ALETHINOPHIDIA: AFROPHIDIA: HENOPHIDIA
Out of Africa: Old Snakes
The Afrophidia, snakes of African evolutionary origin, contains almost 99 percent of all alethinophidian snakes, the majority belonging in the Caenophidia (shown here). The remaining 185 species are the Henophidia (Heno = old, true; -ophidia = snakes), the Old Snakes. The Henophidia contains three families of fossorial, small-mouthed Southeast Asian snakes—Anomochilidae, Cylindrophiidae, and Uropeltidae, the pipesnakes and shieldtails from Southeast Asia, often grouped as the superfamily Uropeltoidea—and two incertae sedis (of unknown position) families: the Indian Ocean Bolyeriidae and Southeast Asian Xenophidiidae.
The Henophidia also contains two major macrostomatan (big-mouthed) superfamilies. Widely distributed and well known, these clades include the world’s largest snakes capable of taking large vertebrate prey. Superfamily Pythonoidea contains the Afro-Asian-Australasian Pythonidae, the Southeast Asian Xenopeltidae, and the Mexican Loxocemidae. Superfamily Booidea contains the American families Boidae and Charinidae, the African Calabariidae, Afro-Asian Erycidae, Malagasy Sanzinidae, and the Pacific-New Guinea Candoiidae. Afrophidian snakes are distributed worldwide, including back in the Americas where they occur alongside amerophidian snakes. All are nonvenomous and many are constrictors.

ANOMOCHILUS MONTICOLA
KINABALU LESSER PIPESNAKE
DAS, LAKIM, LIM & HUI, 2008
ADULT LENGTH
201/2 in (520 mm)
The Kinabalu Lesser Pipesnake is the largest known species of Anomochilus. Endemic to the Malaysian state of Sabah, Borneo, it is only known from a few specimens collected on Mt. Kinabalu, at elevations around 4,920 ft (1,500 m asl). It is an inhabitant of rainforest leaf litter where it adopts a semi-fossorial existence. Lesser pipesnakes lack a mental groove under the chin, the result being that they have a narrow mouth gape and can only swallow small or slender prey, presumably invertebrates but possibly also elongate vertebrates. Their reproductive strategy is also very poorly known. While the Asian pipesnakes (Cylindrophiidae) and shieldtails (Uropeltidae) are viviparous, it is thought that Anomochilus may be oviparous, the only known gravid female (A. leonardi) containing four soft-shelled eggs.
RELATED SPECIES
The genus and family contain two other localized species, the Malayan Lesser Pipesnake (Anomochilus leonardi) from Peninsular Malaysia and Sabah, Borneo, and the Sumatran Lesser Pipesnake (A. weberi) from Sumatra and Kalimantan, Borneo. This family of three species is considered more primitive than the Asian pipesnakes of genus Cylindrophis, but recent research suggests they are close to the Sri Lankan Pipesnake (C. maculatus).
FAMILY |
Anomochilidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Southeast Asia: Malaysian Borneo (Sabah) |
ELEVATION |
4,760–4,970 ft (1,450–1,515 m) asl |
HABITAT |
Low to medium elevation montane rainforests |
DIET |
Not known, but presumed to comprise small or slender invertebrates, or possibly vertebrates |
REPRODUCTION |
Possibly oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Data Deficient |


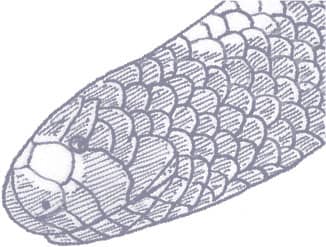
The Kinabalu Lesser Pipesnake is a cylindrical-bodied snake with a short, rounded head, indistinct from the neck, and a short tail. Its scales are smooth and glossy. The snake is iridescent blue-black in color with pale yellow spots on the flanks, larger blotches on the venter, a broken band of pale yellow across the snout, and an orange band around the tail.
CYLINDROPHIS BOULENGERI
BOULENGER’S PIPESNAKE
ROUX, 1911
ADULT LENGTH
13 in (330 mm)
Boulenger’s Pipesnake is only known from approximately a dozen specimens collected in Indonesian West Timor, independent Timor-Leste (East Timor), and the Indonesian islands of Wetar to the northeast and Babar to the east. These small snakes are infrequently encountered, usually in lowland forest or riverine forest, but also in bamboo stands and cultivated areas such as banana groves. They are semi-fossorial to fossorial, specimens also being found under large rocks near rivers. Although there are no natural history records for this species, it probably feeds on blindsnakes and other slender vertebrates, and in common with the much better documented Red-tailed Pipesnake (Cylindrophis ruffus) it is probably viviparous. The true taxonomic status of the populations from different islands has yet to be fully determined.
RELATED SPECIES
Several other poorly known Asian pipesnakes occur on islands in southeastern Indonesia: the Lesser Sunda Pipesnake (Cylindrophis opisthorhodus) on Lombok, Sumbawa, Komodo, and Flores, the Tanahjampea Island Pipesnake (C. isolepis), the Yamdena Island Pipesnake (C. yamdena), and the Aru Island Pipesnake (C. aruensis).
FAMILY |
Cylindrophiidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Southeast Asia: Indonesia and Timor-Leste |
ELEVATION |
395–1,280 ft (120–390 m) asl |
HABITAT |
Lowland forest, riverine forest, banana groves, and bamboo stands |
DIET |
Presumed slender vertebrates such as blindsnakes |
REPRODUCTION |
Viviparous, litter size unknown |
CONSERVATION STATUS |
IUCN not listed |

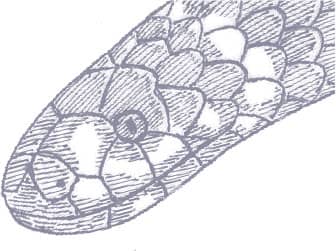
Boulenger’s Pipesnake is smooth-scaled and glossy, with a rounded head and short tail. It is black above and cream on the venter, with a series of irregular upward extensions of this pale pigment onto the flanks, each with an orange spot at its terminus, those of the neck, anterior body, and tail meeting or almost meeting to form orange rings. Cream bars also extend onto the sides of the head from the chin region. The pale venter is black-spotted.
CYLINDROPHIS MACULATUS
SRI LANKAN PIPESNAKE
(LINNAEUS, 1758)
ADULT LENGTH
28 in (715 mm)
The Sri Lankan Pipesnake is a nocturnal and semi-fossorial inhabitant of lowland plains and low montane rainforests, but it may also be found in cultivated areas such as gardens or paddy fields. It is often found under large stones or logs, or in piles of leaf litter. It hunts small snakes such as shieldtails (Uropeltidae), blindsnakes (Asiatyphlopinae), or roughsides (Aspidura), but is also reported to feed on earthworms and insects. It is a live-bearing species, and females may produce up to 15 neonates. When it feels threatened by a potential predator the Sri Lankan Pipesnake will flatten its body, exposing its red spots to best advantage, hide its head, and elevate its tail like a cobra, the tip inverting to expose the contrasting black and white undersides.
RELATED SPECIES
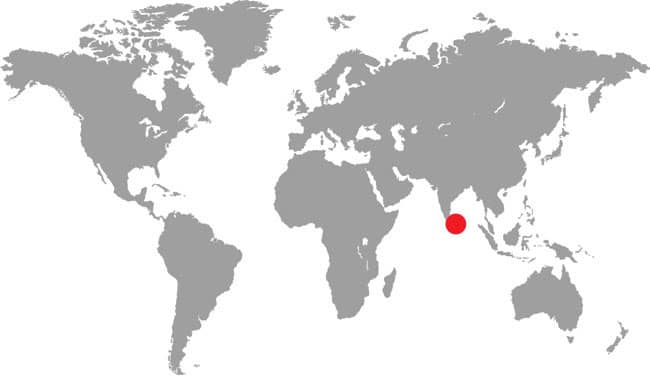
Asian pipesnakes are primarily a Southeast Asian group, occurring from Myanmar to Sulawesi and the Tanimbar and Aru islands in Indonesia, with Cylindrophis maculatus being the only South Asian member of the genus. They occur on Sri Lanka along with members of the related shieldtail family Uropeltidae.
FAMILY |
Cylindrophiidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South Asia: Sri Lanka |
ELEVATION |
0–3,940 ft (0–1,200 m) asl |
HABITAT |
Lowland and low montane forested hills and plains, gardens, and cultivated areas |
DIET |
Small snakes, earthworms, and insects |
REPRODUCTION |
Viviparous, with litters of 1–15 neonates |
CONSERVATION STATUS |
IUCN not listed |


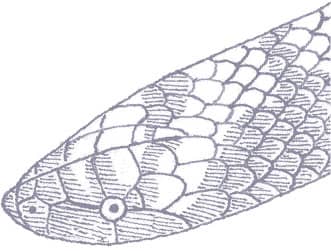
The Sri Lankan Pipesnake is a smooth-scaled snake with a slightly pointed and flattened head, only just distinct from the neck, and a very short tail. Dorsally it is black with a double series of brick red spots that reduce the black pigment to fine cross-bars. Ventrally it is white with black cross-bars, including under the tail.
CYLINDROPHIS RUFFUS
RED-TAILED PIPESNAKE
(LAURENTI, 1768)

ADULT LENGTH
271/2–351/2 in (700–900 mm)
The Red-tailed or Common Asian Pipesnake is a widely distributed and frequently encountered member of the genus Cylindrophis, being recorded from south China and mainland Southeast Asia, to Sumatra, Java, Borneo, Sulawesi, and their satellite archipelagos. It is found in a wide variety of lowland habitats. The prey of the Red-tailed Pipesnake consists of cylindrical vertebrates such as snakes or eels. Pipesnakes are live-bearers, a large Red-tailed Pipesnake producing litters of up to 13 neonates. When threatened the pipesnake will bury its head in its coils and elevate its tail, inverting the tip to expose the red pigment beneath. This may be intended to mimic a head and distract attention from the real head, or to intimidate the predator.
RELATED SPECIES
The genus Cylindrophis contains 14 species, 13 of which occur in Southeast Asia, only the Sri Lankan Pipesnake (C. maculatus) occurs in South Asia. Cylindrophis ruffus occurs in sympatry with the Burmese Pipesnake (C. burmanus) in Myanmar, the Lined Pipesnake (C. lineatus) and Engkari Pipesnake (C. engkariensis) in Sarawak, Borneo, Mirza’s Pipesnake (C. mirzae) on Singapore, Jodi’s Pipesnake (C. jodiae) in Vietnam, the Suboculate Pipesnake (C. subocularis) on Java, and the Sulawesi Black Pipesnake (C. melanotus).
FAMILY |
Cylindrophiidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Southeast Asia: south China, Myanmar, Thailand, Laos, Vietnam, Cambodia, Indonesia, and Malaysia |
ELEVATION |
0–5,500 ft (0–1,675 m) asl |
HABITAT |
Lowland rainforest, swamps, rice paddies, and saltwater lagoons |
DIET |
Snakes and eels |
REPRODUCTION |
Viviparous, with litters of 5–13 neonates |
CONSERVATION STATUS |
IUCN Least Concern |


The Red-tailed Pipesnake is a smooth-scaled, cylindrical snake with a rounded head, only slightly distinct from the neck, very small eyes, and a short tail. It is glossy black or brown above with a series of white or cream transverse bars, which may coalesce dorsally as a complete or broken collar and series of rings. The venter is black and white, except for the underside of the tail, which is bright red, this pigment continuing onto the dorsum of the tail as a red ring.
PLECTRURUS PERROTETII
NILGIRI EARTHSNAKE
DUMÉRIL & BIBRON, 1854

ADULT LENGTH
171/4 in (440 mm)
The Nilgiri Earthsnake is endemic to southwestern India, where it inhabits the Nilgiri Mountains of western Tamil Nadu, Karnataka, and Kerala, in the Western Ghats. It spends the majority of its time underground, only moving onto the surface after heavy rain, which is quite a common occurrence in this part of India. It is a rainforest floor species and reputedly common where it occurs, but it is also found in cultivated fields, especially those manured with horse dung. Such habitats will accumulate large populations of earthworms, the primary prey of uropeltid snakes. At night the Nilgiri Earthsnake is said to move out of the ground and under the horse dung as the air and ground temperature drops. Gustave Samuel Perrotet (1793–1867) was a French explorer and naturalist collector.
RELATED SPECIES
The genus Plectrurus contains two other species, both endemic to south India: the Kerala Earthsnake (P. aureus) and Günther’s Earthsnake (P. guentheri).
FAMILY |
Uropeltidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South Asia: southwestern India |
ELEVATION |
3,490–7,380 ft (1,065–2,250 m) asl |
HABITAT |
Rainforest |
DIET |
Presumed earthworms |
REPRODUCTION |
Viviparous, with litters of 3–6 neonates |
CONSERVATION STATUS |
IUCN not listed |


The Nilgiri Earthsnake is a cylindrical snake with smooth, glossy scales, a narrow, pointed head, small eyes, and a short, blunt tail that tapers and terminates as two small points, one above the other. The general coloration is light or dark brown but the scales may be marked with a red or yellow central streak, and the underside of the tail is often orange.
RHINOPHIS HOMOLEPIS
TREVELYAN’S SHIELDTAIL
HEMPRICH, 1820

ADULT LENGTH
8–113/4 in (200–300 mm)
Trevelyan’s Shieldtail occurs at low elevations in central Sri Lanka, where it inhabits hillside forest, forest edges, paddy fields, gardens, farms, and cattle pens. Often small colonies are found together in agricultural habitats, especially those with soft soil and an abundant earthworm fauna, their primary prey. This species is also common near watercourses and swampy areas. Like all shieldtails for which reproductive strategy is known, this species is a live-bearer. At first glance it is easy to mistake which is the head and which is the tail. The head is the sharp, pointed end of the snake while the bulbous, bright yellow end with a pink center is the tail.
RELATED SPECIES
The genus Rhinophis contains 19 species, 14 endemic to Sri Lanka, four endemic to India, and one (R. oxyrhynchus) occurring in both countries. This genus includes most of the Sri Lankan shieldtails. This species was also known as R. trevelyanus but the older name R. homolepis has priority. Similar species include Drummond-Hay’s Shieldtail (R. drummondhayi), a species considered Near Threatened by the IUCN.
FAMILY |
Uropeltidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South Asia: Sri Lanka |
ELEVATION |
2,460–3,120 ft (750–950 m) asl |
HABITAT |
Forest edges, paddy fields, farms, and gardens |
DIET |
Earthworms |
REPRODUCTION |
Viviparous, with litters of 2–4 neonates |
CONSERVATION STATUS |
IUCN not listed |

