CHAPTER 46
Pacemakers and Implantable Defibrillators
History and General Overview of Pacemakers and Implantable Defibrillators
Four years after production of the first transistor in 1954, C. W. Lillehei, a cardiothoracic surgeon, and Earl Bakken, an electrical technician, developed the first battery-operated system to pace the heart. This was followed just 2 years later by the introduction of the first implantable, battery-powered permanent pacemaker (PM). Further advancements in technology ultimately led to the development of the implantable cardioverter-defibrillator (ICD) in the early 1980s, which was subsequently approved for use by the Food and Drug Administration (FDA) in 1985. PMs and ICDs are collectively called cardiovascular implantable electronic devices (CIEDs) and will be referred to this way throughout this chapter; remember, a “CIED” may be either a PM or an ICD. These devices have become extraordinarily sophisticated and bear only a slight resemblance to their early predecessors. As an anesthesia technician, you will encounter CIEDs frequently in your practice: you should understand what kinds of devices exist, why patients have them, how they can help patients or cause problems, how anesthesia providers plan perioperative care for patients with CIEDs, and your role in their use.
Today, more than 3 million people in the United States have a PM and more than 300,000 have an ICD. People with CIEDs span a large age range, from the very young to the elderly. Adults with CIEDs frequently have coronary artery disease (50%), hypertension (20%), and diabetes (10%). Generally speaking, pacing is indicated for patients with disorders of the sinoatrial (SA) node (i.e., unable to initiate a sinus beat) and/or atrioventricular (AV) node (i.e., unable to properly conduct a sinus impulse). ICDs are implanted for people who have a history of, or are at risk for, malignant ventricular arrhythmias, such as ventricular tachycardia (VT) and ventricular fibrillation (VF). It is important to understand that all modern conventional ICDs can also function as a PM.
CIED technology has continued to evolve. To treat heart failure, in 2001, devices capable of pacing the right and left ventricles simultaneously (i.e., termed biventricular pacing) were FDA approved. Biventricular pacing is also called cardiac resynchronization therapy (CRT); the term CRT-D describes a CRT-capable ICD, while CRT-P describes a CRT-capable pacemaker.
In addition to the aforementioned permanently implanted devices, several temporary modalities are commonly used to pace the heart and are described in more detail below.
Brief Review of the Cardiac Cycle
Understanding the function of cardiac pacemakers and defibrillators requires knowledge of the cardiac cycle. Chapter 4, Cardiovascular Anatomy and Physiology, discusses cardiac electrophysiology and the cardiac cycle in detail. In brief, the heart must coordinate the contractions of the atria and ventricles to pump effectively. The impulse for this coordinated series of contractions originates in the SA node, an area of the heart often referred to as the intrinsic cardiac pacemaker. Located near the lateral aspect of the superior cavoatrial junction, the SA node is responsible for initiating the wave of electrical depolarization that leads to atrial contraction and, ultimately, ventricular filling. Firing of the SA node produces the “P” wave on the electrocardiogram (ECG) tracing. The signal is next propagated to the AV node, located in the base of the right atrium, where it is delayed before being conducted to the ventricles via the bundle of His and the Purkinje fibers (collectively known as the His-Purkinje system). Depolarization and contraction of the ventricles produces the “QRS” complex on the ECG tracing. The “T” wave represents the ventricular repolarization (recovery) period and follows the QRS complex on the ECG tracing. A perturbation at any point along the conducting pathway may disrupt the heart’s coordinated timing and lead to hemodynamic compromise (bradycardia, heart block, hypotension, etc.). In some instances, patients with conduction disease are candidates for either temporary pacing or a CIED.
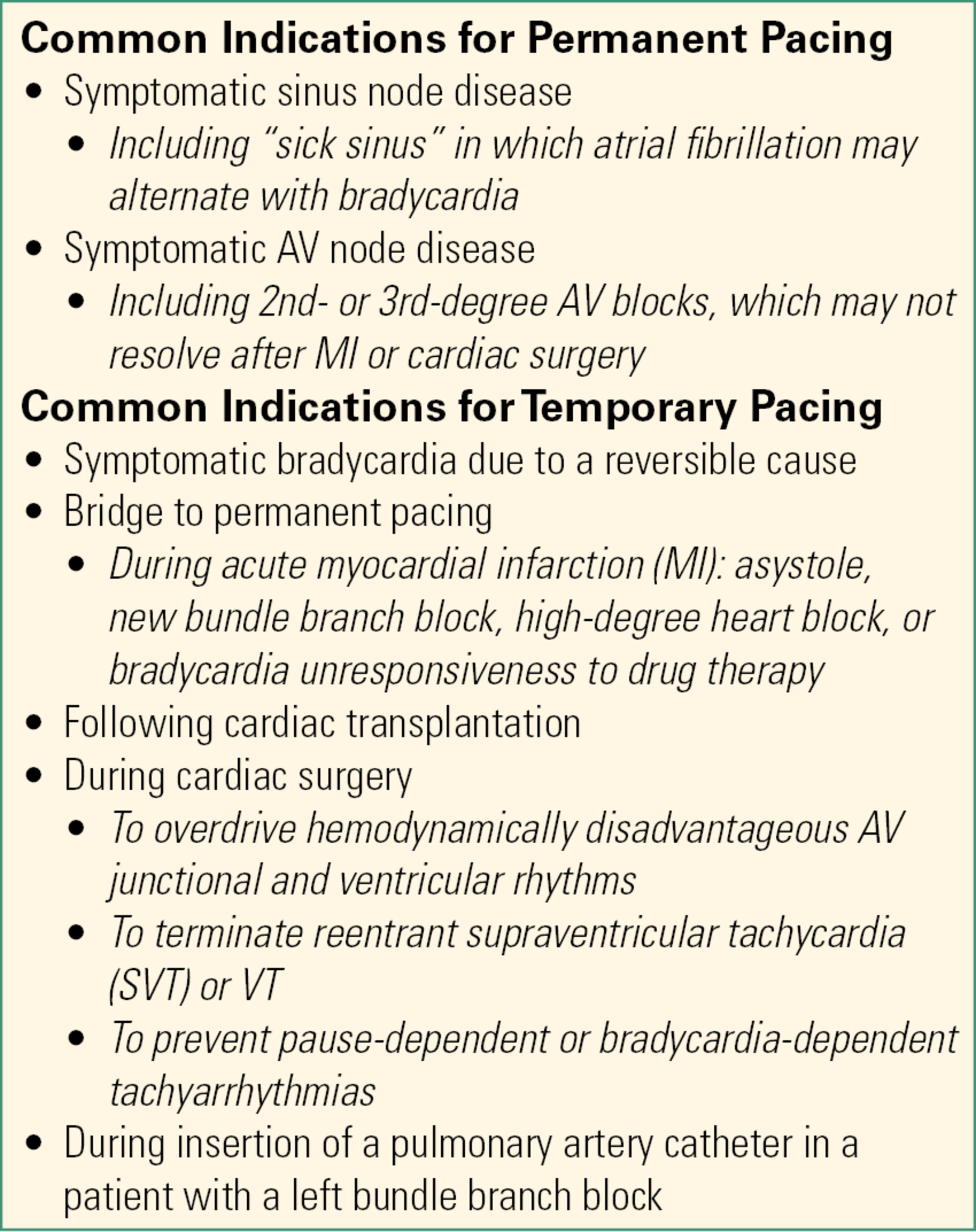
A list of some common indications for ICD placement is provided in Table 46.1. Common indications for both permanent and temporary pacing are provided in Table 46.2.
