CHAPTER 9
Respiratory Pharmacology
Pharmacology
The anesthesia provider must understand the medications required during induction, maintenance, and emergence from anesthesia. This knowledge goes beyond medication dosing and administration, the provider must understand the mechanism of action, physiologic effects, and side effects of these medications as well as their effect on various organ systems. In this chapter, we will focus on the most commonly used medications used specifically for the respiratory system and discuss also the effect that anesthetics have on the respiratory system.
Respiratory pharmacology includes medications that affect the bronchioles and pulmonary blood vessels, medications used to anesthetize the airway, medications that use the airways as a route of administration, and medications that are metabolized or cleared by the lung.
Bronchodilators and Bronchoconstrictors
As discussed in Chapter 7, Respiratory Anatomy and Physiology, the bronchi and bronchioles contain smooth muscle that may constrict causing these small airways to narrow. Some of the most important respiratory medications used in anesthesia relax these muscles and keep airways open. Constriction of bronchial smooth muscle decreases airway diameter, increases resistance to airflow, and increases the work of breathing. Patients may have chronic constriction of their bronchioles (e.g., patients with chronic obstructive pulmonary disease [COPD]) or may develop acute bronchoconstriction (e.g., in acute asthma, or in response to allergic or drug-induced histamine release) (Fig. 9.1). Medications may affect bronchial smooth muscle by directly interacting with beta-adrenergic, cholinergic, or histamine receptors present on bronchiolar smooth muscle. The lung is innervated by the autonomic nervous system, which is covered in detail in Chapter 13. Most of the bronchodilators and bronchoconstrictors described below have their effects through the receptors of the sympathetic and parasympathetic nervous system. Sympathetic stimulation causes dilation of bronchioles, while parasympathetic stimulation causes bronchoconstriction. Medications may cause changes in bronchiolar smooth muscle tone by their effects on the autonomic nervous system. Many medications, both systemic and inhaled, affect the smooth muscle surrounding the bronchi or bronchioles. Medications that cause direct bronchial smooth muscle relaxation are referred to as bronchodilators and include beta-adrenergic receptor agonists (e.g., albuterol, terbutaline, epinephrine), methylxanthines (e.g., theophylline), and histamine receptor antagonists (e.g., diphenhydramine). Medications may cause bronchodilation indirectly by inhibiting neural signals that promote bronchoconstriction. For example, ipratropium prevents the parasympathetic nervous system from sending signals to the bronchial smooth muscles to cause them to constrict.
