Chapter 1
What Is Life?
Erwin Schrödinger (1887–1961), reluctant cofounder of quantum mechanics, 1933 Nobel laureate in physics, and author of a famous thought experiment involving cruelty to cats, was used to speaking his mind. So much so that, after the Nazis came to power in 1933, he resigned from his chaired professorship at Berlin University and emigrated first to Oxford, then to his native Austria, from where he was exiled again after the Anschluss. In 1939, the government of neutral Ireland invited him to take up a chair of theoretical physics at the newly founded Dublin Institute for Advanced Studies, which became his home for the next 17 years.
One of the obligations of Schrödinger’s new job was to give an annual public lecture. In 1943, he held a series of three such lectures at Trinity College Dublin where he discoursed on the topic “What Is Life?” Two aspects of life, heredity and thermodynamics, took center stage in these talks. Schrödinger framed these as the basic questions of how life creates “order from order” and how it creates “order from disorder.” In his analysis of genetics (order from order), he estimated the number of atoms contained in a gene (“genes” as physical things still being a relatively abstract concept). He proposed that the genetic information might be encoded in something resembling an aperiodic crystal: a combination of a regular structure with stable, information-bearing variations—an idea that, given modern knowledge of the structure of DNA, seems startlingly prescient. In the second half of his discourse, Schrödinger clarified that organisms can create ordered arrangements of molecules and cells by creating even greater disorder in the environment (e.g., by converting ordered carbohydrate molecules into less ordered water and carbon dioxide), keeping life in line with the immutable second law of thermodynamics.
Schrödinger’s lectures, and the brief book that followed, were hugely influential, marking a significant turning point in how scientists look at biology. At a time when there was no such thing as “biophysics,” Schrödinger was asking a question that is simultaneously simple and profoundly deep: how do the physics of the Universe constrain its biology? In the decades following Schrödinger’s lectures, many aspects of this question have been resolved, such that today, in this opening chapter, we can take a stab at not only defining what life is but also listing some of its most fundamental requirements.
Definition of Life
Life scientists have long skirted the issue of defining what life is using the same approach US Supreme Court Justice Potter Stewart (1915–1985) once used in a case about public standards of decency: although, he admitted, he could not precisely and unambiguously define pornography, “I know it when I see it.” If we want to embark, though, on a deep and rigorous evaluation of life’s origins and their relationship to the underlying physics of the Universe, this sort of empirical approach is not going to cut it. All the more so if we are interested in alternatives to the approach life (and its origins) took here on Earth. That is, in order to understand what range of forms life could have taken elsewhere in the Universe, we will have to start with a defensible working definition of what life is. So, fellow travelers: just what is life?
The most striking property that distinguishes living systems from the inanimate world is their ability to copy themselves, an attribute scientifically described by the term self-replicating. Among Homo sapiens, the process is more colloquially captured in the phrase “find a partner, settle down, and have kids.” The fact that living things copy themselves is so central to all of biology that some wit once pointed out that “life is just a DNA molecule’s way of reproducing itself.” All of biology, from the slimy mats of bacteria living in your sink drain through to warring nations, can be described as tools for or consequences of the ceaseless lust of our genes to copy themselves.
Another key limit to our discussion is to define life as a chemical system (as opposed to a mechanical or electronic system). Over decades, writers of science fiction and putative nonfiction that extrapolates current (nano)technological trends into the future have suggested that self-replicating, microscopically small robots may someday clean out our arteries, degrade our toxic waste, and generally make themselves useful. Irrespective of the accuracy of these predictions, it seems likely that the physical laws of the Universe allow the creation of mechanical beings able to copy themselves, thus meeting our first criterion for life. Cyberspace hosts similarly viable organisms, known as worms. Although such “malware” requires a computer, an internet connection, and typically some poorly written software in order to reproduce, one might argue that these simply constitute its ecosystem, just as we require an ecosystem for our reproduction. When working out a universal definition of life, it’s not entirely clear that these “artificial” examples don’t count. Perhaps all the more so given that, as technology progresses, the boundaries between biological, mechanical, and electronic systems continue to erode.
Considering the origins and distribution of life in the Universe, however, it is difficult to imagine that living computer viruses or mechanoid life could have arisen spontaneously from nonlife. Engineered things employ parts larger than molecules; after all, systems consisting of molecular parts are, by definition, chemical. Before the creation of the first organisms, these parts would have to be moved around by Brownian motion, the random fluctuations of molecules moving to and fro. And even at the molecular level, Brownian motion isn’t all that fast: if you open a bottle of perfume in still air, how long does it take for the fragrance to diffuse across the room? At the mechanical level, it is even slower: Brownian motion slows with the square root of mass, meaning it would take far longer than the age of the Universe for a bucket of watch parts to spontaneously assemble into a functioning watch, much less into a machine capable of self-replication. Thus, while mechanically based life and computer viruses might ultimately arise through the design efforts of intelligent, chemical life forms, it seems unlikely that either could arise spontaneously, absolving us from including self-replicating robots in our working definition of living beings.
