CHAPTER 44
Autotransfusion Devices
Introduction
The anesthesiologist and the anesthesia team can save a patient’s life with a properly timed blood transfusion. The discovery of the ABO blood groups by Landsteiner, which won him the Nobel Prize in 1930, meant that massive blood loss from trauma was no longer uniformly fatal (see Chapter 20, Transfusion Medicine). When a patient is bleeding to death from a traumatic injury or from an injured blood vessel during a surgical operation, blood transfusion remains the only treatment available. No matter how well the heart and blood vessels are filled and working to keep blood supplied to all the cells of the body (see Chapter 4, Cardiovascular Anatomy and Physiology) and no matter how well the lungs are working to supply oxygen to the blood (see Chapter 7, Respiratory Anatomy and Physiology), red blood cells are needed to carry the oxygen within the bloodstream. Without red blood cells, oxygen is not delivered to the tissues, and they die.
Conversely, a poorly timed blood transfusion can increase a patient’s likelihood of death or complications. As you have seen discussed throughout this book, every procedure and intervention in health care has associated risks and side effects. The risks and side effects associated with blood transfusions include hemolytic and nonhemolytic transfusion reactions, allergic reactions, transfusion-related acute lung injury, hypothermia, fluid overload, electrolyte abnormalities such as hyperkalemia and hypocalcemia, and transmission of viral infections such as HIV and hepatitis viruses. Because of all of these risks, the decision to transfuse allogeneic blood (blood stored in the blood bank that was donated by another person) should be made when the benefit of transfusion outweighs the risks. This decision can be very difficult; modern studies try to determine the exact threshold whereby transfusion becomes beneficial as compared to withholding transfusion. Studies from the intensive care unit suggest that we should be transfusing LESS blood than we have in the past. However, good scientific studies on this are very difficult to do in the rapid blood loss setting of the operating room. Patients would need to be randomly assigned, before surgery, to receive blood transfusion according to two different strategies—one resulting in more transfusion and one less. How could this be sorted out in the case of rapid blood loss? Knowing that at some point lack of transfusion is fatal, how could this be ethical? As of now, the arguments surrounding this topic are heated and no consensus has been reached; it is unlikely a clear consensus will be reached on this topic in the near future. For now, it is generally accepted that blood transfusions are best avoided unless the patient clearly needs it. In order to avoid allogeneic transfusions, there are numerous techniques that can be performed before, during, and after surgery. This chapter covers some of those techniques, including preoperative blood donation, intraoperative cell salvage, acute normovolemic hemodilution (ANH), and the implication of these techniques in different types of surgery, Jehovah’s Witness (JW) patients, and blood saving protocols incorporating many techniques in an effort to reduce allogeneic transfusions.
Cell Salvage
Cell salvage, commonly referred to as “cell saver,” is a technique whereby blood that is suctioned from the patient during surgery is sent to a reservoir, then the red blood cells are washed, repackaged in a blood collection bag, and reinfused via the patient’s IV tubing. Since the blood comes from the patient who will receive it, cell salvage avoids many of the problems with allogeneic RBC transfusions, such as viral transmission and immune-related complications like ABO mismatch and lung injury. Early attempts of cell salvage were associated with very high mortality. The first recorded use of this technique was performed in 1818 when a gynecologist named Blundell treated patients with postpartum hemorrhage by washing blood-soaked swabs in saline and then re-infusing the blood. Arnold Griswald developed the first cell salvage device in 1943, a device where suctioned blood was strained through cheesecloth before being reinfused. The first “modern” cell salvage device was available in the 1970s; since its introduction, many of the problems associated with this technique have been refined, and cell salvage is felt to be extremely safe. Figure 44.1 shows a modern cell salvage machine.
