Chapter 28 NURSING MANAGEMENT: obstructive pulmonary diseases
1. Outline the aetiology, pathophysiology, clinical manifestations and multidisciplinary care of asthma.
2. Discuss the nursing management of the patient with asthma.
3. Differentiate between the aetiology, pathophysiology, clinical manifestations and multidisciplinary care of the patient with chronic obstructive pulmonary disease (COPD).
4. Outline the effects of cigarette smoking on the lungs.
5. Identify the indications for oxygen therapy, methods of delivery and complications of oxygen administration.
6. Explain the nursing management of the patient with COPD.
7. Describe the pathophysiology, clinical manifestations, multidisciplinary care and nursing management of the patient with cystic fibrosis.
8. Describe the pathophysiology, clinical manifestations, multidisciplinary care and nursing management of the patient with bronchiectasis.
‘When you can’t breathe, nothing else matters’™ is the message from the Australian Lung Foundation to draw attention to the problems experienced by people who live with obstructive pulmonary diseases every day.1 Chronic respiratory diseases impose a significant burden on resources within Australia and New Zealand and are a growing problem. Approximately 2.1 million Australians are estimated to have chronic obstructive pulmonary disease (COPD)—of these, about 1.2 million have moderate to severe COPD and approximately 900,000 have a mild form of the disease.2 Obstructive pulmonary diseases, the most common chronic lung diseases, include diseases characterised by increased resistance to airflow as a result of airway obstruction or airway narrowing. Types of obstructive lung diseases are asthma, COPD, cystic fibrosis and bronchiectasis. Asthma is a chronic inflammatory lung disease that results in airflow obstruction but is usually reversible, particularly in the early stages of disease. COPD is an obstructive pulmonary disease that is characterised by progressive limitation in airflow that is not fully reversible.1,3 The patient with asthma has variations in airflow over time, with normal lung function between exacerbations, whereas the limitation in expiratory airflow in the patient with COPD is generally more constant. The pathology of asthma and response to therapy differ from COPD. However, the patient with a diagnosis of obstructive pulmonary disease may have features of both asthma and COPD.1 Patients with asthma who have less-responsive reversible airflow obstruction are very difficult to distinguish from COPD patients. Cystic fibrosis, which is another form of obstructive pulmonary disease, is a genetic disorder that produces airway obstruction because of changes in exocrine glandular secretions, resulting in increased mucus production. Bronchiectasis is an obstructive disease characterised by dilated bronchioles, most frequently due to untreated or poorly treated pulmonary infections that cause an increase in sputum production.
Asthma
Asthma is a chronic inflammatory disorder of the airways, resulting in varying degrees of obstruction in the airways.4 This inflammation causes airway hyperresponsiveness with widespread but variable airflow obstruction, presenting as recurrent episodes of wheezing, breathlessness, chest tightness and cough, particularly at night and in the early morning. The airway obstruction may reverse spontaneously or with treatment. The clinical course of asthma is unpredictable, ranging from paroxysms of dyspnoea and wheezing to unremitting symptoms.
Asthma is a growing global problem affecting all ages of the population. New Zealand is estimated to have the second highest prevalence of asthma in the world (after the UK), with one in six New Zealand adults affected by the disease.5 The rate of asthma is much higher in Māori adults (22%) than in non-Māori adults (15%). In Australia, the prevalence is also among the highest in the world: 8–12% of adults have asthma—about 2 million people.4,6 The incidence of asthma is increasing in both countries. This may be due in part to improved case finding and changes in diagnostic classification, but these cannot account for all the increase. The morbidity associated with asthma is dramatic. It affects workplace attendance, occupational choices, physical activity and many other aspects of life.4
The healthcare costs and growing burden of asthma in Australia and New Zealand have been widely publicised. Hospitalisation rates have increased significantly over the past 30 years and in New Zealand alone there were almost 1000 admissions to hospital for asthma in the late 1990s.5 Total hospitalisations for Australia were almost 38,000 in 2007–2008,6 although rates vary across the country. Since the late 1980s there has been a substantial decline in the number of deaths attributed to asthma and in 2008 (the latest statistics available) there were 449 deaths in Australia.7 However, there do appear to be fluctuations in the number of deaths, particularly in those aged over 65 years, and so asthma remains a cause for concern. Underdiagnosis and inappropriate therapy are the major contributors to asthma morbidity and mortality. The high morbidity rates related to asthma may be attributed to limited access to healthcare, an inaccurate assessment of disease severity, a delay in seeking help, inadequate medical treatment, non-adherence to prescribed therapy and an increase in allergens in the environment.
RISK FACTORS FOR ASTHMA AND TRIGGERS OF ASTHMA ATTACKS
Risk factors for asthma and triggers of asthma attacks can be related to the patient (e.g. genetic factors) or the environment (see Box 28-1 and Fig 28-1). Male gender is a risk factor for asthma in children (but not adults), although it is as yet unclear why this is so. Obesity has been shown to be a risk factor for asthma.4 Other risk factors and triggers are discussed below.
BOX 28-1 Triggers of acute asthma attacks
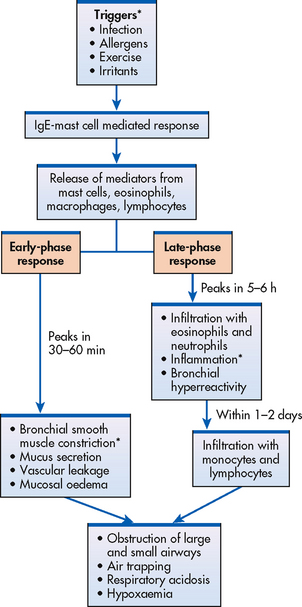
Figure 28-1 Early- and late-phase responses of asthma. Items with an asterisk (*) are primary processes.
Genetics
Asthma has a component that is inherited, but the genetics are complex. Numerous genes may be involved in the development of asthma and different ethnicities may have different genes.4 Atopy, the genetic predisposition to develop an allergic (immunoglobulin E [IgE]–mediated) response to common allergens, is a major risk factor for asthma.8
Immune response
The hygiene hypothesis suggests that a newborn baby’s immune system must be educated so that it will function properly during infancy and the rest of life. Those exposed to certain infections early in life and who use few antibiotics, are exposed to other children (e.g. siblings, day care) or live in the country or with pets will have a lower incidence of asthma. People who do not have these factors present in childhood have a higher rate of asthma.8
Allergens
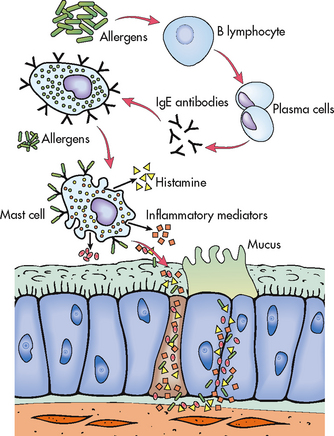
In some persons with asthma, an exaggerated IgE response to certain allergens occurs.4 These allergens attach to IgE receptors on mast cells (see Fig 28-2). The IgE–mast cell complexes remain for a long time so that a second exposure to the allergen triggers mast cell degranulation even years after the initial exposure to the allergen. (Allergic reactions are discussed in Ch 13.) Allergic asthma may be seasonal and related to allergies such as tree or weed pollen. It is seen mainly in young adults and children. Non-seasonal forms of asthma may be year round (perennial) and related to allergens such as dust mites, moulds, animals, feathers and cockroaches.
Exercise
Asthma that is induced or exacerbated during physical exertion is called exercise-induced asthma (EIA).9 Typically, EIA occurs after several minutes of vigorous exercise (e.g. jogging, aerobics, walking briskly, climbing stairs) and is characterised by bronchospasm, shortness of breath, cough and wheezing. Symptoms of EIA are pronounced during activities where there is exposure to cold air. For example, swimming in an indoor heated pool is less likely to produce symptoms than downhill skiing. Airway obstruction may occur due to changes in the airway mucosa caused by the hyperventilation occurring during exercise with either cooling or rewarming of air and capillary leakage in the airway wall.
Air pollutants
Various air pollutants, cigarette or wood smoke, vehicle exhaust, elevated ozone levels, sulfur dioxide and nitrogen dioxide can trigger asthma attacks. In heavily industrialised or densely populated areas, climatic conditions often lead to concentrated pollution in the atmosphere, especially with thermal inversions and stagnant air masses. Ozone alert days are regularly noted in news reports and patients should minimise outdoor activity during these times. Cigarette smoking is associated with an accelerated decline of lung functioning in a person with asthma; it also increases the severity of the disease, may cause the patient to be less responsive to treatment with corticosteroids (either systemic or inhaled) and reduces the chance of the asthma being controlled.10
Occupational factors
Occupational asthma is the most common form of occupational lung disease. Exposures in the workplace can also aggravate pre-existing asthma.11 These agents are diverse, such as wood and vegetable dusts (e.g. flour), pharmaceutical agents, laundry detergents, animal and insect dusts, secretions and serums (e.g. chickens, crabs), metal salts, chemicals, paints, solvents and plastics. Individuals can become sensitised to these agents. Characteristically patients will give a history of arriving at work feeling well but with gradual development of symptoms by the end of the day.
Can physical training improve respiratory and general health in persons with asthma?
EVIDENCE-BASED PRACTICE
Clinical question
In patients with asthma (P), does physical training (I) as compared to a control group (C) improve respiratory and general health (O)?
Critical appraisal and synthesis of evidence
• Meta-analysis of 13 RCTs (n = 455).
• Subjects with any degree of asthma severity and who were 8 years and older were included. Physical training (aerobic exercise) had to be undertaken for at least 20–30 minutes, two to three times a week, over a minimum of 4 weeks.
• No adverse effects were noted on lung function and wheeze in asthmatic patients.
• Physical training was found to have no effect on resting lung function or the number of days of wheeze.
• Physical training can improve cardiopulmonary fitness in individuals with asthma.
Implications for nursing practice
• Patients with asthma should be encouraged to engage in regular physical exercise.
• Patients should be advised about prevention and treatment of exercise-induced asthma.
P, patient population of interest; I, intervention or area of interest; C, comparison of interest or comparison group; O, outcome(s) of interest; T, time.
Respiratory infections
Respiratory infections (especially viral infections) are one of the most common precipitating factors of an acute asthma attack.4 Influenza and rhinovirus are the major pathogens in older children and adults. Infections cause inflammatory changes in the tracheobronchial system and alter the mucociliary mechanism. Therefore, they increase the hyperresponsiveness of the bronchial system. Increased airway responsiveness can last from 2 to 8 weeks after the infection in both normal and asthmatic persons. It is thought that viruses cause asthma exacerbations by activating the immune system. This ultimately results in the production of inflammatory mediators, leading to the onset of asthma symptoms (see Fig 28-1).
Nose and sinus problems
Allergic rhinitis is a major predictor of adult asthma.12 Treatment of allergic rhinitis may reduce the frequency of asthma exacerbations.4 Some patients with asthma have chronic sinus problems that cause inflammation of the mucous membranes. Although the cause is usually non-infectious (e.g. allergies), bacterial infections may also be a cause. Sinusitis must be treated and large nasal polyps removed for the asthma patient to have good control. (Sinusitis is discussed in Ch 26.)
Drugs and food additives
Sensitivity to specific drugs may occur in some asthmatic persons, especially those with nasal polyps. Some people with asthma have what is sometimes termed the asthma triad or ‘Samter triad’—nasal polyps, asthma and sensitivity to aspirin and non-steroidal anti-inflammatory drugs (NSAIDs).13 Salicylates can be found in some over-the-counter preparations and some foods, drinks and flavourings. In some asthmatics who ingest aspirin or NSAIDs (e.g. ibuprofen, indomethacin), wheezing will develop in approximately 2 hours accompanied by severe rhinorrhoea, orbital oedema, red eyes and flushing of the head and neck.13
β2-adrenergic blockers (e.g. metoprolol) taken orally or by topical application such as eye drops (e.g. timolol) may trigger asthma because they inhibit adrenergic stimulation of the bronchioles and thus prevent bronchodilation. Angiotensin-converting enzyme (ACE) inhibitors may produce cough in susceptible individuals, thus making asthma symptoms worse. Other agents that may precipitate asthma in the susceptible patient are tartrazine (yellow dye no. 5 found in many foods) and sulfiting agents (food preservatives commonly found in fruits, beer and wine and used extensively in salad bars to protect vegetables from oxidation). Asthma exacerbations have been reported after the use of sulfite-containing preservatives found in topical ophthalmic solutions, intravenous (IV) corticosteroids and some inhaled bronchodilator solutions. Monosodium glutamate (MSG) can also trigger an asthmatic episode and is found widely in commercially produced foods.
Gastro-oesophageal reflux disease
The exact mechanism by which gastro-oesophageal reflux disease (GORD) triggers asthma is unknown. It is postulated that reflux of stomach acid into the oesophagus can be aspirated into the lungs, causing local irritation and inflammation. Alternatively, it may be that the oesophageal acidity causes reflex vagal stimulation and bronchoconstriction, resulting in irritation in other locations such as the lungs.14 Although GORD is primarily involved in nocturnal asthma, it can trigger daytime asthma as well. Patients with hiatus hernia, excessive stress and a prior history of reflux or ulcer disease may have acid reflux as an asthma trigger. (GORD is discussed in Ch 41.)
Psychological factors
Asthma is not a psychosomatic disease. However, emotional factors associated with crying, laughing, anger and fear can lead to hyperventilation and hypocapnia, which can cause airway narrowing.4 An asthma attack caused by any trigger can produce panic, stress and anxiety, which are not unexpected emotions during this experience. Anxiety is a very normal response to not being able to breathe. The extent to which psychological factors contribute to the induction and continuation of any given acute exacerbation is unknown, but it probably varies from patient to patient and in the same patient from episode to episode.
