Would it be too bold to imagine, that in the great length of time since the earth began to exist, perhaps millions of ages before the commencement of the history of mankind,…that all warm-blooded animals have arisen from one living filament, which THE GREAT FIRST CAUSE endued with animality, with the power of acquiring new parts, attended with new propensities, directed by irritations, sensations, volitions and associations, and thus possessing the faculty of continuing to improve by its own inherent activity, and of delivering down those improvements by generations to its posterity!
—Erasmus Darwin, Zoonomia
Although Charles Darwin (fig. 4.1) deserves most of the credit for bringing about the scientific revolution in biology, he was by no means the first to suggest that life had changed through time. As early as the fifth century B.C.E., Greek philosophers such as Empedocles promoted the idea that life is constantly transforming. In 50 B.C.E., the Roman philosopher Lucretius wrote the poem De rerum naturae (“On the nature of things”), which postulated the existence of atoms and argued that everything in nature was in flux. With the fall of the Roman Empire, however, this bold style of thinking was suppressed by church orthodoxy, and the Genesis account of earth and life history ruled for almost 1,300 years. By the early 1700s, most people in Europe and North America still believed in a literal interpretation of the Bible and in the idea that the earth had been formed about 6,000 years ago, with no further change except for decay and corruption due to the sins of Adam and Eve.

FIGURE 4.1. We are familiar with pictures of Darwin as a sickly, bearded old man, but when he traveled the world on the Beagle voyage, climbed the Andes, and came up with natural selection, he was a vigorous young man in his twenties. Darwin’s 5-year research expedition would have been equivalent to a Ph.D. project, because there were no modern doctoral programs back in 1836. The original caption reads, “How Charles Darwin might have looked as a modern graduate student just back from five years of field work. The picture is intended to fix in readers’ minds that Darwin was at his most innovative at this age, and readers should remember that Darwin might now be denied admission to a good graduate school because of his deficiencies in languages and math.” (From P. R. Darlington Jr., 1980, Evolution for Naturalists: The Simple Principles and Complex Reality, frontispiece. Reprinted with permission of John Wiley & Sons, Inc.)
During the next century, however, it became increasingly difficult to reconcile the Genesis accounts of the origin of earth and life with the expanding knowledge of nature. As we discussed in chapter 3, the discovery of faunal succession between 1795 and 1805 made Noah’s flood explanations of geology more and more implausible. By 1840 flood geology had been abandoned altogether by devout Christian geologists. By the mid-1700s, natural historians like Linnaeus and his successors had already recognized over 6,000 species of animals and many more plants, far too many to squeeze into Noah’s ark. At the same time, exploration of exotic places—Africa, South America, Australia, and Southeast Asia—was producing still more new species previously unknown to Europeans, and the ark story became ludicrous. Instead of showing a pattern that reflected dispersal from Mount Ararat in Turkey, the distribution of animals showed a completely nonbiblical pattern. There were many biogeographic puzzles, like the fact that many islands (such as New Zealand, Madagascar, and Hawaii) were inhabited by unique species not found anywhere else on earth or the fact that Australia was dominated by peculiar beasts such as the egg-laying platypus and the pouched marsupials but had no native placental mammals. Why had nothing but marsupials migrated from Mount Ararat to Australia?
In addition to all these new facts from natural history, new attitudes were prevailing in the 1700s. Often called “the Enlightenment,” it was a period of skepticism and questioning of dogmatic authority (especially royal authority and religious dogmas), as scholars and philosophers sought rational, nonsupernatural explanations for the world. Inspired by the breakthroughs in science and explaining nature pioneered by Bacon, Newton, Leibniz, Pascal, and Galileo in previous centuries, Enlightenment scientists and philosophers explored new and daring ideas, unfettered by the shackles of the powers that be. In France, the Enlightenment was led by Rousseau, Diderot, and Voltaire, who questioned the authority of the king of France and the church, and by Lavoisier and Buffon, who made scientific breakthroughs in chemistry (Lavoisier) and proposed daring nonbiblical explanations of nature (Buffon). In England, John Locke and Thomas Hobbes examined political and economic systems and laid the foundation for the American experiment in democracy; George Berkeley broke new ground in philosophy (as did Spinoza and Kant in Holland and Germany); and the Industrial Revolution began with steam engines, textile manufacturing, and canal systems transforming England from an agrarian nation to an industrialized one. In 1764, the Lunar Society (so named because they met for dinner on the Monday night nearest the full moon; they called themselves the “Lunatics”) was formed in Birmingham and promoted new scientific and technological ideas. The original founders included Erasmus Darwin (Charles Darwin’s grandfather), William Small (Jefferson’s mentor), and the industrialist Matthew Boulton. Soon the “Lunatics” included many of the great minds in Britain (including Benjamin Franklin when he visited). In Scotland, the brilliant men who met in Edinburgh and Glasgow pubs included the philosopher David Hume (discussed in chapter 2), the pioneering economist Adam Smith (the “father of capitalism”), the inventor of the first practical steam engine, James Watt, and the father of modern geology, James Hutton. Eventually, these new ideas and challenges to royal authority helped lead to the American Revolution against Britain in 1775. American patriots such as Jefferson were inspired largely by British and French political philosophers, such as Locke and Rousseau. These ideas also triggered the French Revolution in 1787, which overthrew the centuries-long domination of France by the Bourbon monarchy and the church.
The Enlightenment had the greatest effect on natural history in France, where Buffon had proposed daring nonbiblical ideas as early as 1749. The foremost French naturalist of the late 1700s was Jean-Baptiste Antoine de Monet, the chevalier de Lamarck (1744–1829). He began his career as a Linnaean-style botanist, but when the French Revolution swept through the king’s gardens (les Jardins du Roi), he was reassigned to study the less glamorous “insects, shells, and worms,” which had been neglected by naturalists for centuries. Lamarck soon revolutionized invertebrate zoology, laying out the foundation of our modern understanding of these “spineless wonders.” Because he had started as a botanist, he soon recognized that zoology and botany made an integrated whole, which he named “biology.” His ideas culminated in 1809 with the publication of Philosophie Zoologique.
In this work, Lamarck points out how all of life is highly variable and interconnected, not composed of discrete fixed species from the original Creation. He arranged animals in the traditional “scale of nature” (scala naturae) from the primitive sea jellies and corals to worms to mollusks and insects to vertebrates, with humans at the top of the scale. In some versions, the upper rungs of the “ladder of nature” were occupied by divine beings, with the ranks of cherubim, seraphim, angels, and archangels culminating in God. Like many people of his time, Lamarck believed that life was constantly being spontaneously generated out of the mud. Once formed into sea jellies, life would be transformed and move up the ladder, so that lineages that had originated long ago had already climbed to the higher rungs of fish or mammals or humans.
This “ladder of nature” notion from the late 1700s is still a common misconception that leads to all sorts of philosophical and biological absurdities. We will talk more about this topic in the next chapter, but the main point is that the concept of the ladder has been replaced by the metaphor of the bushy, branching “family tree of life.”
Although Lamarck’s ideas were evolutionary, they were not very similar to modern concepts of evolution. Instead of the bushy family tree of life we now recognize, Lamarck’s concept was that of many different “blades of grass” independently arising by spontaneous generation out of the mud and climbing the ladder of complexity and not interconnected by common ancestry. Typical of the works of its time, Philosophie Zoologique was highly speculative and philosophical and not supported by much hard evidence from nature or experimental data. A minor idea in Lamarck’s book was the inheritance of acquired characters. Like most people of that time (including Charles Darwin 50 years later), Lamarck believed that the characteristics that you developed during your lifetime (such as the muscles of a blacksmith or a bodybuilder) could be passed directly to your offspring. According to Lamarck, the giraffe would keep stretching and stretching its neck and pass those improvements to its descendants until they all had long necks. After Lamarck’s death, this idea was lampooned by his enemies as “Lamarckism” or “Lamarckian inheritance” (even though Charles Darwin believed it, too). Consequently, most of Lamarck’s great achievements are largely forgotten, and his name is now attached to a minor part of his ideas that has become disreputable.
In the survival of favoured individuals and races, during the constantly-recurring struggle for existence, we see a powerful and ever-acting form of selection.
—Charles Darwin, On the Origin of Species
Charles Darwin (fig. 4.1) was born on the same date as Abraham Lincoln (but about 15 hours earlier)—February 12, 1809. Like Lincoln, he was a liberating force for humankind, but instead of freeing people from slavery, he freed biology from the bondage of supernaturalism. Philosophers of science have long pointed to Darwinian evolution as the greatest scientific revolution within biology, comparable to the role of Isaac Newton’s or Albert Einstein’s revolutionary ideas in physics or the plate tectonics revolution in geology. Before Darwin, it was possible (although increasingly difficult) to see nature as divinely created as we see it, unchanged over thousands of years. After Darwin, all of life was subject to natural law, just as Newton had shown that the stars and planets followed natural laws and did not require God to move them. As Sigmund Freud (1917) commented,
In the course of centuries the naïve self-love of men has had to submit to two major blows at the hands of science. The first was when they learnt that our earth was not the center of the universe but only a tiny fragment of a cosmic system of scarcely imaginable vastness. This is associated in our minds with the name of Copernicus, though something similar had already been asserted by Alexandrian science. The second blow fell when biological research destroyed man’s supposedly privileged place in creation and proved his descent from the animal kingdom and his ineradicable animal nature. This revaluation has been accomplished in our own days by Darwin, Wallace and their predecessors, though not without the most violent contemporary opposition.