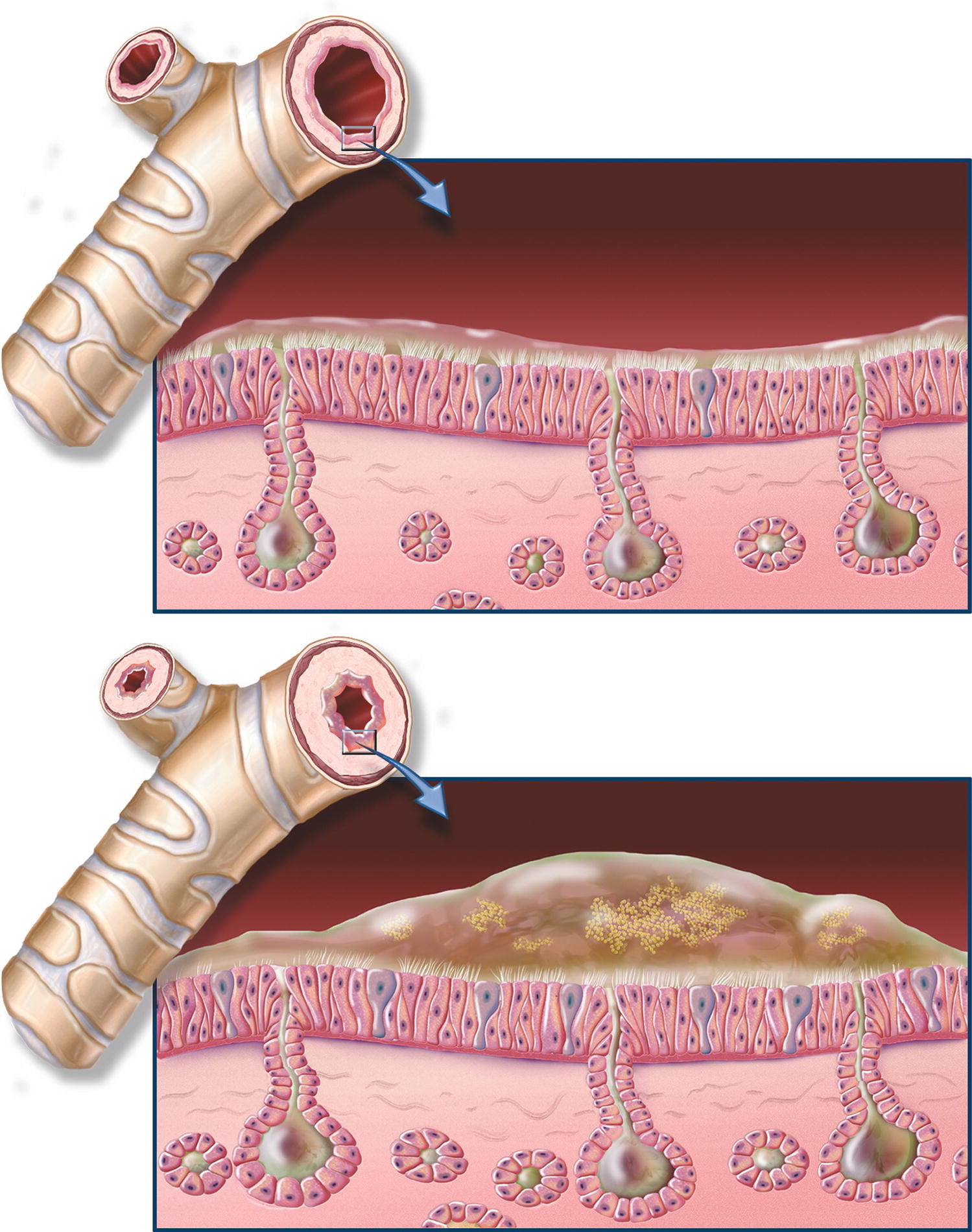
FIGURE 9.1. Chronic bronchitis and normal bronchial anatomy. Chronic obstructive pulmonary disease (COPD) close-up of cross-section of healthy bronchi next to normal bronchial tube; close-up of cross-section of bronchi with chronic bronchitis next to narrowed bronchial tube. (From Chronic Obstructive Pulmonary Disease (COPD) Anatomical Chart. Batimore, MD: Lippincott Williams & Wilkins; 2009, with permission.)
Inhaled short-acting β2 agonists, which interact with sympathetic adrenergic receptors, are the most widely used bronchodilators due to their direct mechanism of antagonism of bronchoconstriction and their safe side effect profile. β2-agonists produce bronchodilation by direct stimulation of β2 receptors in airway smooth muscle. The most commonly encountered short-acting inhaled β2 agonist, albuterol, can be used as needed, driven by symptoms. Antagonism of the effects of pulmonary parasympathetic nervous system can also treat bronchoconstriction. These agents are competitive antagonists of acetylcholine binding to muscarinic cholinergic receptors. The effects of acetylcholine on the respiratory system include not only bronchoconstriction but also increased tracheobronchial secretion and stimulation of the chemoreceptors of the carotid and aortic bodies. Mechanistically, one would expect anticholinergics to reduce airway mucus secretion and reduce mucus clearance, but this is generally not observed. The most commonly used of these “antimuscarinics” is ipratropium bromide, which is topically active and not significantly absorbed from the respiratory or gastrointestinal (GI) tracts. The onset of bronchodilation is relatively slow and is usually maximal 30-60 minutes after inhalation but may persist for 6-8 hours.
Medications may also cause bronchoconstriction, often as an unwanted side effect. Examples of bronchoconstrictors include beta-adrenergic receptor antagonists (e.g., propranolol), medications that stimulate the parasympathetic nervous system (e.g., neostigmine), and medications that cause release of histamine (e.g., morphine, vancomycin).
Medications and Pulmonary Blood Flow
Respiratory pharmacology also encompasses medications that modulate blood flow through the lungs and by doing so may alter ventilation-perfusion matching. Medications like nitroglycerin that decrease systemic blood pressure may decrease blood pressure in the pulmonary artery and decrease perfusion to some alveoli, causing increased dead-space ventilation. Conversely, medications like epinephrine that increase systemic blood pressure can increase pulmonary artery blood pressure and perfusion to alveoli, which can decrease dead-space ventilation. In most circumstances, the administration of a medication and its effect on ventilation-perfusion matching is not the primary goal.