A final, but critical, element in our definition of life emerges from the observation that not all self-replicating chemical systems are alive. Wildfires are, for those of us who live in California, an all too frequently occurring example of a self-replicating chemical system that is not alive. Specifically, fire is a self-replicating network of thermally induced radical reactions* that, given more fuel and oxygen, readily produce “offspring” that can likewise reproduce (fig. 1.1). Fire, though, is not alive in any sense that is useful to our discussion. Crystals can likewise “direct” the formation of more copies of themselves. Take, for example, a supersaturated solution—that is, a solution in which a solute is, transiently, at a higher concentration than its equilibrium (i.e., long-term) solubility. If we were to smash a growing crystal into smaller pieces in this solution, each of those pieces would serve as a nucleus and grow into a new, larger crystal. Clearly, then, replication alone is insufficient to distinguish between the animate and the inanimate. What is missing? In a word, evolution.
Living beings produce offspring in their own image via the replication of their genetic material. But this replication is not absolutely perfect: random genetic mutations produce inheritable differences that may improve or impede the offspring’s viability. These inheritable differences give natural selection a chance to shape the fate of future generations and the evolution of a species. Evolution, the ability to adapt under selective pressures, is a fundamental property of life and clearly distinguishes it from inanimate, if sometimes self-replicating, materials. A crystal makes perfect copies of itself. The first crystal of quartz that condensed out of a giant cloud of gas and dust that form the Solar System some 4.57 billion years ago is identical to the quartz that crystallized last week in the Corning glassware plant in upstate New York. Crystals and crystallization are changeless and thus are incapable of evolving into new and more complex forms. They are not alive.
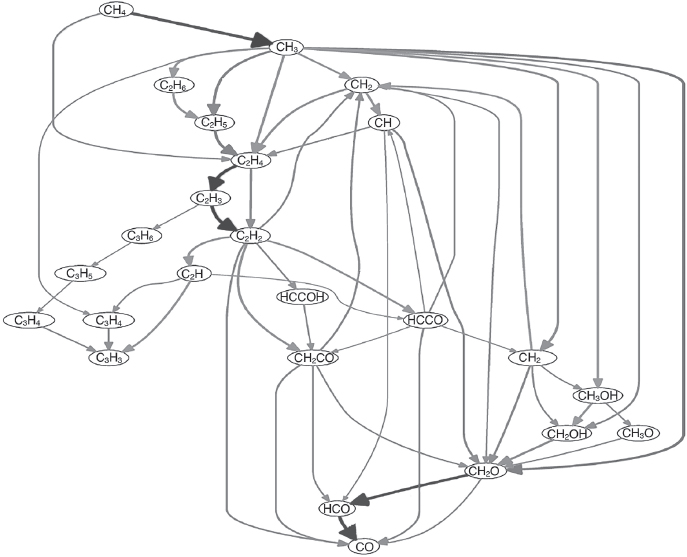
Figure 1.1 Fire is a complex, self-replicating reaction network. It is not, however, alive. Shown is a schematic of the carbon species involved in the burning of methane (CH4) at low oxygen to produce carbon monoxide (CO). Not shown are the highly reactive hydrogen- and oxygen-containing radicals—molecular species with an odd number of electrons—that both produce these reactions and are produced by them, driving this self-replicating behavior.
As middle-aged academic types well past our reproductive years, we feel obliged to add a note here: our definition of life involves self-replicating systems, not necessarily self-replicating individuals. Mules, for example, are generally sterile, and yet anyone who has tried to lead one down to the bottom of the Grand Canyon will attest to their stubbornly animate nature. But mules are always part of a larger system (one that includes reproductively competent female horses and male donkeys) that, as a whole, is capable of reproducing and evolving. More generally, reproduction often requires the combined efforts of two organisms and not just pairs of males and females; viruses and their hosts are an example. By the same token, evolution, too, generally acts on the level of populations rather than on individuals. Neither observation, however, degrades the usefulness of our definition.
Now that we’ve settled on a specific definition of what life is, the next question is: what are the minimal—and thus fundamental—conditions required to support it? In answering this, we must inevitably be somewhat parochial: our understanding of the conditions under which life can arise and evolve is almost certainly going to be flavored by deeply held preconceptions based on our understanding of life on Earth. But as long as we are aware of this underlying bias, we can at least tackle each of the seemingly necessary conditions in as unbiased and logical a fashion as is (even the word itself is telling) humanly possible. That is, we should cast our net wide, making an effort not to mistake constraints predicated on what we observe regarding life on Earth for constraints that are truly universal.
The Chemistry of Life
A whopping 98% of the matter in our Universe is either hydrogen (H) or helium (He). Life as we have defined it, however, requires chemistry far more complex than that available to these two atoms, the chemistry of which is limited to the hydrogen molecule (H2), “protonated hydrogen” (H3+), and helium hydride (HeH+). And while the latter two are stable in extreme isolation in the laboratory and have, in 1996 and 2019, respectively, been confirmed to form in interstellar space, they cannot be prepared in bulk due to their propensity to react violently with any other type of molecule they contact. Even taking into account our potentially narrow-minded, Earth life–centric biases, it seems certain that a self-replicating chemical system cannot be built based on such meager chemical complexity. But, as we’ll see, while hydrogen and helium were formed in great abundance in the first minutes of the Universe, the formation of heavier, more complex atoms was rather a more delicate matter.
What atoms are required for life? Here we must begin to speculate, but not without a lot of well-established science to back us up. There are, after all, only a finite number of elements in the periodic table (fig. 1.2), and many of these are poorly suited to support life for a fair list of reasons. Consequently, many of the 90 or so naturally occurring elements can be ruled out. So many, in fact, that in the end there may very well be only a single element—carbon (C), the basis for all life here on Earth—that is able to support the complex chemistry presumably required to create a self-replicating chemical system. The easiest way to appreciate the special, perhaps even unique, qualities of carbon is to compare it with silicon (Si), the chemically similar “cousin” that sits immediately below it on the periodic table.
