Neurology
ANATOMY AND PHYSIOLOGY
Neurohistology
The nervous system is composed of two general components, neurons (nerve cells), which are the functional units involved in nerve transmission, and glial cells, which are the supporting (non-neuronal) cells and consist of various subtypes. Glial cells are generally known as the glue that holds the nervous system together, but serve much greater functions, such as modulating nerve transmissions and myelinating nerves.
Neurons: These are the nondividing functional unit of the nervous system, which can be classified according to function (motor, sensory, or interneurons). Each neuron is broken into a cell body, receiving dendrites, and a single projecting axon. Of the three components, clumps of rough endoplasmic reticulum (RER) and polyribosomes (referred to as Nissl bodies) are only found in the cell body and dendrites (not axon).
Glial cells: There are four central nervous system (CNS) glial cell types (astrocytes, oligodendrocytes, microglia, and ependymal cells) and one peripheral nervous system (PNS) glial cell type (Schwann cells).
 Astrocytes: These are the most abundant and largest of the glial subtypes. Their most notable role is the metabolism and recycling of certain neurotransmitters (glutamate, serotonin, and gamma-aminobutyric acid [GABA]). They also buffer the extracellular potassium concentration, respond to injury (gliosis), and make up the blood-brain barrier. They contain glial fibrillary acidic protein (GFAP), which is a marker used in diseases such as astrocytoma and glioblastoma.
Astrocytes: These are the most abundant and largest of the glial subtypes. Their most notable role is the metabolism and recycling of certain neurotransmitters (glutamate, serotonin, and gamma-aminobutyric acid [GABA]). They also buffer the extracellular potassium concentration, respond to injury (gliosis), and make up the blood-brain barrier. They contain glial fibrillary acidic protein (GFAP), which is a marker used in diseases such as astrocytoma and glioblastoma.
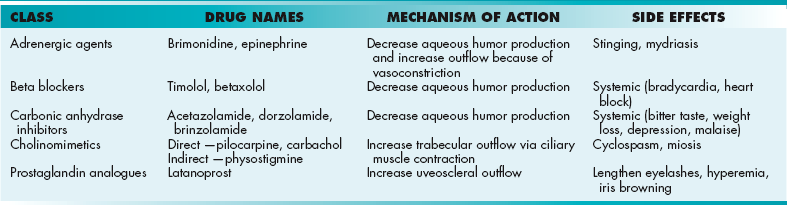
 Oligodendrocytes: These cells myelinate neurons within the CNS (one cell myelinates multiple neurons; Fig. 13-1A) and are damaged in disease processes such as multiple sclerosis (MS) and leukodystrophies.
Oligodendrocytes: These cells myelinate neurons within the CNS (one cell myelinates multiple neurons; Fig. 13-1A) and are damaged in disease processes such as multiple sclerosis (MS) and leukodystrophies.
Figure 13-1 A, One oligodendrocyte myelinates multiple central nervous system (CNS) neurons. B, One Schwann cell myelinates only one peripheral nervous system (PNS) neuron. (From Boron WF, Boulpaep EL. Medical Physiology. 2nd ed. Philadelphia: Elsevier; 2008.)
 Microglia: These cells arise from monocytes (hematopoietic precursor) and thus are the resident macrophages of the CNS. Their function is to protect the CNS. When the brain is damaged or infected, they become activated and multiply quickly to perform functions such as phagocytosis and presenting antigen. Microglia cells are implicated in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, as well as infections, such as human immunodeficiency virus (HIV) infection.
Microglia: These cells arise from monocytes (hematopoietic precursor) and thus are the resident macrophages of the CNS. Their function is to protect the CNS. When the brain is damaged or infected, they become activated and multiply quickly to perform functions such as phagocytosis and presenting antigen. Microglia cells are implicated in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, as well as infections, such as human immunodeficiency virus (HIV) infection.
 Ependymal cells: These ciliated cells line the cavities of the CNS (ventricular system) in the choroid plexus, where they are involved in the production of cerebrospinal fluid (CSF) and are part of the blood-CSF barrier. They are implicated in disease processes such as ependymomas and syringomyelia.
Ependymal cells: These ciliated cells line the cavities of the CNS (ventricular system) in the choroid plexus, where they are involved in the production of cerebrospinal fluid (CSF) and are part of the blood-CSF barrier. They are implicated in disease processes such as ependymomas and syringomyelia.
 Schwann cells: These cells are derived from neural crest origin and are similar to oligodendrocytes, but instead myelinate neurons of the PNS (one cell myelinates one neuron; Fig. 13-1B). They are implicated in diseases such as Guillain-Barré syndrome (GBS), Charcot-Marie-Tooth disease (CMT), chronic inflammatory demyelinating polyneuropathy (CIDP), schwannomas, and acoustic neuromas.
Schwann cells: These cells are derived from neural crest origin and are similar to oligodendrocytes, but instead myelinate neurons of the PNS (one cell myelinates one neuron; Fig. 13-1B). They are implicated in diseases such as Guillain-Barré syndrome (GBS), Charcot-Marie-Tooth disease (CMT), chronic inflammatory demyelinating polyneuropathy (CIDP), schwannomas, and acoustic neuromas.
Sensory Receptors
Sensory neurons receive signals from external or internal stimuli via numerous sensory receptors. Each type of sensory receptor conveys a unique type of sense such as vibration, pressure, pain, and temperature. In addition to classifying receptors based on the sense they convey, we can also classify them according to location (cutaneous versus muscle), morphology (free, nonencapsulated versus encapsulated), and rate of adaptation to a stimulus (slow-adapting versus fast-adapting). Slow-adapting receptors (e.g., muscle spindles, Merkel disks, Ruffini corpuscles) steadily detect the stimulus and steadily produce a signal over the duration of the stimulus. In contrast, fast-adapting receptors (e.g., Meissner corpuscles, Pacinian corpuscles) quickly generate action potentials that diminish soon after the onset of the stimulus. This gives us a sense of the stimulus duration and intensity. This is why we stop feeling our clothes soon after we have them on.
Cutaneous Receptors
 Free nerve endings: These are nonencapsulated nerve endings, located throughout the epidermis and viscera. They convey information regarding pain and temperature. Some of these nerve endings are associated with C fibers, which are slow (unmyelinated), convey warm temperature, and are involved in referred pain. Others are associated with Aδ fibers, which are fast (myelinated), convey cold temperature (see also Krause end bulbs, below), and are involved in localized pain.
Free nerve endings: These are nonencapsulated nerve endings, located throughout the epidermis and viscera. They convey information regarding pain and temperature. Some of these nerve endings are associated with C fibers, which are slow (unmyelinated), convey warm temperature, and are involved in referred pain. Others are associated with Aδ fibers, which are fast (myelinated), convey cold temperature (see also Krause end bulbs, below), and are involved in localized pain.
 Merkel disks: These are nonencapsulated, large, myelinated fibers located in hair follicles. These convey the senses of position (location) and static touch (e.g., textures).
Merkel disks: These are nonencapsulated, large, myelinated fibers located in hair follicles. These convey the senses of position (location) and static touch (e.g., textures).
 Pacinian corpuscles (Fig. 13-2, left): These are encapsulated, large, myelinated fibers found in the dermis, ligaments and joints. They convey the senses of vibration and deep pressure.
Pacinian corpuscles (Fig. 13-2, left): These are encapsulated, large, myelinated fibers found in the dermis, ligaments and joints. They convey the senses of vibration and deep pressure.
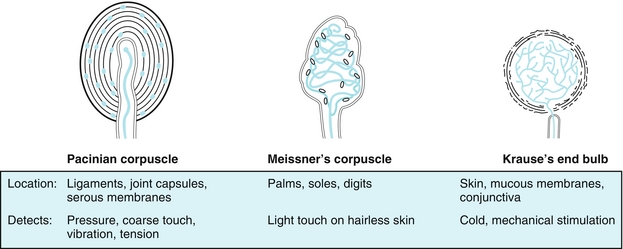
Figure 13-2 Left, Pacinian corpuscles are for pressure and vibration. Middle, Meissner’s corpuscles are for light touch. Right, Krause’s end bulbs are for cold (like Santa Claus). (From Burns R, Cave MD. Rapid Review Histology and Cell Biology. 2nd ed. Philadelphia: Elsevier; 2007.)
 Meissner corpuscles (Fig. 13-2, middle): These are encapsulated, large, myelinated fibers found in the epidermis of hairless skin (fingers and lips). These convey the senses of position (location) and dynamic touch (e.g., light touch).
Meissner corpuscles (Fig. 13-2, middle): These are encapsulated, large, myelinated fibers found in the epidermis of hairless skin (fingers and lips). These convey the senses of position (location) and dynamic touch (e.g., light touch).
 Krause end bulbs (Fig. 13-2, right): These are primarily for the sensation of cold; think Krause sounds similar to Santa Claus, who is always cold in the North Pole.
Krause end bulbs (Fig. 13-2, right): These are primarily for the sensation of cold; think Krause sounds similar to Santa Claus, who is always cold in the North Pole.
 Ruffini corpuscles: These are encapsulated fibers found in the dermis that convey the senses of pressure and skin stretch.
Ruffini corpuscles: These are encapsulated fibers found in the dermis that convey the senses of pressure and skin stretch.
Muscle Receptors
The main sensory muscle receptors are muscle spindles, which detect change in the length of skeletal muscle fibers and Golgi tendon organs, which are placed at the junction of the tendon and muscle fibers and sense the force of contraction.
 Muscle spindles: The muscle spindles are present in intrafusal fibers, which run parallel to the actual contractile muscle fibers (the contractile muscle fibers are also referred to as extrafusal fibers). By running in parallel (along with) the contraction of the muscle, intrafusal fibers can detect when the length of the muscle shortens or lengthens. This can be better understood with a clinical example, such as the myotactic reflex, in which hitting the patellar tendon with a reflex hammer causes the knee to jerk (Fig. 13-3). The tendon is stretched with the hammer, pulling on the muscle spindle. This then sends information to the spinal cord, which stimulates the knee extensor muscles to contract (causing the knee to jerk). It also inhibits the knee flexor muscles from contracting. A quick way to remember which nerve roots are tested by which reflexes is simply to count to eight; ankle (S1-2), patellar (L3-4), biceps (C5-6), triceps (C7-8).
Muscle spindles: The muscle spindles are present in intrafusal fibers, which run parallel to the actual contractile muscle fibers (the contractile muscle fibers are also referred to as extrafusal fibers). By running in parallel (along with) the contraction of the muscle, intrafusal fibers can detect when the length of the muscle shortens or lengthens. This can be better understood with a clinical example, such as the myotactic reflex, in which hitting the patellar tendon with a reflex hammer causes the knee to jerk (Fig. 13-3). The tendon is stretched with the hammer, pulling on the muscle spindle. This then sends information to the spinal cord, which stimulates the knee extensor muscles to contract (causing the knee to jerk). It also inhibits the knee flexor muscles from contracting. A quick way to remember which nerve roots are tested by which reflexes is simply to count to eight; ankle (S1-2), patellar (L3-4), biceps (C5-6), triceps (C7-8).
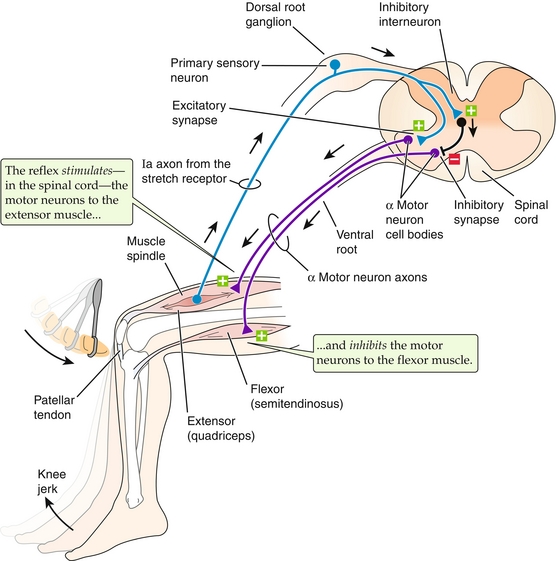
Figure 13-3 Muscle spindles sense muscle stretch and send a signal to the spinal cord. In the case of the patellar tendon reflex (shown), this causes activation of the extensor muscles and inhibition of the flexor muscles, causing the knee to jerk. (From Boron WF, Boulpaep EL. Medical Physiology. 2nd ed. Philadelphia: Elsevier; 2008.)
 Golgi tendon organ: Located at the junction of muscle fibers, with its tendons arranged perpendicular to extrafusal muscle fibers (Fig. 13-4). This receptor conveys a sense of muscle tension via afferent nerves and provides an autogenic inhibition reflex (also called the inverse myotatic reflex), which causes muscle relaxation before a tendon can be torn. This is why weight lifters may drop a heavy weight before it’s too late, and why this sensory receptor overrules the muscle spindle.
Golgi tendon organ: Located at the junction of muscle fibers, with its tendons arranged perpendicular to extrafusal muscle fibers (Fig. 13-4). This receptor conveys a sense of muscle tension via afferent nerves and provides an autogenic inhibition reflex (also called the inverse myotatic reflex), which causes muscle relaxation before a tendon can be torn. This is why weight lifters may drop a heavy weight before it’s too late, and why this sensory receptor overrules the muscle spindle.
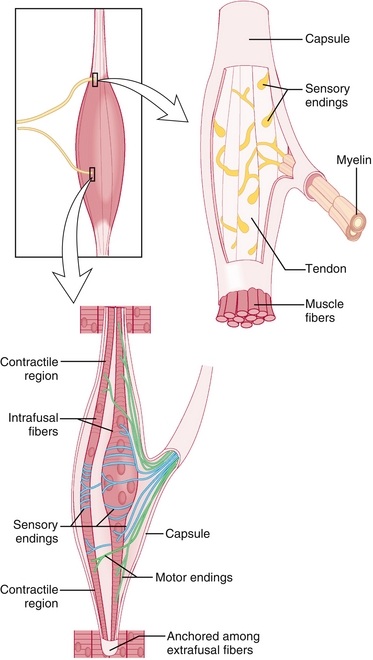
Figure 13-4 Location and anatomy of Golgi tendon organs (top right) and muscle spindles (bottom). The Golgi tendon organs are located at the junction of the muscle and tendon and are perpendicular to the extrafusal (contractile) fibers to be able to sense the force that the contractile fibers are generating. If this force is too high and places the tendon at risk of rupture, it will send inhibitory signals to stop exerting force. The muscle spindles are in parallel with the extrafusal fibers and therefore can sense length. (From Nolte J. Elsevier’s Integrated Neuroscience. Philadelphia: Elsevier; 2007.)
Neurotransmitters
Neurotransmitters (NTs) are substances found in synaptic vesicles that are secreted into the synaptic cleft from a presynaptic neuron to a postsynaptic neuron. They are classified according to chemical composition and the action elicited by a NT, which can be exictatory or inhibitory. See Chapter 7 for details of synaptic transmission and metabolism of many of these neurotransmitters.
Amino Acids
 Glutamate: As part of the glutamatergic pathway, glutamate has an excitatory effect, specifically on N-methyl-d-aspartate (NMDA) receptors, and is involved in cognition functions (learning and memory) in the hippocampus. In brain injury or disease, such as a stroke or seizure, excitotoxicity from excess glutamate release can lead to neuronal damage and death from the resulting excess calcium influx into the neuron.
Glutamate: As part of the glutamatergic pathway, glutamate has an excitatory effect, specifically on N-methyl-d-aspartate (NMDA) receptors, and is involved in cognition functions (learning and memory) in the hippocampus. In brain injury or disease, such as a stroke or seizure, excitotoxicity from excess glutamate release can lead to neuronal damage and death from the resulting excess calcium influx into the neuron.
 Gamma-aminobutyric acid (GABA): This is an inhibitory NT found in the nucleus accumbens and is involved in regulating excitability throughout the nervous system. It is decreased in anxiety and Huntington’s disease.
Gamma-aminobutyric acid (GABA): This is an inhibitory NT found in the nucleus accumbens and is involved in regulating excitability throughout the nervous system. It is decreased in anxiety and Huntington’s disease.
 Glycine: This is an inhibitory NT used by the Renshaw cells of the spinal cord. Strychnine, which can be fatal to humans, blocks its action.
Glycine: This is an inhibitory NT used by the Renshaw cells of the spinal cord. Strychnine, which can be fatal to humans, blocks its action.
 Acetylcholine: Found in the basal nucleus of Meynert, this NT is involved in functions such as learning, short-term memory, arousal, and reward. In Alzheimer’s disease, there is a loss of neurons in the nucleus of Meynert and thus the amount of NT released is reduced.
Acetylcholine: Found in the basal nucleus of Meynert, this NT is involved in functions such as learning, short-term memory, arousal, and reward. In Alzheimer’s disease, there is a loss of neurons in the nucleus of Meynert and thus the amount of NT released is reduced.
 Opioid peptides: Include endorphins, enkephalins, and dynorphins; involved in analgesia.
Opioid peptides: Include endorphins, enkephalins, and dynorphins; involved in analgesia.
Monoamines and Catecholamines
Dopamine (DA):
This NT is involved in functions such as nausea, reward, cognition, the motor system, and the endocrine system through four discrete pathways:
 Nigrostriatal pathway is part of the motor system. It projects from the substantia nigra to the striatum; destruction of these neurons can lead to parkinsonism.
Nigrostriatal pathway is part of the motor system. It projects from the substantia nigra to the striatum; destruction of these neurons can lead to parkinsonism.
 Mesolimbic pathway is found projecting from the ventral tegmentum to the nucleus accumbens; this is generally considered the “reward pathway” of the brain. It has also been linked to the positive symptoms of schizophrenia (hallucinations and delusions).
Mesolimbic pathway is found projecting from the ventral tegmentum to the nucleus accumbens; this is generally considered the “reward pathway” of the brain. It has also been linked to the positive symptoms of schizophrenia (hallucinations and delusions).
 Tuberoinfundibular pathway is found projecting from the arcuate nucleus to the portal vessels of the infundibulum; DA in this case inhibits prolactin release in the anterior pituitary. As a result, symptoms such as gynecomastia, galactorrhea, and menstrual dysfunction result from DA-blocking agents.
Tuberoinfundibular pathway is found projecting from the arcuate nucleus to the portal vessels of the infundibulum; DA in this case inhibits prolactin release in the anterior pituitary. As a result, symptoms such as gynecomastia, galactorrhea, and menstrual dysfunction result from DA-blocking agents.
 Mesocortical pathway is found projecting from the arcuate nucleus to the frontal lobes; this has been linked to negative symptoms of schizophrenia (causing the characteristic hypoactive, flat affect).
Mesocortical pathway is found projecting from the arcuate nucleus to the frontal lobes; this has been linked to negative symptoms of schizophrenia (causing the characteristic hypoactive, flat affect).
Norepinephrine (NE): Found in the locus ceruleus, lateral tegmental areas, reticular formation, and solitary tracts, this NT is involved in the arousal, reward, and maintenance of mood. In depression, NE is decreased. In states such as mania, anxiety, or stimulant drug use (amphetamines and cocaine), NE is elevated. Of note, in Alzheimer’s disease, there is substantial loss of the locus ceruleus.
Serotonin (5-HT): Found only in the raphe nucleus of the brainstem, this NT is involved in functions such as mood, sleep, and pain. It is elevated during mania and reduced in depression and insomnia.
Others
Adenosine: Generally acts as an inhibitory neurotransmitter. Caffeine acts as a stimulant by antagonizing adenosine receptors. Of note, adenosine is used in the treatment of supraventricular tachycardia (SVT).
Nitric oxide (NO): Formed from the conversion of arginine to citrulline, this NT is involved in memory formation through paracrine signaling. NO has been linked to reperfusion injury when blood flow is reestablished in an ischemic region.
ANATOMY
Each cerebral hemisphere is broken grossly into four lobes—frontal, parietal, occipital, and temporal (Fig. 13-5A). Each lobe specializes in certain functions (Fig. 13-5B). The frontal lobe provides crucial executive functions such as cognition, planning, decision making, error correction, and troubleshooting. The frontal lobe houses the frontal eye fields, premotor area (generates execution or plan of movement), and primary motor cortex. The frontal lobe also houses Broca’s area in the dominant hemisphere (usually the left hemisphere, regardless of whether or not the person is right- or left-handed), to which the motor aspect of speech production is linked. The function of the parietal lobe includes integrating sensory modalities and housing the principal sensory areas. The temporal lobe is involved in auditory perception; it is home to the primary auditory cortex. It also houses Wernicke’s area (associative auditory cortex) in the dominant hemisphere, in which written and spoken language is understood. Broca’s and Wernicke’s areas are interconnected by the arcuate fasciculus, which aids in language processing. The occipital lobe is involved in the processing and integration of visual information.
