ANSWERS TO STUDY QUESTIONS
Chapter 1
- Jenner’s method of using cowpox infection to confer immunity to smallpox was superior to earlier methods because it carried a significantly reduced risk of serious disease. The earlier method of using material from the lesions of smallpox victims conferred immunity but at the risk of acquiring the potentially lethal disease.
- The Pasteur method for treating rabies consists of a series of inoculations with attenuated rabies virus. This process actively immunizes the recipient, who then mounts an immune response against the virus to stop the progress of infection. A simple test for active immunity would be to look for antibodies specific to the rabies virus in the recipient’s blood at a time after completion of treatment, when all antibodies from a passive treatment would have cleared from the circulation. Alternatively, one could challenge the recipient with attenuated rabies to see whether a secondary response occurred (this test may be precluded by ethical ramifications).
- The immunized mothers would confer passive immunity on their offspring because the anti-streptococcal antibodies, but not the B cells, cross the placental barrier and are present in the babies at birth. In addition, colostrum and milk from the mother would contain antibodies to protect the nursing infant from infection.
- (a) H; (b) CM; (c) B; (d) H; (e) CM.
- The four immunologic attributes are specificity, diversity, self/nonself recognition, and memory. Specificity refers to the ability of certain membrane-bound molecules on a mature lymphocyte to recognize only a single antigen (or a small number of closely related antigens). Rearrangement of the immunoglobulin genes during lymphocyte maturation gives rise to antigenic specificity; it also generates a vast array of different specificities, or diversity, among mature lymphocytes. The ability of the immune system to respond to nonself molecules, but (generally) not to self molecules (self/nonself recognition), results from the elimination during lymphocyte maturation of immature cells that recognize self antigens. After exposure to a particular antigen, mature lymphocytes reactive with that antigen proliferate, differentiate, and adapt, generating a larger and more effective population of memory cells with the same specificity; this expanded population can respond more rapidly and intensely after a subsequent exposure to the same antigen, thus displaying immunologic memory.
- The secondary immune response is faster (because it starts with an expanded population of antigen-specific cells), more effective (because the memory cells have learned and adapted during the primary response), and reaches higher levels of magnitude than the primary response (again, because we begin with many more cells that have already honed their strategy).
- Consequences of mild forms of immune dysfunction include sneezing, hives, and skin rashes caused by allergies. Asthma and anaphylactic reactions are more severe consequences of allergy and can result in death. Consequences of severe immune dysfunction include susceptibility to infection by a variety of microbial pathogens if the dysfunction involves immunodeficiency, or chronic debilitating diseases, such as rheumatoid arthritis, if the dysfunction involves autoimmunity. The most common cause of immunodeficiency is infection with the retrovirus HIV-1, which leads to AIDS.
- (a) True. (b) True. (c) False. Most pathogens enter the body through mucous membranes, such as the gut or respiratory tract. (d) True. (e) False. Both are involved in each case. Innate immunity is deployed first during the primary response, and adaptive immunity begins later during that first encounter. During the secondary response, innate and adaptive immunity are again both involved. While innate responses are equally efficient, the second time around adaptive immunity uses memory cells to pick up where it left off at the end of the primary response and is therefore quicker and more effective in pathogen eradication during a secondary response. (f) False. These are two different types of disorder: autoimmunity occurs when the immune system attacks self, and immunodeficiency occurs when the immune system fails to attack nonself. The one caveat occurs in cases of immune deficiency involving immune-regulatory components. Just like broken brakes, these can result in an overzealous immune attack on self structures that thus presents as autoimmunity. (g) False. The intravenous immunoglobulin provides protection for as long as it remains in the body (up to a few weeks), but this individual has not mounted his or her own immune response to the antigen and will therefore not possess any memory cells. (h) True. (i) False. The genes encoding a T-cell receptor are rearranged and edited during T-cell development in the thymus so that each mature T cell carries a different T-cell receptor gene sequence. (j) False. The innate immune response does not generate memory cells (as the adaptive immune response does), so it is equally efficient during each infection. (k) False. Fragments of foreign antigen are not retained. Memory cells will continue to express the same T-cell receptor that originally bound antigen during the primary response, leading to clonal selection and expansion. Likewise, memory cells retain their differentiation profile and effector functions going into the secondary response (e.g., TH1-specific cytokines and effector capabilities).
- Pasteur had inadvertently immunized his chickens during the first inoculation, using an old, attenuated bacterial strain. The old strain was no longer virulent enough to be fatal, but it was still able to elicit an adaptive immune response that protected the chickens from subsequent infections with fresh, virulent bacteria of the same type.
- Viruses live inside host cells and require the host cell’s machinery to replicate. Fungi are extracellular and are often kept in check by the immune system, but they can be a problem for people with immune deficiencies. Fungi are the most homogeneous in form. Parasites are the most varied in form and can range in size from single-celled, intracellular microorganisms to large macroscopic intestinal worms. Some parasites also go through several life-cycle stages in their human host, altering their antigenic structures and location so significantly between stages that they require completely different immune eradication strategies. Bacteria can cause intracellular or extracellular infections, which require different immune targeting and elimination methods. Many bacteria express cell surface molecular markers (PAMPs) that are recognized by receptors that are part of our innate immune system (PRRs).
- Herd immunity is what occurs when enough members of a population have protective immunity to a pathogen, either through vaccination or prior infection, that they act as a buffer against spread and help protect those without immunity. The efficacy of herd immunity depends on pathogen characteristics such as how the pathogen is transmitted (airborne, fecal/oral, etc.) and whether it can survive for long outside the host. Herd characteristics include how we interact with one another as a “herd” (e.g., crowding indoors versus sparse populations and outdoor encounters) and the primary target population (e.g., young children versus adults). (Other answers are also acceptable.)
- Ehrlich’s original theory had each cell expressing many different receptors and this was refined to many different cells, each one making many copies of a unique receptor. With Ehrlich’s original conception, “selection” of this receptor would somehow need to trigger the cell to secrete only this receptor and not the others. In the refined and current version of the theory, once a receptor is selected that cell will make many copies to secrete. Since this is the only version of the antigen-specific receptor made by this cell, there is no confusion over which receptor received the signal and needs to be secreted.
- (a) I; (b) A; (c) A; (d) A; (e) I; (f) A; (g) A; (h) A; (i) I; (j) A; (k) A.
- Tolerance means that our immune system can discern between ourselves and foreign antigens, and does not attack self antigens. Lymphocytes learn tolerance by being exposed to self antigens during development, when most potentially self-reactive cells are either destroyed or inhibited from responding.
- An antigen is anything that elicits an adaptive immune response—most commonly a part of some foreign protein or pathogen. An antibody is a soluble antigen-specific receptor molecule released by B cells that binds to an antigen and labels it for destruction. One interesting twist: antibodies can be antigens if they come from another species and are recognized as foreign by the host. This occurs in some instances of passive immunization, when antibodies from an animal such as a horse are given to humans, who then mount an adaptive immune response against horse-specific chemical patterns in the antibodies.
- Pattern recognition receptors (PRRs) are germ line-encoded receptors expressed in a variety of immune cells. They are designed by evolution to recognize molecules found on common pathogens, and they initiate the innate immune response when they bind these molecules. In contrast, B- and T-cell receptors are expressed in lymphocytes, and the genes that encode these are produced by DNA rearrangement and editing, so that the receptor locus in each B or T cell’s genome has a different sequence and encodes a different receptor. B- and T-cell receptors are diverse and have the ability to recognize a much greater assortment of antigens than PRRs, including those never before encountered by the immune system. B- and T-cell receptors are part of the adaptive immune response.
- Cytokines are soluble molecular messengers that allow immune cells to communicate with one another. Chemokines are a subset of cytokines that are chemotactic, or have the ability to recruit cells to the site of infection.
- (a) Yes. The problem is that T and B cells rearrange their DNA and each cell makes a unique receptor, which means they must have a unique DNA sequence for creating that molecule. (b) No. Since DNA rearrangement does not happen in germ cells, only in somatic cells (B and T cells, specifically), this is not passed on to progeny.
- Only like making replicas of the weapons. There is no “saving” pieces or all of the pathogen to remember for later. The immune response saves the solution and not the problem.
- No. Since memory occurs only in B and T cells and these cells are not passed on to the progeny, there is no inheritance of memory. Babies receive maternal antibodies in the womb and through breast milk, but not the cells that produce them. The antibodies themselves do not last more than a few months. This is why babies need to be vaccinated, to make their own memory B and T cells.
- Extracellular bacteria and fungi are more alike. They are both extracellular and of similar size. Helminths are large extracellular worms, and viruses are small obligate intracellular pathogens. The immune system is likely to sort the latter two into very different bins based on where they are found, inside versus outside of cells, and their size or structure.
- Clonal selection occurs in the lymphoid organs, not the site of infection (unless the site of infection is a lymphoid organ). The lymphoid organs are where innate and adaptive immunity meet, and where clonal selection occurs. There are sites in the body where the immune response is all but off limits. These include places like the central nervous system and the eye. Inflammation in these areas is likely to do more damage than good, and could result in permanent loss of function in the host if left unchecked.
- Inflammation-mediated destruction of healthy tissue or possibly a blood cell cancer. In fact, uncontrolled replication of B or T cells leads to leukemia or lymphoma, cancers of the white blood cells and lymphatic system, respectively.
- Immune imbalance disorders, especially allergy and autoimmunity. Both of these are overreactions of the immune system (to benign foreign structures or self structures, respectively). What they have in common is a lack of or break in tolerance. This can be caused by a decrease in the immune-suppressing or inhibiting side of the immune balance equation (less brake).
- The self/nonself theory of immune reactivity would suggest that we should recognize and attack microbes in our gut, including commensal (i.e., beneficial) bacteria. Typically this does not occur, although we will see later in the book that we do in fact recognize many of our “old friend” microbes without attacking them. The damage or danger theory suggests that we will not attack commensals unless (1) they cause damage to host cells, which then triggers an immune response, and/or (2) they induce host cells to express warning signs that set off an immune reaction. In most instances, this is true. We do not attack benign microbes in our body. Damage to the epithelial cells lining the gut can lead to reactions against otherwise benign microbes, even if the microbes themselves did not cause this damage, also consistent with the danger model.
Clinical Focus Answers
- Considerations in developing nations vary as much as the nations themselves, but might include cost to create or purchase the vaccine, spaces to preserve the vaccine (usually cold), tools and expertise for administering the vaccine (e.g., needles and trained personnel), safety or religious concerns, mistrust of the industry and medical intervention, and so on. The concerns in developing nations, such as the United States, are very different and usually revolve around issues of personal choice or safety. Myth can be a barrier in both situations.
- The target population is really the developing embryo or fetus, but that is a difficult stage to target. Administration of antibodies against Zika to women who are either trying to become pregnant or have just conceived might help to protect them from becoming infected, thereby saving the baby from complications. These antibodies might also cross the placenta and reach the child, which could also protect the baby in utero from the infection and consequences. Of course, neither the mother nor the child would be immune, so the risk of later infection and adverse outcomes is still present.
Chapter 2
- (a) Although T cells complete their maturation in the thymus, not the bone marrow, mature CD4+ and CD8+ T cells will recirculate back to the bone marrow. (b) Pluripotent hematopoietic stem cells (HSCs) are rare, representing less about 0.05% of cells in the bone marrow. (c) HSCs can be mobilized from the bone marrow and circulate in the blood, which is now used as a source of stem cells for transplantation. (d) Macrophages will increase both MHC class I and class II expression after activation; however, TH cells are CD4+ and recognize antigenic peptide bound to MHC class II. (e) T cells develop in the thymus; B cells develop in the bone marrow and achieve full maturity in the spleen. (f) Lymphoid follicles are found in all secondary lymphoid tissues, including that associated with mucosal tissues (MALT). (g) The follicular dendritic cell (FDC) network guides B cells within follicles. The follicular reticular cell (FRC) system guides T cells within the T-cell zone (although in some cases it may also help B cells to get to follicles, so aspects of this statement are true). (h) Infection and associated inflammation stimulates the release of cytokines and chemokines that enhance blood cell development (particularly to the myeloid lineage). (i) FDCs present soluble antigen on their surfaces to B cells, not T cells. (j) Dendritic cells can arise from both myeloid and lymphoid precursors. (k) B and T lymphocytes have antigen-specific receptors on their surface, but NK cells, which are also lymphocytes, do not. (l) B cells are generated outside the bone marrow in birds and ruminants. (m) All vertebrates have a thymus (even the jawless vertebrates have a rudimentary thymus). (n) Recent data suggest that at least one jawless vertebrate, the lamprey, has T- and B-like cells.
- Myeloid: a, b, d, e. Lymphoid: c, f, g, h.
- The primary lymphoid organs are the bone marrow (bursa of Fabricius in birds) and the thymus. These organs function as sites for B-cell and T-cell maturation, respectively. The secondary lymphoid organs are the spleen, lymph nodes, and barrier-associated lymphoid tissue (including skin and mucosal-associated lymphoid tissue, MALT). All these organs trap antigen and provide sites where lymphocytes can interact with antigen and subsequently undergo clonal expansion.
- HSCs are stem cells, which, unlike mature fully differentiated cells, are (1) multipotent and (2) capable of self renewal.
- The thymus helps us avoid autoimmune responses by negatively selecting thymocytes expressing T-cell receptors that bind to self peptide–MHC complexes with high affinity. Thymocytes with new TCRs scan the surfaces of epithelial cells in the cortex and medulla of the thymus; if they bind too tightly to surface MHC molecules presented by these cells they are eliminated (typically but not exclusively by clonal deletion).
- In humans, the thymus reaches maximal size during puberty (b). During the adult years, the thymus gradually atrophies.
- Immunodeficient mice are missing one or more immune cell type (either because of genetic mutations or chemotherapy). Injection of stem cells will restore these cell types—an easily measured outcome. As HSCs are successively enriched in a preparation, the total number of cells that must be injected to restore these cell populations decreases.
- Monocytes are the blood-borne precursors of macrophages. Monocytes have a characteristic kidney bean–shaped nucleus and limited phagocytic and microbial killing capacity compared with macrophages. Macrophages are much larger than monocytes and undergo changes in phenotype to increase phagocytosis, antimicrobial mechanisms (oxygen dependent and oxygen independent), and secretion of cytokines and other immune system modulators. Tissue-specific functions are also found in tissue macrophages.
- The bursa of Fabricius in birds is the primary site where B lymphocytes develop. Bursectomy would result in a lack of circulating B cells and humoral immunity, and it would probably be fatal.
- (a) The spleen is classically considered an organ that “filters” antigens from blood. The lymph node receives antigens principally from the afferent lymphatics. (b) Both the lymph node paracortex and the splenic periarteriolar lymphoid sheath are rich in T cells. B cells are found primarily in the follicles. (c) Germinal centers are found wherever there are follicles, which are present in all secondary lymphoid tissue, including lymph node, spleen, and the wide variety of mucosal-associated lymphoid tissues. (d) True. (e) Afferent lymphatics are associated with lymph nodes, not the spleen. (Efferent lymphatics can be found in both.) (f) Ikaros is required for lymphoid development, which occurs primarily in the bone marrow (and thymus). However, lymphocytes populate the spleen and mount an immune response there, so the spleen would clearly be compromised in function in the absence of Ikaros and lymphocytes.
- (1) d; (2) l; (3) c; (4) m, n; (5) g; (6) i; (7) n; (8) b; (9) o; (10) h; (11) c, f, g; (12) c, f; (13) c, e, f; (14) j, k; (15) e.
Research Focus Answer
This is an open-ended question that describes real observations; several answers could be considered correct. The model you advance “simply” has to be logical and consistent with all the data presented. Perhaps the most straightforward possibility is the one that turns out to be true: Notch regulates the decision of a progenitor (the common lymphoid progenitor [CLP], in fact) to become either a B cell or a T cell. If Notch is active, CLPs become T cells (even in the bone marrow). If Notch is turned off, CLPs become B cells (even in the thymus). Other possibilities? Notch could induce apoptosis of B cells. However, if this were the case, why would there be more T cells in the bone marrow? Alternatively, Notch could influence the microenvironments that the cells develop in and, when active, make the bone marrow niches behave like thymic niches. When off, it could allow the thymic niche to “revert” to a bone marrow–like environment. This would work (even if it is a more complex scenario) and deserves full credit as an answer. Experimental approach depends on the hypothesis proposed.
Clinical Focus Answer
(a) This outcome is unlikely; since the donor hematopoietic cells differentiate into T and B cells in an environment that contains antigens characteristic of both the host and donor, there is tolerance to cells and tissues of both. (b) This outcome is unlikely. T cells arising from the donor HSCs develop in the presence of the host’s cells and tissues and are therefore immunologically tolerant of them. (c) This outcome is unlikely for the reasons cited in (a). (d) This outcome is a likely one because of the reasons cited in (a).
Chapter 3
- (a) Cytoplasm. (b) Nucleus. (c) By dephosphorylation with the enzyme calcineurin phosphatase, activated by binding to the calcium-calmodulin complex. Activation of the cell results in an increase in intracytoplasmic calcium ion concentration. (d) There are many possible answers to this question. For example, there may be multiple forms of NFAT, and so these drugs may just interact with particular forms of the enzyme. Other cells may have alternative pathways that can bypass the need for calcineurin, whereas T cells may not. In fact, cyclosporin binds to the protein immunophilin, which is specifically expressed in activated T cells, and it is the complex of cyclosporin and immunophilin that inhibits the activity of calcineurin.
- Immunoglobulin proteins and the T-cell receptor share a common motif: the immunoglobulin fold, which may be the binding target for these antibodies.
- (a) False. Receptors and ligands bind through noncovalent interactions such as hydrogen bonds, ionic bonds, hydrophobic interactions, and van der Waals interactions. (b) True. Receptor-ligand binding can activate the receptor and result in phosphorylation and receptor cross-linking.
- (a) Reduction enabled the breakage of disulfide bonds between the heavy and light chains and the pairs of heavy chains in the IgG molecule. Alkylation ensured that the bonds between the separated chains would not reform. Scientists could then separate the chains and figure out their molecular weight and how many chains belonged to each molecule. (b) Papain digested the molecule in the hinge region, releasing two antigen-binding fragments (Fabs) and one non–antigen-binding fragment, which spontaneously crystallized (Fc). This told investigators that there were two antigen-binding sites per molecule and suggested that part of the molecule was not variable in sequence (since it was regular enough in structure to crystallize). Pepsin isolated the divalent antigen-binding part of the molecule from the rest. (c) Antibodies were generated that specifically recognized the Fab and Fc fragments. Antibodies to Fab were found to bind to both heavy and light chains, thus indicating that the antigen-binding sites had components of both chains. Antibodies to Fc fragments bound only to the heavy chain.
- An ITAM is an immunoreceptor tyrosine-based activation motif. It is a motif with a particular sequence containing phosphorylatable tyrosines in the cytoplasmic regions of several immunologically important proteins. Iga and Igb are part of the signaling complex in B cells and are phosphorylated by the Src-family kinase Lyn, when the B-cell receptor (BCR) moves into the lipid raft regions of the membrane on activation.
- An adapter protein bears more than one binding site for other proteins and serves to bring other proteins into contact with one another without, itself, having any enzymatic activity. Activation of the TCR results in activation of Lck, a Src family kinase. Lck phosphorylates the tyrosine residues on the ITAMs of the CD3 complex associated with the TCR. ZAP-70 then binds to the phosphorylated tyrosine (pY) residues via its SH2 regions, and is then itself phosphorylated and activated.
- I would expect IgM to be able to bind more molecules of antigen than IgG, but perhaps not five times more. Steric hindrance/conformational constraints may prevent all 10 antigen-binding sites of IgM from being able to simultaneously bind to 10 antigenic sites.
- Because you know that, in at least one case, activation of a cell can result in an alteration of the phenotype of the cytokine receptor, resulting in a dramatic increase in its affinity for the cytokine. For example, the IL-2 receptor (IL-2R) exists in two forms: a moderate-affinity form consisting of a βγ dimer and a high-affinity form, synthesized only following cell activation that consists of an αβγ trimer.
