22.3
Amino Acid Metabolism
22.3.1 Introduction
The biosynthesis of amino acids and their polymers (i.e., proteins) essential for yeast growth represents a major nitrogen sink during fermentation. Proteins are necessary for catalysis of key metabolic pathways (enzymes), and glycoproteins act as a structural component of yeast cell walls (mannoproteins). As compared to other nutrients such as carbohydrates, sulfur, and phosphorus, grape must usually contains low concentrations of nitrogen in a form that is assimilable (i.e., able to be incorporated) by yeast. Thus, available nitrogen is often a limiting factor for both yeast growth and fermentation rate, and its deficiency can result in suboptimal (sluggish or stuck) fermentations and off‐aroma formation. Winemakers can address this inadequacy to an extent by supplementing musts with nitrogen (usually an inorganic form), but overcorrection may lead to microbial instability, production of toxic compounds, or undesirable changes to wine flavor.
22.3.2 Nitrogen uptake and catabolite repression
As described earlier (Chapter 5) and listed below, grape must contains several classes of nitrogenous compounds, but not all are equally useful to yeast as a nitrogen source under anaerobic conditions [1].
- Ammonium (NH4+) and most primary amino acids (e.g., leucine, glutamate, valine) can be utilized by yeast under fermentation conditions. The sum of these sources (i.e., inorganic nitrogen and free amino nitrogen, FAN) is referred to as yeast assimilable nitrogen (YAN).
- Secondary amino acids (proline and hydroxyproline) are not well utilized under anaerobic conditions because metabolism of these compounds requires oxygen. As an example, <10% proline was consumed as compared to >90% of the primary amino acids in a Chardonnay fermentation [2].
- Proteins and oligopeptides (with the exception of glutathione) are typically not well utilized as nitrogen sources, since S. cerevisiae has low protease activity at wine pH. However, certain yeast strains (particularly spoilage yeast like Candida) possess aspartic acid‐rich proteases with higher activity under acidic conditions [3].
YAN compounds do not enter yeast cells by passive diffusion, but are instead actively taken up through membrane proteins called permeases. These include general amino acid permease (Gap1p), which has a broad affinity for a range of amino acids, and several selective permeases with high affinity for particular nitrogen sources; for example, Mep1p, Mep2p, and Mep3p are selective for NH4+ [4,5]. Although yeast can grow on a large range or inorganic and organic nitrogen sources, not all forms of YAN in must are equally preferred, and different sources will be utilized at different rates [5]. Classically, assignment of preferred versus non‐preferred nitrogen sources was based on relative growth rates or rates of amino acid depletion, but a more modern approach is to classify a nitrogen source as preferred if it decreases the expression of genes necessary for uptake or metabolism of other nitrogen sources, for example, GAP1 [6]. These changes are collectively referred to as nitrogen regulation or nitrogen catabolite repression (NCR) [5].
The preferred sources that cause the strongest NCR effects are asparagine and glutamine, followed by NH4+ and glutamate [6]. The advantage of these nitrogen sources to yeast appears to be related to the fact that they have a central role in amino acid metabolism, and thus require fewer intermediary steps to be utilized for de novo synthesis of amino acids (Figure 22.3.1). More thorough treatments of yeast amino acid synthesis can be found elsewhere [1,5,7], and are summarized here. Briefly, glutamate (85%) and glutamine (15%) serve as the major nitrogen donors for amino acid biosynthesis. These two compounds can be regenerated from α‐ketoglutarate and glutamate, respectively (Figure 22.3.1). Alternatively, other α‐amino acids can act as nitrogen donors to regenerate glutamate from α‐ketoglutarate, as described in the next paragraph. In combination, these reactions function as a continuous cycle to convert generic nitrogen sources into a desired amino acid product by incorporation of nitrogen into carbon skeletons, as described next.

Figure 22.3.1 Schematic overview of nitrogen uptake and metabolism in yeast. 1a, Amino acid permease (e.g., Gap1p); 1b, ammonium permease (Mep1p/2p/3p); 2, aminotransferase (Bat1p/2p, Gdh1p); 3, glutamine synthetase (Gln1p); 4, glutamate synthetase, GOGAT (Glt1p); 5, glutamate dehydrogenase (Gdh2p)
22.3.3 Amino acid anabolism, catabolism, and carbon skeletons
Unlike humans who require certain “essential” amino acids in their diets, yeast can synthesize all amino acids necessary for their function and growth if they are provided with carbon and nitrogen sources and necessary co‐factors. The anabolic pathway for amino acid biosynthesis begins with glucose and leads to a penultimate step of α‐keto acid formation (also called a “carbon skeleton”) [1,5,7]. The α‐keto acid can subsequently accept an amine group from either glutamate or glutamine to form an amino acid (Figure 22.3.2).