For such a revolutionary idea, it is surprising that it came from such a conventional, outwardly conservative man. Charles Darwin was born of a family of doctors, including his grandfather Erasmus, who was the king’s physician, and his father, Robert, also a distinguished physician. But evolution was in Charles’s blood as well. Erasmus wrote a 1794 poem entitled Zoonomia, which contained some of the most advanced evolutionary speculation of its time but was made less threatening by its poetic format and its religious references. Young Charles could not help but be influenced by his grandfather’s ideas. When Charles was a teenager, his father sent him to medical school in Edinburgh. Charles proved to have no stomach for dissecting rotting corpses robbed from graves or watching people scream through surgery (as was common in medicine then, because there were no anesthetics). He was, however, exposed to the ideas of Robert Grant (the man who proved that sponges are animals), who was in turn influenced by the French evolutionists like Lamarck and Geoffroy. After Charles had dropped out of medical school, his father next sent him to Cambridge University, where he could become a clergyman and do something useful with his life while he pursued his mania for natural history collecting. There he neglected his theological studies and was instead influenced by botanist John Stevens Henslow and the geologist Adam Sedgwick (first professor of geology anywhere in the world). Several years later, Darwin had the opportunity to sail on the oceanographic voyage of the HMS Beagle. At first his father was horrified at the prospect, but eventually he relented.
The voyage lasted 5 years (1831–1836) and the Beagle traveled completely around the world. Darwin collected fossils of huge extinct animals in Argentina, climbed the Andes in Chile, and was one of the first to study the anthropology of the natives of Tierra del Fuego, in the freezing southern tip of South America. When the ship stopped at the Galapagos Islands for water and food, Darwin ended up making many observations and collections, yet not until he returned did he appreciate the importance of what he saw. The Beagle continued on its journey to Australia and South Africa before finishing its survey of the Brazil-Argentina coast and then returning home. When Darwin returned home, his father said, “Why, even the shape of his head has changed!”
Even more remarkable is what had changed inside his head. As a Cambridge-educated, wealthy gentleman, Darwin didn’t need to work for a living; he could conduct research and access the elite scientific societies of the time. He set about publishing a book about his Beagle voyage and a book about coral atolls in the Pacific, which immediately made his reputation. He also made arrangements to have his specimens studied by the eminent specialists of the day. He married his first cousin Emma Wedgwood (of the Wedgwood pottery family), settled in the town of Down (just southeast of London), and began to raise a family. As he began to work, he read the writings of the Reverend Thomas Malthus, who pointed out that human populations were prone to exponential size increase unless held in check by death and disease. Darwin also became a fancier of domestic pigeons and soon developed a network of other gentlemen who raised and bred exotic domestic animals to develop unusual varieties, practicing a form of artificial selection. These ideas, and many others, combined to give Darwin his first notions of evolution by natural selection.
Only 6 years after returning from the Beagle voyage, Darwin had written his first sketch of his evolutionary ideas but put it under lock and key with instructions to his wife to open it and publish it only after his death. Darwin had good reason to be worried about his reputation if he published ideas about evolution. The entire concept was associated with radical French thinking and revolutionary politics of the lower-class medical schools in London. It was an anathema to the powerful conservative elites of the wealthy, the nobility, and the Anglican Church. In 1844, a Scottish publisher named Robert Chambers wrote an anonymous tract called Vestiges of the Natural History of Creation, which caused a national furor at its evolutionary thinking. Although the science in Vestiges was amateurish and easy to discredit, clearly evolutionary thinking was in the air in the 1840s. But it was still too controversial for a respectable Cambridge-educated gentleman like Darwin to touch.
So Darwin sat on his dangerous idea for 15 years, working quietly at home on barnacles. At that time, these animals were poorly understood, but Darwin discovered that they were highly modified crustaceans related to shrimp, showing amazing adaptations. He published several scientific books on barnacles, which gave him an impeccable scientific reputation. Darwin accomplished this despite working only a few hours a day because he was wracked by a mysterious illness, which may have been a psychosomatic response to the dangerous ideas he was considering. All the while, he was amassing notes for a gigantic book on the “species problem.” He would have procrastinated for many more years had he not received a letter in the mail from a younger British naturalist, Alfred Russel Wallace, who was collecting specimens in what are now Malaysia and Indonesia. During a bout of malarial fever, Wallace had come up with a similar concept of natural selection and had sent his ideas to Darwin, of all people. Horrified, Darwin went to his friends Charles Lyell (the famous geologist) and Joseph Hooker (a famous botanist), searching for an honorable solution to the dilemma of who would get credit for the idea. They arranged to have Darwin’s 1842 abstract and Wallace’s letter read at an 1858 meeting of the Linnean Society. The president of the Linnean Society, Thomas Bell, while summarizing the discoveries for the year 1858, wrote: “The year which has passed has not, indeed, been marked by any of those striking discoveries which at once revolutionize, so to speak, the department of science on which they bear.” But Darwin now had to work quickly or he would be scooped. He abandoned his planned gigantic work and wrote a shorter (155,000 words, which was short by Victorian standards) summary of his ideas, On the Origin of Species by Means of Natural Selection. It sold out all 1,250 copies on the day it was published and eventually went through six editions while Darwin was alive.
The argument of On the Origin of Species is very simple yet powerful. First, Darwin drew an analogy to the artificial selection of domesticated animals practiced by animal breeders. He argued that if they could modify the ancestral wolf into dogs as different as a Chihuahua and a Great Dane, then species were not as fixed and stable as commonly believed. Then he borrowed the idea from Malthus that natural populations are capable of exponential growth, yet they remain stable in nature because of high mortality rates. From this he deduced that more young are born than can survive. Darwin next described the variability of natural populations and pointed to the evidence from domesticated animals that these variations are highly heritable. He concluded that organisms that inherit favorable variations are more likely to survive and breed, and he called this process natural selection (called by others “survival of the fittest”).
As we saw, Darwin was not the originator of the concept of evolution, and at least two others proposed something like natural selection. Why, then, does Darwin deserve most of the credit? For one thing, Darwin was the right man at the right time. In 1844, the idea was still too controversial and Chambers’s amateurish efforts only made people scoff at the idea of evolution. By 1859, however, the time was right and many people were thinking along these lines (as Wallace’s independent inspiration shows). In addition, Darwin had worked hard to build a sterling scientific reputation and was also a member of the Oxford-Cambridge elite. He was not a radical from the lower-class medical schools of London. Most importantly, Darwin put all the pieces together in one book and provided two important concepts: the evidence that life had changed through time (the “fact” of evolution) and a mechanism for how it occurred, natural selection (the “theory” of evolution). He overwhelms the reader with example after example, so that by the end the conclusion is inescapable.
Darwin’s ideas were controversial at first, but by the time he died in 1882, the fact that life had evolved was universally accepted in all educated parts of the world (including most of the United States). When he died, Darwin was hailed as one of Britain’s greatest scientists. He was buried in “Scientists’ Corner” of Westminster Abbey, next to Isaac Newton and the rest of Britain’s scientific geniuses. However, his mechanism of natural selection initially did not fare so well. Many of Darwin’s critics could not imagine how it was sufficient to shape organisms. Some argued that if favorable variations occurred, they would be blended out of existence in a few generations by backcrossing with the normal strains of animals. Darwin never solved this problem by the time he died in 1882.
Ironically, the solution had already been discovered in 1865 by an obscure Czech monk named Gregor Mendel. He found that by breeding strains of pea plants in his garden, he could produce very simple and mathematically predictable inheritance patterns. More importantly, he showed that inheritance does not blend the genes of both parents but is discrete, so that rare genes from one parent can seem to vanish for a generation but then reappear fully functional in the next generation if the genes are recombined in a certain way. Mendel’s work remained unknown until it was independently rediscovered by three different lab groups in 1900, when the time was ripe for appreciating his insights. Genetics made enormous strides over the next 50 years, culminating with the discovery of the DNA molecule and its role in inheritance in 1953.
Evolution is a change in gene frequencies through time.
—Theodosius Dobzhansky, Genetics and Origin of Species
Evolution is merely a reflection of changed sequence of bases in nucleic acid molecules.
—John Maynard Smith, The Theory of Evolution
Although the world had accepted the fact that life has evolved by the time of Darwin’s death in 1882, it was less convinced that his mechanism, natural selection, was sufficient to explain all of evolution. Natural selection slowly lost favor as several different genetics labs rediscovered Mendelian inheritance and then built new ideas of how evolution occurred. Paleontologists, meanwhile, became even more heterodox, with some following Darwin’s mechanism, others subscribing to some sort of inheritance of acquired characters (“neo-Lamarckians”), and still others completely agnostic as to what mechanism drove evolution. Although the fossil record continued to provide more and more evidence for how life had evolved, paleontologists were not at the forefront for theoretical mechanisms of how evolution occurred. Meanwhile, systematists (biologists who study the naming and relationships of organisms) were busy describing new species, but few thought of the evolutionary implications of their work. There was simply no common thread among them, and there appeared to be no way to show that Darwinian natural selection was compatible with genetics, paleontology, and systematics.