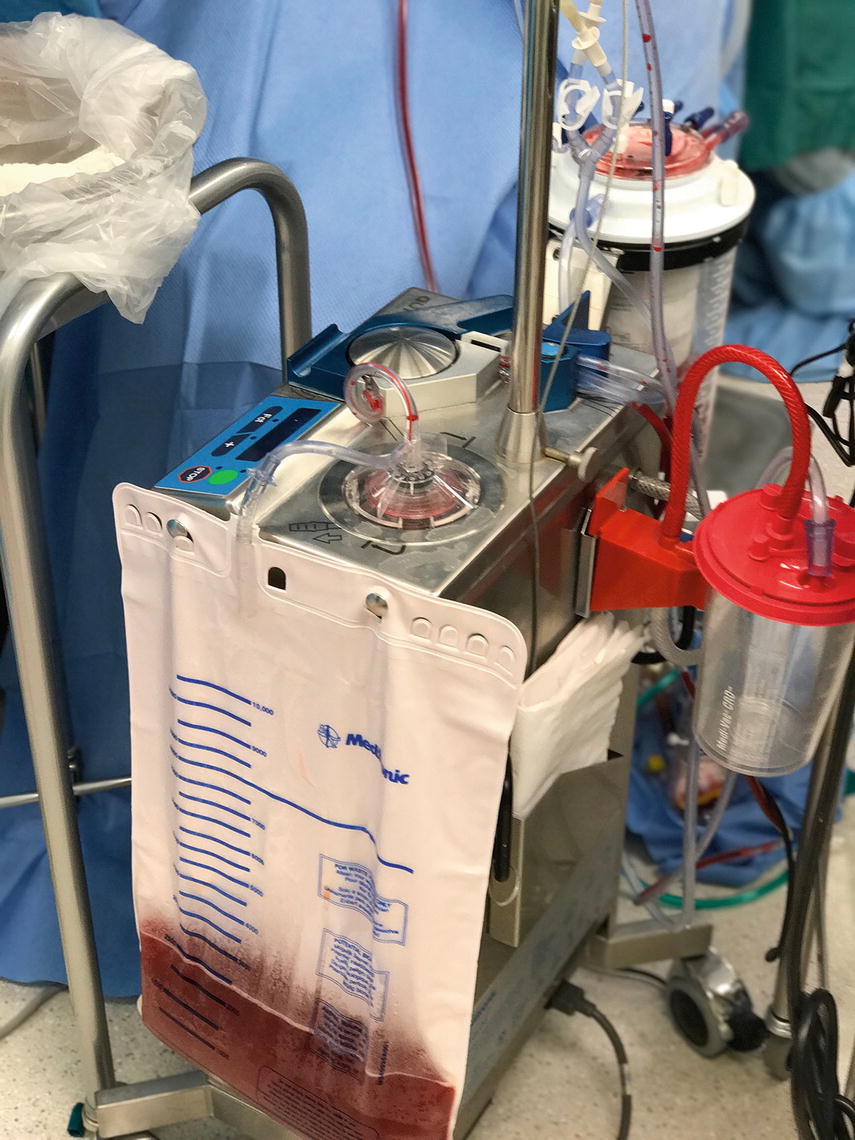
FIGURE 44.1. A modern autotransfusion machine.
There are three phases involved with cell salvage: collection of blood, washing, and reinfusion. Collection of RBCs from the operative field is done with a dedicated suction device that has two lumens. One lumen is for suctioning blood, while the second lumen continuously adds anticoagulant saline to the blood, so that it does not form clots inside the tubing or the collection canister. The blood suctioned from the field passes through a filter and then sits in the collection reservoir. Once enough blood volume has been collected from the surgical field into the reservoir, the blood is ready to be prepared for reinfusion. The blood is sent through a centrifuge to separate out the cellular components. Those components are then washed in a semipermeable membrane with saline solution to separate the components that are meant to be reinfused (the red blood cells) from those that are not (white blood cells, free hemoglobin, plasma, platelets, and heparin). The salvaged red blood cells are then resuspended in normal saline solution in a bag; the resulting hematocrit (the percentage of the volume that is red blood cells) is 50%-80%. The packaged cell salvage blood can be given immediately by the anesthesia team through the IV tubing or within 6 hours. See Figure 44.2 for a picture of a cell salvage setup.
