OOCATOCHUS RUFODORSATUS
RED-BACKED RATSNAKE
(CANTOR, 1842)
ADULT LENGTH
193/4–271/2 in, rarely 351/2 in (500–700 mm, rarely 900 mm)
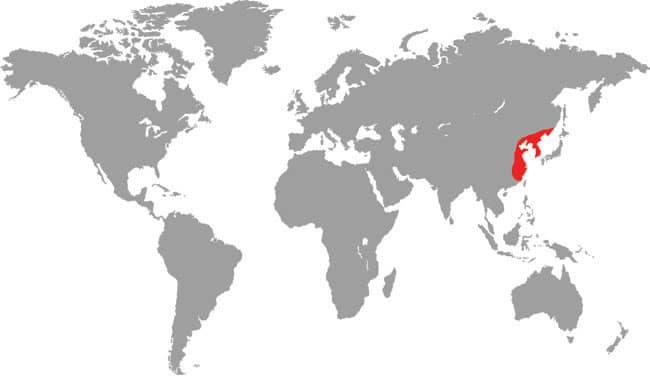
Also known as the Frog-eating Ratsnake, the Red-backed Ratsnake occurs from far-eastern Russia, south through Korea, to eastern China and Taiwan. It occurs in low-lying freshwater aquatic habitats such as swamps, marshes, rice paddies, ponds, and streams. It is also encountered in meadows and it enters gardens. It can be a very common species. If threatened it flees to the water, where it remains submerged. A terrestrial-aquatic species, it rarely climbs. Its prey is also primarily aquatic, consisting of fish and frogs, but lizards, mice, other snakes, and some insects are also taken. The Red-backed Ratsnake is Asia’s only known viviparous member of the Colubrinae, all other species being oviparous.
RELATED SPECIES
Originally included in the ratsnake genus Elaphe, but now the only species in the genus Oocatochus, O. rufodorsatus is related to the equally viviparous Smooth Snake (Coronella austriaca), rather than other ratsnakes.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
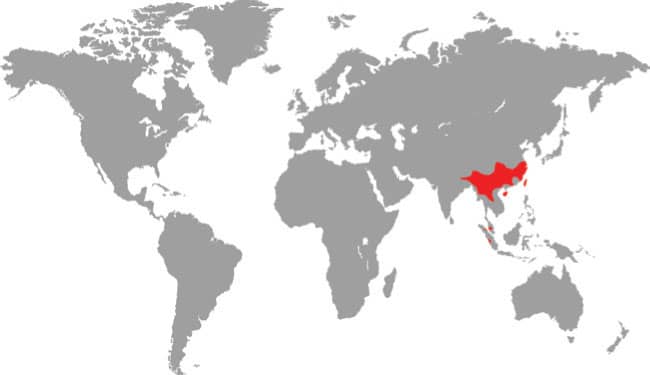
East Asia: northeast China, eastern Russia, Korea, and Taiwan |
ELEVATION |
245–3,280 ft (75–1,000 m) asl |
HABITAT |
Swamps, rice paddies, ponds, streams, meadows, and gardens |
DIET |
Fish, frogs, lizards, small mammals, other snakes, and insects |
REPRODUCTION |
Viviparous, with litters of 8–25 neonates |
CONSERVATION STATUS |
IUCN Least Concern |

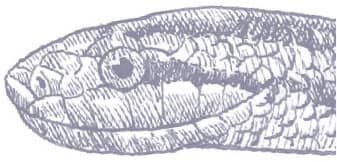
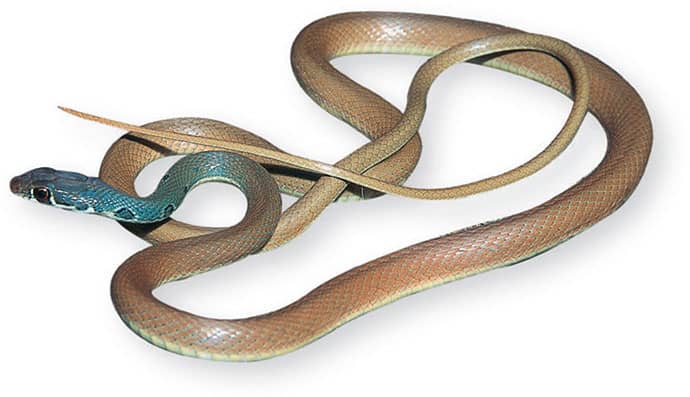
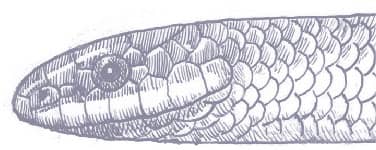
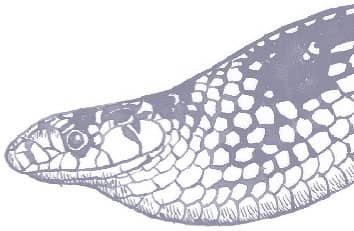
The Red-backed Ratsnake is a small, slender snake with smooth scales, a short tail, and a narrow head, hardly broader than the neck, with slightly protruding eyes and round pupils. It is a boldly but variably marked species with a gray or brown background color. Patterning usually comprises a brown, orange, or yellow vertebral stripe, which continues onto the tail, with lateral patterning consisting of either stripes or rows of blotches.
OPHEODRYS AESTIVUS
ROUGH GREENSNAKE
(LINNAEUS, 1766)
ADULT LENGTH
233/4–291/2 in, rarely 3 ft 10 in (600–750 mm, rarely 1.16 m)
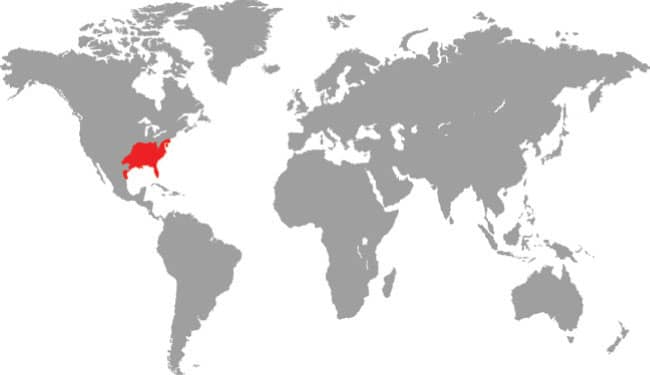
Distributed throughout the southeastern USA, from New Jersey to Texas, including the Florida Peninsula, and entering northwestern Mexico, the Rough Greensnake is primarily an arboreal species. It is found in a wide variety of wet habitats, from swamps to woodland, and from meadows to the banks of canals and rivers. It is an agile diurnal climber, very well camouflaged for arboreal life, being rendered almost invisible in the crowns of even sparsely foliated trees. It sleeps aloft and this is probably when it is most easily discovered. Rough Greensnakes feed on a wide variety of invertebrates, with insects and spiders their primary prey. They also take snails, millipedes, woodlice, and occasionally also small frogs. The use of pesticides may have an adverse effect on Rough Greensnake populations by reducing prey availability.
RELATED SPECIES
Two subspecies are recognized, the Northern Rough Greensnake (Opheodrys aestivus aestivus) and the Florida Rough Greensnake (O. a. carinatus). The closest relative to O. aestivus is the Smooth Greensnake (O. vernalis), a species with smooth scales from northern and northeastern USA and southeastern Canada. Some authors place one or both species in the genus Liochlorophis.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: southeastern USA and northwest Mexico |
ELEVATION |
0–5,000 ft (0–1,525 m) asl |
HABITAT |
Marshland, swamps, lake edges, river and canal banks, wet grasslands and meadows, and wet woodland edges |
DIET |
Insects, spiders, millipedes, isopods, snails, and small frogs |
REPRODUCTION |
Oviparous, with clutches of 1–14 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

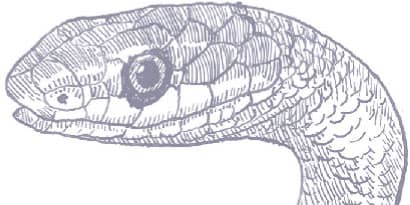
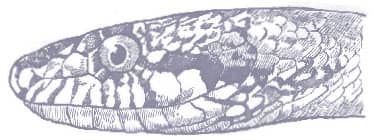
The Rough Greensnake is a slender snake with keeled scales, a long tail, and a head broader than the neck, with relatively large eyes and round pupils. It is dorsally green, darker on the dorsum than on the flanks, and the venter may be white or yellow, and with yellow-green lips and a yellowish throat.
OREOCRYPTOPHIS PORPHYRACEUS
RED MOUNTAIN RATSNAKE
(CANTOR, 1839)
ADULT LENGTH
311/2–351/2 in, rarely 4 ft (800–900 mm, rarely 1.25 m)
Also known as the Red Bamboo Trinket Snake, this distinctly patterned snake is found throughout East and Southeast Asia. It inhabits rainforest, montane, and hill forest to elevations up to 8,530 ft (2,600 m) asl. It is crepuscular and terrestrial in habit, and a rare and secretive species that burrows into deep mossy beds or hides on rocky slopes with stands of bamboo and tussock grass. Its prey consists of small mammals, mostly voles and shrews, which are killed by constriction. The Red Mountain Ratsnake is a slow-moving species and inoffensive, rarely biting even when handled. The generic name Oreocryptophis means “secretive mountain snake” (Oreo = mountain, -crypto = secretive, -ophis = snake).
RELATED SPECIES
Oreocryptophis is a monotypic genus, previously included in Elaphe. Up to eight subspecies are recognized, the nominate form (O. porphyraceus porphyraceus) occurring in southwest China, northeast India, Nepal, northern Myanmar, and Thailand. Other subspecies are found in south and east China, Laos, Cambodia, and Vietnam (O. p. vaillanti); central China (O. p. pulchra); Taiwan (O. p. kawakamii); Hainan Island (O. p. hainana); northeastern Thailand (O. p. coxi); Peninsular Malaysia and Sumatra (O. p. laticincta), and Hong Kong, southern China, and possibly Laos and Vietnam (O. p. nigrofasciata).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
East and Southeast Asia: northeast India to eastern China and Taiwan, south to Singapore and Sumatra |
ELEVATION |
380–8,530 ft (115–2,600 m) asl |
HABITAT |
Low to medium montane forest, rainforest, forest edge situations, mossy forest floors, and bamboo thickets |
DIET |
Small mammals |
REPRODUCTION |
Oviparous, with clutches of 2–7 eggs |
CONSERVATION STATUS |
IUCN not listed |

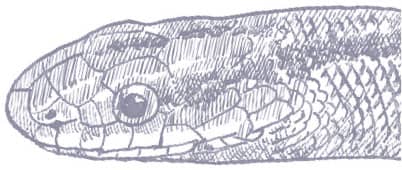
The Red Mountain Ratsnake has a laterally compressed, smooth-scaled body, a narrow, elongate, squarish head, and small eyes and round pupils. Two color morphotypes are defined. One is dark red-brown with broad, pale-edged black bands, a black stripe through each eye, a black dorsal stripe on the head, and fine dorsolateral black stripes passing from band to band. The other is similar but the broad bands are red or orange, edged with black, and the background color is red or orange. Some specimens are striped rather than banded.
ORIENTOCOLUBER SPINALIS
SLENDER RACER
(PETERS, 1866)
ADULT LENGTH
193/4–221/2 in (500–570 mm)
The Slender Racer is a very poorly documented species. Its range largely lies within northern China and Mongolia but it also occurs in far-eastern Russia, on the Korean Peninsula, and in Kazakhstan, where it is listed in the Red Data Book of Endangered Species. In the interior of Asia it is found in arid habitats such as rocky or gravel semidesert, or rocky hillsides with shrubby cover, but it is also reported from forests and close to streams, and on the far-eastern coast it inhabits thickets. Its prey preferences are documented as comprising geckos and lacertid lizards. This species is nervous and fast-moving, but inoffensive if handled. It is oviparous with clutches of up to nine eggs.
RELATED SPECIES
Orientocoluber is a monotypic genus most closely allied to the Eurasian whipsnakes and racers of genera Dolichophis (shown here) and Hierophis (shown here), in which genus it was once listed, and the dwarf snakes (Eirenis).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
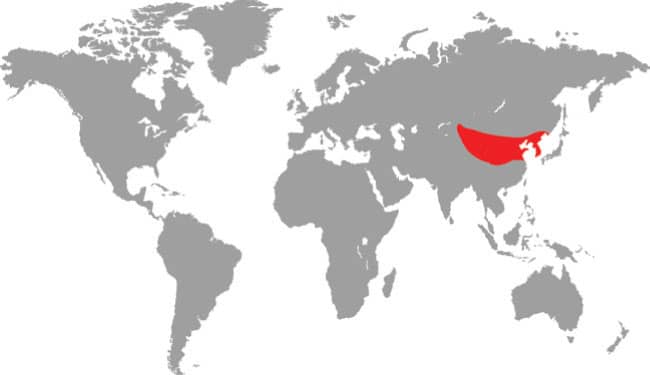
Southeast Asia: Russia, Mongolia, Kazakhstan, northern China, and Korean Peninsula |
ELEVATION |
4,080–6,230 ft (1,245–1,900 m) asl |
HABITAT |
Rocky or gravel semidesert, vegetated mountain slopes, streams, forests, and coastal thickets |
DIET |
Lizards |
REPRODUCTION |
Oviparous, with clutches of 4–9 eggs |
CONSERVATION STATUS |
IUCN not listed, listed in Kazakhstan Red Data Book |

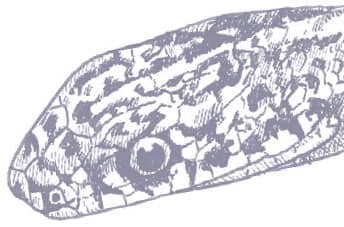
The Slender Racer is, as its name suggests, an extremely slender snake with weakly keeled scales, a long tail, and a head only slightly wider than the neck, with large eyes, round pupils, and shelved supraocular scales that present a scowling expression. It is gray to brown above, darker dorsally than laterally, and white below. A distinctive white vertebral stripe extends from the dorsum of the head to the tail, black flecks are present on the flanks, and there is black marbling on the head.
OXYBELIS AENEUS
BROWN VINESNAKE
(WAGLER, 1824)
ADULT LENGTH
3–5 ft 7 in (0.9–1.7 m)
The Brown Vinesnake is recorded from every territory from southwestern USA to eastern Bolivia, including many islands off the Caribbean and Pacific coasts. It inhabits both pristine and disturbed habitats, and is often found hunting small lizards, such as anoles, in low vegetation. It is also reported to take small birds, mice, frogs, and insects. When stalking prey the diurnal Brown Vinesnake elevates its head and anterior body and moves in a ponderous, punctuated way, with a swaying motion. This form of movement may assist it to locate camouflaged prey, and also blend in with the movements of the vegetation. Its venom is weak and designed for lizards, although one bite to a human caused localized swelling and blistering. Its main defense is to gape widely and expose its blue-black mouth lining.
RELATED SPECIES
The American vinesnakes bear a strong resemblance to the Asian vinesnakes (Ahaetulla) and the African twigsnakes (Thelotornis), which are both genera of the Colubridae, although Oxybelis has round pupils while the other two genera possess horizontally elliptical pupils. In this respect Oxybelis more resembles the Caribbean treesnakes of genus Uromacer (shown here), although that genus is in the Dipsadidae.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
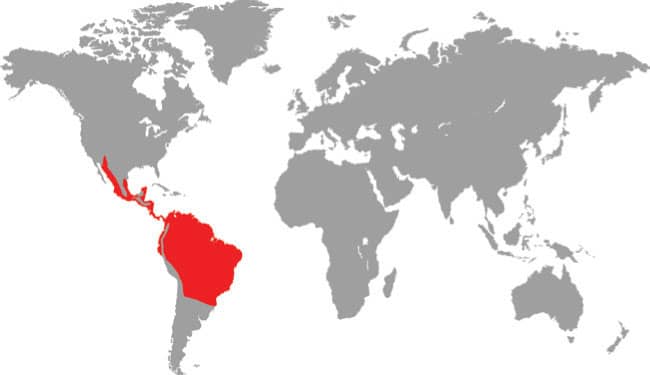
DISTRIBUTION |
North, Central, and South America: southwest USA and Mexico, to eastern Bolivia, including Trinidad and Tobago, and Aruba Island |
ELEVATION |
0–6,280 ft (0–1,915 m) asl |
HABITAT |
Primary and secondary forest, gallery forest, woodland, disturbed habitats, and gardens |
DIET |
Lizards, small birds, frogs, small mammals, and insects |
REPRODUCTION |
Oviparous, with clutches of 3–8 eggs |
CONSERVATION STATUS |
IUCN not listed |

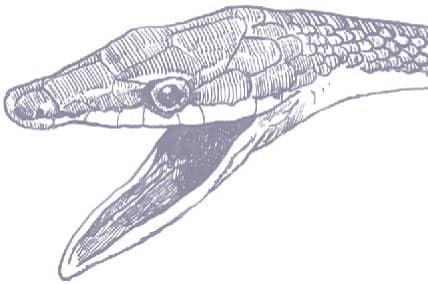
The Brown Vinesnake is an extremely slender, laterally compressed snake with smooth, obliquely arranged scales, a long prehensile tail, and an elongate, pointed, narrow head, and eyes with round pupils. It is brown or gray-brown above and white below, with the supralabials and throat also white, the line between brown and white exhibiting a stark demarcation around the lower edge of the eye.
OXYBELIS FULGIDUS
GREEN VINESNAKE
(DAUDIN, 1803)

ADULT LENGTH
4 ft 3 in–6 ft 7 in, rarely 7 ft 7 in (1.3–2.0 m, rarely 2.3 m)
The Green Vinesnake occurs in sympatry with the Brown Vinesnake (Oxybelis aeneus) throughout much of its range, which extends from southern Mexico to northeastern Bolivia. A larger species, it is found in various forest habitats, from lowland to montane, primary to secondary, and wet to dry, but is also found in small trees and lower vegetation, and may be met with on the ground. It feeds on frogs, lizards, birds, and small mammals, killing them with venom injected through the enlarged rear fangs. Although like the Brown Vinesnake it is diurnal, it is less frequently encountered. Its venom is effective on lizards, frogs, and even small mice, but although bites to humans have caused localized pain and swelling there have been no more serious effects.
RELATED SPECIES
There are two other greenish Oxybelis vinesnakes in tropical America, the Short-headed Vinesnake (O. brevirostris), which occurs from Honduras to western Ecuador, and the endemic Isla de Roatán Vinesnake (O. wilsoni), which is more mustard yellow in color and which occurs on the largest of the Honduran Islas de la Bahía, while O. fulgidus is found on neighboring Isla de Utila.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
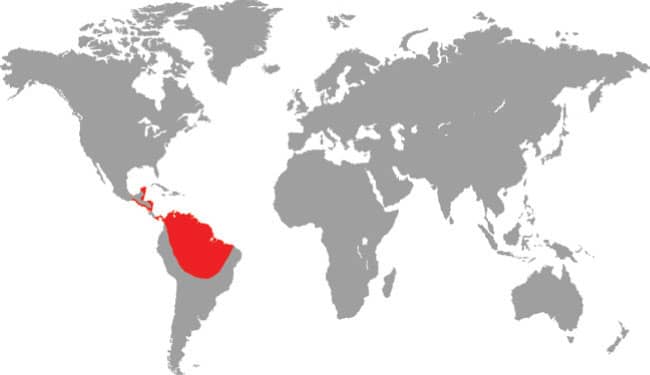
DISTRIBUTION |
North, Central, and South America: southern Mexico to northeastern Bolivia |
ELEVATION |
0–5,250 ft (0–1,600 m) asl |
HABITAT |
Primary and secondary rainforest, montane forest, and riverine forest |
DIET |
Frogs, lizards, birds, and small mammals |
REPRODUCTION |
Oviparous, with clutches of 8–14 eggs |
CONSERVATION STATUS |
IUCN not listed |

The Green Vinesnake is slender with a long, moderately laterally compressed body, smooth scales in oblique rows, a long prehensile tail, and a long head, which terminates in a sharp, pointed snout. The small eyes have round pupils. The dorsum is emerald green, the lower flank and the lower half of the head are light green, and the undersides are green. A pale yellow-green stripe runs from the snout tip, under the eye to the angle of the jaw, and a white stripe runs along the lower flanks.
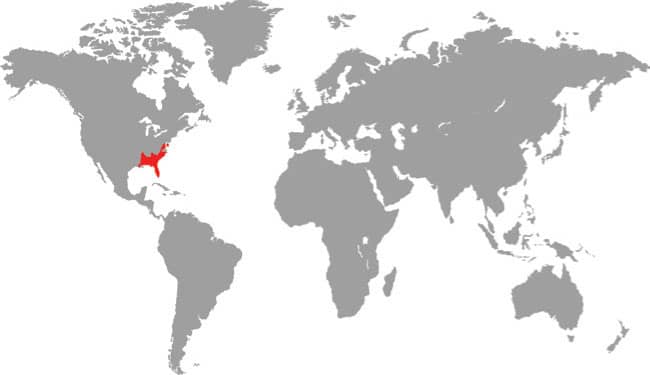
PANTHEROPHIS ALLEGHANIENSIS
EASTERN RATSNAKE
(HOLBROOK, 1836)
ADULT LENGTH
3 ft 3 in–6 ft, occasionally 7 ft 7 in (1.0–1.8 m, occasionally 2.3 m)
The recently redefined Eastern Ratsnake occurs along the Atlantic seaboard, east of the Appalachians and Apalachicola River, from New England to the Florida Keys. A powerful constrictor, it is found in many habitats, including deciduous and mixed woodland, pinewoods, and hardwood hammocks on sawgrass plains. It also occurs in farmland, in coastal mangrove entanglements, and on small islands, such as the Keys. It is an adept climber, even of tall palms or pines, using its keeled ventral scales and powerful coils to scale the smoothest of trunks. Its prey preferences include most vertebrates small enough to overpower and swallow, especially mammals and birds. It sometimes takes domestic chickens and their eggs, hence the origin of the old name “Chicken Snake.” Lizards, other snakes, and frogs are also included in the diet.
RELATED SPECIES
The American Ratsnake (Elaphe obsoleta) contained five to eight subspecies defined by color pattern, but modern molecular analysis led to the genus Elaphe being confined to Eurasian ratsnakes, with Pantherophis resurrected for American ratsnakes. It was determined that three separate species existed, P. alleghaniensis east of the Appalachians and Apalachicola River, the Midland Ratsnake (P. spiloides) between these barriers and the Mississippi, and the Western Ratsnake (P. obsoletus) west of the Mississippi. The former subspecies E. o. obsoleta had comprised the melanistic northern populations of all three taxa.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North America: eastern USA |
ELEVATION |
0–1,970 ft (0–600 m) asl |
HABITAT |
Deciduous and mixed woodland, pinewoods, cypress stands, hardwood hammocks in sawgrass plains, mangrove thickets, farmland, and small islands |
DIET |
Mammals, birds, lizards, snakes, and frogs |
REPRODUCTION |
Oviparous, with clutches of 8–20, rarely 30 eggs |
CONSERVATION STATUS |
IUCN not listed |

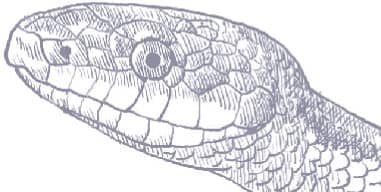
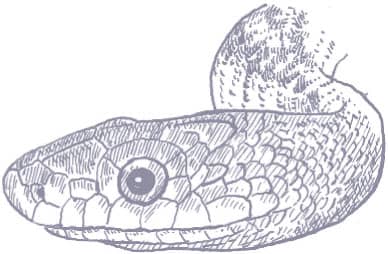
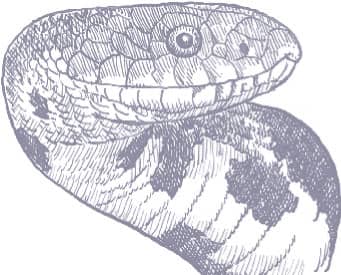
The Eastern Ratsnake is large snake, with a long head, large eyes, and round pupils. Its color pattern varies, northern populations being black with only faint stripes, central populations yellow with four black stripes, and southern populations yellow-gray with brown stripes (Gulf Hammock), orange with faint stripes (Everglades), or buff with brown stripes and blotches (Florida Keys). Juveniles are spotted, the origin of the generic name Pantherophis.
PANTHEROPHIS BAIRDI
BAIRD’S RATSNAKE
(YARROW, 1880)
ADULT LENGTH
4 ft–4 ft 7 in, occasionally 5 ft 2 in (1.2–1.4 m, occasionally 1.6 m)
Baird’s Ratsnake is found in southwestern Texas, USA, and across the border into Coahuila, Nuevo León, and Tamaulipas in northeast Mexico. It is an inhabitant of arid upland habitats, including rocky hillsides and wooded limestone canyons, but is also found in riparian habitats and wetland areas on desert fringes. It also occurs around human habitations. Baird’s Ratsnake is nocturnally active, especially following rainfall, and although primarily terrestrial it also climbs into low vegetation or onto rocky outcrops. It feeds on rodents, bats, birds and their eggs, and occasionally lizards, but given its secretive nocturnal existence it has been little studied in nature. It will vibrate its tail on dead leaves as a warning if confronted, and may bite if handled. Spencer Fullerton Baird (1823–87) was an American ornithologist and herpetologist.
RELATED SPECIES
Pantherophis bairdi is related to the Cornsnake (Pantherophis guttatus), Western Ratsnake (P. obsoletus), and Eastern Foxsnake (P. vulpinus).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North America: southern USA and northern Mexico |
ELEVATION |
2,950–5,910 ft (900–1,800 m) asl |
HABITAT |
Semiarid woodland, rocky hillsides, wooded limestone canyons, and riparian habitats |
DIET |
Small mammals, birds and their eggs, and lizards |
REPRODUCTION |
Oviparous, with clutches of 4–15 eggs |
CONSERVATION STATUS |
IUCN not listed |