PATHOPHYSIOLOGY
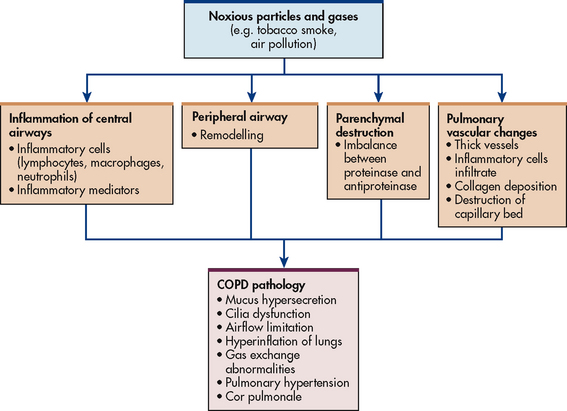
The primary pathophysiological process in asthma is persistent but variable inflammation of the airways. Airflow is limited because the inflammation results in bronchoconstriction, hyperresponsiveness (hyperreactivity) and oedema of the airways. Exposure to allergens or irritants initiates the inflammatory cascade (see Fig 28-1). A variety of inflammatory cells are involved including mast cells, macrophages, eosinophils, neutrophils, T and B lymphocytes, and epithelial cells of the airways.8
The early-phase response is triggered when an allergen or irritant cross-links IgE receptors on mast cells found beneath the basement membrane of the bronchial wall (see Figs 28-1 and 28-2). The mast cells become activated, with subsequent release of granules and disruption of the phospholipid cell membrane. Both processes result in the release of inflammatory mediators, including histamine, bradykinin, leukotrienes, prostaglandins, platelet-activating factor and chemotactic factors.15 (A similar process can occur in a susceptible patient after exercise.) These mediators cause intense inflammation associated with the classic immediate reaction of asthma, which consists of bronchial smooth muscle constriction, increased vasodilation and permeability and epithelial damage. Clinically the effects are bronchospasm, increased mucus secretion, oedema formation and increased amounts of tenacious sputum (see Fig 28-3). This immediate response peaks within 30–60 minutes of exposure to the trigger (e.g. allergen, irritant) and subsides in another 30–90 minutes. Clinically the patient has wheezing, chest tightness, dyspnoea and cough.
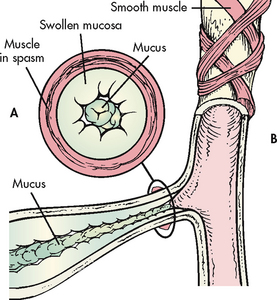
Figure 28-3 Factors causing expiratory obstruction in asthma. A, Cross-section of a bronchiole occluded by muscle spasm, swollen mucosa and mucus in the lumen. B, Longitudinal section of a bronchiole.
Symptoms can recur 4–10 hours after the initial attack because of eosinophil and lymphocyte activation and further release of more inflammatory mediators. The epithelial cells also produce cytokines and other inflammatory mediators. This delayed response is called the late-phase response. It can be more severe than the early-phase response and persist for 24 hours or more. It is characterised by a self-sustaining cycle of inflammation. Airflow may be limited from the swelling of the airways with or without bronchoconstriction. Corticosteroids are effective in treating this inflammation.
Alterations in neural control of the airways occur in asthma. The autonomic nervous system, consisting of the parasympathetic and sympathetic nervous systems, innervates the bronchi. Airway smooth muscle tone is regulated by the parasympathetic nervous system. In asthma, there is overactivity of the parasympathetic nervous system. When airway nerve endings are stimulated by mechanical or chemical stimuli (e.g. air pollution, cold air, dust, allergens), increased release of acetylcholine results in increased smooth muscle contraction and mucus secretion, ultimately leading to bronchoconstriction.
Chronic inflammation may result in structural changes in the bronchial wall, which is known as remodelling. A progressive loss of lung function occurs that is not prevented or fully reversed by therapy. The changes in structure may include fibrosis of the subepithelium, smooth muscle hypertrophy of the airways, mucus hypersecretion, continued inflammation and angiogenesis (proliferation of new blood vessels). Remodelling is thought to explain why some individuals have persistent asthma and limited response to therapy.3,4 There is some evidence that remodelling occurs in even mild asthma of recent onset, but can be prevented by early introduction of inhaled corticosteroids.5
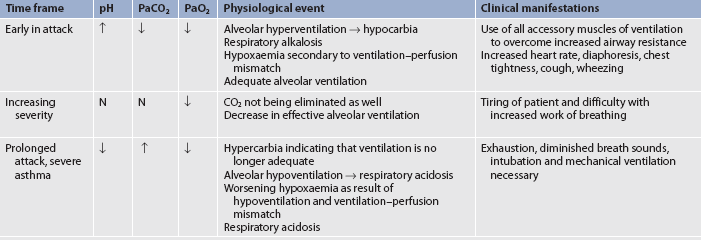
Hyperventilation also occurs during an asthma attack as lung receptors respond to increased lung volume from trapped air and airflow limitation. Decreased perfusion and ventilation of the alveoli and increased alveolar gas pressure lead to ventilation–perfusion abnormalities in the lungs.16 The patient will be hypoxaemic early on with decreased PaCO2 and increased pH (respiratory alkalosis) as they are hyperventilating. As the airflow limitation worsens with air trapping, the patient works much harder to breathe. The PaCO2 will normalise as the patient gets tired and then it will rise to produce respiratory acidosis, which is an ominous sign signifying respiratory failure.
CLINICAL MANIFESTATIONS
Asthma is characterised by an unpredictable and variable course, from minor interferences in breathing to life-threatening episodes—affected individuals may experience both extremes during their lifetime. There may be recurrent episodes of wheezing, breathlessness, chest tightness and cough, particularly at night and in the early morning; these are typical of asthma. An attack of asthma may have an abrupt onset, but usually symptoms occur more gradually. Attacks may last for a few minutes to several hours. Between attacks the patient may be asymptomatic with normal or near-normal pulmonary function, depending on the severity of disease. However, in some persons, compromised pulmonary function may result in a state of continuous asthma and chronic debilitation characterised by irreversible airway disease.
The characteristic clinical manifestations of asthma are wheezing, cough, dyspnoea and chest tightness after exposure to a precipitating factor or trigger. Expiration may be prolonged. Instead of a normal inspiratory:expiratory ratio of 1:2, it may be prolonged to 1:3 or 1:4. Normally the bronchioles constrict during expiration. However, as a result of bronchospasm, oedema and mucus in the bronchioles, the airways become narrower than usual.16 Thus it takes longer for air to move out of the bronchioles. This produces the characteristic wheezing, air trapping and hyperinflation.
Wheezing is an unreliable sign by which to gauge the severity of an attack. Many patients with minor attacks wheeze loudly, whereas others with severe attacks do not wheeze. The patient with severe asthmatic attacks may have no audible wheezing because of the marked reduction in airflow. For wheezing to occur, the patient must be able to move enough air to produce the sound. Wheezing usually occurs first on exhalation. As asthma progresses the patient may wheeze during inspiration and expiration.
In some patients with asthma, cough is the only symptom and this is termed cough variant asthma. The bronchospasm may not be severe enough to cause airflow obstruction but it can increase bronchial tone and cause irritation and stimulation of the cough receptors. The cough may be non-productive. Mobilising secretions may be difficult. Secretions may be thick, tenacious, white, gelatinous mucus.
The person with asthma has difficulty with air movement in and out of the lungs, which creates a feeling of suffocation. Therefore, during an acute attack, the person with asthma usually sits upright or slightly bent forwards using the accessory muscles of respiration to try to get enough air. The more difficult the breathing becomes, the more anxious the patient feels.
Examination of the patient during an acute attack usually reveals signs of hypoxaemia, which may include restlessness, increased anxiety, inappropriate behaviour, increased pulse and blood pressure, and pulsus paradoxus (a drop in systolic pressure during the inspiratory cycle >10 mmHg). However, reliance on pulsus paradoxus as an indicator of the severity of asthma is no longer recommended by the Scottish Intercollegiate Guidelines Network (SIGN) for practical reasons.17 The respiratory rate is significantly increased (usually >30 breaths/min) with the use of accessory muscles. Percussion of the lungs indicates hyperresonance and auscultation indicates the presence of inspiratory or expiratory wheezing.
Diminished or absent breath sounds may indicate a significant decrease in air movement resulting from exhaustion and an inability to generate enough muscle force to ventilate. Severely diminished breath sounds, often referred to as the ‘silent chest’, is an ominous sign, indicating severe obstruction and impending respiratory failure.18
CLASSIFICATION OF ASTHMA
Asthma can be classified as mild intermittent, mild persistent, moderate persistent or severe persistent (see Table 28-1). The classification system is used at diagnosis to determine the initial treatment; it is based on the patient’s current impairment (i.e. symptoms, lung function measurements) and the risk for future exacerbations that require oral corticosteroids. Patients may move to different asthma classifications over the course of their disease.
TABLE 28-1 Classification of asthma severity: clinical features before treatment
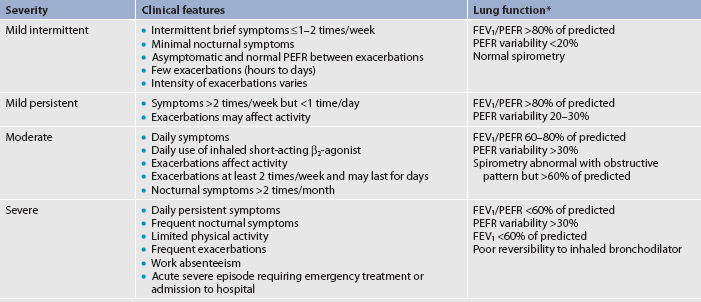
Percentage predicted values for forced expiratory volume in 1 second (FEV1) and percentage of personal best for peak expiratory flow rate (PEFR). Notes:
• Patients should be assigned to the most severe step in which any feature occurs. Clinical features for individual patients may overlap across steps.
• An individual’s classification may change over time.
• Patients at any level of severity of chronic asthma can have mild, moderate or severe exacerbations of asthma. Some patients with intermittent asthma experience severe and life-threatening exacerbations separated by long periods of normal lung function and no symptoms.
• Patients with two or more asthma exacerbations per week (i.e. Progressively worsening symptoms that may last hours or days) tend to have moderate-to-severe asthma.
Source: Adapted from: New Zealand Guidelines Group (NZGG). The diagnosis and treatment of adult asthma. Wellington: NZGG; 2002. Available at www.nzgg.org.nz/guidelines/0003/Full_text_Guideline.pdf, accessed 22 January 2011.
COMPLICATIONS
Severe acute asthma
Severe asthma exacerbations occur when the patient is dyspnoeic at rest and speaks in words, not sentences, because of the difficulty in breathing. The patient will usually sit forwards to maximise the diaphragmatic movement and have prominent wheezes, a respiratory rate >30 breaths/min and pulse >120 beats/min. Accessory muscles in the neck may be straining in order to lift the chest wall, and the patient is often agitated. The peak flow (peak expiratory flow rate [PEFR]) will be 40% of the personal best or <150 mL. Arterial blood gas (ABG) changes are listed in Table 28-2. Neck vein distention and pulsus paradoxus of 40 mmHg or more may result. Usually it is difficult to auscultate pulsus paradoxus secondary to a noisy chest or increased work of breathing. These patients usually are seen in emergency departments (EDs) or are hospitalised.8,10
Life-threatening asthma
A few patients perceive asthma symptoms poorly and may have a significant decrease in lung function without any change in symptoms. Patients with life-threatening asthma are typically too dyspnoeic to speak and will be perspiring profusely. They may even be drowsy or confused as the ABGs further deteriorate. Their breath sounds may be very difficult to hear and no wheezing may be apparent if the airflow is exceptionally limited. The peak flow is <25% of the personal best. Such patients will have bradycardia and be close to respiratory arrest. They require ED or hospital care and are often admitted to an intensive care unit.
DIAGNOSTIC STUDIES
Underdiagnosis of asthma is common. A detailed history is important in determining whether a person has had previous attacks of a similar nature, often precipitated by a known cause. Since wheezing and cough are seen with a variety of disorders this complicates the diagnosis of asthma. These disorders include COPD, pulmonary embolism, GORD, obesity, vocal cord dysfunction and heart failure.
Some controversy exists about how best to diagnose asthma. Common diagnostic measures are presented in Box 28-2. In general, the nurse should consider the diagnosis of asthma if various indicators (i.e. clinical manifestations, health history and peak flow variability) are positive. However, pulmonary function tests are necessary to determine lung reversibility and thus establish the diagnosis of asthma.
MULTIDISCIPLINARY CARE
Diagnostic studies
History and physical examination
Pulmonary function studies including response to bronchodilator therapy
Peak expiratory flow rate (PEFR)
Measurement of ABGs or oximetry (if severe exacerbation)
Allergy skin testing (if indicated)
Collaborative therapy
Mild, moderate asthma
Identification and avoidance/elimination of triggers
Desensitisation (immunotherapy) if indicated
Drug therapy (see Tables 28-4, 28-6, 28-7)
Asthma action plan (see Fig 28-7)
The PEFR, measured with the peak flow meter, generally correlates with forced expiratory volume (FEV) and is a helpful tool in the diagnosis and management of asthma. A variety of peak flow meters are on the market, but to confirm the diagnosis, spirometry is preferred, as there are no standardised PEFR reference values. In general, peak flow meters are best designed as monitoring and not diagnostic devices.15
Pulmonary function is usually within normal limits between attacks if the patient has no other underlying pulmonary disease. However, the patient with asthma may show an obstructive pattern including a decrease in forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), PEFR, the FEV1 to FVC ratio (FEV1/FVC) and forced expiratory flow rate measured during the middle of FVC (FEF25–75%), with the degree of obstruction depending on the values obtained. (The normal values for pulmonary function tests are discussed in Ch 25.)
