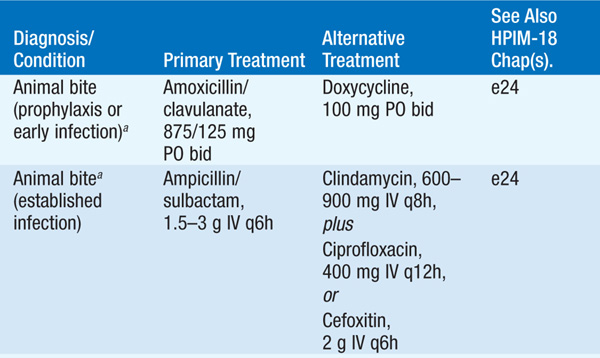
Skin and soft tissue infections are diagnosed principally by a careful history (e.g., temporal progression, travel, animal exposure, bites, trauma, underlying medical conditions) and physical examination (appearance of lesions and distribution). Treatment of common skin infections is summarized in Table 93-1; parenteral treatment is usually given until systemic signs and symptoms have improved. Types of skin lesions include the following:
TABLE 93-1 TREATMENT OF COMMON INFECTIONS OF THE SKIN




1. Vesicles: due to proliferation of organisms, usually viruses, within the epidermis (e.g., VZV, HSV, coxsackievirus, poxviruses, Rickettsia akari)
2. Bullae: caused by toxin-producing organisms. Different entities affect different skin levels; for example, staphylococcal scalded-skin syndrome and toxic epidermal necrolysis cause cleavage of the stratum corneum and the stratum germinativum, respectively. Bullae are also seen in necrotizing fasciitis, gas gangrene, and Vibrio vulnificus infections.
3. Crusted lesions: Impetigo caused by either Streptococcus pyogenes (impetigo contagiosa) or Staphylococcus aureus (bullous impetigo) usually starts with a bullous phase before development of a golden-brown crust. Crusted lesions are also seen in some systemic fungal infections, dermatophytic infections, and cutaneous mycobacterial infections. It is important to recognize impetigo contagiosa because of its relation to poststreptococcal glomerulonephritis.
4. Folliculitis: Localized infection of hair follicles is usually due to S. aureus. “Hot-tub folliculitis” is a diffuse condition caused by Pseudomonas aeruginosa. Freshwater avian schistosomes cause an allergic reaction after penetrating hair follicles, resulting in “swimmer’s itch.”
5. Papular and nodular lesions: Raised lesions of the skin occur in many different forms and can be caused by Bartonella (cat-scratch disease and bacillary angiomatosis), Treponema pallidum, human papillomavirus, mycobacteria, and helminths.
6. Ulcers, with or without eschars: can be caused by cutaneous anthrax, ulceroglandular tularemia, plague, and mycobacterial infection. Ulcerated lesions on the genitals can be caused by chancroid (painful) or syphilis (painless).
7. Erysipelas: abrupt onset of fiery red swelling of the face or extremities, with well-defined indurated margins, intense pain, and rapid progression. S. pyogenes is the exclusive cause.
• Pathogenesis Bacteria gain access to the epidermis through breaks in the skin, whether accidental (e.g., cuts, scratches, burns) or iatrogenic (e.g., surgical incisions, IV catheters). The expanding area of erythema may be due to extracellular toxins and/or the host immune response rather than to increasing bacterial numbers.
• Microbiology Etiologic causes include commensal flora (e.g., S. aureus, S. pyogenes) or a wide variety of exogenous flora. With the latter, a thorough history and epidemiologic data may help identify the cause.
– Examples of exogenous bacteria causing cellulitis include the following: Pasteurella multocida after a cat or dog bite; Capnocytophaga canimorsus after a dog bite; Eikenella corrodens after a human bite; P. aeruginosa in association with ecthyma gangrenosum in neutropenic pts, a penetrating injury (stepping on a nail), or hot-tub folliculitis; Aeromonas hydrophila after a laceration sustained in fresh water; or Erysipelothrix rhusiopathiae after contact with domestic swine and fish.
• Clinical manifestations This acute inflammatory condition of the skin is characterized by localized pain, erythema, swelling, and heat.
– Cellulitis due to S. aureus often spreads from a central site of localized infection, such as an abscess or an infected foreign body.
– S. pyogenes can cause a rapidly spreading, diffuse process, often with fever and lymphangitis.