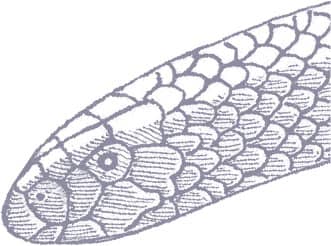
Trevelyan’s Shieldtail is a small, cylindrical snake with a long, pointed head and very small eyes. The tail terminates as a slightly bulbous pink and yellow shield, which contrasts with the glossy blue-black coloration of most of the body. Small yellow triangles or spots are distributed along the flanks and a yellow collar may encircle the neck, while the lower flanks may be white with dark flecks.
UROPELTIS MACROLEPIS
LARGE-SCALED SHIELDTAIL
(PETERS, 1862)
ADULT LENGTH
113/4–123/4 in (300–323 mm)
Shieldtails are also known as rough-tails or thorntails. Some species have gradually tapering tails, but most look as if they have been cut acutely through the tail with a sharp knife and the wound has healed with a rough scab or scar. This is the shield that earns them their common name. They are expert burrowers and the terminal shield effectively plugs the burrow behind them, even collecting dirt on its rough surface. The Large-scaled Shieldtail is found in southern Gujarat and Maharashtra states, western India, where it is reputedly relatively common. It feeds on earthworms and soft-bodied invertebrates and is encountered on the surface in the monsoon season when its burrows are flooded. Shieldtails are inoffensive snakes that have many enemies and only one defense: to burrow.
RELATED SPECIES
Two subspecies are recognized, the Bombay Large-scaled Shieldtail (Uropeltis macrolepis macrolepis) and the Mahabaleshwar Shieldtail (U. m. mahableshwarensis), which occurs farther south in the northern Western Ghats. Uropeltis contains 23 of the 55 known species of shieldtails and earthsnakes. The genus is endemic to south India. Similar species include Elliot’s Shieldtail (U. ellioti).
FAMILY |
Uropeltidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South Asia: western India |
ELEVATION |
0–4,490 ft (0–1,370 m) asl |
HABITAT |
Forests, paddy fields, and agricultural fields |
DIET |
Earthworms and insects |
REPRODUCTION |
Presumed viviparous, litter size unknown |
CONSERVATION STATUS |
IUCN Least Concern |


The Large-scaled Shieldtail is stout-bodied, with smooth, iridescent scales and a rounded head with relatively large eyes. The tail looks as if it has been cut with a knife, the shield being covered in strongly bicarinate and tricarinate scales. Coloration is black above, while the flanks are often marked with yellow spots or bars that may form a longitudinal lateral stripe, extending under the throat, or onto the dorsum.
CASAREA DUSSUMIERI
ROUND ISLAND KEEL-SCALED BOA
(SCHLEGEL, 1837)
ADULT LENGTH
3 ft 3 in–4 ft 2 in (1.0–1.28 m)
The Round Island Keel-scaled Boa belongs to the Bolyeriidae, the primitive split-jawed snakes, with a split maxillary bone that hinges into anterior and posterior halves. It lacks the vestigial pelvic spurs of true boas and lays eggs rather than giving birth to neonates. It hunts sleeping day geckos and skinks at night, killing them by constriction, and shelters under palm fronds or in seabird burrows. Extirpated from Mauritius, possibly by rats, it now only occurs on tiny Round Island 14 miles (22.5 km) to the north. With its highest point only 920 ft (280 m) asl and a total area of just 2/3 sq miles (1.69 sq km), it is a protected area with a unique and highly endemic fauna and flora, but in the past it was devastated by introduced goats and rabbits, causing habitat loss and soil erosion on a large scale.
RELATED SPECIES
Casarea dussumieri has only one close relative, the Round Island Burrowing Boa (Bolyeria multocarinata), a fossorial species that suffered more from the erosion of the island’s soil than did C. dussumieri. This second species has not been seen since 1975 and is officially listed as Extinct by the IUCN. Casarea dussumieri has been saved from extinction by a captive breeding program run by the Jersey Wildlife Conservation Trust, working in collaboration with the Government of Mauritius.
FAMILY |
Bolyeriidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Indian Ocean: Round Island (Mauritius) |
ELEVATION |
920 ft (280 m) asl |
HABITAT |
Dry forest |
DIET |
Lizards |
REPRODUCTION |
Oviparous, with clutches of 3–11 eggs |
CONSERVATION STATUS |
IUCN Endangered, CITES Appendix I |


The Round Island Keel-scaled Boa is a slender snake with small, keeled scales and an angular, pointed head with small eyes and vertically elliptical pupils. Its coloration is gray-brown or orange, with or without a dorsal stripe of darker brown pigment.
XENOPHIDION SCHAEFERI
MALAYAN SPINE-JAWED SNAKE
GÜNTHER & MANTHAY, 1995

ADULT LENGTH
101/4 in (263 mm)
The Malayan Spine-jawed Snake is known only from its holotype, collected in the 42/3 sq mile (12.14 sq km) Templer Park, Selangor, 131/2 miles (22 km) from Kuala Lumpur. Its habitat is lowland primary rainforest. This small snake is nocturnal, terrestrial, and thought to feed on earthworms and insect larvae, although small lizards may also feature in its diet (see below). Its reproductive strategy is unknown, but it is probably oviparous. The name “spine-jawed snake” originates from the presence of a unique elongate palatine process on the maxilla. The IUCN list this species as Data Deficient, but it is perhaps severely endangered, locally extirpated, or even extinct, as its type locality, 11/4 miles (2 km) south of the park entrance, was cleared and planted with bananas in 1990.
RELATED SPECIES
The only other species in the Xenophidiidae, also only known from its holotype, is the Borneo Spine-jawed Snake (Xenophidion acanthognathus) from Mt. Kinabalu at 1,970 ft (600 m) asl in Sabah, Borneo. Slightly larger than its Malayan congener, this species feeds on skinks and lays eggs, but its type locality has also been severely damaged.
FAMILY |
Xenophidiidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Southeast Asia: Peninsular Malaysia |
ELEVATION |
330 ft (100 m) asl |
HABITAT |
Lowland rainforest |
DIET |
Believed to comprise earthworms, and possibly insect larvae |
REPRODUCTION |
Probably oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Data Deficient |
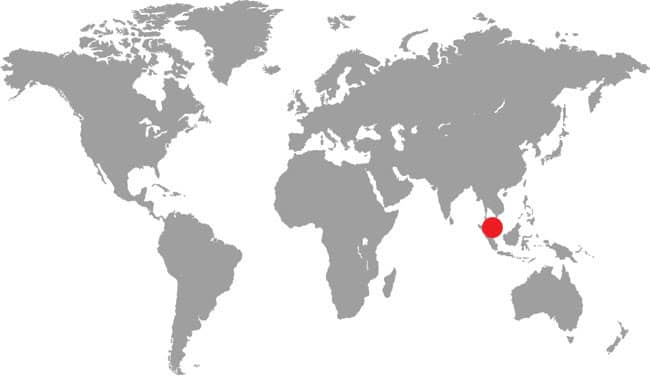

The Malayan Spine-jawed Snake is a small snake with a compressed body, weakly keeled scales, a short tail, and an elongate squarish head with small round-pupilled eyes and large prefrontal scales. Coloration and patterning consist of a dark brown dorsum with a broad grayish-white zigzagging dorsal stripe, itself overlain by a finer vertebral dark brown stripe. The venter is pale gray.
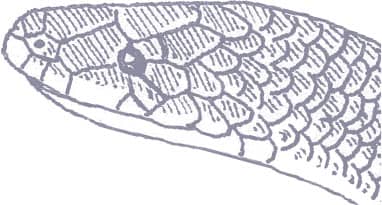
XENOPELTIS UNICOLOR
SUNBEAM SNAKE
REINWARDT, 1827
ADULT LENGTH
2 ft 7 in–3ft 7 in (0.8–1.1 m)
Also known as the Iridescent Earthsnake, the Sunbeam Snake is found throughout mainland Southeast Asia, Sumatra, Java, Borneo, and the Philippines. An inhabitant of lowland rainforest, freshwater swamps, and cultivated habitats such as rice paddies, it is a terrestrial to semi-fossorial species that lives in leaf litter and animal burrows, and may even become fossorial, burrowing into soft mud. Secretive and nocturnal by nature, Sunbeam Snakes are usually only encountered abroad, crossing tracks or roads, after monsoonal rain. The Sunbeam Snake has a rather catholic diet, preying on rodents, birds, lizards, snakes, and frogs, all of which are killed by constriction.
RELATED SPECIES
The only species in the family Xenopeltidae are Xenopeltis unicolor and its close relative the Hainan Sunbeam Snake (X. hainanensis) from China’s Hainan Island. The nearest relatives to the sunbeam snakes are the pythons and the Mexican Burrowing Python (Loxocemus bicolor).
FAMILY |
Xenopeltidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Southeast Asia: Myanmar to southern China, south to Sumatra, Java, and Borneo, and east to the Philippines |
ELEVATION |
0–4,590 ft (0–1,400 m) asl |
HABITAT |
Lowland rainforest, swamps, and rice paddies |
DIET |
Small mammals, birds, lizards, snakes, and frogs |
REPRODUCTION |
Oviparous, with clutches of 3–17 eggs |
CONSERVATION STATUS |
IUCN Least Concern |


The Sunbeam Snake is well named, its glossy-scaled body having the iridescence of a rainbow or sunbeam with every color in the spectrum represented in daylight. It has a cylindrical body, a short tail, and a flattened, compressed head with small eyes. Juveniles often bear a white or yellow collar, but this disappears with increased maturity.
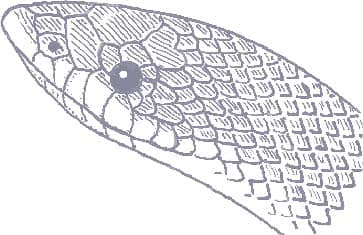
LOXOCEMUS BICOLOR
MEXICAN BURROWING PYTHON
COPE, 1861
ADULT LENGTH
3–5 ft (0.9–1.5 m)
The Mexican Burrowing Python is found in Central America, from central Mexico to Guatemala, Honduras, Nicaragua, and Costa Rica. It is particularly common on the Pacific versant but there are also isolated records from the Atlantic versant. It is nocturnal and semi-fossorial, hiding in leaf litter during the day. The Mexican Burrowing Python feeds on small mammals, lizards, and frogs, which are killed by constriction, but also appears to be a reptile egg specialist. It actively searches out the nests of iguanas and sea turtles, devouring most of the eggs whole by pressing them against its body coils and working its jaws over the egg. During the breeding season males will combat and bite one another for access to available females. Unlike the boas of the Americas, the Mexican Burrowing Python lays eggs.
RELATED SPECIES
Loxocemus bicolor has no close relatives in the Americas, although it shares some characteristics with the boas. The closest relatives of Loxocemus are the Old World pythons and the Sunbeam Snake (Xenopeltis unicolor), hence this New World “python” is placed in its own monotypic family, Loxocemidae.
FAMILY |
Loxocemidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North and Central America: central Mexico to Costa Rica |
ELEVATION |
66–1,970 ft (20–600 m) asl |
HABITAT |
Arid lowland seasonal and deciduous forests, thorn forest, and lower montane forests |
DIET |
Small mammals, lizards, frogs, and reptile eggs |
REPRODUCTION |
Oviparous, with clutches of 4–12 eggs |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |


The Mexican Burrowing Python does not look like a boa or a python. It is uniform gray above and white below, with the change of color strongly demarcated on the flanks. The head is pointed, with small eyes and dorsal scutes. The tail is non-prehensile.
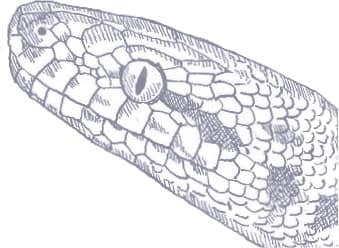
ANTARESIA CHILDRENI
CHILDREN’S PYTHON
(GRAY, 1842)
ADULT LENGTH
2 ft 7 in–3 ft 7 in (0.8–1.1 m)
Children’s Python is a small, inoffensive species. However, it is not so named because it makes a suitable pet species for children, but rather because it was named in honor of the British naturalist John George Children (1777–1852). Children’s Python is found across northern Australia, west of the Great Dividing Range from the Kimberley of Western Australia to Mt. Isa in Queensland, and is usually associated with arid rocky escarpments, where it may be found hunting on roosting bats. It is also frequently encountered in wooded, forested, or grassland habitats, and even in urban areas, such as Darwin, where it hunts small terrestrial mammals, and a wide variety of other small vertebrates, from birds to lizards and frogs.
RELATED SPECIES
The genus Antaresia contains three other species: the Spotted Python (A. maculosa) from Queensland and southern New Guinea, Stimson’s Python (A. stimsoni), which has western and eastern subspecies across Australia, and the Anthill Python (A. perthensis), the smallest python in the world at a maximum length of 24 in (610 mm).
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australia: northern Australia |
ELEVATION |
0–195 ft (0–60 m) asl |
HABITAT |
Arid rocky escarpments, especially around caves, dry savanna woodland, coastal forest, and urban environments |
DIET |
Small mammals, especially bats, and birds, lizards, and frogs |
REPRODUCTION |
Oviparous, with clutches of 7–20 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


Children’s Python is a small, gracile snake with a variable gray-brown to red-brown body, speckled with darker pigment, and a small head, small, light-colored eyes, and a non-prehensile tail.
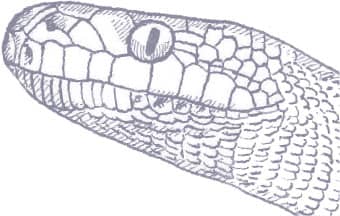
APODORA PAPUANA
PAPUAN PYTHON
(PETERS & DORIA, 1878)
ADULT LENGTH
6 ft 7 in–14 ft 9 in, rarely > 16 ft 5 in (2.0–4.5 m, rarely > 5.0 m)
The Papuan Python is the heaviest and largest of the New Guinea pythons, with rare specimens exceeding 13 ft (4 m), even 16 ft 5 in (5 m) in length, and weighing over 591/2 lb (27 kg). It is a stoutly built snake capable of overpowering and constricting wallabies of its own weight, but it is also a noted predator of reptiles, especially snakes, even taking pythons of similar lengths to itself. Although widely distributed throughout New Guinea and its satellite islands, this large python is one of the least frequently encountered species, being usually associated with heavily forested areas near large rivers, where it may be found crossing roads on wet nights. However, it also occurs in gardens, where it hunts bandicoots and rats, which are killed by constriction.
RELATED SPECIES
This python is sometimes placed in the genus Liasis with its closest relatives, the Australian Olive Python (Liasis olivaceus), the Brown Water Python (L. fuscus), and Macklot’s Water Python (L. mackloti).
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Australasia: New Guinea (mainland and many offshore islands) |
ELEVATION |
0–985 ft (0–300 m) asl |
HABITAT |
Lowland rainforest, monsoon forest, riverine forest, and also gardens |
DIET |
Mammals and reptiles |
REPRODUCTION |
Oviparous, with clutches of up to 22 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Papuan Python is a stout-bodied snake with a broad, chunky head. It is usually two shades of brown, the back being darker than the flanks, with a gray head, every scale demarcated by black interstitial skin. The iris of the eye is also light gray. The python is soft and velvety to the touch, due to the small size of the body scales.
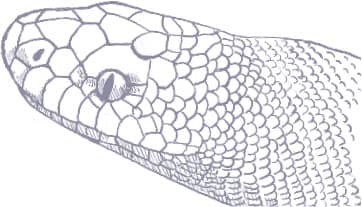
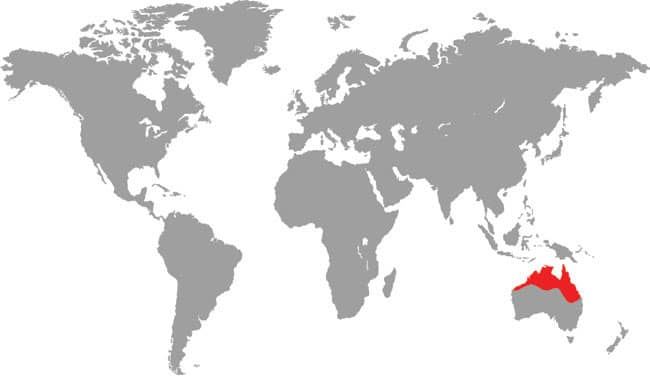
ASPIDITES MELANOCEPHALUS
BLACK-HEADED PYTHON
(KREFFT, 1864)
ADULT LENGTH
5–10 ft (1.5–3.0 m)
The Black-headed Python, and its relative the Woma (see below), are considered the most basal of Australasian pythons. These are the only pythons to lack heat-sensitive pits in their labial and rostral scales, a sign that they do not primarily hunt warm-blooded prey. Although the Black-headed Python will take mammals and birds, it is much more attuned to preying on reptiles, including “goannas” (monitor lizards) and snakes, even venomous species. Across its range, the Black-headed Python may be found in wooded habitats and coastal forests, but it is especially common on rocky escarpments or in semidesert habitats, although it gives way to its congener in true desert. Black-headed Pythons are recorded from Port Headland, Western Australia, to Rockhampton in Queensland.
RELATED SPECIES
The only close relative of Aspidites melanocephalus is the Woma (A. ramsayi), an arid habitat specialist found across the center of Australia. It is banded brown and lacks the black head of A. melanocephalus.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Australasia: northern Australia (Western Australia to Queensland) |
ELEVATION |
50–195 ft (15–60 m) asl |
HABITAT |
Humid coastal forest, dry woodland, and arid rocky desert |
DIET |
Mammals, ground-dwelling birds, and reptiles |
REPRODUCTION |
Oviparous, with clutches of 6–18 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