The breakthrough occurred in the 1930s, when three scientists introduced a set of mathematical models known as population genetics. Two were British (Sir Ronald Fisher and J. B. S. Haldane) and one was an American, Sewall Wright, whom I had the great fortune to meet in 1983 while he was still active and alert at age 94. These mathematical simulations allowed evolutionists to describe changing gene frequencies through many generations and simulate the effects of mutation and selection. Population genetics clearly showed that even slight selection pressure can quickly change gene frequencies and made evolution by Darwinian natural selection plausible again. By the late 1930s, several books tied population genetics to the various subdisciplines of evolutionary biology. In 1937, geneticist Theodosius Dobzhansky published Genetics and the Origin of Species, which updated and synthesized all the genetics known at the time, and showed how selection in fruit fly experiments gave amazing demonstrations of evolution in action. In 1942, ornithologist Ernst Mayr published Systematics and the Origin of Species (discussed in chapter 3), which dealt with the problem of speciation in nature and showed how the allopatric speciation model was consistent with natural selection. And in 1944 (written in 1941, but delayed by World War II), paleontologist George Gaylord Simpson published Tempo and Mode in Evolution, which attempted to show that nothing in the fossil record was inconsistent with Darwinian natural selection. Together, these works brought the major threads of evolutionary biology—genetics, systematics, and paleontology—back into the Darwinian fold. By the late 1940s, biologist Julian Huxley was referring to this new consensus as the “modern synthesis” or the “neo-Darwinian synthesis.” By the 1959 centennial of the publication of On the Origin of Species, the neo-Darwinian synthesis completely dominated evolutionary biology, and most biologists thought that all the major problems had been solved and questions had been answered. After almost 60 years, most current textbooks on evolution still reflect this dominance of neo-Darwinism.
What are the main ideas of the neo-Darwinian synthesis? Its central core comes from genetics (with its focus on the genotype of the organism), which has shown just how effective natural selection can be in changing the frequencies of genes in populations. From this, neo-Darwinists define evolution in an extremely reductionist manner as a change in gene frequencies through time, without regard to the embryology or development of the organism or the influence of the body (phenotype). Some extreme neo-Darwinists argue that the body is simply a device for genes to make more copies of themselves. Population genetics and fruit fly experiments showed that most variation is due to the recombination of genes from both parents but that additional variation is the result of slight mutations. These random variants are then weeded out by natural selection, and the stronger the selection, the more rapid the genetic change. In some extreme versions of neo-Darwinism, natural selection was treated as an all-powerful, all-pervasive force that, in Darwin’s words, “is daily and hourly scrutinizing throughout the world every variation, even the slightest; rejecting all that which is bad, preserving and adding up all that is good; silently and insensibly working.” Some even argued that all changes are adaptive in some way, even if we can’t detect how. To them, there are no features of an organism unaffected by natural selection. Such views are often called panselectionism.
Along with the reductionist attitude that organisms are nothing more than vessels to carry their genes came the extrapolation that the tiny genetic and phenotypic changes observed in fruit flies and lab rats were sufficient to explain all of evolution. This defines all evolution as microevolution, the gradual and tiny changes that cause different wing veins in a fruit fly or a slightly longer tail in a rat. From this, neo-Darwinism extrapolates all larger evolutionary changes (macroevolution) as just microevolution writ large. These central tenets—reductionism, panselectionism, extrapolationism, and gradualism—were central to the neo-Darwinian orthodoxy of the 1940s and 1950s and are still followed by the majority of evolutionary biologists today.
As we shall see later in this chapter, the evidence for microevolutionary change is abundant throughout nature, and we see evolution in action all the time. Neo-Darwinian evolutionary biology has had many great successes (detailed in all the textbooks), so there is no reason to doubt that natural selection is the most important engine for evolution. But is it the only factor involved? Is evolution truly reducible to changes in gene frequencies through time?
The variations detected by electrophoresis may be completely indifferent to the action of natural selection. From the standpoint of natural selection they are neutral mutations.
—Richard Lewontin, The Genetic Basis of Evolutionary Change
When neo-Darwinism swept through the profession in the 1940s and 1950s, it achieved almost a complete consensus. Many evolutionary biologists thought that the major problems were all solved; only the details needed to be worked out. But it is not a good thing when a field in science seems to have all the answers and is no longer questioning its assumptions. A continuing critical attitude, new unsolved problems, and skepticism and controversy are essential to the health of good science. If a science does not continue to test its ideas and views all essential problems as solved, then it soon stagnates and dies.
Fortunately, the neo-Darwinian synthesis has been continually scrutinized and challenged by legitimate biological and paleontological data, so the field is rife with healthy controversy. Most of these challenges only question some of the more extreme neo-Darwinian tenets or argue that natural selection is not the only mechanism by which life evolves. Despite the misleading misquotations of creationists, none of these ideas challenges the well-established fact that life has evolved or that natural selection is an important (if not exclusive) mechanism for evolution. For example, creationists frequently quote Stephen Jay Gould saying that “neo-Darwinism is dead” and suggest that Gould does not believe in evolution! In fact, Gould is arguing for the importance of non–neo-Darwinian mechanisms for evolution. A typical Gould quote in context reads:
I well remember how the synthetic theory beguiled me with its unifying power when I was a graduate student in the mid-1960’s. Since then I have been watching it slowly unravel as a universal description of evolution. The molecular assault came first, followed quickly by renewed attention to unorthodox theories of speciation and by challenges at the level of macroevolution itself. I have been reluctant to admit it—since beguiling is often forever—but if Mayr’s characterization of the synthetic theory is accurate, then that theory, as a general proposition, is effectively dead, despite its persistence as textbook orthodoxy. (Gould 1980b:120)
As the full quotation clearly shows, Gould is talking about the neo-Darwinian synthesis and is not expressing doubts that evolution occurred. Creationists either can’t tell the difference or are deliberating misquoting Gould to mislead their readers.
Now that we have cleared up the creationist misunderstanding that a challenge to neo-Darwinism is not a denial that evolution has occurred, let us look at the legitimate scientific issues that are raised by Gould’s words.
As we discussed already, most naturalists of the nineteenth century, including Lamarck and Darwin, concluded that features acquired during one’s lifetime could be passed directly to descendants (the inheritance of acquired characters). This type of inheritance is unfortunately known as “Lamarckian inheritance”; as we mentioned already, it was an old notion from before Lamarck and only a minor part of Lamarck’s ideas and was accepted by Darwin as well. It is obvious why this idea is appealing. Instead of the wasteful Darwinian mechanism of the death of many offspring, just so a few favorable variants can survive, Lamarckian inheritance allows new variations to be passed on directly in a single generation and allows organisms to adapt more readily.
By the 1880s, however, some geneticists began to doubt whether Lamarckian inheritance was real. Instead, they asserted the primacy of Darwinian natural selection. The German biologist August Weismann performed a series of experiments that seemed to discredit the idea of the inheritance of acquired characters. He cut off the tails of twenty generations of mice, but each new generation developed a tail, despite this rather extreme form of selection pressure. From this, Weismann concluded that anything that happens to our bodies (“soma” in Weismann’s terminology) during our lifetimes does not get back into the genome (“germ line”). This became known as “Weismann’s barrier” or the central dogma of genetics: the flow of information is a one-way flow, from genotype to phenotype, but not the reverse. When James Watson and Francis Crick discovered DNA, the central dogma was redefined to mean the one-way flow of information from DNA to RNA to proteins to phenotype—but never back to the DNA.
For decades, the central dogma seemed to work, and even the hint of Lamarckism was considered highly controversial and unorthodox. But as early as the 1950s, embryologist Conrad Waddington showed that repeated environmental stresses could cause abrupt genetic change without direct selection, or what he called genetic assimilation. The best evidence, however, comes from immunology. When we are born, our immune system is functional but does not yet recognize all the foreign germs and pathogens it must defend against. We acquire immunity through our lifetimes each time our immune system is exposed to a germ and develops an antibody to defend against it. However, a series of experiments showed that laboratory mice could pass on their immunity directly to their offspring (Steele et al. 1998). It is hard to see how this is explained by anything other than Lamarckian inheritance.
More recently, molecular biologists have found that acquired inheritance is the norm, rather than the exception, in most microorganisms. Viruses work entirely this way, inserting their DNA into the cell of a host and making more copies of themselves. Many bacteria and some other organisms (including plants such as corn) seem to have “jumping genes” that exchange gene fragments between strains of organisms without sex or even recombination. One group of viruses (the retroviruses that cause HIV, among other infections) copies their own genetic information from host to host and may be capable of carrying the DNA of one organism into another.
All of these new mechanisms of inheritance suggest the genome is not as simple and “one-way” as we thought only 40 years ago. John Campbell (1982) summarized a full range of genetic interactions, starting with the simple “structurally dynamic” genes that respond to a certain environmental stimulus by producing a particular response. At a more sophisticated level are genes that apparently sense their environment and change their response. Automodulating genes change their future responsiveness to stimuli when stimulated. The most Lamarckian of all are “experiential genes,” which transmit specific modifications induced during their lifetimes into the genome of their descendants. The example from immunology may fit this, as does bacterial and viral DNA swapping.
Clearly, the simplistic “central dogma” no longer applies to microorganisms, which are remarkably promiscuous in swapping DNA around. It may also not apply to many multicellular organisms either, if the immunologic experiments are correctly interpreted.
One of the first challenges to neo-Darwinism came when molecular biology began to understand the details of the genome in the 1960s. Prior to this, geneticists had assumed that each gene in the chromosome coded for only one protein (and the structures built from them), so that inheritance would be simple (the “one gene, one protein” dogma). They also asserted that every gene was under the constant scrutiny of natural selection (panselectionism) and no gene was selectively neutral (even if we can’t detect how selection operates). But in the 1960s, a series of discoveries shattered this simplistic idea of the genome. Using a newly developed technique called electrophoresis, Lewontin and Jack Hubby (1966) found that organisms had far more genes than they actually use or that can be expressed in the phenotype. Soon, geneticists were discovering that as much as 85–97 percent of the DNA in some organisms (including about 90 percent of human DNA) is not critical for expression of a phenotypic feature and is either “silent” DNA or “junk” DNA left over from the distant past when it had some function. If it is not expressed, it cannot be detected by natural selection and is neutral with respect to selective advantages or disadvantages. This new idea of neutralism completely shattered the old belief in panselectionism. In recent years, a few geneticists have tried to salvage the idea that there is less “junk DNA” than once thought, and Project ENCODE made the claim that most of the DNA was minimally functional. However, these claims have been debunked by many different lines of evidence. Most of our DNA is indeed “junk” that is never read or used in any real functional sense.
