![]()
This chapter deals with the fungi dispersed and propagated by means of swimming spores called zoospores. At their simplest they resemble primitive animal forms and, except in the absence of photosynthetic pigments, some algae, which are primitive plants. They are the cause of some very important economic diseases of the present day, including clubroot of brassicas and other members of the Cruciferae, potato blight and downy mildew of grape-vine (Vitis vinifera), and are of interest to the naturalist for their interaction with many wild flowering plants. Phytophthora cinnamoni, cause of jarrah dieback in the eucalyptus forests of Australia, is one of the most destructive pathogens known.
Zoospores move by means of one or more flagella, which push or pull the individual cells forward through the water by an undulating motion and a sort of rowing or swimming action. The flagella function in much the same way as those of the motile cells of Protozoa, algae and the sperm of animals. The flagella in all of these are apparently constructed in a closely similar fashion with a bundle of eleven fibres (or tubules), two situated centrally and nine equally spaced parallel to the outer surface. The fibres run the length of each flagellum, and are all contained within an undifferentiated background of flagellar substance. Each fibre is formed from a protein capable of contracting and expanding, the molecules of which are activated enzymatically. By sliding on one another they impart a rapid undulating movement to the flagellum. Although this mechanism is biochemically distinct from the mechanism for muscle function in animals it bears certain very close similarities to it. If zoosporic organisms were progenitors of the animals, as some believe, the seeds of muscular function were already present at the very early stages of evolution.
The first groups we describe are not always easy to see because they attack below ground. Roots become infected by zoospores swimming in the water held between the soil crumbs or in water held at the surface of the soil, and this results in a lack of vigour, discoloration or collapse of the above-ground portion of the plant.
ZOOSPORE FUNGI WITHOUT WALLS
If you live on acid soil and grow brassica crops in your garden you are almost sure to encounter the clubroot or finger and toe disease caused by the biotroph Plasmodiophora brassicae (Protozoa, Plasmodiophoromycota – Table 1.1). This was first recorded in Scotland in 1795, and was at that time thought to be caused by watering cabbage plants with the bath water of syphilitic patients because of the strange shapes assumed by the roots. In cool climates it is a common pathogen of the many vegetables derived from Brassica species, including cabbages, cauliflowers, broccoli, turnips, swedes, radishes, rape grown for sheep feed and oilseed rape. It also attacks many ornamental and wild members of the family Cruciferae. It can be recognised by a bronzing of the leaves, stunting and wilting or collapse of the affected plants, which when they are pulled up show swollen or clubbed and otherwise misshapen roots (Fig. 4.1). Surprisingly for a soil-inhabiting fungus, Plasmodiophora is genetically very variable and has produced a number of physiologic races, attacking different Brassica species and their varieties. Some success has been achieved in producing plants showing resistance to attack but none is completely effective.

FIG. 4.1
Distorted growth of a cabbage root (Brassica oleracea) following infection by Plasmodiophora brassicae, cause of clubroot disease, which leads to increased levels of growth regulators in the diseased tissues. (D.S. Ingram.)
As we have seen in Chapter 2 (see here), Plasmodiophora has evolved a remarkable mechanism for infecting its host. First a single zoospore emerges from a thick-walled, spiky resting spore (Fig. 4.2) in the soil. It has two whiplash flagella which appear to be situated fore and aft of the zoospore body, but in fact arise laterally, with one pointing forwards and the other backwards. Having swum in the soil water towards a host root, being attracted by distinctive chemicals leaking from the cells, it becomes attached to the surface of a root hair and differentiates within itself a bullet-shaped stachel contained within a membranous tubular ‘gun-barrel’. The stachel, with a small fragment of cytoplasm containing a nucleus attached to it by a membrane, is forcibly ‘shot’ into the living root hair cell, where it becomes enclosed by the plasmalemma. The stachel is powered by a vacuole within the zoospore that expands rapidly as liquid is pumped into it, the energy being provided by stored lipids which are rapidly digested. Polymixa betae, a pathogen closely related to Plasmodiophora which attacks the roots of beet and its relatives (Beta spp.), has evolved an almost identical infection mechanism. Polymixa is important as a carrier (vector) of an important virus disease of sugar beet called Rhizomania.
Following infection of the root hair, Plasmodiophora moves towards the second stage of its life cycle. Its nuclei divide to form a multi-nucleate plasmodium. This eventually divides into 10 to 20 zoospores, each with a single, haploid nucleus. Some of the zoospores probably reinfect adjacent root hairs, but others appear to act as gametes (‘sexual’ spores) and fuse together in pairs to form larger cells each containing two nuclei, which then infect the living cells of the cortex, the body of the root. Drawing nutrients from the cells, the cytoplasm of the fungus enlarges and the nucleus divides, eventually producing another large multinucleate plasmodium in the enlarged tissues of the roots. It is during the development of these plasmodia that the nuclei from different parents come together in pairs and fuse to form large diploid nuclei. Eventually these undergo meiosis to produce haploid nuclei, each of which becomes surrounded by lipid-rich cytoplasm, a membrane and a thick wall, to produce a thick-walled resting spore covered with dead material that gives it its spiky appearance. When the root dies and rots the resting spores are released into the soil to begin the cycle again.
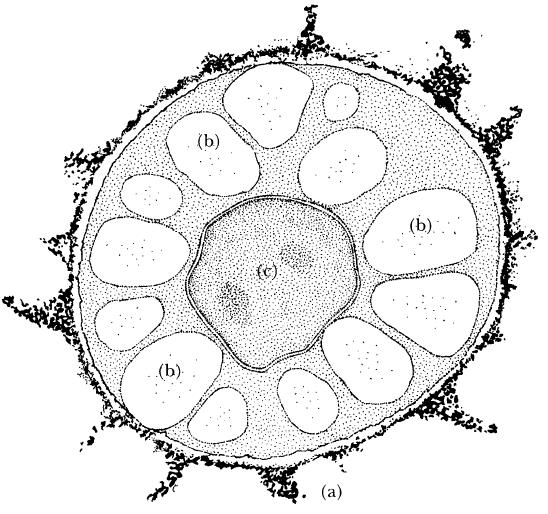
FIG. 4.2
A resting spore of Plasmodiophora brassicae, cause of clubroot disease of members of the cabbage family (Cruciferae). Note the spiky appearance of the thick outer wall (a), the large lipid droplets (b) and the single central nucleus (c). Each resting spore is approximately 3–5 μm diameter. (Illustration by Mary Bates, based on an electron microscope photograph of an ultra-thin section.)
This brief excursion into the esoteric area of life cycles illustrates the complexity of studying the zoospore fungi that grow in the soil, a medium that makes direct examination very difficult. There have been well over a dozen different life cycles proposed for Plasmodiophora over the years. The studies that led to the one outlined here were facilitated by growing the host cells and the obligately parasitic pathogen together as infected tissue cultures in the laboratory. Although nobody has made a counter-proposal over the past 25 years, that does not mean it is correct in every detail – time will tell.
Infection by Plasmodiophora leads to a multiplication of the number of root cells and also an increase in size of the individual cells, caused by disturbance of the levels of the host’s growth hormones, particularly the cytokinins and auxins. This is reflected externally in the galls and swellings which give the disease its common names, ‘clubroot’ and ‘finger and toe’. Masses of starch also accumulate in the infected tissues, derived from the excess of sugars imported into the clubbed root to nourish the growing pathogen. An interesting feature of the life cycle is that the root hair phase can occur in plants of many different families, but the clubroot stage is restricted to members of the Cruciferae. The reason for this specificity is not known.