Table 46.1. Some Indications for Implantable Cardioverter-Defibrillator (ICD) Implantation
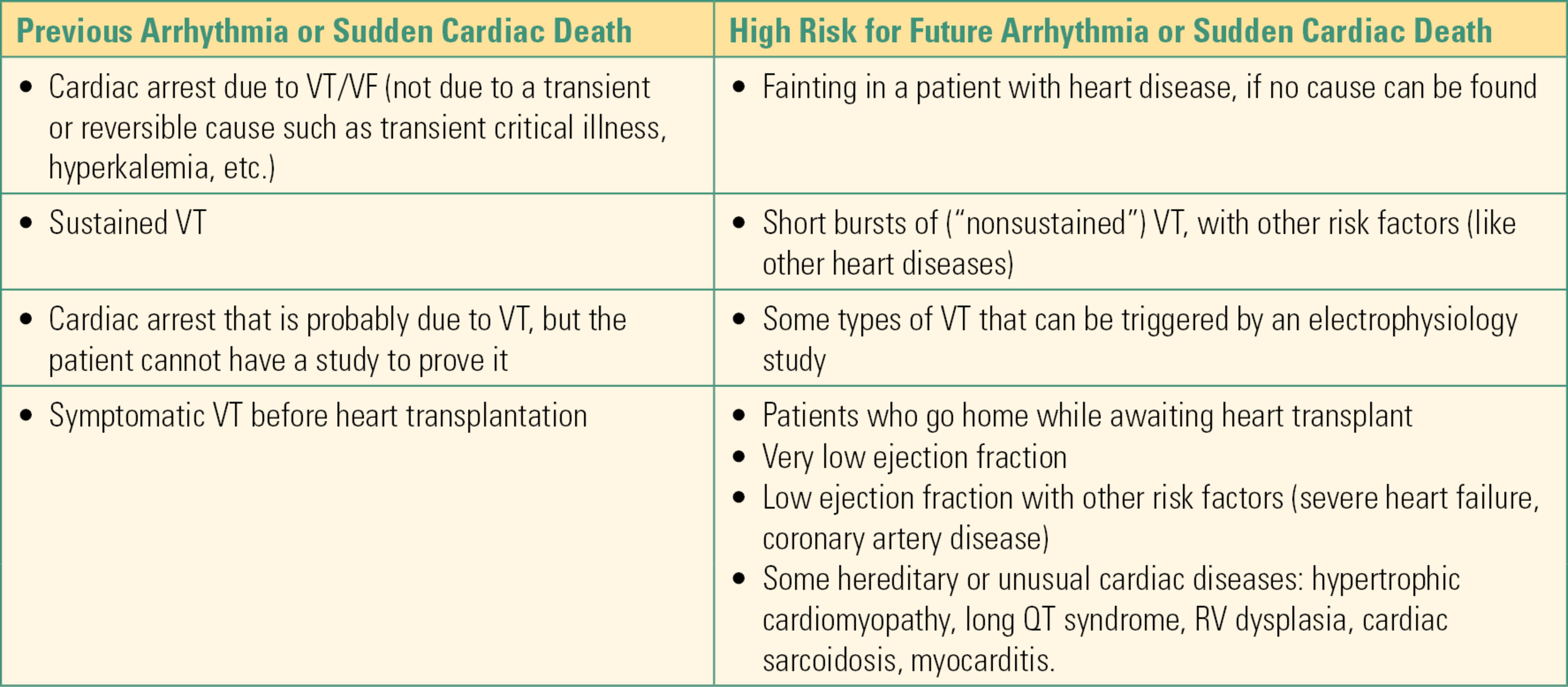
Table 46.2. Indications for Permanent Vs Temporary Pacing
Pacemaker and ICD Insertion and Function
Transvenous (i.e., conventional) CIEDs have two main components, a pulse generator (“can”) and one to three lead(s). The pulse generator is typically implanted in the upper chest area just underneath the clavicle (collar bone). The leads are connected to the pulse generator. They are inserted through a central vein into the heart and then make contact with the heart muscle (myocardium) where they are then fixed in position.
Depending on the number of leads placed and their location, transvenous CIEDs can pace one to three heart chambers. A lead is almost always implanted in the right ventricle, often implanted in the right atrium, and might also be implanted in the coronary sinus to facilitate pacing the left ventricle when indicated (for CRT).
As stated previously, ICDs are generally implanted in patients who have experienced or are at risk of developing a malignant ventricular arrhythmia. The ICD senses VT or VF and responds by delivering high-energy therapy (either antitachycardia pacing [ATP] or shock) to terminate the arrhythmia. In the awake patient, shocks are painful and can cause adverse psychological effects. Since ATP (sometimes called “overdrive pacing”) is effective at terminating VT, ICDs are often programmed to deliver ATP before a shock.
Two relatively new, more minimally invasive CIEDs are now being implanted. A subcutaneous ICD (S-ICD) was FDA approved in 2012. Unlike conventional transvenous ICDs that have lead(s) in the heart, this device has a single-coil electrode tunneled subcutaneously (under the skin). Because this electrode is implanted subcutaneously, the potential for both acute (i.e., pneumothorax, lead dislodgement, perforation, and endocarditis) and long-term (i.e., lead failure, infection) complications are reduced or avoided. However, at present the downside of the S-ICD is its limited functionality; unlike transvenous ICDs, this system is not capable of sophisticated antitachycardia or antibradycardia pacing. A percutaneously implanted leadless pacemaker was just FDA approved in April 2016. Currently, this device can only be used to pace the right ventricle.
Other Pacing Modalities
Although the term pacemaker generally brings to mind a permanently implanted device as described above, several temporary pacing modalities are also available, which can be used during emergencies when long-term pacing is unlikely to be necessary or until a permanent device can be implanted. Temporary pacing can be accomplished by applying pads over the chest and/or back (transcutaneous pacing), by inserting a lead into the heart through a central vein (transvenous pacing), by inserting a probe into the esophagus (esophageal pacing), or for the patient undergoing cardiac surgery by temporarily affixing leads to the external surface of the heart (epicardial pacing). As an anesthesia technician, you will see temporary pacing often, since it is sometimes required during or after surgery; you should develop a basic understanding of how each of these modalities function, why each might be chosen, and how they are utilized.