In the previous example, nitroglycerin might have been administered to improve coronary blood flow. The effects on the pulmonary circulation were a side effect. In other cases, the primary goal may be to dilate pulmonary arteries and decrease pulmonary vascular resistance. Numerous medical conditions can increase pulmonary vascular resistance to the point where the right ventricle begins to fail. Severe COPD, primary pulmonary hypertension, severe sarcoidosis, some forms of congenital heart disease, and many varieties of severe lung disease fall into this category. In these conditions, pulmonary vasodilators may be beneficial. Nitrates (e.g., nitroglycerin), calcium channel blockers (e.g., nicardipine, nifedipine), phosphodiesterase inhibitors (e.g., milrinone), nitric oxide, endothelin receptor blockers, and prostacyclin have all been shown to dilate pulmonary arteries. Most of them dilate systemic arteries as well. Only nitric oxide, prostacyclin, and endothelin receptor blockers affect the pulmonary circulation more than the systemic circulation. One method of making a vasodilator more specific to the pulmonary circulation is to administer the drug as an inhalant. Prostacyclin and nitric oxide have been administered in this manner. Interestingly, there are virtually no clinical indications to administer medications to intentionally constrict pulmonary vessels.
Metabolism and Uptake of Medications by the Respiratory System
Some medications are altered or metabolized by enzymes in the lung. For example, methadone, an opioid, is metabolized in the lung, which reduces the concentration available to act as an analgesic. Norepinephrine and dopamine, two medications that raise blood pressure, are also metabolized in the lung. The lung also participates in uptake of medications, which reduces plasma concentration. For instance, the lungs extract propofol and fentanyl from the blood. Finally, respiratory pharmacology may refer to the effect of medication on ventilation: many anesthetic medications alter control of ventilation due to effects on the brain’s ability to monitor and react to disturbances in carbon dioxide levels.
The Respiratory System as a Route of Administration
Medications may be administered into the airways in the form of solids, liquids, or gases. These medications may be delivered directly to the airways by applying the medication topically or may be carried into the lungs by a spontaneous or mechanical inhalation. Because lung tissue is extremely vascular and has a large surface area, it can be an excellent route of administration, even for medications destined for the systemic circulation. Lidocaine, epinephrine, and atropine have all been shown to be absorbed into the systemic circulation from pulmonary administration. Unfortunately, there is a wide variability in absorption and subsequent blood levels; dosing for systemic administration is not precise.
Medications may be delivered to the respiratory system using different technologies. Intravenous medications reach the lung via the bloodstream and can exert effects on the pulmonary vasculature or the smooth muscles of the bronchioles. Conversely, drugs intended for systemic absorption or topical effects can be delivered directly to the large airways in their liquid form. Liquids and solids delivered to the airways may also be aerosolized. Aerosolization means creating very small particles of a medication in liquid or solid form, then delivering them suspended in gas flow so that these small, precisely sized particles are carried by the gas into the smallest airways to act directly on the bronchioles, instead of being deposited entirely in the pharynx and large airways. Some of these medications come in metered-dose inhalers, devices that are primarily designed to aerosolize the medication in measured doses to awake patients; these can be adapted for the ventilator circuit (Fig. 9.2A). Inhaled medications may also be delivered as liquids via a nebulizer or atomizer (Fig. 9.2B). Gas is used to aerosolize the medication for topical application or inhalation (e.g., lidocaine or bronchodilators). Like metered-dose inhalers, nebulizers can be used by an awake patient via mask or mouthpiece or in-line in the ventilator circuit for an intubated patient.