Control of clubroot is not easy. The most important element is an extended rotation so that the susceptible crop does not appear more than once in five or more years in the same field, thereby allowing resting spore numbers to decline, although some can survive for forty years or more. Particular brassica varieties show resistance, such as the swede cultivar ‘Marian’. The acidity of the soil is also an important element in control, acid soils showing greater infection of susceptible crops than those that are more alkaline. However, from a farming point of view it is difficult to use this as a control measure, because in areas where potatoes are also being grown, raising the pH with lime encourages the development of another disease, potato scab (Streptomyces scabies). The extent to which clubroot occurs on wild members of the family Cruciferae is difficult to estimate and any records would be of interest.
Clubroot can easily be examined by the amateur with access to a compound microscope. Cut thin sections of clubbed root with a sharp blade or scalpel, mount in a drop or two of water, cover with a cover slip and examine with the microscope (see Appendix). The swollen cells, large plasmodia and masses of starch grains will easily be visible in younger tissues. In older tissues resting spores with their characteristic spiky walls can be seen at higher magnifications (x40 objective and above). Root hair infections can be produced by growing seedlings in silver sand and watering them with ground-up ‘clubs’. If the sand is kept wet the spores will germinate and infect. Seven to fourteen days later the seedlings may be lifted, washed gently in water and examined with the microscope. The most easily seen stage is the cleavage of the plasmodium into groups of zoospores, which have the appearance of a row of pearls strung out along the root hair (Fig. 4.3).
Another crop disease that does not show above-ground symptoms is powdery scab of potato tubers caused by the biotroph Spongospora subterranea, a fungus closely related to Plasmodiophora. The clusters of resting spores, which form characteristic spore-balls, can survive in the soil for many years. They are induced to germinate by the renewed presence of the host, which releases stimulatory chemicals, causing the spores to produce biflagellate zoospores resembling those of Plasmodiophora. These infect the cells of the potato and form plasmodia, again like those of Plasmodiophora. The symptoms of the resulting powdery scab are cork-filled pitted lesions on the tubers from which spore-balls can be retrieved and readily identified with the aid of a microscope (Fig. 4.4). Look for Spongospora on freshly dug tubers. It appears as raised scabs which later become depressed shallow cavities as the surface breaks away to reveal the brownish balls of single-celled circular spores. Many other surface organisms, including common scab, caused by Streptomyces scabies, a member of the Actinomycetes, and black scurf, caused by Rhizoctonia solani, a member of the Basidiomycota, can be mistaken for powdery scab, so beware. There may be other symptoms, including a cankerous form in wet soils where whole tubers may be misshapen, making them unsaleable. The fungus can also attack tomatoes, producing small corky lesions on the roots.

FIG. 4.3
Root hair plasmodia and zoosporangia of Plasmodiophora brassicae, cause of clubroot disease of the cabbage family (Cruciferae) in the root hairs of wallflower (Cheiranthus cherii). The individual spherical zoosporangia are approximately 6–10 μm diameter. (Phase contrast light microscope photograph, D.S. Ingram.)
Spongospora is of special interest because, like Polymixa, it is a vector of viruses, particularly potato mop top virus (see here). Another Spongospora attacks watercress (Nasturtium officinale). In the 1950s the English watercress industry expressed concern at the decline in productivity in certain beds. Investigation showed a characteristic bending of the roots and the condition was given the name ‘crook-root disease’ and shown to be caused by a hitherto unknown species of Spongospora, which was eventually considered to be a variety of the potato pathogen. The disease occurs on wild watercress, although it is hard to find until you get your eye in. The use of zinc ‘frits’, fragments of glass carrying zinc, which is toxic to Spongospora, controlled the spread of the disease, which otherwise would probably have destroyed the watercress industry.
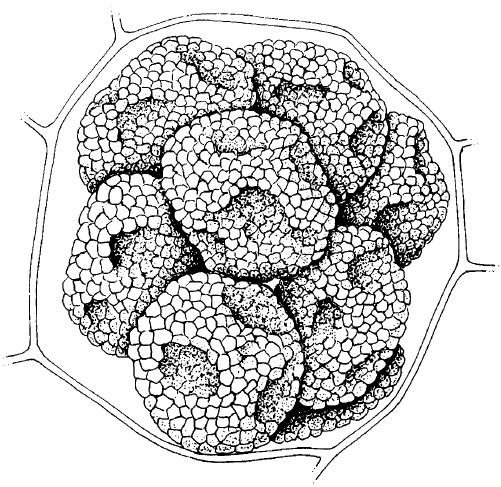
FIG 4.4
Spore balls (each approximately 40 μm diameter) of Spongospora subterranea, cause of powdery scab of potatoes (Solanum tuberosum), within a single tuber cell. (Illustration by Mary Bates, after Butler & Jones, 1961.)
Plasmodiophora, Polymixa and Spongospora, having both zoospores and plasmodia without walls, are now classified along with primitive animal life in the Kingdom PROTOZOA (see Table 1.1). The zoosporic fungi dealt with next, from the moment that the zoospore encysts, possess walls of chitin and glucans and are therefore classified in the Kingdom FUNGI as the Chytridiomycota (Synchytrium spp. and Olpidium and its relatives – see Chapter 1 and Table 1.1). We shall also consider the Oomycota, which possess hyphae with walls containing cellulose as the principal structural component. They were once thought to be related to the Chytridiomycota but are now classified in an entirely separate Kingdom, the CHROMISTA. Included in the Oomycota are such major groups of pathogens as the Peronosporales: Pythium spp; Phytophthora spp; the downy mildews – Peronospora spp. and their relatives; and the white blisters – Albugo spp.
ZOOSPORE FUNGI WITH WALLS BUT WITHOUT HYPHAE
Perhaps the most spectacular disease caused by a fungus in this group is wart of potatoes, caused by yet another biotroph Synchytrium endobioticum (Fig. 4.5). It was first identified in Hungary in 1895, but was probably present in Britain from about 1860, for at that date a model of a potato tuber with cauliflower-like growths, the characteristic manifestation of the disease, was presented to the Royal Scottish Museum in Edinburgh. In these growths tiny thick-walled spherical resting spores, resistant to decay, are eventually formed, and are incorporated into the surrounding soil when the growths turn black and rot. This ensures the contamination of the soil and the insidious contamination of the surface of otherwise healthy ‘seed’ tubers before lifting; when planted elsewhere to produce a crop, these spread the disease.
Germination of the resting spores gives rise to spherical zoospores, each with a single posterior whiplash flagellum. These swim to the surface of a developing ‘eye’ of a tuber and penetrate a surface cell, where they develop to produce further infective zoospores and stimulate the division and enlargement of adjacent cells. A continuous cycle of vegetative reinfection gives rise to the very large outgrowths which are so characteristic and so destructive. At certain times some of the zoospores appear to act as gametes and fuse in pairs to give diploid zoospores which enter fresh cells and cause further cell division. Meiosis to restore the haploid condition is believed to occur immediately after germination.

FIG. 4.5
Fig. 4.5 A gall of Synchytrium endobioticum, cause of wart disease of potato (Solanum tuberosum), growing up from an artificially inoculated, pot-grown tuber of the cultivar ‘Arran Chieftain’ grown by Mary Noble. (D.S. Ingram.)
