Chikungunya Virus and Zika Virus Transmission Cycles
Stephen Higgs; Dana L. Vanlandingham Kansas State University, Manhattan, KS, United States
Abstract
With respect to epidemics of CHIKV and ZIKV in humans, clearly the most, if not the only, significant mosquitoes are the anthropophagic and peridomestic species Aedes aegypti and Ae. albopictus. Although Ae. albopictus is a competent vector for ZIKV, its involvement in human transmission cycles of the virus has been very limited. This is not the case with CHIKV. Largely because of the effects of a viral mutation, Ae. albopictus played a highly significant role as a vector on Indian Ocean Islands, following the emergence of CHIKV from East Africa, in parts of Asia, and in isolated European outbreaks (de Lamballerie et al., 2008). The more anthropophilic species, Ae. aegypti, implicated in all previous epidemics, has been the predominant vector, for example, in the Americas. Virus isolates have been obtained from, and specific antibodies detected in, a number of different vertebrate species; however, the importance of these as maintenance hosts outside of Africa is unknown. Nonhuman primates are likely the only significant hosts other than people. Domestic animals seem to play no significant role in viral amplification. Despite apparent suitable ecological conditions, the lack of significant zoonotic cycles in Asia is a mystery that demands further investigations.
Keywords
Chikungunya; Zika; Virus; Mosquitoes; Aedes aegypti; Aedes albopictus; Vertebrate hosts
Introduction
Although chikungunya virus (CHIKV) is in the family Togaviridae, genus Alphavirus, and Zika virus (ZIKV) is in the family Flaviviridae, genus Flavivirus, these viruses share a number of important common characteristics. Both viruses were isolated in Africa over 65 years ago with ZIKV identified in 1947 in Uganda and CHIKV identified during the 1952 epidemic in Tanzania (Dick, 1952; Robinson, 1955; Mason and Haddow, 1957). Since their first isolation, these viruses have caused sporadic outbreaks in limited geographic areas until recently (see Diallo et al., Chapter 4; Sam, Chapter 5). Historically, CHIKV has caused more human cases than have been caused by ZIKV, with major epidemics occurring in Asia and India from the 1950s to 1980s. The apparent periodicity of CHIKV human epidemics at approximately 40-year intervals is not fully understood. These may be preceded by unrecognized large-scale epizootics in nonhuman primates; however, field investigations need to be performed to test this hypothesis (Halstead, 2017). In 2004, CHIKV spread out of Africa and into islands in the Indian Ocean, commencing a significant global expansion into new geographic regions, where it has caused numerous outbreaks (Chastel, 2005; Consigny et al., 2006; Enserink, 2006; Higgs, 2006; Ligon, 2006; Paganin et al., 2006; Pialoux et al., 2007; de Lamballerie et al., 2008; Gerardin et al., 2008). In contrast to CHIKV, ZIKV had caused very limited human clinical cases until the recent outbreaks (Macnamara, 1954; Simpson, 1964; Moore et al., 1975; Fagbami, 1979). However, in 2007, ZIKV was found for the first time outside Africa and Asia after its spread from Asia into islands in Micronesia (Duffy et al., 2009; Hayes, 2009). There were further outbreaks of both CHIKV and ZIKV in the Pacific, before the viruses spread into the New World in 2013 and 2016, respectively, as detailed in Chapter 7.
In addition to similar patterns of geographic spread and associated outbreaks, another characteristic that is shared between CHIKV and ZIKV is a transmission cycle that primarily involves container-breeding Aedes species mosquitoes and either nonhuman primates or humans. This chapter will focus on the mosquitoes and where appropriate, different species of vertebrates, involved maintaining the virus in various geographic areas, by examining both field investigations and laboratory studies of CHIKV and ZIKV transmission.
Transmission of Arboviruses Between Vectors and Vertebrates
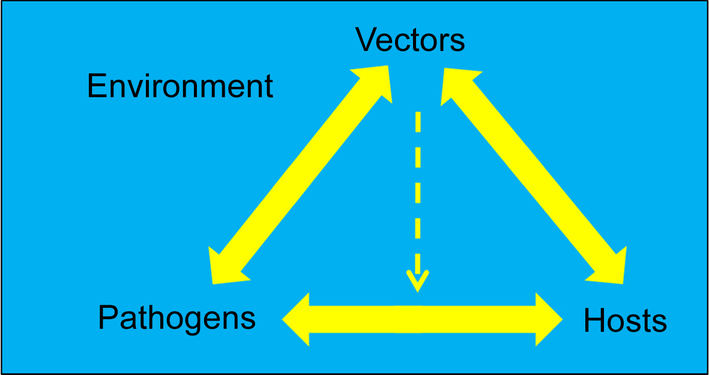
In this section, we describe virus, vector, and vertebrate host interactions and research conducted to improve our understanding of these, with specific examples using CHIKV or ZIKV. A driving force of the transmission cycle is the fact that certain arthropods are hematophagous, i.e., they feed on blood. An autogenous female mosquito requires blood meals to obtain proteins needed to produce eggs. A female mosquito becomes infected with an arbovirus while feeding on a viremic vertebrate host, with successful infection dependent on the titer of virus in the blood of the vertebrate host and the susceptibility to infection of the mosquito species biting the host (Higgs, 2004).
The transmission of mosquito-borne arboviruses requires several biological criteria to be satisfied. With few exceptions, a vertebrate host is needed for the female mosquito to feed upon in order to provide a blood meal for egg production. To support a successful transmission cycle, the susceptible vertebrate host must produce a viremia with high enough viral titer in the blood of suitable duration for mosquitoes to feed on them and become infected (Anderson et al., 2010). To be regarded as a competent vector for a specific virus, the mosquito must be susceptible to the arbovirus, and live long enough to allow the virus to replicate and be secreted in the saliva—the so-called extrinsic incubation period (EIP)—so that the mosquito is able to transmit the virus to another vertebrate upon which she feeds.
The basic transmission cycle can be thought of as beginning when the uninfected mosquito feeds on an infected viremic vertebrate host. While the mosquito is feeding, the blood enters into the mosquito’s midgut where the blood is digested. In a susceptible mosquito (meaning that the mosquito is able to be infected with that particular virus), the virus will pass through the midgut and enter into the mosquito hemolymph. From the hemolymph, the virus typically replicates in, for example, fat body and nervous tissues but ultimately must infect the mosquito salivary glands, replicate, and be released into saliva, where it is secreted into another host during a subsequent feed. The EIP varies between different virus-vector species relationships, and is influenced by vector and viral factors and importantly by environmental conditions especially temperature (Richards et al., 2007; Anderson et al., 2010). Fig. 1 shows the components of a generalized arbovirus transmission cycle.
Vector Characteristics: Mosquito Species and Intraspecies Variation in Competence as Vectors for Arboviruses
From studies of mosquitoes collected from different geographic locations, it has long been recognized that not only is there variation in susceptibility to infection with a specific arbovirus among species, but also there is variation within a species. For example, as described in the natural transmission cycle “Transmission Cycles of CHIKV” section, a number of species are susceptible to infection by CHIKV. However, some species are refractory to infection, such as Anopheles gambiae, which cannot be infected with CHIKV, but is susceptible to infection with the closely related O’nyong nyong virus (Vanlandingham et al., 2006).
Variation in vector competence is well documented but not fully understood. As part of their studies to better understand the genetic determinants of vector competence of Ae. aegypti for yellow fever virus (YFV), Miller and Mitchell (1991) selected isofemale lines from the Rexville line of Ae. aegypti that differed phenotypically with respect to their susceptibility to infection. Experiments to compare the resultant susceptible Rexville D (RexD) line and resistant Rex7D lines, included challenge with the prototype 1947 Ugandan Rhesus monkey MR 766 isolate of ZIKV used as a third suckling mouse brain passaged stock. Interestingly, the YFV-susceptibility phenotype was retained for ZIKV with a 100% dissemination rate in the RexD line and no dissemination in the 7D line. Based on the data, it was hypothesized that virus dissemination from the midgut is governed by a single major mosquito gene and modifying minor genes or a group of closely linked genes. Although it was felt that this experimental approach with inbred mosquito lines held great promise in discovering the molecular basis for flavivirus resistance in Ae. aegypti, despite significant technological advances since 1991 and sequencing of the Ae. aegypti genome (Nene et al., 2007), we still do not know the genetic determinant of mosquito susceptibility to infection with arboviruses.
As described in the section on natural transmission cycles, the relative importance of different species of arthropods and the roles that they play in the transmission of CHIKV and ZIKV reflect considerable species specificity. Some species, for example, Ae. aegypti, play a major role in transmitting the virus to humans, while others like Culex quinquefasciatus do not (Van den Hurk et al., 2017). Furthermore, there exists intraspecific variation with, for example, Ae. aegypti from one location being highly susceptible, but those from another being relatively resistant. There are well-documented, if not well-understood, genetic variations in vector competence of both Ae. aegypti and Ae. albopictus for different CHIKV isolates (Tesh et al., 1976; Mourya et al., 1987). As described later and in Chapter 8, the importance of Ae. albopictus as a vector of CHIKV has been largely attributed to viral mutation that has increased both infectivity and dissemination of CHIKV in Ae. albopictus but has not had the same effect in Ae. aegypti. This increased capacity of the virus to infect and be transmitted by Ae. albopictus has been exacerbated by the continuing spread of this invasive mosquito into new areas. Other contributing factors that make Ae. albopictus a species of significance are its susceptibility to infection by multiple viruses (Vanlandingham et al., 2016), its willingness to feed on a range of vertebrate species and peridomestic habitats (Richards et al., 2006). These aspects of the vector-virus relationship are described in more detail later. A dedicated issue of the Journal of Medical Entomology (Reisen, 2016) provides an excellent resource describing the history of Ae. albopictus spread in the United States.
Vector Characteristics: Host Preference
Host preference of a susceptible mosquito is important, since it is an initial determinant of whether or not a mosquito will feed on a viremic host and be exposed to a virus. Some species of mosquito are quite specific on the species of vertebrates on which they feed. For example, Ae. aegypti is regarded as strongly anthropophilic, feeding preferentially on humans, and often taking multiple meals on different individuals in a relatively short period of time. Although the Asian tiger mosquito Ae. albopictus is regarded as having similar anthropophagic feeding tendencies, a study by Delatte et al. (2010) of Ae. albopictus collected on La Reunion revealed that although the species had a significant preference for feeding on people, it was opportunistic and, depending on host availability, would feed on a wide range of vertebrate species. As described later, many researchers have studied these basic processes regarding the transmission cycles of specific viruses, and many general overviews have been published (Higgs, 2004).
Vertebrate Characteristics: Host Factors Involved in the Transmission Cycle
There are many factors that can inhibit the transmission cycle such as a vertebrate host that only produces a low-titer viremia, a mosquito which is not susceptible to be infected with the particular virus, or a virus that is unable to disseminate to other tissues within the mosquito, specifically the salivary glands, in order for the mosquito to transmit the virus to another vertebrate host. To perpetuate a transmission cycle, the vertebrate host must not only be susceptible to infection, but must also produce a viremia (virus in the blood) of sufficient magnitude and duration to infect a mosquito that feeds upon it. There is a threshold quantity of virus in the blood (expressed as titer) that must be met in order for a susceptible mosquito to become infected. If the threshold of virus in the blood is not met, then the vertebrate is regarded as a “dead end” host, because mosquitoes that feed upon it do not become infected. Even though these hosts do not contribute to the maintenance of the transmission cycle, they may still develop potentially fatal disease symptoms, as is the case for humans and horses infected by West Nile virus (WNV). Duration of viremia above the threshold also influences transmission dynamics. With sustained high-titer viremias, the likelihood that an animal will be fed upon by a susceptible mosquito is increased. Depending on conditions, a vertebrate that is viremic for 5 days will probably infect more mosquitoes that one with a viremia that only lasts for 1 day.
Early studies providing viremia data designed to determine the susceptibility of two nonhuman primates for CHIKV involved the experimental infection of vervet monkeys (Cercopithecus aethiops) and baboons (Papio ursinus) (Paterson and McIntosh, 1964; Jupp et al., 1981). Following CHIKV infection, viremias of up to 8 log10 plaque forming units (PFU)/mL were detected. This titer is more than sufficient to infect a high proportion of susceptible mosquitoes that subsequently fed upon them, which indicates that these nonhuman primates could be involved in the CHIKV transmission cycle. In another experiment, Rhesus macaques were experimentally infected by exposure to infected Ae. apicoarenteus and proceeded to developed viremias of up to 4.5 log10 lethal dose 50% (LD50) in 2 days postfeeding (Sempala and Kirya, 1973). Both Ae. apicoargenteus and Ae. africanus mosquitoes were successfully infected by feeding on viremic rhesus monkeys. The Ae. apicoargenteus mosquitoes remained infected for the 14-day duration of this experiment and were able to transmit CHIKV at 2 days postinfection (dpi). These investigations, and many other similar experiments, indicate that nonhuman primates are involved in the CHIKV transmission cycle because they produce a high enough viremia, following virus challenge, to infect mosquitoes that feed on them. Laboratory experiments are discussed later, summarized in Table 5, and nonhuman primate studies are discussed more thoroughly by Morrison in Chapter 10.
