![]()
An early morning poke about in the garden during late summer or early autumn is an exciting experience for a plant pathologist, especially if the weather is wet and the owner has neglected to thin his vegetables or apply fungicide sprays to his fruit trees. Disease will be found everywhere: rots on the fallen apples, shot-holes in the cherry leaves, scab on the pears, black spot on the late-flowering roses, blight on the potatoes, downy mildew on the lettuces and cabbages, rust on the leeks and antirrhinums and a fine white covering of powdery mildew on almost everything, but especially noticeable on the vegetable marrows and roses. Even the underground parts of the plants may show symptoms if dug up: rots on the carrots, evil-smelling clubroot (finger and toe) on the cabbages and other brassicas, rot and scab on the potatoes and, if blight is present, a foxy red discoloration when cut open. The glasshouse will provide a rich harvest too: rots and wilt on the tomatoes, powdery mildew on the cucumbers and damping-off in the pan of seedlings sown too densely and then overwatered. And if the owner has neglected to weed his patch, even the weeds will be afflicted: rust on the groundsel and annual grasses, white blister on the shepherd’s purse and bright red target spots on the dock leaves. The old sycamore in the far corner may have tar spot on its leaves, and the gap beyond may be the only reminder of the elms that once stood at the very end and shaded everything before falling victim to the devastating wilt, Dutch elm disease. The same trail of death and decay, albeit less obvious but no less varied, awaits the naturalist who explores the meadow or the wood, who studies the verges and hedgerows of a country lane, who climbs the high hills or descends to the sand dunes and salt marshes of the coastal plain. The remarkable, often unrecognised, fact is that plant diseases are everywhere, an integral and often even essential part of the natural scene, and the variety of symptoms resulting from these diseases is simply enormous.
This variety is not, however, without pattern. In every case it will be found that particular pathogens and groups of pathogens elicit the same type of symptoms, although the severity of the attack may vary according to the host species, the general state of vigour of the host and the prevailing weather conditions. For example, Monilinia fructigena (2.1), a member of the Ascomycota (see here), will cause a brown rot if it infects an apple fruit injured, perhaps, by a pecking bird or a rubbing branch. It will, however, cause rot more quickly in ‘Cox’s Orange Pippin’ apples than in ‘Bramley’s Seedlings’, and it will always be more destructive if the weather is cool and wet. By contrast, the powdery mildews, Erisyphe spp. and other members of the Erysiphaceae (see here and Plate 5), also in the Ascomycota, rarely seem to kill the host tissues, whether the host be an apple tree or a vegetable marrow, despite the presence of an enormous amount of fungal mycelium on the surface of the leaves. However, if the weather is dry the host will be much more stunted than usual, will probably produce a smaller crop, may lose its leaves early and will certainly be more heavily infected.

FIG. 2.1
An apple fruit (var. Golden Delicious) rotted by the necrotrophic fungus Monilinia fructigena. Note the wound where the pathogen gained entry to the fruit. (B. Goddard, University of Cambridge.)
To understand what is going on it is necessary to know something about the ecology and physiology of the fungi and bacteria that cause plant disease. This information has been gathered over the last hundred years or more by plant pathologists studying crops of agricultural and economic importance such as wheat, potatoes, lettuce, grapes or apples; but the same principles apply and the same patterns are evident in diseases on wild, uncultivated plants.
STRATEGIES OF PARASITISM
Until about 20 years ago, plant pathogenic fungi and bacteria were classified, for the purpose of study, according to a scheme devised by the great nineteenth century German plant pathologist Anton de Bary. The basis of this classification was ecological, and fungi and bacteria were grouped according to whether they were pure saprophytes, facultative parasites or obligate parasites. Pure saprophytes grow and reproduce only on tissues that are already dead, and are completely incapable of infecting living plants. They are familiar, for example, as moulds on bread or cooked food. It is interesting to note that frequently they possess exactly the same armoury of cell-destroying enzymes and toxins as pathogens; clearly, therefore, the capacity to infect a host and cause disease requires more than the mere ability to destroy living tissues. Facultative parasites, such as Monilinia fructigena mentioned above, are able to infect living plants, kill them and then live on the dead tissues as saprophytes. Obligate parasites, however, such as the powdery mildews, are much more limited in their ecology and are unable to grow in nature in the absence of a living host (see Chapter 8 and Plate 5). P.W. Brian, in 1967, drew a distinction between two sorts of obligate parasites. Those that are restricted to a living host in nature, but can be grown on their own on an artificial culture medium in the laboratory, he called ecologically obligate parasites. Phytophthora infestans, cause of potato blight, fits this category (see Chapter 2 and Plate 1). In such cases obligate parasitism results from the inability of the pathogen to compete on dead host tissue with pure saprophytes, which invade the tissues after death. Others, those so specialised in their parasitism that they are unable to grow in the absence of a host, even in the laboratory, he called physiologically obligate parasites. The powdery mildews are of this type. By implication, the physiologically obligate parasites possess some biochemical or physiological deficiency that can only be supplied by a living host, and this prevents independent growth.

FIG. 2.2
Classification of plant parasitic fungi. After A. de Bary (1867) and P.W. Brian (1967).
De Bary’s classification was the basis of the revolution in the study of the ecology of pathogenic fungi led by Denis Garrett in the 1940s and 50s, and Brian’s modification was a response to the growing interest in the 1950s and 60s in the physiology and biochemistry of the interaction between pathogens and their hosts, led in the UK by R.K.S. Wood of Imperial College, London and stimulated by Brian himself in the University of Glasgow. The de Bary classification as modified by Brian is still very helpful, and will be used later in the present volume, a summary being set out in Fig. 2.2 for ease of reference. However, for the purpose of considering the mechanisms by which fungi and bacteria interact with a host and cause the visible symptoms of disease, it is much more convenient to use another scheme, first devised by the Australian plant pathologist Neville White in 1957, defined in detail by David Lewis in 1973 and placed in an ecological context by Parbery in 1997. The basis of this classification is the mechanism by which fungi and bacteria obtain nutrients from their hosts, for there is a direct relationship between the strategy employed and the symptoms of disease that result.
In this scheme three broad categories of pathogen are recognised.
1. Necrotrophs
Having penetrated a host, these pathogens rapidly kill the cells and tissues and break them down with enzymes to release the sugars and other nutrients required for growth and reproduction. They are therefore destructive parasites that cause symptoms such as damping-off, rots, wilts, necrotic spots on leaves and so on, all of which result from the death and breakdown of cells and tissues. Monilinia fructigena belongs to this category.
2. Biotrophs
Having penetrated a plant, these pathogens derive their energy from the living cells and tissues by tapping into the plant’s own sugar and nutrient supply. The relatively balanced relationship required for this form of parasitism may last for a long time, with the host plant not being killed for several weeks, months or even years. The powdery mildews (Plate 5) are typical biotrophic fungi. The host leaves may be covered with the surface-growing hyphae of the pathogen bearing long chains of spores, yet still remain green and intact, albeit somewhat stunted and debilitated. Other biotrophs, such as the downy mildew, rust and smut fungi, may permeate host tissues but the infected organs remain relatively green and healthy, only being damaged where spores erupt through the surface layers or when the load of infection is too great. Symptoms include a fine down of hyphae and spores on the green leaf in the case of downy mildews, or pustules on leaves or other organs where the spores of rusts or smuts break through the cuticle (the outermost layer), prior to release. Yellowing and dead areas of tissue may indicate that the host cells in a limited area have given up under the strain. Biotrophs also cause tumours and other symptoms indicating disturbance of cell and organ growth.
