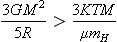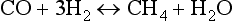The Origin and Early Evolution of the Earth
2.1 Introduction
The subject of this book is the Earth, and its cycles and processes. The specific focus is the Earth in its present state, but to understand how it came to this point and predict how it may change in the future, it is important to examine the full history of the Earth. We need to examine its origin and evolution as a planetary body. Advances in planetary science, including models and abundant new data provide a valuable framework for understanding fundamental aspects of development of the Earth. The recent discovery of planets outside the solar system extends the scope of our understanding of planetary processes and properties. Many of the Earth’s properties are taken for granted as simply being “natural,” but comparison with other planets and models of planetary evolution indicates that the character of the Earth is actually quite odd. The unusual properties include life, an ocean, “moderate” surface temperature and the presence of plate tectonics. We will discuss the origin and evolution of the Earth to provide a planetary perspective and insight into some of the fundamental Earth processes. We will start with the origin of the elements that the Earth and solar system formed from.
2.2 Pre-Solar Evolution: The Origin of the Elements
The Earth is the end product of a series of evolutionary processes that began at the time the first elements were produced, 15 billion years ago. The Earth’s composition, its evolution and many of its chemical and physical cycles described in this book are influenced to various degrees by processes that occurred during or before the formation of the solar system. These processes include nuclear reactions to produce the elements, gravitational collapse to produce stars and protoplanetary systems, condensation to produce solid grains, and accretion to accumulate grains into planets. The basic framework of this scheme is believed to be generally well understood, although many of the details of even the most fundamental processes such as condensation and accretion are highly uncertain.
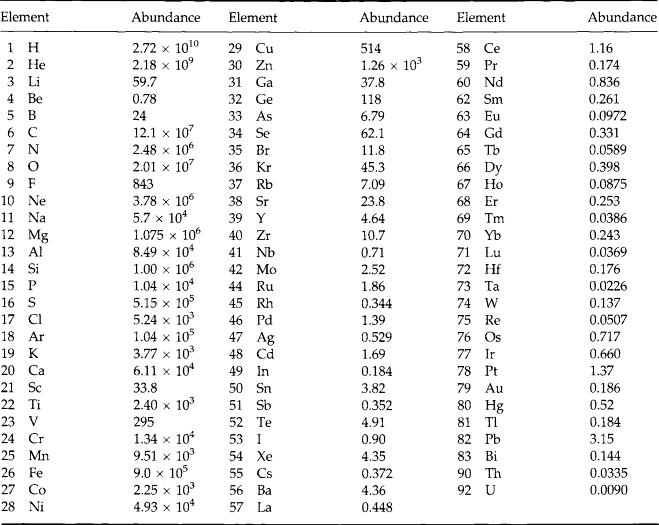
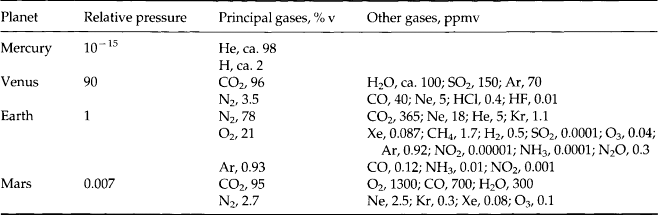
The composition of the Earth was determined both by the chemical composition of the solar nebula, from which the sun and planets formed, and by the nature of the physical processes that concentrated materials to form planets. The bulk elemental and isotopic composition of the nebula is believed, or usually assumed to be identical to that of the sun. The few exceptions to this include elements and isotopes such as lithium and deuterium that are destroyed in the bulk of the sun’s interior by nuclear reactions. The composition of the sun as determined by optical spectroscopy is similar to the majority of stars in our galaxy, and accordingly the relative abundances of the elements in the sun are referred to as “cosmic abundances.” Although the cosmic abundance pattern is commonly seen in other stars there are dramatic exceptions, such as stars composed of iron or solid nuclear matter, as in the case with neutron stars. The best estimation of solar abundances is based on data from optical spectroscopy and meteorite studies, and in some cases extrapolation and nuclear theory. The measured solar abundances are listed in Fig. 2-1 and Table 2-1. It is believed to be accurate to about 10% for the majority of elements. The major features of the solar abundance distribution are a strong decrease in abundance for heavier elements, large deficiency of Li, Be, and B, and broad abundance peak centered near Fe. The factor of 10 higher abundance of even atomic number nuclei relative to their immediate odd atomic number neighbors is due to the higher binding energy of nuclei with even numbers of protons. The abundance curve of odd nuclei plotted against mass is a very smooth function. The cosmic abundance pattern is the net result of nuclear reactions that occurred during the origin of the universe and in the interiors of later generations of stars.
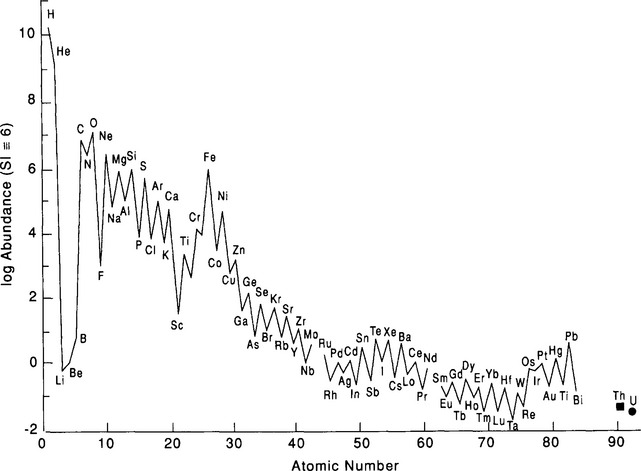
Fig. 2-1 Cosmic (solar) abundances of the elements, relative to Si, which is given the arbitrary value of 106.
