William Henry Bragg (1862–1942).
At the same time that Thomas Hunt Morgan was getting to grips with the role of genes in heredity, experimenters in a seemingly unrelated field were developing the techniques that would eventually unveil the molecular mechanism of heredity. This is also a story of how a new scientific discovery can be quickly turned to experimental use, opening the way for more discoveries.
X-rays had been discovered in 1895 (see here), but at first their nature was something of a mystery. In 1912, however, a team headed by Max von Laue, working at the University of Munich, found that X-rays could be diffracted by crystals, making interference patterns like the patterns made by light diffracted through the double-slit experiment (see here). In a key experiment, a beam of X-rays was shone through a crystal of copper sulphate and the diffraction pattern was recorded photographically. The photograph showed many distinct spots arranged in a pattern of intersecting circles, centred on the spot produced by the main beam. This helped to establish that X-rays are a form of electromagnetic radiation, like light but with shorter wavelengths, and von Laue received the Nobel Prize in 1914 ‘for his discovery of the diffraction of X-rays by crystals’. At the time, it was still necessary to establish the wave nature of X-rays, because many people, including a certain William Bragg, still preferred, prior to 1912, the explanation of X-rays in terms of a stream of particles, rather like cathode rays (electrons). But like any good physicist, Bragg knew that ‘if it disagrees with experiment, then it is wrong’.

When news of the German experiments reached England in 1912, William Henry Bragg was an established physicist working at Leeds University. His son, William Lawrence Bragg (always known as Lawrence) was just starting out as a research physicist in Cambridge. Intrigued by von Laue’s work, and open to new ideas, they discussed the implications with one another, and realized that it ought to be possible to work out the structure of a crystal by analysing the pattern of bright and dark spots produced by diffraction. They decided to divide up the task. Lawrence worked out the rules which made it possible to predict exactly where the bright and dark spots would be located if a beam of X-rays with a particular wavelength struck a crystal made up of atoms spaced a certain distance apart from one another. This became known as Bragg’s law. It meant that if you know the way atoms are spaced out in a crystal you can use diffraction to measure the wavelength of the X-rays, or if you know the wavelength of the X-rays you can use diffraction to work out how the atoms in a crystal are arranged. He was immediately able to use his law to make a partial interpretation of the diffraction patterns obtained in Munich, but proper analysis required more information about the wavelengths of the X-rays. So William concentrated on the experimental side, including inventing the first X-ray spectrometer, an instrument to measure the wavelengths to be plugged in to Bragg’s Law.
Interpreting the data is horribly difficult for complicated structures in which there are large numbers of different kinds of atoms. But simpler crystals are much easier to understand, and this kind of work showed, for example, that crystals of sodium chloride (common salt, NaCl) are made up not of lots of distinct NaCl molecules but of an array of sodium (Na) and Chlorine (Cl) with equal spacing, alternating in a lattice. The work of the two Braggs, which established that X-rays could be used as a tool to investigate the structure of matter, culminated in a book, X-rays and Crystal Structure, published in 1915, while Lawrence was serving in the British Army in France. It was there that he learned that had shared the Nobel Prize for 1915 with his father ‘for their services in the analysis of crystal structure by means of X-rays’. Lawrence Bragg is the youngest person, at 25, to receive the Nobel Prize in Physics. As he said in his Nobel Lecture: ‘The examination of crystal structure, with the aid of X-rays has given us for the first time an insight into the actual arrangement of the atoms in solid bodies … There seems to be hardly any type of matter in the condition of a true solid which we cannot attempt to analyse by means of X-rays. For the first time the exact arrangement of the atoms in solids has become known; we can see how far the atoms are apart and how they are grouped.’34
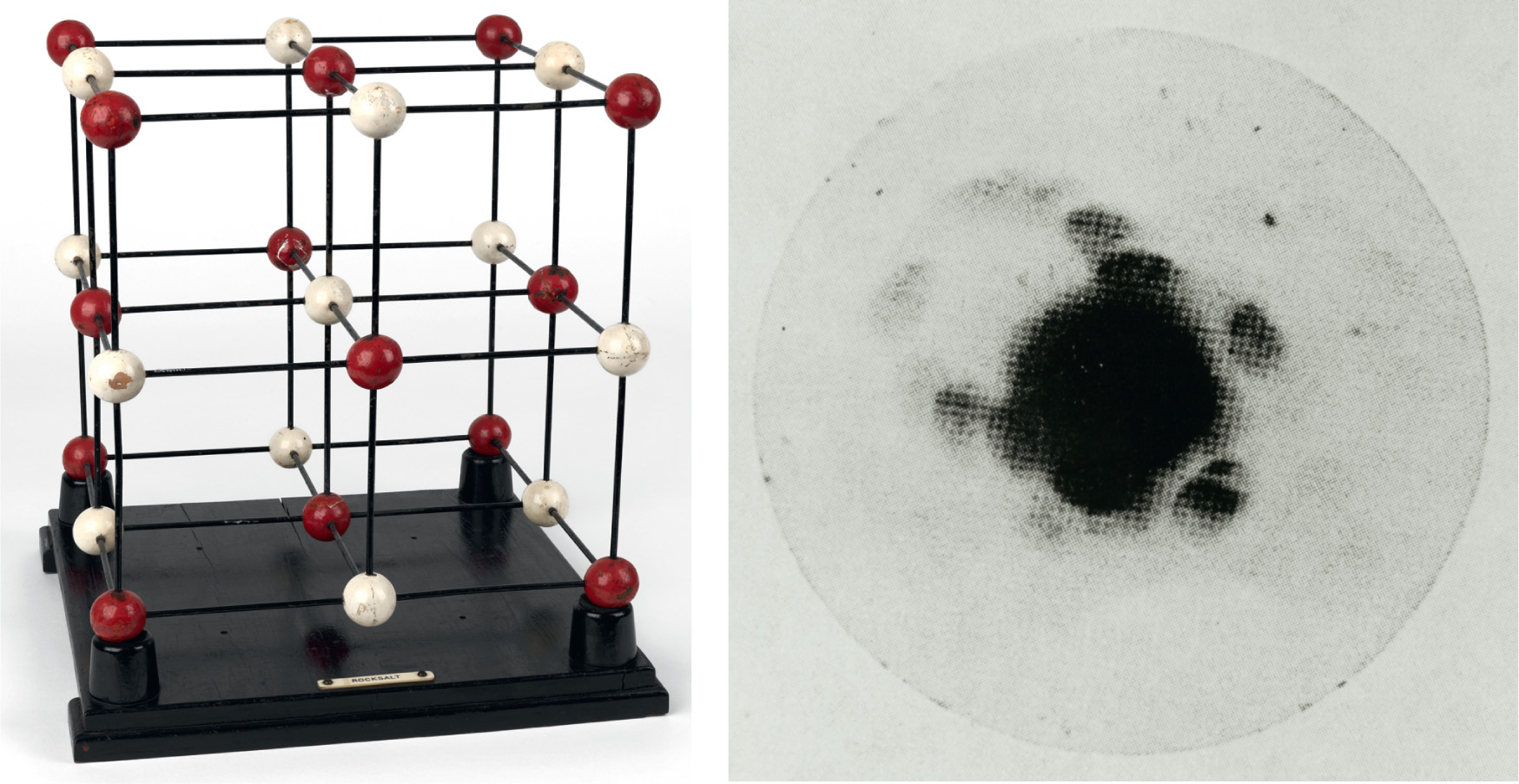
It was this ability that would, in the decades that followed, lead to an understanding of the structure of proteins (see here) and DNA itself (see here).