CHAPTER 4
Cardiovascular Anatomy and Physiology
Introduction
Any surgical procedure can trigger the surgical stress response. The stress response is characterized by hypothalamic activation of the autonomic nervous system resulting in the increased secretion of catecholamines. These catecholamines have direct and indirect effects on the heart and cardiovascular system. The majority of anesthetics interact with the cardiovascular system by depressing sympathetic outflow, dilating blood vessels, or directly interacting with the heart. Surgical stimuli, blood loss, and anesthetic procedures have the further ability to interact with the patient’s cardiovascular system. Thus, much of the anesthesia provider’s work, and much of the support the anesthesia technician provides, revolves around this important system. This chapter introduces the anesthesia technician to the fundamental anatomy and physiology of the heart and vasculature. This basic science will form the foundation of further study throughout this book.
Surface Anatomy of the Heart
The heart is a set of hollow muscular pumps combined into a single organ. It is located in the middle of the chest (the mediastinum) between the lungs and their pleural coverings. It is shaped like an upside-down pyramid that is tilted to the patient’s left. The base of the heart lies superiorly, and the apex lies inferiorly and leftward (Fig. 4.1). The heart itself is covered in a thick sac called the pericardium.
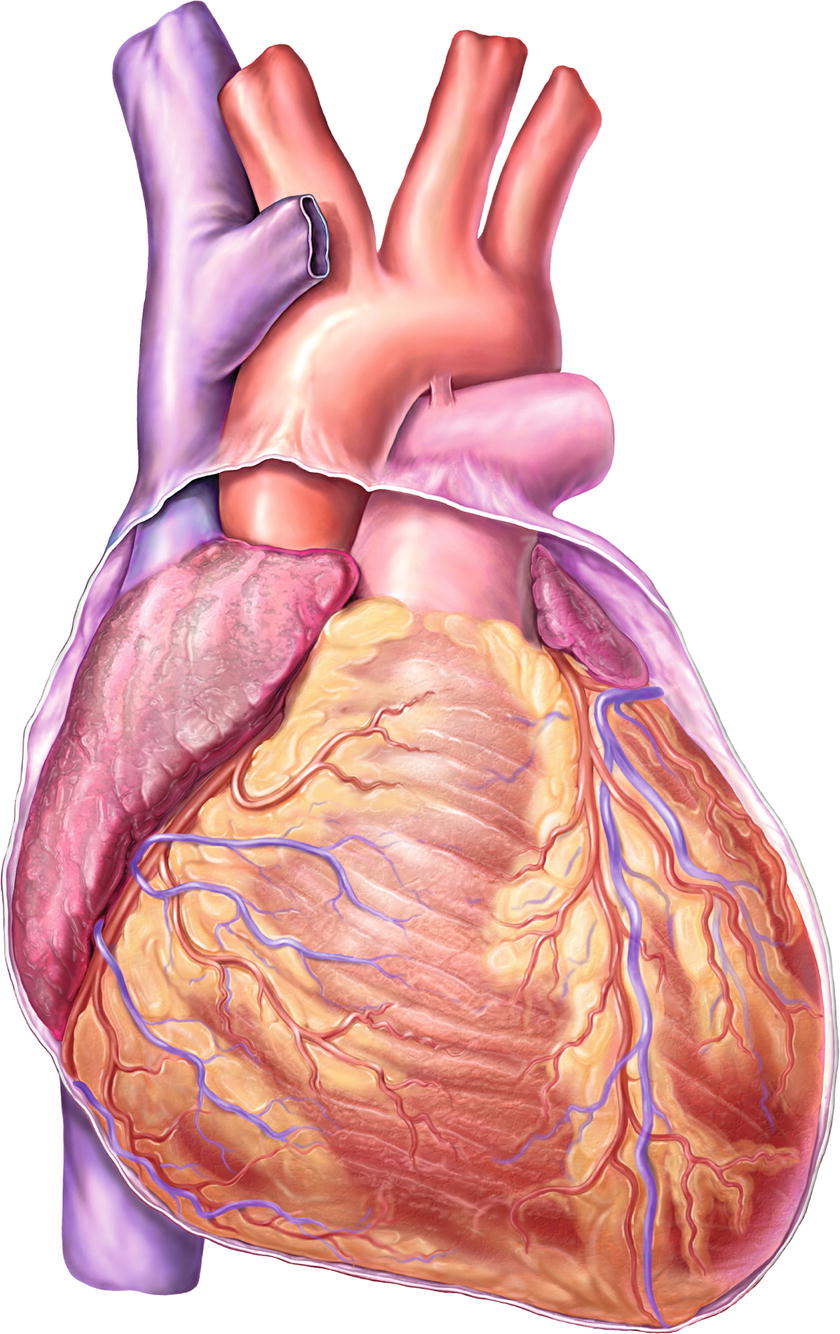
FIGURE 4.1. Normal heart anatomy, anterior view.
The heart is divided into two sides, a right and a left, and consists of a total of four chambers. The upper two chambers are the right and left atria, while the lower chambers are called the right and left ventricles. The inferior and superior vena cava are connected to the right atrium (RA), and the pulmonary artery is connected to the right ventricle (RV). On the left side of the heart, the pulmonary veins are connected to the left atrium (LA), and the aorta is connected to the left ventricle (LV) (Fig. 4.2). The heart is rotated slightly to the left, and the apex is tilted slightly toward the front of the chest. Thus, the RA sits forward of the LA, and the RV is the front portion of the large muscular ventricular mass. The LV is positioned on the left side of the heart and is larger than the RV, but because of the rotation and the tilting, it is also positioned slightly behind and under to the RV. An understanding of the position of the heart is important to properly read the electrical signals that are generated by the heart and recorded on an electrocardiogram (ECG). The ECG is discussed in more detail later in Chapter 5, Cardiovascular Monitoring.
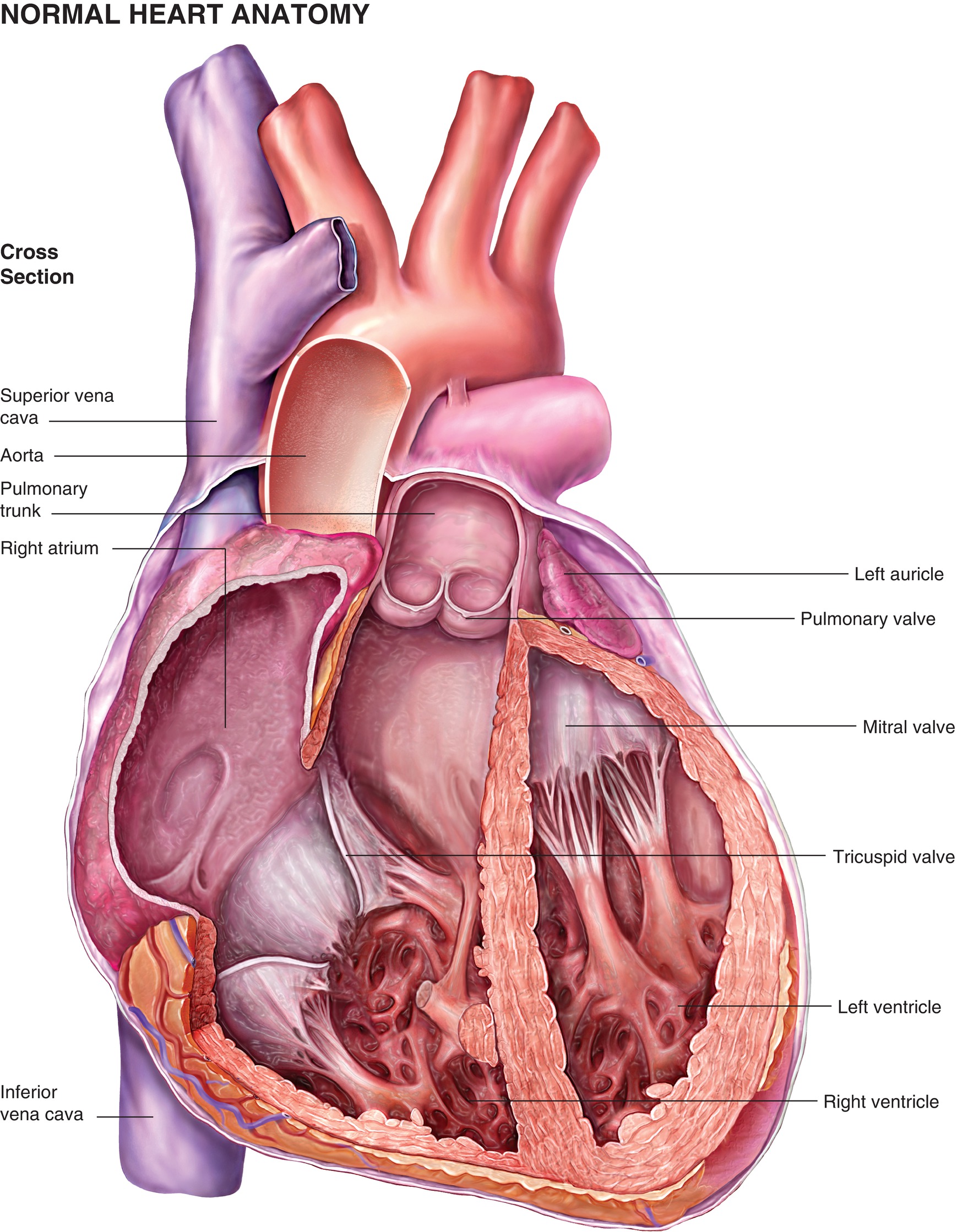
FIGURE 4.2. A coronal cut through the heart demonstrating the four chambers of the heart and their vascular connections.
Fetal Development
Major organ development occurs between the 4th and 8th week of fetal life. The heart is the first organ to complete its development during fetal growth. The heart begins as a primitive vascular tube that curves back on itself and bends anteriorly and rightward to form three distinct portions called the single-chambered primitive atrium, a ventricle, and the truncus. These develop further into a right heart and a left heart, atria and ventricles with intervening atrioventricular (AV) valves, and the aortic and pulmonary trunks. Errors in fetal development of the heart can result in significant malformations. For example, the heart may fail to form two ventricles, or the aorta and pulmonary trunks may be fused into a single trunk arising from both ventricles. An average adult heart is 12 cm from base to apex, 9 cm at its broadest transverse diameter, and 6 cm anteroposteriorly. Its weight varies with average 300 g in male and 250 g in females, with adult weight achieved between the ages of 17 and 20 years.
Pericardium
The heart is enclosed in a three-layered sac called the pericardium. The outer fibrous layer of the pericardium is attached to the great vessels, the sternum, and the diaphragm. It secures the heart to the sternum in the front and to the diaphragm below. The inner serous layer consists of a visceral and parietal portion. The visceral pericardium, also known as the epicardium, covers the entire heart and great vessels. The parietal pericardium forms an inner lining to the fibrous pericardium. The space between the two layers of the serous pericardium is called the pericardial space. Normally, the pericardial space contains 30-50 mL of serous fluid that acts as a lubricant to decrease friction while the heart beats. The fibrous layer of the pericardium is noncompliant (not easily expandable) and helps to prevent dilation of the heart and restrict cardiac filling beyond the normal range. When there is an acute collection of fluid or blood in the pericardial space, a clinical condition called cardiac tamponade develops. Rapidly accumulating fluid in the pericardial space can put pressure on the heart chambers, severely restricting blood from entering the heart, and can result in circulatory collapse. Surgical intervention is often necessary to relieve cardiac tamponade.
Myocardium
The heart wall consists of three layers. The outermost layer of the heart is the epicardium, consisting of epithelial cells that form a serous membrane that covers the entire heart. The innermost layer of the heart is the endocardium. It lines the inner surface of the heart, its valves, the papillary muscles, and the fibrous cords that connect the valves and continues into the major cardiac veins and arteries. The middle layer of the heart is the muscular layer known as the myocardium. It is responsible for the contractile action of the atria and ventricles. The human body contains three types of muscle: cardiac muscle, smooth muscle (found in other hollow organs like the bowel or bronchi), and skeletal muscle. Cardiac muscle cells are specialized muscle cells with amazing resiliency and capabilities. At an average heart rate (HR) of 60 beats/min, the heart beats 3 billion or more times without resting in one’s lifetime. To accomplish this incredible feat, myocardial cells function in a slightly different manner than do skeletal muscle cells. Myocardial cells are rich in mitochondria, the energy engine of the cell. The large number of mitochondria makes the cells resistant to fatigue. This allows myocardial cells to perform almost continuous work as long as they have a rich supply of oxygen and nutrients. Unlike skeletal muscle, myocardial cells rapidly become unable to function if their blood supply is interrupted. In addition to extra mitochondria, myocardial cells have other unique properties. For example, they have the intrinsic ability to contract in the absence of stimuli in a rhythmic manner and have the ability to conduct electrical impulses from one myocardial muscle cell to the next. Both of these functions play an important role in the cardiac conduction system described below.