Over 99% of the atoms in the sun are H and He and are believed to have formed in the “Big Bang,” the origin of the universe (Silk, 1989). Nucleosynthesis that occurred during the Big Bang produced basically the cosmic abundance of 1H, and 4He and small amounts of 2H, 3He, and 7Li but essentially no heavier elements. The Li/H ratio produced by the Big Bang was about 10−9. Element formation occurred over a quarter hour time period during which temperatures dropped from very high values to less than about 109 K. After this brief period of expansion and cooling the universe no longer contained matter that was hot and dense enough for nuclear reactions to occur. The fundamental nuclear reaction that occurred in the Big Bang was the fusion of hydrogen to form helium. Synthesis stopped at 7Li because formation of the next abundant element, carbon, required higher densities than existed in the universe at the appropriate temperature range. Inside stars the temperatures and densities are sufficiently high for synthesis of heavy elements. Without future generations of hot dense matter, in the form of stars, there would have never have been any elements that were heavier or more chemically interesting than H, He, and Li (Penzias, 1979).
The H and He produced in the Big Bang served as “feed stock” from which all heavier elements were later created. Less than 1% of the H produced in the Big Bang has been consumed by subsequent element production and thus heavy elements are rare. Essentially all of the heavier elements now in the Earth were produced after the Big Bang inside stars. Following the Big Bang, the universe expanded to the point where instabilities formed galaxies, mass concentrations from which up to 1014 stars could develop.
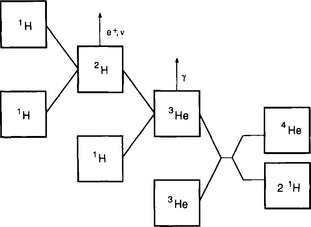
The reaction that is the major energy source for stars similar to the sun is the fusion of H to He. The basic reactions (the proton-proton chain) in the sun are shown in Fig. 2-2. Although these reactions are the major source of energy in the solar system, they proceed at a remarkably slow and uniform rate. In the sun’s core where the temperature is 14 × 106 K, the lifetime of a proton before it is fused to deuterium is 1010 years. The average energy generation rate for the entire sun is only 200 µW/kg. Hydrogen burning occurs in stars whose interior temperatures are in the 107 to 108 K range and while there are several reaction chains depending on the stellar mass, the general fusion reactions forming He are similar to those that occurred during the Big Bang.
For stars like the sun, the burning of H to He occurs deep in stellar interiors at a fairly stable rate for over 90% of the star’s lifetime. For the sun this “main sequence” stage will last for about 1010 years before H depletion and rising core temperature initiate a set of more energetic nuclear reactions that occur in the final stages of its evolutionary lifetime. Throughout the geologic record of Earth the sun has been a main sequence star burning H to He. On the basis of theory and observations of similar stars it is expected that the total luminosity (total radiated power) emitted from the sun should not undergo large changes. Short- and long-term changes do occur, however, and they may have had significant effects on the Earth and its physical and chemical cycles. Short-term variability, at the level of 0.1% per year is observed by spacecraft. The most important change, and the one that is the most predictable, is the gradual increase in the solar luminosity over geologic time. As hydrogen is burned in the sun’s core, the mean molecular weight of nuclei results in a slow but relentless increase in temperature of the core. To maintain the star in hydrostatic equilibrium the internal pressure must remain constant to support the weight of overlying matter. To maintain constant pressure as the mean molecular weight of the gas increases, the temperature must rise. The temperature rise results in increased energy generation and it has been estimated that over the past four billion years the “solar constant” (the intensity of sunlight at Earth) has increased by about 30%. Over the next three billion years it will increase by more than an additional 30%. Astronomically such a change seems minor, but the stress that the increased brightness places on terrestrial processes is very large. In fact it is quite remarkable the surface temperature of the Earth has remained as constant as it has while the solar luminosity has increased. A 30% decrease in the sun’s output should decrease the temperature of a 300 K airless body heated by sunlight by 20 K. In the case of the Earth, this lesser illumination would lower the mean surface temperature to the freezing point, if there were no other compensating processes occurring. There is no evidence that the Earth’s oceans have ever frozen over. The remarkable stability of the Earth’s temperature in light of solar changes and large changes in the composition of the atmosphere has led to the “Gaia” hypothesis (Lovelock, 1979). This theory suggests that biological organisms on the Earth collectively act to moderate the long-term atmospheric environment to the mutual benefit of terrestrial life. An alternative hypothesis, not involving organisms, is described by Kasting et al. (1988). The next chapter discusses the Gaia hypothesis in detail.
Following the long duration of hydrogen burning, stars enter the red giant phase where increasingly heavier elements are produced. Increasing temperatures in the stellar cores allow more massive, highly charged nuclei to collide with sufficient energy to penetrate coulomb barriers and initiate fusion reactions. The first major step is the fusion of He to form C, a reaction that takes place above 108 K. This occurs by the triple alpha process, an interaction that requires essentially a three-body collision between He nuclei. The nearly simultaneous collision of three particles requires high densities and is the reason why this reaction did not occur in the Big Bang. In the triple alpha sequence two He nuclei collide to form a highly unstable 8Be nucleus. The 8Be must then interact with a third He to form 12C on a very short time scale because of the 10−16 s decay time of 8Be. The reaction is very temperature dependent and the He burning phase is violent, unstable, and relatively short-lived. At temperatures above 108 K fusion reactions can produce elements up to Fe. From He to Fe the binding energy per nucleon increases with atomic number and fusion reactions are usually exothermic and provide an energy source. Beyond Fe the binding energy per nucleon decreases and exothermic reactions do not occur. Up to Fe many of the nuclei are products of alpha reactions, which involve fusion with a He nucleus. Because of this and the fact that there is high binding energy for nuclei that are multiples of 4He all of the most abundant isotopes for elements up to Fe are multiples of 4He (i.e. 12C, 16O, 32S, 24Mg, 28Si, etc.); see Fig. 2-1.