Reversibility of lung function is an important component in diagnosing asthma, therefore when pulmonary function tests (PFTs) are undertaken, the patient is asked to withhold taking any bronchodilator medications for 6–12 hours prior to the scheduled test. However, PFTs can be undertaken before and after the administration of a bronchodilator to determine the degree of response. A positive response is an increase of 12% between pre-administration and post-administration values, which equates to around 200 mL increased volume.
Lung function parameters decrease from their baseline levels during exacerbation. Some patients may have symptoms of asthma, but have normal lung function. Therefore, measures of airway responsiveness to known bronchial irritants, such as methacholine, histamine or exercise, may assist in establishing the diagnosis of asthma.
An elevated serum eosinophil count and elevated serum IgE levels are highly suggestive of atopy (allergic tendency), which may be the aetiology of a person’s asthma. Allergy skin testing may be of some value to determine sensitivity to specific allergens. However, a positive skin test does not necessarily mean that the allergen is causing the asthma attack. On the other hand, a negative allergy test does not mean that the asthma is not allergy related. A radioallergosorbent test, which is a blood test, is sometimes used to identify allergic causes in certain patients who show negative skin tests and in those who should not be skin tested (e.g. patients with severe eczema). (Allergy testing is discussed in Ch 13.)
A chest X-ray in an asymptomatic patient with asthma is usually normal, but needs to be obtained as a baseline upon initial diagnosis. A chest X-ray obtained during an acute attack usually shows hyperinflation and may reveal other complications of asthma, such as mucoid impaction, pneumothorax, atelectasis or pneumomediastinum.
If the patient has wheezing and acute distress, it is not feasible to obtain a detailed health history (although a family member may supply some pertinent information). During an acute attack of asthma, bedside spirometry (specifically FEV1 or FVC, but usually PEFR) may be used to monitor obstruction. Pulmonary function test results, serial spirometric parameters, oximetry and measurement of ABGs help provide information about the severity of the attack and the response to therapy. A full blood count (FBC) and serum electrolytes are also obtained to help monitor the course of therapy.
A sputum specimen for culture and sensitivity may be obtained to rule out the presence of bacterial infection, especially if the patient has purulent sputum, a history of upper respiratory tract infection, a fever or an elevated white blood cell count. However, the vast majority of asthma exacerbations are viral in nature and sputum cultures are rarely done on an outpatient basis.
A hand-held, point-of-care device called the Niox Mino is available in Australia and New Zealand to measure airway inflammation related to asthma, by calculating fractional exhaled nitric oxide (FENO) levels. FENO levels are increased in the breath of people with asthma, and changes in the levels may indicate whether inflammation is present and whether or not treatment for asthma is working. However, studies have not been done to determine the effectiveness of FENO levels as an aid in the diagnosis of asthma.
MULTIDISCIPLINARY CARE
The rising incidence of asthma morbidity and mortality up to the late 1980s highlighted the need for evidence-based multidisciplinary clinical practice guidelines and treatment protocols, with the subsequent development of reports prepared by asthma experts worldwide and the creation of the Global Initiative for Asthma (GINA).10 The goals of GINA are to decrease asthma morbidity and mortality and improve the management of asthma worldwide. In Australia and New Zealand, the Asthma management handbook was developed by the National Asthma Council in conjunction with the Thoracic Society of Australia and New Zealand4 and this remains the key reference for healthcare professionals involved in the care of patients with asthma.
Education for an active partnership with patients remains the cornerstone of asthma management and should be carried out by healthcare providers delivering asthma care. Education should start at the time of asthma diagnosis and be integrated into every step of clinical asthma care. Asthma self-management should be tailored to the needs of each patient, maintaining sensitivity to cultural beliefs and practices. Emphasis should be placed on evaluating outcomes in terms of the patient’s perceptions of improvement, especially quality of life and the ability to engage in usual activities. The Asthma Foundation of New Zealand provides an example of a plan for managing asthma on its website (see Resources on pp 727–728). Desirable therapeutic outcomes include: (1) control or elimination of chronic symptoms such as cough, dyspnoea and nocturnal awakenings; (2) attainment of normal or nearly normal lung function; (3) restoration or maintenance of normal levels of activity; (4) reduction in the number or elimination of recurrent exacerbations; (5) reduction in the number or elimination of emergency department visits and acute care hospitalisations; and (6) elimination or reduction of side effects of medications.4 An example of asthma education programs for adults can be accessed through the websites of the Asthma Foundation of New Zealand and the National Asthma Council Australia websites (see Resources on pp 727–728).
The goal of asthma treatment is to achieve and maintain control of the disease. Once the patient is diagnosed, guidelines give direction on the classification of severity (see Table 28-1) and which medications the patient requires (see Table 28-3 and Fig 28-4). The current guidelines focus on (1) assessing the severity of the disease at diagnosis and initial treatment and then (2) monitoring periodically to achieve control of the disease. At initial diagnosis, a patient may have severe asthma and require step 4 of asthma medication. After treatment, the patient is assessed as to the level of control (i.e. well controlled, not well controlled or very poorly controlled). As the patient achieves control of the symptoms the healthcare provider steps down the medication—or steps it up if the symptoms worsen. Achieving rapid control of the symptoms is the goal in order to return the patient to daily functioning at the best possible level.4 The level of control is based on the patient’s responses to symptoms, night-time wakening, interference with normal activities and use of rescue or reliever medication. The level of control is also determined by the patient’s current peak flow or FEV1, as well as any exacerbations or adverse effects of treatment. Patients respond individually to treatment and thus are in a state of flux as they seek to achieve control and minimise the risk for future exacerbations.
TABLE 28-3 Stepwise approach for managing asthma

* The intensity of treatment will depend on the severity of exacerbation; up to three treatments at 20-minute intervals or a single nebuliser treatment as needed. A course of systemic corticosteroids may be needed. Use of short-acting β2-agonists more than two times a week in intermittent asthma (daily or increasing use in persistent asthma) may indicate the need to initiate (increase) long-term control therapy.
Source: Quick Reference of the national Asthma Education and Prevention Program (nAEPP) Expert Panel Report. Guidelines for the diagnosis and management of asthma—update on selected topics 2002. Available at www.nhlbi.nih.gov/guidelines/asthma/asthsumm.htm, accessed 22 January 2011.
Mild exacerbation and persistent asthma
The choice of drug therapy depends on the severity of symptoms (see Table 28-3). Patients in all classifications of asthma will require a short-term (rescue or reliever) medication. The short-acting β2-adrenergic agonists (SABAs) (e.g. salbutamol) are the gold standard and most effective. For any classification of asthma, in a ‘rescue plan’ patients are instructed to take 2–4 puffs of salbutamol every 20 minutes three times to gain rapid control of the symptoms. Occasionally, a short course of oral corticosteroids is needed to decrease airway inflammation.
Patients with persistent asthma must be on a long-term or controller medication (see Box 28-3). Inhaled corticosteroids (ICSs) (e.g. fluticasone) are the most effective class of drugs to combat the inflammation. Some patients may also require oral corticosteroids. Information sheets and self-management plans are widely available on the internet to help patients to take control of their asthma.4,18
BOX 28-3 Long-term control versus quick relief of asthma
DRUG THERAPY
Long-term control medications
*Considered quick-relief drugs when used in a short burst (3–10 days) at the start of therapy or during a period of gradual deterioration. Corticosteroids are not used for immediate relief of an ongoing attack.
With a mild exacerbation, the patient has difficulty breathing only with activity and may feel that they ‘can’t get enough air’. The peak flow is greater than 70% of the patient’s personal best, and usually the symptoms are relieved at home promptly with a SABA such as salbutamol delivered via a nebuliser or metered-dose inhaler (MDI) with a spacer.
Acute asthma episode
With a moderate exacerbation, dyspnoea interferes with usual activities and peak flow is 40–60% of the patient’s personal best. In this situation, the patient usually comes to the ED or the healthcare provider’s office to get help. Relief is provided with the SABA delivered as in the mild exacerbation and oral corticosteroids as needed. Oral routes are usually as effective as IV routes, as well as being less invasive and less expensive. The patient’s symptoms may persist for several days even after the corticosteroids as started. Oxygen can be used with both mild and moderate exacerbations to maintain SpO2 at 90% or greater. The patient’s symptoms and peak flow are monitored and lung auscultation is done to ensure the patient is moving air. A good response would be measured by the peak flow (or FEV1) returning to 70% of personal best, normal airflow on physical examination, alleviation of patient’s distress and findings sustained more than 1 hour after the last treatment.8
Severe and life-threatening exacerbation
Management of the patient with severe and life-threatening exacerbation focuses on correcting hypoxaemia and improving ventilation. The goal is to keep the O2 saturation at 90% or greater. Continuous monitoring of the patient is critical. Obtaining a PEFR during a severe asthma attack is usually not possible. However, if it can be obtained and it is less than 200 L/min, it indicates severe obstruction in all but very small adults. Many of the therapeutic measures are the same as those for acute asthma. Repetitive or continuous SABA administration is provided in the ED. Initially three treatments of a SABA (spaced 20–30 minutes apart) are given. Then more SABA is given depending on the patient’s airflow, improvement and side effects from the SABA. Patients with severe exacerbations usually find partial relief from the SABA plus ipratropium. However, patients with life-threatening asthma will get minimal, if any, relief from the same medications. After the initial treatment, ipratropium is not given during the inpatient stay as it has not been found to deliver any added benefit. Nebulised SABA is continued for several days, even after clinical improvement is noted.8
In severe asthma, oral systemic corticosteroids are given to patients who do not respond to the initial SABA. It is no longer recommended that patients double the dose of ICSs in times of an exacerbation as this is not effective. In life-threatening asthma, corticosteroids are administered intravenously and are usually tapered rapidly. IV corticosteroids (e.g. methylprednisolone) are administered every 4–6 hours, although their peak effect is not apparent for 4–12 hours. Then the patient is started on the oral corticosteroids. The length of oral prednisone treatment for both severe and life-threatening asthma after discharge is usually about 10 days. Inhaled corticosteroids are usually added while the patient is still in hospital. High-dose ICSs prevent asthma relapse and may be prescribed until the patient can step down to lower doses. In severe and life-threatening asthma, adjunctive medications such as IV magnesium sulfate may be administered in patients with a very low FEV1 or peak flow (less than 40% of predicted or personal best at presentation) or those who fail to respond to initial treatment. In addition, when the patient is in the hospital, heliox (a mixture of helium and oxygen) may be used to deliver the nebulised albuterol, as helium has a low density and may improve the bronchodilation of the albuterol.8,10
Supplemental O2 is given by mask or nasal prongs to achieve a PaO2 of at least 8 kPa (60 mmHg) or an O2 saturation greater than 90%. An arterial catheter may be inserted to facilitate frequent ABG monitoring. Because the patient’s insensible loss of fluids is increased and the metabolic rate is increased, moderate rates of IV fluids are given to provide optimal hydration. Sodium bicarbonate administration is usually limited to treatment of severe metabolic or respiratory acidosis (pH <7.29) in the mechanically ventilated patient because effective bronchodilation by β-adrenergic agonists is not possible if the patient has extreme acidosis. Bronchoscopy, although rarely performed during an acute attack, may be necessary to remove thick mucous plugs.
When asthma exacerbations are life-threatening and respiratory arrest is pending or actually occurring, the patient will require intubation or mechanical ventilation if there is no response to treatment. The patient should be provided with 100% oxygen, hourly or continuously nebulised SABAs, IV corticosteroids and possibly other adjunctive therapies as noted above.
Theophylline, mucolytics and sedatives are no longer recommended for asthma exacerbations. Sedatives can result in depression of the respiratory drive and possible death. Antibiotics are not recommended for asthma treatment unless there are signs of bacterial pneumonia, fever and purulent sputum, suggesting bacterial infections. Chest physiotherapy has no role and is generally not recommended for asthma because it is too stressful for the breathless patient.8,10,17 Although it is no longer listed in the guidelines for usual asthma exacerbation management, adrenaline is occasionally administered for acute treatment of anaphylaxis if selective β2-adrenergic agonists are not available. If adrenaline is administered, patients need close blood pressure and electrocardiogram monitoring.
Bronchial thermoplasty, a bronchoscopic procedure in which controlled thermal energy is applied to the airway wall to decrease the smooth muscle, is currently being tested to determine its effectiveness as a treatment for patients with severe and persistent asthma. Results suggest that it may improve asthma-specific quality of life with a reduction in severe exacerbations in the post-treatment period. The FDA approved the procedure for use in the US in April 2010, but approval has not yet been given for routine use in Australia or New Zealand.19
DRUG THERAPY
The Thoracic Society of Australia and New Zealand, the National Asthma Council Australia and the New Zealand Guidelines Group support a stepwise approach to drug therapy, with the type and amount of medication dictated by asthma severity (see Table 28-3). This emphasises that persistent asthma requires daily long-term therapy in addition to appropriate medications to manage acute asthma exacerbations. To clarify this concept, medications can be categorised into two general classifications: (1) long-term control medications to achieve and maintain control of persistent asthma; and (2) quick-relief medications to treat symptoms and exacerbations.4,18 Because inflammation is considered an early and persistent component of asthma, drug therapy for persistent asthma must be directed towards long-term suppression of the inflammation (see Box 28-3).
Anti-inflammatory drugs
Corticosteroids
As chronic inflammation is a primary component of asthma, corticosteroids are widely used to reduce bronchial hyperresponsiveness, block the late-phase reaction and inhibit migration of inflammatory cells. Corticosteroids are more effective in improving asthma control than any other long-term drug. Inhaled corticosteroids are first-line therapy for patients with persistent asthma (see Table 28-4). Usually inhaled corticosteroids must be administered for 1–2 weeks before maximum therapeutic effects can be seen. Some inhaled corticosteroids (e.g. fluticasone, budesonide) begin to have a therapeutic effect in 24 hours.