Transcutaneous Pacing
Transcutaneous pacing involves placing two cutaneous (skin) pads on the chest and/or back that are then connected to an external defibrillator. This modality is relatively easy to employ and is noninvasive; for these reasons, it is part of the advanced cardiac life support (ACLS) algorithm. However, some key disadvantages include the following: (1) it is not always reliable, (2) it is painful and thus not well tolerated in the awake patient, and (3) it can only be used to pace the ventricle (i.e., there is no atrial pacing). This type of pacing is most frequently used to emergently stabilize a patient until a more definitive therapy can be instituted. It is also occasionally used during surgery when there is an urgent, unplanned need for backup pacing. Since this is the most common pacing modality used during a code, it is particularly important for the anesthesia technician or technologist to understand the setup of this system. Please see Chapter 45, Defibrillators, and Chapter 58, Cardiac Arrest, for additional information. Transcutaneous pacing is available as an option on most modern defibrillators.
Temporary Pacing from an External Pulse Generator (Pacemaker)
As an anesthesia technician, many of the pieces of equipment you supply are essential to the life of the anesthetized or critically ill patient, and must not fail; laryngoscopes, positive pressure ventilation devices, and resuscitation drugs come to mind. None, however, is as rapidly life-critical as the external temporary pacemaker. A patient whose heart is not beating properly and is pacemaker dependent may not tolerate even a few second pause in pacing. A patient may have an immediate cardiac arrest because a pacemaker malfunctions, has a battery failure, is disconnected, or is unavailable. Whenever temporary pacing might be needed, it is essential to ensure necessary equipment is readily available and functioning properly. Many external pacemakers are powered by a replaceable battery and have an on/off switch on the front or side panel. The device must be tested (by turning the unit on) prior to use. If the device does not power on, try replacing the battery. Ideally, a second (backup) pulse generator with a known working battery should be immediately available. These devices generally have a low battery indicator.
With the exception of transcutaneous pacing (which is commonly performed using an external defibrillator), temporary pacing systems require the use of an external temporary pacemaker. Temporary transvenous or epicardial pacing is delivered via a temporary pacemaker such as in Figure 46.1. Esophageal pacing requires a temporary pacemaker such as in Figure 46.2. The anesthesia technician or technologist should be familiar with these devices and be able to confirm that they are functioning properly.
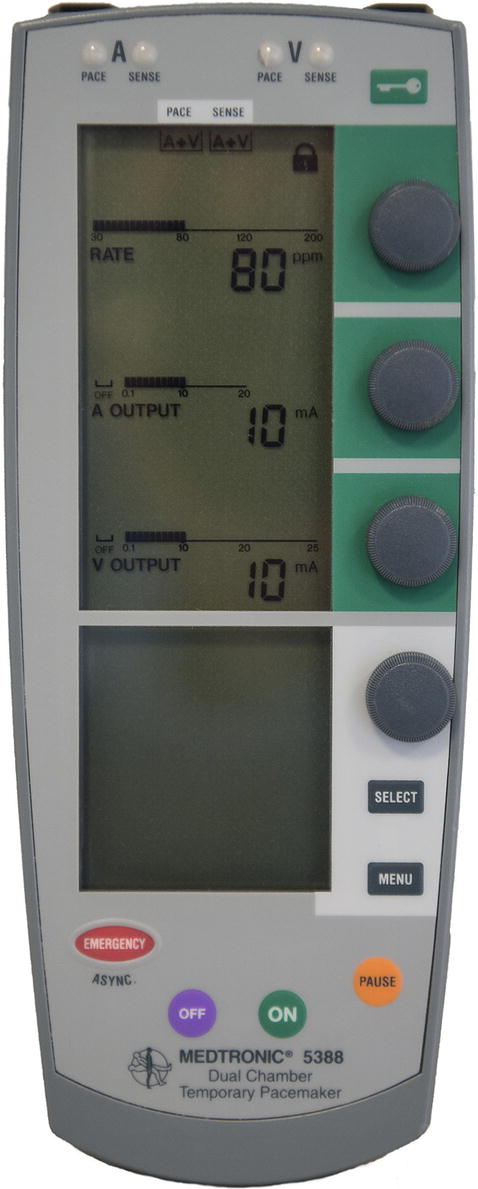

FIGURE 46.1. Examples of two external pacemaker controllers: displays show the heart rate and current output settings for the atrial and ventricular leads. (Reproduced with permission of Medtronic, Inc.)
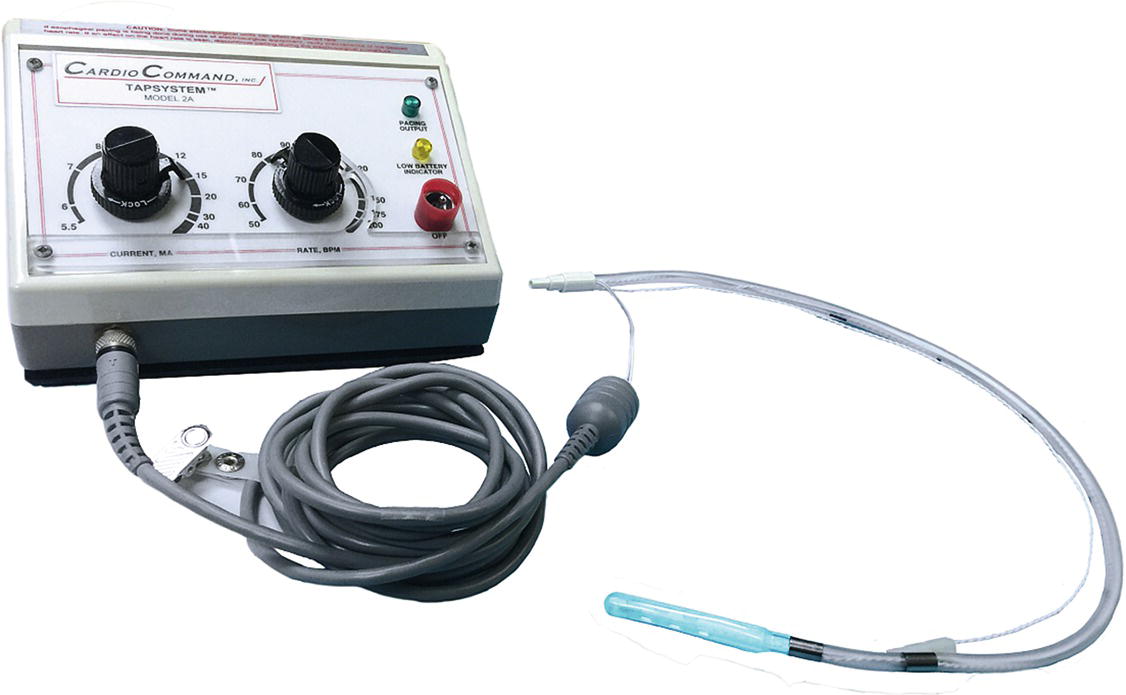
FIGURE 46.2. Esophageal pacemaker. (Courtesy of CardioCommand, Inc.)