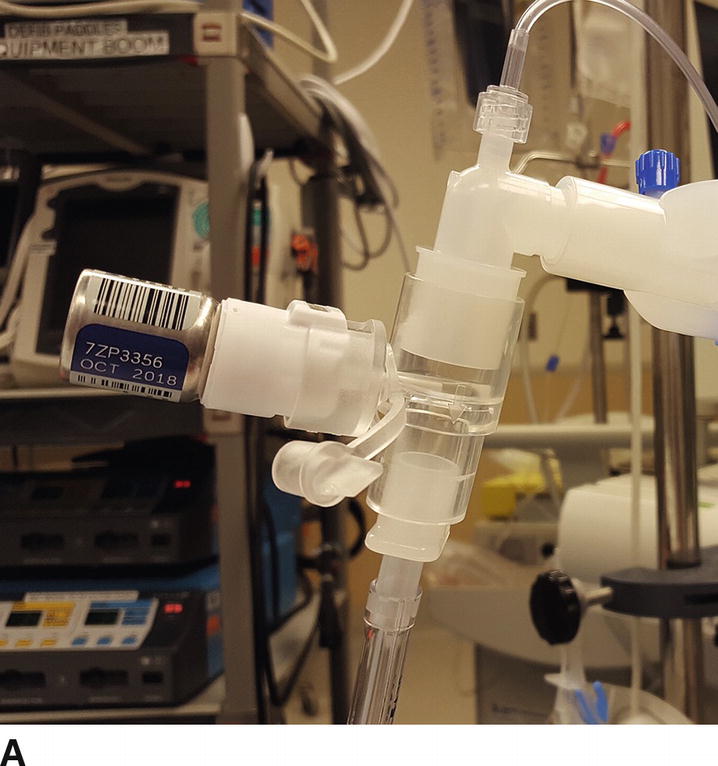
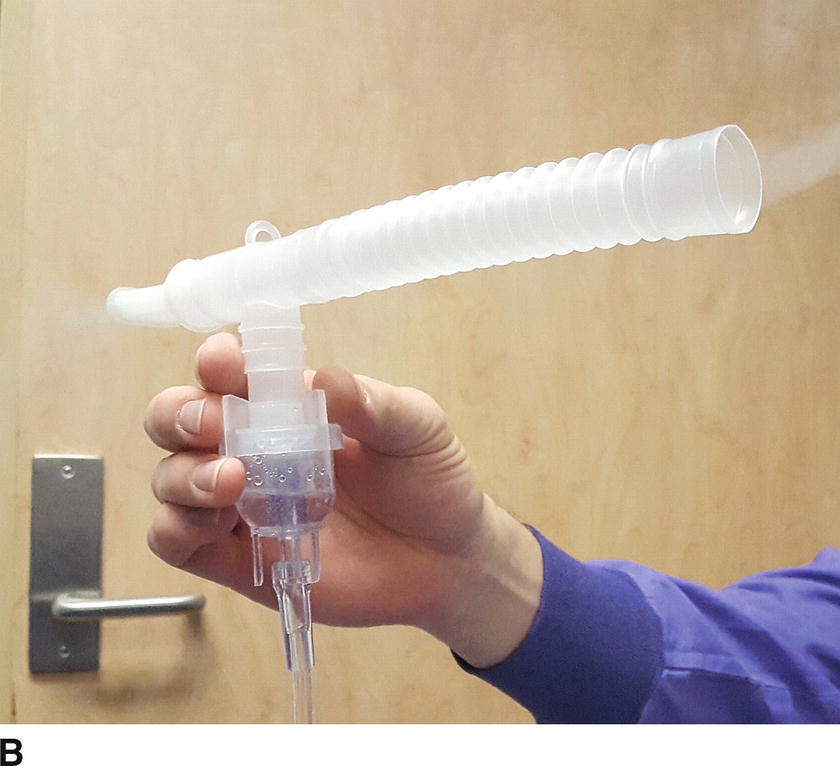
FIGURE 9.2. An inhaled bronchodilator, delivered to the airway by aerosol. A: Metered-dose inhaler containing albuterol. The metal canister contains the drug, dispenses a metered dose, and aerosolizes the liquid into particles of calibrated size. This in-line adapter permits attachment of a metered-dose inhaler into a positive pressure ventilation circuit; an awake patient can use a mouthpiece adapter, which many asthma patients carry (not shown). Only a few drugs are available in a metered-dose inhaler. B: A nebulizer assembly, which can be used for albuterol or other aerosolized airway medications. Gas (either oxygen or air) passes under pressure into a chamber containing liquid, through a valve that creates of droplets of liquid and suspends them into the gas flow. Shown is a mouthpiece adapter: this nebulizer assembly can also be used with a face mask or in-line in the ventilator circuit. The nebulizer permits administration of a variety of drugs via inhalation and provides more reliable and uniform delivery.