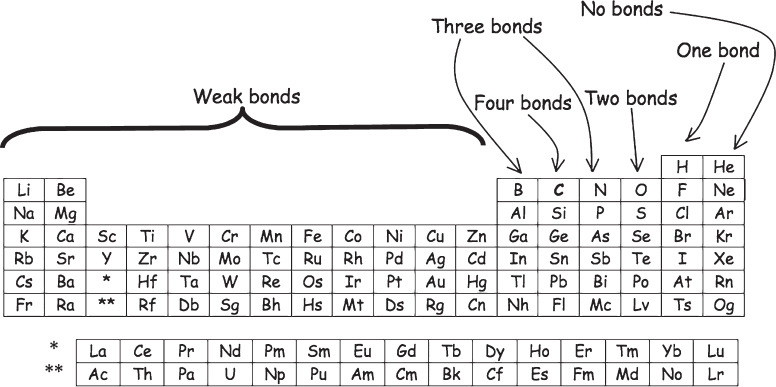
Figure 1.2 The atomic building blocks of life. Even a quick perusal of the periodic table leads us to the conclusion that relatively few elements are likely to support the complex chemistry required to form life here on Earth, or anywhere. In particular, only carbon (C) is tetravalent (forms four bonds), forms strong covalent bonds with itself and with its neighbors in the table, and is kinetically stable. These properties render the element uniquely well suited to support the complex chemistry presumably necessary for life.
Some of the properties that suit carbon so well to its central role in Terrestrial life are shared or even exceeded by silicon. For example, silicon, like carbon, is tetravalent—that is, each atom forms four bonds, allowing for the formation of a rich array of complex molecular structures. And, while a silicon-silicon bond is weaker than a carbon-carbon bond, the discrepancy is only about 35%. Consistent with this, both silicon and carbon can form long molecular chains: compounds of silicon and hydrogen, called silanes, with up to 28 consecutive silicon-silicon bonds have been reported in the scientific literature. Likewise, while carbon is the fourth most common element in the Solar System (fig. 1.3), silicon is far more common in the Earth’s crust, where it is second only to oxygen (O) in terms of its abundance (fig. 1.4). Nevertheless, silicon simply cannot support the same rich chemistry as its “upstairs” neighbor on the periodic table. The problem lies in both the thermodynamics (equilibrium stability) of silicon’s interactions with other atoms and the kinetics (rates) with which those interactions form and break.
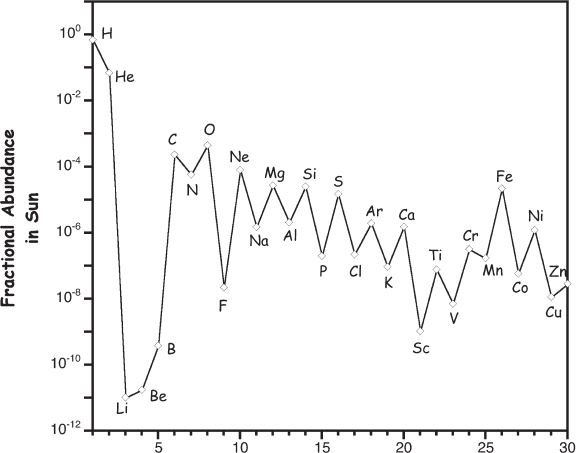
Figure 1.3 The relative abundance of the various elements available in the pre-solar nebula—and thus, ultimately, available to serve as the building blocks of the planets—can be discerned by from the relative abundances of elements in the Sun, which are shown here. Of note, the Sun is relatively enriched in elements heavier than helium relative to other stars its size. Nevertheless, this qualitative pattern is common among stars of the Sun’s generation (more on this in chapter 2).
A perusal of the relative stability (thermodynamics) of various bonds formed by carbon and silicon provides the first fodder for our arguments. Because typical carbon-carbon, carbon-nitrogen, and carbon-oxygen single bonds are all equally stable (to within about 10%), nature does not have to invest much energy when swapping between, say, a carbon-carbon bond and a carbon-oxygen bond, or vice versa (fig. 1.5). The same is also true for carbon when it participates in double or triple bonds; typical carbon-carbon, carbon-oxygen, and carbon-nitrogen double bonds are all more or less equally stable (to within 30%), as are carbon-carbon and carbon-nitrogen triple bonds. Better yet, a typical carbon-carbon double bond is approximately twice as stable and a triple bond very nearly three times as stable as the corresponding single bond (fig. 1.6). That is, their stabilities per bond are all about the same, and thus, again, nature can shift carbon between various single-, double-, and triple-bonded configurations (e.g., converting two single bonds into one double bond) without much change in energy. This attribute is a distinct advantage when it comes to generating the sort of complex chemistry likely to support life; because of this property, it is relatively easy for biology to shuffle carbon from molecule to molecule across a bewildering array of compounds (fig. 1.7).

Figure 1.4 An ingredient list for life on rocky, terrestrial planets. Shown is the relative abundance of elements in the Earth’s crust. Note that this pattern differs dramatically from the relative abundances in the Sun (a proxy for the Solar System at large) shown in Figure 1.3.