All external pacing devices have default settings (i.e., settings that will be active when the device is first powered on). As an example, Table 46.3 provides the default settings for two commonly used Medtronic dual-chamber pacemakers (Models 5388 and 5392), which are both capable of right atrial and ventricular pacing and sensing.
Table 46.3. Default Settings for a Commonly Used Temporary Dual-Chamber Pacemaker (Medtronic, Model 5388)
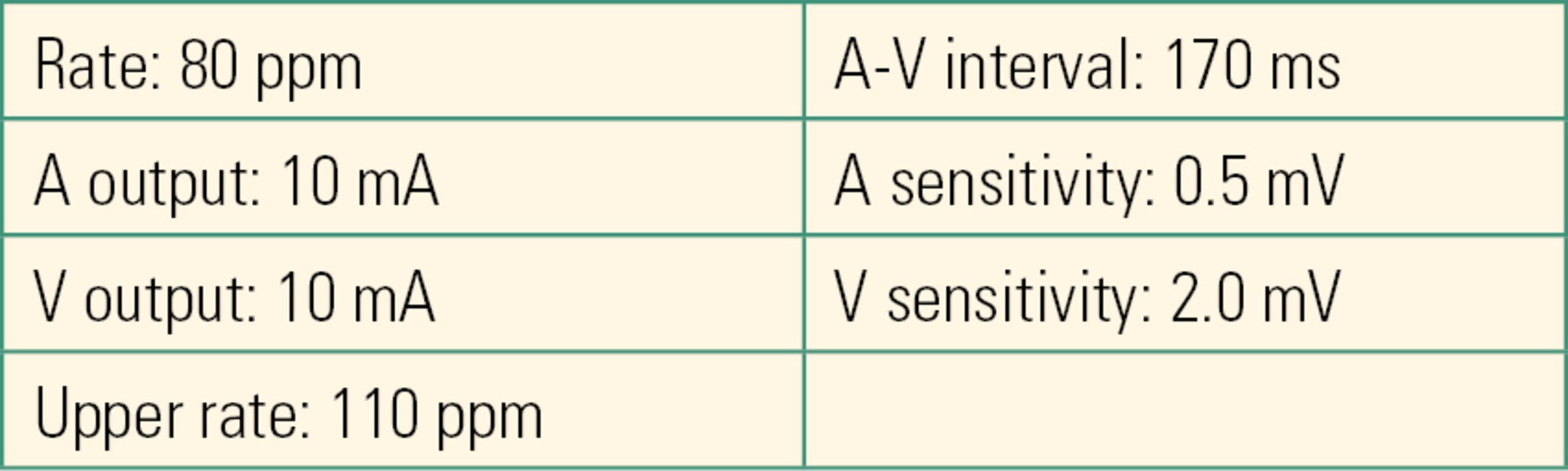
Once the device is turned on, dual-chamber pacing and sensing begin at these default values.
The clinician typically programs the device by altering its settings based upon the number of pacing leads, indication(s) for pacing, and patient’s medical conditions.
Settings that are changed most frequently include “mode” (see below for more details) and “rate” (also called “lower pacing rate” or “lower rate limit”). The sensitivity is sometimes, but less frequently, adjusted. The minimum energy required for the device to consistently initiate a paced heart beat (“threshold”) is noted by the clinician. This is also referred to as the energy required to achieve “capture.” A change in the pacing threshold might indicate impending loss of the device’s ability to support the patient by producing paced heart beats when needed. Pacing-dependent patients (i.e., those with no underlying / intrinsic rhythm) are at highest risk. However, other patients who require temporary pacing support are likely to have significant cardiovascular problems such as heart failure and cardiogenic shock, which cause their rhythm problems; even if not pacemaker dependent, these patients may also not tolerate an interruption in their paced rhythm.
Transvenous Pacing
Temporary transvenous pacing is delivered via a pacing lead placed percutaneously (through the skin, using a needle and guide wire) into the internal jugular, subclavian, or femoral vein (Fig. 46.3). Inserting a transvenous pacing lead is akin to central venous catheter placement (see Chapters 36 (Vascular Access) and 37 (Intravascular Monitoring Equipment). The procedure requires identifying appropriate vascular anatomy, utilizing sterile technique (including sterile prep, full body drape, gown, gloves, etc.), and a commercially available kit. An ultrasound machine is often utilized to facilitate vascular cannulation. While still a temporary modality, temporary transvenous pacing has some distinct advantages over transcutaneous pacing: (1) it is not painful, (2) it is more reliable and stable, and (3) it can be used for a longer duration. Despite these advantages, there are potential complications associated with this modality, and certain precautions must be taken when caring for the patient with a temporary transvenous pacing lead in place. Care must be taken when moving the patient because the lead might be easily dislodged, and some patients may not tolerate even a brief pause in pacing. Another serious complication from disturbance of the position of the wire is myocardial perforation causing pericardial effusion or tamponade. For the patient with a temporary transvenous pacing lead in place, magnetic resonance imaging (MRI) is contraindicated due to the potential for dislodgement, inability to pace the patient, and thermal injury. Patients may also have thrombosis of the vein used for lead placement.
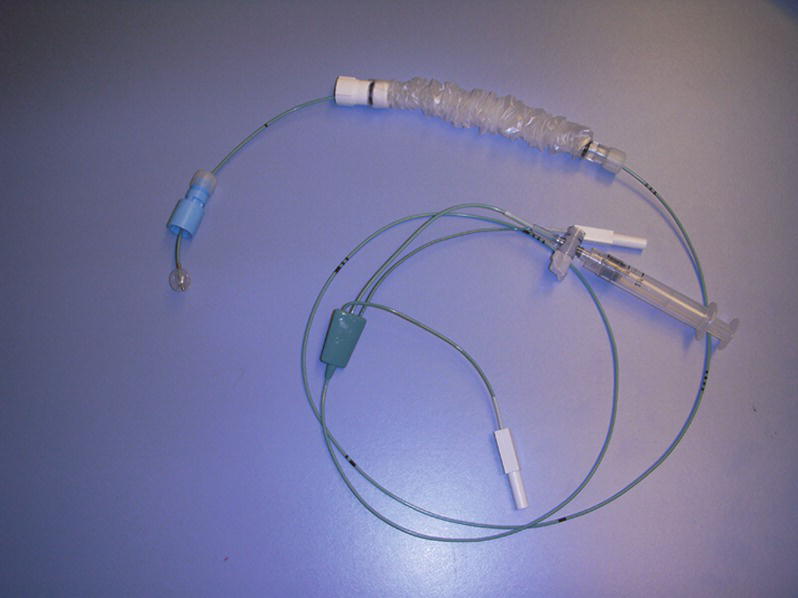
FIGURE 46.3. Balloon-guided transvenous pacing electrode.
