggesting independent evolution within Sri Lanka.
Bangladesh
The first recorded outbreak in Bangladesh occurred in December 2008 in two villages in the Rajshahi and Chapianawabganj districts, which border West Bengal, India (International Centre for Diarrhoeal Disease Research, 2009). The 39 patients were from a community of potters, and larvae of Ae. albopictus were found in water contained in numerous pots kept in and around houses. In 2011, outbreaks were reported in Dohar subdistrict in Dhaka district. Investigations focused on Char Kushai village showed a high attack rate of 29% (1105/3840 inhabitants), with the highest rates in adult women, likely because they spend most of their time at, or near, home (Khatun et al., 2015). Most (89%) of the hatched mosquitoes collected from affected households were Ae. albopictus, with no Ae. aegypti. All age groups were similarly affected, suggesting little preexisting immunity despite proximity to Calcutta and West Bengal, which have had several past outbreaks. No CHIKV sequences were reported, but a Bangladesh strain imported into Taiwan in October 2008 was an ECSA (E1-A226V) strain related to sequences from India (Yang et al., 2016).
Maldives
CHIKV was reported in 121/197 (61%) of the inhabited islands between December 2006 and April 2007 (Yoosuf et al., 2009). There were 11,879 reported cases out of a population of 300,000. A traveler from the Maldives was diagnosed with CHIKV in Singapore in January 2007, and the sequenced isolate was found to be of the ECSA genotype (E1-226A), clustering with isolates from India and Sri Lanka in 2006–07 (Ng et al., 2009). In December 2008, an outbreak of acute febrile disease was reported to have started on several islands in the Laamu Atoll (ProMED-mail, 2009). Two German tourists returning home from Ari Atoll in September 2009 were infected with ECSA-type CHIKV, this time carrying the E1-A226V mutation and related to Sri Lankan strains from 2008 (Pfeffer et al., 2010). Because many employees of tourist resorts in the Maldives come from India and Sri Lanka, it is likely that there were at least two introductions of CHIKV into the Maldives through this route, leading to outbreaks in 2006 and 2008.
Bhutan
CHIKV was reported for the first time in July 2012. Over 200 suspect cases were notified from Samtse and Chukha districts in southwest Bhutan (which borders West Bengal, India) and travelers from those districts to the capital, Thimphu (Wangchuk et al., 2013). Viral sequences from patient samples showed that the Bhutan strain was of the ECSA genotype and carried E1-226A, and was most related to similar strains from India isolated in 2010.
Nepal
Nepal shares a 1800-km border with northeast India. Three serologically diagnosed cases of CHIKV were reported for the first time from Dhading district in March-June 2013 (Pun et al., 2014). Later, increasing numbers of patients with fever and joint pain were reported in several districts in the Terai lowlands region (Parsa, Dang, and Kanchanpur) between March and November 2013; of 169 samples tested serologically, 27.8% were diagnosed as dengue, while 3.6% were diagnosed as CHIKV (Pandey et al., 2015). There is no routine surveillance for CHIKV, and dengue was first reported only as recently as 2006 (Dhimal et al., 2015). With the presence of both Ae. aegypti (which predominates) and Ae. albopictus at up to 2000 m above sea level, increasing population movement and climate change, there will be continued expansion of areas at risk of arboviral disease from the urban lowlands into the Middle and High Mountain regions in Nepal (Dhimal et al., 2015).
Pakistan
A small serosurvey in Pakistan only detected low levels of complement fixation test antibodies to CHIKV in 1/43 (2%) human samples (Darwish et al., 1983). In 2011, of 75 children with dengue-like illness in Lahore, 33 (44%) were diagnosed with DENV and 3 (4%) with CHIKV by ELISA (Afzal et al., 2015). Starting in November 2016, an outbreak of CHIKV was reported to have affected 30,000 people in Karachi and other towns in Sindh province (Rauf et al., 2017). Sequencing revealed that the circulating CHIKV was of the ECSA genotype, carried E1-226A, and was closely related to recent isolates from India (Shi et al., 2017).
Chikungunya in East Asia
China
The first reported isolation of CHIKV in China was from bats (Rousettus leschenaulti) collected in Xishuangbanna, Yunnan province in 1986 (Chen and Tao, 1996). Over the following 2 years, CHIKV was also isolated from Ae. albopictus, Culex tritaeniorhynchus and a single human case of fever in the same province (Zhang et al., 1992). CHIKV antibodies were also detected in 1%–44% of healthy individuals from different areas in Yunnan, with the highest rates seen around Lincang and Xishuangbanna (Wang et al., 2013). Yunnan province is in southwest China and borders Vietnam and Burma, which had previously reported CHIKV outbreaks. These early reports indicate that CHIKV was established in this province and had caused disease.
Several imported cases have been described. In 2008, five CHIKV cases were imported into Guangzhou, Guangdong Province, from Malaysia and Sri Lanka (Zheng et al., 2010). The CHIKV strains were from the Indian sublineage and phylogenetically matched the epidemiological history; the Malaysian strain carried E1-A226V, while the Sri Lankan strains had parental E1-226A. In June 2010, a febrile Nigerian traveling from Ethiopia was detected during airport screening in Guangzhou, and found to be infected with CHIKV of the Central African lineage of the ECSA genotype (Bai et al., 2014). This was unusual because virtually all other ECSA isolates in Asia were from the epidemic Indian Ocean lineage (Indian sublineage), which had become established in Asia (Chen et al., 2016). In mid-2012, two sporadic imported cases of Asian CHIKV were reported in Zhejiang province, in east China; the first in a traveler from the Philippines (reported in GenBank with the accession numbers KC352904 and KC488650), and the second in a sailor who returned after visiting several countries in Southeast Asia (Sun et al., 2013). The two Asian sequences clustered strongly with strains from the ongoing epidemic in the Philippines (Sy et al., 2016; Lanciotti and Valadere, 2014). These imported cases of four different clades of virus from two genotypes (ECSA and Asian), from three different geographical regions, clearly illustrate the epidemic threat to nonendemic countries posed by the globalization of CHIKV.