TABLE 28-4 Asthma and chronic obstructive pulmonary disease
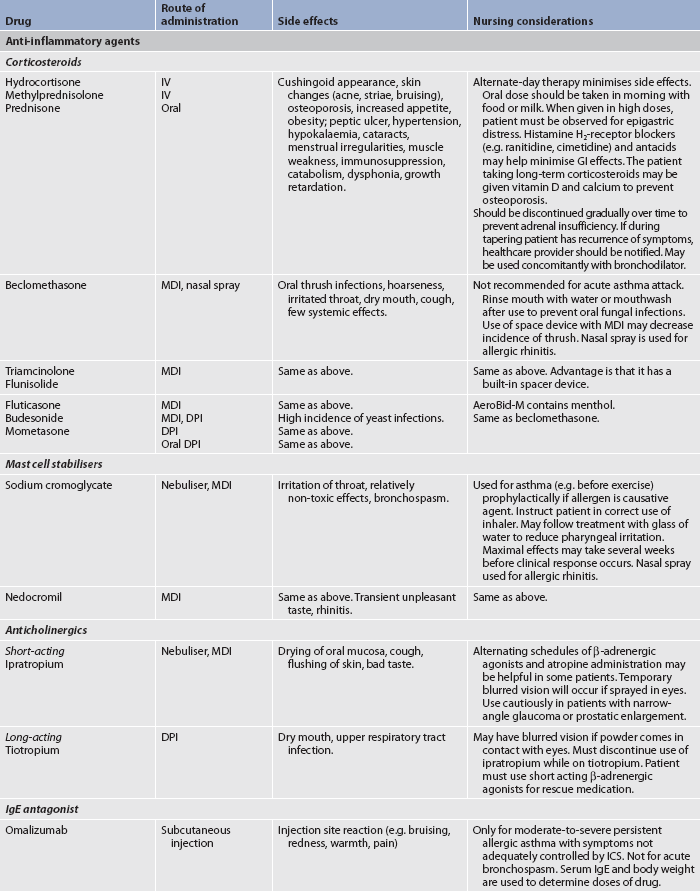
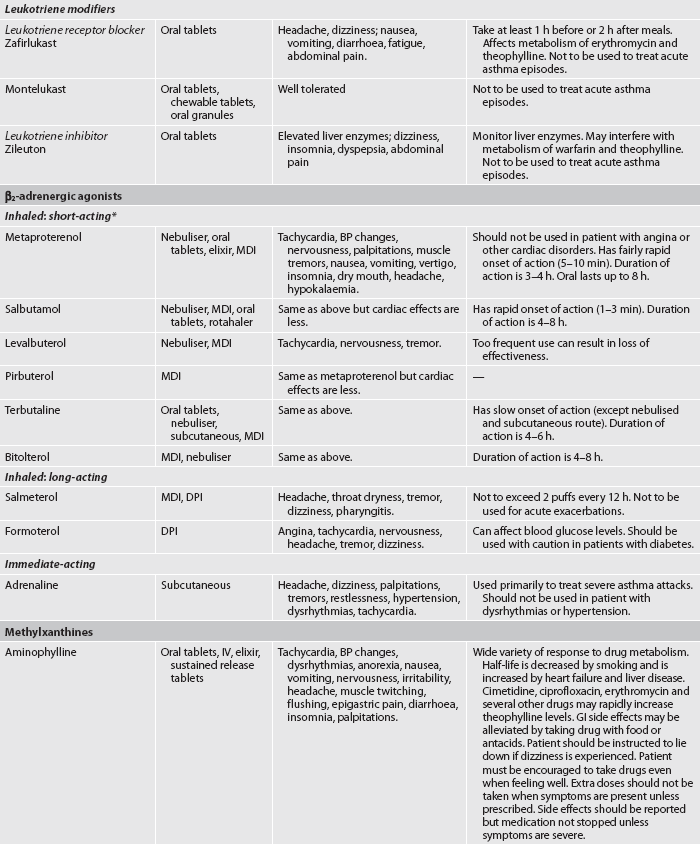

* Although these drugs are available in oral preparations, they are classified in this table based on their onset and duration of action as an inhaled drug. BP, blood pressure; CNS, central nervous system; DPI, dry powder inhaler; GI, gastrointestinal; HFA, hydrofluoralkane (propellant); ICS, inhaled corticosteroid; IV, intravenous; MDI, metered-dose inhaler.
Corticosteroids given by inhalation are active topically and can usually control the disease without systemic side effects. When administered in the aerosol form using MDIs, little systemic absorption occurs, thus eliminating the side effects that result from adrenal suppression seen with oral or IV corticosteroids. The National Institute for Health and Clinical Excellence has appraised the clinical and cost-effectiveness of inhaled corticosteroids for adults and children over the age of 12 years with chronic asthma.20 The findings do not support one make of drug over another and the guidance recommends a combination device for those people who need an ICS and long-acting β2-agonists (LABA).
Oropharyngeal candidiasis, hoarseness and dry cough are local adverse effects caused by inhalation of corticosteroids. These problems can be reduced or prevented by using a spacer (see Fig 28-4) with the MDI and by rinsing the mouth with water after each use. Using a spacer or holding device for inhalation of inhaled corticosteroids can be helpful in getting more medication into the lungs and less into the gastrointestinal tract, thus decreasing systemic side effects. However, newer drugs (e.g. ciclesonide) that are activated in the lungs (not the pharynx) appear to minimise these side effects without the need for a spacer or mouth rinsing.10
Short courses of orally administered corticosteroids are indicated for acute exacerbations of asthma. Side effects associated with short-term therapy include insomnia, heartburn, mood swings, blurry vision, headache, increased appetite and weight gain. Maintenance doses of oral corticosteroids may be necessary to control asthma in a minority of patients with severe chronic asthma when long-term therapy is required. A single dose in the morning to coincide with endogenous cortisol production and alternate-day dosing are associated with fewer side effects. Side effects of long-term corticosteroid therapy are discussed in Chapter 49; however, inhaled corticosteroids, particularly at the highest dosage levels, have been associated with the development of side effects such as easy bruising, accelerated bone loss and suppression of the hypothalamic–pituitary–adrenal axis.18 Women, especially postmenopausal women, who have asthma and who use corticosteroids should take adequate amounts of calcium and vitamin D and participate in regular weight-bearing exercise. (Osteoporosis is discussed in Ch 63.)
Leukotriene modifiers
Leukotriene modifiers include leukotriene receptor antagonists (zafirlukast, montelukast) and leukotriene synthesis inhibitors. These types of drugs interfere with the synthesis or block the action of leukotrienes.21 Leukotrienes are produced from arachidonic acid metabolism (see Fig 12-4). Leukotrienes are potent bronchoconstrictors and some also cause airway oedema and inflammation, thus contributing to the symptoms of asthma. Because these drugs block the release of some substances from mast cells and eosinophils, they have both bronchodilator and anti-inflammatory effects. These drugs are not indicated for use in the reversal of bronchospasm in acute asthma attacks. They are used for prophylactic and maintenance therapy. One advantage of leukotriene modifiers is that they are only administered orally.17 Leukotriene modifiers can be successfully used as add-on therapy to reduce (not substitute for) the doses of inhaled corticosteroids. They are less effective than long-acting β2-agonists as add-on therapy. There are few adverse effects from these drugs, but liver function should be monitored during treatment with zileuton.
Monoclonal antibody to IgE
Omalizumab is a monoclonal antibody to IgE that decreases circulating free IgE levels. Omalizumab prevents IgE from attaching to mast cells, thus preventing the release of chemical mediators16 and has been approved for use in both Australia and New Zealand for adults and adolescents with moderate allergic asthma that cannot be controlled with inhaled corticosteroids.17,22
Bronchodilators
Three classes of bronchodilator drugs currently used in asthma therapy are β2-adrenergic agonists, methylxanthine derivatives and anticholinergics (see Table 28-4).
β2-adrenergic agonist drugs
Short-acting inhaled β2-adrenergic agonists, such as terbutaline and salbutamol, have an onset of action within minutes and are effective for 4–8 hours. Inhaled β2-adrenergic agonists are indicated for the short-term relief of bronchoconstriction and are the treatment of choice for acute exacerbations of asthma.18 β2-adrenergic agonists are also useful in preventing bronchospasm precipitated by exercise and other stimuli because they prevent mediator release from mast cells. They do not inhibit the late-phase response. If used frequently, inhaled β2-adrenergic agonists may produce tremors, anxiety, tachycardia, palpitations and nausea. Overuse of β2-adrenergic agonists may cause rebound bronchospasm, especially common with salbutamol. Too frequent use of β2-adrenergic agonists indicates poor asthma control, may mask asthma severity and may lead to reduced drug effectiveness. Inhaled β2-agonists are not first-choice drugs for long-term control and they should not be used alone. They should be added to the treatment regimen when control has been inadequate with a preferred long-acting drug (e.g. inhaled corticosteroid). Oral β2-agonists are used for long-term control. However, they should not be used alone or as first-line therapy in treating asthma.
Longer-acting (8–12 hours or 24 hours) inhaled β2-adrenergic agonists include salmeterol and formoterol. These drugs are useful for nocturnal asthma. Patient teaching should stress that these drugs are used only once every 12 hours and are not to be used to obtain quick relief from bronchospasm. Long-acting inhaled β2-agonists may increase the risk of severe asthma and asthma-related death, but only when used incorrectly (i.e. when used alone as first-line monotherapy for long-term control).23 Patients should be told about the safety risks and that salmeterol should not be used when symptoms are significantly worsening or in acutely deteriorating asthma. Patients should have a short-acting β2-agonist available for acute breathing problems. If patients need more medicine than short-acting β2-agonists, then inhaled corticosteroids should be added first, with salmeterol added on if that is not enough to control symptoms. Long-acting β2-agonists should be used only in patients taking a recommended medication for long-term control, and only if that medication has been inadequate by itself.
Combination therapy using an inhaled corticosteroid and an inhaled long-acting β2-adrenergic agonist (salmeterol) is one of the outlined treatment options in internationally accepted asthma guidelines.4,18 Adding an inhaled corticosteroid to an inhaled long-acting β2-adrenergic agonist results in greater improvement in lung function and overall asthma control compared with higher-dose inhaled corticosteroids.
![]() DRUG ALERT—long-acting β2-adrenergic agonists
DRUG ALERT—long-acting β2-adrenergic agonists
• Should not be the first medicine used to treat asthma.
• Should never be used as the only medication to treat asthma; should be added to the treatment plan only if other controller medicines do not control asthma.
• Should not be used to treat wheezing that is getting worse.
• Should always use short-acting β2-agonists to treat sudden wheezing.
Methylxanthines
Methylxanthine (theophylline) preparations are less effective bronchodilators than inhaled β2-adrenergic agonists. The trend is now towards introducing theophylline as an additional bronchodilator later in the therapeutic regimen. Theophylline may have a synergistic effect with β2-adrenergic agonists. It is not effective as an inhalant and must be given orally or intravenously as aminophylline. Sustained-release theophylline preparations are preferable for maintenance therapy.
Although the exact mechanism of action is unknown, the main therapeutic action of methylxanthine derivatives is bronchodilation, which is useful in the early-phase response. Only minimal bronchodilation occurs at therapeutic theophylline concentrations.
Theophylline alleviates the early phase of asthma attacks and the bronchoconstrictive portion of the late-phase asthmatic response. However, it has no effect on bronchial hyperresponsiveness. Long-acting theophylline products administered at bedtime may be used to treat the patient with nocturnal asthma. The main problem with theophylline is the relatively high incidence of side effects, which include nausea, headache, insomnia, gastrointestinal distress, tachycardia, arrhythmias and seizures. Theophylline has a narrow margin of safety and serum blood levels should be monitored regularly to determine whether the drug is within therapeutic range.4
Anticholinergic drugs
Airway diameter is predominantly controlled by the parasympathetic division of the autonomic nervous system. The effects of acetylcholine on the airways are increased mucus secretion and smooth muscle contraction, resulting in bronchoconstriction. Anticholinergic agents (e.g. ipratropium) inhibit only the component of bronchoconstriction related to the parasympathetic nervous system. Thus these drugs are less effective than β2-adrenergic agonists and are usually used in combination with other bronchodilators. Anticholinergic agents produce most of their bronchodilation in larger airways, in contrast to β2-adrenergic agonists, which act primarily in smaller airways. Anticholinergics are not useful in routine asthma management but may be used as alternative bronchodilators for patients with severe adverse effects from β2-adrenergic agonist inhalers. They may also provide additive effects used in combination with β2-adrenergic agonists (e.g. formoterol and salmeterol).
The onset of action of anticholinergics is slower than β2-adrenergic agonists, peaking at 1 hour and lasting longer, usually up to 4–6 hours. Systemic side effects of inhaled anticholinergics are uncommon because they are poorly absorbed. The most common side effect of anticholinergic drugs is a dry mouth.
Inhalation devices for drug therapy
One of the major factors determining success in asthma management is the correct administration of drugs.4,18 There are multiple devices for asthma drug administration and their use can be confusing. The majority of asthma drugs are administered only or preferably by inhalation, because in many cases a lower dose is needed, systemic side effects are reduced and the onset of action is faster. Inhalation devices include nebulisers, MDIs and dry powder inhalers (DPIs).24
Nebulisers are devices that deliver a suspension of fine particles of liquid in a gas. Medication is nebulised, or reduced to a fine spray. Nebulisers are usually powered by a compressed-air or O2 generator. At home the patient may have an air-powered compressor; in the hospital, wall O2 or compressed air is used to power the nebuliser. Nebulisers generally deliver a larger dose of medication and are usually used for more severe asthma attacks. Aerosolised medication orders must include the medication, the dose, the diluent and whether it is to be nebulised with O2 or compressed air. The advantage of nebulisation therapy is that it is easy to use. Medications that are routinely nebulised include albuterol and ipratropium.