By the early part of the twentieth century the disease was widespread in Britain and throughout Europe. In the beginning its spread was so rapid and mysterious that farmers who grew potatoes feared for their livelihood, and those charged with the oversight of the country’s food supply feared for the future of the potato as a staple food. For example, 594 holdings in Scotland became infected between the first identification in 1907 and 1922. Gradually facts to aid the understanding of the disease were established. It became apparent that the disease was very severe in gardens and allotments (where rotation of crops was limited) and that fields adjacent to gardens were particularly prone to infestation. Moreover, across the British Isles the disease was most prevalent in the northwest and west midlands of England and in southern Scotland, areas of intensive potato cultivation. Once fields had been contaminated subsequent potato crops showed the disease and the severity of the infestation increased according to the frequency of the planting of potatoes. But none of this helped control the disease in any dramatic fashion and the situation looked hopeless until the report in 1920 of the observation by English growers that certain varieties of potato appeared to escape infection. Subsequent study showed that these varieties were in fact very resistant (popularly said to be ‘immune’) to attack, a resistance controlled by a single gene, with one or more subsidiary genes sometimes acting as modifiers (see here).
Once an understanding of the disease had been gained, the strategy for control was determined. Firstly a series of administrative orders was promulgated to control the planting of non-immune potatoes in designated areas and their movement about the country; secondly, planting susceptible varieties on land that had contained diseased plants was prohibited; and finally, resistant varieties grown on such land were not allowed to be used for ‘seed’. Even the growing of susceptible varieties in gardens was prohibited (with special dispensations for some early varieties). As the genetics of inheritance of resistance and susceptibility was understood, it was possible to ensure that resistance was bred into all new potato varieties. Indeed, new varieties could not be released to commerce until they had undergone a statutory test for resistance and susceptibility to wart disease. These measures reduced the importance of the disease and allowed normal farming to proceed over much of the country. The effect of these measures can be seen in the period 1923–57, when only 223 fresh outbreaks were notified, and since then the disease has continued to decline.
In Canada the disease was first identified in Newfoundland in 1909, on the Canadian mainland in 1918 and at this time too in the USA, but in all these cases initial severe quarantine measures and the development of orders preventing the indiscriminate use of susceptible varieties prevented the spread of the disease.
The resting spores of this devastating disease lie a long time in the soil and some are still capable of infecting potatoes up to 50 years from the initial outbreak. The number of viable individuals gradually falls over this period but how many remain infective after periods in excess of 50 years is not known. Moves are afoot to de-schedule the fields earliest affected by wart disease, but the approach must be cautious. The disease still turns up on a regular basis in gardens when permitted non-immune susceptible varieties are grown, and many new owners of productive cottage gardens are horrified and mystified at the deformed tubers and cauliflower-like growths which are the products of their carefully planted early potato varieties, until they learn to grow only immune varieties.
Experimental work has shown that Synchytrium endobioticum will affect wild species of Solanum such as black nightshade (S. nigrum) and woody nightshade (S. dulcamara). It may have crossed over to the cultivated potato in Europe from such wild hosts, but evidence on this point is lacking. It is also known to exist in the Andes, but whether as a native or an introduction is not clear. The roots of tomato, a close relative of potato, can be infected to form small growths quite different in scale from the gigantic protuberances on the potato tuber, which is not a root but a stem. There are, however, no reports of the organism attacking other domesticated or wild members of the potato family (Solanaceae).
The genus Synchytrium contains a good number of other species that attack a variety of wild flowering plants in Britain; curiously, in most cases these attacks are at or near ground level and not below ground, and none of the hosts show infected growths of the extravagant size of the potato wart disease. Among the most striking of the infections are those of S. taraxaci, which forms yellow, orange or red bumps on the midrib of dandelion (Taraxacum officinale); S. anemones, which causes blackish galls on the above ground parts of wood anemone (Anemone nemorosa), and S. mercurialis, which attacks dog’s mercury (Mercurialis perennis), producing glassy whitish-yellow swellings on the bases of stems and leaves, which can be discovered initially by running the fingers up and down the stems. If viewed with a microscope, thin sections of these galls can often be seen to contain large black resting spores. Lastly, look for S. aureum. This is a portmanteau name given to several Synchytrium species that have not yet been separated taxonomically. We have seen S. aureum on common chickweed (Stellaria media) and common loosestrife (Lysimachia vulgaris), where it forms myriads of small golden galls on the infected plants.
The biology and taxonomy of Synchytrium species on wild plants is still poorly understood and would be an excellent subject for study by the amateur naturalist with endless patience and access to a good compound microscope.
Another unforgettable disease resulting from infection by a unicellular organism related to Synchytrium is the crown wart disease of lucerne (Medicago sativa), caused by Urophylyctis alfalfae. This is seen in areas where lucerne is grown on soils that hold water (much lucerne is grown on very free-draining soils). The galls, which form at the crown of the plant, look like leafy lumps of cauliflower and contain elaborately decorated resting spores. The vigour of infected plants is much reduced.
A final genus, Olpidium, which, like Synchytrium, is a member of the Chytridiales, invades the surface cells of roots and leaves but causes minimal damage to the host. O. brassicae, for example, is widespread on the roots of brassicas and other plants (Fig. 4.6) and although it may cause problems for infected seedlings it has a limited effect on the adult plant. O. radicale is similar but is said to attack a range of host plants. The importance of these pathogens lies in their capacity to carry important viruses from plant to plant, including lettuce big vein, tobacco necrosis, cucumber necrosis and melon necrotic spot.
Most of the fungi described above can cause severe problems in crops; they contaminate the land and effectively limit its use for many years into the future; they can spread to new sites on the feet of animals or human beings, on vehicles and implements, in blown soil or in water and on tubers and plants; but that being said, they have only comparatively limited powers of spatial movement.

FIG. 4.6
Resting spores (approximately 12 μm diameter) of Olpidium sp. in root cells of common bluebell, Hyacinthoides non-scripta. (Dr H.J. Hudson, University of Cambridge.)
PYTHIUM, THE SECRET DESTROYER
Pythium, a member of the Oomycota in the Kingdom CHROMISTA (see Chapter 1 and Table 1.1) has many of the characteristics of the non-mycelial fungi we have just been discussing. It is disseminated by zoospores but these, on reaching the surface of a host plant, germinate to form germ tubes which penetrate the tissues and produce a mycelium of ramifying hyphae without septa. These hyphae grow into and through the cells and, in their turn, break out into the surrounding water or air and bear zoosporangia, which release zoospores whenever free water is present. In some species the sporangia become detached and are distributed locally. Some are possibly wind-borne. They may also produce thick-walled oospores, resting spores which have very considerable powers of survival in the soil in the absence of a host. There are many surprising and interesting characteristics of Pythium species, but perhaps the most remarkable is their ability to destroy the tissues of the plants they invade.