3. Hemibiotrophs
These are intermediate in their parasitism and exhibit features of both necrotrophy and biotrophy. An example is Venturia inaequalis (see here and Plate 4), the cause of apple scab, a disease that manifests itself as dark, scabby spots on the leaves and fruit of apples. Spores infect the young leaves of the apple tree in spring, forming a mycelium that grows biotrophically just below the cuticle. The infected areas may be paler green than normal, but there is little more to see. Later, as the season advances, the hyphae may grow deeper into the leaf, killing cells as they go, so that the relationship then becomes necrotrophic and areas of dead tissue develop. Also, spores erupt through the cuticle and a dark scab is formed. Eventually the tissues die completely and the leaf falls to the ground, yet the pathogen continues to grow on the dead tissue on the surface of the soil and, after surviving the winter, may produce spores which will reinfect the new apple leaves in the spring.
It is probable that many of the plant pathogens thought by plant pathologists to be necrotrophs are, in fact, hemibiotrophs with a very short biotrophic phase (e.g. a few hours to a few days) at the start of their life history. Unfortunately, the early stages of infection of too few diseases have been studied in sufficient detail to provide an unequivocal answer to this question. A better understanding of hemibiotrophy would greatly help in the interpretation of the host specificity and symptom development of many plant diseases like apple scab or light leaf spot of brassicas (Pyrenopeziza brassicae) that are still poorly understood.
Necrotrophs and biotrophs are very different in their physiologies and food-gathering strategies. The features that characterise these two groups merit further consideration, for this will help in understanding the nature of the diseases they cause, and these are listed and discussed below, with some simplification for reasons of clarity.
THE NECROTROPHS, CAUSE OF ROTS, WILTS AND NECROSES
1. Saprophytic growth
The pathogen is capable of saprophytic growth on the tissues it has killed, or on other substrates in the absence of a suitable host. The more extreme necrotrophs, like the Pythium spp. that cause damping-off of seedlings, may also be able to compete reasonably well with pure saprophytes, which grow on dead tissues and nothing else. They therefore equate with the facultative parasites of the de Bary-Brian classification. Increasing specialisation as a necrotrophic pathogen usually means a progressive loss of this ‘competitive saprophytic ability’ (a term coined by the fungal ecologist Denis Garrett) and some necrotrophs may therefore equate with the ecologically obligate parasites of de Bary-Brian. Competitive ability depends on the capacity to grow rapidly or produce antibiotic(s) and/or other substances such as the enzymes needed to kill or inhibit rival fungi or bacteria competing for a food source. This capacity is sacrificed in the evolution of increasing ability as a pathogen invading living host tissues, where competitors are fewer.
2. Weak parasitism
It is a paradox that the most destructive necrotrophs are capable of attacking only the young tissues of seedlings, or more mature tissues of plants that have been weakened by stress, injury or old age. Moreover, a large number of pathogen spores or a large amount of mycelium is normally required to gain entry to the host. This probably reflects the high energy levels required for killing host cells and tissues, overcoming in-built mechanisms by which pathogen attacks are resisted, and breaking down cell walls.
3. Wide host range
Most necrotrophic parasites are capable of attacking a wide range of host species. Pythium ultimum (see here), for example, not only causes damping-off of seedlings, but will also cause soft rots on such unrelated species as cabbages (Brassica oleracea), potatoes and cucumbers (Cucumis sativus). This may result from a lack of specialised nutritional requirements, but may also reflect the fact that the relationship with the host is unsophisticated and therefore has not necessitated the evolution of features geared specifically to growth in the tissues of a particular plant genus or species. Moreover, since host tissues are killed before the pathogen makes any appreciable growth in them, host resistance mechanisms triggered by infection do not have to be avoided using specific strategies (see Chapter 3).
It is interesting to note, however, that the host ranges of necrotrophs are not infinite, with some plant species being resistant to each pathogen. Pre-formed fungitoxins present in the tissues of some but not all species may be responsible in some cases (e.g. in onion skins), and many toxic substances such as phenols accumulate more rapidly in some plant tissues than others as a result of wounding. Moreover, the parasite may lack the appropriate enzymes to release nutrients from the tissues of some potential hosts, a matter that will be referred to further below, or the environmental conditions may be unsuitable for infection to occur. Finally, as suggested above, many pathogens currently believed to be necrotrophs may in fact be hemibiotrophs, with a short biotrophic phase at the start of the disease cycle. Since a characteristic feature of biotrophy is considerable specificity in host range (see here), this could explain the limited host range of many apparent necrotrophs.
4. Production of plant cell-destroying enzymes
This attribute is especially important and is largely responsible for the cell death and cell and tissue breakdown that lead to the rots and necroses that are the typical symptoms of disease caused by necrotrophic pathogens (Fig. 2.1 and Plate 3). Investigations suggest that the production of such enzymes is normally triggered by the presence of the chemical to be broken down. They are then released from the tips of the hyphae or cells of the pathogen and may diffuse for a considerable distance into the host tissues. Cells are killed by the enzymes, thus negating any active resistance response, and nutrients are released to nourish the advancing pathogen by erosion of the components of the cell walls and the cell contents. The necrotroph thus marches through the host tissues into a nutrient-rich ‘soup’ that supplies all the energy required for its continued growth and reproduction.
A wide variety of enzymes may be produced by a necrotroph, each capable of attacking a different component of the plant cell. Indeed, the production of different enzymes may occur sequentially during growth of the pathogen as different cellular components are uncovered. Before considering the nature of these enzymes, however, it is necessary to examine the structure of the plant cell wall, for despite its apparent simplicity it is, in fact, very complex and research to elucidate its finer detail is still going on.
Plant tissues are made up of individual cells, each surrounded by a rigid wall, and joined to one another where they touch by a glue-like layer called the middle lamella. There may also be spaces between cells, lined with a film of fluid. The wall has two zones: an outer primary wall, produced as the cell grows, and an inner secondary wall, produced after cell expansion has ceased. Both contain fibres; those of the primary wall are loosely packed, while those of the secondary wall are densely packed. The fibres are embedded in amorphous (non-fibrous) material which is continuous with the middle lamella. The fibres consist of cellulose, a substance composed of bundles of parallel, unbranched chains (polymers) of glucan molecules. These may be crystalline and are extremely resistant to enzyme attack.
One major group of components of the amorphous material and the middle lamella are pectic substances. These too are polymers of sugar molecules and are found in decreasing amounts from the middle lamella, through the primary wall into the secondary wall. The basic chains are known as pectic acids (e.g. polygalacturonic acid) and are soluble in water. They are especially plentiful in the tissues of fruit and are responsible for the setting of fruit jam. Frequently these pectic acids combine with calcium ions (Ca++) to give insoluble substances called pectates (polygalacturonates), or they may react with alcohol to give pectins (polymethyl galacturates).
A second major group of components of the amorphous parts of the wall are the hemicelluloses. These are complex forms of mainly linear, but sometimes branched, polymers of various sugars, mainly xylose. Proteins are also present throughout the wall, especially those containing a sugar component, called glycoproteins. There may also be enzyme proteins present with a role in regulating the growth of the wall. The hemicellulose molecules that coat the cellulose fibres and are bonded to them are in turn chemically bonded to the pectic molecules and the glycoproteins, binding the whole cell wall together.
Other substances may be deposited in the walls of cells modified during growth and maturation to perform special functions in the plant. Lignin, for example, a tough, three-dimensional amorphous polymer of complex molecules such as coniferyl, sinapyl and cinnamyl alcohols, is deposited in the cells that conduct water and gives strength to the xylem cells, which are the major component of woody tissues. Fatty, hydrophobic (water-repelling) polymers such as cutin are a major component of the waterproof cuticle that covers the outer surface of the plant. Another hydrophobic fatty polymer, suberin, forms corky layers in the bark and in wounded tissue, which it seals against infection by necrotrophs.