The first locally transmitted outbreaks occurred almost simultaneously in Dongguan and Yangjiang cities in Guangdong, in September 2010, and affected over 250 patients (Qiaoli et al., 2012; Wu et al., 2013). These cities are 280 km apart, and phylogenetic analysis of the outbreak strains indicated two likely separate and undocumented introductions of ECSA-type CHIKV from Southeast Asia. Yunnan and Guangdong, the two provinces that have been affected by CHIKV to date, are located in south China in the subtropical zone, and have endemic Ae. albopictus. In addition, Guangdong receives many visitors from Southeast Asia, making it at high risk of further CHIKV epidemics.
An entomological study in the Beiwan region, northwest Xinjiang Uigur Autonomous Region, detected CHIKV in wild Ae. vexans, which is the most common species in the area (Guo et al., 2015). This species feeds on various vertebrate hosts, including farm animals as well as humans. While far less efficient as a vector for CHIKV than Ae. albopictus (Talbalaghi et al., 2010), the species may be important if present in high densities in the absence of the main Aedes vectors.
Taiwan
A 1965 seroprevalence survey of arboviruses in Wun-Li village, just north of Tainan city, showed neutralizing CHIKV antibodies present in 3% of those < 40 years, and 90% of those > 40 years, a strongly age-associated pattern that was also reported from India and Sri Lanka in the 1960s (Clarke Jr et al., 1967). This suggests that intense CHIKV circulation occurred at least four decades previously, followed by a long period of quiescence. Laboratory-confirmed autochthonous transmission of CHIKV has not yet been reported. The distribution of Ae. aegypti mirrors the areas most affected by endemic dengue in the southern regions of the country (such as Tainan and Kaohsiung municipalities), while Ae. albopictus is present throughout (Yang et al., 2014). Both vectors are capable of transmitting CHIKV in Taiwan, with the ECSA E1-A226V strain replicating most effectively in Ae. albopictus (Chen et al., 2015).
Analysis of 78 imported travel-associated CHIKV cases in Taiwan between 2006 and 2014 reflected the changing epidemiology around Asia (Yang et al., 2016). The ECSA genotype predominated from 2008 to 2010, introduced from the ongoing Southeast Asian epidemics in Singapore, Malaysia, Indonesia, and Thailand. After 2010, there were mainly Asian strains introduced from Indonesia and the Philippines, the latter being closely related to the subsequent outbreak strains in Yap and the Caribbean. Indonesia in particular was a rich source of diverse strains, from both ECSA and Asian genotypes (forming two separate clusters in the latter).
Japan
Japan has only had imported cases of CHIKV described, at a rate of 10–20/year between 2011 and June 2016 (National Institute of Infectious Diseases, 2016). Ae. aegypti is absent from Japan, but Ae. albopictus is present in areas south of Aomori. The first locally transmitted DENV cases in 70 years occurred in Tokyo in 2014, with over 100 cases linked to a large park (Arima et al., 2014), which indicates the potential risk of CHIKV as well.
Hong Kong
Hong Kong has only had imported cases of CHIKV, which was made a notifiable disease in March 2009. There is a risk of CHIKV outbreaks because of the widespread presence of Ae. albopictus (but not Ae. aegypti), and the precedence of the first local DENV outbreak centered around a construction site in Ma Wan Island in 2002 (Ma et al., 2011).
South Korea
South Korea has endemic Ae. albopictus (Takhampunya et al., 2014), particularly in the southern parts of the country that lie in the subtropics. Ae. albopictus on Jeju Island, which has two busy seaports, was found to have close genetic relatedness with Vietnamese strains (Lee et al., 2013). Imported CHIKV cases have been reported, and there remains a risk of local transmission.
Chikungunya in Oceania
Pacific Islands
A seroprevalence study in certain islands in the Pacific (Solomon Islands, New Hebrides (now Vanuatu), and Palau) between 1960 and 1974 found no CHIKV seropositivity in 220 samples (Tesh et al., 1975). Seropositivity to CHIKV was very low (0% to 7%) in most sites sampled in New Guinea (Tesh et al., 1975; Kanamitsu et al., 1979). Rates were > 10% in a few sites in New Guinea, but crossreactivity with Ross River virus (RRV) could not be excluded because RRV is endemic in the region; indeed, seropositivity to RRV was even higher (Tesh et al., 1975).
The Pacific Public Health Surveillance Network (PPHSN), with participation of 22 Pacific Island countries and territories, reported an unprecedented wave of arbovirus outbreaks starting in January 2012 (Fig. 2). Until September of 2014, there were 18 outbreaks of DENV, 7 of CHIKV and 3 of Zika virus (ZIKV), affecting at least 120,000 people (Roth et al., 2014a). CHIKV had previously never been detected in the Pacific. Conditions for its spread were ideal, since the human populations are susceptible, Ae. aegypti is present throughout most of the Pacific (causing previous dengue outbreaks) while Ae. albopictus is present in several countries (including Papua New Guinea, Fiji, Tonga, and the Solomon Islands) and vector control is inadequate (Horwood et al., 2013a).
