CONTENTS
24.2 Nanotechnology and Drug Delivery
24.2.1 Passive Targeting: The EPR Effect
24.2.2.1 Nonspecificity of Current Targeting Ligands
24.2.2.2 Targeting Ligands Do Not Deliver Drugs to the Site of Action
24.2.2.3 Access Barriers Limit the Effectiveness of Targeting Ligands
24.2.3 Future Directions for Nanotechnology and Drug Delivery
24.2.3.1 Alter the Pharmacokinetics
24.2.3.2 Study Other (Easier) Pathologies
24.2.3.3 Investigate Drug Combinations
24.2.3.4 Use Nature’s Own Processes
24.2.3.5 Imaging and Theranostics
24.3 Overcoming the Epithelial Barrier
24.3.1 Nanotechnology and Epithelial Transport
24.3.2 Successful Strategies for Epithelial Transport
24.4 Future Directions For Drug Delivery
24.4.4 Personalized and Precision Medicines
24.4.6 A Need for Improved In Vitro/In Vivo Testing
24.4.7 A Need for Collaborative, Transparent Research
As described in Chapter 1, the early years of drug delivery research (from about 1950 to 1980) saw the development of various mechanisms to achieve controlled drug release. The principal physical approaches to achieve controlled release were based on diffusion- and dissolution-control. Osmotic pressure and ion-exchange mechanisms were also used. Technologies at this time included sustained-release, once-a-day, formulations for oral delivery, and transdermal patches suitable for daily or weekly administration. The thrust of the research at this time was based on adjusting the physicochemical properties of the formulation, which resulted in the desired sustained-release pharmacokinetic profiles in vivo. This research was highly successful and resulted in the introduction of a large number of commercial products.
The period from the 1980s to the present time is marked by a slower pace of research, as evidenced by the lower numbers of products that have been brought to commercial fruition. During this time, more complicated challenges have emerged, which have proved much more difficult to resolve. Increasingly, formulators must work with molecules derived from drug discovery programs that have challenging physicochemical properties for satisfactory delivery, including poor water solubility, large size, and metabolic lability. Additionally, drug delivery scientists have to address a multitude of highly complex biological barriers, including epithelial and endothelial barriers, the complexity and variability of the biological environment in vivo, access and penetration barriers en route to the target, the complexity of the pathologies being treated (for example, cancer), and, in the case of implantable drug delivery systems (DDS), problems of biocompatibility and host-response reactions (Yun et al. 2015). The combination of formulation and biological barriers has, in many cases, limited successful efforts at delivery.
This book describes current technologies and emerging trends in drug delivery science. It comes 15 years after the publication of the first edition (Hillery et al. 2001). Significant progress, greater in some areas than in others, has been made in the last decade and a half. The preceding chapters of this book bear witness to the excellence of current research being carried out worldwide in the field, as well as to the considerable range and scope of this work. In this chapter, further discussion is confined to two important areas of drug delivery research: (1) nanotechnologies for drug delivery and (2) transport across epithelial barriers.
24.2 NANOTECHNOLOGY AND DRUG DELIVERY
Since the 1990s, the synthesis and testing of novel drug nanocarriers has constituted the most active area of R&D in the drug delivery field. A vast amount of literature is now available on the subject—for example, an Internet search for “nanotechnology and drug delivery” currently yields 1.75 million hits. Initially, nanoparticles (NPs) were defined as particles smaller than 100 nm. However, for drug delivery, NPs less than 100 nm typically do not have enough space to load drug (an exception being when NPs are made of the drug per se, e.g., DNA or RNA condensed by interaction with cationic polymers), so that NPs used in drug delivery are usually larger than 100 nm. The definition was therefore modified, to describe engineered particles that have at least one dimension in the nanoscale range (Nanotechnology Safety and Health Program 2014). Thus, current nanotechnologies for drug delivery are classified purely on the basis of their size. There is no scientific rationale for defining NPs based on their size alone—the usefulness of NPs should be due to their unique properties and functions, rather than their size. In fact, many of the current “nanotechnologies” described in this book are actually the selfsame carriers that were simply called “microparticles” and “drug-targeting systems” in the first edition of this text.
An important aspect of nanotechnologies is their targeting potential. The term “targeting” is a broad one, meaning different things depending on the context. For example, local/topical application is a type of regional targeting, e.g., antiallergy drugs can be given by nasal spray, for the topical treatment of allergic rhinitis. By delivering the drug locally, it is effectively targeted to its site of action, maximizing the therapeutic effect, while minimizing unwanted side effects. Alternatively, some drugs, such as antibiotics and hormones, possess an intrinsic targeting ability in that they distribute through the entire body but only interact with their target receptors or cells, without affecting other cells. In the context of nanotechnology, targeting is defined as either (1) “passive,” taking advantage of the “enhanced permeability and retention” (EPR) effect (Maeda et al. 1985; Matsumura and Maeda 1986), or (2) “active,” using a specific targeting ligand, which is attached to a drug, or drug-loaded DDS, in order to improve uptake and sequestration into the cell or tissue (see also Chapter 5). Both passive and active targeting strategies are considered further here.
24.2.1 PASSIVE TARGETING: THE EPR EFFECT
The idea that the EPR effect facilitates “passive targeting” to tumors (i.e., that nanocarriers can passively enter the leaky blood supply of a tumor and thus preferentially accumulate in the tumor tissue) is one that has gained near universal acceptance in the field. It is widely referred to throughout this book. However, in recent years there have been doubts expressed about the clinical applicability of the phenomenon, the limited evidence for the effectiveness of the mechanism, the low amount of uptake generally achieved, and the heterogeneity of the effect (Stirland et al. 2013; Nichols and Bae 2014; Bae and Park 2011). In Chapter 5, the authors further add to this note of caution (Section 5.2.1).
In spite of the doubts that are increasingly being raised, there nevertheless remains a widespread misconception in the field that, due to the EPR effect, nanocarrier DDS are taken up exclusively by tumors. It should be stressed that, even in the most favorable circumstances, this is far from the case – in contrast, most of the administered dose (i.e. ≈95%), still ends up in the reticuloendothelial system (RES) organs of the liver and spleen and, to a lesser extent, in other, nontumor tissues.
