YOU MAY HAVE HEARD ABOUT CONTROVERSIES surrounding genetically modified plants. These controversies have even caused street demonstrations and riots in several countries. You may also be assured that genetically modified food plants or their products find their way, on a daily basis, onto your breakfast, lunch, or dinner plates. Thus, it is important to understand what genetically modified plants are and how they are made. This scientific knowledge will allow you to form an informed opinion about the use of genetically modified plants.
The words “genetically modified” constitute a serious misnomer. Humans have bred plants and animals for thousands of years. In selective breeding, we have built into animals and plants gene combinations that are not normally found in nature and that probably would not survive without human intervention. For example, Chihuahuas and Great Danes did not appear spontaneously, nor can these or other dog breeds be maintained without human intervention. We also know that both breeds are quite different from “primeval” dogs domesticated thousands of years ago. The latter looked like wolves.
Similarly, several types of food plants look quite different from their progenitors. Corn, for example, derives from plants that produced very small purple kernels, not large, yellow ones. Progenitors of modern corn, however, apparently appeared spontaneously from wild parents and were propagated and bred to enhance the characteristics that we enjoy in modern corn. On the other hand, plant breeders mutated the genes of the progenitors of modern barley with chemicals or radiation and selected for mutants with desirable characteristics to produce the high-yielding, short-stemmed strains of barley cultivated today. Thus, we see that humans have modified the genes of the creatures all around us, starting a long time ago in some cases, either by mixing genes from different parents, by selecting interesting variants, or by directly acting on genes through chemical or physical agents. All these plants and animals can legitimately be called “genetically modified organisms” relative to their progenitors.
When the term “genetically modified organism” is used, however, it does not refer to the examples mentioned above. Rather, the term “genetically modified” (GM) is currently applied to plants and animals that result from adding genes, in particular genes from completely unrelated organisms, to preexisting plants or animals. In other words, GMOs are regarded as life forms that could not have appeared naturally. This is indeed true for the types of genetically engineered plants that we will discuss in this chapter. Genetic modification of animals, including humans, is discussed in chapter 13.
Genetically modified plants are commonly grown today in the United States (see table 6.1). Most of the corn (including corn products found in breakfast cereals) and soybean- and canola-derived products sold in this country are the result of plants engineered with recombinant genes. The most common genetic modifications are those that confer resistance to certain insect pests and those conferring resistance to certain herbicides. Let us first see how genetic modification of plants is achieved. Two techniques are used to genetically engineer plants: Agrobacterium-mediated gene transfer and biolistics.
This first method relies on the ability of the soil bacterium Agrobacterium tumefaciens to transfer some of its plasmid DNA to plant cells. Agrobacterium is a plant pathogen that, after infecting its host, causes the disease called crown gall. This disease is characterized by the formation of tumors at the point of infection. In the infection process, Agrobacterium transfers its plasmid DNA to the plant cell, and this DNA is subsequently integrated within the DNA double strand of the infected plant cells. Within this plasmid are tumor-causing genes, so the plasmid is called the Ti (for tumor inducing) plasmid. The important point here is that Agrobacterium is a natural genetic engineer, since it can naturally transfer its DNA to a plant host. In order to insert foreign genes into plants, scientists remove the tumor-causing genes from the Ti plasmid and insert a foreign gene in their place. The Ti plasmid is thus a natural plasmid vector useful for plant transformation.
A typical plant transformation experiment consists in harvesting plant tissues (for example, leaf pieces) and incubating them with Agrobacterium cells containing the gene of interest in its Ti plasmid. After just a few hours, Agrobacterium has transferred its Ti plasmid with the foreign genes to the plant cells. Whole plants are then regenerated from these treated plant pieces. This is accomplished by cultivating the plant pieces in a petri dish with a nutrient medium containing plant hormones that promote both root and stem growth. Thus whole fertile plants can be regenerated from the treated tissues. These plants can then be propagated by the usual means, seeds. The complete genetic modification process is shown in figure 6.1.