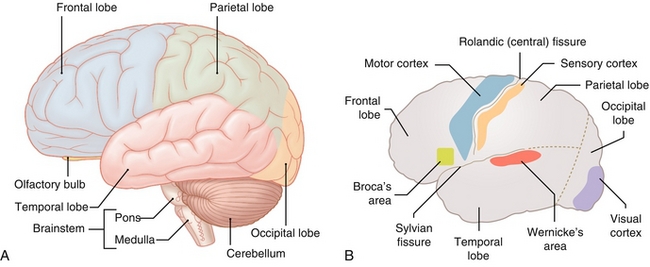
Figure 13-5 A, Gross anatomy of the brain. B, Selected areas of importance. (A from Moses K, Nava P, Banks J, Petersen D. Atlas of Clinical Gross Anatomy, 2nd ed. St. Louis: Elsevier; 2012; B from Lim EKS. Medicine and Surgery. Philadelphia: Elsevier; 2007.)
The cortical homunculus is a pictorial representation of the anatomic divisions of the primary motor cortex (frontal lobe) and primary somatosensory cortex (parietal lobe); it shows the portion of the human body involved in sensory and motor function mapped onto the cerebral hemisphere (Fig. 13-6). It is important to note that some regions (e.g., hands, face, lips) are disproportionately larger in comparison to the rest of the body. This is because fine motor control and skills are needed in these particular parts of the body; more neurons need to be devoted to fine motor control of the hand when compared with the hip. The homunculus is important in allowing one to localize a lesion based on the specific defects noted on neurologic exam. The middle cerebral artery (MCA) provides circulation to the lateral aspect of the cerebral hemisphere (resulting in neurologic deficits in the face and upper extremities if occlusion occurs), whereas the anterior cerebral artery (ACA) provides circulation to the medial aspect of the cerebral hemisphere (resulting in lower extremity and trunk neurologic deficits if occlusion occurs).
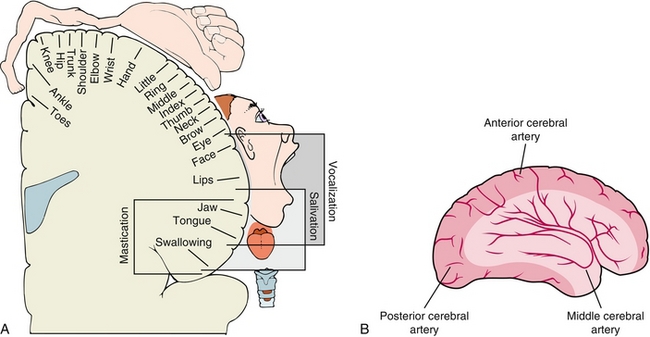
Figure 13-6 A, The motor homunculus mapped out onto the cerebral cortex. The medial side is supplied by the anterior cerebral artery, leading to trunk and leg weakness in anterior cerebral artery strokes. The rest is supplied by the middle cerebral artery, leading to arm weakness in middle cerebral artery strokes. B, Depiction of the anterior and middle cerebral artery vascular distributions. Note that A is a coronal view and B is a sagittal view. (A from Levy MN, Bruce M, Koeppen BM, Stanton BA. Berne & Levy Principles of Physiology, 4th ed. Philadelphia: Elsevier; 2005; B from Lawlor MW. Rapid Review USMLE Step 2. Philadelphia: Elsevier; 2006.)
Basal Ganglia
This subcortical structure is a group of nuclei whose main function is to modulate voluntary motor control. The basal ganglia are also involved in procedural learning, eye movement, and cognition. It consists of the following components (Fig. 13-7):
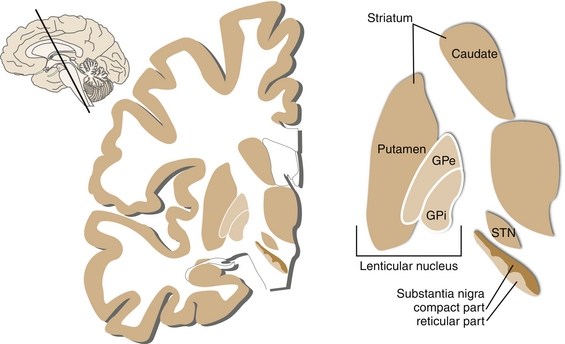
Figure 13-7 Nomenclature of the basal ganglia. The striatum includes the putamen and caudate nucleus. The lentiform nucleus includes the putamen and globus pallidus. (From Nolte J. Essentials of the Human Brain. Philadelphia: Elsevier; 2009.)
 Striatum, which is subdivided into the caudate (deals with cognition) and putamen (deals with motor control).
Striatum, which is subdivided into the caudate (deals with cognition) and putamen (deals with motor control).
 Globus pallidus, which is subdivided into the externa (lateral external segment) and interna (medial internal segment), abbreviated GPe and GPi, respectively. Both segments have inhibitory GABAergic neurons that operate using a disinhibition principle. They are steadily firing at a high rate in the absence of signal, but may pause or reduce the rate in response to a signal from the striatum. As a result, there is a net reduction of this tonic inhibition on their targets.
Globus pallidus, which is subdivided into the externa (lateral external segment) and interna (medial internal segment), abbreviated GPe and GPi, respectively. Both segments have inhibitory GABAergic neurons that operate using a disinhibition principle. They are steadily firing at a high rate in the absence of signal, but may pause or reduce the rate in response to a signal from the striatum. As a result, there is a net reduction of this tonic inhibition on their targets.
 Subthalamic nucleus, which is the only portion of the pathway to produce the excitatory NT glutamate.
Subthalamic nucleus, which is the only portion of the pathway to produce the excitatory NT glutamate.
 Substantia nigra, which is subdivided into the pars compacta (SNc; DA-producing) and pars reticulata (SNr), which works in unison with the GPi.
Substantia nigra, which is subdivided into the pars compacta (SNc; DA-producing) and pars reticulata (SNr), which works in unison with the GPi.
The basal ganglia consist of a complex circuit that ultimately aids in communication between the cortex, thalamus, and basal ganglia (see Fig. 13-7). The signal starts at the primary motor cortex (precentral gyrus), which projects excitatory (glutaminergic) cortical neurons onto the striatum. From there, the signal can take two different directions, giving rise to two major pathways—the direct excitatory or indirect inhibitory pathways. These are involved in triggering motion; the direct pathway stimulates it, and the indirect pathway inhibits it.
Direct (stimulatory) pathway: See Figure 13-8. Initially, the cortex stimulates the striatum. The striatum, however, inhibits the globus pallidus interna (GPi). Normally, the GPi tonically inhibits the thalamus, so therefore the direct pathway inhibits the inhibition of the thalamus. Therefore, this leads to increased thalamic output to the cortex.
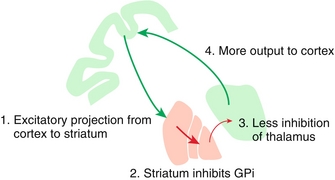
Figure 13-8 The direct (stimulatory) pathway, where the striatum prevents the globus pallidus interna (GPi) tonic inhibiton of the thalamus, therefore leading to stimulation (inhibition of inhibition). This leads to the thalamus sending more signals to the cortex for movement. (From Nolte J. Elsevier’s Integrated Neuroscience. Philadelphia: Elsevier; 2007.)
Indirect (inhibitory) pathway: Remember that indirect inhibits. This pathway is more complicated (Fig. 13-9). Again, the cortex stimulates the striatum to cause inhibition. However, here the striatum inhibits the globus pallidus externa (GPe) instead. In turn, the subthalamic nucleus undergoes inhibition of inhibition and therefore is stimulated to excite the GPi. Recall that the GPi normally inhibits the thalamus, so therefore increased GPi activity leads to increased thalamic inhibition. Thus, the thalamus will have less output to the cortex.
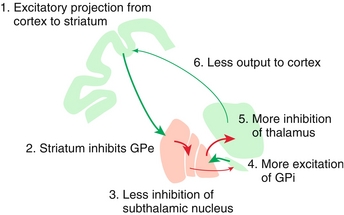
Figure 13-9 The indirect (inhibitory) pathway starts the same as the direct pathway (cortex excites striatum) but the difference is that the indirect pathway stimulates the GPe instead, leading to increased thalamic inhibition. (From Nolte J. Elsevier’s Integrated Neuroscience. Philadelphia: Elsevier; 2007.)
In summary, whereas the direct (stimulatory) pathway allows for increased thalamic transmission to the cortex by inhibiting the GPi inhibition of the thalamus, the indirect (inhibitory) pathway leads to increased GPi inhibition of the thalamus and therefore less thalamic transmission to the cortex. The interplay between the excitatory and inhibitory signals is mediated by dopamine via the substantia nigra pars compacta (SNc). Pathology with the basal ganglia therefore, unsurprisingly, leads to movement disorders such as Wilson’s disease, tardive dyskinesia, Parkinson’s disease, and Huntington’s disease (see later).
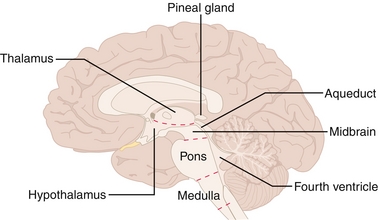
Hypothalamus
The hypothalamus is a major subcortical structure that consists of distinct nuclei that are involved in various functions.
Mnemonic: The hypothalamus makes me hungry for HAM BEETS (Hunger, Autonomic nervous system, Memory, Behavior, Endocrine, Emotion, Temperature, Sleep-Wake cycle, and Sexual urges).
Instead of memorizing all the distinct nuclei and their individual functions, a better way to look at the hypothalamus is to break it into two contrasting regions (anterior and posterior) and two contrasting areas (lateral and medial), with distinct functions.
Anterior: Deals with parasympathetics and cooling.
Posterior: Deals with sympathetics and heating (e.g., shivering).
 Destruction leads to a poikilothermic (cold-blooded) individual (posterior destruction, poikilothermic).
Destruction leads to a poikilothermic (cold-blooded) individual (posterior destruction, poikilothermic).
 A functioning posterior hypothalamus keeps your posterior warm, like a functioning heater.
A functioning posterior hypothalamus keeps your posterior warm, like a functioning heater.
Lateral: Deals with thirst and hunger. Inhibited by leptin.
Mnemonic: Lateral makes you hungry for a late night snack and makes your waist grow laterally.
Medial: Deals with satiety. Stimulated by leptin.
The following are distinct nuclei of which you should be aware:
 Supraoptic nucleus and paraventricular nucleus: See Chapter 9 for more details. These two nuclei play a significant role in the posterior pituitary’s release of antidiuretic hormone (ADH) (supraoptic) and oxytocin (paraventricular).
Supraoptic nucleus and paraventricular nucleus: See Chapter 9 for more details. These two nuclei play a significant role in the posterior pituitary’s release of antidiuretic hormone (ADH) (supraoptic) and oxytocin (paraventricular).
 Arcuate nucleus: This nucleus plays a significant role in releasing hormones from the anterior pituitary.
Arcuate nucleus: This nucleus plays a significant role in releasing hormones from the anterior pituitary.
 Suprachiasmatic nucleus: Receives input from the retina via the optic chiasm; this plays a significant role in circadian rhythm. (You need enough sleep via the suprachiasmatic nucleus to be charismatic.)
Suprachiasmatic nucleus: Receives input from the retina via the optic chiasm; this plays a significant role in circadian rhythm. (You need enough sleep via the suprachiasmatic nucleus to be charismatic.)
Thalamus
The thalamus is a subcortical structure that functions like a switchboard in relaying sensory information to the cortex. It can be divided into functional nuclei (Fig. 13-10).
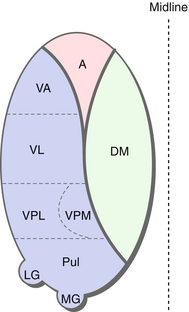
Figure 13-10 Anatomy of the thalamus. Note that this thalamus has been compressed into one layer; each nucleus is not actually present at each level. A, anterior nuclear group; DM, dorsomedial nucleus; LG, lateral geniculate nucleus; MG, medical geniculate nucleus; Pul, pulvinar; VA, ventral anterior; VL, ventral lateral; VPL, ventral posterior lateral; VPM, ventral posterior medial. (From Nolte J. Essentials of the Human Brain. Philadelphia: Elsevier; 2009.)
Anterior nuclear group (A): Relays input from the fornix to the cingulate gyrus as part of the Papez circuit; this plays a role in learning and memory.
Dorsomedial nucleus (DM): Relays input from the prefrontal cortex and the limbic system; plays a crucial role in memory, attention, planning, organization, and abstract thinking. A lesion of this nucleus is associated with Korsakoff syndrome (see later).
 Ventral anterior, lateral nuclei relay MOTOR input from basal ganglia and cerebellum to the primary motor and premotor cortex and functions in coordination and planning of movement.
Ventral anterior, lateral nuclei relay MOTOR input from basal ganglia and cerebellum to the primary motor and premotor cortex and functions in coordination and planning of movement.
 Ventral posterior medial (VPM), and ventral posterior lateral (VPL) nuclei relay SENSORY input from the face (VPM) via the trigeminal nerve (cranial nerve [CN] V) and from the body (VPL) via dorsal columns and spinothalamic tract.
Ventral posterior medial (VPM), and ventral posterior lateral (VPL) nuclei relay SENSORY input from the face (VPM) via the trigeminal nerve (cranial nerve [CN] V) and from the body (VPL) via dorsal columns and spinothalamic tract.
Medial geniculate nucleus (MGN): Relays auditory input from the inferior colliculus to the primary auditory cortex. Medial for music.
Lateral geniculate nucleus (LGN): Relays visual input from the retina to the optic cortex via the optic radiations. Lateral for looking.
Pulvinar: Integration of visual, auditory and somatosensory input.
Limbic System
The limbic system (Fig. 13-11), which consists of the hippocampus, amygdala, limbic cortex, fornix, and mammillary body, provides myriad functions, such as memory, emotion, award, fear, pleasure, addiction, and olfaction.
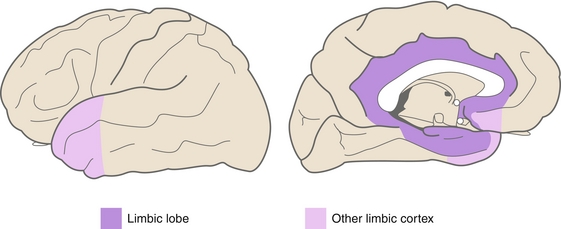
Figure 13-11 The limbic system. (From Nolte J. Essentials of the Human Brain. Philadelphia: Elsevier; 2009.)
Mnemonic for limbic system functions: Five Fs = Feeding, Fleeing, Fighting, Feeling and… Sex.
Ventricular System
The ventricular system (Fig. 13-12) is a set of caves connected by tunnels in the brain that is continuous with the central canal of the spinal cord and subarachnoid space. It contains CSF, which functions as a cushion in protecting the brain, providing buoyancy in suspending the brain against gravity and maintaining chemical stability. CSF starts in two lateral ventricles and moves into the third ventricle via the interventricular foramina of Monro and then into the fourth ventricle via the cerebral aqueduct of Sylvius.
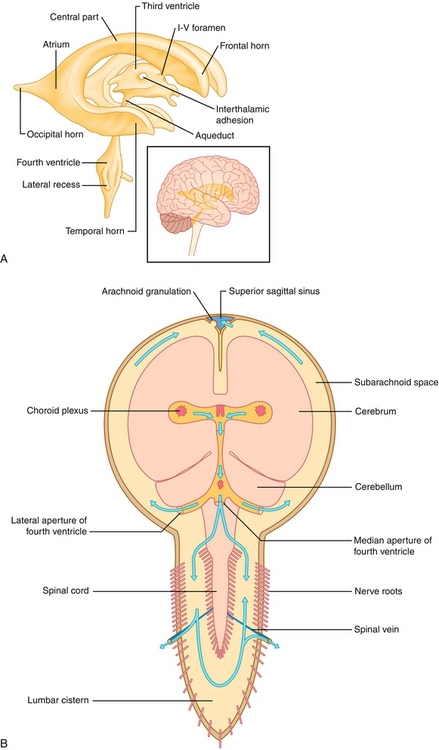
Figure 13-12 A, The ventricular system. B, Its anatomic relationship to the brain. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
From there it can continue to pass straight into the central canal of the spinal cord or go into the cisterns of the subarachnoid space via three small foramina: the median aperture (foramen of Magendie) and the right and left lateral apertures (foramina of Luschka). Because the foramen of Magendie is the median aperture, there is only one (but there are two lateral apertures of Luschka). Once the CSF is in the subarachnoid space, it can flow down the spinal cord into the lumbar cistern at the end of the cord around the cauda equina (where lumbar punctures are performed) or flow around the superior sagittal sinus to be resorbed via the arachnoid villi into the venous system. The ventricular system is implicated in pathologies such as hydrocephalus (abnormal enlargement of ventricles), meningitis, ventriculitis, and subarachnoid hemorrhage, which will be discussed in more detail later (see “Pathology”).
Cerebellum
The cerebellum (Fig. 13-13) is a structure located below the cerebral cortex and behind the pons component of the brainstem, where it plays a crucial role in the coordination, accuracy, and timing of our movements. It houses four deep nuclei, which from lateral to medial are the dentate, emboliform, globose, and fastigial nuclei.
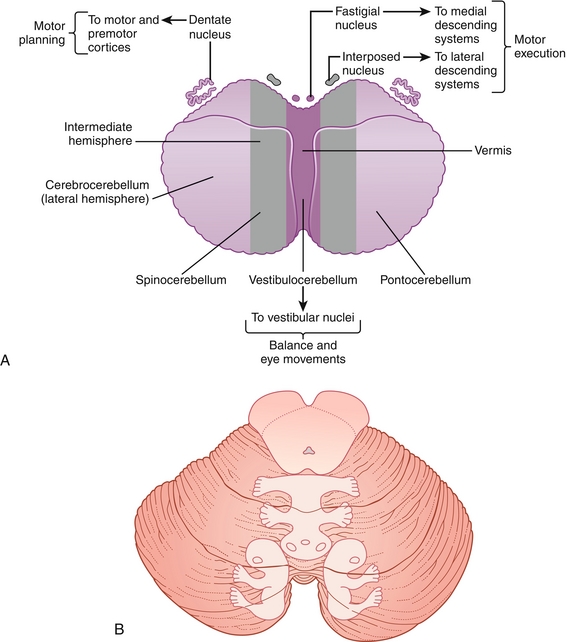
Figure 13-13 A, Cerebellar anatomy and function. B, Cerebellum homunculus. (A from FitzGerald MJT, Gruener G, Mtui E: Clinical Neuroanatomy and Neuroscience. 5th ed. Philadelphia: Saunders; 2007; B from FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
Mnemonic “Don’t Eat Greasy Foods.”
These nuclei receive inhibitory GABAergic input from Purkinje cells and excitatory glutaminergic input from mossy and climbing fibers via the inferior cerebellar peduncle (ipsilateral proprioception input) and medial cerebellar peduncle (contralateral cortical input). Once input is received, modulated output signals are projected from the dentate nuclei by Purkinje fibers via the superior cerebellar peduncle to the contralateral VA and VL nuclei of the thalamus. Because of these connections, it is important to note that a lesion of the cerebellum affects the ipsilateral side of the body. Based on anatomy, the cerebellum can be subdivided to understand the functional denomination:
1. Lateral cerebellar hemisphere: Dentate nuclei aid in voluntary movement of the extremities as part of the cerebrocerebellum pathway.
2. Midline-medial vermis: Interposed (emboliform and globose) and fastigial nuclei aid in balance and fine-tuning of body and limb movements as part of the spinocerebellum pathway.
3. Flocculonodular lobe: Fastigial nuclei aid in balance and eye movement as part of the vestibulocerebellum pathway using visual and vestibular input from the retina and semicircular canals.
Blood Supply
Cerebral circulation is provided by two main arteries, the internal carotid artery and vertebral artery. The anterior circulation is provided by the internal carotid arteries, which branch into the anterior and middle cerebral arteries. The posterior circulation is provided by the vertebral arteries, which fuse to form the basilar artery (supplies brainstem and cerebellum) which in turn branches into the posterior cerebral arteries. Both internal carotid arteries connect via the anterior communicating artery at the anterior cerebral artery in the cerebral vault. The internal carotid is interconnected with the posterior circulation via the posterior communicating arteries in the cerebral vault. Together, this forms the circle of Willis (Fig. 13-14), which provides interconnections between the internal carotid arteries and basilar artery, allowing for collateral (backup) circulation if one of the artery supplies becomes stenosed or occluded. The circle of Willis includes the anterior cerebral artery, anterior communicating artery, internal carotid artery, posterior cerebral artery, and posterior communicating artery.