A detailed study on ZIKV infection dynamics in different tissues and shedding into different body fluids demonstrated infection in both rhesus macaques (Macaca mulatta) and cynomolgus (Macaca fascicularis) monkeys, with viral RNA being detectable in the blood as early as 1 dpi and peaking at 2–3 dpi (Osuna et al., 2016).
Sempala and Kirya (1973) collected adult Ae. apicoargenteus mosquitoes in the Zika forest and allowed their progeny to feed on mice that had been inoculated with CHIKV strain E103, 2 days earlier, or on a rhesus macaque monkey that had been infected by subcutaneous inoculation. As controls, Ae. africanus females were also allowed to feed on the infected animals. Both species became infected and were capable of transmission to naïve rhesus macaques. Infection in the mosquito was sustained for at least 14 days, when the experiment was terminated.
In addition to the involvement of nonhuman primates in the CHIKV and ZIKV transmission cycles, humans are commonly involved as the primary vertebrate host in the urban transmission of both of these viruses if a susceptible mosquito feeds on a viremic person with a suitably high virus titer (this chapter). Bearcroft (1956) collected blood from an experimentally infected human volunteer with ZIKV and observed that when serum was collected four days postinfection and inoculated into mice, some of the mice died. This suggested that virus was present in the volunteer at 4 dpi. In a comprehensive clinical study of chikungunya fever patients in La Reunion, Thiberville et al. (2013) described an acute viremic phase at 1–4 days following the infection, with average peak viral loads of 1.2 × 109 RNA copies/mL, a titer that would be sufficient to infect many susceptible mosquito species. For more information on human viremia and the disease symptoms associated with human infections see Chapter 3.
Viral Characteristics: Infectivity for Mosquitoes
An obvious but important factor that contributes to the success of the transmission cycle is the ability of the virus to infect the mosquito that feeds on the infected vertebrate. This may appear to be synonymous with species specificity and the concept that the mosquito must be susceptible to infection. The consequences of incompatibility between a particular virus-mosquito species combination are the same regardless of the underlying mechanism. It is, however, important to distinguish between viral infectivity and vector susceptibility, and realize that both viral and vector genetics can play a role in determining the success or failure of the infection process.
Although the mechanisms underlying virus and vector specificity have not been determined, there are multiple laboratory studies that have attempted to define them. Early research examining the virus proteins responsible for vector specificity focused on La Crosse virus in Ae. triseriatus (Sundin et al., 1987). More recent experiments that focused on viral genetics and the encoded viral proteins of CHIKV and a closely related virus, o’nyong nyong, were conducted to examine virus/vector specificity in Ae. aegypti mosquitoes and Anopheles gambiae (Vanlandingham et al., 2006). Of particular importance are the studies demonstrating that a mutation within the CHIKV structural envelope E1 gene that substituted the amino acid alanine with a valine at position 226 resulted in a highly significant enhancement of infectivity for the hitherto relatively unimportant mosquito, Ae. albopictus. No change in infectivity for Ae. aegypti resulted from this mutation. This is discussed further later and more extensively in Chapter 8. Working with Brazilian, Dominican Republic, and United States Ae. aegypti, Roundy et al. (2017) reported variation in the infectivity of ZIKV strains from Senegal, Cambodia, and Mexico. Only the Senegalese DAK AR41525 strain was transmitted by all three mosquito populations; however, the underlying cause of the differences between the viruses was not elucidated. Liu et al. (2017) determined that a recent clinical isolate, GZ01 from the Americas, was significantly more infectious for Ae. aegypti that the Cambodian FSS13025 strain that was isolated in 2010. The mechanism for this enhanced infectivity is a spontaneous alanine to valine amino acid mutation in the NS1 gene at position 188, in the GZ01 strain, which results in secretion of NS1 protein into the host’s blood. This effect of elevated NS1 antigenaemia on virus acquisition of ZIKV by mosquitoes feeding on a viremic host was previously reported for dengue and Japanese encephalitis viruses (Liu et al., 2016). This enhancing mutation is described more fully by Huang (Chapter 8).
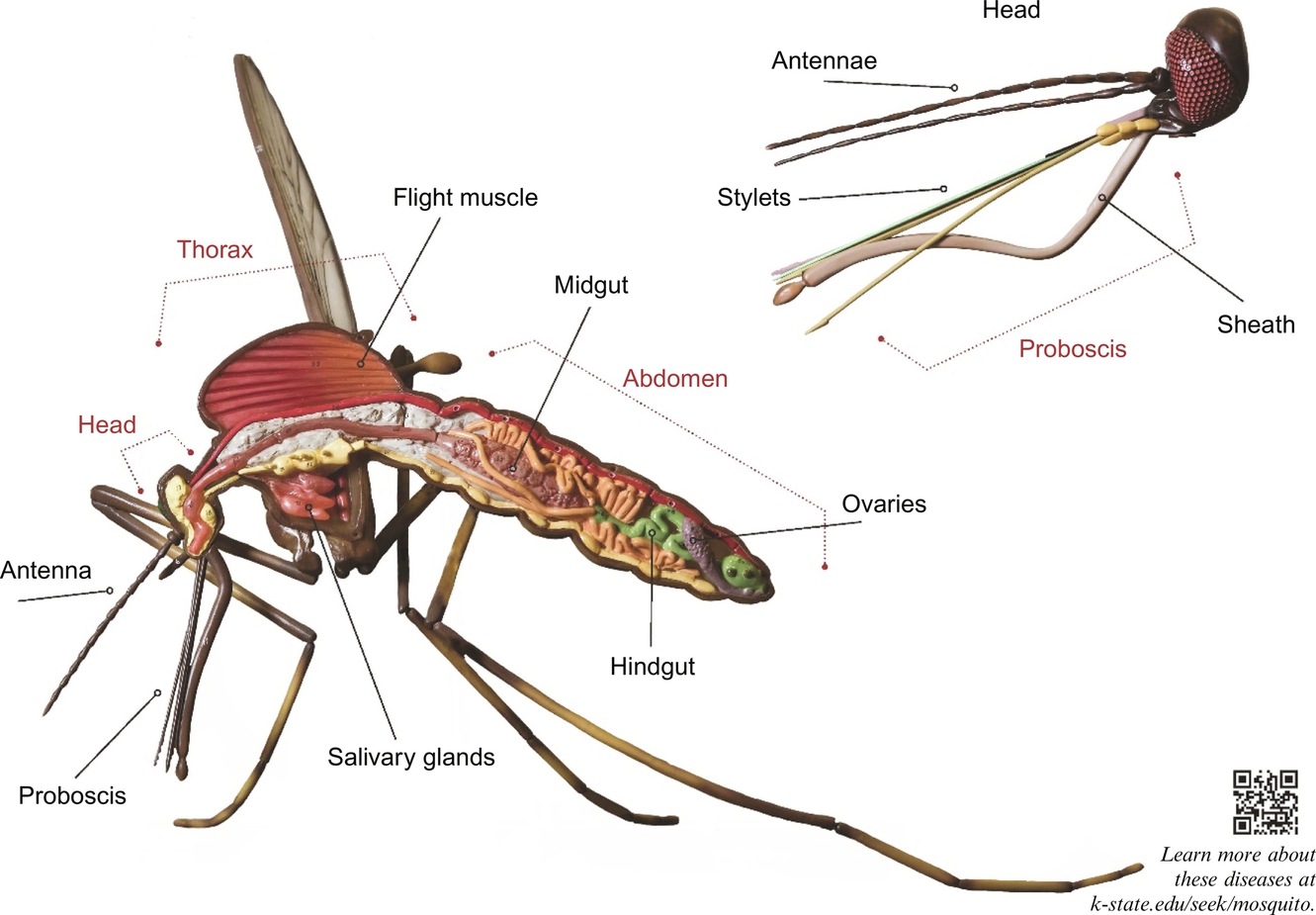
Mosquito Anatomy and Virus Infection
To fulfill the role as a competent vector, after exposure to a virus by feeding on a viremic host, a mosquito must ultimately become infectious, meaning it is able to infect another host on which it feeds. This requires sequential infection of specific tissues to amplify and disseminate the virus. The primary anatomical structures and tissues involved in virus transmission are illustrated in cross section in Fig. 2.
Virus Infection of the Mosquito Midgut
Soon after blood is imbibed, a peritrophic matrix is secreted around the meal. Although there has been discussion on whether or not this may be a midgut infection barrier, experimentally this has been shown not to be the case (Kato et al., 2008). There has also been discussion on whether or not different types of cell in the midgut vary with respect to their susceptibility to infection. Epithelial cells are the predominant cell type found in the mosquito midgut, although other cell types, with varying functions, are present (Lehane and Billingsley, 1996). The exact mechanisms of mosquito midgut epithelial cell infection are still not fully understood. For enveloped viruses such as CHIKV and ZIKV, it is assumed that the virus attaches to a receptor; however, the identity of the receptor remains unknown.
Nuckols et al. (2013) compared early midgut infection of CHIKV in Ae. albopictus mosquitoes infected orally using artificial blood meals with mosquitoes infected by enema. Using this bidirectional exposure to a genetically engineered infectious clone of CHIKV, expressing green fluorescent protein (GFP), in the midgut enabled spatial and temporal characterization of CHIKV midgut infection. At 3 dpi, the enema-delivered CHIKV was associated with a more anterior distribution of CHIKV-infected cells as compared to the CHIKV infection delivered as a blood meal. Virus was retained in the midgut cells in this region at seven and 14 days; however, there was no evidence to suggest involvement of a particular cell type within the midgut that was more susceptible to virus infection.
To further investigate whether or not all midgut cells were equally susceptible to CHIKV infection or if only a subset of specific cell types are involved, sophisticated experiments using genetically modified CHIKV, expressing two different fluorescent proteins, were performed on Ae. aegypti and Ae. albopictus mosquitoes (Nuckols, 2012). Equivalent titers of two virus types, one expressing GFP and a second expressing red fluorescent protein, were presented in artificial blood meals to the mosquitoes. Midguts were removed at early time points (0, 3, 6, 9, 12, 18, and 24 h postinfection (p.i.)) and at later intervals, examined for fluorescent signal and also titrated to determine viral titers. The rationale for this investigation was that colocalization of green and red signals in individual cells at a statistically higher rate than expected, based on number of cells infected, would be indicative that some cells were more susceptible than others. Results found that the signals from the two genetically altered CHIK viruses (i.e., red or green) were not significantly colocalized, supporting the conclusion that different midgut cell types do not differ with respect to their susceptibility to CHIKV infection. This is in contrast for two other alphaviruses, VEEV and EEEV, for which preferential infection of cells in the posterior midgut was reported (Weaver et al., 1988; Smith et al., 2008).
Dissemination of Virus from the Mosquito Midgut
Following infection of midgut epithelial cells and replication of the virus in the cytoplasm of these cells, the next step in a successful mosquito infection is dissemination of the virus to secondary amplification tissues and then ultimately into the salivary glands. How the virus exits the midgut epithelial cells through the basement membrane is not fully understood. For some viruses, it has been proposed that the tracheal system may provide a conduit to the hemocoel. For CHIKV, dissemination is not threshold-titer dependent and can occur within 1–2 dpi (Dong et al., 2016). Although tracheal cell infection has been observed, this is not believed to be the major route of dissemination from the midgut (Dong et al., 2016). The exact mechanism of virus dissemination from the midgut via the basement membrane to tissues within the hemocoel is still not fully understood. Secondary amplification tissues, commonly infected by arboviruses, include the fat bodies and neural tissues (Higgs, 2004). For most mosquito-borne viruses, the EIP, which is dependent on vector, virus, and environmental factors, is typically 3–10 days after taking the initial blood meal. This EIP can even occur earlier than 3 dpi depending on the virus and vector combination. For instance, Dubrulle et al. (2009) detected CHIKV in the saliva of Ae. aegypti within 2 dpi (Alto et al., 2017). Detailed experiments using a modified CHIKV infection clone (Tsetsarkin et al., 2006) expressing the Renilla luciferase reporter gene (Ziegler et al., 2011) found that on day 3 p.i., 37% of orally infected Ae. albopictus mosquitoes had a disseminated infection as compared to none of the day three postinfection Ae. aegypti mosquitoes (Ziegler et al., 2011). Interestingly, at 7 dpi, 74% of the Ae. aegypti had a disseminated infection and only 20% of the Ae. albopictus mosquitoes had a disseminated infection (Ziegler et al., 2011). Arias-Goeta et al. (2013) reported similar results in dissemination rates of different CHIKV variants at the so-called midgut barrier level.