There is a pressing need to resolve outstanding issues, such as the extent (currently only a few percent uptake), applicability (relevance in humans, as opposed to mouse models), and reproducibility (due to problems of intra- and interpatient tumor heterogeneity) of the EPR effect. It would be highly beneficial for future research to precisely define these parameters, as well as define the optimal nanocarrier physicochemical properties that could improve tumor uptake via the EPR effect.
Research into the EPR effect is hampered by the lack of appropriate animal models. The limitations of preclinical cancer models have been widely reviewed and are largely acknowledged by the field (Begley and Ellis 2012; Crommelin and Florence 2013). Although veterinary cancers and transgenic models would be the most physiologically relevant animal models to use, they are not usually employed in research because of their low availability, or the prolonged time necessary to generate tumors. It should be noted that a recent review concluded that most of the frequently used rodent models (such as the subcutaneous flank tumor xenograft) can lead to an overestimation of the potential usefulness of the EPR effect (Prabhakar et al. 2013). This is because animal tumors grow much faster than human ones, so that blood vessels in rodent tumors do not develop properly and are inherently much leakier, which favors NP uptake. There is obviously a pressing need to develop more predictive animal models, as discussed further in Section 24.4.6.
Crucially, the EPR effect needs to be systematically and rigorously investigated in human patients. To date, the information available in human subjects is scant and not standardized. It is also, thus far, not very convincing. Even within a single tumor, large differences with regard to vascular permeability have been shown (Lammers et al. 2012; Lammers 2013). Harrington et al. used radiolabeled PEGylated liposomes in conjunction with whole body gamma camera imaging to study liposome biodistribution in vivo (Harrington et al. 2001, 2002). The levels of tumor liposome uptake estimated from regions of interest on gamma camera images were again low—approximately 0.5%–3.5% of the injected dose at 72 h. They also showed considerable variation in the amount taken up depending on the tumor type, as well as for the same tumor type, but between different patients.
The use of active targeting strategies is widely referred to throughout this book, for example, using folic acid to target cancer cells, lectins and vitamins to target enterocytes, and the use of monoclonal antibodies to target specific receptors. In spite of all this research and focus, there are currently no actively targeted nanomedicines in clinical use. To understand the poor success of the strategy to date, it is important to remember the inherent limitations associated with this approach.
24.2.2.1 Nonspecificity of Current Targeting Ligands
There is currently a lack of specificity associated with the approach. Targeting ligands are often chosen for receptors that are merely overexpressed on tumor cells, rather than exclusive to tumor cells. For example, folic acid is widely chosen as a targeting motif because the folic acid receptor is known to be overexpressed on cancer cells. However, the folic acid receptor is also present on normal cells. Since normal cells far outnumber tumor cells in vivo, the overexpression differential advantage of tumor cells is lost (Bae and Park 2015). To achieve greater specificity, ligands unique to the target must be used, for example, in the case of cancer targeting, by using tumor-associated antigens (i.e., antigenic structures specifically occurring at the surface of tumor cells). However, ligands of such unique specificity are difficult to find.
24.2.2.2 Targeting Ligands Do Not Deliver Drugs to the Site of Action
Targeting vectors have zero ability to deliver their cargo to a specific cell or tissue. These ligands are not satellite navigation “homing” devices that direct a nanocarrier specifically to a tumor or any other site. In vivo, there is no greater accumulation of actively targeted NPs at a tumor site in comparison to their nontargeted counterparts. On the contrary, they are merely cell surface–recognition moieties, which are still dependent on the random encounter of a nanocarrier with the appropriate receptor, during its circulation lifetime in vivo (Crommelin and Florence 2013). There is no guarantee of this interaction. In this sense, the use of the word “active” is misleading, as it suggests an active (i.e., energy driven) process at work, to seek out the corresponding receptor, but this is absolutely not the case.
24.2.2.3 Access Barriers Limit the Effectiveness of Targeting Ligands
The likelihood of a random encounter of a targeting ligand with its corresponding receptor is severely compromised in vivo due to the difficulty in accessing target receptors (Lammers et al. 2012; Crommelin and Florence 2013). In the case of tumor targeting, the vectors are for receptors on tumor cells, which means the nanocarrier must first extravasate from the bloodstream to access these cells. If the nanocarrier does manage to extravasate from the bloodstream (and this is in no way guaranteed—see the problems associated with the EPR effect, described in Section 24.2.1), it still has to diffuse, against a high interstitial fluid pressure, through the perivascular space (which contains layers of pericytes, smooth muscle cells, and fibroblasts), in order to reach the tumor cells. If the nanocarrier does succeed in navigating through this complex tumor microenvironment, targeting vectors will then bind to the first receptors they encounter and so may not penetrate very deeply into the tumor (this phenomenon is known as the binding-site barrier).
In the limited environment of a cell line, a targeted NP may indeed show enhanced uptake by tumor cells (up to 100× greater uptake, than for nontargeted controls)—but these are in vitro studies, in which the NPs are pipetted directly on top of the test cells, so that ligand–receptor interactions are highly favored. The situation in vivo is much more complex, and nanocarriers must overcome the accompanying access and penetration barriers, before uptake can take place. Thus, increased uptake of a nanocarrier in a cell line is actually a very poor indicator of efficacy in vivo.
A number of approaches are currently being taken to address the problems of access to tumor cells, including actively targeting the nanocarrier to tumor endothelium, rather than the tumor cells (reviewed in Lammers et al. 2012). By binding and killing tumor endothelial cells, the tumor becomes deprived of oxygen and nutrients, resulting in tumor death. This approach has many advantages over targeting tumor cells: (1) it is easier to find the target in vivo, (2) it eliminates the need for the DDS to extravasate, and (3) the nanocarrier does not have to penetrate the perivascular space.