- Src family kinases have two tyrosine sites on which they can be phosphorylated: an inhibitory site and an activating site. In the resting state, the inhibitory site is phosphorylated by the kinase Csk, and the kinase folds up on itself, forming a bond between an internal SH2 group and the inhibitory phosphate, shielding the active site of the enzyme. Cleavage of the phosphate group from the inhibitory tyrosine allows the enzyme to open its structure and reveal the active site. Further activation of the enzyme then occurs when a second tyrosine is phosphorylated and stabilizes the activated state.
- Since Lck lies at the beginning of the signaling pathway from the T-cell receptor, a T cell with an inactive form of Lck will not be able to undergo activation to secrete IL-2.
- The signaling kinase Bruton’s tyrosine kinase (Btk) is defective in 85% of cases of X-linked agammaglobulinemia. It is encoded on the X chromosome, and hence most cases of this disease occur in boys. Phosphorylation by Btk activates PLCγ, which cleaves phosphatidylinositol bisphosphate (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DAG) following activation of pre-B cells through the pre-B-cell receptor, or of B cells through the immunoglobulin receptor. This leads eventually to activation of the NFAT and MAP kinase transcription factor pathways, culminating in B-cell differentiation and proliferation, which cannot occur in the absence of a functioning Btk protein.
- Both the BCR and the TCR are noncovalently associated on their respective cell membranes, with signal transduction complexes that function to transduce the signal initiated by antigen binding to the receptor and into the interior of the cell. In the case of the B-cell receptor, the signal transduction complex is composed of Iga/Igb. ITAMs on Iga/Igb are phosphorylated by tyrosine kinases that are brought into close proximity with the B-cell receptor complex on the oligomerization of the receptor and its movement into the lipid raft regions of the membrane, which occur on antigen binding. The phosphorylated tyrosines of Iga/Igb then serve as docking points for downstream components of the signal transduction pathway. In the case of the TCR, the signal transduction complex is the CD3 set of proteins, which contains six chains and serves a similar function to Iga/Igb. ΙTAMs on CD3 are phosphorylated by Src family kinases, particularly Lck, and then serve as docking points for downstream components of the TCR signaling pathway. Lck in turn is associated with the TCR coreceptors CD8 and CD4.
- There are multiple possibilities for this answer, and we offer a few here: (a) The antibody is a Y-shaped molecule with the antigen-binding regions located at the two tips of the Y. At the junction of the three sections is a flexible hinge region that allows the two tips to move with respect to one another and hence to bind to antigenic determinants arranged at varying distances from one another on a multivalent antigen. (b) Within the variable region domains of the heavy and light chains, a common b-pleated sheet scaffold includes multiple antiparallel b strands in a conserved conformation. However, at the turns between the b strands, there are varying numbers and sequences of amino acids, corresponding to hypervariable regions in the immunoglobulin variable region sequences. These enable the creation of many different antigen-binding sites. (c) The constant regions of antibody molecules form the bridge between the antigen-binding region and receptors on phagocytic cells that will engulf antigen-antibody complex, or components of the complement system that will bind to the Fc regions of the antibody and aid in the disposal of the antigen. Different constant region structures bind to different Fc receptors and complement components.
- Src family kinases such as Lck are located at the beginning of many signal transduction pathways, and their activities are subjected to rigorous control mechanisms. Lck is maintained in an inactive state by being phosphorylated on an inhibitory tyrosine residue. This phosphorylated tyrosine is then bound by an internal SH2 domain that holds Lck in a closed, inert conformation. If the DNA encoding this tyrosine residue has been mutated or eliminated, such that this phosphorylation cannot occur, then there will be a constitutive level of Lck activity. Since the enzyme that phosphorylates this inhibitory tyrosine is Csk, a reduction in Csk activity would have the same effect.
- Pleiotropy is the capacity to bring about different end results in different cells.
Synergy is the ability of two or more cytokines affecting a cell to bring about a response that is greater than the sum of each of the cytokines.
Redundancy is the property that describes the fact that more than one cytokine can bring about the same effect.
Antagonism is the tendency for two cytokines binding to the same cell to bring about opposite effects, or to reduce/eliminate the response to the other.
Cascade induction is the ability of a cytokine to bind to one cell and to induce that cell to secrete additional cytokines.
- A cytokine may induce the expression on the cell surface of new chemokine receptors and/or new adhesion molecules that would cause the cell to move to a new location and, once present, to be retained there.
- When a type I interferon binds to its receptor on a virally infected cell, the interferon signal results in the activation of a ribonuclease that breaks down cytoplasmic RNA. It is particularly effective against double-stranded RNA.
- (a) The IL-2R is made up of three components: a, b, and g. Quiescent (unactivated) T cells bear only the bg dimer, which binds IL-2, but at intermediate affinity insufficient to result in a signal under physiological conditions. On antigen stimulation, the T cell synthesizes the a subunit, which binds to the bg dimer and converts it into a high-affinity receptor capable of mediating the IL-2 signal at physiological cytokine concentrations.
(b) See the following figure:

- The receptors for these cytokines share a common g chain, which functions as a signal-transducing unit following recognition of each of these cytokines by cytokine-specific a-chain receptor components. The receptors for IL-2 and IL-15, but not the others, also contain a b chain, to form heterotrimeric receptors. The IL-4 and IL-7 receptors are heterodimeric.
Chapter 4
- (a) Defensins, lysozyme, psoriasin; (b) phagocytosis, C-reactive protein, ficolins, MBL, surfactant proteins A and D, C-reactive protein; (c) phagosome NADPH oxidase, O2, ROS, NO, iNOS, arginine, RNS; (d) interferons-α and -b, NK cells; (e) PRRs, PAMPs, antibodies, T-cell receptors; (f) TLR2, TLR4; (g) TLR3, TLR7, TLR9; (h) TLR4, MyD88, TRIF; (i) NLRs; (j) cGAS; (k) STING; (l) NF-κB, IRFs; (m) IL-1, PRRs, caspase-1, inflammasomes, NLRs; (n) IFIT proteins, Mx proteins, 2´, 5´-oligoadenylate A synthetase, protein kinase R; (o) PAMPs, PRRs, dendritic cells; (p) cytokines, dendritic cells; (q) PRRs, defensins.
- The first line of defense is anatomical barriers. Examples: physical barriers of epithelial layers; mechanical mechanisms of pathogen elimination (peristalsis, sneezing, coughing); chemical barriers (acidic pH, enzymes, antimicrobial proteins and peptides). The second line of defense is induced cellular responses. Examples: induced production of antimicrobial proteins and peptides; phagocytosis and killing by enzymatic degradation and ROS and RNS; formation of NETs; regulated cell death.
- CLRs (such as mannose receptor, dectin-1, and DC-SIGN) directly activate signaling pathways that induce phagocytosis. Scavenger receptors such as SR-A and SR-B also directly activate phagocytosis. Opsonin receptors bind opsonins that have bound to the bacteria. Examples are CD91/calreticulin that binds collectins, MBL, and so on; complement receptors that bind complement components; and Fc receptors that bind immunoglobulins.
- B. Beutler showed that lpr mice were resistant to endotoxin (LPS) and that the genetic difference in these mice was lack of a functional TLR4 because of a single mutation in the TLR4 gene. R. Medzhitov and C. Janeway demonstrated that a protein with homology to Drosophila Toll (which turned out to be TLR4) activated the expression of innate immunity genes when expressed in a human cell line.
- Inflammation is characterized by redness, heat, swelling, pain, and sometimes loss of local function. Cytokines made by PRR-activated resident innate cells act on the vascular endothelium, causing vascular dilation (producing redness and heat) and increasing permeability, resulting in the influx of fluid and swelling (producing edema). Prostaglandins generated following the induced expression of COX2, together with mediators such as histamine, lead to the activation of local pain receptors. The swelling and local tissue damage can result in loss of function. The increased vascular permeability allows an influx of fluid containing protective substances, including opsonins and complement (as well as antibodies, if present). Local production of chemokines, together with induced expression of adhesion molecules on vascular endothelial cells, recruits to the site additional innate cells, such as neutrophils and macrophages, which contribute further to innate responses and pathogen clearance through phagocytosis and release of antimicrobial mediators. Proinflammatory cytokines made during this innate response may also act systemically, triggering the acute-phase response.
- Regulated cell death is cell death induced by receptor-activated signaling pathways. One example in innate immunity is NETosis, the death of neutrophils due to the extrusion of neutrophil extracellular traps (NETs), which trap and kill bacteria. A second example is pyroptosis, the induced death of macrophages; it is beneficial in that it results in the killing of any intracellular bacteria and also allows the release of mature IL-1 and IL-18 following their processing by inflammasomes.
- ILCs have a variety of functions. While NK cells kill (by inducing apoptosis) cells that have become altered due to malignancy, infection, or stress, most ILCs function through the production of cytokines that activate other cells to release mediators that are protective against pathogens. Some ILCs also produce factors that are important for the normal development of lymphoid tissues.
- The innate immune system plays key roles in activating and regulating adaptive immune responses. Dendritic cells are key mediators of these roles. They bind pathogens at epithelial layers and deliver them to secondary lymphoid organs such as lymph nodes. After activation/maturation induced by TLR signaling, they present peptides from processed antigen to activate naïve CD4+ and CD8+ T cells. Depending on the pathogen and the PRRs to which it binds, dendritic cells are activated to produce certain cytokines that differentially induce naïve CD4+ cells to differentiate into TH subsets with different functions, usually appropriate for the particular pathogen. Also, PAMP binding to TLRs expressed on B and T lymphocytes can contribute to their activation by specific antigens to generate adaptive responses. Example of an adaptive response enhancing innate immune responses: The cytokine IFN-γ, produced by activated TH1 cells, is a potent macrophage activator, including activating them to kill intracellular bacteria such as Mycobacterium tuberculosis.
- A major potential disadvantage of the adaptive immune system, with the de novo generation of diverse antigen receptors in each individual’s B and T cells, is the possibility of autoimmunity, which may result in disease. Adaptive responses are also slow. Conserved PRRs that have evolved to recognize PAMPs are less likely to generate destructive responses to self components, and innate responses are activated rapidly. The disadvantages of potential autoreactivity and slow response are overwhelmed by the advantages of having adaptive as well as innate immunity. While the innate response is rapid and helps initiate and regulate the adaptive response, innate immunity cannot respond to new pathogens that may have evolved to lack PAMPs recognized by PRRs. Also, in general (except for NK cells), there is no immunological memory, so the innate response cannot be primed by initial exposure to pathogens. Given their complementary advantages and disadvantages, both systems are essential to maintaining the health of vertebrate animals.
Clinical Focus Answer
Children with genetic defects in MyD88 and IRAK4 are particularly susceptible to infections with Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa, and children with genetic variants of TLR4 are susceptible to gram-negative urinary tract infections. Children with defects in the pathways activating the production or antiviral effects of IFN-α and IFN-β are susceptible to herpes simplex virus encephalitis. These individuals are thought not to be susceptible to a broader array of infectious diseases because other innate and adaptive immune responses provide adequate protection. The supporting evidence for the adaptive immune response protecting against infections is that this limited array of susceptibilities is seen primarily in children, who become less susceptible as they get older, presumably as they develop adaptive immunological memory to these pathogens.
Analyze the Data Answer
- The innate responses are important for survival. From the high mortality in TLR7−/− and MyD88−/− mice, we can conclude that TLR7 and MyD88 are required to induce the innate immune response that plays a positive role in protecting mice from RV infection. It is very likely that viral single-stranded RNA (ssRNA) is the component that activates the immune response, as that is the PAMP that TLR7 recognizes, and TLR7 utilizes the MyD88 adaptor.
- The virus is endocytosed by leukocytes in the inflammatory infiltrate of the infected bladder epithelium. TLR7 in endosomes following endocytosis and partial degradation binds the viral ssRNA and activates the MyD88 signaling pathway, leading to activation of IRF7, which induces IFN-α and IFN-β production. The IFNs bind to the IFN-α receptor (IFNAR) on virus-infected cells, activating expression of several proteins that inhibit viral replication.
- Cytosolic dsRNA in the infected bladder epithelial cells should activate the RIG-I-like receptor (RLR) pathway, leading to the activation of IRF3 and IRF7 and type I interferon production. The fact that inflammatory infiltrate leukocytes are required for protection suggests that the RLR pathway is not being activated in the infected cells. RV probably has developed a mechanism to block this pathway in infected cells, such as the proteins expressed by other viruses that block dsRNA and prevent it from binding to RLR (see Table 4-7).
Chapter 5
- (a) True. (b) True. (c) True. (d) True. (e) False. The outer membrane of a virus is derived from the outer membrane of the host cell and is therefore susceptible to complement-mediated lysis. However, some viruses have developed mechanisms that enable them to evade complement-mediated lysis. (f) True.
- IgM undergoes a conformational change on binding to antigen, which enables it to be bound by the first component of complement C1q. In the absence of antigen binding, the C1q-binding site in the Fc region of IgM is inaccessible.
- (a) The initiation of the classical pathway is mediated by the first component of complement, and C3 does not participate until after the formation of the active C3 convertase C4aC2b. The initiation of the alternate pathway begins with spontaneous cleavage of the C3 component, and therefore no part of the alternate pathway can operate in the absence of C3. (b) Clearance of immune complexes occurs only following opsonization of complexes by C3b binding, followed by phagocytosis or binding to the surface of erythrocytes via CR1 binding. Therefore, immune complex clearance is inhibited in the absence of C3. (c) Phagocytosis would be diminished in the absence of C3b-mediated opsonization. However, if antibodies specific for the bacteria are present, some phagocytosis would still occur.
- Any combination of the following answers is acceptable. (1) Complement opsonizes pathogens, thus facilitating the binding of immune system cells via complement receptors. (2) The membrane attack complex (MAC) can induce lysis of pathogens. (3) C3a, C4a, and C5a act as chemoattractants to bring leukocytes to the site of infection, increasing inflammatory response. (4) Binding of C3 fragments by CD21 enhances B-cell activation by complement-coated antigen.
- (a) The classical pathway is initiated by immune complexes involving IgM or IgG; the alternate pathway is generally initiated by binding of C3b to bacterial cell wall components, and the lectin pathway is initiated by binding of lectins (e.g., MBL to microbial cell wall carbohydrates). (b) The terminal reaction sequence after C5 convertase generation is the same for all three pathways. The differences in the first steps are described in part (a) above. For the classical and lectin pathways, the second step involves binding of the serine protease complexes C4b2a (classical) and MASP-1, MASP-2 (lectin). These each act as a C3 convertase in each respective pathway, and the two pathways are identical from that point on. The alternative pathway uses a different C3 convertase, C3bBb. The formation of Bb requires factor D, and the C3 convertase is stabilized on the cell surface by properdin. The alternative pathway C5 convertase is C3bBbC3b. (c) Healthy cells contain a variety of complement inhibitor proteins that prevent inadvertent activation of the alternative pathway. Some of these proteins are expressed only on the surface of host (not on microbial) cells; others are in solution, but are specifically bound by receptors on host cells. Mechanisms of action are explained in the answer to question 7.
- (a) Induces dissociation and inhibition of the C1 proteases C1r and C1s from C1q. Serine protease inhibitor. (b) Accelerates dissociation of the C4b2aC3, C3 convertase. Acts as a cofactor for factor I in C4b degradation. (c) Accelerates dissociation of C4b2a and C3bBb C3 convertases. (d) Acts as cofactor for factor I in degradation of C3b and C4b. (e) Binds C5b678 on host cells, blocking binding of C9 and formation of the MAC. (f) Cleaves and inactivates anaphylatoxins.
- There are many options here and the readings describe several alternatives. The bacterium Staphylococcus aureus has evolved many ways to evade complement action. A small protein called Staphylococcus complement inhibitor (SCIN) binds and blocks the activity of the two C3 convertases, C4b2a and C3bBb. Variola and Vaccinia viruses express complement-inhibitory proteins that bind C3b and C4b and act as cofactors for factor I, thus preventing complement activation. Fungi have evolved mechanisms to destroy complement proteins. The opportunistic human pathogen Aspergillus fumigatus, a fungus that tends to attack patients who are already immunocompromised, secretes an alkaline protease, Alp1, that is capable of cleaving C3, C4, C5, C1q, and IgG.
- Immune complexes are cleared from the body following opsonization by C3b. In the absence of the early components of complement, C3b convertases are not formed, and hence no C3b will be available.
- a. False. Long before the emergence of the adaptive immune system, the early complement components were already functional, although the appearance of functional components of the MAC coincides approximately with the development of the adaptive immune system.
b. False. The various gene families each have unique domain structures.
c. True.
d. True.
- See the following table.
| Complement component knocked out | |||||||
|---|---|---|---|---|---|---|---|
| C1q | C4 | C3 | C5 | C9 | Factor B | MASP-2 | |
| Formation of classical pathway C3 convertase | A | A | NE | NE | NE | NE | NE |
| Formation of alternative pathway C3 convertase | NE | NE | A | NE | NE | A | NE |
| Formation of classical pathway C5 convertase | A | A | A | NE | NE | NE | NE |
| Formation of lectin pathway C3 convertase | NE | A | NE | NE | NE | NE | A |
| C3b-mediated opsonization | D | D | A | NE | NE | D | D |
| Neutrophil chemotaxis and inflammation | D | D | D | D | NE | D | D |
| Cell lysis | D | D | A | A | A | D | D |
Clinical Focus Answer
(a) The two complement regulatory proteins DAF and protectin are both attached to plasma membrane surfaces by glycosylphosphatidylinositol linkages. In paroxysmal nocturnal hemoglobinuria (PNH), a defect in the enzyme PIG-A, which synthesizes these linkages, causes decreased surface expression of both of these proteins. (b) Defects in PIG-A tend to be expressed somatically in cells early in hematopoietic development. A given individual may express red blood cells that are wholly deficient, partially deficient, or wholly competent in PIGA expression. (c) Patients with PNH are essentially unable to express CD16 or CD66abce, indicating that those antigens are also most probably attached to the membranes using GPI linkages. Similarly, patients with PNH are unable to express CD14, so it is also probably a GPI-linked protein. In contrast, patients with PNH as well as normal control individuals are both able to express CD64, which is therefore most likely to be a transmembrane protein.
Analyze the Data Answers
- The arrow shows the population of cells that is undergoing apoptosis. CD46 is rapidly lost from the surface of cells undergoing apoptosis. C1q binds to the DNA that appears on the surface of apoptotic cells, and in the absence of the regulatory component CD46, the cell is susceptible to C3b deposition and C3b-mediated opsonization.
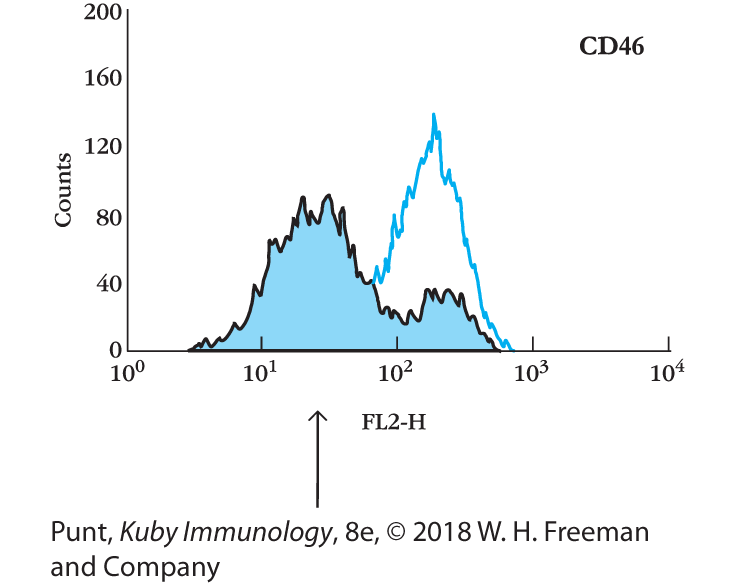
- Each of the circles shown represents cell populations with the indicated surface expression of C1q and CD46. T cells undergoing apoptosis begin to express DNA on their cell surfaces, which is bound by the first component of the classical pathway, C1q. Healthy T cells, on the other hand, express relatively high levels of the regulatory complement component CD46, which is a cofactor for factor I.