Medications may also be administered as gases or vapors delivered via the airways to the bloodstream (e.g., anesthetic agents). The rate of ventilation will determine how quickly the medication enters or leaves the body. Inhaled drugs are almost instantaneously available in the blood, avoiding first-pass metabolism in the liver.
Inhalation is the preferred route of delivery for many drugs that exert their effect directly on the airways, particularly for asthma and COPD. The major advantage of inhalation is the delivery of drug to the airways in doses that are effective with a much lower risk of systemic side effects. This is particularly important with the chronic outpatient use of inhaled corticosteroids, which largely avoids systemic side effects. In addition, drugs such as inhaled bronchodilators have a more rapid onset of action than when taken orally, so that more rapid control of symptoms is possible.
Anesthetic Agents and Pulmonary Function
Several medications used to sedate patients, induce general anesthesia, maintain general anesthesia, or provide analgesia significantly alter respiratory function. We will now review the pulmonary effects of the most commonly used anesthetic agents, which are covered in Chapter 17, Overview of a General Anesthetic.
Propofol, a GABAa agonist, is a rapidly acting intravenous anesthetic agent, which has many advantageous properties, making it useful for both bolus dose for induction of general anesthesia and administration in smaller doses by continuous intravenous infusion for sedation or larger doses for maintenance of general anesthesia. The half-life of propofol has been estimated to be between 2 and 24 hours; however, duration of clinical effect is much shorter due to its rapid redistribution into peripheral tissues. When used for IV sedation, a single dose of propofol typically wears off within minutes. Propofol is one of the most effective agents at reducing systemic vascular resistance and causes hypotension. The pulmonary effect of this vasodilation is an alteration in ventilation-perfusion matching, causing dead-space ventilation. Propofol is a potent bronchodilator and makes gas movement into the lungs easier. Propofol decreases patients’ drive to breathe and can cause respiratory depression or even apnea. Propofol thus requires extreme care because its combination of depression of respiratory drive, altered ventilation-perfusion matching, and hypotension can severely compromise patients.
Ketamine is another intravenous medication used for sedation, induction, or maintenance of general anesthesia. In high doses, ketamine produces anesthesia and analgesia; and in low doses, it acts as an analgesic drug. It has a rapid onset after IV/IM administration (<5 minutes), with a recovery time between 45 and 120 minutes. Ketamine is reported to have minimal effect on the central respiratory drive and is a potent bronchial smooth muscle relaxant, though it increases upper airway secretions. Despite being a direct cardiac depressant, it does not cause significant hypotension as propofol does.
The most commonly used agents to maintain general anesthesia are the volatile anesthetics. All currently used volatile anesthetics are potent bronchodilators. Isoflurane, sevoflurane, and desflurane at concentrations of up to 1 MAC have the same bronchodilating effect in laboratory animals. Although it produces bronchodilation, desflurane is also irritating to the airways and can cause breath holding and coughing. In human models, sevoflurane demonstrated a 15% reduction in airway resistance of patients undergoing elective surgery, while desflurane did not significantly alter resistance.
Inhaled anesthetics remain effective bronchodilators, even in the presence of severe bronchospasm due to anaphylaxis, refractory to β2-antagonists. Volatile anesthetics relax airway smooth muscle by directly depressing smooth muscle contractility; they are not dependent on the autonomic nervous system for their effect.
Dexmedetomidine is an intravenous α2-adrenergic receptor agonist. It decreases pain and produces sedation. Dexmedetomidine is safe from a respiratory point of view, as it has not been shown to cause respiratory depression or prolonged apnea leading to desaturation. Bradycardia and hypotension are common, but both blood pressure and heart rate return slowly to normal when the infusion is turned off. It is not a pulmonary vasodilator and does not worsen ventilation-perfusion matching.
Opioids (e.g., morphine, hydromorphone, and fentanyl) are the most commonly used medications for pain relief in the perioperative period. Opioid receptors are present in respiratory control centers of the central nervous system and in receptors in the airway. Opioids are known to depress both the drive to breathe and the drive to keep the upper airway open; this depression is directly dependent on the dose the patient receives. The respiratory depression caused by opioids is a common cause of postoperative respiratory failure. The effect of opioids on the respiratory system can be reversed with the use of naloxone, a specific opioid receptor antagonist. However, it also reverses the pain-relieving effect of opioids. When given intravenously, it works within 2 minutes; and when given intramuscularly, it works within 5 minutes. Naloxone may precipitate rapid opioid withdrawal symptoms, or it may make postoperative pain difficult to treat. Anesthesia providers will often titrate small doses to avoid these adverse effects if a patient can be ventilated safely. The effects of naloxone can be seen up to 45 minutes after administration, but naloxone has a shorter half-life than many opioids, and patients must be observed closely for return of respiratory depression.