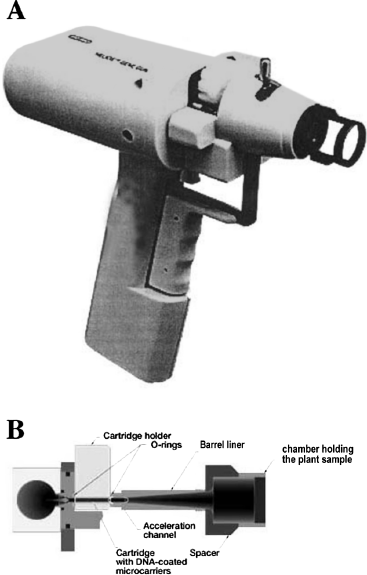
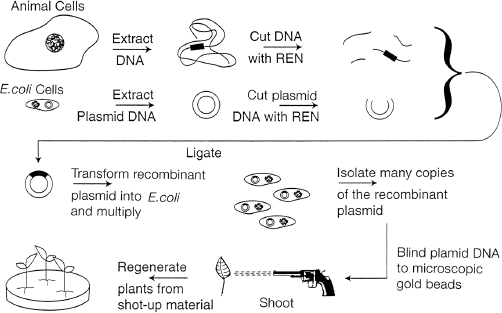
Biolistics, the other gene transfer technique, does not rely on Agrobacterium. Here, a physical means is used to force foreign DNA into plant cells. Back in 1987, researchers came up with an idea that must have found its origins in the Wild West. They reasoned that DNA, somehow absorbed on the surface of microscopic metal particles, could actually be shot at plant cells by using a gun. In this way, a DNA “bullet” of sorts would penetrate the plant cell wall, and that DNA would become expressed in the plant cell. And it worked! DNA can indeed stick temporarily to microscopic gold or tungsten beads. This shot is loaded into a gun (in early models, the barrel of a .22 caliber was sawed off and the gun welded to a thick metal lid covering a metal firing chamber), and the gun is fired at the plant target. The discharge must be powerful enough for the beads to penetrate the plant cells, but not so powerful as to splatter the sample all over the chamber. You might guess that this was rather tricky to achieve with the explosives used in a regular shotgun. Modern “gene guns,” as they are called, accelerate metal beads through the quick release of helium gas under pressure (figure 6.2). Basically, once the DNA-coated beads find themselves inside the plant cells, DNA is released from them, migrates to the cell nucleus, and ends up integrated within the plant DNA. Biolistics thus achieves the same result as Agrobacterium-mediated gene transfer, without the use of live bacteria, however. As in the case of Agrobacterium, shot-up plant tissues must be regenerated into fertile plants. The biolistic process is summarized in figure 6.3.
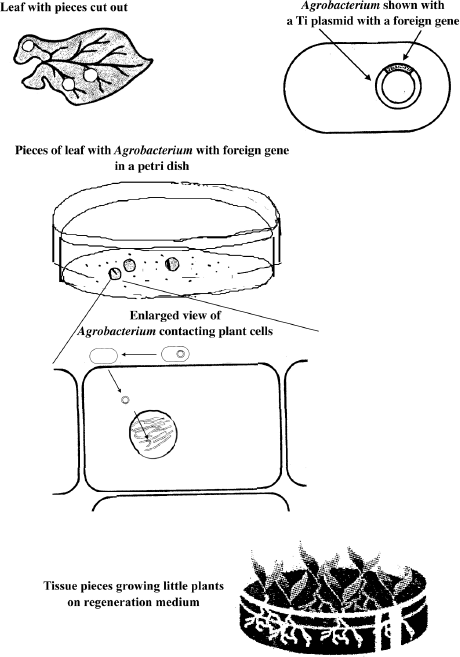
Figure 6.1 Genetically Modifying Plants Using Agrobacterium-Mediated Gene Transfer. The process begins by using a piece of plant tissue, for example, a leaf. A gene for the desired trait is cloned into the Ti plasmid of Agrobacterium. The pieces of leaf are then placed in a petri dish together with Agrobacterium containing the foreign gene. The bacterial cells make physical contact with the plant cells and transfer the plasmid-borne foreign gene to the plant cells’ chromosomes. The tissue pieces are then placed on regeneration medium. Each tissue piece will grow into several little plants containing the foreign gene.