Pacing Pulmonary Artery (Swan-Ganz) Catheter
Pacing pulmonary artery catheters (“PAC” or “Swan”) allow atrial, ventricular, or AV sequential pacing in addition to the other sophisticated cardiac monitoring capabilities these devices provide. Depending on the manufacturer, there may be atrial and/or ventricular pacing/sensing electrodes integrated into the catheter (Fig. 46.4), or there may be proximal and distal ports through, which separate atrial and ventricular pacing wires are inserted. The benefit of using a pacing PAC is the ability to also obtain invasive hemodynamic measurements (cardiac output, pulmonary artery pressure, systemic vascular resistance, etc.). As with a standard PAC, a pacing PAC is inserted through an introducer sheath. Once inserted, if pacing is indicated, the catheter must be connected to an external pulse generator. This system is sometimes used in minimally invasive “robotic” cardiac surgery instead of epicardial pacing.
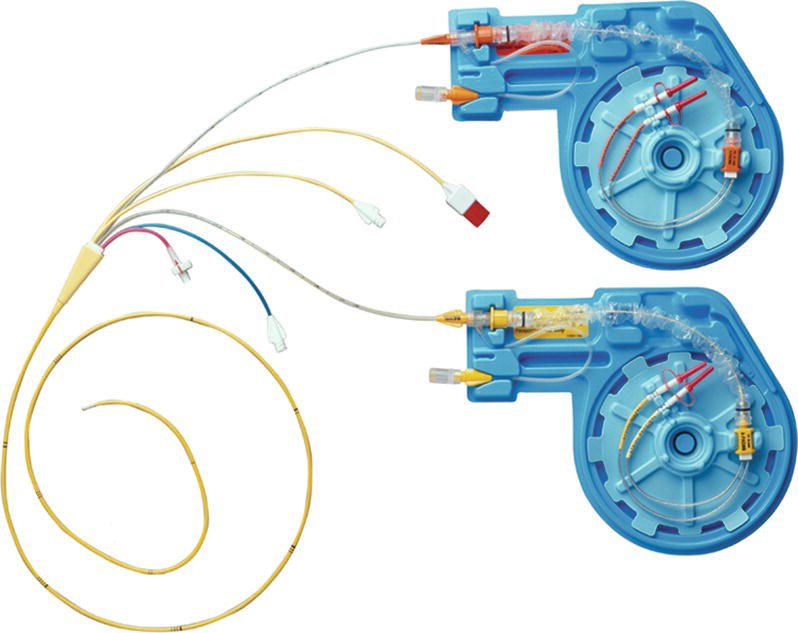
FIGURE 46.4. Pacing Swan-Ganz catheter.
Epicardial Pacing
Epicardial pacing leads are often affixed to the heart by the surgeon during open heart surgery. The leads are sewn onto the surface of the heart muscle and connected to an external pulse generator (Fig. 46.1). Epicardial pacing is frequently indicated in the immediate postcardiopulmonary bypass and postsurgical periods because patients are at risk for conduction abnormalities (such as severe bradycardia and heart block) during these times due to hypothermia, electrolyte imbalances, or the surgical repair itself. Leads may be placed in the right atrium, right ventricle, or both and are tunneled through the chest wall via a small incision. These leads are loosely sutured in place to facilitate eventual removal at the bedside using gentle traction.
Transesophageal Pacing
Transesophageal pacing is achieved via a pacing lead inserted into the esophagus, since the esophagus is in close proximity to the left atrium. After lead insertion (through either the nose or mouth), it is attached to an external pulse generator (Fig. 46.2) and then advanced (with the pulse generator turned on) until capture (i.e., pacing impulses produce paced heart beats) is achieved. Note that a different pulse generator is required than that used for transvenous or epicardial pacing. This modality is exclusively used for atrial pacing; hence, it is indicated for SA node dysfunction; it cannot be used in AV node dysfunction (i.e., heart block), since the ventricle cannot be paced.
Pacemaker Code
Integral to understanding pacemakers and their function is an understanding of the generic pacemaker code. The code (or NBG, “N” from NASPE, “B” from BPEG, and “G” for generic) is used to describe the basic behavior of a pacing device. For a full description of the pacemaker code, see Table 46.4.
Table 46.4. NASPE/BPEG Revised (2002) Generic Pacemaker Code (NBG)
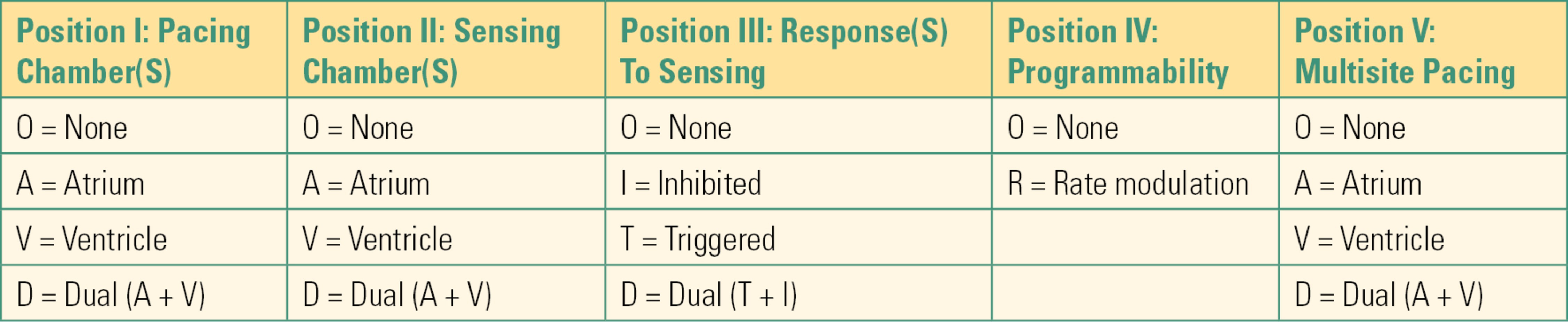
- Position I in the code (the first letter) describes the chamber(s) paced.
- Position II describes the chamber sensed (which chamber(s) the pacemaker is monitoring for intrinsic cardiac activity).
- Position III describes the pacemaker’s response to a sensed event, either inhibiting or triggering the pacemaker’s ability to deliver an impulse. In the inhibited (“I”) mode, if the pacemaker senses intrinsic myocardial activity within the programmed time interval, the pacemaker is inhibited from pacing. In the triggered (“T”) mode, the pacemaker will deliver a ventricular impulse in response to a sensed atrial event, in an effort to preserve AV synchrony (coordinated contraction between the upper and lower cardiac chambers). For example, in DDD pacing (the most common mode), the pacemaker will pace and sense in both chambers and will respond to a sensed atrial event by pacing the ventricle when required.
- Position IV describes programmability. Because of underlying rhythm disturbances, patients are sometimes unable to increase their native heart rate. This renders the patient incapable of meeting metabolic demand (meaning not enough oxygen is delivered to the vital organs). Most modern implantable pacemakers have “smart” sensors, which can be programmed to increase the heart rate with activity to meet increased metabolic needs. Sensors detect different parameters in an attempt to detect exercise, however, and this can lead to intraoperative artifacts. The most common sensor detects body acceleration. Others detect changes in minute ventilation or thoracic impedance. Pacing sensitivities and thresholds can change during surgery due to changes in oxygenation, ventilation, and perceived motion by the device.