The Black-headed Python is instantly recognizable from the jet-black head and neck that earn it both its common and scientific names (melano = black, -cephalus = headed). The lips and snout do not bear the thermosensitive pits found in other pythons.
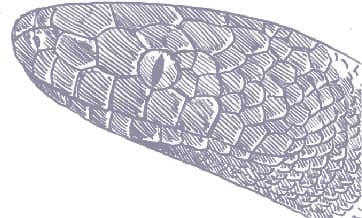
BOTHROCHILUS BOA
BISMARCK RINGED PYTHON
(SCHLEGEL, 1837)
ADULT LENGTH
2 ft 7 in–4 ft 3 in (0.8–1.3 m)
The Bismarck Ringed Python is one of only two pythons found in the Bismarck Archipelago, Papua New Guinea, the other being the much larger Amethystine Python (Simalia amethistina). Although primarily a rainforest species, the Bismarck Python is also commonly encountered in oil-palm and coconut plantations, where it hunts small mammals, lizards such as skinks, and smaller snakes, and it is not above the act of cannibalism. This is probably the most secretive of the New Guinea pythons, being semi-fossorial, often hiding inside coconut husk piles or oil-palm frond rows, and rarely seen in the open, except after heavy rain, when it may be found on roads at night.
RELATED SPECIES
The closest relatives to Bothrochilus boa are the white-lipped pythons of genus Leiopython (shown here). In the past all these species have been included in one or other of these genera. This is one of only two New Guinea python species not found on mainland New Guinea, the other being the Biak Island White-lipped Python (L. biakensis).
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australasia: Bismarck Archipelago (New Britain, New Ireland, and satellite islands) |
ELEVATION |
0–985 ft (0–300 m) asl |
HABITAT |
Primary and secondary forest, and also oil-palm and coconut plantations |
DIET |
Small mammals and reptiles |
REPRODUCTION |
Oviparous, with clutches of up to 9 eggs |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |


The Bismarck Ringed Python is often boldly patterned with orange or brown rings that alternate with black rings. The elongate head is often glossy black, like that of the white-lipped pythons. The patterning is most evident in juvenile specimens and may fade in adults.
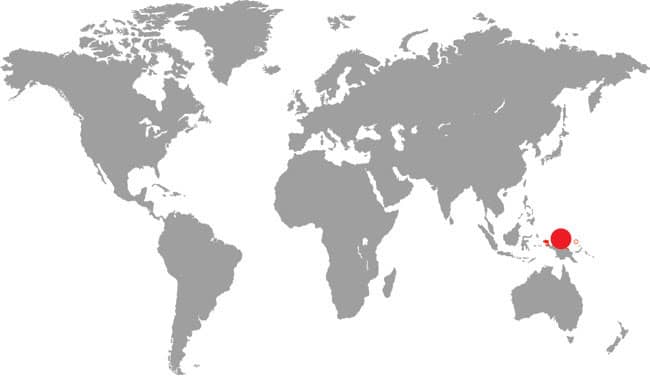
LEIOPYTHON ALBERTISII
NORTHERN WHITE-LIPPED PYTHON
(PETERS & DORIA, 1878)

ADULT LENGTH
3 ft 3 in–5 ft 7 in (1.0–1.7 m)
Often called the D’Albertis Python, having being named in honor of the Italian naturalist Luigi Maria d’Albertis (1841–1901) who collected in New Guinea, the Northern White-lipped Python inhabits the lowland forests and plantations of northern New Guinea. It does not occur in the Bismarck Archipelago to the east, which is inhabited by the related Bismarck Ringed Python (Bothrochilus boa), but there is an isolated island population in the St. Matthias Islands to the north of New Ireland. Where it occurs, this python may be the most frequently encountered python species, especially on roads at night after heavy rain when it is out hunting bandicoots and rats. It is an inoffensive species that rarely bites, but like all pythons is capable of delivering a harmless but bloody bite.
RELATED SPECIES
Leiopython albertisii is closely related to the other white-lipped pythons. These include the Biak Island White-lipped Python (L. biakensis) from the Schouten Islands, west New Guinea; Karimui White-lipped Python (L. fredparkeri) from Simbu, Papua New Guinea; Huon White-lipped Python (L. huonensis) from Morobe, Papua New Guinea, Wau White-lipped Python (L. montanus) from Eastern Highlands Province, and the much larger (8 ft 2 in/2.5 m) Southern White-lipped Python (L. meridionalis) from the southern coastal lowlands. This genus is most closely related to the Bismarck Python (Bothrochilus boa).
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australasia: New Guinea (northern mainland and St. Matthias Archipelago) |
ELEVATION |
0–5,410 ft (0–1,650 m) asl |
HABITAT |
Lowland rainforest, riverine forest, swamps, and plantations |
DIET |
Small mammals |
REPRODUCTION |
Oviparous, with clutches of up to 17 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

The Northern White-lipped Python is usually chestnut brown on the body, merging to yellow-brown on the flanks and white below, with a strongly contrasting glossy black head and distinctive black and white “piano-key” markings along the lips. It is much more stunningly marked than its larger, maroon, southern relative L. meridionalis.
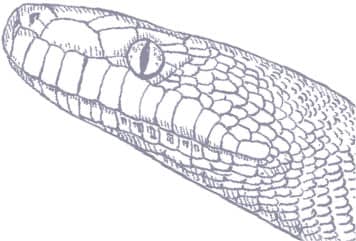
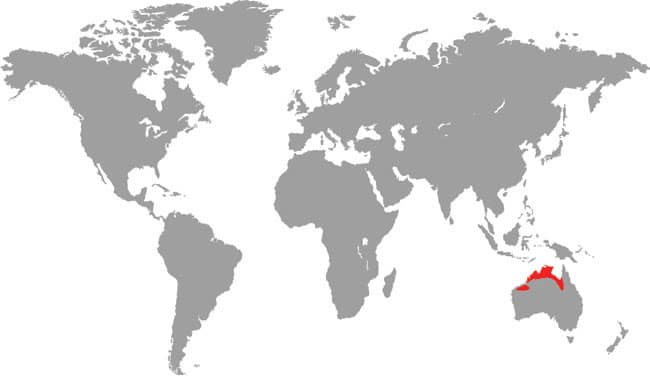
LIASIS MACKLOTI
MACKLOT’S WATER PYTHON
DUMÉRIL & BIBRON, 1844
ADULT LENGTH
3 ft 3 in–7 ft 3 in (1.0–2.2 m)
Macklot’s Water Python, also known as the Freckled Python, is found on the islands of the eastern Lesser Sundas and there is evidence to suggest it may also occur in northwestern Australia, which was very close to the island of Timor during glacial times when sea-levels were lower. Macklot’s Water Pythons are associated with low-lying aquatic habitats such as seasonally flooded grasslands, wet forest, and rice paddies. They may venture into towns using creeks and culverts, and they are most often encountered in the wet season when many of these slow-moving snakes are killed on the roads. Prey probably ranges from rats to birds, and possibly lizards or frogs, but these are understudied pythons in nature. Heinrich Christian Macklot (1799–1832) was a German naturalist who was killed in an uprising in Java.
RELATED SPECIES
Liasis mackloti comprises three subspecies, which some authors treat as species. Two are island endemics, the 5 ft (1.5 m) Sawu Python (L. m. savuensis) and the 7 ft 3 in (2.2 m) Wetar Python (L. m. dunni). The islands between Sawu and Wetar are inhabited by the 5 ft 2 in (1.6 m) nominate form. The Brown Water Python (L. fuscus) of northern Australia and southern New Guinea is its closest relative. Northwestern Australian water pythons may also belong to L. mackloti.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Indo-Australia: Indonesia (eastern Lesser Sunda Islands), Timor-Leste, and possibly northwestern Australia |
ELEVATION |
0–1,480 ft (0–450 m) asl |
HABITAT |
Lowland forest and wetlands, creeks, plantations, and rice paddies |
DIET |
Small mammals, birds and their eggs, and possibly lizards, frogs, or fish |
REPRODUCTION |
Oviparous, with clutches of 8–20 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

Macklot’s Water Python is gray-brown with a scattering of darker scales, presenting a freckled appearance. It is off-white or yellowish below and on the lips. The head is moderately elongate and the iris of the eye may contrast with the body color, especially so in white-eyed Sawu Pythons.
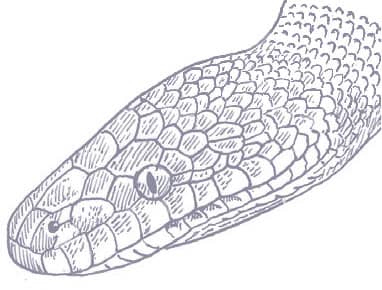
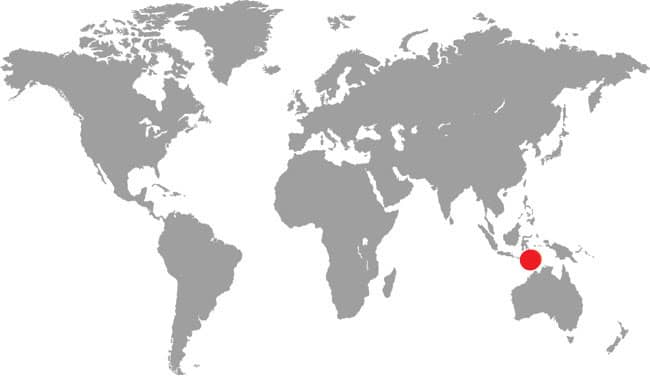
LIASIS OLIVACEUS
AUSTRALIAN OLIVE PYTHON
GRAY, 1842
ADULT LENGTH
10 ft–21 ft 3 in (3.0–6.5 m)
The Australian Olive Python is Australia’s second largest python, after the Scrub Python (Morelia kinghorni) of Cape York Peninsula, Queensland. It is a powerful constrictor, capable of subduing and swallowing large mammals such as wallabies, but it also preys on birds, lizards such as frilled lizards and goannas (monitor lizards), and snakes, including other pythons. Australian Olive Pythons are particularly common in rocky habitats, especially those with permanent water sources, where it lingers in ambush for animals coming to drink, but it may also be encountered in dry tropical forests and wooded savannas. There are two main ranges for this species, with a northern population from the Kimberley of Western Australia, through the Northern Territory to western Cape York Peninsula, Queensland, and a separate, more southern population in the Pilbara of Western Australia.
RELATED SPECIES
Liasis olivaceus is related to the water pythons (L. fuscus and L. mackloti) and the Papuan Python (Apodora papuana). There are two subspecies, the nominate Northern Olive Python and the isolated Western Olive Python (L. o. barroni) in the Pilbara, which may be a full species.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Australia: northern Australia (Western Australia to Queensland) |
ELEVATION |
0–2,130 ft (0–650 m) asl |
HABITAT |
Lowland rainforest, riverine forest, coastal lowlands, and rocky outcrops with permanent water |
DIET |
Mammals, birds, and reptiles |
REPRODUCTION |
Oviparous, with clutches of 7–31 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

The Australian Olive Python is uniform brown above and white or yellow below. It has a robust body, and an elongate head with large scutes rather than granular scales on the dorsum.
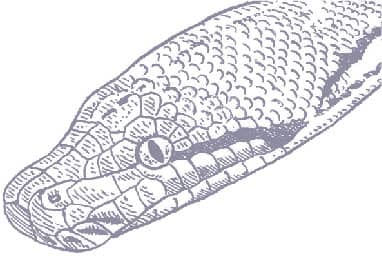
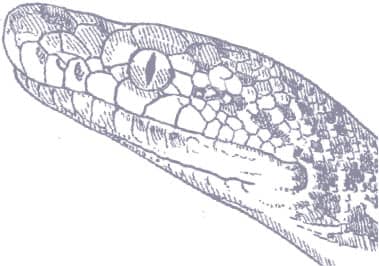
MALAYOPYTHON RETICULATUS
RETICULATED PYTHON
(SCHNEIDER, 1801)
ADULT LENGTH
Male
20–23 ft (6.0–7.0 m)
Female
20–33 ft (6.0–10 m)
The Reticulated Python is the longest snake species alive today, outsized females achieving 33 ft (10 m) and 165 lb (75 kg), although giant specimens are now rare. Primarily a mammal-predator, it has highly visible, heat-sensitive pits along the lips and on the tip of the snout, to enable it to hunt warm-blooded prey. It can take large prey, such as an adult female sun bear in Borneo, and humans are also occasionally taken. Young pythons sleep on branches over rivers, choosing sites above deep pools into which they plunge to escape predators. The female lays up to 100 leathery-shelled eggs that adhere together as a pile. She incubates them for 65–105 days and defends her nest against predation by egg-thieves. Annually, thousands of Reticulated Pythons are collected and slaughtered for their skins and meat.
RELATED SPECIES
Two small island populations are recognized as subspecies of Malayopython reticulatus, from Selayar (M. r. saputrai) and Tanahjampea (M. r. jampeanus), south of Sulawesi, Indonesia. The closest relative of this species is the Lesser Sunda Python (M. timoriensis), while recent research demonstrates a closer relationship to Australo-Papuan pythons (Simalia), rather than Afro-Asian pythons (Python).
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Southeast Asia: Bangladesh to the Philippines, south to Timor-Leste |
ELEVATION |
0–4,270 ft (0–1,300 m) asl |
HABITAT |
Riverine forest, rainforest, and mangrove swamp |
DIET |
Mammals from rodents to deer; rare accounts of sun bears or humans being taken as prey |
REPRODUCTION |
Oviparous, with large clutches of 50–100 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Reticulated Python exhibits an instantly recognizable pattern of oranges, blacks, whites, and buffs, arranged in a reticulate or netlike design. Some specimens also have bright yellow heads. The head is more elongate than in other pythons, and bears a black and orange postocular stripe. The iris of the eye is orange with a black, vertically elliptical pupil.
MALAYOPYTHON TIMORIENSIS
LESSER SUNDA PYTHON
(PETERS, 1876)
ADULT LENGTH
3 ft 3 in–4 ft 3 in (1.0–1.3 m)
This species is often called the “Timor Python” due to its scientific name, but this is an error based on a lack of collection data. The first specimen sent to the Berlin Museum, where it was described by Wilhelm Carl Hartwig Peters (1815–83), was shipped from Kupang in West Timor, leading Peters to believe it originated from Timor. However, Kupang was then an important Dutch East Indian trading and shipping port, and it is now believed that this specimen originated from elsewhere in the Lesser Sundas, but not Timor, where it does not appear to occur and where locals are unfamiliar with photographs of this species. However, they do recognize the other two Timorese pythons, Macklot’s Water Python (Liasis mackloti) and the Reticulated Python (Malayopython reticulatus). The Lesser Sunda Python occurs in a variety of forest habitats where it uses its heat-sensitive pits to hunt warm-blooded mammals, and possibly also birds.
RELATED SPECIES
The only closely related species is the much larger and more widespread Reticulated Python, Malayopython reticulatus (shown here). Recent research has demonstrated that these two pythons are more closely related to Australo-Papuan pythons of the genus Simalia than they are to Afro-Asian pythons of the genus Python.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous constrictor |
DISTRIBUTION |
Southeast Asia: Lesser Sunda Islands from Lombok to Alor, Indonesia, but not Timor |
ELEVATION |
0–1,640 ft (0–500 m) asl |
HABITAT |
Dry and wet deciduous forest, montane forest |
DIET |
Small mammals, possibly also birds |
REPRODUCTION |
Oviparous, with clutches of 4–6 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