A different approach is to use triggered drug delivery, using stimuli-sensitive carrier materials. Once at the tumor, stimuli-sensitive release facilitates the release of the low-molecular-weight payload, which can diffuse more rapidly through the perivascular space than a (much larger) nanoparticulate carrier. For example, Chapter 23 describes the use of ThermoDox®, low temperature–sensitive liposomes that release their doxorubicin (Dox) payload in the vicinity of a tumor, when the tumor is subjected to localized heat treatment.
Although at a very early stage of development, a further research avenue involves the use of energy-driven mechanisms to actively propel drugs toward a target or deep into a tumor. One recent example (reminiscent of the sci-fi film Fantastic Voyage) is the use of gold–mesoporous silica nanorods that use the surrounding biological environment as a source of fuel, to self-propel themselves through the body (Wang et al. 2015). Multiple fuel sources have been explored to power these nanomotors; one model uses gastric acid, which produces bubbles of hydrogen gas for nanomotor thrust and propulsion.
24.2.3 FUTURE DIRECTIONS FOR NANOTECHNOLOGY AND DRUG DELIVERY
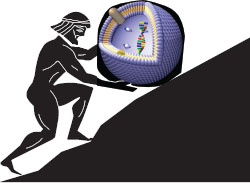
At the preclinical level, we are churning out nanocarriers at a prolific rate. Literally thousands of different constructs have been described in the literature, yet they all share remarkably similar baseline properties: typically, they incorporate a (nonspecific) targeting ligand, are loaded with Dox, show uptake in a cell culture study, and are variously described as “targeted,” “smart,” and “advanced,” with the potential to “cure cancer.” Although this activity keeps pharmaceutical research humming along and contributes toward the vast amount of literature now available in the field, endlessly producing new nanocarriers for the sake of newness is not enough and will not help the field to progress (Figure 24.1).
Producing ever-more complex nanoparticulate DDS will not, per se, guarantee clinical success. Increased complexity does not necessarily provide improved NP performance in vivo (Raemdonck and de Smedt 2015), also, complex DDS are expensive; they require specialized manufacturing know-how and facilities, and possibly difficult reconstitution procedures. In general, the more complex the system, the greater the scale-up, manufacturing, and regulatory challenges will be (see Section 24.4.5).
A more productive approach might be to focus on developing a more rational design strategy for nanocarriers in drug delivery, which prioritizes their ultimate purpose. The next generation of nanocarriers, developed by rational design, would consider a number of key aspects at the outset of their development, including
• The clinical need
• The scientific basis, principles, and justification for development, that underpins this research
• How this delivery system improves on all the (literally thousands of) previous nanocarriers already developed and described in the literature
• Biocompatibility and toxicity issues, which, by extension, impact on the likelihood of the DDS receiving regulatory approval (see Section 24.4.5)
• How this system will be meaningfully tested, to find relevance to clinical applications
In developing a rational design strategy, one possible way forward is to try and set more easily attainable goals. Rather than trying to find a single, omnipotent “magic bullet” that will single-handedly cure cancer, the field might be better served by progressing in smaller, less ambitious, more incremental steps, which focus on carving out specific niche areas of expertise. Some suggestions are outlined here.

FIGURE 24.1 There is a danger that drug delivery scientists may become like Sisyphus, continually toiling to produce new nanocarrier constructs, which, without the ability to overcome biological barriers, may not translate into clinical formulations.
24.2.3.1 Alter the Pharmacokinetics
Many commercially available nanotechnology formulations offer improved drug pharmacokinetics, diverting drugs away from vulnerable organs (e.g., heart, brain, kidneys). For example, Dox can cause cardiomyopathy, which may lead to congestive heart failure and death. Doxil®, the PEGylated liposomal form of Dox, was found in clinical trials for ovarian cancer to have “comparable efficacy, favorable safety profile” to the control treatment, topotecan (Gordon et al. 2001). So Doxil® was actually no better than the current treatment—its added value was in the reduction of cardiotoxicity caused by Dox. Similarly, DaunoXome® improves the cardiotoxicity of Dox, rather than imparting any improved therapeutic effect, and the liposomal product AmBisome® minimizes the nephrotoxicity associated with the antifungal drug, amphotericin B. Abraxane®, an albumin-based NP formulation of paclitaxel, is—as stated by the European Medicines Agency—“a new formulation developed to overcome the water insolubility of the active component paclitaxel and prevent hypersensitivity reactions associated with solvent-containing formulations.” In this case, the advantage of reformulation as NPs was to facilitate increased solubility for a practically insoluble drug. Researchers might thus consider switching emphasis away from the development of increasingly elaborate nanoparticulate DDS for tumor targeting (which, as outlined in Sections 24.2.1 and 24.2.2, is fraught with difficulties and limitations) and look instead at drug reformulation in order to improve problems such as unfavourable pharmacokinetics, or poor solubility.
24.2.3.2 Study Other (Easier) Pathologies
Nanotechnology research is currently heavily skewed toward cancer therapy. Given the inherently difficult nature of this disease (characterized as it is, by a large inter- and intrapatient tumor heterogeneity, drug toxicity, and access and penetration barriers), another way forward is to address other therapeutic needs, which afford less delivery challenges and are more inherently suited to NPs. A prime example would be the use of nanotechnology in the treatment of inflammatory disorders. The inflammatory response is characterized by increased vascular permeability and increased blood flow. Thus, the treatment of inflammatory disorders is ideally suited to passive targeting strategies using nanocarriers, with the advantage of having none of the access issues and other problems associated with tumor therapy. Focusing research here would be of use in the treatment of a wide variety of inflammatory diseases and conditions, including atherosclerosis, arthritis, Alzheimer’s, hypersensitivity reactions, asthma, and infectious diseases.
Similarly, cardiovascular diseases, as well as cancers of the blood, are more amenable to DDS targeting concepts, because the nanocarrier does not have to leave the circulation for the drug to exert its effect, so the issues outlined in Section 24.2.2.3 (extravasation, penetration of the perivascular space, tumor penetration, etc.) do not arise.
A further promising research direction is the use of nanotechnologies in vaccine delivery. As described in Chapter 17, there is considerable evidence, albeit still mostly in laboratory animal studies, that antigens presented as micro- and nanoparticulate delivery systems stimulate better immune responses than soluble antigens. NPs also show considerable promise for use in imaging procedures, as described in Section 24.2.3.5.