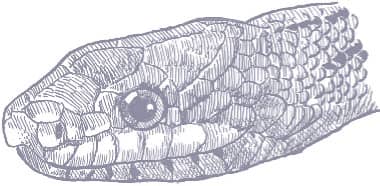
Baird’s Ratsnake is a moderately large snake with smooth scales, a prehensile tail, a head that is distinct from the neck, and large eyes with round pupils. Its ground color varies from yellow to yellow-gray, gunmetal, or silver, with broad, pale gray longitudinal stripes. The yellow pigment predominates on the anterior body while the posterior body and tail are darker, but coloration varies across the geographical range. Juveniles are gray with darker gray bands rather than striped.
PANTHEROPHIS GUTTATUS
CORNSNAKE
(LINNAEUS, 1766)
ADULT LENGTH
2–5 ft, rarely 6 ft (0.6–1.5 m, rarely 1.8 m)
The Cornsnake, or Red Ratsnake, is found throughout southeastern and eastern USA, from New Jersey to Florida, and west to Texas, in a wide variety of habitats including dry woodlands, pine barrens on sandy soil, freshwater swamps, hammocks in sawgrass plains, and mangrove swamps. Cornsnakes are also commonly encountered around farm buildings and even in suburban areas. This is a nocturnal predator, primarily of endothermic (warm-blooded) prey, rodents, and birds and their eggs, but it will also feed on frogs, lizards, sometimes other snakes, or insects. With its keeled ventral scales and muscular body, it is an excellent climber of both trees and buildings. Cornsnakes are inoffensive, rarely bite, and are popular in captivity. Many cultivars or color morphotypes are bred specifically for the vast pet trade.
RELATED SPECIES
Formerly called Elaphe guttata, Pantherophis guttatus is related to the Great Plains Ratsnake (P. emoryi), a former subspecies, and Slowinski’s Cornsnake (P. slowinskii), from Louisiana and Texas, named for the late Joseph Slowinski, killed by a Many-banded Krait (Bungarus multicinctus) in 2001. The former Rosy Ratsnake (E. g. rosacea), from the Florida Keys, is no longer recognized.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North America: southeastern and eastern USA |
ELEVATION |
0–4,530 ft (0–1,380 m) asl |
HABITAT |
Hardwood forests, pine barrens, swamps, grassy plains, mangrove swamps, and around human habitations |
DIET |
Mammals, birds, lizards, snakes, frogs, and insects |
REPRODUCTION |
Oviparous, with clutches of 3–40 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

The Cornsnake varies in patterning across its range. The ground color may be gray, tan, or orange with a series of large, dark-edged, dark red rhomboid or oval saddles across the back, smaller markings on the flanks, and similarly colored postocular and dorsal stripes on the dorsum and sides of the head. The undersides are usually checkerboard black and white. Juveniles are gray with brown dorsal saddles. Adult amelanistic (lacking black) and anerythristic (lacking red) partial albinos are also encountered in nature.
PANTHEROPHIS VULPINUS
EASTERN FOXSNAKE
(BAIRD & GIRARD, 1853)
ADULT LENGTH
3 ft 3 in–4 ft 7 in, occasionally 6 ft (1.0–1.4 m, occasionally 1.8 m)
The Eastern Foxsnake occurs around the Great Lakes, in Ontario, Canada, and Ohio, Michigan, Illinois, and Indiana, USA. Other American ratsnakes show a preference for forested or wooded habitats, but the Eastern Foxsnake prefers open habitats such as grasslands, swamps, and agricultural habitats. It also inhabits open woodlands, and occurs around buildings. Mammals form the bulk of the adult’s diet, with birds also taken. Juveniles feed on lizards, frogs, and insects. Prey is killed by constriction. The Eastern Foxsnake is less studied in nature than its more commonly encountered relatives. It is an inoffensive species that rarely bites.
RELATED SPECIES
There were two subspecies of foxsnakes, the Western Foxsnake (P. vulpinus), from Michigan to Nebraska, and the Eastern Foxsnake (P. gloydi), from Michigan, northern Ohio, and Ontario. Molecular analysis has demonstrated that the division between the two populations is the Mississippi Valley, so P. gloydi was synonymized with P. vulpinus as the Eastern Foxsnake, and a new species was described as the Western Foxsnake (P. ramspotti).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North America: northern USA and southeastern Canada |
ELEVATION |
500–1,500 ft (152–457 m) asl |
HABITAT |
Grasslands and pastures, open woodland, agricultural habitats, marshes, beaches, and around buildings |
DIET |
Mammals, birds and their eggs, lizards, frogs, and also snakes, salamanders, and earthworms |
REPRODUCTION |
Oviparous, with clutches of 7–20, rarely 29 eggs |
CONSERVATION STATUS |
IUCN Least Concern |


The Eastern Foxsnake is gray to yellow, with patterning comprising a series of brown or black irregular dorsal blotches on the back and a small series on either flank. The head may bear a small dark marking in the center. The adult livery of some specimens is quite similar to that of the juveniles of other American ratsnakes, with dark blotches on a pale gray background.
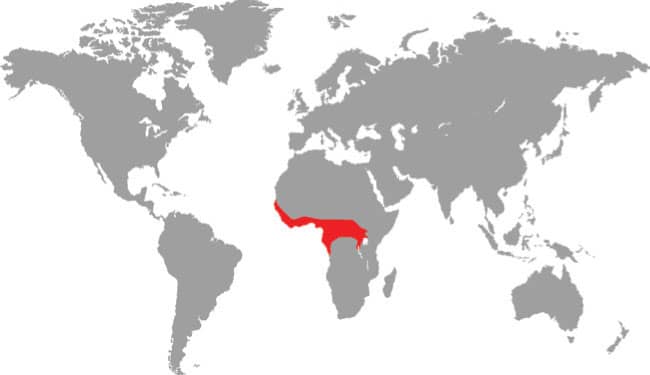
PHILOTHAMNUS HETERODERMUS
FOREST GREENSNAKE
(HALLOWELL, 1857)
ADULT LENGTH
173/4–271/2 in, rarely 351/2 in (450–700 mm, rarely 900 mm)
The Forest Greensnake is not always green, some specimens being brown. It inhabits deep forest, including rainforest, evergreen forest, gallery forest, and also deciduous woodland and savanna woodland, in a broad band through West, Central, and East Africa, from Guinea-Bissau to Kenya and south to Angola. It occurs in lowland and low montane forests to elevations of almost 6,560 ft (2,000 m). It is diurnal, and both arboreal and terrestrial in habit. The prey preferences of the Forest Greensnake comprise frogs, with toads reportedly refused. It is not known if lizards feature in the diet. This is generally a poorly known species.
RELATED SPECIES
The African genus Philothamnus contains 20 species of smooth-scaled treesnakes, which are closely related to the keel-scaled African treesnakes of genus Hapsidophrys (shown here). The closest relative of P. heterodermus is its former subspecies, the Thirteen-scaled Greensnake (P. carinatus), from Central Africa, which despite its name also has smooth dorsal scales. Harmless green treesnakes in Africa are often mistaken for green mambas (Dendroaspis), and killled.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
West, Central, and East Africa: Guinea-Bissau to Angola, and Kenya |
ELEVATION |
33–6,230 ft (10–1,900 m) asl |
HABITAT |
Rainforest, gallery forest, evergreen forest, deciduous woodland, and savanna woodland |
DIET |
Frogs |
REPRODUCTION |
Oviparous, with clutches of 1–4 eggs |
CONSERVATION STATUS |
IUCN not listed |

The Forest Greensnake is a slender snake with obliquely arranged, smooth scales, a long tail, and a narrow, elongate head with large eyes and round pupils. Two color morphotypes exist, a green morphotype with a yellow-green venter, and a dark brown morphotype with a light brown venter. White spots are present on the anterior body scales but these remain concealed unless the snake inflates its throat defensively.
PHILOTHAMNUS SEMIVARIEGATUS
SPOTTED BUSHSNAKE
(SMITH, 1840)
ADULT LENGTH
21/4–31/2 ft, occasionally 4 ft 3 in (0.7–1.1 m, occasionally 1.3 m)
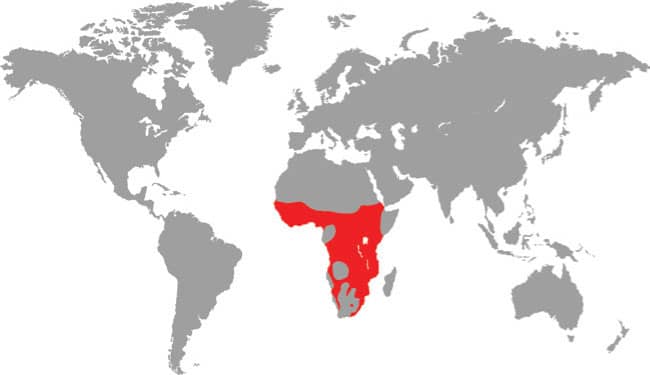
The Spotted Bushsnake is an extremely attractive arboreal snake distributed very widely through Sub-Saharan Africa. It occurs in a range of wet and dry woodland, forest, and savanna habitats, from semidesert to riverine gallery forest and coastal bush. It is one of the most frequently encountered bushsnakes in Africa. It is alert, agile, fast-moving, and well suited to life aloft, being able to climb the trunks of trees swiftly using the keels on its ventral scales to obtain a purchase, and using its slender body to bridge caps between branches. Its prey consists of geckos, chameleons, and frogs. When threatened, the Spotted Bushsnake inflates its throat to expose the contrasting blue edges to its scales.
RELATED SPECIES
The closest relatives of the Spotted Bushsnake include the Western Green Snake (Philothamnus angolensis), which has a patchy distribution from KwaZulu-Natal to Cameroon, and the Elegant Greensnake (P. nitidus) from West, Central, and East Africa.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Sub-Saharan Africa: Ethiopia and Sudan to Guinea and South Africa |
ELEVATION |
0–6,560 ft (0–2,000 m) asl |
HABITAT |
Wet and dry forest and savanna, karoo scrub, coastal bush and forest, savanna woodland, semidesert, and riparian habitats |
DIET |
Lizards and frogs |
REPRODUCTION |
Oviparous, with clutches of 3–12 eggs |
CONSERVATION STATUS |
IUCN not listed |
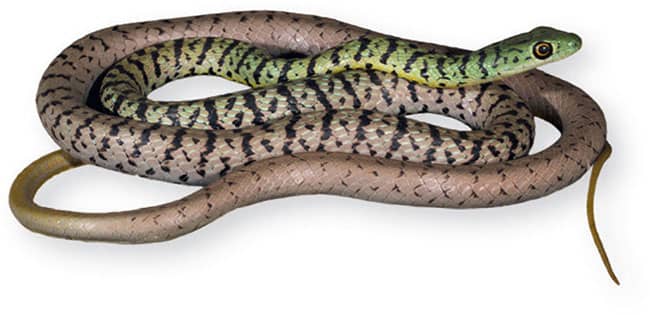
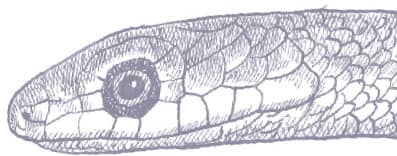
The Spotted Bushsnake is a slender snake with smooth, obliquely arranged scales, a long tail, and a head broader than the neck, with very large eyes and round pupils. Specimens may be bright green, blue-green, gray-green, or yellow-green, with a yellowish or greenish-white venter. The anterior of the body is heavily spotted or barred with black, and the head is green even in gray specimens.
PHRYNONAX POECILONOTUS
PUFFING SNAKE
(GÜNTHER, 1858)
ADULT LENGTH
5–6 ft, rarely 8 ft (1.5–1.8 m, rarely 2.4 m)
The Puffing Snake, also known as the Northern Birdsnake, is so named because of its defensive display, which consists of gaping widely, hissing loudly, and inflating the neck. It is found from southern Mexico to Honduras and Nicaragua, along the Caribbean versant, and onto the Pacific versant in Costa Rica. It primarily inhabits wet forest habitats from lowland rainforest to low montane moist forest, and where it occurs in dry forests it inhabits gallery forest along rivers. This species is diurnal, and both terrestrial and arboreal. It preys primarily on birds, but also takes their eggs, arboreal small mammals, bats, and lizards. Puffing Snakes are large nonvenomous snakes with a long reach, and they will bite if confronted but they are nonvenomous.
RELATED SPECIES
Three subspecies are recognized: the nominate form (Phrynonax poecilonotus poecilonotus) from southern Mexico to Honduras; a northern subspecies (P. p. argus) from the Yucatan Peninsula, Mexico; and a southern Central American form (P. p. chrysobronchus) from Nicaragua and Costa Rica. Specimens from Panama and northern South America are now attributed to the former subspecies P. polylepis. Close relatives are Shropshire’s Puffing Snake (P. shropshirei) from Costa Rica, which may be a synonym of P. poecilonotus. These snakes were previously placed in the genus Pseustes.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North, Central, and South America: southern Mexico to Costa Rica |
ELEVATION |
0–4,660 ft (0–1,420 m) asl |
HABITAT |
Rainforest, lowland and low montane wet forest, and gallery forest in lowland dry forest |
DIET |
Birds and their eggs, small mammals, and lizards |
REPRODUCTION |
Oviparous, with clutches of 7–14 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

The Puffing Snake is a large, laterally compressed snake with smooth scales, a long tail, a broad head, and large eyes with round pupils. The dorsum is glossy olive, brown, or green with occasional scattered reddish or orange scales and obscure bands. The venter is yellowish, particularly on the throat and lips, while the lower flanks are pale orange. Juveniles may be banded, and almost melanistic specimens are known.
PHYLLORHYNCHUS BROWNI
SADDLED LEAFNOSE SNAKE
STEJNEGER, 1890
ADULT LENGTH
93/4–153/4 in, rarely 20 in (250–400 mm, rarely 510 mm)
The Saddled Leafnose Snake inhabits southern Arizona, Sonora, and Sinaloa. It is found in desert habitats with rocky, stony, or sandy substrates and with cover provided by mesquite, creosote bushes, saltbush, thorn scrub, or cacti such as saguaro. A small, nocturnal snake, the Saddled Leafnose Snake emerges from underground burrows at night, especially following rain. It hunts lizards, possibly geckos or side-blotched lizards, but also feeds on lizard eggs, excavated from the sand using the large, leaf-shaped rostral scale on its snout. Juveniles may feed on insects. It is rarely seen, with most specimens encountered crossing desert highways, being well camouflaged on the desert floor. Although small they will defend themselves by loud hissing, inflating their throats, and making mock but ineffectual strikes. Herbert Brown (1848–1913) was president of the Audubon Society of Arizona.
RELATED SPECIES
The closest relative of Phyllorhynchus browni is the Spotted Leafnose Snake (P. decurtatus), with which it occurs in sympatry, although the Spotted Leafnose Snake also occurs in southern California and Baja California. Four subspecies are recognized, from Maricopa, Arizona (P. b. lucidus), southern Arizona and northern Sonora (P. b. browni), southern Sonora (P. b. fortitus), and Sinaloa (P. b. klauberi). The leafnose snakes are closely related to the lyresnakes (Trimorphodon), also from southwest USA and northwest Mexico.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: southwestern USA and northwestern Mexico |
ELEVATION |
985–3,000 ft (300–915 m) asl |
HABITAT |
Rocky, sandy, or stony desert with mesquite, saltbush, thorn scrub, creosote, and saguaro cacti |
DIET |
Lizards and their eggs, insects |
REPRODUCTION |
Oviparous, with clutches of 2–6 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

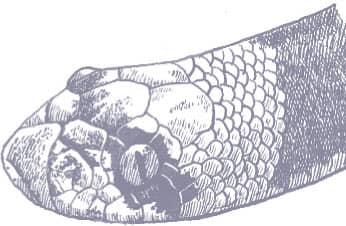
The Saddled Leafnose Snake is a short, stocky little snake, with smooth scales, a short tail, and a short head, barely distinct from the neck, with an enlarged, protruding rostral scale and moderately large eyes with vertical pupils. Coloration is pinkish brown, white below, with regular large round dorsal saddles of mid- to dark brown, those on the anterior body being darkest and the others having dark edges, and a dark brown band across the anterior head and eyes.
PITUOPHIS CATENIFER
GOPHERSNAKE
(BLAINVILLE, 1835)
ADULT LENGTH
4 ft–5 ft 2 in, rarely 9 ft 2 in (1.2–1.6 m, rarely 2.8 m)
The Gophersnake is a large, widely distributed snake, from southwestern Canada, through western and Midwestern USA, into northwestern and central Mexico. Among North American colubrids, probably only the indigo snakes (Drymarchon) are larger than the Gophersnake, although island populations are often much smaller than mainland forms. It may be found in a wide variety of habitats, from desert to marsh, grassland to forest, and agricultural habitats, where it adopts a diurnal, crepuscular, or nocturnal habit depending on the season and climate. Gophersnakes are accomplished burrowers that dig to locate potential mammalian prey in subterranean burrows. Prey comprises mammals, from mice to rabbits, which are constricted on the surface or pinioned between coils and crushed against tunnel walls underground. Lizards, and birds and their eggs are also taken.
RELATED SPECIES
There are up to ten subspecies, including Pituophis catenifer sayi, the loud hissing Bullsnake. Island endemics occur on Santa Cruz Island (P. c. pumilus) off California, and Coronado Island (P. c. coronalis), Cedros Island (P. c. insulanus), and San Martín Island (P. c. fuliginatus) in the Gulf of California. Other Pituophis include the Pinesnake (P. melanoleucus), of southeastern USA, Louisiana Pinesnake (P. ruthveni), Mexican Bullsnake (P. deppei), Cape Gophersnake (P. vertebralis), from Baja California, and Middle American Gophersnake (P. lineaticollis).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North America: southwestern Canada, western and central USA, to central Mexico |
ELEVATION |
0–9,500 ft (0–2,895 m) asl |
HABITAT |
Desert and semidesert, prairies, deciduous woodlands, coniferous forests, agricultural land, and swamps |
DIET |
Mammals, birds and their eggs, and lizards |
REPRODUCTION |
Oviparous, with clutches of 2–24 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

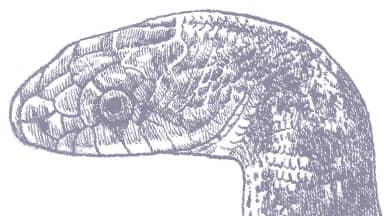
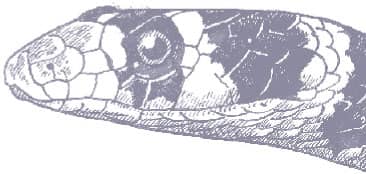
The Gophersnake is a powerfully muscular but relatively slender snake with keeled scales, a slightly pointed head, an enlarged rostral scale, and small eyes with round pupils. It is variably colored, being yellow, tan, brown, or gray, darker dorsally than laterally, with a pattern consisting of a bold series of dark dorsal blotches and a lateral pattern comprising several rows of irregular dark spots. The venter is usually immaculate yellow or tan.
PLATYCEPS ELEGANTISSIMUS
ELEGANT RACER
(GÜNTHER, 1878)
ADULT LENGTH
193/4–233/4 in, rarely 271/2 in (500–600 mm, rarely 700 mm)
The attractive Elegant Racer occurs in southern Israel and Palestine, southwestern Jordan, and northern and northwestern Saudi Arabia. It is an inhabitant of rocky or stony habitats such as dry wadis or rocky hillsides, and is only occasionally found in predominantly sandy habitats. It was believed that due to the high daytime temperatures the Elegant Racer was more nocturnal than diurnal, in stark contrast to its congeners, but recent research now suggests that it is diurnal, but cryptic in habits. It is an alert and fast-moving species that is rarely encountered in nature and therefore poorly known. Although a terrestrial species, one specimen was caught swimming in a wadi. The Elegant Racer feeds on small lizards, especially terrestrial geckos, but small rodents may also feature in its diet.
RELATED SPECIES
The genus Platyceps contains as many as 31 western Palearctic racers formerly included in the genus Coluber, which is now confined to the North American Racer (C. constrictor). Closely related species include the Sinai Racer (P. sinai), Thomas’ Racer (P. thomasi), and Variable Racer (P. variabilis), from the Arabian Peninsula. The Sinai and Thomas’ Racer are almost identical to vertebral-striped P. elegantissimus, while the Variable Racer is most like the unstriped forms.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Middle East: southern Israel, Palestine, southwestern Jordan, and northern Saudi Arabia |
ELEVATION |
0–5,410 ft (0–1,650 m) asl |
HABITAT |
Rocky and stony wadis and hillsides |
DIET |
Lizards, possibly small mammals |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Least Concern |

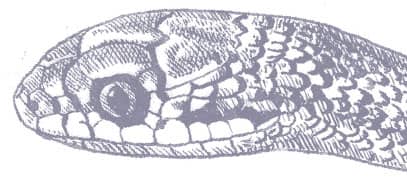
The Elegant Racer is a small, slender snake with smooth scales, a long tail, a narrow pointed head with a projecting snout, large eyes, and round pupils. The patterning comprises a series of regular transverse black bands on an olive to cream background. The bands are broader dorsally than laterally, and become complete rings on the tail. The head bears two narrow black bands, followed by a broad nape band, and the venter is off-white or cream. Some specimens exhibit a narrow orange vertebral stripe, which may only be present in the pale interspaces.
PLATYCEPS NAJADUM
DAHL’S WHIPSNAKE
(EICHWALD, 1831)
ADULT LENGTH
311/2–381/2 in, occasionally 3 ft 3 in (800–980 mm, occasionally 1.0 m)
Dahl’s Whipsnake, also known as the Slender Racer, is a common and wide-ranging species, occurring from the Balkans, to Turkey, the Caucasus, and south to Iran. It is found in a variety of lowland and low montane habitats from semidesert to rocky hillsides, river valleys, abandoned buildings, forest edges, and scrubland. The highly alert and fast-moving Dahl’s Whipsnake is active by day and hunts by pursuing and chasing down its lizard prey, which is either consumed alive or pressed against a rock by a body coil until it is dead. This species rarely employs constriction as a means of killing prey. Although lizards are the primary prey, nestling or juvenile mice, and large insects, may also be taken on occasion.
RELATED SPECIES
Up to six subspecies are recognized, from the Caucasus (Platyceps najadum najadum); southeastern Europe and Turkey (P. n. dahlii); Turkmenistan and Iran (P. n. atayevi); Zagros Mountains, Iran (P. n. schmidtleri); and southeast Azerbaijan (P. n. albitemporalis), while the status of the Kalymnos Island population (P. n. kalymnensis) and those on other Greek islands is open to question. The Glossy-bellied Racer (P. ventromaculatus), of western Asia and the Middle East, is a close relative.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Southeastern Europe and southwestern Asia: the Balkans, Greece, Cyprus, Turkey, southwest Russia, Caucasus, Syria, Lebanon, Iraq, Turkmenistan, and northern Iran |
ELEVATION |
0–7,220 ft (0–2,200 m) asl |
HABITAT |
River valleys, rocky slopes, forest edges, scrubby hillsides, semidesert, and abandoned buildings |
DIET |
Lizards, rarely mice or insects |
REPRODUCTION |
Oviparous, with clutches of 3–16 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

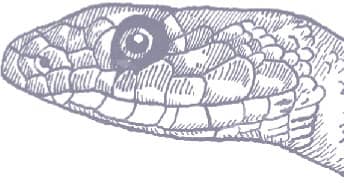
Dahl’s Whipsnake is extremely slender, and smooth-scaled, with a whiplike tail, a narrow, slightly pointed head, large eyes, and round pupils. The patterning, at least of western specimens, comprises a gray-brown head and a gray neck and anterior body, with a black, white-edged collar, and a series of black eyespots on the flanks that decrease in size posteriorly. Beyond the gray anterior section, the body and tail are uniform brown. The venter, throat, and lips are white, while white also extends onto the preoculars and postoculars. No other racer or whipsnake exhibits this pattern.
PLATYCEPS RHODORACHIS
WADI RACER
(JAN, 1865)
ADULT LENGTH
233/4–271/2 in, occasionally 4 ft 3 in (600–700 mm, occasionally 1.3 m)
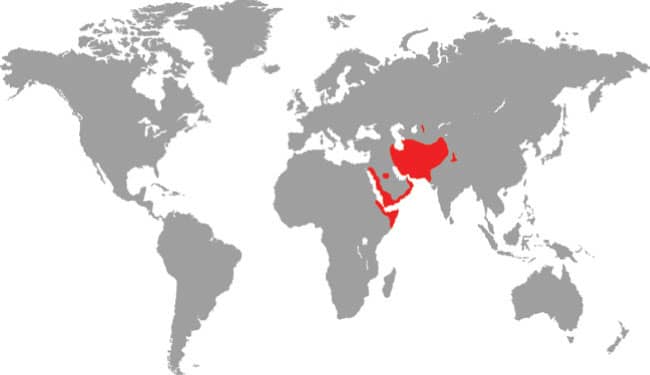
Also known as Jan’s Cliff Racer, the Wadi Racer is a widely distributed species, found throughout the Middle East, from Israel to Yemen and Oman, and in western Asia to as far east as India. It also occurs in northeastern Africa, in Somalia, Ethiopia, and Eritrea. It inhabits arid habitats, rocky or stony plains, wadis, or hills, and although it is not reliant on water it is commonly encountered near watercourses. The Wadi Racer is diurnal, alert, and fast-moving, but crepuscular in hot weather. A skilled climber and swimmer, it preys on frogs, tadpoles, lizards, rodents, bats, birds, and snakes, including conspecifics, but it is said to avoid toads. Although not strictly venomous, the Wadi Racer chews to introduce a mildly neurotoxic saliva that subdues its prey. Bites to humans cause only localized itching.
RELATED SPECIES
Up to four subspecies are recognized, from Iran (Platyceps rhodorachis rhodorachis); Iran and Kazakhstan to Pakistan and India (P. r. ladacensis); Kashmir (P. r. kashmirensis); and northeast Africa (P. r. subnigra). The close relatives of P. rhodorachis include Rogers’ Racer (P. rogersi) from North Africa and the Middle East. It also resembles the Spotted Desert Racer (P. karelini) from Iran and Turkmenistan.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, but possesses mildly toxic saliva |
DISTRIBUTION |
Northeastern Africa, Middle East and southwest Asia: Eritrea, Ethiopia, Somalia, Yemen, Oman, Saudi Arabia, UAE, Israel, Jordan, Iraq, Iran, Afghanistan, Kazakhstan, Tajikistan, Turkmenistan, Uzbekistan, Pakistan, and northern India |
ELEVATION |
0–9,020 ft (0–2,750 m) asl |
HABITAT |
Rocky hillsides and wadis, stony coastal plains, and cultivated or wet habitats |
DIET |
Lizards, frogs, tadpoles, small mammals, birds, and snakes |
REPRODUCTION |
Oviparous, with clutches of 4–8 eggs |
CONSERVATION STATUS |
IUCN not listed |