Cardiac Chambers and the Circulation of Blood
As described above, the heart has four chambers: the RA, the RV, the LA, and the LV. The purpose of the heart is to circulate blood throughout the body where it can deliver oxygen and other nutrients, as well as pick up waste products like carbon dioxide. The blood circulates through the body in two loops or systems: the pulmonary circulation and the systemic circulation (Fig. 4.3).

FIGURE 4.3. Blood circulates through the heart in two loops forming the pulmonary and systemic circulations.
Beginning with the terminal portion of the systemic circulation, blood is drained into the venous system from the various organs and extremities. Blood from the lower portion of the body eventually drains into a large vein, the inferior vena cava (IVC). Blood from the upper portion of the body, including the upper extremities and the head and neck, drains into the superior vena cava (SVC). This venous blood has had much of its oxygen removed (deoxygenated) by the tissues and contains extra waste products like additional carbon dioxide (CO2). The systemic venous blood from both large veins drains into the RA. Contraction of the RA pushes the deoxygenated blood through the tricuspid valve into the RV. Contraction of the RV forces the blood forward past the pulmonic valve into the pulmonary artery, the beginning of the pulmonary circulation. The pulmonary arteries branch into ever smaller arteries and arterioles before turning into capillaries. Capillary blood flows close to the pulmonary alveoli. The blood can now add oxygen from the alveoli and discharge CO2 into the alveoli (see Chapter 7, Respiratory Anatomy and Physiology). Once the blood has been oxygenated and discharged some of its CO2, it begins to collect from the capillaries into venules and eventually into the large pulmonary veins. The four large pulmonary veins drain into the left side of the heart and the LA. Contraction of the LA forces the oxygenated blood forward through the mitral valve and into the LV. The forceful contraction of the LV can generate high pressures, and the blood is ejected out of the LV through the aortic valve and into the aorta. From the aorta, the blood flows out to the systemic circulation, where it can perfuse the body. The blood can then return to the heart to complete the two loops. It is the continuous forward pumping action of the heart that keeps blood flowing through the body where it can deliver nutrients and pick up waste products.
Right Atrium
The RA is a thin-walled muscular chamber. It receives deoxygenated venous blood from the SVC, the IVC, and the coronary sinus. The coronary sinus empties into the RA just above the tricuspid valve. In addition to the main chamber of the atrium, the RA has a small muscular pouch, the right atrial appendage. The RA is separated from the LA by the septum. The septal wall has an oval depression, the fossa ovalis. There are no true one-way valves in the venae cavae; thus, when the right atrial pressure rises, elevated pressure is reflected into the IVC and the SVC. Venous distention and systemic venous congestion are commonly seen when pressures are elevated in the heart as in congestive heart failure. Normal right atrial pressure ranges from 2 to 10 mm Hg.
Right Ventricle
The RV extends from the tricuspid valve nearly to the apex of the heart. The tricuspid valve prevents flow of blood backward from the RV into the RA. At the base of the heart, the RV extends to the left to form the infundibulum. The pulmonary valve prevents the flow of blood from the pulmonary artery backward into the RV. In addition to the main muscular walls of the RV, the RV contains two major papillary muscles and a third smaller papillary muscle. The papillary muscles connect the chordae, fibrous collagenous cords, to the leaves (cusps) of the tricuspid valve. The RV receives blood from the RA through the tricuspid valve and ejects it through the pulmonic valve into the pulmonary artery where it can flow to the lungs. The resistance to flow in the pulmonary circulation is approximately 1/10th that of the systemic circulation. Therefore, the RV does not need to generate as much pressure to pump blood to the lungs as the LV needs to pump blood to the body. This also explains why the RV muscle is much thinner than the thick muscular wall of the LV. Systolic pressure in the RV ranges from 15 to 25 mm Hg with end-diastolic pressures from 0 to 8 mm Hg (the concepts of systolic and diastolic pressures are discussed in detail below).
Left Atrium
The LA is the smaller of the two atria but has thicker walls. Its cavity and walls are mostly formed by the proximal part of the pulmonary veins, which are incorporated into the atria during fetal development. The left atrial aspect of the septum between the atria has a rough appearance and marks the site of the foramen ovale. In fetal life, the foramen remains open and is essential for fetal circulation. At birth, this foramen closes spontaneously, but in about 20%-30% of the normal population, a small defect may persist without any symptoms. Although asymptomatic, a patent foramen ovale presents a potential passageway for gas bubbles or debris to pass from the right side of the heart to the left side without passing through the lungs. Under normal circumstances, most debris, like small blood clots or gas bubbles, will be stopped by the pulmonary microcirculation before they can reach the left side of the heart. Under abnormal circumstances (i.e., conditions in which the right atrial pressure is elevated), passage of gas or debris from the RA across the foramen ovale to the LA becomes a real possibility. This is important as any debris or bubbles in the left side of the heart could flow out of the heart and directly into the brain, causing a stroke. The LA receives oxygenated blood from the lungs through pulmonary veins. Normal filling pressure ranges from 4 to 12 mm Hg. Like the RA, the LA also has an appendage. The LA appendage forms a portion of the left heart border as seen on a chest x-ray. In atrial fibrillation (disorganized electrical activity of the heart), it can be a source of intracardiac blood clots. These clots can embolize systemically, causing a stroke or limb ischemia. When the atria fibrillates, it loses its contractile function and the left atrial appendage is less able to empty blood into the atrial main cavity. Blood pooling in the atrial appendage is prone to clotting.
Left Ventricle
The LV wall is almost three times thicker than the RV wall. It is designed to be a powerful contractile chamber that can maintain flow in the high-pressured systemic arteries. The LV cavity extends from the AV groove to the cardiac apex. The mitral valve forms the inlet to the LV; the aortic valve forms the outlet. The septum dividing the right and LVs is thick and is functionally more a part of the LV than the RV (Fig. 4.4). A ventricular septal defect is the most common congenital heart defect in children younger than 2 years of age. Most small defects close spontaneously. Only the larger defects need surgical correction. The normal thickness of the LV wall is between 0.8 mm and 1.1 cm. In hypertrophic cardiomyopathy, the septum may get disproportionately thickened. When the ventricle contracts, the hypertrophic septum can obstruct the outflow of blood from the ventricular cavity into the aorta (dynamic subaortic obstruction). This clinical condition may cause sudden death and requires medical treatment. The LV receives blood from the LA through the mitral valve and ejects blood through the aortic valve to the systemic circulation via the aorta. The mitral valve prevents the flow of blood backward into the LA during contraction of the LV. There are several fibrous chords attached to papillary muscles in the ventricle that support the mitral valve cusps. These chordae are essential for the proper functioning of the mitral valve. Pressure in the LV is high during muscular contraction (“systole”) and equals systemic blood pressures. When the ventricle relaxes (“diastole”), the pressure falls. Normal end-diastolic pressures in the LV range from 4 to 12 mm Hg. The smooth left ventricular outflow tract ends at the cusps of the aortic valve. The cusps of the aortic valve are attached in part to the aortic wall and in part to the supporting ventricular structures. The aortic valve in its closed position has three coronary sinuses (outpouching between the aortic wall and the cusp).
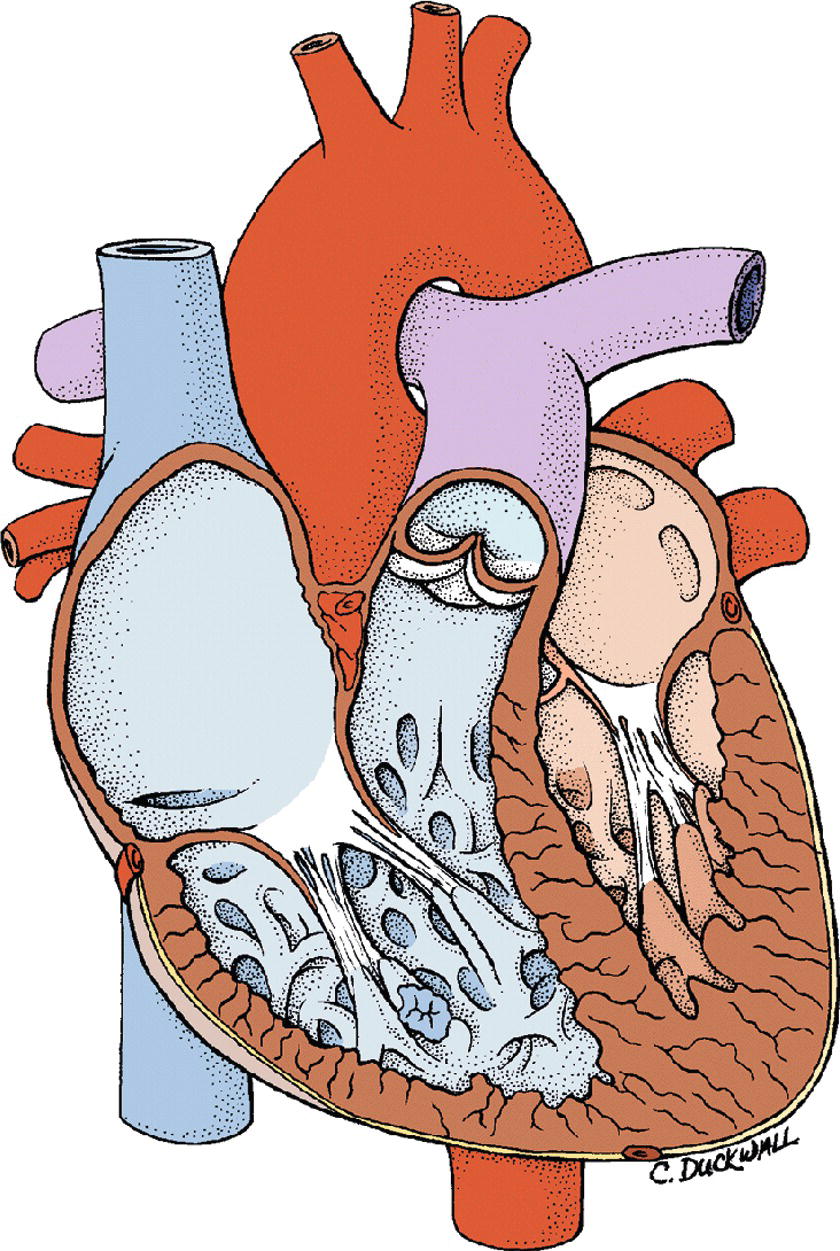
FIGURE 4.4. Cross-section of the heart demonstrating right and left ventricles.
Cardiac Skeleton
The heart’s fibrous skeleton, the “annulus fibrosus,” is a firm anchor to which most of the heart’s muscles and valves are attached. The annulus fibrosus gives structure to the heart and acts as an insulator. Because the annulus fibrosus is not made up of myocardial muscle cells, it does not conduct electrical impulses from one region of the heart to another. This ensures that electrical impulses traveling from the atrial myocardial cells to the ventricular myocardial cells must move through a specialized pathway, the AV node. This system helps the heart regulate electrical signaling to coordinate contraction of the atria and ventricles as well as to help prevent abnormal electrical heart rhythms (arrhythmias).
Cardiac Conduction System
Myocardial muscle cells differ from skeletal muscle cells because of their inherent ability to spontaneously depolarize and repolarize in a rhythmic fashion (automaticity). Ventricular muscle cells spontaneously depolarize at a lower frequency (30-40 beats/min) than atrial muscle cells, but in the intact heart, both are synchronized to a more rapid rhythm, generated by pacemaker cells in the RA. The pacemaker cells, called the sinoatrial (SA) node, and the specialized myocardial cells, which conduct the electrical signals to synchronize the contraction of all myocardial cells, make up what is known as the “cardiac conduction system” (Fig. 4.5).