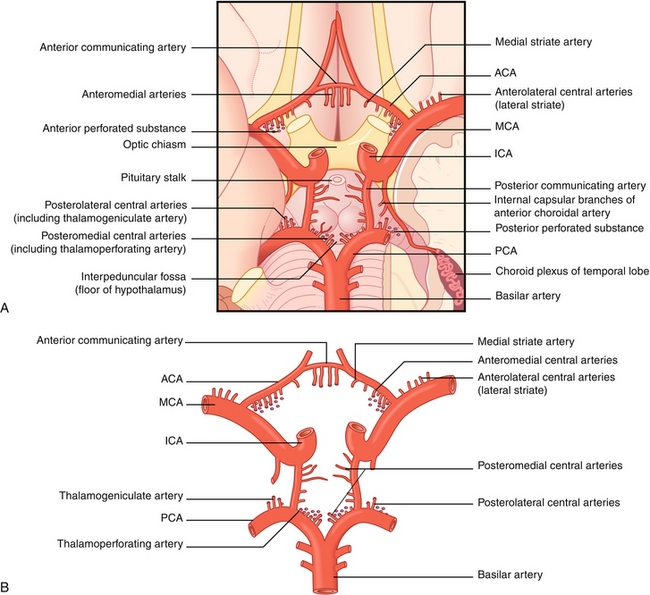
Figure 13-14 A, The circle of Willis as viewed on the inferior surface of the brain. B, The circle of Willis viewed in isolation. ACA, anterior communicating artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior communicating artery. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
The cerebral circulation is supplied by three main arteries—the anterior, middle, and posterior cerebral arteries. Each artery supplies distinct parts of the cerebral hemisphere (Fig. 13-15). The anterior cerebral artery supplies the anteromedial surfaces, which include the frontal and parietal lobes, anterior portion of the basal ganglia and internal capsule, and medial motor homunculus. The middle cerebral artery supplies the lateral surfaces, which include the anterior and inferior temporal lobes, insular cortices, lateral surfaces of the hemispheres, and deep branches of the basal ganglia. The posterior cerebral artery supplies the posterior and inferior surfaces, which are primarily formed by the occipital lobe.
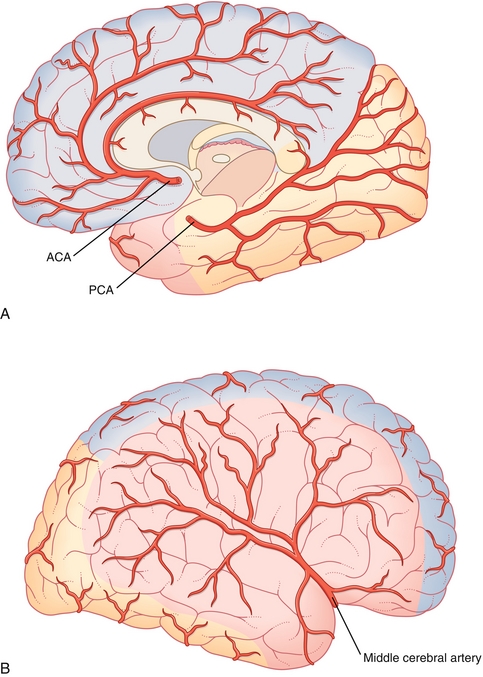
Figure 13-15 Cerebral circulation with anterior communicating artery (ACA) territory in blue, middle cerebral artery territory in red, and posterior communicating artery (PCA) territory in orange. A, Medial view. B, Lateral view. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
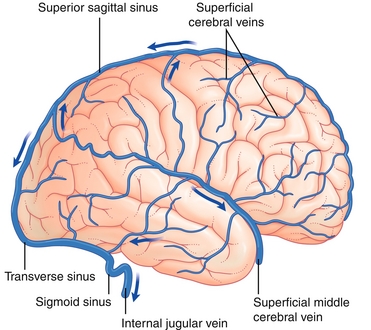
Venous drainage of the brain includes superficial (superior sagittal sinus) and deep subdivisions (inferior sagittal sinus) that connect at the confluence of sinuses before bifurcating into two transverse sinuses (Fig. 13-16). The transverse sinuses travel laterally and inferiorly in an S-shaped curve that forms the sigmoid sinuses, which go on to form the jugular veins.
Brainstem
The brainstem (Fig. 13-17), which consists of the medulla, pons, and midbrain, is a continuous structure adjoining the brain to the spinal cord and has conductive and integrative functions. It includes many of the motor and sensory tracts (corticospinal, spinothalamic, and posterior column) from the spinal cord, as well as motor and sensory innervations from the face via CNs III to XII).
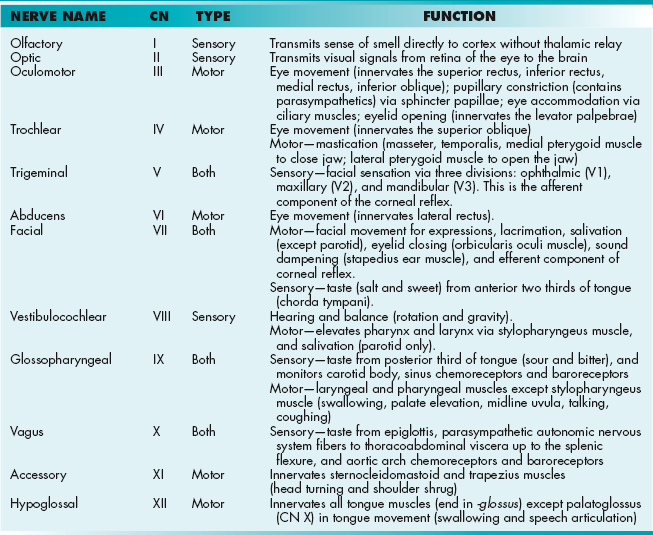
Cranial Nerves
See Table 13-1.
Unlike spinal nerves, which emerge from the spinal cord, CNs emerge directly from the brain (brainstem or cerebrum; see Table 13-1). A good way to remember where each cranial nerves emerge is the “2, 2, 4, 4” rule, where the first two CNs emerge above the brainstem (CNs I, II), the next two emerge in the midbrain (CNs III, IV), the next four in the pons (CNs V, VI, VII, VIII), and the final four in the medulla (CNs IX, X, XI, XII).
Mnemonic for the names of each CN: “On Old Olympus’ Towering Top, A Friendly Viking Grew Vines And Hops.”
Mnemonic of whether the cranial nerve (listed in order of CNs I through XII) carries sensory signals, motor signals, or both: “Some Say Marry Money, But My Brother Says Big Business Makes Money.”
In addition to knowing the name and functions of each CN, it is important to know the pathway they take in the base of the skull (Fig. 13-18). These pathways become important when discussing pathology.
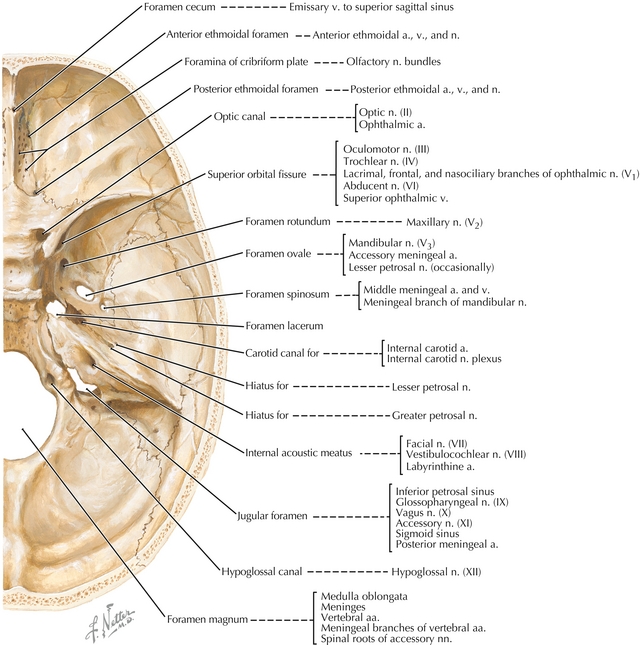
Figure 13-18 Fossa in the cranium and the structures that pass through it. (From Hansen J. Netter’s Clinical Anatomy, 2nd ed. Philadelphia: Elsevier; 2009.)
Cribriform plate: CN I goes through this and can be injured or transected in a trauma (e.g., nasal fracture), which can lead to temporary or permanent anosmia.
Middle Cranial Fossa
Optic canal: CN II, ophthalmic artery and central retinal vein.
Superior orbital fissure: CN III, IV, V1, VI, ophthalmic vein, and sympathetic fibers. Nerve blocks can be performed here for lacerations in the V1 distribution.
Foramen rotundum: CN V2: Nerve blocks can be performed here for lacerations in the V2 distribution.
Foramen ovale: CN V3: Nerve blocks can be done here for lacerations in the V3 distribution.
The trigeminal nerve (CN V) is packed full of so many branches of nerves that there is Standing Room Only (V1: superior orbital fissure; V2: foramen rotundum; V3: foramen ovale).
Foramen spinosum: Middle meningeal artery: Damage to this artery leads to an epidural hematoma.
Posterior Cranial Fossa
Internal auditory meatus: CNs VII, VIII: Schwannomas growing near this cause hearing loss.
Jugular foramen: CNs IX, X, XI, jugular vein: Lemierre’s syndrome—thrombophlebitis of the internal jugular vein caused by head and neck infections.
Foramen magnum: CNs XI spinal roots, brainstem and vertebral arteries.
Cavernous Sinus
A collection of veins within the skull located lateral to the pituitary gland and superior to the sphenoid sinus (Fig. 13-19). It drains blood from the ophthalmic vein and superficial cortical veins into the internal jugular vein. It is important to know the structures running through this sinus because pathology affecting the cavernous sinus can affect the structures running through it.
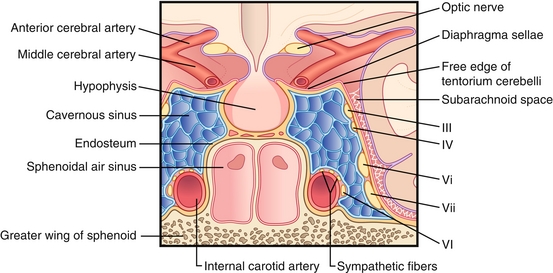
Figure 13-19 Anatomy of the cavernous sinus. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
One mnemonic for remembering the contents is “O TOM CAT”: Oculomotor nerve (CN III), Trochlear nerve (CN IV), Ophthalmic nerve (CN V1), Maxillary nerve (CN V2), Carotid artery (internal), Abducens nerve (CN VI), and Trochlear nerve (repeat).
When looking at the orientation of these structures, it is important to note that the abducens nerve and carotid artery run through the middle of the sinus, whereas other structures run along the lateral walls:
Mnemonic: CN “six sticks” to the carotid.
All the nerves pass through the superior orbital fissure, with the exception of CN V2 (exits via the foramen rotundum). The most commonly tested pathology of the cavernous sinus is the cavernous sinus thrombosis, where a blood clot forms in the cavernous sinus, usually as a result of an infection nearby spreading into the sinus. The classic symptoms include visual changes, exophthalmos (from an enlarged cavernous sinus pushing on the eye), headache, and cranial nerve palsies. The most commonly affected cranial nerve is the abducens nerve (CN VI).
Spinal Cord
The spinal cord houses the major motor and sensory tracts that interconnect the rest of the body to the brain (Fig. 13-20). It consists of three major motor and sensory tracts—dorsal (posterior) columns (Fig. 13-21), lateral corticospinal tracts (Fig. 13-22), and spinothalamic tracts (Fig. 13-23). Each specializes in conducting specific sensory information to the brain. The dorsal column provides ascending pressure, vibration, touch, and proprioceptive sensory information. The dorsal column is organized with the arms outside and legs inside, where the fasciculus cuneatus is lateral (upper body and extremities, C2-T6) and the fasciculus gracilis is medial (lower body, extremities, T7 and below).
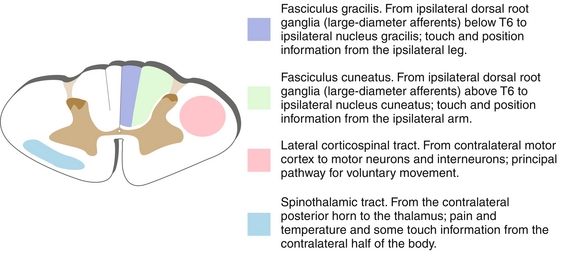
Figure 13-20 Cross section of the spinal cord. The dorsal columns are composed of the fasciculus gracilis (legs) and fasciculus cuneatus (arms). (From Nolte J. Essentials of the Human Brain. Philadelphia: Elsevier; 2009.)
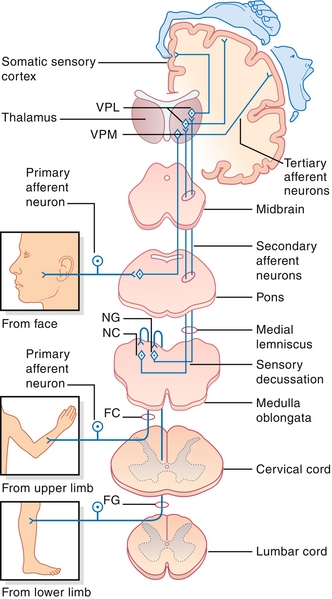
Figure 13-21 Posterior (dorsal) column, medial lemniscal pathway. As shown, the decussation is in the brainstem; therefore, spinal lesions will cause ipsilateral loss of proprioception and vibratory sensation, whereas cortical lesions will cause contralateral loss. FC, Fasciculus cuneatus; FG, fasciculus gracilis; NC, nucleus cuneatus; NG, nucleus gracilis; VPL, VPM, ventral posterior lateral, ventral posterior medial nuclei of thalamus. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
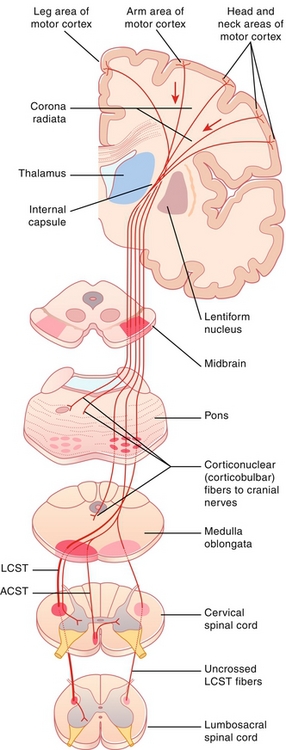
Figure 13-22 Lateral corticospinal tract pathway. As shown, the decussation is in the brainstem; therefore, spinal lesions will cause ipsilateral paralysis, but lesions above the medulla will cause contralateral paralysis. ACST, anterior corticospinal tract; LCST, lateral corticospinal tract. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
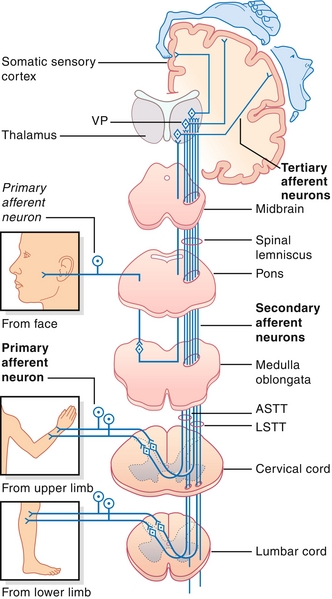
Figure 13-23 Spinothalamic tract. The decussation of these fibers is different than that of the posterior (dorsal) column, medial lemniscal pathway, and lateral corticospinal tract pathway in that the decussation of the fibers is in the spinal cord itself. This becomes important in conditions such as Brown-Séquard syndrome. ASTT, anterior spinothalamic tract; LSTT, lateral spinothalamic tract; VP, ventral posterior nucleus of thalamus. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
Mnemonic: Dancers are graceful because they know where their legs are, thanks to the fasciculus gracilis.
The spinothalamic tract provides ascending pain and temperature sensory information. The lateral corticospinal tract provides descending voluntary motor information to the contralateral limbs. These latter two tracts are organized as if someone is diving into the spinal cord, where the hands are medial and legs are lateral. The dorsal (posterior) columns and lateral corticospinal tracts cross sides (decussate) in the brainstem, whereas the spinothalamic tracts cross in the spinal cord. This nuance is important when talking about injuries to just half of the spinal cord (Brown-Séquard syndrome; see later).
Spinal nerves are a part of the PNS, where they exit the spinal cord carrying a mix of motor, sensory, and autonomic signals. There are 31 pairs of spinal nerves, which include 8 cervical spinal nerve pairs (C1-C8), 12 thoracic pairs, 5 lumbar pairs, 5 sacral pairs, and 1 coccygeal pair. Cervical spinal nerves (C1-7) exit above the corresponding vertebra, whereas the remaining spinal nerves exit below. The clinical significance of these nerves is that each spinal root supplies a specific myotome and dermatome, which can be used to localize lesions depending on the neurologic deficits seen on exam. For example, if vertebral disk herniation occurred at the nerve roots between L5 and S1 (the most common site of disk herniation), this could lead to difficulty with toe walking.
Important dermatomes to memorize in localizing lesions (Fig. 13-24) include T4 (the nipple), T10 (the umbilicus), L1 (inguinal ligament), and the various parts of the feet. The medial foot is L4, the top of the foot is L5, and the lateral foot is S1.
Auditory Pathway
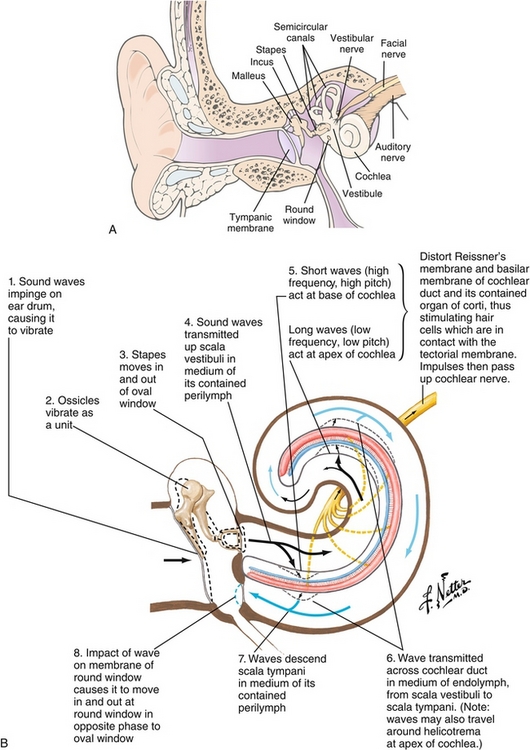
The ear is divided into three sections, with each playing a unique role in detecting sound. The external ear acts like a satellite dish to capture pressure waves (sound) and focus them on the eardrums. The air-filled middle ear (Fig. 13-25A) contains three ossicles (malleus, incus, and stapes). These ossicles mechanically convert the low pressure vibrations at the ear drum into amplified high pressure waves to cause fluid (perilymph) movement in the inner ear via the oval window. This fluid movement stimulates hair cells in the inner ear (cochlea), which transforms this mechanical movement into electrical signals in neurons (Fig. 13-25B). The electrical nerve impulses are now transmitted down cochlear fibers to the brain via the vestibulocochlear nerve. Before reaching the thalamus (medial geniculate nucleus [MGN]), and being relayed to the primary auditory cortex on the temporal lobe, they are processed at intermediate stations, such as the cochlear nuclei and superior olivary complex of the brainstem and inferior colliculus of the midbrain.
Vestibular Pathway
The vestibular system (Fig. 13-26) is another component of the inner ear, which is dedicated to balance. Three canals, oriented perpendicular to each other, provide sensory input for rotary movements, with the horizontal canal detecting horizontal head movement (e.g., spinning), and the superior and posterior canals detecting vertical head movement (e.g., nodding head). Each canal opens into the utricle and has a dilated sac at one end (ampulla), which houses the crista ampullaris (hair cells and supporting cells) in a gelatinous structure (cupula). Each canal is filled with endolymph, which lags behind as the head moves. This lag pushes opposite of the cupula, causing hair cells to bend, and depending on the tilt of the hair cells, excitatory (depolarizing) or inhibitory neural electrical signals are generated.
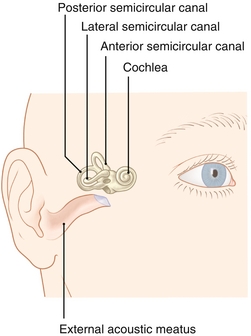
Figure 13-26 The vestibular system is composed of three semicircular canals. The horizontal canal detects horizontal head movements, whereas the superior and posterior canals detect vertical head movements. (From Moses K, Nava P, Banks J, Petersen D. Atlas of Clinical Gross Anatomy, 2nd ed. St. Louis: Elsevier; 2012.)