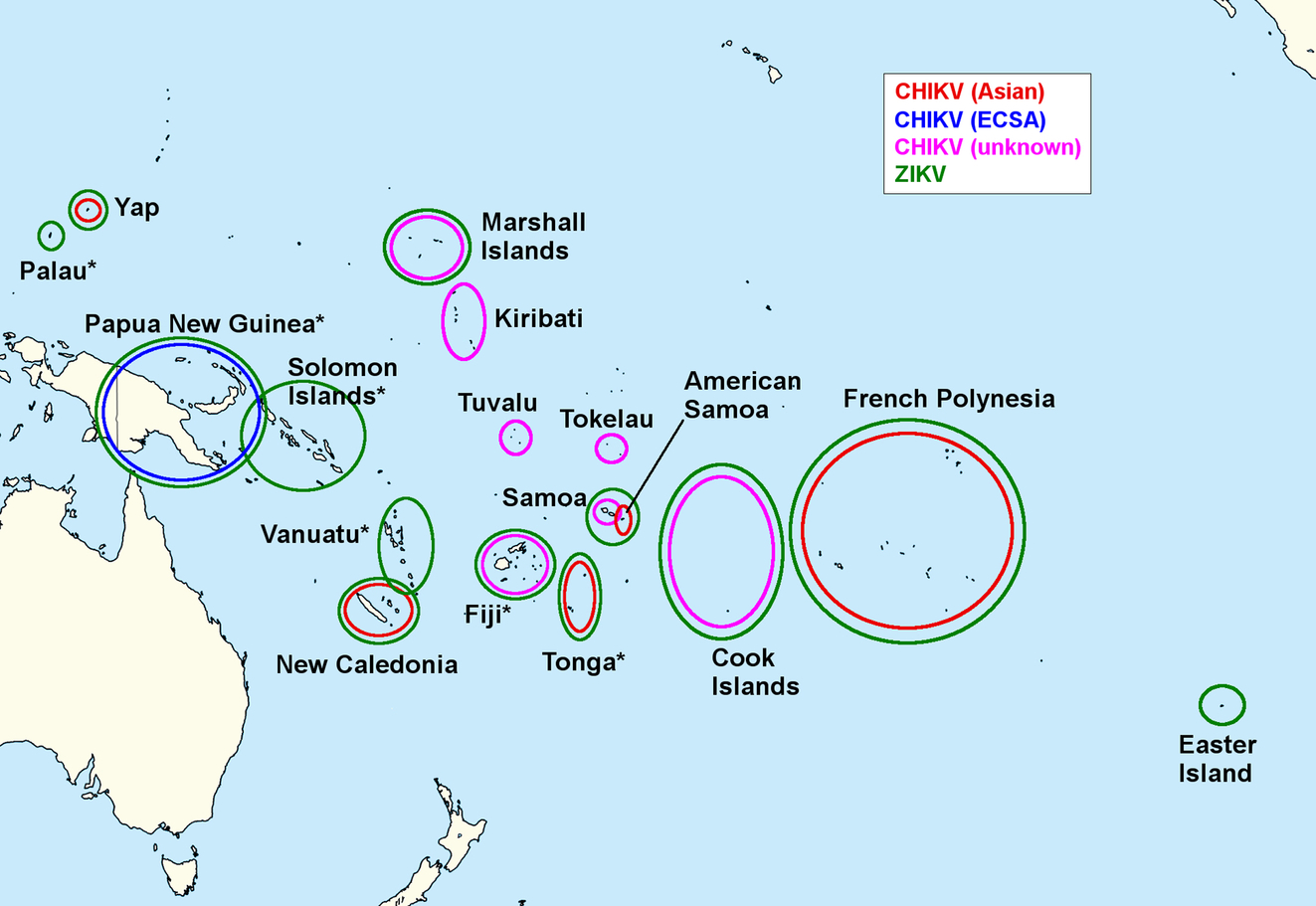
The first autochthonous CHIKV transmission in the Pacific was reported in New Caledonia, with 33 cases occurring between March and June 2011. The virus was of the Asian genotype, consistent with the recent arrival from Indonesia of the suspected index case (Dupont-Rouzeyrol et al., 2012). The likely vector was Ae. aegypti, since Ae. albopictus has not yet been found in New Caledonia. A small outbreak affecting 30 cases occurred in February 2013, again due to an Asian strain imported from Indonesia (Roth et al., 2014b).
In June 2012, an outbreak of CHIKV of the ECSA genotype (carrying E1-A226V) was reported in Vanimo, Sandaun Province, in the west of Papua New Guinea (Horwood et al., 2013b). By May 2013, the disease was still spreading eastwards, with thousands of syndromic cases (Roth et al., 2014b). An entomological survey in Vanimo found all collected larvae and 99.4% of adults to be Ae. albopictus, but CHIKV was not detected in these samples.
An outbreak started in August 2013 in Yap state, Federated States of Micronesia, causing at least 1700 suspected cases (Roth et al., 2014a) out of a total population of 11,376. CHIKV of the Asian genotype was detected, and sequences clustered with Philippine strains from 2011 to 2013, and the earliest CHIKV strains from the subsequent Caribbean outbreak in December 2013 and January 2014 (Lanciotti and Valadere, 2014). CHIKV was also detected in captured wild mosquitoes of both Ae. hensilli, which is native, and Ae. aegypti, an introduced species (Savage et al., 2015). Ae. albopictus is not present. In laboratory experiments, Ae. hensilli was found to be very susceptible to infection by CHIKV, with infection rates of 62% and dissemination rates to the head of 80% (Ledermann et al., 2014). As Ae. hensilli is far more abundant throughout the Yap islands than Ae. aegypti (which is sporadically distributed), it was most likely to play the predominant vector role in this outbreak. This finding was interesting as it showed that other Aedes species less well characterized as CHIKV vectors may act as principal vectors if abundant relative to the usual vectors.