Figure 6.2 The Gene Gun. A. A photograph of one commercially available biolistic instrument called a gene gun. B. A diagram of the gene gun.
Figure 6.3 The Biolistic Process of Genetically Modifying Plants. First, DNA is isolated from the cells of an organism. Plasmid DNA is isolated from E. coli. Both the plasmid and the DNA from the other organism are cut with the same restriction enzyme (labeled REN). The cut pieces of DNA and plasmid are mixed and ligated (glued) back together. The plasmids are introduced into E. coli to make many copies of the recombinant plasmid. The recombinant plasmid containing the desired gene is isolated and attached to gold beads. The beads are loaded into a gene gun and shot into plant tissue. The tissue is then placed on growth medium as in the Agrobacterium method.
Note that when the foreign genes used in these experiments are of a nonplant origin, they must be equipped with a promoter and a terminator that function in plants. A commonly used promoter is derived from the cauliflower mosaic virus (CaMV), a virus that infects cauliflower, broccoli, and other members of the cabbage family. A commonly used terminator is called a “nos terminator,” a terminator for a plant-hormone gene found in Agrobacterium Ti plasmid. These specific pieces of DNA are cut and put together using the recombinant DNA techniques explained in chapter 5.
For a variety of reasons, it is important to know whether plant products are genetically modified. For example, some farmers grow and specifically sell non-GM crop plants to their customers; some countries require that only non-GM products be imported; other countries require labeling products containing GM plants. Since GM and non-GM plants or products made from them cannot be distinguished by outward appearance, genetic tests are necessary to determine whether a product is or is not genetically modified. Since it is not always known what foreign gene might have been used to engineer a particular plant, a genetic test was developed based on the detection of the promoter of the foreign gene.
Foreign genes, if they are not of plant origin, must be engineered with plant-expressible promoters for proper functioning in a plant host. Cauliflower mosaic virus promoter, isolated from a virus that infects plants in the cabbage family, is commonly used because it is very active in many different plants. Thus, regardless of the nature of the foreign gene itself, it is possible to distinguish between GM and non-GM plants if the genetic engineer used the CaMV promoter. Detection of the CaMV promoter is done by polymerase chain reaction (PCR; see chapter 1). Since the sequence of the CaMV promoter is known, we can design primers for it. We can test the DNA isolated from plants or plant products using these primers in a PCR reaction. The length of the CaMV promoter is 195 base pairs. If the promoter is present in a plant product, the PCR reaction will amplify a 195-base-pair piece of DNA that can be easily detected by gel electrophoresis (chapter 1). Of course, if the CaMV promoter was not used, this technique will not determine whether or not the plant was genetically modified. However, if the promoter is detected, and the cauliflower mosaic virus does not normally infect the tested plant, one can be quite confident that there was some genetically modified plant product in the sample.
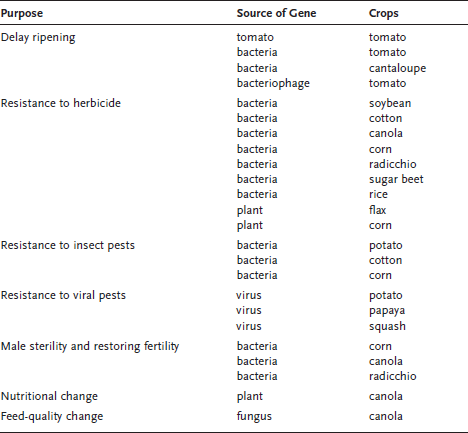
The most common GM food plants on the U.S. market are insect-resistant corn, herbicide-resistant soybeans and canola, and virus-resistant papaya. table 6.1 lists all the genetically modified plants that have been approved as of 2002 by the U.S. Food and Drug Administration.