24.2.3.3 Investigate Drug Combinations
An important feature of nanotechnologies is their ability to incorporate more than one drug as payload, even drugs that possess highly different physicochemical characteristics (see, for example, Chapter 5, Figure 5.9). However, there is a tendency in preclinical research to use a single drug (typically Dox)—with the focus primarily on increasing the complexity of the carrier, rather than paying attention to the encapsulated drug. Future research could investigate nanotechnologies as carriers for multiple drug combinations. In particular, this approach would be advantageous for cancer treatments, as multidrug chemotherapy is associated with synergistic anti-cancer effects, reduced individual cytotoxicites, and suppression of drug resistance.
24.2.3.4 Use Nature’s Own Processes
There has been a recent interest in echoing nature’s own processes to design novel DDS. For example, Chapter 23 describes the approach of “putting the drug in the cancers food”—i.e., utilizing the NP composition, structure and properties of the low-density lipoprotein, and its receptor-based natural-targeting properties, as an endogenous mechanism to achieve greater uptake of anticancer drugs by tumor cells. A further example is a recent study that describes the development of platelet-mimetic NPs, comprising biodegradable polymeric NPs enclosed in the plasma membrane of human platelets (Hu et al. 2015). As well as demonstrating reduced cellular uptake by macrophage-like cells, the cloaked NPs displayed platelet-mimicking properties, which resulted in an enhanced therapeutic efficacy in a model of coronary restenosis and a systemic bacterial infection.
It would seem a highly efficient approach to work in synergy with existing physiological processes in the body. Furthermore, studying natural processes increases current understanding of fundamental cell biology and cellular uptake processes. An example here is the recent research into exploiting receptor-mediated transcytosis (RMT) via the transferrin receptor, for improving CNS delivery. The research has yielded important information on the fundamental physiology of the RMT process (see Chapter 15, Section 15.2.2), which opens avenues for further research and optimization in this area. Such biologically inspired delivery technologies also offer the advantages of biocompatibility and biodegradability, which positively impact on subsequent clinical translation and FDA regulatory approval. This is in contrast to the use of synthetic nanocarriers, which can be associated with toxicity and biocompatibility issues (see Section 24.4.5). Synthetic nanocarriers are also susceptible to recognition and capture by the organs of the RES, typically resulting in high carrier uptake by the liver, which compromises targeting to other sites.
24.2.3.5 Imaging and Theranostics
A promising future direction for nanotechnology is in the field of theranostics, which is the subject of Chapter 18. The field has evolved in recent years and now is focused on the simultaneous delivery of both a therapeutic drug and a diagnostic agent, within a single multifunctional nanoparticulate platform. Although the field offers a promising future direction for nanotechnology and the development of personalized medicine, the limitations of the approach also need to be clarified, to help guide future research. The premise of using a single NP construct is based on the concept that both imaging agent and drug remain within the nanocarrier and can be followed concurrently. However, it has not been confirmed that both imaging agent and drug reach the target tumor simultaneously—both can be released before reaching the target, resulting in different biodistributions in the body (Hollis et al. 2013). The differing biodistributions of the nanocarrier, free drug, and free probes need to be resolved, before the potential of this application can be realized.
Again, this might be an area that could benefit from adopting a less complex strategy—for example, focusing on the use of nanocarriers as imaging agents per se, rather than as dual diagnostic and therapeutic platforms. This in itself would be a vital service—nanocarriers could play an important role in ex vivo tissue analysis, for the detection of early cancer and the profiling of molecular biomarkers. Many radionuclide-loaded nanocarriers, as well as antibody–radionuclide conjugates, are demonstrating their superiority for nuclear imaging techniques (Lammers et al. 2012; see also Chapter 5, Section 5.4.1). Development of advanced imaging nanotechnologies should better assist oncologists in the early diagnosis of cancer and in the early detection of metastases, and also provide guidance on when is the most appropriate time to stop therapy.
24.3 OVERCOMING THE EPITHELIAL BARRIER
The noninvasive delivery of biologics remains one of drug delivery’s most elusive and sought-after goals, that necessitates overcoming the epithelial barrier. As described in Chapter 4, the protective barrier function of epithelial interfaces is essential to our survival but also presents a formidable challenge to the entry of drugs and DDS. An understanding of the nature of the epithelial barrier then, is crucial to the success of achieving transepithelial transport. For this reason, an entire chapter of this book (Chapter 4) is dedicated to understanding epithelial barriers in general. Additionally, each chapter of Section 3 (nonparenteral routes) begins with a detailed consideration of the relevant anatomical and physiological barriers pertaining specifically to the route in question, as well as the implications therein to successful drug delivery and targeting via this route.
In particular, researchers joining the drug delivery field from nonbiology-based backgrounds might do well to consider these issues very carefully. Sophisticated nanoengineering of DDS cannot translate into clinical success without an appreciation of the relevant anatomical and physiological challenges (Figure 24.1). It is worth briefly summarizing some of the challenges of transepithelial transport here, to fully appreciate the challenges ahead (see also Chapter 4).
1. Access barriers: There are considerable problems associated with gaining access to the absorbing surface of an epithelial interface. For example, for the oral route, a DDS must negotiate through chyme, intestinal fluids, pancreatic secretions, bile salts, and sloughed off intestinal cells before reaching the absorbing surface. The enterocytes themselves are coated with a mucus layer, about 500 μm thick. Access is further compromised by the relatively short, and highly variable, intestinal transport time. As described earlier, targeting moieties on the DDS surface do not deliver the nanocarrier to the enterocyte—as always, targeting is only relevant if the ligand system can gain proximity to the enterocyte surface, which, given all the access barriers present, is a formidable task.