In this they contrast with the zoosporic pathogens considered previously, which are all biotrophs. Having overcome the intrinsic resistance mechanism of the threatened host, they develop an intimate relationship with it. Minimum damage is inflicted and the living cells provide the pathogen with food, protection and the ability to multiply and produce spores over a long period of time. Pythium does not fit into this pattern of behaviour. It is a necrotroph, a plant destroyer which lies in wait for its host (either as an oospore or as mycelium growing saprophytically on dead tissue) and typically enters the tissue of a young seedling, causing disintegration of the middle lamella, death of the cells and in some cases disintegration of the cellulose cell walls. The consequent collapse of the host is called damping-off, and is most commonly seen in pots of seedlings contaminated by unsterile soil containing oospores of the pathogen (Plate 2). A beautiful crop of young seedlings will begin to die at the point of initial infection, and the collapse then spreads to all the seedlings in the pot until after some time almost nothing visible remains. This pattern may sometimes also be seen in cartons of cress seedlings purchased as salad and kept for a day or so on the kitchen window sill. The likelihood of damping-off occurring is increased if seedlings are stressed by being planted too close together, kept at too low a temperature or overwatered. The presence of excess water reduces the availability of oxygen to the roots and also facilitates the dispersal of the zoospores. Pythium species have a very wide host range, a characteristic of necrotrophs, although there are limits, as will become apparent.
The Pythium species associated with damping-off include the commonest, P. debaryanum, a species on which many taxonomists cast doubt but which is often referred to; P. ultimum, which also causes a tip blight of garden peas and watery wound rot of potato tubers, and P. aphanidermatum, which additionally occurs as a storage rot of vegetables and causes cottony leak of cucumbers. Each can cause damping-off, death and destruction of seedlings of almost any plant species while at the same time being implicated in more specific attacks on adult tissues of particular hosts. Other Pythium species cause root rots of plants as diverse as wheat, sugar cane (Saccharum officinarum) and garden violas, or rots of storage organs as varied as ginger (Zingiber officinale) rhizomes and sugar beet (Beta vulgaris) roots. It is possible that in seedlings resistance mechanisms are poorly developed and the susceptible tissues are not protected by a thick cuticle and corky skin. In older plants, where resistance and protective layers are well developed, particular strategies must be evolved to circumvent these in each host. Hence a measure of host specificity is achieved. A new dimension is given to the genus by the discovery that some species are even capable of attacking shrimps and young fish, and can become important impediments to good home aquarium management and efficient fish hatchery production.
Some Pythium species also appear to have the ability to cause disease without infecting the host plant. Toxins are produced at the root surface and these cause reduced growth of roots and collapse of leaves. This characteristic may be responsible for the occurrence of a number of diseases in which Pythium spp. interact synergistically with other pathogens such as Fusarium oxysporum (see Chapter 6) to produce diseases more severe than those caused by either organism alone.
Infection by Pythium species is very difficult to identify in wild plants. Damping-off can easily be produced at home, however, by sowing seeds of cress (Lepidium sativum) very thickly in a pot of old unsterile soil, placing in a cool corner and overwatering (or even better, standing the pot in a dish of water). Soon after the crowded seedlings have started to grow disease will probably set in. Infected seedlings should be mounted on a microscope slide in a drop of water and squashed under a cover slip. The wide hyphae without septa can then be observed with a microscope. Sporangia and oospores may sometimes also be seen.
If some pieces of infected tissue are placed in a dish of sterile tap water with a few healthy seedlings the hyphae, which look like strands of cotton wool, will grow out and attempt to invade the new hosts. As zoosporangia form, zoospores may be released into the water.
INTO THE AIR
In many Pythium spp. the zoosporangia are produced in water as slightly modified hyphae from which an undifferentiated protoplasmic mass surrounded by a membrane is extruded. The zoospores are differentiated inside this before being discharged to infect neighbouring plants. Other Pythium spp. have well differentiated zoosporangia which are often globular. Sometimes, as in P. ultimum, these are borne on the ends of hyphae which may project a short distance into the air. Being detachable, they are taken up in air currents and dispersed. Water is required, however, before they can germinate, release zoospores and infect a new host.
The closely related genus Phytophthora in the Oomycota (see Chapter 1 and Table 1.1) is characterised by the zoospores being fully formed in the sporangium before being discharged. Its sporangia are distributed in a variety of ways. Those of some species, like P. cinnamoni on Eucalyptus spp., are released in the soil and their zoospores distributed through the soil water to attack neighbouring plants. Others, such as those of P. palmivora on cacao pods (Theobroma cacao), are formed on the upper parts of the plant, detached by rain and distributed by splash and drip. At least two species, P. infestans, the cause of potato blight, and P. phaseoli on beans (Phaseolus spp.), have sporangia that are readily detached without the intervention of water, being removed from the plant into moving air currents. Phytophthora species can be found in a variety of situations in farms and gardens worldwide. Some are necrotrophs, but others show characteristics of hemibiotrophy or biotrophy (Plates 1 and 2).
A typical necrotrophic Phytophthora carried in soil water first appeared in strawberry fields in Scotland in 1921. During the economic depression that followed the First World War, encouragement was given to the establishment of horticultural holdings on which the growing of soft fruit, particularly strawberries (Fragaria x ananassa) and raspberries (Rubus idaeus), proved a useful enterprise. Then, quite suddenly, areas in strawberry fields became yellow with dieback of the plants and eventually the diseased areas spread to wipe out whole crops. Examination of the roots showed a dark red discoloration of the xylem, and the disease was named ‘red-core’ (‘red stele disease’ in the USA). A characteristic Phytophthora, P. fragariae, was isolated from the roots. Although chemical means were found to limit the disease and resistant varieties such as ‘Auchincruive Climax’ were eventually bred, the effect of the disease was to cause a relocation of strawberry growing and sometimes of strawberry growers from Lanarkshire in the west of Scotland to Perthshire and Angus in the east. The disease was contained in these new areas by strict statutory attention to the production of disease-free planting stock.
In the USA red core disease was found on strawberries in the 1930s and a similar disease appeared in the Pacific northwest in the 1950s. This, or something very like it, attacked raspberries in the 1980s in Scotland, other European countries and Australia in quick succession, but how it travelled such vast distances is not known. A great many names have been given to the necrotrophic Phytophthoras causing diseases of soft fruit, such as P. fragariae, P. megasperma, P. erythroseptica and P. cryptogea, but no definitive name has yet been agreed. There is evidence that the disease organisms of strawberries and raspberries are distinct but closely related and that they probably both arose on the Pacific coast of the Americas. These diseases are now among the most important of soft fruit worldwide, and control is being sought by strict monitoring of planting stock. At the same time, a search has begun for resistance in new varieties.
Another Phytophthora that infects from the soil, P. cinnamoni, is extremely important. In gardens and nurseries in temperate climates worldwide it causes a dieback of dwarf conifers and various species of Rhododendron, Erica and Calluna, with characteristic attacks on the growing roots, yellowing and dieback of the above ground portions and sometimes very characteristic brown segments in conifers. P. cinnamoni is also responsible for other diseases, and has been recorded on over 1,000 host plants. It is a serious problem on avocados (Persea americana) in California and has become particularly important, not to say devastating, as the introduced cause of dieback of the jarrah forests of Western Australia, where it is threatening the very survival of important natural ecosystems over vast tracts of country. Spread of the disease in the jarrah forests is especially rapid along roads and tracks, the spores being carried by vehicles and in water torrents during rainy periods.