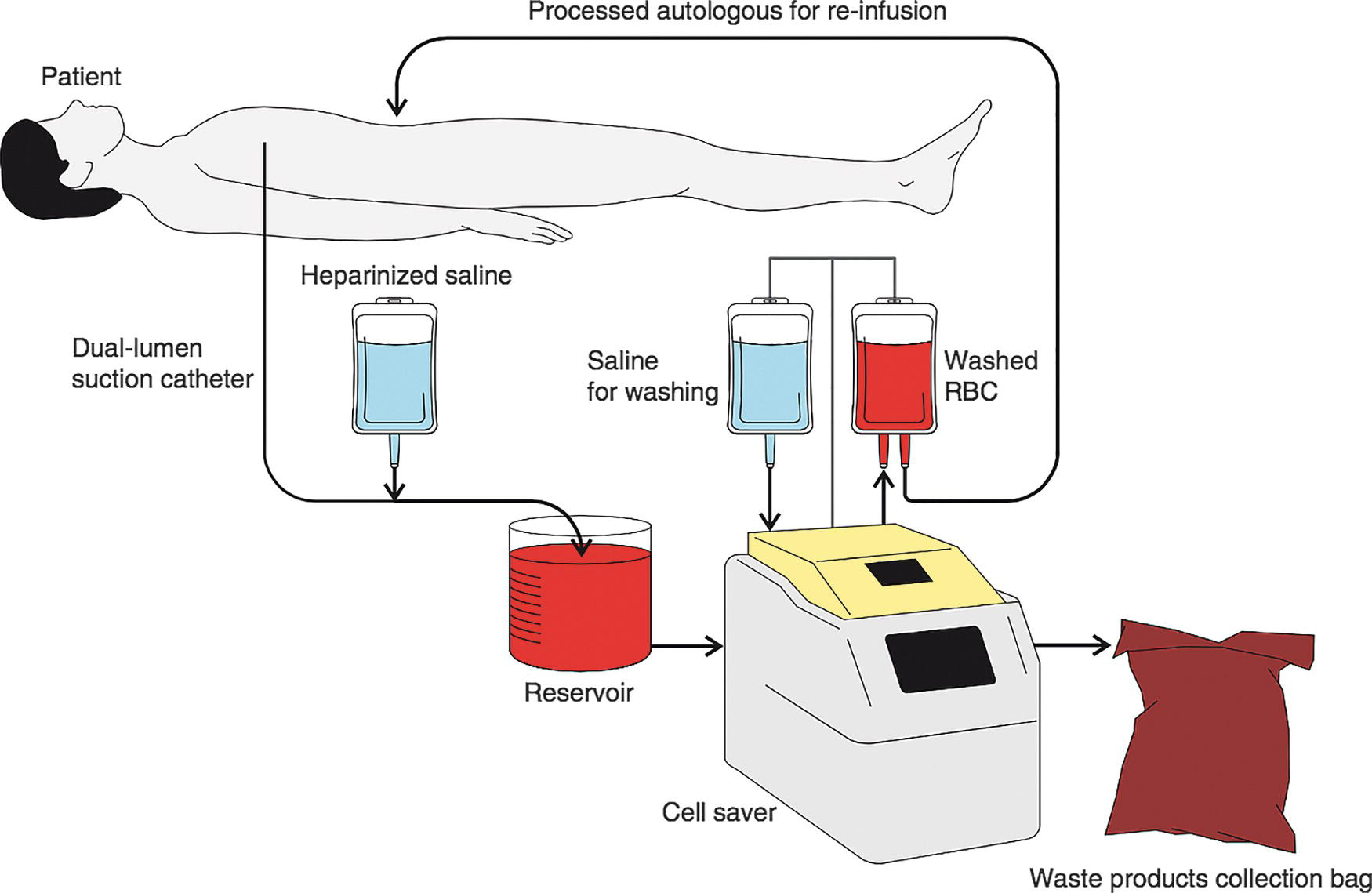
FIGURE 44.2. General setup for autotransfusion. In most cases, the washed RBCs are collected in a bag and then given to the anesthesia provider to be transfused as needed during the case. During setup for a Jehovah’s Witness case, however, the black lines in this diagram are connected by IV tubing to create a closed continuous loop.