Further outbreaks in 2014 were reported in Tokelau, Samoa, American Samoa, and Tonga (with over 10,000 suspected cases) (Roth et al., 2014a). Of interest, a patient from Tongatapu, Tonga, was diagnosed with CHIKV encephalitis on his return to the United States (Nelson et al., 2014). The Cook Islands experienced an outbreak starting in October 2014, which had affected over 700 cases by July 2015 (Radio New Zealand, 2015b). Other countries affected in 2015–16 include Kiribati (with over 3000 cases) (Radio New Zealand, 2015a), the Marshall Islands, Tuvalu, and Fiji (World Health Organisation Western Pacific Region, 2016).
As described later in more detail, a ZIKV outbreak had occurred in French Polynesia between October 2013 and April 2014. In September 2014, patients in Tahiti, the main island, were confirmed positive for CHIKV. The strains were from the Asian genotype, and were more closely related to strains from the ongoing epidemics in the Caribbean (including a sporadic case imported from Guadeloupe in May 2014) (Nhan et al., 2014) than the other Asian strains previously identified in the Pacific in Yap, Tonga, and New Caledonia (Aubry et al., 2015a). This suggested that CHIKV had been introduced from the Caribbean rather than as a result of the ongoing outbreaks in the Pacific, a route which previously had been seen in a DENV-3 outbreak. The outbreak lasted until March 2015, affecting an estimated 66,000 out of the 270,000 population (about 24%), and was associated with 18 deaths and severe complications in 50 patients, including a cluster of 9 patients with Guillain-Barré syndrome (Oehler et al., 2015). Laboratory studies showed that Ae. aegypti is a more efficient vector for CHIKV than Ae. polynesiensis, which is endemic in French Polynesia. Ae. polynesiensis may have played an important role in CHIKV outbreaks in Pacific regions where it is the most widespread species, such as the Cook Islands and Samoa (Richard et al., 2016a).
Australia and New Zealand
CHIKV seropositivity was absent in Queensland in the 1970s (Kanamitsu et al., 1979). Imported but not autochthonous cases have been reported in Australia, the first being in a traveler from East Nusa Tenggara, Indonesia, in 1989 (Harnett and Bucens, 1990). There were 160 cases reported between 2008 and 2012, coinciding with the main period of the Southeast Asian outbreaks. The main countries of origin were Indonesia (28.0%), India (18.5%), and Malaysia (10.0%) (Viennet et al., 2013). Vector competence studies have shown that native and introduced species in Australia including Ae. aegypti, Ae. albopictus, Ae. vigilax, and Ae. notoscriptus can transmit CHIKV (van den Hurk et al., 2010). These studies are useful, but relevance to public health also requires vectorial capacity modeling, which incorporates variables such as vector density and distribution, behavior in biting humans, extrinsic incubation period, and mosquito biological factors. Such modeling predicts that the latter two species, while widespread in Australia, are unlikely to contribute to sustained transmission causing outbreaks (Jansen et al., 2015). The most likely to be involved in CHIKV transmission would be Ae. aegypti and Ae. albopictus, although these are currently limited to north Queensland and the Torres Strait Islands (north of Australia), respectively. CHIKV in the Pacific, particularly the outbreak occurring in neighboring Papua New Guinea in 2012 (Roth et al., 2014a), would have been a major concern. In New Zealand, endemic vectors such as Opifex fuscus and Ae. antipodeus and imported vectors such as Ae. notoscriptus and Ae. australis can transmit CHIKV in the lab (Kramer et al., 2011), but have never been incriminated as vectors in the wild.
Chikungunya in Western Asia/Middle East
Yemen
The first reported CHIKV outbreak in the Eastern Mediterranean region of the WHO (which includes the Middle East) involved 15,000 suspected cases in the Al-Hudaydah governorate, in southwest Yemen, between October 2010 and April 2011. The outbreak had two peaks, and the later peak was accompanied by DENV circulation (Malik et al., 2014). The reported attack rate of 7/1000 was likely underestimated since active surveillance and case detection was not conducted. Situated on the Red Sea and close to northeast Africa, Al-Hudaydah is a busy port and center for sea trade and refugees from neighbouring countries. Interestingly, the outbreak CHIKV strain was found to carry E1-226A, which may be consistent with the identification of Ae. aegypti as the causative vector (Zayed et al., 2012), and outbreak sequences clustered with the E1-226A clusters of the ECSA Indian sublineage (Ciccozzi et al., 2014).
Saudi Arabia
Dengue viruses and their Aedes vectors are widespread in Saudi Arabia. Despite being the site of the annual mass religious gatherings of the Hajj and Umrah, attended by millions of pilgrims from all around the world, there has only been a single reported case of locally acquired CHIKV of the ECSA genotype, diagnosed in May 2011 in Jeddah (Hussain et al., 2013). Although a partial sequence of E1 was stated to have the 226A residue, as did the strains from the Yemen outbreak that occurred earlier in the year, the full Saudi sequence is not available for further analysis. Two household members had serological evidence of CHIKV. DENV importation has previously been strongly inferred from phylogenetic similarities between DENV-2 isolates circulating in Saudi Arabia and strains from countries which send the highest number of pilgrims, such as Indonesia, Pakistan, and India (El-Kafrawy et al., 2016). Nevertheless, surveillance systems are in place whereby all dengue-negative samples are screened for other relevant viral fevers (Hussain et al., 2013).
Others
Israel has reported numerous imported cases of CHIKV and is at risk of autochthonous cases since Ae. albopictus is present in the major cities (Leshem et al., 2012). Ae. albopictus has been present in Lebanon since 2003, and local strains are capable of transmitting CHIKV (Haddad et al., 2012). In Iraq, a seroprevalence study carried out in 2012–13 in Nasiriyah showed negligible seroprevalence to CHIKV (2/399, or 0.5%), but the recent status of CHIKV infections or Aedes vectors is not known (Barakat et al., 2016). There is therefore a risk of further outbreaks in the Middle East, as happened in Yemen.