- Position V refers to multisite pacing, also known as biventricular pacing or CRT, and is used to help treat heart failure. By pacing both ventricles, the heart’s timing is more coordinated and physiologic. Biventricular devices can be pacemakers (CRT) or pacemakers combined with an ICD (CRTD, “D” for defibrillator).
Common Pacemaker Modes
The pacemaker modes you are most likely to encounter are described below:
Asynchronous: These are “fixed” pacing modes. Because there is no sensing, pacing occurs without regard for the underlying, native heart rate.
- AOO: Asynchronous atrial pacing
- VOO: Asynchronous ventricular pacing
- DOO: Asynchronous dual-chamber pacing
Synchronous: These modes sense the intrinsic rate, causing the pacemaker to inhibit or trigger pacing as appropriate.
- AAI: Atrial sensing and pacing. The pacemaker will be inhibited from pacing in response to a sensed beat.
- VVI: Ventricular pacing and sensing. The pacemaker will be inhibited from pacing in response to a sensed beat. This mode is used when intrinsic AV conduction is lost (i.e., heart block) or when atrial pacing is undesirable or not possible (i.e., atrial tachyarrhythmias such as atrial fibrillation/flutter).
- DDD: This is the most versatile and commonly used mode because it allows for dual-chamber (atrium and ventricle) pacing and sensing. In addition, in this mode, the pacemaker will respond to sensed (native) beats by either inhibiting pacing from occurring or triggering ventricular pacing following atrial sensed events (AV tracking). This mode will preserve AV synchrony and can be used for patients with SA and/or AV node dysfunction. It is not appropriate for patients with atrial tachyarrhythmias such as atrial fibrillation (because in this case AV tracking is detrimental).
Perioperative CIED Management
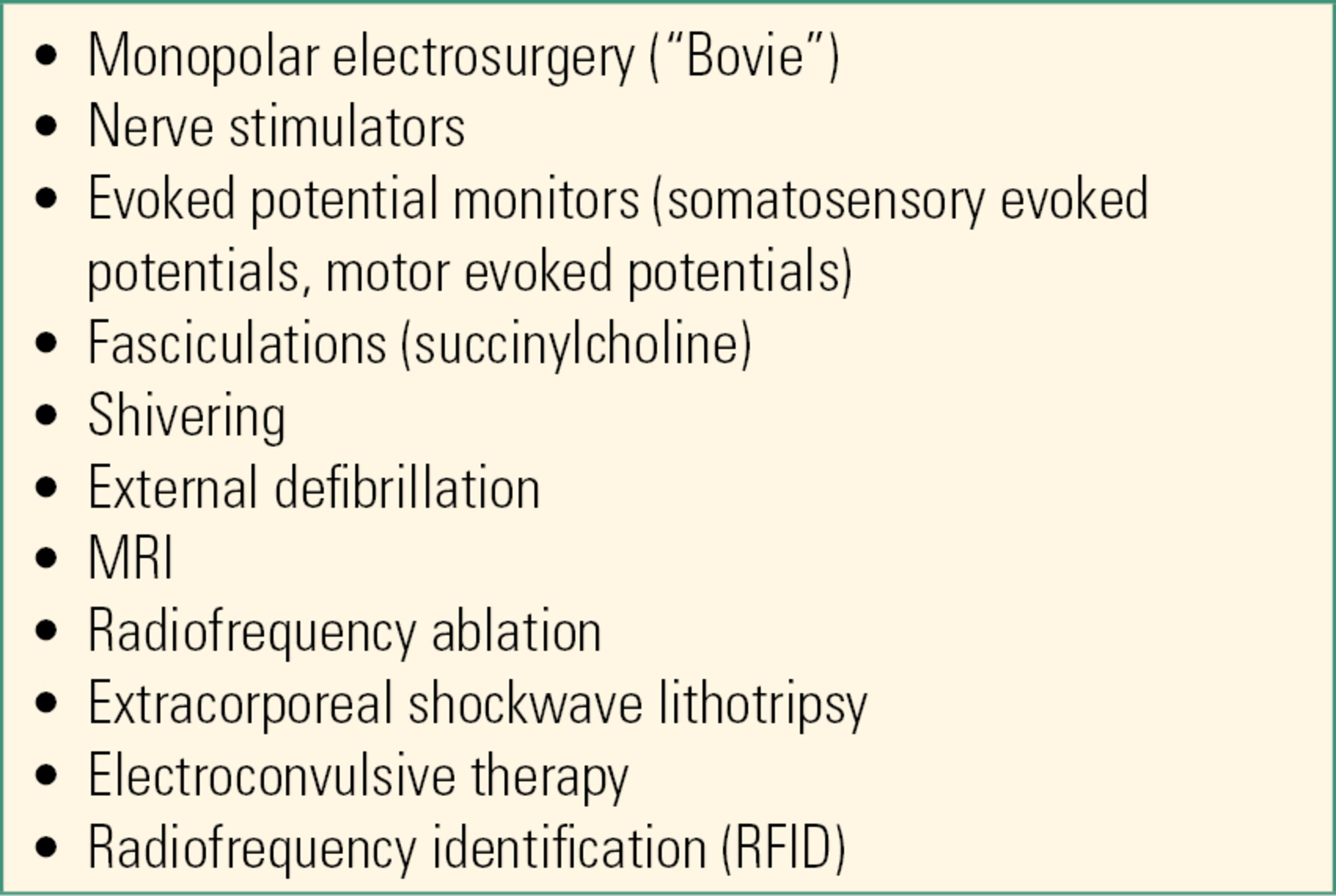
Preoperatively, it is important to identify patients with a CIED. For the CIED patient scheduled for surgery, the operative care team should determine the following information: (1) device type (i.e., PM, ICD, CRT-P, CRT-D, S-ICD), (2) device manufacturer, (3) the patient’s underlying rhythm and whether the patient is pacing-dependent, and (4) date of the last CIED interrogation. If the CIED’s battery is nearing the end of its life, consideration should be given to replacing the pulse generator prior to surgery, especially for the pacing-dependent patient or if intraoperative hemodynamic problems are anticipated. It is also important for the operative care team to determine whether there is a risk of intraoperative electromagnetic interference (EMI), since EMI often adversely affects CIED function. In the operating room, EMI is most often caused by the use of monopolar electrosurgery (i.e., “the Bovie”). Bovie, however, is not the only cause of EMI (Table 46.5). EMI frequently causes “oversensing,” which then can result in pacing inhibition (i.e., pacing impulses are not delivered when needed). EMI also might cause an ICD to deliver unwanted (i.e., inappropriate) high-energy therapy (i.e., a shock or ATP). Other potential sources of EMI encountered during the perioperative period can be found in Table 46.5.