Salivary Glands and Virus Transmission
As described in more detail (“Transmission Cycles of CHIKV in Africa” section), early laboratory infection and transmission experiments with CHIKV were performed by feeding laboratory-reared mosquitoes on febrile patients (Ross, 1956a). For ZIKV, transmission experiments involved feeding Ae. aegypti mosquitoes on either mice or a rhesus monkey (Boorman and Porterfield, 1956). Criterion used by these researchers to indicate successful transmission was either death of the animals or seroconversion of these animals following exposure of the virus by mosquitoes feeding. A relatively recent laboratory experiment involving CHIKV and Aedes mosquito vectors was conducted by Dubrulle et al. (2009). A CHIKV, with the E1-A226V mutation found in the Indian Ocean Linage (IOL) (see Chapter 8 for further details), was orally presented to Ae. aegypti and Ae. albopictus mosquitoes. The mosquito saliva was analyzed by quantitative RT-PCR and by plaque assay. Infectious CHIKV and CHIKV RNA were identified in both Ae. aegypti and Ae. albopictus mosquito saliva at 2 dpi.
Impact of Saliva on Virus Transmission
During the process of feeding, the female mosquito uses her proboscis to penetrate the skin and to probe for a blood vessel. Mosquito saliva is a pharmacologic cocktail of secreted molecules, principally composed of proteins that can affect vascular constriction, blood coagulation, platelet aggregation, inflammation, immunity, and angiogenesis. These proteins enable rapid blood acquisition, and importantly these antiinflammatory and other immunomodulatory activities can influence the efficiency and severity of arboviral infection.
Building on multiple transmission studies with mosquito-borne viruses, and also following leads from investigations on tick vectors and tick-borne pathogens (Wikel, 2017), researchers have conducted experiments examining mosquito saliva and its relationship to virus transmission (Higgs and Vanlandingham, 2016; Higgs et al., 2017). Of considerable significance is the demonstration that mosquito saliva has been shown to cause more severe disease in the vertebrate host when inoculated with an arbovirus as compared to simply inoculating the virus into the host without the saliva (Osorio et al., 1996; Edwards et al., 1998; Limesand et al., 2000, 2003; Schneider et al., 2004; Schneider et al., 2006; Styer et al., 2006; Schneider et al., 2007; Styer et al., 2011; Pingen et al., 2016; Schmid et al., 2016; Higgs et al., 2017). An experiment comparing the immune response of mice to a virus inoculated with Ae. aegypti salivary gland extract (SGE) mixed with the Alphavirus, Sindbis (SINV), and the SINV alone found that these routes of exposure are significantly different, indicating that saliva does alter the immune response to an arbovirus (Schneider et al., 2004). Other similar experiments have been conducted using Ae. aegypti saliva and the Flavivirus, WNV (Schneider et al., 2010). These experiments demonstrated that mosquito saliva enhances transmission of arboviruses and can lead to more severe disease in the vertebrate host.
With respect to CHIKV, Thangamani et al. (2010) conducted a similar experiment comparing the natural route of CHIKV transmission using infected Ae. aegypti to feed on mice, or infection of mice by inoculation of CHIKV using needle injection. These studies provided the first analysis of cutaneous cytokines at the early stage of mosquito-transmitted CHIKV in experimental mice and demonstrated the significant impact of saliva on virus establishment. The results of this research indicate that the different routes of virus exposure lead to different immune outcomes (Thangamani et al., 2010). In addition to understanding how salivary proteins affect the vertebrate host, there are many researchers that have conducted studies on the proteins themselves in order to identify the types of proteins found in saliva.
Consequences of Virus Infection on Mosquitoes
Once a mosquito is infected it is infected for life with few, if any, deleterious effects. This enables the mosquito to transmit the virus for the rest of its life, which can be a relatively long period of time. A ZIKV transmission study found that laboratory-maintained mosquitoes remain infected and capable of transmission for 72 days (Boorman and Porterfield, 1956). A study using another Flavivirus, WNV, found that the mosquito will transmit virus up to 21 dpi and that the amount of virus transmitted by individual mosquitoes on this day varies greatly from 3 to 198,866 pfu with mean titers ranging from 5846 to 30,532 pfu/sample (Vanlandingham et al., 2004). A recent study (Grubaugh et al., 2017) reported how the predominant WNV populations that are transmitted during different feeding events of an individual mosquito can be genetically diverse (Grubaugh et al., 2016). This reflects genetic drift during replication of the virus in the mosquito.
Transmission Cycles of CHIKV
There have been numerous studies of virus and vector interactions examining various mosquitoes and CHIKV throughout its geographical range. These studies have led to the discovery of which mosquitoes are susceptible and also which mosquitoes are the primary vectors of these viruses.
Transmission Cycles of CHIKV in Africa
Early outbreaks that were attributed to CHIKV in Africa occurred throughout the continent starting in 1952 in Tanganyika (merged with Zanzibar in 1964, and subsequently renamed the United Republic of Tanzania). As is befitting when an epidemic with unknown etiology occurs, investigators took an objective and open-minded approach so that during the 1952–53 epidemic in the southern province of Tanganyika territory, multiple types and species of blood-feeding insects and ticks were collected, especially from native huts in the area (Lumsden, 1955). Lumsden (1955) commented that disease distribution in huts was consistent with arthropod populations usually isolated in one hut or a group of huts, rather than correlated with insects that were breeding outside of huts a considerable distance away from the huts. With this in mind, the authors concluded that Ae. aegypti was the most likely main vector.
Various collection techniques were employed during the investigation, including knock-down catches using pyrethrum-in-kerosene sprays, 24-h human-baited catches in huts, indoor and outdoor human and chicken catches, mosquito larvae collections, castor oil paper catches, hut floor collections, and collections from wildlife. Insect groups collected included Reduvidae (Lisarda rhodesiensis), Cimidae (Cimex hemipterus), Psychodidae (Phlebotomus sp. and Sergentomyia sp.), Culicidae (Anopheles funestus, An. gambiae, Toxorhynchites sp., Taeniorhynchus africanus, T. uniformis, Ae. aegypti, Ae. simpsoni, Ae. albothorax, Eretmapodites subsimplicipes, E. quinquevittatus, and Culex fatigans, which is now classified as Cx. quinquefasciatus), Tabanidae (Haematopota fasciatapex, Tabanus unilineatus, T. taeniola, Atylotus fuscipes), Muscidae (Stomoxys sitiens, Glossina pallidipes), and Ixodidae (Rhipicephalus neavei, Haemaphysalis leachi). Lumsden’s research produced considerable entomological data and reports that “virus was isolated from all the mosquito groupings tested (Anopheles spp., Ae. aegypti, and Cx. fatigans), and possibly also from bed bugs (Cimex hemipteran)” but refers the reader to a manuscript in preparation by Ross. This paper (Ross, 1956a) details the inoculation of homogenates of collected mosquitoes and bed bugs into suckling mice and also two trials feeding laboratory-reared Ae. aegypti mosquitoes on febrile patients in Newala. In the first trials using laboratory-reared Ae. aegypti mosquitoes that were fed on febrile patients (Ross, 1956a), five of 28 fed mosquitoes contained a lethal agent that was repeatedly passaged, homogenates of 12 mosquitoes killed inoculated mice, and one was referred to as rapidly toxic. In the second trial, two of nine mosquitoes contained a passageable agent, five others probably contained an agent, and the remaining two failed to kill inoculated mice. Ross reported “numerous,” an undefined number, transmission experiments by feeding these mosquitoes on mice and a human volunteer without observable effects.
In experiments with field-collected arthropods, pools were produced using all the individuals in a specific genus that were collected in a single day (three Ae. aegypti pools, three Culex pools, one Anopheles pool). These pools of mosquitoes were ground in saline and the homogenate was inoculated into mice and intraperitoneally into one monkey. Overall, 59% of 83 mice inoculated with wild-caught mosquito homogenates died and two of three Ae. aegypti pools and one of three Culex pools produced virus. One Ae. aegypti and one Culex pool were regarded as toxic since inoculated mice died within 24 h. Virus was not isolated from Anopheles. Interestingly, five groups of mice died when inoculated with bed bugs collected from the beds of patients. Some baby mice fed upon by collected bedbugs died but no virus could be isolated from them. Overall 8.5% of mice inoculated with bed bugs died. Repeated mouse brain-to-brain passage confirmed infection of three bedbugs.
Interestingly, it was noted that “the population of Ae. aegypti on the plateau contained a considerable proportion of the pale form: in cases of this form all of the normally black mesonotal scales were pale golden yellow, but intergradations between the normal condition and the fully pale form were commonly met with.” It is not clear if this form was included in the field isolations and experiments, and whether or not it was used in the experimental infections with laboratory-reared mosquitoes. A study by McIntosh et al. (1963) described experimental cyclical transmission of CHIKV by Ae. aegypti formosus between vervet monkeys (Cercopithecus aethiops pygerythrus), thus confirming this subspecies’ ability to serve as a vector.
An important comment on the pioneering inoculation experiments described is that Ross recognized some of the mosquitoes were transmitting Makonde virus rather than CHIKV. These were distinguished based on the age of mice that could be killed and time to death. Despite this comment, CHIKV was confirmed in both wild caught and laboratory-reared mosquitoes. Further details are provided (“Transmission Cycles of CHIKV” and “Transmission Cycles of ZIKV” sections). Ultimately, Spence and Thomas (1959) concluded that Makonde virus was a previously named virus, Uganda S (Dick and Haddow, 1952), a virus known to be transmitted by Ae. aegypti (Boorman, 1958).
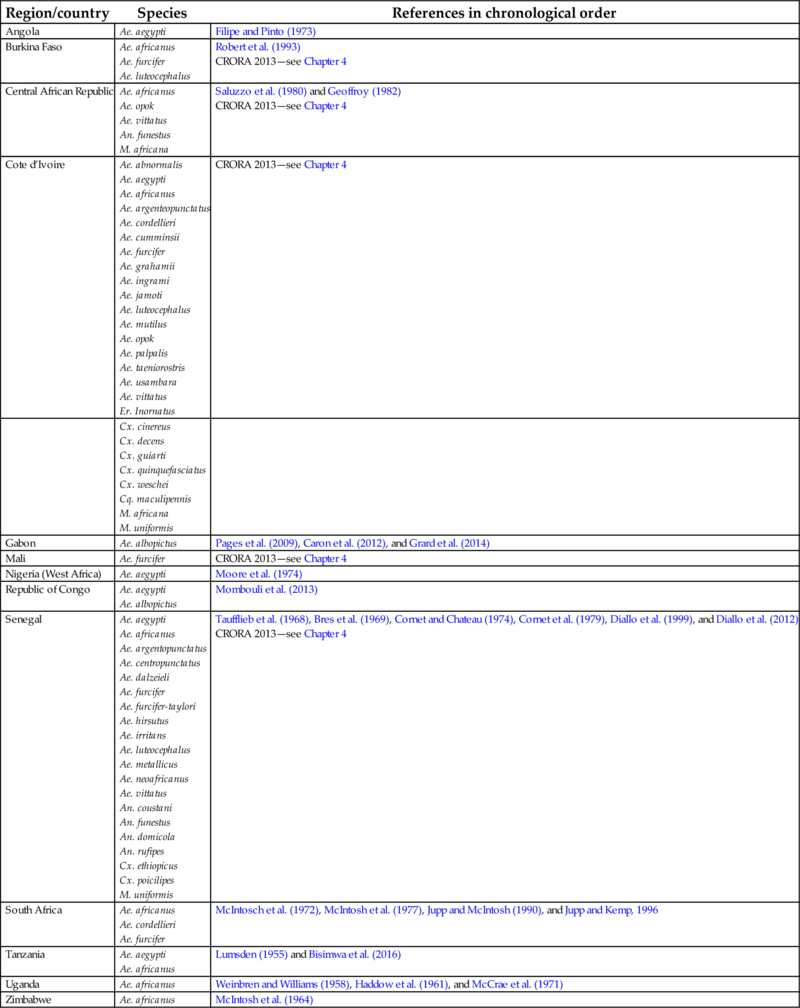
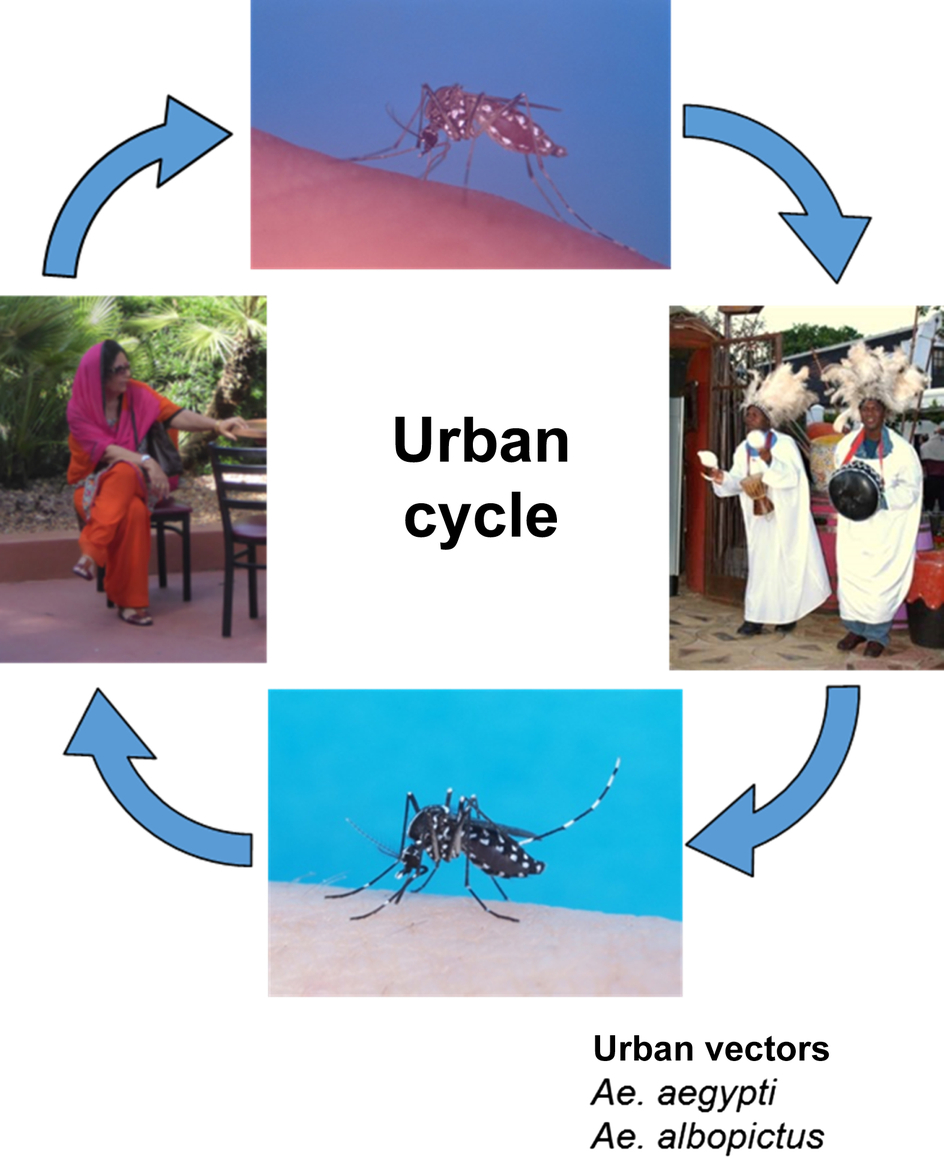
In addition to Ae. aegypti, the other species that was collected from huts in both the lowlands and plateau regions was Cx. fatigans. This was described as being active mainly at night and, based on precipitin tests to distinguish between human, goat, and chicken blood, fed upon humans and fowl. An observation was made that this species seemed to host seek even with considerable remnants of previous undigested meals; thus, virus detected in pools was contained in this blood meal and not in the mosquito. Based on later epidemiological evidence, it was suggested that the species involved may not be important as a vector (Carey et al., 1969) and experiments have suggested that CHIKV may not replicate in, or be transmitted by, this species (Rao, 1964). McIntosh et al. (1963) were unable to experimentally infect Cx. pipiens quinquefasciatus with CHIKV. Table 1 provides a summary of potential African vectors based on field studies (see also Chapter 4). The human-peridomestic mosquito transmission cycle, often referred to as an urban cycle, is depicted in Fig. 3.