Let’s first consider genetic modification for insect resistance. The gene for insect resistance is isolated from the bacterium Bacillus thuringiensis, a natural pathogen of certain insects. This gene codes for a toxin, called Bt toxin, that kills the insects’ intestinal cells. Insects infesting plants with this Bt toxin gene ingest the toxin as they feed, and they die. Corn, potato, and cotton plants have been engineered with this gene. Interestingly the Bt toxin gene in use today is synthetic, just like the insulin gene discussed in chapter 5. That is, the amino acid sequence of the toxin protein was decoded into the corresponding DNA sequence, and that sequence made in the test tube.
Two examples of herbicides for which plants have been genetically modified for resistance are Roundup® (produced by Monsanto) and Liberty® (produced by AgrEvo). Both herbicides are kill-all, meaning that they kill all plants. Herbicide resistance can be achieved by inserting genes for enzymes that have the ability to break down the herbicides. Such genes have been isolated from bacteria. Thus, plants engineered for resistance to either herbicide will survive being sprayed by the herbicide, while any plants without this gene, including weeds, will not.
Interestingly, resistance to viral infection has been achieved by inserting into plants one of the genes of the virus itself. Viruses are typically composed of a protein coat and a DNA or an RNA genome. A generic virus can be visualized as a molecule of nucleic acid “wrapped” into a layer of proteins and sometimes lipids. The shape of the protein coat is extremely variable and is virus-dependent. Think of this protein coat as “packaging” the viral genome and containing the necessary ingredients to deliver it inside host cells. For reasons not entirely clear, if a plant is genetically modified with the gene for the coat protein of the virus, the genetically modified plant exhibits resistance to infection by that virus.
All the examples discussed so far deal with plants engineered for resistance to some kind of injury, inflicted either by viruses, insects, or herbicides. Some researchers, however, have attempted to and succeeded in changing the nutritional quality of canola by changing its oil composition. Another early example of changing the quality of the food is delayed ripening in tomatoes, as in the case of a tomato called the Flavr-Savr Tomato. Delayed ripening can be achieved by different genetic modifications. One approach is to reduce the activity of the enzyme that produces ethylene, a natural ripener. Another method is to reduce the activity of an enzyme that softens fruit by breaking down pectin. Delayed-ripening tomatoes did reach the grocery stores in the late 1980s. However, they were a market flop and are no longer available.
Table 6.1 Genetically Modified Crops Approved by the Food and Drug Administration Through 2002
Note: although the above have been approved, they are not necessary in commercial production.
A genetic modification in progress aims to improve the nutritional qualities of rice. Polished, or white, rice, which is commonly consumed in Asia and elsewhere, contains no vitamin A. Vitamin A deficiency is a serious problem for people whose diet is based mostly on rice, such as poor people in Asia. It can lead to blindness in children and exacerbates other pathological conditions. A Swiss/German university team decided to engineer rice plants to make them able to produce vitamin A (actually provitamin A, which is converted to vitamin A in the human body) in their seeds, the edible part of rice. Rice is actually able to synthesize compounds that can be metabolized into provitamin A. The problem is that rice does not contain the proper enzymes to do this in the seeds. The researchers isolated two genes coding for the relevant enzymes, one gene from the soil bacterium Erwinia ureidovora and another gene from daffodils. Both genes were transferred to rice plants using the Agrobacterium method, and, sure enough, the rice grains started making provitamin A, giving the grains a golden color, the natural color of provitamin A. For this reason, this rice variety has been dubbed “golden rice.” Golden rice is still in its developmental phase. In order to produce commercial rice that makes provitamin A, the transformed plants must be regenerated from tissue cultures and then crossed with varieties that have the growth and edibility qualities favored by farmers and consumers. Field trials of these golden-rice hybrids are underway.