At the most basic level, the fundamental structure of the genetic code guarantees that a high percentage of mutations will be invisible to natural selection. The genetic code (fig. 4.2) consists of a three-letter “triplet” sequence of nucleotides (adenine, cytosine, guanine, and uracil). As the DNA is transcribed by tRNA, it interprets each three-letter sequence as the code for one of the 20 amino acids (plus a few codes are used to stop the transcription of DNA). Notice in figure 4.2 that of the 64 possible combinations of three letters, many of them specify the same amino acid. It is usually the first two letters of the triplet that count, and the third letter makes no difference. For example, if the first two nucleotides are cytosine and uracil, it produces the amino acid leucine, no matter what the third letter is. Clearly, most mutations in the third-letter position (every third nucleotide in the DNA) are invisible to natural selection and must be neutral as a result.
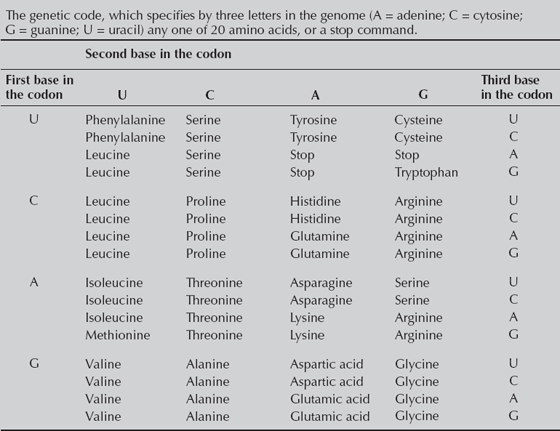
FIGURE 4.2. The genetic code. Each protein is specified by a three-letter “triplet” codon combination of adenine, guanine, cytosine, or uracil. Note how most amino acids can be specified by just the first two letters, and the third letter makes no difference—it is adaptively neutral, and most mutations at this locus are silent and nonselective.
From these discoveries, geneticists have come to realize that many mutations are adaptively neutral and continue to occur without interference from natural selection. This has led to the discovery of the molecular clock. When molecular biologists began to compare the DNA of closely related organisms, they found that there seemed to be a regular, predictable amount of change in their DNA that depended only upon how long ago the two lineages had been separated. When they calibrated their divergence points on the molecular family tree with the fossil record, they found that they could determine how long ago various lineages branched off, even in the absence of fossil evidence. All of this works because so much of the genome is invisible to selection and can constantly change by random mutation without interference. Although the molecular clock has had some great successes, mutation rates can vary unpredictably, so scientists are cautious about putting too much weight on molecular clock estimates for the age of a lineage when all the other evidence disagrees.
More importantly, the fact that 80–97 percent of the DNA in most organisms codes for nothing at all (so far as we know) says that evolution and selection must work entirely on the remaining few percent of the DNA that does code for something. Those remaining genes are known as regulatory genes. They are the master switches that control the reading of the rest of the DNA, some of which is used to make the basic structures of life (structural genes) and therefore does not differ between organisms. So from the assertion in the 1950s that every gene codes for one protein, we now know that most genes don’t code for anything, and only a few regulatory genes exert almost complete control over every other gene in the DNA. By tiny changes in those “switches” or regulatory genes, the organism can make big evolutionary leaps.
We can see the importance of these regulatory genes when something goes wrong, and a bizarre atavistic mutant, or “evolutionary throwback” occurs. Humans still have the genes for the long tail of our monkey ancestors, and every once in a while, the suppression of those genes fails and a human is born with an external tail (fig. 15.9). A simple failure in the transcription in the genes of a horse, and you get a horse with three toes (fig. 4.3). The side toes are poorly developed, but they still resemble the condition of the ancestral horses, which had two functional side toes. This experiment shows that the genes for the ancestral side toes are not lost in modern horses, only suppressed by the regulatory genes, and when there is a mistake in regulation, these ancient features reappear. Such freakish “horned horses” were thought to have great powers, and Julius Caesar rode one into battle.
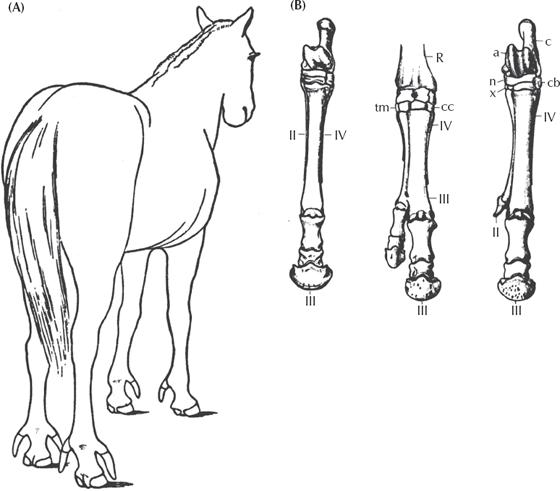
FIGURE 4.3. (A) A famous example of a rare mutant horse that has three toes, rather than one. (B) Bone structure of the feet of the mutant horses. On the left is a normal horse foot; in the middle is an extra toe formed by duplicating the central toe; on the right is an extra toe formed by enlarging the reduced side toes (splint bones), which were functioning side toes in earlier horses. (From Marsh 1892)
The most striking example was an experiment that showed that birds still have the genes for teeth, even though no living bird has teeth. The embryonic mouth tissues of a chick were grafted into the mouth area of a developing mouse. When the mouse grew teeth, they were not normal mouse teeth, but conical peg-like teeth similar to those of the earliest toothed birds, or the dinosaurian ancestors of birds. All it took was the removal of the regulatory genes that a chick would normally have (by grafting tissues into a mouse) and the long-suppressed genes for reptilian teeth carried by all birds finally emerged. Other embryonic studies have managed to change the genes that code for the birds’ short, stumpy, bony tail, resulting in the development of a long bony tail like a dinosaur. Another genetic modification experiment in developing chickens gave rise to chickens with dinosaur-like feet, not bird feet. Yet another experiment produced a bird with a dinosaurian snout with teeth instead of a normal beak. Birds have nearly all their old dinosaurian genes residing in their genome, just not expressed.
You have loaded yourself with an unnecessary difficulty in adopting Natura non facit saltum [Nature does not make leaps] so unreservedly.
—Thomas Henry Huxley, in an 1859 letter to Charles Darwin
The importance of regulatory genes goes far beyond neutralism and junk DNA. It raises the question again of whether microevolution, which is so successful at making small changes (such as the number of bristles or wing veins in a fruit fly or the length of the beak of a Galapagos finch) is sufficient to explain macroevolution (the development of large-scale changes in evolution, such as new body plans). If you just keep accumulating tiny microevolutionary changes through time, would this produce wholly novel organisms?
This debate goes back to the earliest days of evolutionary biology. Darwin was a convinced gradualist, but his friend and defender Huxley warned him (in the quotation at the start of this section) that he need not tie his evolutionary ideas to gradualism or rule out evolutionary “leaps” to new body forms. When neo-Darwinism became dominant in the 1940s and 1950s, Richard Goldschmidt, a German-born geneticist at Berkeley, protested the strict gradualist position. He argued from his studies of gypsy moths that the changes required to build new body plans and new species were not the same as those he found within the normal variation within a species. Goldschmidt argued that some sort of large-scale genetic change was needed (a “systemic mutation” in his words) to jar species out of their normal range of variation and into new body plans. These changes were due to slight changes in “controlling genes” (what we now call regulatory genes). According to Goldschmidt, speciation was a discontinuous, rapid process that was caused by changes in controlling genes, not by accumulation of small microevolutionary changes. If a new macromutation appeared that gave the individual a big advantage, it might produce a “hopeful monster” that could establish a new species or a new adaptive zone.
Naturally, such opinions were highly unorthodox with respect to the gradualistic ideas of the newly dominant neo-Darwinians, and they subjected Goldschmidt to ridicule and scorn. When I was taking evolution classes from hard-core neo-Darwinians in graduate school, they would scoff “How does the hopeful monster find a mate?” Without more than one hopeful monster, there is no possibility of breeding or establishing a new population, and thus there would be no chance of a new species forming.
Ironically, the past 20 years have vindicated Goldschmidt to some degree. With the discovery of the importance of regulatory genes, we realize that he was ahead of his time in focusing on the importance of a few genes controlling big changes in the organism, not small-scale changes in the entire genome as neo-Darwinians thought. In addition, the hopeful monster problem is not so insurmountable after all. Embryology has shown that if you affect an entire population of developing embryos with a stress (such as heat shock), it can cause many embryos to go through the same new pathway of embryonic development, and then they all become hopeful monsters when they reach reproductive age (Rachootin and Thomson 1981).
The more we learn about regulatory genes, the more we realize their primary importance to evolution. A common example is the study of heterochrony, where organisms change the sequence of their developmental timing. This allows evolution to take advantage of the changes already encoded in our embryology and development. For example, nature frequently makes changes through neoteny, where an organism retains its juvenile body form while achieving reproductive maturity. The most famous case involves the salamanders (such as the Mexican axolotl) that do not complete their metamorphosis into lunged salamanders but hold on to their juvenile gills and body form, yet they can breed like adults (fig. 4.4). Whenever these salamanders are exposed to stagnant water conditions, they can complete their metamorphosis into lunged adults and walk to the next fresh pool of water. Thus, this ability to choose to breed either as the juvenile or adult body form gives them great ecological flexibility, all with a few tiny changes in the regulation of their development.