During the red giant phase of stellar evolution, free neutrons are generated by reactions such as 13C(α,n) 16O and 22Ne(α,n) 25Mg. (The (α,n) notation signifies a nuclear reaction where an alpha particle combines with the first nucleus and a neutron is ejected to form the second nucleus.) The neutrons, having no charge, can interact with nuclei of any mass at the existing temperatures and can in principle build up the elements to Bi, the heaviest stable element. The steady source of neutrons in the interiors of stable, evolved stars produces what is known as the “s process,” the buildup of heavy elements by the slow interaction with a low flux of neutrons. The more rapid “r process” occurs in explosive environments where the neutron flux is high. The mechanism of the s process is illustrated in Fig. 2-3. Starting with a seed isotope, successive neutron captures build up increasingly neutron-rich isotopes of the same element until an unstable isotope is reached. The typical decay of this neutron-rich isotope is beta decay, which produces the next element in the periodic table. The new element will have one more proton and one less neutron than its radioactive parent. In beta decay a neutron disintegrates into a proton, a neutrino and an ejected electron (beta particle). The new element created in the s process will then add new neutrons until it reaches a neutron-rich isotope that undergoes beta decay to form yet the next element. The s process can produce the isotopes along the “valley of beta stability” in the chart of the nuclides, the chart of isotopes plotted on a graph of total neutrons in nuclei versus atomic number. The relative abundance of an isotope produced by the s process is proportional to its binding energy and inversely proportional to its neutron capture cross-section. Tightly bound nuclei have small cross-sections and are slower to absorb neutrons to form heavier isotopes. The s process cannot produce isotopes that are particularly neutron rich or neutron poor, but it can produce most of the cosmically abundant elements between 56Fe and 209Bi.
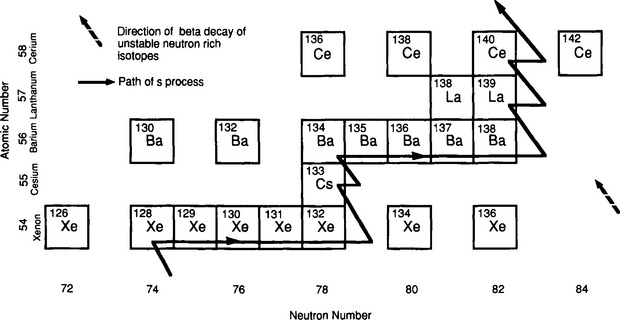
Fig. 2-3 Schematic showing the path of the s process. The isotopes 134Xe, 136Xe, and 142Ce are beyond the reach of s process nucleosynthesis and are only produced by the r process.
Because the path of the s process is blocked by isotopes that undergo rapid beta decay, it cannot produce neutron-rich isotopes or elements beyond Bi, the heaviest stable element. These elements can be created by the r process, which is believed to occur in cataclysmic stellar explosions such as supernovae. In the r process the neutron flux is so high that the interaction time between nuclei and neutrons is shorter that the beta decay lifetime of the isotopes of interest. The s process chain stops at the first unstable isotope of an element because there is time for the isotope to decay, forming a new element. In the r process, the reaction rate with neutrons is shorter than beta decay times and very neutron-rich and highly unstable isotopes are created that ultimately beta decay to form stable elements. The paths of the r process are shown in Fig. 2-3. The r process can produce neutron-rich isotopes such as 134Xe and 136Xe that cannot be reached in the s process chain (Fig. 2-3).
Thus the origin of the elements began in the Big Bang, but the formation of most of the elements important for physical and chemical processes on the Earth occurred in stars. The element production process required cycles of star formation, element formation in stellar cores, and ejection of matter to produce a gas enriched in heavy elements from which new generations of stars could form. The atoms in the Earth are products of reactions in a large number of stars; typical atoms have been cycled through several generations of stars. The synthesis of material and subsequent mixing of dust and gas between stars produced the solar mix of elements in the proportions that are called cosmic abundances. Although some isotopes are exceedingly rare, nuclear processes in stars have produced every known stable isotope.
In addition to stable elements, radioactive elements are also produced in stars. The unstable but relatively long-lived isotopes 40K, 232Th, 235U, and 238U make up the internal heat source that drives volcanic activity and processes related to internal convection in the terrestrial planets. The short-lived transuranium elements such as Rn and Ra that are found on the Earth are all products of U and Th decay. These isotopes are sometimes used as tracers of natural terrestrial processes and cycles. Long-lived isotopes, such as 87Rb and 147Sm are used for precise dating of geological samples. When the solar system formed it also contained several short-lived isotopes that have since decayed and are now extinct in natural systems. These include 26Al, 60Fe, 244Pu, 107Pd, and 129I. 26Al with a half-life of less than a million years is particularly important because it is a potentially powerful heat source for planetary bodies and because its existence in the early solar system places tight constraints on the early solar system chronology.
2.3 The Origin of the Solar System
The sun and planets formed 4.55 × 109 years ago from interstellar gas and dust. This interstellar material had a bulk elemental composition similar to that of the sun, but the elements in the initial material were highly fractionated between the solid and gaseous phases. Most of the condensable materials were in the form of submicrometer dust grains, while materials that do not condense under astrophysical conditions, such as H, He, and the noble gases, were gaseous. The grains are believed to be mixtures of silicates and carbonaceous matter. In popular models for grains they have silicate cores about 100 nm in diameter coated with 100 nm thick mantles of compounds composed primarily of H, C, N, and O. Irradiation by ultraviolet light and charged particles likely leads to the formation of complex cross-linked polymers from condensed low-atomic-weight compounds in space.