2. Large hydrophilic molecules do not passively diffuse through epithelial membranes: The physicochemical properties of a drug molecule that favor epithelial transport are described in Chapter 4. To summarize here, in order to be absorbed via transcellular passive diffusion, a drug molecule must have a low molecular weight, be lipophilic and uncharged; it must also demonstrate some aqueous solubility and be metabolically stable. These properties are virtually the antithesis of the physicochemical properties of the new biologics that are coming through drug development programs, which means these agents demonstrate very poor epithelial transport. Although small amounts (<0.1%) of protein or peptide drugs can sometimes be absorbed orally in an intact form, this is likely to occur through the Peyer’s patches, frank lesions, and senescent cell replacement events that are part of normal gut physiology.
3. Carrier-mediated transport is restricted to small molecules: Carrier-mediated transport is a possible alternative means of transepithelial absorption for biologics. But typically, carrier-mediated transport is confined to small molecules (see also Chapter 4, Figure 4.4). For example, in the GI tract, nutrient transporters have evolved for the absorption of the digested products of protein nutrients, i.e., amino acids and di- and tripeptides. Most peptide and protein drugs are much too large for uptake via such transporters.
4. Increasing the contact time at the absorption surface does not automatically improve absorption: Merely increasing the contact time of a protein drug (e.g., insulin) with the absorbing surface (for example, by incorporating a mucoadhesive or incorporating nanoto-pography into the DDS) does not automatically mean that the protein will be absorbed in a bioactive form. No matter how long the contact time with the absorbing surface has been extended, the fundamental characteristics of the biologic remain the same—it is still a large, charged, and labile macromolecule that demonstrates poor transepithelial transport. Maintaining the drug at the absorption surface for prolonged periods will not change these fundamental properties and in order to improve absorption, additional strategies will also be required (e.g., via the inclusion of an absorption enhancer, enzyme inhibitor, etc.).
24.3.1 NANOTECHNOLOGY AND EPITHELIAL TRANSPORT
Micro- and nanoparticulate DDS have been intensively researched and developed for the last two decades in attempts to enhance the oral bioavailability of peptide and protein drugs, in particular, insulin. A wide variety of targeting ligands, including lectins, sugars, and vitamin B12, have been conjugated to NPs, in order to facilitate uptake by enterocytes. However, as concluded in Chapter 7, real progress remains disappointingly slow—nanoparticulate DDS are all still at an early stage of development and have not shown a significant enhancement of oral bioavailability thus far. Reasons for the slow progress include, as described earlier, difficulties in accessing the enterocyte surface, the limited targeting opportunities that are possible, and the constraints conferred by the physicochemical properties of the drug. A further important consideration is that nanoparticulate DDS will typically be taken up across epithelia by endosomal vesicular transport (see also Chapter 4, Section 4.3.3). Endosomes are generated by a variety of receptor and nonreceptor mechanisms. At the apical surface of enterocytes, these are typically clathrin mediated and result in endosomes of ≈120–150 nm in diameter. Thus, particles that do not fit into this size of structure will not be efficiently taken into the cell. But even for those nanocarriers that do succeed in being internalized, the endosomes will then typically fuse with lysosomes, and the endosomal contents are destroyed, rather than transported across the cell and into the body.
24.3.2 SUCCESSFUL STRATEGIES FOR EPITHELIAL TRANSPORT
In the light of these challenges, it is worth highlighting strategies that are showing promise in overcoming the epithelial barrier, as a guide for future research. As described in Chapter 7, formulations that have thus far proven successful in improving oral bioavailability are typically relatively simple formulations, that include formulation excipients such as an absorption enhancer and an enzyme inhibitor, incorporated in an enteric-coated tablet or capsule.
A variety of mechanisms have been successfully developed to improve the solubility and dissolution rate of poorly soluble drugs, which can have the knock-on effect of improving transepithelial absorption (as described in Chapter 3). Strategies such as the development of NanoCrystals® and amorphous solid dispersions (ASDs) can be considered a real success story in the field. In fact, solubility enhancement technologies, which constituted a mere paragraph in the first edition of this book, have now matured sufficiently to warrant a dedicated chapter in this edition.
The emergence of alternative epithelial routes to the oral one for systemic absorption is another success story in the field. As described in the preceding chapters, the buccal, transdermal, nasal, and pulmonary routes all have licensed products for systemic delivery. Alternative epithelial sites to the GI tract offer fewer difficulties for systemic absorption, as they feature favorable conditions such as a relatively lower metabolic activity; in some cases, higher permeability; and the avoidance of hepatic first-pass metabolism. These routes also offer ease of access, ease of administration, prolonged retention, and the potential for controlled release from long-acting devices (e.g., transdermal patches, buccal films, intravaginal rings [IVRs]). Mechanisms are also being developed that actively force drug molecules across the epithelium, via the application of current or ultrasound, as for example in transdermal and ophthalmic delivery. Other mechanisms are used to physically breach the epithelial barrier, for example, using long-acting injections (LAIs) and implants, microneedles arrays, and gene guns.
The successful methods of achieving transepithelial transport described in this book all have in common that they integrate device and formulation optimization with close attention to both the physicochemical characteristics of the drug and the prevailing environmental conditions of the route.
24.4 FUTURE DIRECTIONS FOR DRUG DELIVERY
The future directions in the field are described in detail in the relevant chapters of this book. Moving forward, the following points are also worth considering.
The rather disappointing truth is that our ability to achieve drug “targeting” by nanocarriers, or any other type of carriers, is currently very limited. There is no still no “magic bullet,” capable of delivering a drug directly to its target. Rather, as outlined earlier (Section 24.2), both passive and active targeting strategies are associated with many short-comings and weaknesses. In spite of this, the term “targeting” is widely used in the context of drug delivery, perhaps creating a somewhat misleading impression that the concept is already highly successful. There is a risk of hubris hampering meaningful progress. Moving forward, the rhetoric surrounding the issue needs to be scaled back, to more realistically reflect the reality of the situation. For this reason, we have removed the word “Targeting” from the title of this edition of the book, reverting from “Drug Delivery and Targeting,” to the more representative title, “Drug Delivery.”