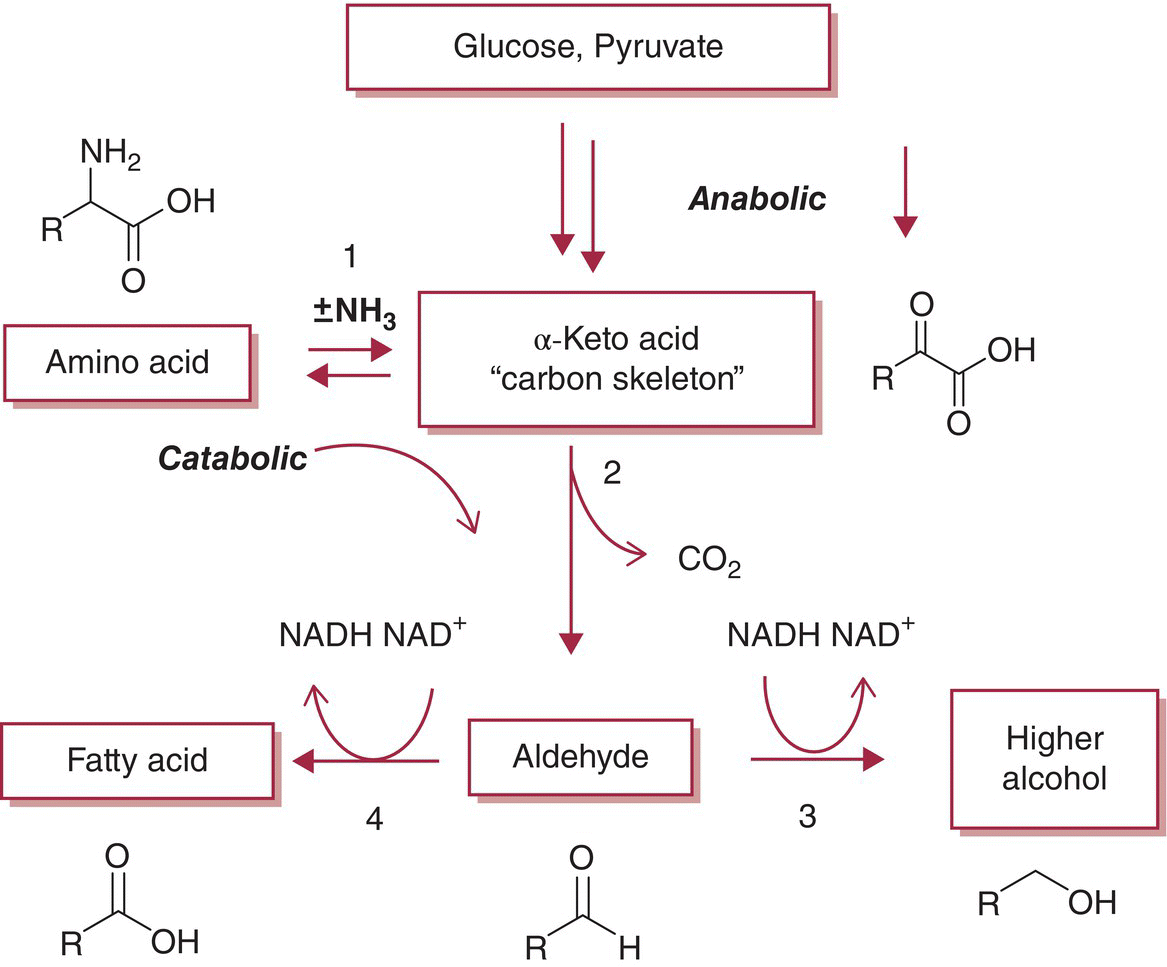
Figure 22.3.2 Schematic of anabolic and catabolic amino acid pathways leading to higher alcohol formation via α‐keto acid carbon skeletons. A small percentage of carbon skeletons (~1%) will also be diverted to form corresponding fatty acids. 1, Aminotransferase (e.g., Bat1/2p); 2, α‐keto acid decarboxylase (e.g., Pdc1p); 3, alcohol dehydrogenase (e.g., Adh1p); 4, aldehyde dehydrogenase (e.g., Ald1p)
The final transamination reaction in Figure 22.3.2 is reversible, and the equilibrium will shift to favor the catabolism of amino acids to corresponding α‐keto acids under nitrogen‐limited conditions [8]. The catabolic pathway liberates nitrogen (e.g., by reforming glutamate), which can then be utilized in the synthesis of different amino acids. The catabolic pathway thus allows yeast to utilize nearly any amino acid as a nitrogen source.