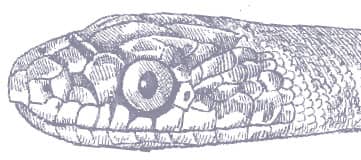
The Wadi Racer is a slender snake, with smooth scales, a long tail, and a narrow, pointed head with large eyes and round pupils. It is generally tan, greenish gray, or olive-green in color, patterned with a series of dark bands or spots that may be present on the anterior body, the entire body and tail, or absent completely. The venter is whitish pink.
PSEUDELAPHE FLAVIRUFA
TROPICAL RATSNAKE
(COPE, 1867)
ADULT LENGTH
4–5 ft, rarely 5 ft 9 in (1.2–1.5 m, rarely 1.76 m)
Also known as the Mexican Night Snake, this ratsnake is distributed down the Caribbean coast of Mexico, Belize, and Honduras, excluding the Yucatán Peninsula but including the Honduran Islas de la Bahía, and Big Corn Island, Nicaragua. It also occurs in Guatemala and on the Pacific coast of Oaxaca and Chiapas, Mexico. It inhabits both mesic and xeric habitats, from swamps and evergreen forests to dry forest and thorn scrub. It is especially common in the coastal lowlands. The Tropical Ratsnake is a nocturnal predator of mice, rats, bats, birds, and sometimes lizards, which are killed by constriction. Although generally placid it will defend itself vigorously by flattening its head, raising its coils into an S-shape, vibrating its tail on dead leaves, and, if necessary, lunging with strikes and bites.
RELATED SPECIES
Three subspecies are recognized: the Northern Tropical Ratsnake (Pseudelaphe flavirufa flavirufa); Matuda’s Ratsnake (P. f. matudai) from southern Chiapas; and the Central American Tropical Ratsnake (P. f. pardalina). A fourth subspecies was elevated to specific status, the Yucatán Ratsnake (P. phaescens). The snakes most closely related to P. flavirufa are the glossy snakes (Arizona elegans) and long-nosed snakes (Rhinocheilus), but P. flavirufa is unlikely to be confused with the American Green Ratsnake (Senticolis triaspis), with which it occurs in sympatry.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North and Central America: southern Mexico, Guatemala, Belize, Honduras, and Nicaragua |
ELEVATION |
0–4,920 ft (0–1,500 m) asl |
HABITAT |
Tropical evergreen forest, semi-xeric thorn scrub, deciduous woodland, karst limestone escarpments, and lowland coastal swamps |
DIET |
Small mammals, birds, bats, and lizards |
REPRODUCTION |
Oviparous, with clutches of 4–9 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

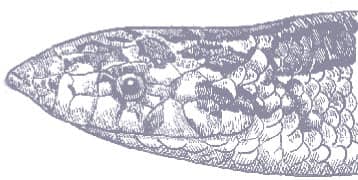
The Tropical Ratsnake is a moderately large snake with smooth scales, a long tail, a broad head, and large eyes with round pupils that become vertically elliptical in bright light. It is pale gray or brown with dorsal and lateral rows of irregular blotches, which may be red, brown, or black, often with black edges. Two similarly colored stripes run onto the back of the head. The venter is yellow-gray with small dark spots. The patterning of juveniles is lighter and more contrasting in its pigmentation.
PSEUDOFICIMIA FRONTALIS
SOUTHWESTERN HOOKNOSE SNAKE
(COPE, 1864)
ADULT LENGTH
153/4–193/4 in, rarely 28 in (400–500 mm, rarely 710 mm)
The Southwestern Hooknose Snake is a Mexican endemic, found from southern Sonora and Sinaloa to Guerrero and Puebla, western Mexico, where it inhabits lowland and low montane thornbush woodland, tropical semiarid and dry forest, and deciduous forest. Its upturned snout is used for excavation of the substrate, and stomach contents reveal that this species preys on moth caterpillars and tarantula spiders. Female Southwestern Hooknose Snakes are oviparous and possess well-developed hemipenes like those of a male, a condition known as pseudohermaphroditism. This is a relatively rare species and poorly known in nature. Although it is a rear-fanged venomous snake, the Southwestern Hooknose Snake is not dangerous to humans.
RELATED SPECIES
This species is sometimes referred to as the “False Ficimia” because its generic name is Pseudoficimia, but this is a misinterpretation. The generic name indicates that while this species does not belong in Ficimia it is similar to snakes of that genus. Southwestern Hooknose Snake is a better name because it occurs to the south of the western hooknose snakes (Gyalopion), and to the west of the southern hooknoses (Ficimia). This is a monotypic genus.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, very mildly venomous; harmless to humans |
DISTRIBUTION |
North America: western Mexico |
ELEVATION |
0–3,610 ft (0–1,100 m) asl |
HABITAT |
Thornbush woodland, tropical semiarid and dry forest, and tropical deciduous forest |
DIET |
Spiders and insects |
REPRODUCTION |
Oviparous, with clutches of 3–30 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

The Southwestern Hooknose Snake is a moderately stout snake with a pointed head, upturned snout, and small eyes with round pupils. It is gray-brown with a pair of darker brown or red-brown stripes that run off the back of the head to initiate a series of similarly colored dorsal blotches connected by a broad yellow line, with smaller dark spots on the flanks, a dark stripe at an angle below the eye, and a dull orange venter.
PTYAS MUCOSA
DHARMAN RATSNAKE
(LINNAEUS, 1758)
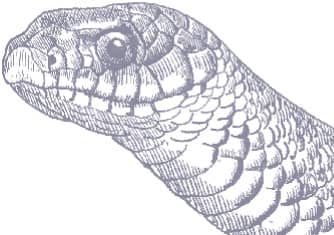
ADULT LENGTH
5 ft–6 ft 3 in, rarely 12 ft 2 in (1.5–1.9 m, rarely 3.7 m)
The Dharman Ratsnake is a large, common, terrestrial and arboreal snake found widely across Asia, from Iran to China and south to Sri Lanka, Peninsular Malaysia, and Taiwan. It is found in many habitats, from lowland and montane forest, both wet and dry, to paddy fields and plantations, and even parks and gardens. It is a diurnal predator of vertebrates ranging from rodents to birds, frogs, lizards, and other snakes, with prey being pinioned by the coils, but it is itself the frequent prey of King Cobras (Ophiophagus hannah). Male Dharmans engage in combat during the mating period, entwining their bodies as they attempt to wrestle each other to the ground (see here). Females will actively guard their nests of eggs during incubation. Although nonvenomous, a large Dharman can deliver bloody bites.
RELATED SPECIES
No subspecies are recognized across the vast range of Ptyas mucosa. The genus contains seven other species, with five centered on mainland Southeast Asia: the Malayan Keeled Ratsnake (P. carinata); Black-striped Ratsnake (P. dhumnades); White-bellied Ratsnake (P. fusca); Indo-Chinese Ratsnake (P. korros); and the stunning Asian Green Ratsnake (P. nigromarginata). The Luzon Mountain Ratsnake (P. luzonensis) is found in the north Philippines and the Sulawesi Black Ratsnake (P. dipsas) in Indonesia.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Asia: Iran and Turkmenistan to China and Taiwan, south to Sri Lanka and Peninsular Malaysia |
ELEVATION |
0–13,100 ft (0–4,000 m) asl |
HABITAT |
Wet and dry, lowland and montane forests, riverine forest, agricultural habitats, parks, and gardens |
DIET |
Small mammals, birds, lizards, frogs, and other snakes |
REPRODUCTION |
Oviparous, with clutches of 5–25 eggs |
CONSERVATION STATUS |
IUCN not listed |

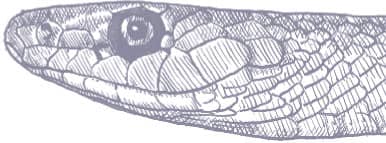
The Dharman Ratsnake is a powerful, slightly laterally compressed snake, with smooth scales, a long tail, a slightly pointed head, large eyes, round pupils, and shelved supraocular scales. It is glossy brown, olive, or greenish with scattered black flecks on the dorsum, and white or yellow underneath. The supralabial scales and the scales of the throat are black-edged.
PTYAS NIGROMARGINATA
ASIAN GREEN RATSNAKE
(BLYTH, 1854)
ADULT LENGTH
2 ft 4 in–3 ft 3 in, rarely 8 ft 2 in (0.7–1.0 m, rarely 2.5 m)
The stunning Asian Green Ratsnake is a diurnal, terrestrial, and arboreal inhabitant of open woodland at low to medium elevations, on plains and hills from Nepal and Bhutan to Sichuan and Yunnan in western China, and south to northern Myanmar, Thailand, Laos, and Vietnam. It also occurs in disturbed habitats. Its primary prey consists of rodents, which are killed by constriction or by being pinioned against solid objects by the ratsnake’s coils, but it also takes lizards, birds, and other snakes. When cornered, its defenses may include lunging bites and exuding the foul-smelling contents of its cloacal glands, but it is nonvenomous and harmless to humans.
RELATED SPECIES
Ptyas nigromarginata is related to the Dharman Ratsnake (P. mucosa) and six other species of Indo-Chinese and Southeast Asian ratsnakes. Many of these species were previously included in the genus Zaocys.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
South and Southeast Asia: northeast India, Nepal, Bhutan, Bangladesh, western China, northern Myanmar, Laos, Vietnam, and Thailand |
ELEVATION |
1,640–7,710 ft (500–2,350 m) asl |
HABITAT |
Open woodland on plains and low hills; also disturbed areas |
DIET |
Small mammals, lizards, birds, and other snakes |
REPRODUCTION |
Oviparous, with clutches of 8–10 eggs |
CONSERVATION STATUS |
IUCN not listed |

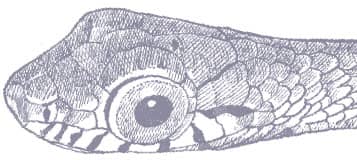
The Asian Green Ratsnake is a slender snake with smooth scales, a long tail, an elongate head, large eyes, and round pupils. The dorsum is bright green to olive-green with black scale edging. Juveniles bear four broad, black longitudinal stripes on the body and tail, but these are confined to the posterior body and tail in adults. The venter is yellow-green and the head is reddish brown with a white throat.
RHAMNOPHIS AETHIOPISSA
SPLENDID DAGGER-TOOTHED TREESNAKE
(GÜNTHER, 1862)
ADULT LENGTH
3 ft–3 ft 7 in, rarely 5 ft (0.9–1.1 m, rarely 1.5 m)
Also known as the Large-eyed Green Treesnake, this is a diurnal, arboreal inhabitant of rainforests and other tropical forest habitats, but not open habitats, from Guinea to Kenya, and south to Angola. It is also recorded from Bioko Island, formerly Fernando Pó, in the Gulf of Guinea. It is characterized by an enlarged vertebral scale row that assists the treesnake in bridging gaps. It also has enlarged rear maxillary teeth that are flanged anteriorly and posteriorly like those of the Dagger-toothed Vinesnake (Xyelodontophis uluguruensis), only slightly less so, hence its common name. It feeds on tree frogs and toads. The large eyes dominate the head and suggest that it is a very alert species with good eyesight, even in the low light conditions of the rainforest.
RELATED SPECIES
Snakes of the genus Rhamnophis are closely related to the black treesnakes (Thrasops) and were originally included in that genus, but they also bear a strong resemblance to the dangerously venomous Boomslang (Dispholidus typus). Rhamnophis aethiopissa is represented by three subspecies. The nominate form (R. a. aethiopissa) occupies most of the range while two other subspecies occur in northern Angola and Zambia (R. a. ituriensis) and Uganda, Kenya, and Tanzania (R. a. elgonensis). The genus also contains Bates’ Dagger-toothed Treesnake (R. batesii) from Central Africa.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
West, Central, and East Africa: Guinea to Cameroon, south to Angola and west to Kenya, and on Bioko Island |
ELEVATION |
0–6,560 ft (0–2,000 m) asl |
HABITAT |
Rainforest, and other forest habitats |
DIET |
Frogs and toads |
REPRODUCTION |
Oviparous, with clutches of up to 17 eggs |
CONSERVATION STATUS |
IUCN not listed |
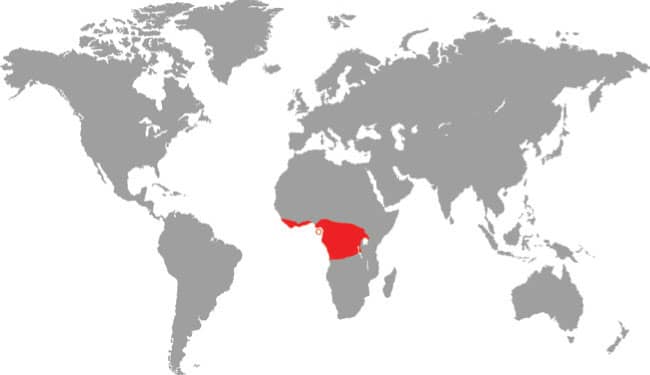

The Splendid Dagger-toothed Treesnake is a slender snake with obliquely arranged smooth scales, a long tail, and a head distinct from the neck, with especially large eyes and round pupils. Dorsally it is yellowish green with every scale black-edged, and ventrally it is green with or without black edges to the ventral and subcaudal scales.
RHINOBOTHRYUM LENTIGINOSUM
AMAZON BANDED SNAKE
(SCOPOLI, 1788)
ADULT LENGTH
2 ft 7 in–5 ft 2 in (0.85–1.6 m)
The Amazon Banded Snake is an infrequently encountered species. It is found widely across northern South America, from Venezuela to Paraguay and the Guianas to Peru, but it only inhabits tropical rainforest and in this habitat it occurs primarily in the rainforest canopy far above the forest floor, where its presence may go unnoticed. It is nocturnal and arboreal and it hunts primarily arboreal lizards such as geckos, anoles, and tree runners. This is a rear-fanged venomous species, but it is docile when handled and its venom is designed to subdue lizards and is believed harmless to humans. It is oviparous but clutch sizes appear to be small, with three eggs being reported from one specimen. This is a very rare snake that requires much more study in nature.
RELATED SPECIES
A second species of Rhinobothryum is known, the Central American Banded Snake (R. bovallii). The genus Rhinobothryum is contained in a clade with the scorpion-eating snakes (Stenorrhina), shovelnose snakes (Chionactis), sandsnakes (Chilomeniscus), and groundsnakes (Sonora).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
Northern South America: Colombia, Venezuela, the Guianas, Brazil, Peru, Bolivia, and Paraguay |
ELEVATION |
33–1,610 ft (10–490 m) asl |
HABITAT |
Tropical rainforest |
DIET |
Lizards |
REPRODUCTION |
Oviparous, with clutches of up to 3 eggs |
CONSERVATION STATUS |
IUCN not listed |
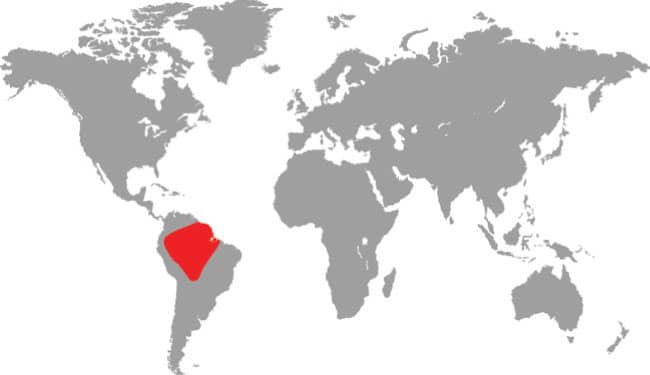

The Amazon Banded Snake is an extremely slender snake with smooth scales, a long tail, a very narrow neck, and a broad, rounded head with large eyes and vertically elliptical pupils. It is a matte black snake with regularly spaced triads comprising a red band, tipped with black, between two white bands. The head is black with white scale suturing and scattered red spots.
RHINOCHEILUS LECONTEI
LONG-NOSED SNAKE
BAIRD & GIRARD, 1853
ADULT LENGTH
153/4–30 in, occasionally 5 ft (400–760 mm, occasionally 1.5 m)
The Long-nosed Snake is a common nocturnal snake of the southwestern deserts of the USA, and may often be encountered crossing roads. It also ranges south into northern Mexico. Its arid habitats range from thornbush or mesquite semidesert to dry prairies. It prefers sandy soils into which it may easily dig using its pointed snout and enlarged rostral scale. Prey consists of lizards and small mammals, but birds and grasshoppers are also eaten. Mice are constricted or pinioned against solid objects to kill them. Long-nosed Snakes rarely bite, even when handled, but they may twist their bodies and void the contents of their cloacal glands. Some specimens are said to defensively discharge blood from their nostrils. John Lawrence LeConte (1825–83) was a Civil War physician and naturalist.
RELATED SPECIES
Three subspecies are currently recognized, the Western Long-nosed Snake (Rhinocheilus lecontei lecontei), the Texas Long-nosed Snake (R. l. tessellatus), and the Pacific Coastal Long-nosed Snake (R. l. antonii). The Isla Cerralvo Long-nosed Snake (R. etheridgei), from the Gulf of California, was a former subspecies, but is now treated as a full species. The genus Rhinocheilus belongs to a clade that also includes the glossy snakes (Arizona elegans) and the Tropical Ratsnake (Pseudelaphe flavirufa), within a larger clade of ratsnakes and kingsnakes.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: southwestern and southern USA, and northern Mexico |
ELEVATION |
0–6,230 ft (0–1,900 m) asl |
HABITAT |
Lowland desert, thornbush, acacia or mesquite semidesert, and dry prairie |
DIET |
Lizards, small mammals, birds, and large insects |
REPRODUCTION |
Oviparous, with clutches of 3–11 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
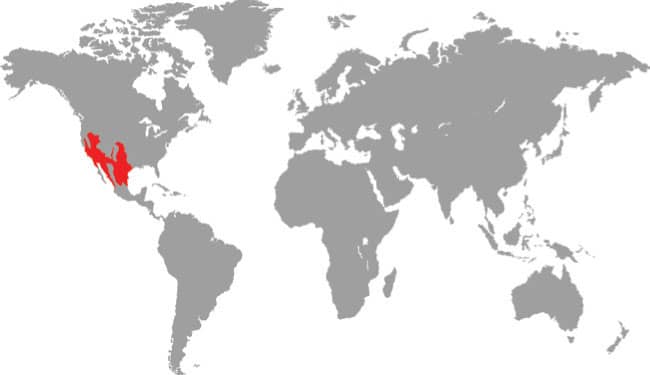

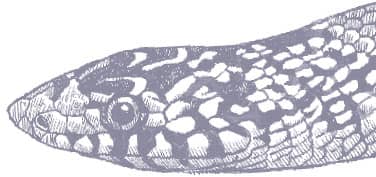
The Long-nosed Snake is a slightly laterally compressed snake with smooth scales, a narrow, strongly pointed head, and small eyes with round pupils. Its patterning comprises alternating vertebral square blotches of black and red or orange, on a whitish-gray background, almost every pale scale being heavily infused with black, while the pale pigment also invades the black or red middorsal markings. The iris of the eye is also reddish.
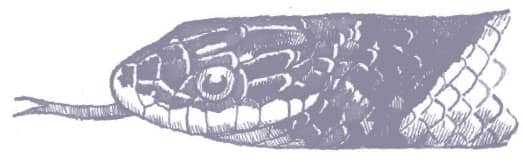
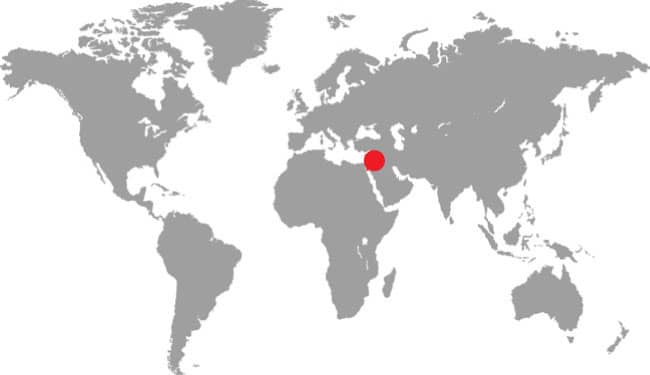
RHYNCHOCALAMUS MELANOCEPHALUS
PALESTINE BLACK-HEADED SNAKE
(JAN, 1862)
ADULT LENGTH
153/4–19 in (400–480 mm)
The diminutive Palestine Black-headed Snake is a secretive fossorial species that shelters under stones during the day and becomes active at night, when it hunts a wide variety of insects, from ant larvae to locusts, and also feeds on woodlice, centipedes, and small geckos. It is distributed from Syria to the Sinai of Egypt and northern Saudi Arabia, generally in semidesert habitats such as wadis, rocky slopes, gravel pans, and arid steppes, but it may also be found in light oak woodland, agricultural areas, and abandoned buildings. Its natural history is poorly documented and although it is known to be oviparous its clutch size is not known.
RELATED SPECIES
Four other species of Rhynchocalamus are recognized, the Aden Black-headed Snake (R. arabicus) from Yemen and Oman, Baran’s Black-headed Snake (R. barani) from southern Turkey, Dayan’s Black-headed Snake (R. dayanae) from the Negev Mountains of Israel, and a former subspecies, Satunin’s Black-headed Snake (R. satunini), from eastern Turkey, the Caucasus, Iraq, and Iran. Rhynchocalamus is the sister taxon to the leafnose snake genus Lytorhynchus (shown here).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
Middle East: Syria, Israel, Palestine, Jordan, Egypt (Sinai), and Saudi Arabia |
ELEVATION |
165–5,910 ft (50–1,800 m) asl |
HABITAT |
Dry steppe, semidesert, wadis, rocky slopes, and gravel plains with sparse vegetation; also light oak forest, agricultural habitats, and abandoned buildings |
DIET |
Insects, crustaceans, centipedes, and small lizards |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN not listed |

The Palestine Black-headed Snake is a small, slender, cylindrical snake with smooth scales, a narrow head, just distinct from the neck, and moderately large eyes and round pupils. The body is uniform light brown above, and white below. The dorsum of the head and neck is glossy jet black, except for the contrasting white supralabials in some specimens.
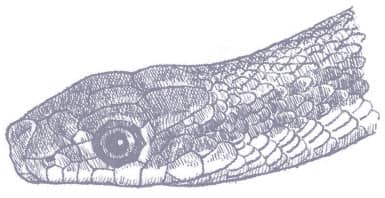
SALVADORA HEXALEPIS
WESTERN PATCHNOSE SNAKE
(COPE, 1867)
ADULT LENGTH
26–351/2 in, occasionally 3 ft 10 in (660–900 mm, occasionally 1.17 m)
The Western Patchnose Snake is a common desert species found in both rocky and sandy habitats, from northern California and Nevada, USA, south to Baja California, Sonora, Chihuahua, and Sinaloa, Mexico. It may inhabit rocky slopes, canyons, or dry arroyos, especially those with desert vegetation. The enlarged rostral scale on the snout enables it to dig for prey, but it primarily hunts on the surface, during the day, being one of the few snakes active in the heat of midday. Its prey consists mostly of diurnal lizards, but small snakes, rodents, and birds are also taken. The Western Patchnose Snake is an alert snake with good eyesight, which largely avoids trouble by fleeing, but if cornered it will inflate its neck and make far-reaching strikes. It is nonvenomous and harmless to humans.
RELATED SPECIES
Four subspecies of Salvadora hexalepis are recognized: Desert Patchnose (S. h. hexalepis); Mohave Patchnose (S. h. mojavensis); Baja California Patchnose (S. h. klauberi); and Coastal Patchnose (S. h. virgultea). Two additional species, the Eastern or Mountain Patchnose (S. grahamiae) and Big Bend Patchnose (S. deserticola), also occur in the USA, while a further four species are found in Mexico: Mexican Patchnose (S. mexicana); Baird’s Patchnose (S. bairdi); Oaxaca Patchnose (S. intermedia); and Pacific Patchnose (S. lemniscatus).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: southwestern USA and northwestern Mexico |
ELEVATION |
785–7,280 ft (240–2,200 m) asl |
HABITAT |
Rocky desert arroyos, canyons, and hillsides, especially with cacti, thorn scrub, creosote, or saltbush vegetation; also chaparral |
DIET |
Lizards, small snakes, reptile eggs, small mammals, and birds |
REPRODUCTION |
Oviparous, with clutches of 3–12 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