The formation of the solar system is believed to have begun when a cloud of gas and dust became unstable to gravitational collapse and started an essentially unconstrained freefall. During the freefall, the dimensions of the cloud decreased by a factor of nearly 1000 by the time a stable rotating lenticular nebula was established. The nebula was stable to further collapse because gravitational forces were countered by gas pressure and centrifugal forces. The condition for gravitational collapse of a cloud is that the internal gravitational potential energy must exceed twice the kinetic energy. This is expressed by
where k is the Boltzmann constant, R the initial cloud radius, G the universal gravitational constant, M the cloud mass, T the temperature, µ the mean molecular mass, and mH the mass of the proton. The collapse of interstellar material to form the solar nebula takes on the order of 105 to 106 years depending on the initial conditions. During most of the collapse the cloud is transparent to its own emitted infrared radiation and it cools to a temperature near 10 K until nebular densities are reached and the cloud becomes opaque. During this isothermal collapse phase, any condensable matter not originally on grains certainly condensed. The solar nebula that formed is believed to have been a stable rotating disk somewhat larger than the present planetary system. The sun formed in the center and the planets formed from materials that accumulated in the disk. The planets Jupiter and Saturn must have formed by some variant of gravitational collapse, because to a good approximation their elemental compositions match that of the bulk nebula. Most of their mass is H and He, elements that could only have been in gaseous form. The other planets apparently formed out of solids. The outer planets Uranus and Neptune formed from icy and rocky materials, while the terrestrial planets, Mars, Earth, Venus, and Mercury, formed exclusively from rocky and metallic particles.
The solar nebula was hot and dense near its center and became cooler and more diffuse with increasing radial distance. Modern theories of evolution in the nebula indicate that the major heat source was frictional viscosity within the rotating disk of gas. The viscosity and associated redistribution of energy and angular momentum was the result of convective movement of gas in a gas disk that had differential rotation with radial distance from its center. In the outer regions of the nebula, it is likely that heating was never sufficient to vaporize pre-existing interstellar dust grains. In the inner regions of the nebula the original grains were apparently vaporized or extensively altered and the inner (terrestrial) planets must have formed from second-generation solids that condensed from the nebular gas. This condensation process is temperature dependent and it influenced the composition of the planets that formed. The bulk of the mass of each planet appears to have formed largely from local material in a “feeding zone,” an annular ring of nebular material. Grains condensed and then were eventually swept up to form planetary bodies. The composition of material in feeding zones depended on temperature and radial distance from the center of the nebula and this determined planetary compositions.
2.4 Condensation
The sequence of condensation of solids from a solar composition at a nebular pressure of 10 Pa (about 10−4 atm) is shown in Fig. 2-4. This sequence is calculated for what solids could exist in equilibrium with the solar nebula at various temperatures. Attainment of true equilibrium requires the lack of nucleation barriers, and efficient diffusion within solids so that grain interiors can maintain equilibrium with the gas phase. While strict equilibrium condensation may have occurred at higher temperatures, perfect equilibrium probably did not occur with grains larger than a few micrometers in size or at lower temperatures where diffusion is slow. At temperatures sufficiently above 1500 K all elements were in the gas phase. The first solid grains to condense would be the highly refractory but cosmically rare elements like Pt, Os, Ir, and Re. The first abundant solids to form are oxides and silicates of Ca, Al, and Ti. Inclusions in certain meteorites are rich in these elements, although it is still not clear whether they are actually preserved condensates or refractory residues of volatilized material. Around 1400 K compounds of the most abundant elements in the Earth, Mg, Si, and Fe, condense. At this high temperature Mg and Si condense as Fe-free silicates and Fe condenses as an FeNi metal alloy. At lower temperatures, silicates that maintain equilibrium with the gas can incorporate FeO. At 750 K, independent of pressure, Fe metal in contact with the nebula should react with H2S gas and form FeS, the first sulfur-bearing solid. Iron remaining in contact with gas below 450 K should react with H2O forming magnetite, Fe3O4. Also at this temperature the first water can be incorporated into solids in the form of bound water in hydrated silicates. At temperatures below 250 K, water and clathrates of methane and ammonia can form. Ultimately, if the temperature ever drops as low as 40 K pure methane ice can exist. The approximate temperatures at which materials condensed and accreted to form the planets are indicated on the right side of Fig. 2-4. While there certainly must have been complications, such as radial mixing of material and non-equilibrium condensation and grain destruction, the equilibrium condensation sequence is generally consistent with the observed properties of the planets and meteorites, at least for compounds that form at high and moderate temperatures. The reality of low-temperature gas-grain reactions such as hydration of silicates and formation of magnetite from metal is in question because these low-temperature equilibrium processes may not have occurred in the time scales available in the rather short-lived solar nebula.