Chapter 6
- (a) False. Vκ gene segments and Cλ are located on separate chromosomes and cannot be brought together during gene rearrangement. (b) True. (c) False. Naïve B cells produce a long primary transcript that carries the variable region and the mRNA for both the μ and δ constant regions. The switch in expression from the μ to the δ heavy chain occurs by mRNA splicing, not by DNA rearrangement. Switching to all other heavy-chain classes is mediated by DNA rearrangements. (d) True. (e) False. The variable regions of the β and δ TCR genes are encoded in three segments, analogous to the V, D, and J segments of the Ig heavy-chain variable region. The Vα and Vγ regions are each encoded in two segments.

- (a) This is Figure 6-9a.
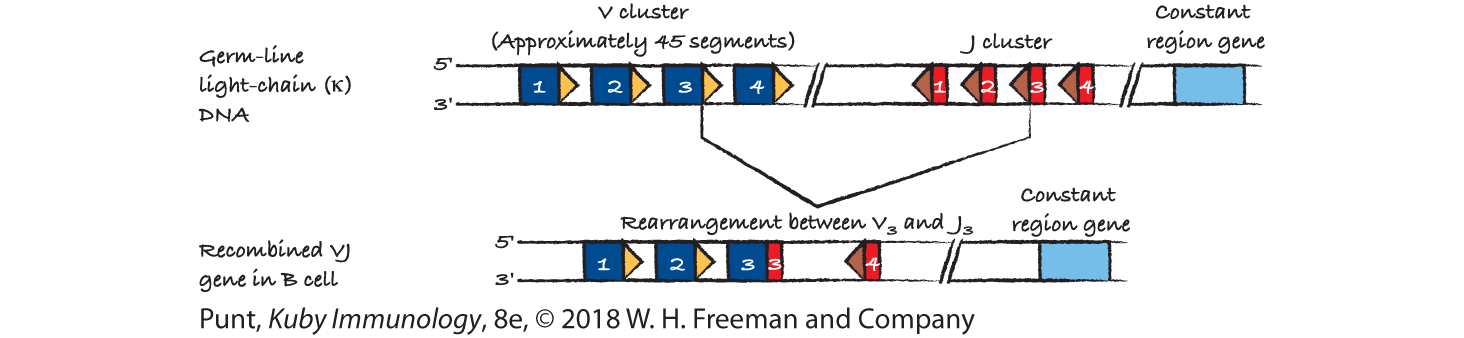
(b) This is Figure 6-9b.
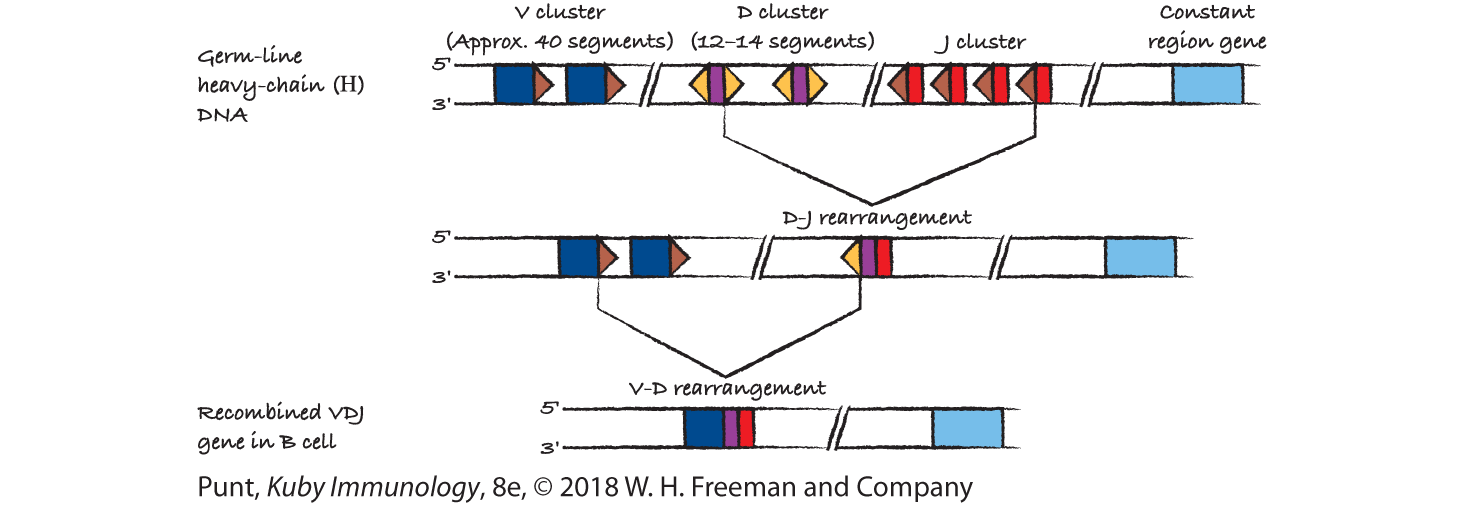
(c) Start with Figure 6-19 (the mouse β locus). Recombination may occur between any D and J segments, and then between any V segment and the DJ segment.

(d) Start with Figure 6-19, top line showing the α and δ loci. Draw a second line that shows Vα2 contiguous with Jα3. The intervening sequences lost on this rearrangement include all the δ gene sequences.

- VH and JH gene segments cannot join because both are flanked by recombination signal sequences (RSSs) containing a 23-bp (two-turn) spacer (see Figure 6-8b). According to the one-turn/two-turn joining rule, signal sequences having a two-turn spacer can join only with signal sequences having a one-turn (12-bp) spacer.
- (a) 1, 2, 3; (b) 5; (c) 2, 3, 4; (d) 5; (e) 1, 3, 4, 5.
- RAG1/2—Responsible for breaking the DNA at the junction of the V, D, or J region sequences and the relevant recombination signal sequences. It also expresses some endonuclease activity.
TdT (terminal deoxyribonucleotidyltransferase)—Randomly adds nontemplated nucleotides (N nucleotides) at V(D)J junctions in the heavy chain of Ig genes and TCR genes. Note that TdT activity is low during Ig light-chain rearrangement and absent in the fetal thymus, when waves of gδ T cells leave the thymus for the periphery. However, gδ T cells generated in the adult thymus do display pronounced N-region diversity.
Artemis—Cleaves the hairpin loop formed when a single-strand DNA break generated by RAG1/2 is converted into a double-strand break with subsequent generation of a hairpin loop between the top and bottom DNA strands of the coding sequences of TCR and BCR gene segments.
DNA PKcs—Forms a complex with Ku70/80. This protein kinase phosphorylates and actives Artemis. It recruits the ligation machinery.
DNA ligase IV—Catalyzes the formation of phosphodiester bonds between the recombined V(D)J gene segments of BCRs and TCRs.
- (a) P: Productive rearrangement of heavy-chain allele 1 must have occurred since the cell line expresses heavy chains encoded by this allele. (b) G: Allelic exclusion forbids the second heavy-chain allele from undergoing either productive or nonproductive rearrangement. (c) NP: In mice, the κ genes rearrange before the λ genes. Since the cell line expresses λ light chains, both κ alleles must have undergone nonproductive rearrangement, thus permitting λ-gene rearrangement to occur. (d) NP: Same reason as given in (c) above. (e) P: Productive rearrangement of the first λ-chain allele must have occurred since the cell line expresses λ light chains encoded by this allele. (f) G: Allelic exclusion forbids λ-chain allele 2 from undergoing either productive or nonproductive rearrangement (see Figure 6-15).
- The κ-chain DNA must have the germ-line configuration because a productive heavy-chain rearrangement must occur before the light-chain (κ) DNA can begin to rearrange.
- Random addition of N nucleotides at the D-J and V-DJ junctions contributes to the diversity within the CDR3 regions of heavy chains, but this process can result in a nonproductive rearrangement if the triplet reading frame is not preserved.
- (1) Those segments not yet ready to engage in DNA recombination are located toward the center of the nucleus, whereas those ready to engage in DNA recombination move close to the nuclear membrane. (2) Epigenetic control: the histone mark H3K4me3 placed on chromatin increases the affinity of that chromatin for the RAG1/2 complex (specifically RAG2), and associated histone acetylation opens up the chromatin for access by the recombinational machinery. (3) The Ig and TCR genes in chromatin are arranged in rosettes, with specialized proteins determining the centers of those rosettes. Recombination can occur between gene segments located in the same rosettes, but not between gene segments located in different rosettes. The three-dimensional arrangement of these rosettes is altered during the DNA recombination process to allow recombination first between D and J regions, and then later, between V and D regions.
- Whereas N-region addition occurs at the joints of Ig heavy- but not light-chain variable regions, all TCR variable region joints may include N-region nucleotides. Somatic mutation adds diversity to the BCR, following antigen stimulation, but does not contribute to TCR diversity.
- The two heavy-chain constant regions that are closest to the 5´ end of the Ig gene [and therefore closest to the recombined V(D)J regions] are μ and δ. All constant regions can be expressed in both a membrane-bound form, which has a hydrophobic sequence at its 3´ end suitable for crossing the membrane in an α-helical conformation, and a soluble form, in which the membrane-crossing region has been replaced by a short segment of hydrophilic amino acids. IgM and IgD antibodies, which can be expressed as membrane-bound or soluble forms, are generated following differential RNA splicing as shown in Figure 6-17a. The decision to express membrane-bound IgM versus membrane-bound IgD is made by differentially splicing the RNA.
In contrast, the decision to express soluble IgM versus membrane-bound IgM is made by differentially splicing the RNA as shown in Figure 6-17b.
In order to generate antibodies of any heavy-chain class other than IgM or IgD, the DNA encoding the constant regions of upstream heavy chains is deleted and the recombined V(D)J segment is reassigned to a location in close proximity to the newly expressed heavy-chain constant region. However, in the case of IgM and IgD, all the DNA remains intact, and so a cell can simultaneously express all four forms of Ig: IgM membrane-bound, IgM secreted; IgD membrane-bound and IgD secreted.
- They used the fact that the receptor is a membrane-bound protein to isolate membrane-bound polysomes and used the RNA associated with the polysomes to generate cDNA probes specific for membrane receptor genes. They used the fact that the TCR is expressed in T cells but not B cells to remove all cDNAs that were expressed in both B and T cells. They hypothesized that the gene for the TCR would be encoded in recombining segments and that the pattern of DNA fragments encoding the receptor genes would be differentially arranged in different T-cell clones.
- (a) It must be a heavy chain because light chains do not have D regions. (b) The RSS. The heptamer of the heptamer-spacer-nonamer sequence directly abuts the end of the V region. (c) (1) P nucleotides are formed by asymmetric cleavage of the hairpin at the coding joint prior to DNA ligation. The italicized GA residues on the coding strand, and the CT residues on the noncoding strand, could have been generated by that mechanism. (c) (2) We cannot know for certain that they were formed by P-nucleotide addition, as they could just as easily have been randomly inserted by TdT. (c) (3) The residues shown in boldface have no place of origin in the original sequence, and so must have been added by N-nucleotide addition. (c) (4) Yes, we can, if no corresponding nucleotides can be found in the germ-line sequence, and they could not be accounted for by asymmetric hairpin joining.
- Answer (a) is true. The heavy-chain gene segments are recombined prior to those of the light chain, enabling each recombined heavy chain to form many different antibodies with different light chains.
- On average, two out of three attempts at rearrangements at the first heavy-chain chromosomal locus will result in an unproductively rearranged heavy chain. This is because the V- and J-chain gene segments must be read in the proper phase. The same is true for rearrangement at the second heavy-chain locus. Therefore, the probability of successfully generating a functional Ig heavy chain is only 1/3 (at the first allele) + (2/3 [probability the first rearrangement is not successful] 3 1/3 [probability that the second rearrangement is successful]) × 0.55 or 55%.
- See the completed table:
V(D)J Rearrangement V-J Rearrangement D-D Joining 12/23 Rule Obeyed N-Nucleotide Addition More Than One C Region Allelic Exclusion Perfect Ig heavy chain + − − + + + + Ig light chain − + − + (−) + + TCR a − + − + + − − TCR b + − − + + + + TCR g − + − + + (in adults) + + TCR d + − + + + (in adults) − +
Analyze the Data Answer
(a) The restriction endonuclease must cut at a site within the constant region, as well as at sites upstream and downstream from it. (b) Recombination has occurred at only one of the two alleles. The germ-line bands complementary to both the constant and the variable region probes most probably derive from the other allele. (c) Given that the additional bands have remained in the same positions in the gel in both the germ-line and the myeloma DNA, it seems likely that there was a successful rearrangement at the first allele. (d) I would clone and sequence the DNA upstream from the Cκ region from both alleles and prove that one displayed a successful arrangement, although the other was still in the germ-line configuration.
Chapter 7
- (a) True. (b) True. (c) False: MHC class III molecules are soluble proteins that do not function in antigen presentation. They include several complement components, TNF, and lymphotoxin. (d) False: The offspring of heterozygous parents inherit one MHC haplotype from each parent and thus will express some molecules that differ from those of each parent; for this reason, parents and offspring are histoincompatible. In contrast, siblings have a one in four chance of being histocompatible (see Figure 7-8c). (e) True. (f) False: Most nucleated cells express MHC class I molecules, but neurons, placental cells, and sperm cells at certain stages of differentiation appear to lack class I molecules. (g) True.
- (a) Liver cells: Class I Kd, Kk, Dd, Dk, Ld, and Lk. (b) Macrophages: Class I Kd, Kk, Dd, Dk, Ld, and Lk. Class II Aαkβk, Aαdβd, Aαkβd, Aαdβk, Eαkβk, Eαdβd, Eαdβk, and Eαkβd.
- (a) SJL macrophages express the following MHC molecules: Ks, Ds, Ls, and As. Because of the deletion of the Eα locus, Es is not expressed by these cells. (b) The transfected cells would express one heterologous E molecule, Eαkβs, and one homologous E molecule, Eαkβk, in addition to the molecules listed in (a).
- See Figure 7-1, Figure 3-7, and Figure 3-10.
- (a) The polymorphic residues are clustered in short stretches primarily within the membrane-distal domains of the MHC class I and class II molecules (see Figure 7-10). These regions form the peptide-binding groove of MHC molecules, or the surfaces most in contact with antigen. (b) MHC polymorphism is thought to arise by gene conversion of short, nearly homologous DNA sequences within unexpressed pseudogenes in the MHC to functional class I or class II genes. MHC diversity in the population is likely maintained via selective pressure. If inheritance of more MHC molecules (heterogeneity) is favorable for survival, then this will lead to a bias toward diversity at the locus. Likewise, if mate selection is indeed influenced by the olfactory detection of MHC difference (see Evolution Box 7-1), then offspring with more diverse MHC alleles might be expected.
- (a) The proliferation of TH cells and IL-2 production by them are detected in assay 1, and the killing of LCMV-infected target cells by cytotoxic T lymphocytes (CTLs) is detected in assay 2. (b) Assay 1 is a functional assay for MHC class II molecules, and assay 2 is a functional assay for MHC class I molecules. (c) Class II Ak molecules are required in assay 1, and class I Dd molecules are required in assay 2. (d) You could transfect K cells with the Ak gene and determine the response of the transfected cells in assay 1. Similarly, you could transfect a separate sample of L cells with the Dd gene and determine the response of the transfected cells in assay 2. In each case, a positive response would confirm the identity of the MHC molecules required for LCMV-specific activity of the spleen cells. As a control in each case, L cells should be transfected with a different MHC class I or class II gene and assayed in the appropriate assay. (e) The immunized spleen cells express both Ak and Dd molecules. Of the listed strains, only the A.TL strain expresses both of these MHC molecules, and thus these are the only strains from which the spleen cells could have been isolated. A positive IL-2 and killing response for (BALB/c × B10.A) F1 suggests these might be a possible source as well. However, if that were the case, they should be able to kill LCMV-infected targets that express Kk as in the infected C3H and B10.A (4R) cells. Since infected target cells from these strains are not killed in assay 2, these F1 mice could not have been the original source.
- It is not possible to predict. Since the peptide-binding cleft is identical, both MHC molecules should bind the same peptide. However, the amino acid differences outside the cleft might prevent recognition of the second MHC molecule by the T-cell receptor on the TC cells.
- If RBCs expressed MHC molecules, then extensive tissue typing would be required before a blood transfusion, and only a few individuals would be acceptable donors for a given individual.
- (a) No. Although those with the A99/B276 haplotype are at significantly increased relative risk, there is no absolute correlation between these alleles and the disease. (b) Nearly all of those with the disease will have the A99/B276 haplotype, but depending on the exact gene or genes responsible, this may not be a requirement for development of the disease. If the gene responsible for the disease lies between the A and B loci, then weaker associations to A99 and B276 may be observed. If the gene is located outside of the A and B regions and is linked to the haplotype only by association in a founder, then associations with other MHC genes may occur. (c) It is not possible to know how frequently the combination will occur relative to the frequency of the two individual alleles; linkage disequilibrium is difficult to predict. However, based on the data given, it may be speculated that the linkage to a disease that is fatal in individuals who have not reached reproductive years will have a negative effect on the frequency of the founder haplotype. An educated guess would be that the A99/B276 combination would be rarer than predicted on the basis of the frequency of the A99 and B276 alleles.
- By convention, antigen-presenting cells are defined as those cells that can display antigenic peptides associated with MHC class II molecules and can deliver a costimulatory signal to CD4+ TH cells. A target cell is any cell that displays peptides associated with MHC class I molecules to CD8+ TC cells.
- (a) Self MHC restriction is the attribute of T cells that limits their response to antigen associated with self MHC molecules on the membrane of antigen-presenting cells or target cells. In general, CD4+ TH cells are MHC class II restricted, and CD8+ TC cells are MHC class I restricted, although a few exceptions to this pattern occur. (b) Antigen processing involves the intracellular degradation of protein antigens into peptides that associate with MHC class I or class II molecules. (c) Endogenous antigens are synthesized within altered self cells (e.g., virus-infected cells or tumor cells), are processed in the endogenous pathway, and are presented by MHC class I molecules to CD8+ TC cells. (d) Exogenous antigens are internalized by antigen-presenting cells, processed in the exogenous pathway, and presented by MHC class II molecules to CD4+ TH cells. (e) Anchor residues are the key locations (typically, positions 2/3 and 9) within an 8- to 10-amino-acid-long antigenic peptide that make direct contact with the antigen-binding cleft of MHC class I. The specific residues found at these locations distinguish the peptide fragments that can bind each allelic variant of class I. (f) An immunoproteasome is a variant of the classical proteasome, found in all cells, and is expressed in antigen-presenting cells and in infected target cells. The presence of this variant increases the production of antigenic fragments optimized for binding to MHC class I molecules.
- (a) EN: Class I molecules associate with antigenic peptides and display them on the surface of target cells to CD8+ TC cells. (b) EX: Class II molecules associate with exogenous antigenic peptides and display them on the surface of APCs to CD4+ TH cells. (c) EX: The invariant chain interacts with the peptide-binding cleft of MHC class II molecules in the rough endoplasmic reticulum (RER), thereby preventing binding of peptides from endogenous sources. It also assists in folding of the class II α and β chains and in movement of class II molecules from the RER to endocytic compartments. (d) EX: Lysosomal hydrolases degrade exogenous antigens into peptides; these enzymes also degrade the invariant chain associated with class II molecules, so that the peptides and MHC molecules can associate. (e) EN: TAP, a transmembrane protein located in the RER membrane, mediates transport of antigenic peptides produced in the cytosolic pathway into the RER lumen, where they can associate with MHC class I molecules. (f) B: In the endogenous pathway, vesicles containing peptide–MHC class I complexes move from the RER to the Golgi complex and then on to the cell surface. In the exogenous pathway, vesicles containing the invariant chain associated with MHC class II molecules move from the RER to the Golgi and on to endocytic compartments. (g) EN: Proteasomes are large protein complexes with peptidase activity that degrade intracellular proteins within the cytosol. When associated with LMP2 and LMP7, which are encoded in the MHC region, and LMP10, which is not MHC encoded, proteasomes preferentially generate peptides that associate with MHC class I molecules. (h) B: Antigen-presenting cells internalize exogenous (external) antigens by phagocytosis or endocytosis. (i) EN: Calnexin is a protein within the RER membrane that acts as a molecular chaperone, assisting in the folding and association of newly formed class I α chains and β2-microglobulin into heterodimers. (j) EX: After degradation of the invariant chain associated with MHC class II molecules, a small fragment called CLIP remains bound to the peptide-binding cleft, presumably preventing premature peptide loading of the MHC molecule. Eventually, CLIP is displaced by an antigenic peptide. (k) EN: Tapasin (TAP-associated protein) brings the transporter TAP into proximity with the MHC class I molecule and allows the MHC molecule to acquire an antigenic peptide (see Figure 7-15).