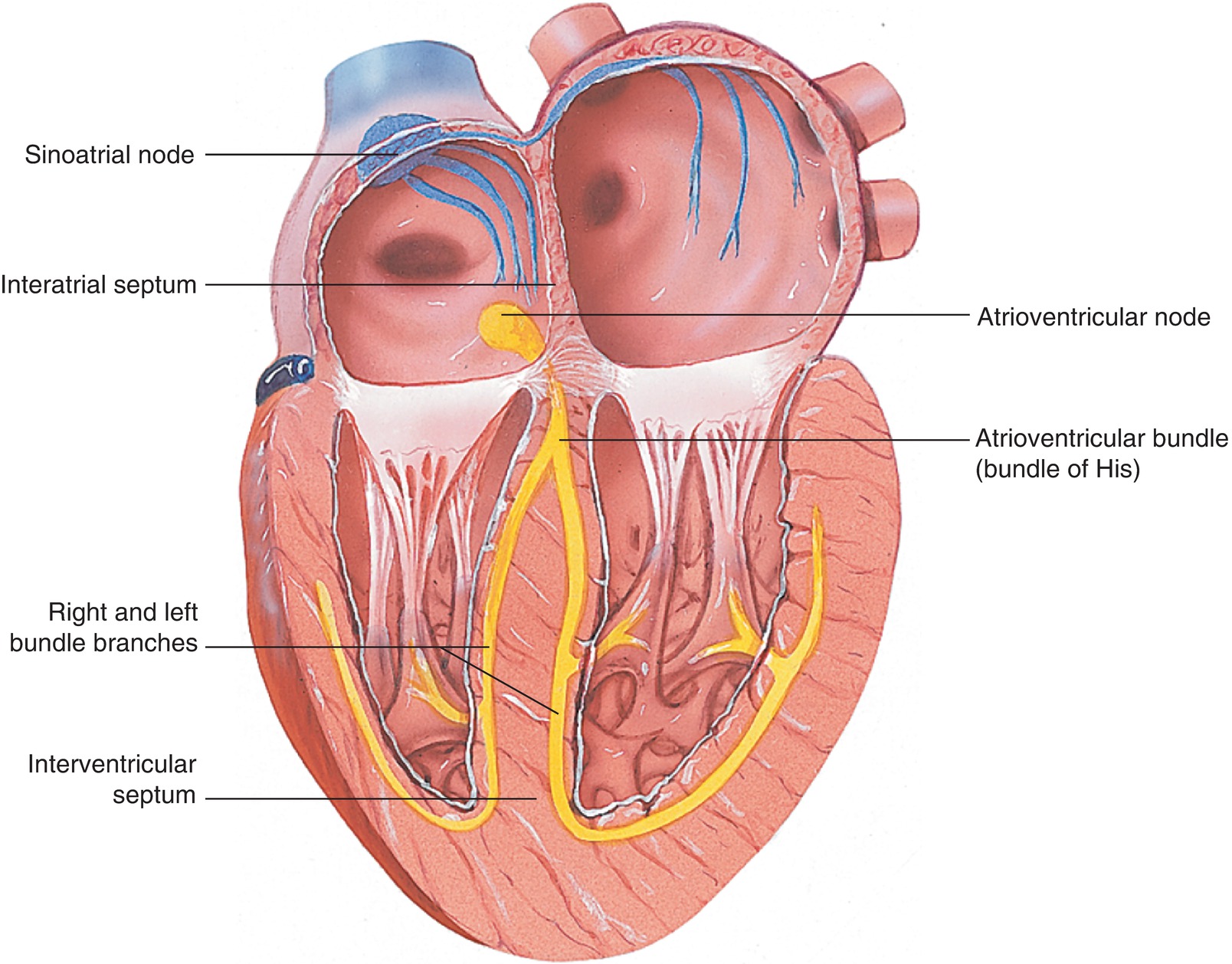
FIGURE 4.5. The myocardial conduction system is made of specialized myocardial muscle cells forming the pacemaker region (the SA node), the AV node, the bundle of His, and the Purkinje fibers.
The SA node is located in the high RA near the junction of the SVC and the RA. These “pacemaker cells” spontaneously depolarize and initiate contraction of the myocardial cells in the atria. Because all myocardial cells can conduct the electrical impulse and cause adjacent myocardial cells to depolarize, the electrical depolarization and subsequent contraction of atrial cells spread like a wave beginning at the SA node. Several other specialized myocardial conduction cells conduct the electrical impulse from the RA to the LA (Bachmann bundle). The depolarization must also be conducted from the atria to the ventricles and more importantly the timing of atrial and ventricular contractions synchronized (see “Cardiac Cycle” below). If the atria and ventricles contract simultaneously, blood would not flow from the atria into the ventricles. The speed of conduction of electrical impulses through normal atrial and ventricular cells is about 0.5 m/s. The speed of conduction of the electrical signal is much faster than the time it takes for muscular contraction; thus, the electrical signal from the SA node would reach the ventricle rapidly, long before the atrium has contracted. This would cause near simultaneous contraction of the atria and ventricles.
In the normal functioning heart, this does not happen because the fibrous annular tissue that separates the atria from the ventricles does not conduct the electrical impulse. Instead, the impulse must pass through a region of cells in the inferior-posterior portion of the atrial septum called the AV node. This group of specialized myocardial cells conducts the impulse at 1/10th the speed of normal myocardial cells. This has the effect of slowing the electrical signal before reaching the ventricles. This gives the atria a chance to contract and eject blood into the relaxed ventricle before the ventricle begins its contraction. In order to efficiently eject blood and generate pressure within the ventricle, the septum and apex of the ventricles must contract first, followed by the base of the heart. This coordination of contraction would not occur if the depolarization wave spread from the AV node through the ventricles from one cell to another. The heart utilizes additional specialized myocardial cells to rapidly conduct the electrical signals to the different portions of the ventricle to achieve effective contraction and blood ejection. These cells conduct the electrical signal 10 times faster than normal myocardial cells. After leaving the AV node, the electrical signal passes through the bundle of His to reach the base of the heart. It then passes on the endocardial side of the interventricular septum using specialized myocardial conduction cells (Purkinje fibers). It then moves along the endocardial side of each of the ventricles from apex to base using right and left branches of Purkinje fibers. The SA node, Bachman bundle, the AV node, the bundle of His, and the Purkinje fibers make up the myocardial conduction system.
Wolff-Parkinson-White and other syndromes have abnormal electrical pathways in the heart that lead from the atria directly to the ventricles bypassing the AV node. These conditions can lead to very fast heartbeats (tachycardia) and even to life-threatening abnormal heart rhythms.
Coronary Arterial Supply
The right and left coronary arteries arise from the ascending aorta just above the aortic valve. The coronary arteries supply the capillaries of the myocardium with oxygenated blood. One might think that the LV would not need any arteries because it could obtain oxygen directly from the oxygenated blood that flows through the ventricular cavities. However, the walls of the ventricles are too thick to obtain sufficient oxygen or eliminate waste products, like carbon dioxide, by diffusion. Arteries branching into arterioles and then capillaries must do the job. The blood is then collected into veins, which drain into the coronary sinus. The left coronary artery (LCA) has two main branches: the left anterior descending (LAD) and the left circumflex (LCX) arteries (Fig. 4.6).
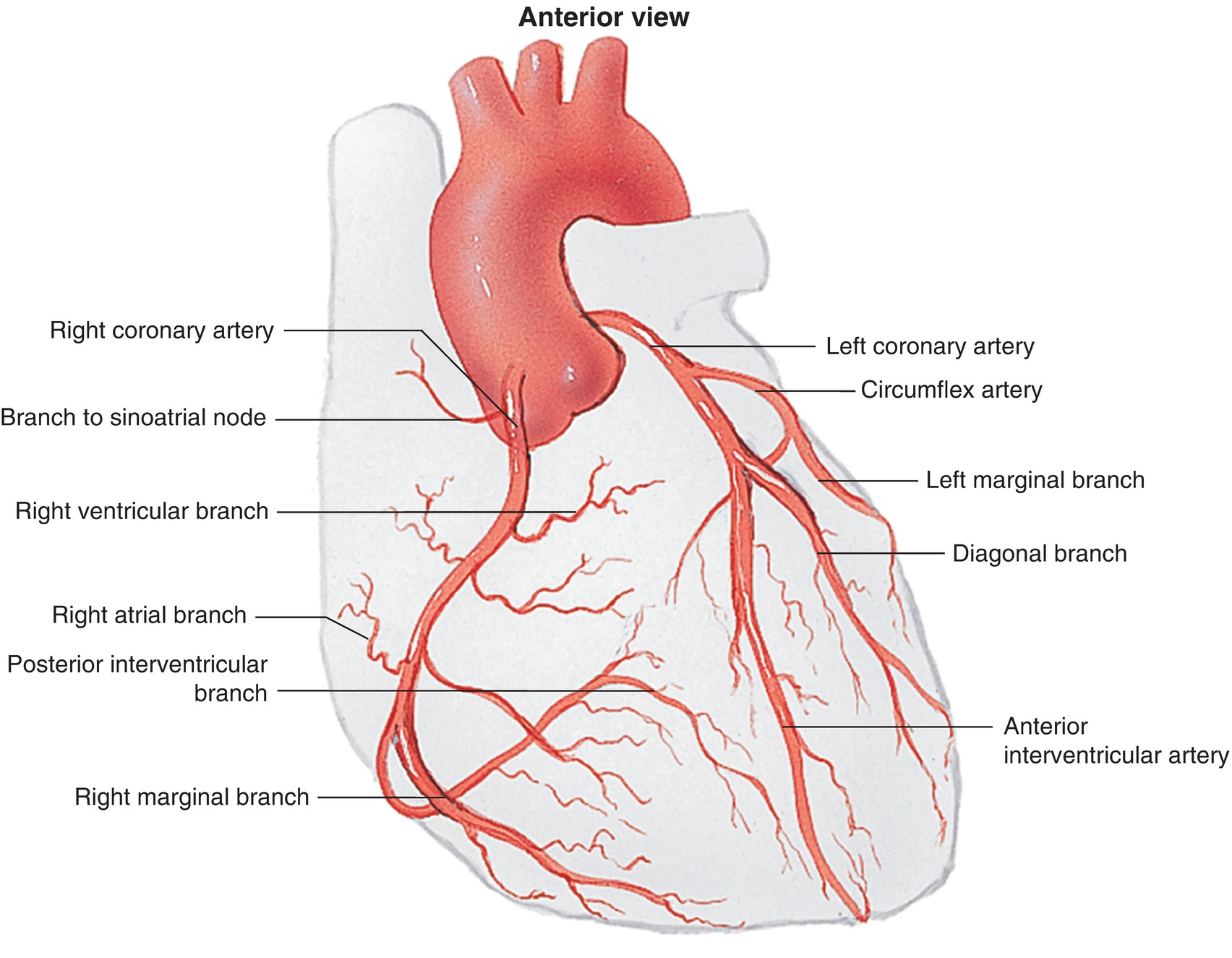
FIGURE 4.6. Coronary artery anatomy.
The right coronary artery (RCA) and the LCX course around the heart in opposite directions in the AV groove. These two arteries throw off branches, with the terminal portions of the arteries meeting on the posterior aspect of the heart at an important landmark known as the crux of the heart. At this point, either the RCA or the LCX supplies the posterior descending artery (PDA), which descends in the interventricular groove toward the apex of the heart. This terminal branch supplies the posterior and inferior parts of the heart. Right or left coronary dominance is determined by which artery supplies the PDA. Seventy percent of people are RCA dominant, 10%-15% are LCA dominant, and 10%-15% have mixed right and left dominance. This is an important anatomical fact as the PDA supplies the crux and the posterior third of the ventricular septum. The AV node is located at the crux and is nourished by the PDA. Obstruction of the blood supply to the AV node can cause malfunction of the node and prevent electrical impulses from traveling from the atrium to the ventricle properly (AV block). The RCA supplies the RA, RV, and inferior wall of the LV (if right coronary dominant). The LAD runs in the anterior interventricular groove and supplies the anterior wall of the LV. Along its course, it gives off diagonal arteries, which supply the lateral LV wall, and septal arteries, which supply the anterior two-thirds of the ventricular septum. The LCX artery supplies the LA and the lateral and posterior walls of the LV. The LAD, LCX, and RCA are considered major arteries because of their large area of distribution. Blockage in the proximal portion in any of these arteries can cause a large amount of myocardial cell death (myocardial infarction) and can significantly affect the heart’s ability to contract.
Coronary Venous Circulation
The venous system of the heart consists of the thebesian veins, the anterior cardiac veins, and the coronary sinus (Fig. 4.7). The coronary sinus is located in the posterior AV groove near the crux and collects about 85% of the blood from the LV. It opens into the RA at the coronary sinus ostium near the orifice of the IVC. During cardiac surgery, the coronary sinus must often be cannulated. Anomalies in coronary sinus anatomy can present significant challenges for both the anesthesiologist and the cardiac surgeon.