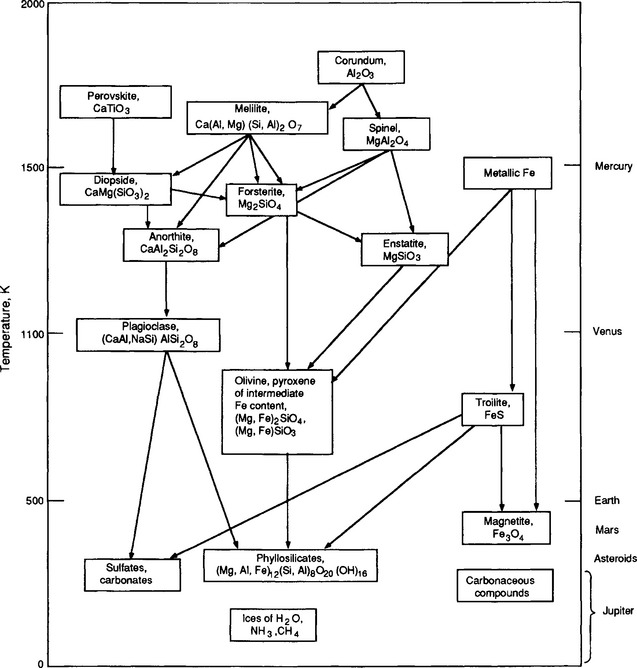
Fig. 2-4 The sequence of condensation of solids from a solar composition gas at a nebular pressure of 10 Pa (ca. 10−4 atm). (Modified with permission from J. A. Wood, “The Solar System,” p. 162, Copyright © 1979, Prentice-Hall, Englewood Cliffs, NJ.)
Evidence for condensation is seen in the meteorites, fragments of the asteroids that formed in the region between Mars and Jupiter. The stony meteorites that have elemental compositions that closely match those of the sun (except for volatile elements such as H, He, N etc.) are called chondrites after the presence of small spherical particles called chondrules. Although the chondrites generally contain close to undifferentiated solar compositions, there are elemental fractions in these objects that are related to condensation processes. Different chondrite groups are distinguished by Fe/Si ratios that vary by 50%, ratios of Ca, Al, and Ti to Si that vary by about 40%, and abundances of volatile elements, such as Cd, Bi, In, and Pb, that vary by orders of magnitude. The depletion of volatile elements is believed to be due to incomplete condensation. The correlated depletion of Ca, Al, and Ti in all but the most primitive meteorites is most likely due to the loss of an early condensate that was composed of these elements. An early condensate of Ca, Al, Ti, and other highly refractory elements could have been separated by accretion from the region of the nebula where grains condensing later formed the Al-depleted chondrites. The Fe/Si fractionation may be the result of the different accretion efficiencies of metal and silicate grains. But even this process is affected by condensation temperature because it determines how Fe is divided between metal, sulfide, oxide, and silicate phases.
Effects of condensation are also seen in the bulk compositions of the planets and their satellites. The outer planets, Uranus and Neptune, have overall densities consistent with their formation from icy and stony solids. The satellites of Uranus have typical densities of 1.3 g/cm3, which would tend to indicate a large ice component. The inner planets, which are composed of silicates and metal have uncompressed densities ranging from 3.4 for Mars to 5.5 for Mercury. The range in densities among the terrestrial planets is largely due to differences in the oxidation state of Fe. Most of the mass of these planets is composed of Fe, Mg, and Si with sufficient oxygen to totally oxidize Si and Mg. The elements Fe, Mg, and Si appear to occur near solar atomic abundances of approximately 1:1:1. The oxidation state of iron ranges from almost completely oxidized in the case of Mars to completely reduced as in Mercury. This range in oxidation state is consistent with equilibration with nebular gas at low and high temperatures, respectively. The oxidation state of Fe in chondrites also ranges from completely reduced in the case of the enstatite chondrites to completely oxidized in the carbonaceous chondrites. Even though the meteorites probably formed beyond the region of the terrestrial planets, the range of oxidation state is undoubtedly the result of nebular pressures and temperatures.
Water and carbon play critical roles in many of the Earth’s chemical and physical cycles and yet their origin on the Earth is somewhat mysterious. Carbon and water could easily form solid compounds in the outer regions of the solar nebula, and accordingly the outer planets and many of their satellites contain abundant water and carbon. The type I carbonaceous chondrites, meteorites that presumably formed in the asteroid belt between the terrestrial and outer planets, contain up to 5% (m/m) carbon and up to 20% (m/m) water of hydration. Comets may contain up to 50% water ice and 25% carbon. The terrestrial planets are comparatively depleted in carbon and water by orders of magnitude. The concentration of water for the whole Earth is less that 0.1 wt% and carbon is less than 500 ppm. Actually, it is remarkable that the Earth contains any of these compounds at all. As an example of how depleted in carbon and water the Earth could have been, consider the moon, where indigenous carbon and water are undetectable. Looking at Fig. 2-4 it can be seen that no water- or carbon-bearing solids should have condensed by equilibrium processes at the temperatures and pressures that probably were typical in the zone of the solar nebula that produced the Earth. Water of hydration does not occur in silicates until the temperature is below about 350 K, and ice could not exist in nebular conditions until the temperature was below 200 K. Temperatures low enough for even the formation of hydrated phases probably did not exist within the region of the terrestrial planets. The origin of carbonaceous materials is even more mysterious. In the nebula the distribution of carbon between CO and CH4 should be controlled by the following equilibrium:
Above 650 K carbon in the nebula should be primarily in gaseous CO but below 650 K, if equilibrium persisted, nearly all carbon would be reduced to CH4. The condensation temperature of methane is 50 K and if all carbon were in this form then it could not have been efficiently incorporated into solids except perhaps at the extreme outer edges of the solar nebula. It is likely, however, that equilibrium between CO and CH4 did not occur on the available time scale and CO was probably an important reservoir of carbon throughout the nebula. With abundant CO, catalytic reactions on grain surfaces could form carbonaceous coatings. It has been suggested that Fischer–Tropsch reactions similar to the following, produced some of the solid carbonaceous matter in meteorites that have delivered organic solids to the Earth throughout its history:
Carbonaceous solids also reach Earth in the form of organic and icy materials that condensed in the cold outer regions of the solar nebula and also as organic materials preserved in interstellar grains.