The obvious benefits of cell salvage are that the patients will receive their own red blood cells back, increase their blood’s oxygen-carrying capacity and oxygen delivery to vital organs, and will hopefully avoid an allogeneic RBC transfusion and all the potential complications that come with it. Studies show that salvaged RBCs have a higher viability, as high as 88%, compared to allogeneic RBCs and have higher levels of 2,3-DPG and ATP, which are markers of cellular health. The RBCs transfused are fresh and have not aged or undergone significant temperature changes associated with storage. They also maintain their normal biconcave disc shape better than allogeneic RBCs, which assume an echinocyte shape after about 14 days. This likely helps the cells pass through capillary beds and deliver oxygen to tissues. The data supporting cell salvage are very favorable. A Cochrane Database systematic review performed in 2010 composed of 75 different randomized trials showed that there were significantly fewer allogeneic transfusions needed when cell salvage was utilized compared to when it was not used. This benefit seemed best in cases of orthopedic surgery, spine surgery, and cardiac surgery.
Cell salvage is very safe, and there are typically no untoward effects when the patient is reinfused with relatively small amounts of salvaged blood. However, there are some risks associated with cell salvage. The cost of a cell salvage setup is significant; because of this, cell salvage should only be considered for surgeries where expected blood loss is large. The 2009 AAGBI guidelines recommend cell salvage for surgeries where anticipated blood loss exceeds 1,000 mL or greater than 20% of the patient’s blood volume, where the risk of transfusion is greater than 50%, or for patients who cannot receive allogeneic RBC transfusions (such as patients with rare blood types for excessive antibodies or for JW patients, discussed below). Studies show that cell salvage is cost-effective when at least 2 units of salvaged blood are reinfused into the patient.
Another risk is that cell salvage can cause suction-related mechanical shear stress on the blood cells, causing them to rupture, this is called hemolysis. Hemolysis can result in significant loss of functional red blood cells and a reduced yield of return on the cell salvage. To reduce this effect, the suction on the cell salvage unit is typically kept lower than other surgical suctions; this may result in a surgical aversion to the device if the surgeon is used to higher suction rates. Some cell salvage devices have a mechanism that varies the suction pressure based on how much air is being suctioned; this has shown to reduce the amount of hemolysis.
A potential complication related to cell salvage is the problems related to incomplete washing. Depending on what component is not washed out properly, multiple problems can occur, including febrile reactions (if white blood cells are infused), pulmonary embolus (if clots are infused), and worsened bleeding (if heparin is infused). Also, if the surgery is for removal of a cancerous mass, there is a possibility of cancer cells being left in the blood canister and reinfused, which can theoretically cause spread of cancer throughout the body. In this case, a leukocyte depletion filter should be applied to the system; studies show that these filters remove a large majority of cancer cells, and it is generally safe to use cell salvage for cancer surgery. Randomized studies done mostly in urology and gynecology patients showed no difference in cancer recurrence rates and a reduction in allogeneic RBC transfusions. Additionally, large volumes of cell salvage blood administered can cause problems with clotting function (coagulopathy). Because the washed blood cells do not contain any other components found in blood, large volumes administered can result in dilution of the patient’s clotting factors, platelets, calcium levels, and fibrinogen count, all of which can interfere with clot formation. However, studies show that coagulation function remains normal if surgical blood loss is less than 3,000 mL.
Lastly, cell salvage should be avoided in cases where the surgical site is in a frankly infected area or when fecal contamination of the surgical field has occurred. This is because the bacteria present would return to the cell saver and potentially be reinfused into the patient’s bloodstream causing disseminated infection. Although studies have shown that leukocyte depletion filters can remove up to 97.6%-100% of the bacteria present, the benefit of using cell salvage in this situation does not outweigh the risk of causing infection elsewhere.
Common Types of Surgery for Cell Salvage
As mentioned above, cell salvage is an excellent technique for cases where large amounts of blood loss is expected. Certain types of surgery will have cell salvage routinely setup for every case. In other types of surgery, the use of cell salvage is more controversial, and it will be less common or nonexistent.
There are good data to support the use of cell salvage in cardiac surgery. Despite advances in surgical techniques, medications, and cardiopulmonary bypass (CPB) equipment, blood transfusion rates for cardiac surgery remain very high. In the United Kingdom, 10% of all transfusions were for cardiac surgical patients; in other countries, this number can be up to 20%. Although approximately 50% of cardiac surgical patients receive no transfusions, a minority (10%-20%) use 80% of the total blood transfusions for all of cardiac surgery. This is important to reduce, and because studies show that with increasing transfusion, there is a dose-dependent depression of immune function and increase in infectious complications, renal dysfunction, pneumonia, wound infections, and sepsis. A 2009 meta-analysis of cell salvage in cardiac surgery showed that its use reduced RBC transfusions by 35%-40% and was not associated with any increased risk of mortality, stroke, acute MI, atrial fibrillation, renal dysfunction, infection, or reoperation for bleeding. The 2007 clinical practice guidelines regarding perioperative blood conservation from the Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists give a class I recommendation (the highest) for routine use of cell salvage in cardiac surgery, except in cases of infection or malignancy.