Conclusions
Chikungunya has had a long historical association with Asia, causing large outbreaks in the 1960s. Subsequent decades were characterized by smaller, sporadic outbreaks or sporadic cases, interspersed with years of apparent nonactivity, although it must be said that few (if any) countries routinely tested for CHIKV outside of prominent outbreaks.
The explosive re-emergence of CHIKV in Asia starting in 2005 was in many ways inevitable, as many countries already have a history of intractable problems with dengue and Ae. aegypti and Ae. albopictus, vectors shared with CHIKV. The distribution of these mosquito species has been expanding to include new areas due to changes in climate and environmental use (Kraemer et al., 2015). With increasing global movement of people and goods (and accompanying mosquitoes at various life cycle stages), there were countless opportunities for introductions of CHIKV into old and new areas. Human populations were largely naïve and therefore, immunologically susceptible, as CHIKV in prior decades seemed to have caused large outbreaks before disappearing for long periods. Certain social factors in Asia, such as the storage of rainwater because of the lack of piped water, inadequate disposal of rubbish (Fig. 3), and large numbers of people living in a single dwelling, greatly contributed to the propagation of mosquitoes and transmission of CHIKV. Importantly, the virus itself was able to evolve in response to new conditions, with key mutations increasing adaptation to Ae. albopictus, previously considered of secondary importance to Ae. aegypti as a vector of CHIKV in Asia. This allowed CHIKV to exploit the wider distribution and abundance of Ae. albopictus, particularly in rural areas with plantations that are common in Asia. While the ability of the ECSA genotype to evolve adaptive changes is now well described, the previously endemic Asian genotype, thought to have been displaced by the aggressive invasion of the ECSA strains, mounted a resurgence and showed its capacity to spread to new regions and continents. The genetics of CHIKV are more fully discussed in Chapter 8. It is likely that distribution of favored vectors will influence circulating strains, and there is much to be understood about the importance of area-specific alternative vectors, such as Ae. hensilii and Ae. polynesiensis in the Pacific islands. The lack of awareness, surveillance, and diagnostic capability also meant that once the disease was established in a given area, failure to recognize it (or clinical attribution to other tropical infections with similar symptoms) meant a failure to implement early public health control measures.

In the absence of licensed antivirals or vaccines (which also raises the question of whether a vaccine would be considered cost effective in resource-limited countries in Asia), CHIKV will undoubtedly continue to be a major problem. There are also several countries that have not yet reported CHIKV but that have the conditions and vectors conducive for transmission cycle establishment; over 270 million people are estimated to be at risk in Asia (Nsoesie et al., 2016). Areas with little or no protective immunity will be at especially high risk of the characteristically explosive outbreaks seen over the last 10 years. For the other countries that have already experienced outbreaks, and where a degree of population immunity exists, it is reasonable to wonder whether the well-documented pattern of outbreaks followed by long periods of quiescence may still hold true, as it did in the 1960s to the 1980s (Burke et al., 1985; Retuya et al., 1998; Arankalle et al., 2007). This is because of the now wider distribution of CHIKV and increased number of travelers within countries, within regions, and between continents. Asia may start to see CHIKV causing low-level sporadic and endemic disease, with continuous reintroductions from other areas. The propensity of CHIKV to surprise remains; it is imperative that Asia learns from its experiences and puts into place the public health infrastructure to prevent and control further outbreaks, which will pose immense economic and clinical burdens.
Zika Virus in Asia
Introduction
After the original isolation of Zika virus in Uganda in 1947, ZIKV was not reported outside of Africa until 1966 when an isolate was made from Ae. aegypti collected in Malaysia. Serosurveys in various countries had suggested that ZIKV was present in Asia, but ZIKV was only confirmed in humans for the first time outside of Africa during the outbreak in Yap, Federated States of Micronesia, in 2007. There is no doubt that ZIKV was present before this, but simply not diagnosed. Clinical signs and symptoms can easily be confused with other tropical infections that present in a similar way, especially dengue fever, and ZIKV was neither routinely looked for nor considered. A lack of clinical awareness was accompanied by lack of diagnostics. However, the abundance of Ae. aegypti and other secondary vectors (notably Ae. albopictus), and the conditions that enable the endemicity of DENV and CHIKV, make Asia an ideal region for ZIKV transmission.
After the Yap outbreak, further outbreaks occurred in the Pacific islands from 2011 onwards, and small clusters and sporadic cases were reported in Southeast Asia. The recently isolated ZIKV strains from the Pacific and Southeast Asia cluster within the Asian genotype, for which the Malaysian isolate from Ae. aegypti in 1966 represents the ancestor (Lanciotti et al., 2016). Phylogenetic analysis implies that ZIKV spread from Asia to the Pacific islands and then to the Americas, which mirrors the route taken by CHIKV when it reached the Americas in late 2013 (Musso et al., 2015).
As of March 10, 2017, the WHO have reported locally mosquito-borne ZIKV infections in two countries in South Asia (Bangladesh and Maldives), 8 countries in Southeast Asia (Cambodia, Indonesia, Laos, Malaysia, Philippines, Singapore, Thailand, and Vietnam) and 13 in the Pacific (American Samoa, Cook Islands, Federated States of Micronesia, Fiji, French Polynesia, Marshall Islands, New Caledonia, Palau, Papua New Guinea, Samoa, Solomon Islands, Tonga, and Vanuatu) (World Health Organization, 2017).