The major components of plants that are attacked by most necrotrophs are the pectic substances of the middle lamella and primary wall. Much of the research on the enzymes used by necrotrophs to break these down into simple sugars has been done with Monilinia fructigena, but the results are widely applicable. There are two major groups of enzymes involved, those that cut the chains (polymers) by a chemical process called hydrolysis and those that cut the chains by a different chemical process called transelimination. Both groups include enzymes that attack the chains at the ends (exo-enzymes) and enzymes that attack the body of each chain (endo-enzymes). Also, both groups contain enzymes that specifically attack the pectins or the pectates. Together, or in sequence, these pathogen-produced enzymes act upon the middle lamella and primary walls of the host to solubilise the amorphous materials, allowing unrestricted growth of the fungus through the tissues and releasing all the sugars required for continued growth and reproduction. No single necrotroph produces all these enzymes, however, and it is important to realise that the particular range and sequence of enzymes deployed, and the symptoms that result, vary according to the host and pathogen species involved and the complex interaction between the enzymes, the chemicals predominating in the wall and the environment.
The enzymes produced by the pathogen determine whether a rot will or will not occur, but the chemical and physical environment of the host ‘shapes’ the interaction. For example, tissues that are relatively acid are more favourable for the process of hydrolysis, whereas neutral to alkaline tissues favour transelimination. Transeliminase enzymes are far more destructive than hydrolases and more severe rots result from their activity. If the plant cell walls are rich in free calcium or magnesium ions, this leads to the inhibition of hydrolysis but the stimulation of transelimination. If the water level of the host tissues is high, rotting is more likely, for this facilitates diffusion through the tissues of both groups of enzymes, an observation that fits well with the fact that rots are usually more severe in wet weather conditions.
Finally, inhibitors of pectic enzymes may be present in host tissues, especially certain glycoproteins and the dark products of oxidised phenols formed when cells are killed. The presence of inhibitors may explain why some fungi cause firm rots and others soft rots, a difference which is very obvious in apples infected by either Monilinia fructigena or another necrotroph causing rots in apples, Penicillium expansum (Fig. 2.3). Both are capable of producing a wide range of pectic enzymes, yet M. fructigena causes a dark, firm rot in apple fruits while P. expansum causes a pale, soft rot. It is likely that the pectic enzymes of the former are inhibited by the large quantities of the dark oxidised phenols produced by wounded apple flesh, the brown discoloration we see when apples are cut, while the enzymes of the latter are unaffected because it has evolved to produce an inhibitor of phenol oxidation.
It has already been noted that the cells of tissues disrupted by pectic enzymes invariably die. This probably occurs because the plasmalemma, the membrane that surrounds all living cells, is disrupted by the shear forces at the point where the plasmodesmata, the thin threads of membrane and cytoplasm that link each cell with its neighbours, pass through the cell walls. It is likely that such shearing results from the movement of one cell against another as the whole tissue mass is destabilised following breakdown of the middle lamella. A further factor may be the accumulation of toxic substances released or formed during the breakdown of the cell walls.
Enzymes capable of degrading the other structural components of the cell wall, described earlier, may also have a role in the breakdown of tissue to release nutrients for pathogen growth. Although, as we have seen, hemicelluloses are widespread in the cell wall, pathogen enzymes capable of breaking them down (called hemicellulases) are probably only of secondary importance in most diseases, but assume greater significance in those cases where pectic enzymes are inhibited, as in the rot of apples caused by M. fructigena. Cellulose, the other major wall material, is a relatively intractable substance because of its fibrous and crystalline nature. For most pathogens causing rots, quite sufficient carbohydrate for growth and sporulation may be obtained from the pectic substances and hemicellulose. Nevertheless, cellulases are important in softening the cellulose during the penetration of the cell wall, where the fibres are so tightly packed that the pathogen cannot simply push through. In the case of some necrotrophic pathogens such as Pythium spp. the ability to produce cellulases in great quantities may allow the fungus to continue to thrive on the dead tissues of the host long after the pectic substances and hemicelluloses have all gone. The production of cellulases thus confers on such pathogens increased ability to compete with pure saprophytes for nutrients.

FIG. 2.3
Apple fruit (var. Bramley’s Seedling) infected by Monilinia fructigena (dark, firm rot) and Penicillium expansum (pale, soft rot). (B. Goddard, University of Cambridge.)
The most intractable of all the constituents of the wall is lignin, the major component of woody tissues. Some necrotrophs have, however, evolved the ability to parasitise trees and many of these produce enzymes capable of degrading lignin. Two of the most important are Heterobasidion annosum, (Fomes annosus) cause of butt rot of conifers, especially those grown for timber, and Armillaria mellea, the honey fungus, which is most troublesome in hardwoods, especially in gardens (see Chapter 11). Breaking down wood is a very complex process, requiring a large range of lignin-degrading enzymes, so these fungi are very slow-growing. Although poor as competitors because of this, they are not at too great a disadvantage since as pathogens they enter the dead wood ahead of competing saprophytes. They thus have an assured supply of foodstuffs that will last for a very long time indeed and on which they can grow and thrive long after the initial pathogenic phase is over. H. annosum and A. mellea are often known as white rotters because, after breaking down the brown lignin, they leave only a bleached skeleton of residual cell wall material. This is in contrast with colonisers of dead wood, called brown rotters, which degrade only the cellulose, leaving the lignin in place.
Factors other than the chemical complexity of lignin combine to render woody tissues particularly resistant to pathogen attack. The close-knit structure of wood creates a physical barrier to pathogen growth, although fungi and bacteria are quite good at growing along the water-conducting xylem vessels. The water content of living wood is often low, which means that wood-rotters need to be capable of withstanding considerable desiccation. The low nitrogen levels of wood require strict nitrogen conservation strategies, especially the reabsorption by the pathogen of any residual enzymes (rich in nitrogen) not used in cell wall breakdown. Finally, because woody tissue is protected by aromatic natural fungicides such as phenols, terpenoids, tropolenes, flavanoids and stilbenes, the potential pathogen must be capable of producing the enzymes that detoxify these substances. It is small wonder that there are so few wood-rotting pathogen species, and that they grow so slowly.
5. Production of pathotoxins
In addition to producing cell-destroying enzymes, some but not all necrotrophs produce toxins that kill host cells in advance of infection, thus negating active defence processes. These are called pathotoxins. This is analogous to the situation in certain diseases of humans: as long ago as the nineteenth century, Louis Pasteur showed that the symptoms of tetanus and diphtheria are caused largely by toxins produced by the bacterial pathogens responsible for these diseases. Toxins produced by necrotrophic plant pathogens may kill cells so rapidly that areas of dead tissue develop on leaves, stems or roots; or they may affect the plant’s basic metabolism, causing yellow halos to form around the point of infection; or some may be transported in the plant, affecting the water relations of cells and tissues, thus causing wilting, often followed by death.