To ensure adequate penetration and deposition of the aerosolised medication, the patient is placed in an upright position that allows for most efficient breathing. The patient must breathe slowly and deeply through the mouth and hold inspiration for 2 or 3 seconds. Deep diaphragmatic breathing helps ensure deposition of the medication. Instruct the patient to breathe normally in between these large forced breaths to prevent alveolar hypoventilation and dizziness. After the treatment, instruct the patient to cough effectively.
A disadvantage of nebuliser equipment use is the potential for bacterial growth. A frequently used and effective home-cleaning method is to wash the nebuliser daily in soap and water, rinse it with water and soak it for 20–30 minutes in a 1:1 white vinegar–water solution, followed by a rinse with water and air drying. Commercial respiratory cleaning agents may also be used if directions are followed carefully. Cleaning the nebuliser in the top shelf of an automatic dishwasher saves time, and the hot water destroys most organisms.
MDIs are small, hand-held pressurised devices that deliver a measured dose of drug with each activation. Dosing is usually accomplished with one or two puffs. Some patients, particularly older adults, may have problems with the coordination required to activate the MDI and thus inhale the medication (see Fig 28-5). Poor coordination can be solved by the use of spacer devices (see Fig 28-4) or the use of a breath-activated MDI. If the patient is still unable to receive adequate medication, a nebuliser may be used.
The MDI should be cleaned by removing the dust cap and rinsing it in warm water (see Fig 28-5). Patients who need to use several MDIs are often unclear about the order in which to take the medications. Historically it was recommended that short-term β2-adrenergic agonists be used first to open up the airway and improve the delivery of subsequent medications. However, this is no longer recommended because there is no evidence demonstrating that this is beneficial and it is a potential source of confusion to patients, as short-term β2-adrenergic agonists are usually used on an ‘as required’ basis.21
One of the major problems with metered-dose drugs is the potential for overuse (i.e. using them much more frequently than prescribed [>2 canisters/month] rather than seeking medical care; see Box 28-4). As patients develop additional asthmatic symptoms, they may use the β2-adrenergic agonist MDI repeatedly. β2-adrenergic agonists help by relieving bronchospasm; they do not treat the inflammatory response. Therefore, patients must receive explicit instructions in the correct therapeutic use of these drugs. Also, patients need to know the correct way to determine whether the MDI is empty (see Fig 28-5). In the past, floating the MDI in water was an appropriate way to determine if medication remained in the MDI; however, this is no longer recommended as it is not accurate and water can enter the MDI. Patients should also be taught that shaking the canister is not an accurate way to determine if the MDI is empty, as they may be hearing only the propellant when the MDI is nearly empty.
BOX 28-4 Problems encountered with MDI use
• Failing to coordinate activation with inspiration
• Activating MDI in the mouth while breathing through nose
• Not holding the breath for 10 s (or as close to 10 s as possible)
• Holding MDI upside down or sideways
• Inhaling more than one puff with each inspiration
• Not waiting a sufficient amount of time between each puff
• Not opening mouth wide enough, causing medication to bounce off teeth, tongue or palate
DPIs are simpler delivery systems than MDIs and they are widely available (see Box 28-5 and Fig 28-6). The DPI contains dry, powdered medication and is breath activated. No propellant is used; instead an aerosol is created when the patient inhales through a reservoir containing a dose of powder. This convenient-to-carry discus has several advantages over MDIs: (1) less manual dexterity is needed; (2) the patient does not need to coordinate device puffs with inhalation; (3) an easily visible colour or number system indicates the number of doses left in the discus; and (4) its use does not require a spacer. Disadvantages are that commonly prescribed drugs are not yet available in DPIs and the medication may clump if exposed to humidity. Since the medicine is only delivered by the patient’s inspiratory effort, patients with a low FEV1 (<1 L) may not be able to inspire the medication adequately.
PATIENT & FAMILY TEACHING GUIDE
1. Remove mouthpiece cap or open the device according to manufacturer’s instructions. Check for dust or dirt. If there is an external counter, note the number of doses remaining.
2. Load the medicine into the inhaler or engage the lever to allow the medicine to become available. Some DPIs should be held upright while loading; others should be held sideways or in a horizontal position.
3. Do not shake your medicine.
4. Tilt your head back slightly and breathe out, getting as much air out of your lungs as you can (see Fig 28-6). Do not breathe into your inhaler because this could affect the dose.
5. Close your lips tightly around the mouthpiece of the inhaler.
6. Breathe in deeply and quickly. This will ensure that the medicine moves down deeply into your lungs. You may not taste or sense the medicine going into your lungs.
7. Hold your breath for 10 s or as long as you can to disperse the medicine into your lungs.
8. If there is an external counter, note the number of doses remaining as it should be one less than the number in point 1 above.
9. Do not keep your DPI in a humid place, such as a bathroom, as the medicine may clump.
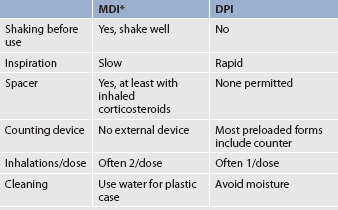
Differences between MDI and DPI are presented in Table 28-5. Aerosolised medication delivery systems, when used with comparable drug doses, provide equivalent efficacy. Therefore the device used should be that which is best suited to the individual patient.
Patient teaching related to drug therapy
Information about medications should include the name, purpose, dosage, method of administration and schedule, taking into consideration activities of daily living (ADLs) that require energy expenditure and thus oxygen, such as bathing. Teaching should also include side effects, appropriate action if side effects occur, how to properly use and clean the device, and consequences for breathing if not taking medications as prescribed.
Poor adherence with asthma therapy is a major challenge in the long-term management of chronic asthma. Lack of adherence often occurs because patients have no symptoms—thus they do not realise that the inflammatory process is ongoing and that they need inhaled corticosteroids. In addition, the inhaled drugs are expensive and patients may not be able to afford them. Patients will use β2-adrenergic agonist inhalers because they provide immediate relief of symptoms. However, if patients are symptom-free, they often do not use the long-term therapy (e.g. inhaled corticosteroids) regularly because no immediate benefit is felt. It is vital to explain to patients the importance and purpose of taking the long-term therapy regularly, emphasising that maximum improvement may take more than a week. It is also important to emphasise that without regular use, the swelling in the airways may progress and the asthma will likely worsen over time.
In addition to the typical MDI and DPI devices, a variety of other devices are used to deliver inhalant pulmonary medications. It is important to be certain that the patient understands exactly how to use the device and printed instructions should be given. Most inhalant drugs have very clear patient instructions, but the nurse needs to use either a placebo device or the actual drug to assess the patient’s ability to deliver the medication. The patient’s understanding of how to deliver the drug needs to be reassessed at every visit.
Since suboptimal inhaler techniques can reduce the effectiveness of inhaler therapy, research into the development of new devices is ongoing. One such device that is being trialled in Australia and New Zealand is Respimat®. This novel propellant-free metered-dose device releases a mist that appears to last longer and is slower moving than that released from traditional MDIs. The device appears to be easier to use than traditional inhalers.
Non-prescription combination drugs
Several non-prescription combination drugs are available over-the-counter. They are usually combinations of a bronchodilator and an expectorant. These agents are advertised as drugs to relieve bronchospasm. In general, they should be avoided. Many patients consider these drugs to be safe because they can be obtained without a prescription. However, dangers exist in drugs containing adrenaline, as it acts only for a short time and may increase the patient’s heart rate and blood pressure. This drug is not recommended for use. Drugs containing adrenaline (found in many over-the-counter decongestants) cause stimulation of the central nervous and cardiovascular systems. Side effects include nervousness, heart palpitations and dysrhythmias, tremors, insomnia and increases in blood pressure.
An important teaching responsibility is to warn patients about the dangers associated with using non-prescription combination drugs. These drugs are especially dangerous to patients with underlying cardiac problems because elevated blood pressure and tachycardia often result. Caution the patient who persists in taking one of these medications to read and follow the accompanying directions on the label. Often patients seek over-the-counter drugs as they are less expensive than prescription medication.
 NURSING MANAGEMENT: ASTHMA
NURSING MANAGEMENT: ASTHMA
 Nursing assessment
Nursing assessment
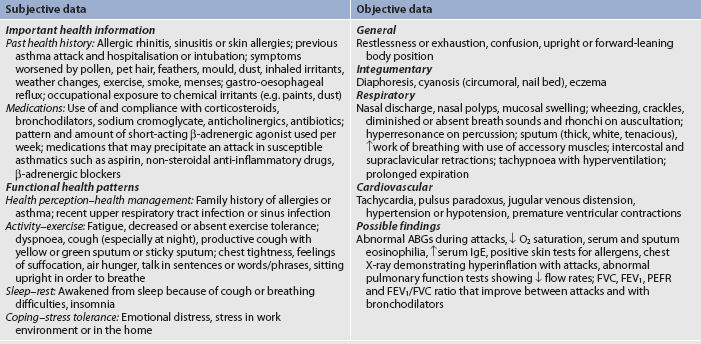
If a patient can speak and is not in acute distress, a detailed health history, including identification of any precipitating factors and what has helped alleviate attacks in the past, can be taken. Subjective and objective data that should be obtained from a patient with asthma are presented in Table 28-6.
 Nursing diagnoses
Nursing diagnoses
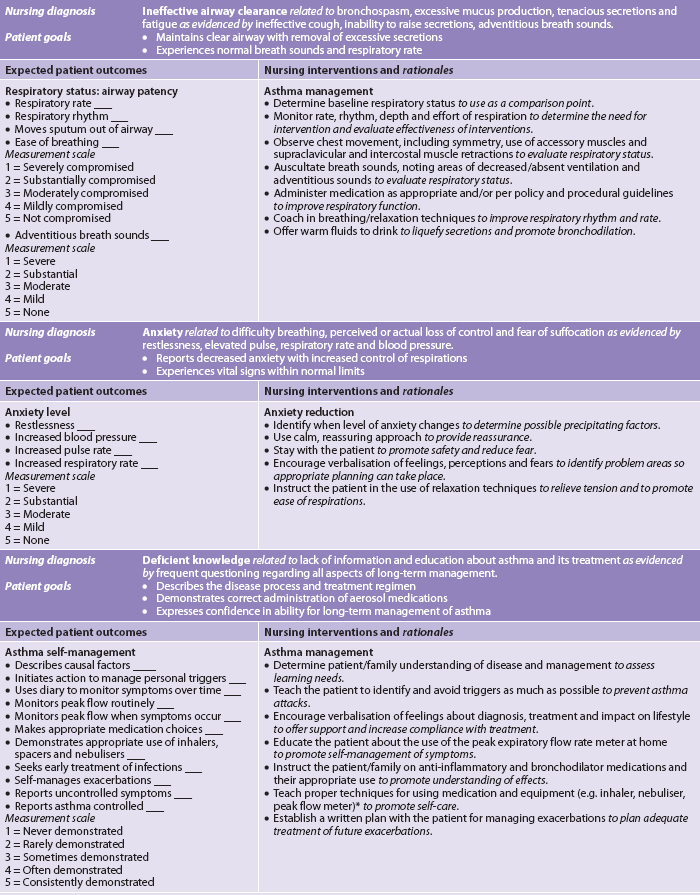
Nursing diagnoses for the patient with asthma may include, but are not limited to, those presented in NCP 28-1.
 Planning
Planning
The overall goals are that the patient with asthma will have: (1) normal or near-normal pulmonary function; (2) minimal symptoms during the day and night; (3) normal activity levels (including exercise and other physical activity); (4) no recurrent exacerbations of asthma, or decreased incidence of asthma attacks; and (5) adequate knowledge to participate in and carry out management.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The nursing role in preventing asthma attacks or decreasing their severity focuses primarily on teaching the patient and family/caregiver. The patient should be taught to identify and avoid known personal triggers for asthma (e.g. cigarette smoke, pet hair) and irritants (e.g. cold air, aspirin, foods, cats, indoor air pollution; see Box 28-1). Use of special dust covers on mattresses and pillows is thought to significantly reduce exposure to dust mites and improve symptoms, and some evidence supports this.25 However, another large-scale review of studies showed that chemical and/or physical methods aimed at reducing house dust mites did not change the asthma symptoms.26 Further studies are needed to determine methods that can reduce dust mite exposure and thus reduce symptoms in those allergy-prone individuals. If cold air cannot be avoided, dressing properly with scarves or using a mask helps to reduce the risk of an asthma attack. Aspirin and NSAIDs should be avoided if they are known to precipitate an attack. Many over-the-counter preparations contain aspirin and patients should be instructed to read the labels carefully. β2-adrenergic receptor blockers (e.g. propranolol) are contraindicated because they inhibit bronchodilation. Desensitisation (immunotherapy) may be partially effective in decreasing the patient’s sensitivity to known allergens (see Ch 13).
Prompt diagnosis and treatment of upper respiratory tract infections and sinusitis may prevent an exacerbation of asthma. If occupational irritants are involved as aetiological factors, the patient may need to consider changing jobs. Treatment of GORD and preventive measures for it may increase asthma control.14 The patient should be encouraged to maintain a fluid intake of 2–3 L per day, good nutrition and adequate rest. If exercise is planned, the healthcare provider can suggest a medication regimen for pre-treatment or long-term control of symptoms to prevent bronchospasm.
 Acute intervention
Acute intervention
One goal in asthma care is to maximise the patient’s ability to safely manage acute asthma episodes via an asthma action plan developed in conjunction with the healthcare provider (see Fig 28-7). Action plans are particularly important for those individuals with moderate to severe persistent asthma or severe exacerbations. The action plan will dictate what symptoms or peak flow reading necessitates a change in asthma care to gain control. The patient can take 2–4 puffs of a short-acting β2-agonist every 20 minutes three times or one nebulised treatment as a rescue plan. Depending on the response with alleviation of symptoms or improved peak flow, continued SABA use and/or oral corticosteroids may be a part of the home management plan at this point. If symptoms persist or if the patient’s peak flow is less than 50% of the personal best, the patient’s healthcare provider or emergency services (EMS) needs to be contacted immediately.