As evidenced by their low abundances, carbon compounds, water, and other volatiles such as nitrogen compounds were probably not significantly abundant constituents of the bulk of the solids that formed near the Earth. Many of the carriers of these volatiles condensed in cooler, more distant regions and were then scattered into the region where the Earth was forming. Fragments of comets and asteroids formed in the outer solar system still fall to Earth at a rate of 1 × 107 kg/yr and early in the Earth’s history the rate must have been higher. Certainly some of Earth’s volatile elements were accreted from comets and other bodies from the outer solar system but it is yet unknown if this was the major source of the more volatile elements that comprise the atmosphere and oceans. One possible tracer that could be used to determine the cometary input is the noble gas composition of comets and the atmosphere. Once outgassed, noble gases tend to remain in the atmosphere and are not influenced by subsequent planetary activity. Unfortunately, the noble gas composition of comets is not presently known. An argument against a common source such as comets for volatiles on the terrestrial planets is based on the differences in the 36Ar contents of planetary atmospheres directly measured using spacecraft. The primordial 36Ar content on Venus is nearly 100 times higher than that for Earth, which is in turn nearly 100 times the Mars value. If this volatile species were carried by the same material that brought water and carbon, then all three could not have been derived from a common comet source.
2.5 Accretion of the Planets
Condensed nebular solids, along with possible pre-solar solids, accumulated by the processes of accretion to form planetary bodies. The process began with low-velocity collisions of micrometer-sized dust and terminated with bodies as large as the Earth and Mars colliding with velocities of over 5 km/s. The first stage involved low-velocity collision of dust grains to form bodies of centimeter size. The movements of dust-sized objects were strongly coupled to gas motions but centimeter and larger particles could de-couple from local gas motions and settle to form a layer. The larger particles, with smaller surface area to mass ratios, are dominated by gravity, not nebular winds. Pulled by the vertical component of gravity, these rock-sized particles would attempt to settle to the central plane of the nebula, forming a thin disk with a relatively high concentration of dust, rocks and boulders. Energy dissipation in the disk by collisions and gas friction should produce an extremely thin disk system somewhat analogous to the rings of Saturn. The particles should initially have almost perfectly circular orbits about the sun at near zero inclinations so that the relative velocity between particles was quite low. It was from this “cold disk” of small bodies with similar orbits that the accretion of larger bodies, and ultimately planets, began.
As the bodies grew by collisions and accretion, gravitational perturbations from each other and from nearby forming planetesimals caused orbits to become less circular and more inclined to the plane of the solar system. As orbits become less similar to each other, their relative impact velocities rise, ultimately up to the km/s range. The increase in velocity dispersion is due to gravitational effects of the larger bodies stirring up the relative motions of all planetesimals and make their orbits less circular. Bodies that pass near another body are scattered, usually into a less circular orbit. In the higher-velocity regimes, collisions are often destructive. An impact at 4 km/s has the same energy per mass and release rate as the chemical energy in high explosives. The accretion process is complex and involves both destruction and net growth. The process is also highly competitive with the largest body in the growing swarm having a selective advantage because of its gravitational field. Gravity expands the capture cross-section to areas much larger than the geometrical cross-section and it helps in retaining rebounded material from high-velocity impacts. At one time, the material that formed the Earth was in the form of a million or so bodies a hundred kilometers in diameter. In the end all of these were incorporated into one object or were ejected out of the feeding zone. The final stages of accretion involved collision with very large bodies perhaps as large as Mars.
A collision with a Mars-sized object may have resulted in the formation of the Earth’s moon. Our moon is by no means the largest satellite in the solar system, but it is unusual in that it and the moon of Pluto are the largest moons relative the mass of the planets they orbit. Geochemical studies of returned lunar samples have shown that close similarities exist between the bulk composition of the moon and the Earth’s mantle. In particular, the abundances of siderophiles (“iron loving” elements such as Ir and Au that concentrate in planetary iron cores) in the moon are similar to those in the Earth’s mantle. These observations have lead to the popular hypotheses that the moon formed from parts of a large impactor and Earth mantle material ejected into orbit by the impact. The collision must have occurred early in the Earth’s history but after separation of its core.
The formation of the large moon has had a profound effect on Earth history. The massive moon has provided an important stabilizing effect on the Earth’s obliquity, the tilt of its spin axis relative to the orbit plane. The obliquity has changed only a few degrees over geologic time but without the stabilizing effects of the moon, the obliquity could have varied as much as 90°. Such change would have caused substantial changes in the atmosphere, climate and ocean circulation. As an example, if the obliquity were over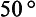 , the equatorial regions would receive less solar energy over the year than the polar regions. It is believed that the obliquity of Mars has changed significantly over its history and this may have played a role in the apparent large degree of variability of its atmosphere. Mars cannot presently support liquid water at its surface but there is evidence that it could in the past. The moon also plays a large role in producing tides, an effect important to a variety of biological and chemical cycles. Early in the Earth’s history the moon was closer and the tidal effects were much larger. The tidal interactions between the Earth and moon result in a slowing of the Earth’s spin rate and an increase in the Earth-moon distance. Over the past 500 000 years the Earth’s spin rate has decreased about 10% by this effect.
, the equatorial regions would receive less solar energy over the year than the polar regions. It is believed that the obliquity of Mars has changed significantly over its history and this may have played a role in the apparent large degree of variability of its atmosphere. Mars cannot presently support liquid water at its surface but there is evidence that it could in the past. The moon also plays a large role in producing tides, an effect important to a variety of biological and chemical cycles. Early in the Earth’s history the moon was closer and the tidal effects were much larger. The tidal interactions between the Earth and moon result in a slowing of the Earth’s spin rate and an increase in the Earth-moon distance. Over the past 500 000 years the Earth’s spin rate has decreased about 10% by this effect.