FIGURE 4.4. The Mexican salamander Ambystoma, known to the Aztecs as the axolotl. In normal conditions, it retains its larval gills into sexual maturity, enabling it to remain aquatic. However, if the water becomes stagnant, it completes its metamorphosis into an adult salamander with lungs, so it can crawl out and find a new lake to live in. By using different parts of its natural developmental cycle, it has great evolutionary flexibility. (After Dumeril 1867)
As Stephen Jay Gould pointed out in his book Ontogeny and Phylogeny (1977), this mechanism is extremely common in nature, especially when the juvenile and adult body forms have radical differences in shape and ecology, and allows the organism to “switch-hit” for whatever works best. Those pesky aphids that invade your flowers each spring are a classic example. When the food resources are abundant (in the spring and summer), they multiply rapidly, with each female giving birth to immature daughters as asexual clones (no males are born at all). Those offspring, in turn, also reproduce asexually as juveniles, so they can make literally hundreds of daughters in a short period of time (which is why they can infest your flowers so quickly). When the fall comes and the food resources dry up and cold weather approaches, they switch to sexual reproduction. A few males are born and mature into adults and then quickly mate with adult females. These lay normal eggs that can survive the winter and hatch out next spring to start the process all over again. All of this evolutionary flexibility does not require big changes in the genome, just small changes in regulating the normal sequence of embryonic development already encoded in the organism.
The most important recent development, however, has been the discovery of the master regulatory genes, known as the homeotic genes (especially the “Hox” genes). These genes are found in nearly all multicellular organisms and regulate the fundamental development of the body plan and how major organ systems develop. They were first discovered with experiments on fruit flies that had unusual mutations. Some had legs growing on their heads instead of antennae (fig. 4.5A); this is known as the “antennipedia” mutation. Some flies developed two pairs of wings instead of the usual single pair (fig. 4.5B). Normal flies have tiny knob-like balancing organs called halteres where the second pair of wings would be, but these mutant flies have apparently changed their regulatory genes so that ancestral wings appeared instead of the halteres.

FIGURE 4.5. Homeotic mutants show that big developmental changes can result from small genetic mutations, giving rise to dramatic differences in body plan. (A) The antennipedia mutation, where a leg grows instead of an antenna. (Photo courtesy F. R. Turner, Biology Department, Indiana University) (B) The bithorax mutant fly, which has a second pair of wings instead of the halteres normally found behind the front pair of wings. (Photo courtesy W. Gehring and G. Backhaus)
From these early discoveries, molecular biologists have identified most of the Hox genes in a number of organisms and found that nearly all animals (including flies, mice, and humans) use a very similar set of Hox genes, with slight variations, subtractions, and additions. Each Hox gene is responsible for the development of part of the organism and all its normal organ systems (fig. 4.6). Small changes in the Hox genes can put different appendages on a segment of a fly (like the leg where the antenna would go or the wing where a haltere belongs) or even multiply the number of segments. Clearly, then, a tiny change in Hox genes can make a big evolutionary difference. In the arthropods (the “jointed legged” animals, such as insects, spiders, scorpions, and crustaceans), for example, a small change in the Hox genes can multiply the number of segments or reduce them and switch one appendage (e.g., a leg) on each segment with another (e.g., a crab claw or an antenna or mouth parts). Arthropods are a classic example of this modular development with interchangeable parts; with a small change in Hox genes, whole new body plans can easily evolve to exploit new resources.

FIGURE 4.6. Map of the locus of action of the Hox genes in the fly and in the mouse. Note that the basic Hox genes are similar in almost all bilaterally symmetrical animals, so the system goes back to the very origin of complex animals. Small changes in any of these Hox genes make big differences in body plans. (Illustration by Carl Buell)
All of these ideas are part of the exciting new research field known as “evolutionary development” (nicknamed “evo/devo”), and it is now the hottest topic in evolution. From the neo-Darwinian insistence on every gene gradually changing to make a new species, we now realize that only a few key regulatory genes need to change to make a big difference, often in a single generation. This circumvents many of the earlier problems with ideas about macroevolution and makes it entirely possible that the processes that build new body plans and allow organisms to develop new ecologies are not the small-scale microevolutional changes extrapolated upward. Some evolutionary biologists still see evo/devo as just an extension of the neo-Darwinian synthesis (e.g., Carroll 2005), but others argue that it is an entirely different type of process than neo-Darwinists envisioned in the 1950s (e.g., Gould 1980a, 2002).
When Gould wrote his 1980 article, “Is a New and More General Theory of Evolution Emerging?,” he was pointing to these provocative new ideas (heterochrony, regulatory genes, homeotic mutants, and the stability of species through time as shown by punctuated equilibrium). He argued that the neo-Darwinian synthesis, with its emphasis on tiny changes in the genotype adding up to new species by microevolutionary change, was not sufficient to explain macroevolution but that these new developments showed how macroevolution could occur.
There are many hard-core neo-Darwinians who do not agree, of course, so evolutionary biology is in an interesting, controversial time where new ideas are being intensely debated. It may turn out that we understand less about how evolution works than we thought we did back during the heyday of the synthesis in the 1950s and 1960s. But the important point is that this is how normal science operates. Even if we knew nothing about the mechanisms that drive evolution, it would not change the factual data that show it has occurred and still is occurring (as discussed in the next section). We still don’t know exactly how gravity works, but it does not change the fact that objects still fall to the ground. We may never know completely how evolution works, but life keeps evolving. And to repeat our earlier point: even if “neo-Darwinism is dead” (as the creationists like to misquote Gould), that’s only one possible explanatory mechanism for evolution. Neo-Darwinism is not all there is to evolution. Evolution happened in the past and is still happening right now.
Well, evolution is a theory. It is also a fact. And facts and theories are different things, not rungs in a hierarchy of increasing certainty. Facts are the world’s data. Theories are structures of ideas that explain and interpret facts. Facts do not go away when scientists debate rival theories to explain them. Einstein’s theory of gravitation replaced Newton’s, but apples did not suspend themselves in mid-air, pending the outcome. And humans evolved from apelike ancestors whether they did so by Darwin’s proposed mechanism or by some other, yet to be discovered…. Scientists regard debates on fundamental issues of theory as a sign of intellectual health and a source of excitement. Science is—and how else can I say it?—most fun when it plays with interesting ideas, examines their implications, and recognizes that old information might be explained in surprisingly new ways. Evolutionary theory is now enjoying this uncommon vigor. Yet amidst all this turmoil no biologist has been lead to doubt the fact that evolution occurred; we are debating how it happened. We are all trying to explain the same thing: the tree of evolutionary descent linking all organisms by ties of genealogy. Creationists pervert and caricature this debate by conveniently neglecting the common conviction that underlies it, and by falsely suggesting that evolutionists now doubt the very phenomenon we are struggling to understand.
—Stephen Jay Gould, “Evolution as Fact and Theory”
How can we talk about “the fact that evolution has occurred”? On what evidence can we make that statement? As we saw in chapter 1, scientists must use the word fact cautiously, as a description of nature, an observation, or hypothesis that has accumulated so much overwhelming evidence without falsification that it is a fact in common everyday parlance. As Gould (1981) put it,
Moreover, “fact” does not mean “absolute certainty.” The final proofs of logic and mathematics flow deductively from stated premises and achieve certainty only because they are not about the empirical world. Evolutionists make no claim for perpetual truth, though creationists often do (and then attack us for a style of argument that they themselves favor). In science, “fact” can only mean “confirmed to such a degree that it would be perverse to withhold provisional assent.” I suppose that apples might start to rise tomorrow, but the possibility does not merit equal time in physics classrooms.
In this sense, the idea that life has evolved and is evolving is “confirmed to such a degree that it would be perverse to withhold provisional assent.” We see life evolving all around us, and we have abundant evidence that it has done so in the past. Creationists may put religious blinders on and refuse to face reality, but to the unbiased observer, the fact of evolution is as clear as the fact that the sun rises in the east or that objects fall to the earth. Some “evolution deniers” cannot live with this reality, but their denial won’t stop viruses and bacteria from evolving new ways to attack us.
Ironically, the evidence that life had evolved was accumulating long before Darwin, as we saw with our discussion of faunal succession (chapter 3), Chambers’s premature efforts to document evolution in 1844 (this chapter), or the evidence that Philip Henry Gosse tried to explain away with his Omphalos hypothesis (chapter 1). But the great strength of Darwin’s book is that it accomplished two different functions: it laid out a huge amount of evidence that life had evolved, thereby establishing the fact of evolution; and it proposed a mechanism for how it had occurred (the “theory” of evolution), which was Darwinian natural selection. As we have just discussed, Darwin’s mechanism is still being debated as to whether it explains everything, but Darwin’s evidence that life has evolved is still valid. Much new evidence has accumulated in the past 150 years that Darwin could only have dreamed about. What was Darwin’s evidence? Why does it demand an evolutionary explanation? How does creationism fail to explain it?
The first line of evidence had been emerging ever since the days of the Linnaean classification of animals in 1758, a full century before On the Origin of Species. The purpose of Linnaeus’s classification scheme was to document God’s handiwork by discovering the “natural system” of classification that God had used. Inadvertently, Linnaeus stumbled on an obvious fact of nature: each group (such as a species) of animals and plants clusters with other groups into larger groups (called taxa in the plural, taxon in the singular), such as a genus or order, and those higher-level supergroups cluster into even larger groups (such as classes or phyla) with additional taxa. For example, humans are part of a taxon (the family Hominidae) that also includes chimps, gorillas, orangutans, and gibbons. The apes, in turn, cluster together with the Old World monkeys (family Cercopithecidae) and New World monkeys, lemurs, and bush babies into a larger group, the order Primates. The primates are clustered with cows, horses, lions, bats, and whales in the class Mammalia. The mammals are clumped with fishes, birds, reptiles, and amphibians in the subphylum Vertebrata. Together with sponges, corals, mollusks, and other invertebrates, the vertebrates are part of the kingdom Animalia. The natural system for arranging and classifying life is a hierarchical system of smaller groups clustered into larger groups, which is best represented as a branching tree of life.