This situation with silicon is quite different, and far less favorable. A single bond between two silicon atoms, for example, is about 40% less stable than a typical silicon-nitrogen single bond and less than half as stable as a typical silicon-oxygen single bond (fig. 1.5). As a consequence, silicon readily oxidizes to silicon dioxide, limiting the chemistry available to this atom whenever oxygen is present. And oxygen is almost always present, it being both the third most common atom in the Universe and the single most common atom in our planet’s crust. The case for silicon grows even worse when we consider double and triple bonds. Molecules containing silicon-silicon double bonds and even a silicon-silicon triple bond have been prepared in the laboratory, but they are so unstable that they spontaneously decay unless kept at cryogenic temperatures (i.e., in liquid nitrogen at −200°C) or “protected” by big, bulky groups blocking the silicon atoms from attack by other molecules. This is because, unlike the carbon-carbon double bond, which is almost twice as stable as a carbon-carbon single-bond, a silicon-silicon double bound is less stable than a silicon-silicon single bond. To understand why this is so, we’re going to have to spend a bit of time thinking about atomic and molecular orbitals.
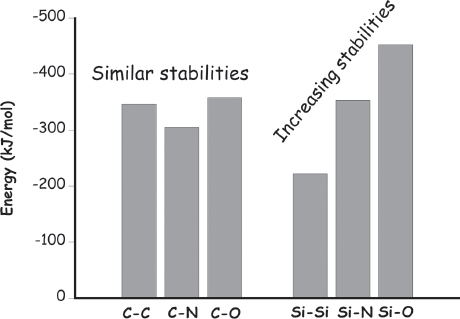
Figure 1.5 Single bonds between carbons or between carbon and nitrogen or oxygen are typically all of similar stability, and thus biology does not have to expend much energy to substitute one for another. This is far less true for silicon, which strongly prefers to be bound to oxygen atoms rather than to atoms of its own kind.
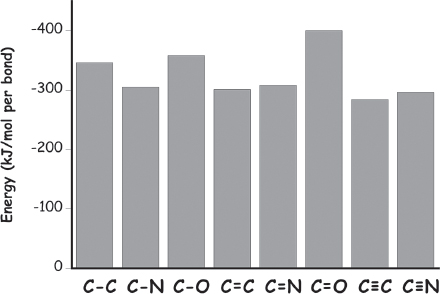
Figure 1.6 Multiple bonds (double bonds, triple bonds) between carbons or between carbon and nitrogen or oxygen are typically of similar stability per bond, and thus biology does not have to expend much energy to substitute, say, two carbon-carbon single bonds for one carbon-carbon double bond. Silicon, in contrast, simply does not form stable double or triple bonds.
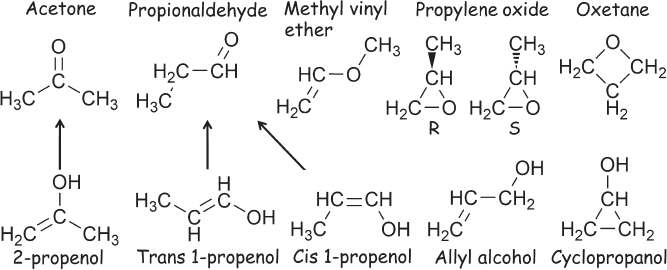
Figure 1.7 The diversity of stable carbon compounds is unparalleled. Shown, for example, are the 11 isomers (i.e., different bonded arrangements) that share the C3H6O empirical formula of acetone. Save for the three “vinyl” alcohols (a hydroxyl group on a carbon involved in a carbon-carbon double bond) at the lower left, which quickly transfer a hydrogen to form the carbonyl (C=O) compounds above them, all these molecules are stable enough that they can be purchased commercially and shipped and stored at room temperature.
The four outermost electrons of a carbon atom, the electrons that can form bonds, reside in four atomic orbitals, one designated 2s and three designated 2p. These orbitals are each capable of holding two electrons, and thus there are four “spaces” left over for binding partners to fill, which is energetically favorable.* In a double-bonded carbon molecule, such as ethylene (CH2=CH2), the geometry of the bonds suggests that the distinction between the 2s orbital and two of the 2p orbitals has disappeared: these orbitals “hybridize” to form three sp2 molecular orbitals. These hybrid orbitals, arranged in a plane with identical (120°) angles between them, form sigma molecular orbitals that bond them to neighboring atoms—in ethylene, that is with the two hydrogens and the other carbon (fig. 1.8). This latter bond, the bond between the two carbons, is approximately 0.13 nm (a nanometer is a billionth of a meter)—short enough that the remaining 2p atomic orbitals on the two carbons, which are sticking out of the plane at a right angle, overlap one another sufficiently to form a second bond (called a pi bond) that is nearly as strong as the first (sigma) bond. Silicon, the next atom below carbon in the periodic table, also has four bonding electrons, but they are in 3s and 3p atomic orbitals, which are significantly farther from the nucleus than the 2s and 2p orbitals of carbon. The greater diameter of these orbitals stretches the silicon-silicon bond distance to 0.22 nm, long enough that there is no longer any significant overlap between the two remaining 3p orbitals. Because of this, the p-orbital electrons tend to stay as single, solitary electrons rather than paired electrons working together to form a bond (fig. 1.8). This leaves silicon-silicon double bonds unstable and highly reactive.
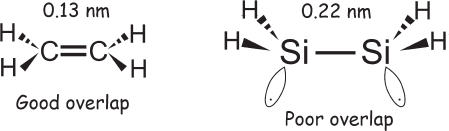
Figure 1.8 A single bond between two carbon atoms is short enough that the two electrons in the remaining p orbitals still overlap, causing the creation of a stable second (double) bond. Single bonds between two silicon atoms, in contrast, are much longer, limiting overlap of the p orbitals and thus precluding the formation of a stable double bond.