There are also good data to support the use of cell salvage in major orthopedic (such as joint replacements) and major spine surgery (such as multilevel fusions or scoliosis surgery). Studies show that use of cell salvage reduces the need for allogeneic transfusions in revision hip and knee surgery. Reductions in transfusion rates in this population are associated with less infection, which is very important for this surgical population where hardware is being inserted. In spine surgery, surgical times are very long, and there is a slow, continuous oozing from the bony tissue; transfusion rates remain very high. For patients undergoing spine surgery without ANH (see below) or cell salvage, the rate of transfusion is 86%. If both ANH and cell salvage are used, that rate is dramatically reduced to 3.4%.
In other types of surgery, the use of cell salvage is more controversial, such as in caesarean sections and obstetric surgery. Although postpartum hemorrhage (bleeding after childbirth) is the most common cause of peripartum mortality worldwide, the use of cell salvage is not mainstream practice. The reason for this is the concern of inducing amniotic fluid embolism (AFE). AFE is a peripartum syndrome of rapid-onset dyspnea, hypoxemia, and cardiovascular collapse; it has a mortality rate as high as 60% in developed countries and occurs at a rate of 1 in 13,000 in the United States. As the name suggests, it is thought to occur via amniotic fluid traveling via the veins into the vasculature of the lungs. If amniotic fluid were to be suctioned into a cell salvage device, it is possible that the amniotic fluid could be reinfused with the red blood cells, possibly causing the syndrome to occur. Although it is not mainstream to use cell salvage for caesarian sections, the American College of Obstetricians and Gynecologists and the American Society of Anesthesiologists recommend the use of cell salvage in cases of postpartum hemorrhage where allogeneic blood is not available or is refused by the patient. If used, a leukocyte depletion filter is highly recommended, as is a separate “waste” suction for amniotic fluid and blood prior to the delivery of the placenta. Cell salvage can be initiated after delivery of the placenta, which may coincide with most profound uncontrolled bleeding. For more discussion of your role in obstetric surgery and anesthesia, see Chapter 49.
Acute Normovolemic Hemodilution
Acute normovolemic hemodilution is another technique that can be performed in the operating room by the anesthesiologist in an effort to reduce the need for allogeneic red blood cell transfusions. The meaning of the terms is as follows: acute (performed with immediate effect), normovolemic (the patient is left with a normal amount of blood volume), hemodilution (the patient’s blood volume is diluted with nonblood solution). In this technique, which was first performed in 1946, the patient’s blood is withdrawn in a controlled fashion immediately prior to surgery in the operating room via IV tubing into a CPDA blood donation bag, which is stored in the room for administration to the patient after surgery. The blood must be removed prior to blood loss from surgery, so it is typically done after induction of anesthesia. After blood removal, the patient is hypovolemic (low blood volume), so crystalloid or colloid solutions are given in volumes of one to three times the amount of blood removed in order to make the patient’s blood volume normal again (normovolemia). Ensuring normovolemia after blood removal is essential so that the patient has normal cardiac output to support circulation of blood to vital organs and tissues. To compensate for the lost red blood cell mass, cardiac output and tissue oxygen extraction both increase in an effort to maintain oxygen delivery.
After blood removal and volume administration, the resulting blood volume of the patient has a lower concentration of red blood cells per volume of blood (hemodilution), so any bleeding during surgery will result in less overall red blood cells lost. The target hematocrit of the patient’s blood after ANH is 25%-30%. A hematocrit lower than this range can predispose the patient to increased risk from dilution of coagulation factors and reduced oxygen-carrying capacity in the blood. A typical amount withdrawn is 1-2 units of blood, which is based on the patient’s size and preoperative hematocrit. Obviously, smaller patients with a lower overall blood volume and those patients with lower preoperative hematocrits will not tolerate as much blood withdrawn as patients who are larger or have higher hematocrits.