A variety of suggestions have been made above to address targeting problems, such as researching more specific niche areas, rather than looking for panaceas. Local targeting has been more successful and various new technologies have emerged to improve local delivery. These technologies are described in the relevant chapters and include improved nasal delivery devices, to target the posterior nasal cavity; improved pulmonary technologies, to facilitate delivery to the alveoli for systemic absorption; the use of microneedle arrays to facilitate transdermal and transscleral delivery; and microelectronic drug delivery for programmed targeting to specific regions of the GI tract.
Many parenteral depot formulations are characterized by an initial burst release, resulting in an initial peak blood concentration much larger than the therapeutically effective concentration. As technology moves toward controlled release of drugs for longer duration times (up to 1 year or longer), research, as described in Chapter 6, is focusing on the need to more precisely control the release kinetics and avoid burst effects, as well as to improve host responses.
The Section IV: Emerging Technologies part of the book describes increasingly sophisticated methods currently under development to effect highly precise control over drug release from DDS. Stimuli-sensitive systems (Chapter 14) allow drug carriers to release their contents upon exposure to external stimuli, such as heat, light, ultrasound, and magnetic fields; or internal triggers, for example, near infected or inflamed tissues that have altered high temperature, acidic or alkaline pH, high reactive oxygen species levels, or high glutathione levels. Chapter 23 describes the heat-sensitive release of Dox from Thermodox®. Many controlled-release systems are being developed by leveraging techniques from the microelectronics industry (Chapter 19), which has led to the development of, for example, microelectromechanical systems (MEMS; Chapter 14) and technologies such as the InteliSite® and IntelliCap® systems, which allow electronically programmed drug release in the GI tract (Chapter 7).
Such great strides in controlled drug release have many applications. One, of great importance in the field, is the development of a glucose-sensitive transient insulin delivery device, with on–off switching capability. There are many hurdles to be overcome in the development of such a device (Park 2014), but increasingly sophisticated technology and know-how is being applied to the problem, so that this objective is becoming a near-term reality (see also Chapter 14, Section 14.4.2).
Another future direction for the field is toward improving compliance and efficacy via the development of multidrug DDS. The potential of nanotechnologies as carriers for multiple drug combinatons in cancer therapy has been outlined in Section 24.2.3.3. For pulmonary drug delivery, combination Drug-Aerosol products (containing drugs from different therapeutic classes within the same aerosol) are the top-selling aerosol products worldwide (Chapter 11). Drug delivery to the female reproductive tract is moving toward “multipurpose prevention technologies (MPT),” i.e., biomedical interventions designed to simultaneously address multiple reproductive health needs. For example, segmented dual-reservoir design IVRs can carry both an antiviral agent and a contraceptive; many other systems are under study (Chapter 12). Three-dimensional (3D) extrusion printing has recently been used to combine complex medication regimes into a single personalized tablet, or “polypill,” for oral delivery (Khaled et al. 2015). This multiactive solid dosage form contained five compartmentalized drugs, with two independently controlled-release profiles.
24.4.4 PERSONALIZED AND PRECISION MEDICINES
There will be increasing emphasis placed on personalized medicine, to provide customized treatment to patients. In the treatment of cancer and other diseases, a patient’s genomic data can be used to identify the best treatment mode, known as precision medicine. There are great opportunities, and challenges, for drug delivery science to provide better, more personalized, and precise treatments and services for individual patients. The emerging field of Theranostics (another entirely new subject area for this edition of the book), and the promise it holds for the development of personalized medicine, is described in Chapter 18. An interesting new development is the alignment of the field of information technology (which is being used to collect and analyze patient health data) with the pharmaceutical and health-care fields, to establish a broad foundation for personalized medicines.
A new DDS must be approved by the relevant regulatory body in order to be marketed. It should be stressed that regulatory agencies are focused on ensuring the “safety and efficacy” of drug products (rather than the ingenuity and sophistication of a particular nanocarrier design). For DDS that use complex formulation designs and manufacturing processes, the task of ensuring consistency in safety and efficacy is much more challenging than for traditional simple dosage forms (Wen et al. 2015).
New DDS require extensive preclinical and clinical studies in order to gain regulatory approval. Chapter 21 describes the plethora of extra studies necessary for the regulatory approval of injectable nanomedicines such as liposomal/NP products and colloidal iron/gold conjugate products—far more than the basic product characterization criteria required for conventional parenteral products. Chapter 6 describes the issues of host response/biocompatibility and immunogenicity, and the extensive testing necessary to gain regulatory approval, for implants and long acting injections (LAIs).
Potentially promising preclinical research may have to be discontinued because of subsequent stringent regulatory demands. In order to achieve successful regulatory approval and clinical translation, preclinical drug delivery research needs to be more cognizant of potential regulatory hurdles further down the line and be more concerned with safety and toxicology issues. For example, the field has seen the recent introduction of a diverse array of nanoscale materials and processes, such as carbon nanotubes, fullerene derivatives, and quantum dots. The hazard potential and biocompatibility of many of these new synthetic nanocarriers are important concerns, as they may involve agents that are toxic, carcinogenic, have long half-lives (of the order of decades), and show widespread tissue distribution in vivo (Hardman 2006; see also Chapter 18). Again, rather than prioritizing the design of evermore sophisticated and complex nanostructures based on these materials, research should first address these inherent toxicological and biocompatibility problems.
Similarly, in considering the development of absorption enhancers for transepithelial permeation, it should be remembered that getting regulatory approval for a new absorption enhancer can be as formidable as getting approval for a new chemical entity (NCE). The required safety studies are costly and time consuming, and there is reluctance in the pharmaceutical industry to fund trials for what may have originally been intended as merely an inexpensive formulation additive.
Another hurdle: if a drug is chemically conjugated to a DDS, regulatory agencies will consider that the conjugated drug is a NCE; as such, clinical trials will again be required. Similarly, a prodrug is considered to be an NCE by regulatory bodies, even though it is bioreversible to the original drug (FDA Guidance for Industry 1999).
As drug delivery technologies increase in sophistication, the concomitant regulatory challenges become increasingly complex. For example, in the case of the “polypill” described in Section 24.4.3, each 3D-printing device that produces a personalized polypill could in principle be treated as an independent manufacturing machine, making regulatory approval highly complicated. A close collaboration between industry, academia, and regulatory agencies is therefore necessary to address these complex challenges and allow drug delivery science to progress, while also ensuring patient safety.