- (a) Chloroquine inhibits the exogenous processing pathway, so that the APCs cannot display peptides derived from native lysozyme. The synthetic lysozyme peptide will exchange with other peptides associated with class II molecules on the APC membrane, so that it will be displayed to the TH cells and induce their activation. (b) Delay of chloroquine addition provides time for native lysozyme to be degraded in the endocytic pathway.
- (a) Dendritic cells: constitutively express both MHC class II molecules and costimulatory signals. B cells: constitutively express class II molecules but must be activated before expressing the CD80/86 costimulatory signal. Macrophages: must be activated before expressing either class II molecules or the CD80/86 costimulatory signal. (b) See Table 7-4. Many nonprofessional APCs function only during sustained inflammatory responses.
- (a) R; (b) R; (c) NR; (d) R; (e) NR; (f) R.
- (a) Intracellular bacteria, such as members of the Mycobacterium family, are a major source of nonpeptide antigens; the antigens observed in combination with CD1 are lipid and glycolipid components of the bacterial cell wall. (b) Members of the CD1 family associate with β2-microglobulin and have structural similarity to MHC class I molecules. They are not true MHC molecules because they are not encoded within the MHC, but rather on a different chromosome. (c) The pathway for antigen processing taken by the CD1 molecules differs from that taken by MHC class I molecules. A major difference is that CD1 antigen processing is not inhibited in cells that are deficient in TAP, whereas MHC class I molecules cannot present antigen in TAP-deficient cells.
- (b) The TAP1-TAP2 complex is located in the endoplasmic reticulum.
- The offspring must have inherited HLA-A3, HLA-B59, and HLA-C8 from the mother. Potential father 1 cannot be the biological father because although he shares HLA determinants with the offspring, the determinants are the same genotype inherited from the mother. Potential father 2 could be the biological father because he expressed the HLA genes expressed by the offspring that are not inherited from the mother (HLA-A43, HLA-B54, HLA-C5). Potential father 3 cannot be the biological father because although he shares HLA determinants with the offspring, the determinants are the same genotype inherited from the mother.
- Considering HLA-A, HLA-B, and HLA-C only, a maximum of six different class I molecules are expressed in individuals who inherit unique maternal and paternal alleles at each locus. In the case of class II, considering only HLA-DP, -DQ, and -DR molecules where any α chain and β chain of each gene can pair to produce new maternal/paternal combinations, a maximum of 12 different class II molecules can be expressed (4 DP, 4 DQ, and 4 DR). Since humans can inherit as many as three functional DRβ genes, each of which is polymorphic, in practice fully heterozygous individuals have the ability to express more than four HLA-DR proteins.
- Polygeny is defined as the presence of multiple genes in the genome with the same or similar function. In humans, MHC class I A, B, and C or class II DP, DQ, and DR are both examples of this (see Figure 7-6). Polymorphism is defined as the presence of multiple alleles for a given gene locus within the population. HLA-A1 versus HLA-A2 (e.g., see Table 7-3) are examples of polymorphic alleles at the class I locus. Codominant expression is defined as the ability of an individual to simultaneously express both the maternal and the paternal alleles of a gene in the same cell. This process is what allows a heterozygous individual to express, for instance, both HLA-Cw2 and HLA-Cw4 alleles (see Figure 7-9). Polygeny ensures that even MHC homozygous individuals express a minimum of three different class I and class II proteins, each with a slightly different antigen-binding profile, expanding their repertoire of antigens that can be presented. MHC polymorphism and codominant expression in outbred populations help facilitate the inheritance and expression of different alleles at each locus, further increasing the number of different antigens that one individual can present. Codominant expression at the class II loci carries an added bonus: since these proteins are generated from two separate genes/chains, new combinations of α and β chains can arise, further enhancing the diversity of class II protein isoforms, or the number of unique MHC class II antigen-binding clefts.
- The invariant chain is involved in MHC class II folding and peptide binding. Cells without this protein primarily retain misfolded class II proteins in the RER and are therefore unable to express MHC class II molecules on the cell surface. Since APCs are the primary cell types that express class II, cells with this mutant phenotype would be incapable of presenting exogenously processed antigens to naïve CD4+ T cells.
- Cross-presentation is the process by which some APCs can divert antigens collected from extracellular sources (exogenous pathway) to processing and presentation via MHC class I proteins (typically the realm of the endogenous pathway). This process is important for activation of naïve CD8+ T cells to generate CTLs capable of detecting and lysing virally infected target cells. Dendritic cells, or a subset of this cell type, are thought to be the major players in this process, although “licensing” by antigen-specific CD4+ TH cells may first be required in order for DCs to engage in cross-presentation.
Clinical Focus Answer
Human TAP deficiency results in a lack of class I molecules on the cell surface or a type I bare-lymphocyte syndrome. This leads to partial immunodeficiency in that antigen presentation is compromised, but there are NK cells and γδ T cells to limit viral infection. Autoimmunity results from the lack of class I molecules that give negative signals through the killer-cell inhibitory receptor (KIR) molecules; interactions between KIR and class I molecules prevent the NK cells from lysing target cells. In their absence, self cells are targets of autoimmune attack on skin cells, resulting in the lesions seen in TAP-deficient patients. The use of gene therapy to cure those affected with TAP deficiency is complicated by the fact that class I genes are expressed in nearly all nucleated cells. Because the class I product is cell bound, each deficient cell must be repaired to offset the effects of this problem. Therefore, although the replacement of the defective gene may be theoretically possible, ascertaining which cells can be repaired by transfection of the functional gene and reinfused into the host remains an obstacle. In the case of Tasmanian devils and facial cancer, it was found that the cancer cells being transferred between devils lack surface MHC class I expression, allowing them to avoid destruction by CD8+ T cells. Transcripts for several key proteins involved in class I synthesis and assembly (TAP1, TAP2, and b2-microglobulin) were all found to be down-regulated in these cancer cells thanks to high levels of histone acetylation at these MHC class I–associated loci, enough to silence gene expression. Thus these cancer cells appear to be accepted much like an isograft, passed from one animal to another during biting incidents. No one is sure why NK cells in Tasmanian devils do not attack these cancer cells.
Analyze the Data Answer
(a) Yes. Comparing the relative amounts of Ld and Lq molecules without peptides, there are about half as many open Lq molecules as Ld molecules. The data suggest that Lq molecules form less stable peptide complexes than Ld molecules. (b) Part (a) in the figure shows that 4% of the Ld molecules don’t bind MCMV peptide compared with 11% of the Ld after a W-to-R mutation. Thus, there appears to be a small decrease in peptide binding to Ld. It is interesting to note that nonspecific peptide binding increases severalfold after mutagenesis, based on the low amount of open-form Ld W97R (mutated Ld) versus native Ld. (c) Part (b) in the figure shows that 71% of the Ld molecules don’t bind tum− P91A14–22 peptide after a W-to-R mutation, compared with 2% for native Ld molecules. Thus, there is very poor binding of tum− P91A14–22 peptide after a W-to-R mutation. (d) You would inject a mouse that expressed Ld because only 2% of the Ld molecules were open forms after the addition of tum− P91A14–22 peptide compared with 77% free forms when Lq were pulsed with peptide. Therefore, Ld would present peptide better and probably activate T cells better than Lq. (e) Conserved anchor residues at the ends of the peptide bind to the MHC, allowing variability at other residues to influence which T-cell receptor engages the MHC class I–antigen complex.
Chapter 8
- a. Knockout mice lacking MHC class II molecules fail to produce mature CD4+ thymocytes, or those clacking MHC class I molecules fail to produce mature CD8+ thymocytes, because at some level lineage commitment requires engagement between the MHC and the appropriate CD4/8 receptor.
b. β-Selection initiates maturation to the DN4 stage, proliferation, allelic exclusion, maturation to the DP stage, and TCR α-chain locus rearrangement.
c. Negative selection of tissue-specific antigens occurs only in the medulla of the thymus, by medullary thymic epithelial cells (mTECs) and some DCs that pick up antigens produced by mTECs.
d. Most thymocytes (>90%) die of neglect in the thymus because they either did not produce viable TCR, or because they do not bind to self MHC.
e. Thymocyte precursors express neither CD4 nor CD8 and enter the thymus from the bone marrow at the corticomedullary junction.
f. Thymocytes that bind peptide-MHC complexes with high affinity are negatively selected.
g. Double-negative (DN) thymocytes progress through several stages distinguished by expression primarily of CD44 and CD25.
h. Some thymocytes with autoreactive T-cell receptors mature to become TREG cells.
i. Regulatory T cells help maintain peripheral tolerance.
j. Commitment to the CD4+ T-cell lineage is regulated by Th-Pok. Runx3 regulates commitment to the CD8+ lineage.
- Precursors of thymocytes enter the thymus at the corticomedullary junction. Interactions with Notch ligands are required to commit them to the T-cell lineage. If positively selected, double-positive (DP) thymocytes travel from the thymic cortex to the medulla. Upregulation of S1P receptor allows them to leave the thymus and enter circulation.
- Many if not most γδ T cells have receptors that have a more restricted (invariant) specificity and can recognize a variety of antigens, including lipids (they do not always require their antigens to be peptide presented by MHC). In this respect their response to antigen is more akin to that of innate immune cells, which use pattern recognition receptors to respond rapidly to antigen. γδ T cells also develop differently—many are generated in waves during fetal development and populate mucosal tissues. The decision to become a γδ T cell occurs early, during the DN3 stage, and most γδ T cells do not go through the conventional positive and negative selection process in the thymus.
- See the following figure:

- (a) There would be no CD4+ SP, but all other stages would be present. The absence of MHC class II would prevent positive selection and lineage commitment of CD4+ T cells. (b) All stages would be present, but some of the mature cells would be reactive to tissue-specific antigens. (This would be revealed only by functional experiments.) AIRE regulates the expression of self tissue–specific antigens by medullary epithelial cells. (c) All DN and DP cells would be present (β-selection would proceed unhindered). However, none of the DP cells would express normal TCR-αβ dimers and could not be positively selected. (For the advanced, TCR-γδ cell development would proceed normally—many of these are DN in phenotype, but a few are CD4+ and CD8+ SP.)
- The first are CD3− TCR β-chain− thymocytes and could simply be immature DN thymocytes. The second group are CD3+ TCR β-chain− and could be TCR-γδ T cells!
- (a) Flow cytometry. (b) Higher. (c) Lower. (Positive selection would occur in the H2k but not H2d background [MHC class II].) Most cells in the TCR transgenic would have the receptor specific for this MHC haplotype, and therefore would get more than the normal number of CD4+ SP cells when positive selection occurs.) (d) No mature single-positive cells; may have a reduced number of DP cells (negative selection is going to occur).
Must speculate with this question—no absolutely clear answer. The cortical epithelium may not be able to mediate clonal deletion because it doesn’t express the right costimulatory molecules. Investigators who have done experiments like this find evidence for negative selection of a sort, however. SP T cells develop, but they appear not to be easily activated.
- (a) Thymocytes in experiment A developed in a thymus whose cortical epithelial cells expressed H2d MHC molecules and therefore became restricted to that MHC (via positive selection). They are not restricted to H2d, and thus ignore targets that express this MHC. (b) Same reasoning as above.
- (a) The immature thymocytes express both CD4 and CD8, whereas the mature CD8+ thymocytes do not express CD4. To distinguish these cells, the thymocytes are double-stained with fluorochrome-labeled anti-CD4 and anti-CD8 and analyzed by FACS. (b) See the following table.
H-Y TCR Transgenic Immature Thymocytes Mature CD8+ Thymocytes H2k female + + H2k male + − H2d female + − H2d male + − (c) Because the gene encoding the H-Y antigen is on the Y chromosome, this antigen is not present in females. Thymocytes bearing the transgenic T-cell receptor, which is H2k restricted, would undergo positive selection in both male and female H2k transgenics. However, subsequent negative selection would eliminate thymocytes bearing the transgenic receptor, which is specific for H-Y antigen, in the male H2k transgenics. (d) Because the H2d transgenics would not express the appropriate MHC molecules, T cells bearing the transgenic T-cell receptor would not undergo positive selection.
- (a) Class I K, D, and L molecules and class II IA molecules. (b) Class I molecules only. (c) The normal H2b mice should have both CD4+ and CD8+ T cells because both MHC class I and class II molecules would be present on thymic stromal cells during positive selection. H2b mice with knockout of the IA gene would express no class II molecules; thus, these mice would have only CD8+ cells.
- (a) Because the pre-T-cell receptor, which does not bind antigen, is associated with CD3, cells expressing the pre-TCR as well as the antigen-binding T-cell receptor would stain with anti-CD3. It is impossible to determine from this result how many of the CD3-staining cells are expressing complete T-cell receptors. The remaining cells are even more immature thymocytes that do not express CD3. (b) No. Because some of the CD3-staining cells express the pre-TCR or TCR-αβ instead of the complete TCR-γδ, you cannot calculate the number of TC cells by simple subtraction. To determine the number of TC cells, you need fluorescent anti-CD8 antibody, which will stain only the CD8+ TC cells.
Clinical Focus Answers
- AIRE (regulates tissue-specific expression of antigens by mTECs), FoxP3 (involved in development of TREG cells), any of the TCR signaling molecules (which regulate TCR signal strength and therefore the outcome of thymic selection), MHC (which presents self peptides), or anything that prevents T cells from getting to the medulla (e.g., CCR7).
- (a) The authors appear to be saying that the MHC variant, which would be expressed by medullary epithelial cells and dendritic cells in the thymus, may not be able to present (bind to) certain brain-specific self peptides (or does so inefficiently). Therefore, some autoreactive CD4+ T cells in the thymus may not be deleted. (Note that HLA-DR is an MHC class II molecule.) (b) There is no one right question to pose. Here are two possibilities. (1) If this were the case, wouldn’t this also mean that peripheral dendritic cells would not be able to present the peptide, and therefore wouldn’t activate the autoreactive T-cell escapees? (2) If you let an autoreactive helper T-cell escape, don’t you still need an autoreactive cytotoxic T cell to escape also? (This question depends on your knowing that autoimmune diseases like multiple sclerosis are caused in part by CD8+ T-cell–mediated damage.) (c) Given that immunologists are still pondering the issue, this is a challenging question. However, here are a couple of possibilities. (1) One accepts the possibility that this is a problem with negative selection. However, to understand how this leads to autoimmune disease, one can propose, for instance, that there is a difference between antigen presentation in the thymus and antigen presentation in the periphery. Presentation of brain self peptides by this MHC variant may be inefficient and allow autoreactive CD4+ T cells to escape. But in the periphery this inefficiency can be overcome by enhancements in costimulatory molecule expression, levels of MHC expression, and so on among antigen-presenting cells that have been stimulated by pathogen or cell damage. (2) In addition (or alternatively), one can propose that this MHC variant compromises the development of regulatory T cells, not just the deletion of autoreactive CD4+ T cells. Specifically, if the MHC class II variant is unable to present the self peptides, then it will neither mediate deletion of autoreactive CD4+ T cells nor select for suppressive, autoreactive regulatory T cells. Note that we need to assume, in all cases, the existence of autoreactive CD8+ T-cell escapees. This is not a radical assumption. As you now know, negative selection in the thymus is never perfect, and our ability to maintain tolerance depends to a significant extent on peripheral mechanisms.
Chapter 9
- Fetal liver cells; B-1 B-cell progenitors are highly enriched in the fetal liver and are the first B cells to populate the periphery. The B-1 B cells would be harvested from the peritoneal and pleural cavities, as well as the spleen.
- T2 cells have intermediate levels of IgD, whereas T1 cells have no to low amounts of IgD. T2 cells bear both CD21 and CD23, whereas neither antigen is expressed on T1 cells. T2 cells have higher levels of the receptor for the B-cell survival factor BAFF than do T1 cells. Interaction of antigen with T1 cells results in apoptosis; interaction of antigen with T2 cells sends survival and maturation signals.
- Cell division at this stage allows the repertoire to maximize its use of B cells in which a heavy chain has been productively rearranged. Each daughter cell can then rearrange a different set of light-chain gene segments, giving rise to multiple B-cell clones bearing the same heavy chain, but different light-chain genes.
- (1) They can undergo apoptosis, in a process called negative selection. This occurs for B cells in the bone marrow and for T cells in the thymus. (2) They can become anergic—refractory to further stimulation—and eventually die. This occurs for both T and B cells. (3) Their receptors can undergo receptor editing. This occurs quite frequently in B cells. In T cells, the extent to which receptor editing occurs varies according to the nature of the animal (the nature of the transgene used to study editing), and therefore whether it is a meaningful mechanism in T-cell receptor development and selection is so far unclear.
- To test the hypothesis: first, knock out the ability of the animal to express that transcription factor, and then analyze the bone marrow for the occurrence of B-cell progenitors at each stage of development, using flow cytometry. One would not expect to see any progenitors after the pre-pro-B-cell stage if the transcription factor is expressed then and is necessary for further B-cell development. Second, make a fusion protein in which the promoter of the transcription factor is fused to a fluorescent protein, such as green or yellow fluorescent protein. Correlate the expression of the fluorescent proteins with the cell-surface markers. One would expect to see the fluorescent protein show up first in cells bearing markers characteristic of the pre-pro-B-cell stage. To test the status of heavy- and light-chain rearrangement: Rearrangement has begun at this stage, with D-to-JH rearrangement occurring on the heavy chain. Test it by PCR, with primers complementary to sequences upstream of the D regions and downstream of the J regions, followed by sequencing, if necessary.
- Create an animal in which the CXCL12 promoter is fused to a marker fluorescent protein, such as GFP. Make slides of bone marrow, taking care that the conditions did not break up cell attachments, and label the cells with markers characteristic of the target stage of development. Look for cell pairings between the CXCL12-labeled cells and progenitor cells labeled with the markers characteristic of the target stage of development.
- Rearrangement starts first at the heavy-chain locus, beginning with D to JH and proceeding with VH to D. If the rearrangements at the first allele are not productive, then rearrangement starts again on the second heavy-chain locus and proceeds in the same order. Successful rearrangement at a heavy-chain locus results in the expression of a heavy chain at the surface of the B-cell progenitor in combination with the surrogate light chain, to form the pre-B-cell receptor. This occurs at the beginning of the large pre-B-cell stage. Expression of the heavy chain at the cell surface signals the cessation of further heavy-chain rearrangement.
At the light-chain locus in mice, rearrangement begins at one of the κ loci, and again, if it is not productive, it starts again on the other κ locus. If this is also not productive, the process repeats at the λ loci. In humans, the process is similar, but rearrangement may start at either the κ or the λ loci. Light-chain rearrangement is completed by the end of the small pre-B-cell stage, and the expression of the complete Ig receptor on the surface of the cell signals the beginning of the immature B-cell stage.
In T cells, rearrangement begins on successive β-chain loci. In possession of V, D, and J segments, the β-chain locus is analogous to the heavy-chain Ig locus. Successful rearrangement of the β chain results in expression of a pre-TCR on the cell surface, just as for the pre-BCR on B cells, coupled with the cessation of further β-chain gene segment recombination. Rearrangement at the α-chain locus follows. One major difference between the processes of rearrangement in T and B cells is that allelic exclusion at the T-cell α-chain locus is incomplete.
- The membrane HEL can cross-link the HEL-specific BCR of immature B cells in the bone marrow, giving a strong negative selection signal. This should induce light-chain receptor editing to change the specificity of the BCR, and if not successful, the HEL-specific B cells probably would undergo apoptosis. No HEL-specific B cells should be found in the periphery (or were in the studies done), and hence these mice should not generate HEL-specific antibodies after immunization. (See the reference cited in Table 9-4.)