Other necrotrophic and hemibiotrophic species of Phytophthora do not attack from the soil but produce their sporangia on the surface of twigs or fruits, and are important as the cause of severe rots in tropical crops. They include P. heveae and P. meadii on rubber (Hevea brasiliensis) and P. palmivora (a name covering a wide taxonomic group) attacking palm buds (Palmae), pepper vines (Piper nigrum) and other tropical species. In cacao, P. palmivora causes ‘black pod disease’. The melon-shaped fruits, full of beans, are normally harvested as the pods turn orange or red. The beans are then removed and fermented to get rid of the surrounding pulp and improve the flavour. When P. palmivora is present, infection of the green pods leads to the development of lesions which rapidly spread to involve whole fruits. Cacao pods are rich in phenolic substances; as these are released from the dying cells they are rapidly oxidised to form dark compounds which turn the infected pods completely black. Sporangia are borne on the surface of such pods, washed off in drips or splashes and thereby carried to adjacent pods. There is a relationship between rainfall, yield and the rate of infection: in periods of heavy rainfall, the more crowded the fruits are the more vulnerable they are to infection. Traditionally control has been by means of chemical sprays, but frequent picking not only reduces the possibility of transmission but also removes infected pods before the infection involves the beans they contain. As we write P. palmivora is again threatening world chocolate supplies.
The Phytophthora species discussed so far produce their sporangia on simple, often very short stalks. In contrast, the hemibiotrophic (some would say biotrophic) potato blight fungus (P. infestans) and P. phaseoli (important on Lima bean, Phaseolus lunatus) both have elongating sporangiophores which swell at the end to produce a lemon-shaped sporangium which is pushed to the side to allow a new apex to grow forward to form the next sporangium. This process is repeated to produce a long, jointed sporangiophore with sporangia attached laterally (Fig. 1.1). The attachment is very delicate, and the sporangia are released when the sporangiophores, with their differentially thickened walls, twist rapidly with changes in atmospheric humidity. It is easy to see this movement if an infected leaf is observed with a low power microscope or lens in a drying atmosphere.
On arrival at the leaf surface of a leaf of potato or tomato, the sporangia germinate. If water is present and the temperature low, swimming zoospores are released and these rapidly encyst and produce tiny germ tubes (Fig. 1.1). At higher temperatures zoospores do not form and direct germination occurs by means of a much larger germ tube. The germ tube, however produced, first swells at the apex to form an appressorium which becomes cemented to the leaf surface. An infection peg then penetrates the leaf either by breaching the young epidermis or by passing through a stomatal pore. After entering the leaf the hyphae grow out from a central point without at first producing any effect visible to the naked eye. The microscope reveals that they grow largely between the living cells and sometimes, but not always, produce small, peg-like hausto-ria which penetrate the cell walls without killing the cytoplasm. The cells often respond by encasing the haustoria in callose, presumably in an attempt to limit the growth of the pathogen. After about three days the centre of the infected area takes on a water-soaked appearance and branching hyphae bearing sporangia appear from the stomatal apertures. Since stomata are commonest on the lower leaf surface, this is where the fungus is most in evidence, particularly at the edge of the lesion. Later, the centre of the lesion collapses and becomes necrotic, while the sporulating edge advances until, depending on the genotype of the host, the whole or part of the leaf has been colonised. Clearly the fungus grows and sporulates in the living tissues of the potato or tomato and may therefore be regarded as a biotroph. Whether it then goes on to kill the cells, growing and sporulating on them as a hemibiotroph would, is not known. It is possible that the cells of the host simply die from the stress imposed by the demands of the fungus, which then dies with them. It is capable of growth on artificial media in the laboratory, but whether it grows saprophytically in nature is a different matter.
When sporangia are washed down into the soil from the leaves, Phytophthora infestans may also infect potato tubers through the ‘eyes’. Again, the hyphae grow between the cells, once more producing characteristic peg-like haustoria. The tuber responds to the presence of the pathogen by encasing haustoria in callose and laying down lignin and other phenolic substances in cell walls adjacent to the infection, giving cut tissues a characteristic bright fox-red colour. The fungus may overwinter in infected tubers, growing into the new shoots as they emerge in spring. Sporangia produced on these shoots infect adjacent healthy plants. P. infestans is also capable of producing male and female sex organs in the tissues of the plant, especially the tuber. Here the haploid male nuclei, in a poorly differentiated male organ, the antheridium, pass into the larger, spherical female organ, the oogonium. This becomes a diploid resting structure, the thick walled oospore (Fig. 4.7), which overwinters in or on the soil, ultimately producing sporangia containing zoospores with one or more nuclei. Until recently this phase had rarely, if ever, been seen in Britain but the situation may now be changing (see below).
THE MYSTERY OF MEIOSIS
A biotrophic organism that can be artificially cultured is of great interest to experimentalists attempting to puzzle out the nature of parasitism. If, for example, mutants of the fungus could be produced which either varied in pathogenicity or in some attribute thought to be important in pathogenicity, they would shed a light on the nature of pathogenicity itself. On the assumption that, like many simple organisms, Phytophthora was haploid for most of its life cycle with a short diploid phase in the resting spore formed after sexual union, many attempts were made by the authors and others during the 1960s to create mutants by irradiating the zoospores with ultraviolet light or by treating them with chemical mutagens. These were all singularly unsuccessful. Meanwhile Eva Sansome, from cytological work in itself difficult because of the minute size of the nuclei involved, claimed that the Phytophthora species and their allies (Pythium speices and downy mildews – see below) were diploid organisms which only produced haploid nuclei by meiosis immediately before the final sexual union. Her work was ignored for many years but, thanks to the persistence of other scientists, has now been proved correct. This explains the difficulty in detecting mutants, for most mutational changes are deletions of genetic information from one but not both copies of a gene within a chromosome and are therefore masked by the unmutated copy of the gene on the sister chromatid. Once the position of meiosis in the life cycle was understood, more detailed genetic analyses were made. These suggest that the Phytophthora species and their allies in the Oomycota are quite distinct from all other fungi (see below).

FIG 4.7
An oospore of Phytophthora infestans, cause of late blight of potatoes (Solanum tuberosum). Note that the antheridium, the ‘male’ sex organ, encircles the base of the oospore (approximately 40–50 μm diameter). (Photograph supplied by the Scottish Crop Reseach Institute, Dundee.)
THE TRAVELLERS
We have seen how soil-borne fungi with swimming spores can mount attacks in localised areas and can spread by the movement of resting spores with the soil and by the dissemination of zoospores in surface water. Among the Phytophthora species, many behave as soil-borne fungi. Others, which attack above-ground portions of the plant, spread through the canopy by splash and drip but can only move further afield in infected planting material. In P. infestans and P. phaseoli, the possession of aerially disseminated zoosporangia introduces a new dimension to the capacity to spread. P. infestans can spread locally in all the ways demonstrated by the species we have just described. In addition it can move around the globe in tubers used as planting material. Moreover, its capacity for aerial spread allows it to travel swiftly and widely from an original focus of infection such as a plant growing from an infected tuber (Plate 1).
We know that the cultivated potato probably had its origin in the high Andes and was imported into Europe in the sixteenth and seventeenth centuries, becoming an agricultural crop in the eighteenth century. There is no evidence that P. infestans was carried with it and potato crops in Europe and in America were apparently free of the disease until the early nineteenth century. The disease probably appeared for the first time in 1843 on the eastern seaboard of the USA and Canada, either in planting material or more likely by aerial spread. There is good documentary evidence that it then appeared in Europe in 1845, almost certainly carried in tubers for planting. It was first noted at Courtrai in Belgium in late June to early July. From that point it spread eastwards into France and Switzerland, westwards into southern England (16 August) and southern Ireland (6 September) and at about the same time into Scotland south of the Highlands, all by aerial spread. We cannot observe aerial spread on this scale now because there is annual local reinfection from infected tubers, but there is clear evidence for extensive aerial spread in Holland, where potatoes on islands at a distance of 11 km from the mainland have developed blight in the absence of any infected planting material. In Britain there is good circumstantial evidence in most years for the simultaneous appearance of discrete outbreaks of blight over an area of several square kilometres from a single original focus.