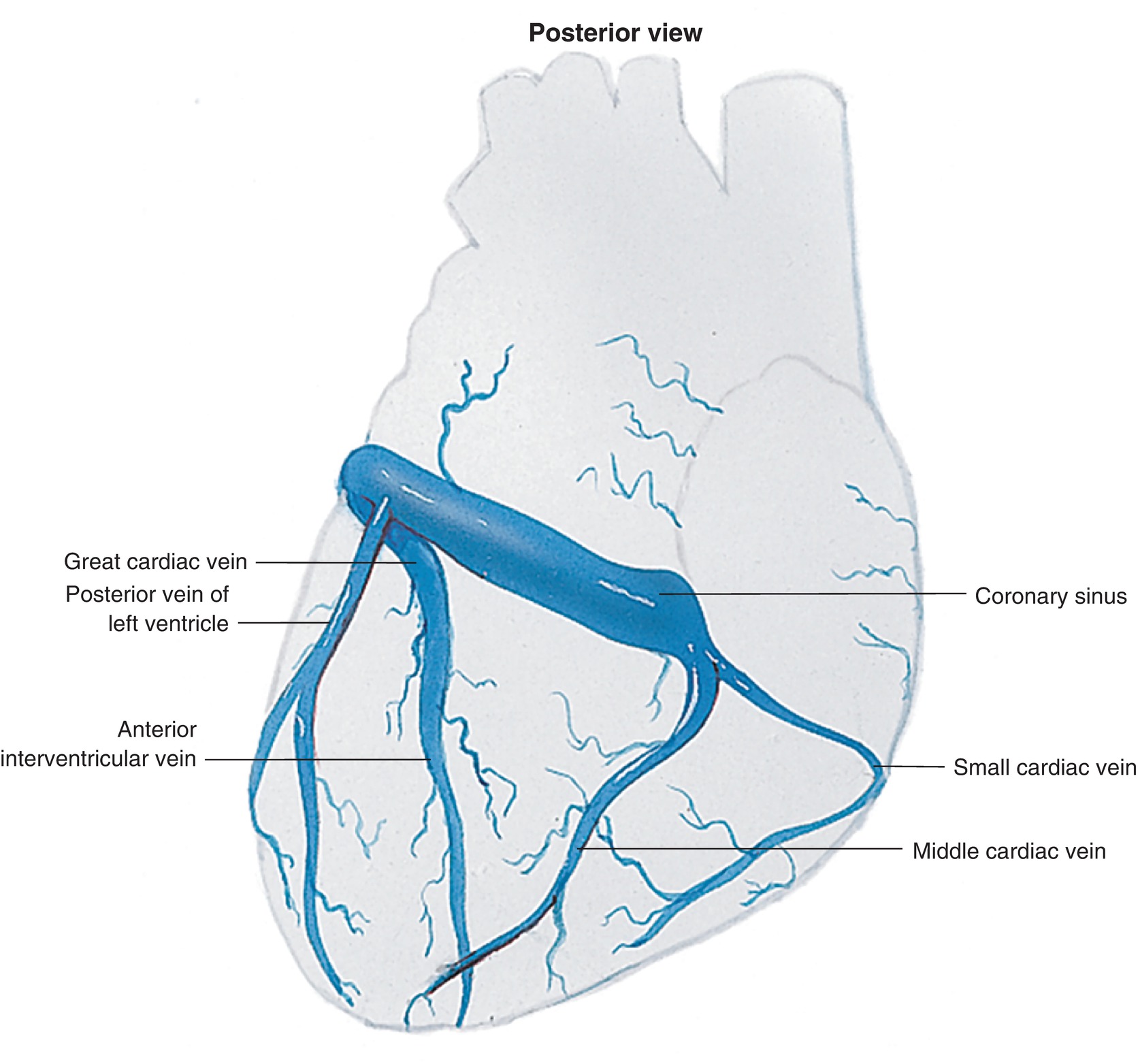
FIGURE 4.7. The coronary venous circulation.
Innervation
The heart is richly innervated by the autonomic nervous system to provide control of HR and the force of cardiac contractions (see Chapter 13).
Autonomic influence on the heart can modify cardiac function appropriately to meet the changing supply and demand of the body for blood flow. All portions of the heart are richly innervated by sympathetic fibers. When active, these sympathetic nerves release norepinephrine to act on the myocardial cells. Norepinephrine binds to beta1-adrenergic receptors on cardiac muscle cells to increase the HR (chronotropy), increase the conduction velocity of electrical signals (dromotropy), increase the force of contraction (inotropy), and increase the speed of contraction and relaxation (lusitropy). Cholinergic parasympathetic innervation to the heart arises from the right and left vagal nerves and innervates the SA node, the atria, and the AV node. When active, these parasympathetic nerves release acetylcholine to act on myocardial cells. Acetylcholine interacts with muscarinic receptors on these cells to decrease the HR (SA node) and decrease the velocity of electrical signals moving through the AV node. Parasympathetic nerves may also act to decrease the force of contraction of atrial (not ventricular) muscle cells. Overall, parasympathetic activation acts to decrease cardiac pumping.
Cardiac Physiology
Cardiac Action Potential
Like skeletal muscle, myocardial cells are able to contract due to the interaction of cellular proteins, actin, and myosin. This interaction and subsequent contraction is dependent upon calcium for it to occur. As discussed above, the initiation of normal myocardial contraction begins with an electrical signal from the SA node. The electrical signal travels through the myocardial conduction system before reaching the rest of the myocardium. Upon reaching the myocardial cells, the electrical signal depolarizes the cell membrane, which opens channels to allow extracellular sodium and calcium to flow into the cell (Fig. 4.8). The inward flow of calcium causes additional calcium stores within the cell to be released. The calcium causes the cell to contract by promoting a temporary binding between actin and myosin. The calcium must be actively sequestered back into storage units within the cell to allow the muscle cell to relax. In addition, the myocardial membrane must be repolarized to prepare for another electrical signal. This sequence of events is called the cardiac action potential.
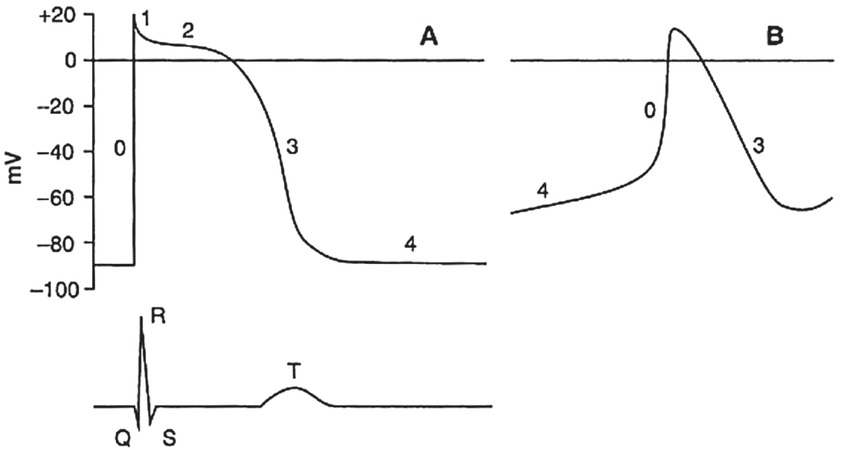
FIGURE 4.8. The cardiac action potential demonstrates cell membrane potential changes over time caused by changing flows of ions into and out of the cell. A: Standard action potential. B: Action potential from a cell with automaticity-automatic depolarization. Note how phase 4 shows the cell slowly depolarizing until it reaches a threshold to initiate phase 0. (From Topol EJ, Califf RM, et al. Textbook of Cardiovascular Medicine. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006, with permission.)
The relative ratios of ions (molecules with strong positive or negative charges) like sodium, potassium, and calcium, inside and outside of the myocardial cell membrane create small electrical potentials or voltages across the membrane. For example, if there are more net positive ions outside of the cell than inside the cell, the cell would have a negative potential (the cell is polarized). By custom, the “positive” or “negative” potential of a cell is determined by the charge of the interior of the cell compared to the outside. At rest, myocardial cells have a negative potential called the resting membrane potential. Because of the charge differential between the inside and outside of the cell, the resting myocardial cell is a miniature battery. Ions are kept inside and outside of the cell because the cell membrane is not permeable to ions. If an appropriate cellular channel for that ion is open, ions will flow into or out of the cell, depending upon the charge and concentration gradients. Once a sufficient electrical signal reaches the myocardial cell, it triggers a sequence of actions that open and close ion channels. The flow of ions across the channels causes the cell membrane potential to change over time resulting in the cardiac action potential. The phases of the cardiac action potential are as follows (Fig. 4.9):
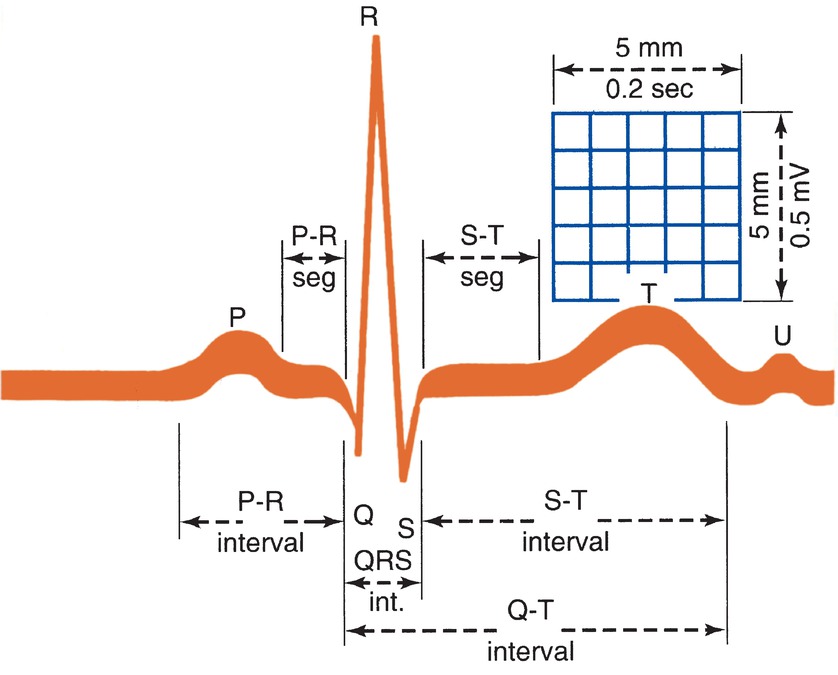
FIGURE 4.9. The ECG wave is formed by the P wave, the QRS complex, the ST segment, and the T wave. (From Weber J, Kelley J. Health Assessment in Nursing. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003, with permission.)
- Phase 4: Resting membrane potential.
- Phase 0: An electrical signal has caused the cell to open sodium channels and sodium rushes into the cell, depolarizing the membrane.
- Phase 1: Fast sodium channels close. Small movements of potassium and chloride cause a slight dip in the membrane potential.
- Phase 2: The membrane is kept depolarized by the inward flow of calcium ions balanced by an outward flow of potassium.
- Phase 3: Repolarization occurs as the calcium channels close, but potassium continues to flow out of the cell.
The cell must reset the ion balance by actively pumping sodium out of the cell and potassium into the cell. The cell must also restore calcium gradients by actively pumping calcium into storage. The cardiac action potential is much longer than that of skeletal muscle, and during this phase, cells remain unresponsive to further excitation. This is the refractory period.
Cardiac Electrophysiology
During the phases of the cardiac action potential, ions move in and out of the cell, causing changes in membrane potential and causing small electrical currents. When the tiny electrical currents from multiple myocardial cells are added up, the currents can be measured with electrodes to generate the electrocardiogram or ECG. The next chapter, Cardiovascular Monitoring, will discuss ECG monitoring in much greater detail.
Abnormal Heart Rhythms
Normal adult hearts beat between 60 and 85 beats/min according to the rhythm set by the SA node. The HR can be accelerated or slowed by actions of the autonomic nervous system. For example, a vigorously exercising adult can have an HR of 140 beats/min. HRs above 100 beats/min are referred to as tachycardia. HRs below 60 beats/min are referred to as bradycardia. Adults are rarely symptomatic until the HR decreases below 50 beats/min. Some degree of bradycardia and tachycardia is normal in healthy populations (i.e., bradycardia during deep sleep and in a well-trained athlete; tachycardia following extreme emotion, excitement, after strenuous exercise, or with a fever).
The SA node is the normal pacemaker for the heart. Several conditions are characterized by abnormalities with the origin of the pacemaker signal in the atria. One common condition is atrial fibrillation. In the normal heart, the SA node pacemaker in the RA initiates the signal, which then spreads like a wave across the atria. In atrial fibrillation, the individual atrial myocardial cells are depolarizing and contracting completely independent of one another. The end result is that there is no coordination between the cells, a wave of depolarization is not produced, and the atria fail to contract. The independently depolarizing and contracting atrial muscle cells produce quivering atria.