Table 1
| Region/country | Species | References in chronological order |
|---|---|---|
| Angola | Ae. aegypti | Filipe and Pinto (1973) |
| Burkina Faso | Ae. africanus Ae. furcifer Ae. luteocephalus | Robert et al. (1993) CRORA 2013—see Chapter 4 |
| Central African Republic | Ae. africanus Ae. opok Ae. vittatus An. funestus M. africana | Saluzzo et al. (1980) and Geoffroy (1982) CRORA 2013—see Chapter 4 |
| Cote d’Ivoire | Ae. abnormalis Ae. aegypti Ae. africanus Ae. argenteopunctatus Ae. cordellieri Ae. cumminsii Ae. furcifer Ae. grahamii Ae. ingrami Ae. jamoti Ae. luteocephalus Ae. mutilus Ae. opok Ae. palpalis Ae. taeniorostris Ae. usambara Ae. vittatus Er. Inornatus | CRORA 2013—see Chapter 4 |
| Cx. cinereus Cx. decens Cx. guiarti Cx. quinquefasciatus Cx. weschei Cq. maculipennis M. africana M. uniformis | ||
| Gabon | Ae. albopictus | Pages et al. (2009), Caron et al. (2012), and Grard et al. (2014) |
| Mali | Ae. furcifer | CRORA 2013—see Chapter 4 |
| Nigeria (West Africa) | Ae. aegypti | Moore et al. (1974) |
| Republic of Congo | Ae. aegypti Ae. albopictus | Mombouli et al. (2013) |
| Senegal | Ae. aegypti Ae. africanus Ae. argentopunctatus Ae. centropunctatus Ae. dalzeieli Ae. furcifer Ae. furcifer-taylori Ae. hirsutus Ae. irritans Ae. luteocephalus Ae. metallicus Ae. neoafricanus Ae. vittatus An. coustani An. funestus An. domicola An. rufipes Cx. ethiopicus Cx. poicilipes M. uniformis | Taufflieb et al. (1968), Bres et al. (1969), Cornet and Chateau (1974), Cornet et al. (1979), Diallo et al. (1999), and Diallo et al. (2012) CRORA 2013—see Chapter 4 |
| South Africa | Ae. africanus Ae. cordellieri Ae. furcifer | McIntosch et al. (1972), McIntosh et al. (1977), Jupp and McIntosh (1990), and Jupp and Kemp, 1996 |
| Tanzania | Ae. aegypti Ae. africanus | Lumsden (1955) and Bisimwa et al. (2016) |
| Uganda | Ae. africanus | Weinbren and Williams (1958), Haddow et al. (1961), and McCrae et al. (1971) |
| Zimbabwe | Ae. africanus | McIntosh et al. (1964) |
African Sylvatic Cycle and Bridge Vectors
The three CHIKV lineages that were circulating between 1952 and 2004 were transmitted by mosquito vectors, including Ae. aegypti and Ae. furcifer-taylori in the epidemic cycle (Haddow, 1953; Lumsden, 1955; Robinson, 1955; Ross, 1956a; Mason and Haddow, 1957; McIntosh et al., 1964; Moore et al., 1974); and various species, including Ae. africanus, in the enzootic cycle (Weinbren et al., 1958). A list of mosquito species from which CHIKV has been isolated/identified during field investigations is provided in Table 1 (Africa) and also in Chapter 4.
In sub-Saharan Africa, CHIKV is presumably maintained in a constant enzootic and sylvatic transmission cycle between arboreal mosquitoes and nonhuman primates that McIntosh et al. (1964) referred to as a feral cycle (Fig. 4). Outbreaks occurred when the numbers of arboreal mosquitoes, such as Ae. furcifer, increased during the rainy season and spread into village populations. These so-called bridge vector mosquitoes initially became infected by feeding on infected forest primates and then transmitting the virus to humans once they take another blood meal (Fig. 5). This is an example of how host preferences described earlier in this chapter can influence a transmission cycle, since the mosquitoes that feed on multiple hosts may be more involved in arbovirus transmission cycles involving humans and other vertebrates. In their 1958 paper, Weinbren et al. (1958) describe the isolation of CHIKV from Ae. africanus collected in Uganda. These were pooled by species and triturated in saline that was then inoculated into mice and/or rhesus monkeys. Isolation of CHIKV in this study provided the first evidence of this virus in Uganda.

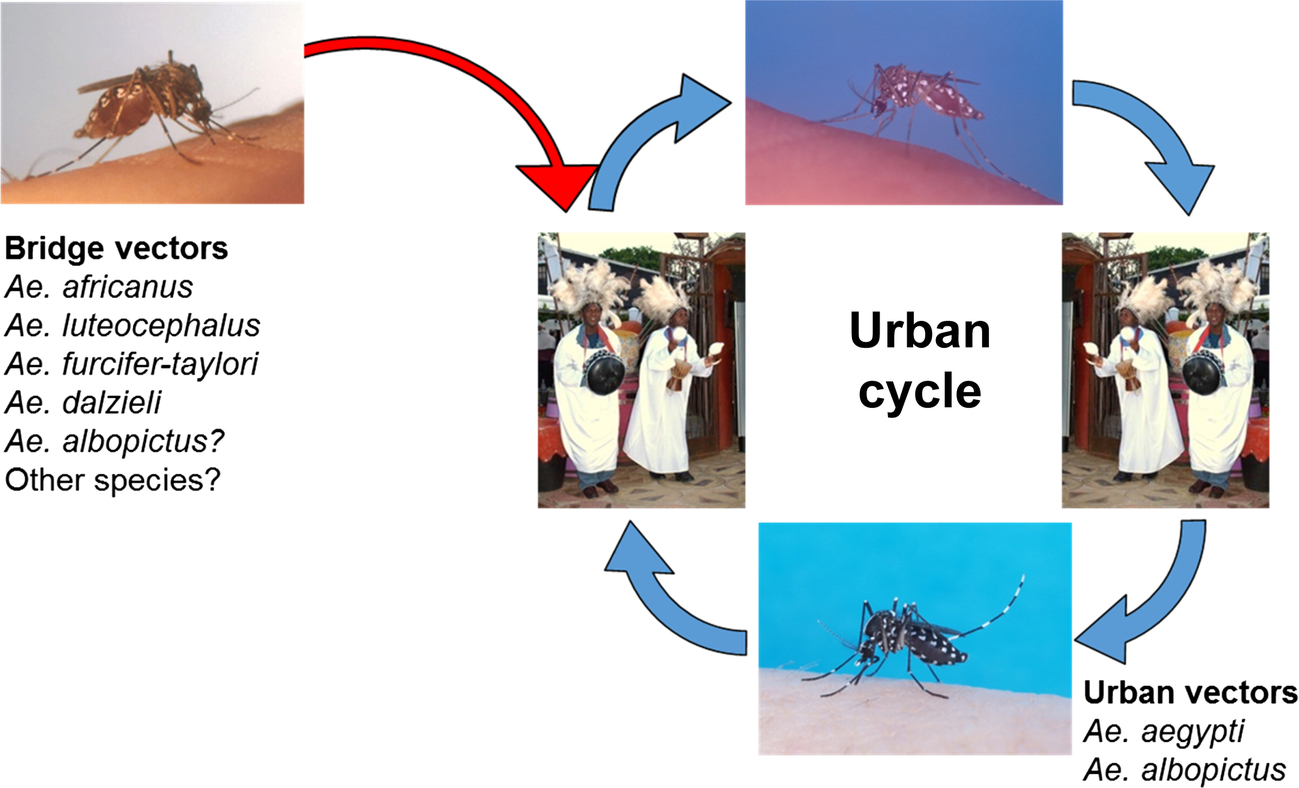
For a more complete list of potential CHIKV vectors in Africa, see Table 1 in this chapter.
With regards to bridge vectors, Chandler et al. (1975), working in the Kano Plain in Kenya, collected mosquitoes and performed 12,168 precipitin tests to determine the host from which blood meals had been taken. Seven species, An. gambiae s.l., Ae. funestus, Ae. pharoensis, Mansonia uniformis, M. africana, Cx. antennatus, and Cx. univittatus, were found to enter houses and feed on the human occupants. Eight species Ae. pharoensis, Ae. ziemanni, M. uniformis, M. africana, Cx. antennatus, Cx. univittatus, Ae. circumluteolus, and Ae. ochraceus, fed on humans and also domestic animals, and as such, this host-switching behavior was regarded as being of potential importance for CHIK arbovirus transmission. Based on the analyses, it was concluded that “more likely to be involved in arbovirus transmission and in this group the following species must be considered: Ae. pharoensis, Ae. ziemanni, M. uniformis, M. africana, Cx, antennatus, Cx. univittatus and Ae. circumluteolus.”
As mentioned elsewhere, because of the range of vertebrate host species on which Ae. albopictus may feed, this species may also play a role as a bridge vector (Delatte et al., 2010). This may become increasingly important in future epidemics of chikungunya, due to the ongoing spread of this highly invasive species, and the presence of the E1 A226V mutant CHIKV in many geographic locations (see Chapter 8).
Africa and Aedes albopictus
The highly invasive, anthropophilic Asian tiger mosquito, Ae. albopictus, was first detected in Africa in 1991 (Cornel and Hunt, 1991) but it was not until the 2004–05 outbreak, which began in east Africa and spread to islands in the Indian Ocean, that the species was identified as being significantly involved in the epidemic spread of CHIKV into the Indian Ocean and beyond (Schuffenecker et al., 2006). Prior to this, CHIKV had previously been isolated from Ae. albopictus collected in China (Chen and Tao, 1996). From 2004 to 2013, the geographic range for CHIKV expanded and in 2013, CHIKV entered the New World where Ae. aegypti and Ae. albopictus are present (Chapter 7).