As if the poor thermodynamics of silicon bonding weren’t bad enough, silicon-containing molecules are also kinetically unstable. For example, in an equilibrium (thermodynamic) sense, both methane (CH4) and silane (SiH4) are unstable in air as all three elements involved are much more stable in their oxides (water, carbon dioxide, and silica). Like most carbon compounds (fig. 1.7), however, methane is kinetically stable. For example, although methane burns vigorously in air, before the striker on your stove ignites it, the methane and other like molecules in the natural gas do not spontaneously burst into flame. Indeed, at room temperature, methane reacts so slowly that its half-life in air is approximately seven years, thus allowing methane from, say, rice paddies to build up in the atmosphere and contribute to greenhouse warming (it absorbs infrared light much more effectively than does carbon dioxide, and thus, molecule for molecule, methane is about 20 times more potent as a greenhouse gas). Silane, in contrast, spontaneously bursts into flame upon contact with air (fig. 1.9), reflecting the much more rapid reaction kinetics of silicon compounds relative to the corresponding compounds of carbon. To see why this is true, we again have to think about orbitals.
To swap one of the substituents attached to a carbon for another substituent, we have two choices. We can add the new substituent, making a five-bond (pentavalent) carbon, and only then break the bond to the substituent being replaced. Or, we can first break the bond to the substituent undergoing replacement to produce a three-bond (trivalent) carbon intermediate before then adding the new substituent (fig. 1.10). And while the trivalent carbon intermediate required by the second mechanism is very high in energy, thus slowing such reactions (because collisions with the requisite energy are rare), the energy of the pentavalent intermediate of the first mechanism is higher still. The problem is that the lowest-energy orbital available to accept bonding electrons to make that fifth bond is a 3s orbital, which is quite high in energy. In contrast, the lowest-energy empty orbitals in silicon are 3d orbitals, which aren’t much higher in energy than the 3s and 3p orbitals that this element normally uses to “perform its chemistry.” Because of this, silicon is relatively stable when bonded to five substituents; the fifth substituent simply forms a bond with a low-lying, empty 3d orbital before the bond to the old substituent is broken (fig. 1.10). The stability of pentavalent silicon reduces the energy required for silicon to react, thus making its reactions many, many orders of magnitude faster than those of the equivalent carbon compounds.

Figure 1.9 Unlike methane (CH4), which has about a seven-year half-life in our atmosphere, silane (SiH4) instantly bursts into flame upon contact with air, clearly illustrating the far greater kinetic reactivity of silicon compounds.
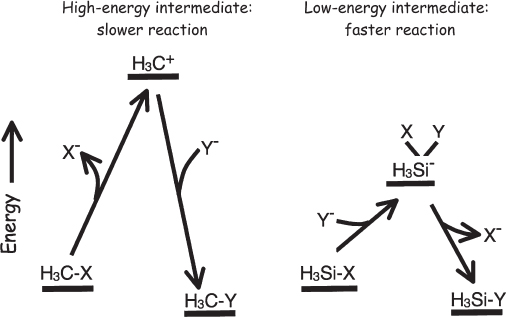
Figure 1.10 To replace a substituent on a carbon atom, a bond must be broken. If bond breakage happens at the beginning of the reaction mechanism, it leads to the formation of a highly unstable, trivalent (three-bond) intermediate. Because of this, carbon tends to react only slowly; relatively few molecular collisions are energetic enough to provide the necessary energy. Unlike carbon, however, silicon supports the formation of up to five bonds. The resultant low-energy intermediate renders silicon far more rapid in its reactions.
And there we have it. Carbon presents a fairly level playing field in which nature can shuffle around carbon-carbon, carbon-nitrogen, and carbon-oxygen single, double, and triple bonds without paying too great a cost to convert any one of these into another. Equally important, carbon’s reaction kinetics are slow and thus, once parked in a given molecule, it tends to stay there. Silicon, in contrast, reacts vigorously whenever oxygen is present, rapidly moving “downhill” (energetically speaking) to form very stable silicon-oxygen bonds. Given all this, it’s no wonder that on the order of 100 million unique carbon compounds have been described by chemists, which is more than all the described non-carbon-containing compounds put together. And this is not even counting all the carbon-containing biological entities, such as proteins and chromosomes, of which there are even more due to evolution’s ceaseless creation of diversity.
Do you still think there’s a chance that, somewhere out there, beasts based on silicon are having this same debate, but in reverse? Well, then, consider one final issue: the second bond in a carbon-oxygen double bond is more stable than the first, and thus carbon is perfectly happy to make double bonds to two oxygen atoms to form the triatomic—and gaseous—oxide, carbon dioxide (O=C=O). Silicon, as we explored above, does not form double bonds and, thus, to satisfy its desire to form four bonds, each silicon atom must link up with four, rather than two, oxygen atoms. The problem is, oxygen wants to form two bonds, a situation that can be satisfied only if each oxygen links to two silicon atoms to form a bridge between them. Because of this, silicon dioxide forms a rigid, three-dimensional network that does not dissolve without breaking bonds. The contrast is stark: carbon forms a wide range of molecules that can be solid (e.g., cellulose), liquid (e.g., alcohols), or gas (e.g., carbon dioxide) all under the same conditions of temperature and pressure, allowing the atom to be easily transported and exchanged between these states (e.g., the excretion of carbon via the exhalation of carbon dioxide). Carbon can likewise form many compounds that are highly soluble in water (e.g., carbohydrates) and many compounds that are only sparingly so (e.g., lipids), permitting both its transport and use in structural and organizational roles. In short, we eat water-soluble carbon compounds such as sugar and other carbohydrates, oxidize them, and then excrete the water-soluble gas carbon dioxide. None of these properties hold for silicates, which are highly insoluble solids such as quartz (pure silicon dioxide) and thus would be exceedingly difficult to mobilize and eliminate.