There are numerous advantages to ANH. Numerous studies have shown that ANH is effective in reducing allogeneic red blood cell transfusions, which can reduce the myriad of complications from transfusion. Additionally, ANH is the only method that provides fresh autologous blood, where the blood has not been diluted, washed, or stored in a refrigerator. Because it is fresh and undiluted, platelet function is intact, coagulation factors and fibrinogen are present and undiluted, and the red blood cells are minimally affected. This is of particular advantage in cardiac surgery, where the inflammatory response and mechanical stresses on the blood caused by the CPB machine can result in profound coagulation problems after bypass; the ANH blood that has been “protected” from the CPB machine has excellent clotting function and can help to avoid transfusions of platelets or plasma. Another advantage is that ANH is well suited for patients with rare blood types or a large amount of antibodies present. It can be used instead of finding rare units of donated blood, which can cause delays and increased costs. ANH can be used for patients who object to receiving allogeneic transfusions, such as JW patients (discussed below). Lastly, the logistics of performing ANH are very simple and easy; ANH can be done at the time of surgery with minimal preparation, is simple to perform, and is very low in cost.
There is one major drawback to ANH: not uncommonly, the harvested blood from ANH is unable to be given back to the patient. This can occur from a variety of reasons, including thrombus formation, disconnection of the tubing, and contamination. Some studies have shown that this can occur as frequently as 1 in 30 attempts. If this occurs, the patient will have been bled intentionally, which predisposes them to needing an allogeneic transfusion. Aside from this downside in a hopefully uncommon situation, there are small downsides to ANH. The technique is labor-intensive and requires some preparation and requires staff familiar with its use. Also, some studies show that it is associated with longer anesthesia times due to the time required to perform it and associated with an increased use of vasopressors (medications infused to increase blood pressure).
There are some contraindications to patients receiving ANH. Some patients are too medically unstable to tolerate the withdrawal of that much blood from their circulation. This technique is absolutely contraindicated in patients who have recent myocardial infarction, unstable angina, or cardiogenic shock. Withdrawal of a large amount of blood in patients with these conditions could result in dramatic hemodynamic instability. There are some other conditions where ANH is relatively contraindicated. These include patients with preoperative anemia who do not have the blood count to tolerate the blood removal, patients with a preexisting septic infection where bacterial growth could occur in the harvested blood, and patients with congestive heart failure or aortic stenosis, whose heart function may not tolerate the blood removal.
Preoperative Autologous Blood Donation
Given the scarcity of resources, associated costs, and complications from allogeneic red blood cell transfusions, every effort should be made to reduce transfusions when possible. Another method of reducing allogeneic transfusions is preoperative autologous blood donation (PABD). With PABD, patients are sent to a blood donation center 4-6 weeks prior to surgery after undergoing, if necessary, medical therapy to boost their hematocrit. They will donate 1-2 units of blood to be saved only for them for their operation, which is then reinfused at the appropriate time during or after surgery. This technique requires significant planning and time prior to surgery and is done only for operations where significant blood loss is expected or if the likelihood of transfusion is high. In order to be successful, the patient must be able to donate prior to surgery. Similar to ANH, it requires the patient to have a normal hematocrit; the lowest tolerable hematocrit for donation is about 33%. Frequently these patients are having surgery for cancer, and their blood counts are low due to bone marrow suppression from neoadjuvant chemotherapy. If this is the case, the patient will require medical therapy to boost their blood count before and after donation, typically with a combination of oral or intravenous iron supplements, erythropoietin (a hormone that stimulates bone marrow production of red blood cells), vitamin B12, and folate. In addition to the medication to boost RBC production, the act of donating will stimulate the production of more red blood cells.
Patients are contraindicated from PABD if they have significant heart disease. This includes recent myocardial infarction in the past 3 months, unstable angina, left main coronary stenosis, congestive heart failure, and aortic stenosis. There are some drawbacks to PABD. Although the data show that PABD can help avoid allogeneic transfusions, PABD is not clearly cost-effective. A large portion, up to 40% of the donated blood, is wasted because of expiration or because the patient does not require it in the operation. Additionally, because lost RBCs can take 21-30 days to regenerate, the patient may still require an allogeneic transfusion if their blood count has not recovered by the time of surgery.