By Darwin’s time, this branching pattern of life was even more strongly supported and led many people toward the notion that life had undergone a branching pattern of evolution (although not as boldly as Darwin suggested it). All of this was deduced by comparison of features visible to the naked eye or simple magnifier, primarily in the anatomy of the organism. But not even Darwin could have dreamed that the genetic code of every cell in your body also shows the evidence of evolution. Whether you look at the genetic sequence of mitochondrial DNA or nuclear DNA or cytochrome c or lens alpha crystallin or any other biomolecule, the evidence is clear: the molecules show the same pattern of nested hierarchical similarity that the external anatomy reveals (fig. 4.7). Our molecules are most similar to those of our close relatives, the great apes, and progressively less similar to those more distantly related to us.
FIGURE 4.7. Branching diagram of the similarities in cytochrome c among various organisms. Nearly every biochemical system shows a similar branching pattern, which is identical to the branching pattern of life during its evolution. (From Fitch and Margoliash 1967. Copyright © 1967 American Association for the Advancement of Science. Reprinted with permission.)
Teasing out the details of this molecular similarity shows us a simple fact: every molecular system in every cell reveals the fact that life has evolved! If we were specially created and unrelated to the apes, why would we share over 98 percent of our genome with the chimpanzee and progressively less shared genome with primates who are less closely related to us? If God created it to look that way, then we are back on the “deceptive God” problem that faced Gosse’s Omphalos hypothesis. No, the simplest interpretation is that the molecules tell the truth: life has a common origin and exhibits a branching pattern of ancestry and descent.
As comparative anatomy became a science in the early 1800s, anatomists were struck by how animals were constructed. Organisms with widely differing lifestyles and ecologies used the same basic building blocks in their anatomy but had modified those parts in remarkably different ways. For example, the basic vertebrate forelimb (fig. 4.8) has the same basic elements: a single large bone in the upper arm (the humerus), a pair of two long bones in the forearm (the radius and ulna), a number of wristbones (carpals and metacarpals), and multiple bones (phalanges) supporting five digits (fingers). But look at the wide array of ways that some animals use this basic body plan! Whales have modified them into a flipper, while bats have extended the fingers out to support a wing membrane. Birds also developed a wing, but in an entirely different way, with most of the hand and wrist bones reduced or fused together, and feather shafts providing the wing support instead of finger bones. Horses have lost their side toes and walk on one large finger, the middle finger. None of this makes any sense unless these animals inherited a standard body plan in place from their distant ancestors and had to modify it to suit their present-day function and ecology. These common elements (bones, muscles, nerves) that serve different functions despite being built from the same basic parts are known as homologous structures. For example, the finger bones of a bat wing are homologous with our finger bones, and so on.
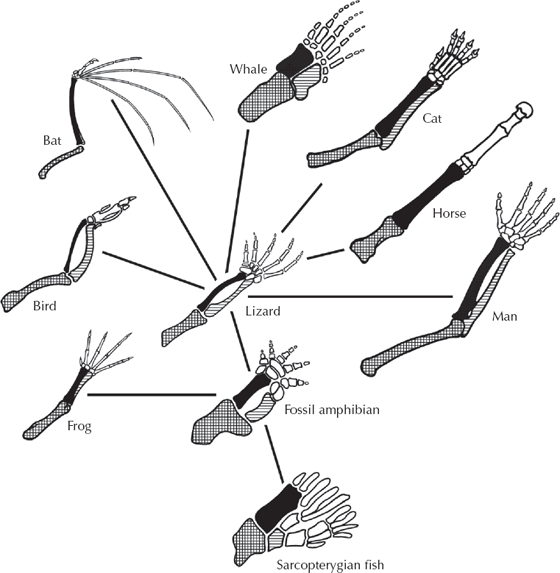
FIGURE 4.8. The evidence of homology. All vertebrate forelimbs are constructed on the same basic plan with the same building blocks, even though they perform vastly different functions. The basic vertebrate forelimb has been modified into flippers in whales, a wing in bats, and a one-fingered running hand in horses, yet the basic bony structure remains the same. (Drawing by Carl Buell)
If the system had been divinely created by an “intelligent designer,” why would there be this underlying similarity? A good engineer would create all wings the same best possible way from scratch, rather than jury-rig the structure using bones that the animal inherited from its ancestors. In fact, nature uses a variety of nonhomologous ways to build a wing. We have already seen how vertebrates build wings in two completely different ways, even though bats and birds started out with the same bones from a common ancestor—and neither of their solutions resembles the wing of a pterodactyl (which is supported only by the bones of the fourth finger), let alone the completely different structure of the wings of an insect. The flippers of a whale may perform the same function as the paddles of a marine reptile or the fins of a fish, but all three structures with a common function have completely different bony structures. These different types of wings and flippers found in unrelated organisms are analogous organs that perform the same function but have a fundamentally different structure. The fact is that organ systems are jury-rigged with whatever bones the animal inherited from its ancestors and not built from scratch in the optimal shape for its current use. This only makes sense if life had evolved to use what anatomy is already available.
In chapter 2, we already alluded to the fact that organs that are remnants of past structures but no longer serve a function were evidence against an intelligent designer. The list of such vestigial organs (fig. 4.9) is overwhelming. They include not only the appendix, tonsils, and tailbones of humans (none of which have a function now), but the tiny splint bones in the feet of horses, which are remnants of the time when horses had three toes. When these bones break, the horse is crippled for life. Whales and snakes both have tiny hips and thigh bones buried deep in their bodies, with no function whatsoever. Why would they have these features unless they evolved from ancestors that did have hind limbs? Any creationist attempt to explain these remnants of ancient history falls into the same trap that Gosse did: a God who would plant these vestiges of the past is a deceiver, tricking us by making it look like life evolved.

FIGURE 4.9. The evidence from vestigial organs. (A) Both whales and snakes retain tiny remnants of their hind legs and hip bones in their bodies, although they are normally not visible externally, nor do they have any function. These facts only make sense if whales and snakes had four-legged ancestors. Horses also retain vestiges of their ancestral side toes, known as splint bones. (B) Close-up of the hip regions of a mounted fin whale skeleton, showing the tiny vestigial hip bones and thigh bones. (Photo by the author) (C) In 1921, Roy Chapman Andrews documented a specimen of a humpback whale that actually had atavistic hind limbs that extended from its body. These are the bones of those hind limbs. (From Andrews 1921)
A related issue is all the organ systems that are poorly or suboptimally designed, or jury-rigged so they work just well enough for the organism to survive. We discussed these in chapter 2 (especially the panda’s thumb and the fishing lures used by the anglerfish and the clam Lampsilis). Once again, these organ systems do not look like they were intelligently designed and only make sense if the organism must use whatever building blocks its ancestors provided.
Even before Darwin, the studies of embryos began to provide important evidence for evolution. In the 1830s, the great German embryologist Karl Ernst von Baer documented that the embryos of all vertebrates show a common pattern (fig. 4.10). Whether they develop into fish, amphibians, or humans, all vertebrate embryos start out with a long tail, well-developed gill slits, and many other fishlike features. In adult fish, the tail and gills develop further, but in humans, they are lost during further development. Von Baer was simply trying to document how embryos developed, not to provide evidence of evolution, which had not yet been proposed.
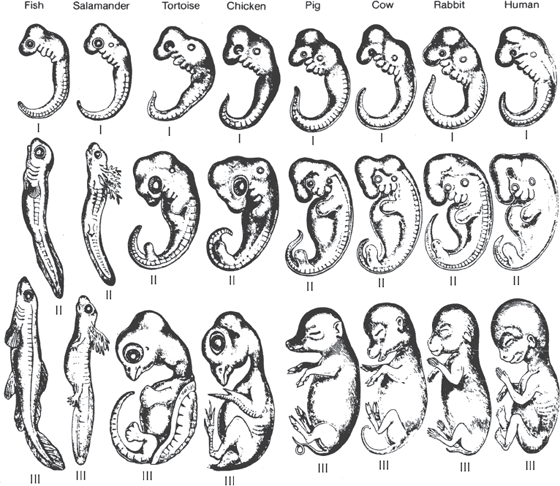
FIGURE 4.10. The evidence from embryology. As embryologist Karl Ernst von Baer pointed out in the 1830s, long before Darwin published his ideas about evolution, all vertebrates start out with a very fishlike body plan early in embryology, including the predecessors of gills and a long tail. As they develop, many lose their fishlike features on their way to becoming reptiles, birds, and mammals. (From Romanes 1910)
Darwin used this evidence in On the Origin of Species, and embryology soon developed into one of the growth fields of evolutionary biology. One of the foremost advocates of evolution was the flamboyant German embryologist Ernst Haeckel. He not only promoted Darwinism in Germany, but he went so far as to argue that we could see all details of evolutionary history in embryos and reconstruct ancestors from embryonic stages of living animals. His most famous slogan, the “biogenetic law,” was “Ontogeny recapitulates phylogeny.” This is simply a fancy way of saying embryonic development (“ontogeny”) repeats (“recapitulates”) evolutionary history (“phylogeny”). To the limited extent that von Baer had shown 40 years earlier, this is true. But embryos also have many unique features (yolk sac, allantois, amniotic membranes, umbilical cords) that have nothing to do with the evolutionary past and are adaptations to their developmental environment. Thus it is dangerous to overextend the evolutionary implications of the stages in an embryo, but they are useful guides nonetheless.