Zika Virus in Asia Between 1947 and 2006
Seroprevalence studies have long suggested the likely presence of ZIKV in Asia. In the 1950s, neutralizing ZIKV antibodies were described in Malaysia, in 15/79 (19%) long-time residents around the capital city, Kuala Lumpur, and 9/50 (18%) residents of East Malaysia (although 27%–37% also had antibodies to other flaviviruses including DENV and Ntaya virus) (Smithburn, 1954), and in over 70% of 358 residents in a rubber estate in Selangor (Pond, 1963). In the latter group, seropositivity increased gradually with age, but it was also noted that many of the older people had migrated from India, making it impossible to determine where exposure had occurred. Low rates of seropositivity were also described in 2/50 (4%) subjects in Tonkin, North Vietnam, and 8/50 (16%) in Bangkok and Chiangmai, Thailand (Pond, 1963). In India, 33/196 (17%) seropositive people were recorded from six states, with highest reported rates in three districts in modern-day Gujarat and Maharashtra states (Smithburn et al., 1954). A total of 19/153 (12%) positive individuals were identified from Manila and nearby rural villages in the Philippines (Hammon et al., 1958); and 9/71 (13%) of the residents of Lombok, Indonesia were positive (Olson et al., 1983). A later study of people living or working by a forest reserve in Sabah, East Malaysia, found that 9/30 (30%) of natives and 40/81 (49%) of migrants from the region had neutralizing antibodies to ZIKV, but there was also similarly high rates to DENV and JEV (Wolfe et al., 2001).
As described in Chapters 2 and 4, ZIKV is likely to have an enzootic cycle in nonhuman primates in Africa, but currently there are few firm data for this in Asia. Marchette et al. (1969) cited their own unpublished findings that “there is strong serologic evidence that it (ZIKV) occurs naturally in wild monkeys in Malaysia,” but did not provide further details (Marchette et al., 1969). Neutralizing antibodies have otherwise been described in 6/71 (8%) orangutans sampled in 1996–98 in Sabah, East Malaysia (Wolfe et al., 2001). Using complement fixation tests, very low (1.2%–3.8%) positive rates were found in various mammals sampled in Pakistan, including Indian gerbils (Tatera indica), Indian desert jirds (Meriones hurrianae), a sheep, and a goat (Darwish et al., 1983); the significance of this is unclear. In Lombok, no neutralizing antibodies to ZIKV were found in 28 domestic and wild animals (Olson et al., 1983). Recent work by Ragan et al. (2017) casts doubt that domestic animals can play any significant role in the transmission cycle of ZIKV (Ragan et al., 2017).
Detection of ZIKV antibodies is difficult to interpret as there are considerable and poorly characterized crossreactions within the flaviruses. Even with the plaque reduction neutralization test, which is considered the most specific assay, seropositivity to ZIKV is not considered specific if neutralizing antibodies to other flaviviruses (either from past infection or immunization) are concurrently detected (Rabe et al., 2016). This sometimes makes it difficult to interpret the significance of seroprevalence studies in Asia, where residents are exposed to endemic flaviviruses such as dengue and Japanese encephalitis viruses. Furthermore, the few published serosurveys are often limited in area, and have small samples with insufficient age-stratified data that are useful to infer patterns of past disease. Nevertheless, some of the reported subjects had antibodies only to ZIKV and not to other group B arboviruses (flaviviruses), suggesting specific evidence of past infection.
These seroprevalence studies were strongly supported by the first ZIKV culture in Asia in 1966. An extensive entomological study in Malaysia led to ZIKV isolation from Ae. aegypti collected from shophouses in Bentong town, in Pahang state (Marchette et al., 1969). ZIKV was not isolated in 4492 Ae. albopictus or 27,636 mosquitoes from other Aedes species throughout the country. The isolation of ZIKV from urban anthropophilic mosquitoes meant that human cases must almost certainly have occurred, but were not diagnosed.
The first serologically confirmed human cases of ZIKV in Asia were reported in Java, Indonesia, in 1977–78, in seven febrile patients who had a four-fold rise in microneutralization antibodies of ZIKV only, and not of other flaviruses (DENV-2, Tembusu, Japanese encephalitis and Murray Valley encephalitis viruses) (Olson et al., 1981). A further seven had antibodies to DENV-2 only, and eight had diagnostic rises in both DENV-2 and ZIKV antibodies. This suggests that ZIKV may be circulating undiagnosed with DENV in endemic areas, but also that diagnosis is difficult in the presence of other flaviviruses, as neutralization assays are not routinely available.
Zika Virus Outbreaks in the Pacific From 2007 Onwards
Starting in April 2007, astute physicians on Yap Island, Federated States of Micronesia, reported an outbreak of mild febrile illness; some patients tested positive for dengue IgM, but the disease appeared to be clinically distinct from dengue (Duffy et al., 2009). A screen of patient samples for various arboviruses revealed positive PCR results for ZIKV. There were no deaths or hospitalizations. This first PCR-confirmed outbreak of ZIKV also provided important diagnostic information, in that serological diagnosis (by IgM or neutralization titres) was relatively straightforward in patients without existing antibodies to heterologous flaviviruses, as the primary antibody response was specific. However, in those that had experienced previous flavivirus exposure, high crossreactive secondary responses to other flaviviruses were observed, making it difficult to definitively confirm ZIKV by serological assay alone (Lanciotti et al., 2008). This crossreactivity makes it difficult to rely on serological diagnosis in the many countries with endemic dengue that are also at high risk of ZIKV. Data from a household survey indicated a high infection rate of 5005/6982 (73%) among Yap residents above 3 years, and also found Ae. hensilli in 36% of water-holding containers in houses, with no other species found in more than 3% (Duffy et al., 2009). Ae. hensilii was also the most frequently collected mosquito species during the outbreak at 41%, while Ae. aegypti made up just 0.1% (Ledermann et al., 2014). Although ZIKV was not detected in any wild caught mosquitoes, laboratory studies showed that ZIKV had an infection rate of 86% and dissemination rate of 23% in Ae. hensilii, suggesting that it could have been the main vector in Yap. The relatively low dissemination rate could have been compensated for by high mosquito densities.