Table 46.5. Factors Causing EMI during the Perioperative Period
According to the most current American Society of Anesthesiologists (ASA) Practice Advisory on the perioperative management of patients with pacemakers and ICDs, it is important for the perioperative care team to ensure that a patient’s CIED is functioning properly prior to surgery. If there is a risk of EMI and the patient is pacing-dependent, the CIED should be temporarily reprogrammed to an asynchronous pacing mode. When EMI is likely in a patient with an ICD, the ICD’s high-voltage therapy (including ability to deliver shocks) should be temporarily programmed “off.” The following algorithm summarizes the steps involved in the preoperative management of CIED patients (Fig. 46.5).
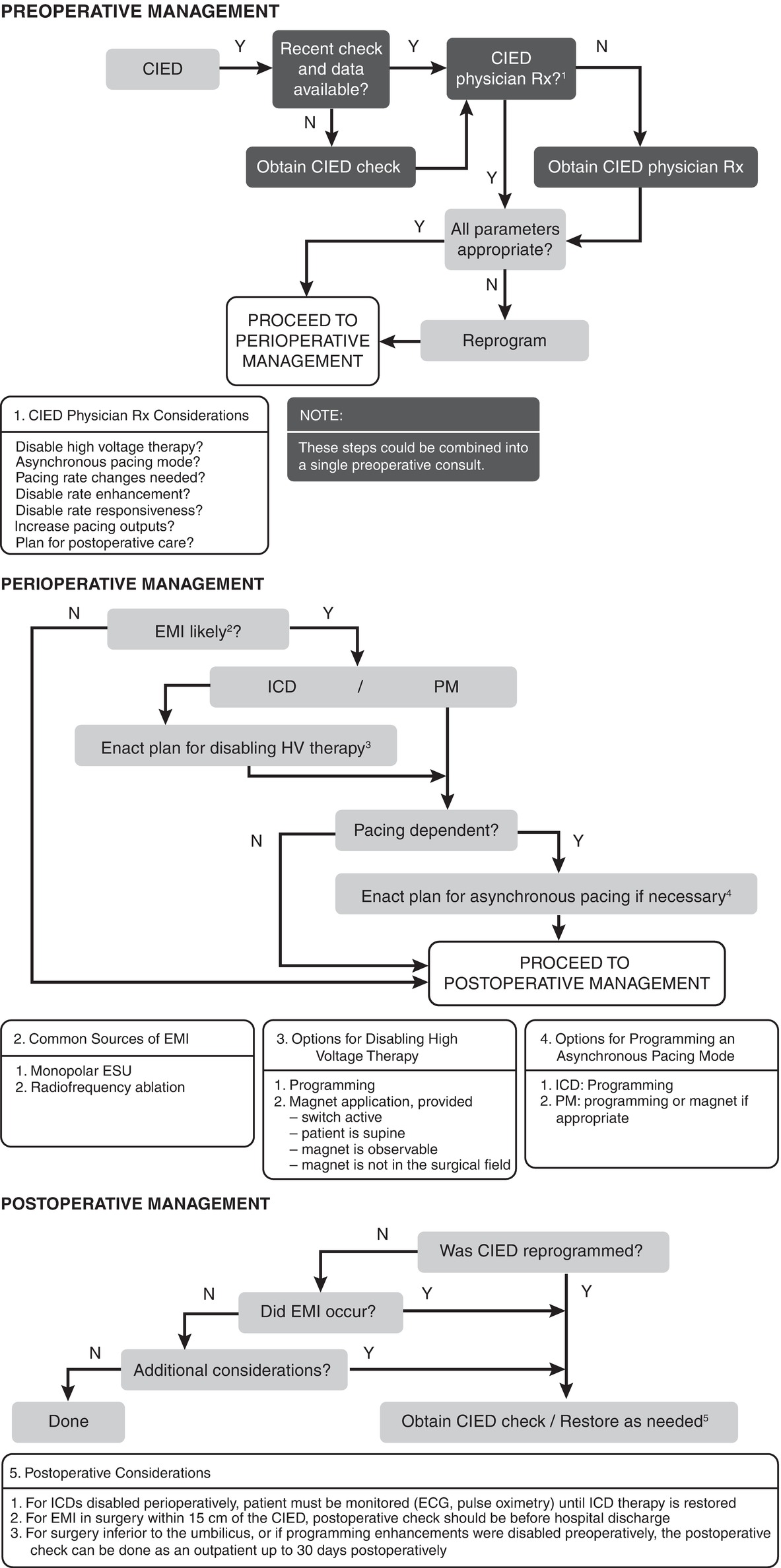
FIGURE 46.5. An algorithm for perioperative management of a patient with a CIED. HV, high voltage. (Reprinted from Fleisher LA. Evidence-Based Practice of Anesthesia. 3rd ed. Philadelphia: Elsevier; 2013:95. Fig. 13.2. Copyright © 2013 Elsevier, with permission.)
Whenever high-voltage ICD therapy is suspended, the patient must be monitored continuously and external pacing and defibrillation equipment must be immediately available. It is important to note that CIED reprogramming will not prevent the system from potential damage from EMI. Although rare, EMI-related system damage has been reported. In addition, asynchronous pacing prevents a CIED from sensing the patient’s underlying rhythm. A rare but serious consequence of asynchronous pacing is “R-on-T”-induced VT/VF (i.e., cardiac arrest). “R-on-T” occurs when a pacing artifact is delivered on a T wave, which is during the refractory or vulnerable period of the cardiac cycle.
A manufacturer-specific programming machine is needed to assess (i.e., interrogate) and alter (i.e., program) the function of CIEDs. There are five main CIED manufacturers: Medtronic, Boston Scientific, St. Jude Medical, Biotronik, and LivaNova (formerly Sorin) (Fig. 46.6). Qualified personnel often use these programming machines before, during, and after surgery to ensure proper CIED function and make programming changes. The anesthesia technician or technologist should be familiar with these programming machines and where they are located.

FIGURE 46.6. Manufacturer-specific programming machines for implantable pacemakers and cardioverter-defibrillators. (Bottom left: Courtesy of Biotronik. Top: Image provided courtesy of Boston Scientific. ©2018 Boston Scientific Corporation or its affiliates. All rights reserved.)
Intraoperative CIED Management
Key intraoperative considerations of particular importance to the anesthesia technician and technologist are:
1. Device management is individualized based on the device, the underlying rhythm of the patient, the planned surgery and its location, and the planned sources of EMI. As the anesthesia technologist, you should anticipate multiple possibilities, but be alert early when a patient with a CIED presents. Some devices may require preoperative or postoperative planning or reprogramming, some may require specialized intraoperative management, and some may require only small changes in intraoperative monitoring, depending on the assessment of the anesthesiologist.
2. The ECG monitor should be programmed to detect rather than filter pacing artifacts (“spikes”).
3. When a procedure involves monopolar electrosurgery, the dispersive electrode (“Bovie pad”) should be positioned so that the current return path is directed away from the pulse generator and leads. Ideally, current should travel from the surgical site to the Bovie pad without ever going near the heart or pacemaker box.