Neuromuscular Blockers
Neuromuscular blockers are commonly used during general anesthesia (see Chapter 14, Neuromuscular Anatomy and Physiology). Muscle relaxation not only helps facilitate surgical exposure but also facilitates endotracheal intubation by relaxing the vocal cords. Neuromuscular blockers are the most common cause of intraoperative anaphylaxis, which can lead to profound hypotension, bronchoconstriction, and cardiovascular collapse. Neuromuscular blockers come in two forms: depolarizing and nondepolarizing. Succinylcholine is the prototypical depolarizing muscle relaxant, which acts by binding to the acetylcholine receptors of the neuromuscular junction and causing rapid contractions of the muscles called fasciculations. Succinylcholine is useful in treating laryngospasm (described in Chapter 18, Principles of Airway Management) and other airway emergencies but has several contraindications. The second category of muscle relaxants is nondepolarizing. There are several drugs in this class of medications, which have been linked to significant histamine release (e.g., atracurium, pancuronium, mivacurium) and can cause bronchospasm. Note that muscle relaxants do not have any effect on the smooth muscle of the bronchial tree, as they are only active on skeletal muscle.
Reversal of nondepolarizing muscle relaxants with the acetylcholinesterase inhibitor neostigmine may cause bronchoconstriction, increased secretions, and bradycardia. However, neostigmine is always combined with an anticholinergic such as glycopyrrolate to mitigate these responses. Approved for use in the United States in December 2015, sugammadex is a cyclodextrin that encapsulates the nondepolarizing muscle relaxants rocuronium and vecuronium rapidly. It does not have the unwanted cholinergic effects of neostigmine and may become the reversal of choice, if its availability and cost allow.
Muscle relaxants have several respiratory effects. The skeletal muscles of the chest wall, diaphragm, pharynx, and larynx are essential for ventilation once the patient is extubated and in recovery. One of the challenges to the anesthesia provider at emergence is timely full reversal of the patient requiring neuromuscular blockade, because of the long-acting nature of most current neuromuscular blockers and the limitations of neostigmine. Patients can appear fully recovered from neuromuscular blockade by all clinical criteria and even by manual train-of-four measurement (see Chapter 32, Neurologic Monitoring), and yet subtle degrees of neuromuscular weakness may still be present in some circumstances. If these go undetected, the respiratory effects of partial neuromuscular blockade can present themselves. Patients may take low-normal tidal volumes prior to extubation but tire easily after extubation, appear unable to complete sentences, become hypoxemic or hypercarbic, become either anxious or obtunded, or require reintubation. More commonly and more subtly, they may aspirate or develop pneumonias because (although they are otherwise strong) only the muscles of the larynx, which protect the airway, are weak, and they may have obstructive apneas in the recovery area because the muscles of the pharynx are weak.
Vasoactive Medications
Chapter 6 (Cardiovascular Pharmacology) described medications that acted on the cardiovascular system. Many of these same medications have effects on the pulmonary blood vessels and on the bronchioles because the heart and lungs share the same autonomic receptors.
Epinephrine, the first experimentally manipulated vasopressor, has a dose-dependent effect on both heart rate and blood pressure. Epinephrine is a nonselective agonist of all adrenergic receptors, including α1, α2, β1, and β2. At low doses, it acts primarily to increase heart rate and stroke volume. As the dose is increased, a profound effect on blood pressure is noted. Epinephrine acts to increase blood flow to the pulmonary vasculature. At doses that increase systemic vascular resistance, it also increases pulmonary vascular resistance. Epinephrine is one of the most effective of bronchodilators and is given intravenously for severe bronchospasm or for the bronchospasm associated with anaphylaxis. Epinephrine can be delivered subcutaneously, intravenously, or instilled directly into the endotracheal tube, making it an ideal bronchodilator/vasopressor in an emergency setting. Epinephrine is occasionally given via nebulization for stridor, the severe constriction and threatened closure of the upper airway.