Figure 28-7 Asthma self-management plan. Note: Emergency number in Australia is 000.
Source: Asthma and Respiratory Foundation of New Zealand.
When the patient experiences an acute exacerbation in the healthcare facility, it is important for the nurse to monitor the patient’s respiratory and cardiovascular systems. This includes auscultating lung sounds; taking the pulse rate, respiratory rate and BP; and monitoring ABGs, pulse oximetry, FEV1 and PEFR. Signs or symptoms that warrant urgent medical intervention to avoid respiratory failure in a severe asthma attack are heart rate >120 beats/min, respiratory rate >30 breaths/min, wheezes heard on chest auscultation which turn silent, speaking in words (not sentences), oxygen saturation < 90%, PaO2 <8 kPa (60 mmHg), PaCO2 >6 kPa (45 mmHg), PEFR <100 L/min and agitation.4
Nursing interventions include administering O2, bronchodilators and medications (as ordered) and ongoing patient monitoring (especially lung auscultation), including the effectiveness of these interventions. It is important to note that louder wheezing may actually occur in airways that are responding to therapy as airflow in the airways increases. As improvement continues and airflow increases, breath sounds increase and wheezing decreases. As the patient begins to respond to therapy and symptoms begin to subside, it is important to remember that despite the disappearance of most of the bronchospasm, the oedema and cellular infiltration of the airway mucosa and the viscous mucus plugs may take several days to improve. Thus intensive therapy must be continued even after clinical improvement has occurred.
An important nursing goal during an acute attack is to decrease the patient’s sense of panic. Having a calm, quiet, reassuring attitude may help the patient to relax. The patient should be positioned comfortably (usually sitting) to maximise chest expansion. The nurse should stay with the patient, as this provides additional comfort. The technique of ‘talking down’ can help the patient to remain calm. In talking down, the nurse gains eye contact with the patient and calmly and firmly encourages slow breathing using pursed lips, which keeps the airways open for longer by promoting positive pressure (pursed-lip breathing is explained on p 709) and abdominal breathing, which slows the respiratory rate and encourages deeper breaths.
When the acute attack subsides, the patient should be encouraged to rest in a quiet, calm environment. When the patient has recovered from exhaustion, the nurse can obtain information about the patient’s health history and pattern of asthma. If family members are present, they may be able to provide information about the patient’s health history. A thorough physical assessment should be completed (see Table 28-6). This information is important in planning an individualised nursing care plan for the patient. Well-thought-out written plans involving the patient and significant others increase the patient’s knowledge and control of the situation and may help improve confidence and compliance.
 Ambulatory and community care
Ambulatory and community care
It is important to remember that asthma is potentially controllable and that every effort should be made to keep the patient free of symptoms. Patients with asthma usually take several medications with different routes of administration and time frames for dosage (e.g. tapering corticosteroid schedules, using several different inhalers with different indications). The drug regimen itself can be confusing and complex. Patients must learn about the numerous medications and develop self-management strategies. The patient and healthcare professional need to monitor the patient’s responsiveness to medication. It is easy to undermedicate or overmedicate a patient with asthma unless careful monitoring is ongoing. Some patients may benefit from keeping a diary to record medication use, the presence of wheezing or coughing, the PEFR, drug side effects and their activity level. This information will be valuable in helping the healthcare provider to adjust the medication. Patients need to understand the importance of continuing the medication even when symptoms are not present. If worsening bronchospasm or severe drug side effects occur, patients should seek medical attention.
Good nutrition is important. Physical exercise (e.g. swimming, walking) within the patient’s limit of tolerance is also beneficial. If dyspnoea occurs on exertion, it can often be prevented with the use of a β2-adrenergic agonist MDI, sodium cromoglycate or nedocromil. Sleep that is uninterrupted by asthma symptoms is important. If patients wake up because of asthma symptoms, their asthma is not under sufficient control and their therapeutic plan should be re-evaluated.
A written asthma management plan (see Fig 28-7) should be developed with the patient and family. Most plans are developed based on the patient’s asthma symptoms, activity levels and peak flow readings. A management plan can be undertaken when the patient’s best peak flow is established and the patient has good asthma control (e.g. not waking up at night with asthma symptoms, able to perform some type of aerobic exercise or strenuous activity, not having frequent daily symptoms).
To follow the management plan, patients must measure their peak flow at least daily. Patients with asthma frequently do not perceive changes in their breathing. Therefore, peak flow monitoring, when done correctly, can be a good objective measurement of asthma (see Box 28-6). Using the PEFR is similar to using BP monitoring in a person with hypertension.
BOX 28-6 How to use your peak flow meter
PATIENT & FAMILY TEACHING GUIDE
• A peak flow meter helps you check how well your asthma is controlled and can provide an early warning of an attack. Peak flow meters are most helpful for people with moderate-to-chronic asthma.
• It is important that you see your healthcare professional to help you develop a good technique and to identify your personal best peak flow reading.
• You need to sit upright or be standing up; slide the marker to the end of the scale; hold the peak flow meter horizontally; keep your fingers away from the marker and take a deep breath; close your lips around the mouthpiece and blow out as hard and as fast as you can; take a note of the reading and repeat this whole process three times; take a note of your highest reading. To check your asthma each day, take your peak flow in the morning and evening at the same times each day.
Your peak flow zones
Your peak flow zones are based on your usual best peak flow number. The zones will help you check your asthma and take the right actions to keep it controlled. The colours used with each zone come from the traffic light.
Green zone (80–100% of your usual best) signals good control. Take your usual daily long-term control medicines, if you take any. Keep taking these medicines even when you are in the yellow or red zones.
Yellow zone (50–79% of your personal best) signals caution: your asthma is getting worse. Add quick-relief medicines. You might need to increase other asthma medicines as directed by your doctor.
Red zone (<50% of your personal best) signals medical alert! Add or increase quick-relief medicines and call your doctor now.
Ask your healthcare professional to write an action plan for you that tells you:
Source: Adapted from National Asthma Education and Prevention Program (NAEPP) Expert Panel Report. Guidelines for the diagnosis and management of asthma—update on selected topics, 2002. Available at www.nhlbi.nih.gov/guidelines/asthma/asthsumm.htm, accessed 22 January 2011.
If a patient’s PEFR is within the green zone (usually 80–100% of personal best), the patient should remain on their usual medications. If the PEFR is within the yellow zone (usually 50–80% of personal best), it indicates caution. Something is triggering the patient’s asthma. Patients who get a cold or sinus infection, which may trigger asthma, should have a written asthma action plan that prescribes an increase in medications during the acute phase of the infection.18 The dose is usually decreased once the cold subsides. Different strategies, based on the asthma management plan, may be employed by the patient. For example, the patient could use the β2-adrenergic agonist inhaler more frequently. If the PEFR is in the red zone (50% or less of personal best), it indicates a serious problem. A rescue plan should be a part of the asthma action plan. Definitive action must be taken, such as take 4 puffs of a short-acting inhaled β2-adrenergic agonist, wait 4 minutes and if no improvement take a further 4 puffs. If no improvement or symptoms become worse, call an ambulance and continue to take the 4 puffs every 4 minutes until assistance arrives. In addition to increasing the use of β2-adrenergic agonist inhalers, oral corticosteroids may be indicated. The patient needs to contact or be seen by the healthcare provider.
It is important to emphasise to the patient the need to monitor the PEFR daily, because asthma tends to worsen gradually over time. It is unusual for a patient’s PEFR to drop quickly, although it may happen. Usually the patient has time to make changes in medications, avoid triggers and notify the healthcare provider.
When developing a management plan, it is important to involve the patient’s family. Often family members feel frustrated and do not know how to help. Family members should be taught what can be done to help the patient during an asthmatic attack. They should know where the patient’s inhalers, oral medications and emergency phone numbers are located. They can also be instructed on how to decrease the patient’s anxiety if an asthma attack occurs. When the patient is stabilised or controlled, they can gently remind the patient about monitoring the daily PEFR by asking questions such as, ‘How’s your peak flow today?’
An increased number of older adults are diagnosed with asthma and they have more complicated health issues than younger patients with asthma. Issues that older adults (particularly from urban and minority backgrounds), caregivers and health professionals have identified as problematic in this group are the negative impact of asthma on quality of life, costly medications, non-adherence to the medical regimen and difficulty in accessing the health system. These factors should be kept in mind when implementing a management plan for older adults.27
Counselling may be indicated to help the patient and the family to resolve personal, family, social and occupational problems that have resulted from asthma. Relaxation therapies (e.g. yoga, meditation, relaxation techniques, breathing techniques) may be of value in helping the patient to relax respiratory muscles and decrease the respiratory rate. A healthy emotional outlook can also be important in preventing future asthma attacks. Resources that can be used when teaching the patient about asthma include fact sheets provided by the Australian Lung Foundation and the National Asthma Council Australia (see Resources on pp 727–728). These associations provide educational materials about asthma, including asthma management handbooks and asthma management plans. Table 28-7 is a patient and family teaching guide for the patient with asthma.
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease state characterised by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking. Although COPD affects the lungs, systemic consequences also develop.3 The term ‘chronic obstructive pulmonary disease’ is used to encompass two types of obstructive airway diseases: chronic bronchitis and emphysema. Chronic bronchitis is the presence of chronic productive cough for 3 months in each of 2 consecutive years in a patient in whom other causes of chronic cough have been excluded. Emphysema is an abnormal permanent enlargement of the airspaces distal to the terminal bronchioles, accompanied by destruction of their walls and without obvious fibrosis. Only about 10% of patients with COPD have pure emphysema. Patients with COPD may have a predominance of one of these conditions, but in reality it is often difficult to determine as the conditions usually coexist. In this section COPD is discussed as one disease state from the standpoint of pathophysiology and management.
Patients with COPD may have asthma and some patients with asthma may go on to develop fixed or irreversible airflow obstruction. It may be nearly impossible to differentiate asthma from COPD, especially if the individual has a history of cigarette smoking.1,3
Current statistics indicate that approximately 2.1 million Australians have some form of COPD,2,28 but this figure is expected to more than double by 2050 to approximately 4.5 million.2 It is estimated that 1.2 million Australians have moderate to severe stages of the disease (stages II–IV), where their lives are affected by symptoms, and a further 900,000 have a milder form (stage I).2,28 The latter group may ignore their symptoms, yet many will go on to develop more severe stages if they do not take appropriate action to manage their condition.
The situation is similar in New Zealand: COPD affects an estimated 15% of the adult population over the age of 45 years (at least 200,000 New Zealanders) and is the fourth leading cause of death.1 There is a disparity between Māori and non-Māori populations, with Māori men aged 45 years and over having a hospitalisation rate from COPD more than four times that of non-Māori men in the same age group, and Māori women having a hospitalisation rate five times that of non-Māori women. A similar pattern is seen with COPD mortality rates.29
Worldwide, it is predicted that COPD will become the third leading cause of death by 2020 because of the increase in cigarette smoking and increased life spans.
AETIOLOGY
The many factors involved in the aetiology of COPD are discussed in this section; however, exposure to cigarette smoke is the primary cause of COPD in Australia and New Zealand.30
Cigarette smoking
Although the prevalence of cigarette smoking by males in Australia and New Zealand has decreased, it is rising among females and is still a major public health concern among young people. It is estimated that if smoking were eliminated, many deaths from COPD could be prevented. For most people who die of lung diseases related to cigarette smoking, death is preceded by a long period of debilitation characterised by frequent hospitalisations and loss of many years of productivity. Cigarette smoking is extremely costly to both the individual and society and cigarette smoking remains one of the most preventable causes of premature death. In addition to being linked with COPD and lung cancer, cigarette smoking has also been implicated as a factor in cancers of the mouth, pharynx, larynx, oesophagus, pancreas, kidney, stomach, cervix and bladder.
When cigarettes are smoked, approximately 4000 chemicals and gases are inhaled into the lungs. More than 60 carcinogens have been isolated from cigarette smoke, including cyanide, formaldehyde and ammonia.1 Nicotine is probably not a carcinogen but it has other deleterious effects. It acts by stimulating the sympathetic nervous system, resulting in increased heart rate, increased peripheral vasoconstriction, increased BP and increased cardiac workload. These effects of nicotine compound the problems in a person with coronary artery disease. (The effects of nicotine are discussed in Ch 10.)
Cigarette smoke has several direct effects on the respiratory tract (see Table 28-8). The irritating effect of the smoke causes hyperplasia of cells, including goblet cells, which subsequently results in increased production of mucus. Hyperplasia reduces airway diameter and increases the difficulty in clearing secretions. Smoking reduces ciliary activity and may cause actual loss of ciliated cells. Smoking also produces abnormal dilation of the distal air space with destruction of alveolar walls. Many cells develop large, atypical nuclei, which are considered precancerous states.
TABLE 28-8 Effects of tobacco smoke on the respiratory system
| Area of defect | Acute effects | Long-term effects |
|---|---|---|
| Respiratory mucosa | ||
| Nasopharyngeal | ↓ sense of smell | Cancer |
| Tongue | ↓ sense of taste | Cancer |
| Vocal cords | Hoarseness | Chronic cough, cancer |
| Bronchus and bronchioles | Bronchospasm, cough | Chronic bronchitis, asthma, cancer |
| Cilia | Paralysis, sputum accumulation, cough | Chronic bronchitis, cancer |
| Mucus glands | ↑ secretions, ↑ cough | Hyperplasia and hypertrophy of glands, chronic bronchitis |
| Alveolar macrophages | ↓ Function | Incidence of infection |
| Elastin and collagen fibres | ↑ Destruction by proteases, ↓ function of antiproteases (α1-antitrypsin),↓ synthesis and repair of elastin | Emphysema |
After only a short time of smoking, changes in small airway function can develop. In the early stages these changes are mostly inflammatory with mucosal oedema and an influx of inflammatory cells. In later stages, however, thickening of the airway wall occurs by a remodelling process related to tissue repair and the inability of cilia to clear mucus, thus resulting in an accumulation of inflammatory exudates in the airway lumen. Quitting smoking can prevent or delay the development of airflow limitation or reduce its progression.3 (See Ch 10 for more detail about cigarette smoking.)