Analyze the Data Answer
- (a) In the spleen, the Dicer knockout animal shows no mature B cells, indicated by the loss of the B220hiCD19+ population. In the top bone marrow population, in the absence of Dicer, there are no sIgM-bearing B220hi cells. The second bone marrow panel shows retention of the progenitor cell marker, c-Kit, in the Dicer knockout. The third panel shows that in the absence of Dicer there is a loss of CD25, a marker characteristic of the point in development at which the pre-BCR is expressed on the cell surface. This suggests that the cells cannot get past this checkpoint.
(b) Since no IgM is expressed on the cell surface of the Dicer knockout animals, miRNAs must be necessary for progression to the pre-B-cell stage, at which IgM is first expressed on the cell surface. The presence of c-Kit and of low amounts of CD25 suggests that miRNAs may be acting at the pre-BCR checkpoint.
- (a) No. The fraction of cells labeled with annexin A5 (Annexin V in the figure) and therefore in the pre-apoptotic state is identical in the control and Dicer knockout populations. (b) Yes. The fraction of cells labeled with annexin A5 increases from 9.2% in the control to 65% in the Dicer knockout. (c) Pulling together the data from the preceding two questions, I would hypothesize that miRNAs aid in controlling expression of the pre-BCR on the cell surface, thereby allowing the cells to pass through the first checkpoint in development. Cells that cannot express pre-BCR die by apoptosis, and that is the process described in the second set of slides.
Chapter 10
- (a) Anergy: Signal 1 (if TCR is engaged) without costimulatory signal 2 because CTLA-4 Ig will block the ability of CD28 to bind CD80/86. (b) No anergy: Signal 1 and signal 2 are both generated. (c) Anergy: Signal 1 without signal 2. (d) No anergy, but no activation either: Neither signal 1 nor signal 2 is generated.
- (a) Very likely: Any activated professional APC, like a dendritic cell, up-regulates MHC molecules and costimulatory ligands, making them ideal activators of T cells. (b) Very unlikely: Activated dendritic cells travel to the draining lymph nodes (or spleen) and encounter naïve T cells there, not in peripheral tissues. Naïve T cells travel among secondary lymphoid organs, not peripheral tissues. However, effector T cells, and some memory T cells, do travel to peripheral tissues and can be activated by dendritic cells there. (c) Very likely: TCR stimulation rapidly induces Ca2+ mobilization. (d) Very unlikely: The virus induced dendritic cells to make IL-12, one of the central polarizing cytokines for the TH1 lineage. (e) Very unlikely: Central memory cells were certainly generated, too. (f) False: This response is likely to be a type 1 response.
- A mouse without GATA-3, the master regulator for TH2 lineage commitment, will be unable to generate TH2 cells, which are instrumental in mounting the immune response to worm infections. TH2 cells help B cells to produce IgE, which has potent antiparasite activity.
- You will need to supply signal 1 (anti-TCR), signal 2 (anti-CD28), and signal 3 (IL-12). CTLA-4 Ig and anti-CD80 antibody both bind to the ligands for the costimulatory receptors and would not engage your T cells.
- (a) Dendritic cells are best at activating naïve T cells—they express a high density of costimulatory ligands and MHC molecules. (b) ICOS is a positive costimulatory receptor (it is expressed on some effector T cells, including TFH cells). (c) Most cells do not express costimulatory ligands. Professional APCs (and thymic epithelial cells) are among the only cells that do. (d) ICOS and CTLA-4 also bind B7 family members (CD80 and CD86). PD1 also binds a B7-like molecule, PD-L1. (e) Signal 3 is provided by cytokines, which include the polarizing cytokines that induce helper T-cell lineage differentiation. (f) It is a disease caused by T-cell response to superantigens (bacterial and/or viral), not autoantigens. (g) They mimic some TCR–MHC class II interactions. (h) They do not have any receptor for MHC class I and do not interact directly with CD8+ T cells via their TCRs, which bind to MHC class II. (i) Naïve T cells do not produce any effector cytokines. (j) They are master transcriptional regulators of T helper cell lineage differentiation. (k) APCs can make some polarizing cytokines, but many of these cytokines originate from other cells, including other T cells, B cells, mast cells, and NK cells. (l) Bcl-6 is a master transcriptional regulator of TFH lineage differentiation. (m) TFH and TH2 cells are classically the major sources of B-cell help, although all helper subsets can interact with B cells and influence Ig class switching. (n) They inhibit T-cell activation. (o) Effector cytokines have many different cellular targets, including B cells, endothelial cells, stromal cells in tissues, innate immune cells, and so on, as well as other T cells. (p) Central memory cells tend to reside in secondary lymphoid organs. (q) CCR7 attracts cells to secondary lymphoid tissue, and effector memory cells tend to rove the periphery. They typically down-regulate CCR7.
- (a) True. It stimulates production of both FoxP3 and RORγt. (b) False. IL-6 in combination with TGF-β polarizes cells to the TH17 lineage, an event that requires RORγt. IL-6 acts in part by inhibiting expression of FoxP3.
- Tyrosine kinases initiate the TCR signaling cascade. Two of the first enzymes activated by TCR engagement are tyrosine kinases: Lck and ZAP-70. These phosphorylate several molecules, activating new kinases and providing sites for interaction with other signaling proteins.
- Lck, ZAP-70, LAT, Ca2+, NFAT
- TH22 differentiation should be regulated by a distinct transcriptional protein (master regulator), which is induced by distinct polarizing cytokines and should result in production of a unique panel of effector cytokines.
- There are many different “right” answers. Benefits (in theory): TSCM can provide protection to the individual indefinitely because they self-renew. They can also differentiate into several different effector subsets (they are not restricted to one type). Risks: Stem cells are often more vulnerable to transformation (they may become cancerous more easily); they may also differentiate into effector cells that might not be as useful.
Clinical and Experimental Focus Answer
The data show that LIF inhibits TH17 polarization (in its presence, the frequency of IL-17+ cells is reduced by 50% after cells are exposed to TH17 polarization conditions). TH1 differentiation appears unaffected (the same frequency of IFN-γ+ cells are present after exposure to TH1 polarization conditions). This suggests that LIF has a specific effect on the pathways that induce TH17 differentiation or on those responsible for production of IL-17. It could interfere with any of the steps involved including (from outside to inside) (1) signaling induced by TGF-β or by IL-6 polarizing cytokines, (2) RORγt expression itself, or (3) IL-17 expression itself. A reduction in TH17 cells could result in less inflammation and the amelioration in disease that is seen in this model.
(It turns out that LIF acts in opposition to IL-6 and blocks its downstream signaler, STAT3. This abrogates the inhibitory effect that IL-6 has on FoxP3 expression, shifting the balance to TREG rather than TH17 lineage commitment. So, disease amelioration is not just a consequence of fewer activated T cells, but a result of the increase in cells that quell T-cell responses.)
Chapter 11
- TI-1 antigens are mitogenic and induce activation through both the BCR and innate immune receptors. TI-2 antigens bind tightly to the complement components C3d and C3dg, and so are bound by both the BCR and the complement receptor CD21 (CR2).
- See the following figure:
(a and b) B-2 (follicular) B cells bear relatively high levels of IgM but do not express CD5. The majority of B-1 B cells, known as the B-1a fraction, does express CD5. However, there is a minority fraction of B-1 B cells, the B-1b fraction, that does not express CD5. (c and d) B-2 B cells express normal levels of CD21, the complement receptor, whereas marginal zone (MZ) B cells express particularly high levels of CD21.
- No to both. Both class switch recombination and somatic hypermutation require the ability of T cells and B cells to interact with each other through the binding of B-cell CD40 molecules by the CD40L molecule on T cells.
- Since activation-induced cytidine deaminase (AID) is required for both class switch recombination and for somatic hypermutation, I would expect my knockout mouse to be unable to express any classes of antibody other than IgM. Furthermore, I would expect the average affinity of the antibodies produced by the knockout mouse to be unchanged between primary and secondary stimulation, since, in the absence of AID, the antibodies’ genes will not be subject to somatic hypermutation.
- See the following figure:

During SHM, the deamination of cytidine on one strand of the DNA encoding antibody variable regions leads to the formation of a mismatched G-U pair. The mismatch can then be recognized by a number of DNA repair mechanisms in the cell and resolved in one of several different ways. The simplest mechanism is the interpretation of the deoxyuridine as a deoxythymidine by the DNA replication apparatus. In this case, one of the daughter cells would have an A-T pair instead of the original G-C pair found in the parent cell. Alternatively, the mismatched uridine could be excised by a uracil-DNA glycosylase enzyme. Error-prone polymerases would then fill the gap as part of the cell’s short-patch base excision repair mechanism. Third, mismatch repair mechanisms could be induced that result in the excision of a longer stretch of DNA surrounding the mismatch. The excised strand could then be repaired by error-prone DNA polymerases, leading to a series of mutations in the region of the original mismatch.
In the case of class switch recombination (CSR), AID deaminates several cytidine residues in the switch (S) regions upstream of the two heavy-chain constant regions between which the class switch will occur (the donor and acceptor S regions). The resulting uridine residues are excised by uridine glycosylases, and the abasic sites are then nicked by endonucleases that create single-strand breaks at the abasic sites. These single-strand breaks are converted to double-strand breaks suitable for end joining by mismatch repair mechanisms. A constellation of enzymes then faithfully reconnects the two S regions, with the excision of the intervening DNA.
- In order to survive, B cells need to receive signals from T cells. Since there are many more antigen-specific B cells than T cells within the germinal centers, B cells must compete with one another for T-cell binding. Since T cells are specific for peptide antigen displayed in the groove of MHC class II molecules, B cells that have internalized and displayed more antigen will have a selective advantage in attracting T-cell attention. B cells with higher-affinity receptors will bind, internalize, and display more antigen than B cells with lower-affinity receptors, and therefore compete successfully for T-cell help and survival signals. B cells with higher-affinity receptors have even been demonstrated to strip antigen from lower-affinity B cells.
- The presence of circulating immune complexes serves as an indicator that the host organism has made a high concentration of antigen-specific antibodies and has succeeded in neutralizing the antigen. Therefore, no more antibody production is needed, and the host should not expend further energy in generating antibodies of this specificity. IgG-containing immune complexes are recognized by the Fc receptor FcγRIIb (CD32), and coligation of the immune complex by FcγRIIb and by the BCR results in phosphorylation of the ITIM domain on the cytoplasmic tail of FcγRIIb. Docking of the SHP phosphatase at this receptor molecule allows it to dephosphorylate PIP3 to PIP2. This interferes with transmission of antigen signals at the B-cell receptor, resulting in the down-regulation of B-cell activation.
- B-10 B cells have recently been shown to secrete the immunosuppressive cytokine IL-10 on antigen stimulation.
- (a) Small, soluble antigens can be directly acquired from the lymphatic circulation by follicular B cells, without the intervention of any other cells. These antigens enter the lymph node via the afferent lymph and pass into the subcapsular sinus (SCS) region. Some small antigens may diffuse between the SCS macrophages that line the sinus to reach the B cells in the follicles. (b) Other small antigens leave the sinus through a conduit network. Follicle B cells can access antigen through gaps in the layer of cells that form the walls of the conduits. (c) Larger antigens are bound by complement receptors on the surfaces of SCS macrophages. Antigen-specific B cells within the follicles can acquire the antigens directly from the macrophages and become activated.
Analyze the Data Answer
- The two left-hand plots show considerable numbers of cells that are high in B220 but low in CD138. This suggests that these mice have considerable numbers of B cells that have not formed plasma cells during the time allowed by the experiments. The plot on the right also shows some B220high cells, but not as many as in the other two plots. Looking at the CD138high populations, the fractions of cells that are CD138high in the plots derived from the IgM+ and IgG+ naïve populations are very similar (13.2% and 10.7%, respectively) whereas the fraction of CD138high cells that is derived from the IgG1+ memory B-cell populations is much bigger (66%). I also note that the CD138high population in the IgG1+ memory B-cell fraction appears to be lower in B220 expression than the CD138high cells in the other two populations.
- It more closely resembles that of the mouse that received the naïve, IgM-bearing cells.
- I would conclude that the cytoplasmic region of the IgG receptor alone is insufficient to confer memory status on a B cell.
- I would want to know what the cell populations looked like prior to antigen stimulation.
- There are many “right” answers here. For example, this experiment appears to confirm that the extra piece of the IgG receptor does not confer memory status on a cell, but I would like to engineer a B cell that carries a constant region heavy-chain γ1 gene that does not contain the extra segment and study its memory response.
Chapter 12
- FcγRIII and CD23 are both Fc receptors (see Table 12-2). Neutralization and complement fixation are antibody functions that do not rely on Fc receptors (although FcR can help mediate the clearance of neutralized antibody-pathogen complexes). Opsonization and antibody-dependent cell-mediated cytotoxicity (ADCC) are mediated by cells that express activating FcRs, including FcγRIII. CD23, however, is an inhibitory FcR that regulates (inhibits) the activity of other activating FcRs. So, in short, antibodies to FcγRIII would block opsonization and ADCC, but not neutralization and complement fixation. Antibodies to CD23 would not inhibit any process. (Because they block inhibitory signals they could theoretically enhance opsonization and ADCC; it is not clear, however, that this would occur in all contexts.)
- (a) False: Some FcγRs, such as FcγRIIB, are inhibitory receptors. Others are expressed on cells that are not phagocytes and can function in transport across tissues (e.g., FcγRn transporting IgG across the placenta). (b) False: IgE mediates degranulation and activation of mast cells, basophils, and eosinophils. (c) True. (d) False: IgM is a polymeric immunoglobulin that can also be transported across epithelial layers. (e) True. (f) True. (g) False: There are two pathways by which cytotoxic T cells kill target cells. One pathway is perforin- and granzyme-dependent, and the other uses Fas ligand expressed by the CTL to induce death of Fas-expressing cells. (h) False: Dendritic cells can be licensed by TLR signals instead of by TH cells. T-cell help is required for optimal proliferation and memory-cell generation. (i) True. (j) True.
- The monoclonal antibody to LFA-1 should block formation of the CTL–target cell conjugate. This should inhibit killing of the target cell.
- (a) If anti-Zobola antibodies contribute to protection against Zobola virus, then transferring antibodies to mice before infecting the mice with the virus should provide protection. As a control, transfer antibodies from a nonimmunized mouse.
(b) Isolate CD8+ T cells from previously immunized and nonimmunized mice, using fluorescent anti-CD8 antibodies and a fluorescence-activated cell sorter, and transfer them into mice prior to infection with Zobola virus. If immunization had stimulated the production of CTLs (or memory CTLs) specific for Zobola, mice receiving CD8+ cells from the immunized mice, but not from the nonimmunized mice, would be protected.
(c) Isolate NK cells from previously immunized and nonimmunized mice, using antibodies to the NK1.1 marker (or other markers) found on mouse NK cells and the fluorescence-activated cell sorter, and transfer them into mice prior to infection with Zobola virus. If immunization had stimulated increases in NK cells or NK memory cells effective against Zobola, then mice receiving NK cells from the immunized mice, but not from the nonimmunized mice, would be protected.
- (a) All; (b) all; (c) CTLs; (d) CTLs; (e) none; (f) all; (g) some NK and NKT cells; (h) some NKT cells; (i) CTLs and NKT cells; (j) NKT cells; (k) all; (l) CTLs and NKT cells; (m) NK cells; (n) none; (o) all; (p) some CTLs.
- See the following table:
Killing of LCMV-infected target cells Source of primed spleen cells B10.D2 (H2d) B10 (H2b) B10.BR (H2k) (BALB/c 3 B10) F1 (H2b/d) B10.D2 (H2d) + − − + B10 (H2b) − + − + B10.BR (H2k) − − + − (BALB/c × B10) F1 (H2b/d) + + − + T cells will lyse targets expressing peptides from the antigen to which they were primed (LCMV) and expressing the MHC to which they are restricted (syngeneic MHC). These requirements are met in all cases where there is a positive symbol. The very observant student might also recognize that T cells of one strain will also react to alloantigens (cells from another strain expressing a distinct MHC haplotype). In fact, this is true, and there will be some background death in all cases of MHC “mismatch” (in other words, you would also notice some “background” killing in conditions that are labeled with a “−”). However, because the T cells have been primed by immunization to LCMV, the LCMV-specific MHC-restricted response would be a secondary response and would dominate the primary alloresponse.
- To determine TC activity specific for influenza, perform a cell-mediated lympholysis (CML) reaction by incubating spleen cells from the infected mouse with influenza-infected syngeneic target cells. To determine TH activity, incubate spleen cells from the infected mouse with syngeneic APCs presenting influenza peptides; measure IL-2 production.
- The “missing self” model has been used to explain how NK cells detect infected or tumor cells. If a potential target cell expresses normal levels of MHC class I molecules, inhibitory receptors on the NK cell (KIR, CD94, NKG2A) induce a signal transduction cascade that abrogates NK lytic activity. These negative signals override prokilling signals generated via ligands binding to activating receptors on the NK cell (NKR-P1 and others). Some tumor cells and virally infected cells, however, reduce their expression of MHC class I and no longer stimulate NK inhibitory receptors. In this case, the activating (prokilling) receptor signal dominates.
- There are two pathways by which cytotoxic T cells kill target cells: one that is perforin-dependent and one that uses Fas ligand (FasL) to induce death in Fas-expressing target cells. (And, as always, T cells will lyse only cells expressing the MHC-peptide combinations to which they are specific and restricted.) T cells from immunized perforin knockout H2d mice will be able to lyse (d). (These T cells will depend on FasL-Fas interactions to kill. Target cells don’t need to express perforin to be susceptible, but they do need to express Fas.) T cells from immunized FasL knockout H2d mice will be able to lyse (d), but they will also be able to lyse (e). (These cells will depend on perforin-mediated pathways. Targets do not need to express perforin or Fas to be susceptible.) T cells from H2d mice in which both perforin and Fas ligand have been knocked out will not be able to lyse any of the cell types.
- If the HLA (human MHC) type is known, MHC tetramers bound to the peptide generated from gp120 and labeled with a fluorescent tag can be used to specifically label all of the CD8+ T cells in a sample that has T-cell receptors capable of recognizing this complex of HLA and peptide.
- (a) True. (b) False: They need to express Fas, which transmits the proapoptotic signal. (c) False: Both mechanisms induce caspase activation. (d) False: Only the perforin-mediated pathway depends on granzyme activity. (e) True. (f) False: Perforin is responsible for the development of surface membrane and endocytic membrane pores.
Clinical Focus Answers
- Arthritis is characterized by inflammation of a joint leading to damaged tissue, swelling, and pain. Psoriatic arthritis is accompanied by skin lesions caused by immune attack (psoriasis). Association of KIR-MHC combinations with susceptibility to arthritic disease would likely stem from a deficiency of inhibitory signals (e.g., absence of MHC alleles that produce inhibitory ligands for specific KIR molecules), leading to damage of host cells and tissues. Diabetes is another autoimmune disease; exhibiting destruction of the host pancreatic islet cells that produce insulin, the same mechanisms predicted for arthritis could operate in diabetes. In both cases, the absence of inhibitory NK signals could lead to damage inflicted by NK cells, directly or through their recruitment of other effector cells.
- Rab27A is a GTPase that regulates the transport of intracellular vesicles (granules) to the cell membrane. Transport is required for the release of vesicular contents into the extracellular space. Many cells depend on this ability to function, including cytotoxic T cells, which release perforin and granzyme from internal vesicles, and melanocytes, which release pigment from vesicles (melanosomes). Without this capacity, an individual will be unable to kill infected cells and will exhibit a form of albinism. Many other cells could be affected, including granulocytes (eosinophils, basophils, and mast cells), although it is important to recognize that some express other Rab variants that compensate for the loss of Rab27A function.