Visual System
The eye muscles (Fig. 13-27) are innervated by three cranial nerves (CNs III, IV, and VI). A good way to memorize which muscles are innervated by which cranial nerves is to think of the fictional molecule LR6SO4R3, where the Lateral Rectus is innervated by CN 6, Superior Oblique is innervated by CN 4, and the Rest innervated by CN 3. Damage to a cranial nerve leads to specific findings when looking at the eye and testing these extraocular muscles (see later, “Eye Pathology”).
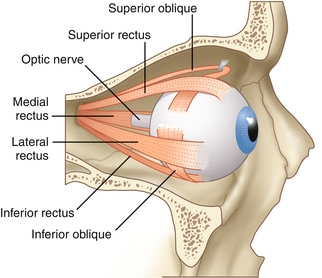
Figure 13-27 Extraocular muscles. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
The eyes convert light waves into electrochemical signals via the retina. Light is first refracted by the cornea, goes through the pupil (opening that is controlled by the iris), and is further refracted by the lens (shape changed by ciliary body) to project an inverted image on the retina (Fig. 13-28).
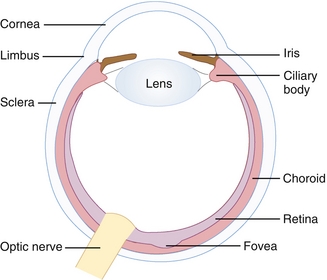
Figure 13-28 Basic structures in the eye. (From Telser AG, Young JK, Kate M. Baldwin KM. Elsevier’s Integrated Histology. Philadelphia: Elsevier; 2007.)
The retina consists of layers that house two types of photoreceptor cells, cones (found in high density in the central retina [fovea], responsible for color perception, high visual acuity) and rods (found in retinal periphery, responsible for monochromic [black-white] and night vision, low visual acuity). It also houses bipolar cells, which are an intermediary in transmitting signal from photoreceptors to ganglion cells. Photoreceptors contain rhodopsin (rods) or photopsin (cones), which contain a large plasma membrane protein (opsin) bound to retinal (a vitamin A derivative), that is very important in the visual phototransduction process (light → electrochemical signal). Retinal exists as cis-retinal, which changes configuration into trans-retinal on light exposure and leads to activation of transducin (G protein), which activates cyclic guanosine monophosphate (cGMP) phosphodiesterase. This enzyme breaks down cGMP, leading to closure of sodium channels, hyperpolarizing the cell and stopping the release of NTs. These NTs generally inhibit the bipolar cells in the dark, but in the light they allow bipolar cells to transmit the signal to the optic nerve.
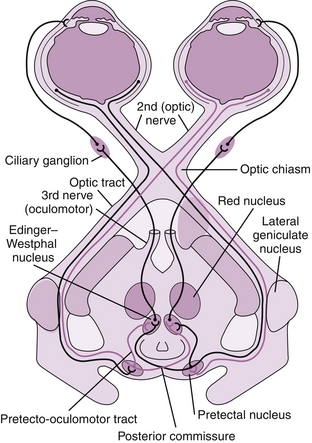
The signal is carried from the optic nerve to the optic chiasm, where nasal retinal fibers cross over. Nasal retinal fibers are those fibers on the retina closest to the nose, whereas temporal fibers are those on the lateral retina, closest to the temples. Beyond the optic chiasm, the optic nerve continues as the optic tract, which carries the crossed and uncrossed fibers to the LGN of the thalamus. From the LGN, the signal is carried to the pretectal nucleus of the midbrain (involved in pupillary reflex) and the visual cortex (occipital lobe) by optic radiations (Fig. 13-29). The optic radiations are split into two parts. Fibers from the inferior retina (Meyer’s loop) carry information from the superior part of the visual field, passing through the temporal lobe by looping around the inferior horn of the lateral ventricle. Fibers from the superior retina (Baum’s loop) carry information from the inferior part of the visual field on a shorter pathway (less susceptible to damage) through the parietal lobe. Lesions along this pathway, from the retina to the visual cortex, can lead to various visual field defects(see later, “Eye Pathology”).
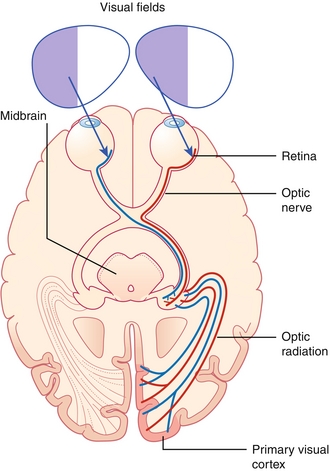
Figure 13-29 The visual pathway, from the retina to the visual cortex. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
In the pupillary light reflex (Fig. 13-30), neurons from the pretectal nucleus run to the Edinger-Westphal nucleus, which receives bilateral input from the pretectal nuclei. From here, presynaptic neurons synapse at the ciliary ganglion and postsynaptic neurons innervate the pupillary sphincter muscle (sphincter pupillae), causing pupillary constriction (miosis).
Electroencephalogram (EEG)
Similar to how an electrocardiogram (ECG) records the electrical activity of the heart, an EEG records the electrical activity of the brain. Clinically, neurologists can use the EEG for the diagnoses of epilepsy (ictal discharges), coma, encephalopathies (triphasic waves), and brain death. It is important to keep the following in mind:
Sleep: As one goes into deeper stages of sleep, these waves slow down.
 Stage 1 → theta waves (presleep, or nodding off).
Stage 1 → theta waves (presleep, or nodding off).
 Stage 2 → sleep spindles (most prominent form; accounts for ≈ 50% of sleep during the night).
Stage 2 → sleep spindles (most prominent form; accounts for ≈ 50% of sleep during the night).
 Stage 3-4 → delta waves (this is the stage where sleep walking and night terrors occur).
Stage 3-4 → delta waves (this is the stage where sleep walking and night terrors occur).
 Rapid eye movement (REM) → beta waves (EEG looks as if the patient were awake, but muscles are paralyzed. REM sleep is when dreams occur. Disrupted REM sleep is thought to be the cause of sleep paralysis).
Rapid eye movement (REM) → beta waves (EEG looks as if the patient were awake, but muscles are paralyzed. REM sleep is when dreams occur. Disrupted REM sleep is thought to be the cause of sleep paralysis).
PATHOLOGY
Developmental Disorders
Disruption during the development of the nervous system can be disastrous, leading to a variety of conditions listed below.
Neural tube defects
Failure of the neural tube to close can lead to a continuum of these defects extending from anencephaly to subtypes of spina bifida (see Chapter 4).
Hydrocephalus
This literally translates to “water in the brain” caused by the excess accumulation of CSF in the ventricular system of the brain from impaired CSF flow (e.g., obstruction), resorption, or excessive production of various causes. Because of the enclosed space of the skull, signs of increased intracranial pressure (ICP) develop, such as headaches, nausea, vomiting, papilledema, sleepiness, coma, and even death caused by herniation. It is important to note that in infants, ICP symptoms present as irritability, poor feeding, muscle hypertonia, and hyperreflexia. Treatment often includes opening up the ventricles (ventriculostomy) or placement of cerebral shunts. Shunts can bypass outflow obstructions or drain excessive CSF into body cavities, such as the abdominal peritoneal cavity, where it can be resorbed. Based on the underlying mechanism, hydrocephalus can be categorized into communicating and noncommunicating categories. They can be further subdivided into congenital or acquired.
Noncommunicating, Obstructive
There is CSF outflow obstruction in the ventricular system.
Congenital: Caused by atresia or webs within the ventricular system (e.g., within the cerebral aqueduct of Sylvius), Arnold-Chiari malformation, or Dandy-Walker syndrome.
 Arnold-Chiari malformation: Malformation of the brain in which there is a downward herniation of the cerebellar tonsils through the foramen magnum. Conditions associated with this malformation include syringomyelia and connective tissue disorders, such as Ehlers-Danlos and Marfan’s syndrome.
Arnold-Chiari malformation: Malformation of the brain in which there is a downward herniation of the cerebellar tonsils through the foramen magnum. Conditions associated with this malformation include syringomyelia and connective tissue disorders, such as Ehlers-Danlos and Marfan’s syndrome.
 Dandy Walker syndrome (DWS): Consists of partial or complete absence of the cerebellar vermis, enlargement of the fourth ventricle, and cyst formation near the internal base of the skull. DWS is associated with corpus callosum absence and karyotype abnormalities.
Dandy Walker syndrome (DWS): Consists of partial or complete absence of the cerebellar vermis, enlargement of the fourth ventricle, and cyst formation near the internal base of the skull. DWS is associated with corpus callosum absence and karyotype abnormalities.
 Following subarachnoid hemorrhage, causes obstruction within the channels of the ventricular system (e.g., stenosis of aqueduct of Sylvius).
Following subarachnoid hemorrhage, causes obstruction within the channels of the ventricular system (e.g., stenosis of aqueduct of Sylvius).
 Underlying brain tumor (e.g., ependymoma, medulloblastoma, colloid cyst) within the ventricular system or one causing external compression of the ventricular system.
Underlying brain tumor (e.g., ependymoma, medulloblastoma, colloid cyst) within the ventricular system or one causing external compression of the ventricular system.
Communicating, Nonobstructive
There is impaired CSF resorption by arachnoid granulations or villi caused by scarring or fibrosis following infectious, inflammatory, or hemorrhagic events, such as postmeningitis or arachnoid bleeds.
Normal pressure hydrocephalus (NPH): This disease entity is often misdiagnosed as Parkinson’s disease or Alzheimer’s disease because of the chronic, insidious nature of the symptoms. Because of the chronic dilation of the ventricular system, NPH presents classically with the triad of urinary incontinence, gait disturbance (ataxia), and dementia in older adults, despite having normal CSF pressures on lumbar puncture. This constellation of symptoms is often termed wet, wobbly, and wacky. Treatment is with a ventriculoperitoneal shunt that drains the excess CSF to the abdominal peritoneal cavity. If performed early, shunting can reverse the symptoms.
Hydrocephalus ex vacuo: There is a compensatory enlargement of CSF because of atrophy or loss of brain parenchyma caused by various diseases, despite no increased CSF pressure (e.g., post-traumatic brain injury) and dementias (e.g., Alzheimer’s, Pick’s, Huntington’s dementia).
Signs of increased ICP, such as papilledema, can also be confused with another disease process known as idiopathic intracranial hypertension (IIH), formerly known as pseudotumor cerebri. IIH is seen in young obese females, who present with headache, nausea and vomiting. They may also complain of pulsatile tinnitus (described as whooshing or buzzing), diplopia, and deteriorating vision, eventually leading to blindness if not treated. The mechanism of this disease process is poorly understood, with speculation that it is caused by increased CSF production or decreased venous drainage from the brain. On funduscopy, these patients have papilledema. Computed tomography (CT) scans will reveal no mass, but sometimes small slitlike ventricles and an empty sella sign can be seen. Diagnosis is usually made by measuring the opening lumbar puncture (LP) pressure, which is greatly elevated.
Treatment of this condition starts with drainage of excess CSF by LP; serial LPs may be necessary. Acetazolamide also decreases CSF production. Patients should also discontinue medications that increase ICP, such as high-dose vitamin A derivatives (e.g., isotretinoin for acne), tetracycline, and hormonal contraceptives. Surgery is a last resort, in which a CSF shunt (lumboperitoneal) and optic nerve decompression can be performed.
Syringomyelia: Also known as a syrinx. A cystic cavity forms within the spinal cord, which expands over time, leading to pain, weakness, and paralysis. Upper extremities are usually affected because of a mass effect on the spinal tracts. The dorsal column is usually spared (pressure, vibration, touch and proprioception remain intact). Typically, this disease presents as a capelike loss of pain and temperature sensation in the back and arms. A syrinx can be congenital or associated with Arnold-Chiari malformation. It can also be acquired as a complication of trauma, meningitis, or tumor.
Neurocutaneous syndromes: Also known as phakomatoses, theses syndromes present with lesions on the skin or eye.
 NF type 1: Autosomal dominant mutation of neurofibromin on chromosome 17, which is a tumor suppressor that inhibits p21 ras oncoprotein. This mutation leads to uncontrolled cell proliferation. Characteristics include neurofibromas, groin and axillary freckling, café au lait spots (light brown macules), Lisch nodules (iris hamartomas), optic gliomas, and epilepsy.
NF type 1: Autosomal dominant mutation of neurofibromin on chromosome 17, which is a tumor suppressor that inhibits p21 ras oncoprotein. This mutation leads to uncontrolled cell proliferation. Characteristics include neurofibromas, groin and axillary freckling, café au lait spots (light brown macules), Lisch nodules (iris hamartomas), optic gliomas, and epilepsy.
 NF type 2 (central NF): Autosomal dominant mutation of merlin on chromosome 22, which is also a tumor suppressor gene. This leads to bilateral acoustic neuromas (schwannomas) that cause sensorineural hearing loss. Think NF 2: 2-22-2 (NF 2 is chromosome 22 and can cause 2 [bilateral] acoustic neuromas).
NF type 2 (central NF): Autosomal dominant mutation of merlin on chromosome 22, which is also a tumor suppressor gene. This leads to bilateral acoustic neuromas (schwannomas) that cause sensorineural hearing loss. Think NF 2: 2-22-2 (NF 2 is chromosome 22 and can cause 2 [bilateral] acoustic neuromas).
Tuberous sclerosis: Autosomal dominant mutation of hamartin or tuberin, which are tumor suppressors, leading to a multisystem disease that has a variable penetrance. Signs include facial angiofibromas (adenoma sebaceum), hypomelanic macules (ash leaf spots), Shagreen patches, cortical tubers, cardiac rhabdomyomas, and renal angiomyolipoma.
Sturge-Weber Syndrome: Occurring sporadically because of embryonal development anomalies, this syndrome presents with seizures at birth and is associated with port wine stain of the face (forehead and upper eyelid), glaucoma, mental retardation, and ipsilateral leptomeningioma.
von Hippel-Lindau (VHL) disease: Autosomal dominant mutation of VHL tumor suppressor gene on chromosome 3 leads to hemangioblastomas (retina, kidney or cerebellum), often associated with renal angioma, renal cell carcinoma, and pheochromocytoma.
Brain Lesions
Understanding anatomy and relating it to the respective function is key in localizing neurologic lesions. Table 13-2 is a list of common pathologic examples that can be encountered.
Table 13-2
Brain Lesions and Their Resulting Symptoms
| Location | Result |
| Frontal lobe | Lack of executive functions leads to disinhibition and reemergence of primitive reflexes. |
| Nondominant parietal lobe | Hemispatial neglect (agnosia of the contralateral side of the world) |
| Dominant parietal lobe | Gerstmann syndrome—agraphia, acalculia, finger agnosia |
| Amygdala | Klüver–Bucy syndrome—disinhibition, psychic blindness (visual agnosia), hyperorality, hypersexuality |
| Hippocampus | Unable to form new memories (anterograde amnesia); unable to form new long-term memories if bilateral lesion |
| Basal ganglia | Motor symptoms, such as tremor, chorea, athetosis |
| Mammillary bodies | Wernicke-Korsakoff syndrome, which consists of Wernicke encephalopathy (confusion, ophthalmoplegia, ataxia) and Korsakoff psychosis (memory loss, confabulation, personality changes); petechial hemorrhages seen in limbic system on pathology. |
| Subthalamic nucleus | Contralateral hemiballismus |
| Cerebellar hemisphere | Ipsilateral deficits, including tremor and limb ataxia |
| Cerebellar vermis | Dysarthria and truncal ataxia |
| Frontal eye fields | Eyes deviate toward lesion. |
| Paramedian pontine reticular formation | Eyes deviate away from lesion. |
| Superior colliculus | Parinaud syndrome—upward gaze paralysis |
| Reticular activating system | Reduced level of conscious, leading to difficulty in arousal and wakefulness |
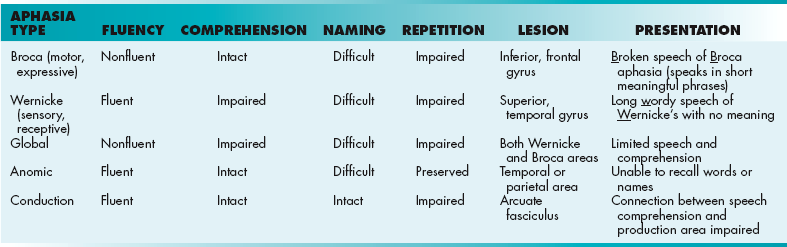
Aphasia
Aphasia is an impairment of language ability that ranges from not remembering words to being completely unable to speak, read, or write, depending on the area and extent of the dominant side of the brain affected (Table 13-3). It usually follows a stroke, but can develop from conditions such as an infection, tumor, or brain injury. In contrast, dysarthria is the motor inability to speak.
Vascular Dysfunction
Similar to a myocardial infarction (MI), a stroke is caused by impeded blood flow leading to specific neurologic deficits, depending on the area of the brain affected. Just as time is muscle in a myocardial infarction, time is brain in a stroke. Irreversible damage develops within a few minutes, so reperfusion with therapies such as tissue plasminogen activator (tPA; see later, “Pharmacology”) quickly is important. Most strokes are ischemic because of embolic phenomenon from atherosclerotic lesions or atrial fibrillation. Carotid dissection (primary or secondary to aortic dissection), endocarditis, or a patent foramen ovale (allowing a blood clot in the leg that breaks off to pass from the right to the left atrium, and then to the left ventricle and brain, called paradoxical emboli) also cause ischemic strokes. Approximately 15% of strokes are hemorrhagic, in which intracerebral bleeding results directly from vessel rupture or hemorrhagic conversion from an ischemic stroke because of increased vessel fragility. A CT scan is used to differentiate between ischemic and hemorrhagic stroke initially, followed by magnetic resonance imaging (MRI; diffusion-weighted imaging). Prior to a stroke, a patient may have experienced a transient ischemic attack (TIA), in which they experience brief neurologic dysfunction that lasts less than 24 hours. For example, some patients experience amaurosis fugax, which is a temporary loss of vision caused by atherosclerotic embolization to the retinal arteries.
Stroke
 Anterior cerebral artery: Supplies anteromedial hemispheric surface, where leg-foot motor and sensory areas are affected.
Anterior cerebral artery: Supplies anteromedial hemispheric surface, where leg-foot motor and sensory areas are affected.
 Middle cerebral artery: Supplies lateral hemispheric surface, where face-arm motor and sensory areas are affected. In addition, patients may show aphasias (Broca or Wernicke’s aphasia) if the dominant lobe is involved (usually the left).
Middle cerebral artery: Supplies lateral hemispheric surface, where face-arm motor and sensory areas are affected. In addition, patients may show aphasias (Broca or Wernicke’s aphasia) if the dominant lobe is involved (usually the left).
 Posterior cerebral artery: Supplies posterior and inferior hemispheric surface, where homonymous hemianopsia with macular sparing can occur.
Posterior cerebral artery: Supplies posterior and inferior hemispheric surface, where homonymous hemianopsia with macular sparing can occur.
Aneurysm: An aneurysm is a balloon-like bulge of a blood vessel that involves all three layers of an artery (intima, media and adventitia). An aneurysm may rupture, causing subarachnoid hemorrhage.
 Saccular or berry aneurysm: Occurs at bifurcations in the circle of Willis, where rupture leads to hemorrhagic strokes or subarachnoid hemorrhage. Often associated with Marfan’s syndrome, Ehler-Danlos syndrome, and adult polycystic kidney disease.
Saccular or berry aneurysm: Occurs at bifurcations in the circle of Willis, where rupture leads to hemorrhagic strokes or subarachnoid hemorrhage. Often associated with Marfan’s syndrome, Ehler-Danlos syndrome, and adult polycystic kidney disease.
 Anterior communicating artery: Most common location of aneurysm, where lesions lead to bitemporal hemianopsia
Anterior communicating artery: Most common location of aneurysm, where lesions lead to bitemporal hemianopsia
 Posterior communicating artery: Common site of aneurysm, which can lead to CN III palsy (eyes down and out)
Posterior communicating artery: Common site of aneurysm, which can lead to CN III palsy (eyes down and out)
 Charcot-Bouchard microaneurysm: Chronic hypertension leads to rupture of small vessels, usually within the basal ganglia and thalamus.
Charcot-Bouchard microaneurysm: Chronic hypertension leads to rupture of small vessels, usually within the basal ganglia and thalamus.
 Dural sinus thrombosis: Rare stroke that results from the thrombosis of the dural venous sinus, resulting in strokelike symptoms, headaches, weakness and seizures. A CT scan shows an empty delta sign, where there is a filling defect in the sagittal sinus.
Dural sinus thrombosis: Rare stroke that results from the thrombosis of the dural venous sinus, resulting in strokelike symptoms, headaches, weakness and seizures. A CT scan shows an empty delta sign, where there is a filling defect in the sagittal sinus.