Where did potato blight originate? A centre of origin for P. infestans has been identified in Mexico, where a number of wild species of Solanum have been found infected by the disease. But an historical connection between this area and the centre of origin of the cultivated potato in the Andes has not been made and recent outbreaks of potato blight on modern varieties in South America are considered to have been imported with those varieties. Historical evidence is accumulating for an association between blight and the original Andean home of the potato, but the matter is by no means concluded.
The travels of potato blight did not finish with its establishment as a worldwide pathogen. After its appearance in Europe and the USA and its step by step colonisation of other potato-growing regions, references to its sexual reproduction are scarce, contrary to expectation. There are one or two notes of oospores having being observed, but so rarely as to suggest that the fungus was functioning around the world without the intervention of a sexual cycle, a situation which made its ability to produce new races in response to the genes for resistance introduced into cultivated potatoes by plant breeders a matter of wonder. When the fungus on wild potatoes in Mexico was studied in detail it was found to produce abundant oospores following the conjugation of oogonia and antheridia, but this only happened when fungi of two mating types, A1 and A2, were brought together. P. infestans was therefore heterothallic. (This is the condition in which mycelia of opposite mating type are required to conjugate to produce the zygote. In fungi like Phytophthora spp. each mycelium is capable of producing both male antheridia and female oogonia, but these will only conjugate with an appropriate organ from mycelium of opposite mating type). Experimental crosses showed that the P. infestans in the rest of the world was all of the A1 mating type. Then in the early 1980s the A2 mating type was observed in Holland, the UK, Egypt and subsequently worldwide. It was often present at fairly low levels, suggesting that it could in the past have been found anywhere if sufficient search had been made, but in some areas such as southern Brazil and the former Soviet Union it was very common. Was this a migration of the A2 mating type, perhaps aided by the export of potatoes from Mexico, was it a mutation from A1 to A2, or had the A2 mating type been present all the time and overlooked? The balance of opinion seems to favour the migrational theory. What is strange, however, is that there does not seem to have been any increase in the capacity of potato blight to vary after the ‘arrival’ of the A2 mating type. One might have expected the capacity for sexual variation to have led to the appearance of highly pathogenic races of potato blight which threatened the present level of resistance in commercial potatoes. This has not happened so far, although there are reports of the identification in the USA of a new strain of potato blight that is able to resist control by all current fungicides. It is probable that P. infestans is capable of parasexual recombination (see here) and that this is responsible for variation, but the critical evidence to prove this point is still being collected.
Since local epidemic spread of potato blight each summer is dependent on the weather, forecasting methods have been developed which not only predict the progress of the epidemic but also indicate the appropriate times for effective spraying to control the disease. Probably the earliest attempt to formulate a set of predictive rules was that of two Dutchmen, Lohnis and van Everdingen, in the early 1920s. They found that at least four hours of dew during the night, a minimum night temperature of 10°C, with mean cloudiness the following day of 0.8 and at least 0.1 mm of rain on the same day led to a cycle of infection. Later, attempts were made to simplify the criteria and adapt them for particular geographic areas. As understanding increased and meteorological data became ever more sophisticated, elaborate computerised systems came into being, and most recently the forecasting system has been turned round to predict not when blight will occur but when it will not occur. The use of any of these systems allows the grower to apply fungicide only when it is likely to be needed, lessening costs and environmental contamination.
THE DOWNY MILDEWS
The downy mildews, although closely related to Phytophthora infestans, all differ from it in having sporangiophores that, although branched, are of limited growth, sporangia being formed at the apex of each branch (Figs. 4.8 and 4.9). They are also obligate biotrophs, incapable (so far at least) of growth even on complex culture media. All are grouped together in the order Peronosporales. Some members of this group, such as Pseudoperonospora spp. and Plasmopara spp., produce zoospores, although the ‘sporangia’ of others, such as Bremia spp., appear to germinate exclusively by means of germ tubes. Even so, the traces of a zoosporangial exit pore are usually visible in the wall of the ‘sporangia’ of these species. All the downy mildews form well-developed haustoria in the living cells of their hosts.
Many of the downy mildews are important pathogens of crops worldwide. The downy mildew of vines, Plasmopara viticola, is another traveller from America; introduced into Europe in about 1878, it caused severe epidemics in European vineyards, leading to loss of production and worthless grapes. The development of control measures followed from the accidental discovery by Millardet in France in 1882 that vines sprinkled with verdigris (copper acetate) – it is said to make them taste bitter to those who might steal them – did not catch the disease. His invention, Bordeaux mixture (copper sulphate and lime), could be produced to stick to the vines and minimise any copper toxicity. It went on to become one of the main fungicides used to control not only downy mildews but also potato blight in the first half of the twentieth century.

FIG. 4.8
Sporangia and branched sporangiophores of Peronospora parasitica, cause of downy mildew of the cabbage family (Cruciferae). Note that the sporangia (which rarely produce zoospores and therefore function as conidia) are formed at the tips of the branches of the sporangiophore. The sporangia are approximately 20 μm diameter. (Light microscope photograph, D.S. Ingram.)
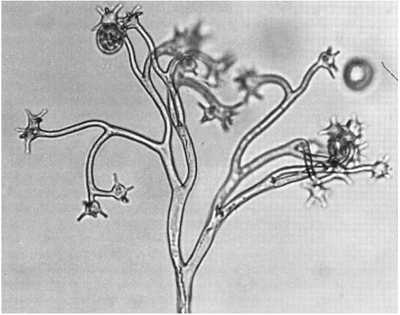
FIG. 4.9b
Sporangia and sporangiophores of Bremia lactucae, cause of downy mildew of lettuce (Lactuca sativa) and its relatives in the daisy family (Compositae). In (a) the sporangia (which do not form zoospores and therefore function as conidia) are still attached. Each is approximately 20 μm diameter. In (b) the sporangia have become detached and the ends of the branches of the sporangiophore can be seen to be expanded into discs, each with 4–6 pegs around its margin. The sporangia were once attached singly to each peg. (Light microscope photographs by Dr P.A. Mason, Institute of Terrestrial Ecology, Edinburgh.)
Another world traveller in this group is Peronospora tabacina, the blue mould of tobacco (Nicotiana spp.). It has been present in the USA and in Australia for a long time. Because of the greater variability of the fungus and the greater spread of resistance factors in the Australian Nicotiana spp., it is postulated that the fungus may have had its origin there. In the USA it is a disease of the seed bed. Young tobacco seedlings being grown for planting out are particularly prone to attack, and if the weather is warm and moist whole seed beds may succumb. In other countries the disease may be more important in field crops of tobacco. The exact form of the attack is determined by the interaction of environmental and cultural conditions. Infection may be minimised by strict attention to hygiene, by the use of chemical fumigants for seedlings and by spraying with fungicides, but care must be taken to avoid damage to the quality of the tobacco leaf by these procedures.