GM crop plants are not grown in Europe or Japan. In both Europe and Japan, there is strong opposition to GM plant products. The European Union has had a ban on foods containing GMOs. They are now considering new rules to allow foods containing GMOs if they are labeled as such. However, in China sixteen GM plant varieties are either in production or soon to be. These varieties include rice, wheat, potato, corn, soybean, canola, peanut, cabbage, tomato, chili, and others, genetically modified for insect, virus, or herbicide resistance. Given all the controversy surrounding GM food plants, it is unclear what their future will be.
Not all potential applications of genetic modification of plants concern food plants, however. In this section, we will describe two other potential applications of genetically modified plants: phytoremediation and human vaccine production in plants.
Phytoremediation is the act of cleaning up polluted soils with plants. As we know, vast stretches of land, worldwide, are heavily polluted with petrochemicals or toxic heavy-metal salts such as mercury and cadmium. It so happens that soil microbes are often able to detoxify these pollutants by degrading petrochemicals and immobilizing heavy metals. However, there are potential problems with spraying contaminated soils with these microbes, for fear of creating yet another environmental problem. This is where GM plants may come to the rescue. Why not isolate the relevant bacterial genes and transfer them to plants? Plants do not move, can grow fast, and, through their roots, absorb polluted water, thereby concentrating toxic chemicals inside their cells. When engineered with bacterial genes, these GM plants would be able to survive. After their detoxification job was achieved, they could be harvested and burned under controlled conditions. And yes, this has been done. There now exist fast-growing GM poplar trees able to detoxify petrochemicals and salts of various heavy metals. Their positive effects have been tested on a small scale, and research is being pursued for other pollutants using poplar trees as well as other plants.
Finally, plants, one day, may be a source of readily accessible human vaccine. Vaccines are made from disabled pathogens, such as viruses or bacteria, so that they can no longer cause disease but instead provoke an immune reaction and thus provide protection from the pathogen. When people are injected with a part of a pathogen called an antigen, their immune system produces specific antibodies that block the antigen. Vaccinated people, when exposed to the pathogen, will also block it, thanks to their newly acquired antibodies. Vaccination works very well to avert a host of viral and microbial diseases. Vaccines, though, typically require refrigeration and must be injected using sterile syringes. These are not always easy to come by in developing countries: hence the idea of producing vaccines in edible plants. This concept has been successfully demonstrated: a gene from the Newcastle virus (a pathogen that causes severe diarrhea) has been cloned and expressed in potatoes. Volunteers who ate these GM potatoes developed antibodies against the virus. These GM potatoes had to be eaten raw, because boiling or frying would have destroyed the antigen. Since most people would not enjoy chewing on raw potatoes, the banana is now being considered for genetic modification with antigen genes. Vaccination campaigns with GM plants have not yet started, however. Amusingly, since vaccines are pharmaceuticals, the idea of producing them in plants has been dubbed “pharming.” Clearly, other proteins of medical importance could potentially be produced in GM plants. One great advantage is that plants cannot be contaminated with human viruses, while plant viruses are harmless to humans.
Well before the introduction of GM plants, some people voiced concerns about the impact these plants could have on the environment. We will consider two major ecological issues associated with genetically modified plants: the appearance of resistance to Bt toxin among target insects and the possible creation of “superweeds.”
When insect populations are sprayed with an insecticide, resistance to this chemical invariably occurs. One famous example is DDT. This insecticide has completely lost its effectiveness in many parts of the world because so many insect species (including mosquitoes that carry the malaria parasite and the tsetse fly that carries the parasite causing sleeping sickness) have developed resistance to it. Fears that insects that are pests for cotton would develop resistance to the Bt toxin made by GM cotton have been borne out. Just two years after the introduction of cotton modified with the Bt toxin gene, such resistance has appeared in the field.