The study of pathotoxins is fraught with difficulty. When grown in culture in the laboratory, most pathogenic fungi and bacteria produce toxic chemicals, many of which will kill plant cells. These are not, however, true pathotoxins, but simply the by-products of metabolism in culture. Only after exhaustive experimentation can it be concluded that a toxic chemical produced by a pathogen in the laboratory is a true pathotoxin and actually has a role in disease. The first pathotoxin to be identified with certainty was Victorin, a cyclic peptide with a terpenoid side chain produced by Bipolaris victoriae, cause of Victoria blight of oats (Avena sativa) in the United States. Many others have now been identified. Some pathotoxins, like Victorin and the toxin produced by Ophiostoma (Ceratocystis) ulmi, cause of Dutch elm disease, and T-toxin produced by Bipolaris maydis, cause of southern corn leaf blight of maize, are highly specific in their activity (Fig. 2.4). Others, such as the tripeptide derivative produced by the bacterium Pseudomonas syringae pv phaseoli, which causes yellow halos around the points of infection in the halo blight disease of French bean (Phaseolus vulgaris), are non-specific; the halo blight toxin (phaseolotox-in) will cause yellowing in a wide range of plant species to which it is applied artificially in the absence of the pathogen, even though the host range of the pathogen itself is limited to Phaseolus spp.. The effects of another non-specific toxin are often seen not in green plants but in the fruit bodies of a fungus, for fungi as well as plants have their pathogens. The brown blotches that sometimes appear on older mushrooms (Agaricus hortensis, a saprophytic fungus) are caused by a bacterium, Pseudomonas tolasii, which produces two toxic peptides. These chemicals disrupt the membranes of the mushroom hyphae and kill them, but they are totally non-specific in their action and will kill the hyphae of a wide range of other fungi as well as the cells of plants if applied artificially.

FIG 2.4
Lesions of Bipolaris maydis, cause of southern corn leaf blight. The fungal toxin, T-toxin, causes cell death in plants carrying the Texas gene for male sterility (used in breeding hybrid maize). (P.H. Williams)
In summary, necrotrophs are unsophisticated pathogens, intent on breaking down cells and tissues to release carbohydrates and other nutrients, but in the process indiscriminately killing young and weakened plants and causing rots, necrotic spots and wilts. At the same time, however, they are highly sophisticated organisms that have evolved to exploit a particular ecological niche. In a mixed micro-flora of pure saprophytes and facultative parasites, being the first to colonise a virgin substrate and extract food from it confers a considerable competitive advantage. By evolving the mechanisms to become pathogens, kill their hosts and then break down the tissues to release foodstuffs, necrotrophs have ensured that they occupy a lead position in the race for the energy to reproduce.
THE BIOTROPHS
1. Normally unable to grow in the absence of a living host in nature
Most biotrophic pathogens lack completely the ability to compete successfully with pure saprophytes whether in the soil, on the surface of a dead leaf or in dead tissue they have killed. They appear to have sacrificed competitive saprophytic ability for a more sophisticated form of parasitism and therefore equate with ecologically obligate parasites (Fig. 2.2), restricted to a living host by their ecological specialisation. Many biotrophs, such as the Erisyphe spp. which cause powdery mildews (Plate 5), cannot even be grown in culture, and therefore equate with physiologically obligate parasites. This inability to grow outside living host tissues may in part be due to the loss of the ability to produce extracellular, cell wall degrading enzymes. If the host cells are to remain alive to supply carbohydrates and other nutrients, the production of enzymes that break down and kill cells would be a significant disadvantage, like killing the goose that laid the golden egg.
The lack of the ability to grow as a saprophyte may also be due to increased adaptation to the host environment in other ways. Hyphal membranes may be very leaky, for instance, to facilitate nutrient exchange with the host, or the pathogen may have lost the ability to make an essential chemical itself, thereby becoming dependent upon its host to supply it. Thus the biotrophic fungus Puccinia graminis, cause of the black stem rust of grasses and cereals (see Chapter 9), depends on its host for a supply of sulphur-containing amino acids. P. graminis was regarded as a physiologically obligate parasite until a group of Australian workers showed that it was capable of very slow growth on a culture medium containing complex protein extracts and yeast extract (Fig. 2.5). Even on these rich food sources colonies took many months to reach a size attained by other fungi in a few days. It was later shown that P. graminis could not manufacture its own sulphur amino acids and that it was these that were required from the rich medium. The slow growth was due to the fact that the membranes were so leaky that nutrients were lost almost as rapidly as they were taken up. Growth in a liquid form of the culture medium was very much better than on a medium solidified with the jelly-like agar, perhaps because the former, which completely surrounded the hyphae, more closely resembled the intercellular fluid of the plant.

FIG. 2.5
Colonies of Puccinia graminis, cause of black stem rust of wheat, growing very slowly on nutrient-rich culture medium in test tubes. The largest colony has reached a diameter of approximately 1.5 cm in 48 days. (D.S. Ingram.)
2. Minimal structural damage to the host
This results largely from the controlled production of cell wall degrading enzymes. Biotrophs produce such enzymes because they require them for penetration of the host and to facilitate growth between cells by dissolving limited areas of the middle lamella, but their production is triggered only by the presence of the chemical to be broken down and is then rapidly switched off again. Moreover, they are produced in only very small quantities. It may also be that in some cases such enzymes are actually bound to the walls of the pathogens’ hyphae and are only active upon direct contact with host cell walls, having no capacity to diffuse further into the tissues. Those that are not bound in this way may be of higher than normal molecular weight (i.e. may be composed of very large molecules) so that they diffuse slowly and therefore move only short distances through cell walls from the point of production.
Tissues infected by biotrophs remain intact, the only evidence of disease visible to the plant pathologist often being the appearance of spores erupting through the surface of the leaf or other organ, accompanied by some chlorosis (yellowing).
3. Intercellular hyphae with haustoria
The hyphae of fungal biotrophs usually grow between cells and absorb nutrients from the intercellular fluid, but many also establish direct contact with the living contents of cells by forming specialised feeding branches called haustoria (Fig. 2.6). These penetrate the walls of the cells and induce the cell membrane lying just inside the wall to grow into loose folds. Then, as the haustorium grows into the cell, the membrane is unravelled to accommodate and enclose the expanding structure. A series of layers, the product of both the host cell and the pathogen, accumulate between the haustorium and the surrounding host cell membrane, and create an interface both for the uptake of nutrients by the pathogen and for the secretion into the host cell of substances that modify the cell’s metabolism to benefit the pathogen. Haustoria increase very considerably the capacity of the pathogen to extract nutrients from living cells. This intimate contact may persist for a very long time in many biotrophic host-pathogen interactions such as the downy mildews (e.g. Bremia lactucae on lettuce (Lactuca sativa), (see here and Plate 2) and the rusts (e.g. Puccinia antirrhini on antirrhinum, see here and Plate 6). In the case of the powdery mildews (e.g. Erysiphe pisi on pea (Pisum sativum, Plate 5 and see here)), because the mycelium grows only on the surface of the host’s leaves and stems, the haustoria which penetrate the surface epidermal cells represent the only channel of communication between host and pathogen.

FIG 2.6a

FIG 2.6b
Haustoria: (a) intercellular hypha and club-shaped haustoria of Bremia lactucae, cause of downy mildew of lettuce; (b) branched haustorium of Peronospora farinosa, cause of downy mildew of sugar beet. (Photograph (a), P.A. Mason, Institute of Terrestrial Ecology, Edinburgh; photograph (b), D.S. Ingram.)
It should be added that some hyphal biotrophs, such as the smut fungi (see here), do not produce haustoria but must absorb all their nutrients through the hyphae. Non-mycelial biotrophs establish direct contact with the cells of the host because the fungal body actually enters the cell and is surrounded by the host’s membrane. This is the case with Plasmodiophora brassicae, the cause of clubroot on brassicas (see here). Again, a zone of interaction between the pathogen and the host’s plasma membrane develops and creates an interface for the exchange of nutrients and other substances.