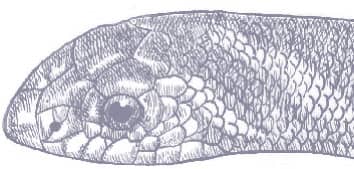
The Western Patchnose Snake is a muscular snake with smooth scales, a long tail, a head just distinct from the neck, large eyes and round pupils, and a large saddle-like rostral scale. It is generally patterned with pastel desert colors. The head is brown above, paler below, followed by a broad yellow to sandy stripe that runs the length of the body and tail. The flanks may be uniform brown or pale sand-colored with two brown longitudinal stripes, the uppermost being the broadest. The venter is white.
SCAPHIOPHIS ALBOPUNCTATUS
GRAY HOOKNOSED SNAKE
PETERS, 1870
ADULT LENGTH
3 ft 3 in–4 ft 3 in, occasionally 5 ft 2 in (1.0–1.3 m, occasionally 1.6 m)
The Gray Hooknosed Snake is distributed from Sierra Leone to Kenya and south to Zambia. It inhabits coastal thickets and woodland, but farther inland it occurs in both wet and dry woodland and savanna habitats. It is primarily fossorial, burrowing in loose soil using its enlarged, shovel-like rostral scale, the slit-like nostrils and tight-fitting lip scales preventing the ingress of soil during the burrowing process. It also uses animal burrows, hunting rodents below ground and killing them by pinioning them against the tunnel walls with its coils. When it feels threatened the Gray Hooknosed Snake will elevate its anterior body, gape widely to display the blue-black interior of its mouth, extend its tongue, and make mock strikes with such force that it may throw its body forward, but this is all bluff, it is nonvenomous and harmless to man.
RELATED SPECIES
A second species, the Ethiopian Hooknosed Snake (Scaphiophis raffreyi), occurs in Ethiopia, South Sudan, Eritrea, Uganda, and northwest Kenya. The African hooknosed snakes may be distantly related to the Asian kukri snakes (Oligodon). They may also be may be confused with the beaked snakes (Rhamphiophis), although the hooknosed snakes’ rostral scales are more pronounced, their bodies are more robust, and their eyes are smaller.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
West, Central, and East Africa: Sierra Leone and Ghana to Kenya, south to Zambia and Angola |
ELEVATION |
0–4,840 ft (0–1,475 m) asl |
HABITAT |
Coastal thicket, and wet and dry woodland and savanna |
DIET |
Small mammals |
REPRODUCTION |
Oviparous, with clutches of up to 48 eggs |
CONSERVATION STATUS |
IUCN not listed |

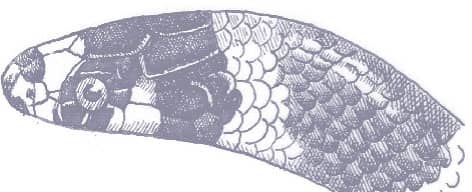
The Gray Hooknosed Snake is a stocky-bodied snake with smooth scales, a short tail, and a narrow head, which is indistinct from the neck and terminates in a pronounced, pointed rostral scale. The eyes are small and the pupils are round. This species is gray or brown above with or without black spotting, and orange, cream, or white below.
SCOLECOPHIS ATROCINCTUS
BLACK-BANDED CENTIPEDE SNAKE
(SCHLEGEL, 1837)
ADULT LENGTH
173/4–191/4 in (450–490 mm)
The Black-banded Centipede Snake occurs from Guatemala to northern Costa Rica along the Pacific versant of Central America, in both wet and dry tropical forests, in lowland and low montane locations. It is a terrestrial and semi-fossorial species that is also occasionally found climbing in low vegetation. Authorities differ as to whether this rare snake is diurnal, crepuscular, or nocturnal. It appears to prey exclusively on large, venomous, scolopendrid centipedes, which are usually eaten backward. It may also prey on insects or arachnids. Although a rear-fanged venomous snake, its mouth is too small to administer a bite to a human. The Black-banded Centipede Snake may be confused with the false coralsnakes (Pliocercus) and the true coralsnakes (Micrurus), but most corals and coral mimics have fully encircling red bands.
RELATED SPECIES
Scolecophis is a monotypic genus, and most closely related to the black-headed and centipede snakes of genus Tantilla (shown here).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
Central America: Guatemala, El Salvador, Honduras, Nicaragua, and Costa Rica |
ELEVATION |
0–5,020 ft (0–1,530 m) asl |
HABITAT |
Dry and wet, lowland and montane tropical forest |
DIET |
Centipedes |
REPRODUCTION |
Oviparous, with clutches of up to 7 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

The Black-banded Centipede Snake is a small, cylindrical snake with smooth scales, a short tail, and a rounded head, indistinct from the neck, with small eyes and round pupils. It is distinctively patterned with alternating black and white bands, the scales of the latter being black-tipped. A broad vivid red vertebral stripe runs the length of the body and tail between the black rings. The dorsum of the head is black-capped, with black rings through the nape, the eye, and the snout.
SENTICOLIS TRIASPIS
AMERICAN GREEN RATSNAKE
(COPE, 1866)
ADULT LENGTH
Male
2 ft 4 in–3 ft 3 in (0.7–1.0 m)
Female
3–4 ft, rarely 6 ft (0.9–1.2 m, rarely 1.8 m)
The American Green Ratsnake is a medium-sized constrictor that just enters the United States in the Chihuahuan Desert of southern Arizona and New Mexico, where it is listed as Endangered. From the US border it is distributed throughout Mexico and Central America, as far south as Costa Rica. Preferred habitats are predominately arid, from rocky canyons to dry chaparral and thorn scrub, but it also occurs in moist habitats, such as low montane mixed forest. It is an alert and secretive terrestrial and arboreal species that avoids confrontation by fleeing, but if cornered it will defend itself vigorously. Adult American Green Ratsnakes prey on rodents and also birds and their eggs, shrews, and bats, while juveniles take lizards and small mice, prey being killed by constriction.
RELATED SPECIES
Three subspecies are recognized: the Western Green Ratsnake (Senticolis triaspis intermedia) from Arizona and Mexico, the nominate Yucatán Green Ratsnake (S. t. triaspis), and the Honduran Green Ratsnake (S. t. mutabilis) from Central America. Senticolis triaspis is unlikely to be confused with the Tropical Ratsnake (Pseudelaphe flavirufa), with which it occurs in sympatry.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North and Central America: southwestern USA, Mexico, Belize, Guatemala, El Salvador, Honduras, Nicaragua, and Costa Rica |
ELEVATION |
0–7,960 ft (0–2,425 m) asl |
HABITAT |
Rocky canyons, semiarid chaparral, low montane mixed woodland, mesquite grassland, tropical dry forest, and thorn scrub |
DIET |
Small mammals, birds and their eggs, and lizards |
REPRODUCTION |
Oviparous, with clutches of 3–9 eggs |
CONSERVATION STATUS |
IUCN Least Concern, Endangered in New Mexico |

The American Green Ratsnake is a muscular-bodied snake with a long tail and a long head, distinct from the neck, with moderately sized eyes and round pupils. Most of its scales are smooth but the median rows are weakly keeled. It is generally unicolor as an adult, usually green or olive and darker posteriorly than anteriorly, but some specimens are brown or red. Juveniles are gray with red or brown saddles.
SIMOPHIS RHINOSTOMA
SÃO PAULO FALSE CORALSNAKE
(COPE, 1866)
ADULT LENGTH
2 ft 7 in–3 ft 3 in (0.8–1.0 m)
The São Paulo False Coralsnake occurs in the southern Brazilian states of São Paulo, Minas Gerais, Goiás, and Mato Grosso do Sul, including Ilha de São Sebastião off the coast of São Paulo, as well as northeastern Brazil in Bahia, and west into Paraguay. It is associated with Atlantic coastal forest habitats but is also found in Cerrado savannas. It is a poorly known species with only limited natural history data available from nature, but it has a broad rostral scute of the kind associated with fossorial snakes. Although only frogs have been recorded as prey, some authors suggest it may also take small mammals or lizards. The São Paulo False Coralsnake mimics the aposematic patterning (warning colors) of the Amazonian Coralsnake (Micrurus spixii) and the Cerrado Coralsnake (M. frontalis).
RELATED SPECIES
Simophis is a monotypic genus. It bears a strong resemblance to several coralsnakes and false coralsnakes, but a suite of characters separate it from other colubrids, including other coral mimics.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South America: Brazil, including Ilha de São Sebastião, and Paraguay |
ELEVATION |
590–3,490 ft (180–1,065 m) asl |
HABITAT |
Cerrado savanna, and Atlantic Forest habitats |
DIET |
Frogs, and possibly small mammals and lizards |
REPRODUCTION |
Oviparous, with clutches of 2–7 eggs |
CONSERVATION STATUS |
IUCN not listed |

The São Paulo False Coralsnake is a smooth- scaled, cylindrical snake with a relatively long tail and a long, pointed head, indistinct from the neck, which terminates in a large rostral scute. Its patterning consists of glossy triads comprising black-white-black-white-black between broad red interspaces. The scales of the white bands are black-tipped. The head is white with black suturing, followed by a curving black band and a narrow red band before the first triad.
SONORA SEMIANNULATA
WESTERN GROUNDSNAKE
BAIRD & GIRARD, 1853
ADULT LENGTH
8–121/2 in, occasionally 18 in (180–320 mm, occasionally 460 mm)
Widely distributed across southwestern USA, from Oregon and California to Texas and Missouri, and into Mexico as far south as Durango, the Western or Variable Groundsnake inhabits a range of arid habitats from desert and semidesert to dry grassland, rocky hillsides, and riparian habitats in arid areas. It is nocturnal and feeds almost exclusively on invertebrates, including not only beetles and grasshoppers, but also dangerous species such as black widow spiders, buthid scorpions, and scolopendrid centipedes. Small geckos also feature in the diet on occasion. As a small snake the Western Groundsnake has many predators and adopts a variety of defensive tactics ranging from thanatosis and spraying musk, to forming a loop by holding its own tail in its mouth, making itself hopefully harder to ingest.
RELATED SPECIES
Sonora semiannulata comprises two subspecies, the Western Groundsnake (Sonora semiannulata semiannulata) and the South Texas Groundsnake (S. s. taylori). Sonora also contains three endemic Mexican species: the Mexican Groundsnake (S. mutabilis); Michoacán Groundsnake (S. michoacanensis); and Filetail Groundsnake (S. aemula). Sonora belongs to a clade that includes the sandsnakes (Chilomeniscus) and the shovelnose snakes (Chionactis).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: western USA and northern Mexico |
ELEVATION |
0–6,820 ft (0–2,080 m) asl |
HABITAT |
Desert and semidesert, dry grassland, rocky slopes, scrubland, and riparian habitats |
DIET |
Insects, spiders, scorpions, centipedes, earthworms, and occasional lizards |
REPRODUCTION |
Oviparous, with clutches of 3–6 eggs |
CONSERVATION STATUS |
IUCN Least Concern, protected in Oregon and Arkansas |

The Western Groundsnake is a small, smooth-scaled, cylindrical snake with a relatively short tail, a narrow, pointed head, and small eyes with round pupils. It is one of the most variably patterned snakes in North America, but patterning often comprises alternating black and white bands, the upper portions of the white bands being red. The snout may be white or red, followed by a black band.
SPALEROSOPHIS DIADEMA
DIADEM SNAKE
(SCHLEGEL, 1837)
ADULT LENGTH
2 ft 7 in–3 ft 3 in, occasionally 4 ft 3 in (0.8–1.0 m, occasionally 1.3 m)
Also known as the Royal Snake, the Diadem Snake occurs across a vast swath of arid habitats from Morocco to Egypt, parts the Arabian Peninsula, and from Turkey to Kazakhstan and northwest India. The Diadem Snake is diurnal, but crepuscular or nocturnal in hot weather, sheltering in animal burrows when conditions are too hot. It is found in vegetated steppe and semidesert, on rocky, gravel, and sandy substrates. It is also common in cultivated habitats and around buildings where its primary prey, rats and mice, are most abundant. Birds and lizards also feature in its diet. Prey is killed by constriction, although toxic saliva is thought to play a part in subduing it. Due to its rodent-killing capacity, the Diadem Snake should be encouraged in agricultural communities.
RELATED SPECIES
Three subspecies are recognized: the nominate form (Spalerosophis diadema diadema) occurs in Pakistan and northeast India; a western form (S. d. cliffordi) is found in North Africa, the Arabian Peninsula, Turkey, and Iraq; while Iran, Turkmenistan, and western Pakistan are inhabited by a third subspecies (S. d. schirasianus). The genus Spalerosophis also contains five other species across North Africa and western Asia. Its closest relatives include the Palearctic racers of genus Platyceps (shown here).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North Africa, Middle East, and western Asia: Morocco to Egypt, Turkey to Oman, Turkmenistan to India |
ELEVATION |
0–7,960 ft (0–2,425 m) asl |
HABITAT |
Steppe, semidesert, wadis, rocky hillsides, sand dunes, and cultivated habitats |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Oviparous, with clutches of 3–16 eggs |
CONSERVATION STATUS |
IUCN not listed |

The Diadem Snake has a muscular body, with keeled scales, a distinctive head, and large eyes with round pupils. Its body color is gray-brown, every scale spotted with dark brown. Patterning comprises a series of brown vertebral rhomboid markings and a second series of smaller brown spots on the flanks.
SPILOTES PULLATUS
TIGER RATSNAKE
LINNAEUS, 1758
ADULT LENGTH
3 ft 3 in–6 ft 7 in, occasionally 8 ft 8 in (1.0–2.0 m, occasionally 2.65 m)
Also known as the Thunder and Lightning Snake, or Chicken Snake, the Tiger Ratsnake is a common diurnal snake of lowland coastal or riverine habitats from southern Mexico, Central America, and South America, as far as the Guianas, Brazil, and Paraguay. It is terrestrial and arboreal and is frequently sighted sleeping in trees over lagoons or swimming across rivers. Primarily a snake of wet forest habitats, it inhabits dry forest in riparian situations. It is a powerful constrictor of mammals, from rats and bats to porcupines, but supplements this diet with birds, their eggs, and lizards. A large and bold snake, the Tiger Ratsnake will defend itself vigorously by inflating its throat and making far-reaching strikes. But it is nonvenomous, and because of its rodent-killing capabilities, it should be protected.
RELATED SPECIES
Genus Spilotes contains two other species, the Amazonian Puffing Snake (S. sulphureus), and a former subspecies elevated by some authors to specific status, the Ecuadorian Tiger Ratsnake (S. megalolepis). Although S. pullatus is often referred to as a “ratsnake” it is not closely related to any of the other American ratsnake genera, being closer to the puffing snakes of genus Phrynonax (shown here).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
North, Central, and South America: southern Mexico to Paraguay, including Trinidad, Tobago, and Isla Margarita |
ELEVATION |
0–6,560 ft (0–2,000 m) asl |
HABITAT |
Gallery forest, lowland and low montane wet forest, secondary growth |
DIET |
Mammals, birds and their eggs, and lizards |
REPRODUCTION |
Oviparous, with clutches of 5–12 eggs |
CONSERVATION STATUS |
IUCN not listed |

The Tiger Ratsnake is a powerful snake with smooth scales, a laterally compressed body, a long tail, a large head, and large eyes with round pupils. Its body, both dorsally and ventrally, is yellow with irregular black markings, the black markings being more dominant posteriorly, and the tail being all black. The head is yellow with black suturing and bands.
STEGONOTUS BATJANENSIS
NORTH MOLUCCAN GROUNDSNAKE
(GÜNTHER, 1865)
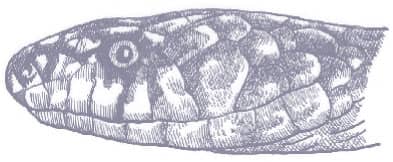
ADULT LENGTH
2 ft 7 in–3 ft 3 in, occasionally 5 ft (0.8–1.0 m, occasionally 1.55 m)
The largest member of the genus Stegonotus, the North Moluccan Groundsnake occurs in the Moluccas of eastern Indonesia. The holotype was collected on the island of Batjan, which is now known as Bacan. The North Moluccan Groundsnake inhabits rainforests, plantations, and low-lying wetland areas where it preys on frogs, lizards, and rodents. This species has enlarged rear teeth that are designed to shear through reptile eggs, another prey item, and these teeth may deliver bloody bites to humans picking them up. Although some discomfort may be felt, these snakes are technically nonvenomous.
RELATED SPECIES
The genus Stegonotus contains more than 20 other species, at least 12 from the New Guinea region, and species in Borneo, the Philippines, and Flores and Timor, in the Lesser Sunda Islands. The groundsnakes of genus Stegonotus are related to the wolfsnakes of genus Lycodon (shown here).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, possibly mildly toxic saliva |
DISTRIBUTION |
Southeast Asia: Indonesia |
ELEVATION |
0–2,130 ft (0–650 m) asl |
HABITAT |
Rainforest, plantations, and wetland areas |
DIET |
Frogs, lizards, small mammals, and reptile eggs |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN not listed |


The North Moluccan Groundsnake is a stout snake with smooth scales, a large, rounded head, and small eyes with vertically elliptical pupils. The head, body, and tail are brown, gray, or black above and white below, with a pattern comprising upward extensions of white, yellow, or orange pigment onto the flanks, these markings in some cases meeting at the midbody to form pale rings. There may be white or yellow bands around the neck and body and many of the scales on the sides of the head are pale-centered with dark edges.
STEGONOTUS RETICULATUS
RETICULATED GROUNDSNAKE
BOULENGER, 1895
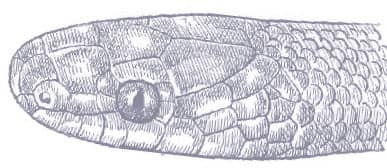
ADULT LENGTH
2 ft 7 in–3 ft 3 in, occasionally 4 ft 3 in (0.8–1.0 m, occasionally 1.3 m)
The Reticulated Groundsnake has been removed from the synonymy of the Slatey-gray Snake (S. cucullatus). It is distributed throughout the Papua New Guinea mainland and islands of Milne Bay, and is also thought to occur in northern Australia. One of the largest Stegonotus in New Guinea, achieving over 3 ft 3 in (1 m) in length, it inhabits low-lying wetland habitats, rainforest, and plantations, but also occurs in disturbed habitats and around human dwellings. Nocturnal and terrestrial, it can also climb, and it is often found crossing roads at night. Prey consists of frogs, lizards, mice, and reptile eggs. Groundsnakes bite vigorously, drawing blood but causing only local effects.
RELATED SPECIES
Stegonotus cucullatus was a species-complex comprising several cryptic species, with S. reticulatus the most distinctive. The New Guinea mainland is home to numerous other Stegonotus species, with others in the D’Entrecasteaux and Trobriand archipelagos (S. guentheri), the Bismarck Archipelago (S. heterurus), Admiralty Islands (S. admiraltiensis), Raja Ampat Islands (S. iridis, S. derooijae), and Schouten Islands (S. parvus). Some species may be confused with the highly venomous New Guinea Small-eyed Snake (Micropechis ikaheka).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, possibly mildly toxic saliva |
DISTRIBUTION |
Australasia: Papua New Guinea, possibly northern Australia |
ELEVATION |
0–4,000 ft (0–1,220 m) asl |
HABITAT |
Rainforest, riverine forest, disturbed areas, wetland areas, plantations, and around human habitations |
DIET |
Frogs, lizards, small mammals, and reptile eggs |
REPRODUCTION |
Oviparous, with clutches of 6–12 eggs |
CONSERVATION STATUS |
IUCN not listed |
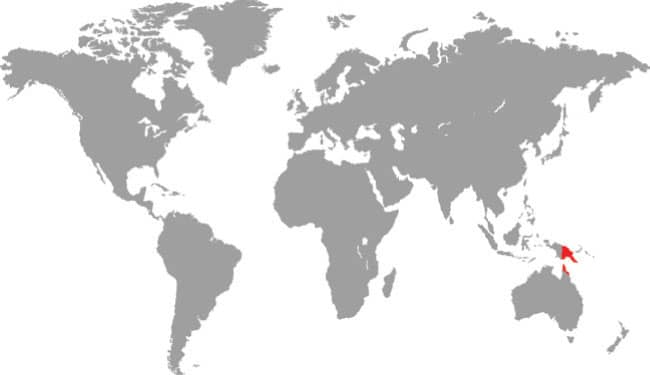

The Reticulated Groundsnake is a smooth-scaled snake with a head distinct from neck, and small, dark, protruding eyes. Every pale scale of the body is dark-edged, resulting in a “reticulate” pattern. The undersides are immaculate white. The head is usually dark above with pale lips, chin, and throat.
STENORRHINA FREMINVILLEI
BLOOD SNAKE
(DUMÉRIL, BIBRON & DUMÉRIL, 1854)
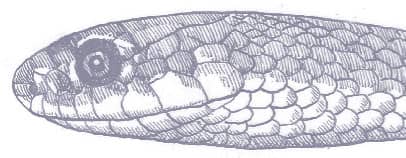
ADULT LENGTH
133/4–181/2 in, occasionally 331/2 in (350–470 mm, occasionally 850 mm)
The Blood Snake is a member of the Central American scorpion-eating genus Stenorrhina. It inhabits lowland and low montane arid or semi-moist forest and also occurs in swampy areas where it adopts a secretive, diurnal, terrestrial, or semi-fossorial habit, living under logs and in leaf litter. Prey consists of scorpions and spiders, including tarantulas, but insects such as beetles and crickets are also eaten. Females are oviparous and may lay two clutches during the dry season. Although it is harmless, Costa Rican folklore holds that anybody bitten by this snake will die following massive hemorrhaging through the skin. This is hard to disprove as this inoffensive species cannot be induced to bite. Christophe-Paulin de La Poix Chevalier de Fréminville (1787–1848) was a French naval officer and naturalist.
RELATED SPECIES
Another species, Degenhardt’s Scorpion-eating Snake (Stenorrhina degenhardtii), occurs as three subspecies, from southern Mexico to Venezuela and Peru. Stenorrhina differ from other snakes within their range due to their fused internasal and anterior nasal scales. Stenorrhina is most closely related to the sandsnakes (Chilomeniscus), shovelnose snakes (Chironius), and ground-snakes (Sonora).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North and Central America: southern Mexico, Guatemala, Belize, El Salvador, Honduras, Nicaragua, Costa Rica, and Panama |
ELEVATION |
0–7,220 ft (0–2,200 m) asl |
HABITAT |
Lowland and low montane wet and dry forest, savanna woodland, and swampy areas |
DIET |
Arachnids and insects |
REPRODUCTION |
Oviparous, with clutches of 4–19 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
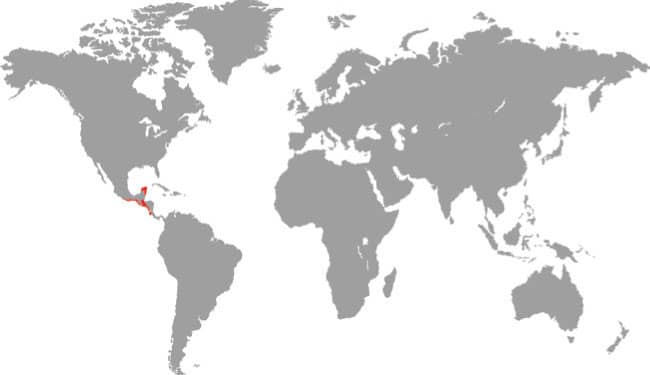

The Blood Snake is a small, smooth-scaled snake with a narrow, squarish head that is indistinct from the neck, and small eyes with round pupils. Dorsally it is blood red, orange, or brown, either unicolor or longitudinally striped, with an unmarked venter.
SYMPHIMUS LEUCOSTOMUS
TEHUANTEPEC WHITE-LIPPED SNAKE
COPE, 1869
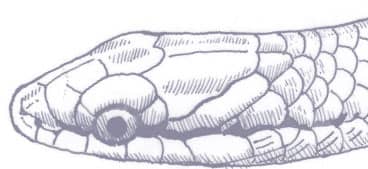
ADULT LENGTH
223/4–32 in (580–810 mm)
The Tehuantepec or Pacific White-lipped Snake inhabits tropical deciduous forest, thorn forest, and pine–oak forest, in the southwestern Mexican states of Jalisco, Michoacán, Guerrero, Oaxaca, and Chiapas. It is a rare snake, found at lowland or low montane elevations, and is probably known from fewer than 20 specimens in museum collections. Its natural history is also poorly documented. It is known to be diurnal, terrestrial, and semi-arboreal, and is also believed to feed on insects, although it may also take scorpions and spiders like its congener, the Mayan White-lipped Snake (Symphimus mayae). Like that species, it is probably oviparous. Although this species is listed as Least Concern by the IUCN it is considered Endangered in Chiapas by the Mexican SEMARNAT System, and afforded Special Protection.
RELATED SPECIES
The only other species of Symphimus is the Mayan White-lipped Snake (S. mayae), which occurs on the Yucatán Peninsula of Mexico and Belize. The genus Symphimus may be closely related to the American greensnakes (Opheodrys) and at one time the Mayan White-lipped Snake was included in that genus.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: Mexico |
ELEVATION |
655–3,280 ft (200–1,000 m) asl |
HABITAT |
Tropical deciduous forest, thorn forest, and pine–oak forest |
DIET |
Presumed insects and/or arachnids |
REPRODUCTION |
Presumed oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Least Concern, Special Protection in Mexico |