Passive smoking is the exposure of non-smokers to cigarette smoke, also known as environmental tobacco smoke or second-hand smoke. In adults, involuntary smoke exposure is associated with decreased pulmonary function, increased respiratory symptoms and severe lower respiratory tract infections such as pneumonia. Environmental tobacco smoke is also associated with increased risk for lung cancer and nasal sinus cancer.31 The cardiovascular system is affected by environmental tobacco smoke with increased heart rate and blood pressure and decreased levels of high-density lipoproteins (HDL). Deaths can be attributed to cardiovascular disease related to second-hand smoke; for example, this caused the deaths of approximately 244 New Zealanders in 200032 and in Australia the figure is estimated to be about 1500 deaths per year, although exact data are unavailable.
Occupational chemical and dusts
If a person has intense or prolonged exposure to various dusts, vapours, irritants or fumes in the workplace COPD can develop independently of cigarette smoking. If the person smokes, the risk of COPD increases. Exposure to these irritants causes the airways to be hyperresponsive.1
Air pollution
High levels of urban air pollution are harmful to persons with existing lung disease. However, the effect of outdoor air pollution as a risk factor for COPD appears to be small compared to the effect of cigarette smoking. Another risk factor for COPD development is fossil fuels that are used for indoor heating and in some cases cooking. Many women worldwide, who have never smoked, are developing COPD because of using these fuels in poorly ventilated areas.1
Infection
Infections are a risk factor for developing COPD. Severe recurring respiratory tract infections in childhood have been associated with reduced lung function and increased respiratory symptoms in adulthood. Recurring infections impair normal defence mechanisms, making the bronchioles and alveoli more susceptible to injury. The person with COPD is prone to acute exacerbations of the disease, with 50–75% of cases thought to be caused by bacteria. These respiratory infections subsequently intensify the pathological destruction of lung tissue and the progression of COPD. The most common causative organisms are Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis.1
Genetics
The fact that a relatively small percentage of smokers get COPD strongly suggests that there are genetic factors that influence which smokers get the disease. Two people may have the same smoking history, yet because of the gene–environment interaction only one develops COPD. Another possible explanation is that because of genetic predisposition, certain people live longer and this influences which people develop COPD.3,33
Alpha1-antitrypsin (AAT) deficiency is the only known genetic abnormality that leads to COPD.1 AAT deficiency accounts for a very small percentage of cases of COPD in Australia and New Zealand, but it has been suggested that the deficiency may not be so rare as first thought—rather it is underreported or undertested. Also known as alpha1-protease inhibitor, AAT is a serum protein produced by the liver and is normally found in the lungs.34 Severe AAT deficiency leads to premature emphysema, often with chronic bronchitis and occasionally with bronchiectasis. Emphysema results when lysis of lung tissues by proteolytic enzymes from neutrophils and macrophages occurs because of AAT deficiency. Normally AAT inhibits the action of these enzymes. Therefore, lower levels of AAT result in insufficient inactivation and subsequent destruction of lung tissue. Smoking greatly exacerbates the disease process in these patients.
HEALTH DISPARITIES
The level of AAT is controlled by a pair of autosomal codominant genes. Low levels of AAT are related to homozygosity for the deficiency gene (ZZ), intermediate levels to heterozygosity (MZ) and normal values to homozygosity for the normal gene (MM). In the recessive gene homozygous group, onset of symptoms often occurs by age 40 years and the disease is found as frequently in women as in men. People with this type of COPD are primarily of northern European origin, but it has also been found in their descendants in Australia and New Zealand.
IV-administered AAT augmentation therapy is used for those with AAT deficiency. The infusions are administered weekly. Its effectiveness in slowing the progression of the disease continues to be evaluated.34
Ageing
Some degree of emphysema is common in the lungs of the older person, even a non-smoker. Ageing results in changes in the lung structure, the thoracic cage and the respiratory muscles. As people age there is gradual loss of the elastic recoil of the lungs. The lungs become more rounded and smaller. The number of functional alveoli decreases as a result of the loss of the alveolar supporting structures and loss of the intra-alveolar septum. These changes are similar to those seen in patients with emphysema. Clinically significant emphysema, however, is usually not caused by ageing alone.
Thinner alveolar walls contribute to loss of alveolar septal tissue and alveolar capillaries. With fewer capillaries available for gas exchange, arterial oxygen levels decrease. The PaO2 falls at a rate of 0.5 kPa (4 mmHg) for each decade of life, beginning after 20 years of age. The surface area available for gas exchange decreases from 80 m2 at 20 years of age to 65–70 m2 by 70 years of age.35
Thoracic cage changes result from osteoporosis and calcification of the costal cartilages. The thoracic cage becomes stiff and rigid and the ribs are less mobile. The shape of the rib cage gradually changes because of the increased functional residual capacity (FRC), causing it to expand and become rounded. Decreased chest compliance and elastic recoil of the lungs caused by ageing affect the mechanical aspects of ventilation and increase the work of breathing. Changes in the elasticity of the lungs reduce the ventilatory reserve, and the ability to clear secretions decreases with age.35
PATHOPHYSIOLOGY
COPD is characterised by chronic inflammation found in the airways, lung parenchyma (gas exchanging surfaces of the lung [respiratory bronchioles and alveoli]) and pulmonary blood vessels (see Fig 28-8). The pathogenesis of COPD is quite complex and involves many mechanisms. The defining features of COPD are irreversible airflow limitation during forced exhalation caused by loss of elastic recoil, and airflow obstruction caused by mucus hypersecretion, mucosal oedema and bronchospasm. COPD results in an uneven distribution of pathological changes leaving severely destroyed lung areas existing with areas of relatively normal lung.36
The inflammatory process starts with inhalation of noxious particles and gases (e.g. cigarette smoke, air pollution). Then the predominant inflammatory cells, macrophages and lymphocytes (primarily CD8 cells), increase and release inflammatory mediators including leukotrienes, interleukins and tumour necrosis factor. In addition, growth factors are recruited into the area and activated, resulting in structural changes in the lungs. The inflammatory process may be magnified by oxidative stress. Oxidants produced by cigarette smoke and other inhaled particles are released from the inflammatory cells, such as macrophages and neutrophils, during inflammation. The oxidative stress adversely affects the lungs as it inactivates antiproteinases (which prevent the natural destruction of the lungs), stimulates mucus secretion and increases fluid in the lungs.3 Therefore the natural balance of proteinase/antiproteinase is tipped in favour of destruction of the alveoli and loss of the elastic recoil of the lung.3,37
In COPD, as the supporting structures of the lung are destroyed, there is no pull or traction on the walls of the bronchioles. Like air being blown into a paper bag, air goes into the lungs easily but is unable to come out on its own; it remains in the lung. Thus the bronchioles tend to collapse (especially on expiration) and air is trapped in the distal alveoli, resulting in hyperinflation and overdistension of the alveoli. This trapped air gives the patient the typical barrel-chested appearance. Thus the lungs can be inflated easily but can only partially deflate.
Destruction of the lung parenchyma is thought to be due to an imbalance of proteinases/antiproteinases. This occurs as a consequence of inflammation, but there can be a genetic basis to the proteinase imbalance. The parenchyma contains elastin, which provides the structural make-up of the connective tissue of the alveolar wall. In a healthy person proteinases (elastase is the main component) in the parenchyma function to break down the alveolar walls (elastin). Normally, proteinase inhibitors (i.e. AAT) prevent this destructive process. In smokers the numbers of neutrophils, macrophages and other inflammatory cells are increased and, with the inflammatory process, overwhelm the body’s normal AAT defence. Therefore there is destruction of the basic elastin structure of the parenchyma. In addition, inflammatory cells in COPD release a variety of inflammatory mediators, such as leukotrienes and interleukins. In the genetic form of emphysema there is a deficiency of AAT and an imbalance of proteinases/antiproteinases occurs. Gas exchange abnormalities result in hypoxemia and hypercarbia (increased CO2) as the disease worsens. As the air trapping worsens and alveoli are destroyed, bullae (large air spaces in the parenchyma) and blebs (air spaces adjacent to pleurae) can form (see Fig 28-9).
Bullae and blebs are not effective in gas exchange as the capillary bed that normally surrounds each alveolus does not exist in the bullae or bleb. Therefore there is a significant ventilation–perfusion (V/Q) mismatch and hypoxaemia results. Peripheral airway obstruction also results in V/Q imbalance and, combined with the respiratory muscle impairment, can result in CO2 retention, particularly in severe disease.3,37
Excess mucus production, resulting in a chronic productive cough, is a feature of individuals with predominant chronic bronchitis and is not necessarily associated with limitation in airflow. However, not all COPD patients have sputum production. Excess mucus production is a result of an increased number of mucus-secreting goblet cells and enlarged submucosal glands, which respond to the chronic irritation of smoke or other inhalants. In addition, dysfunction of cilia leads to chronic cough and sputum production. Some of the inflammatory mediators also stimulate mucus production.
Pulmonary blood vessel changes resulting in mild to moderate pulmonary hypertension may occur late in the course of COPD. The small pulmonary arteries vasoconstrict due to hypoxia and their structure changes, resulting in thickening of the vascular smooth muscle as the disease advances. Because of the loss of alveolar walls and the capillaries surrounding them, there is increased pressure in the pulmonary circulation. Typically, the patient does not have difficulty with hypoxaemia at rest until late in the disease. However, hypoxaemia may develop during exercise, and the patient may benefit from supplemental O2.
Pulmonary hypertension may progress and lead to hypertrophy of the right ventricle of the heart, or cor pulmonale, with or without right-sided heart failure. COPD has been shown to have systemic effects, especially in severe disease. These extrapulmonary changes contribute greatly to the clinical findings of the patient and affect their survival and management. The mechanisms that cause the changes are unclear and are likely multifaceted, but systemic inflammation and inactivity of the patient are likely key factors.38 Cachexia is common with a loss of skeletal muscle mass, and weakness is likely due to increased apoptosis (programmed cell death) and/or muscle disuse.3 Patients may have weakness in all muscles in the upper and lower extremites.39 They also have exercise intolerance, deconditioning and osteoporosis. Patients with severe COPD may develop chronic anaemia, anxiety, depression and an increased incidence of cardiovascular (CV) disease. The latter is likely due to an increase in C-reactive protein (another inflammatory marker linked with CV disease).3,38
Clinically it is common to find a combination of emphysema and chronic bronchitis in the same person, often with one condition predominating. Patients with COPD may also have asthma and if they experience poorly reversible airflow limitation, their condition may be indistinguishable from COPD, but clinically are treated as asthma.
CLINICAL MANIFESTATIONS
Clinical manifestations of COPD typically develop slowly around 50 years of age after 20 pack-years of cigarette smoking.35 A diagnosis of COPD should be considered in any patient who has symptoms of cough, sputum production or dyspnoea, and/or a history of exposure of risk factors for the disease. A chronic intermittent cough usually occurs in the morning and may or may not be associated with the production of small amounts of sticky mucus. Typically, dyspnoea is progressive, usually occurs with exertion and appears every day. However, patients may dismiss the symptoms as they rationalise, ‘I’m getting older’ or ‘I’m out of shape’. They change behaviours to avoid dyspnoea, such as taking the lift. Gradually the dyspnoea interferes with daily activities, such as carrying shopping bags, and they cannot walk as fast as their spouse or peers. Patients often seek medical help when they have an acute respiratory infection and their dyspnoea worsens.
In late stages of COPD, dyspnoea may be present at rest. As more alveoli become overdistended, increasing amounts of air are trapped. This causes a flattened diaphragm and an increased anterior–posterior diameter of the chest, forming the typical barrel chest. Effective abdominal breathing is decreased because of the flattened diaphragm from the overdistended lungs. The person becomes more of a chest breather, relying on the intercostal and accessory muscles. However, chest breathing is not efficient breathing.
Wheezing and chest tightness may be present, but may vary by time of the day or from day to day, especially in patients with more severe disease. The wheeze may arise from the laryngeal area or wheezes may not be present on auscultation. Chest tightness, which often follows activity, may feel similar to muscular contraction.
The person with advanced COPD frequently experiences weight loss and anorexia. Even when the patient has adequate kilojoule intake, weight loss is still experienced. Fatigue is a highly prevalent symptom that affects the patient’s activities of daily living.40 Haemoptysis can occur during respiratory tract infections.
During physical examination a prolonged expiratory phase of respiration, wheezes or decreased breath sounds are noted in all lung fields. The patient may need to breathe louder than normal for auscultated breath sounds to be heard. The anterior–posterior diameter of the chest is increased (‘barrel chest’) from the chronic air trapping. The patient may sit upright with arms supported on a fixed surface such as an over-bed table (tripod position). The patient may naturally purse lips on expiration (pursed-lip breathing) and use accessory muscles, such as those in the neck, to aid with inspiration. Oedema in the ankles may be the only clue to right heart involvement.
Over time, hypoxaemia (PaO2 <8 kPa [60 mmHg] or SaO2 <88%) may develop with hypercapnia (PaCO2 >6 kPa [45 mmHg]) later in the disease. The bluish-red colour of the skin results from polycythaemia and cyanosis. Polycythaemia develops as a result of increased production of red blood cells as the body attempts to compensate for chronic hypoxaemia. Haemoglobin concentrations may reach 200 g/L or more. Cyanosis develops when there is at least 50 g/L or more of circulating unoxygenated haemoglobin.