We must minimize the rise of resistance if Bt toxin is to remain an effective tool to fight pest damage. One such measure, instituted since the initial introduction of Bt modified crops, is that farmers who plant Bt crops are now required to grow a certain percentage of non-GM crops in close proximity of the GM crop. The idea is to create refuges in which susceptible insects can survive, that is, areas planted with crops that do not contain Bt toxin. This should help to maintain insect populations still susceptible to the toxin. Indeed, if Bt crops were the only food source for insects, only resistant individuals would survive and reproduce. Because susceptible individuals are not killed by Bt toxin in the refuges, availability of the refuges reduces the rise of resistant individuals. The goal of having refuges is to allow many sensitive insects to survive and dilute the effect of rising resistance. We cover more about the rise of resistance in a population in chapter 11. The lesson with Bt-modified crops is that genetic modification of crops with Bt toxin is not a cure-all against insect infestation of crops.
Another potential ecological problem is the transfer of genetically modified traits from GM crops to their weedy relatives. For example, if herbicide resistance in crop plants is transferred to their weedy relatives, it can produce “superweeds.” All cultivated plants are derived from plants found in the wild. Many crops still have wild, weedy relatives with which they can occasionally interbreed. If this breeding happened between herbicide-resistant GM crop plants and their weedy relatives, herbicide-resistant weeds would be created, and they could destroy the effectiveness of the herbicides. Another example is that of transfer of viral resistance from genetically modified crops to their weedy relatives. For example, viral resistance in squash may be transferred to their wild cousin that is a pest, especially in the Southern US. The transfer of genetic modification from GM crops to their wild relatives has not yet been documented in the wild. Yet it is clear that a small amount of interbreeding can occur between domesticated crops and their wild counterparts.
Let us now discuss labeling issues and food safety. People are legitimately concerned about the safety of what they eat. Even though Bt toxin is deadly for insects, it has no effect on human intestinal or other cells, and it is not allergenic because it is quickly destroyed in human gastric juice. Similarly, enzymes that degrade herbicides have been tested for toxicity and allergenicity. GM food plants have been extensively analyzed for the presence of toxic compounds and have been approved by the Food and Drug Administration. One test for transgenic products is to determine whether the foreign protein is degraded in the stomach. If the protein is not degraded in the stomach, it has the potential for triggering allergic reaction. Nevertheless, not everybody is convinced that GM food plants are entirely safe.
One solution to this question of food safety is to label foods that contain GM plant products. Indeed, the European Union is considering allowing the importation of products containing GM plants if the products are labeled. If this were done, the public could choose between GM and non-GM foods. There are two obstacles to labeling genetically modified foods: One is that biotech companies have fought hard to prevent the labeling of these products in the United States, and so far they have won this battle. This means that the public has no way of knowing which foods contain GM plant products and which do not. Another looming obstacle is the fact that so many staple crops in the United States are genetically modified. If processed foods are to be labeled, even if only a small portion of the contents is genetically modified, a major transformation of farming and distribution practices in the United States is necessary to keep genetically modified crops separate from nonmodified crops in the food stream.
This issue of labeling of genetically modified foods promises to be contentious for years to come. The increasingly global market of foods will further heighten the need to be aware of issues related to the genetic modification of crops.
We have seen how plants can be genetically modified by the addition of genes from another organism. Two methods are commonly used to introduce foreign genes into plants. The first method uses a natural genetic engineer in plants, the bacterium Agrobacterium tumefaciens. For this, the foreign gene is first inserted into Agrobacterium’s Ti plasmid. Then the Agrobacterium naturally transfers the recombinant plasmid into the plant cells when the two are mixed. Another technique, called biolistics, consists of bombarding plants with microscopic, DNA-coated metal particles. Currently, genetically modified plants are commercially produced and marketed in the United States. Ecological, food safety, and labeling concerns surround the growing and selling of genetically modified plants.