Atrial fibrillation can be easily distinguished from a normal rhythm generated from the SA node on the ECG. (A detailed explanation of ECG monitoring is found in the next chapter, Chapter 5, Cardiovascular Monitoring.) The SA node generates a smooth atrial depolarization visible as a “P” wave; this is absent in atrial fibrillation (Fig. 4.10). The disorganized atrial muscle cell depolarizations can be seen on the ECG as a fine wavy line. Because the atria are not depolarized in a wave, the P wave is absent on the ECG. Besides lack of atrial contraction, another consequence of disorganized atrial depolarizations is that these electrical signals can be conducted through the AV node and produce irregular ventricular depolarizations and contractions. This might not seem like a bad thing; however, the atria can produce electrical signals at a rate of over 500 per minute. If these signals were conducted to the ventricles, the heart would beat too fast. At rates above 180-200 beats/min, the ventricles do not have enough diastolic time to fill and forward flow begins to fall. The faster the rate, the more forward flow falls. The heart will rapidly reach the point where it cannot produce enough cardiac forward flow to perfuse the major organs. The heart attempts to protect itself from too many atrial signals reaching the ventricles by blocking them at the AV node. With very fast atrial signals, the AV node is unable to conduct every signal and begins “dropping” signals (beats) so that the ventricular rate is much slower than the atrial rate. In addition, the pattern of dropped or conducted signals can be variable, producing irregular ventricular contractions. Other atrial arrhythmias include atrial flutter and multifocal atrial tachycardia. Both of these rhythms have abnormal P waves. Because the atrial signal is conducted through the myocardial conduction system to the ventricles, the shape of the QRS complex will be relatively normal. These atrial arrhythmias usually produce fast heart rates with normal (narrow) QRS complexes and are termed narrow complex tachycardias.
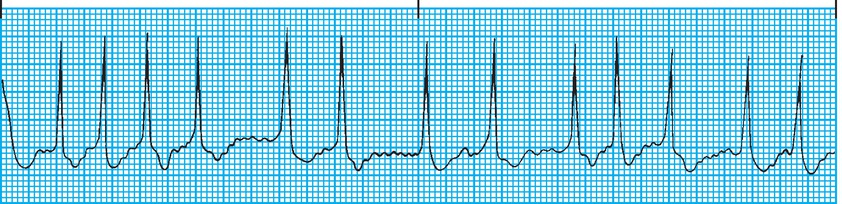
FIGURE 4.10. ECG demonstrating the lack of a P wave and an irregular heart rate characteristic of atrial fibrillation. (From Springhouse. ECG Facts Made Incredibly Easy. 2nd ed. Ambler, PA: Wolters Kluwer Health; 2010, with permission.)
Disease or drug-induced reductions in conduction velocity through the AV node can cause serious problems. When conduction through the AV node slows to the point where the PR interval exceeds 0.2 seconds or the AV node fails to conduct normal atrial beats, it is referred to as heart block or AV block. When the AV node fails to conduct any atrial signals to the ventricle, the patient has “third degree” or “complete heart block.” This is an emergency requiring a pacemaker.
Disease in the Purkinje bundles can cause abnormal transmission of the electrical signal to the ventricles (a “bundle-branch block”). Often, one or the other of the bundles is malfunctioning and failing to conduct the signal. In this condition, the normal bundle conducts the signal to its ventricle; and then, the signal must spread to the other ventricle through the heart muscle. Because the heart muscle conducts the signal 10 times slower than the myocardial conduction system, the ventricle supplied by the blocked bundle will depolarize later and more slowly than the other ventricle. This will result in a broad QRS complex, reflecting the slowed conduction, and an abnormally shaped QRS complex, reflecting the altered timing in depolarization of the ventricles.
Arrhythmias can also originate in the ventricles. When an organized fast rhythm originates in the ventricle, it is called ventricular tachycardia (V tach) (Fig. 4.11). As mentioned above, fast ventricular rhythms can be associated with low cardiac output (CO) due to insufficient time to fill between contractions. In V tach, the ECG demonstrates a wide QRS complex tachycardia reflecting the slow conduction of the electrical signal through the heart muscle, even though the HR is fast. V tach is life threatening and requires immediate treatment. The most dangerous arrhythmia is ventricular fibrillation (V fib). This condition is physiologically similar to atrial fibrillation. The ventricular muscle cells depolarize and contract in a completely disorganized and independent fashion, producing a quivering ventricle that cannot generate any forward blood flow. The ECG demonstrates a completely disorganized electrical signal (Fig. 4.12). V fib produces no forward blood flow and is a life-threatening arrhythmia. Treatment for V fib requires application of an immediate electrical shock to the heart that causes all of the ventricular muscle cells to simultaneously depolarize. In many cases, after the shock (defibrillation) is delivered to the heart, the heart’s natural SA node pacemaker can take over and begin sending signals to coordinate the depolarization of the ventricles. One way to illustrate this concept is to think about a stadium filled with people. Each person represents an individual muscle cell. Beginning at one end of the stadium, the fans stand up and initiate the “wave.” As fans stand up and sit down raising their arms in order, the wave travels around the stadium pushing a beach ball in front of it. This is a coordinated contraction. Now, imagine that every fan in the stadium is standing up and down randomly in a completely disorganized fashion. The stadium would appear to be quivering or fibrillating. A wave would not be produced, and the beach ball would not be pushed smoothly around the stadium. Now, imagine someone got on the public address system and screamed, “SIT DOWN.” All the fans suddenly sit (depolarized by the shock), and the stadium is quiet. Then, the fans at one end of the stadium stand up and initiate the wave again (the SA node takes over as the pacemaker). The stadium has been successfully “defibrillated,” and we have the return of normal sinus rhythm. V fib is a life-threatening event, and if not treated promptly, the patient will die.

FIGURE 4.11. ECG demonstrating wide QRS complexes at a fast rate characteristic of ventricular tachycardia. (From Springhouse. ECG Facts Made Incredibly Easy. 2nd ed. Ambler, PA: Wolters Kluwer Health; 2010, with permission.)
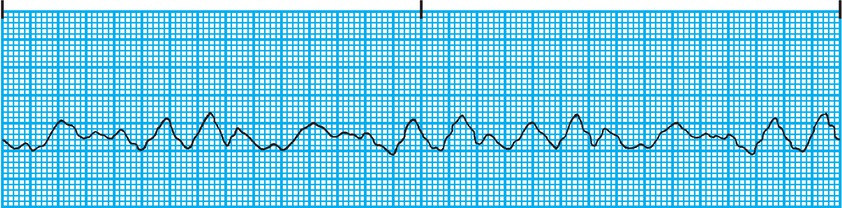
FIGURE 4.12. ECG demonstrating a bizarre disorganized signal characteristic of ventricular fibrillation. (From Springhouse. ECG Facts Made Incredibly Easy. 2nd ed. Ambler, PA: Wolters Kluwer Health; 2010, with permission.)
Although many things can cause arrhythmias, some of the most common causes include the following:
- Myocardial ischemia or infarction: Myocardial cells starved of oxygen do not function normally. They will not contract normally or conduct electrical impulses normally. The same is true for dead myocardial cells that have turned into a scar. These abnormal cells can be the origin of an arrhythmia or can alter conduction of a signal, causing an arrhythmia.
- pH or electrolyte imbalances: Normal pH and electrolyte levels are important for the myocardial cells to maintain their membrane potentials. Abnormalities in membrane potentials can cause abnormalities in the cardiac action potential and subsequent arrhythmias.
- Overstretching of the heart due to valvular disease: Incompetent cardiac valves can cause blood to flow backward in the heart, stretching and overfilling chambers. These stretched and overfilled chambers are prone to abnormal heart rhythms.
Cardiac Cycle
The cardiac cycle is defined as the period from the beginning of one heartbeat to the beginning of the next heartbeat. It includes systole (i.e., contraction) and diastole (i.e., relaxation). The duration of each cycle is variable depending upon the HR. For example, with an HR of 72 beats/min, the cardiac cycle is 0.8 seconds (e.g., 60 seconds per minute divided by 72 beats/min = 0.8 seconds per beat). During this 0.8 seconds, the ventricles are in systole 0.3 seconds and in diastole 0.5 seconds. Although cardiac muscle contracts and relaxes faster at higher HRs, there is a limit. In general, as the HR increases, forward blood flow also increases. However, at HRs above 180-200 beats/min, the heart does not have enough time in diastole to fill before the next contraction begins. At HRs above this range, cardiac function progressively declines. Another important aspect of the HR is that the amount of time the heart spends in diastole affects myocardial perfusion. As mentioned earlier, blood flows from the aorta through the coronary arteries and then into the small arterioles and capillaries that feed the heart muscle itself. The capillaries are where the distance between the myocardial cells and blood flowing through the capillaries is short enough that gas and nutrient exchange can occur. During systole, ventricular pressures are high and essentially obstruct blood flow though the small arterioles and capillaries. This means that ventricular heart muscle is only supplied with oxygen and nutrients during diastole. Higher HRs can eventually lead to insufficient diastolic times to perfuse the heart muscle.
To better understand the cardiac cycle, examine Figure 4.13. This figure depicts several simultaneous things happening during the course of two heartbeats. The top line represents the pressure in the aorta. The next line, the blue line, represents the pressure inside the LV, while the gray line below it represents the pressure in the LA. The red line represents the volume of blood within the LV. Although similar things are happening with the RA and RV, in order to simplify the diagram, the figure depicts only the LA and LV pressures. The dark blue line represents the ECG tracing from a single lead. Finally, the last gray line shows the phonocardiogram and depicts the sounds of the heart during the cardiac cycle. The mitral and tricuspid valves are often referred to as the AV valves, and they are labeled on the diagram as the AV valves.
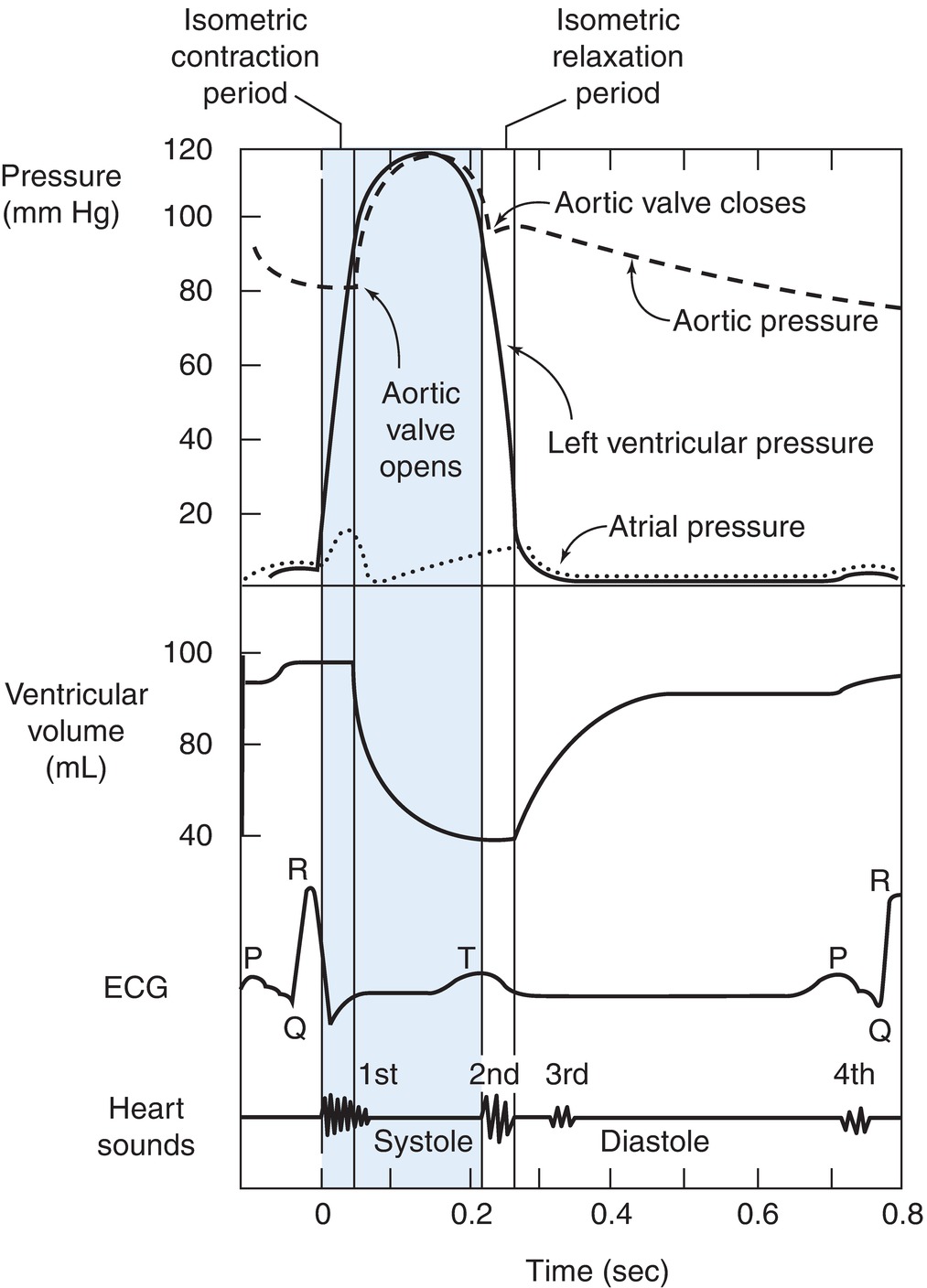
FIGURE 4.13. The cardiac cycle depicted by measuring the pressures in the cardiac chambers during a heartbeat. (From Porth CM. Pathophysiology: Concepts of Altered Health States. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005, with permission.)