The Lesser Sunda Python exhibits the same body shape and elongate head of its much larger relative, the Reticulated Python. The body has a brown or yellow-brown background, with black reticulations, at least anteriorly, and a pale yellow underside. The iris of the eye is brown, and less contrasting than in the Reticulated Python, and the postocular stripe is faint or absent.
MORELIA CARINATA
ROUGH-SCALED PYTHON
(SMITH, 1981)
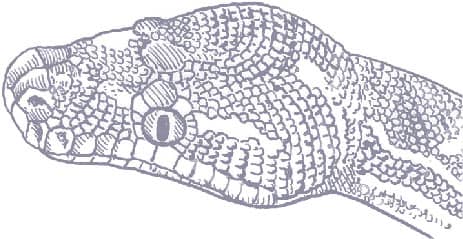
ADULT LENGTH
5 ft–6 ft 7 in (1.5–2.0 m)
The Rough-scaled Python is the only python with strongly keeled scales. It is also characterized by a single large, round scale in the center of the head, surrounded by small granular scales. It has the smallest range of any Australian python, being confined to the lower reaches of the Mitchell, Hunter, and Moran rivers, in the Kimberley, Western Australia, where it inhabits stands of monsoon forest in the steep rocky, riverine gorges. This is an understudied python in nature; until 2000 only six specimens were known. It could have been threatened by poaching for the reptile trade, or a local natural disaster, but thanks to captive breeding efforts by the Australian Reptile Park in New South Wales, its future seems secure. Its wild prey preferences and biology are still unknown.
RELATED SPECIES
The closest relatives of Morelia carinata are the widespread Carpet Python (M. spilota) and the Centralian Python (M. bredli) of central Australia.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australia: Western Australia (Kimberley) |
ELEVATION |
33–670 ft (10–205 m) asl |
HABITAT |
Monsoon forest in rocky riverine gorges |
DIET |
Probably small mammals and/or birds |
REPRODUCTION |
Oviparous, with clutches of 10–14 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

The Rough-scaled Python is easily recognized by its strongly keeled scales, and the single round scale in the center of its head. In coloration and patterning it rather resembles the widespread Carpet Python (Morelia spilota).
MORELIA SPILOTA
CARPET PYTHON
(LACÉPÈDE, 1804)

ADULT LENGTH
5 ft–8 ft 2 in (1.5–2.5 m)
Across Australia, except the arid center, Carpet Pythons exist as six subspecies. The Diamond Python, the nominate subspecies, inhabits the Hawkesbury sandstone outcrops of New South Wales. Carpet Pythons are also common in southern New Guinea. Habitats include wet and dry forests, savanna-woodland, rocky habitats, and even cities. The IUCN lists it as Least Concern, but some subspecies receive Australian state protection.
RELATED SPECIES
Close relatives are the Centralian Python (Morelia bredli) and the Rough-scaled Python (M. carinata). The other subspecies are the Eastern Carpet Python (M. s. mcdowelli) and Inland Carpet Python (M. s. metcalfei), east and west of the Great Dividing Range; Jungle Carpet Python (M. s. cheynei), Atherton Tablelands, Queensland; Top End Carpet Python (M. s. variegata), Arnhem Land; and Western Carpet Python (M. s. imbricata), south-western Australia. The status of Papuan populations is undetermined.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australasia: Australia and southern New Guinea |
ELEVATION |
0–3,690 ft (0–1,125 m) asl |
HABITAT |
Tropical rainforest, dry woodland, savanna woodland, rocky outcrops, and urban environments |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Oviparous, with clutches of 9–52 eggs |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |


The Carpet Python is generally reddish or brown with dark-edged yellow cross-bars and blotches, but the Diamond Python (Morelia spilota spilota) is yellow or gray with every scale edged with black and a series of dark-edged cross-bars, while the Jungle Carpet Python (M. s. cheynei) exhibits a bold pattern of black and yellow cross-bands or stripes.
MORELIA VIRIDIS
SOUTHERN GREEN TREE PYTHON
SCHLEGEL, 1872

ADULT LENGTH
3 ft 3 in–5 ft (1.0–1.5 m)
The Southern Green Tree Python’s bright green livery makes it one of the most iconic and attractive snake species in the world, although the yellow or orange juveniles are even more stunning. This highly arboreal python has a slender, muscular body and a prehensile tail. It sleeps looped over a branch like a coiled rope. The head is large, the mouth can gape widely, and long teeth will secure struggling prey. Prey consists of small mammals such as mice, rats, and small bandicoots, but birds and lizards are also taken. It is nocturnal and may be encountered hunting on the ground, after rain. Although a tropical forest species, it has adapted to live in oil-palm plantations.
RELATED SPECIES
Morelia viridis was split into two species separated by the central cordillera of New Guinea. They appear morphologically identical, but are genetically different. The Northern Green Tree Python (M. azurea) inhabits northern New Guinea and nearby islands. To the layman, green tree pythons also closely resemble the emerald tree boas (Corallus caninus, and C. batesii) of South America, providing a classic example of convergent evolution.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australasia: southern New Guinea, Indonesia (Aru Islands), and Australia (Queensland) |
ELEVATION |
0–5,910 ft (0–1,800 m) asl |
HABITAT |
Rainforest, monsoon forest, and oil-palm plantations |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Oviparous, with large clutches of 8–30 eggs |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |


The Southern Green Tree Python is a stunning snake, being vivid green with markings of yellow, white, or blue, with some specimens almost entirely blue or blue-green. Hatchlings start life bright yellow or orange, with markings of black and white, changing to the adult livery at 15 months of age.
PYTHON BIVITTATUS
BURMESE PYTHON
KUHL, 1820

ADULT LENGTH
10–22 ft (3.0–6.7 m)
The Burmese Python is the second-largest snake species in Asia, after the Reticulated Python (Malayopython reticulatus), but it achieves a greater girth than Reticulated Pythons of the same length. It inhabits tropical dry forests and riverine grasslands in Indo-China and along the Ganges Valley of southern Nepal, but is absent from the rainforests of Malaysia, Borneo, and Sumatra. Isolated populations occur in Java, Bali, and Sulawesi, which are remnants of a wider population from glacial times, when sea-levels were lower and dry forest more extensive. Mammals are the main prey, up to the size of deer, and are ambushed and constricted. Burmese Pythons were accidentally introduced into the Florida Everglades during a hurricane, where they thrive in habitat similar to their native Asian grasslands. The population has grown despite thousands being captured and culled.
RELATED SPECIES
Python bivittatus is closely related to the Indian Python (Python molurus) and was treated as a subspecies for many years. The isolated population on Sulawesi is now recognized as a separate subspecies, P. b. progschai.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Indo-China: Myanmar to Thailand and China, west to Nepal, with isolated populations in Java, Bali, and Sulawesi; introduced into Florida, USA |
ELEVATION |
0–3,940 ft (0–1,200 m) asl |
HABITAT |
Tropical dry forest, and riverine grassland and woodland |
DIET |
Mammals from rodents to deer, and sometimes birds |
REPRODUCTION |
Oviparous, with large clutches of 30–100 eggs |
CONSERVATION STATUS |
IUCN Vulnerable, CITES Appendix II |

The Burmese Python’s patterning comprises brown saddles on a yellowish-brown background, with similarly colored blotches on the flanks and a pale brown V-shape on the dorsum of the head. The best character to distinguish it from the similar Indian Python (Python molurus) is the presence of a subocular scale under the eye.
PYTHON BREITENSTEINI
BORNEO SHORT-TAILED PYTHON
STEINDACHNER, 1881

ADULT LENGTH
3 ft 3 in–6 ft 7 in (1.0–2.0 m)
The Borneo Short-tailed Python is endemic to the island of Borneo, where it is widely distributed and relatively common. This is a heavy-bodied terrestrial python that is not built for climbing into vegetation, but rather leads a secretive existence in the leaf litter. Although primarily an inhabitant of low montane and lowland rainforest and wetland areas, it has adapted well to living in oil-palm plantations, where it feeds on small mammals and birds, especially the abundant rodents. Rat damage is one of the most serious threats faced by the oil-palm industry and snakes like the python are some of the best biological controls available, yet this does not save the python, which is often killed on sight by workers.
RELATED SPECIES
Python breitensteini was formerly one of three subspecies of the Short-tailed or Blood Python (P. curtus). Python curtus is from western and southern Sumatra and known as the Sumatran Short-tailed Python, while the other former subspecies, the Malayan Short-tailed or Blood Python (P. brongersmai), occurs on the Malay Peninsula and eastern Sumatra. This last species is well named, being red in color and willing to draw blood from anybody who tries to handle it. A new and related species has been described from Thailand (P. kyaiktiyo).
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australasia: Borneo (Sarawak, Sabah, Brunei, and Kalimantan) |
ELEVATION |
0–3,280 ft (0–1,000 m) asl |
HABITAT |
Lowland and mid-montane rainforest, riverine forest, swamps, and plantations |
DIET |
Small mammals and birds |
REPRODUCTION |
Oviparous, with clutches of 10–15 eggs |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |

The Borneo Short-tailed Python is a relatively short but very stout-bodied snake with a short tail. It is generally tan in color with a patterning of irregular dark brown saddles and blotches. The dorsum of the head is tan while the sides of the head are much darker.
PYTHON MOLURUS
INDIAN PYTHON
(LINNAEUS, 1758)
ADULT LENGTH
10–22 ft (3.0–6.7 m)
The Indian Python inhabits dry forests and grasslands and spends the day sheltering in porcupine burrows, emerging at night to hunt mammals, from bats and rats to jackals, civets, wild boar, and deer, which are ambushed and killed by constriction. Although large, they do not hunt humans. The IUCN does not currently list the Indian Python, but it is placed on Appendix I (most protected) of CITES, and India also protects the species domestically. The Indian Python occurs throughout Sri Lanka, India, and Pakistan, but gives way to its close relative, the Burmese Python (Python bivittatus), to the north in Nepal and in Assam, northeastern India.
RELATED SPECIES
Python molurus is related to its former subspecies, the Burmese Python (P. bivittatus). The Sri Lankan population was also once treated as a subspecies (P. m. pimbura), but the subspecies is generally not recognized today. Python molurus and P. bivittatus can be distinguished by a subocular scale between the eye and the supralabials, present in P. bivittatus but absent in P. molurus.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Asia: India, Pakistan, and Sri Lanka |
ELEVATION |
0–8,200 ft (0–2,500 m) asl |
HABITAT |
Dry forest, rainforest, grasslands, and scrubland |
DIET |
Mammals, from rodents to deer |
REPRODUCTION |
Oviparous, with large clutches of up to 107 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix I |

The Indian Python is similar to the Burmese Python (Python bivittatus), but it has a pale gray rather than yellowish ground color, and a less distinct arrowhead on the dorsum of its head.
PYTHON NATALENSIS
SOUTHERN AFRICAN PYTHON
SMITH, 1840

ADULT LENGTH
13 ft–16 ft 5 in (4.0–5.0 m)
The Southern African Python is Africa’s second-longest snake, after the Central African Python (Python sebae), which may achieve 24 ft 7 in (7.5 m). It is an inhabitant of low-lying coastal thickets, savanna woodland, and rocky outcrops, often in close proximity to water, but does not occur in arid deserts or cool montane habitats. In South Africa it is found in the north and east, but despite its scientific name, ironically, it is rare in KwaZulu-Natal. A large and powerful constrictor, it preys on mammals from rats to antelopes and monkeys, and on rare occasions has even been documented preying on humans. Recently fed pythons are themselves vulnerable to attack by humans or carnivores such as African wild dogs or hyenas. They will seek a burrow in which to digest their meals in safety.
RELATED SPECIES
The closest relative of Python natalensis is the Central African Python (P. sebae), from West, Central, and East Africa, of which P. natalensis was formerly a subspecies. The two can be distinguished by the granular dorsal head scalation of P. natalensis, compared to the large head scutes of P. sebae.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Southern and East Africa: Namibia to southern Kenya, Mozambique, and eastern and northeastern South Africa |
ELEVATION |
0–5,910 ft (0–1,800 m) asl |
HABITAT |
Lowland grassland and woodlands, coastal thickets, and rocky outcrops |
DIET |
Mammals and birds |
REPRODUCTION |
Oviparous, with large clutches of up to 100 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

The Southern African Python is a stoutly built python with a body pattern of dark brown irregular saddles on a light brown background, and a brown arrowhead marking on the dorsum of its head.
PYTHON REGIUS
BALL PYTHON
(SHAW, 1802)
ADULT LENGTH
3 ft 3 in–5 ft (1.0–1.5 m)
Although referred to as the Ball Python in the United States, this species is called the Royal Python in the UK, a literal translation of its specific name. The name Ball Python is derived from the defensive posture these pythons adopt, of coiling into a ball with their heads buried in the center. Ball Pythons are inoffensive snakes and rarely bite. This species is found across a wide swath of West and Central Africa, and spend much of their time in animal burrows, emerging at night to hunt. Hundreds of thousands of Ball Pythons were imported into the West for the pet trade, but hopefully this unsustainable harvest has declined due to the massive captive breeding market, which has also seen the development of many color morphs known as “designer morphs.” If the snake-keeping public prefer to acquire the unusual albino color morphs, this may take the pressure off the wild populations.
RELATED SPECIES
Python regius is one of Africa’s two smallest python species, the other species being the Angolan Python (P. anchietae) of Angola and Namibia.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
West and Central Africa: Senegal to Sudan |
ELEVATION |
0–3,280 ft (0–1,000 m) asl |
HABITAT |
Open savannas and savanna woodland or scrub |
DIET |
Small mammals |
REPRODUCTION |
Oviparous, with clutches of 5–15 eggs |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |


The Ball Python is a stout but relatively short python with a distinctive pattern of pale brown saddles and stripes on a dark brown to black background, and a dark brown head with a pale stripe through each eye.
SIMALIA AMETHISTINA
AMETHYSTINE PYTHON
(SCHNEIDER, 1801)
ADULT LENGTH
16 ft 5 in–19 ft 8 in (5.0–6.0 m)
The Amethystine Python was a very widely distributed species, until the Queensland subspecies was elevated to species status and the Indonesian island populations were described as separate species. The remaining New Guinea–Bismarck Archipelago Simalia amethistina may still be a species complex. This is a slender inhabitant of both closed and open habitats, and it feeds on a variety of mammals, from rats and bandicoots to small wallabies, as well as birds, although juveniles may also take skinks. Adults are often found in the vicinity of flying fox rookeries where the pythons find easy prey. The common and scientific names of this python originate from the “oil-on-water” iridescent sheen that is visible on the scales in daylight, and which may serve to camouflage the outline of a sleeping python.
RELATED SPECIES
The genus Simalia contains those species formerly in Morelia that exhibit regular dorsal head scutes, rather than granular scales. The closest relatives of S. amethistina are the former subspecies, the Scrub Python (S. kinghorni) from Queensland, and the former island populations, the Southern Moluccan Python (S. clastolepis), Halmahera Python (S. tracyae), and Tanimbar Python (S. nauta), all from Indonesia.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
New Guinea: west New Guinea and Papua New Guinea, including Bismarck Archipelago (New Britain, New Ireland) |
ELEVATION |
0–5,580 ft (0–1,700 m) asl |
HABITAT |
Rainforest, riverine forest, mangrove and freshwater swamps, savannas, and plantations |
DIET |
Mammals and birds; also lizards as juveniles |
REPRODUCTION |
Oviparous, with clutches of 10–11 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Amethystine Python is fairly variable in coloration, ranging from dark brown to straw yellow, with or without darker markings, but all specimens exhibit the iridescent sheen in daylight. Amethystine pythons are slender snakes with elongate heads and numerous thermosensitive labial pits.
SIMALIA BOELENI
BOELEN’S PYTHON
(BRONGERSMA, 1953)
ADULT LENGTH
6 ft 7 in–19 ft 8 in (2.0–3.0 m)
Boelen’s Python, named for the Dutch surgeon who collected the holotype, is a stunning species also known as the Black Python due to its unique coloration. Unlike other New Guinea pythons, it only occurs in montane rainforest above 4,270 ft (1,300 m) asl, in cool, humid conditions. Endemic to New Guinea, it is distributed along the length of the Central Cordillera, but is nowhere common. Sought after by the pet trade, it is also threatened by the wholesale clearance of montane forest for timber, slash-and-burn agriculture, oil prospecting, and gold mining. Its natural history is poorly documented, with even its prey preferences an open question, although it probably feeds on rats, bandicoots, or ground-nesting birds.
RELATED SPECIES
The closest relative of Simalia boeleni is probably the Amethystine Python (S. amethistina).
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
New Guinea: west New Guinea and Papua New Guinea |
ELEVATION |
4,270–9,840 ft (1,300–3,000 m) asl |
HABITAT |
Montane rainforest |
DIET |
Poorly known; mammals, and possibly birds |
REPRODUCTION |
Oviparous, with clutches of 14–20 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |

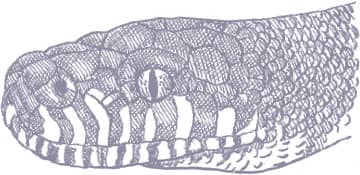
Boelen’s Python is iridescent black or blue-black with white or yellow markings on the lips, throat, and anterior underside, with fingers of white extending upward onto the flanks. The iris of the eye is light gray. It is a stockily built snake with a broad, chunky head. Juveniles are red-brown in color.
SIMALIA OENPELLIENSIS
OENPELLI PYTHON
(GOW, 1977)

ADULT LENGTH
10 ft–16 ft 5 in (3.0–5.0 m)
Named for the small town of Oenpelli on the East Alligator River, the iridescent Oenpelli Python is believed by some to be the “Rainbow Serpent” of Aboriginal Dreamtime. It certainly inhabits the right habitat—sandstone escarpments in the Kakadu National Park and surrounding Arnhem Land, where it is known as “Nawaran.” This is a very rarely encountered species that hunts bandicoots, possums, rock rats, and flying foxes in the crevices and caves of the escarpments at night, although lizards may feature in the diets of juveniles. As with any species that inhabits small, limited ranges, there are concerns for the future of this python, and it is listed as Threatened in the Northern Territory. The Oenpelli Python is the subject of a captive breeding and conservation project involving local people.
RELATED SPECIES
The Australian python that is closest to Simalia oenpelliensis is probably the much larger Scrub Python (S. kinghorni) of Queensland.
FAMILY |
Pythonidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Australia: Northern Territory (west Arnhem Land) |
ELEVATION |
0–950 ft (0–290 m) asl |
HABITAT |
Sandstone escarpments |
DIET |
Small mammals, and possibly lizards |
REPRODUCTION |
Oviparous, with clutches of 6–10 eggs |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Oenpelli Python is very slender with an elongate head and almost forward-facing eyes. It is silver-gray with markings confined to irregular, rather subdued mottling of darker pigment, although this species can change color depending on the prevailing conditions, from grayish at night to reddish in the daytime.
CALABARIA REINHARDTII
CALABAR GROUND BOA
(SCHLEGEL, 1851)
ADULT LENGTH
2 ft–3 ft 3 in (0.6–1.03 m)
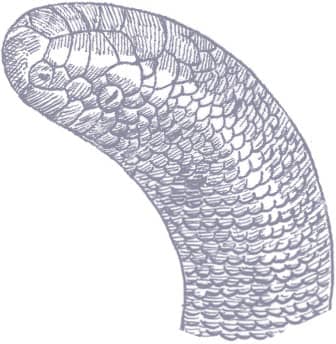
The Calabar Ground Boa, named for a region of Nigeria, was previously thought to be a python because it lays elongate eggs while boas are live-bearers. It is found in the rainforests of West and Central Africa, and also inhabits savanna woodland and human-mediated habitats, such as oil-palm plantations. Nocturnal and fossorial, the Calabar Ground Boa is usually only seen after heavy rain, when it emerges to hunt. Prey comprises mainly small mammals, such as rodents and shrews. When it feels threatened the Calabar Ground Boa will coil into a tight ball, its head in the center and its tail acting as a pseudo-head, distracting attention away from the real head. The scientific name honors Johannes Theodor Reinhardt (1816–82), a Danish naturalist.
RELATED SPECIES
As the only member of the Calabariidae, Calabaria reinhardtii does not have any close relatives. In the past it has been linked with the pythons (Pythonidae), the rosy and rubber boas (Charinidae) of western USA, Canada, and Mexico, and the Afro-Asian sand boas (Erycidae), but current opinion is that the true boas (Boidae) are its closest relatives and it may represent a basal element within the boas.
FAMILY |
Calabariidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
West and Central Africa: Guinea, Sierra Leone, Liberia, Côte d’Ivoire, Ghana, Togo, Benin, Nigeria, Cameroon, Central African Republic, Equatorial Guinea, Gabon, Congo, and DRC |
ELEVATION |
10–3,440 ft (3–1,050 m) asl |
HABITAT |
Rainforests, evergreen and deciduous forests, savanna woodland, and plantations |
DIET |
Small mammals, shrews, and rodents |
REPRODUCTION |
Oviparous, with clutches of 1–4 elongate eggs |
CONSERVATION STATUS |
IUCN not listed |


The Calabar Ground Boa is a cylindrical-bodied snake with a rounded head, indistinct from the neck, small eyes, and a short stumpy tail. Its scales are smooth and tight-fitting, and its coloration and patterning comprise orange and brown blotches and scattered scales on a dark brown to dark gray background.
BOA CONSTRICTOR
COMMON BOA
LINNAEUS, 1758
ADULT LENGTH
6 ft 7 in–10 ft, rarely 16 ft 5 in (2.0–3.0 m, rarely 5.0 m)
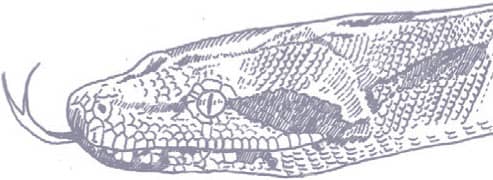
The Common Boa, or Boa Constrictor, inhabits tropical rainforests, woodland, grassland, semidesert, cultivated land, and human settlements. Nocturnal, it hunts aloft or on the ground, feeding mostly on mammals, birds, and lizards. It has been known to take vampire bats, village dogs, porcupines, coatimundis, green iguanas, deer, and even an ocelot. In Manaus, Brazil it is an important control on the disfiguring human disease leishmaniasis, because it preys on the common opossum that acts as a disease reservoir. It is not large enough to endanger humans.
RELATED SPECIES
Amazonian and Guianan boas belong to the nominate subspecies, and three other subspecies are recognized: the Long-tailed Boa (B. c. longicauda), the Peruvian Red-tail Boa (B. c. ortonii), and Argentine Boa (B. c. occidentalis). Four other Boa species also exist: the Central American or Imperial Boa (B. imperator), and Western Mexico Boa (B. sigma), and from the Lesser Antilles, the St. Lucia Boa (B. orophias) and Dominican Clouded Boa (B. nebulosa).
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
South America: Colombia, Venezuela, Trinidad, the Guianas, Brazil, Peru, Ecuador, Bolivia, Paraguay, Uruguay, and Argentina |
ELEVATION |
0–3,280 ft (0–1,000 m) asl |
HABITAT |
Rainforest, grassland, semidesert, islands, cultivated land, and human settlements |
DIET |
Mammals, birds, and lizards |
REPRODUCTION |
Viviparous, with litters of 10–64 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II (except B. c. occidentalis Appendix I) |


The Common Boa is a powerfully built snake with a prehensile tail, angular head, and relatively small eyes. It is gray or brown with a series of distinctive dark brown dorsolateral saddles. The head is gray-brown with a faint brown midline and a dark brown postocular stripe. The tail is ringed red in juveniles, but usually turns brown with maturity.
CHILABOTHRUS ANGULIFER
CUBAN BOA
(COCTEAU & BIBRON, 1840)

ADULT LENGTH
6 ft 7 in–10 ft, rarely 13 ft (2.0–3.0 m, rarely 4.0 m)
The Cuban Boa is the largest snake in the Caribbean, excluding Trinidad, which is home to the Green Anaconda (Eunectes murinus) and Common Boa (Boa constrictor). It is found throughout the island of Cuba, including Guantánamo Bay, and on the Isla de la Juventud. It inhabits woodland habitats, but is also associated with rocky outcrops, and especially caves where it hunts bats. Adults also hunt rodents, village chickens, iguanas, and woodsnakes (Tropidophis), while juveniles take anole lizards and mice. Most specimens are less than 10 ft (3 m) in total length, but one specimen collected at Guantánamo Bay was reported to measure 16 ft (4.85 m). Cuban Boas are noted for their irascible temperament, biting easily, but they are not dangerous to humans.
RELATED SPECIES
The West Indian boas were all once contained in the South American genus Epicrates but were relatively recently transferred to the resurrected endemic West Indian genus Chilabothrus. Chilabothrus angulifer is the largest of the 12 species. It has no subspecies. Other species that reach over 6 ft 7 in (2 m) include the Haitian Boa (C. striatus) from Hispaniola (Haiti and Dominican Republic), the widely distributed Bahamian Boa (C. strigilatus), and the recently described Silver Boa (C. argenteum), from the Conception Island Bank, in the Bahamas.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
West Indies: Cuba and Isla de la Juventud |
ELEVATION |
0–1,070 ft (0–325 m) asl |
HABITAT |
Moist and dry woodland, rocky outcrops, and caves |
DIET |
Mammals, birds, lizards, and snakes |
REPRODUCTION |
Viviparous, with litters of 1–7 neonates |
CONSERVATION STATUS |
IUCN Near Threatened, CITES Appendix II |


The Cuban Boa is a muscular snake with a prehensile tail and a broad, rounded head with moderately large eyes. It is usually brown above and paler gray on the lower flanks, with variable patterning, western specimens being boldly marked with a black or dark brown angular pattern, while eastern specimens exhibit indistinct or no markings.
CHILABOTHRUS MONENSIS
MONA ISLAND BOA
(ZENNECK, 1898)

ADULT LENGTH
311/2–351/2 in, rarely 3 ft 3 in (800–900 mm, rarely 1.0 m)
The Mona Island Boa is endemic to the tiny Puerto Rican island of Isla Mona, with an area of 22 sq miles (57 sq km), located in the Mona Passage, between Puerto Rico and the Dominican Republic. Habitat loss and the introduction of feral cats, which kill the small boas, are among the reasons for its decline. The Mona Island Boa is an inhabitant of dry subtropical forest but it is also found on rocky outcrops and it may enter caves. It is also said to utilize termitaria and even the rafters of houses. It is a nocturnal predator of sleeping anole lizards and occasionally takes large ameiva lizards, rodents, and small bats, although these may also be declining on Isla Mona.
RELATED SPECIES
Chilabothrus monensis from the west of Puerto Rico is most closely related to the Virgin Islands Boa (C. granti), a former subspecies occurring on the Isla Culebra and Cayo Diablo, and the US and British Virgin Islands, to the east of Puerto Rico.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
West Indies: Puerto Rico (Isla Mona) |
ELEVATION |
0–165 ft (0–50 m) asl |
HABITAT |
Dry subtropical forest or rocky outcrops |
DIET |
Small lizards, rarely mammals |
REPRODUCTION |
Viviparous, with litters of 4 neonates |
CONSERVATION STATUS |
IUCN Endangered, CITES Appendix I |

The Mona Island Boa is a slender snake with a distinct head, moderately large eyes, and a prehensile tail. It is dorsally gray-brown with a pattern comprising transverse dark brown angular blotches, which may be broken at the midline. The head is patternless, apart from a postocular stripe in some specimens, and the undersides are immaculate white or stippled with brown.
CHILABOTHRUS SUBFLAVUS
JAMAICAN BOA
(STEJNEGER, 1901)
ADULT LENGTH
5 ft–6 ft 7 in (1.5–2.0 m)
Known locally as “yellow snake,” the Jamaican Boa is listed as Appendix I of CITES, a status level only applied to ten other snake species. Once found throughout Jamaica, it has been virtually extirpated in the wild. Causes for this decline include active persecution, habitat loss to housing, agriculture and mining, and the introduction of pigs, dogs, cats, and the small Indian mongoose. Today it may only survive in the wild on Great Goat Island, off the south of Jamaica. However, captive breeding of this species by zoos has created a large captive population and prevented its ultimate extinction. It is a woodland inhabitant that also inhabits rocky outcrops and caves, preying on rodents, bats, and lizards, but there is also a record of a parrot being eaten. Jamaica has no dangerous snakes.
RELATED SPECIES
Many of the West Indian island-dwelling boas are also Endangered like Chilabothrus subflavus. The Puerto Rican Boa (C. inornatus) and the Mona Island Boa (C. monensis) are also on CITES Appendix I.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Caribbean: Jamaica and Great Goat Island |
ELEVATION |
0–130 ft (0–40 m) asl |
HABITAT |
Woodland, forest, and rocky outcrops, including caves |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Viviparous, with litters of 3–39 neonates |
CONSERVATION STATUS |
IUCN Vulnerable, CITES Appendix I |