Analyze the Data Answer
(a) Epitopes 2, 12, and 18 generated high CTL activity, and epitopes 5 and 21 generated medium activity. (b) It is possible that different peptides use distinct anchor residues, which would make this prediction more difficult. However, if we make the assumption that the same amino acids would be bound by HLA-A2, it appears that a leucine (L) on the amino-terminal side separated by four amino acids from a threonine (T) is the only common motif for the five most immunogenic peptides (2, 12, 18, 5, and 21). All of the peptides that generate high CTL activity have two consecutive leucines on the amino-terminal side as well. The problem with the threonines serving as anchors is that peptide 2 has four amino acids at the carboxyl side of the T, which seems to result in the end of the peptide extending out of the binding pocket. This would be a very unusual configuration. Peptide 12 may have a less dramatic but similar problem. Therefore, a leucine in pocket 2 of HLA-A2 would be consistent with the data generated by M. Matsumura and colleagues in 1992 (Emerging principles for the recognition of peptide antigens by MCH class molecules. Science 257:927). Thus, the main anchor may be a leucine residue at the amino end of the binding pocket, with possible contribution by threonine at the carboxyl end under some circumstances. (c) It is possible that there are no T cells specific for those peptides, even if they are presented in complex with MHC. Therefore, you would not see a CTL response. (d) CTLs recognize antigen only in the context of self MHC molecules. Therefore, in order to assess CTL activity, the T2 cells also had to express HLA-A2. (e) MHC class I molecules typically bind peptides containing 8 to 10 residues (see Figure 7-5). Peptide 2 is 11 residues long, suggesting that it bulges in the middle when bound. Since it appears to be a major epitope for CTL killing, bulging does not seem to interfere with CTL interaction and may contribute.
Chapter 13
- An open-ended question, but essentially one can conclude that the colonization of germ-free mice with norovirus almost fully restores intestinal epithelium health—and immune cell number and activity—to its normal state. Many questions can be asked to follow up on this observation. Does this virus restore the intestinal immune system as well as a bacterial species? What is the mechanism behind this? Is IFN-γ involved in restoring health? The original article (Kernbauer, E., et al. Nature 2014; 516:94) is quite accessible and can be used to answer and explore questions.
- This is an image of a stained section of the small intestine, not the skin. Several features give this away quickly, including the single, rather than multiple layers of epithelium, the villi, and the presence of goblet cells (white, mucus-filled cells in the epithelial layer).
- Many possible answers.
- (a) Goblet cells. (b) Langerhans cells, dendritic cells, ILCs, IELs, eosinophils. (c) Tuft cells. (d) Langerhans cells, dendritic cells. (e) IELs. (f) Probably all! (g) Paneth cells (intestine); M cells (intestine and respiratory tract); enterocytes (intestine); tuft cells (intestine and probably respiratory tract); IELs (intestine and probably respiratory tract; skin has resident lymphocytes, but they are not strictly referred to as IELs); goblet cells (intestine and respiratory tract); eosinophils (all); Langerhans cells (skin); club cells (respiratory tract); ILCs (all); keratinocytes (skin); dendritic cells (all).
- (a) IL-4, IL-10, IFN-γ, IL-13. (b) TSLP and IL-33 initiate a type 2 response; IL-4 and IL-13 are effector cytokines. (c) TSLP and IL-33 are two alarmins (IL-25 is another). (d) IL-10.
- (a), (c), and (d)
- (c)
- (a) and (e)
Chapter 14
- You have not designed an experiment that allows you to focus on antigen-specific T cells—which will represent only a fraction of the whole population. You will need to find a better way to track these. (What are the possibilities? Use TCR transgenics specific for influenza [most, if not all, cells will be antigen specific], or modify the virus so that it expresses another antigen [e.g., OVA] that can be seen by TCR-transgenic cells. Or, try to isolate influenza-specific T cells [by tetramer staining? Difficult, but possible in theory].)
- (a) False: All leukocytes respond to chemokines—they are one of the central regulators of immune cell migration. (b) False: Naïve B cells do not express CCR7 (which helps to send cells to the paracortex), but when activated by antigen binding, they up-regulate it so that they travel to the paracortex to find T-cell help. (c) False: Small antigens (typically opsonized [by complement, for instance]) arrive on their own via the afferent lymphatics. (d) False: They “arrest” their migrating behavior. (e) False: They crawl along the fibroblastic reticular cell network. (f) False: It appears from recent data that these networks can be established at sites of infection. (g) False: Strong adhesion requires chemokine activation. Rolling is the first event (mediated by selectins). (h) True.
- The movement of an antigen-activated B cell from the follicle to the border between the follicle and paracortex is a classic example. However, there are many others, including the response of innate cells to signals generated by inflammation at the site of infection (e.g., neutrophils are attracted to IL-8 produced by other innate cells, including other neutrophils).
- Both receive help in the lymph node cortex. B cells travel to the interface between follicle and paracortex to receive T-cell help and remain loosely associated with the follicle during their interaction with the T cell. The CD8+ T cells receive help in the paracortex, where they interact with APCs and CD4+ T cells.
- Recall that CCL3 is produced by antigen-presenting cells that have been activated by CD4+ helper T cells in the lymph node. CCL3 attracts CD8+ T cells to form a tricellular complex so that they can receive optimal help. Without this cytokine, CD8+ T cells may not find their way to antigen-presenting cell/CD4+ T-cell pairs and may not be optimally activated. On the other hand, other chemokines (e.g., CCL4) may be able to compensate.
- (a) Rolling, chemokine interactions, adhesion, transmigration. (b) Adhesion: Adhesion molecules such as LFA-1 and VLA-4 are converted to their high-affinity forms by chemokine receptor signals (via an inside-out process). (c) The homing and chemokine receptors expressed by naïve lymphocytes attract them to secondary lymphoid tissues. For example, they express L-selectin (CD62L), which interacts with ligands on specialized endothelial structures (high-endothelial venules) located in the cortex of the lymph node.
- Both the pattern of expression of chemokine receptors and chemokines regulate the compartmentalization of T and B cells in the lymph node. For example, naïve T cells express CCR7, which interacts with chemokines that decorate the fibroblastic reticular cell network in the paracortex. Naïve B cells express CCR5, which is expressed by cells in the follicle and by the follicular DC network. T-cell and B-cell movement is guided by the routes laid down by these networks.
- True: Germinal center B cells are more motile and extend unexpectedly long processes within the germinal center.
- (a) − ; (b) ✓ ; (c) ✓ ; (d) − ; (e) ✓ ; (f) − ; (g) ✓ .
- (a) Naïve T and B cells would not home properly to the HEV. (The individual would be significantly immunocompromised, although possibly able to compensate with some innate immune activity and splenic T-cell and B-cell activity.) (b) Naïve T cells would not home properly to the paracortex. Activated B cells would not home properly to the follicle-paracortex border. Animal would not be able to develop optimal adaptive immune responses unless compensated by other chemokines. (The individual would be immunocompromised.) (c) Naïve B cells would not home properly to the follicle. Animal would not be able to develop T cell–dependent antibody responses unless compensated by other chemokines. (The individual would be partially, but still significantly, immunocompromised.) (d) Naïve T and B cells, effector T and B cells, and effector memory T and B cells would not be able to leave the lymph node (and other tissues). (The individual would be immunocompromised unless compensated by other egress regulators and extralymphoid immune cell activity.)
- Note that all cells can transit via blood and lymphatics.
Resident memory T cell: Barrier organs, possibly brain
Central memory T cell: T-cell zones of secondary lymphoid organs, secondary lymphoid organs
Effector T cell: Secondary lymphoid organs, barrier organs, brain (sometimes), T-cell zones and sinuses of secondary lymphoid organ (sometimes)
Plasma cell: Bone marrow, sinuses of secondary lymphoid organ, follicles (for a short time)
Naïve lymphocyte: Secondary lymphoid organs, bone marrow
Dendritic cell: All sites
CD169+ macrophage: Sinuses of secondary lymphoid organs
TFH cell: Follicle, secondary lymphoid organ, T-cell zone of secondary lymphoid organ (for a time)
Analyze the Data Answer
(a) This should be clear from the video. (b) Differences in expression of homing receptors and/or chemokine receptors would be reasonable hypotheses. Make sure to state specific possibilities (based on the information in Appendix III and examples in the text). (c) This requires creative (and rigorous) speculation on your part—we do not really know. Both these subpopulations are effector memory cells—consider what each will do if reactivated. Will they react with different kinetics? Will they stay in the same area of the tissue? Will they serve the same cell populations? Read the authors’ discussion for their view of the possibilities.
Experimental Design Answer
The best experimental designs will include your question, your prediction, and your experimental design. The design must include controls (both positive and negative, if possible—and more than one at times). You must also identify what you will measure and how you will interpret that measurement.
Question: Do naïve B cells require CCR5 to localize to B-cell follicles? Prediction: Yes, they are absolutely dependent on this chemokine receptor; or no, they can use other chemokine receptors, although perhaps less efficiently.
Experimental Design: Fundamentally, you must compare the in vivo activities of B cells that can use CCR5 with those that cannot. One possibility: Compare the behavior of labeled, wild-type versus labeled, CCR5−/− B cells in two groups of mice. Alternatively, or in addition, track the behavior of labeled, wild-type B cells in the presence or absence of the CCR5-blocking antibody.
Once you decide on your design, you must develop a protocol using intravital two-photon microscopy (dynamic imaging). You must be able to trace naïve B-cell movements (and, ideally, identify the B-cell follicle, too). Therefore, you need to fluorescently label those B cells: in vitro CFSE staining (see Chapter 20) is probably the best approach in this case because you will be examining the behavior of B cells from different mice. It is not the only approach, however. (Note: To define the follicular area, you could also co-inject T cells that are labeled with a different color [few if any should be in the follicles] or come up with another more clever, original idea. Some investigators [as you may have noticed] do not directly label the follicle, but infer its location from the behavior of the cells.)
Isolate, label, and inject the cells. Wait a specified time (based on previous studies in the literature or on your own experiments), anesthetize the mice, and record cell behavior in an exposed lymph node over time.
What will you measure? Go back to your question. Identifying the number of B cells that end up in a follicle over a period of time would answer your question directly. The figure you sketch could be a bar graph comparing these numbers in each experimental condition. Direction and speed of the cells may be two other useful parameters that could be generated from an analysis of trajectories—and you can describe how they will contribute to your understanding of the question.
Clinical Focus Answer
There are many different possibilities, in theory. CD18 is part of the LFA-1 complex, which regulates extravasation of multiple subsets of leukocytes (see text and Advances Box 14-1). A CD18 deficiency could, therefore, inhibit the ability of innate immune cells to travel to the site of infection, naïve lymphocytes to enter secondary lymphoid organs, effector cells to recirculate effectively, and so on. All of these problems would severely decrease the ability of a child to fight off infection. Treatment could include genetic modification of bone marrow stem cells (reintroducing the CD18 gene into hematopoietic stem cells), but should also include judicious antibiotic use. Check online resources for what is possible. (Wikipedia [https://www.wikipedia.org/] and any government- or university-based clinical site will likely provide good information.)
Chapter 15
- (a) No type I (which is mediated by IgE/FcεRI interactions), normal type II (which is mediated by IgG or IgM). (b) Same as part (a). (c) May have some type I reaction, but given that this receptor is important in regulating (both enhancing and suppressing) B-cell production of IgE, the response may be abnormal. (d) Type II responses would be most impaired because IgG and IgM exert their effects, in part, by recruiting complement, as well as by inducing ADCC. (e) Type I responses are likely to be suppressed. When bound by soluble versions of FcεRII on B cells, CD21 enhances IgE production. In its absence, the animal may not be able to generate as much IgE antibody as a wild-type mouse.
(Note: All these answers assume that there are no other similar or redundant genes and proteins that could compensate for the absence of the gene in question.)
- Primary mediators are found in mast cell and basophil granules and are released as soon as a mast cell is activated. These include histamine, proteases, serotonin, and so on (see Chapter 15 text). Secondary mediators are generated by mast cells and basophils in response to activation and are released later in the response. These include cytokines, leukotrienes, prostaglandins, and so on (see Chapter 15 text).
- Histamine binds to at least four different histamine receptors. Binding to H2 receptors inhibits mast cell degranulation and therefore inhibits its own release.
- Through the induction of IgG antibodies instead of IgE antibodies and through the activation of regulatory T cells that inhibit the IgE response.
- Rh-mismatched moms and dads can generate both Rh+ and Rh− fetuses. An Rh+ mother will be tolerant of the Rh antigen and will not produce antibodies that could harm either an Rh− or Rh+ fetus. However, an Rh− mother has the potential to generate an antibody response against an Rh+ fetus and could generate a harmful secondary response to a second, Rh+ fetus. RhoGAM (anti-Rh antibodies) will clear B cells (and antibodies) generated during the first pregnancy, preventing such a secondary response.
- See answer to question 5. Rh− babies are not at risk, but Rh+ fetuses are.
- Type III hypersensitivities are disorders brought about by immune complexes that cannot be cleared. They can activate innate immune cells that express Fc receptors and can activate complement, both of which induce inflammation. Such immune complex–mediated inflammation occurs in blood vessels, resulting in vasculitis, as well as in tissue where the complexes are deposited when they pass through inflamed, vasodilated capillaries. Multiple insults can cause these hypersensitivities, including insect bites and inhalation of fungal spores or animal protein (see Chapter 15 text).
- (a) IV; (b) I, II, III, IV; (c) I, mainly, but III also results in mast cell activation and histamine release; (d) IV; (e) I; (f) II; (g) I, mainly (and others can benefit, too); (h) I; (i) II; (j) II; (k) I.
- Chronic infections can cause damaging chronic inflammation. For example, viral hepatitis causes liver damage. The text of Chapter 15 mentions gum disease due to periodontal bacteria, which can cause damage to gums and teeth. The example extensively discussed earlier is tuberculosis, where the unresolved M. tuberculosis infection results in recruitment of many inflammatory cells such as macrophages and tissue damage from their release of inflammatory mediators. TB can be a long-term chronic disease.
- (a) In the absence of inflammation, insulin signaling will not be impaired by cytokine stimulation. Cytokine signals activate kinases, including JNK, that phosphorylate and inactivate insulin receptor substrate (IRS), a key downstream mediator of insulin receptor signaling. (Interestingly, this observation suggests that free fatty acids, alone, may not be enough to induce insulin resistance.) (b) This question requires speculation—there is no right answer. Some possibilities include (1) differences in IRS that make it less able to be phosphorylated at the serine residue, (2) differences in JNK that make it less likely to bind IRS, and (3) differences in gene regions that regulate cytokine production by adipocytes, and so on. See Chapter 15 text and use your imagination!
Analyze the Data Answer
- Eosinophils are recruited to the sites of allergic responses (by eosinophil chemotactic factor, a primary mediator released by mast cell degranulation); the eosinophils release mediators that increase the local response. Co-injection of OVA with Acinetobacter lwoffii reduces the number of eosinophils relative to OVA alone or to OVA with Staphylococcus epidermidis. IL-5 and IL-13 are both TH2 cytokines that play roles in allergic responses; the lower levels of mRNAs seen when OVA was co-administered with OVA indicate a reduced TH2 response, which should lead to a less severe allergic response.
- Mice that had been sensitized with OVA plus A. lwoffii had reduced IgE anti-OVA antibodies relative to those receiving OVA alone or OVA plus S. epidermidis. Injection of either bacterial species resulted in some reduction in IgG2a anti-OVA antibodies, but this is probably not relevant to the IgE response.
- The induction of higher IFN-γ levels after co-injection with A. lwoffii indicates skewing of sensitized effector CD4+ cells to the TH1 subset (some of which will home back to the site of allergen exposure in the skin), rather than the TH2 subset that induces IgE production and allergic responses. IL-10, which inhibits immune and inflammatory responses, can be made by monocytes, macrophages, dendritic cells, and T cells (especially TREGs). In this case it may be suppressing the TH2 response as well as the overall inflammatory response.
- OVA alone induces differentiation of naïve T cells to the allergy-promoting subset TH2. Co-injection of A. lwoffii with OVA probably induces dendritic antigen–presenting cell production of the TH1-inducing cytokine IL-12. With lower numbers of TH2 cells and hence lower levels of IL-4 and/or IL-13 cytokines, there is less heavy-chain class switching to IgE, necessary for activating the allergic response to OVA.
- Yes. From the researchers’ earlier studies it is known that children raised near farms and forests, who are less likely to develop allergies, have higher levels of Acinetobacter species on their skin. The results of this study in mice demonstrate that skin exposure to Acinetobacter during initial allergen sensitization to skewing immune responses away from the TH2 responses that are necessary to drive IgE production and allergic responses.
Chapter 16
- The process called central tolerance eliminates lymphocytes with receptors displaying affinity for self antigens in the thymus or in the bone marrow. A self-reactive lymphocyte may escape elimination in these primary lymphoid organs if the self antigen is not encountered there or if the affinity for the self antigen is less than what is needed to trigger the induction of apoptotic death. Self-reactive lymphocytes escaping central tolerance elimination are kept from harming the host by peripheral tolerance, which involves three major strategies: induction of cell death or apoptosis, induction of anergy (a state of nonresponsiveness), or induction of an antigen-specific population of regulatory T cells that keeps the self-reactive cells in check.
- Tolerance is necessary to remove or regulate the many self-reactive B and T lymphocytes that we know escape negative selection in the thymus. We also need to control responses against some foreign substances that enter the body, which are either beneficial (e.g., gut commensals and components of food) or benign (e.g., tree pollen and animal dander). Without tolerance, which is defined as unresponsiveness to an antigen, unnecessary and pathogenic inflammation can arise against foreign or self antigens, such as during life-threatening hypersensitivity reactions (e.g., allergic asthma, anaphylaxis from food allergies, etc.) or any of a number of autoimmune diseases (e.g., SLE, lupus, RA, type 2 diabetes, etc.), respectively.
- Receptor editing is a process by which B cells exchange their existing light-chain V region for another, via RAG-mediated recombination. This occurs late in B-cell development and leads to modified antigen specificity that, in some cases, will rescue a self-reactive B cell from negative selection or anergy. Because most B-cell responses are T-dependent, T-cell tolerance can override some B-cell self reactivity. If the relevant self antigen–specific TH cells are absent or regulated, this will indirectly regulate or inhibit B-cell self reactivity.
- (a) 6, TH cells and CTLs; (b) 9, TREG cells; (c) 8, antibodies; (d) 10, TH cells and CTLs; (e) 7, antibodies; (f) 2, antibodies; (g) 3, TREG cells; (h) 1, antibodies; (i) 5, TH cells and CTLs; (j) 4, antibodies.
- (a) EAE is induced by injecting mice or rats with myelin basic protein in complete Freund’s adjuvant. (b) The animals that recover from EAE are now resistant to EAE. If they are given a second injection of MBP in complete Freund’s adjuvant, they no longer develop EAE. (c) If T cells from mice with EAE are transferred to normal syngeneic mice, the mice will develop EAE.
- This theory for the cause of autoimmune myocarditis posits that bacterial antigens found in group A streptococcus share similar molecular structures with host proteins found in cardiac muscle. This is believed to be the reason why some individuals develop a myocarditis shortly after infection with group A strep, presumably due to the activation of lymphocytes recognizing the streptococcus that cross react with host cell structures.
- (1) A virus might express an antigenic determinant that cross-reacts with a self component. (2) A viral infection might induce localized expression of IFN-γ. IFN-γ might then induce inappropriate expression of MHC class II molecules on non–antigen-presenting cells, enabling self peptides presented together with the MHC class II molecules on these cells to activate TH cells. (3) A virus might damage an organ, resulting in release of antigens that are normally sequestered from the immune system.
- Anti-CD3 monoclonal antibodies have been used to block T-cell activity in type 1 diabetes mellitus (T1DM). Rituximab, a monoclonal antibody against the B cell–specific antigen CD20, depletes a subset of B cells and has been used to treat patients with rheumatoid arthritis (RA). Monoclonal antibodies against CD4, which deplete TH cells, and one against IL-6, which blocks this pro-inflammatory cytokine, have also been used to treat RA. For psoriasis, a monoclonal antibody that recognizes the p40 subunit shared by IL-12 and IL-23 blocks this signaling pathway. Likewise, the fusion protein CTLA-4Ig blocks interactions between CD28 on T cells and CD80/86 on APCs, as treatment for RA, lupus, and inflammatory bowel disease. (See Table 16-5.)
- (a) True. (b) False: IL-12, which promotes the development of TH1 cells, increases the autoimmune response to MBP plus adjuvant. (c) False: The presence of HLA-B27 is strongly associated with susceptibility to ankylosing spondylitis but is not the only factor required for development of the disease. Most HLA-B27+ individuals will not develop the disease. (d) True. (e) False, or only partially true: The elimination of autoreactive T cells in the thymus, which occurs during negative selection, will still occur. However, the tTREG cells generated in the thymus will be absent. Likewise, pTREG cells, which are induced in the periphery and help to regulate or suppress immune reactivity, will also be absent. Therefore, parts of both central and peripheral tolerance will be missing.