4. A very limited host range
Most biotrophic fungi are able to parasitise only a relatively narrow range of hosts, and in many cases may even have evolved races or strains capable of infecting only certain cultivars or genotypes of a host species. For example, Plasmodiophora brassicae only causes disease in members of the cabbage family (Cruciferae), to which brassicas belong. Erysiphe graminis, cause of powdery mildew in grasses and cereals, infects only members of the grass family (Gramineae). Moreover, E. graminis exists as a series of structurally identical strains, each called a forma specialis, and each capable of infecting only one genus or species within the Gramineae. Those attacking cereals are, respectively, f.sp. tritici (wheat, Triticum aestivum), f.sp. hordei (barley, Hordeum vulgare) and f.sp. avenae (oats). In each case there are races within each forma specialis that will affect only certain cultivars of wheat, barley or oats. Similarly, high levels of specialisation with respect to host range are found in many other biotrophs, including the rusts, smuts and downy mildews.
A high level of specificity is related to the degree of sophistication of the relationship of biotrophs with their hosts. This reflects not only nutritional specialisation and the fact that the requirements of the pathogen are closely integrated with the environment of a particular host, but also the sophisticated nature of the mechanisms that have evolved to enable the pathogen to bypass the resistance mechanisms of particular hosts without actually killing the cells. Over the millennia, each time a pathogen has evolved to overcome the resistance of a host, the host has responded by evolving yet more sophisticated mechanisms of resistance. This has been followed by further pathogen evolution and so on. The artificial production by plant breeders of cultivars of crop species carrying resistance to disease has speeded up this evolutionary tit for tat in some host-pathogen combinations, leading, for example, to the array of formae speciales and races seen in E. graminis.
5. Hormonal disturbance of the host
Most biotrophs appear to induce significant morphological disturbance of the host, which may take the form of tumours (e.g. crown gall on fruit trees – Agrobacterium tumefaciens), distorted stems or leaves (e.g. peach leaf curl – Taphrina deformans), swollen roots (e.g. clubroot of brassicas – Plasmodiophora brassicae) and so on (Fig. 4.1). In the case of crown gall it has been shown that a nucleic acid plasmid moves from the bacterium into the host cells and there induces the abnormal production of the growth hormone indoleacetic acid. In the case of the clubroot disease of brassicas, P. brassicae causes an increase in the levels of both indoleacetic acid and another growth hormone, cytokinin, in the brassica root cells, which increase in both number and size. In other cases, for example where host tissues do not respond to high levels of hormones by growing abnormally, the evidence of hormonal disturbance takes the form of green islands (Plate 6). These are areas of green tissue around the lesion that continue to look healthy long after the rest of the leaf has started to yellow and die. It is thought that production of plant growth regulators such as cytokinins by the pathogen, or by the host following stimulation by the pathogen, are responsible for this delay in the onset of senescence.
Almost all infections by biotrophs reveal some evidence of hormonal disturbance of the host tissue, which suggests that plant growth hormones produced by biotrophic pathogens or by hosts in response to biotrophic pathogens are very important in delaying the senescence of infected tissue and in stimulating host metabolism in such a way as to serve the needs of the pathogen. Plant growth hormones may also have a role in suppressing host resistance to disease.
6. Diversion of the sugar transport pathways of the host
It has already been seen that biotrophic pathogens do not obtain the sugars they need for growth and sporulation by breaking down host cells, as necrotrophs do. Because they require a supply of carbohydrates, however, they divert the normal sugar-transporting pathways of the plant for their own use. Instead of exporting the sugars made during photosynthesis to nourish the growing roots and stem tips, the infected leaf itself imports sugars produced in the plant’s uninfected leaves. Within the infected leaf, the sugars travel towards the point of infection. In this way the fungus is assured of a continuous supply of food, but this is at the expense of the normal growth of the plant. This explains in part why one of the symptoms of infection of plants by biotrophs is stunted growth.
At the site of infection the sugars of the plant are taken up into the fungus and are there converted first to polyols (polyhydric alcohols) and then to the lipids, both storage forms of food that the plant itself is unable to utilise. This conversion is therefore a form of non-return valve, ensuring that once a fungus has extracted carbohydrate from the plant it cannot leak back again, even though it may not be required for immediate use. If the supply of sugars arriving at the infection site becomes too great for the fungus to convert to polyols and lipids it may induce the host plant itself to convert the sugar to starch, a storage form of carbohydrate in plants. Later, when the infected leaf is dying and the supply of sugar from other leaves is drying up as the plant ages, the fungus may induce breakdown of this starch to re-establish its supply.
The mechanisms by which biotrophs are able to change the pattern of sugar transport within an infected plant is not known, but it is thought that interference with growth hormone levels may be involved.
7. Increased metabolic activity of host cells around the site of infection
All plant pathogens, whether necrotrophs or biotrophs, cause an increase in the respiration rate of the host as it struggles to release sufficient energy to make both the fungitoxic chemicals needed to resist attac0k and the structural chemicals required to repair the severe damage caused by the pathogen. In the case of infection by biotrophs this increased respiratory activity may have another function in that it may supply, as by-products, chemicals required by the fungus for the conversion of plant sugars to polyols and lipids. The increased respiratory activity is accompanied by the enhanced formation by the host, in response to signals from the pathogen, of a whole range of other substances required by the pathogen for its nourishment. All this requires a massive increase in the formation of the enzymes that drive these processes and this, in turn, is reflected in an increase within the nucleus of the nucleic acids that control their formation. Examination of the cells of infected plants with a microscope reveals, almost invariably, that the nuclei are enlarged.
Ironically photosynthesis, which generates carbohydrates in green plants, is inhibited by biotrophs around the point of infection despite the frequent occurrence of green islands. The reasons for this are not known, but the problem is overcome by the diversion of the sugar transport pathways as described above.
In summary, biotrophy is a very sophisticated and very successful form of parasitism. The host cells remain alive for a long time and the pathogen is thus protected by the host’s innate resistance mechanisms from the competition that would result if the tissues were invaded by pure saprophytes. In order to achieve this, however, biotrophic pathogens have sacrificed some of their ecological flexibility by reducing their ability to produce cell-degrading enzymes and toxins, thus limiting them to a parasitic mode of life. Once a host dies, since the biotroph is unable to compete as a saprophyte, it must survive in the form of resting spores or be dispersed to infect some other living host plant.
Hemibiotrophs, which appear to combine the features of both biotrophy and necrotrophy, may be the most sophisticated pathogens of all, for when the biotrophic phase of development comes to an end as the host succumbs to the ever-increasing demands of the pathogen, the pathogen remains in the tissue, moving into a necrotrophic phase of growth, and is thus first in the succession of fungi and bacteria that can break down dead tissues.
THE EVOLUTION OF BIOTROPHY AND NECROTROPHY
Theories abound as to how biotrophy and necrotrophy may have evolved. One school of thought suggests that necrotrophs are primitive in an evolutionary sense, having first evolved from saprophytes through the development of the appropriate enzymes and other factors required to overcome the resistance of plants to attack by fungi and bacteria. Subsequently, it is argued, biotrophs evolved from necrotrophs as they became increasingly dependent on a supply of sugars from the host’s own photosynthetic processes, rather than breaking down the cell wall. The acquisition of the ability to produce plant growth hormones, which are said to play a central role in the diversion of the host’s sugar-transporting pathways, is thought to have been a key evolutionary event. Such evolving biotrophs would be at a disadvantage if they produced enzymes that actually killed plant cells, and it is suggested that this ability was first suppressed by the accumulation of sugars at the infection site in the interests of maintaining the host tissues in a living and active state. The presence of the end products of a reaction are well known to inhibit the activity of the enzyme controlling it. At a later stage of evolution, selection of mutants with modified enzyme production more suited to the biotrophic existence would have occurred. According to this theory hemibiotrophs are fungi that have not yet reached the stage of becoming true biotrophs.