The Jamaican Boa is an agile-bodied snake with a long, angular head, large eyes, and a prehensile tail. The anterior body and head are olive to yellow with black scale tips, with black transverse bands appearing by the midbody, which increase in intensity until the posterior of the body and the tail are virtually black. All scales are iridescent. Juveniles are often yellowish or pinkish with dark bands.
CORALLUS CANINUS
GUIANAN EMERALD TREEBOA
(LINNAEUS, 1758)
ADULT LENGTH
4 ft 7 in–6 ft, rarely 6 ft 7 in (1.4–1.8 m, rarely 2.0 m)
Until recently the Emerald Treeboa was a single species distributed throughout northern South America, east of the Andes, but today this species has been split into two separate species, with Corallus caninus the name applied to emerald treeboas from north of the Amazon River and east of the Rio Negro, in northeastern Brazil, eastern Venezuela, and the Guianas. Emerald treeboas are extremely similar in appearance to the green tree pythons (Morelia azurea and M. viridis) of New Guinea, an example of convergent evolution where two unrelated and distant species resemble one another. Emerald treeboas are nocturnal, arboreal lowland rainforest snakes that hunt and constrict rodents and lizards. Their long front teeth maintain a grip on the struggling prey following the strike.
RELATED SPECIES
The Amazonian Emerald Tree Boa (Corallus batesii) inhabits the remainder of the original range, in Colombia, western and central Brazil, Peru, and northern Bolivia. The species most closely related to the two emerald treeboas are the Annulated Treeboa (C. annulatus) of Central America and northwestern Colombia, and Blomberg’s Treeboa (C. blombergi) of western Ecuador.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Northern South America: Venezuela, Guyana, Suriname, French Guiana, and Brazil |
ELEVATION |
0–3,280 ft (0–1,000 m) asl |
HABITAT |
Lowland tropical rainforest |
DIET |
Small mammals, and lizards |
REPRODUCTION |
Viviparous, with litters of 6–15 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Guianan Emerald Treeboa is a muscular snake with a laterally compressed body, a long head, vertically elliptical pupils, and a prehensile tail. The adult livery is bright green with irregular white transverse bars across the back, while juveniles may be yellow, green, or orange. Emerald treeboas can be distinguished from green tree pythons by their longer heads and the presence of heat-sensitive pits on all the supralabials rather than just a few.
CORALLUS RUSCHENBERGERII
CARIBBEAN COASTAL TREEBOA
(COPE, 1875)
ADULT LENGTH
4 ft 7 in–6 ft 7 in, occasionally 7 ft 7 in (1.4–2 m, occasionally 2.3 m)
The Caribbean Coastal Treeboa is found on the American mainland from Pacific coastal Costa Rica to Caribbean coastal Venezuela, Trinidad, Tobago, and many smaller offshore islands. It is the largest member of the genus Corallus. Treeboas are nocturnal and, curiously, they are the only snakes known that can be located by their eyeshine, reflecting a flashlight beam from hundreds of feet away. Although largely arboreal, treeboas often hunt terrestrial prey from a head-down posture, dangling close to the ground, ready to strike anything running underneath. The prey of the Caribbean Coastal Treeboa comprises bats, birds, rodents, mouse opossums, and lizards. Often large numbers of treeboas may be found in a relatively small area such as a mangrove swamp. Commodore Dr. William Samuel Waithman Ruschenberger (1807–95) was a U.S. Navy and Army physician.
RELATED SPECIES
Corallus ruschenbergerii is most closely related to the Amazonian Treeboa (C. hortulanus) of northern South America, and the Grenadian Treeboa (C. grenadensis) and Cook’s Treeboa (C. cookii), both from the Lesser Antilles. A more distant relative is the extremely rare Cropani’s Treeboa (C. cropanii) from the Brazilian Atlantic Forests. Not seen alive since 1953, it was believed extinct until a specimen was captured in 2017. This snake is now being radio-tracked in the wild.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Northern South and southern Central America: Costa Rica, Panama, Colombia, Venezuela, and Trinidad |
ELEVATION |
0–3,280 ft (0–1,000 m) asl |
HABITAT |
Lowland rainforest, wet and dry forests, flooded grassland, and mangrove swamps |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Viviparous, with litters of 9–15 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |
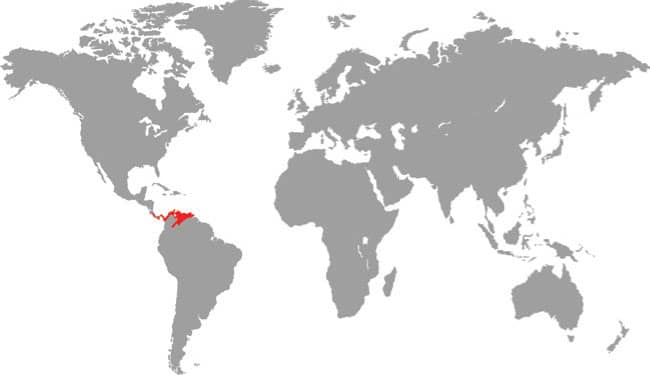

The Caribbean Coastal Treeboa is a large, slender but powerful snake with a long head, with distinctive labial pits, and a long prehensile tail. Specimens may be uniform buff, orange-brown, or gray, with or without black-tipped scales, or they may be patterned with hollow, brown diamond markings along the flanks. The undersides are off-white while the sides of the head are pale.
EPICRATES CENCHRIA
BRAZILIAN RAINBOW BOA
(LINNAEUS, 1758)

ADULT LENGTH
5 ft–6 ft 7 in (1.5–2.0 m)
The name “Rainbow Boa” originates from the highly visible iridescent sheen on the scales of this species, which presents an “oil-on-water” appearance. This may be a defensive adaptation to aid the crypsis of a nocturnal snake in daylight, because it is reflected in the names of other, unrelated nocturnal snakes, such as the Sunbeam Snake (Xenopeltis unicolor) and Amethystine Python (Simalia amethistina). The Brazilian Rainbow Boa inhabits the Amazon Basin and the Guiana Shield. It achieves a relatively large size and can capture and constrict a wide variety of small to medium-sized mammals and birds. The presence of heat-sensitive pits on its lips enables it to efficiently hunt endotherms at night. Brazilian Rainbow Boas shelter in holes or under logs during the day.
RELATED SPECIES
Epicrates cenchria was previously viewed as a single species with nine subspecies, but now three of these are recognized as full species, while the others are no longer recognized. These are the Argentine Rainbow Boa (E. alvarezi), Paraguayan Rainbow Boa (E. crassus), and Caatinga Rainbow Boa (E. assisi) from northeastern Brazil.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Amazonian South America: Colombia, Venezuela, the Guianas, Brazil, Peru, Ecuador, and Bolivia |
ELEVATION |
0–9,020 ft (0–2,750 m) asl |
HABITAT |
Rainforest, flooded forest, and savanna woodland |
DIET |
Small mammals and birds |
REPRODUCTION |
Viviparous, with litters of 6–28 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Brazilian Rainbow Boa has a powerful body, long, arrow-shaped head, and prehensile tail. It is orange or red above, with a pattern of hollow black rings along the back, and yellow-centered black spots along the flanks. The head is orange with black stripes, while the undersides are white with black spots.
EPICRATES MAURUS
NORTHERN RAINBOW BOA
GRAY, 1849
ADULT LENGTH
5 ft–6 ft 7 in (1.5–2.0 m)
Also known as the Brown Rainbow Boa or Colombian Rainbow Boa, the northernmost member of the genus Epicrates inhabits a wide variety of habitats, from dry woodland to thorn forest, savanna woodland, and swamps. Where it occurs in sympatry with the Brazilian Rainbow Boa (E. cenchria) it inhabits more open habitats or forest-savanna edge, leaving the rainforest proper to the other species. Prey comprises a variety of small mammals, birds, and lizards, which are captured at night and killed by constriction. Females may produce up to 20 neonates and facultative parthenogenesis has been reported in this species. Females have also been known to ingest undeveloped eggs or stillborn embryos as a means of recovering resources lost to reproduction.
RELATED SPECIES
Of all the forms of rainbow boa, Epicrates maurus was elevated from subspecific status long before the most recent revisions of the E. cenchria group, which resulted in three new species, and no subspecies. The former subspecies E. c. barbouri, from Ilha de Marajó, Pará, Brazil, is now synonymized with E. maurus.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Northern South and southern Central America: Costa Rica, Panama, Colombia, Venezuela, Trinidad, the Guianas, and northeastern Brazil |
ELEVATION |
0–8,630 ft (0–2,630 m) asl |
HABITAT |
Lowland deciduous woodland, gallery forests, palm groves, marshes, non-flooded forests, savannas, and thorn forest |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Viviparous, with litters of 6–20 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |
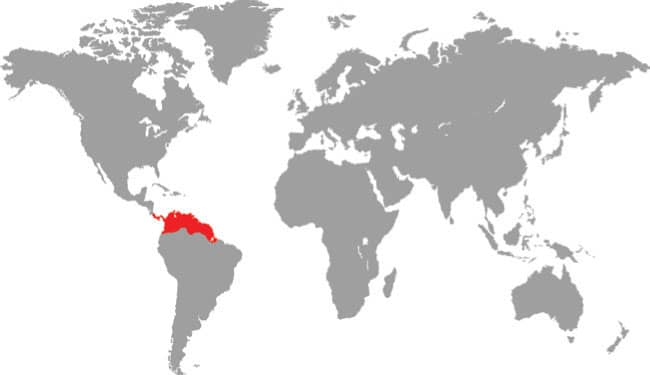

The Northern Rainbow Boa is relatively stout-bodied with a long head, with pits on the labial scales, and a prehensile tail. Juveniles are light brown with lateral speckling and a series of large, light-centered ocelli markings on the back, whereas adults are uniform orange-brown with only the indistinct remnants of their juvenile patterning. The scales are highly iridescent, hence the name “rainbow boa.”
EUNECTES MURINUS
GREEN ANACONDA
(LINNAEUS, 1758)
ADULT LENGTH
Male
10–13 ft (3.0–4.0 m)
Female
23 ft–26 ft 3 in (7.0–8.0 m)
This is the heaviest snake in the world, with female specimens over 220 lb (100 kg) recorded. A large female can hunt, eat, mate, and give birth in water, without needing to venture onto land. Her weight may make crawling difficult but does not affect swimming. Females grow much larger than males. In the breeding season, “mating balls” may form with several smaller males courting a single female. Green Anacondas prey on mammals as large as brocket deer, capybaras, and young tapirs, and on waterbirds and caimans. Prey is constricted to death. Cannibalism is also documented. There are no confirmed records of humans being eaten. Green Anacondas in some habitats will estivate through the dry season, while those in rainforest rivers may be active all year round.
RELATED SPECIES
Three other anacondas are recognized: the Yellow Anaconda (Eunectes notaeus) from southern Brazil, Paraguay, and northern Argentina; De Schauensee’s Anaconda (E. deschauenseei) from the mouth of the Amazon; and the recently described Beni Anaconda (E. beniensis) from Bolivia.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, powerful constrictor |
DISTRIBUTION |
Northern South America: Colombia, Venezuela, Trinidad, the Guianas, Brazil, Peru, Ecuador, and Bolivia |
ELEVATION |
0–785 ft (0–240 m) asl |
HABITAT |
Rivers in rainforest, lakes, seasonally flooded savannas |
DIET |
Mammals from agouti to tapir, waterbirds, and caimans |
REPRODUCTION |
Viviparous, with large litters of 20–40 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |
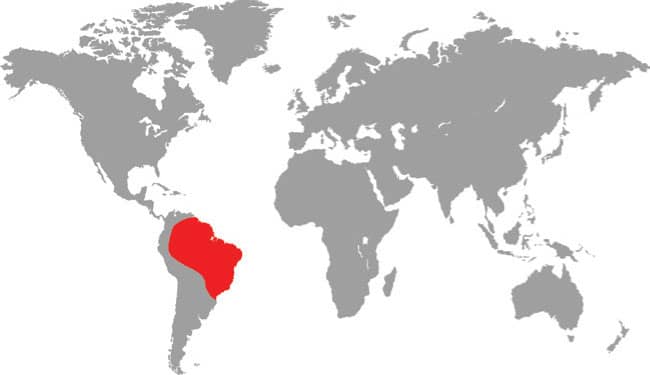

The Green Anaconda is characteristically patterned dark green with large black ocelli markings. The head exhibits a pair of curved, black-edged, orange postocular stripes. In large specimens these stripes move more dorsally, as the head broadens with size and age, and in clear sunlit water they may resemble a pair of horns, their outlines “drop-shadowed” by the black edges.
EUNECTES NOTAEUS
YELLOW ANACONDA
COPE, 1862

ADULT LENGTH
Male
6 ft 7 in–8 ft (2.0–2.4 m)
Female
10–13 ft (3.0–4.0 m)
Also known as the Paraguayan Anaconda, the Yellow Anaconda inhabits the vast seasonally flooded grasslands of the Pantanal in southwestern Brazil and neighboring countries, sometimes in sympatry with the Green Anaconda (Eunectes murinus). It hunts mammals, waterbirds, and Broad-snouted Caiman, which are killed by constriction. Turtles and fish are also occasionally taken. The largest prey animals are probably young peccaries and brocket deer. Although not found in Amazonia, the Yellow Anaconda may occur in closed-canopy riverine forest within its range. The larger adult females attract a number of smaller males as suitors, and form “mating balls” during the breeding season. Although not as large as its northern relative, this species may still achieve weights of 110–120 lb (50–55 kg).
RELATED SPECIES
Eunectes notaeus is closely related to two similarly patterned species, De Schauensee’s Anaconda (E. deschauenseei) from Ilha de Marajó, in the mouth of the Amazon, and French Guiana, and the Beni Anaconda (E. beniensis) from Beni River, Bolivia, which was originally thought to be the result of hybridization between Yellow and Green Anacondas.
FAMILY |
Boidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Central South America: Paraguay, Bolivia, southwestern Brazil, northeastern Argentina, and Uruguay |
ELEVATION |
260–490 ft (80–150 m) asl |
HABITAT |
Seasonally flooded savannas, and riverine gallery forest |
DIET |
Mammals, waterbirds, caimans, turtles, and possibly fish |
REPRODUCTION |
Viviparous, with litters of 10–40 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Yellow Anaconda’s patterning is less distinctive than that of the Green Anaconda. The background color is yellow to brown, with black spots coalescing to form irregular zigzag or dumbbell patterns on the back. The head bears a black arrowhead marking, and a dark postocular stripe is present from the eye to the angle of the jaw. The undersides are yellow.
CANDOIA ASPERA
NEW GUINEA GROUND BOA
(GÜNTHER, 1877)