• Diagnosis If there is drainage, an open wound, or an obvious portal of entry, Gram’s staining and culture may identify the etiology. Aspiration or biopsy of the leading edge of the cellulitic tissue yields a diagnosis in only 20% of cases.
• Treatment See Table 93-1.
• Pathogenesis Infection, either apparent or inapparent, results from a breach in integrity of the skin or mucous membrane barriers and can be associated with malignancy, a diverticulum, hemorrhoids, or an anal fissure.
– In the case of infections with no obvious portal of entry, transient bacteremia is thought to seed sites of nonpenetrating trauma (e.g., bruise, muscle strain).
– Infection spreads to the deep fascia and along fascial planes through venous channels and lymphatics.
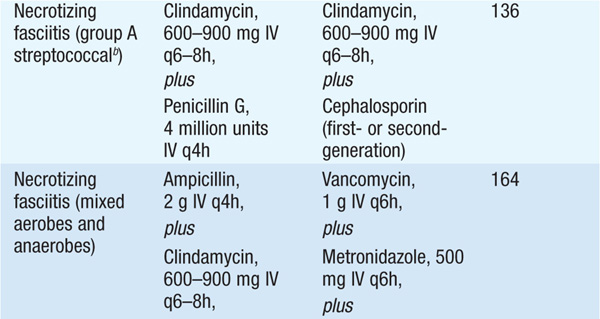
• Microbiology Necrotizing fasciitis is caused by S. pyogenes, mixed aerobic and anaerobic bacteria, or Clostridium perfringens; methicillin-resistant S. aureus (MRSA) strains that produce the Panton-Valentine leukocidin have also been reported as an occasional cause.
• Clinical manifestations The timing of cutaneous manifestations (e.g., violaceous bullae; friable, necrotic skin; induration; brawny edema) depends on whether the infection began superficially (rapid onset) or in deeper structures (slower onset).
– Early in the disease course, severe pain and unexplained fever may be the only findings.
– Thrombosis of blood vessels in dermal papillae leads to ischemia of peripheral nerves and anesthesia of the affected area.
– In later stages, pts appear toxic and often develop shock and multiorgan failure.
• Diagnosis Diagnosis is based on clinical presentation. Other findings may include gas detected in deep tissues by imaging studies (particularly with clostridial species but rarely with S. pyogenes) and markedly elevated serum creatine phosphokinase levels (in the case of concomitant myositis).
• Treatment Emergent surgical exploration to deep fascia and muscle, with removal of necrotic tissue, is essential. Table 93-1 provides recommendations for adjunctive antibiotic therapy.
• Clinical manifestations and microbiology Infections involving the muscle have differing manifestations, depending on the etiology.
– Myositis: can be caused by bacteria (clostridia, streptococci), viruses (influenza virus, dengue virus, coxsackievirus), or parasites (Trichinella, Taenia solium, Toxoplasma). This condition usually manifests with myalgias, but pain can be severe in coxsackievirus, Trichinella, and bacterial infections.
– Pyomyositis: a localized muscle infection usually due to S. aureus, common in tropical areas, and typically with no known portal of entry.
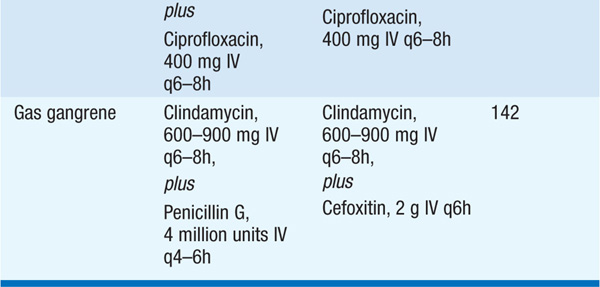
– Myonecrosis: can be caused by clostridial species (C. perfringens, C. septicum, C. histolyticum, C. sordellii) or by mixed aerobic and anaerobic bacteria. Myonecrosis is usually related to trauma; however, spontaneous gangrene—usually due to C. septicum—can occur in pts with neutropenia, GI malignancy, or diverticulosis. Myonecrosis of the uterus, typically due to C. sordellii, occurs in women after spontaneous or medically induced abortion and in healthy postpartum women; infection is rapidly and almost uniformly fatal as there are few or no localizing clinical findings.
• Diagnosis and Treatment Emergent surgical intervention to visualize deep structures, obtain materials for culture and sensitivity testing, remove necrotic tissue, and reduce compartment pressure is both diagnostic and therapeutic.