Another school of thought argues that biotrophs are evolutionarily primitive and that the earliest pathogens lived in close association with the living cells of evolving plants. It is further suggested that some biotrophs might then have acquired by evolutionary selection the enzymes for killing host cells, breaking them down and living upon them saprophytically. Such changes would confer a selective advantage in evolution for they would increase the range of ecological niches available to the pathogens concerned.
Since there are few fungal pathogens preserved within fossilised plant tissues it is difficult to accumulate sufficient evidence from the geological record to support either one of these hypotheses. Some have attempted to assemble evidence by the examination of living pathogens, but that available so far is not completely convincing and certainly does not allow an unequivocal distinction to be made between the two evolutionary strategies proposed. It is our belief that necrotrophy, biotrophy and hemibiotrophy have evolved many times since life on earth began, with evolution progressing from necrotrophy to hemibiotrophy or biotrophy and vice versa on different occasions in different pathogens interacting with different hosts. The genetic make up of the fungi and bacteria make them so plastic that very rapid evolution is possible. It is inconceivable to us that any one theory for the evolution of biotrophy or necrotrophy should be regarded as universally applicable. Moreover, hemibiotrophy is not necessarily a stage in the evolution of either biotrophy or necrotrophy; it may often be an evolutionary end point in its own right.
THE INFECTION PROCESS
We have not yet described how pathogens actually breach the outer surface of a potential host and establish themselves within the tissues. A consideration of the complex processes involved must begin with some observations on spores, for although mycelium growing from an infected plant often infects another potential host, spores are the most frequent initiators of the infection process.
Spores
The spore is the ‘seed’ of the pathogen. It may have a thick wall and be a resting spore, or it may be thin-walled, having recently been carried to the surface of the plant by wind or water. In either case it will contain food storage compounds such as the fatty lipids and will be packed full of cytoplasm.
The spore will usually be dormant, held in that state of suspended animation by a number of factors. For example, it may lack the nutrients it requires for germination. It may contain water-soluble inhibitors of germination, synthesised during its formation to prevent it germinating while still on the host plant on which it was produced, and will only be able to germinate when water is present to wash the inhibitors out. Alternatively, the fungal spore may be held in a state of dormancy called fungistasis, induced by the toxic products of other microorganisms (fungi and bacteria) growing as saprophytes on or near the surface of the plant. In the case of resting spores, more complex factors like the requirement for a cold shock or for a specific chemical signal from a potential host may be required to trigger germination. Such strategies have evolved to ensure that resting spores germinate only at a time when a susceptible host is likely to be present. Once the spore is provided with the appropriate nutrients, water and the necessary triggers of germination it may, if it is on the surface of a susceptible host, germinate and begin the process of infection (Fig. 2.7). The precise strategy utilised for infection varies from species to species, but a clear distinction can be drawn between the necrotrophs and the biotrophs.
FIG. 2.7
A germinating urediospore (approx 20 μm diameter) of the yellow rust fungus (Puccinia striiformis) on a leaf of barley (Hordeum vulgare). The germ tube is growing over the epidermal cells towards a stomatal pore. (Scanning electron microscope photograph supplied by N. Read, University of Edinburgh.)
Cutinase
A key enzyme required for penetration is cutinase, which breaks down the cuticle of the plant. Recent research has shown that minute quantities of cutinase are produced by fungal infection hyphae. Once cuticle is encountered the cutinase begins to break it down into simpler substances. As soon as these are detected by the fungus it switches on the production of very much larger quantities of enzyme which then accelerate the process, enabling the cuticle to be breached. As soon as this process is complete, when no more breakdown products are detected, cutinase production ceases. The other cell wall degrading enzymes referred to above are then deployed to continue the penetration of the plant cell wall.
Some pathogens do not appear to produce cutinase. These either enter the host through natural openings such as stomata or wounds, or penetrate the cuticle by mechanical means – it is known that enormous pressures may be generated at the hyphal tip.
Infection by necrotrophs
As a rule, necrotrophic pathogens must build up a large mass of spores or mycelium at the surface of a host before infection can occur. This ‘massing of the troops before attack’ is necessary because considerable quantities of cell-destroying enzymes and sometimes toxins are required to kill and breach the host tissues, thus preventing the activation by the host of the machinery of resistance. Such a strategy is consistent with the unspecialised nature of the interaction between necrotrophs and their hosts. In the less specialised necrotrophs this build up of mycelium is not organised and may simply result from the accumulation and branching of hyphae growing together, or of spores collecting on the plant surface. In more specialised necrotrophs, however, an organised infection structure may be involved.
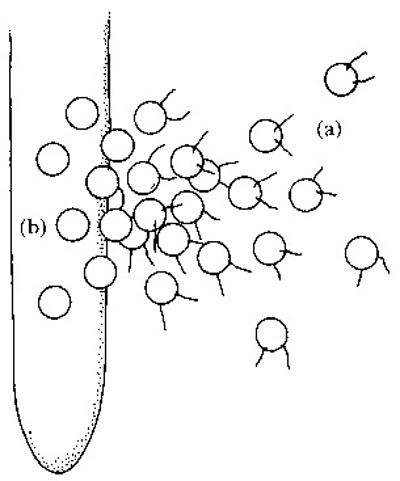
FIG. 2.8
The infection strategy of a necrotrophic Pythium sp., causing damping-off in seedlings. Zoospores (a) swim along a concentration gradient of leaked nutrients towards a wounded or weakened root. They cluster at the wound site or just behind the apex (b), germinate and send out hyphae which colonise and kill the root tissues beneath. (Illustration by Mary Bates.)
Pythium spp., cause of the damping-off of seedlings, produce large numbers of zoospores with which to mount an attack on the host (Fig. 2.8). These are attracted towards the wounded or weakened roots of a potential host from which chemical nutrients such as sugars and amino acids have leaked into the wet soil that favours growth by this pathogen. The zoospores swim in the films of water around the soil particles, along the concentration gradient of the leaking nutrients, towards the surface of the root, a process known as chemotaxis. The zoospores cluster around the wound site or alternatively aggregate just behind the root tip, for this is the region from which nutrients leak most readily. They then germinate, using the leaked nutrients as a food source, and form a network of hyphae over the whole surface of the root, continuing to absorb leaked nutrients. This network provides a major energy source from which multiple penetrations of the root may be effected by individual hyphae. By contrast, Rhizoctonia solani (the asexual form of Thanatephorus cucumeris), also a cause of damping-off in seedlings, has a slightly more sophisticated strategy (Fig. 2.9). Here the fungus does not infect from a spore but from a sclerotium. The sclerotium is a consolidated mass of hyphae that has secreted a hard, water-resistant outer surface, thus enabling the structure to serve as a kind of resting spore. The sclerotium is induced to germinate by chemical nutrients leaking from wounded or weakened roots, again into wet soil, and the emerging hyphae then grow either towards the wound site or to the point just behind the root tip, guided by chemotaxis. Once at the roots, instead of forming a network of hyphae all over the surface, a mass of mycelium aggregates at a single point and proliferates by branching. This serves as the energy base for a massive attack on the root tissue below. Once inside the root tissue, both P. ultimum and R. solani grow extensively by forming a network of mycelium that grows through and between cells, killing and breaking them down indiscriminately.