Norepinephrine, also a vasopressor and a major adrenergic neurotransmitter in humans, has a profound effect on blood pressure and pulmonary vascular resistance at clinically relevant doses. Due to its action on adrenergic receptors, norepinephrine may have some bronchodilatory effects.
Milrinone, a phosphodiesterase inhibitor, acts in the cardiac myocytes to increase calcium concentrations, thereby exerting both an inotropic (contraction) and lusitropic (relaxation) effect independent of the adrenoreceptors. Patients exhibiting right heart dysfunction after cardiac surgery often respond well to intravenous milrinone due to its ability to reduce right ventricular afterload via its effect as a pulmonary arterial vasodilator.
There are many medications in the armamentarium of the anesthesia provider, many of which are commonly used and have effects on the respiratory system. Anesthesia providers are constantly aware of the patient’s ventilation, both how anesthetics impact it and how to improve it with medications. As an anesthesia technician, you should be familiar with and anticipate the effects of medications on the respiratory system, so that you can anticipate how your help may be needed to support providers and patients in optimizing ventilation. The authors have retained several portions of the first edition’s chapter, written by Mark Burno, Casey A. Harper, Matthew Chao-Ben Chia, and M. Christine Stock with permission of the authors we were able to contact.
Review Questions
1. Which of the following medications causes bronchoconstriction?
A) Albuterol
B) Epinephrine
C) Neostigmine
D) Diphenhydramine
E) Theophylline
Answer: C
Medications that cause direct bronchial smooth muscle relaxation are referred to as bronchodilators and include beta-adrenergic receptor agonists (e.g., albuterol, terbutaline, epinephrine), methylxanthines (e.g., theophylline), and histamine receptor antagonists (e.g., diphenhydramine). Neostigmine causes stimulation of the parasympathetic nervous system and thus causes bronchoconstriction.
2. Which of the following pulmonary vasodilators can be delivered via an inhaled route?
A) Nitroglycerin
B) Prostacyclin
C) Milrinone
D) Nifedipine
E) Nicardipine
Answer: B
Only nitric oxide, prostacyclin, and endothelin receptor blockers affect the pulmonary circulation more than the systemic circulation. One method of increasing the specificity of the effect on the pulmonary circulation is to administer the drug as an inhalant. Prostacyclin and nitric oxide have been administered in this manner.
3. Which of the following medication has been shown to be absorbed systemically after pulmonary administration?
A) Epinephrine
B) Norepinephrine
C) Dopamine
D) Vasopressin
E) Phenylephrine
Answer: A
Lidocaine, epinephrine, and atropine have all been shown to be absorbed into the systemic circulation from pulmonary administration. Unfortunately, there is a wide variability in absorption and subsequent blood levels.
4. Which of the following inhaled anesthetics has been associated with lung irritation, coughing and breath holding?
A) Sevoflurane
B) Nitrous oxide
C) Isoflurane
D) Desflurane
E) None of the above
Answer: D
Although it produces dilation of bronchial smooth muscle, desflurane is notable among the volatile anesthetics in that it is irritating to the airways and can cause breath holding and coughing.
5. Which of the following neuromuscular blocker is most likely to cause rapid muscle contractions referred to as fasciculations?
A) Atracurium
B) Mivacurium
C) Rocuronium
D) Vecuronium
E) Succinylcholine
Answer: E
Succinylcholine is the prototypical depolarizing muscle relaxant, which acts by binding to the acetylcholine receptors of the neuromuscular junction and causing rapid contractions of the muscles called fasciculations. All the others listed are nondepolarizing neuromuscular blockers that do not cause fasciculations.
SUGGESTED READINGS
Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, Ortega R. Section IV: Anesthetic agents, adjuvants, and drug interaction. In: Clinical Anesthesia. 7th ed. Philadelphia, PA: Wolters Kluwer Health; 2013.
Boer F. Drug handling by the lungs. Br J Anaesth. 2003; 91(1):50-60.
Butterworth J, Mackey D, Wasnick J, eds. Respiratory physiology and anesthesia. In: Clinical Anesthesiology. 5th ed. New York, NY: McGraw-Hill Medical; 2013:Section II: Clinical Pharmacology.
Deegan RJ. Propofol: a review of the pharmacology and applications of an intravenous anesthetic agent. Am J Med Sci. 1992;304(1):45-49.
Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.