Brainstem: Unlike cerebrum strokes, brainstem strokes often involve cranial nerves (Table 13-4). Most notably, brainstem strokes show alternating signs; CN involvement and hemiparesis are on opposite sides.
Table 13-4
| Occlusion Syndrome | Vasculature Affected | Symptoms |
| Medial medullary syndrome | Vertebral, vertebral branch, lower basilar artery | Contralateral hemiparesis (arm + leg), decreased proprioception, ipsilateral tongue (hypoglossal nerve) paralysis; facial sparing |
| Lateral medullary syndrome (Wallenberg syndrome) | Posterior inferior cerebellar artery (PICA) | Contralateral loss of pain and temperature, ipsilateral facial pain and temperature (sensory signs and symptoms only) |
| Lateral inferior pontine syndrome | Anterior inferior cerebellar artery (AICA) | Ipsilateral facial pain and temperature loss, ipsilateral facial paralysis |
| Locked-in syndrome | Basilar artery | Anterior pons infarction leads to quadriparesis (face and eyes also), but vertical gaze (CN III), pontine tegmentum, and reticular formation spared (consciousness preserved). |
| Foville’s syndrome | Perforating basilar arteries | Contralateral hemiparesis, hemisensory loss, and internuclear ophthalmoplegia (INO); ipsilateral horizontal gaze and facial paralysis |
| Weber’s syndrome | Paramedian branches of posterior cerebral artery (PCA) | Contralateral parkinsonism, hemiparesis, lower facial muscle paralysis, and hypoglossal nerve paralysis; ipsilateral CN III palsy. |
Trauma
Traumatic brain injury (TBI) is a major cause of death and disability worldwide, especially for young adults. In TBI, or any situation of altered mental status, a patient’s level of consciousness (eye, verbal and motor response) is graded based on the Glasgow Coma Scale (GCS). The scale ranges from 3 to 15 and classifies brain injuries as mild (13 to 15), moderate (9 to 12), or severe (3 to 8).
Classification
 Diffuse: Diffuse axonal injury is a devastating type of TBI as a result of shearing forces from a sudden acceleration or deceleration of the head. Diagnosis is usually made via MRI because it is difficult to detect on a CT. Patients generally end up staying in a coma and never regaining consciousness.
Diffuse: Diffuse axonal injury is a devastating type of TBI as a result of shearing forces from a sudden acceleration or deceleration of the head. Diagnosis is usually made via MRI because it is difficult to detect on a CT. Patients generally end up staying in a coma and never regaining consciousness.
Epidural hematoma: As a result of temporal bone fracture from trauma at the pterion (weakest part of skull; Fig. 13-31A), the middle meningeal artery can rupture, leading to a buildup of the blood between the dura mater and skull. Patients generally have a lucid interval, but soon rapidly deteriorate because of transtentorial herniation (see later) as a result of expansion of the hematoma under systemic arterial pressure. CT generally shows a “biconvex” lens that does not cross suture lines, but can cross the falx and tentorium (dural reflections).
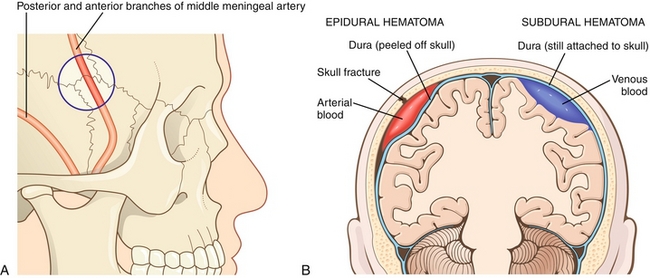
Figure 13-31 A, The middle meningeal artery is injured in epidural hematomas. The blue circle is over the pterion. B, Comparison between epidural and subdural hematomas. Note that because the dura is so firmly adherent to the skull at the suture lines, epidural hematomas are not strong enough to peel the dura off the sutures and therefore cannot cross suture lines. However, subdural hematomas are under the dura (hence, subdural) and therefore can cross suture lines. (A from FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011; B from Kumar V, Abbas AK, Fausto N, Aster J. Robbins & Cotran Pathologic Basis of Disease. 8th ed. New York: Elsevier; 2009.)
Subdural hematoma (SDH): Bleeding between the dura and arachnoid membrane (Fig. 13-31B) from the rupture of small bridging veins as a result of rapid changes in velocity (acceleration or deceleration in whiplash or shaking). It is commonly seen in older adults and alcoholics because of brain atrophy, which increases the subdural space and results in a wider distance in which the veins have to travel, making them vulnerable to tear. The same principle applies to children from shaken baby syndrome, who tend to have larger subdural spaces because of their smaller brains. Unlike an epidural hematoma, subdural bleeds are slow venous bleeds with delayed onset of symptoms. As pressure (ICP) gradually increases, patients begin to have increasing headache and confusion. CT generally shows blood collecting in a crescent-shaped moon pattern that crosses suture lines, but cannot cross the dural reflections.
Subarachnoid hemorrhage (SAH): Bleeding between the arachnoid membrane and pia mater surrounding the brain results from rupture of a cerebral aneurysm (especially berry aneurysms in Marfan’s syndrome, Ehlers-Danlos syndrome, and polycystic kidney disease) or arteriovenous malformation (AVM), spontaneously or from trauma (Fig. 13-32). Patients generally complain of sudden onset of the “worst headache of my life” or may present beforehand with warning leaks as general headaches. Most SAHs are detected on CT after the onset of bleeding, which shows blood in the cisterns and filling of blood along the sulci and fissures. If a CT scan is negative, a lumbar puncture is performed and CSF collected, which may show elevated blood cells equally in all tubes collected, or the CSF may appear yellow (xanthochromic) because of the bilirubin (breakdown of heme from red blood cells). Treatment consists of preventing rebleeding (clipping or coiling aneurysms), seizures, vasospasm (treat with calcium channel blocker nimodipine), and hydrocephalus.
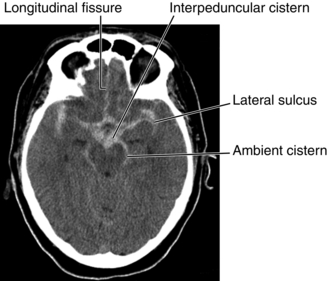
Figure 13-32 CT scan of subarachnoid hemorrhage showing blood (the more dense white signal) in the areas normally occupied by the cerebrospinal fluid (normally a less dense and more black signal), such as the cisterns shown in the image. This is why a lumbar puncture can be used for diagnosis; the blood is in the CSF itself. (From Nolte J. The Human Brain. 6th ed. Philadelphia: Elsevier; 2008.)
Intracerebral hemorrhage (ICH): This is bleeding within the brain tissue itself that can occur as a result of trauma (e.g., skull fracture, AVM), stroke, bleeding within a tumor, or amyloid angiopathy. ICH risk factors include hypertension, diabetes, menopause, current cigarette smoking, and alcoholism.
Basilar skull fracture: Traumatic fracture involving the base of the skull (temporal, occipital, sphenoid and ethmoid bone), leading to damage to the meninges. As a result, there can be CSF rhinorrhea or otorrhea and ecchymosis of the mastoid process of the temporal bone (Battle sign) or periorbital ecchymosis (raccoon eyes) (Fig. 13-33).
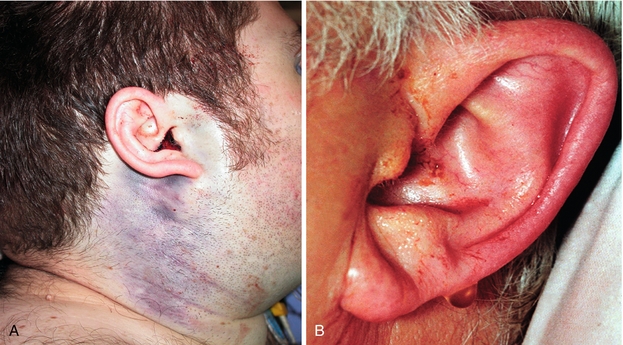
Figure 13-33 Basilar skull fracture causing Battle sign (A) and CSF otorrhea (B; literally, CSF leaking out of the ear). (From Swash M, Glynn M. Hutchison’s Clinical Methods. 22nd ed. Edinburgh: Elsevier; 2007.)
Cerebral contusion: This is literally a bruise to the brain caused by multiple microhemorrhages from small blood vessel leaks. An example is coup contrecoup injury, in which the coup injury occurs at the site of impact and the contrecoup injury occurs on the side opposite the impact, where the brain is forced against the skull.
Herniation
This is a serious consequence of very high ICP or mass effect that results from a traumatic brain injury, tumor, or stroke. The skull is a closed cavity that cannot expand to accommodate increasing ICP, so the brain shifts through structures such as the falx cerebri, tentorium cerebelli, and foramen magnum in an attempt to decompress. This places extreme pressure on delicate neural structures, which often leads to coma and death. Herniated patients typically exhibit the following two abnormal postures (Fig. 13-34):
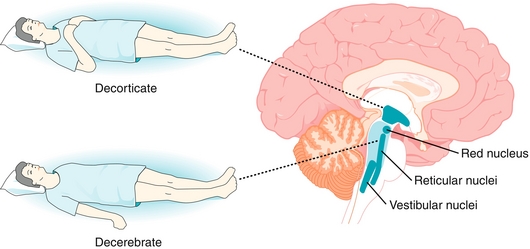
Figure 13-34 Decorticate posturing (above the red nucleus), and decerebrate posturing (below the red nucleus). The red nucleus sends signals to the upper extremity that favor flexion of the arms; therefore, lesions below the red nucleus will sever these flexor tracts and lead to decerebrate posturing. (From Weyhenmeyer J, Gallman E: Rapid Review Neuroscience. Philadelphia: Mosby; 2007.)
 Decorticate posturing: As a result of lesions above the red nucleus in the midbrain, a patient presents with arms flexed and hands clenched and bent inward over chest and the legs extended, with feet inward. The former is caused by red nucleus disinhibition, which favors motor neurons to the flexor muscles of the upper extremities. The latter is caused by lateral corticospinal tract disruption, which normally supplies motor neurons to the flexor muscles of the lower spinal cord.
Decorticate posturing: As a result of lesions above the red nucleus in the midbrain, a patient presents with arms flexed and hands clenched and bent inward over chest and the legs extended, with feet inward. The former is caused by red nucleus disinhibition, which favors motor neurons to the flexor muscles of the upper extremities. The latter is caused by lateral corticospinal tract disruption, which normally supplies motor neurons to the flexor muscles of the lower spinal cord.
 Decerebrate posturing: This is a more serious lesion involving damage below the red nucleus (usually a brainstem lesion). Patients present rigid with clenched teeth, arched head back, and arms, elbows, and legs extended.
Decerebrate posturing: This is a more serious lesion involving damage below the red nucleus (usually a brainstem lesion). Patients present rigid with clenched teeth, arched head back, and arms, elbows, and legs extended.
Herniation (Fig. 13-35) can be categorized into two major classes, supratentorial and infratentorial, depending on whether the lesion is above or below the tentorium cerebelli, consisting of subtypes according to which direction the brain moves in response to increasing pressure.
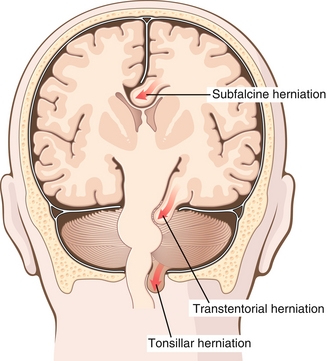
Figure 13-35 Selected herniation syndromes. Shown are subfalcine herniation under the falx cerebri, uncal transtentoral herniation under the tentorium cerebelli, and tonsillar herniation, in which the cerebellar tonsils move downward through the foramen magnum. (From Kumar V, Abbas AK, Aster J. Robbins Basic Pathology, 9th ed. St. Louis: Elsevier; 2012.)
Supratentorial
Uncal: Downward transtentorial herniation of the innermost part of the temporal lobe (uncus) through the tentorium cerebelli (dura that separates the cerebellum from the cerebrum), which can be associated with the following:
 Kernohan’s notch: Uncal herniation causes an indentation of the contralateral cerebral peduncle or crus, which contains descending corticospinal fibers, leading to ipsilateral hemiparesis at the side of herniation because it is above the decussation (crossing of the fibers). This is often termed a false localizing sign because the herniation creates injury on the opposite side of the brain.
Kernohan’s notch: Uncal herniation causes an indentation of the contralateral cerebral peduncle or crus, which contains descending corticospinal fibers, leading to ipsilateral hemiparesis at the side of herniation because it is above the decussation (crossing of the fibers). This is often termed a false localizing sign because the herniation creates injury on the opposite side of the brain.
 Compression of the ipsilateral posterior cerebral artery leads to contralateral homonymous hemianopsia.
Compression of the ipsilateral posterior cerebral artery leads to contralateral homonymous hemianopsia.
 “Blown pupil”: This is one of the earliest signs of uncal herniation. As the third cranial nerve (CN III) is compressed, parasympathetic neurons surrounding the nerve are affected first, resulting in unopposed sympathetic dilatation (mydriasis). As compression progresses, the innermost motor part of CN III is affected, leading to ptosis (CN III innervates levator palpebrae) and eye deviation (down and out).
“Blown pupil”: This is one of the earliest signs of uncal herniation. As the third cranial nerve (CN III) is compressed, parasympathetic neurons surrounding the nerve are affected first, resulting in unopposed sympathetic dilatation (mydriasis). As compression progresses, the innermost motor part of CN III is affected, leading to ptosis (CN III innervates levator palpebrae) and eye deviation (down and out).
 Duret hemorrhages are parenchymal bleeds in the pons and midbrain caused by tearing and bleeding in the small paramedian arteries of the basilar artery, leading to abnormal posturing, coma (reticular formation), and death.
Duret hemorrhages are parenchymal bleeds in the pons and midbrain caused by tearing and bleeding in the small paramedian arteries of the basilar artery, leading to abnormal posturing, coma (reticular formation), and death.
Central: Downward transtentorial herniation of both parts of the cerebral hemispheres through a notch in the tentorium cerebelli. Duret hemorrhages can also result from this.
Cingulate (subfalcine): Most common type of herniation, in which the innermost part of the frontal lobe herniates under the falx cerebri (dura that separates the two brain hemispheres). The frontal lobe can then press on the anterior cerebral artery and mimic symptoms of anterior cerebral artery ischemia or stroke.
Transcalvarial: Brain squeezes through a fracture or surgical site (e.g., craniectomy).
Infratentorial
Upward cerebellar: As a result of increasing posterior fossa pressure, the cerebellum can move up.
Downward cerebellar (tonsillar): “Coning” of the cerebellar tonsils downward through the foramen magnum. The cerebellar tonsils can compress the brainstem and cause the medullary respiratory centers to cease to function, leading to apnea or abnormal breathing. As noted, this can also occur in Arnold-Chiari malformation.
Spinal Cord
Motor neuron signs: Differentiating between upper (descending) and lower motor neurons can assist in localizing the damage/lesion along the motor tracts.
 Upper motor (UMN) signs: Hyperreflexia and increased tone with positive Babinski.
Upper motor (UMN) signs: Hyperreflexia and increased tone with positive Babinski.
 Lower motor (LMN) signs: Hyporeflexia and decreased tone with atrophy and fasciculations.
Lower motor (LMN) signs: Hyporeflexia and decreased tone with atrophy and fasciculations.
Spinal Cord Lesions
Brown-Séquard syndrome: Hemisection of the spinal cord leads to the following findings (Fig. 13-36):
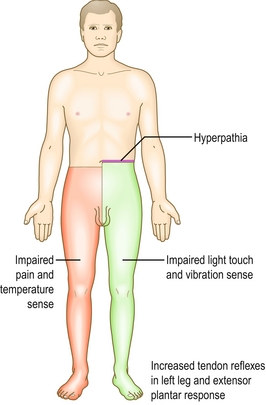
Figure 13-36 Brown-Séquard syndrome, showing the characteristic ipsilateral loss of vibration and proprioception as well as loss of motor control, with contralateral loss of sensation of pain and temperature. (From Douglas G, Robertson C. MacLeod’s Clinical Examination. 11th ed. Edinburgh: Elsevier; 2005.)
 Ipsilateral UMN signs (corticospinal tract)
Ipsilateral UMN signs (corticospinal tract)
 Ipsilateral dorsal column signs (loss of tactile, vibration, and proprioception)
Ipsilateral dorsal column signs (loss of tactile, vibration, and proprioception)
 Contralateral spinothalamic signs (pain and temperature loss); recall that the spinothalamic tract crosses in the spinal cord and not in the brainstem, so there are contralateral signs.
Contralateral spinothalamic signs (pain and temperature loss); recall that the spinothalamic tract crosses in the spinal cord and not in the brainstem, so there are contralateral signs.
Poliomyelitis: Spread through the fecal-oral route, the polio virus replicates in the oropharynx and small intestine before spreading hematogenously to the CNS, where it leads to destruction of anterior horn cells and to LMN signs.
Tabes dorsalis: Degeneration of the dorsal columns of the spinal cord as a result of tertiary syphilis. Patients can present with a variety of symptoms, such as weakness, paresthesias (shooting, lightning, burning, pricking pain), tabetic gait (high-stepping gait, with foot striking ground), loss of coordination, impaired response to light (Argyll Robertson pupil; see later, “Eye Pathology”), progressive joint degeneration (Charcot joint caused by neuropathic osteoarthropathy—destruction → resorption → deformity → ulceration → infection; can also be caused by diabetes), positive Romberg test (tests vestibular dysfunction, cerebellar dysfunction, and proprioceptive sensory loss), absence of deep tendon reflexes (DTRs; Westphal sign), and tabetic ocular crisis (sudden, intense ocular pain with lacrimation and photophobia, known as Pel crisis). Treatment often consists of IV penicillin, with pain control and physical therapy.
Horner’s syndrome: Ipsilateral presentation of facial rubor (flushing), anhidrosis (absence of facial sweating), miosis (pupil constriction), and ptosis (superior tarsal muscle) [mnemonic—RAMP], which results from lesions of the sympathetic outflow on the affected side (Fig. 13-37). Many diseases can be the cause, including apical lung tumors (e.g., Pancoast tumor), Brown-Séquard syndrome, lateral medullary syndrome, cluster-migraine headache, trauma, thoracic aortic aneurysm, multiple sclerosis, late-stage syringomyelia, and carotid artery dissection.
Infection
Meningitis: This is inflammation of the meninges, which houses CSF; it is the protective membrane surrounding the brain and spinal cord. Patients typically present complaining of headache and neck stiffness associated with fever, altered level of consciousness (e.g., confused and lethargic), photophobia, and phonophobia. In meningococcal meningitis (caused by Neisseria meningitidis), a rapidly spreading petechial rash (nonblanching) can be seen on the trunk, extremities, mucous membranes, and conjunctiva. On examination, patients generally have signs of meningismus, which include nuchal rigidity (inability to flex the neck passively), Kernig knee sign (knee pain with passive extension), and Brudzinski neck sign (neck flexion causes involuntary flexion of the knee and hip). Ultimately, diagnosis is made by lumbar puncture (LP), which can differentiate the various causes of meningitis, ranging from bacterial to aseptic (nonbacterial) causes, such as viral and fungal, by looking at specific factors within the CSF (Table 13-5). CSF can also be sent for more specialized tests to look for certain causes, such as VDRL (syphilis), India ink (Cryptococcus), viral polymerase chain reaction (PCR) assay (enterovirus, herpes simplex virus [HSV]), and titers (neurosyphilis, Lyme disease, Coccidioides).
Initial treatment should include IV fluid resuscitation if hypotension or shock is present. Because bacterial meningitis can be life-threatening, antibiotics should be immediately started if there is a high clinical suspicion prior to obtaining blood work, LP, or blood cultures. Adjuvant treatment with corticosteroids should also be given to reduce overall inflammation, leading to reduced mortality, hearing loss, and neurologic sequelae (e.g., learning and behavioral disabilities). Viral meningitis typically requires supportive therapy alone (e.g., fluids, analgesics) because of the benign course. However, if a patient suffers a seizure during your care, suspect HSV, which has a predilection for affecting the temporal lobes and causing seizures. In this case, the addition of acyclovir is warranted. Despite ideal management of meningitis, complications can arise, such as deafness, epilepsy, seizures, hydrocephalus, disseminated intravascular coagulation (DIC), Waterhouse-Friderichsen syndrome (adrenal hemorrhage with hypotension and hyperkalemia), and sepsis, leading to death.
Encephalitis: Encephalitis is an acute inflammation of the brain that presents as fever, headache, confusion, drowsiness, and fatigue, with a viral or bacterial cause. Some common viruses to keep in mind are herpes simplex, rabies virus, poliovirus, and West Nile virus. Some common bacterial causes include syphilis, Lyme disease, and pathogens that cause meningitis.