Jehovah’s Witnesses
Cell salvage has a particular role in the blood-free treatment required by observant JW. JW are a denomination of Christians founded by Charles Taze Russell in 1881. The group claims more than 8 million evangelist adherent members worldwide. The main principles of the religion are that the Bible is infallible and that the problems with other religions are related to its misinterpretation. JW prefer their own interpretation of the Bible, the New World Translation of Holy Scriptures. Their core beliefs are (1) Christ’s second coming and Armageddon are imminent and God will restore the Earth to the perfection of Eden, (2) a righteous 144,000 will be resurrected to a royal priesthood in Heaven and the rest of humanity resurrected to a lower plane, (3) the soul is not immortal, and (4) death is a state of unconscious nonexistence. One of the beliefs of the religion, through their interpretation of Biblical passages, is that blood transfusions are unacceptable.
Many patients will refuse blood products at the time of surgery, even understanding that the alternative may be death. JW almost universally refuse core blood components, such as red blood cells, plasma, and platelets. Other derivatives of blood products, such as albumin and clotting factor concentrates, are considered matters of conscience and may be refused or accepted by an individual witness. In a meta-analysis of JW patients undergoing heart surgery, it was seen that most JW have a variation in what they will accept in their medical care, so that Witnesses are asked prior to surgery, if possible, what other interventions and medications they will allow to be administered.
Most Witness communities provide education on blood products, fractions, and derivatives and support from community elders for patients and families facing major surgical decisions. Alternately, many states have a legal interest in transfusion of minors or the sole caregivers of minor children (in order to prevent those children from becoming wards of the state) and occasionally there are legal issues involved. Anesthesia providers and surgeons also attend carefully to the ethics involved: it is extremely rare, but possible, that a patient may die under an anesthesia provider’s care or later in the intensive care unit because the patient has refused a life-saving transfusion. Just as no patient should receive care that they refuse, no patient can ask a provider to do something they feel is unethical. If you feel it is wrong not to transfuse someone who is dying because of their religious beliefs when a transfusion could save them, it is okay to ask someone else to take your place on that case.
Because of their refusal to accept RBC transfusions, meticulous attention must be paid to surgical blood loss and preventing the need to transfuse blood products. Some patients will accept preoperative erythropoietin and iron supplements. Some will accept ANH during surgery. JW usually find cell salvage acceptable, but consent needs to be obtained on a person-to-person basis because of individual variation in beliefs about blood being removed from the body and left without continuity. In these cases, some JW patients will accept cell salvage blood if the setup is continuous with their circulation; everything from the suction tip to the transfusion bag must be spiked and primed and connected to the patient’s IV before incision is made. Also, most JW patients will accept infusions of antifibrinolytic medications (medications that prevent clot breakdown by the enzyme plasmin).
Blood Saving Protocols
The techniques mentioned in this chapter are not meant to be exclusive. Some components can be used together to optimize the chances of success in the surgery and avoid transfusion of allogeneic blood products, or reduce the need for allogeneic products if it is likely the patient will be transfused. Frequently, a JW patient undergoing a large operation will have two or more of these techniques used together, because there is no option to transfuse these patients with allogeneic products if needed. Preoperatively, the patient can be given medications to increase their RBC mass and referred to a donation center for PABD. A week prior to surgery, the surgeon, in conjunction with the patient’s primary doctor, may elect to have the patient discontinue the antiplatelet or anticoagulant medications to prevent excessive bleeding during the operation.
After induction of anesthesia, the patient can have ANH performed and another unit of whole blood prepared for after the bloody portion of the operation. The surgeon can use a cell salvage suction device for additional blood to be returned to the patient throughout the operation. Additionally, newer surgical techniques can be utilized to prevent unnecessary blood loss, such as elevating the surgical site to prevent venous congestion, the use of topical hemostatic glue to prevent blood loss, and performing a staged operation where a part of the operation is done, followed by a period of rest for the patient to recover and then completing surgery at a later date. The anesthesiologist can perform additional techniques to prevent blood loss, including maintenance of normal body temperature to enhance clotting function and controlled hypotension with infusion of agents to lower blood pressure to prevent excessive arterial bleeding. After surgery, the doctors may choose that the patient undergo fewer blood tests than they would otherwise to avoid unnecessarily taking blood volume from the patient. Ultimately, all of the health care team works together to take the best care of the patient and prevent unnecessary blood product transfusions.