- (a) 7; (b) 3, 4, and 5; (c) 8; (d) 3 and 4; (e) 6; (f) 3 and 4.
- (a) Polyclonal B-cell activation can occur as a result of infection with gram-negative bacteria, cytomegalovirus, or Epstein-Barr virus (EBV), which induce nonspecific proliferation of B cells; some self-reactive B cells can be stimulated in this process. (b) If normally sequestered antigens are exposed, self-reactive T cells may be stimulated. (c) The immune response against a virus may cross-react with normal cellular antigens, as in the case of molecular mimicry. (d) Increased expression of TCR molecules should not lead to autoimmunity; however, if the expression is not regulated in the thymus, self-reactive cells could be produced. (e) Increased expression of MHC class II molecules has been seen in T1DM and Graves’ disease, suggesting that inappropriate antigen presentation may stimulate self-reactive T cells.
- (a) False: Acute rejection is cell mediated and probably involves the first-set rejection mechanism (see Figures 16-11b and 16-13. (b) True. (c) False: Passenger leukocytes are donor dendritic cells that express MHC class I molecules and high levels of MHC class II molecules. They migrate from the grafted tissue to regional lymph nodes of the recipient, where host immune cells respond to alloantigens on them. (d) False: A graft that is matched for the major histocompatibility antigens, encoded in the HLA, may be rejected because of differences in the minor histocompatibility antigens encoded at other loci. (e) True.
- See the following table:
Donor Recipient Response Type of Rejection BALB/c C3H R FSR BALB/c Rat R FSR BALB/c Nude mouse A BALB/c C3H, had previous BALB/c graft R SSR BALB/c C3H, had previous C57BL/6 graft R FSR BALB/c BALB/c A BALB/c (BALB/c × C3H) F1 A BALB/c (C3H × C57BL/6) F1 R FSR (BALB/c × C3H) F1 BALB/c R FSR (BALB/c × C3H) F1 BALB/c, had previous F1 graft R SSR - (a) Graft-versus-host disease (GvHD) develops as donor T cells recognize alloantigens on cells of an immune-suppressed host. The response develops as donor TH cells are activated in response to recipient MHC-peptide complexes displayed on APCs. Cytokines elaborated by these TH cells activate a variety of effector cells, including NK cells, CTLs, and macrophages, which damage the host tissue. In addition, cytokines such as TNF may mediate direct cytolytic damage to the host cells. (b) GvHD develops when the donated organ or tissue contains immunocompetent lymphocytes and when the host is immune suppressed. (c) The donated organ or tissue could be treated with monoclonal antibodies to CD3, CD4, or the high-affinity IL-2 receptor (IL-2R) to deplete donor TH cells. The rationale behind this approach is to diminish TH-cell activation in response to the alloantigens of the host. The use of anti-CD3 will deplete all T cells; the use of anti-CD4 will deplete all TH cells; the use of anti–IL-2R will deplete only the activated TH cells.
- The use of soluble CTLA-4 or anti–CD40 ligand to promote acceptance of allografts is based on the requirement of a T cell for a costimulatory signal when its receptor is bound. Even if the recipient T cell recognizes the graft as foreign, the presence of CTLA-4 or anti-CD40L will prevent the T cell from becoming activated because it does not receive a second signal through the CD40 or CD28 receptor (see Figure 16-18). Instead of becoming activated, T cells stimulated in the presence of these blocking molecules become anergic. The advantage of using soluble CTLA-4 or anti-CD40L is that these molecules affect only those T cells involved in the reaction against the allograft. These allograft-specific T cells will become anergic, but the general population of T cells will remain normal. More general immunosuppressive measures, such as the use of CsA or FK506, cause immunodeficiency and subsequent susceptibility to infection.
- Azathioprine is a mitotic inhibitor used to block proliferation of graft-specific T cells. Cyclosporin A, FK506 (tacrolimus), and rapamycin (sirolimus) are fungal metabolites that block activation and proliferation of resting T cells. Ideally, if early rejection is inhibited by preventing a response by specific T cells, these cells may be rendered tolerant of the graft over time. Lowering the dosage of the drugs is desirable because of decreased side effects in the long term.
- (a) IL-2 receptor—underexpression of IL-2 or the IL-2R would be associated with a reduction in the generation and maintenance of TREG cells (a key polarizing cytokine for this cell type), favoring inflammation over regulation and the development of autoimmunity. (b) CTLA-4—this negative regulatory molecule is found on T cells and is involved in suppression of the immune response. Dysfunction in this molecule that biases toward absence or underperformance will lead to overactivation or prolonged activation of T cells, again resulting in a tendency toward sustained inflammation and autoimmune reactions. (c) CD40—this is a costimulatory molecule involved in activation of antigen-presenting cells on B cells. Enhanced activity of this molecule (a variant of which has been associated with RA and Graves’ disease) would lower the threshold for costimulation in APCs and B cells, allowing increased APC activation and antibody production that can, at times, be directed against autoantigens. (d) ERAP1—this aminopeptidase is involved in antigenic trimming in the ER during MHC class I peptide loading. Therefore, variants of this gene can have a role in which peptides are presented to the immune system. In particular, when there is a strong association of a particular MHC allele and autoimmunity (e.g., ankylosing spondylitis), certain variants of ERAP could lead to more efficient trimming of self peptides into fragments that can be presented to the immune system and stimulate autoimmunity. Therefore, rather than more or less ERAP, it is the variant form of this enzyme combined with the MHC alleles of the host that can bias toward autoimmunity. (e) FoxP3—suppression or inactivation of this gene would result in a reduction or absence of both tTREG and pTREG cells. This tips the inflammation:regulation balance toward inflammation and could result in attacks on self tissues (autoimmunity) or benign foreigners (e.g., food or gut commensals).
- Local innate inflammatory responses, including complement activation and phagocytosis, will lead to local inflammation, especially in the blood vessels of the newly engrafted organ. This can lead to occlusion of blood vessels and poor perfusion (low oxygen), with further cell death. Adaptive responses will likely occur both locally and in the draining lymph node, where direct (via donor APC) and indirect (via recipient APC) presentation of MHC alloantigens will lead to selection and activation of recipient T cells capable of recognizing foreign MHC (whole or processes, respectively). Without immune suppression, the damage and resulting danger signals that accompany surgery and cell death in the transplanted tissue will result in APC activation and enhanced MHC expression and costimulatory potential. These activated T cells will then produce cytokines (mostly TH1) that recruit and further activate immune cells and that help encourage activation of B cells with immunoglobulins specific for allo- or tissue-specific antigens. Finally, TH1 cells can produce cytokines (IL-2 and IFN-γ) and license DCs, allowing activation of alloantigen-specific CTLs. Finally, graft-specific CTLs, TH1 cells, and antibody will collaborate to induce cell death and inflammation, which leads to the death of the engrafted tissue.
Clinical Focus Answers
- The observations that women mount more robust immune responses and more TH1 pathway–directed responses than men, as well as the effects of female sex hormones on the immune response, may in part explain sex-based differences in susceptibility to autoimmunity. Since the TH1 type of response is proinflammatory, the development of autoimmunity may be enhanced. However, this also means that women should be, in general, less likely to mount TH2-mediated reactions (recall from Chapter 10 that TH1 and TH2 pathways are antagonistic). This could result in a lower prevalence of allergy (hypersensitivity) in women, as well as a disadvantage during encounters with helminths and other parasites that require the action of IgE (see Chapter 17).
- (Multiple answers are possible; here are a few examples.) In the case of most T cell–mediated autoimmune diseases, anything that boosts or induces TREG cells (especially those specific for the autoantigen), or is aimed at building tolerance and suppressing inflammation against autoantigens, would be an example of induction of peripheral tolerance. Example drugs/procedures include addition of IL-10 (to encourage TREG cell production or expansion, and suppression of the immune response), blocking TH17 cytokines (such as IL-17 or IL-23) to enhanced action of TREG cells, or interference with costimulation (e.g., using a CTLA-4 fusion protein, such as Abatacept and Belatacept). These same procedures could be used to treat transplant rejection, with the addition of specific tolerance-inducing procedures as the autoantigens in this case are known (alloantigens). Donor leukocyte infusion is one example of a preparatory tolerizing procedure, again, likely working through peripheral tolerance, which can prime the immune system for allograft acceptance.
- The ideal donor animal for xenotransplantation would have organ sizes roughly equivalent to those of humans, would grow quickly, and would either be similar in genetic make-up to humans (e.g., nonhuman primates) or highly amenable to genetic alteration (e.g., removal of antigens associated with rejection). It should be free of any disease that can be passed to humans, and this is favored by phylogenetic distance (e.g., nonhuman primates are not very distant but pigs are). The test of the organs must include transplantation into nonhuman primates first and observation periods that are sufficiently long to ascertain that the organ remains fully functional in the new host and that no disease is transmitted. You need to consider ethical controversies related to experimentation in animals, but especially nonhuman primates. Finally, even after jumping these hurdles, you must consider that some agricultural animals are forbidden as food or revered (e.g., pigs, cows, etc.), and their use might directly conflict with specific cultural and/or religious beliefs. By considering and addressing these issues at the outset, you are practicing greater cultural and religious inclusion and sensitivity; in other words, you are working toward a goal of application of this new technique or resource for everyone, not just a specific segment of society with which you are most familiar.
Chapter 17
- Nonspecific host defenses include ciliated epithelial cells, bactericidal substances in mucous secretions, complement split products activated by the alternative pathway that serve both as opsonins and as chemotactic factors, and phagocytic cells.
- Specific host defenses include humoral immunity, which targets the destruction of extracellular infections (bacterial, fungal, or parasitic) or neutralization of all types of pathogens during extracellular stages, CTLs that identify and eliminate virally infected host cells, and T helper cells that secrete cytokines to assist other leukocytes in the elimination of both intracellular and extracellular pathogens.
- When the majority of a population is immune to a particular pathogen—that is, there is herd immunity—then the probability of the few susceptible members of the population contacting an infected individual is very low. Thus, susceptible individuals are not likely to become infected with the pathogen. If the number of immunized individuals decreases sufficiently, most commonly because of reduction in vaccination rates, then herd immunity no longer operates to protect susceptible individuals and infection may spread rapidly in a population, leading to an epidemic.
- Humoral antibody peaks within a few days of infection and binds to the influenza HA glycoprotein, blocking viral infection of host epithelial cells. Because the antibody is strain specific, its major role is in protecting against re-infection with the same strain of influenza.
- (a) African trypanosomes are capable of antigenic shifts in the variant surface glycoprotein (VSG). The antigenic shifts are accomplished as gene segments encoding parts of the VSG are duplicated and translocated to transcriptionally active expression sites. (b) Influenza is able to evade the immune response through frequent antigenic changes in its hemagglutinin and neuraminidase glycoproteins. The antigenic changes are accomplished by the accumulation of small point mutations (antigenic drift) or through genetic reassortment of RNA between influenza virions from humans and animals (antigenic shift).
- (a) Ab. (b) Because antigen-specific, MHC-restricted TH cells participate in B-cell activation.
- (a) BCG (bacille Calmette-Guérin); (b) antigenic shift, antigenic drift; (c) gene conversion; (d) tubercles, TH cells; activated macrophages; (e) toxoid; (f) IFN-α, IFN-γ; (g) secretory IgA; (h) IL-12, IFN-γ.
- Most fungal infections prevalent in the general population do not lead to severe disease and are dealt with by innate immune mechanisms and lead to protective adaptive responses. Problematic fungal infections are more commonly seen in those with some form of immunodeficiency, such as patients with HIV/AIDS or those with immunosuppression caused by therapeutic measures.
- One possible reason for the emergence of new pathogens is the crowding of the world’s poorest populations into very small places within huge cities, because population density increases the spread of disease. Another factor is the increase in international travel. Other features of modern life that may contribute include mass distribution of food, which exposes large populations to potentially contaminated food, and unhygienic food preparation.
- (a) Influenza virus changes surface expression of neuraminidase and hemagglutinin. (b) Herpesviruses remain dormant in nerve cells. Later emergence can cause outbreaks of cold sores or shingles (chickenpox virus). (c) Neisseria secretes proteases that cleave IgA. (d) False. (e) Several gram-positive bacteria resist complement-mediated lysis. (f) Influenza virus accumulates mutations from year to year. (g) False.
- (a) No, IgE is raised against allergens and some parasites. (b) No, autoreactive T cells are activated only by intracellular infections. The statements in (c) through (f) are correct.
- (a) Large, granular cells such as mast cells and eosinophils. Neutrophils and macrophages will also be involved. (b) Therapeutic cytokines such as IL-4 would help encourage IgE and the TH2 response, which is already present. However, cytokines that drive a TH1 response, such as IL-12 or IFN-γ, may be more beneficial to longer-term immunity.
- The answer comes from the concept of original antigenic sin, which posits that we only mount a primary response once we have exhausted the potential to use memory cells to eradicate the infection. Since most of our first encounters with influenza will vary, the years in which “all” of the key influenza epitopes are significantly “new” to each of us will also vary. It is only in these years that we experience a new primary response to influenza virus, and therefore symptoms of the flu are most severe.
- (a) True. (b) True. (c) False: Because DNA vaccines allow prolonged exposure to antigen, they are likely to generate immunologic memory. (d) True. (e) False: A DNA vaccine contains the gene encoding an entire protein antigen, which most likely contains multiple epitopes.
- Because attenuated organisms are capable of limited growth within host cells, they are processed by the cytosolic pathway and presented on the membrane of infected host cells together with MHC class I. These vaccines, therefore, usually can induce a cell-mediated immune response. The limited growth of attenuated organisms within the host often eliminates the need for booster doses of the vaccine. Also, if the attenuated organism is able to grow along mucous membranes, then the vaccine will be able to induce the production of secretory IgA. The major disadvantage of attenuated whole-organism vaccines is that they may revert to a virulent form. They also are more unstable than other types of vaccines, requiring refrigeration to maintain their activity.
- (a) The antitoxin was given to inactivate any toxin that might be produced if Clostridium tetani infected the wound. The antitoxin was necessary because the girl had not been previously immunized and, therefore, did not have circulating antibody to tetanus toxin or memory B cells specific for tetanus toxin. (b) Because of the treatment with antitoxin, the girl would not develop immunity to tetanus as a result of the first injury. Therefore, after the second injury 3 years later, she will require another dose of antitoxin. To develop long-term immunity, she should be vaccinated with tetanus toxoid.
- The Sabin polio vaccine is live and attenuated, whereas the Salk vaccine is heat killed and inactivated. The Sabin vaccine thus has the usual advantages of an attenuated vaccine compared with an inactivated one (see the answer to question 15). Moreover, since the Sabin vaccine is capable of limited growth along the gastrointestinal tract, it induces production of secretory IgA. The attenuated Sabin vaccine can cause life-threatening infection in individuals, such as children with AIDS, whose immune systems are severely suppressed. Now that polio is rarely if ever seen in the United States, continuing use of a vaccine with the potential to revert to a more virulent form introduces an unwarranted element of risk to both the vaccinee and others who might contract the disease from them.
- The virus strains used for the nasally administered vaccines are temperature-sensitive mutants that cannot grow at human body temperature (37°C). The live attenuated virus can grow only in the upper respiratory tract, which is cooler, inducing protective immunity. These mutant viruses cannot grow in the warmer environment of the lower respiratory tract, where they could replicate and mutate into a disseminated influenza infection.
- T-cell epitopes generally are internal peptides, which commonly contain a high proportion of hydrophobic residues. In contrast, B-cell epitopes are located on an antigen’s surface, where they are accessible to antibody, and contain a high proportion of hydrophilic residues. Thus, synthetic hydrophobic peptides are most likely to represent T-cell epitopes and induce a cell-mediated response, whereas synthetic hydrophilic peptides are most likely to represent accessible B-cell epitopes and induce an antibody response.
- In this hypothetical situation, the gene can be cloned into an expression system and the protein expressed and purified in order to test it as a recombinant protein vaccine. Alternatively, the gene can be cloned into a plasmid vector that can be injected directly and tested as a DNA vaccine. Use of the cloned gene as a DNA vaccine is more efficient, because it eliminates the steps required for preparation of the protein and its purification. However, the plasmid containing the gene for the protective antigen must be suitably purified for use in human trials. DNA vaccines have a greater ability to stimulate both the humoral and cellular arms of the immune system than protein vaccines do and thus may confer more complete immunity. The choice must also take into consideration the fact that recombinant protein vaccines are in widespread use whereas DNA vaccines for human use are still in early test phases.
- Pathogens with a short incubation period (e.g., influenza virus) cause disease symptoms before a memory-cell response can be induced. Protection against such pathogens is achieved by repeated immunizations to maintain high levels of neutralizing antibody. For pathogens with a longer incubation period (e.g., polio virus), the memory-cell response is rapid enough to prevent development of symptoms, and high levels of neutralizing antibody at the time of infection are unnecessary.
- Bacterial capsular polysaccharides, inactivated bacterial exotoxins (toxoids), and surface protein antigens. The latter two commonly are produced by recombinant DNA technology. In addition, the use of DNA molecules to direct synthesis of antigens on immunization is being evaluated.
- A possible loss of herd immunity in the population. Even in a vaccinated population of children, a small percentage may have diminished immunity to the disease target due to differences among MHC molecule expression in a population, providing a reservoir for the disease. In addition, most vaccinated individuals, if exposed to the disease, will develop mild illness. Exposure of unvaccinated individuals to either source of disease would put them at risk for serious illness. Epidemics within adult populations would have more serious consequences, and infant mortality due to these diseases would increase.
- (a) 1 or 2; (b) 2; (c) 3; (d) 4; (e) 1; (f) 2; (g) 2.
- (a) No. The antiserum you received 1 year ago protected you temporarily, but those antibodies are now gone and you have no memory B cells to produce new antibodies during this second exposure. (b) Antibodies in the antiserum bound to the snake venom and neutralized its ability to cause damage. Phagocytes then engulfed and destroyed this antibody-coated venom. Because the snake venom was coated with antibodies, naïve B cells were not activated during this first exposure and therefore no adaptive immune response was mounted. (c) Equally sensitive. There are no residual cells or antibodies that were involved in the original encounter with this snake venom and, therefore, no recall response.
Clinical Focus Answers
- (a) Because the infected target cells expressed H2k MHC molecules, but the primed T cells were H2k restricted. (b) Because the influenza nucleoprotein is processed by the endogenous processing pathway and the resulting peptides are presented by MHC class I molecules. (c) Probably because the transfected MHC class I Db molecule is only able to present peptide 365–380 and not peptide 50–63. Alternatively, peptide 50–63 may not be a T-cell epitope. (d) These results suggest that a cocktail of several immunogenic peptides would be more likely to be presented by different MHC haplotypes in humans and would provide the best vaccines for humans.
- Any connection between vaccination and a subsequent adverse reaction must be evaluated by valid clinical trials involving sufficient numbers of subjects in the control group (those given a placebo) and experimental group (those receiving the vaccine). This is needed to give a statistically correct assessment of the effects of the vaccine versus other possible causes for the adverse event. Such clinical studies must be carried out in a double-blind manner; that is, neither the subject nor the caregiver should know who received the vaccine and who received the placebo until the end of the observation period. In the example cited, it is possible that the adverse event (increased incidence of arthritis) was caused by an infection occurring near the time when the new vaccine was administered. Determining the precise cause of this side effect may not be possible, but ascertaining whether it is likely to be caused by this vaccine is feasible by appropriate studies of the vaccinated and control populations.