Ventricular Systole
The first section of the diagram in Figure 4.13 represents ventricular systole, which is divided into three phases: isometric contraction, rapid ejection, and slow ejection.
- Isometric contraction phase: This phase represents the beginning of ventricular contraction. The increase in pressure within the ventricle causes the mitral valve to close, preventing the flow of blood backward into the LA. The beginning of ventricular contraction is seen on this ECG as the R wave, a large positive upstroke created by the depolarization of the muscular ventricle. The sound of the closure of the AV valves can be detected on the phonocardiogram or heard with a stethoscope. AV valve closure produces the first heart sound (S1). S1 is a low, slightly prolonged “lub” caused by the vibrations of the sudden closure of the AV valves. They can best be heard with a stethoscope over the apex of the heart. The isometric contraction phase is also called the isovolumetric contraction phase because all the valves are closed and there is no ejection of blood. The volume of blood in the ventricle does not change until the ejection phase. During the isometric contraction phase, the ventricular pressure rises. The pressure in the ventricle must rise above the aortic pressure to get the blood to flow into the aorta. The atrial pressure has also started to rise, not because of atrial contraction but rather because the blood pouring in from the vena cava is filling up in the RA and the blood from the pulmonary veins is filling up the LA.
- Rapid ejection phase: When left ventricular pressure exceeds aortic pressure and right ventricular pressure exceeds pulmonary artery pressure, the aortic and pulmonic valves open. Blood ejects out of the ventricles into the aorta and pulmonary arteries, respectively. The majority of the ventricles’ blood is emptied during the first third of the ejection period, rapid ejection. The aortic pressure curve peaks sharply because the rapid flow of blood flow out of the ventricle into the aorta soon and causes the pressure within the ventricle to fall.
- Slow ejection phase: A small amount of additional blood is ejected during the latter two-thirds of the ejection phase. This is referred to as the slow ejection phase. Even though the ventricles continue to contract during this phase, very little blood is ejected during this period. The total volume of blood ejected during the rapid and slow ejection phases is called the stroke volume (SV). During the slow ejection phase, a slow broad wave appears on the ECG, the T wave. The T wave is the detection of the electrical currents created by the repolarization of the ventricles. Once repolarization occurs, the ventricular muscle starts to relax.
Ventricular Diastole
Ventricular diastole can be divided into four phases: isovolumetric relaxation, rapid ventricular filling, slow ventricular filling, and atrial systole.
- Isometric relaxation phase: The isometric or isovolumetric relaxation phase is the beginning of diastole. The ventricular pressure has fallen due to left ventricular relaxation to a point where it is lower than that in the aorta (lower than that in the pulmonary artery for the RV). The aortic valve and the pulmonary valves snap shut due to the pressure gradient and prevent the backward flow of blood. This pressure reversal and closure of the aortic and pulmonary valves produce the second heart sound (S2) as shown on the phonocardiogram. S2 is a shorter, high-pitched “dup,” caused by the vibrations of the closing aortic and pulmonic valves just after the end of ventricular systole. They can be easily heard with a stethoscope at the left second intercostal space. During the isometric relaxation phase, the ventricular pressure curve falls close to 0 mm Hg.
- Rapid ventricular filling phase: When ventricular pressure falls below atrial pressure, the AV valves open and blood enters rapidly from the atria into the ventricles. The rapid flow of blood out of the atria into the ventricles causes the pressure in the atria to fall. This is the downward slope of the V wave on the atrial pressure tracing.
- Slow ventricular filling phase: The slow ventricular filling phase is also known as diastasis or the last part of diastole. During this phase, only a small amount of blood drains from the lungs and peripheral circulation into the atria and into the ventricle. The rising pressure in the ventricles reduces the pressure gradient from the atria to the ventricles, resulting in reduced flow. Toward the end of this phase, atrial depolarization occurs and is seen as a small upward deflection on this ECG (the P wave).
- Atrial systole phase: Atrial contraction begins during the last phase of ventricular diastole and contributes 10%-25% of the total amount of blood that fills the ventricles in a normal individual. Atrial contraction begins about the time of the peak of the P wave. When individuals lose their regular atrial contraction (e.g., atrial fibrillation), they often underfill their ventricles, leading to a reduction in cardiac function. The “a” wave of the central venous tracing correlates to atrial contraction just before the closure of the AV valves.
Cardiac Volumes and Cardiac Output
The blood volume in the ventricles at the end of diastole is approximately 120 mL. This volume is called end-diastolic volume (EDV). Sixty percent of the total blood volume in the ventricles is ejected out during systole, and the residual volume after ventricular systole is approximately 50 mL. The difference between the EDV and the end-systolic volume (approximately 70 mL) is known as the SV and is equal to the amount of blood ejected after each ventricular contraction. To determine ejection fraction (EF), SV is divided by EDV. The normal adult EF is approximately 50%-70% depending upon hemodynamic and volume status. The EF is a good indicator of cardiac function because cardiac disorders (e.g., ischemic heart disease, cardiomyopathies, valvular heart disease, or congestive heart failure) can markedly reduce the EF.
The volume of blood the heart pumps in liters per minute is called CO. It is equal to the SV multiplied by the HR. A person with an HR of 72 beats/min and an SV of 70 mL has a CO of 5.0 L (CO = HR × SV). This value is within the normal range for a resting average-size adult; however, CO can vary significantly with exercise, fever, or metabolic conditions like hyperthyroidism or hyperthermia. During periods of strenuous exercise, a well-trained athlete’s CO can reach as high as 35 L/min. Decreases in CO can be produced by a variety of physiologic and pathologic conditions. For example, arrhythmias (abnormal heart rhythms), ischemic heart disease, or valvular heart disease can produce significant reductions in CO. CO can also vary based on the size of the individual. The cardiac index (CI) is a measurement used by clinicians to adjust for individual differences in body size (CI = CO/body surface area [BSA] or SV × HR/BSA) and is the CO per square meter of BSA. The CI is therefore expressed in liters per minute per meter squared. The CI gives a better representation of perfusion than CO alone. The normal CI is 2.5-4.0 L/min/m2 of BSA.
Factors Affecting Cardiac Output
There has been a great deal of research into the major physiologic factors that affect CO. These include the HR, the EDV of the ventricle (preload), the force with which the ventricle can contract (contractility), and the resistance against which the heart must eject blood (afterload). The description of how these factors affect cardiac function is the cornerstone of cardiac physiology.
Preload
The total volume of circulating blood affects preload. The greater the venous return to the heart, the more the myocardial fibers will stretch to accommodate the load on the heart. According to the Frank-Starling law, the greater the initial myocardial fiber length, the greater will be the force of contraction. This mechanism has been compared to the increased recoil of a rubber band when stretched. The increase in contractile force is related to an increase in sarcomere length (the contractile unit of a cardiac muscle cell). After a certain point, the sarcomere can become overstretched and the contractile force will decrease. In conditions where there is decreased filling of the heart (e.g., hypovolemia), the sarcomeres are short and the force of contraction is diminished. With increasing blood volume (increasing preload), the contractile force increases. When the blood volume is too high and there is excessive filling and stretching of the heart, the force of contraction begins to fall and is referred to as congestive heart failure. The ventricular function curve as depicted in Figure 4.14 shows the relationship between fiber length and the force of contraction.
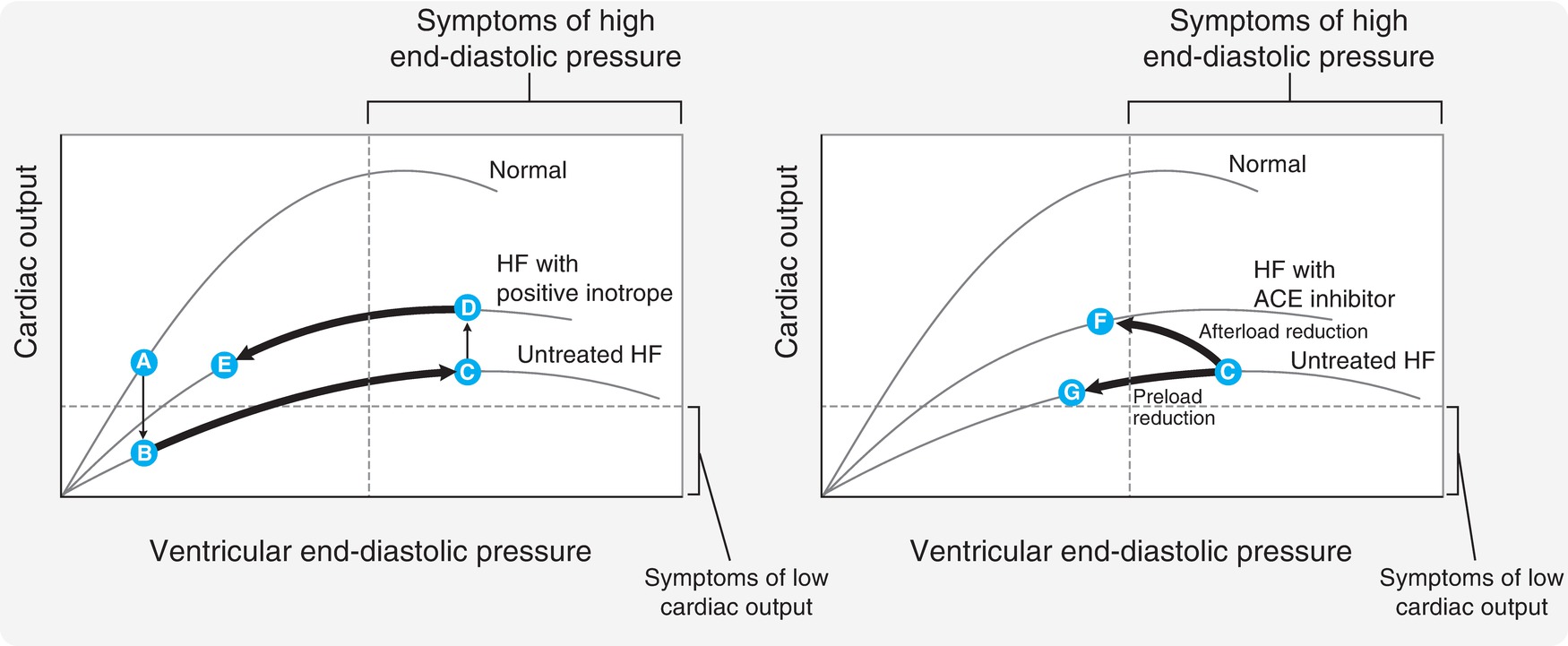
FIGURE 4.14. The physiologic relationship between the Starling law of the heart and venous return pressure and volume. (Adapted from Harvey RA, Champe PC, eds. Lippincott’s Illustrated Reviews: Pharmacology. Philadelphia, PA: Lippincott Williams & Wilkins; 1992:157, Figure 16.6, with permission.)
The left panel of the figure depicts ventricular end-diastolic pressure graphed against CO on the y axis. The upper “normal” curve demonstrates that increasing end-diastolic pressure (a measure of the preload volume in the ventricle) increases to a point. After that point, even with increasing end-diastolic pressure, the CO begins to fall as the ventricle becomes overstretched.
Clinically, preload is estimated by measuring the pulmonary capillary wedge pressure. A catheter is placed in the pulmonary artery (pulmonary artery catheter or PAC), and a small balloon is inflated (see Chapter 37). The balloon wedges in a small pulmonary artery. The pressure from the LA is transmitted backward through the pulmonary circulation and is measured by the pulmonary artery capillary wedge pressure (PCWP). Higher PCWP can correspond to a higher preload. Another way to measure the volumes of the heart (preload) is to use echocardiography (see Chapter 34). Echo machines can use sound waves to image the heart and examine the size and contractile function of the cardiac chambers.