Abscess: A brain abscess is a collection of infected material formed from distant infectious sources or local spread (e.g., meningitis, mastoiditis, dental abscess, otitis, sinusitis). Patients will have increased ICP and focal neurologic signs caused by this space-occupying lesion. As a result, a patient will appear very ill, with a fever, headache, and focal neurologic findings. Diagnosis is established by CT, which typically will show a ring-enhancing lesion. LP is contraindicated because of risk of herniation. Treatment ultimately consists of a combination of antibiotics and surgical drainage of the abscess, depending on the location.
Viral Infections
West Nile Virus is caused by Flaviviridae, usually affecting humans through infected mosquitoes. At first, a patient may be asymptomatic, but can eventually develop a fever and encephalitis.
Progressive multifocal leukoencephalopathy (PML) is a disease caused by the John Cunningham virus (JCV; polyomavirus) leading to quickly progressing demyelination of oligodendrocytes. Normally, JCV is kept under control by the immune system. It causes disease only when the immune system has been severely weakened, especially in AIDS patients or those receiving chemotherapy.
Parasitic Infections
Toxoplasmosis is a parasitic disease caused by Toxoplasma gondii that affects humans through contaminated cat feces or ingestion of meats (pork, lamb, venison) infested with cysts. The disease spectrum includes encephalitis, chorioretinitis, cutaneous lesions, and congenital lesions.
Taenia solium, also known as pork tapeworm, is the most common tapeworm infection of the brain worldwide. It causes neurocysticercosis, which is the leading cause of seizures and epilepsy in the developing world. This disease develops from eating raw or undercooked pork or from the ingestion of tapeworm eggs via the fecal-oral route. The eggs hatch in the intestine and pass through the intestine wall to migrate preferentially toward the brain and muscles, where they form cysts that can remain dormant for years. However, once active, they may cause a local inflammatory reaction causing myalgias or seizures. Treatment with albendazole and steroids is only warranted if a patient is symptomatic (e.g., having seizures). Otherwise, if one coincidentally finds an asymptomatic patient with neurocysticercosis on CT, no treatment is needed.
Prion: A prion is an infectious misfolded protein that accounts for transmissible spongiform encephalopathies, such as bovine spongiform encephalopathy (mad cow disease) in cows and Creutzfeldt-Jakob disease (CJD), kuru, fatal familial insomnia, and Gerstmann-Straussler-Scheinker (GSS) syndrome in humans. Prions can arise from sporadic mutation or genetic transmission, or they can be acquired. Once acquired, these misfolded proteins act as a template to induce preexisting, properly folded proteins in neural tissues to convert into misfolded ones (α helix → β sheets), which continue the same process in a chain reaction cycle that exponentially grows the population of misfolded proteins. Patients typically present with rapid neurologic deterioration, starting with personality and psychiatric changes and ataxia, which leads to dementia and loss of the ability to speak. Findings on exam generally include myoclonus (involuntary jerking movements), with electromyography (EMG) showing triphasic spikes. Once acquired, the end result is universally fatal, and patients are generally given supportive and comfort care. Strict sterilization in denaturing the protein’s tertiary structure is key in preventing further transmission. The mainstay of sterilization is the combination of heat, pressure, and chemical cleaners.
Demyelinating Diseases
Multiple sclerosis (MS): MS is an autoimmune disorder of the CNS, mostly affecting women in their 20s and 30s, which eventually leads to demyelination (Fig. 13-38) and inflammation of the CNS. As the name implies, multiple sclerosed plaques involving the white matter (cerebellum, brain stem, basal ganglia, spinal cord, and optic nerve) result from repeated damage and destruction to the oligodendrocytes over time. In addition to demyelination, inflammation results from T cell entry into the brain via the blood-brain barrier (BBB), where it recognizes myelin as a foreign molecule and triggers inflammatory cytokines. The BBB is normally not permeable to any type of cell unless the integrity of the tight junctions forming the barrier becomes compromised.
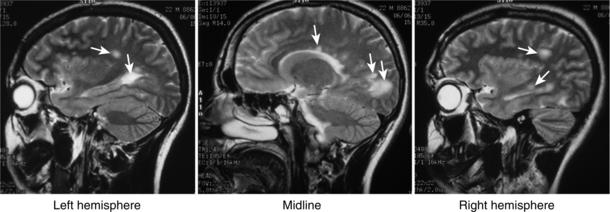
Figure 13-38 Multiple demyelinating plaques (arrows) can be seen on this MRI image of the brain. (From Weyhenmeyer J, Gallman EA. Rapid Review Neuroscience. Philadelphia: Elsevier; 2006.)
MS can present as a variety of neurologic symptoms, such as visual problems (e.g., nystagmus, optic neuritis, diplopia), dysarthria, dysphagia, muscle weakness, spasms, or paralysis, hypoesthesia or paresthesia, or bowel and bladder difficulties (constipation, diarrhea, frequency, retention, incontinence). Sometimes patients may complain of worsening of symptoms when the body gets overheated (e.g., during hot weather or exercise). The increased temperature slows or blocks nerve impulses. In other cases, some complain of electrical shock sensation down the spine (Lhermitte sign). Typically, a patient presents with Charcot triad (SIN mnemonic) of scanning-telegraphic speech, intention tremor (incontinence and internuclear ophthalmoplegia), and nystagmus. The most common presentation is optic neuritis, where the patient complains of sudden vision loss (partial or complete), blurry or foggy vision, pain with eye movement, and some subtle loss of color vision, especially red.
Because of the wide array of symptoms, MS can be difficult to diagnose. The McDonald criteria combine clinical, laboratory and radiologic data. Neuroimaging using MRI with gadolinium contrast is the gold standard, in which multiple lesions or plaques of demyelination can be seen anywhere from the brain down to the spinal cord. Lesions around the ventricle-based brain veins are classic (Dawson fingers). LP can show signs of chronic inflammation and typically shows increased oligoclonal IgG bands on electrophoresis. In addition, there is increased p100 latency of nerves when doing visual, auditory, and sensory evoked potentials caused by demyelination. Treatment of an acute attack of MS generally consists of high doses of IV corticosteroids for the inflammation and symptomatic treatment of pain, spasticity (e.g., baclofen), and fatigue (e.g., amantadine). Long-term disease-modifying treatments include immunomodulators, such as interferon-β, fingolimod, glatiramer acetate, mitoxantrone, and natalizumab. Deficits suffered during an acute attack may resolve or persist. Ultimately, the overall treatment goal is to prevent the increasing disability that results over time from recurrent episodes of relapsing-remitting MS and prevent development into progressive MS.
Acute disseminated encephalomyelitis (ADEM): Similar to MS, ADEM is an autoimmune disease that produces multiple inflammatory lesions in the brain and spinal cord. However, it generally affects children and adolescents following viral, bacterial, or parasitic infection and requires an extended period of time to recover completely
Transverse myelitis: This demyelinating inflammatory process occurs across the thickness of the spinal cord following infection, immunization, or the development of MS. Depending on the level of the spinal cord and the tracts involved, patients present with motor, sensory, and sphincter (bowel, bladder) deficits. Transverse myelitis should be on the differential of acute onset of extremity weakness and numbness. Other conditions on the differential should include Guillain-Barré syndrome, acute spinal cord trauma, compressive spinal cord lesions, and spinal cord infarction.
Central pontine myelinolysis (CPM): CPM, also known as osmotic demyelination syndrome, is a complication of overly rapid correction of severe hyponatremia. The serum osmolarity is low in hyponatremia; with chronic hyponatremia, the cells make intracellular adaptations to equalize the osmolarity between the serum and inside the cell. However, if the hyponatremia is rapidly corrected, the serum osmolarity will now be significantly higher than the intracellular osmolarity. This rapidly draws water out of the cell and damages the myelin. Patients generally develop an acute onset of dysphagia, dysarthria, and paralysis. No specific treatment other than supportive care is available once demyelination occurs. Although most patients die, some may live with disability, ranging from mild tremors and ataxia to spastic quadriparesis and locked-in syndrome.
Leukodystrophy: This is a group of inherited disorders that leads to impaired growth and development of the myelin sheath. Leukodystrophy can be inherited in a recessive, dominant, or X-linked manner.
Guillain-Barré syndrome (GBS): GBS, also known as acute inflammatory demyelinating polyneuropathy, is an autoimmune disorder affecting the PNS. Because of molecular mimicry following infections (often C. jejuni or CMV), there is an autoimmune attack on myelin of the peripheral sensory and motor nerves. A patient may present with symmetric ascending weakness starting distally in the hands and feet and migrating toward the trunk. Bulbar cranial nerves can also be affected, leading to facial weakness or paralysis, dysphagia, drooling, and difficulty swallowing and maintaining an open airway. Commonly, respiratory support is needed (e.g., endotracheal intubation) because of impending respiratory failure. Sensory loss is manifested as loss of proprioception and reflexes, along with bladder dysfunction. Autonomic dysfunction can also be seen in severe cases (e.g., cardiac arrhythmia, fluctuating blood pressure). Suspect GBS if a patient presents with rapid onset of muscle paralysis, areflexia, absence of fever, and a GI or respiratory infection in the past 30 days. Diagnosis is confirmed with LP, which shows CSF with an albuminocytologic dissociation (increased protein without increased cell count), and nerve conduction study (NCS) or EMG showing prolonged distal latencies and conduction slowing. There are two treatment options: (1) plasmapheresis to filter antibodies out of the blood system; and (2) IV immune globulin (IVIG) to neutralize antibodies. Most patients recover weeks to months after onset, but some may have residual disability. Some patients may have one or more relapses of similar symptoms, classifying them as chronic inflammatory demyelinating polyneuropathy (CIDP).
Charcot-Marie-Tooth (CMT) disease: An incurable, hereditary, sensorimotor neuropathy that consists of a group of clinical and genetic subtypes, in which there is a defect in the production of neuronal proteins involved in the myelin sheath and axon of peripheral nerves. Initially there are intermittent severe painful muscle contractions that are disabling, but over time there is loss of muscle tissue and touch sensation in the extremities. Generally, high-arched cavus feet are associated with this disease.
Subacute combined degeneration (Lichtheim disease): As discussed in the spinal cord pathologies, there is patchy loss of myelin in the dorsal and lateral columns caused by vitamin B12 deficiency. Patients typically present with weakness, paresis, numbness, and/or tingling in the legs, arms and trunk. If the B12 deficiency is not corrected, damage may be irreversible. If a patient has B12 and folic acid deficiencies, the vitamin B12 deficiency should be corrected first to avoid precipitating this condition.
Neurodegenerative Diseases
Alzheimer disease’s (AD): AD is the leading cause of dementia, in which there is reduced overall synthesis of the neurotransmitter acetylcholine and diffuse cortical atrophy (especially in the hippocampus) because of the loss of neurons and synapses. Although the exact cause of AD is not known, higher amounts of extracellular β-amyloid core (amyloid β [Aβ] protein or senile plaques), and intracellular abnormally phosphorylated tau protein (neurofibrillary tangles) are seen on histology.
AD develops sporadically in most cases; 10% of cases are familial and tend to be earlier in onset. Early-onset AD is also seen in Down syndrome patients (trisomy 21), who carry an extra copy of the gene for Aβ precursor protein (APP). Early-onset AD is also seen in mutations in presenilin-1 and -2 (chromosomes 1 and 14, respectively), which are subcomponents of an enzyme responsible for converting APP to Aβ protein. Late-onset AD is seen in those carrying apolipoprotein E4 (ApoE4), which is an isoform allele of the gene found on chromosome 19. Another isoform, ApoE2, serves a protective role in AD. AD is a diagnosis of exclusion that is clinically diagnosed. Short-term memory loss is usually the initial symptom. As the disease progresses, problems with long term memory, executive function, and behaviors develop.
Amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease): ALS is a fatal disorder that affects both lower motor neurons and upper motor neurons. The autonomic nervous system and cognition are spared. It is characterized by a rapidly-progressive weakness, muscle atrophy and fasciculations (LMN signs), spasticity (UMN sign), dysphagia, dysarthria, and eventually respiratory compromise and death. Although the pathophysiology is not completely understood, it is linked to a mutation in the superoxide dismutase (SOD) enzyme, which is an antioxidant that generally neutralizes superoxide, a toxic free radical. Other than symptomatic treatment, riluzole is the only treatment known to improve survival by preventing glutamate excitotoxicity indirectly.
Spinal muscle atrophy (SMA): This autosomal recessive disease is caused by a mutation in a gene coding a crucial protein (SMN) for motor neuron survival. As a result, anterior motor neuron death results, leading to diffuse, whole-body atrophy and LMN signs (e.g., fasciculations). SMA can manifest from infants to adults in varying forms of severity. In infants, it is most severe and called Werdnig-Hoffman disease, or floppy baby syndrome because of the abnormally low muscle tone observed. In addition to palliative care, no cure exists for SMA, but gene therapy, stem cell therapy, and SMN activation may eventually play a role in treatment.
Movement Disorders
These are neurologic disorders characterized by various abnormal or dysfunctional movements. Chorea, for example, is a sudden, jerky, purposeless movement generally seen with lesions to the basal ganglia, which can evolve into athetosis, which is a slow, writhing movement, especially of the fingers. In contrast to other movements, hemiballismus is a sudden wild flailing of one arm caused by a subthalamic nucleus lesion. Other movements can include myoclonus (sudden, brief involuntary muscle contraction, such as jerks or hiccups), and dystonias, which are sustained, involuntary muscle contractions.
Parkinson’s disease (PD): PD is a degenerative disorder of the CNS in which the dopamine-producing cells of the substantia nigra in the midbrain undergo cell death for unknown reasons. It has been postulated that the accumulation of Lewy bodies (alpha-synuclein intracellular inclusions) and its progression in location from the brainstem to the cortex plays a role in PD development. Referring back to the basal ganglia section in physiology, keep in mind that dopamine plays a significant role in motor movement modulation. In general, high dopamine levels promote motor activity, whereas low levels, such as those in PD, decrease it. As a result of the loss of dopaminergic neurons, patients develop a collection of motor symptoms, known as parkinsonism. Classic symptoms include rigidity (increased muscle tone), bradykinesia-akinesia (slowness of or no movement), postural instability (impaired balance and frequent falls), and tremor (pill-rolling tremor at rest only). Other common symptoms include a festinating shuffling gait (short steps) with decreased arm swing and turning en bloc, hypophonia (soft speech), micrographia (small, cramped handwriting), and hypomimia (masklike emotionless face). In addition to motor symptoms, patients also develop sleeping difficulties (REM disorders precede motor findings) and neuropsychiatric symptoms affecting mood, cognition, behavior, or thought (e.g., depression, apathy, anxiety). Similar to AD, primary idiopathic PD is diagnosed clinically with a medical history and neurologic examination. When dealing with a patient with parkinsonism, secondary or acquired causes must also be ruled out. Methyl-phenyl-tetrahydropyridine (MTPT), a contaminant in illicit street drugs, can cause selective destruction of dopaminergic neurons, whereas drug-induced parkinsonism can result from antipsychotics (e.g., haloperidol) because of dopamine D2 receptor blockade. Parkinson plus syndromes are conditions in which parkinsonism exists with some other dominating feature. For example, multiple system atrophy (MSA), also known as Shy-Drager syndrome, is a combination of parkinsonism, ataxia, and autonomic dysfunction. Progressive supranuclear palsy (PSP) is a combination of parkinsonism with predominantly visual symptoms (especially inability to move the eyes vertically), causing early falls (Fig. 13-39).
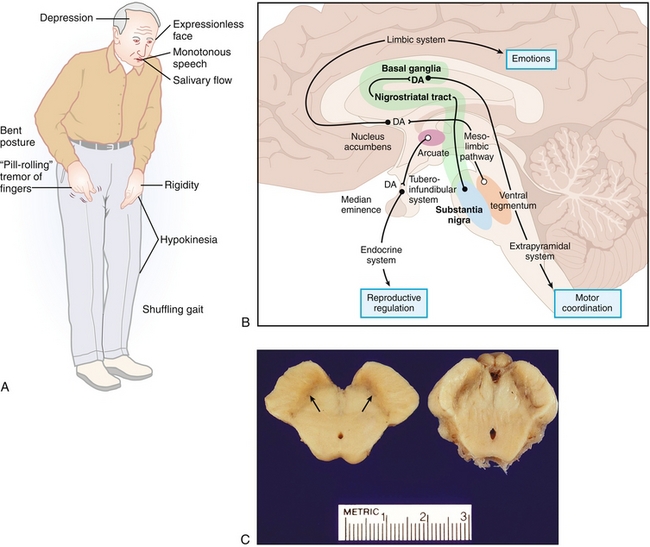
Figure 13-39 Parkinson’s disease. A, Classic symptoms of Parkinson’s disease. B, Dopaminergic pathways that are altered in Parkinson's disease, leading to the symptoms shown in A. C, Cross section of the midbrain showing the substantia nigra (arrows, “black substance”; left), with significantly fewer dopaminergic neurons; compare with a normal midbrain and normal substantia nigra (right). DA, dopamine. (A from Damjanov I. Pathophysiology. Philadelphia: Elsevier; 2008; B from Kester M, Karpa KD, Quraishi S, Vrana KE. Elsevier’s Integrated Pharmacology. Philadelphia: Elsevier; 2007; C from Adkison LR, Brown MD. Elsevier’s Integrated Genetics. Philadelphia: Elsevier; 2007.)
Huntington’s disease (HD): An autosomal dominant neurodegenerative genetic trinucleotide repeat disorder. The Huntington gene on chromosome 4 contains a sequence of CAG (glutamine) that is repeated. This polyglutamine sequence normally consists of fewer than 36 glutamine repeats in normal individuals; once it is 36 or more repeats, the Huntington protein possesses different characteristics and is considered a mutant form. This mutant form leads to neuronal death via NMDA receptor binding and glutamate excitotoxicity. Patients typically present with a triad of early dementia (ages, 20s to 50s), behavioral changes (e.g., emotional lability, aggression, hypersexuality), and choreiform movement. CT scanning of the brain generally shows atrophy of the striatum, which consists of the caudate nucleus (cognition) and putamen (motor). Once the disease is suspected, genetic testing is crucial in confirming physical findings, and providing the patient with genetic counseling is crucial. The trinucleotide CAG is unstable during replication (only with paternal spermatogenesis), and its instability increases with the number of repeats present. As a result, genetic anticipation is seen—with each successive generation, the numbers of repeats increases, along with the severity and earlier onset of HD. There is no cure for HD, and symptoms are treated as appropriate (Fig. 13-40).
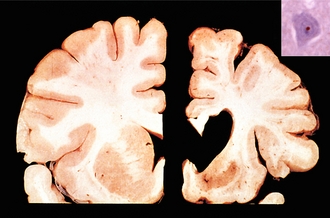
Figure 13-40 Huntington’s disease, showing a normal brain with normal striatum (left) and the brain of a patient with Huntington’s disease (right). Inset, Histology slide with an intranuclear inclusion that is strongly immunoreactive for ubiquitin (H&E stain, 6.25 micrometers). (From Kumar V, Abbas AK, Aster J. Robbins Basic Pathology. 9th ed. St. Louis: Elsevier; 2012.)
Friedreich ataxia: An autosomal recessive inherited disorder in which trinucleotide repeats (GAA) lead to decreased transcription of the frataxin protein. As a result, mitochondrial dysfunction occurs because of free radical damage from cytoplasmic iron buildup. Patients present from the age of 5 to 30 years with signs and symptoms such as kyphoscoliosis, ataxia (wide-based gait), dysarthria (slurred speech), nystagmus, and high plantar arches (pes cavus). Patients develop heart disease (e.g., conduction defects, cardiomyopathy) and diabetes. Death is usually secondary to hypertrophic cardiomyopathy.
Dementia
Dementia (Table 13-6) is an irreversible global decrease in cognitive ability beyond what is expected with age. It is important to differentiate it from delirium, which is often reversible and waxing and waning. Further differentiation is discussed in Chapter 14. Before making the diagnosis of dementia, other organic causes must be ruled out, such as syphilis, HIV, vitamin B deficiency, Wilson’s disease, and hypothyroidism.
Table 13-6
| Disease | High-Yield Facts |
| Alzheimer’s disease | Most common form of dementia; diagnosis of exclusion |
| Pick’s disease (frontotemporal dementia) | Caused by frontotemporal atrophy; characterized by personality changes (disinhibition, emotional blunting); aphasia is also a common feature |
| Lewy body dementia | Visual hallucinations are a key feature, but others include parkinsonism and mental status fluctuations. |
Seizures
A seizure is an episode in which there is an abnormal excess of synchronous neuronal firing that leads to transient symptoms, such as tonic-clonic movements, behavioral alterations, or sensory alterations (e.g., déjà vu, burning rubber olfactory hallucination). Many conditions can bring about an episode of a seizure, including hypoglycemia, hyponatremia, fever, delirium tremens, infection, stroke, and brain tumors. Epilepsy is a term used specifically to describe recurrent, spontaneous, unprovoked seizures from an underlying alteration of brain function. Epilepsy is a continuum of seizure disorders that differ in terms of clinical presentation, underlying causes, and pathophysiology, which is classified according to site of origin and pattern of spread (see Fig. 13-41).