4. Backup pacing and defibrillation equipment (transcutaneous pacing pads and external defibrillator) must be immediately available.
5. A magnet should be available, since a magnet can be used during surgery to cause temporary asynchronous pacing (pacemaker only), or temporarily suspend the high-voltage therapy of an ICD. Note that a magnet will never change the pacing mode of an ICD. The indiscriminate (i.e., “blind”) use of a magnet over a CIED is ill-advised since the effect of applying a magnet can be unreliable and magnets can produce serious adverse outcomes (Fig. 46.7).

FIGURE 46.7. Magnet. (Reproduced with permission of Medtronic, Inc.)
Postoperative CIED Management
Typically any CIED that was reprogrammed preoperatively should have its preoperative settings restored in the immediate postoperative period (i.e., before the patient leaves a monitored setting). In addition, sometimes a complete CIED interrogation is needed after surgery to recheck the device and ensure the settings are still optimal and its components were not damaged during the procedure.
Summary
Anesthesia technicians and technologists will encounter a substantial number of surgical patients with CIEDs or those who require temporary pacing support. Consequently, it is important to have a basic understanding of the indications for pacing and how CIEDs function. Ensuring that all equipment needed for temporary and permanent pacing/defibrillation is readily available and functioning properly is a critical role of the anesthesia technician or technologist.
Acknowledgment
The authors acknowledge Dr. Jeffrey Mako for his contributions to the prior edition of this chapter.
Review Questions
1. When care is being provided for the CIED patient undergoing surgery, the anesthesia technician or technologist should do all of the following EXCEPT:
A) Set up the ECG monitor to detect pacing activity (“spikes”).
B) Ensure that a magnet is readily available.
C) Ensure that temporary pacing and defibrillation equipment is readily available.
D) Offer to assist with magnet placement as part of placing monitors on the patient.
E) Anticipate that an anesthesia provider might choose not to make any changes in a patient’s management as a result of their CIED.
Answer: D
“Blind” placement of a magnet over a CIED is not recommended and may have undesired effects. It is important for the anesthesia technician or technologist to ensure that appropriate monitoring and emergency equipment is immediately available. Not all procedures involve EMI risk to a CIED, and many anesthesiologists may proceed without any changes to a CIED in minor procedures, procedures remote from the heart and pacemaker generator, or procedures performed without EMI.
2. Temporary pacing can be achieved by which of the following modalities?
A) Transcutaneous
B) Transesophageal
C) Transvenous
D) Via a PAC
E) All of the above
Answer: E
All of the listed modalities may be used to provide temporary pacing support.
3. Which of the following modalities can only be used to provide pacing of the atria of the heart and cannot be used to pace the ventricle directly?
A) Transcutaneous.
B) Transesophageal.
C) PA catheter.
D) Epicardial.
E) All of the above can pace either chamber.
Answer: B
The esophageal lead sits directly behind the left atrium and cannot directly pace the ventricle. It can only pace the atrium and relies on intact AV conduction to successfully pace the heart. Transcutaneous leads conduct current to the ventricle, which is both the largest part of the heart, and the closest to the chest wall, by passing a large amount of current through the skin. The PA catheter permits separate wires to be passed into both ventricle and atrium. Epicardial wires are sewn directly onto the surface of the ventricle.
4. After identifying a patient has a CIED, it is important for the clinical care team to determine:
A) Proper CIED function
B) The device manufacturer
C) Date of the last CIED interrogation
D) A, B, and C
E) A and C
Answer: D
The device manufacturer, date of last CIED interrogation, and proper CIED function should always be established prior to elective surgery. Consulting a CIED expert may be required. Device manufacturer information is required in order to understand how the device will respond to a magnet and to obtain the manufacturer-specific reprogramming machine.
5. According to the ASA Practice Advisory on the perioperative management of patients with CIEDs, which of the following statement is FALSE?
A) In the pacing-dependent patient with an ICD, a magnet will not make the pacemaker asynchronous: if EMI is likely, the pacemaker must be reprogrammed.
B) When intraoperative EMI is likely, an ICD’s high-voltage therapy should be temporarily suspended.
C) A magnet should always be applied to a pacemaker or ICD during surgery.
D) Backup pacing and defibrillation equipment should be immediately available.
E) All ICDs are also pacemakers.
Answer: C
If intraoperative EMI is likely, the CIED of a pacing-dependent patient should be programmed to an asynchronous pacing mode, and the high-voltage therapy of an ICD should be suspended. In both instances, backup equipment for defibrillation and pacing should be readily available. Although blind magnet placement is not advised (especially over an ICD), a magnet can sometimes be used in lieu of reprogramming to mitigate the effects of intraoperative EMI. All ICDs have pacemaker functions: even if the patient never requires a pacemaker under normal circumstances, it is possible they could require pacing after a shock.
6. When a patient with a CIED is undergoing a procedure involving monopolar electrosurgery, the dispersive electrode (“Bovie pad”) should:
A) Not be used.
B) Be placed directly over the pulse generator.
C) Be placed so the current return path is directed through the pulse generator and leads.
D) Be placed so the current return path is directed away from the pulse generator and leads.
E) Always be on the lower extremities.
Answer: D
To minimize EMI effects, the dispersive electrode should be placed so that the current return path is diverted away from the pulse generator and leads.
7. Which of the scenarios below is LEAST likely to result in EMI?
A) A patient is given succinylcholine for muscle relaxation.
B) A patient undergoes a radiofrequency ablation procedure to minimize nerve pain.
C) A patient who complains of feeling cold.
D) A patient undergoing a CT scan to obtain images of their lungs.
E) A patient who has been given external defibrillation.
Answer: D
Factors that have been recognized to cause EMI during the perioperative period include Bovies, nerve stimulators, evoked potential monitors, fasciculations (e.g., succinylcholine), shivering (e.g., from being cold), external defibrillation, MIR, radiofrequency ablation, extracorporeal shockwave lithotripsy, electroconvulsive therapy, and radiofrequency identification. Ionizing radiation is not commonly a cause of EMI, and CT is a common alternative to MRI if providers are concerned about pacemaker malfunction.
SUGGESTED READINGS
American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Implantable Electronic Devices. Practice advisory for the perioperative management of patients with cardiac rhythm management devices: pacemakers and implantable cardioverter-defibrillators: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Implantable Electronic Devices. Anesthesiology. 2011; 114(2):247-261.
Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Heart Rhythm. 2011;8:e1-e18.
Rozner MA. Implantable cardiac pulse generators: pacemakers and cardioverter-defibrillators. In: Miller RD, Eriksson LI, Fleisher LA, et al., eds. Chapter 48: Miller’s Anesthesia. 8th ed. Philadelphia, PA: Elsevier; 2015:1460-1486.
Schulman PM, Rozner MA. The perioperative management of implantable pacemakers and cardioverter-defibrillators. Adv Anesth. 2016;34:117-141.