ADULT LENGTH
Male
153/4–173/4 in (400–450 mm)
Female
193/4–271/2 in, rarely 361/2 in (500–700 mm, rarely 930 mm)
Colloquially known as the “Viper Boa,” the New Guinea Ground Boa is found throughout most of the island of New Guinea, the Bismarck Archipelago to the east, and the northern Moluccas of Indonesia to the west. It inhabits both rainforest and man-made environments such as coconut or oil-palm plantations, where it has a preference for dark, cool, damp locations, such as overgrown creeks or inside piles of discarded coconut husks. It is a sedentary, terrestrial sit-and-wait ambusher of rodents, bandicoots, lizards, and frogs, and it is itself preyed upon by the New Guinea Small-eyed Snake (Micropechis ikaheka). It is so static in its habits that Papuans often refer to it as the “sleepy snake.” However, large specimens can deliver painful, bloody but otherwise harmless bites if handled.
RELATED SPECIES
Most of the range is occupied by Candoia aspera schmidti, while the nominate subspecies, C. a. aspera, is found in New Ireland, at the eastern extreme of the range. Candoia aspera may be confused with the Solomon Islands Ground Boa (C. paulsoni) and the two do occur together in parts of its range. It is also sometimes mistaken for the venomous Smooth-scaled Death Adder (Acanthophis laevis), but it more closely resembles the Rough-scaled Death Adder (A. rugosus) of the Trans-Fly region, though C. aspera is absent from that area.
FAMILY |
Candoiidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Melanesia: eastern Indonesia and New Guinea |
ELEVATION |
0–3,280 ft (0–1,000 m) asl |
HABITAT |
Rainforest, and coconut and oil-palm plantations |
DIET |
Small mammals, frogs, and lizards |
REPRODUCTION |
Viviparous, with litters of 5–48 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |
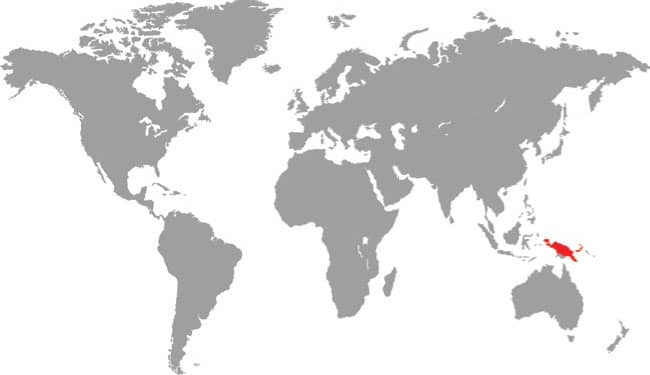

The New Guinea Ground Boa is an extremely stocky snake with heavily keeled scales and a short, non-prehensile tail. The head is angular and viper-like and covered in small granular scales, and the eyes have vertically elliptical pupils. Cloacal spurs are present in males, but reduced in size or absent in females. Coloration is generally dark or light brown, or yellow, with a pattern of darker squarish saddles. The undersides may be dark or light and are often speckled with brown or red.
CANDOIA CARINATA
NEW GUINEA TREEBOA
(SCHNEIDER, 1801)

ADULT LENGTH
Male
153/4–223/4 in (400–575 mm)
Female
233/4–28 in (600–715 mm)
The New Guinea Treeboa, also known as a “bevel-nosed boa” due to its overhung snout, occurs throughout New Guinea, the Bismarck Archipelago to the east, and in eastern Indonesia, the Moluccas, and Sulawesi. Its pattern is so similar to the lichen-like colors of the tree bark that if it remains motionless it is virtually invisible. Unlike the terrestrial New Guinea Ground Boa (Candoia aspera), with which it occurs in sympatry, the New Guinea Treeboa is arboreal, where it hunts lizards, either tree-dwelling species or terrestrial species that are ambushed from above. Prey includes skinks, geckos, and possibly tree frogs. It is an extremely docile snake. Females produce small litters of neonates as thick as matches.
RELATED SPECIES
Two subspecies are recognized, the nominate form (Candoia carinata carinata) occurring through most of the range, with a newly described subspecies (C. c. tepedeleni) in the Bismarck Archipelago, east of New Guinea. Candoia carinata is most similar to the equally slender Palau Treeboa (C. superciliosa), which was once included in this species. The Solomon Islands Ground Boa (C. paulsoni) was also treated as a subspecies of C. carinata, despite the physical differences in their appearances.
FAMILY |
Candoiidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Melanesia: eastern Indonesia and New Guinea |
ELEVATION |
0–5,000 ft (0–1,525 m) asl |
HABITAT |
Rainforest, and coconut and cocoa plantations |
DIET |
Lizards and frogs |
REPRODUCTION |
Viviparous, with litters of 5–6 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |
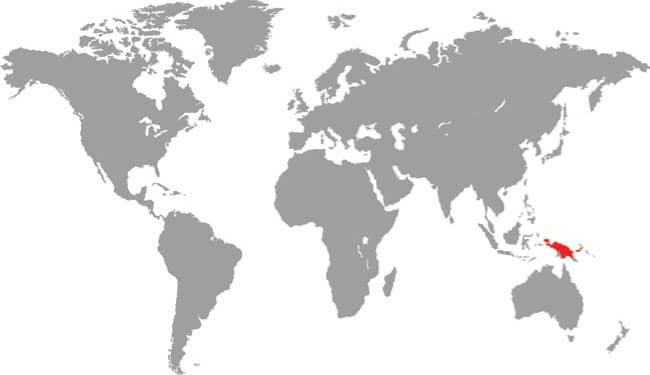

The New Guinea Treeboa is an extremely slender snake with a long, prehensile tail, and a long, angular head, covered in granular scales, with very small eyes. It may be either light gray with dark gray saddles, or light brown with dark brown saddles or stripes. There is often a yellow-brown saddle over the cloaca and a large white spot posterior to the cloaca. Males have large cloacal spurs; those of females are tiny.
CANDOIA PAULSONI
SOLOMON ISLANDS GROUND BOA
(STULL, 1956)

ADULT LENGTH
Male
271/2–33 in (700–840 mm)
Female
3 ft 3 in–4 ft 3 in (1.0–1.3 m)
The Solomon Islands Ground Boa is found from eastern Indonesia to the Solomon Islands, a distance of 3,730 miles (6,000 km). It is nocturnal and terrestrial, and is sometimes found in sympatry with the equally terrestrial but stouter and more rugose New Guinea Ground Boa (Candoia aspera). Unlike its congener it climbs and may be found in oil palms. Juveniles feed on small lizards such as skinks or geckos, while adults prey on small mammals and frogs. In New Guinea the boa is the occasional prey of the Eastern Brownsnake (Pseudonaja textilis). The Solomon Islands Ground Boa was once treated as a subspecies of the much more slender New Guinea Treeboa (C. carinata). Being larger, female Solomon Islands Ground Boas produce much larger litters of neonates. John Paulson was a Swedish herpetologist.
RELATED SPECIES
The nominate subspecies (Candoia paulsoni paulsoni) inhabits the Solomon Islands and the islands east of the Bismarck Archipelago, while another subspecies (C. p. mcdowelli) occurs in Papua New Guinea and the Milne Bay Islands. Island endemics are found on Bougainville (C. p. vindumi), Misima (C. p. rosadoi), and Woodlark (C. p. sadlieri). This species appears to be absent from Indonesian west New Guinea, but a sixth subspecies (C. p. tasmai) occurs further west, in the north Moluccas. Candoia paulsoni resembles the Pacific Boa (C. bibroni), which occurs from the Solomons to Fiji.
FAMILY |
Candoiidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Melanesia: eastern Indonesia, New Guinea, and the Solomon Islands |
ELEVATION |
0–6,000 ft (0–1,830 m) asl |
HABITAT |
Rainforest and oil-palm plantations |
DIET |
Small mammals, frogs, and lizards |
REPRODUCTION |
Viviparous, with litters of 16–48, rarely 60 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |
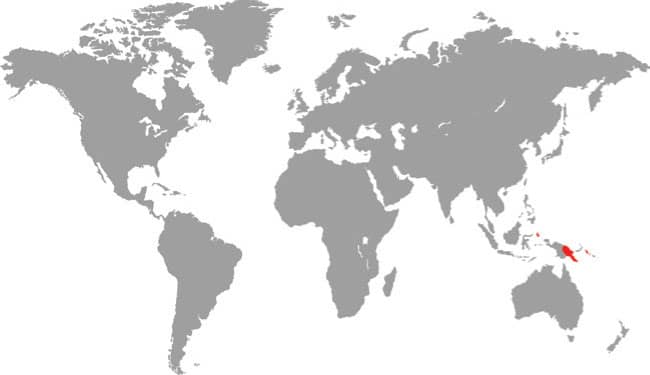

The Solomon Islands Ground Boa is a moderately stout snake, midway between Candoia aspera and C. carinata. Its long angular head is covered by granular scales, it has small eyes with vertical pupils, and its moderate tail is shorter than that of C. carinata though much longer than that of C. aspera. Coloration and patterning are extremely variable, with browns or grays predominating and a dark zigzag vertebral line common.
ERYX JACULUS
JAVELIN SAND BOA
(LINNAEUS, 1758)
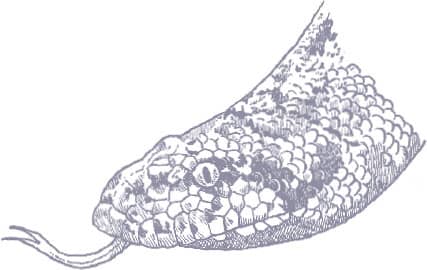
ADULT LENGTH
153/4–233/4 in, rarely 311/2 in (400–600 mm, rarely 800 mm)
Also known as the Western Sand Boa, the Javelin Sand Boa is probably Europe’s only boa, occurring in the southeast of the continent, but it is also found in North Africa and the Middle East, while the eastern extent of its range in Iran and the Caucasus is undetermined. This is one of the smallest sand boas, and Greek island specimens are even smaller. It inhabits sandy habitats, from land behind beaches to dry valleys and semidesert, but is also found in arable land. It likes rocky, scrubby land and avoids entirely sandy soils. Hiding under cover during the day, it lies in ambush under the sand at night, waiting for small rodents, lizards, and crickets, or forages for nestling birds or reptile eggs. This is a live-bearing species.
RELATED SPECIES
Three subspecies may be recognized, a nominate form (Eryx jaculus jaculus) in North Africa, a Eurasian subspecies (E. j. turcicus) from the Balkans to Syria, and a Caucasian subspecies (E. j. familiaris) from Armenia and Iran. Some authorities do not recognize any subspecies. The related Dwarf Desert Sand Boa (E. miliaris) occurs from Afghanistan to the northern Caucasus and may just enter Europe. Eryx jaculus also occurs in sympatry with the Central Asian Sand Boa (E. elegans) in Iran.
FAMILY |
Erycidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Southeastern Europe, North Africa and Middle East: Balkans to Greece, Turkey to northern Saudi Arabia and Iran, Egypt to Morocco |
ELEVATION |
0–4,920 ft (0–1,500 m) asl |
HABITAT |
Beaches, arable land, dry valleys, rocky scrub, and semidesert |
DIET |
Small mammals, birds, reptile eggs, lizards, and some invertebrates |
REPRODUCTION |
Viviparous, with litters of 6–20 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Javelin Sand Boa is moderately stout with a short tail, smooth scales, and a head that terminates in a pointed rostral scale for digging. The eyes are small with vertical pupils. It is generally gray to gray-brown, darker above than on the flanks, with an irregular pattern of darker brown or orange transverse bars, which may coalesce to form a zigzag pattern or vertebral stripe. Males have larger cloacal spurs than females.
ERYX JAYAKARI
ARABIAN SAND BOA
(BOULENGER, 1888)
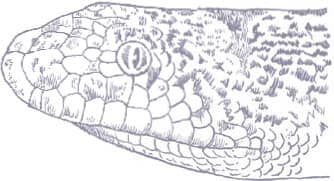
ADULT LENGTH
113/4–173/4 in, rarely 251/4 in (300–450 mm, rarely 640 mm)
The Arabian Sand Boa is a true desert-adapted snake, occurring in the Arabian Peninsula from Kuwait to Oman and Yemen. It inhabits sandy deserts and is marvelously adapted for life below the surface. It does not inhabit rocky or montane desert. Its head is shaped for digging and the almost dorsal eyes enable it to keep watch without exposing its whole head. Juveniles take soft-bodied invertebrates and small geckos while adults take adult geckos and small mammals like shrews or mice. This species may be fairly common, although it is nocturnal and rarely seen on the surface. The Arabian Sand Boa is an egg-laying species, producing a small number of large eggs. Colonel Atmaram Jayakar (1844–1911) was an Indian Army surgeon who collected the holotype in Oman.
RELATED SPECIES
No subspecies of Eryx jayakari are recognized. Its range may overlap that of the Javelin Sand Boa (E. jaculus) in the north. There is also a record of the East African or Egyptian Sand Boa (E. colubrinus) in Yemen. At night, in the dark, sand boas also bear a superficial resemblance to the dangerously venomous carpet or saw-scale vipers (Echis).
FAMILY |
Erycidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Arabia: Saudi Arabia, Kuwait, Yemen, Oman, and UAE |
ELEVATION |
0–3,610 ft (0–1,100 m) asl |
HABITAT |
Sandy desert |
DIET |
Lizards, small mammals, and invertebrates |
REPRODUCTION |
Oviparous, with clutches of up to 4 eggs |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |
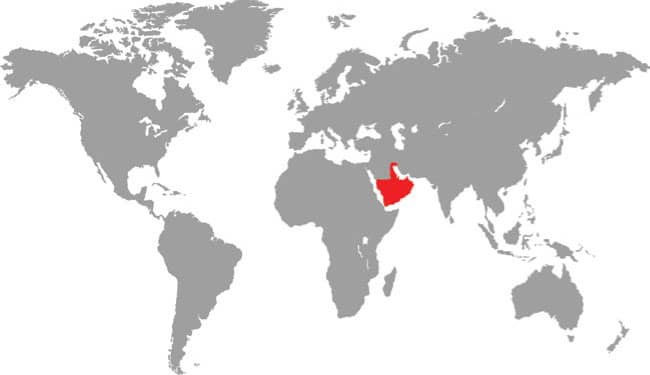

The Arabian Sand Boa is a moderately stout snake, with a short tail and a flattened head that terminates with a rounded flat rostral, which is used for excavation in the sand. The eyes are extremely small, with vertically elliptical pupils, and more dorsally positioned than in other sand boas. Cloacal spurs are present, larger in males than females. Coloration is tan, orange, or yellow with irregular darker transverse markings.
ERYX JOHNII
RED SAND BOA
(RUSSELL, 1802)

ADULT LENGTH
2 ft 5 in–3 ft 3 in (0.75–1.0 m)
The Red Sand Boa, also known as John’s Sand Boa, is one of the largest sand boas. It may be encountered in farmland. It is fossorial and nocturnal, ambushing rodents, birds, lizards, large invertebrates, and even other snakes, from a position just below the surface. Its stumpy tail resembles its head, leading to the colloquial but mistaken name of “two-headed snake.” This is a live-bearing species. Sand boas are popular with snake charmers, being large and impressive, but safe and docile to handle. The Red Sand Boa was described by the Honourable East India Company physician-naturalist and snake expert Patrick Russell (1726–1805), in honor of the missionary-medic and herpetologist Rev. Christoph John (1747–1813).
RELATED SPECIES
Eryx johnii occurs in sympatry with the Common Indian or Rough-scaled Sand Boa (E. conicus), an almost equally large species but with extremely rough, keeled scales. The Rough-scaled Sand Boa is also found in Sri Lanka, where E. johnii is absent. The Iranian–Afghan population of E. johnii may warrant subspecific status.
FAMILY |
Erycidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
South Asia: eastern Iran and Afghanistan, Pakistan, India, and Nepal |
ELEVATION |
0–3,150 ft (0–960 m) asl |
HABITAT |
Sandy soil habitats, semidesert, and agricultural habitats |
DIET |
Small mammals, birds, lizards, snakes, and invertebrates |
REPRODUCTION |
Viviparous, with litters of 6–8 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |


The Red Sand Boa is an extremely stout-bodied snake with a rounded head, indistinct from the neck, small eyes with vertical pupils, an enlarged shovel-shaped rostral for burrowing, and a short, stumpy tail, which resembles the head. It is smooth-scaled throughout and usually red or reddish-brown in color, although some specimens are gray or yellow and others have broad black bands that increase in definition on the posterior of the body and the tail.
CHARINA BOTTAE
NORTHERN RUBBER BOA
(BLAINVILLE, 1835)