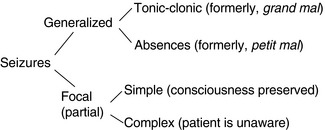
Figure 13-41 Classification of seizure disorders. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
Partial seizures: Arise in a localized, focal area of the brain, but can spread to nearby areas that can ultimately become secondarily generalized seizures with diffuse brain involvement. For example, a Jacksonian seizure starts in one area of the brain and moves along the motor homunculus. Typically, a “Jacksonian march” starts with tingling in the fingers moving more proximally, leading to arm, head, and eye movements. Sometimes the seizure can cross to the other side of the brain, leading to a secondarily generalized seizure.
 Simple partial: Seizures present as motor, sensory, autonomic, or psychologic symptoms, depending on origin. Consciousness is not impaired; however, the seizure can spread, leading to a complex partial seizure or generalized seizure.
Simple partial: Seizures present as motor, sensory, autonomic, or psychologic symptoms, depending on origin. Consciousness is not impaired; however, the seizure can spread, leading to a complex partial seizure or generalized seizure.
 Complex partial: A seizure preceded by an aura, in which consciousness is impaired. Patients may remain motionless or engage in repetitive behaviors, called automatisms (e.g., grimacing, lip smacking). Although it may arise from any lobe of the brain, it most commonly arises from the mesial temporal lobe. Occasionally, mesial temporal sclerosis may be seen on imaging, indicating gliosis and atrophy from neuronal loss.
Complex partial: A seizure preceded by an aura, in which consciousness is impaired. Patients may remain motionless or engage in repetitive behaviors, called automatisms (e.g., grimacing, lip smacking). Although it may arise from any lobe of the brain, it most commonly arises from the mesial temporal lobe. Occasionally, mesial temporal sclerosis may be seen on imaging, indicating gliosis and atrophy from neuronal loss.
Generalized seizures: Diffuse brain involvement that impairs consciousness.
 Absence: Formerly known as petit mal seizures, these are brief seizures of sudden onset and termination, with no postictal confusion. Because this often presents with staring spells in children it may be confused with attention deficit disorder. EEG shows 3-Hz generalized spike and slow wave discharges. Treatment is mainly with ethosuximide or valproic acid.
Absence: Formerly known as petit mal seizures, these are brief seizures of sudden onset and termination, with no postictal confusion. Because this often presents with staring spells in children it may be confused with attention deficit disorder. EEG shows 3-Hz generalized spike and slow wave discharges. Treatment is mainly with ethosuximide or valproic acid.
 Myoclonic: Rapid involuntary contraction of muscles described as “jumps.” In juvenile myoclonic epilepsy, seizures begin between puberty and adulthood, where seizures affect the neck, shoulders, and upper arms. They generally occur shortly after waking.
Myoclonic: Rapid involuntary contraction of muscles described as “jumps.” In juvenile myoclonic epilepsy, seizures begin between puberty and adulthood, where seizures affect the neck, shoulders, and upper arms. They generally occur shortly after waking.
 Tonic-clonic: This is the most common form of epilepsy, formerly known as grand mal seizures. Episodes are divided into two phases, the tonic phase and clonic phase. It first begins with the tonic phase, which will cause the patient to fall down. Muscles suddenly contract, causing the extremities to be rigidly pulled toward or away from the body. Following this, the clonic phase consists of rapid muscle contractions and relaxations, causing convulsions. Clinically, the patient may be rolling on the ground with the extremities violently shaking.
Tonic-clonic: This is the most common form of epilepsy, formerly known as grand mal seizures. Episodes are divided into two phases, the tonic phase and clonic phase. It first begins with the tonic phase, which will cause the patient to fall down. Muscles suddenly contract, causing the extremities to be rigidly pulled toward or away from the body. Following this, the clonic phase consists of rapid muscle contractions and relaxations, causing convulsions. Clinically, the patient may be rolling on the ground with the extremities violently shaking.
 Atonic: Also known as drop seizures, in which patients have a brief lapse of muscle tone leading to drop attacks, and they collapse to the ground. These seizures must be differentiated from similar looking attacks that may occur in cataplexy or syncope.
Atonic: Also known as drop seizures, in which patients have a brief lapse of muscle tone leading to drop attacks, and they collapse to the ground. These seizures must be differentiated from similar looking attacks that may occur in cataplexy or syncope.
When encountering a patient that presents with seizure-type symptoms, further history and physical examination needs to be done to confirm if it was a seizure, and if so, what the underlying cause may be (epilepsy vs. metabolic vs. tumor vs. infection vs. stroke). In general, the patient’s age may shed light on possible causes. For children, it is typically genetic or infectious (febrile), which differs from adults or older patients, in whom tumors and strokes are more common.
Headache (HA)
Primary Headaches
These exist independently from other medical conditions or diseases.
Tension-type HA: This is the most common type of HA, often described as a bilateral pressure or “bandlike” pain around the head. Precipitating factors often include stress, sleep deprivation, hunger, and eye strain. Although painful, these HAs are not harmful, and symptoms improve with nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen. It is important to note that frequent use of pain medication to relieve tension-type HA can lead to the development of medication overuse HA (rebound HA).
Migraine HA: This typically presents as a disabling unilateral, pulsating HA associated with autonomic symptoms, such as nausea, vomiting, phonophobia, and photophobia for 4 to 72 hours. An aura generally proceeds the HA in a third of sufferers, in which the patient may have transient visual deficits, sensory hallucinations, or even motor and language disturbances. Although the exact cause is highly debated, it is believed to be a neurovascular disorder, in which the brain blood vessels undergo constriction and neurogenic inflammation because of changes in the levels of serotonin, histamine, and substance P.
Cluster HA: Presents rapidly as a severe, excruciating unilateral orbital-supraorbital eye pain associated with autonomic symptoms, such as ptosis, miosis, conjunctival injection, rhinorrhea, lacrimation, facial swelling, flushing, and sweating, lasting 15 minutes to 4 hours or longer. A distinguishing feature is the regularity of the attacks, speculating a connection with circadian rhythm. It is often referred as “alarm clock HA” because of the ability to wake a person from sleep with regular timing. Generally, many patients respond to inhalation of 100% oxygen.
Trigeminal neuralgia: Also known as tic douloureux. It presents as episodes of severe pain described as stabbing electrical shocks, burning, crushing, or shooting pain in the face originating from the trigeminal nerve. Although it may occur paroxysmally, with no simulations, it can be triggered by common activities, such as brushing teeth, shaving, combing hair, eating, or talking. External stimuli, such as the wind or loud noises, may also trigger attacks. Trigeminal neuralgia is usually treated with carbamazepine, which prevents depolarization by inhibiting voltage-gated sodium channels.
Secondary Headaches
These are headaches caused by an underlying condition.
Subarachnoid hemorrhage (SAH): Presents as a “thunderclap” HA. Typically described as the “worst headache of my life” or “like being kicked in the head.” SAH is usually associated with vomiting, neck stiffness, confusion, altered level of consciousness, oculomotor palsy, neurologic deficits, or seizures. See earlier (“Trauma”) for further details.
Idiopathic intracranial hypertension (IIH): Also known as pseudotumor cerebri, IIH results from increased intracranial pressure, with the absence of tumor or disease. The HA is usually associated with nausea, vomiting, visual changes (double vision and visual loss from long-term untreated papilledema), and pulsatile tinnitus (whooshing sensation or buzzing in ear). See earlier (“Developmental Disorders”) for further details.
Meningitis: Always consider this diagnosis when a patient presents with a HA and fever. See earlier (“Infection”) for further details.
Giant cell arteritis (GCA): Generally known for affecting the temporal artery in temporal arteritis, GCA is a form of vasculitis that affects large and medium-sized vessels, such as the aorta. It is named according to the type of inflammatory cells found on biopsy (giant cell from union of several macrophages). Generally affecting women more than men, patients present with symptoms such as HA, jaw-tongue claudication, tinnitus, and visual changes (diplopia or blurred vision). If left untreated, the inflammation can affect the ophthalmic artery, leading to sudden blindness, so GCA is considered a medical emergency. On exam, a patient may have prominent temporal arteries, which are exquisitely tender to palpation. Definitive diagnosis is made via biopsy, but elevated inflammatory markers (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]) support it. If GCA is suspected based on the history and physical examination, treatment with high-dose steroids should be started prior to biopsy confirmation. It is important to note that GCA is associated with polymyalgia rheumatica, which presents as pain and stiffness in the neck, shoulders and hips, and treated similarly.
Brain tumors: Similar to IIH, brain tumors can raise ICP to cause a HA. However, depending on the location, surrounding brain structures can be damaged, leading to focal neurologic deficits. This will be discussed in further detail in the brain tumor section (see later).
Closed-angle glaucoma: Raised intraocular pressure leads to a painful red, mid-dilated eye causing a HA associated with visual abnormalities, nausea, and vomiting. More details are given later.
Carbon monoxide (CO) poisoning: This colorless, odorless, tasteless, toxic gas is usually emitted from gasoline-powered tools, heaters, furnaces, and cooking equipment. Typically, a cherry red patient with altered mental status presents with a history of living in a garage (using a heater). Also consider CO poisoning when several members of a family have simultaneous onset of headaches and nausea. Patients with CO poisoning may have normal oxygen saturation, but CO oximetry is used to determine carboxyhemoglobin levels, which misrepresent oxyhemoglobin. Treatment consists of administration of 100% oxygen via a nonrebreather mask to displace CO from hemoglobin; hyperbaric oxygen therapy is also used.
Brain Tumors
Primary brain tumors are slow-growing cancers that usually are not noticed until they begin to affect nearby structures. Patients can present with seizures, unrelenting headaches, altered mental status, or dementia. Primary brain tumors rarely metastasize. In general, brain tumors are supratentorial in adults (Table 13-7) and infratentorial in children (Table 13-8).
Table 13-7
| Adult Tumors | Description | Key Histologic Features |
| Glioblastoma multiforme | Most common primary brain tumor; very aggressive tumor with poor prognosis; called “butterfly glioma” because it can cross corpus callosum | Stains GFAP; “pseudopalisading” pleomorphic tumors with central necrosis, hemorrhage |
| Meningioma | Resectable tumors because they occur outside hemispheric convexity | Psammoma bodies (laminated calcification) |
| Schwannoma | Schwann cell origin affecting CN VIII (acoustic schwannoma) at cerebellopontine angle | S-100 positive. A cause of sensorineural hearing loss. |
| Oligodendroglioma | Frontal lobe tumor with chicken wire capillary pattern | “Fried egg” cells; often calcified |
| Pituitary adenoma | Presents as prolactinoma or bitemporal hemianopsia | Rathke’s pouch |
Table 13-8
| Childhood Tumors | Description | Key Histologic Features |
| Pilocytic (low-grade) astrocytoma | Well-circumscribed cystic-solid tumor found in posterior fossa | GFAP-positive; Rosenthal fibers (eosinophilic corkscrew fibers) |
| Medulloblastoma | Solid cerebellar tumor that is highly malignant; form of a primitive neuroectodermal tumor (PNET); seen in Turcot syndrome; presents as obstructive hydrocephalus (fourth ventricle compressed) | Homer Wright rosettes; cells are very radiosensitive, so can be used in therapy |
| Ependymoma | Ependymal cells typically found in fourth ventricle leading to obstructive hydrocephalus; can seed (metastasize to) the cerebrospinal fluid | Perivascular pseudorosettes; rod-shaped blepharoplasts near nucleus (basal ciliary bodies) |
| Hemangioblastoma | Cerebellar tumor associated with von Hippel-Lindau syndrome, which can produce EPO (polycythemia vera) | Foamy cells with high vascularity. |
| Craniopharyngioma | Benign tumor that is calcified and rich in cholesterol; often confused with pituitary adenoma. | Derived from Rathke’s pouch remnants |
Cranial Nerve Lesions
See Table 13-9 and Figure 13-42.
Table 13-9
| Cranial Nerve (CN) | Presentation |
| Trigeminal (CN V) | Jaw deviates toward the side of the lesion because there is an unopposed lateral pterygoid, which receives bilateral cortical input. |
| Vagus (CN X) | Uvula deviates away from the side of the lesion because the affected side has weak muscles compared with the nonaffected side. |
| Accessory (CN XI) | Weakness on turning head to contralateral side of lesion for the sternocleidomastoid muscle (SCM) and shoulder droop on same side of lesion for trapezius |
| Hypoglossal (CN XII) | Tongue deviates toward the side of the lesion (“lick your wounds”) because the opposite side overpowers the affected side. |
Figure 13-42 Right facial nerve paralysis. The patient is unable to smile (arrow 2) or furrow his brow on the affected side (arrow 1). (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
Facial nerve paralysis: CN VII lesions most commonly affect the muscles of facial expression, but it is important to keep in mind the other functions that may be compromised, such as lacrimation (dry eyes), corneal reflex (cannot blink), taste, and sound dampening (hyperacusis). When a facial nerve lesion is suspected, it is very important to classify it as an UMN lesion (usually from stoke) or an LMN lesion (e.g., compression from a parotid tumor). On physical examination, forehead involvement should be assessed by asking the patient to raise the eyebrows. The forehead is spared (e.g., patient can furrow forehead) in UMN lesions because it is innervated by both cerebral hemispheres. The following are some causes of facial nerve paralysis:
 Supranuclear, nuclear: Infarcts to internal capsule (lacunar stroke), pons (CN VII nucleus), or face area of motor cortex homunculus.
Supranuclear, nuclear: Infarcts to internal capsule (lacunar stroke), pons (CN VII nucleus), or face area of motor cortex homunculus.
 Infranuclear: Bell’s palsy (linked to herpes simplex so treated with steroids and acyclovir), Lyme disease (bilateral facial palsy; associated with cardiovascular arrhythmias in stage 2), GBS (bilateral facial palsy), herpes zoster oticus (Ramsay-Hunt syndrome), AIDS, sarcoidosis (bilateral facial palsy; sign of neurosarcoidosis), tumors (acoustic neuroma, parotid, cholesteatoma), and diabetes mellitus.
Infranuclear: Bell’s palsy (linked to herpes simplex so treated with steroids and acyclovir), Lyme disease (bilateral facial palsy; associated with cardiovascular arrhythmias in stage 2), GBS (bilateral facial palsy), herpes zoster oticus (Ramsay-Hunt syndrome), AIDS, sarcoidosis (bilateral facial palsy; sign of neurosarcoidosis), tumors (acoustic neuroma, parotid, cholesteatoma), and diabetes mellitus.
Mnemonic: My Lovely Bella Has An STD: Lyme disease, Bell’s palsy, Herpes Zoster, AIDS, Sarcoidosis, Tumors-Trauma, Diabetes mellitus.
Ear Pathology
Vertigo: An illusion of movement, despite being in a stationary position (sensation that “the room is spinning”). When evaluating a patient with vertigo, it is crucial to categorize vertigo as peripheral or central. Peripheral causes affect the vestibular system in the inner ear. Central causes affect the CNS. Central causes include migraine headaches, lateral medullary syndrome, vertebrobasilar TIA, cerebellar infarction, and multiple sclerosis. Peripheral causes are discussed here.
 Benign paroxysmal positional vertigo (BPPV): This is the most common cause of vertigo. It presents as short, intermittent episodes of dizziness provoked by head movements and associated with nausea and nystagmus. BPPV is caused by calcium crystals (otoliths) that affect the cupula or cause abnormal endolymph movement. Otoliths are typically found in the utricle, but over time they may migrate into one of the semicircular canals (posterior is most common). BPPV is diagnosed clinically, but can be confirmed by the Dix-Hallpike and roll tests, which test the posterior and horizontal semicircular canals, respectively. Treatment aims to reposition the otoliths out of the semicircular canals through the Epley maneuver. Antivertigo medications that include antihistamines (meclizine) and anticholinergics (scopolamine) can be given for symptomatic management.
Benign paroxysmal positional vertigo (BPPV): This is the most common cause of vertigo. It presents as short, intermittent episodes of dizziness provoked by head movements and associated with nausea and nystagmus. BPPV is caused by calcium crystals (otoliths) that affect the cupula or cause abnormal endolymph movement. Otoliths are typically found in the utricle, but over time they may migrate into one of the semicircular canals (posterior is most common). BPPV is diagnosed clinically, but can be confirmed by the Dix-Hallpike and roll tests, which test the posterior and horizontal semicircular canals, respectively. Treatment aims to reposition the otoliths out of the semicircular canals through the Epley maneuver. Antivertigo medications that include antihistamines (meclizine) and anticholinergics (scopolamine) can be given for symptomatic management.
 Ménière’s disease: Typically, this presents as vertigo, tinnitus, hearing loss, and fullness or pressure in one or both ears, but not all patients experience the same symptoms. It is believed to be caused by excess endolymph buildup in the inner ear, which over time causes dilation and blockage to cochlear structures. Herpesvirus has also been linked to this disease, and acyclovir can be used as a possible treatment. It is a diagnosis of exclusion; physical examination, brain MRI, and audiometry should be done to rule out other entities such as schwannoma. Treatment aims to reduce further fluid buildup (low-salt diet or diuretics [acetazolamide]) and symptomatic control of nausea and vomiting. Surgery is a last resort; destructive (vestibular neurectomy) and nondestructive (shunt) procedures can be done.
Ménière’s disease: Typically, this presents as vertigo, tinnitus, hearing loss, and fullness or pressure in one or both ears, but not all patients experience the same symptoms. It is believed to be caused by excess endolymph buildup in the inner ear, which over time causes dilation and blockage to cochlear structures. Herpesvirus has also been linked to this disease, and acyclovir can be used as a possible treatment. It is a diagnosis of exclusion; physical examination, brain MRI, and audiometry should be done to rule out other entities such as schwannoma. Treatment aims to reduce further fluid buildup (low-salt diet or diuretics [acetazolamide]) and symptomatic control of nausea and vomiting. Surgery is a last resort; destructive (vestibular neurectomy) and nondestructive (shunt) procedures can be done.
Vestibular neuritis: This generally presents postviral or during a viral syndrome, with a sudden onset of vertigo associated with nystagmus, nausea, vomiting, and gait instability. Unlike labyrinthitis, there are no hearing symptoms. Because of the clinical overlapping picture, it is crucial to differentiate it from acute vascular events, particularly cerebellar strokes. Treatment consists of a short corticosteroid taper.
Infection
Acute otitis externa: A common cause of an earache, or otalgia, this is an inflammation of the outer ear (pinna) and external auditory canal caused by pathogens such as Pseudomonas aeruginosa and Staphylococcus aureus. Typically, it is known as “swimmer’s ear”, occurring after swimming, in which the remaining water serves as a moist environment for bacterial growth. It can also be caused by trauma (ear scratching or cotton swabs), occlusive ear devices (ear phones, hearing aids), and dermatologic conditions (psoriasis). In addition to otalgia, swelling can cause conductive hearing loss and, in severe cases, canal obstruction. Treatment consists of thoroughly cleaning the ear canal (remove cerumen) and treating inflammation and infection with topical antibiotics and steroids.
Of note, when dealing with older diabetic individuals or immunocompromised patients, consider malignant (necrotizing) external otitis, which can spread to the skull base and requires emergent ENT evaluation.
Acute otitis media (AOM): Also a common cause of otalgia, this is an inflammation of the middle ear usually caused by pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. The most important factor in the pathogenesis of AOM is eustachian tube dysfunction, which occurs secondary to allergic rhinitis or upper respiratory tract infections. Treatment strategy is controversial, but generally consists of watchful waiting or antibiotics, depending on the patient’s age and overall clinical picture.
Cholesteatoma: A cyst that is an abnormal accumulation of keratinizing squamous epithelium, which if not surgically excised, expands over time, causing recurrent ear infections, mucopurulent drainage, hearing loss, vertigo, facial nerve palsy, and potentially life-threatening complications (e.g., meningitis, brain abscess).
Ramsay-Hunt syndrome: This is caused by reactivation of herpes zoster virus (shingles) in the geniculate ganglion, which affects cranial nerve VII. As a result, facial muscle movement, sensation of part of the ear and ear canal, and taste function of the anterior two thirds of the tongue can be affected. Typically, a patient presents with a seventh nerve palsy (Bell’s palsy), but on inspection of the tympanic membrane, tongue, and/or hard palate, eruptive erythematous vesicles are seen.