As noted previously, it is sometimes quite difficult for the healthcare provider to distinguish COPD from asthma. However, there are some clinical features that are different (see Table 28-9).
TABLE 28-9 Comparison of asthma and COPD*
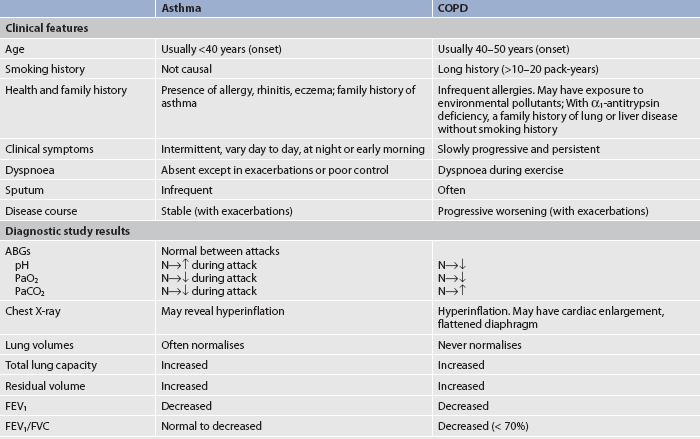
ABGs, arterial blood gases; AP, anteroposterior; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; N, normal.
* Persons may have features of both asthma and COPD.
Source: Adapted from Barnes P, Drazen J, Rennard s et al., eds. Asthma and COPD: basic mechanisms and clinical management. London: Academic Press; 2002.
CLASSIFICATION OF COPD
COPD should be considered in any person with an exposure to risk factors such as cigarette smoking and/or environmental or occupational pollutants and/or chronic cough and dyspnoea. The diagnosis is confirmed by spirometry. COPD can be classified as mild, moderate and severe (see Table 28-10). The FEV1/FEV <70% establishes the diagnosis of COPD and the severity of obstruction (as indicated by FEV1) determines the stage of COPD. The management of COPD is primarily based on the patient’s symptoms, but the staging provides a general guideline for the type of interventions.
TABLE 28-10 Classification of severity of COPD
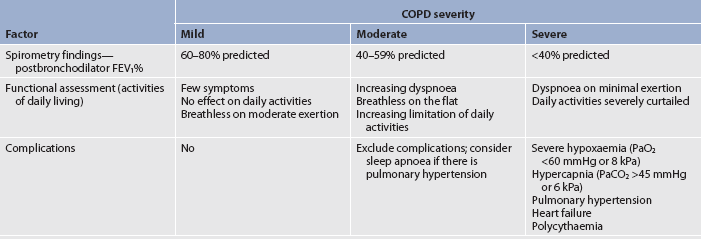
FEV1, forced expiratory volume in 1 second; PaO2, partial pressure of oxygen, arterial; PaCO2, partial pressure of carbon dioxide, arterial. Source: http://copdx.org.au/guidelines/index.asp, accessed 22 January 2011.
COMPLICATIONS
Cor pulmonale
Cor pulmonale is hypertrophy of the right side of the heart, with or without heart failure, resulting from pulmonary hypertension. In COPD, pulmonary hypertension is caused primarily by constriction of the pulmonary vessels in response to alveolar hypoxia, with acidosis further potentiating the vasoconstriction (see Fig 28-10). Cor pulmonale is a late manifestation of chronic pulmonary heart disease. The patient benefits most when a diagnosis of pulmonary heart disease can be made early so therapy can be instituted. In patients with severe COPD, 40% demonstrate cor pulmonale and the prognosis is poor.41 Chronic alveolar hypoxia causes vascular remodelling. Chronic hypoxia also stimulates erythropoiesis, which causes polycythaemia. This results in increased viscosity of the blood. In COPD there may be an anatomical reduction of the pulmonary vascular bed as seen in emphysema with bullae. These patients would have increased pulmonary vascular resistance.
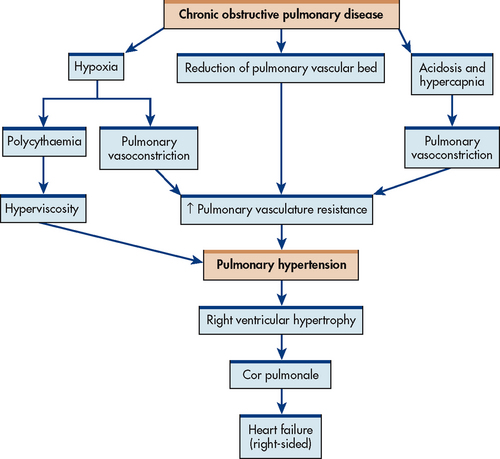
Figure 28-10 Mechanisms involved in the pathophysiology of cor pulmonale secondary to chronic obstructive pulmonary disease.
Normally the right ventricle and pulmonary circulatory system are low-pressure systems compared with the left ventricle and systemic circulation. When pulmonary hypertension develops, the pressures on the right side of the heart must increase to push blood into the lungs. Eventually, right-sided heart failure develops.
The clinical manifestations of chronic pulmonary heart disease and cor pulmonale are related to dilation and failure of the right ventricle with subsequent intravascular volume expansion and systemic venous congestion. Dyspnoea is a usual symptom and is associated with hypoxaemia and hypercarbia. Lung sounds are normal or crackles may be heard in the bases of the lungs bilaterally. Heart sound changes include accentuation of the pulmonic component of the second heart sound, right-sided ventricular diastolic S3 gallop and a loud pulmonic component of S2 along the left sternal border. ECG changes include increased P wave amplitude (P pulmonale), a tendency for right axis deviation and incomplete right bundle branch block. Overt manifestations of right-sided heart failure may develop, which include distended neck veins (jugular venous distension), hepatomegaly with right upper quadrant tenderness, ascites, epigastric distress, peripheral oedema and weight gain.41
Management of cor pulmonale includes continuous low-flow O2. Long-term O2 therapy improves survival of hypoxaemic patients, especially when used more than 15 hours per day. Vasodilator therapy has not demonstrated sustained benefit and is not recommended on a routine basis. Although the use of digoxin is not indicated for right-sided heart failure, it may be used when left-sided heart failure is present. Diuretics are generally used, but serum creatinine and blood urea nitrogen (BUN) are needed to monitor renal function as diuretics can cause volume depletion.41 (Cor pulmonale is discussed further in Ch 27.) Electrolytes must be monitored to assess for hypokalaemia, which can predispose to dysrhythmias.
Acute exacerbations of COPD
According to the guidelines of the Global Initiative for Chronic Obstructive Lung Disease (GOLD), exacerbations of COPD are signalled by a change in the patient’s usual dyspnoea, cough and/or sputum that is different from the usual daily patterns. These flare-ups require changes in management. Patients present with an increase in dyspnoea, sputum volume and/or sputum purulence. They may also have non-specific complaints of malaise, insomnia, fatigue, depression, confusion, decrease in exercise tolerance, increased wheezing, increased cough or fever without other causes.1
Exacerbations of COPD are typical in the course of the disease, with increasing frequency (average 1–2/year) as the disease progresses, and are associated with poorer outcomes. The primary causes of exacerbations are bacterial (50%) or viral infections and air pollution/other environmental sources (15% or 20%).42 Exacerbations may be treated at home or in the hospital, depending on the severity. The severity is determined by the patient’s medical history before the exacerbation, the presence of other diseases, symptoms, ABGs and other laboratory tests. Typically in the later stages of COPD the patient has a low-normal pH, high-normal or above-normal PaCO2 and high-normal HCO3−. This indicates compensated respiratory acidosis as the patient has chronically retained CO2 and the kidneys have conserved HCO3− to increase the pH to within the normal range.
Careful assessment of the patient’s ABGs for any movement towards respiratory acidosis and further hypoxaemia indicating respiratory failure is essential. Also assess the patient’s medical history for the stage of COPD as noted by the level of FEV1. Assess for new symptoms or worsening of usual symptoms. Determine the number of previous exacerbations per year and whether treatment occurred in the home or hospital. The presence of other comorbid conditions will complicate the exacerbation. In addition, the current type of treatment will affect the exacerbation management. Be alert for signs of severity, such as use of accessory muscles, central cyanosis, development of oedema in the lower extremities, unstable blood pressure, signs of right-sided heart failure and altered alertness.3 All of these signs affect patient management decisions (i.e. to treat as an inpatient or outpatient).
Medications, such as bronchodilators and oral systemic corticosteroids, are used to decrease airway resistance during exacerbations of COPD.1 If the patient has clinical signs of airway infection (e.g. increased volume and change in colour of sputum and/or fever, especially in the severe stages of COPD with more than 3–4 exacerbations per year), antibiotic treatment is usually used. Therapies used to treat exacerbations of COPD in the hospital are similar to home management, except supplemental oxygen therapy titrated by ABG measurement may be used.1 Attempts should be made to use non-invasive mechanical methods (e.g. continuous positive airway pressure [CPAP] to support ventilation rather than invasive ventilatory support [e.g. intubation]).43 Teaching the patient and family early recognition of signs and symptoms of exacerbations is important to promote early treatment and to prevent hospitalisation and possible respiratory failure.
Useful information for sufferers and health professionals can be found on the Australian and New Zealand COPD reference site (see Resources on pp 727–728).
Acute respiratory failure
Patients with severe COPD who have exacerbations are at risk for the development of respiratory failure.43 Frequently COPD patients wait too long to contact their healthcare provider when they develop fever, increased cough and dyspnoea or other symptoms suggestive of exacerbations of COPD. An exacerbation of cor pulmonale may also lead to acute respiratory failure. Discontinuing bronchodilator or corticosteroid medication may also precipitate respiratory failure. The use of β2-adrenergic blockers (e.g. propranolol) may also exacerbate acute respiratory failure in the patient with an asthmatic component to the COPD. However, cardioselective β2-adrenergic blockers (e.g. atenolol, metoprolol) should not be withheld from patients with mild to moderate diseases because they do not produce clinically significant problems with respiration.44
The indiscriminate use of sedatives, benzodiazepines and opioids—especially in the preoperative or postoperative patient who retains oxygen—may suppress the ventilatory drive and lead to respiratory failure. The patient with COPD who retains carbon dioxide should be treated with low-flow rates of oxygen with careful monitoring of ABGs to avoid hypercarbia, to ensure that adequate oxygenation occurs and to monitor for acidosis. It has been thought that high-flow rates of oxygen depress the respiratory centre and that the patient’s respirations will diminish or cease. However, it is vital to provide adequate oxygen while assessing the ABGs, rather than not providing oxygen because of the fear of carbon dioxide narcosis.
Surgery or severe, painful illness involving the chest or abdominal organs may lead to splinting and ineffective ventilation and respiratory failure. Careful preoperative screening, which includes pulmonary function tests and ABG monitoring, is important in the patient with a heavy smoking history and COPD to prevent postoperative pulmonary complications. (Respiratory failure is defined and discussed in Ch 67.)
Peptic ulcer and gastro-oesophageal reflux disease
The incidence of peptic ulcer disease is increased in people with COPD. The reason for this occurrence is partly explained by hypersecretion of gastric acid resulting from increased arterial carbon dioxide and decreased arterial oxygen tension. This occurs only in patients who chronically retain carbon dioxide. The ulcers are more commonly in the duodenum rather than the stomach and do not present with pain.45 It is important to test gastric aspirates and faeces for occult blood.
GORD, which may or may not be associated with a hiatus hernia, occurs frequently in patients with COPD and may aggravate respiratory symptoms in a similar way to that of asthma (see above). The reflux and accompanying heartburn may be aggravated or even precipitated by theophylline or β2-adrenergic agonists. As a result of oesophageal irritation or aspiration into the tracheobronchial tree, reflux airway constriction and obstruction may occur. The presence of acid in the oesophagus can cause a vagally mediated reflex bronchoconstriction. (Treatment of hiatus hernia and GORD is discussed in Ch 41.)
Depression/anxiety
Patients with COPD experience many losses as the disease progresses over time. They can feel helpless with low self-esteem and be unable to vent their emotions for fear of compromising their breathing. Cognitive and behavioural therapy along with COPD education has been found to improve quality of life.46 The reported prevalence of depression in COPD varies, but may be four times more frequent than in the general population. Anxiety can complicate respiratory compromise and may precipitate dyspnoea and hyperventilation. When a person is exceptionally dyspnoeic, and particularly if this occurs suddenly, they become anxious and try to breathe faster, thus affecting their oxygenation status. Proper screening for anxiety and depression by healthcare providers is needed for a proper diagnosis.
After diagnosis, treatment consists of cognitive and behavioural psychotherapy and/or pharmacotherapy. Selective serotonin reuptake inhibitors (SSRIs) are often used for both depression and anxiety. Buspirone, which is one medication used to treat anxiety, has few if any respiratory depression effects. Benzodiazepines are avoided as they may depress the respiratory drive and may be habit forming. Explore the psychological realm of the patient’s disease and the impact it has on the patient’s quality of life. It may also help the patient to learn about progressive muscle relaxation exercises that can reduce anxiety, as can learning more about the disease and treatment. This may give patients a sense of control that they can manage daily activities along with the often complex medication regimens. It is important to include the patient’s caregiver or family in the teaching so that they can help the patient to cope physically and emotionally. Providing sufficient time for nursing interventions during an acute exacerbation is important to help reduce anxiety. When the patient becomes anxious because of dyspnoea, the use of pursed-lip breathing and short-acting bronchodilators may be appropriate.
DIAGNOSTIC STUDIES
The diagnosis of COPD is confirmed by pulmonary function tests. Goals of the diagnostic work-up are to determine the major disease component of COPD, the severity of the disease and the impact of the disease on the patient’s quality of life. These factors enable an individualised treatment plan to be devised for the patient. In addition to pulmonary function tests, other diagnostic studies are performed (see Box 28-7). Chest X-rays taken early in the disease may not show abnormalities. Later in the disease the findings presented in Table 28-11 may be present.