From 2012, an unprecedented wave of different arboviral outbreaks spread across the Pacific (Fig. 2) (Roth et al., 2014a). DENV had been the only known circulating arbovirus before CHIKV first appeared in New Caledonia in 2011. ZIKV then spread into French Polynesia between October 2013 and April 2014 (Cao-Lormeau et al., 2014), with an estimated 28,000 cases (11.5% of the population) reported (European Center for Disease Prevention and Control, 2014). A preoutbreak seroprevalence study of 593 blood donors found ZIKV IgG antibodies in only 5/593 (0.8%) (Aubry et al., 2015b), but after the outbreak in 2014, seropositivity was 50%–66% in the most inhabited islands, including Tahiti (Aubry et al., 2015a). This suggested the true attack rate was greatly underestimated, likely due to asymptomatic or mild disease that did not require medical attention. An increased number of babies born with microcephaly and cerebral abnormalities were reported in association with the outbreak (Besnard et al., 2016), and using a mathematical modeling approach, infection with ZIKV in the first trimester of pregnancy gave the best fit for the timing of the cases (Aubry et al., 2015a). An unusually high number of cases of Guillain-Barré syndrome cases (42, compared to < 10/year for the previous 4 years) occurred at the same time as the outbreak, leading to a hypothesized link with ZIKV (European Center for Disease Prevention and Control, 2014; Cao-Lormeau et al., 2016). A detailed description of disease symptoms due to ZIKV infection is provided by Halstead in Chapter 3. In the laboratory, French Polynesian Ae. aegypti was easily infected with ZIKV but it took at least 14 days before the virus was detectable in saliva, while virus was not detectable in the saliva of Ae. polynesiensis, a widely distributed native species (Richard et al., 2016b). This raises the possibility of involvement of other vectors, particularly in some remote islands where Ae. aegypti is absent. In Yap, where Ae. hensilii was directly implicated in CHIKV transmission and believed to be involved in ZIKV transmission, Culex quinquefasciatus was the second most frequently trapped species (Savage et al., 2015; Ledermann et al., 2014). Its competence for ZIKV was not studied. As described in Chapter 2, there is currently conflicting evidence for the role of Cx. quinquefasciatus as a ZIKV vector (Leal, 2016).
Following imported cases from French Polynesia in November 2013, New Caledonia had its first locally acquired ZIKV cases in January 2014, with over 1300 cases (Dupont-Rouzeyrol et al., 2015). The Cook Islands outbreak between February and May 2014 affected over 900 people (Roth et al., 2014a). Outbreaks were also reported in early 2014 in Easter Island, which is part of Chile (Tognarelli et al., 2016). In 2015, outbreaks were confirmed in the Solomon Islands (324 cases), Vanuatu, Fiji, and Samoa; and in early 2016, in Tonga (596 cases) (Craig et al., 2016). During this period of ZIKV outbreaks in the South Pacific, over 63,000 Australians visited affected islands, which is a concern because Ae. aegypti is now endemic in Northern Queensland (Taylor and Paterson, 2016). During January to July 2016, an outbreak in American Samoa affected 756 people (out of a population of 55,502) (Healy et al., 2016).
Zika Virus in Southeast, South and East Asia From 2012 Onwards
Numerous countries in Southeast Asia are considered to have endemic ZIKV by virtue of sporadic cases or exported cases reported in other countries (Fig. 4). To date, only Singapore has reported a major outbreak. In Kampong Speu Province, Cambodia, a 3-year-old boy with self-limiting fever was confirmed to have ZIKV by PCR and seroconversion (Heang et al., 2012). An ongoing study of febrile illness had started in 2006, and this was the only ZIKV diagnosis made in over 10,000 enrolled cases. As part of a prospective study of febrile illness in Cebu City, Philippines, 1/270 patients were diagnosed with ZIKV by PCR (Alera et al., 2015). Several imported cases from Thailand have been reported, including the first into Europe in November 2013, which was serologically diagnosed (Tappe et al., 2014). This prompted the Thai Ministry of Public Health to retest negative DENV- and CHIKV-samples collected from febrile patients with rash and no history of travel outside their province in 2012–14 (Buathong et al., 2015). Seven cases were then diagnosed as ZIKV positive, four by PCR and three by neutralization. These cases, and those imported cases reported in the literature, came from provinces distributed all around Thailand. An imported case to Australia from Jakarta, Indonesia, was reported in 2012 (Kwong et al., 2013). During a dengue outbreak in Sumatra, Indonesia, in 2014–15, 103 DENV-negative specimens were tested for other arboviruses, and ZIKV was cultured from one patient (Perkasa et al., 2016). In September 2014, a traveler returning to Germany from Sabah, Malaysia, was diagnosed with ZIKV serologically, although an acute serum was negative by PCR (Tappe et al., 2015). As of November 2016, only 7 cases had been reported in Malaysia (including several imported from Singapore), and an ongoing surveillance of DENV-negative samples has not yielded any positives from 1502 tested since July 2015 (Ministry of Health, Malaysia, 2016). A first ZIKV case acquired in Vietnam was reported in a returning Israeli traveler in December 2015 (Meltzer et al., 2016); since then, sporadic numbers of locally acquired cases have been reported in several provinces in Vietnam (ProMED-mail, 2016a).