Hearing impairment: This can be a concerning symptom for many, but careful examination (Weber and Rinne tests) should be done to differentiate between conductive hearing loss, which is often reversible, from sensorineural hearing loss, which is not. Conductive hearing loss is caused by a problem with sound wave conduction, which can occur in the external ear (e.g., cerumen, otitis externa, tympanic membrane perforation) or internal ear (e.g., AOM or otosclerosis). The Weber test, in which the tuning fork is touched to the midline of the forehead, localizes to the affected ear, whereas the Rinne test, which tests air conduction (AC) versus bone conduction (BC), is negative (abnormal). Normally AC is greater than BC (positive), but because of the conduction defect, BC is greater than AC (negative). On the other hand, sensorineural hearing loss results from dysfunction of the inner ear, cochlea (e.g., noise- induced), or vestibulocochlear nerve (e.g., ototoxic drugs). The Weber test localizes to the normal ear, whereas the Rinne test is positive.
Eye Pathology
External
Hordeolum: Also known as a stye, this acute painful external red bump on the eye is an infection of the sebaceous glands of Zeis at the base of the eyelashes, or an infection of the apocrine sweat glands of Moll. It can also occur internally, infecting the meibomian (sebaceous) gland lining the inside of the eyelids. Treatment generally consists of a combination of warm compresses, incision and drainage, oral antibiotics, and pain control.
Chalazion: Painless internal nodule caused by chronic inflammation of a blocked meibomian gland. Initially, topical antibiotics can be used in the acute phase, but chalazions generally go away with time. Further treatment options include corticosteroid injections and surgery.
Coloboma: Present from birth, this defect in the eye is caused by the failure of the choroid fissure to close, resulting in a hole in of the eye structures (retina, optic disc, choroid, and iris).
EOM abnormalities: See Figure 13-43. Remember the mnemonic LR6SO4R3.
Figure 13-43 Extraocular movement abnormalities. A, Third nerve palsy with a down and out pupil, mydriasis (caused by lack of parasympathetic tone from the third nerve), severe ptosis (caused by denervation of the levator palpebrae muscle). B, Sixth nerve palsy with lateral rectus muscle paralysis. The eye looks medially because of the unopposed medial rectus muscle. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
 CN III damage: Eye looks down and out because of unopposed superior oblique and lateral rectus. Ptosis caused by levator palpebrae superioris muscle disruption. Mydriasis and loss of accommodation because of parasympathetic disruption.
CN III damage: Eye looks down and out because of unopposed superior oblique and lateral rectus. Ptosis caused by levator palpebrae superioris muscle disruption. Mydriasis and loss of accommodation because of parasympathetic disruption.
 CN IV: Downward gaze defective, so turn head toward side of lesion.
CN IV: Downward gaze defective, so turn head toward side of lesion.
 CN VI: Eye looks medially because of unopposed medial rectus.
CN VI: Eye looks medially because of unopposed medial rectus.
Cataracts: Crystalline clouding of the lens or lens capsule that results in opacification and obstruction of light. The two most common causes include senile cataracts (because of old age) or congenital cataracts.
 Marcus Gunn: Often seen in optic neuritis, this afferent pupillary defect (APD) is seen during the swinging flashlight test, in which shining light in the affected eye produces less pupillary constriction relative to the unaffected eye.
Marcus Gunn: Often seen in optic neuritis, this afferent pupillary defect (APD) is seen during the swinging flashlight test, in which shining light in the affected eye produces less pupillary constriction relative to the unaffected eye.
 Argyll Robertson: This highly specific sign of neurosyphilis, known as prostitute’s pupil, presents as pupils that constrict to accommodation (“they accommodate”), but do not react to light.
Argyll Robertson: This highly specific sign of neurosyphilis, known as prostitute’s pupil, presents as pupils that constrict to accommodation (“they accommodate”), but do not react to light.
Glaucoma: An eye disease characterized by damage to the optic nerve because of increased pressure in the eye (intraocular pressure). It is typically divided into two main categories based on the angle between the iris and cornea.
 Open-angle (90%) glaucoma: Insidious onset of painless vision loss, which is not noticed until disease has significantly progressed. The only signs of this disease include visual field loss and optic nerve changes (increased cup-to-disc ratio).
Open-angle (90%) glaucoma: Insidious onset of painless vision loss, which is not noticed until disease has significantly progressed. The only signs of this disease include visual field loss and optic nerve changes (increased cup-to-disc ratio).
 Closed-angle (10%) glaucoma: A sudden onset of pain, a midsized, nonreactive pupil and injected conjunctiva (red eye), which requires emergency treatment to lower the high intraocular pressure (usually IOP > 30 mm Hg). Further discussion regarding medical treatment to lower intraocular pressure will be discussed later (“Pharmacology”). If medical management fails, surgical methods, such as iridotomy or iridectomy, canaloplasty, and trabeculectomy or trabeculoplasty are available.
Closed-angle (10%) glaucoma: A sudden onset of pain, a midsized, nonreactive pupil and injected conjunctiva (red eye), which requires emergency treatment to lower the high intraocular pressure (usually IOP > 30 mm Hg). Further discussion regarding medical treatment to lower intraocular pressure will be discussed later (“Pharmacology”). If medical management fails, surgical methods, such as iridotomy or iridectomy, canaloplasty, and trabeculectomy or trabeculoplasty are available.
Posterior Eye Pathology
Retinal detachment: An ocular emergency characterized by “peeling” of the retina and described as a veil or curtain being drawn over the visual field, which is preceded by flashes of light, eye heaviness, and floaters. Diagnosis is usually made by funduscopy or ultrasound, and treatment generally consists of sealing retinal breaks.
Papilledema: Asymptomatic optic disc swelling caused by increased ICP, which warrants further workup to discover and treat the underlying cause; otherwise, vision loss can result. On fundoscopic exam, typical findings include venous engorgement (earliest sign), loss of venous pulsations, blurring of optic margins, and elevation of the optic disc.
Optic neuritis: Demyelinating inflammation of the optic nerve usually associated with multiple sclerosis (first manifestation), which can lead to complete or partial vision loss. On fundoscopic exam, the optic disc is usually pale, but can be normal. Treatment generally consists of steroids.
Central retinal artery occlusion (CRAO): Acute onset of unilateral painless vision loss caused by occlusion (embolic, thrombotic, inflammatory, or traumatic) of the central retinal artery that can be irreversible if not corrected within 90 minutes. On exam, there can be an APD, Hollenhorst plaque (cholesterol embolus), pale optic nerve, and a cherry red spot (retinal whitening caused by ischemia; note that this can also be seen in carbon monoxide poisoning). Treatment generally includes eye massage, eye paracentesis (lowers IOP to allow embolus to move), oxygen therapy, and consideration of thrombolytics.
Central retinal vein occlusion (CRVO): Acute onset of unilateral painless vision loss caused by occlusion of the central retinal vein, leading to macular edema, ischemia, and neovascular glaucoma. Typically on fundoscopic exam, there is extensive hemorrhage, giving a so-called blood and thunder appearance. Treatment generally includes intravitreal administration of steroids.
Visual Field Defects
Depending on the lesion location in the visual pathway, a characteristic visual field loss is typically seen. Below are common visual field anomalies and their corresponding lesions (Fig. 13-44).

Figure 13-44 Visual field defects and their corresponding lesions. (From Felten DL, Shetty AN. Netter’s Atlas of Neuroscience. Philadelphia: Elsevier; 2009.)
 Unilateral macular degeneration → central scotoma
Unilateral macular degeneration → central scotoma
 Optic chiasm → bitemporal hemianopsia
Optic chiasm → bitemporal hemianopsia
 Optic tract → left homonymous hemianopsia
Optic tract → left homonymous hemianopsia
 Meyer’s loop (located in temporal lobe) → left upper quadrantic anopsia
Meyer’s loop (located in temporal lobe) → left upper quadrantic anopsia
 Dorsal optic radiation (located in parietal lobe) → left lower quadrantic anopsia
Dorsal optic radiation (located in parietal lobe) → left lower quadrantic anopsia
 Posterior cerebral lesion → left hemianopsia with macular sparing
Posterior cerebral lesion → left hemianopsia with macular sparing
Internuclear ophthalmoplegia (INO): Also known as MLF syndrome. INO is a disorder in which there is a lesion to the medial longitudinal fasciculus (MLF), which essentially connects the abducens nucleus (CN VI) to the contralateral oculomotor nucleus (CN III) in the brainstem. As a result, there is medial rectus nerve palsy, with nystagmus and horizontal diplopia in the abducting eye on attempted lateral gaze, but convergence remains normal (Fig. 13-45). In young patients with bilateral INO, multiple sclerosis is often the cause. In older patients with unilateral INO, stroke is usually the cause.
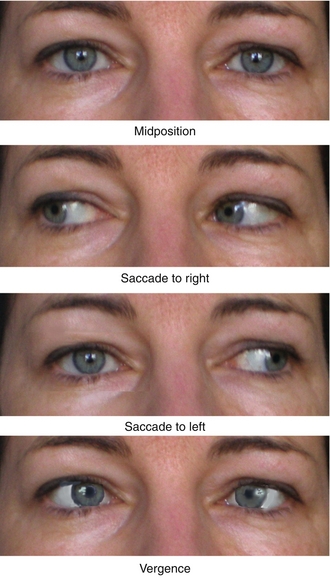
Figure 13-45 Internuclear ophthalmoplegia. The patient’s right eye is unable to perform conjugate left lateral gaze because of destruction of the medial longitudinal fasciculus. Convergence remains normal. (From FitzGerald MJT, Gruener G, Mtui E. Clinical Neuroanatomy and Neuroscience. 6th ed. Philadelphia: Saunders; 2011.)
PHARMACOLOGY
Glaucoma
As noted, the goal of glaucoma therapy is to lower IOP to prevent further damage to the optic nerve. Five pharmacologic groups can be used (Table 13-10).
Opioids
Opioids are a large class of drugs that act as agonists by binding to opioid receptors (mu [μ] = morphine, delta [δ] = enkephalin, kappa [κ] = dynorphin), which are found in the central and peripheral nervous system and the GI tract, leading to decreased synaptic transmission of various neurotransmitters (e.g., acetylcholine [Ach], serotonin [5-hydroxytryptamine—5-HT], norepinephrine [NE], glutamate, substance P). Common opioids that you will encounter in the clinical setting include morphine, fentanyl, codeine, hydromorphone, oxycodone, dextromethorphan, methadone, and meperidine. Clinically, they are used for analgesia, but some are used for cough suppression (dextromethorphan), diarrhea (loperamide, diphenoxylate), maintenance therapy for heroin addicts (methadone), and acute pulmonary edema (morphine). As a result of frequent or excessive dosing, side effects can develop, such as miosis (pinpoint pupils), constipation, respiratory depression, and CNS depression. Over time, patients can develop opioid tolerance by neuroadaptation through receptor desensitization, which leads to reduced analgesia effect. No tolerance develops to constipation and miosis. In case of opioid overdose, an opioid receptor antagonist can be used. Naloxone is short-acting and is used intravenously (low oral bioavailability). Naltrexone is longer acting and can be used orally.
Newer opioids have been created to reduce side effects. Tramadol, a very weak opioid agonist, is commonly used for chronic pain, but is also known to decrease the seizure threshold. Butorphanol, a kappa agonist and partial mu agonist, is used for migraine headaches and labor and delivery (less respiratory depression).
Epilepsy
Anticonvulsants (also known as antiepileptic drugs [AEDs]) were created with the goal of suppressing rapidly firing neurons that start a seizure. In addition to seizures, anticonvulsants are also used as mood stabilizers to treat bipolar disorder (see Chapter 14). The two general mechanisms of action of AEDs are inactivation of voltage-gated sodium channels or increased GABA signaling (GABAA receptors, transaminase, or transporter). Table 13-11 lists common AEDs.
Table 13-11
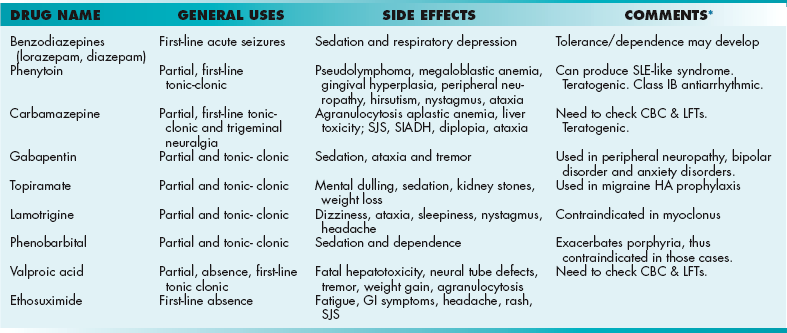
CBC, complete blood count; LFT, liver function test; SIADH, syndrome of inappropriate antidiuretic hormone secretion; SJS, stiff joint syndrome; SLE, systemic lupus erythematosus.
*Note: CYP inducers – Phenytoin, phenobarbital, and carbamazepine. CYP inhibitors – Valproic acid
Anesthetics
Anesthetics provide a reversible loss of sensation. They can be classified as local (e.g., injected into a wound) or general (e.g., inhaled anesthetics that cause whole-body anesthesia).
General anesthetics are used to cause a reversible loss of consciousness through the inhalation or intravenous administration of the anesthetic to induce and maintain a state of unconsciousness. Inhaled anesthetics, in order from highest to lowest potency, include methoxyflurane, halothane, enflurane, isoflurane, and nitrous oxide. IV anesthetics include barbiturates, benzodiazepines (used in conjunction with opioids), ketamine (phencyclidine [PCP] analogue causing dissociative amnesia), etomidate, and propofol. The more lipid-soluble the drug, the more potent it is and the faster the induction and recovery time. This is inversely related to the MAC (minimal alveolar concentration), which is used as a marker to compare various anesthetics. Thus, the more lipid-soluble and potent anesthetics have a low MAC. Anesthesiologists use the MAC of an anesthetic along with other variables (ventilation rate, tidal volume, atrioventricular [AV] concentration gradient, and blood solubility) to control general anesthesia during surgeries.
Local anesthetics act at a distinct site to prevent transmission of nerve impulses by binding to fast acting sodium channels. The two general classes are esters (procaine, cocaine, tetracaine) and amides (have two Is in the name → lidocaine, bupivacaine, mepivacaine). General side effects include cardiovascular toxicity (arrhythmias seen with cocaine or bupivacaine), seizures, and hypotension or hypertension. It is important to note that if a patient has an allergic reaction to an ester anesthetic, it is all right to give an amide.
Malignant hyperthermia (MH) is a rare, life-threatening condition that results from inhaled anesthetics and succinylcholine. It is caused by an autosomal dominant inherited mutation in the ryanodine receptor (RYR1) gene, which results in uncontrolled increased skeletal muscle metabolism. When MH presents, patients are in a hypercatabolic state, resulting in a high body temperature, tachycardia, tachypnea, rigid muscles, rhabdomyolysis, and increased O2 consumption and CO2 production. Treatment consists of stopping the triggering agent, administering dantrolene, and providing supportive therapy aimed at correcting the hyperthermia, acidosis, and organ dysfunction.
Neuromuscular Blockade
Neuromuscular blocking drugs inhibit neuron transmission to muscles by blocking postsynaptic nicotinic acetylcholine (ACh) receptors. Clinically, they are used to produce paralysis, which aids in intubation for mechanical ventilation. They are generally classified as nondepolarizing or depolarizing neuromuscular blocking drugs based on whether or not they depolarize the motor endplate (Table 13-12).
Table 13-12
| Class | Comments |
| Nondepolarizing (atracurium, pancuronium, rocuronium, cisatracurium) |
Drug names generally end in -curonium or -curium. Competitive antagonists that can be reversed with increase in ACh by acetylcholinesterase inhibitor drugs (AchEIs; e.g., neostigmine) Exhibit tetanic fade (failure of muscles to maintain a fused tetany at high electrical stimulation frequencies) |
| Depolarizing (succinylcholine) |
Resistant to degradation by acetylcholinesterase (fast onset), but short duration because of metabolism by blood cholinesterase (pseudocholinesterase) Noncompetitive antagonist with two phases: • Phase I (depolarizing phase) —sudden muscle fasciculations, twitches followed by flaccidity • Phase II (desensitizing phase)—less responsive to depolarization After phase II, full neuromuscular blockade (paralysis) complete; AChEI potentiates phase I, but can reverse phase II Does not exhibit tetanic fade Complication of hyperkalemia caused by upregulation of receptors as a result of nerve damage or demyelinating disease (e.g., spinal cord injury, MS, chronic burns) |
Pharmacologic Treatment of Other Neurologic Disorders
Parkinson’s Disease
In general, treatment of Parkinson’s disease involves increasing the amount of dopamine (DA) in relationship to acetylcholine. Once pharmacotherapy (Table 13-13) has been exhausted, surgical therapies are available, such as deep brain stimulation (DBS).
Alzheimer’s Disease
Current therapy is aimed at counteracting the reduction in cholinergic activity and excitotoxicity of glutamate seen in Alzheimer’s disease. In addition to pharmacologic therapy (Table 13-14), caregiving and psychosocial intervention needs to be addressed.
Table 13-14
Pharmacotherapy for Alzheimer’s Disease
| Name | Mechanism | Side Effects |
| Acetylcholinesterase inhibitors (donepezil, rivastigmine, galantamine) | Reduces rate of acetylcholine (ACh) breakdown, thus increasing ACh concentration | Nausea and vomiting because of cholinergic excess |
| Memantine | Noncompetitive NMDA receptor antagonist | Hallucinations, confusion, dizziness |
Huntington’s Disease
Symptomatic management of chorea and optimizing quality of life are the main two goals. Chorea is treated in a stepwise system, starting with tetrabenazine (Table 13-15) and then moving to atypical neuroleptics, typical neuroleptics, and eventually a combination of tetrabenazine and a neuroleptic.
Table 13-15
Pharmacotherapy for Huntington’s Disease
| Drug Name | Mechanism | Side Effects |
| Tetrabenazine | VMAT inhibitor,* so promotes dopamine degradation. | Similar to antipsychotic side effects, such as akathisia, depression, dizziness and parkinsonism, but does not cause tardive dyskinesia |
*VMAT, vesicular monoamine transporter. By preventing dopamine’s repackaging into presynaptic vesicles, it instead will be degraded.
Multiple Sclerosis
See Table 13-16.
Table 13-16
Pharmacotherapy for Multiple Sclerosis
| Drug Name | Mechanism | Side Effects |
| Interferon-β | Has anti-inflammatory properties; improves blood-brain barrier integrity | Flulike symptoms and injection site reactions most common |
| Glatiramer acetate | By resembling myelin basic protein, it shifts proinflammatory Th1 cells to regulatory Th2 cells to suppress inflammatory response by substituting itself as a target of immune system attack.. | Injection site and injection reactions; least likely to have flulike symptoms |
| Mitoxantrone | Topoisomerase inhibitor generally used in cancer chemotherapy | Nausea, vomiting, hair loss, immunosupression, cardiomyopathy |
| Natalizumab | Monoclonal antibody against cellular adhesion molecules, which reduces influx into the CNS.. | Fatigue, allergic reaction, hepatotoxicity, PML development |
| Fingolimod | Sphingosine analogue; derived from fungus that modulates sphingosine-1-phosphate receptor to sequester lymphocytes in lymph nodes | Fatal infections, bradycardia, skin cancer, hemorrhagic focal encephalitis. |
Headaches
First-line treatment in acute attacks is typically NSAIDs, such as ibuprofen, naproxen, ketorolac, or aspirin. Triptans such as sumatriptan may also be used to treat acute migraines. These are often combined with antiemetics, such as prochlorperazine (Compazine), metoclopramide, or ondansetron. Medications used for migraine prophylaxis include tricyclic antidepressants (TCAs; e.g., amitriptyline or nortriptyline), antiepileptics (valproic acid, valproate, divalproex, topiramate), beta blockers (propranolol), and calcium channel blockers (verapamil).
Stroke
Once a thrombus (blood clot) occurs in an ischemic stroke, removing the blockage by mechanically removing it (thrombectomy) or by pharmacologic thrombolysis (clot busting) with tPA are the two definitive therapies available. tPA catalyzes the conversion of plasminogen (inactive enzyme, called a zymogen) to plasmin (active enzyme), which degrades fibrin clots. Therefore, tPA activates the body’s own clot degradation system. Before giving tPA, a list of inclusion and exclusion criteria is reviewed and risks and benefits discussed with the patient and family. Multiple contraindications exist and include severe hypertension, recent bleeding (GI, intracranial bleeding), and recent surgery. To be most effective, tPA should be given as early as possible. Generally, it is given within 3 hours of the start of neurologic symptoms, but this window can be extended to 4.5 hours depending on certain patient characteristics (age < 80 years, no history of stroke or diabetes, not on anticoagulants). Once given, close observation is done to evaluate for hemorrhage, especially intracerebral hemorrhage.