Summary
Autotransfusion serves as an important and necessary purpose in the operating room, it can save lives, time, and money if used properly and appropriately. There are also risks involved, like any procedure or transfusion. Understanding these risks and weighing them against any benefits is crucial to responsibly using autotransfusion.
Review Questions
1. The first modern cell salvage machines were made available in what time period?
A) 1870s
B) 1920s
C) 1940s
D) 1970s
Answer: D
Although the idea of reinfusing surgical blood has been around since the early 1800s, devices were not developed until the 1940s. Even then the machines needed further refinement and the machines we now recognize as “cell saver” machines were not released until the 1970s.
2. Which of the following is an absolute contraindication for cell salvage use.
A) Fecal contamination
B) Cancerous tumor
C) Obstetrics
D) Hepatitis infection
Answer: A
Frankly infected sites should be avoided because of the possibility of bacteria returning to cell saver machine and then being reinfused into the bloodstream, potentially causing disseminated infection.
3. Salvaged blood has a higher viability than allogenic (transfused) blood.
A) True
B) False
Answer: A
Salvaged RBCs have as much as 88% greater viability than allogenic RBCs and also have higher levels of other cellular health markers.
4. Expiration time for reinfusion of processed, washed cells is?
A) 6 hours if refrigerated
B) 8 hours at room temperature
C) 6 hours at room temperature
D) 24 hours at room temperature
Answer: C
The packaged cell salvage blood can be given immediately or within 6 hours.
5. For which of the following methods is crystalloid or colloid solutions given in large volumes?
A) Cell salvage
B) ANH
C) PABD
D) Allogenic transfusion
E) None of the above
Answer: B
In acute normovolemic hemodilution (ANH), blood is withdrawn in a controlled fashion immediately before surgery so that it can be administered to the patient after surgery. Crystalloid or colloid solutions are given in volumes of one to three times the amount of blood removed to make the patient’s blood volume normal: this is essential to ensure normal cardiac output.
6. A Jehovah’s Witness patient is experiencing postpartum hemorrhage and has agreed to the use of cell salvage. The provider is concerned about the potential for inducing amniotic fluid embolism, which of the following best describes what you should be watching for?
A) Tachypnea (rapid breathing), hypoxia, and bradycardia
B) Tachypnea (rapid breathing), hypoxia, and tachycardia
C) Bradypnea (abnormally slow breathing), hypoxia, and bradycardia
D) Bradypnea (abnormally slow breathing), hypoxia, and tachycardia
E) None of the above
Answer: B
Amniotic fluid embolism (AFE) is marked by rapid-onset dyspnea (shortness of breath), hypoxia, and cardiovascular collapse. Its signs include rapid breathing, tachycardia (rapid pulse), and a decrease in blood pressure. AFE has a mortality rate as high as 60% in developed countries and occurs at a rate of 1 in 13,000 in the United States. If cell salvage is used in cases of postpartum hemorrhage (such as when allogenic blood is not available or is refused by the patient), a leukocyte depletion filter is highly recommended.
SUGGESTED READINGS
Ashworth A. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105(4):401-416.
Babinowitz MR. Blood-sparing techniques in head and neck surgery. Otolaryngol Clin North Am. 2016;49(3):549-562.
Carless PA. Cell salvage for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;(4):CD001888.
Epstein NE. Bloodless spinal surgery: a review of the normovolemic hemodilution technique. Surg Neurol. 2008;70(6):614-618.
Goucher H. Cell salvage in obstetrics. Anesth Analg. 2015;121(2):465-468.
Jamnicki M. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth. 2003;17(6):747-754.
Lawson T. Perioperative Jehovah’s Witnesses: a review. Br J Anaesth. 2015;115(5):676-687.
Nalla BP. Update on blood conservation for cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26(1):117-133.
Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27-S86.
Vasques F. Outcome of Jehovah’s Witnesses after adult cardiac surgery: systematic review and meta-analysis of comparative studies. Transfusion. 2016;56(8): 2146-2153.
Zhou J. A review of the application of autologous blood transfusion. Braz J Med Biol Res. 2016;49(9):e5493.