24.4.6 A NEED FOR IMPROVED IN VITRO/IN VIVO TESTING
Given the increasing complexity of DDS formulations, as well the variability, heterogeneity, and complexity of the biological milieu that they must operate under, there is definite need to develop better in vitro and in vivo tests, to allow both the accurate assessment, and prediction, of drug activity and toxicity. A particular problem is that even though a nanocarrier may be assiduously characterized in vitro, as soon it is injected, it will be cloaked immediately with a “corona” of plasma proteins, which will fundamentally change the nature of the carrier, including its size, surface properties, stability, and other properties. For this reason, the surface of nanocarriers is frequently PEGylated for extended circulation in the blood, although the overall impact on targeted drug delivery has been modest at best.
As noted in Section 24.2.1, cell lines and the majority of animal models currently used to test nanotechnology DDS are poor predictors of in vivo performance. For tumor-targeting research efforts, it has been proposed to improve the current situation by establishing a well-defined, standardized, panel of animal models, which should include, among others, an orthotopic, metastatic, and transgenic model (Lammers 2013). Having a “gold standard” panel would allow improved testing of novel DDS formulations and provide more predictive analysis of in vivo performance. It would also allow direct head-to-head comparisons of new formulations against other DDS and against the standard therapy. A further interesting possibility is the recently described in vitro tumor model, “tumor-microenvironment-on-chip” (T-MOC) (Kwak et al. 2014). The model comprises 3D microfluidic channels in which tumor cells and endothelial cells are cultured within an extracellular matrix perfused by interstitial fluid. The T-MOC platform has been shown capable of simulating the complex microenvironment around the tumor and allows nanocarrier transport behavior to be studied while systematically and independently varying tumor microenvironmental parameters, such as cutoff pore size, interstitial fluid pressure, and tumor tissue microstructure.
The need for better in vitro and in vivo predictive models is not confined to nanotechnology for cancer research. In practically every chapter of this book, across the entire spectrum of drug delivery research, authors have expressed their concern about the limitations of current testing procedures and stressed the need for improved methodologies.
The development of more predictive in vitro and in vivo models seems an area that would be ideally suited to widespread collaboration within the pharmaceutical industry. Although pharma companies may be reluctant to collaborate on developing new drug leads for fear of loss of competitive advantage, the pooling of resources to develop improved testing procedures and improved in vitro-in vivo correlation would offer significant advantages to all.
24.4.7 A NEED FOR COLLABORATIVE, TRANSPARENT RESEARCH
A striking feature of drug delivery science is the breadth and range of the field, which encompasses pharmaceutics, polymer chemistry, biology, physiology, pathology, physics, immunology, oncology, medicine, and engineering. Overcoming the considerable challenges associated with drug delivery requires a concerted multifaceted effort by scientists from all of these disciplines.
Cross-disciplinary collaboration allows the input of fresh ideas and enables new ways of approaching old problems. Reimagining content in a new context may help drug delivery scientists to think outside the box and even facilitate the kind of creative leap necessary to “think in new boxes” (de Brabendere and Iny 2013). Recent examples of the benefits of cross-fertilization from other disciplines include the leverage of techniques from the microelectronics industry, to precisely fabricate DDS in the nanometer range (Chapter 19); the use of reverse-engineering design to generate liposomal DDS (Chapter 23); the merging of imaging technologies with DDS, to develop the field of theranostics (Chapter 18); the merging of vaccinology with DDS, to develop nanoparticulate vaccine delivery systems (Chapter 17); and the use of cell biology to understand physiological uptake processes in the BBB, to develop drug delivery strategies to the CNS (Chapter 15).
Progress in drug delivery technology, as with all true progress, occurs via trial and error. The key to moving forward in drug delivery is to try many approaches and test them out—those that work can be further improved. But there is also much to be gained by studying what did not work. At the risk of sounding like a motivational wall poster: “There is no failure, only feedback.” Experiments are always an opportunity to learn, adjust, and try again. A careful consideration of why things did not turn out as expected can be the springboard to greater success. It can also save a lot of time, effort, money, and needless repetition, if negative results are shared with the scientific community.
Unfortunately, there are currently extremely limited opportunities for scientists to present negative data, thus this valuable, indeed vital, information is lost. If scientists were able to be more open and transparent about negative findings, progress would proceed far more rapidly than at the current pace. The authors strongly agree with the recent recommendations to improve the reliability of preclinical cancer studies (Begley and Ellis 2012):
There must be more opportunities to present negative data. It should be the expectation that negative preclinical data will be presented at conferences and in publications. Preclinical investigators should be required to report all findings, regardless of the outcome. To facilitate this, funding agencies, reviewers and journal editors must agree that negative data can be just as informative as positive data.
This obviously requires a paradigm shift in current thinking on how research is conducted, at all levels of scientific research, from government funding agencies, right through pharmaceutical industry, academia, and research institutions. Such changes will not happen overnight, although there are already encouraging indicators that the tide is turning. For example, the Journal of Controlled Release (JCR) recently outlined a new editorial policy for the journal (Park 2015):
Understanding why a certain formulation failed in animal studies and/or in clinical studies is more important than seeing another me-too data. Sometimes authors find that a widely used experimental method is misleading, and describing such information is highly valuable despite the negative data. Presenting a new insight into a presumed, and widely accepted, theory, is critical for the progress of the field, especially when the theory has not been able to explain the results in clinical studies. In short, the JCR welcomes those data that do not conform to the widely accepted dogma, proven by carefully designed experiments.
A further practical solution, easily implemented, would be to create an Internet forum for drug delivery, where unsuccessful preclinical experimental data could be freely exchanged, discussed, and analyzed. The site would need careful monitoring by moderators who are familiar with the specific topics under discussion: a lot of information is not necessarily useful information. But a carefully moderated forum, sharing negative and inconclusive results, would be of great service to the drug delivery community—and would also serve as a best practice model for scientific research in general. In particular, a collaborative Internet resource seems ideally suited to the new generation of digital-native millennials who are entering drug delivery research. Having an open forum would also serve to encourage dialog and help to forge the type of multifaceted, cross-disciplinary collaboration that is crucial for future progress in the field.