The addition of Ae. albopictus to the urban CHIKV transmission cycle in 2005 enabled the spread of CHIKV into areas where Ae. albopictus was abundant and widely distributed, including areas where Ae. aegypti was absent or relatively uncommon, such as on the island of Reunion. The predominance of this invasive species coincided with the mutation in one amino acid in the envelope (E1) gene of East-Central-South African (ECSA) genotype of CHIKV, which enabled the virus to infect Ae. albopictus with greater efficiency (Tsetsarkin et al., 2007). This is discussed in more detail later and in Chapter 8. The IOL of CHIKV that emerged during this epidemic was found later in other tropical areas as well as temperate regions in Europe (Powers and Logue, 2007; Rezza et al., 2007; Grandadam et al., 2011). The same mutation in the CHIKV envelope glycoprotein (E1-A226V) is thought to have occurred independently between 2006 and 2008 in three additional outbreaks where Ae. albopictus is present (Vazeille et al., 2016), Interestingly, both Ae. aegypti and Ae. albopictus mosquitoes were vectors in the 2011 outbreak in Congo (Fig. 4) indicating that the CHIKV urban cycle is able to utilize either vector, based on the mosquitoes that are present.
Although the genetic change in the ECSA lineage of CHIKV to the IOL, which harbors the E1-A226V mutation, is best characterized by the events that took place on the island of Reunion, there are indications that this mutation occurred during several independent outbreaks that occurred during approximately the same time period (de Lamballerie et al., 2008).
African Vertebrate Hosts
With respect to vertebrates involved in the transmission cycle, McIntosh et al. (1964) found seropositive vervet monkeys (Cercopithecus aethiops pygerythrus) and baboons (Papio ursinus). Laboratory experiments with cattle, sheep, goats, and horses demonstrated some low-titer hemagglutination-inhibition (HI) antibodies, but no neutralizing antibodies. Moore et al. (1974) reported as a personal communication that virus was isolated from a golden sparrow (Auripasser luteus) captured in Nigeria. Although McIntosh et al. (1964) reported that 17 of 30 sera collected from wild birds were positive, titers were said to be low and details of the species were not provided. A previous study (McIntosh et al., 1963) experimentally attempted to infect five species of birds, cattle, sheep, goats, and horses. None developed viremias, although one goat had a positive HI serum. There are no conclusive data to suggest that birds of any species have any role in the transmission cycles of CHIKV. Other serosurveys in Africa failed to find antibodies in eight species of bats, in cattle, rodents, and birds (Simpson et al., 1968; McCrae et al., 1971), although Bres et al. (1969) apparently isolated virus from a ground squirrel (Xerus erythropus; and a bat (Scotophilus sp.) (Pasteur, 1984). Additional data from laboratory experiments are provided later.
Transmission Cycle of CHIKV in Asia/Pacific Islands
As described in Chapter 5, historically, the primary vector for all CHIKV outbreaks in Asia has been Ae. aegypti. In a somewhat unusual laboratory study, Rao et al. (1968) demonstrated mechanical transmission of CHIKV by Ae. aegypti within 8 h of feeding on viremic mice. Shah et al. (1964) and Rao (1966) suggested that Ae. albopictus was a potential CHIKV vector in India. Experimental studies demonstrated the susceptibility of Indian Ae. albopictus to CHIKV infection (Singh and Pavri, 1967), and indeed based on viral titer to obtain a 50% infection rate, Ae. albopictus was more susceptible than Ae. aegypti. The role of Ae. albopictus as a vector for CHIKV in the region was subsequently reported in Madagascar, India, and Gabon (Thiberville et al., 2013). As described in Chapter 5, the Indian 2008 outbreak involved CHIKV with the E1-A226V mutation (Santhosh et al., 2009). In India, another mutation, E2-L210Q, has been reported (Niyas et al., 2010) that, like the E1A226V, also has increased infectivity for midgut epithelial cells.
Because of the close association between humans and mosquitoes, the main vectors that are associated with transmission of CHIKV in Asia are anthropophilic Aedes species mosquitoes (Fig. 2). These anthropophilic mosquitoes are found near people and are attracted to people for their food source. In contrast to the situation in Africa, CHIKV has not been isolated from arboreal zoophilic mosquitoes. The Ae. aegypti cycle involving humans is referred to as the urban transmission cycle. The urban transmission cycle can result in epidemics that cause an unusually high number of human cases in a specific geographic area or population, which is referred to as the epidemic cycle. If the virus is normally found in a given area or human population, the cycle can be endemic.
Vertebrates and the Transmission Cycle of CHIKV in Asia
In Asia where disease outbreaks with patients displaying symptomatic chikungunya fever can be traced back to the 1700s (Chapter 3), there does not appear to be a sylvatic cycle. As detailed in Chapter 3, investigations of sporadic outbreaks have detected neutralizing antibodies in some wild and domesticated animals and occasionally in a few nonhuman primates; however, the role of these species in maintaining the virus is uncertain and outbreaks seem to be caused by the reintroduction of virus by infected people from affected areas.
Although evidence of CHIKV infection has been reported for Asian primates for example Macaca fascicularis (Halstead and Udomsakdi, 1966; Harrison et al., 1967; Marchette et al., 1978; Inoue et al., 2003; Apandi et al., 2009; Vourc’h et al., 2014; Sam et al., 2015), no naturally occurring sylvatic cycle has been conclusively demonstrated in Asia. Vourc’h et al. (2014) tested 791 sera by PCR and 1051 sera by ELISA from multiple species. Seven ELISA-positive sera were identified: 2/52 from brown lemurs (Eulemur fulvus), 3/186 crab-eating macaques (Macaca fascicularis), and 3/75 black rats (Rattus rattus). Negative sera were from domestic animals including dogs (Canus lupus), cats (Felis catus), horse (Equus ferus), cattle (Bos primigenius), goat (Capra aegagrus), sheep (Ovis aries), pig (Sus scrofa), chicken (Gallus gallus) and from wild vertebrates including shrews (Suncus mutinus), Norway rats (Rattus norvegicus), house mice (Mus musculus), Hamadryas baboon (Papio hamadryas), Southern pig-tailed macaque (Macaca nemestrina), and Campbell’s monkey (Cercopthecus campbelli). Of 17 Panther chameleons (Chamaeleo pardalis) tested, none were positive by PCR. In Pakistan, Darwish et al. (1983) reported CHIKV antibody-positive rodents (Tatera indica, Meriones hurrianae, Rattus rattus, Rattus norvegicus). Halstead and Udomsakdi (1966) reported antibodies in cattle, water buffalo, horses, pigs, dogs, rabbits, and bats, however found cats to be seronegative. Halstead has recently expressed the opinion that seropositivity was most likely due to nonspecific reactive factors (Halstead, 2017).
Working with infected mosquitoes, Paul and Singh (1968) experimentally demonstrated relatively high susceptibility of Indian nonhuman primates (Macaca radiata), a species that is commonly found in urban areas of Southern India. Viremia was sustained for 4 days, with peak titers of 107 mouse LD50/mL of blood, which was sufficient to infect both Ae. aegypti and Ae. albopictus mosquitoes.
In China, CHIKV has been isolated from the fruit bat Rousettus leschenaulti, the significance of these isolations with respect to human epidemics is unknown. Vectors from which CHIKV has been isolated from field collections are show in Table 2.
Table 2
| Region/country | Species | References in chronological order |
|---|---|---|
| Thailand | Ae. aegypti | Halstead et al. (1969) |
| India | Ae. aegypti | Myers, et al. (1965) and Sarkar (1966) |
| Indian Ocean Islands | Ae. aegypti Ae. albopictus Cx. quinquefasciatus | Bessaud et al. (2006), Beesoon et al. (2008), Ratsitorahina et al. (2008), and Sang et al. (2008) |
| Malaysia | Ae. aegypti Ae. albopictus | Kumarasamy et al. (2006) and Noridah et al. (2007) |
| Singapore | Ae. albopictus | Ng et al. (2009) |
Transmission Cycle of CHIKV in the Indian Ocean
As described in Chapter 5, CHIKV was introduced into and was responsible for a major epidemic on islands in the Indian Ocean during 2005, with Ae. albopictus as the predominant mosquito vector. The first observations of Ae. albopictus being a significant vector of CHIKV in a natural outbreak were made during the epidemic that began in 2004 in Kenya, involving Ae. aegypti mosquitoes, which spread to the south eastern islands in the Indian Ocean (Chastel, 2005; Consigny et al., 2006; Enserink, 2006; Higgs, 2006; Ligon, 2006; Paganin et al., 2006). Reunion Island was particularly affected, with ~ 40% population infected by 2007 (Gerardin et al., 2008). The typical primary vector, Ae. aegypti, which had been the vector involved in all previous human epidemics, was virtually absent (Reiter et al., 2006; Schuffenecker et al., 2006) and a study by Delatte et al. (2008) found no Ae. aegypti. Analysis of 240 mosquito pools collected by the Direction Régionale des Affaires Sanitaires et Sociales (DRASS) and processed by the Service de Santé des Armées, Marseille (SSA), found 22 CHIKV-positive pools of Ae. albopictus and, interestingly, two positive pools of Culex quinquefasciatus. Therefore, it was suspected that Ae. albopictus was the main vector of CHIKV on the island (Bessaud et al., 2006).
Schuffenecker et al. (2006) sequenced samples collected from the island of Reunion and the Seychelles during the outbreak from May through December 2005, and identified an amino acid change from alanine to valine in samples collected in late November and early December; this change was only noted from samples obtained from Reunion. Researchers suggested that a mutation of the ECSA lineage of CHIKV of an alanine to valine substitution at position 226 of the E1 protein (referred to as the E1-A226V mutation), could be responsible for a change in infectivity and dissemination of the circulating CHIKV for Ae. albopictus (Enserink, 2006; Reiter et al., 2006; Schuffenecker et al., 2006). Previous development of CHIKV infectious clones used for infection and dissemination experiments in Aedes mosquitoes (Vanlandingham et al., 2005a, 2006) enabled the rapid development of an infectious clone of the virus circulating on the island of Reunion (Tsetsarkin et al., 2006). This infectious clone of a CHIKV isolated from Reunion enabled an experimental approach that proved that the E1-A2206V mutation was a contributing factor in the outbreak on Reunion (Tsetsarkin et al., 2007). This pivotal study indicated that the E1-A226V mutation significantly increased the capacity of the mutant virus to infect Ae. albopictus as compared to Ae. aegypti, which as a consequence enabled Ae. albopictus to be an important vector of CHIKV in this region.
With regards to the two CHIKV-positive pools of Cx. quinquefasciatus reported on Reunion during the outbreak (Bessaud et al., 2006), this species of mosquitoes in this genus has never been reported to play any significant role in previous or subsequent outbreaks. Experimental attempts to infect Cx. pipiens quinquefasciatus collected in Africa failed (McIntosh et al., 1963). Although Cx. pipiens were collected from Saint-Pierre (Vazeille et al., 2007), they were not included in laboratory studies.
Transmission Cycle of CHIKV in Europe
As described in Chapter 6, there have been numerous imported cases in many European countries. The first recorded autochthonous transmission cycle of CHIKV in Europe was due to an infected traveler who arrived from India into Italy in 2007 (Rezza et al., 2007). The ensuing outbreak that involved over 200 cases was entirely due to Ae. albopictus that were first reported in Italy in 1991 (Dalla Pozza and Majori, 1992; Rezza et al., 2007). Subsequent autochthonous cases in other countries were also the result of Ae. albopictus mosquitoes feeding on infectious travelers.
Transmission Cycle of CHIKV in the Americas
When CHIKV was introduced or more correctly reintroduced into the Americas (Halstead, 2015) (and Chapter 3), it has behaved as previously observed in both Africa and Asia. Basically, Ae. aegypti has played the primary role in the transmission cycle with humans as the vertebrate host. To date, there is no evidence that other mosquito species or vertebrates have played any significant role in CHIKV transmission in the Americas. The outbreak in the Americas was due to the Asian genotype, and so since experimentally the E1 A226V mutation does not enhance the infectivity of this genotype for Ae. albopictus, this may explain why transmission in the Americas has involved Ae. aegypti. The first confirmation of Ae. aegypti transmission of the Asian genotype in the Americas was reported in 2015 (Diaz-Gonzalez et al., 2015), while the first report of Ae. aegypti infection with the ESCA genotype that was identified in Brazil in 2015 (Teixeira et al., 2015) was not until 2017 (Costa-da-Silva et al., 2017).
Transmission Cycles of ZIKV
Transmission Cycle of ZIKV in Africa
Zika virus was originally isolated in Uganda in 1947 by Dick et al. (1952) who were conducting surveillance studies for yellow fever virus with caged sentinel primates held on towers with platforms in the forest canopy. The virus caused relatively few human cases until the recent expansion of the virus out of Africa and Asia.