– Empirical antibiotic treatment should target likely etiologies—e.g., vancomycin (1 g IV q12h) for pyomyositis and ampicillin/sulbactam (2–3 g IV q6h) for mixed aerobic-anaerobic infections.
– For treatment of clostridial myonecrosis (gas gangrene), see Table 93-1.
• Pathogenesis Joints become infected by hematogenous seeding (the most common route), by spread from a contiguous site of infection, or by direct inoculation (e.g., during trauma or surgery). Acute bacterial infection can rapidly destroy articular cartilage as a result of increased intraarticular pressure and the elicited host immune response.
• Microbiology The predominant etiologic agents differ with the pt’s age; S. aureus is the most common nongonococcal isolate in adults of all ages.
– In children <5 years old, S. aureus, S. pyogenes, and Kingella kingae predominate.
– In young adults, Neisseria gonorrhoeae is the most common etiology.
– In adults, S. aureus predominates, but gram-negative bacilli, pneumococci, and β-hemolytic streptococci are involved in one-third of cases in older adults.
– Other causes of septic arthritis include Borrelia burgdorferi (Lyme disease), tuberculosis and other mycobacterial infections, fungal infections (e.g., coccidioidomycosis, histoplasmosis), and viral infections (e.g., rubella, mumps, hepatitis B, parvovirus infection).
• Epidemiology and clinical manifestations The risk factors and presentation differ depending on whether N. gonorrhoeae is the cause.
– Nongonococcal bacterial arthritis: Risk is increased in pts with rheumatoid arthritis, diabetes mellitus, glucocorticoid therapy, hemodialysis, malignancy, and IV drug use.
• In 90% of pts, one joint is involved—most often the knee, which is followed in frequency by the hip, shoulder, wrist, and elbow; IV drug users often have spinal, sacroiliac, or sternoclavicular joint involvement.
• Pts have moderate to severe pain, effusion, decreased range of motion, and fever.
– Gonococcal arthritis: Women are 2–3 times more likely than men to develop disseminated gonococcal infection (DGI) and arthritis, particularly during menses and during pregnancy (see Chap. 92).
• DGI presents as fever, chills, rash, and articular symptoms (migratory arthritis). The cutaneous and articular findings result from an immune reaction to circulating gonococci and immune-complex deposition, so that synovial fluid cultures are consistently negative.
• In true gonococcal arthritis (which always follows DGI), a single joint (hip, knee, ankle, or wrist) is usually involved.
– Prosthetic joint infections: complicate 1–4% of joint replacements and are usually acquired intra- or perioperatively.
• Acute presentations are seen in infections caused by S. aureus, pyogenic streptococci, and enteric bacilli.
• Indolent presentations are seen in infections caused by coagulase-negative staphylococci and diphtheroids.
– Reactive arthritis: follows ~1% of cases of nongonococcal urethritis and 2% of enteric infections (e.g., Yersinia enterocolitica, Shigella flexneri, Campylobacter jejuni, Salmonella spp.). Only a minority of pts have the other classic findings associated with reactive arthritis, including urethritis, conjunctivitis, uveitis, oral ulcers, and rash.
• Diagnosis If there is concern about joint infection, examination of synovial fluid from the affected joint is essential. There is considerable overlap in the cell counts due to different etiologies, but synovial fluid culture and examination for crystals (to rule out gout and pseudogout) can help narrow the diagnosis.
– Normal synovial fluid contains <180 cells (mostly mononuclear)/μL. Acute bacterial infection of joints results in synovial fluid cell counts averaging 100,000/μL (range, 25,000–250,000/μL), with >90% PMNs. Synovial fluid in gonococcal arthritis contains >50,000 cells/μL, but results of Gram’s staining are usually negative, and cultures of synovial fluid are positive in <40% of cases. Other mucosal sites should be cultured to diagnose gonorrhea. Pts with septic arthritis due to mycobacteria or fungi can have 10,000–30,000 cells/μL in synovial fluid, with 50–70% PMNs. Synovial fluid cell counts in noninfectious inflammatory arthritides are typically 30,000–50,000/μL.
– Gram’s staining of synovial fluid should be performed, and injection directly into blood culture bottles can increase the yield of synovial fluid cultures.
– Blood cultures are positive in 50–70% of cases due to S. aureus but are less commonly positive with other organisms.