This kind of collaborative, open-forum approach is steadily gaining traction within the scientific community. An interesting recent example of an open-source model for pharmaceutical R&D is the Project Data Sphere (www.projectdatasphere.org). Developed in order to accelerate oncology drug discovery and development, it is an online resource for sharing clinical trial data. The technology platform, built and maintained by business analytics software company SAS, shows how historical cancer research data can be shared, integrated, and analyzed, bringing together diverse groups with common interests, including researchers from academia, industry, hospitals, and institutions. Additionally, Chapter 20 describes many public, web-accessible databases, for drug discovery purposes. A further possibility has been described in Chapter 23, with the idea of “translating drug delivery without profit: open source pharmaceuticals,” i.e., making effective drug delivery formulations open source and freely available.
In conclusion, successful progress in the field is contingent on a number of factors, including
• A full appreciation of the challenges (formulation and biological) that need to be overcome
• The use of a rational design strategy for the development of a new DDS
• An increased collaboration between complimentary disciplines
• A concerted effort to develop more appropriate in vitro and in vivo testing methodologies
• A more open and transparent reporting of preclinical research, including the publication of negative data
Considerable progress has been made in the field and, in spite of the formidable challenges, many new approaches and avenues of research have opened up. The perseverance, verve and ingenuity that has brought us from the Spansule® “tiny little time pills” of the 1950s to the sophisticated nanotechnologies of today continues to drive research forward and assures the development of safe and effective DDS for the future.
Bae, Y.H. and K. Park. 2011. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 153(3):198–205.
Barenholz, Y. 2012. Doxil®—The first FDA-approved nanodrug: Lessons learned. J. Control. Release 160:117–134.
Begley, C.G. and L.M. Ellis. 2012. Drug development: Raise standards for preclinical cancer research. Nature 483:531–533.
Crommelin, D.J. and A.T. Florence. 2013. Towards more effective advanced drug delivery systems. Int. J. Pharm. 454(1):496–511.
de Brabandere, L. and A. Iny. 2013. Thinking in New Boxes: A New Paradigm for Business Creativity. New York: Random House.
FDA Guidance for Industry. Applications covered by section 505(b)(2). 1999. Available from: http://www.fda.gov/downloads/Drugs/Guidances/ucm079345.pdf, accessed March 17, 2016.
Gordon, A.N., D. Guthrie, D.E. Parkin et al. 2001. Recurrent epithelial ovarian carcinoma: A randomized phase III study of PEGylated liposomal doxorubicin versus topotecan. J. Clin. Oncol. 19(14):3312–3322.
Hardman, R. 2006. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 114(2):165–172.
Harrington, K.J., S. Mohammadtaghi, P.S. Uster et al. 2001. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled PEGylated liposomes. Clin. Cancer Res. 7(2):243–254.
Harrington, K.J., K.N. Syrigos, and R.G. Vile. 2002 December. Liposomally targeted cytotoxic drugs for the treatment of cancer. J. Pharm. Pharmacol. 54(12):1573–1600.
Hillery, A.M., A.W. Lloyd, and J. Swarbrick. 2001. Drug Delivery and Targeting: For Pharmacists and Pharmaceutical Scientists. Boca Raton, FL: CRC Press.
Hollis, C.P., H.L. Weiss, M. Leggas et al. 2013. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: Lessons learned of the enhanced permeability and retention (EPR) effect and image-guided drug delivery. J. Control. Release 172:12–21.
Hu, C.-M.J., R.H. Fang, K.-C. Wang et al. 2015. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526:118–121.
Khaled, S.A., J.C. Burley, M.R. Alexander et al. 2015. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 217:308–314.
Kwak, B., A. Ozcelikkale, C.S. Shin et al. 2014. Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip. J. Control. Release 194:157–167.
Lammers, T. 2013. SMART drug delivery systems: Back to the future vs. clinical reality. Int. J. Pharm. 454(1):527–529.
Lammers, T., F. Kiessling, W.E. Hennink et al. 2012. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 161:175–187.
Maeda, H., M. Ueda, T. Morinaga et al. 1985. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein-neocarzinostatin: Pronounced improvements in pharmacological properties. J. Med. Chem. 28:455–461.
Matsumura, Y. and H. Maeda. 1986. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 46:6387–6392.
Nanotechnology Safety and Health Program. National Institute of Health, Technical Assistant Branch. 2014. http://www.ors.od.nih.gov/sr/dohs/Documents/Nanotechnology%20Safety%20and%20Health%20Program.pdf, accessed March 17, 2016.
Nichols, J.W. and Y.H. Bae. 2014. EPR: Evidence and fallacy. J. Control. Release 190:451–464.
Park, K. 2014. Controlled drug delivery systems: Past forward and future back. J. Control. Release 190:3–8.
Park, K. 2015. Editorial: The state of the journal. J. Control. Release 215:A1–A2.
Prabhakar, U., H. Maeda, R.K. Jain et al. 2013. Challenges and key considerations of the enhanced permeability and retention (EPR) effect for nanomedicine drug delivery in oncology. Cancer Res. 73:2412–2417.
Raemdonck, K. and S.C. de Smedt. 2015. Lessons in simplicity that should shape the future of drug delivery. Nat. Biotechnol. 33:1026–1027.
Stirland, D.L., J.W. Nichols, S. Miura et al. 2013. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release 172:1045–1064.
Wang, Y.-S., H. Xia, C. Lv et al. 2015. Self-propelled micromotors based on Au–mesoporous silica nanorods. Nanoscale 7:11951–11955.
Wen, H., J. Huijeong, and X. Li. 2015. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. (Spec. issue Clinical and Commercial Translation of Drug Delivery Systems) 17(6):1327–1340.
Yun, Y.H., B.K. Lee, and K. Park. 2015. Controlled drug delivery: Historical perspective for the next generation. J. Control. Release 219:2–7.