As described in Chapter 4, the first human isolate was in Nigeria in 1954 (Macnamara, 1954). ZIKV is transmitted primarily by Aedes species mosquitoes as evidenced by early isolates of the virus from Ae. aegypti, Ae. albopictus, and Ae. africanus (Dick et al., 1952; Dick, 1953; Weinbren and Williams, 1958; Haddow et al., 1964; Marchette et al., 1969). It was not until the late 1970s that ZIKV was detected in other Aedes species, and other mosquito genera such as Culex, Anopheles, and Mansonia (Lee and Moore, 1972; Cornet et al., 1979; McCrae and Kirya, 1982; Monlun et al., 1993; Akoua-Koffi et al., 2001; Diallo et al., 2014; Ledermann et al., 2014; Guedes et al., 2016; Guo et al., 2016). In Nigeria, two isolates were made from the forest species Aedes luteocephalus (Lee and Moore, 1972), while Kirya et al. (1970) isolated ZIKV from a single pool Ae. apicoargenteus. The African ZIKV transmission cycle is depicted in Fig. 6 and further details of African mosquito vectors are provided in Table 3 and also in Table 3 of Chapter 4.
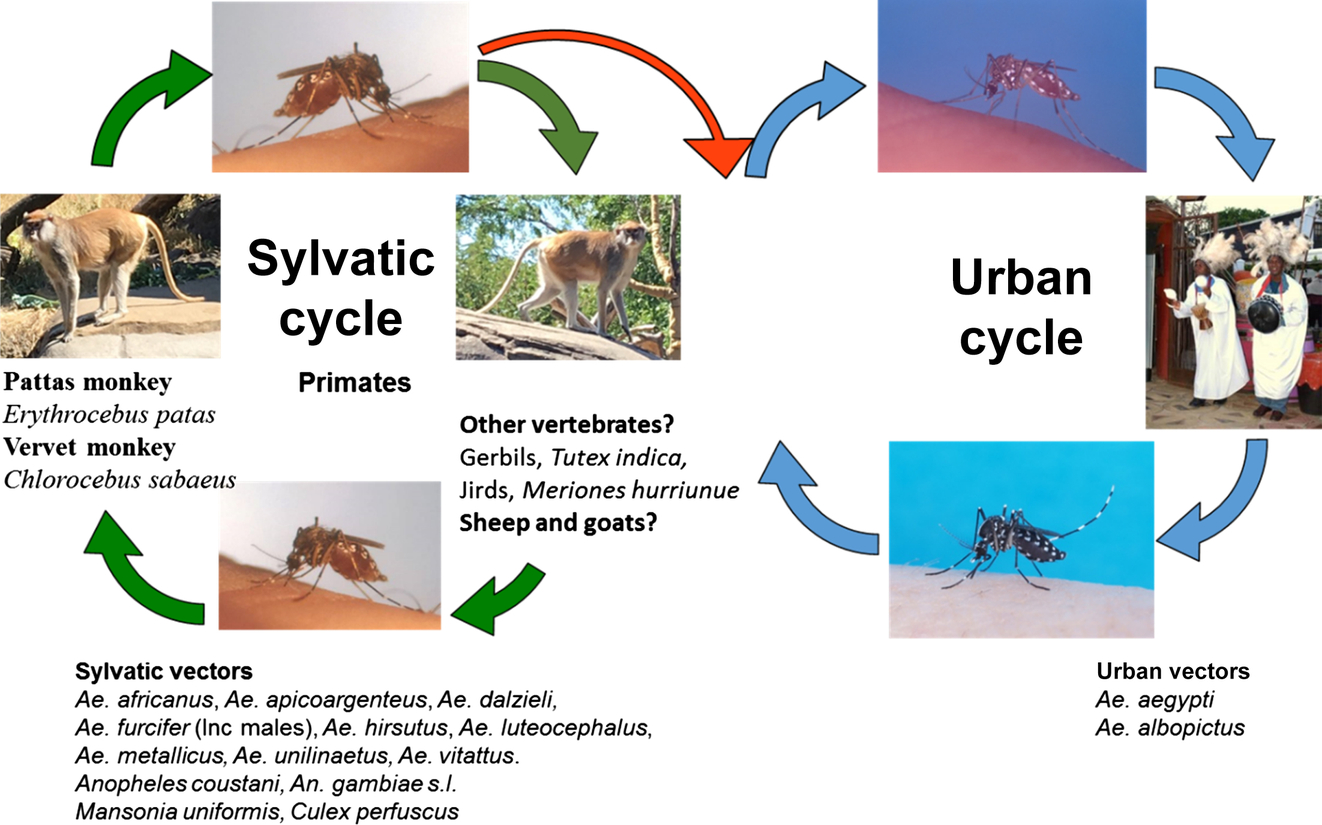
Table 3
ZIKV in African Vertebrates
Serosurveys of African vertebrates have indicated infection in multiple species. Identification of ZIKV was based on isolation from a sentinel rhesus monkey (Macacca mulatta) by Dick et al. (1952). Based on virus isolation or positive serological tests, other nonhuman primates have been implicated as potential maintenance hosts of ZIKV (Andral et al., 1968; Bres, 1970; Carey, 1971; Kirya and Okia, 1977; Renaudet et al., 1978; Geoffroy, 1982; McCrae and Kirya, 1982), including vervet monkeys (Cercopithecus aethiops, Cercopithecus aethiops tantalus, Cercopithecus mona), red-tailed monkeys (Cercopithecus ascanius schmidti), baboons (Papio ursinus), Patas monkeys (Erythrocebus patas), colobus monkeys (Colobus guerza), chimpanzee (Pan troglodytes), and bushbabies (Galago senegalensis, G. demidovi). Serologically positive sera have also been collected from free-tailed bats (Molossidae and Tadaridea), mice (Praomys sp.) a rusty-bellied brush-furred rat (Lophuromys sikapusi), and squirrels (Anomalurus sp.); however, the relevance of these infections with respect to ZIKV maintenance remains uncertain (Geoffroy, 1982; McCrae and Kirya, 1982). As mentioned below under “Transmission Cycle of ZIKV in the Americas,” recent experimental studies by Ragan et al. (2017) tested 16 different vertebrate species and none were found to be suitable as amplifying hosts.
Transmission Cycle of ZIKV in Asia
As described in Chapter 5 with respect to ZIKV in Asia, the virus was first reported in Malaysia in 1969 from a collection of Ae. aegypti mosquitoes (Marchette et al., 1969). Serological evidence suggesting the presence of Zika was reported by Smithburn (Smithburn and Bugher, 1953; Smithburn et al., 1954); however, the virus was not serologically confirmed until 1977–78 during studies in Indonesia (Olson et al., 1981). Despite the intensity of the entomological surveillance reported by Marchette et al. (1969), no isolates of ZIKV were made from 4492 Ae. albopictus or 27,636 other Aedes species. In 2007, cases of Zika were reported from Yap Island, Federated States of Micronesia (Duffy et al., 2009). Based on its relative abundance during the outbreak that resulted in at least 1700 suspected human cases, Ae. hensilli was implicated as the primary vector. Experimental demonstration of the competence of Ae. hensilli for ZIKV was subsequently provided by Ledermann et al. (2014). Although experimentally other species of mosquitoes, for example Ae. polynesiensis, may be infected and capable of transmitting ZIKV (Richard et al., 2016), there are no data to suggest that they played a significant role in the spread of ZIKV toward the Americas.
Although evidence of ZIKV infection has been reported for Asian primates, specifically in orangutans (Pongo pygmaeus) (Wolfe et al., 2001), their significance in the transmission cycle of ZIKV in this region is uncertain, and likely not significant. In Pakistan, Darwish et al. (1983) reported ZIKV antibody-positive rodents (Tatera indica, Meriones hurrianae, Bandicota bengalensis), and interestingly, sheep and goats. Again, these species are unlikely to make a significant contribution as amplification hosts, especially in view of recent data on viremias in similar species (Ragan et al., 2017).
Transmission Cycle of ZIKV in the Americas
The extraordinarily rapid spread of ZIKV since 2015 through the highly susceptible population of the Americas has resulted in the infection of over 500,000 people (PAHO, 2017). The consensus is that this epidemic has been almost exclusively driven by Ae. aegypti as the vector (Table 4). Remarkably, the first isolation from the species was not made until 2015 when the virus reached Mexico (Guerbois et al., 2016). The transmission of ZIKV in Florida during 2016 is assumed to have involved Ae. aegypti and not Ae. albopictus, although both species are present in the state (Hornby et al., 1994).
Table 4
| Species | References in chronological order |
|---|---|
| Aedes aegypti | Ferreira-de-Brito et al. (2016) and Guerbois et al. (2016) |
| Aedes albopictus | Smartt et al. (2017) |
| Culex pipiens quinquefasciatus | Guedes et al. (2016), Guo et al. (2016), and Guedes et al. (2017) |
Chouin-Carneiro et al. (2016) evaluated susceptibility of five populations of Ae. aegypti and Ae. albopictus for Asian genotype ZIKV. The populations included Ae. aegypti from Brazil, French Guiana, Guadeloupe, Martinique, and the United States (Florida); and Ae. albopictus from Brazil and the United States (Florida). All populations were susceptible to infection and when tested, Ae. aegypti from Brazil and Ae. albopictus from the United States were able to transmit ZIKV after 14 dpi. Roundy et al. (2017) tested Ae. aegypti from Brazil, Dominican Republic, and the United States with three different isolates of ZIKV (Cambodia, Mexico, Senegal). As would be predicted based on previous studies, with for example YFV (Tabachnick et al., 1985) and dengue viruses (Bennett et al., 2002), Ae. aegypti populations from different geographical locations display variation in their susceptibility to ZIKV infection and some virus isolates are more infectious than others. As discussed, the explanation for these variations in mosquito competence is yet to be fully explained.
With respect to establishment of a ZIKV transmission cycle in North America, the lack of wild nonhuman primates, essentially precludes this. Recent studies by Ragan et al. (2017) tested 16 different vertebrate species: leopard frog (Lithobates spp.), garter snake (Thamnophis sirtalis), house sparrows (Passer domesticus), chicken (Gallus gallus domesticus), Indian runner duck (Anas platyrhynchos), rock pigeons (Columbia livia), nine-banded armadillo (Dasypus novencintus), deer mice (Peromyscus maniculatus), golden hamster (Mesocricetus auratus), groundhog (Marmota monax), cottontail rabbit (Sylvilagus spp.), American mink (Neovision vision), raccoon (Procyon lotor), boar goats (Capra aegagrus hircus), pig (Sus scrofa), Holstein cattle (Bos taurus), and found none of them capable of producing a viremia that would facilitate transmission of ZIKV to mosquitoes. As stated by Higgs and Vanlandingham (2015), the situation in tropical regions of the Americas, where there are multiple species of new world primates, is uncertain. Recently, Bueno et al. (2016) reviewed literature related to different animals that had been evaluated for ZIKV in the context of the concern that animals in Latin America may be impacted by ZIKV circulation. Favoretto et al. (2016) subsequently published a non-peer-reviewed report of ZIKV infection in Brazilian nonhuman primates, Capuchins (Sapajus libidinosus) and marmosets (Callithrix jaccus).
Potential New Vectors Since ZIKV Emergence
Other North American studies have suggested that Ae. triseriatus can be infected with ZIKV but Cx. pipiens cannot (Aliota et al., 2016), and two studies have reported that Ae. vexans can be experimentally infected with the virus (Gendernalik et al., 2017; O’Donnell et al., 2017). Although Guerbois et al. (2016) did not isolate ZIKV from Cx. quinquefasciatus in Mexico, interestingly this species has been implicated in the ZIKV epidemic in Brazil in two studies (Guedes et al., 2016; Guedes et al., 2017), but in another, Cx. quinquefasciatus from Rio de Janeiro was found not to be competent for ZIKV (Fernandes et al., 2016).
Amraoui et al. (2016) found that Cx. pipiens from Tunisia and Cx. quinquefasciatus from California were not competent vectors for ZIKV. This study confirmed two previous studies of North American Cx. quinquefasciatus that also reported that they were not susceptible to ZIKV infection. To add to the controversy, a study by Guo et al. (2016) in China found them to be extraordinarily susceptible to infection with ZIKV and highly capable of transmission. More recently, Liu et al. (2017) reported that Cx. quinquefasciatus from Guangdong Province were unable to transmit ZIKV. Fu et al. (2017) not only reported detection of ZIKV in field collected Cx. pipiens quinquefasciates from Dejiang, Guizhou Province, China, but also reported for the first time, ZIKV isolation from Armigeres subbalbatus. Huang et al. (2016) used Cx. pipiens from two states, California and New Jersey, and Cx. quinquefasciatus from Florida. The inclusion of Cx. quinquefasciatus that originated from Vero Beach, Florida, was important since, at the time of the study, this was the only state in North America where autochthonous transmission was occurring. The result precluded concern related to the potential for Cx. quinquefasciatus or Cx. pipiens to be acting as a vector. Similarly, a study by Kenney et al. (2017) also found that neither Cx. pipiens nor Cx. quinquefasciatus from North America were competent for ZIKV. A study by Boccolini et al. (2016) found Italian Cx. pipiens to be refractory to ZIKV infection, while Heitmann et al. (2017) reported German Cx. pipiens, Cx. quinquefasciatus biotype modestus and Cx. torrentium to be refractory to infection. As is evident from the earlier content, based on evidence collected to date, it seems that species of Culex mosquitoes are not highly significant as vectors of ZIKV, and most are refractory (van den Hurk et al., 2017, Table 4).