After planetary accretion was complete there remained two groups of surviving planetesimals, the comets and asteroids. These populations still exist and play an important role in the Earth’s history. Asteroids from the belt between Mars and Jupiter and comets from reservoirs beyond the outer planets are stochastically perturbed into Earth-crossing orbits and they have collided with Earth throughout its entire history. The impact rate for 1 km diameter bodies is approximately three per million years and impacts of 10 km size bodies occur on a 100 Myr time scale. The collision of a 10 km asteroid or comet produces a crater nearly 200 km in diameter and the atmospheric and oceanic effects from shock processes and ejecta can produce severe stress on terrestrial organisms. Major global effects can include heavy particulate loading of the atmosphere, production of nitrogen oxides from shock effects and strong infrared radiation from ejected matter. The cretaceous-tertiary extinctions that occurred 65 Myr ago are marked with a global layer that contains shocked quartz grains and the trace element signature of meteoritic material. The total mass of the layer is consistent with ejecta from the hypervelocity impact of a 10 km comet or asteroid. The above impact rates have remained relatively constant over the past 3.9 Gyr, although they were much higher before this time. The surface of the moon provides an excellent record of this impact history. The smooth basalt-filled mare on the Earth’s side of the moon provide a record of cratering over the past 3.5 Gyr while the bright highlands areas reveal the intense era of large impact events that occurred before this time.
2.6 Early Evolution of the Earth
When the Earth was in its early stages of accretion, it was presumably a cool object because the rate of accretional heat added to it was small. In the final stages, accretional energy was appreciable and must have heated the upper regions of the Earth to high temperatures. When the Earth approached its present mass, projectiles would impact at a minimum velocity of 11.2 km/s, the velocity of escape. The kinetic energy at this velocity is high and, at least for very large objects, a substantial fraction of the impact energy can be trapped within the Earth. The oldest rocks on Earth were formed 600 Myr after its formation and unfortunately there are no direct records of what the Earth was like at this stage. The moon, however, does preserve a record of its earliest history, and it provides a glimpse of what it might have been like. The moon actually melted down to a depth of about 400 km, forming a “magma ocean” from which its > 60 km thick anorthositic crust crystallized. It is likely that a major portion of the upper parts of the Earth also melted at some time during the final stages of accretion.
After the planet accreted, additional heat sources were radioactive decay and gravitational energy released by formation of its core. If the Earth contained amounts of 26Al and 60Fe, similar to those seen in primitive meteorites, the decay of these short-lived isotopes would provide an early and intense source of thermal energy within the first few million years of its history. The other radioactive heat sources (U, Th, and K) are longer lived and release heat on time scales of over 108 years. Partial melting of the upper layers leads to the formation of molten iron masses that sink towards the Earth’s interior. The gravitational settling of dense metal releases heat that creates a single pulse of gravitational energy. All of these heat sources lead to the chemical differentiation of the Earth into a core, mantle and crust, and to outgassing of volatiles to form an atmosphere. Apparently all of the terrestrial planets underwent differentiation, although there may have been significant variations in the details of crustal development and subsequent evolution. Later evolution depends on the amount of internal heat available and the thickness of crust through which geologic activity must penetrate. The Earth is still a very active body with volcanic activity and widespread plate movement. Its closest neighbors, Mars and Venus, are less active and have had different evolutionary histories.
It now appears that the early evolution of the Earth may have been much more violent than commonly imagined in the past. The evidence and general agreement that the moon probably formed as the result of the impact of a Mars-sized object or larger, underscores calculations indicating that the Earth’s accretion included impact with bodies of planetary size. These great impacts influenced volatile loss and core formation. It is likely that great disturbances and heat of large impacts caused core formation to occur simultaneously with accretion rather than after the planet was fully assembled, as is commonly assumed. The violence of these events may be responsible of the depletion of volatiles in the Earth. The volatile element potassium, the most important source of radiogenic heat, is only one-fifth as abundant in the Earth as it is in primitive meteorites. This depletion may be related to the composition of the bodies that accreted to from Earth, but it might also be due to volatile loss during great impacts such as that the is believed to have formed the moon.
The evolution of the Earth’s atmosphere and oceans are important for many of the cycles described in this book. Both of these evolved by outgassing but there is little information about the history of these processes. Again, comets could have been a main source of some of the volatiles (H2O, CH4, etc); however, proof of this source is still lacking (see Section 2.4 above). Unfortunately, bombardment of the inner planets by large projectiles pulverized planetary surfaces until about 3.9 billion years ago, erasing essentially all direct records of the Earth’s early history. The Earth’s ocean and its atmosphere are unique in comparison with Mars and Venus. Neither of these planets have oceans and their atmospheres are composed largely of CO2 (Table 2-2). Mars has ice on and in its surface materials but Venus has essentially no water. With a surface temperature of 650 K, water near the surface of Venus could exist only as a gas. It has been suggested that Venus may have had as much water as the Earth but lost it in a catastrophic blow-off process driven by extreme greenhouse heating early in its history. (See Chapter 7 for a discussion of loss by escape from the upper atmosphere.) Another possibility is that Venus could have lost an early atmosphere and hydrosphere as a result of large impacts, a process that may also have “eroded” the atmospheres of Earth and Mars. The CO2 inventory of Venus is similar to that on the Earth except that on Venus it is in the atmosphere; on the Earth, all but trace amounts are trapped in carbonate rocks. Eventually, with continually increasing solar output or changes in the atmosphere, the CO2 content of the Earth’s atmosphere may increase. Increased greenhouse heating and positive feedback would lead to higher surface temperatures. Mars, in contrast, is in a nearly permanent ice age with only transient periods when conditions are sufficient for the existence of liquid water. The most remarkable aspect of the Earth is its atmosphere. It is composed of oxygen and nitrogen in contact with liquid water in highly non-equilibrium proportions. Except for 40Ar produced by decay of 40K, the atmospheric composition is controlled by biological processes that act on time scales that are certainly faster than anywhere else in the solar system. Early in the Earth’s history the atmosphere was probably dominated by CO or CO2, like Mars and Venus. In time the CO2 was incorporated into carbonate rocks and nitrogen and oxygen came under the control of biological processes. The rise of oxygen due to photosynthesis started early in the Earth’s history and reached modern levels before the start of the Cambrian, 600 million years before the present.