Analyze the Data Answer
(a) pSG5DNA-Bcl-xL (i.e., plasmid pSG5 encoding Bcl-xL) targeting calreticulin and pSG5DNA-Bcl-xL targeting LAMP-1 are the most effective vaccines at inducing CD8+ T cells to make IFN-γ. pSG5DNA-Bcl-xL targeting HSP70 also activated CD8+ T cells. However, the pSG5 construct without the anti-apoptosis gene targeting calreticulin also induced a good CD8+ T-cell response. (b) Calreticulin is a chaperone protein associated with partially folded MHC class I molecules in the endoplasmic reticulum. Associating E7 antigen with the chaperone may enhance loading of MHC class I molecules with E7, making the antigen more available to T cells once it is expressed on cells. (c) The DNA vaccines co-injected with pSG5DNA-Bcl-xL were effective in inducing CD8+ T cells, possibly because the expression of anti-apoptotic genes in dendritic cells allowed those cells to survive longer and present antigen to T cells for a longer time. The longer they presented antigen, the longer the host would respond to produce antigen-specific T cells. (d) The data in part (b) of the figure (see Chapter 17 text) indicate that in the absence of CD4+ helper T cells (in CD4 knockout mice), there is ineffective activation of CD8+ T cells. Therefore, T-cell help is necessary to activate the CD8 response; by targeting antigen to MHC class II, you more efficiently activate helper T cells. The Sig/E7/LAMP-1 construct was necessary because most antigens presented by MHC class II molecules are processed by the endocytic pathway, and the Sig/E7/LAMP-1 construct targets antigen to the Golgi, where the E7 peptides can be exchanged with CLIP and inserted into MHC class II. (e) Helper T cells (poor response in the CD4 knockout mice), long-lived dendritic cells (immunization with pSG5DNA-Bcl-xL improves the response), antigen (the absence of peptide failed to induce a response), and the targeting of antigen to MHC class II (immunizing with the Sig/E7/LAMP-1 construct is the only one that induces a CD8+ T-cell response).
Chapter 18
- (a) True. (b) False: X-linked agammaglobulinemia is characterized by a reduction in B cells and an absence of immunoglobulins. (c) False: Phagocytic defects result in recurrent bacterial and fungal infections. (d) True. (e) True. (f) True. (g) True. (h) True. (i) False: These children are usually able to eliminate common encapsulated bacteria with antibody plus complement but are susceptible to viral, protozoan, fungal, and intracellular bacterial pathogens, which are eliminated by the cell-mediated branch of the immune system. (j) False: Humoral immunity also is affected because class II–restricted TH cells must be activated for an antibody response to occur.
- (a) 1 and 3; (b) 1, 2, and 4; (c) 2; (d) 1; (e) 2.
- The defect in X-linked hyper-IgM syndrome is in CD40L expressed on B cells. CD40L mediates binding of B cells to T cells and sends costimulatory signals to the B cell for class switching. Without CD40L, class switching does not occur, and the B cells do not express other antibody isotypes.
- As discussed in Chapter 8, the thymus is the location for differentiation and maturation of helper and cytotoxic T cells. Positive and negative selection occur in this organ as well. Thus, the thymocytes produced in the bone marrow of patients with DiGeorge syndrome do not have the ability to mature into effector cell types. In Chapter 2, we noted that the thymus decreases in size and function with age. In the adult, effector-cell populations have already been produced (peak thymus size occurs during puberty); therefore, a defect after this stage would cause less severe T-cell deficiency.
- As long as the mother is not immunocompromised, maternal antibodies in the mother’s serum will be passively transferred to the baby in utero. After birth, these IgG molecules will supply the newborn with passive immune protection from many common bacterial infections, which can then be quickly dispatched by antibody-mediated mechanisms. In immune-competent babies, these maternal antibodies will eventually be replaced by the child’s own immune response to the infectious agents they encounter. In children with SCID, this does not occur, and they gradually become more susceptible to bacterial infections as their maternally derived antibodies disappear.
- (a) Leukocyte adhesion deficiency results from biosynthesis of a defective β chain in LFA-1, CR3, and CR4, which all contain the same β chain. (b) LFA-1 plays a role in cell adhesion by binding to ICAM-1 expressed on various types of cells. Binding of LFA-1 to ICAM-1 is involved in the interactions between TH cells and B cells, between CTLs and target cells, and between circulating leukocytes and vascular endothelial cells.
- The high-affinity IL-2 receptor is composed of two chains: the α chain and the common γ chain. Later, the γ chain was discovered to be a component of the receptors for five other cytokines: IL-4, -7, -9, -15, and -21. During hematopoiesis, developing lymphocytes require IL-7 signaling, and therefore the complete IL-7R, in order to develop properly. Without the common γ chain, this does not occur and the development of lymphocytes is blocked.
- As there are multiple MHC I genes (for HLA-A, -B, and -C), it is unlikely that a mutation would affect expression of all the genes on both chromosomes. Rare individuals lack MHC class I proteins due to deficiency in b2-microglobulin.
- Some components of the immune system are responsible for regulating or suppressing the activity of leukocytes (e.g., TREG cells). When these pathways are defective, overactive immune responses can occur, leading to breaks in self tolerance that lead to attacks on self molecules, or autoimmune syndromes. One example is a disorder called immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, in which the gene encoding the transcription factor that controls development of TREG cells, Foxp3, is defective. Another example is autoimmune polyendocrinopathy and ectodermal dystrophy (APECED), a disorder that arises from defects in the AIRE gene, encoding a transcription factor found in the thymus. The Aire protein is responsible for the expression of tissue-restricted antigens. Without this protein, the negative selection of T cells in the thymus that recognize self antigen is disrupted and autoreactive T cells emerge and instigate organ-specific autoimmune attacks.
- As the individual’s own HSCs are being used, matching HLA types is not necessary and there will be no graft-versus-host reaction.
- (a) False: HIV-1 is believed to have evolved from a strain of SIV that jumped the species barrier from African chimpanzees to humans, although HIV-2 is thought to have arisen from a separate but similar transfer from SIV-infected sooty mangabeys. (b) False: HIV-1 infects chimpanzees but does not cause immune suppression. (c) True. (d) False: Zidovudine (or azidothymidine, AZT) acts at the level of reverse transcription of the viral genome, whereas saquinavir is an inhibitor of the viral protease. (e) True. (f) False: A diagnosis of AIDS is based on both a low CD4+ T-cell count (below 200 cells/μl, or 14%) and the presence of certain AIDS-defining conditions (see Table 18-4). (g) False: The PCR detects HIV proviral DNA in latently infected cells. (h) True.
- Lysis of active virus-producing cells; killing by cytotoxic T cells; ADCC or antibody-mediated phagocytosis.
- No. For most of the asymptomatic phase of HIV infection, viral replication and the CD4+ T-cell number are in dynamic equilibrium; the level of virus and the percentage of CD4+ T cells remains relatively constant.
- An increase in the viral load and a decrease in CD4+ T-cell levels indicate that HIV infection is progressing from the asymptomatic phase into AIDS. Often, infection with an opportunistic agent occurs when the viral load increases and the CD4+ T-cell level drops. The disease AIDS in an HIV-1–infected individual is defined as a CD4+ T-cell count less than 200 cells/μl and/or the presence of certain opportunistic infections (see Table 18-4).
- Skin-test reactivity is monitored to indicate the functional activity of TH cells. As AIDS progresses and CD4+ T cells decline, there is a decline in skin-test reactivity to common antigens.
- Receptors for certain chemokines, such as CXCR4 and CCR5, also function as coreceptors for HIV-1. The chemokine that is the normal ligand for the receptor competes with the virus for binding to the receptor and can thus inhibit cell infection by blocking attachment of the virus (see Figure 18-14). Cytokines that activate T cells stimulate infection because they increase expression of receptors used by the virus.
- No. There are latently infected cells that reside in lymph nodes or in other sites. These cells can be activated and begin producing virus, thus causing a relapse of the disease. In a true cure for AIDS, the patient must be free of all cells containing HIV DNA.
- Patient L. S. fits the definition of AIDS, whereas patient B. W. does not. The clinical diagnosis of AIDS among HIV-infected individuals depends on both the T-cell count, or percentage, and the presence of various indicator diseases. See Table 18-4.
- One hurdle is the rapid generation of HIV variants through mutation, so that the variants that arise in an individual or to which s/he is exposed will not be recognized by memory B and T cells induced by the vaccine. A second challenge is presented by the extensive glycan coat that covers up the gp120/gp41 Env protein so that BCRs and antibodies have a hard time binding to the Env protein. A third challenge is that the generation of broadly neutralizing antibodies that will react with most viral strains requires many somatic hypermutations that need to occur and be selected for over time.
Analyze the Data Answer
(a) CVID lowers the number and percentage of T helper cells. (b) The table accompanying this question indicates that there are fewer naïve CD8+ T cells in patients with CVID. This could mean chronic activation in CVID has made these T cells less dependent on IL-2. Alternatively, the figure accompanying this question shows that bone marrow cells from patients with CVID make less IL-2. If this is also true of TH cells, or there are fewer TH cells (as seen in the table accompanying this question), then the generation of CTLs may be impaired. This example of the complexity of the immune system demonstrates that specific responses are not always easily predictable. (c) False: The data in the figure accompanying this question show that the kinetics and overall production of TNF-α is greater in CVID than in normal patients, but IL-2 production is lower. Thus, there is no consistent impact. Based on what we know about TNF-α activity, it might be responsible for increased pathology or cell death, perhaps explaining the loss of both CD4+ and CD8+ cells in patients with CVID. (d) According to information in Chapter 18, CVID lowers IgG, IgA, and IgM. However, based on the data presented in the table accompanying this question, one might predict that in the absence of T-cell help, there will be a significant impact on class switching.
Clinical Focus Answer
All individuals needing antiretroviral therapy are supposed to receive combination drugs to lessen the chance that resistant viral variants will survive. These drugs cross the placenta, which will prevent the fetus from becoming infected in utero. Newborns should begin antiretroviral therapy as soon as possible after birth to reduce the chance that infection will occur during birth from the mother’s body fluids. Treatment should continue for several weeks, and longer if the infant is breastfeeding.
Chapter 19
- (a) False: Hereditary retinoblastoma results from inactivation of both alleles of Rb, a tumor-suppressor gene. (b) True. (c) True. (d) True. (e) False: Some oncogenic retroviruses do not have viral oncogenes. (f) True.
- Cells of the pre-B-cell lineage have rearranged the heavy-chain genes and express the μ heavy chain in their cytoplasm (see Figure 9-4). You could perform Southern blot analysis with a Cμ probe to see whether the heavy-chain genes have rearranged. You could also perform fluorescence staining with antibody specific for the cytoplasmic μ heavy chain.
- (a) Early-stage melanoma cells appear to be functioning as APCs, processing the antigen by the exogenous pathway and presenting the tetanus toxoid antigen together with the MHC class II DR molecule. (b) Advanced-stage melanoma cells might have a reduction in the expression of MHC class II molecules, or they might not be able to internalize and process the antigen by the exogenous route. (c) Since the paraformaldehyde-fixed early melanoma cells could present processed tetanus toxoid, they must express MHC class II molecules on their surface. (d) Stain the early and advanced melanoma cells with fluorescent mAb specific for MHC class II molecules.
- Tumor antigens may be encoded by genes expressed only by tumors, may be products of genes overexpressed by the tumor or of genes normally expressed only at certain stages of differentiation, or may be products of normal genes that are altered by mutation. In some cases, viral products may be tumor antigens.
- IFN-α, IFN-β, and IFN-γ enhance the expression of MHC class I molecules on tumor cells, thereby increasing the CTL response to tumors. IFN-γ also increases the activity of CTLs, macrophages, and NK cells, each of which plays a role in the immune response to tumors. TNF-α and lymphotoxin-α have direct antitumor activity, inducing hemorrhagic necrosis and tumor regression. IL-2 activates tumor-infiltrating lymphocytes (TILs), which have antitumor activity.
- (a) Melanoma cells transfected with the CD80/86 gene are able to deliver the costimulatory signal necessary for activation of CTL precursors to effector CTLs, which then can destroy tumor cells (Figure 19-11). Melanoma cells transfected with the GM-CSF gene secrete this cytokine, which stimulates the activation and differentiation of APCs, especially DCs, in the vicinity. The DCs then can present tumor antigens to TH cells and CTL precursors, enhancing the generation of effector CTLs (see Figure 19-10). (b) Because some of the tumor antigens on human melanomas are expressed by other types of cancers, transfected melanoma cells might be effective against other tumors carrying identical antigens.
- (a) 4; (b) 2; (c) 1; (d) 5; (e) 3; (f) 9; (g) 8; (h) 7; (i) 6.
- Part D (M2 macrophages).
- False: Inflammation can be a bad sign when TH2, TREG cells, or M2 macrophages dominate the response, creating a microenvironment that is protumor.
- (a) Adoptive transfer of modified T cells—collection of tumor-infiltrating lymphocytes, which are then expanded ex vivo (e.g., with IL-2) and reinfused into the patient, is one example. While some patients with metastatic melanoma had a positive response to this therapy it did not help all, possibly due to the inadvertent expansion of regulatory T cells. More recently, chimeric antigen receptor (CAR) T cells have been developed for treating cancer. These are T cells collected from patients and transduced to express a chimeric receptor (part immunoglobulin and part T-cell receptor) that recognizes tumor antigens without the need for MHC presentation. This topic is covered in Clinical Focus Box 19-2. (b) Monoclonal antibodies—any mAb treatment listed in Table 19-4. For example, rituximab, an anti-CD20 mAb, can bind to B cells (which express this surface molecule) and induce cell death. This treatment can be used as a form of therapy for some blood cell cancers, such as non-Hodgkin’s lymphoma. (c) Checkpoint blockade—mAbs that recognize CTLA-4 or PD-1/PD-L1 can be used to block the negative regulatory signals (PD-L1) or the receptors of these signals (PD-1 and CTLA-4); basically, blocking the brake pedal on T cells. Collectively, these checkpoint inhibitors allow endogenous T cells (TH1 or CTLs) to perform their natural anti–tumor cell activity, unhindered by regulation (see Figure 19-12). (d) Therapeutic vaccine—the prostate cancer therapy sipuleucel-T (Provenge) is one example (Figure 19-10). In this method, patient DCs are collected and treated with a fusion protein expressing a prostate-specific antigen and GM-CSF, a cell-stimulating cytokine/growth factor. These are expanded ex vivo and reinfused into the patient, as a means to activate T cells specific for this prostate-specific antigen. These T cells should then home to the site of the tumor and help to eradicate prostate cancer cells expressing these antigens at high levels. The anti-HPV vaccine would not be an example here, only because this is considered a prophylactic vaccine, or one that must be administered before infection with this cervical cancer–causing virus, rather than a therapeutic vaccine, or one that can be administered after the onset of disease.
Clinical Focus Answers
- In some instances, Coley’s toxins may have induced a local immune response that helped the immune system to recognize and fight the tumor cells. For instance, introduction of bacteria should elicit local innate responses when PAMPs on the bacteria are recognized by PRRs on immune cells. These danger or damage signals would help to recruit leukocytes to the site (inflammation) and activate local antigen-presenting cells. These activated APCs could then supply the necessary signal 2 components (e.g., up-regulation of MHC class II and CD80/86) to engage with T cells. The antigens presented by these cells might include both bacterial and local tumor antigens, acquired via phagocytosis of dead or damaged tumor cells. If antigens from the tumors were presented to naïve T cells, this could activate antitumor-specific adaptive responses. Even if there are no tumor-specific epitopes recognized by naïve T cells, if the introduced bacteria activate a local TH1-like response the tumor microenvironment would begin to shift away from protumor (M2 macrophages, TH2 cells, and immune-suppressing microenvironment) toward a more antitumor environment (M1 macrophages, IL-12, TH1 cells, and CTLs). One might think of this as an early form of immunotherapy!
- Because cervical cancer is linked to HPV infection, a preventive cancer vaccine may be possible; if HPV infection is prevented, then cervical cancer should also be prevented. Other cancers that may be targets for such prevention include adult T-cell leukemia/lymphoma and the liver cancer that is linked to hepatitis B infection. Most cancers have not been clearly linked to an agent of infection, and therefore a preventive vaccine is not an option.
Chapter 20
- If I wished to precipitate my antigen, I might elect to use a polyclonal preparation, as it would contain antibodies toward multiple different determinants on the antigen and therefore many antibody molecules could bind per antigen molecule, maximizing the chances that at least some of the antibodies could bind more than one antigen and facilitate precipitation.
- With time, the population of B-cell clones that respond to an antigen in an individual will change. Some B-cell clones will die, and different clones will predominate. Overall, the affinity of the antibodies in the serum will increase according to the methods described in Chapter 12. However, this means that the proteins with which individual antibodies will cross-react will change as the range of binding sites modulates, and this is what has happened in your experiment.
- (a) Ideally, polyclonal. I will have the most effective agglutination if antibodies can cross-link multiple sites on the bacterial surface. However, a monoclonal antibody would also work, as most antigens are repeated many times on the bacterial surface. (b) Ideally, polyclonal. To form a precipitate, I will need to cross-link multiple proteins. If each only has a single site at which an antibody can bind, a bivalent antibody can cross-link only two proteins, and that would be insufficient to create a precipitate. If I am precipitating a protein with multiple copies of the same site, then monoclonal antibodies could still work. (c) Here, either would work. The antibody binds to the band, localizing an enzymatic reaction at the band and causing substrate conversion to product. A polyclonal antibody mixture would have the advantage that different antibodies could bind at different antigenic determinants on the target protein and therefore could give rise to a stronger signal. However, different bleeds of polyclonal sera would have different levels of cross-reactivity with other, structurally similar determinants on other proteins. Monoclonal antibodies will bind to predictable determinants, and, although they might still cross-react with structurally similar determinants on other proteins, those cross-reactivities are predictable and will not change from batch to batch. (d) Here, one would use two monoclonal antibodies directed toward different determinants on the cytokine. If the capture antibody were to be polyclonal, such that all the binding sites on the cytokine were bound, this would compete with a polyclonal detection antibody. (e) Monoclonal. Here, reproducibility of reactivity and cross-reactivity is demanded for clinical safety.
- (a) Yes. There is some evidence of hemagglutination in the first well, which represents an antiserum dilution of 1 in 10. (b) By 42 days of age, the baby’s serum has the same capacity for hemagglutination inhibition as the positive-control sample. (c) No. These could be maternal antibodies absorbed through breast milk or colostrum.
- RIAs are orders of magnitude more sensitive than are ELISAs with chromogenic substrates. And yes, chemiluminogenic substrates allow more amplification and greater sensitivity, resulting in an assay that matches the RIA in its ability to measure low concentrations of antibodies or antigens.
- ELISPOT assays measure the number of cells capable of secreting particular molecules, such as antibodies or cytokines. These assays therefore require that when substrate is converted to product, the product remains at the location where the substrate-to-product reaction occurred. Products in ELISPOT reactions are therefore insoluble. Similarly, Western blot assays use antibodies to determine the location of particular bands on a gel. Again, one requires that the product of the enzymatic assay remains localized precisely where the enzyme is bound.
- (a) Since cost is an issue, and I already have the antibody labeled, I would probably use equilibrium dialysis. (b) Surface plasmon resonance experiments will enable me to measure the association, as well as the dissociation rate constant, of the binding reaction between my antigen and antibody.
- By using longer-wavelength, lower-energy light, two-photon microscopy induces less photobleaching of the tissue preparation. Further, since no fluorescence is emitted unless two exciting photons simultaneously impinge on the sample, the focal plane of the observed images can be more tightly defined.
- Magnetic-activated cell sorting is most useful for batch separations of cell populations. It is faster than fluorescence-based methods, but not quite as precise. I would choose fluorescence-based sorting when I need to be sure there are no contaminating cells, because in FACS, cells are quite literally separated one at a time.
- Experimental Design Answer There are many ways to test this idea, but we will offer just one here, which makes use of adoptive transfer and fluorescence labeling: Generate B-1 B cells in culture specific for the virus in question, and load them with CFSE. Inject the cells into the tail of a mouse infected with the virus, allow the cells to home for 12 to 24 hours, and then sacrifice the mouse and search for CFSE-labeled cells in the lung, using tissue sections and immunofluorescence.
- Experimental Design Answer I will use a tamoxifen/Cre fusion protein whose expression is under the control of a B cell–specific promoter, such as that controlling the expression of CD19. Therefore, Cre will be active only in B cells and only if I add the tamoxifen and I can therefore control exactly when the gene targeting will occur in reference to antigen immunization.
I also need to generate an inactive, truncated form of the gene in question that is flanked by loxP sites. Ideally, the gene will have a selectable marker, such as neomycin resistance, and also will bear a sequence external to the loxP sites that will control for insertion of the gene into the correct location (see Figure 20-33a, left).