Laboratory Studies
Most of the details that we have regarding the course of arboviral infection in the mosquito vector were determined by laboratory studies. Some of these studies were conducted in the 1950s and interestingly, studies on CHIKV and ZIKV inspired researchers to resolve problems associated with mosquito infections that resulted in highly innovative solutions that we still use to this day. Although the most natural approach for generating infected mosquitoes for laboratory studies of arboviruses that infect humans is to feed them on viremic patients, this approach has the logistical challenge of having mosquitoes available and willing to feed coincident with availability of a patient with sufficiently high viremia. Ethical issues must also be addressed (Achee et al., 2015). There was thus a need for a method to infect mosquitoes that could be easily employed in a laboratory setting. Not only would such a technique negate the need for human patient volunteers, but it also would permit the use of viral stocks of known titer and provide convenience when developing experimental plans, such as consideration of the availability of mosquitoes. In 1953, Ross (1953) described a new apparatus, which was developed to feed mosquitoes using virus suspension produced as mouse brain homogenates mixed with rabbit serum and 1% rabbit erythrocytes. This mixture was said to be most attractive to mosquitoes and digested more rapidly than the normal blood meal. It was presented in a narrow inverted tube closed with a bat-wing membrane. A thermostatically controlled water bath was used to warm the jacketed tube and mosquitoes were held in vials. Mosquitoes were infected by allowing them to probe through the bat-wing membrane to imbibe the blood meal. Boorman and Porterfield (1956) subsequently developed a simpler technique consisting of blood in tubes with a mouse skin secured with a rubber band over the tube opening. The blood was warmed in a beaker containing 40°C water. The warmed blood in the tube was then inverted onto netted cages of mosquitoes. Using this method, mosquitoes could be infected by presenting them with infected blood and transmission could be studied by allowing infected mosquitoes to probe through a mouse skin to feed on uninfected blood. Transmission of virus would then be detected by injecting the blood into mice intracerebrally. In one experiment, virus was isolated from the skin membrane itself. Readers involved in mosquito-arbovirus research will appreciate these early contributions that have culminated into today’s modern and sophisticated methods to infect mosquitoes using systems such as that produced by Hemotek (Discovery Workshops LLC, Accrington, Lancashire, United Kingdom).
Something that must be emphasized is that the early experiments were performed without the formal regulatory biocontainment and biosafety regulations by which we must all now abide. When conducting mosquito infection experiments, researchers must now work in suitably approved secure containment insectaries and use protocols that fulfill appropriate biosafety requirements. All work with CHIKV requires the use of biosafety three (BSL-3) practices. In the United States, although ZIKV may be handled using BSL-2 practices, for mosquito research this requirement is raised to BSL-3. Information on the design and operation of insectaries and standard operating procedures for working with arboviruses is readily available (Higgs et al., 1997; Duthu et al., 2001; Higgs, 2005a,b; Huang et al., 2017).
Laboratory Studies to Determine Vector Competence of Mosquitoes for CHIKV
Laboratory studies using colony mosquitoes to test susceptibility to various viruses enable a controlled environment to gather data that could be used to predict which vector species may be involved in the virus/vector enzootic cycles or epidemic cycles. An example of how these studies can be used to understand the spread of an arbovirus into a new area is the work conducted examining CHIKV infection in Ae. albopictus mosquitoes almost 30 years before this mosquito was found to be important in the transmission cycle on islands in the Indian Ocean.
Using the prototype ECSA Ross strain and the Barsai strain of CHIKV, Tesh et al. (1976) were able to infect 16 geographic populations of Ae. albopictus with CHIKV under laboratory conditions. Turell et al. (1992) were subsequently able to infect ten populations of Ae. albopictus and seven populations of Ae. aegypti with the Thailand 15561 strain of CHIKV. These data indicated that although Ae. albopictus was not a vector for CHIKV at the time of these studies, it did have the potential to be involved in the transmission cycle, which was found to be the case with the subsequent emergence of the CHIKV in areas that only had the Ae. albopictus and where Ae. aegypti was absent or in very low numbers. As observed for several other viruses, there is variation of susceptibility between different populations of mosquitoes of the same species to CHIKV infection. In a very sophisticated study, Dong et al. (2016) challenged two well-established colonies of new world strains of Ae. aegypti, the Higgs white-eyed (HWE) and Orlando Florida (ORL) strains with CHIKV. The HWE line was developed as a spontaneous mutant in the Rexville D strain, used for Green Fluorescent protein studies (Higgs et al., 1996) and subsequently characterized by Coates et al. (1997). Dong et al. (2016) observed differences in the infection patterns in midguts and salivary glands. Interestingly, although no significant difference was seen with respect to dissemination rate to the salivary glands that occurred within 2 days of infection, at 7 dpi virus was only detected in 60%–65% of infected mosquitoes. The authors suggested that this indicated a salivary gland escape barrier, possibly associated with apoptotic responses to infection. Interplay between infection dynamics in different mosquito populations, environmental factors, for example the temperature at which infected mosquitoes are maintained, has been reported for CHIKV (Mbaika et al., 2016).
Laboratory studies also provide a more controlled examination of field observations. For example, a recent study examining the parental ECSA, E1-226A, CHIKV (see later section and Chapter 8 for details), i.e., prior to the valine substitution characterized during the Reunion outbreak, found that Ae. albopictus mosquitoes from Congo did not select the E1-A226V mutation following passage in the mosquito. Additionally, following artificial feeding of virus with equal amounts of the parental (alanine) and mutated (valine) at the E1-226 position, there was no preferential transmission of the virus with the valine substitution (Vazeille et al., 2016). These data indicate that although this mutation played a vital role in the Reunion outbreak, this mutation does not seem to have the same effect in Congo Ae. albopictus.
In addition to identifying vectors that are currently involved in the CHIKV cycle, it is important to examine vectors that are susceptible to CHIKV if the virus were to be introduced into a new area. A study examining the vector competence of a potential CHIKV vector, the sylvan form of Ae. aegypti, Ae. aegypti formosus, found that this mosquito is a competent vector for CHIKV (Vazeille et al., 2013). For this study, Ae. aegypti formosus was collected on the island of Santiago, which is an island located off the coast of Africa in the Atlantic Ocean. This mosquito was selected because it is becoming more anthropophilic, meaning that the mosquito has an increased preference for biting humans. The results of this study indicate that if CHIKV were to be introduced into this area, there is a competent vector available to transmit the virus among people. This is counterintuitive, because it is generally thought that Ae. aegypti formosus is refractory to arboviral infection (Bosio et al., 1998).
In addition to the examples of laboratory experiments examining CHIKV and various mosquitoes that could be competent vectors for transmission, there have been many other mosquito species that have been challenged with CHIKV under laboratory conditions. These experiments are listed in Table 5, are also described in Chapter 4 (Table 2), and have been described in the review by Coffey et al. (2014).
Table 5
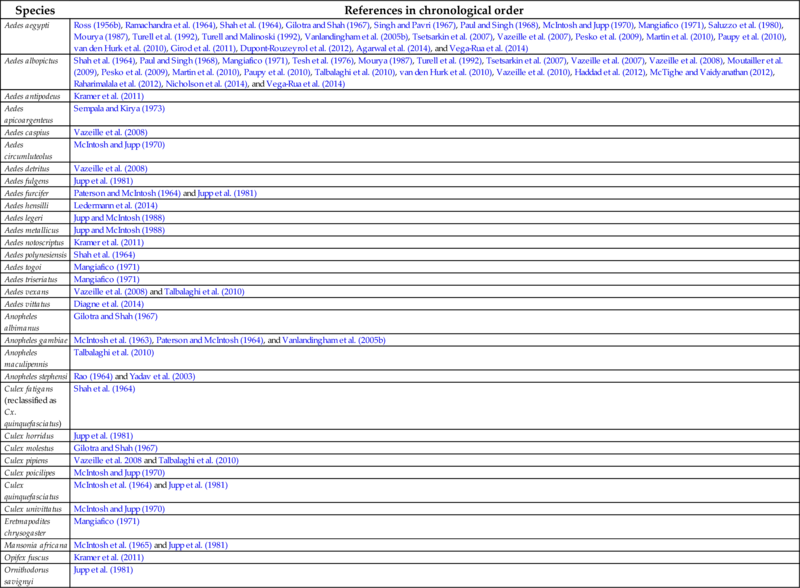
Note that for completeness studies are included that failed to infect mosquitoes.
Jupp et al. (1981) evaluated the competence of several African mosquitoes for CHIKV but also examined the ability of the tick, Ornithodoros savignyi, to transmit the virus. In this study, several mosquitoes were found to be susceptible to infection. Transmission of CHIKV was found to occur when Ae. fulgens were allowed to feed on rodents (Nystromys albicaudatus). Transmission was also observed in vervet monkeys (Cercopithecus aethiops) that were fed on by Ae. furcifer and Mansonia africana. The vector competence of Cx. horridus mosquitoes for CHIKV was found to be low with only one of 17 mosquitoes having a disseminated infection following feeding on an artificial blood meal. This study also found that both Cx. quinquefasciatus and the tick, O. savignyi, were not able to be infected. Another early study examining CHIKV transmission in various species of mosquitoes was conducted by McIntosh et al. (1964). In this study, Ae. aegypti, Ae. a. formosus, and Ae. calceatus were experimentally infected. Interestingly, Ae. a. formosus was able to transmit the virus to mice and a monkey. There was virus replication but no transmission in An. gambiae. Following challenge of Ae. metallicus and Ae. simpsoni, no virus replication was observed. Of 103 tested after a “large number” of Cx. quinquefasciatus were fed on mice with blood titers of 5.5–8.0, none were positive.
Other Transmission Mechanisms Used by CHIKV
Researchers have investigated different modes of transmission of CHIKV, other than the horizontal type, i.e., mosquito to vertebrate, which drives most cycles in nature. These alternative types of transmission can be observed for various virus and vector combinations. One type of transmission is generically referred to as vertical transmission and involves the virus being transmitted from one mosquito to another mosquito. Transmission of virus present in the eggs of infected female to the embryo, known as transovarial transmission (TOT), can be quantified as both a percentage of the females that are fed an infectious meal that transmit virus to the eggs, and also as a percentage of the offspring of these females that become infected—referred to as the filial infection rate. Since virus is passed to eggs regardless of the sex of the embryo, the detection of infected male mosquitoes in field collections can be an indicator of TOT. Venereal transmission from a TOT-infected male mosquito to the female during copulation is probably a relatively rare type of vertical transmission. For some arboviruses, for example, La Crosse, that is transmitted in temperate regions by Ae. triseriatus and Rift Valley fever virus found in arid areas, the deposition of virus by infected females into their eggs may be the critical mechanism for the virus to survive between seasons in these relatively harsh environments (Watts et al., 1973; Linthicum et al., 1985).
With respect to CHIKV, an alphavirus, in their reviews of vertical transmission, both Leake (1984) and Turell (1988) suggested that in comparison with viruses in other families, TOT was relatively rare in alphaviruses. Experiments by Jupp et al. (1981) did not observe any TOT of CHIKV by either Ae. aegypti or Ae. furcifer. In a later report (Jupp and McIntosh, 1990), no virus was detected in 7241 female and 4052 male Ae. furcifer/cordellieri mosquitoes, and not detected in 13,029 newly emerged mosquitoes that included the known competent vectors Ae. aegypti, Ae. fulgens, and Ae. vittatus. In a 1987 experiment conducted by Mourya, vertical transmission was also not found in either Ae. aegypti or Ae. albopictus. Later experiments by Vazeille et al. (2007) detected viral antigen in developing eggs within 6 days of oral infection of mosquitoes, but did not examine progeny for viral infection. A subsequent study (Vazeille et al., 2009) failed to detect virus either by antigen-based assays or PCR in progeny of infected Ae. albopictus females, even after a second gonotrophic cycle. A more comprehensive study by Agarwal et al. (2014) extended the period of maintenance to include examination of progeny resulting from a third gonotrophic cycle induced by two noninfectious blood meals provided after the initial infectious one. This study using Ae. aegypti collected in India and challenged with the ECSA CHIKV strain resulted in a filial infection rate of 1:35.5 for larvae and 1:49.5 in adults. Chompoosri et al. (2016) also reported experimental TOT of CHIKV in both Ae. aegypti and Ae. albopictus, with a greater rate shown in Ae. albopictus. Niyas et al. (2010) reported the detection of CHIKV in adult Ae. albopictus that were hatched from larvae collected from the field. Using intrathoracically infected male Ae. aegypti mosquitoes, Mavale et al. (2010) demonstrated venereal transmission of CHIKV to female mosquitoes with whom they mated. One should be cautious in how an observation from such a proof-on-concept experiment that uses an entirely artificial method of infection can be extrapolated to the transmission cycle in nature.