The Tehuantepec White-lipped Snake is a slender snake with smooth scales, a long tail, and a broad, elongate head with large eyes and round pupils. Dorsally the snake is light brown with a broad, darker brown vertebral stripe. The head is brown above and yellow on the lips, with a yellow stripe running onto the neck, and the two colors separated by a dark stripe.
SYMPHOLIS LIPPIENS
MEXICAN SHORT-TAILED SNAKE
COPE, 1861
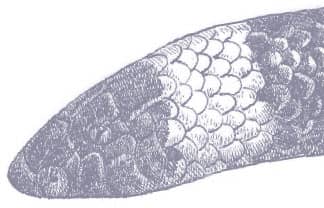
ADULT LENGTH
17–211/4 in (430–540 mm)
The Mexican Short-tailed Snake is distributed from southwestern Chihuahua to Sonora and Sinaloa, and down the northwestern Mexican coast to Jalisco. It is a rare snake that inhabits tropical deciduous forest and thorn scrub, where it is believed to lead a nocturnal and semi-fossorial existence. Given its close apparent relationship to Mexican scorpion- and spider-eating snakes it is likely that this species also feeds on arachnids, but no data regarding its prey preferences are available. It is an instantly recognizable snake, no other species within its range bearing the same distinctive pattern. The etymology of the specific name lippiens, which means “almost blind,” is a reference to the very small eyes of this species.
RELATED SPECIES
The genus Sympholis is monotypic and no subspecies are recognized. It is thought most closely related to the scorpion-eating snakes (Stenorrhina) and the hooknose snakes (Ficimia, Gyalopion, and Pseudoficimia).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North America: northwestern Mexico |
ELEVATION |
1,430–3,000 ft (435–915 m) asl |
HABITAT |
Tropical deciduous forest and thorn scrub |
DIET |
Presumed arthropods |
REPRODUCTION |
Presumed oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN not listed |
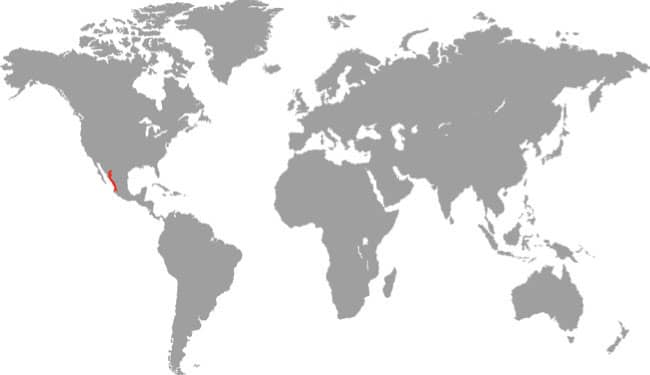

The Mexican Short-tailed Snake is a cylindrical snake with smooth scales, a short tail, a head that is not distinct from the neck, and small eyes. Its patterning is distinctive, comprising regular, ragged-edged yellow or white rings on a black background, the interspaces being two to three times the width of the rings.
TANTILLA MELANOCEPHALA
BLACK-HEADED CENTIPEDE SNAKE
(LINNAEUS, 1758)
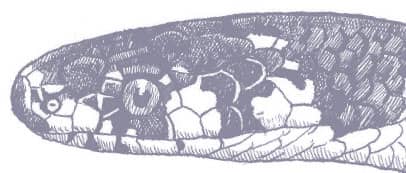
ADULT LENGTH
113/4–153/4 in, occasionally 173/4 in (300–400 mm, occasionally 450 mm)
The Black-headed Centipede Snake was originally thought to occur from Guatemala to northern Argentina, but several former populations have now been described as separate, but related, species and the mainland population of the Black-headed Centipede Snake is now confined to South America, although it is also reported from Trinidad, Tobago, and several islands of the Lesser Antilles. It is nocturnal, terrestrial, and semi-fossorial. It is found in rainforests and open habitats, at low and high elevations, sheltering under leaf litter, palm fronds, or in termite mounds. Where it occurs this is a very common species with several specimens being found under the same fallen palm fronds. Prey includes venomous scolopendrid centipedes and insects. In many parts of South America this is the only Tantilla species represented.
RELATED SPECIES
The American genus Tantilla contains 66 species. The Tantilla melanocephala species group contains at least eight other species: the Amulet Centipede Snake (T. armillata) and Red-headed Centipede Snake (T. ruficeps) in Central America; Honduran Centipede Snake (T. lempira); Andes Centipede Snake (T. andinista), Miyata’s Centipede Snake (T. miyatai), and Mountain Centipede Snake (T. insulamontana) in Ecuador; and Masked Centipede Snake (T. capistrata) in Peru.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
South America: the Guianas to Argentina and Uruguay, Trinidad, Tobago, and Lesser Antilles |
ELEVATION |
0–10,100 ft (0–3,080 m) asl |
HABITAT |
Primary and secondary rainforest, Caatinga, Cerrado, savanna, and cultivated areas |
DIET |
Centipedes and insects |
REPRODUCTION |
Oviparous, with clutches of 1–3 eggs |
CONSERVATION STATUS |
IUCN not listed |

The Black-headed Centipede Snake is a small, smooth-scaled snake with a small head, indistinct from the neck, and small eyes with round pupils. It is dorsally brown with five longitudinal black stripes, the vertebral stripe being the boldest, and a yellow venter. The head is black with white spots, and a black nape band or collar is present.
TANTILLA OOLITICA
RIM ROCK CROWNED SNAKE
TELFORD, 1966
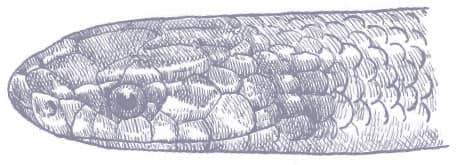
ADULT LENGTH
6–9 in, rarely 111/2 in (150–230 mm, rarely 290 mm)
The Rim Rock Crowned Snake is one of the smallest snakes in the United States, with one of the smallest ranges, inhabiting the hardwood hammocks and pinewoods on an oolitic (formed from rounded grains, or ooliths) limestone ridge running parallel to the coast, through Monroe and Miami-Dade counties, Florida, from Miami to Key Largo and continuing to Key West. Habitat loss due to development is the main threat to this tiny, inoffensive snake, one specimen even being found on a vacant lot in Miami. It is also one of the least studied of Florida’s snakes. It is believed to prey on beetles, spiders, scorpions, centipedes, snails, and possibly smaller snakes. Probably its best chance of survival is the Key Largo wildlife refuge.
RELATED SPECIES
Eleven of the 64 species of Tantilla occur within the United States, but only three occur in the state of Florida: the Southeastern Crowned Snake (T. coronata) in the Panhandle, the Florida Crowned Snake (T. relicta) with three subspecies in the Peninsula, and T. oolitica.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North America: southeastern USA |
ELEVATION |
0–33 ft (0–10 m) asl |
HABITAT |
Hardwood hammocks and pinewoods on oolitic limestone |
DIET |
Insects, spiders, scorpions, centipedes, snails, and small snakes |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Endangered, protected in Florida |

The Rim Rock Crowned Snake is a small, smooth-scaled snake with a cylindrical body, a head indistinct from the neck, and small eyes with round pupils. It is light tan above and pinkish brown or cream below, with a black dorsum to the head and a broad black band around the neck.
TANTILLITA CANULA
YUCATÁN DWARF SHORT-TAILED SNAKE
(COPE, 1875)
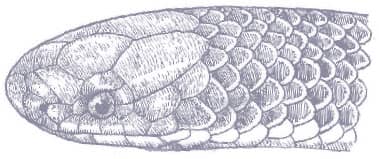
ADULT LENGTH
53/4–7 in (145–180 mm)
The Yucatán Dwarf Short-tailed Snake is found on the Yucatán Peninsula of southern Mexico, in the states of Campeche, Yucatán, and Quintana Roo, and in northern Guatemala and Belize, also on the Yucatán Peninsula. A lowland species, this tiny snake inhabits tropical evergreen forests and thorn forests below 985 ft (300 m). It is terrestrial or semi-fossorial, being found in leaf litter or under logs or rocks. Several live specimens have also been found by archeologists excavating Mayan temples, or by construction crews building new dwellings. Little is known of the natural history of this diminutive species, but it is believed to feed on arthropods, possibly centipedes, and probably also lays eggs.
RELATED SPECIES
The genus Tantillita contains only two other species, the Speckled Dwarf Short-tailed Snake (T. brevissima) from southwestern Mexico and Guatemala, and Linton’s Dwarf Short-tailed Snake (T. lintoni) from southern Mexico, Guatemala, and Belize. Tantillita is closely related to the centipede snakes (Tantilla), and the Tehuantepec Striped Snake (Geagras redimitus).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North and Central America: Mexico, northern Guatemala, and Belize |
ELEVATION |
0–985 ft (0–300 m) asl |
HABITAT |
Tropical evergreen forest and thorn forest |
DIET |
Presumed arthropods, especially centipedes |
REPRODUCTION |
Presumed oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Least Concern |
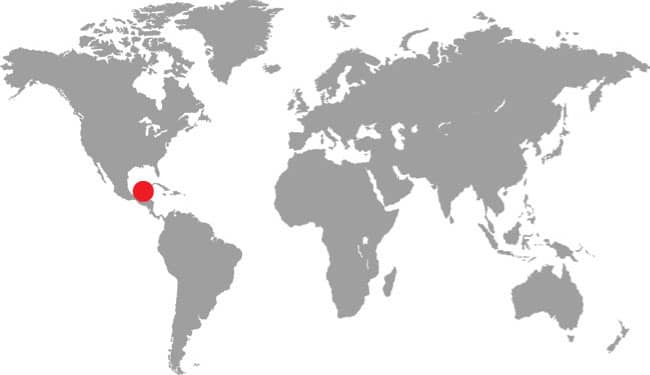

The Yucatán Dwarf Short-tailed Snake is small with smooth scales, a very short tail, a head indistinct from the neck, and small eyes with round pupils. It is brown above, slightly darker on the head, but it lacks the black cap and collar of many Tantilla, the only patterning being a faint pale vertebral stripe in some specimens. The venter is cream.
TELESCOPUS FALLAX
EUROPEAN CATSNAKE
FLEISCHMANN, 1831
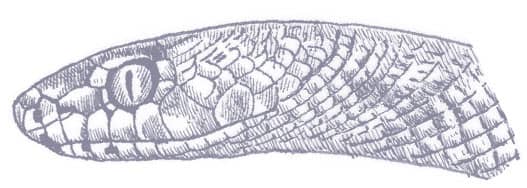
ADULT LENGTH
2 ft 7 in–3 ft 3 in, rarely 4 ft (0.8–1.0 m, rarely 1.2 m)
The European Catsnake is distributed through southeastern Europe, the Middle East, and western Asia, in arid rocky habitats, primarily in coastal regions and on islands. It is a nocturnal and terrestrial species that climbs rocks but not trees. It also inhabits man-made habitats, such as dry stone walls and railway embankments, which are home to large populations of geckos and lacertid lizards, its preferred prey. Small snakes, mice, and even nestling birds are also taken. The European Catsnake is a rear-fanged species that stalks its prey, strikes quickly, and chews to bring the fangs into play, using its coils to restrain the prey while the venom takes effect. Its defensive display involves flattening its already broad head, hissing, and striking, but its venom is not dangerous to humans.
RELATED SPECIES
Seven subspecies are recognized, from mainland southeast Europe, Malta, Rhodes, and Turkey (Telescopus fallax fallax); Antikythera Island, Greece (T. f. intermedius); Crete (T. f. pallidus); Koufonisia Island, Greece (T. f. multisquamatus); Cyprus (T. f. cyprianus); southeast Turkey and the Middle East (T. f. syriacus); and eastern Turkey, northern Iran, and Transcaucasia (T. f. iberus). The genus Telescopus contains another 14 species in Africa, Arabia, and western Asia, including a former subspecies of T. fallax from Israel and Jordan (T. hoogstraali).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
Southwest Europe, Middle East, and western Asia: the Balkans, Albania, Greece, Turkey, Malta, Corfu, Cyprus, Rhodes, Israel, Syria, Lebanon, Sinai, Iraq, Iran, and Caucasus |
ELEVATION |
0–6,560 ft (0–2,000 m) asl |
HABITAT |
Rocky habitats, including perianthropic habitats such as railway embankments and dry stone walls |
DIET |
Lizards, small snakes, small mammals, and birds |
REPRODUCTION |
Oviparous, with clutches of 5–8 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
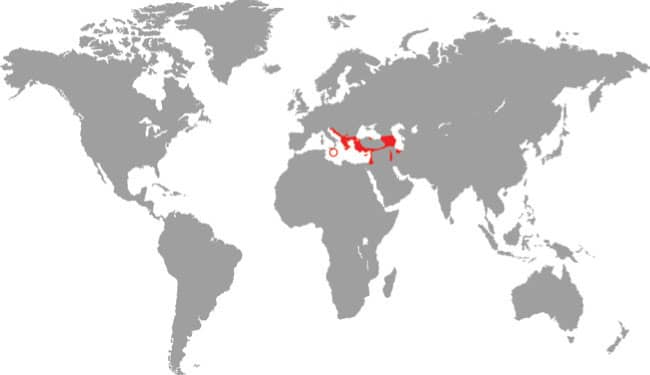

The European Catsnake is a laterally compressed snake with smooth scales, a long tail, and a broad head with protruding eyes and vertically elliptical pupils. It is a gray to brown snake with a pattern comprising distinctive dark dorsal blotches and small lateral bars. The iris of the eye is yellow.
TELESCOPUS SEMIANNULATUS
EASTERN TIGER SNAKE
SMITH, 1849
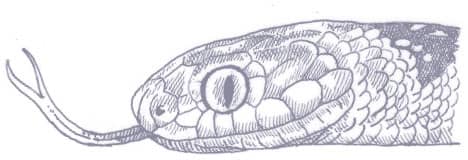
ADULT LENGTH
Male
233/4–311/2 in (600–800 mm)
Female
311/2–351/2 in, rarely 3 ft 9 in (800–900 mm, rarely 1.15 m)
The Eastern Tiger Snake is a common nocturnal African snake, often seen crossing roads after rain. It occurs from South Africa and Namibia to Kenya and Tanzania, in rocky habitats and sandy savanna woodland, where it hunts geckos, sleeping chameleons, small birds, bats, and mice. Its venom is weak, requiring the Eastern Tiger Snake to also employ constriction to restrain its prey while it takes effect. Although primarily terrestrial, it scales rocks, buildings, or trees with ease. When encountered it may adopt a defensive posture, elevating its anterior body to form an S-shaped curve, flattening its already broad head, and making sudden and rapid strikes, with such force that the body is often thrown forward. Females can store the male’s sperm and lay eggs every two months during the summer.
RELATED SPECIES
The nominate subspecies (Telescopus semiannulata semiannulata) occupies most of the range, with the Namibian population recognized as a separate subspecies (T. s. polystictus). A similar, second species, Beetz’s Tiger Snake (T. beetzi), occurs in southern Namibia and into Namaqualand, South Africa. The Arabian Catsnake (T. dhara) occurs to the north in Kenya.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
Southern and East Africa: South Africa, Namibia, Swaziland, Botswana, Zambia, Mozambique, Malawi, Zimbabwe, DRC, Tanzania, and Kenya |
ELEVATION |
0–5,580 ft (0–1,700 m) asl |
HABITAT |
Rocky outcrops, sandveld, bushveld, lowveld, coastal thicket, savanna woodland |
DIET |
Lizards, small mammals, and birds |
REPRODUCTION |
Oviparous, with clutches of 6–20 eggs |
CONSERVATION STATUS |
IUCN not listed |
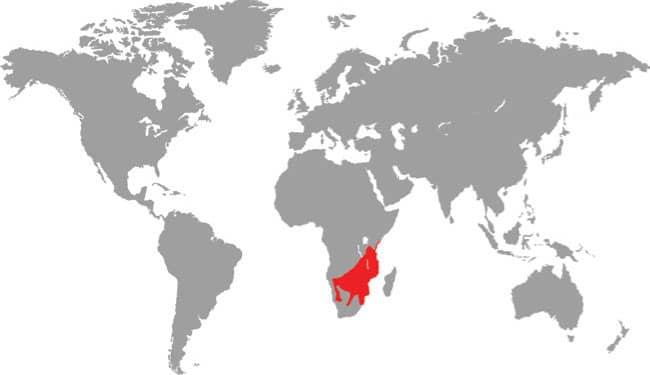

The Eastern Tiger Snake is a slender, smooth-scaled snake with a broad head, distinct from the neck, and protruding eyes with vertically elliptical pupils. It is brown, orange, sandy yellow, or pinkish yellow, with a black nape band and a series of regularly spaced black blotches or bands on the dorsum. These bands are most numerous in the Namibian subspecies. The venter is pale yellow to orange.
THELOTORNIS CAPENSIS
SOUTHEASTERN SAVANNA TWIGSNAKE
SMITH, 1849
ADULT LENGTH
2 ft 7 in–4 ft, rarely 5 ft (0.8–1.2 m, rarely 1.5 m)
The Southeastern Savanna Twigsnake, also known as the Savanna Vinesnake, inhabits open woodland and savanna habitats from Angola to KwaZulu-Natal, but it is absent from most of South Africa. A diurnal and highly arboreal snake, it is perfectly camouflaged for its lifestyle, hunting chameleons and small birds in the trees. The horizontal pupils provide it with amazing vision, enabling it to discern the most camouflaged of chameleons. Prey is killed with venom injected via the enlarged rear fangs. Its threat posture involves inflating its neck and flattening its head, but it is reluctant to bite. However, bites from this snake are extremely serious, the hemotoxins in the venom causing prolonged bleeding and renal failure. The eminent German herpetologist Robert Mertens (1894–1975) died several days after being bitten. There is no antivenom manufactured to treat twigsnake bites.
RELATED SPECIES
Two subspecies are recognized, a nominate eastern form (Thelotornis capensis capensis), and a western form (T. c. oatesi). The Eastern Savanna Twigsnake (T. mossambicanus) was previously a subspecies of T. capensis. Twigsnakes bear a strong resemblance to the Asian vinesnakes (Ahaetulla), American vinesnakes (Oxybelis), Hispaniolan vinesnakes (Uromacer), and the Dagger-toothed Vinesnake (Xyelodontophis uluguruensis).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, highly venomous: procoagulants, and possibly anticoagulants or hemorrhagins |
DISTRIBUTION |
Southern Africa: southern Angola, Namibia, eastern Botswana, DRC, Malawi Mozambique, Zambia, Zimbabwe, Swaziland, and northeast South Africa |
ELEVATION |
0–6,000 ft (0–1,830 m) asl |
HABITAT |
Wet and dry savanna, and riparian woodland |
DIET |
Lizards, small birds, frogs, snakes, and bats |
REPRODUCTION |
Oviparous, with clutches of 4–18 eggs |
CONSERVATION STATUS |
IUCN Least Concern |


The Southeastern Savanna Twigsnake is an extremely slender snake with weakly keeled scales arranged in oblique rows, a long tail, an elongate, narrow head, and large eyes with horizontal pupils. It is pale gray to gray-brown in color, with patterning comprising diagonal pale bands and flecks of black and pink, altogether presenting a twig-like appearance. The dorsum of the head is brown, with a red-brown stripe through the eye.
THELOTORNIS KIRTLANDI
FOREST TWIGSNAKE
(HALLOWELL, 1844)
ADULT LENGTH
4 ft 3 in–5 ft 7 in (1.3–1.7 m)
The Forest Twigsnake, or Vinesnake, inhabits pockets of montane forest in East Africa, but occurs more widely in rainforest and dense woodland habitats farther west, and it also inhabits reed beds on moist savannas. It occurs as far west as Sierra Leone and Guinea and as far south as northern Angola and Zambia. Although sometimes called the “Birdsnake,” it preys primarily on chameleons, geckos, and agamid lizards, with birds, their eggs, amphibians, and other snakes only occasional prey. Terrestrial lizards may be ambushed from above, in a tactic used by New Guinea Treeboas (Candoia carinata). Few bites are known for this species but it must be considered potentially dangerous given the fatalities attributed to the Southeastern Savanna Twigsnake (Thelotornis capensis), and the lack of antivenom. Jared Potter Kirtland (1793–1877) was an American naturalist.
RELATED SPECIES
The genus Thelotornis contains three other species, the Southeastern Savanna Twigsnake (T. capensis), the Eastern Savanna Twigsnake (T. mossambicanus), and the endangered Usumbara Twigsnake (T. usumbaricus) from East Africa.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, highly venomous: probably procoagulants, possibly anticoagulants and hemorrhagins |
DISTRIBUTION |
West and Central Africa: Guinea to Ghana, Nigeria to DRC, Uganda and Tanzania, south to Angola and Zambia |
ELEVATION |
5,250–7,220 ft (1,600–2,200 m) asl |
HABITAT |
Rainforest, dense woodland, thickets, moist savanna reed beds |
DIET |
Lizards, birds, eggs, amphibians, and other snakes |
REPRODUCTION |
Oviparous, with clutches of 4–12 eggs |
CONSERVATION STATUS |
IUCN not listed |
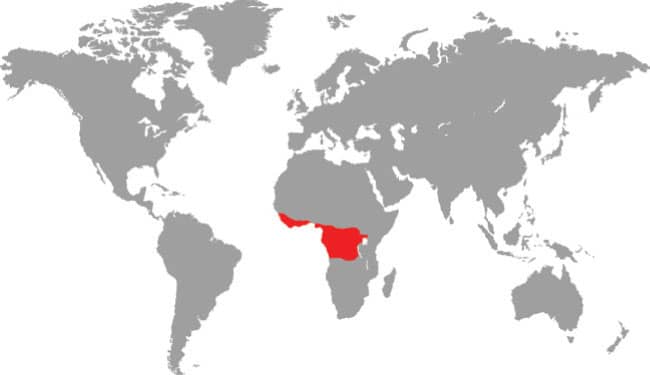

The Forest Twigsnake is an extremely slender snake with weakly keeled scales arranged in oblique rows, a long tail, and an elongate, narrow head and large eyes with horizontal pupils. Its most striking characteristic is the bright green dorsum of the head, a character it shares with the Usumbara Twigsnake, which contrasts with the white of the lip scales. The body is mottled gray, green, and brown with black cross-bar markings.
TOXICODRYAS BLANDINGII
BLANDING’S TREESNAKE
(HALLOWELL, 1844)
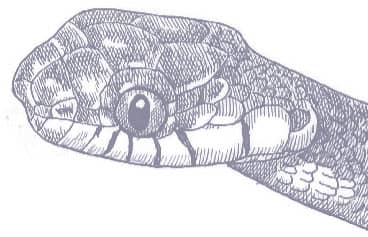
ADULT LENGTH
4 ft 7 in–6 ft 7 in, rarely 9 ft 2 in (1.4–2.0 m, rarely 2.8 m)
Blanding’s Treesnake is Africa’s largest nocturnal treesnake, occurring across West and Central Africa. It is found in tropical rainforest, riverine gallery forest, and savanna woodland. It prefers large trees, and rarely ventures to the ground. It hunts prey ranging from arboreal mammals to sleeping birds, their eggs, lizards, and frogs. Bats are often taken, the treesnake occupying trees near bat roosts or entering roof spaces in search of them, and it is believed able to smell sleeping birds in their nests. The venom of the rear-fanged Blanding’s Treesnake contains powerful postsynaptic neurotoxins that interfere with neuromuscular transmissions. Bites have caused muscle pain and difficulty in breathing and there remains the potential for a serious snakebite. This species should be treated with caution. William Blanding (1772–1857) was an American naturalist.
RELATED SPECIES
There are two species in the African genus Toxicodryas, the other being the smaller Powdered Treesnake (T. pulverulenta). In the past both species were included in the Asian catsnake genus Boiga (shown here). Toxicodryas blandingii is easily confused with the highly venomous tree cobras (Pseudohaje) or Forest Cobra (Naja melanoleuca).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, venomous, potentially dangerous: postsynaptic neurotoxins |
DISTRIBUTION |
West and Central Africa: Senegal to Kenya, south to Angola and Zambia |
ELEVATION |
0–7,220 ft (0–2,200 m) asl |
HABITAT |
Rainforest, woodland, savanna woodland, gallery forest, and parks |
DIET |
Lizards, small mammals, birds and their eggs, and frogs |
REPRODUCTION |
Oviparous, with clutches of 7–14 eggs |
CONSERVATION STATUS |
IUCN not listed |
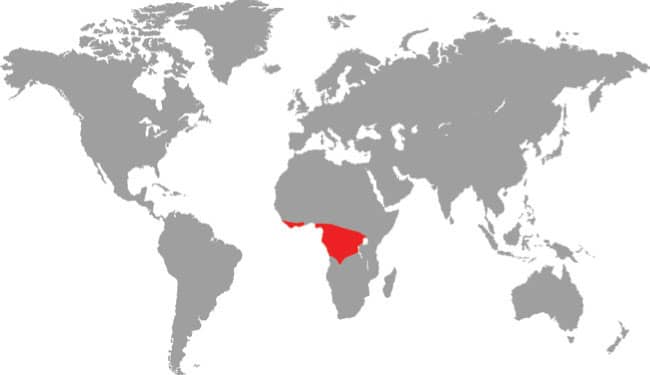

Blanding’s Treesnake is a large snake with smooth scales, a laterally compressed body, a long tail, and a very broad, flattened head, distinct from the neck, with large eyes and round pupils. The species is sexually dichromatic, males being glossy black above and yellowish below and on the lips, while females are matte brown with darker transverse bands.
TRIMORPHODON LAMBDA
SONORAN LYRESNAKE
COPE, 1886
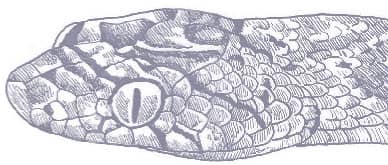
ADULT LENGTH
233/4–351/2 in, occasionally 4 ft (600–900 mm, occasionally 1.2 m)
The Sonoran Lyresnake is found in Sonora, Mexico, and north into Arizona and Nevada, USA. It is found in rocky habitats, from escarpments to boulder-strewn desert floors with growth of creosote, mesquite, and saguaro cacti. Although primarily a terrestrial species, this snake can climb well if required and is at home scaling rocky outcrops and low desert vegetation. This is a relatively large snake that hunts primarily lizards at night, exploring crevices and fissures for prey, which is killed using venom injected via the rear fangs. Other prey is believed to include small rodents, bats, and birds. The warning display of the Sonoran Lyresnake involves elevating the anterior body in a threatening curve. The venom is believed weak and has no effect on humans, but caution is advised when handling large specimens.
RELATED SPECIES
No subspecies of Trimorphodon lambda are recognized, its former subspecies, the Sinaloan Lyresnake (T. paucimaculatus), being elevated to specific status. Trimorphodon lambda was once also a subspecies of the Western Lyresnake (T. biscutatus), along with the California Lyresnake (T. lyrophanes) and Central American Lyresnake (T. quadruplex). Trimorphodon is related to the leafnose snakes (Phyllorhynchus).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North America: southwestern USA and northern Mexico |
ELEVATION |
0–2,620 ft (0–800 m) asl |
HABITAT |
Rocky deserts, rocky escarpments, and mesquite, creosote, and saguaro habitats |
DIET |
Lizards, small mammals, and birds |
REPRODUCTION |
Oviparous, with clutches of 5–20 eggs |
CONSERVATION STATUS |
IUCN not listed |
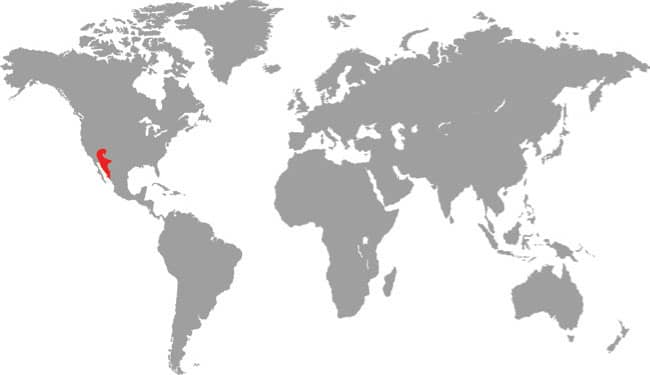