ADULT LENGTH
193/4–233/4 in, rarely 323/4 in (500–600 mm, rarely 830 mm)
The Northern Rubber Boa may be the northernmost boa in the world, since it occurs from California, throughout the northwestern United States, to British Columbia, Canada. It often shelters beneath logs or boulders. Juveniles feed on reptile eggs and lizards, and adults on rodents, moles, birds, lizards, or salamanders. It stalks its prey, often with its mouth agape, strikes, and kills it by constriction. One defense involves coiling into a ball, head in the center, and the blunt tail elevated as a pseudo-head. While raiding mouse nests it has been observed to make mock strikes at the adult mouse with its tail, while it eats the young. Northern Rubber Boas may live for 20 years, and their tails are often scarred, possibly due to the above activity. Paulo Botta (1802–70) was an Italian physician and archeologist who visited California.
RELATED SPECIES
Until recently Charina bottae was treated as a single species, with two subspecies. The isolated southern Californian populations have now been elevated to specific status as the Southern Rubber Boa (C. umbratica). Rubber boas are unlikely to be confused with the only other boas within their range, the rosy boas (Lichanura).
FAMILY |
Charinidae: Charininae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Western North America: northwestern USA and British Columbia (Canada) |
ELEVATION |
1,640–10,000 ft (500–3,060 m) asl |
HABITAT |
Upland coniferous or pine–oak woodlands, grassland, and desert edges |
DIET |
Small mammals, birds, lizards, salamanders, and reptile eggs |
REPRODUCTION |
Viviparous, with litters of 1–10 neonates |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |


The Northern Rubber Boa is a small snake with a cylindrical body, rounded head, small eyes with vertical pupils, and stumpy tail. Coloration is usually mid-brown to olive-brown or green, without any markings, but lighter on the flanks and underbelly.
LICHANURA TRIVIRGATA
ROSY BOA
(COPE, 1861)
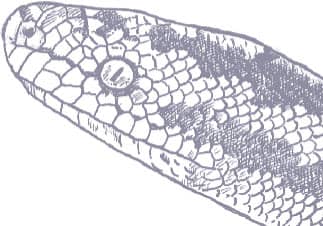
ADULT LENGTH
311/2–351/2 in, rarely 3 ft 7 in (800–900, rarely 1.1 m)
The Rosy Boa is popular in captivity because of its docility and small size. It occurs in a variety of subspecies and forms and inhabits semidesert habitats, usually where there is an abundance of rocks for shelter and hunting, such as talus slopes or rocky valleys. It is most common near water sources. Prey consists of small mammals, small birds, lizards, and other snakes; even a small Sidewinder Rattlesnake (Crotalus cerastes) has been reported as prey. Only males possess cloacal spurs, with which they stroke the female during courtship, a common feature of boa and python behavior. Despite their popularity in captivity, Rosy Boas are little studied in nature.
RELATED SPECIES
The status of the subspecies of Lichanura trivirgata has been the subject of considerable discussion and change. Some authorities recognize three subspecies: Mexican Rosy Boa (L. t. trivirgata) on the Baja California Peninsula, and the Desert Rosy Boa (L. t. gracia) and Coastal Rosy Boa (L. t. roseofusca), both from California. The northern Baja population is also sometimes treated as a separate subspecies (L. t. saslowi).Some authorities do not recognize any subspecies. The Northern Striped Rosy Boa from California is a separate species (L. orcutti).
FAMILY |
Charinidae: Charininae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North America: USA (California and Arizona) and Mexico (Baja California and northwestern Sonora) |
ELEVATION |
0–6,560 ft (0–2,000 m) asl |
HABITAT |
Semidesert, rocky slopes, and talus slopes, usually near water |
DIET |
Small mammals, birds, lizards, and snakes |
REPRODUCTION |
Viviparous, with litters of 1–12 neonates |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix II |
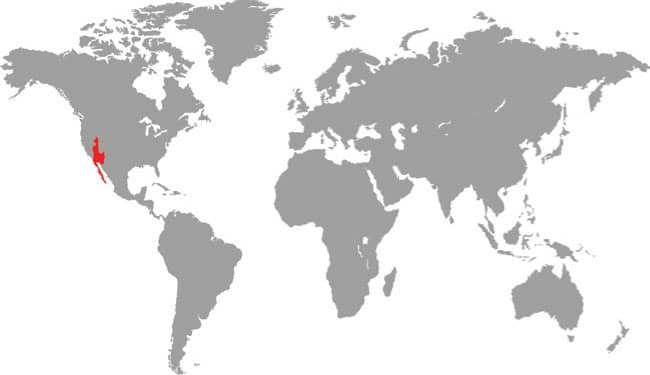

The Rosy Boa is a moderately stout-bodied snake, with smooth scales, a longish head that is only slightly distinct from the neck, small eyes, and a relatively short but prehensile tail. Coloration is variable and is the basis of some of the subspecies definitions. The body may be gray, brown, orange, or pink, overlain by three broad longitudinal stripes, which may be orange, rose-red, brown, or black, the vertebral stripe extending onto the dorsum of the head.
EXILIBOA PLACATA
OAXACAN DWARF BOA
BOGERT, 1968

ADULT LENGTH
153/4–181/2 in (400–470 mm)
Only recorded from the Sierra de Juárez and Sierra Mixe in northern Oaxaca state, Mexico, the Oaxacan Dwarf Boa inhabits cloud forest. The holotype was found under a large boulder and subsequent specimens have been found under large, flat stones, often the same stone yielding specimens over several years. Boas have also occasionally been seen moving around in light rain. The natural history of the Oaxacan Dwarf Boa in nature is poorly known but captive specimens fed on small frogs, though only in darkness, suggesting nocturnal activity. A museum specimen contained a salamander so it is assumed that amphibians are important in the diet. A docile species, it resorts to defensive balling when handled, or emptying the noxious contents of its cloacal glands.
RELATED SPECIES
Exiliboa placata differs from all other neotropical boas in the possession of a single large internasal scale. It does not appear to be closely related to any other species, but is closer to Ungaliophis (shown here) than other neotropical boas. It looks more like a semi-fossorial colubroid snake than a boa, but both sexes possess small cloacal spurs, something not seen in advanced snakes.
FAMILY |
Charinidae: Ungaliophiinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North America: Oaxaca (Mexico) |
ELEVATION |
6,560–8,040 ft (2,000–2,450 m) asl |
HABITAT |
Cool, moist cloud forest |
DIET |
Small frogs, their eggs, and salamanders |
REPRODUCTION |
Viviparous, with litters of 8–16 neonates |
CONSERVATION STATUS |
IUCN Vulnerable, CITES Appendix II |


The Oaxacan Dwarf Boa is a small snake with glossy black scales, a head just distinct from the neck, covered by enlarged scutes rather than granular scales, slightly protruding eyes, and a short tail. The only break in the black pigment is a white cloacal spot.
UNGALIOPHIS CONTINENTALIS
ISTHMIAN BROMELIAD BOA
MÜLLER, 1880
ADULT LENGTH
193/4–30 in (500–760 mm)
The Isthmian Bromeliad Boa, also known as the Northern Bromeliad or Isthmian Dwarf Boa, is a rare species, only known from a few specimens. It is known to inhabit lowland rainforest and upland pine forests, specimens being found on boulders above a stream, under rotten pine logs, and on a bromeliad-laden tree. Such a microhabitat would provide shelter, moisture, and potential prey. Virtually everything known regarding the natural history of this snake comes from captive specimens. Captive specimens have taken neonate mice, lizards, and frogs; in nature they also take birds and bats. The male uses his cloacal spurs to court the female, but he also engages in copulatory biting of her tail as he coils his body around hers.
RELATED SPECIES
Ungaliophis species possess a single large prefrontal scale on their heads, a feature not seen in other boas. A second species, the Panamanian Bromeliad Boa (U. panamensis), occurs from southern Nicaragua to Colombia. The two species exhibit a number of scalation and dental differences, and U. panamensis has triangular rather than ovoid markings.
FAMILY |
Charinidae: Ungaliophiinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North and Central America: southern Mexico, Guatemala, Honduras, and Nicaragua |
ELEVATION |
215–7,550 ft (65–2,300 m) asl |
HABITAT |
Lowland tropical rainforest, montane pine woodlands, and cloud forest |
DIET |
Birds, bats, frogs, and lizards |
REPRODUCTION |
Viviparous, with litters of 2–10 neonates |
CONSERVATION STATUS |
IUCN not listed, CITES Appendix II |
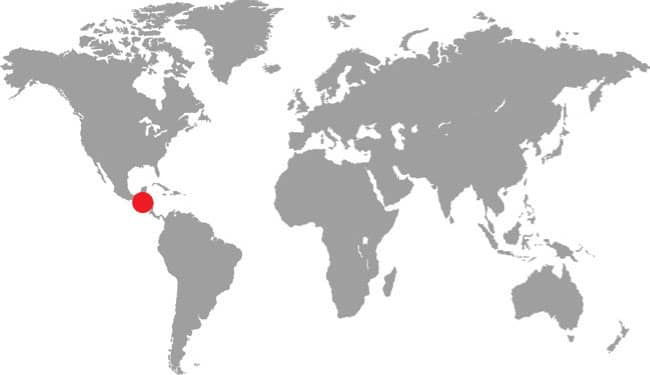

The Isthmian Bromeliad Boa is a small, slender snake with smooth scales, a slightly pointed head, just distinct from the neck, and a short, prehensile tail. It is generally gray, the ground color heavily flecked with black, and with orange on the lower flanks and a pale-edged black arrowhead marking on the dorsum of the head, which continues down the back as a double row of distinctive light-edged, ovoid black ocelli.
ACRANTOPHIS DUMERILI
DUMÉRIL’S BOA
JAN, 1860
ADULT LENGTH
4–5 ft, rarely 10 ft (1.25–1.5 m, rarely 3.0 m)
Duméril’s Boa is the second largest snake in Madagascar after its congener, the Madagascan Ground Boa (Acrantophis madagascariensis), which may achieve 10 ft 6 in (3.2 m). Duméril’s Boa, which was named in honor of the French herpetologist André Marie Constant Duméril (1774–1860), is a common species. Terrestrial and nocturnal, it is often killed on roads. Prey includes mammals, from rats and bats to tenrecs and lemurs, but birds including domestic fowl are also taken. While the Madagascan Ground Boa produces small litters (two to six) of large neonates, Duméril’s Boa produces large litters (6–13) of small neonates. Curiously, males are larger than females, while in most other boas the female is the larger. Both species are referred to in Malagasy as “do.”
RELATED SPECIES
While Acrantophis dumerili occurs in southern and central Madagascar, its closest relative, the Madagascan Ground Boa (A. madagascariensis), inhabits the north of the island, although there are locations on the western coast where both species may occur in sympatry. The two can be distinguished by the enlarged scutes on the anterior head of A. madagascariensis, which are absent in A. dumerili.
FAMILY |
Sanziniidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Indian Ocean: southern and central Madagascar |
ELEVATION |
0–4,350 ft (0–1,325 m) asl |
HABITAT |
Thornbush savanna, dry forest, and cultivated habitats |
DIET |
Mammals and birds |
REPRODUCTION |
Viviparous, with litters of 6–13 neonates |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix I |
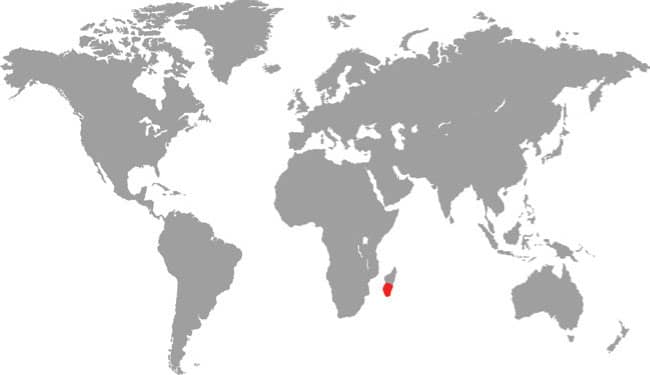

Duméril’s Boa is a stout snake with a powerful, muscular body, a broad, angular head that is distinct from the neck, and a prehensile tail. It is generally gray or brown, with irregular black or dark brown dumbbell-shaped saddles that narrow considerably to cross the back, and a dark postocular stripe following the eye. Acrantophis dumerili and A. madagascariensis bear a striking resemblance to the Common Boa (Boa constrictor) of the Neotropics.
SANZINIA MADAGASCARIENSIS
MADAGASCAN TREEBOA
(DUMÉRIL & BIBRON, 1844)
ADULT LENGTH
5–6 ft, rarely > 6 ft 7 in (1.5–1.85 m, rarely > 2.0 m)
The Madagascan Treeboa, also known as the “Sanzinia,” is a very common snake in eastern Madagascar. It is arboreal during the day, but terrestrial during the night, and found in a wide variety of habitats, ranging from primary rainforest to plantations and even village gardens. As a relatively large constricting snake it can take a wide array of prey, from rodents and tenrecs, to small lemurs, birds, and frogs. Warm-blooded prey may be located using the treeboa’s heat-sensitive labial pits, captured, and constricted. The Madagascan Treeboa has long teeth for securing prey and it will bite if disturbed. Despite this snake being nonvenomous, the teeth can deliver a painful, bloody bite. Stories of 8 ft 2 in–13 ft (2.5–4 m) specimens are not thought credible. The local name for this snake is “mandrita.”
RELATED SPECIES
The name Boa mandrita was used for this species for a period during the late twentieth century because when Sanzinia and the two Acrantophis species were sunk into genus Boa there would have been two species called Boa madagascariensis. Today a second species is recognized (S. volontany), inhabiting the drier western forests. Although the two species look similar they differ genetically.
FAMILY |
Sanziniidae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Indian Ocean: eastern Madagascar |
ELEVATION |
0–5,250 ft (0–1,600 m) asl |
HABITAT |
Primary and secondary rainforest, plantations, and cultivated and inhabited areas |
DIET |
Mammals, birds, and frogs |
REPRODUCTION |
Viviparous, with litters of 1–19 neonates |
CONSERVATION STATUS |
IUCN Least Concern, CITES Appendix I |
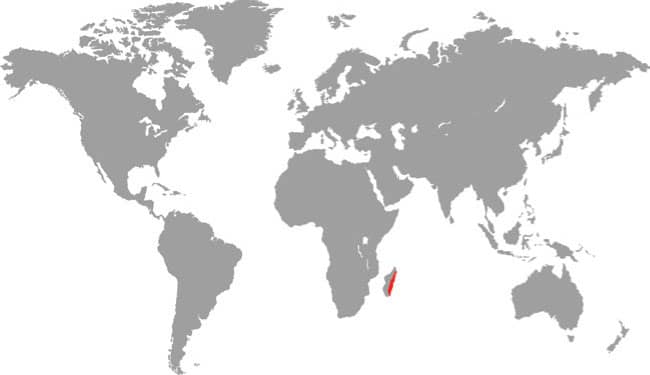

The Madagascan Treeboa has an elongate, muscular body, prehensile tail, and a broad chunky head with obvious labial pits. Coloration is variable but most specimens are brown or olive with a series of transverse, light-edged dumbbell markings, and a black postocular stripe from the eye to the angle of the jaw. Males and some females have cloacal spurs.