For flaviviruses such as dengue, Japanese encephalitis, and yellow fever viruses, vertical transmission has been both experimentally demonstrated and occasionally found in nature, as suggested by virus presence in male adult mosquitoes or in larvae. However, it is difficult to understand how important vertical transmission is for arboviruses transmitted in areas in which adult vectors are present throughout the year, albeit with variation in seasonal abundance. For the arthropod-specific flaviruses that infect mosquitoes but not vertebrate hosts, vertical transmission is the mechanism by which mosquitoes are infected (Calisher and Higgs, 2017).
Interestingly, Rao et al. (1968) demonstrated mechanical transmission of CHIKV by allowing Ae. aegypti to feed on viremic mice, interrupting their feeding and then allowing them to feed on uninfected naive infant mice at various times after the initial feed. The criteria used to indicate successful mechanical transmission were sickness of the naive mice and confirmation of CHIKV in their brains by complement fixation tests. When allowed to recommence feeding immediately after exposure to the viremic mice, 17/29 mosquitoes mechanically transmitted CHIKV, after 30 min. 2/8 transmitted, after 2 h, 3/9 transmitted, after 4 h, 4/8 transmitted and after 8 h, 1/3 transmitted. Mechanical transmission was not observed by mosquitoes tested at 12, 24, and 36 h.
Early Experiments With Aedes Mosquitoes and ZIKV
In order to improve the ability to evaluate vector competence in the laboratory, Boorman and Porterfield (1956), working with ZIKV, used a mouse skin membrane and heparin-treated blood to infect Ae. aegypti. At 5–10 days postfeeding, little or no ZIKV could be detected; however, after 10 days, titers increased, and from 20 to 60 days were sustained at approximately 105 mouse LD50/mosquito. Not only was infection from the blood meal accomplished, but also the technique was used to demonstrate transmission of ZIKV from 12 of 20 of the fed mosquitoes into uninfected blood through the mouse skin membrane. Furthermore, a rhesus monkey became infected when fed upon by three of the artificially infected mosquitoes 72 days postinfection. Bearcroft’s (1956) attempt to infect Ae. aegypti by feeding on a human volunteer that had been inoculated with an Eastern Nigerian isolate of ZIKV seemingly failed since infant mice fed upon these mosquitoes did not die during a 14-day observation period.
Laboratory Studies of Intraspecies Difference of Mosquitoes to ZIKV Infection
As observed for several other viruses, and described briefly in “Transmission of Arboviruses Between Vectors and Vertebrates” and “Transmission Cycles of ZIKV” sections, there is variation of susceptibility between different populations of mosquitoes of the same species, and laboratory experiments with ZIKV are summarized in Table 6 (see also Chapter 4, Table 4). Costa-da-Silva et al. (2017) used a strain of ZIKV (ZIKVBR) that was isolated from a Brazilian clinical case (Cugola et al., 2016) to orally challenge three different laboratory-adapted colonies of Ae. aegypti mosquitoes. The Rockefeller colony was established from mosquito eggs collected in Cuba in 1926 (Kuno, 2010), and the Rexville D colony originated as larvae collected in Puerto Rico (Miller and Mitchell, 1991). Development of the HWE line has been described earlier. Differences were observed with respect to infection, dissemination, and transmission by the three colonies, but all were susceptible, and all were capable of transmission. Although the Higgs strain had the lowest infection rate at 7 dpi, this strain had the highest ZIKV load in saliva at 14 dpi (20% detection in saliva with a mean RNA copy number of 1.88 × 103).
Table 6
Note that for completeness studies are included that failed to infect mosquitoes.
Experiments by Chouin-Carneiro et al. (2016) investigated the differential susceptibilities of Ae. aegypti and Ae. albopictus from the Americas to an Asian lineage ZIKV, strain NC-2014-5132, isolated from a patient in New Caledonia. Ae. aegypti from French Guiana, Guadeloupe, Martinique, and the United States; and Ae. albopictus from Brazil and the United States were all susceptible to infection with ZIKV with dissemination to the salivary glands at 14 dpi. Transmission efficiency was, however, reported to be surprisingly low—10% for Brazilian Ae. aegypti and 3.3% for United States Ae. albopictus. Dissemination rates at 7 dpi were significantly higher for Ae. aegypti from Guadeloupe or French Guiana, than those from Martinique, Brazil, or the United States. Ae. albopictus from Florida were at least twice as susceptible to infection compared to Ae. albopictus from Brazil. In comparison with Ae. albopictus from Singapore, Ae. albopictus from Brazil and the United States were less susceptible to ZIKV. Wong et al. (2013) reported that at 7 dpi, 100% of Singapore Ae. albopictus had disseminated infections, with 73% having ZIKV in the saliva. Remarkably, at 10 dpi, ZIKV was detected in the saliva of all mosquitoes. For these experiments, the researchers used the same ZIKV, Ugandan MR766 as Miller and Mitchell (1991). Working with Brazilian, Dominican Republic, and United States Ae. aegypti, Roundy et al. (2017) reported variation in the infectivity of ZIKV strains from Senegal, Cambodia, and Mexico. Only Dominican Republic mosquitoes transmitted the Cambodian and Mexican viruses. Challenge of mosquitoes by feeding on viremic mice resulted in higher infection rates when compared with presentation of virus in artificial blood meals. It was suggested that variation between the populations could be attributed to genetic differences among the mosquitoes, differences in the microbiome, virome, or immune activation; however, experiments were not performed to identify the underlying mechanism.
Vertical Transmission of ZIKV
A recent description of TOT of ZIKV (Thangamani et al., 2016) depended on intrathoracic inoculation of mosquitoes, which probably results in unnaturally high levels of exposure of female reproductive tissues to ZIKV in the mosquito hemolymph. Diallo et al. (2014) detected ZIKV in one pool of male Ae. furcifer, and suggested that vertical transmission could be an important mechanism in this species. Based on many years of field data for ZIKV, there is no conclusive evidence to support a significant role of vertical transmission for maintenance in nature. It seems likely that in interepidemic periods, the viruses are either being maintained in a sylvatic cycle or in an undetected human cycle. Periodically, the viruses spill over from the enzootic cycles into the human population.
Coinfection of Mosquitoes With CHIKV and ZIKV
As discussed earlier and in several other chapters, CHIKV and ZIKV occupy sympatric geographical ranges, often occur at the same time, have similar transmission cycles involving the same vector species, notably Ae. aegypti and Ae. albopictus, and of course have humans as an amplifying vertebrate host. Furthermore, they also share these characteristics with some other arboviruses, for example dengue (DENV) and YFV. Inevitably, questions have been asked whether either mosquitoes or vertebrates can be coinfected with more than one of these viruses. From the vertebrate perspective, antibodies produced as a result of infection with one of these viruses do not seem to confer complete protection against the others, although some crossreactivity has been reported for more closely related viruses. Although human coinfections have been reported (Myers and Carey, 1967; Dupont-Rouzeyrol et al., 2015; Furuya-Kanamori et al., 2016; Villamil-Gomez et al., 2016a,b; Waggoner et al., 2016; Zambrano et al., 2016), these seem to be relatively uncommon, even for example in Singapore that had concurrent CHIKV and DENV epidemics (Chang et al., 2010). Arbovirus transmission cycles depend on mosquitoes feeding at least twice on vertebrates during their lifetime: once on an infected vertebrate, and then after the completion of the intrinsic incubation period, on a second susceptible vertebrate. Given the hundreds of thousands of human infections that can happen in a relatively short period of time, clearly this sequential feeding occurs very frequently. It therefore seems that when two viruses are circulating in the human population at the same time, some mosquitoes may be exposed to both viruses. Intuitively, it would seem that feeding at different times on two different hosts infected with different viruses is more likely than feeding on one coinfected individual that is viremic for both. For mosquito surveillance, mosquitoes are tested as pools of many individuals of the same species, rather than individually. Although pools have sometimes tested positive for more than one virus, this does not indicate coinfection but more likely reflects the presence of two individual mosquitoes infected with different viruses in the pool. Under experimental conditions, however, coinfection has been demonstrated. Vazeille et al. (2010) detected both CHIKV and DENV-1 in Ae. albopictus exposed simultaneously to a blood meal containing both viruses. When intrathoracically inoculated with DENV-1, and then presented with a blood meal containing CHIKV 7 or 14 days later, all mosquitoes produced a disseminated CHIKV infection. Nuckols et al. (2015) demonstrated simultaneous transmission of CHIKV and DENV by both Ae. aegypti and Ae. albopictus, and more recently, Ruckert et al. (2017) showed that Ae. aegypti could be coinfected with CHIKV, DENV, and ZIKV if simultaneously exposed to an artificial blood meal containing high titers of all three viruses. It is difficult to estimate if these findings have any significant impact in nature and, it was stated that “exposure to three viruses simultaneously is likely to be an extremely rare occurrence.”
Control of CHIKV and ZIKV by Targeting Mosquitoes
Controlling any mosquito-borne disease depends on the integration of multiple approaches. With the demonstration that mosquitoes were involved as vectors of YFV and Plasmodium spp. responsible for human malaria came the realization that disease control could be affected by reducing the abundance and distribution of mosquitoes. This approach was successfully applied in the early 1900s by William Crawford Gorgas to eliminate yellow fever from Panama during the construction of the Panama Canal, and to eradicate malaria from the United States. In these early examples, success was largely dependent on what we refer to as source reduction; basically an environmental strategy to reduce or eliminate the aquatic sites that are an absolute requirement for survival of mosquito larvae. The removal of small pools and puddles, redirection of water accumulation, and drainage of swamps were all part of the mosquito control programs. The development of chemical insecticides augmented source reduction, and when applied in a carefully coordinated and collaborative strategy as in the Americas, these achieved remarkable accomplishments, for example the elimination of dengue from multiple countries between the 1940s through the 1970s (Camargo, 1967). More recently, technology and insecticidal chemicals were combined to produce impregnated bed nets and fabrics that have been effectively used to significantly reduce vectors responsible for malaria in many African countries. With success, however, came challenge as the arthropods evolved in response to chemical selection and developed resistance to insecticides. The development of mosquito repellents and education of the general public have provided approaches for personal protection to prevent or reduce exposure to potentially infected mosquito bites; however, new approaches focusing back on the vector are now being applied. The two approaches at the forefront of combating both CHIKV and ZIKV are the release of mosquitoes infected with the bacterial symbiont Wolbachia, and the release of genetically engineered mosquitoes that is akin to the release of sterile males used for many years to control screw worm flies. The Wolbachia approach can be used as both a population suppression and population replacement strategy, the latter being based on the unexpected discovery that mosquitoes infected with the bacteria are relatively resistant to infection by several arboviruses. Resistance of Wolbachia-infected mosquitoes to arbovirus infection has been reported for CHIKV (van den Hurk et al., 2012) and ZIKV (Dutra et al., 2016). The Wolbachia-based suppression approach is currently being implemented in Singapore as part of the battle against Aedes-transmitted DENV, CHIKV, and ZIKV. The approach known as RIDL—Release of Dominant Lethal—has been used in several countries, including Brazil as part of the fight against ZIKV, and has recently been approved for Florida. Higgs (2013) and Benelli et al. (2016) have published reviews that describe and compare these approaches. Kean et al. (2015) review multiple approaches to engineering the competence of Aedes mosquitoes, including manipulation of immune responses, paratransgenesis, and entomopathogenic fungi.
Conclusion
It seems beyond doubt that both CHIKV and ZIKV should now be regarded as permanently established in multiple countries in the Americas. Similar to dengue viruses, the transmission cycles of both CHIKV and ZIKV are primarily between susceptible people and the competent vectors, namely, Ae. aegypti and Ae. albopictus. As with dengue, it seems likely that despite infection resulting in protective antibodies, there are always enough susceptible people in the region to sustain a transmission cycle. In the absence of effective vaccines and a sustainable vaccination campaign even when they become available, this situation is unlikely to change. Despite new technologies for mosquito control, for example, release of dominant lethal (RIDL) mosquitoes and Wolbachia-infected mosquitoes, the prospects for widespread vector eradication are highly unlikely, even if they were economically viable on a continental scale. With time, we will better understand the dynamics and details of the transmission cycles in the Americas. This may ultimately involve new vector species and new vertebrate hosts; however, even if new species become involved, they are probably not necessary to sustain these viruses in the region.