The Sonoran Lyresnake is a laterally compressed snake with smooth scales and a broad head, distinct from the neck, with large, protruding eyes and vertically elliptical pupils. Its dorsal ground color is gray, brown, or pinkish, with a series of dark brown dorsal blotches, each with a transverse light brown center, the dark area extending almost to the venter on the flanks. The head bears two dark brown chevrons, the lyre-shaped marking, one through the eyes and the other to the angle of the jaw.
TRIMORPHODON VILKINSONII
TEXAS LYRESNAKE
COPE, 1886
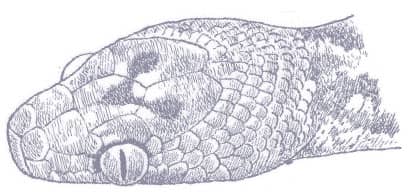
ADULT LENGTH
233/4–351/2 in, rarely 3 ft 5 in (600–900 mm, rarely 1.04 m)
The Texas Lyresnake is found in western Big Bend, Texas, southern New Mexico, and northern Chihuahua, Mexico. It is the easternmost member of the lyresnake genus Trimorphodon. It inhabits rocky bluffs but is also found in flat, open, rock-strewn desert and close to rivers. This is a secretive species that is rarely encountered. It is most active on nights with high humidity due to rainfall, when it searches the fissures and crevices for lizards, both active nocturnal species and sleeping diurnal species, but it also preys on mice, bats, and nestling birds. Prey is killed by the snake’s weak venom, which is injected via its enlarged, grooved rear fangs. If handled the Texas Lyresnake will bite, but it is not dangerous to humans. Edward Wilkinson (1846–1918) was an amateur naturalist.
RELATED SPECIES
Trimorphodon vilkinsonii was once included as a subspecies of the Western Lyresnake complex (T. biscutatus, T. lambda, etc.). Another species, the Mexican Lyresnake (T. tau), which has two subspecies, occurs farther south.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North America: southern USA and northern Mexico |
ELEVATION |
2,950–6,070 ft (900–1,850 m) asl |
HABITAT |
Rocky outcrops and bluffs, and open rocky desert |
DIET |
Lizards, small mammals, and birds |
REPRODUCTION |
Oviparous, with clutches of 6–7 eggs |
CONSERVATION STATUS |
IUCN Least Concern, Threatened in Texas |
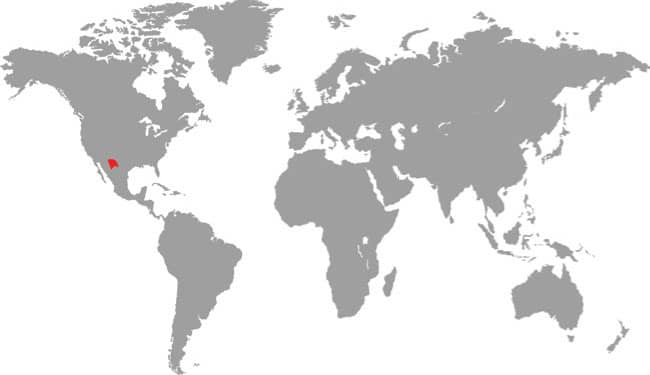

The Texas Lyresnake has a laterally compressed body, with smooth scales, a broad head, distinct from the neck, and large protruding eyes with vertically elliptical pupils. It is dorsally pale gray with a series of dark gray to black middorsal blotches, each edged with white or very pale gray, that extend down the flanks as narrow bands. A dark lyre-shaped marking is present on the dorsum of the head.
XENELAPHIS ELLIPSIFER
ORNATE BROWNSNAKE
BOULENGER, 1900
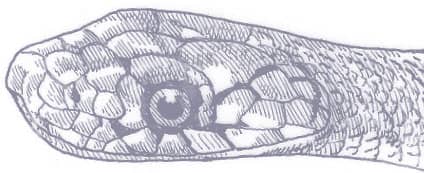
ADULT LENGTH
5 ft–7 ft 7 in, occasionally 8 ft 2 in (1.5–2.3 m, occasionally 2.5 m)
The Ornate Brownsnake is a large but rare species known from only a few specimens or sightings from Borneo, Sumatra, and Peninsular Malaysia. It inhabits lowland and low montane rainforest and is most likely to be encountered near to watercourses. Brownsnakes of genus Xenelaphis are semi-aquatic, and they are reported to feed on fish. Adults also take small mammals to the size of squirrels, and juveniles are said to feed on lizards and frogs. This species can also climb into low bushes. It has only been sighted on rare occasions in nature, so natural history data are scarce. However, one specimen was repeatedly seen near the same stream, suggesting a small home range and site fidelity.
RELATED SPECIES
A second species, the much commoner Malayan Brownsnake (Xenelaphis hexagonotus), occurs in southern Myanmar and Thailand, Vietnam, Malaysia, Sumatra, Java, and Borneo. This genus was originally contained in the Xenodermatinae (odd-scaled snakes), now Xenodermatidae (shown here), but is now thought more closely related to Asian ratsnakes and racers of Coelognathus (shown here), Gonyosoma (shown here), and Ptyas (shown here).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Southeast Asia: Malay Peninsula, Borneo, and Sumatra |
ELEVATION |
490–3,610 ft (150–1,100 m) asl |
HABITAT |
Primary lowland and low montane rainforest, near water |
DIET |
Small mammals, lizards, frogs, and fish |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Least Concern |
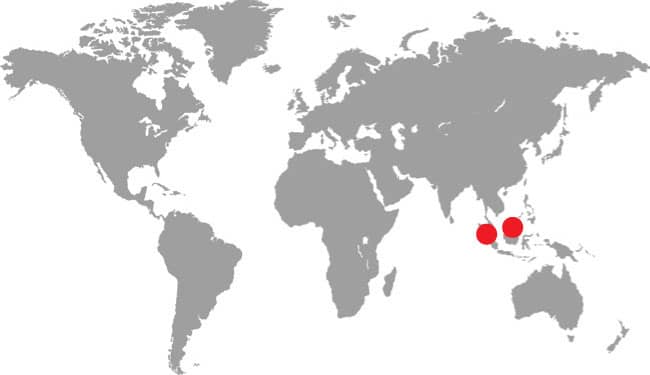

The Ornate Brownsnake is a large, robust snake with smooth scales, a long tail, a large head that is distinct from the neck, and large eyes with round pupils. It is dorsally pale cream with a series of large, red, dorsal ocelli markings with black edges, and a bright red to orange dorsum to the head. The supralabials and neck are yellow while the venter is white to cream.
ZAMENIS LONGISSIMUS
COMMON AESCULAPIAN SNAKE
(LAURENTI, 1768)
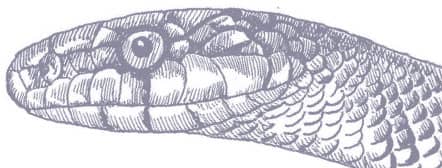
ADULT LENGTH
4 ft 7 in–5 ft 2 in, rarely 7 ft 5 in (1.4–1.6 m, rarely 2.25 m)
The Common Aesculapian Snake is linked with Asclepius, the Roman god of medicine, and featured on the Rod of Asclepius, the symbol of medicine. It is distributed from northeast Spain to Ukraine, and south to the Balkans, Greece, and Turkey. It was proposed that isolated populations in Germany and Austria were remnants of populations established by Roman legions, but they are more likely to represent refugia from a once wider distribution. Isolated populations are also known from eastern Turkey, Iran, and Russia. There is an introduced population in North Wales, which has thrived for decades, with another in London. This is a harmless predator of rodents and birds, with juveniles taking small mice and lizards. It is found in deciduous woodland, near rivers, and in perianthropic habitats.
RELATED SPECIES
No subspecies are currently recognized for Zamenis longissimus. Its nearest relative is the striped former subspecies, the Southern Italian Aesculapian Snake (Z. lineatus), which also occurs on Sicily. The Persian Ratsnake (Z. persicus), from Azerbaijan and Iran, was also a former subspecies.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Europe and western Asia: northeast Spain, France, Italy, Germany, Poland, Czech Republic, the Balkans, Greece, Bulgaria, Romania, Ukraine, Turkey, Russia, and Iran; introduced to UK |
ELEVATION |
0–5,580 ft (0–1,700 m) asl |
HABITAT |
Deciduous woodland, vineyards, orchards, riverbanks, water meadows, dry stone walls |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Oviparous, with clutches of 5–8 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
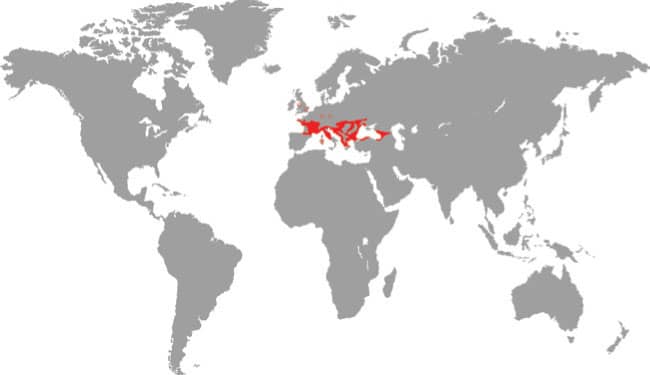

The Common Aesculapian Snake is a stout-bodied snake, with smooth scales, a long tail, a squarish head, just distinct from the neck, and large eyes with round pupils. Adults are fairly uniform olive-green or brown to gray with paler pigmentation on the undersides, throat, lips, and nape of the neck. Juveniles are more contrastingly marked, often with a checkerboard pattern of browns, a dark postocular stripe, and a black and yellow collar, which resembles that of the Western Grass Snake (Natrix helvetica).
ZAMENIS SCALARIS
LADDER SNAKE
(SCHINZ, 1822)
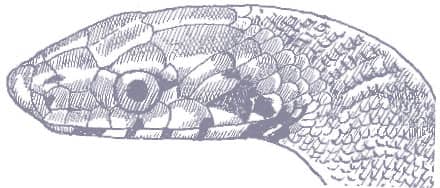
ADULT LENGTH
4 ft 3 in–5 ft 2 in (1.3–1.6 m)
The Ladder Snake is found throughout the Iberian Peninsula, except the extreme north. It also ranges into southern France and is recorded from Minorca in the Balearic Islands. It has a preference for open habitats, especially south-facing rocky slopes and dry, open woodland, but it also inhabits man-made habitats such as olive groves, vineyards, and cork oak plantations. It is generally diurnal and fond of basking, but it may become crepuscular or nocturnal in especially hot weather. The Ladder Snake is a fast-moving and alert predator of small mammals and birds, with juveniles taking mice and lizards. Prey may be pursued back to its own burrow, the Ladder Snake being equally at home underground. It climbs well and may be encountered in trees, hunting birds or their nestlings.
RELATED SPECIES
Zamenis scalaris was included in a separate genus, Rhinechis, due to its pointed snout, but molecular data places it within Zamenis with the Common Aesculapian Snake (Z. longissimus), Southern Italian Aesculapian Snake (Z. lineatus), Leopard Snake (Z. situla), Persian Ratsnake (Z. persicus), and Transcaucasian Ratsnake (Z. hohenackeri). Its closest relative is the Leopard Snake, from southeastern Europe.
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Southwest Europe: Spain, Portugal, and southern France; also Minorca |
ELEVATION |
0–6,890 ft (0–2,100 m) asl |
HABITAT |
South-facing rocky hillsides, open woodland, olive groves, cork oak plantations, and vineyards |
DIET |
Small mammals, birds, and lizards |
REPRODUCTION |
Oviparous, with clutches of 6–12 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
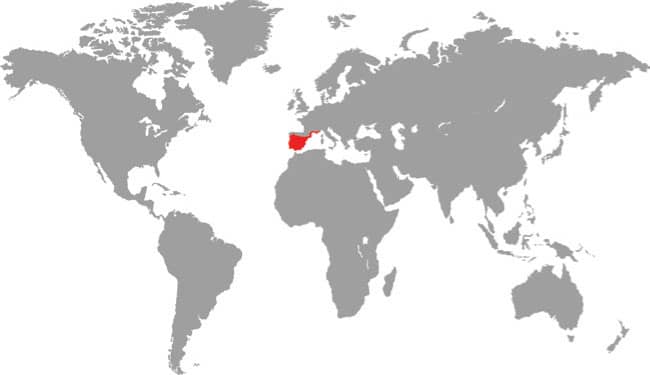

The Ladder Snake is a robust snake with smooth scales, a long tail, and a distinctive pointed head, with large eyes and round pupils. Adults vary from yellow to reddish brown or gray, with a bold pair of dark, longitudinal, dorsolateral stripes that are linked by regular transverse bars, which may be faint, presenting a ladder-like pattern. Juveniles exhibit the transverse bars across the midbody and a scattering of dark spots on the flanks.
ZAMENIS SITULA
LEOPARD SNAKE
(LINNAEUS, 1758)
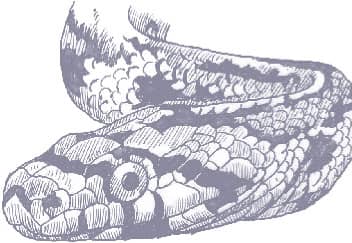
ADULT LENGTH
271/2–311/2 in, rarely 3 ft 3 in (700–800 mm, rarely 1.0 m)
The Leopard Snake has an extremely fragmented distribution from Sicily, Malta, and the heel of Italy, to the coastal strip from Croatia to Albania, Greece, and European Turkey, inland to Macedonia and Bulgaria, and across the Black Sea on the Crimean Peninsula. It is found on many islands, including Corfu, Crete, and Rhodes. This snake is an inhabitant of semiarid habitats such as well-vegetated rocky slopes, open woodland, and groves. Although it is diurnal it does not bask like the Ladder Snake (Zamenis scalaris), being more inclined to shelter close to vegetation. It preys on rodents, but lizards also feature in its diet. Leopard Snakes can climb, and birds or birds’ eggs are also occasionally taken. Prey is killed by constriction although small animals may be eaten alive.
RELATED SPECIES
Zamenis situla is one of six species in the genus Zamenis but appears to be the sister species to the Ladder Snake (Z. scalaris) of the Iberian Peninsula, although it is also related to the Common Aesculapian Snake (Z. longissimus).
FAMILY |
Colubridae: Colubrinae |
RISK FACTOR |
Nonvenomous, constrictor |
DISTRIBUTION |
Southeast Europe: southern Italy, Sicily, Malta, Croatia, Bosnia, Albania, Greece, Macedonia, Bulgaria, Turkey, and Crimea; also Corfu, Crete, and Rhodes |
ELEVATION |
0–5,250 ft (0–1,600 m) asl |
HABITAT |
Mediterranean habitats, from rocky slopes with dense vegetation, to open woodland, and human-mediated habitats such as plantations, groves, and dry stone walls |
DIET |
Small mammals, lizards, and birds |
REPRODUCTION |
Oviparous, with clutches of 2–7 eggs |
CONSERVATION STATUS |
IUCN Least Concern |


The Leopard Snake may be one of the most attractive snakes in Europe. The adults display a series of red, black-edged transverse dorsal bands on a gray background, with a red ocelli marking on the nape of the neck. The flanks exhibit a series of large black spots, and black bands are present on the head, between the eyes, from the eye to the lip, and from the eye to the angle of the jaw. In some specimens the red markings coalesce to form a pair of dorsal stripes.
GRAYIA SMYTHII
SMITH’S AFRICAN WATERSNAKE
(LEACH, 1818)
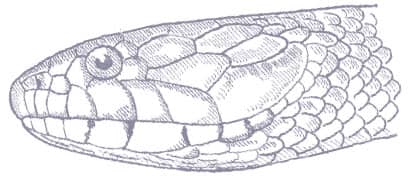
ADULT LENGTH
3 ft 3 in–5 ft 7 in, rarely 8 ft 2 in (1.0–1.7 m, rarely 2.5 m)
Smith’s African Watersnake is a large snake that is found around the periphery of watercourses, such as lakes or rivers, usually in savanna regions, including Lakes Victoria, Albert, and Edward. It feeds on fish, with frogs and tadpoles also in its diet. It will hiss loudly and open its mouth in threat when confronted, but is reluctant to bite. However, its close resemblance to the highly venomous Banded Water Cobra (Naja annulata) counsels caution with wild specimens. Although the specific name is smythii it is believed this species was named for the Norwegian physician-naturalist Christen Smith (1785–1816), who died on the expedition that collected the holotype.
RELATED SPECIES
Grayia is the sole genus in Grayiinae, an endemic African subfamily of the Colubridae. It was named in honor of John Edward Gray (1800–1975), an eminent zoologist at The Natural History Museum in London. Apart from G. smythii the genus contains three other species: Caesar’s African Water Snake (G. caesar) and the Ornate African Watersnake (G. ornata) in Central Africa, and Thollon’s African Watersnake (G. tholloni) in East and Central Africa. This is one of the few Old World watersnake genera not contained in the Natricidae.
FAMILY |
Colubridae: Grayiinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
East, Central, and West Africa: Senegal to South Sudan, south to Angola |
ELEVATION |
0–4,540 ft (0–1,385 m) asl |
HABITAT |
Rivers and lakes in savanna habitats |
DIET |
Fish, frogs, and tadpoles |
REPRODUCTION |
Oviparous, with clutches of 9–20 eggs |
CONSERVATION STATUS |
IUCN not listed |
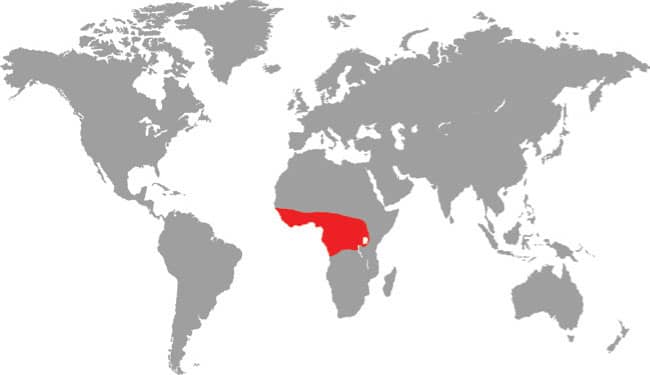

Smith’s African Watersnake is a stout-bodied snake with smooth scales, a long tail, and a robust head, with small eyes and round pupils. The dorsal coloration may be black or yellow-brown with faint, paler blotches, the remnants of the more distinctive bands of the juvenile livery. The head is brown with black-edged scales and a yellow throat and lips.
BLYTHIA RETICULATA
BLYTH’S IRIDESCENT SNAKE
(BLYTH, 1854)
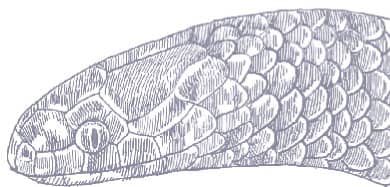
ADULT LENGTH
201/4 in (514 mm)
Blyth’s Iridescent Snake is an inhabitant of wet evergreen forests at low to medium elevations. Its natural history is poorly known due to its secretive nature. It is semi-fossorial to fossorial, being usually discovered under fallen logs, dense leaf litter, or piles of decomposing vegetation. The tail bears a short spine at its terminus. This spine is dug into the ground when the snake is burrowing, giving it an anchor point from which to apply force forward. Whether it is diurnal or nocturnal is not known. Prey consists primarily of earthworms but some authors suggest this species may also eat other soft-bodied soil invertebrates. It lays clutches of up to six eggs and is inoffensive when handled.
RELATED SPECIES
This species was described by the British naturalist Edward Blyth (1810–73), as Calamaria reticulata. When it was later transferred to a new monotypic genus, that genus was named Blythia in his honor. This genus is one of nine genera within the Colubridae that are treated as incertae sedis—“of uncertain placement” at the subfamily level.
FAMILY |
Colubridae: incertae sedis |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South and Southeast Asia: northeast India, Myanmar, southwest China, and Tibet |
ELEVATION |
475–6,560 ft (145–2,000 m) asl |
HABITAT |
Wet evergreen forests |
DIET |
Earthworms and arthropods |
REPRODUCTION |
Oviparous, with clutches of up to 6 eggs |
CONSERVATION STATUS |
IUCN Data Deficient |
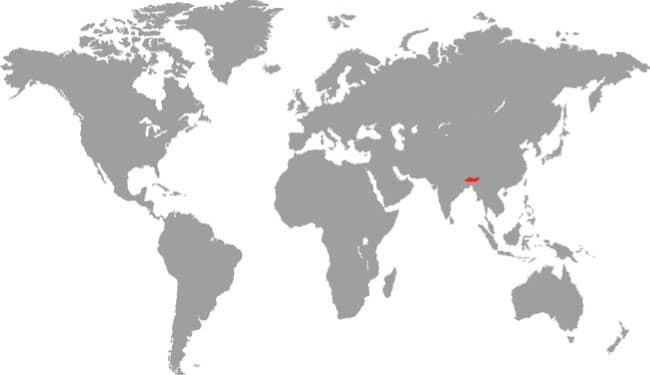

Blyth’s Iridescent Snake is small with a cylindrical body, glossy, smooth scales, a short tail with a pointed tip, a narrow, pointed head that is indistinct from the neck, and small eyes. It is dorsally uniform black, blue-black, or even deep purple or olive, with a faint broken dark vertebral line sometimes present. Juveniles possess a yellow collar that is broken middorsally.
GONGYLOSOMA MUKUTENSE
PULAU TIOMAN GROUNDSNAKE
GRISMER, DAS & LEONG, 2003
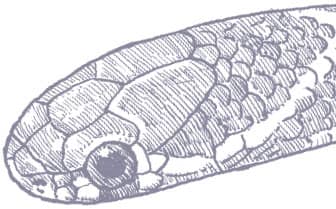
ADULT LENGTH
17 in (430 mm)
The Pulau Tioman Groundsnake, or Mukut Smoothsnake, is endemic to Tioman Island, located off the east coast of the Malaysian Peninsula. Mukut is the small village where the holotype was collected, as it was being swallowed by a juvenile Malayan Keeled Ratsnake (Ptyas carinatus). Members of genus Gongylosoma inhabit lowland and submontane rainforest, where they are found in leaf-litter or beneath rocks or logs. The prey preferences of the Pulau Tioman Groundsnake are unknown but in common with its congenerics it probably feeds on spiders, insects and/or small lizards. The genus is oviparous. This is one of many small leaf-litter dwelling snakes in Southeast Asia, that are themselves the prey of larger ophiophagous snakes such as the coralsnakes (Calliophis) and kraits (Bungarus).
RELATED SPECIES
Gongylosoma is incertae sedis (“of uncertain placement”) within the Colubridae. Four other species are included in the genus Gongylosoma: the Five-striped Groundsnake (G. longicaudum), which occurs in sympatry with this species, the Spotted Groundsnake (G. baliodeirum), Nicobar Groundsnake (G. nicobariensis), and the Indo-Chinese Groundsnake (G. scriptum) from Myanmar and Thailand.
FAMILY |
Colubridae: incertae sedis |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Southeast Asia: Malaysia (Tioman Island) |
ELEVATION |
33 ft (10 m) asl |
HABITAT |
Lowland and submontane rainforest |
DIET |
Prey preferences not known, presumed arthropods and lizards |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Critically Endangered |
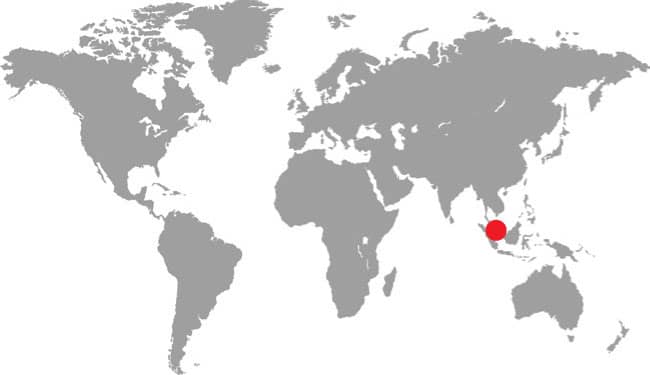