FIG. 2.9
The infection strategy of Rhizoctonia solani, a necrotroph that causes damping-off in seedlings. A hypha (a) growing from a sclerotium in the soil proliferates at a specific point on the root surface (b). This then serves as the energy source for an enzymatic attack on the root tissue beneath (c). (Illustration by Mary Bates.)
Another type of necrotroph attack is that used by Gaeumannomyces graminis, the cause of take-all disease of grasses and cereals (Fig. 2.10). Here the fungus will be growing in soil on the dead tissues of its previous host. Using nutrients from this foodbase, which may comprise old roots, stubble or straw, exploratory hyphae grow out through the soil until they encounter the roots of an uninfected plant. Nutrients transported along the hyphae from the food base are then used to infect the new host and a bridgehead of infected tissue is established at that point. From that bridgehead, using the colonised tissue of the new host as a food source, the pathogen sends out further exploratory hyphae, thicker and darker than normal, over the surface of the root and these mount a series of new attacks on the uninvaded areas of the root, establishing new bridgeheads as they go. Eventually the whole root is colonised in this way.
An even more sophisticated infection strategy is that adopted by Armillaria mellea, the honey fungus that causes rots in trees (Fig. 2.11). Again, the fungus uses the previously infected host as a food base, but instead of sending out exploratory hyphae it forms a more complex structure constructed from an aggregation of hyphae, called a rhizomorph. Rhizomorphs resemble blackened roots, or even bootlaces, to the naked eye, and may be several millimetres in diameter. The hyphae within them are arranged in an ordered pattern, with outer protective layers and inner layers able to transport water and nutrients. From the food base of a dead tree, A. mellea sends out rhizomorphs that can grow great distances through the soil, sometimes many metres, until they encounter the roots of a new host. There a rhizomorph attaches itself to the surface of the new host tree and, using nutrients transported along its entire length, is able to muster the energy required to mount an enzymatic attack that will kill and break down the woody tissues. The fungus spreads through the wood and colonises it completely, eventually killing the whole tree. Further rhizomorphs are produced on the dying tree and grow out into the soil in search of new hosts. This specialised infection strategy means that once a tree in a garden, or a neighbour’s garden, becomes infected, none of the surrounding trees is safe from attack.
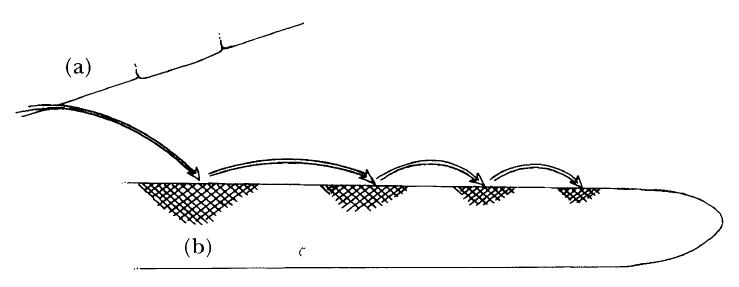
FIG. 2.10
The infection strategy of Gaeumannomyces graminis, a necrotroph that causes take-all disease of cereals and grasses. Hyphae grow out from a food base such as a piece of buried straw (a). When a hypha contacts the root of a potential host a bridgehead of infection is established (b). Using nutrients from the newly colonised tissues, new runner hyphae grow out and establish further infections until the whole root is colonised. (Illustration by Mary Bates.)
Infection by biotrophs and hemibiotrophs
Biotrophs and hemibiotrophs, in contrast to necrotrophs, are normally able to infect from a single spore. In this case, a specialised infection structure is invariably produced and this goes through a series of stages in developing an interface with the living cells of the host. This kind of strategy is consistent with what we have already seen of the highly specialised nature of the relationship between biotrophs and their specific hosts.
Perhaps the most remarkable infection structures formed by biotrophs are those of Plasmodiophora brassicae, the cause of clubroot disease (Fig. 2.12). This non-hyphal fungus produces zoospores that are attracted by chemotaxis and follow a trail of leaked nutrients back to the roots of plants in the cabbage family (Cruciferae). There the individual zoospores attach themselves to the surface of the root hairs, become rounded and develop a thin but firm wall. From a point on the wall of this cyst a special hypha bulges out and glues itself to the surface of the root hair. It then swells slightly and ceases to grow. Attachment structures of this kind are called appressoria. At the same time, the contents of the zoospore begin a remarkable transformation. First a bullet-like structure, the stachel, forms within the cyst. Attached to the rear end of this is a large piece of membrane and this in turn is attached to that part of the cytoplasm containing the nucleus. Simultaneously, the large blobs of lipids that the zoospore contained at the beginning of the attack begin to break down very rapidly as their energy is released and consumed in the manufacture of a huge balloon-like body filled with liquid, called a vacuole. This increases in size very rapidly as water is pumped into it and pressure builds up within the cyst. Eventually this pressure becomes so great that the bullet-like stachel, together with the membrane, cytoplasm and nucleus from the cyst, is shot through the wall of the root hair and into the cell itself. As it goes the membrane of the root hair cell is pushed inwards and eventually closes around the pathogen cytoplasm and nucleus, thus establishing an intimate contact between host and pathogen. This whole process of infection takes only a few hours, with the actual penetration process itself, in which the pathogen is shot into the root hair, occurring over a period of minutes. The pathogen then takes up nutrients from the host root hair cell, its nucleus divides to produce many more nuclei and more cytoplasm is formed. The developing pathogen is called a plasmodium. As this process is repeated a very large plasmodium develops, occupying a significant part of the space within the root hair cell but still continuing to draw nutrients from it. Eventually the root hair plasmodium divides up into a mass of new zoospores. These are then released and reinfect the main body of the root by a process similar to that involved in infection of the root hairs. This second stage of infection is the one that leads to the development of the swollen roots so characteristic of clubroot disease.

FIG. 2.22
Fig. 2.11 The infection strategy of the necrotroph Armillaria mellea (honey fungus), a necrotroph that causes rot in trees. A rhizomorph (a) grows out through the soil from the food base of a heavily colonised tree (b). Water and nutrients are translocated along the rhizomorph and provide the energy for an enzymatic attack on the roots of a new host (c). (Illustration by Mary Bates.)
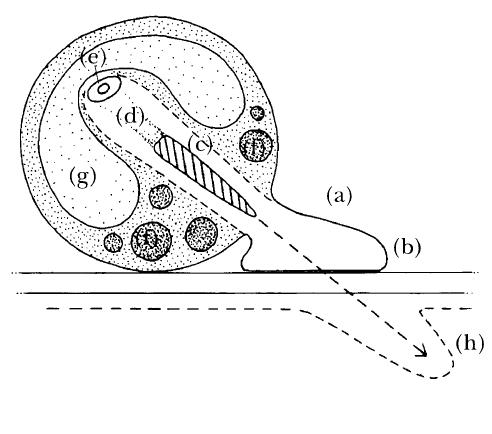
FIG. 2.12
The infection strategy of the biotroph Plasmodiophora brassicae, cause of clubroot disease of members of the cabbage family (Cruciferae). An encysted zoospore forms an appressorium (a) which becomes firmly attached to the root surface (b). Within the zoospore a bullet-shaped stachel develops (c). Attached to it is a membranous structure (d) which in turn is attached to a small part of the cytoplasm and the nucleus (e). Large lipid droplets (f) are metabolised to provide the energy for expansion of a vacuole (g) which drives the stachel, membrane, cytoplasm and nucleus in to the host root hair cell. The root hair cell remains alive and the membrane (h) is not pierced, but is pushed inwards and eventually encloses the pathogen. (Illustration by Mary Bates.)