– Plain radiographs show soft tissue swelling, joint space widening, and displacement of tissue planes by distended capsule. Narrowing of the joint space and bony erosions suggest advanced disease.
TREATMENT Infectious Arthritis
• Drainage of pus and necrotic debris is needed to cure infection and to prevent destruction of cartilage, postinfectious degenerative arthritis, and joint deformity or instability.
• A third-generation cephalosporin (cefotaxime, 1 g IV q8h; or ceftriaxone, 1–2 g IV q24h) provides adequate empirical coverage for most community-acquired infections in adults when smears demonstrate no organisms. Vancomycin (1 g IV q12h) should be used to cover the possibility of MRSA when there are gram-positive cocci on the smear.
– In IV drug users and other susceptible pts, treatment for gram-negative organisms such as P. aeruginosa should be considered.
– If a pathogen is identified by culture, treatment should be adjusted according to the specific bacterial organism and its antibiotic susceptibility.
• Treatment for S. aureus should be given for 4 weeks, that for enteric gram-negative bacilli for 3–4 weeks, and that for pneumococci or streptococci for 2 weeks. Treatment of gonococcal arthritis should commence with ceftriaxone (1 g/d) until improvement; the 7-day course can be completed with an oral fluoroquinolone (e.g., ciprofloxacin, 500 mg bid). If fluoroquinolone resistance is not prevalent, a fluoroquinolone can be given for the entire course.
• Prosthetic joint infections should be treated with surgery and high-dose IV antibiotics for 4–6 weeks. The prosthesis often has to be removed; to avoid joint removal, antibiotic suppression of infection may be tried. A 3- to 6-month course of ciprofloxacin and rifampin has been successful in S. aureus prosthetic joint infections of relatively short duration, although prospective trials confirming the efficacy of this regimen are still needed.
• Pathogenesis Osteomyelitis is typically caused either by direct spread from a contiguous focus of infection or by hematogenous spread. Areas of bone or contiguous surrounding tissue that have abnormal viability, blood supply, sensation, or edema are at increased risk for bacterial infection. Bacteria can colonize and persist in these areas, partly because of the decreased immunosurveillance resulting from compromised blood flow and—in the case of some organisms, such as S. aureus—elaboration of bacterial adhesins and toxins.
• Epidemiology In the U.S., 0.1–1.8% of otherwise healthy adults are affected by acute osteomyelitis; 30–40% of adults with diabetes develop osteomyelitis after a foot puncture. Orthopedic surgery (particularly with implantation of hardware), obesity, diabetes, trauma, bacteremia, poor circulation, and older age are risk factors for osteomyelitis.
• Microbiology Table 93-2 lists organisms that cause osteomyelitis.
TABLE 93-2 MICROORGANISMS THAT CAUSE OSTEOMYELITIS
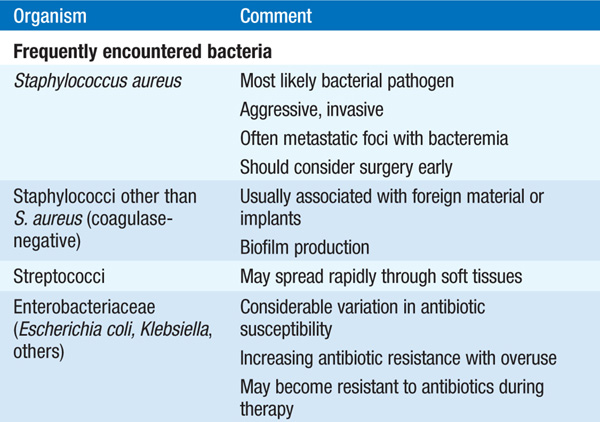

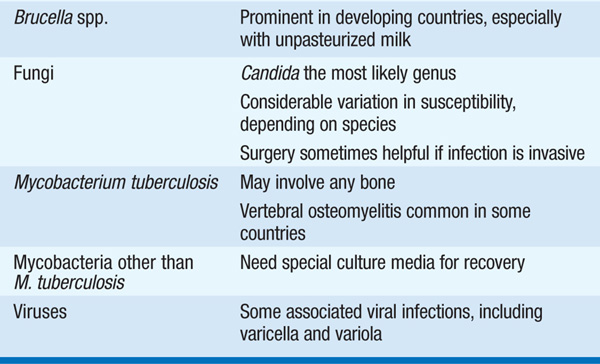
– S. aureus is the most common cause.