Creationists such as Jonathan Wells (2000), in their eternal effort to mislead the uninitiated and miss the forest for the trees, will crow about how the biogenetic law has been discredited. But Haeckel’s overenthusiasm does not negate the careful embryological work of von Baer that shows that many features of our past evolutionary stages are preserved in our embryos. Wells, in particular, nags about how some of Haeckel’s original diagrams had errors and oversimplifications, but this does not change the overall fact that the sequence of all vertebrate embryos shows the same patterns in the early stages, and all of them go through a fishlike stage with pharyngeal pouches (which become the gill slits in fishes and amphibians) and a long fish-like tail, then some develop into fishes and amphibians and others lose these features and develop into reptiles, birds, and mammals.
If you had any doubts that you once had ancestors with fish-like gills and a tail, figure 4.11 shows what you looked like 5 weeks after fertilization. Why did you have pharyngeal pouches (predecessors of gills) and a tail if you had not descended from ancestors with those features?
FIGURE 4.11. This is what you looked like 5 weeks after conception. You still had many fishlike features, such as a well-developed tail and the embryological precursors of gill slits, both of which are lost in most human embryos as they develop. (From IMSI Photo Images, Inc.)
As was pointed out in chapter 3, the great expeditions of discovery in the 1700s and 1800s soon produced a huge diversity of animals and plants that were unfamiliar to Europeans and not anticipated by the authors of either the Noah’s ark story or Linnaeus in 1758. Not only do these animals and plants render any version of the Noah’s ark story impossible, but they created another problem as well. They are not distributed around the earth in pattern from Mount Ararat in Turkey (the supposed landing site of the ark) but instead have their own unique distribution patterns that only make sense in light of evolution.
Darwin got a hint of this on the Galapagos Islands, where each island had a slightly different species of giant tortoise or finch. Instead of populating the islands with the same species as occurred on the mainland, apparently God had seen fit to put new, unique species on each island (and this phenomenon is true of islands in general). Later studies of the unusual animals of exotic places further confirmed the fact that these far-flung locations were largely populated with unique animals found nowhere else, and their distribution patterns made no sense in the context of migration from the ark. For example, Australia is home to its own unique fauna of native pouched mammals, or marsupials (fig. 4.12). These include not only the familiar kangaroo and koala, but many other creatures that evolved to fill the same ecological niches that placental mammals occupy on other continents. There are marsupial equivalents of placental wolves, cats, flying squirrels, groundhogs, anteaters, moles, and mice. If the animals had all migrated away from the ark, why had nothing but marsupials arrived in Australia and then apparently evolved to fill the niches left vacant by the lack of placentals?
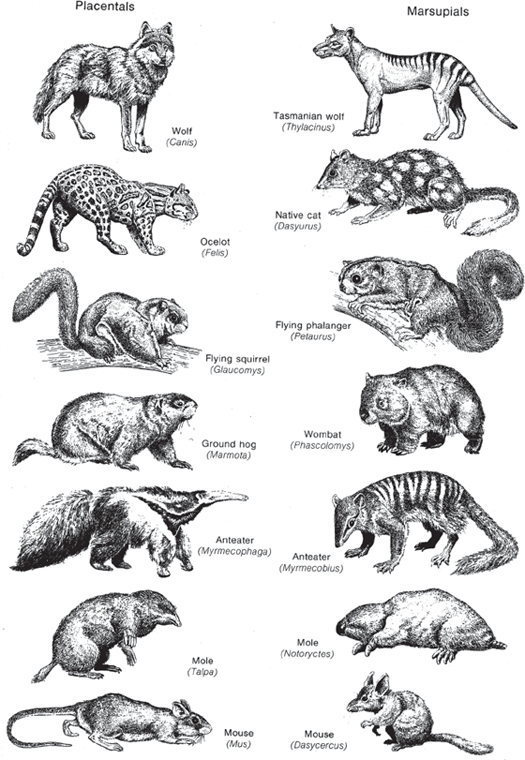
FIGURE 4.12. The evidence from biogeography. The native fauna of Australia consists mainly of pouched marsupials, which have converged remarkably on their placental counterparts from other continents, even though the two groups are not closely related. In Australia, there are marsupials that look vaguely like wolves, cats, flying squirrels, groundhogs, anteaters, moles, and mice—but they are all pouched mammals. (Modified from Simpson and Beck 1965)
Other patterns are equally persuasive. For example, many of the southern continents have at least one flightless bird species, all members of the primitive group known as ratites. Africa has the ostrich, South America the rhea, Australia the cassowary and emu, and New Zealand the kiwi. This distribution makes no sense in the Noah’s ark story but does fit the idea that they were closely related when all these southern landmasses were part of the great Gondwana supercontinent about 100 million years ago. Since the time that these continents have drifted apart, so too have their ratite natives diverged from one another.
Finally, the fossil record provides the details of how life has evolved and is now the strongest piece of evidence for evolution. The remaining chapters of this book will detail the incredible evolutionary stories revealed by fossils, so we will not discuss them further here.
These were all lines of evidence that Darwin mustered in 1859 and they have only grown stronger in the past 150 years with the accumulation of more details and examples. Each alone is strong evidence that life has evolved and is impossible to explain by creationism, and added together they make the case overwhelming. But we have even better evidence: we can see life evolving today, so evolution is as much an observed fact of nature as the fact that the sky appears blue.
Nothing in biology makes sense except in the light of evolution.
—Theodosius Dobzhansky, 1973
Biologists finally began to realize that Darwin had been too modest. Evolution by natural selection can happen rapidly enough to watch. Now the field is exploding. More than 250 people around the world are observing and documenting evolution, not only in finches and guppies, but also in aphids, flies, graylings, monkeyflowers, salmon and sticklebacks. Some workers are even documenting pairs of species—symbiotic insects and plants—that have recently found each other, and observing the pairs as they drift off into their own world together like lovers in a novel by D. H. Lawrence.
—Jonathan Weiner, “Evolution in Action”
If the evidence mustered by Darwin and many other scientists in the past 150 years was not enough to prove that life has evolved, there is an even simpler test: watch life evolve right now! Creationists try to discredit evolution by saying that it all happened in the past; they seem unaware that it continues to happen all around us, even as I write this.
We can see natural selection operating on many different scales and on many different types of organisms. The details of many of these recent studies are provided in Jonathan Weiner’s excellent book, The Beak of the Finch: A Story of Evolution in Our Time (1994) or David Mindell’s The Evolving World: Evolution in Everyday Life (2006). Looking over the shoulders of the hundreds of hardworking, dedicated, self-sacrificing biologists who spend years enduring harsh conditions in the field to observe evolution in action inspires admiration in us real scientists. This is in sharp contrast with the creationists who sit in their comfortable homes and write drivel about subjects they have never studied and do not understand.
The classic example, of course, has long been the finches of the Galapagos Islands (fig. 4.13). Darwin himself collected many of them when he was there in 1835, but they were all so different that he did not notice that the diverse birds he had shot were all finches with highly modified beaks and different color patterns. It was not until he got back to England that ornithologist John Gould (recruited to study Darwin’s specimens) pointed this out to him. In the twentieth century, David Lack did a much more detailed study published in 1947. In recent years, Darwin’s finches have been the focus of research of Peter and Rosemary Grant of Princeton University. The Grants visited the islands year after year, documenting the changes in the finch populations. On one island (Daphne Major), the finch population changed dramatically from year to year. During a 1977 drought, the finches with strong beaks survived, because they could crack the toughest seeds and survive the shortage of food. The next few years, all of the finches on that island were their descendants and had the stronger nut-cracking bills found on other species of Galapagos finches. Since that time, the return of wetter conditions has changed the finches yet again, so forms that have more normal beaks for eating a wide variety of seeds could also survive. From this, it is easy to see how such strong selection pressures could transform the ancestral finches (which still live in South America) into a wide variety of specialized finches that perform the roles that other birds play on the mainland. Instead of nuthatches, there are thick-billed finches; instead of woodpeckers, there are finches with long bills for drilling wood and probing for grubs; instead of warblers, there are finches with similar bills called warbler finches. One finch has even learned to use a twig as a tool for fishing for insects in the hollows of trees! Recent research has identified the genes that control beak shape in these finches and artificially duplicated the pattern seen in nature by adding or subtracting those genes.

FIGURE 4.13. Although Darwin didn’t notice this while he was in the Galapagos, the majority of the birds are finches, which have evolved from a generalized finch ancestor blown over from South America into birds with a wide variety of bills for nutcracking, probing for insects, picking up tiny seeds, and many other tasks performed by different families of birds on the mainland. (Modified from Lack 1947; used by permission)
We don’t have to live in the Galapagos Islands to see evolution happen. We can see evolution in action in our own backyards. The common European house sparrow is found all over North America today, but it is an invader, brought from Europe in 1852. The initial populations escaped and quickly spread all over North America, from the northern boreal forests of Canada down to Costa Rica. We know that members of the ancestral population were all very similar because they were introduced from a few escaped immigrants. Because they have spread to the many diverse regions of North America, they are rapidly diverging and on the way to becoming many new species. House sparrows now vary widely in body size, with more northern populations being much larger than those that live in the south. This common phenomenon, known as Bergmann’s rule, is due to the fact that larger, rounder bodies conserve heat better than smaller bodies. House sparrows from the north are darker in color than their southern cousins, perhaps because dark colors help absorb sunlight and light colors are better at reflecting it in warm climates. Many other changes in wing length, bill shape, and other features have been documented.