FIG. 2.13
Fig. 2.13 The infection strategy of the biotroph Bremia lactucae, cause of downy mildew of lettuce (Lactuca sativa). A spore (a) germinates on the surface of a host leaf and forms a germ tube (b) which swells at the tip to form an appressorium (c) attached firmly to the cuticle. An infection peg (d) penetrates a living epidermal cell and forms first a primary vesicle (e) and then a secondary vesicle (f). An intercellular hypha (g) grows from the secondary vesicle and invades the leaf. The hyphae form haustoria (h) at intervals and these penetrate further living cells. (Illustration by Mary Bates.)
The hyphal biotrophs have evolved very different but equally sophisticated strategies for gaining entry to their hosts. For example, Bremia lactucae, the cause of downy mildew in lettuce, forms a series of infection structures called vesicles (Fig. 2.13). First of all a spore lands on the surface of a host leaf. If water is present a water soluble inhibitor of germination is washed out of the spore and sugars leak out from the surface of the leaf and provide the spore with sustenance. It immediately germinates to produce a special hypha called a germ tube, which grows over the surface of the leaf until it reaches a point where two cells of the outer layers of the leaf, the epidermis, actually meet. There it stops growing, glues itself to the surface of the leaf and swells slightly to produce an appressorium. From this a tiny infection peg emerges and produces powerful cell wall dissolving enzymes over a very small surface area, allowing the infection peg to penetrate the cuticle and the wall, after which enzyme production ceases. Once inside the cell the infection peg grows and expands to form a spherical structure called a primary vesicle. The contents of the spore then travel down through the germ tube and into the primary vesicle, and at this stage the point of penetration is plugged; in effect, the spore has been moved from the harsh environment of the surface of the leaf to the much more protected environment within the host cell.
Meanwhile the host cell does not die but is induced to produce far more plasmalemma than normal, and as the primary vesicle expands in the cell this membrane becomes unravelled to surround it so that the living cytoplasm of the host cell is in intimate contact with the fungus itself. At this point a new hypha begins to grow out from the primary vesicle, again inducing the host’s plasmalemma to grow abnormally. This hypha expands to become the secondary vesicle, which has an even closer contact within the host cell and takes up nutrients from it. The pathogen nuclei divide repeatedly and the cytoplasm increases to fill the expanding vesicle. Eventually the secondary vesicle itself forms a further hypha, which grows out of the initially penetrated cell and into the intercellular spaces of the lettuce leaf. This hypha branches and the resulting mycelium permeates the whole leaf, always growing between the cells. The hyphae take up nutrients from intercellular spaces but also form the haustoria that establish direct contact with individual living cells and increase the capacity for nutrient absorption.
FIG. 2.14
The infection strategy of the biotroph Erysiphe graminis, cause of powdery mildew of cereals and grasses. A spore (a) germinates on the surface of a leaf and swells at the tip to form an appressorium (b) which produces an infection peg that penetrates the cuticle above an epidermal cell. A branched haustorium (c) forms in the living cell and extracts nutrients from it. A hypha (d) next arises from the appressorium and grows out over the leaf surface to establish a new infection, and so on. (Illustration by Mary Bates.)
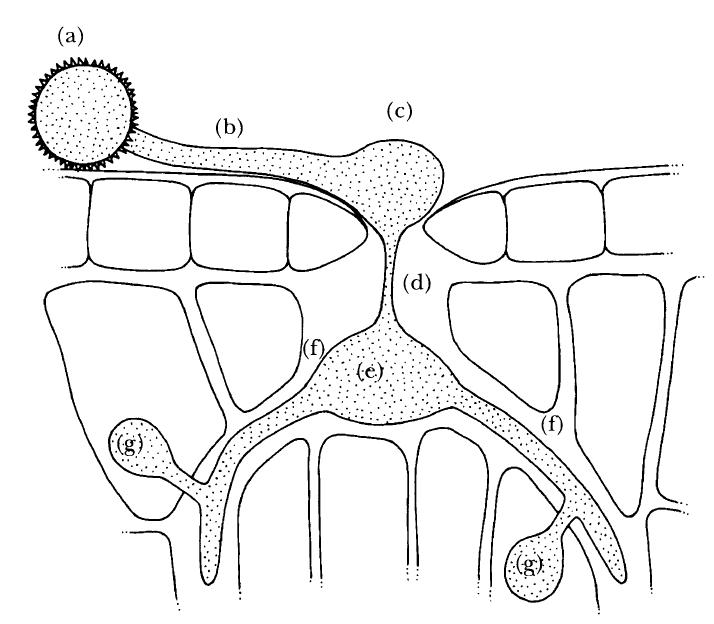
FIG. 2.15
Fig. 2.15 The infection strategy of the biotroph Puccinia graminis, cause of black stem rust of cereals and grasses. A urediospore (a) germinates on the leaf and a germ tube (b) grows out over the surface until it encounters a stomatal pore. It then swells at the tip to form an appressorium (c) which in turn forms an infection peg (d) that penetrates the pore and expands to give a vesicle (e) within the sub-stomatal cavity. Intercellular hyphae (f) arise from the vesicle, producing haustoria (g) which penetrate the living cells of the host. (Illustration by Mary Bates.)
Erysiphe spp., the cause of powdery mildews of a wide range of garden and wild plants, grow on the surface of the host (Fig. 2.14). In this case the spore germinates in the same way as Bremia lactucae spores, and the resulting germ tube grows and produces an appressorium that sticks to the surface of the host. An infection peg is again formed and the cuticle and cell wall of the host breached. In this case, however, no vesicles are formed. Instead the infection peg forms a haustorium directly, within the initially penetrated cell, and there begins to extract nutrients through it. The appressorium then produces a second hypha, which grows out and again produces an appressorium, which once more produces a haustorium in a further epidermal cell, and so on. Eventually the whole surface of the leaf is colonised and haustoria form a complex series of feeding branches that penetrate the surface cells.
A final example is Puccinia graminis (Fig. 2.15), the cause of black stem rust of grasses and wheat. Here the fungus takes advantage of the stomata, the pores in the leaf used for gas exchange, to gain entry to the host. The spore lands on the surface of a leaf and, if water and nutrients are present, germinates in the usual way. The germ tube then grows towards a stomatal pore, perhaps attracted by substances leaking from it, and begins to swell at its tip once it has encountered the pore. The resulting appressorium becomes firmly glued to the rim of the pore and then forms an infection peg that grows down through the pore itself into the substomatal cavity. There it swells to form a vesicle within the cavity and begins to take up nutrients from the intercellular fluid of the host leaf. The vesicle becomes a massive structure, full of cytoplasm and nuclei, and then produces hyphae which grow between the cells of the infected leaf, forming haustoria that establish direct contact for nutrient exchange with individual cells.
Many more examples of infection by necrotrophic, biotrophic and hemibiotrophic fungi might be cited. This small number of examples, however, gives the reader some idea of the huge range of strategies that may be utilised by pathogenic fungi to achieve the most difficult part of the process of pathogenesis, that of gaining entry to the host itself. Both nectrotrophic and biotrophic bacteria, lacking hyphae, usually enter their hosts through wounds or natural openings such as stomata. Viruses sometimes enter through wounds but are usually introduced into host cells by a vector such as an arthropod or a fungus.
In this chapter the development of disease in susceptible hosts has been considered. Plants have, however, evolved a variety of defence mechanisms to resist attack and only those pathogens that can overcome these are able to grow in a plant and cause the symptoms of disease. Defence against attack is the subject of the next chapter.