The first reported case in Singapore was imported from Brazil in May 2016, but strict control measures probably prevented further cases. The first authochtonous outbreak occurred centered around a construction site in August 2016, and as the diagnosis was made only after a few weeks into the outbreak, containment proved difficult. More than 400 cases resulted, including new clusters in different locations (Sadarangani and Hsu, 2016). Full genome analysis indicated that the Singaporean strains clustered separately from Brazilian and French Polynesian sequences, with all sharing ancestral origins with earlier Southeast Asian isolates from Thailand (2013–14), the Philippines (2012), and Cambodia (2010). This suggested that the Singaporean outbreak was due to viruses already circulating in Southeast Asia rather than an introduction from the Americas (Maurer-Stroh et al., 2016). This was consistent with the epidemiological setting, as many of the patients were migrant construction workers (National Environment Agency-Singapore, 2016), who mainly come from other countries in the region. Indeed, a reason that this outbreak was thought to have occurred despite national readiness for ZIKV was that doctors did not consider the diagnosis in the initial patients, as they had not traveled to the Americas, which had well-publicized ongoing ZIKV (Sadarangani and Hsu, 2016).
This outbreak also highlighted the potential for introducing infectious diseases through the movement of migrant workers. It is estimated that there are more than 30 million migrant workers in Asia, both registered and unregistered, who mainly move from countries such as Indonesia, Bangladesh, India, and the Philippines, to work in wealthier countries such as Japan, Hong Kong, Singapore, and Malaysia (Low et al., 2015). Singapore, for example, has the third highest ratio of migrants to total population in Asia, at over 40%; Malaysia, which ranks seventh with 15%, has 2.1 million registered migrants and over a million estimated undocumented migrants (Munoz Moreno et al., 2015). Those employed in the construction industry are at risk of arboviruses because building sites are conducive to mosquito breeding, the workers often live in cramped conditions on-site, and they have less access to medical care (Lysaght et al., 2016). There is therefore a duty of care for governments in destination countries to protect both public health and the rights of this vulnerable population (Tam et al., 2016).
In South Asia to date, locally acquired ZIKV has been reported only in Bangladesh and Maldives. From the former, 1/101 archived samples were tested positive, although the date of the specimen was not given (ProMED-mail, 2016b). From the Maldives, a Finnish man working in Dhiffushi Island was diagnosed by PCR of urine in June 2015 (Korhonen et al., 2016). There are clear concerns about other countries in this region, particularly India and Sri Lanka, which were badly affected by CHIKV outbreaks from 2006 to 2008. Ae. aegypti, the principal vector of CHIKV with the E1-226A residue, is widespread in those countries.
No locally transmitted ZIKV has been reported in East Asia, although imported cases have been detected in China (Zhang et al., 2016), Japan (Shinohara et al., 2016), Korea (Jang et al., 2016), and Taiwan (Huang et al., 2016). ZIKV remains a concern as Ae. albopictus is present in some regions of these countries (and Ae. aegypti less so).
Conclusions
Numerous unanswered questions about ZIKV remain. While a great deal of work is currently in progress, most of this is based in the Americas, and there is a concern that the answers (particularly for epidemiological issues) may not be fully relevant to other ZIKV-endemic regions, including Asia. A study that analyzed travel from ZIKV-affected areas in the Americas, presence of vector species, populations at risk, climate conditions and health expenditure per capita found that for countries in the Asia-Pacific region, those with the highest risks for imported ZIKV and subsequent health impact were India, the Philippines, Indonesia, Vietnam, Pakistan, and Bangladesh (Bogoch et al., 2016). There is no doubt that the occurrence of an epidemic on the scale of ZIKV in the Americas (or, as a precedent, CHIKV in Asia) would have a devastating impact in Asia, particularly with the emerging evidence of long-term neurological complications and possible long-term transmissibility between humans.
A key unknown is that of the definitive range of mosquito vectors of ZIKV across different locations, as Ae. aegypti may not be the main vector as is commonly assumed (Ayres, 2016). In particular, there are some areas where the putative main vector Ae. aegypti is absent or in low numbers, such as certain islands in the Pacific where Ae. hensilii may be important. The role of Culex mosquitoes is under debate, but should not be discounted as they are widely distributed and abundant in many ZIKV outbreak areas, with known capacity to transmit other flaviviruses (Leal, 2016). The roles of other Aedes species need to be determined, such as Ae. albopictus, which was central in the worldwide spread of the ECSA genotype of CHIKV, and which has been directly implicated in a ZIKV outbreak in Gabon (Grard et al., 2014). A laboratory study found that in Singaporean Ae. albopictus, 100% of mosquitoes tested had virus in the saliva by 10 days postinfection, and were thus all potentially infectious (Wong et al., 2013). If the current ZIKV outbreaks can be spread by both Ae. aegypti and Ae. albopictus, the full extent of affected areas could be huge.
A further key question is why there have been no outbreaks in endemic Southeast and South Asia since the likely presence of the virus was established as early as the 1950s, until the outbreak in Singapore in 2016. The lack of suitable diagnostics (itself a critical unmet need) is unlikely to be the only reason, as a large outbreak of febrile illness testing negative for usual causes like DENV would have been noticed as it was in the Pacific islands. Numerous countries considered endemic have shown a sporadic background of ZIKV, obscured by larger numbers of DENV cases; it would be important to know whether outbreaks are held in check by population immunity (again, study of which would require a reliably specific assay), or by other genetic, environmental, vector-specific or viral-specific factors.
Failure to control Aedes vectors has resulted in the chronic endemicity of DENV and the resurgence of CHIKV and now ZIKV. This is due to varying levels of resources, community efforts, and political will throughout the region. Nevertheless, countries with well-regarded vector control programs like Singapore have still found it hard to overcome the many biological, social, and environmental factors that enable emergence of arbovirus outbreaks. Traditional methods, which mainly target larval breeding sites and fogging where there are human cases, may no longer be sustainably effective. New paradigms of vector control are advocated, either with new technologies (e.g., genetically modified mosquitoes and Wolbachia species) or shifts in existing practice (e.g., specific surveillance and targeting of virus-infected mosquitoes) (Vythilingam et al., 2016). Carefully designed trials will then be needed to also demonstrate effects on human cases and not just mosquito indices.