Can we categorically say that life cannot be based on silicon? Of course not. Perhaps we have a “carbon bias” and are simply not creative enough to see how silicon could support life. It is clear, however, that silicon is less well suited to support complex chemistry, and thus it seems much, much less likely that silicon-based life could form than carbon-based life. Thus, if aliens ever do visit us, the smart money says that we should welcome them with carbon-based cakes and not with silicon-based rocks.
Given the above, it’s pretty clear that carbon wins over silicon regarding their suitability to serve as the basis for complex chemistry. But what of the 90 or so other naturally occurring elements? They fare even worse than silicon. At the far right of the periodic table (fig. 1.2), the noble gases, helium (He), neon (Ne), and the like, participate in effectively zero chemistry. Nor can we build a complex chemistry based on the halogens, such as fluorine (F) and chlorine (Cl), as these atoms generally bond to only a single partner, greatly limiting their chemistry. Oxygen and the similar atoms below it in the third-to-last column of the periodic table likewise make only two bonds, and nitrogen (N) and boron (B) and their respective columns make three—numbers that are also handicaps relative to carbon’s tetravalency. Moving farther to the left, we have the transition metals, including iron (Fe), nickel (Ni), and gold (Au), and then the alkaline earth metals, such as calcium (Ca) and magnesium (Mg), and the alkali metals, including sodium (Na), potassium (K), and their brethren. None of these atoms, however, form strong covalent bonds—that is, bonds in which electrons are shared between two atoms (in molecular orbitals). Instead, when these elements bond they tend to transfer electrons almost entirely to themselves or over to their bonding partners, producing ionic bonds, so called because the transfer of electrons produces charged atoms or groups of atoms called ions.* These ionic bonds, however, are far less stable than covalent bonds, thus limiting the ability of metals to participate in chemistry as complex as that seen for the nonmetal elements on the right-hand side of the periodic table. In short, while carbon may not be the only element that can support life, any theory about alternative life forms that relies on elements other than carbon is significantly suspect.
The Solvent of Life
Life almost certainly requires a liquid solvent. More specifically, although chemical reactions can, of course, take place in the gas and solid phases, each of these has disadvantages relative to liquid-phase reactions. Gas-phase chemistry, for example, is limited to molecules that are volatile enough to stay in the gas phase. This likely rules out molecules of sufficient complexity to support self-replicating chemical systems. Likewise, even though molecular species can diffuse through solids to give rise to chemical reactions, such diffusion is extremely slow and would seem unlikely, if not unable, to support life. Consistent with these arguments, the human body contains around 70% water, highlighting the fact that this is the solvent employed by life on Earth. But is water the only liquid that can plausibly serve in this role? Once again, even a cursory exploration of the list of small molecules that one can make using the Universe’s most common atoms suggests that, of all the entrants on the list of potential “biotic solvents,” water may well be the only reasonable choice; many of water’s properties render it particularly well suited to be a biological solvent (table 1.1). So many properties, in fact, that its ability to form the basis of biochemistry may be unique.
Some of the “ideal” properties of water are well known, and others, while less so, are no less critical for life on Earth. An example is taught to almost every elementary school student: water is one of the few substances—and the only known molecular substance (i.e., not a monatomic element)—that expands when it freezes. Because of this, ice floats. If, instead, the ocean was filled with, for example, liquid ammonia (NH3), which is, after all, the fourth most common molecule in the Universe, its winter pack “ice” would sink, where it would be insulated from the summer’s warmth and prevented from seasonally melting. With each passing year, more and more of the ammonia ocean would be locked up in the solid until, in a time frame quite rapid by geological standards, the planet would freeze over with only a thin seasonal veneer of liquid on the surface. Could such a frozen ocean support the origins of life? Perhaps. But a permanently liquid ocean, with its ability to transport nutrients and modulate temperature, seems more likely to do the trick.