2.7 Earth and the Development of Life
The Earth began with processes of great violence, impacts that may have physically torn the planet in two followed by gravitational reassembly. As previously discussed such impacts may have resulted in formation of the moon. The majority of accretion occurred rather quickly and within a 107 year time period, the era of planet distorting impacts was over. Following accretion, the planet entered an era where smaller bodies would occasionally impact, the largest of these being only a few hundred kilometers in diameter and they would produce impact features hundreds to thousands of kilometers in diameter. Typically they were smaller than the impacts that occurred earlier but they were much larger than those that would impact after this period, which is often called the “era of heavy bombardment.” It was the time during which the large impact basins, such as Mare Imbrium, formed on the lunar surface. The era ended 3.9 Gyr ago when the supply of sufficiently large impactors in Earth-crossing orbits was effectively depleted. The largest impact basin known on the moon is South Aitken basin, which is over 2000 km in diameter. Although no record of this period survives for the Earth, extrapolation from the lunar record would suggest that the Earth would have experienced over a thousand basin-forming impacts. The impact rate during the “heavy bombardment era” would have actually been rather low, with tens of thousands to millions of years between major impacts.
It is unclear what the Earth was like during this heavy bombardment era but it is certain that it was punctuated with great impact events. The Earth’s surface may have been warm due to a dense atmosphere and greenhouse heating or it may have been cool due the relative faintness of the early sun. It was largely a water- or ice-covered planet and it is possible there were no continents – only short-lived volcanic islands. The atmosphere would have been transparent to the ultraviolet and the Earth’s surface would have been quite inhospitable for any early life. Life may have formed during this time but the impact of 100 km bodies provides enough global heating to essentially sterilize the planet down to depths, in some cases, of several kilometers. If life formed on the early Earth it may have been destroyed and reformed many times; hence this period has also been called the period of “impact frustration” (Maher and Stevenson, 1988). No long-term life was possible until after 3.9 Gyr ago when the great impacts had ceased.
It is significant that the earliest records of life on Earth start shortly after the period of impact frustration. Apparently life formed as soon as the conditions permitted it. Life originated from compounds produced by prebiotic organic chemistry. The source of the molecules included those produced on Earth by energetic processes such as impacts and electrical discharges as well as those that fell in from space. Whatever processes occurred, they would have had to happen either in the deep ocean or in what might have been rare regions of land and shallow water.
The Earth is a highly unusual planet because life did evolve on it and it thrived to the extent that the surface and atmosphere of the planet were greatly modified. The Earth is unique in this respect relative to all known astronomical bodies (Taylor, 1999). The Earth’s location, composition, and evolutionary history are all significant factors in the planet’s success in nurturing life. Critical factors include its temperature, its atmosphere, its oceans, its long-term stability and its “just right” abundance of water and other light element compounds.
One of the Earth’s most important properties is its location; it lies within the habitable zone (HZ) of the sun. The HZ is the range of solar distances where an Earth-like planet can have liquid water on its surface. Besides its surface temperature, the Earth contains a mix of land and ocean that seem highly suitable for life. The presence of land is critical because of its role in removal of CO2 from the atmosphere and also it ultimately serves as a habitat of the rich diversity of life that occurs on land and in the shallow water surrounding it. The silicate-CO2-weathering cycle, which is discussed in Chapters 8 and 11, is probably the most biologically important chemical process on Earth because it provides a means of keeping the surface temperature in a region appropriate for life. This cycle requires land and shallow water. It is remarkable, though, that the planet contains land at all. If it were not for continents, the Earth would be essentially a water-covered planet with only small islands like Hawaii and Iceland providing contact with the atmosphere.
Overall, the Earth is unique compared to any other planet we know of in its remarkable perfection in supporting life. The integrated systems that allow this to happen started with events occurring billions of years ago. Keeping this historical perspective in mind while studying present-day Earth systems will help complete the picture of the planet.
Lovelock, J. E. Gaia, a New Look at Life on Earth. New York: Oxford University Press; 1979.
Kasting, J. F., Toon, O. B., Pollack, J. B. How climate evolved on the terrestrial planets. Scient. Am. 1988; 258(2):90–97.
Maher, K. A., Stevenson, D. J. Impact frustration of the origin of life. Nature. 1988; 331:612–614.
Penzias, A. A. The origin of the elements. Science. 1979; 205:549–554.
Silk, J. The Big Bang. Oxford: W. H. Freeman; 1989.
Taylor, S. R. On the difficulties of making Earth-like planets. Meteoritics and Planet. Sci. 1999; 34:317–329.
Zahnle, K. J., Sleep, N. H. Impacts and the early evolution of life. In: Thomas P. J., Chyba C. F., McKay C. P., eds. Comets and the Origin and Evolution of Life. New York: Springer; 1997:175–208.