Afterload
Afterload is an indication of the amount of wall tension that is produced by the ventricle. Clinically, afterload cannot be directly measured, and as a surrogate, clinicians think of afterload as the amount of pressure the ventricle must generate to eject blood. Anything that impedes the ability of the heart to eject is referred to as increasing afterload. For example, a constricted aortic valve or narrowed peripheral arteries would make it harder for the heart to eject blood, and thus increase afterload. The LV would compensate by working harder to generate higher pressures to overcome the increased resistance to ejection. Because increasing afterload increases the work of the heart, it also increases myocardial oxygen consumption. Conversely, decreasing afterload makes it easier for the heart to eject blood and decreases myocardial oxygen consumption.
Afterload is an important concept. Many clinical conditions and drugs, including anesthetic agents, can increase or decrease afterload. Clinicians will often administer different medications in order to manipulate afterload. For example, a clinician may administer an arterial vasodilator to decrease afterload in a patient with a failing heart. The goal in this case is to ease the burden on the heart. Figure 4.14 demonstrates the effect of afterload reduction in the panel on the right. The failing heart is depicted in the bottom Frank-Starling curve. When the angiotensin-converting enzyme (ACE) inhibitor is given, the heart changes to the middle curve. Even with a lower end-diastolic pressure, the heart is able to produce a higher CO because of the afterload reduction.
Contractility
Myocardial contractility is the intrinsic ability of the heart to contract. We have already seen how preload and afterload can affect contractile function. Independent of these factors, the heart is able to produce a stronger or weaker contraction depending upon its contractility. In other words, if preload and afterload are kept constant, the “contractility” of the heart can affect the force of contraction. Many drugs change the intracellular amount of myocardial calcium and can increase contractility (e.g., epinephrine, norepinephrine, milrinone). Another word for contractility is “inotropy”; thus, drugs that increase contractility are referred to as positive inotropes. It is also important to understand that increasing contractility increases myocardial oxygen consumption. The left panel of Figure 4.14 demonstrates the effects of giving a positive inotrope to a patient with heart failure. Once the inotrope is given, the patient moves to the middle curve. Now, for the same end-diastolic pressure, the heart is able to produce a greater CO.
Agents that reduce intracellular calcium decrease contractility. Examples of negative inotropes include calcium channel blockers and beta-blockers. Acidosis and hypoxemia also negatively affect the contractility of the heart. One way to quantify contractility is through measurement of the EF with echocardiography. Under resting conditions, normal EF is between 50% and 70%. If afterload and preload are the same, a change in EF is a sensitive indicator of a change in contractility.
Cardiac Reflexes
As discussed above, the heart is innervated by the autonomic nervous system. This innervation is responsible for several reflex changes in cardiovascular function. These reflexes are often feedback loops that help the body maintain normal HR and blood pressure. In the aortic reflex, a rise in blood pressure stimulates baroreceptors (pressure or stretch receptors) in the aortic arch and carotid sinuses. These baroreceptors stimulate the brainstem, causing a reflex parasympathetic outflow through the vagal nerve that slows the HR. Conversely, decreases in blood pressure cause decreases in baroreceptor output, resulting in decreased parasympathetic outflow and a resultant increase in HR in an attempt to restore blood pressure. The vagus nerve is responsible for the effector outflow to the heart. Any stimulus that leads to changes in vagal output can affect the heart. For example, carotid sinus massage (pressure in the neck over the carotid artery) can activate the carotid baroreceptors, resulting in increased vagal output and a slowing HR. In the past, this maneuver was used to intentionally increase vagal outflow to the AV node to attempt to disrupt certain kinds of tachyarrhythmias. The Valsalva maneuver is another method to attempt to increase vagal output. A sustained increase in intrathoracic pressure causes an acute reduction in CO (reduced flow of blood into the heart). The sympathetic system is briefly activated to stimulate the heart and increase CO. When the intrathoracic pressure is released, blood flows rapidly back into the heart, increasing preload and blood pressure. The body responds with parasympathetic outflow, resulting in bradycardia. The sustained increase in intrathoracic pressure can be achieved by asking patients to “bear down” while holding their breath. In intubated patients, the anesthesia provider can deliver a large tidal volume and hold the inspiratory pressure for several extra seconds before releasing it. Other reflexes that result in increased parasympathetic flow include stimulation of ocular structures (pressure on the globe, cornea, eye muscles), stretch of hollow organs in the abdomen, traction on the attachments of the intestines to the abdomen, or even application of ice water to the face.
One example of a reflex that decreases parasympathetic outflow and increases sympathetic outflow is the Bainbridge reflex. This reflex is triggered by high venous blood pressure that stimulates venous stretch receptors in the venae cavae and the RA.
Myocardial Oxygen Supply and Demand
When myocardial cells have insufficient blood supply and begin to dysfunction, it is referred to as myocardial ischemia. When the myocardial cells sustain irreversible damage and die, it is referred to as a myocardial infarction, more commonly known as a heart attack. The sudden onset of either of these conditions is referred to as acute coronary syndrome. Common symptoms include chest pain, shortness of breath, arm pain, and nausea. An ECG can be useful in making a diagnosis. Because the heart muscle is constantly contracting, it requires a continuous supply of blood flow and oxygen to support its metabolic demands. The balance between myocardial oxygen supply and oxygen demand determines whether the oxygen delivered to the heart is sufficient for the work it is doing. This is an important concept in understanding and treating heart disease. The major determinants of myocardial oxygen supply are the blood flow to the heart cells and the oxygen content of the blood.
Blood Oxygen Content
Ventilation and gas exchange in the lungs affect the amount of oxygen in the blood (see Chapter 7). Even with sufficient inspired oxygen concentration and a perfectly functioning respiratory system, very little oxygen dissolves in blood. The body overcomes this limitation with hemoglobin. Hemoglobin is a protein within red blood cells that has a very high affinity for oxygen. Therefore, the total amount of blood oxygen content comes from dissolved oxygen and oxygen bound to hemoglobin. With normal lung function and inspired oxygen, hemoglobin is fully saturated (carries as much oxygen as it can). With hypoxic inspired gas mixtures or abnormal lung function, hemoglobin may not be fully saturated with oxygen. Clinicians frequently monitor the percentage of oxygen saturation of blood with a pulse oximeter, with normal values ranging between 97% and 100%. At this level of saturation and a normal hemoglobin level (14-15 g/dL), there is 20 times as much oxygen bound to hemoglobin as there is dissolved oxygen. Even when fully saturated, if the hemoglobin level falls to less than 6 g/dL, there may be insufficient oxygen to supply the heart. If the oxygen saturation of the hemoglobin is less than 97%, values of higher than 6 g/dL may be required to supply the heart. To summarize, the oxygen content of blood is determined by the inspired oxygen level, the function of the lungs, and the amount of hemoglobin.
Myocardial Blood Flow
Oxygen delivery to the myocardium is determined by the amount of oxygen in the blood and by how much blood flows to the myocardium. As described earlier in this chapter, the coronary arteries deliver blood to the myocardium. The major coronary arteries (RCA, LCA, LCX) branch like a tree to form multiple smaller arteries, arterioles, and eventually capillaries. If a blood vessel supplying the myocardium is obstructed, the myocardium supplied by that artery may become ischemic, or even infarcted. The amount of heart muscle affected is determined by how much myocardium is supplied by that vessel and the presence of any collateral circulation. The closer the obstruction occurs to the root of the tree (the origin of the major artery), the greater the amount of myocardium that will be affected. For example, an obstruction near the origin of the LCA will damage a very large portion of the heart and is often fatal. Even an obstruction in smaller arteries can be important, for example, if they supply a critical portion of the heart such as the myocardial conduction system.
The heart has a backup system to protect against obstructions in arteries. This is accomplished by forming multiple cross-connections between arteries, collateral circulation. This way, if an obstruction occurs, it is possible that another artery is connected to the obstructed artery below the obstruction and can supply blood flow to that part of the heart. Even if coronary arteries are unobstructed, the amount of blood flowing through them will depend upon aortic pressure. Recall that the coronary arteries originate from the proximal aorta. Any condition causing low blood pressure (hypotension) reduces the driving pressure that causes blood to flow through the coronary arteries.
It is important to remember that many drugs can affect the coronary circulation. Vasodilators such as nitrates, including nitroglycerin, can dilate coronary vessels and increase coronary blood flow. Other drugs that are commonly used to raise blood pressure can constrict coronary arteries and reduce coronary blood flow. These drugs are discussed in more detail in the section on cardiovascular pharmacology. Finally, recall the earlier discussion about diastole. The majority of myocardial perfusion occurs during diastole because of compression of arterioles within the myocardium during systole. Therefore, high HRs, which minimize overall diastolic time, can reduce myocardial perfusion.
Myocardial Oxygen Demand
The other side of myocardial oxygen balance is demand. The amount of oxygen the heart consumes is related to the HR (doubling the HR doubles oxygen consumption), the amount of resistance the heart must pump against (afterload), the force and speed with which the heart generates pressure (contractility), and the size of the cardiac chambers. Atria or ventricles that are stretched utilize more oxygen in order to generate the same amount of pressure as normal-sized chambers. Increases in any of these factors increase myocardial oxygen consumption.
Myocardial Infarction
Maintaining the balance between myocardial oxygen demand and supply is crucial to normal cardiac function. One of the most common causes of cardiac dysfunction is an acute interruption of coronary blood flow to the heart. With either aging or disease, lipid material (like cholesterol) can build up within the wall of an artery. The body’s response to the lipid forms what is known as an atherosclerotic plaque. The buildup of plaque within the wall of a coronary vessel can gradually obstruct the vessel. This can gradually lead to ischemia of the region supplied by the vessel. A more dangerous condition occurs when the plaque “ruptures.” The narrowing of the vessel lumen by the plaque causes turbulent blood flow. This turbulence can disrupt the cells covering the plaque and expose material to the blood that initiates clotting of blood. Clot formation by platelets and clotting proteins can rapidly cause complete obstruction of the vessel, leading to ischemia or infarction of myocardial tissue. This often requires emergent treatment. If the region of ischemia or infarction is large enough, it can impair the heart’s ability to pump. In addition, even small regions of ischemic tissue can interfere with the electrical activity of the heart and produce a sudden, life-threatening arrhythmia.
The treatment for myocardial ischemia is to attempt to restore the balance between myocardial oxygen supply and demand. Reducing demand starts with a resting patient and drugs to reduce the HR (e.g., beta-blockers), contractility (e.g., beta-blockers, calcium channel blockers), and afterload (e.g., calcium channel blockers, alpha-blockers, nitrates). Care must be taken in the administration of these drugs because they can also decrease blood pressure and cardiac filling. If oxygen saturation or hemoglobin levels are low, these need to be addressed as well. In many cases, these treatments may not be enough and an attempt will be made to improve coronary blood flow by relieving an obstruction with clot-busting drugs, a percutaneous intervention by a cardiologist, or surgery. Drugs like aspirin (interferes with platelets) or heparin (interferes with clotting proteins) can reduce further clot formation. Other drugs (thrombolytics) directly attack the clot itself. In many cases, testing is necessary to determine which arteries are obstructed. A cardiologist or radiologist can inject dye into the circulation, which can be viewed by fluoroscopy or a computed tomography (CT) scan. Cardiologists can then attempt to expand a narrowing in a coronary artery with a balloon (angioplasty) and then keep it open with an expandable mesh (stent). In other cases, surgery is required to place a graft from one coronary artery to below the obstruction to create an alternative blood supply to the affected area.
Valvular Heart Disease
As described earlier, the valves of the heart perform the important function of preventing backward flow of blood within the heart. Dysfunction of heart valves can occur when they become narrowed (stenotic) or incompetent (allow backward flow of blood). Both conditions can severely impair cardiac function and represent important challenges to the anesthesiologist. In the following section, we briefly discuss some of the more important valvular conditions.