– The overlapping circulations of the urinary tract and the spine may be a source of vertebral osteomyelitis due to urinary tract pathogens such as Escherichia coli and Klebsiella.
• Clinical manifestations Pts generally have a febrile illness, with localized pain and tenderness. A history of surgery or trauma in the affected region—even in the remote past—should raise suspicion.
• Diagnosis Radiographic studies and occasionally invasive sampling of lesions are needed to confirm the diagnosis.
– X-rays of the affected region may demonstrate bone loss, sequestra, periosteal elevation, or swelling, but many of these findings may become apparent only after the infection has continued for several weeks.
– CT and especially MRI scans offer increased sensitivity in detecting osteomyelitis.
– Needle aspiration or biopsy of lesions allows histologic confirmation of disease and may permit identification of the etiologic agent.
• Treatment Table 93-3 lists antibiotics for the treatment of osteomyelitis due to common pathogens. Empirical antibiotic therapy should target staphylococci and often includes cefazolin or an antistaphylococcal penicillin (oxacillin or nafcillin).
TABLE 93-3 ANTIBIOTICS FOR THE TREATMENT OF OSTEOMYELITIS
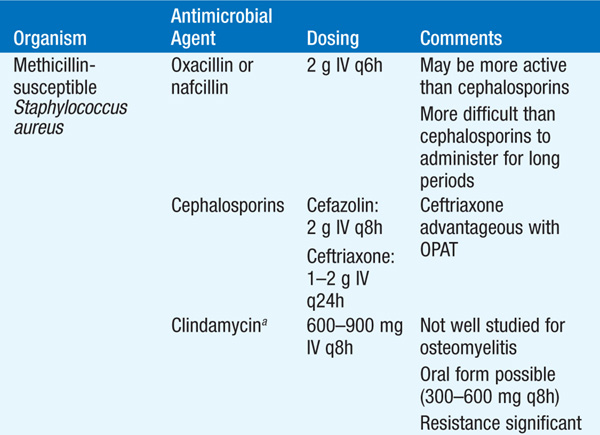

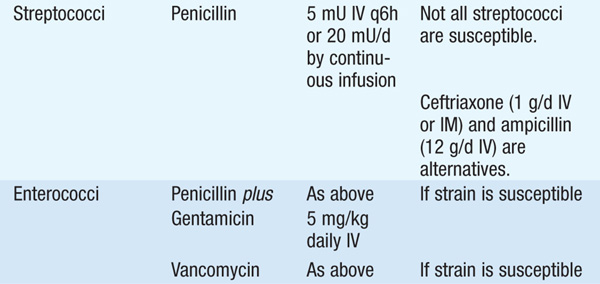
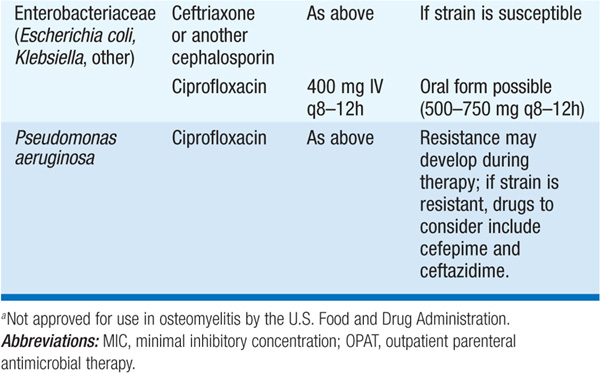
– The optimal route and duration of therapy remain controversial, but a 4- to 6-week course of IV therapy is the usual recommended minimum; pediatric studies are providing increasing evidence that shorter courses and oral agents may be adequate.
– Serial measurement of inflammatory markers (ESR, C-reactive protein) can serve as a surrogate marker of response to treatment in some infections (particularly in cases due to S. aureus).
– Given the prolonged treatment necessary, outpatient parenteral antibiotic therapy is increasingly being used and is both safe and effective.

For a more detailed discussion, see Stevens DL: Infections of the Skin, Muscles, and Soft Tissues, Chap. 125, p. 1064; Tice AD: Osteomyelitis, Chap. 126, p. 1071; Wang F: Molluscum Contagiosum, Monkeypox, and Other Poxvirus Infections, Chap. 183, p. 1476; and Madoff LC: Infectious Arthritis, Chap. 334, p. 2842, in HPIM-18.