Table 1.1
Physical properties of potential biological solvents
Solvent |
Formula |
Liquid range at 1 atmosphere (°C) |
Molar density (mol/L) |
Heat capacity (J/g°C) |
Heat of vaporization (kJ/g) |
Dielectric constant |
Density ratio: solid to liquid |
Water |
H2O |
0 to 100 |
55.5 |
4.2 |
2.3 |
80 |
0.9 |
Hydrogen fluoride |
HF |
−83 to 20 |
48.0 |
2.6 |
1.3 |
84 |
1.8 |
Ammonia |
NH3 |
−78 to −34 |
40.0 |
4.7 |
1.4 |
25 |
1.2 |
Methane |
CH4 |
−182 to −161 |
26.4 |
3.3 |
0.3 |
2 |
1.1 |
Hydrogen |
H2 |
−259 to −253 |
35.0 |
0.008 |
0.2 |
0.2 |
1.3 |
Water also has an extraordinary ability to absorb heat without much of a rise in its temperature, and even more so when it evaporates to form water vapor, which is why water and steam are used as carriers for heat in central heating systems, radiators, and hot water bottles. In more precise terms, the heat capacity of liquid water is 4.2 J/g°C, meaning it requires 4.2 J of heat energy to raise the temperature of 1 g of water by 1°C. This value is about three times that typical of rock or metal. And a whopping 2.3 kJ (i.e., 2,300 J) is required to evaporate 1 g of liquid water (at its boiling point) to form water vapor, which means that water absorbs a great deal of heat when it evaporates under warm conditions and releases (and thus transports) an equivalent amount of heat to a cool place when it condenses. Water’s high heat capacity, which is significantly higher than that of most other common substances, and high heat of vaporization, which is the highest of any known substance, help to moderate the Earth’s climate—a seemingly critical event in the origins and evolution of life that we’ll cover in more detail in chapter 3. On a related note, thanks to its unique ability to form extended hydrogen-bonding networks, water remains liquid over an unusually broad temperature range spanning 100°C (at one atmosphere pressure), thus helping to ensure that, even if the climate does fluctuate radically, liquid solvent will remain available to support life.
In addition to these important physical properties, the chemical properties of water seem to render it uniquely suited as the basis for the sort of complex chemistry necessary to create living things. For example, the dielectric constant of water is around 80, which is significantly higher than that of any other cosmologically abundant liquid. This means that two oppositely charged ions in water are attracted with one-eightieth the force they would feel in a vacuum. Because of this, water can shield charged ions from one another, allowing them to be readily taken into solution where they can perform chemistry. Water also has the highest molar density of any molecular liquid; 55.5 mol of water molecules are crammed into each and every liter of the stuff. Here mole (abbreviated mol) refers to a unit used by chemists to describe a fixed number of molecules. In this regard, “a mole” is completely analogous to “a dozen.” Because molecules are much, much, much smaller than eggs, however, the chemist’s dozen is much, much, much larger than a baker’s dozen: 1 mol corresponds not to 12 or 13 molecules but to 6.023 × 1023 molecules. Thus, there are 3.34 × 1025 water molecules in 1 L of liquid water; no other liquid packs anywhere near as many molecules in a given volume.
Because of its extraordinary molar density, the energy required to arrange water molecules (the solvent) around any molecules dissolved in it (the solute) is quite high (many water molecules need to be rearranged to make room for each cubic nanometer of solute), and thus water tends to force many types of dissolved molecules to organize themselves, minimizing this cost. This organizing effect is called the hydrophobic effect (from the Greek hydro, meaning “water,” and phobos, meaning “fear”). This effect, which is why, for example, “oil and water don’t mix,” plays a critical role in the formation of biomolecular structures on Earth. Lastly, hydrogen and oxygen are the first and third most cosmologically abundant atoms, rendering water second only to hydrogen gas (H2) as the most common molecule in the Universe.
Of course, the fact that water is well suited for life on Earth doesn’t automatically rule out that life elsewhere might be based on some other solvent. Or does it? It would be hard to find an alternative as no other liquid has even a fraction of the seemingly favorable attributes of water. Hydrogen fluoride (HF) comes close. Compared with water, it has a slightly higher dielectric constant (84, to water’s 80) and thus is at least as good a solvent for ionic materials, a slightly wider liquid temperature range of 103°C (at atmospheric pressure it freezes at −83°C and boils at 20°C), and a comparable molar density (48.0 versus 55.5 mol/L for water). But as fluorine is cosmologically quite rare—it is about 100,000 times less abundant in the Solar System than oxygen—it seems very unlikely that there are little purple fish happily swimming in seas of liquid hydrogen fluoride on the planet Zap 7.
Given the above, we are probably safe ruling out hydrogen fluoride as a potential solvent for life. And none of the other molecular liquids formed by the cosmologically abundant elements (methane, ammonia, etc.) come anywhere near as close to the ideal properties of water as does hydrogen fluoride (check out table 1.1 again): their liquid ranges are extremely small, their ability to solvate ionic materials is poor, and their ability to regulate climate is more limited. From such considerations emerges the near certainty that life not only has an absolute requirement for a liquid solvent, but that water is by far the most “qualified” solvent to fulfill that role. This is not to say that life cannot have arisen based on other solvents, simply that the origins of life face a much more significant hurdle in the absence of this remarkable and abundant liquid.
Energy for Life
In addition to the matter from which it is constituted, life also requires energy to drive the reactions that underlie its metabolism and replication machinery. This is obvious to the chemist as living organisms create an implausible amount of order out of disorder, such as when the randomly distributed molecules of carbon dioxide, water, and nitrate fertilizer end up in the highly nonrandom structure of a plant. According to the second law of thermodynamics, living things can achieve this increase in order only if even more entropy (roughly speaking, a measure of disorder) is created elsewhere. By using energy from an external source, life can swap energy for entropy: the living organisms get the order, while the rest of the Universe pays the price and becomes less ordered.