The Pulau Tioman Groundsnake is a small, slender snake with a squarish, blunt head, slightly broader than the narrow neck, and moderately large eyes. The body is red-brown anteriorly, becoming brown-gray by midbody, with the undersides cream. The dorsum of the head and nape are darker brown with a white stripe passing along the lips, posterior to the eye and curving over the nape to form a chevron, this being the only species in the genus with such a marking.
RHABDOPS OLIVACEUS
INDIAN OLIVE FOREST SNAKE
(BEDDOME, 1863)
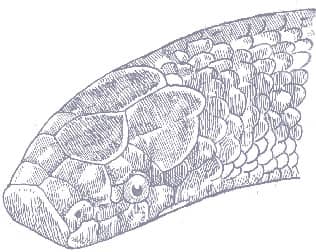
ADULT LENGTH
303/4 in (780 mm)
The Indian Olive Forest Snake is found in the ancient Western Ghats of southwestern India, an area of high rainfall and dense hill forests, with a high degree of endemicity in its fauna. The Indian Olive Forest Snake is found in hill forests, especially close to streams and creeks. At night it forages for earthworms and slugs, its primary prey, in moist conditions, in forest leaf litter or along stream beds. It uses its enlarged rostral scale to dig for its prey. It is terrestrial to semi-fossorial and may also be semi-aquatic, but it is rarely encountered and poorly known in nature. During the day it hides in crevices or under logs. Inoffensive, this snake will not bite, even when handled.
RELATED SPECIES
The genus Rhabdops is incertae sedis within the Colubridae, “of uncertain placement,” which means it cannot be pigeonholed into one of the four subfamilies. There are nine genera in this position, including Blythia (shown here). Rhabdops also contains a second species, the Trapezoid Forest Snake (R. bicolor), which occurs in northeast India, Myanmar, and southwest China.
FAMILY |
Colubridae: incertae sedis |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South Asia: southwestern India |
ELEVATION |
2,000–4,270 ft (610–1,300 m) asl |
HABITAT |
Hill forest, especially in riparian habitats |
DIET |
Slugs and earthworms |
REPRODUCTION |
Presumed oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Least Concern |

The Indian Olive Forest Snake is a stocky snake with smooth, glossy scales, a head slightly broader than the neck, and small eyes with vertically elliptical pupils. It is olive-brown to yellow-green above, and yellow to brown below. The dorsum bears four lateral rows of black spots, and the head is as the body.
SCAPHIODONTOPHIS ANNULATUS
GUATEMALAN SPATULA-TOOTHED SNAKE
(DUMÉRIL, BIBRON & DUMÉRIL, 1854)
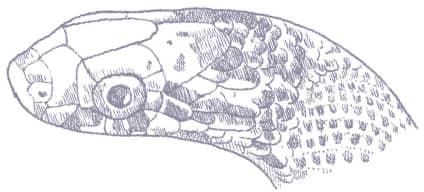
ADULT LENGTH
271/2–361/4 in (700–920 mm)
The Guatemalan Spatula-toothed Snake, sometimes called the Neckband Snake or Half Coralsnake, is widely distributed from central Mexico to Nicaragua. A secretive, diurnal, terrestrial to semi-fossorial snake, it is found in leaf litter or under logs. It hunts lizards, especially smooth-scaled skinks, which possess tough osteoderms (bony plates) in the skin. The Guatemalan Spatula-toothed Snake has evolved specialized, hinged, spatula-like teeth to maintain purchase on a robust, writhing skink, which it then swallows in a few seconds. Many specimens have truncated tails, a possible sign that this species uses the tactic of caudal pseudautotomy to avoid predation, sacrificing part of its tail to save its life.
RELATED SPECIES
A second species of Scaphiodontophis, the Common Spatula-toothed Snake (S. venustissimus), occurs in southern Central America and Colombia. Scaphiodontophis and the Asian black-headed snakes, genus Sibynophis (shown here), comprise the small family Sibynophiidae, which some authors treat as a subfamily of the Colubridae.
FAMILY |
Sibynophiidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North and Central America: southern Mexico, Belize, Guatemala, Honduras, El Salvador, and Nicaragua |
ELEVATION |
0–5,090 ft (0–1,550 m) asl |
HABITAT |
Lowland and low montane wet and dry forest, and gallery forest |
DIET |
Lizards, especially skinks |
REPRODUCTION |
Oviparous, with clutches of 1–10 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

The Guatemalan Spatula-toothed Snake is a slender snake with a long tail, smooth scales, and a head only slightly distinct from the neck, with large eyes and round pupils. Most of the body and tail is brown above, either unicolor or with three fine longitudinal lines of small dark spots. The anterior body is brightly colored, being red with white-black-white or yellow-black-yellow bands, while the head may be brown, red, or black, with a yellow throat and lips.
SIBYNOPHIS COLLARIS
COLLARED BLACK-HEADED SNAKE
(GRAY, 1853)

ADULT LENGTH
30–331/2 in (760–850 mm)
The Collared Black-headed Snake is found from northwest India, through the Himalayan foothills to Myanmar and southwestern China, and south through Southeast Asia to Peninsular Malaysia. It occurs in lowland forests but also to over 9,840 ft (3,000 m) in montane forest, usually in dense vegetation. It may be diurnal or nocturnal, and is terrestrial to semi-fossorial in habit. Prey consists of lizards, especially skinks, for which it possesses specialized dentition, as well as frogs, other snakes, and, in the case of juveniles, insects. When specimens are handled they do not bite, but instead coil around the fingers and constrict. It will also exude a cloacal secretion, which is said to smell of tobacco.
RELATED SPECIES
The genus Sibynophis is the type genus of the Sibynophiidae. Eight other species of Asian black-headed snakes are described. Peninsular India is occupied by the Indian Black-headed Snake (S. subpunctatus), while the Arrow Black-headed Snake (S. sagittarius) also occurs in northeast India. The remaining species occur in Southeast Asia, China, Indonesia, and the Philippines. Some authors treat the Sibynophiidae as a subfamily of the Colubridae.
FAMILY |
Sibynophiidae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South and Southeast Asia: northern India, Nepal, southwestern China, Bhutan, Bangladesh, Myanmar, Thailand, Vietnam, Cambodia, and Peninsular Malaysia |
ELEVATION |
0–10,800 ft (0–3,280 m) asl |
HABITAT |
Lowland and montane forest, preferably with dense vegetation |
DIET |
Lizards, frogs, snakes, and insects |
REPRODUCTION |
Oviparous, with clutches of 4–6 eggs |
CONSERVATION STATUS |
IUCN Least Concern |

The Collared Black-headed Snake is a slender snake with smooth scales, a long tail, and a head no wider than the neck, with small eyes and round pupils. Dorsally it is brown or gray-brown, with a black vertebral line of spots. Ventrally it is yellow, while the head is gray above with a faint darker band posteriorly, the labials are white, and a broad black collar, edged posteriorly with white, is present posterior to the head.
CARPHOPHIS VERMIS
WESTERN WORMSNAKE
(KENNICOTT, 1859)
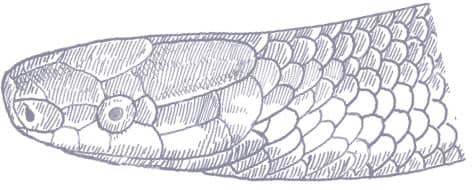
ADULT LENGTH
8–151/2 in (200–390 mm)
The Western Wormsnake occurs in the Midwest USA from southern Iowa, western Wisconsin and Illinois, south to northwestern Texas and northern Louisiana. It is a secretive snake that inhabits rocky, wooded hillsides, and although not an open-country species, it may be found along forested streams that extend into prairie habitats. This is a semi-fossorial species that burrows into damp soil, the habitat of earthworms, its primary prey. It does not survive long when its forested habitat is cleared, being especially vulnerable to desiccation due to excessive water loss through its semipermeable skin. It is unable to burrow into already dry soil. Although earthworms form the majority of the diet, the Western Wormsnake also feeds on soft-bodied insect larvae and even small snakes, such as Ringneck Snakes (Diadophis punctatus).
RELATED SPECIES
A second species of wormsnake, the Eastern Wormsnake (Carphophis amoenus), occurs from New York to Illinois, and south to the Carolinas and Louisiana. The genus Carphophis is most closely related to the mudsnakes (Farancia). The Carphophiinae contains five North American genera, and one from Tibet.
FAMILY |
Dipsadidae: Carphophiinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: central USA |
ELEVATION |
195–2,000 ft (60–610 m) asl |
HABITAT |
Rocky wooded hillsides and wooded streamsides |
DIET |
Earthworms |
REPRODUCTION |
Oviparous, with clutches of 1–12 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
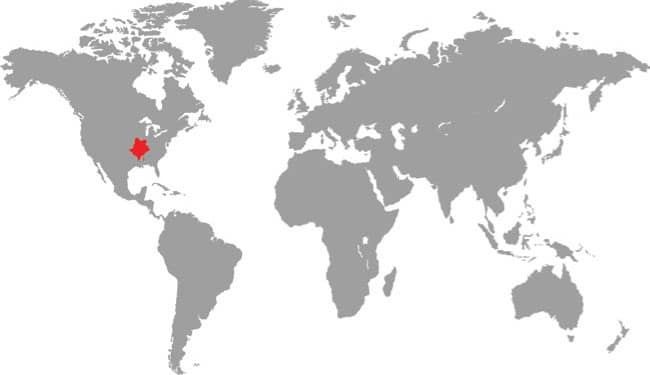

The Western Wormsnake is a slender snake with smooth scales, and a head indistinct from the neck, with small, dark, round-pupilled eyes, and a short tail. It is glossy brown to dark gray dorsally, and yellowish, pinkish, or light brown on the venter and lower flanks.
CONTIA TENUIS
SHARPTAIL SNAKE
(BAIRD & GIRARD, 1852)

ADULT LENGTH
173/4–19 in (450–483 mm)
The Sharptail Snake is distributed from southwest British Columbia, including Vancouver Island, where it is one of only four snake species found, and south to central California. This species occurs in a wide variety of habitats, from moist coniferous and pine-oak woodlands to open grasslands and prairie, and cool montane meadows. It is also found on rocky slopes and in suburban gardens. It may be found under rocks or inside rotten logs where the conditions are damp, but it may venture deeper for hibernation. It uses its sharp caudal spine to force itself forward through the soil. This is a secretive and inoffensive species and its diet appears to consist almost entirely of slugs, or their eggs. Some authors report earthworms, insects, and salamanders in their diet, but this is disputed.
RELATED SPECIES
A related species, the Forest Sharptail Snake (Contia longicaudae), also occurs in California and Oregon, and exhibits a longer tail and much more reduced black ventral markings than C. tenuis. The genus Contia is most closely related to the American hognose snakes (Heterodon).
FAMILY |
Dipsadidae: Carphophiinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: western USA and Canada |
ELEVATION |
0–6,890 ft (0–2,100 m) asl |
HABITAT |
Moist woodland, riparian habitats, open prairies, grasslands and meadows, rocky slopes, and suburban gardens |
DIET |
Slugs |
REPRODUCTION |
Oviparous, with clutches of 2–9 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
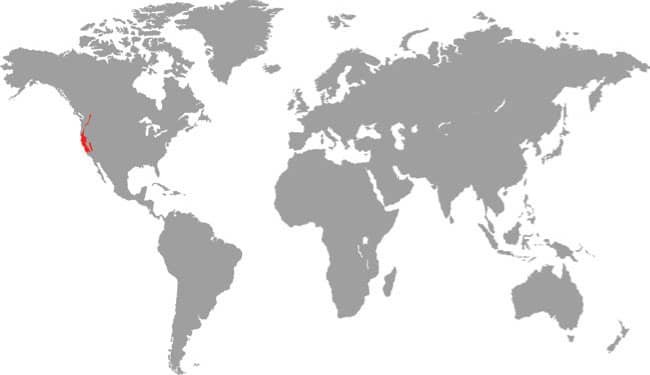

The Sharptail Snake is a slender snake with smooth scales, a rounded head that is indistinct from the neck, small eyes with round pupils, and a short tail. It is brown above, gray on the flanks, and white on the undersides, every ventral scale being edged with black. A pale longitudinal line demarcates the dorsolateral margin. The snout tip and lips are also white.
DIADOPHIS PUNCTATUS
RINGNECK SNAKE
(LINNAEUS, 1766)
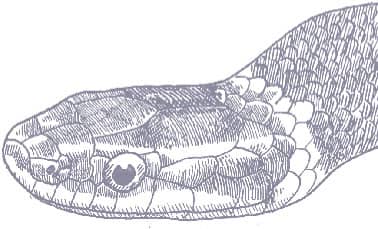
ADULT LENGTH
93/4–233/4 in, rarely 34 in (250–600 mm, rarely 860 mm)
The Ringneck Snake is one of the most widely distributed North American snakes, occurring from Nova Scotia in Canada to San Luis Potosí, eastern Mexico, through eastern and central USA, and on the western seaboard from Washington to Baja California. It has been introduced to Grand Cayman Island. Most Ringnecks are small (less than 233/4 in/600 mm), but the Regal Ringneck (Diadophis punctatus regalis), from Utah, may achieve 34 in (860 mm). Ringnecks inhabit woodland and rocky canyons, and sometimes occur in aggregations of over 100 individuals. They are also found in the scree between railway sleepers. Defensively they may display the tightly coiled, inverted, brightly colored tail, although the purpose of this display is not fully understood. This species is mildly venomous, and some people experience localized effects following bites.
RELATED SPECIES
The genus Diadophis is monotypic, but D. punctatus contains 7 to 14 geographical subspecies, some of which are difficult to tell apart. Some authors consider D. punctatus to be two species. The genus Diadophis is the sister clade to wormsnakes (Carphophis) and the Rainbow Snake and Mudsnake (Farancia).
FAMILY |
Dipsadidae: Carphophiinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North America and West Indies: southeast Canada, USA, and northern Mexico; introduced into the Cayman Islands |
ELEVATION |
0–7,870 ft (0–2,400 m) asl |
HABITAT |
Woodland, chaparral, rocky valleys, and railway lines |
DIET |
Salamanders, small frogs, small lizards, other snakes, earthworms, insect larvae, and slugs |
REPRODUCTION |
Oviparous, with clutches of 1–10 eggs, 18 in D. p. regalis |
CONSERVATION STATUS |
IUCN Least Concern |
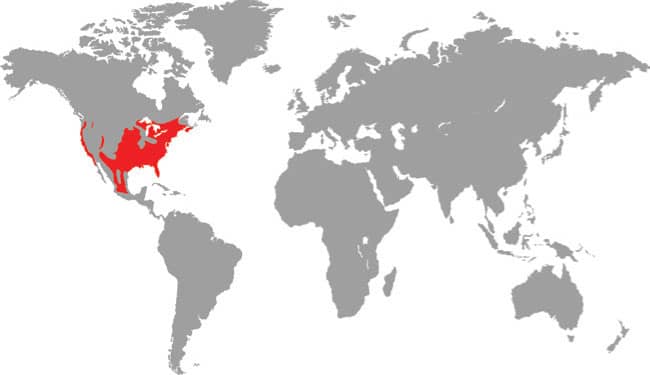

The Ringneck Snake is a small snake with a rounded head, small eyes with round pupils, glossy, smooth scales, and a short tail. The dorsum is usually brown, gray, or blue-gray, but depending on subspecies, the venter may be yellow, orange, or red, while the throat is white, with a black-edged ring around the neck that is the same color as the venter. The undersides of some specimens are immaculate, others lightly spotted, while some subspecies exhibit a distinct line of midventral dark spots. Southern populations often lack the neck band.
FARANCIA ERYTROGRAMMA
RAINBOW SNAKE
PALISOT DE BEAUVOIS, 1802

ADULT LENGTH
4 ft 7 in–5 ft 2 in (1.4–1.6 m)
The Rainbow Snake occurs from southern Maryland to northern Florida and west to Louisiana, with a small isolated population in the Florida Peninsula. It is found in slow-moving freshwater habitats including rivers, lakes, cypress swamps, and canals, and it also occurs in brackish habitats, such as coastal marshes, mudflats, and tidal creeks. The female is oviparous, excavating a cavity in the soil for her eggs, and she may remain with them during incubation. This species is nocturnal in habit and feeds on aquatic vertebrates such as salamanders, sirens, frogs, and fish including eels, with earthworms also being taken. The enlarged rear teeth and Duvernoy’s glands of the Rainbow Snake are used to subdue eels. If handled the Rainbow Snake does not bite, but may probe harmlessly with its caudal spine.
RELATED SPECIES
Two subspecies of Farancia erytrogramma are known. The nominate Northern Rainbow Snake (F. e. erytrogramma) occupies the majority of the range, while the severely endangered South Florida Rainbow Snake (F. e. seminola) is only known from Fisheating Creek, Glades County, Florida. It has not been seen since 1952 and is the subject of a citizen science appeal for sightings. A second species of Farancia is also recognized, the Mudsnake (F. abacura), which has an eastern subspecies (F. a. abacura) and a western subspecies (F. a. reinwardtii), both in the United States.
FAMILY |
Dipsadidae: Carphophiinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North America: southeast USA |
ELEVATION |
0–490 ft (0–150 m) asl |
HABITAT |
Rivers, canals, lakes, cypress swamps, and coastal marshes |
DIET |
Fish (including eels), salamanders, sirens, frogs, and earthworms |
REPRODUCTION |
Oviparous, with clutches of 10–52 eggs |
CONSERVATION STATUS |
IUCN Least Concern, Endangered in Mississippi and Louisiana, possibly Extinct in Florida Peninsula |
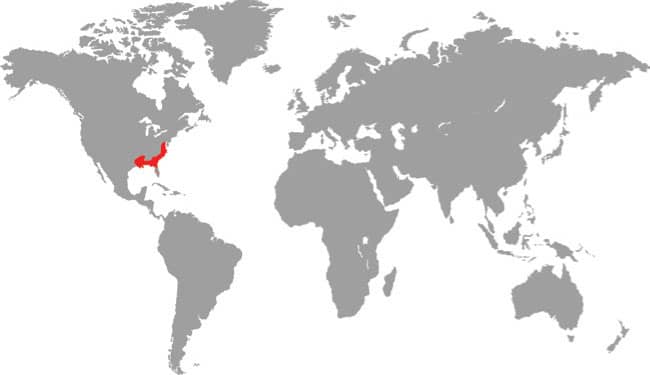

The Rainbow Snake is a slender, glossy, smooth-scaled snake with a rounded head, small eyes with round pupils, and a long tail with a spinous tip. Its color is iridescent blue-black to purple, with three longitudinal red stripes and red edging to the dorsal head scales. The lips and chin are yellow, and the venter is red to yellow with three rows of black spots.
HETERODON KENNERLEYI
MEXICAN HOGNOSE SNAKE
KENNICOTT, 1860
ADULT LENGTH
153/4–233/4 in, occasionally 30 in (400–600 mm, occasionally 760 mm)
The Mexican Hognose Snake is found across northeastern Mexico and also enters the southern USA, in the border region from Texas to Arizona. It likes sandy or gravelly soils into which it can burrow, and it occurs in many habitats from farmland to woodland, floodplains, and creosote bush desert. It preys mostly on toads, with frogs, lizards, salamanders, reptile eggs, small mammals, birds, and even occasional turtles, also taken. The turned-up snout is used for excavating prey from the soil. Toads that inflate themselves, to avoid predation, are deflated by the enlarged rear teeth and subdued by the snake’s Duvernoy’s gland secretions. Hognose snakes rely on bluff and rarely bite, but some people do experience localized reactions to their secretions, so bites should be avoided. They also employ thanatosis, rolling upside down with their mouths agape, tongue protruding loosely, as if dead.
RELATED SPECIES
Four other North American hognose snakes are known: the Eastern Hognose Snake (Heterodon platirhinos), from Ontario to Florida and Texas; the Western Hognose Snake (H. nasicus), from Alberta to Manitoba, and south to Texas; the Dusky Hognose Snake (H. gloydi) in Texas and Oklahoma; and the Southern Hognose Snake (H. simus) from Lousiana to the Carolinas and Florida. The Mexican Hognose Snake is a former subspecies of the Western Hognose Snake.
FAMILY |
Dipsadidae: Carphophiinae |
RISK FACTOR |
Rear-fanged, mildly venomous |
DISTRIBUTION |
North America: northeastern Mexico and extreme southern Texas, New Mexico, and Arizona |
ELEVATION |
0–8,010 ft (0–2,440 m) asl |
HABITAT |
Woodland, flood plains, creosote desert, prairie, cultivated land |
DIET |
Toads, lizards, frogs, salamanders, turtles, reptile eggs, small birds, and small mammals |
REPRODUCTION |
Oviparous, with clutches of 4–25 eggs |
CONSERVATION STATUS |
IUCN not listed |
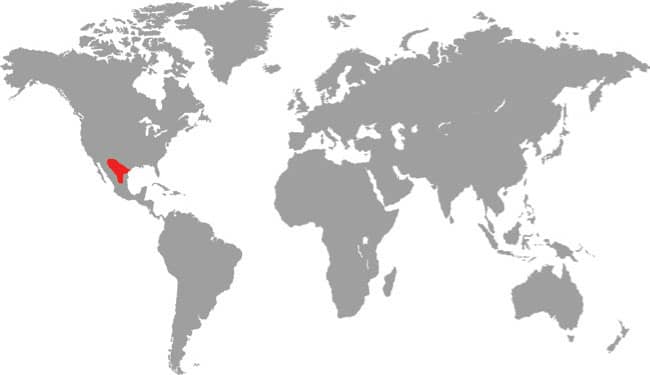

The Mexican Hognose Snake is a stocky snake with keeled scales, a short tail, and a short head that terminates in the upturned rostral scale of the snout. Its dorsal coloration is tan-brown with patterning comprising rows of black-edged dark brown blotches that become bands on the tail, and a series of dark chevron markings on the head. The venter is off-white, heavily speckled with black, and orange or yellow under the tail.
THERMOPHIS BAILEYI
XIZANG HOT-SPRING SNAKE
(WALL, 1907)

ADULT LENGTH
173/4–24 in (450–610 mm)
The Xizang Hot-spring Snake, or Tibetan Hot-spring Snake, is a high-elevation species occurring in the Lhasa region. It inhabits the streams, ponds, meadows, and marshes surrounding the hot springs of the Tibetan Plateau. Poorly known, it is believed to feed on the small alpine frogs and fish that also inhabit this barren landscape. Although it is believed to be oviparous it may be viviparous, a safer strategy for snakes living at high elevations. According to the IUCN, this species is threatened by the development of geothermal hydroelectric stations and habitat disturbance. This species was named in honor of Lieutenant Colonel Frederick Bailey (1882–1967), a British officer and spy.
RELATED SPECIES
The genus Thermophis also contains the Shangri-La Hot-spring Snake (T. shangrila), from Yunnan, China, and the Endangered Sichuan Hot-spring Snake (T. zhaoermii). These snakes are the only known non-American members of the Dipsadidae and may illustrate the radiation of snake families across the Bering land bridge between Siberia and Alaska during the Quaternary glacial period. Their closest relative is the Ringneck Snake (Diadophis punctatus).
FAMILY |
Dipsadidae: Carphophiinae |
RISK FACTOR |
Rear-fanged, mildly venomous |
DISTRIBUTION |
Central Asia: Tibet |
ELEVATION |
9,840–16,100 ft (3,000–4,900 m) asl |
HABITAT |
Hot-spring rivulets, pools, meadows, and marshes |
DIET |
Small frogs and fish |
REPRODUCTION |
Believed to be oviparous, with clutches of up to 6 eggs; possibly viviparous |
CONSERVATION STATUS |
IUCN Near Threatened |
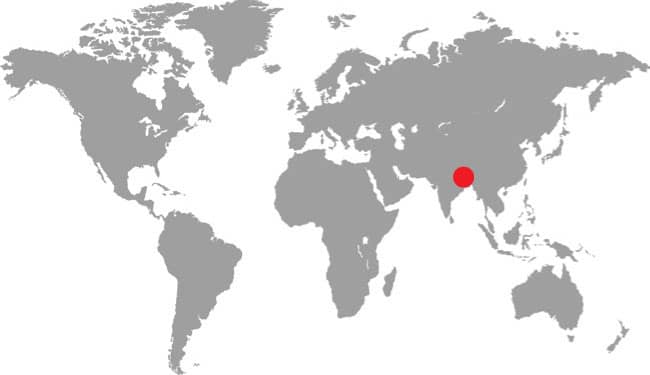

The Xizang Hot-spring Snake is a slender snake with a long tail and keeled scales. The head is slightly pointed, and the eyes relatively small with round pupils. Its dorsal coloration is olive-green to brown with transverse rows of dark brown spots, the middorsal series forming a punctuated longitudinal stripe. The undersides and lips are pale yellow.
ADELPHICOS QUADRIVIGATUM
VERACRUZ EARTHSNAKE
JAN, 1862
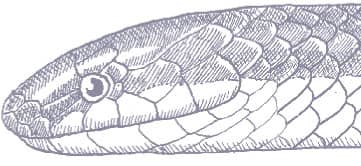
ADULT LENGTH
11–153/4 in, rarely 201/2 in (280–400 mm, rarely 520 mm)
The small Veracruz Earthsnake occurs from the Mexican state of Tamaulipas to Veracruz. It is an inhabitant of lowland and low montane wet forest and upland pine forest, where it shelters and hunts under rotten logs or in decaying leaf litter. It is also found in coffee plantations, in the dense leaf litter at the bases of trees, and in sugar plantations under logs. It is not found in plantations that engage in regular burns of the ground cover. The Veracruz Earthsnake feeds on earthworms and soft-bodied invertebrates, and is, itself, predated by ophiophagous snakes, such as coralsnakes (Micrurus). Eggs are frequently laid in termite mounds, including the arboreal nests of tree-dwelling termites.
RELATED SPECIES
There are three subspecies recognized, although some authors treat them as species. The genus Adelphicos also contains another six species from Oaxaca, Mexico (A. latifasciatum and A. visoninum), Chiapas, Mexico (A. nigrilatum), Guatemala (A. daryi and A ibarrorum), and both Mexico and Guatemala (A. sargii and A. veraepacis). Although A. quadrivigatum is not considered threatened, several of the more localized species are listed as Vulnerable or Endangered by the IUCN.
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: southeast Mexico |
ELEVATION |
0–6,230 ft (0–1,900 m) asl |
HABITAT |
Lowland and low montane wet tropical forest, upland pine forest, coffee and sugar plantations |
DIET |
Earthworms and soft-bodied invertebrates |
REPRODUCTION |
Oviparous, with clutches of 3–5 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
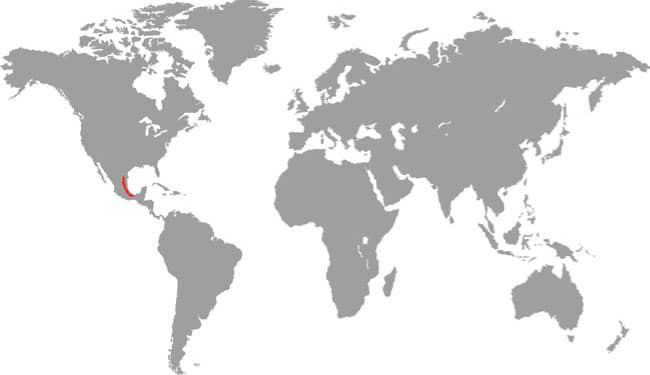