In 1958 the disease was identified in southern England on Nicotiana spp. and some 45 months later it had spread through the tobacco-growing areas of southern Europe, North Africa and west Asia. It reached eastern Iran in 1961–62 but does not appear to have invaded India, tropical Africa, South America or China. There can be little doubt that this spectacular piece of travelling was largely by aerial transport of the sporangia, although whether the original introduction into Britain was by this means must remain in doubt. Some believe that it entered the country on tobacco leaves or seeds imported by an amateur tobacco grower.
Two genera of downy mildew frequently encountered by the naturalist on wild and cultivated species are Peronospora and Bremia (Figs. 4.8 and 4.9 and Plate 2). P. parasitica may be observed on leaves of cultivated brassicas such as cabbage, Brussels sprout (Brassica oleracea var. gemmifera) and cauliflower (Brassica oleracea var. botrytis), as well as on wild brassicas and other members of the Cruciferae such as shepherd’s purse (Capsella bursa-pastoris) and wallflower (Cheiranthus cheirii). Diseased plants often have swollen and distorted stems, usually covered with a grey-white ‘down’ of sporangiophores and sporangia. On leaves, infection may lead to yellowing around the lesion, although the tissues remain alive. Sometimes necrosis occurs in the older, central region where the tissues have apparently succumbed to the pathogen’s demand for nutrients. A ‘down’ of sporangiophores appears on the living tissues of the underside of the lesion. If cross sections of infected tissue are cut with a stiff-backed razor blade or scalpel (see Appendix), mounted in water and examined with a microscope, the hyphae of the fungus are seen to be coarse, without septa (typical of the Oomycota) and growing between the cells. The haustoria, which penetrate the living cells of the host, are club-shaped or lobed (Fig. 2.6).
Peronospora farinosa, another downy mildew (Fig. 4.10), is frequently encountered on the leaves of members of the beet family (Chenopodiaceae), in the garden or farm on beet and in the wild on orache (Atriplex spp.), goosefoot (Chenopodium spp.) and sea beet (Beta maritima). Infected leaves become thickened and distorted as a result of disruption of the hormonal control of cell division and cell enlargement in the host (Fig. 4.10). They also appear paler green than normal, perhaps because cell enlargement does not lead to any increase in the number of chloroplasts (organelles containing chlorophyll) per cell. The sporangiophores and sporangia are a dull grey colour. Peronospora farinosa may also become systemic in infected plants, growing through the living tissue to infect all parts.

FIG. 4.10
Peronospora farinosa, cause of downy mildew of sugar beet (Beta vulgaris) supplied by G. Scott. The plant is systemically infected. The leaves are distorted, paler green than normal and covered with a fine grey ‘down’ of sporangiophores and sporangia. (D.S. Ingram.)
Other Peronospora spp. that may be encountered by the naturalist include the following.
P. arborescens is commonly seen on Welsh poppy (Meconopsis cambrica), on which it appears to be occasionally perennial in the root stock, and on other cultivated poppies. The sporangia are purple in colour, giving the lesion a purplish tinge. P. destructor causes long yellow lesions on onion leaves (Allium cepa) and can seriously reduce the crop. The sporangia are violet-coloured. P. grisea occurs on Veronica species and is especially obvious on brooklime (V. beccabunga). The leaves have a steely, curled look (presumably resulting from hormonal disturbance) and bear dingy violet sporangiophores on lower surfaces.
Bremia lactucae is the cause of downy mildew of lettuce and its relatives (Plate 2). The disease is often observed as a white, crumbly-looking down of sporangiophores and sporangia on the lower surface of unsprayed lettuce plants. The lesions are angular, being confined by the veins of the leaf, and the tissues remain quite green for several days following infection, eventually turning yellow or even necrotic when the fungal growth is too much for the host. The disease may easily be seen on wild lettuce and on ragworts (Senecio spp.).
It is very easy to see B. lactucae inside the host tissues if you have a microscope. Find a young, infected lettuce leaf, preferably on a seedling, and cut a 1 cm square with a pair of scissors. Place this in a drop of water on a microscope slide and crush by rolling gently with a glass rod or round pencil. Add a little more water and a cover slip. At the lowest (or highest, depending on which way up you have placed the leaf piece) plane of focus the branched sporangiophores may be seen emerging from the stomata. The tips of these open out as a saucer-shaped disc with 4 or 5 pegs around the rim, each bearing a sporangium (the sporangia may become detached during preparation) (Fig. 4.9). At a different plane of focus the relatively broad, non-septate hyphae may be seen growing between the living cells of the leaf, producing at intervals short, club-shaped haustoria which penetrate the cell walls, invaginating the plasma membrane to establish an interface for nutrient and chemical exchange (Fig. 2.6). Occasionally the sexually produced thick-walled oospores may be seen.
There are numerous other downy mildew genera occurring on wild and cultivated hosts. Amongst the best known are the following.
Pseudoperonospora. This genus resembles Peronospora except that the sporangia germinate to produce zoospores. P. humuli causes perhaps the most serious disease of cultivated hops (Humulus lupulus) and also occurs on wild hops. Greyish sporangia may be seen on the lower surfaces of the leaves and cones, matched by yellow patches on upper surfaces. Infection eventually becomes systemic in the root stocks. P. urticae causes greyish-lilac downy patches of sporangia on the undersides of the yellowed leaves of nettle (Urtica dioica and U. urens).
Plasmopara. This genus again resembles Peronospora, but the sporangia germinate to produce zoospores. P. viticola occurs on vines and other Vitaceae. It is only occasionally seen in Britain but is endemic in the USA and occurs widely in European vineyards. P. nivea occurs on bishop’s weed (Aegopodium podagraria) and chervil (Chaerophyllum temulum), both members of the carrot family (Umbelliferae). P. pygmaea occurs on wood anemone.
THE TROPICAL DOWNY MILDEWS
In the tropics or subtropics an additional group of downy mildews, the Sclerosporales, occurs on grasses and cereals. Within this group the genus Sclerophthora forms its sporangia in a similar fashion to Phytophthora and discharges its zoospores in a manner somewhat between that of Phytophthora and Pythium. Sclerospora, on the other hand, produces thick, tree trunk-like sporangiophores with the sporangia attached to a crown of small branches; the whole structure has an elephantine quality about it which is quite unmistakeable. Further taxonomic groupings are made on the ability of the sporangia to function as conidia without the production of free-swimming zoospores.
These fungi are widespread on grasses in the tropics, and Sclerospora graminicola is a serious problem on bulrush millet (Pennisetum americanum) and foxtail millet (Setaria italica), where the flower spike may become leafy and other parts of the plant stunted. The oospores, which have highly ornamental walls, frequently develop in the seeds of the host and it is by this means that the disease is spread.
Finally, mention must be made of another genus of the Oomycota related to the downy mildews but distinct from them. Albugo has aseptate hyphae and produces biflagellate zoospores from its zoosporangia. Like the closely related downy mildews, it is an obligate biotroph. The commonest British species, A. candida, causes white blisters (sometimes called white rust) on infected members of the family Cruciferae, such as cabbage, cauliflower and horseradish (Armoracia rusticana). In the wild it also causes spectacular, blister-like infections on shepherd’s purse (Plate 2). Infection usually leads to swelling and distortion of the stems, flowers, seed pods and leaves due to hormonal disturbance of the host tissues. Early on in the disease the tissues remain quite green, but eventually raised, shiny white blisters appear as the chains of sporangia are produced on short club-shaped sporangiophores immediately below the cuticle (Fig. 4.11). Eventually the cuticle bursts and masses of white sporangia are produced, to be borne on the wind or in rain splash to new hosts. Zoospores are produced on germination of the sporangia. Within the host tissues, if examined in section, the hyphae can be seen growing between the cells, producing almost spherical haustoria which are attached to the main hyphae by narrow necks. The oospores, if present, have a brown, warty wall that appears to be folded like the skin of a rhinoceros.