Thus, life requires an external disequilibrium (an “ordered” state), whose tendency to drive toward a more equilibrated (“disordered”) state can be exploited by life for use in organizing its molecules into some pattern capable of reproduction. One of the more abundant sources of disequilibrium in the Universe is the much higher temperature of stars than of the Universe at large. Because of this, the copious number of high-energy photons emitted by a star can be absorbed by the surface of a (much cooler) planet. Here on Earth, plants take advantage of this disequilibrium and use it to feed the striking disequilibrium that is our biosphere. For example, as forest fires remind us, the presence of combustible wood in an atmosphere containing oxygen is a clear deviation from chemical equilibrium with respect to a mixture of water and carbon dioxide (the products of combustion). Indeed, this reaction is out of equilibrium by more than 500 orders of magnitude! Were it at equilibrium, there should not be a single “wood” molecule on the entire planet.* We animals, in turn, take advantage of the latter disequilibrium when we oxidize the carbohydrates in a nice al dente bowl of pasta to generate the energy we use to run our metabolic processes. We should note, however, that while these two “lifestyles” (photosynthesis, and the oxidation of photosynthesis-derived carbohydrates with photosynthesis-derived oxygen) represent the dominant disequilibria that drive life on Earth, they are not the only sources of energy known to support biology—we’ll hold off on this topic, though, until chapter 8, where we delve into this issue more deeply.
Other Constraints on Life
Chemistry, solvents, and thermodynamics aside, there are at least a handful of other constraints that must be met in order for life to arise and thrive. We’ll be discussing these in detail in the following chapters, but let’s take a moment here to look them over.
Life probably requires a condensed state on which—or in which—to form and evolve: it needs land or a liquid ocean. The reason for this was outlined above: molecules of sufficient complexity to form life are inevitably too dense to stay suspended in an atmosphere. This is, of course, a much bigger constraint on the origins of life than on its ability to thrive after it has arisen; if the surface of the Earth were to slowly become uninhabitable, it is a pretty good bet that at least some bacteria would adapt to full-time living in cloud droplets. Indeed, some Earthly bacteria may already have done so. But the limited mass transport and limited size of condensed bodies that can occur in the gas phase make this realm an exceedingly unlikely one for the origins of life. This effectively rules out life on gas giant planets, such as Jupiter, which lack any solid or even liquid surface—if life cannot arise on a planet that, like Jupiter, lacks a surface, then it likewise cannot evolve into giant, hydrogen-filled Hindenburgoids that live their whole lives in the atmosphere. And of course, life is all the more unlikely to have formed in interstellar space. This didn’t stop the cosmologist Fred Hoyle (1915–2001) from writing a wonderful sci-fi novel, The Black Cloud, about an intelligent, interstellar cloud that pays the Solar System a visit and accidentally wipes out most of humanity. The oversight was understandable: having been born and raised in the cold vacuum of space, it simply hadn’t occurred to the cloud that life was possible on the hot, crowded surface of a planet. Hoyle was a proponent of a theory that the Universe is infinitely old, and thus he was able to pass on the question of how life might arise in space by stipulating that life, too, has simply existed forever. As we’ll see in chapter 2, though, Hoyle’s cosmology has been proven wrong; the Universe, and thus life, had a beginning. And where might the latter beginning have begun? It looks as if the surfaces (or oceans) of rocky, solid planets are probably the only hope.
Life also presumably requires time. And the narrower the range of conditions under which life could arise in the first place, the more unlikely it is that sufficiently stable environments would remain within this range long enough for life to develop. The Universe is a dangerous place. The luminosity of a star changes and, with it, the temperature of any planets warmed by its light. Planets are sometimes struck by asteroids so large that the energy imparted by the impact can boil oceans and sterilize entire worlds. Atmospheres escape into space. Rotational axes tilt, plunging planets into million-year winters. Supernovae explode with the power of a billion suns, wiping out life on any planet within a few hundred light-years. Considering these risks, it is clear that not all environments in the Universe that are capable of supporting the formation of life will remain stable long enough for life to arise at all, much less gain a secure footing.
Conclusions
Given a universe containing the right ingredients, the ingredients required for life to originate seem relatively straightforward. All we need is some water, carbon on a solid planetary surface (or in its oceans), an energy source, some time, and we’re off. But is it that easy? What is required to produce a water- and carbon-bearing planetary environment that provides energy sources and yet is stable over eons? And how often are these conditions met? And if we find these conditions, how likely is it that life will arise? The following chapters explore each of these critical questions in turn.
Today, more than 75 years after Schrödinger’s lectures, many of the questions he asked about life have been answered in delightful and amazing detail. What we know less about is how life arose on Earth, and how—and whether—it might have arisen elsewhere, the topics to which we will now turn.
Further Reading
What is Life?
Schrödinger, Erwin. What Is Life? Cambridge: Cambridge University Press, 2012.
Expectations of and Constraints on Life in the Universe
Schulze-Makuch, Dirk, and Louis N. Irwin. Life in the Universe: Expectations and Constraints. 3rd ed. Berlin: Springer-Verlag, 2018.
The History of Origins-of-Life Research
Fry, Iris. The Emergence of Life on Earth: A Historical and Scientific Overview. New Brunswick, NJ: Rutgers University Press, 2000.
- * Radicals are carbon compounds that contain an odd number of electrons. As electrons are most stable when paired up with a partner of opposite spin, radicals are highly reactive. We’ll see them again in chapter 4.
- * All the available orbitals are already filled in a neon atom, hence its near complete inability to bond to anything else. The same also holds true for the outer orbitals of helium, argon, and the rest of the “noble gases” that share the column with neon over on the right-hand edge of the periodic table.
- * Ion comes from the Greek ienai, meaning “to go,” which in turn originated from the name of a Greek tribe, the Ionians; in Ancient Greece, the Ionians migrated from Thessaly to Peloponnese and to the western coast of Asia Minor. Moving ions conduct charge, such as in salt water.
- * Makes you appreciate the challenge that firefighters are up against, doesn’t it?