The Veracruz Earthsnake is small with glossy, smooth scales; a small head that is indistinct from the neck; small, dark eyes, and a short tail. The dorsal coloration is olive-green or red-brown with a series of dark brown longitudinal stripes, the lateral stripes being bolder than the vertebral ones, and all scales are speckled with dark spots. The undersides are yellow, sometimes pink, and also heavily infused with dark spots.
AMASTRIDIUM SAPPERI
NORTHERN RUSTYHEAD SNAKE
(WERNER, 1903)
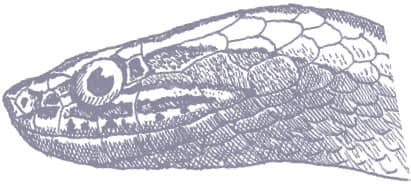
ADULT LENGTH
133/4–153/4 in, rarely 283/4 in (350–400 mm, rarely 730 mm)
The rustyhead snakes are so-called because of the rust-orange nuchal collar on the posterior of the head. The Northern Rustyhead Snake occurs from Tamaulipas to Oaxaca in Mexico, and in Belize, Guatemala, and Honduras, but not the Yucatán Peninsula. This small diurnal snake has been collected in wet tropical forest, on streamsides, and in coffee plantations, where it takes advantage of any ground cover to hide or hunt. Specimens have contained small frogs, lizards, and centipedes, but little is known of their natural behavior. This species’ reproductive strategy is unknown but its congener, the Southern Rustyhead Snake (Amastridium veliferum), is oviparous. Karl Theodor Sapper (1866–1945) was a German volcanologist, linguist, and explorer who traveled widely in Mexico and Central America during the late nineteenth century.
RELATED SPECIES
The Southern Rustyhead Snake (Amastridium veliferum) occurs from Costa Rica and Panama to Colombia. Some authors consider Amastridium to be monotypic and reduce A. sapperi to a subspecies of A. veliferum.
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North and Central America: Mexico, Belize, Guatemala, and Honduras |
ELEVATION |
0–5,250 ft (0–1,600 m) asl |
HABITAT |
Lowland and low montane wet tropical forest, and coffee plantations |
DIET |
Frogs, small lizards, and centipedes |
REPRODUCTION |
Probably oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Least Concern |
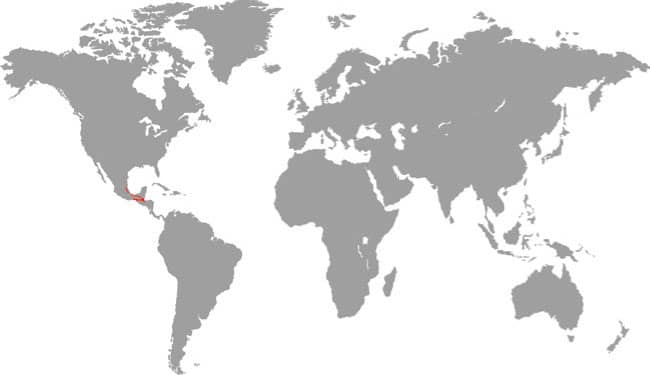

The Northern Rustyhead Snake is a small snake with smooth scales, a squarish head, small eyes with round pupils, and a long tail. It is black or dark brown in color, although the dorsum of the head may be lighter brown or gray. Faint longitudinal stripes may be visible in some specimens. Its most obvious feature is the rust-orange nuchal collar that contrasts strongly with the dorsal color and provides the species with their common names.
ATRACTUS GIGAS
GIANT ARROW EARTHSNAKE
MYERS & SCHARGEL, 2006
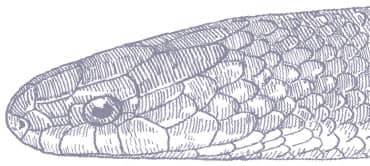
ADULT LENGTH
10–41 in, occasionally 3 ft 7 in (255–1,040 mm, occasionally 1.1 m)
The Giant Arrow Earthsnake is the largest known species of the largest snake genus, and inhabits the Ecuadorian and Peruvian Andes. Described from a single 3 ft 5 in (1.04 m) female specimen as recently as 2006, it is now known from over a dozen specimens. The largest male found so far was a 10 in (255 mm) subadult. The Giant Arrow Earthsnake inhabits montane humid forest, cloud forest, and secondary growth, but is also found in plantations. It is semi-fossorial, in leaf litter or under fallen logs, but may also be seen crossing trails in the early morning or late afternoon. The diet of this moderately large, docile snake is poorly known. It is reported to feed on earthworms but the feces of one specimen contained small mammal remains.
RELATED SPECIES
The South American genus Atractus is the most specious snake genus with 143 species currently described. The name Atractus means “arrow.” The genus exhibits considerable diversity and complexity. Atractus just enters Central America in eastern Panama, being replaced by the closely related genus Geophis (shown here) in Central America.
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South America: Ecuador and Peru |
ELEVATION |
1,970–7,550 ft (600–2,300 m) asl |
HABITAT |
Humid tropical forest, cloud forest, secondary growth, and coffee plantations |
DIET |
Earthworms and possibly small mammals |
REPRODUCTION |
Oviparous, with clutches of up to 12 eggs |
CONSERVATION STATUS |
IUCN not listed |

The Giant Arrow Earthsnake is a robust species with smooth scales, a rounded head that is hardly distinct from the neck, small eyes with round pupils, and a short tail. Adults may be uniform reddish brown or exhibit a reticulate pattern, every scale being spotted with brown on a pale background. Juveniles are dark with a series of irregular transverse reddish cross-bands, and yellowish infusions on the head scales. This species may possess a checkerboard or heavily spotted venter. Some specimens are more melanistic, a possible adaptation for life at higher elevations.
ATRACTUS LATIFRONS
BROADHEAD ARROW EARTHSNAKE
(GÜNTHER, 1868)
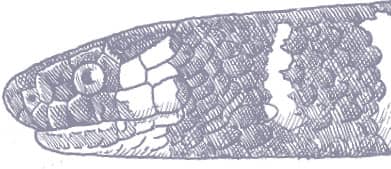
ADULT LENGTH
201/2–24 in (520–610 mm)
The Broadhead Arrow Earthsnake, or the Wedgetail Earthsnake, is an Amazonian member of the largest snake genus, Atractus. It has been recorded from every country in northern South America except Ecuador and Guyana. Its preferred habitat comprises pristine lowland primary rainforest or riverine gallery forest, but it is found more easily in recently cleared forest plots. It is semi-fossorial, living in the forest-floor leaf litter, where it feeds on earthworms and insects. It is a harmless but polymorphic species and caution is advised with specimens whose patterning resembles the highly venomous coralsnakes (Micrurus).
RELATED SPECIES
Tricolor Atractus latifrons and some of its congeners, including the Black Arrow Earthsnake (A. elaps) and the Guianan Arrow Earthsnake (A. badius), resemble other Amazonian coral-mimics such as the Aesculapian False Coralsnake (Erythrolamprus aesculapii) and the true coralsnakes.
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South America: Colombia, Venezuela, Suriname, French Guiana, Brazil, Peru, and Bolivia |
ELEVATION |
295–1,640 ft (90–500 m) asl |
HABITAT |
Primary lowland rainforest, riverine forest, and recently deforested areas |
DIET |
Earthworms and insects |
REPRODUCTION |
Oviparous, with clutches of 3–6 eggs |
CONSERVATION STATUS |
IUCN not listed |
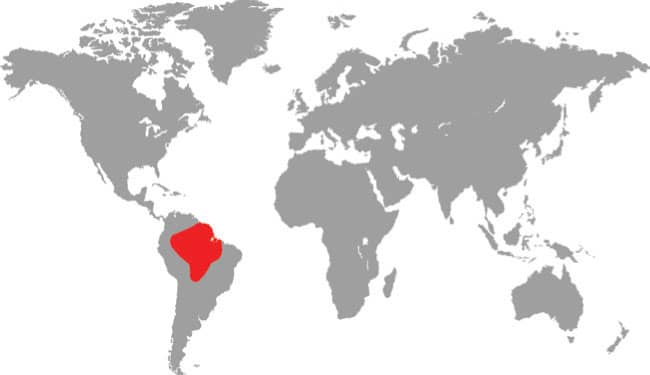

The Broadhead Arrow Earthsnake is a slender species with smooth scales, a slightly pointed head, small eyes, round pupils, and a relatively short tail. It is polymorphic, occurring in several color morphotypes, ranging from bicolor (black with narrow yellow rings), to tricolor (red with black-yellow-black or black-yellow-black-yellow- black rings), to virtually unicolor with rings only present on the head and tail. Juveniles are more brightly marked, while in old adults the markings may be obscured by dark pigment.
CHERSODROMUS RUBRIVENTRIS
RED-BELLIED EARTHRUNNER
(TAYLOR, 1949)
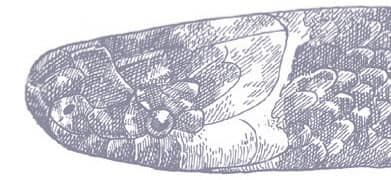
ADULT LENGTH
5–133/4 in (130–350 mm)
The Red-bellied Earthrunner is confined to the Sierra Madre Occidental, in the Mexican states of Querétaro, San Luis Potosí, and Hidalgo, where it inhabits cloud forest and pine–oak forest, on rocky karst slopes at relatively high elevations. It is considered Endangered by the IUCN due to habitat alteration and fragmentation caused by agricultural practices. This is a rare and secretive snake that is infrequently encountered, but it is most likely to be found under rotting logs, boulders, and in leaf litter during the day. Stomach contents of specimens have revealed a diet of beetle larvae and adult ants, which are probably foraged at night under ground cover, although it is possible the Red-bellied Earthrunner may also forage on the surface.
RELATED SPECIES
A second species is recognized, Leibmann’s Earthrunner (Chersodromus liebmanni), a more abundant species from Oaxaca and Veracruz, Mexico. The nearest relatives to Chersodromus may be the coffee snakes (Ninia) of Central America, which both look similar and appear to exhibit a parallel natural history.
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North America: Central Mexico |
ELEVATION |
5,410–6,040 ft (1,650–1,840 m) asl |
HABITAT |
Cloud forest and pine–oak forest on karst limestone |
DIET |
Ants and beetle larvae |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Endangered |
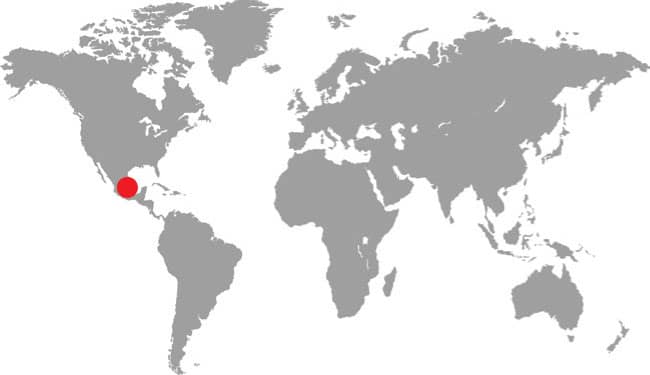

The Red-bellied Earthrunner is small with smooth, shiny scales, a squarish head, small eyes, and a short tail. It is black above and red on the ventral surfaces, with a broad yellow collar around the neck, which continues onto the throat. The chin is black.
CONIOPHANES PICEIVITTIS
MESOAMERICAN STRIPED SNAKE
COPE, 1870
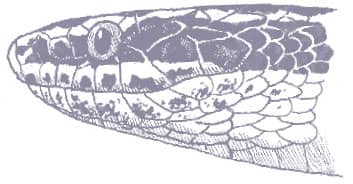
ADULT LENGTH
193/4–221/2 in (500–570 mm)
The Mesoamerican Striped Snake, also called Cope’s Striped Snake, is found from northern Mexico to Costa Rica and is recorded from every country in between except Belize, being replaced on the Yucatán Peninsula by the related and similarly patterned Schmidt’s Striped Snake (Coniophanes schmidti). Preferred habitats include lowland and low montane wet and dry tropical forest, both deciduous and evergreen. This is a terrestrial species that hunts frogs, lizards, and smaller snakes, such as blindsnakes, both diurnally and nocturnally, but it is not commonly encountered, being most likely to be found under rocks or rotten logs. It is also documented to forage for reptile eggs. Although members of the genus Coniophanes are rear-fanged, their venom is mild and they are not considered dangerous to humans.
RELATED SPECIES
Two subspecies are recognized, the nominate form (Coniophanes piceivittis piceivittis) from Mexico to Costa Rica, and a northern form (C. p. frangivirgatus) from Veracruz and Tamaulipas, Mexico. A further 16 species of Coniophanes are found in Mexico, Central America, and on inshore islands in the western Caribbean.Coniophanes piceivittis occurs in sympatry with the Yellow-bellied Snake (C. fissidens), the Spot-bellied Snake (C. bipunctatus), and the Regal Black-striped Snake (C. imperialis). Coniophanes is closely related to the brownsnakes (Rhadinaea).
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
North and Central America: Mexico, Guatemala, El Salvador, Honduras, Nicaragua, and Costa Rica |
ELEVATION |
0–4,270 ft (0–1,300 m) asl |
HABITAT |
Dry tropical and wet tropical deciduous and evergreen forests |
DIET |
Frogs, lizards, small snakes, and reptile eggs |
REPRODUCTION |
Oviparous, with clutches of 1–6 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
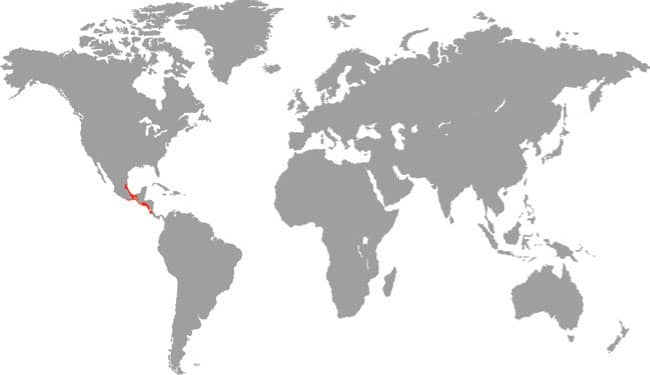

The Mesoamerican Striped Snake is a small, smooth-scaled snake with a pointed head, distinct from the neck, small eyes, and a long tail. It is black above with a pair of yellow dorsolateral stripes running from the snout, over the eyes, and along the body onto the tail. The undersides are pale brown, this coloration extending onto the lower flanks.
DIAPHOROLEPIS WAGNERI
HUMPBACK TERRIER SNAKE
JAN, 1863
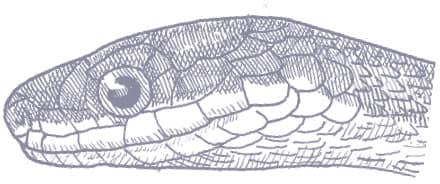
ADULT LENGTH
193/4–27 in (500–690 mm)
The curious common name of this species is a translation from the Spanish “Culebra terrier jorobada.” The only other name available, “Ecuadorian Frog-eating Snake,” may be misleading as authors do not agree on whether this species preys on frogs or lizards. The most distinctive characteristic in this extremely rare snake is its enlarged, bicarinate vertebral scale row. It occurs in the Pacific versant of the Ecuadorian and Colombian Andes, and also in the Darién region of eastern Panama. It is a highly agile inhabitant of lowland and low montane evergreen forests, especially near water, and is reportedly most often encountered after rain, but authors differ as to whether it is diurnal or nocturnal. This species is named after the German naturalist and traveler Moritz Wagner (1813–87), who collected the holotype.
RELATED SPECIES
A second but even rarer species (Diaphorolepis laevis) is endemic to Colombia and may have smooth dorsal scales, based on the epithet laevis (Latin for “smooth”). The genus Diaphorolepis is most closely related to the South American fishing snake genus Synophis (shown here).
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
Central and South America: Panama, Colombia, and Ecuador |
ELEVATION |
330–4,990 ft (100–1,520 m) asl |
HABITAT |
Lowland and low montane evergreen forests and clearings |
DIET |
Undetermined, possibly frogs or lizards |
REPRODUCTION |
Oviparous, clutch size unknown |
CONSERVATION STATUS |
IUCN Data Deficient, locally endangered |
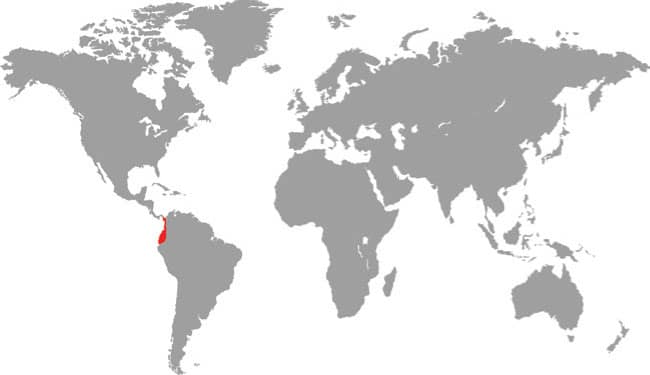

The Humpback Terrier Snake is a slender snake with strongly keeled scales, which are bicarinate on the vertebral row. Its square-nosed head is distinct from the narrow neck, the eyes are large with round pupils, and it has a very long tail. It is brown on the dorsum, and bright yellow on the venter, throat, and supralabials, with a sharp demarcation between the two colors on the outer ventral scales, and brown pigmentation midventrally, the underside of the tail being completely brown.
DIPSAS BREVIFACIES
YUCATÁN THIRST SNAKE
(COPE, 1866)
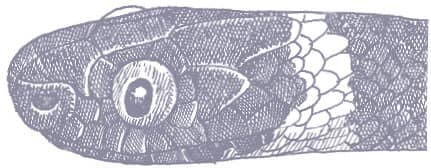
ADULT LENGTH
141/2–203/4 in (370–528 mm)
Central and South America contain 36 species of “thirst snakes” or “snail-suckers.” The Yucatán or Short-faced Thirst Snake is the most northerly species, ocurring on the Yucatán Peninsula of Mexico and Belize. Arboreal and nocturnal, it is common in dry thorn forests. Thirst snakes feed exclusively on slugs and snails. Slugs are swallowed tail first, but snails must be extracted from their shells. This is accomplished by holding the shell firmly in the coils, inserting the lower jaw and hooking the snail with the teeth. Then, applying pressure to the shell, the lower jaw is withdrawn with the prize, a process assisted by infralabial gland secretions that subdue the snail.
RELATED SPECIES
No other Dipsas occur within the range of this species, but it may be confused with Sartorius’ Terrestrial Snail-sucker (Tropidodipsas sartorii), with which it occurs in sympatry. That species has weakly keeled scales and a mental groove, but Dipsas has smooth scales and lacks a mental groove.
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
North and Central America: Mexico and Belize |
ELEVATION |
0–985 ft (0–300 m) asl |
HABITAT |
Dry tropical thorn forest and seasonally wet forest |
DIET |
Snails and slugs |
REPRODUCTION |
Oviparous, with clutches of 2–5 eggs |
CONSERVATION STATUS |
IUCN Least Concern |
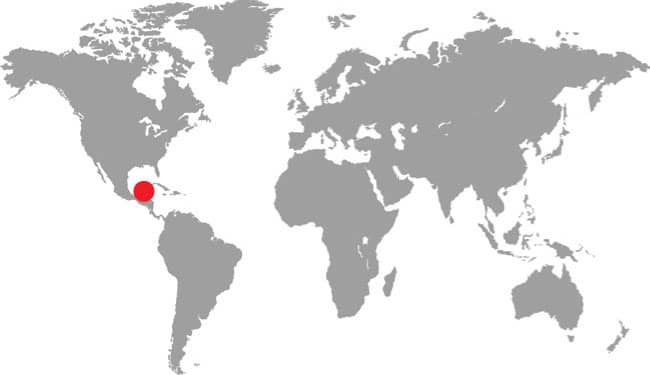

The Yucatán Thirst Snake is an extremely slender, laterally compressed, smooth-scaled snake, with a broad, rounded head, large, bulbous eyes, vertically elliptical pupils, and a long tail. It is shiny black dorsally and ventrally with regularly spaced broad salmon-pink to red bands, half to one-third the width of the black interspaces, the first red band forming a broad nuchal collar.
DIPSAS INDICA
SOUTH AMERICAN THIRST SNAKE
LAURENTI, 1768
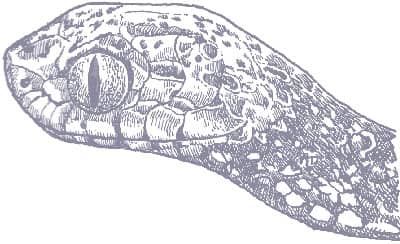
ADULT LENGTH
233/4–311/2 in, rarely 353/4 in (600–800 mm, rarely 910 mm)
The South American Thirst Snake may be the mostly widely distributed member of the genus Dipsas. It may also be one of the largest, with specimens approaching 3 ft 3 in (1 m) known. It is found throughout northern South America, east of the Andes, from the Caribbean coast to northeastern Argentina, but it is not commonly encountered. The infrequency of encounters may be as a result of this species being highly arboreal, often inhabiting the canopy, and nocturnal in the heavily forested Amazon and Guianan forests. As with other thirst snakes, it takes both slugs and snails, but given its larger size it can predate larger snails than its congeners. The method by which snails are extracted from their shells is explained for the Yucatán Thirst Snake (D. brevifacies).
RELATED SPECIES
The nominate subspecies (Dipsas indica indica) occupies most of the range, while the Ecuadorian population is treated as a separate subspecies (D. i. ecuadoriensis). Peter’s Snail-eater (D. petersi), from southeastern Brazil, is also a former subspecies. Thirst snakes, also known as snail-eaters or snail-suckers, are members of a global guild of snakes referred to by herpetologists as “goo-eaters.”
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Nonvenomous |
DISTRIBUTION |
South America: Colombia, Venezuela, the Guianas, Brazil, Ecuador, Peru, Bolivia, Paraguay, and northeastern Argentina |
ELEVATION |
0–3,280 ft (0–1,000 m) asl |
HABITAT |
Amazonian rainforest |
DIET |
Snails and slugs |
REPRODUCTION |
Oviparous, with clutches of 2–6 eggs |
CONSERVATION STATUS |
IUCN not listed |
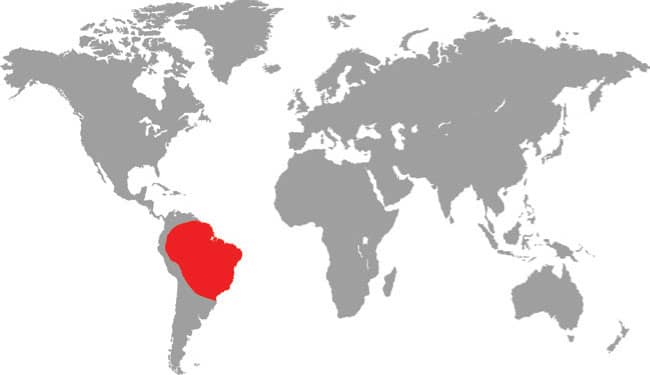

The South American Thirst Snake is a slender snake with smooth scales, a laterally compressed body, and a long, prehensile tail. The head is rounded, with large eyes and vertically elliptical pupils. This species is dorsally light brown with a series of irregular dark brown saddle markings and a scattering of white spots. Some specimens are almost unicolor. The undersides are immaculate white.
ENULIOPHIS SCLATERI
COLOMBIAN LONGTAIL SNAKE
(BOULENGER, 1894)
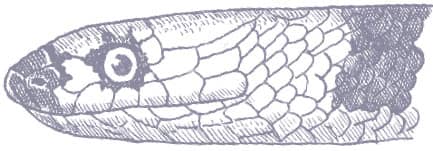
ADULT LENGTH
193/4–213/4 in (500–550 mm)
The Colombian Longtail Snake occurs in Honduras, Nicaragua, Costa Rica, Panama, and Colombia, in lowland wet forests, evergreen forest, and the gallery forests along rivers. It is also found in dry forest and arid savannas. It is active by both day and night but is a secretive species that inhabits forest floor leaf litter and rotten logs. The prey of the Colombian Longtail Snake comprises reptiles and amphibians, and also reptile eggs, which are punctured by the enlarged rear fangs. The long tail of this species is extremely fragile and is often broken. Females are oviparous and lay between two and five eggs. Although mildly venomous and rear-fanged, this species is harmless to humans. Philip Lutley Sclater (1829–1913) was a British ornithologist and biogeographer.
RELATED SPECIES
Enuliophis sclateri was previously included in the genus Enulius (shown here), and some authors still recognize that taxonomic arrangement.
FAMILY |
Dipsadidae: Dipsadinae |
RISK FACTOR |
Rear-fanged, mildly venomous; harmless to humans |
DISTRIBUTION |
Central and South America: Honduras, Nicaragua, Costa Rica, Panama, and Colombia |
ELEVATION |
0–4,220 ft (0–1,285 m) asl |
HABITAT |
Lowland moist forest, evergreen forest, gallery forest, dry forest, and savanna |
DIET |
Frogs, lizards, small snakes, and reptile eggs |
REPRODUCTION |
Oviparous, with clutches of 2–5 eggs |
CONSERVATION STATUS |
IUCN not listed |

The Colombian Longtail Snake is long and slender with smooth scales and a rounded head, which is broader than the neck, and a long tail. It is dark gray to black with a distinctive white head interrupted by black pigment around the eyes and on the snout tip.