Aortic Stenosis
Aortic stenosis (AS) is one of the most common valvular problems. Rheumatic heart disease or degeneration of congenitally malformed valve leaflets is often the cause of the stenosis. The narrowing of the aortic valve impedes the outflow of blood from the LV. The worse the stenosis, the more the LV must work to eject blood. Early on in the progression of this disease, the LV becomes thick (hypertrophied) and generates very large pressures to overcome the obstruction. The thick ventricle is also stiff and does not relax as well as a normal ventricle. This makes it more difficult to fill the ventricle. The heart becomes very dependent upon preload and, in turn, left atrial contraction to fill the stiff ventricle. Patients without AS can often tolerate loss of atrial contraction (e.g., atrial fibrillation), whereas patients with AS are acutely sensitive to changes in preload or atrial fibrillation. In addition, the hypertrophied heart working against high resistance consumes more oxygen and is susceptible to ischemia. As the stenosis becomes worse, the heart can no longer compensate with ventricular hypertrophy and the patients become more symptomatic. The LV begins to dilate and the increased end-diastolic pressure causes the LA to dilate and often fibrillate. In addition, the increased diastolic pressure within the atrium backs up into the lungs, causing congestion. This congestion and the significant decrease in CO are referred to as congestive heart failure. Ischemia is also common with moderate to severe stenosis. These patients can have difficulty with even minimal exertion. Patients reaching this stage require repair or replacement of the stenotic valve.
Whether presenting for valve surgery or noncardiac surgery, anesthetic management of these patients is complex and the stress of surgery can often prove fatal. Anesthetic goals include maintenance of preload at the right level (not too much, not too little), avoidance of arrhythmias (may require urgent cardioversion), maintenance of contractility (must be able to generate enough pressure to overcome the obstruction), avoidance of tachycardia (need time for ventricular filling), and avoidance of peripheral vasodilation and hypotension (maintain sufficient aortic pressure to maintain coronary blood flow). These patients will often require invasive monitors (arterial line, central venous pressure) and in severe cases transesophageal echocardiography (TEE). One of the biggest concerns is the lack of reserve in these patients. They can rapidly go from maintaining CO to severe congestive heart failure.
Aortic Regurgitation
Rheumatic heart disease, trauma, aortic dissection, and congenital abnormalities are the most common causes of an aortic valve regurgitating blood backward into the LV. This regurgitation occurs during diastole when the aortic valve is supposed to be closed. The regurgitating blood overloads the LV, and it dilates over time. The increased EDVs and pressure can back up into the LA and the lungs. The heart must eject a much larger amount of blood (SV) because a significant portion can flow right back into the heart after ejection due to the incompetent valve. The amount of backflow depends on how leaky the valve has become. In addition to increased SV, the heart attempts to compensate by increasing the HR. This increases the CO and reduces the amount of time the heart spends in diastole, thus reducing the amount of regurgitation. Unlike AS, the heart can compensate for aortic regurgitation for some time. By the time symptoms appear, the disease is usually severe and the heart quite dilated. Anesthetic management includes the following: maintain adequate HR (reduces regurgitation and maintains CO), ensure adequate preload, avoid hypertension, and reduce afterload (reduces back pressure causing regurgitant flow). The anesthesia technician should consult with the anesthesia provider about the need for invasive monitoring (e.g., arterial line, central venous line) and vasoactive infusions (vasodilators, inotropes).
Mitral Stenosis
The most common cause of a stenotic mitral valve is rheumatic heart disease. Much like AS, the LA must work harder against the obstruction to flow into the ventricle. The heart compensates with dilation of the LA and pressures within the LA rise. As the disease progresses, congestion in the lungs and atrial fibrillation are common. The good news about this disease is that left ventricular function is preserved. Anesthetic concerns surround avoiding or treating volume overload and tachyarrhythmias that reduce diastolic time. The heart requires adequate diastolic time to fill the LV when the mitral valve is obstructed. The anesthesia technician should be ready for invasive monitoring. Although venodilators to reduce preload may be required, vasoactive infusion is not needed as often as in other valvular conditions.
Mitral Regurgitation
Mitral regurgitation is common and can be caused by multiple factors. Similar to aortic regurgitation, the regurgitant flow from the LV back into the LA overloads the LA. Pressures within the LA are significantly increased, and the LA can be dramatically dilated. The increased LA pressures commonly cause congestion in the lungs. The reduction in forward flow from the LV to the aorta (much is lost due to the backward flow into the LA) reduces CO. As in aortic regurgitation, the heart requires an adequate preload and a normal atrial rhythm and contraction to fill. Afterload should be slightly reduced to promote forward blood flow. The anesthesia technician should be ready for invasive monitors and vasoactive infusions. TEE may be required in severe cases (see Chapter 42).
Summary
Anesthesiologists deal with patients who have cardiac disease on a regular basis. In addition, surgery and multiple drugs, including anesthetic agents, can severely affect cardiovascular function. Anesthesia technicians should have a working knowledge of cardiovascular anatomy and physiology to better understand the effects of surgery and drugs on the cardiovascular system. This knowledge will also help the technician understand why particular forms of cardiovascular monitoring are utilized. This chapter introduces the anesthesia technician to cardiac anatomy, the circulation of blood through the four cardiac chambers, the innervation of the heart, the myocardial conduction system and cardiac rhythms, the cardiac cycle and the pressures within the heart, the factors that affect cardiac function (preload, afterload, contractility), myocardial oxygen balance, and valvular dysfunction.
Review Questions
1. Which of the following is TRUE about how blood flows through the heart?
A) RA to RV to LV to LA to aorta
B) RV to RA to pulmonary artery to LV to LA to aorta
C) RA to RV to pulmonary artery to LA to LV to aorta
D) RA to LA to pulmonary artery to RV to LV
E) None of the above
Answer: C
Blood flows from the RA into the RV where it is ejected into the pulmonary artery and lungs. Oxygenated blood returns from the lungs into the LA and then into the LV where it is ejected into the aorta.
2. Which of the following veins return blood DIRECTLY into the RA?
A) SVC and IVC
B) Pulmonary veins
C) Femoral vein
D) Subclavian vein
E) Internal jugular vein
Answer: A
The SVC collects blood from the upper extremity through the subclavian vein and from the head and neck from the internal jugular vein. The SVC then drains directly into the superior RA. The blood from the lower extremities drains into the femoral vein and eventually into the IVC. The IVC drains directly into the inferior portion of the RA.
3. Which of the following statements are TRUE about the ventricles?
A) The ventricles have thicker walls than the atria.
B) The LV has much thicker walls than the RV.
C) The right and left ventricles are separated by the interventricular septum.
D) The ventricles receive blood from the atria.
E) All of the above are true.
Answer: E
The ventricles receive blood from the atria after which they pump the blood into a major artery. This pumping action requires a larger pressure, and thus, the ventricles have thicker, more muscular walls than the thin-walled atria. The LV must pump blood into the aorta at very high pressures and is much more muscular than the RV, which pumps blood into the lower-pressure pulmonary circulation.
4. Which of the following is TRUE regarding the myocardial conduction system?
A) The system is composed of specialized nerve cells that conduct impulses.
B) The conduction system conducts blood from the LA into the LV.
C) The conduction system conducts electrical impulse from the autonomic nervous system to different portions of the heart.
D) The conduction system is made up of specialized myocardial muscle cells.
E) None of the above.
Answer: D
The myocardial conduction system is made up of specialized myocardial muscle cells that are responsible for pacing the heart and conducting electrical impulses to synchronize and coordinate the contraction of the atria and ventricles.
5. Which of the following statements are TRUE regarding the coronary circulation?
A) The RCA, the LAD coronary artery, and the LCX are the major “trunk” arteries that supply large areas of the heart.
B) The right and left coronary arteries originate from the aorta.
C) The heart protects itself with cross-connections between arteries (collateral circulation).
D) The majority of myocardial blood flow occurs during diastole.
E) All of the above are true.
Answer: E
All of the above statements are true. The right and left coronary arteries originate from the proximal aorta. The LCA branches into the LAD and circumflex arteries. These are all major arteries, and an obstruction in one of these arteries will damage a very large portion of the heart, possibly resulting in death. The heart protects itself from obstructions in its blood supply by connections between arteries (the collateral circulation). The majority of blood flow through the myocardial arterioles and capillaries occurs during diastole, when the ventricular pressure is lower.
6. The cardiac action potential represents the changes in membrane potential in myocardial muscle cells from the flow of ions across the membrane.
A) True
B) False
Answer: A
True. The myocardial cells maintain a resting membrane potential like a battery. When the membrane is depolarized from an electrical signal, ion channels open, allowing the flow of ions across the membrane further depolarizing the membrane. Eventually, the membrane is repolarized by changing which ion channels are open, allowing the flow of ions.
7. The ECG is useful for monitoring which of the following?
A) The pressure in the central venous circulation
B) Arrhythmias
C) The PCWP
D) Cardiac output
E) None of the above
Answer: B
The ECG is useful for monitoring the rhythm of the heart, the function of the myocardial conduction system, and myocardial ischemia (insufficient oxygen delivery to myocardial cells). The central venous pressure and the PCWP are measured with catheters placed in the central circulation and pulmonary artery, respectively. CO can be measured with a PAC or echocardiography.
8. Which of the following statements is FALSE regarding the cardiac cycle?
A) As blood flows from the atria into the ventricles, the ventricular pressure rises.
B) After the atria contract, the ventricles reach their EDV.
C) When the LV contracts, the pressure rises in the ventricle until it overcomes the aortic pressure and it begins ejecting blood into the aorta.
D) Systole is defined as when the ventricles begin to relax.
E) None of the above.
Answer: D
Systole is defined as when the ventricles are contracting. Diastole is when the ventricles are relaxing. Blood flows from the atria into the ventricle. As the volume increases in the ventricle, the pressure rises. Just before systole begins, the atria contract to add more blood into the ventricles. The volume in the ventricles just before they contract is the EDV and determines the end-diastolic pressure.
9. Which of the following statements are TRUE about the cardiac cycle?
A) Valves within the heart prevent the flow of blood backward.
B) EF is defined as the amount of blood ejected from the heart during diastole.
C) CO is equal to the EF times the HR.
D) Diastole is when the heart is contracting.
E) None of the above.
Answer: A
The valves within the heart are very important as they prevent blood from flowing backward into the atria during ventricular contraction and backward into the ventricles from the pulmonary artery and aorta. The EF is the fraction of the end-diastolic ventricular blood that is ejected during systole. In normal resting patients, around 50% of the blood in the heart at end diastole is ejected during systole. CO is equal to the SV (the amount of blood ejected with each heartbeat during systole) times the HR.
10. Which of the following factors DO NOT affect the force of contraction of the heart?
A) The AV node
B) Preload
C) Contractility
D) Drugs
E) Afterload
Answer: A
The AV node is a portion of the myocardial conduction system that regulates the speed of conduction from the atria to the ventricles. Preload, afterload, and contractility are the major determinants of the force of myocardial contraction and can be explained by using Frank-Starling curves. Drugs can both positively and negatively affect contractility as well as affect preload and afterload.
11. Which of the following are potentially lethal cardiac arrhythmias?
A) Ventricular fibrillation
B) Ventricular tachycardia
C) Sinus bradycardia
D) A and B
E) A and C
Answer: D
Both ventricular fibrillation and ventricular tachycardia may not produce any forward blood flow. Unless the ventricular tachycardia is slow, the patient will die.
12. Which of the following is NOT a determinant of myocardial oxygen supply?
A) Hemoglobin level
B) Afterload
C) Blood oxygen saturation
D) Coronary blood flow
E) Diastolic time
Answer: B
Afterload is a determinant of myocardial oxygen demand (the harder the heart works, the more oxygen it uses). Oxygen is supplied to the blood by binding to hemoglobin in the blood. Low hemoglobin levels can result in insufficient oxygen for the heart. In normal humans, hemoglobin is 97%-100% saturated with blood. If insufficient oxygen is loaded onto hemoglobin in the lungs, the heart may not get enough oxygen. Finally, coronary blood flow during diastole is what brings the oxygen in the blood to the myocardial cells. High systolic pressures compress the coronary arterioles and prevent blood from flowing to the heart muscle during systole.
SUGGESTED READINGS
Agur AMR, Dalley AF. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013: Chapter 1. Thorax.
Barrett K, Barman S, Boitano S, Brooks H. Section V: Cardiovascular physiology. In: Ganong’s Review of Medical Physiology. 24th ed. New York, NY: McGraw-Hill Companies; 2012.
Guyton AC, Hall JE. Textbook of Medical Physiology. 12th ed. Philadelphia, PA: Saunders Elsevier; 2011.
Kaplan J, Reich D, Lake C, Konstadt S. Cardiac Anesthesia. 5th ed. St. Louis, MO: Saunders Elsevier; 2006.
Standring S, ed.. The anatomical basis of clinical practice. In: Gray’s Anatomy. 41th ed. Toronto, Ontario, Canada: Elsevier Churchill Livingstone; 2016: Chapter 57.
Widaier EP, Raff H, Strang KT. Vander’s Human Physiology: The Mechanisms of Body Function. 13th ed. London: McGraw-Hill; 2014: Chapter 12. Cardiovascular Physiology.