New species can arise even faster than people once thought. A study by Andre Hendry at McGill University in Montreal analyzed the sockeye salmon near Seattle (fig. 4.14). These salmon tend to breed either in lakes or in streams and have different shapes dependent on the environment in which they breed. In the 1930s and 1940s, sockeye salmon were introduced to Lake Washington east of Seattle and rapidly became established in the mouth of the Cedar River. By 1957, they had also colonized a beach called Pleasure Point. In less than 40 years, these two populations have rapidly diverged. The males of populations that live in the swift-flowing waters of Cedar River are more slender to fight the strong currents; the females are bigger so they can dig deeper holes for their eggs to prevent the river from eroding them away. The populations that live in the warmer, quieter waters of the lakeshore near Pleasure Point have males with deeper, rounder bodies, which are better at fending off rivals for mating privileges, and females with smaller bodies because they do not have to dig deep holes for their eggs. These populations are genetically isolated and already show the differences that would be recognized as separate species in most organisms. Hendry was able to show that this species split started in less than 40 years, and in just a few more generations, they might be genetically isolated and become distinct species.
FIGURE 4.14. Evolution has happened in some groups over just a few decades. In the swift currents of Cedar River, Washington, the sockeye salmon introduced in the 1930s have adapted so that the males can swim in the strong currents and the females dig deeper nests in the sand to lay their eggs. But the salmon that invaded the shallow waters of Pleasure Point in 1957 have developed rounder, deeper bodies in males to help fight off rival males; the females dig shallower nests for their eggs because there are no strong currents. (Modified from Weiner 2005)
Another rapidly evolving fish is the three-spined stickleback (fig. 4.15). Sticklebacks that live in the ocean have heavier body armor than those that live in lakes. In one pond near Bergen, Norway, biologists have been able to document this change in less than 31 years. In Loberg Lake, Alaska, the change took only a dozen years, or just six generations. Sticklebacks also change their spines in response to local conditions. In open water, longer spines are an advantage because they protect against being swallowed by predators. But in shallow water, the long spines are a liability because they make it easier to be captured by dragonfly larvae, which have long pincers. A single Hox gene, Pitx1, turns off and on the switches that regulate spine length. Other studies have shown that when captive sticklebacks are artificially modified so they have unusual new combinations of spines, the females only mate with the males that have new traits, so sexual selection is a driving force in their evolution of novelty. Prior to these studies, ichthyologists would readily assign specimens with different spine counts and different body armor to different species, but these studies show just how easy it is for one stickleback population to transform to another species given the right conditions.
FIGURE 4.15. The three-spined stickleback fish also show rapid evolution. Species that live in the lakes or in the ocean have longer spines that make it harder for a predator to swallow them. But sticklebacks living in shallow streams have evolved shorter spines so predators like dragonfly larvae cannot catch them by their protruding spines. (Photos courtesy D. M. Kingsley and S. Carroll)
There are the classic cases of industrial melanism, so familiar from every textbook. The peppered moth, Biston betularia, normally has a speckled appearance that blends in well with mottled appearance of trunks and branches of trees. During the Industrial Revolution, soot in the air made the tree trunks black, and the normal form was conspicuous. Instead, a dark-colored mutant became dominant, because they were well camouflaged against the dark tree trunks, while the birds picked off the normal speckled varieties. When environmental regulations cleaned up the air and eliminated the sooty tree trunks, the normal speckled varieties returned, and the dark mutants were again selected against.
Examples like these could be multiplied endlessly. In New England, the periwinkles dramatically changed their shell shape and thickness in less than a century, probably due to predation pressure by newly introduced crabs. In the Bahamas, the anole lizards (the common “chameleon” in the pet shops, which are not true chameleons) changed the proportions of their hind limbs after people introduced them to new islands with different vegetation. In Florida, the soapberry bug evolved a significantly longer beak in response to the invasion of its habitat by a nonnative plant with larger fruits. In Hawaii, the honeycreeper birds evolved shorter bills as their favorite food source, the native lobelloids, has disappeared, and the birds switched to another source of nectar. In Nevada, the tiny mosquito fish that lives in isolated desert water holes that were once connected during the last ice age quickly evolved major differences in less than 20,000 years. And in Australia, the introduced wild rabbits (brought by European settlers less than a century ago) modified their body weight and ear size in response to the different conditions of the outback.
Humans are often the strongest agents of selection for many wild animals. In populations of bighorn sheep, trophy hunters killed off most of the rams with spectacular horns, so the smaller males with reduced horns had a better chance of breeding, and the population no longer has many large-horned rams. Rattlesnakes that are too nervous and buzz when humans approach are quickly killed, so in many regions, the rattlers no longer give any warning. Overfishing of the Atlantic cod led to a population crash during the 1980s, and large cod nearly vanished; those that bred quickly while they were small and immature had a better chance of survival.
But the most dramatic and rapid examples of evolution in action occur with microorganisms, especially viruses and bacteria. Every year doctors have new flu strains to battle, because last year’s cold and flu strains have evolved new protein coats that make them unrecognizable to our immune systems and allow them to infect us again. This is why there will never be a cure for the common cold—the virus evolves too fast for any drug to keep up. The heavy use of antibiotics has selected for strains of bacteria that are resistant to every drug we throw at them. When sulfonamides were introduced in the 1930s, resistant strains evolved in only a decade. Penicillin was introduced in 1943, and there were resistant strains by 1946. For this reason, doctors are now much more cautious about issuing antibiotics to sick patients who want the drugs even though they are useless against the viruses that cause cold and flu. Similarly, the heavy use of antiseptic cleansers and wipes has led to strains of bacteria that can resist most antiseptics. Many medical researchers think that our excessive cleanliness in the Western world is to our disadvantage because young people are no longer exposed to many different kinds of germs, and they become vulnerable when a strong strain (that doesn’t infect people in the “dirty” Third World) invades. Now hospitals are worried—when one of these drug-resistant strains appears in a hospital, it can quickly spread to many patients and nothing can stop it.
Likewise, many insects and weeds have evolved resistance to pesticides and herbicides, all within a few decades, causing enormous economic damage to people all over the world. Every modern housefly now carries the genes that make it resistant not only to DDT, but also to pyrethroids, dieldrin, organophosphates, and carbamates, so there are few pesticides left that can suppress them. The mosquitoes that evolved resistance to DDT and other organophosphate insecticides apparently evolved in Africa during the 1960s, spread on to Asia, then reached California by 1984, Italy in 1985, and France in 1986. As entomologist Martin Taylor describes it (in Weiner 1994:255),
It always seems amazing to me that evolutionists pay so little attention to this kind of thing, and that cotton growers are having to deal with these pests in the very states whose legislatures are so hostile to the theory of evolution. Because it is the evolution itself they are struggling against in their fields every season. These people are trying to ban the teaching of evolution while their own cotton crops are failing because of evolution. How can you be a creationist farmer any more?
Evolution is happening all around us. It happens every time a new germ invades your body, a new pest or weed destroys our crops, or a new insecticide-resistant fly or mosquito bites you. Creationists may get some personal comfort from their beliefs, but they cannot change the fact that life is evolving all around us and threatens our survival if we don’t come to terms with that evolution (see fig. 1.3).
For Further Reading
Campbell, J. 1982. Autonomy in evolution, in Perspectives on Evolution, ed. R. Milkman. Sunderland, Mass.: Sinauer, 190–200.
Carroll, S. 2005. Endless Forms Most Beautiful: The New Science of Evo/Devo. New York: Norton.
Desmond, A., and J. Moore. 1991. Darwin: The Life of a Tormented Evolutionist. New York: Warner.
Eldredge, N. 1985. Unfinished Synthesis. New York: Oxford University Press.
Gould, S. J. 1977. Ontogeny and Phylogeny. Cambridge, Mass.: Harvard University Press.
Gould, S. J. 1980. Is a new and more general theory of evolution emerging? Paleobiology 6:119–130.
Gould, S. J. 1982. Darwinism and the expansion of evolutionary theory. Science 216:380–387.
Gould, S. J. 2002. The Structure of Evolutionary Theory. Cambridge, Mass.: Harvard University Press.
Levinton, J. 2001. Genetics, Paleontology, and Macroevolution. 2nd ed. New York: Cambridge University Press.
Mindell, D. P. 2006. The Evolving World: Evolution in Everyday Life. Cambridge, Mass.: Harvard University Press.
Ridley, M. 1996. Evolution. 2nd ed. Cambridge, Mass.: Blackwell.
Schwartz, J. 1999. Sudden Origins: Fossils, Genes, and the Emergence of Species. New York: Wiley.
Stanley, S. M. 1979. Macroevolution: Patterns and Process. New York: Freeman.
Stanley, S. M. 1981. The New Evolutionary Timetable. New York: Basic.
Steele, E.. 1979. Somatic Selection and Adaptive Evolution: On the Inheritance of Acquired Characters. Chicago: University of Chicago Press.
Steele, E., R. Lindley, and R. Blanden. 1998. Lamarck’s Signature: How Retrogenes Are Changing Darwin’s Natural Selection Paradigm. Reading, Mass.: Perseus.
Weiner, J. 1994. The Beak of the Finch: A Story of Evolution in Our Own Time. New York: Knopf.
Weiner, J. 2005. Evolution in action. Natural History 115(9):47–51.
Wesson, R. 1991. Beyond Natural Selection., Cambridge, Mass.: MIT Press.
Wills, C. 1989. The Wisdom of the Genes: New Pathways in Evolution. New York: Basic.