Albugo candida often occurs on shepherd’s purse in the same lesions as the downy mildew Peronospora parasitica, with spores of both fungi being produced on the infected tissues. The coexistence of the two pathogens appears to be temperature-dependent. As the temperature rises in summer the balance may be tipped in favour of Albugo and the Peronospora dies out.

FIG. 4.11
Chains of sporangia (a), emerging zoospores (b) and a single zoospore (c) of Albugo candida, cause of white blister of members of the cabbage family (Cruciferae). Each sporangium is approximately 15–20 μm diameter and the released zoospores are approximately 8–10 μm diameter. (Illustration by Mary Bates, based on drawings by Webster (1980).)
Another, less common, white blister is A. tragopogonis, which infects members of the family Compositae such as salsify and goat’s-beard or Jack-go-to-bed-at-noon (Tragopogon spp.) and ragworts. A. bliti infects members of the purslane family (Portulaceae). Another Albugo, A. ipomoeae-panduratae, occurs in warmer climates, infecting sweet potato (Ipomoea batatas), and the similar A. ipomoeae-aquaticae attacks water spinach (Ipomoea aquatica).
THE CONTRIBUTION TO SCIENCE
Not only are the zoospore-forming fungi important pathogens in their own right, but attempts to understand and control them have led to significant advances in the science of plant pathology.
In Chapter 1, reference was made to the first attempt to understand that fungi were the cause rather than the result of disease and death in plants, and to the role played by Phytophthora infestans in the development of the early hypotheses. It was Anton De Bary’s definitive study of potato blight in the 1870s that finally clinched the argument in favour of the fungal pathogen.
One of the first demonstrations of a clear-cut inherited resistance (or immunity) to disease was shown in the wart disease of potato (Synchytrium endobioticum), an immunity which has been long-lived and of great practical utility. The hope that a similar immunity or resistance could be found against Phytophthora infestans led to crosses of Solanum tuberosum with wild potatoes such as S. demissum, using some elegant breeding techniques to overcome the differences in chromosome number between the parents. The observed breakdown of resistance to blight in these hybrid potatoes and, as new genes for resistance were introduced, their failure, in turn, contributed to the concept of R genes, an understanding of allelic series of resistance genes and studies of the genetics of virulence and avirulence. Perhaps, too, it provided a background against which the gene-for-gene hypothesis could be clarified in flax rust by Flor (see Chapter 3). Later, potato breeding with semi-domesticated potato species led to a further understanding of the broad-based resistance that is now widely used to control disease. Within the last ten years studies of Bremia lactucae on lettuce and Peronospora parasitica on the Cruciferae have added significantly to our understanding of the genetics of resistance and virulence (see Chapter 3).
The interaction between Phytophthora infestans and resistant cultivars of potato was used for early studies of the biochemical basis of resistance. This work led to the identification of the first phytoalexins in the 1940s and 50s, later shown to be the terpenoid compound rishitin and its relatives. Now, work with Peronospora parasitica on thale cress (Arabidopsis thaliana) is contributing to molecular biological studies of recognition of aliens by potential hosts.
The development of forecasting of epidemics from meteorological data began with P. infestans, as did the study of the nature of epidemics and the use of chemical sprays for their control. There is also a number of less immediately practical matters, the study of which began with the zoospore-producing fungi. Taxis in zoospores, for example, which is the way zoospores are attracted or directed to roots, surfaces and so on; the complex series of interactions mediated by sex hormones which controls the mating behaviour in these organisms, and the importance of sterols in controlling the production of sexual spores. Perhaps, however, the most interesting of all these esoteric matters studied in the zoospore-producing fungi is the question of their evolutionary origins and taxonomic position. There has always been considerable uncertainty surrounding the relationship of the Oomycota to other groups of fungi. For example, it has long been recognised that they have much in common with certain groups of algae: diploid life cycles; walls containing cellulose (and glucans); biflagellate zoospores; distinctive sexual structures and mechanisms of fertilization; and the induction of sporulation by mineral solutions. On the basis of such evidence many have argued that the Oomycota should not be regarded as true fungi at all. There has also been much debate about the relationship to one another of the genera within the Oomycota – Pythium, Phytophthora and the downy mildews.
Now, molecular biological techniques are making possible definitive studies of these matters. The new techniques allow comparison of the DNA of different organisms, especially those parts of the genome that are highly conserved (i.e. have remained relatively unchanged through evolution). Most important of these conserved pieces of DNA is the ribosomal RNA-gene repeat (rDNA for short). Ribosomes, subcellular bodies involved in the synthesis of proteins, probably evolved from bacteria very early in the history of life on earth.
Ribosomal DNA has now been sequenced (analysed in detail) for most major groups of living organisms. The rate of mutation within the DNA and the resulting evolutionary divergence seem to provide an excellent ‘clock’ to measure the progress of evolution of all living things. Computer programs have been developed to compare the rDNA sequences of different organisms and to generate phylogenies (evolutionary trees). By these means it has been found that the Oomycota are not in any way related to the rest of the members of the Kingdom FUNGI (Ascomycota, Basidiomycota, Chytridiomycota and Zygomycota) but instead belong to a new Kingdom, the CHROMISTA, which also includes the brown algae (seaweeds) and diatoms (unicellular planktonic algae with silica ‘shells’). Thus Phytophthora and its allies are no longer regarded as fungi and it has become fashionable to call them ‘pseudo-fungi’.
The ‘spacer regions’ of DNA that separate the main rDNA genes mutate more rapidly than the rDNA genes themselves. These mutations persist in populations of individuals carrying them, since they confer no disadvantage (because the spacer DNA appears to have no other function than to separate the main genes). Studies of such mutations provide a means of determining the ‘relatedness’ of one organism to another below the level of the Kingdom, especially at the level of the genus and species. Recent research of this kind has shown that Phytophthora is a clearly defined genus with sporangial form determining the main groupings within it. In contrast, Pythium is a very diverse assemblage, encompassing at least four well-separated groups, each about the size of the genus Phytophthora. The genus Peronospora (a downy mildew) has been shown to be little more than an obligately parasitic offshoot of Phytophthora.
All this rDNA work has had a valuable practical spin-off in disease diagnosis. By isolating and sequencing rDNA it is possible to detect within a day very low levels of infection of hosts by different Phytophthora spp., making it possible to apply control measures in good time. In Scotland, for example, early diagnosis is leading to better control of Phytophthora on the commercially valuable raspberry crop.
Finally, it is important to mention the great diversity of zoosporic fungi that parasitise algae. Studies of such organisms in fresh water has been pioneered by Dr Hilda Canter-hund at the Freshwater Biological Association in the English Lake District. For an enthusiastic amateur with a good microscope, however, a whole world remains to be discovered in almost any ditch, pond or lake. Here will be found underwater biotrophs and necrotrophs, resistance reactions, including the hypersensitive response, and epidemics of disease. References that provide a window on this world are included in the reading list to Chapter 4.