22.3.4 Higher alcohol formation
Both the anabolic and catabolic pathways will generate α‐keto acids, but these compounds are not accumulated in appreciable amounts even under circumstances that limit amino acid formation (e.g., low YAN). Several α‐keto acids including those corresponding to leucine, isoleucine, valine, phenylalanine, and methionine, are metabolized to their corresponding higher alcohols (aka fusel alcohols, Chapter 6) by the pathway shown in Figure 22.3.2.1 Briefly, in an energetically favorable step, α‐keto acids are decarboxylated to their corresponding aldehydes, which can then be enzymatically reduced to form a higher alcohol or enzymatically oxidized to form a carboxylic (fatty) acid [8]. Under anaerobic (reductive) fermentation conditions, the higher alcohol pathway is strongly favored. For example, in a model fermentation using phenylalanine as a YAN source, >99% of phenylpyruvate (α‐keto acid) is diverted to 2‐phenylethanol (higher alcohol) with the remainder forming phenylacetic acid (carboxylic acid) [10]. A review of typical concentrations and sensory properties of higher alcohols is provided earlier in the text (Chapter 6). Several factors are known to affect higher alcohol production as outlined below, and reviewed in more detail elsewhere [1].
- Low YAN increases higher alcohol production by favoring α‐keto acids over amino acids at equilibrium (an example is shown in Figure 22.3.3). Fermentation conditions that stimulate yeast growth but limit nitrogen availability will also increase higher alcohol formation, presumably by increasing demand for amino acid production.
- Yeast strains will differ both in absolute and relative production of higher alcohols. For example, one group observed 2‐fold differences in production of 3‐methyl‐1‐butanol (isoamyl alcohol), 2‐methyl‐1‐propanol (isobutanol), and methionol from the same must (Airén) using three different yeast strains [11].
- High concentrations of a specific precursor amino acid will result in increased formation of the corresponding higher alcohol (Chapter 6) through the catabolic pathway, i.e., using valine as a sole nitrogen source in media will result in formation of 2‐methyl‐1‐propanol as the dominant higher alcohol [12], and methionol concentrations in wine are reported to correlate with methionine in must [13]. However, the concentration of higher alcohols in wine is often weakly correlated with their corresponding amino acid in must [14], and studies with 13C‐labeled tracers indicate that >75% of higher alcohols in wine are formed via the anabolic pathway [15].
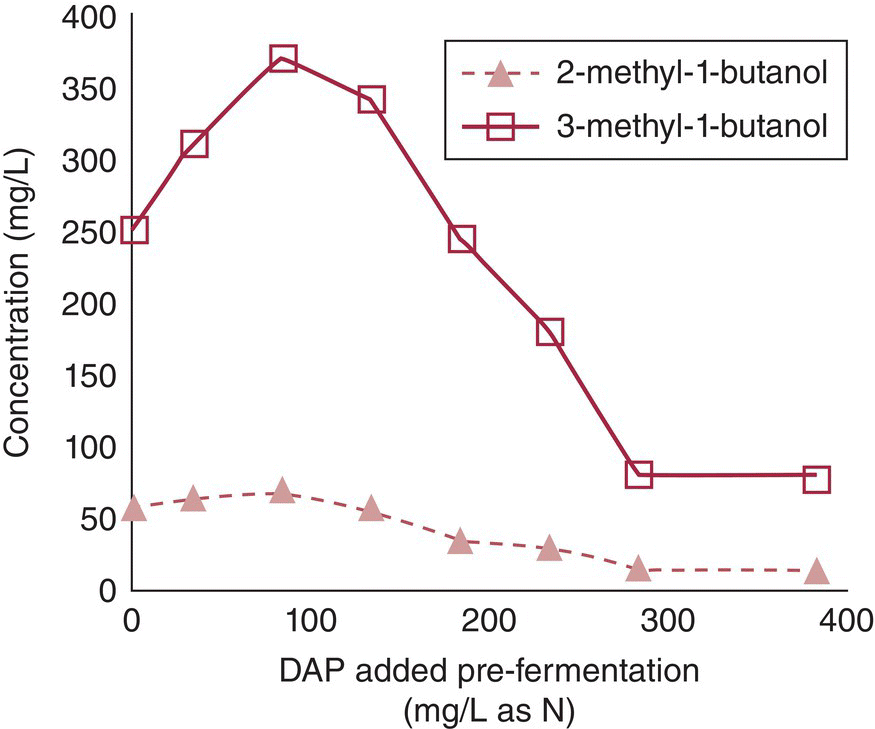
Figure 22.3.3 Effects of increasing YAN on higher alcohol formation. YAN was varied by adding diammonium phosphate (DAP) to a chemically defined medium containing 117 mg/L of YAN prior to fermentation using yeast strain AWRI 796. Other higher alcohols (2‐phenylethanol, 2‐methyl‐1‐propanol) show similar behavior.
Data from Vilanova et al. [16]
The reason that the α‐keto acid carbon skeletons are degraded to higher alcohols rather than recycled to other metabolites is unclear, but several hypotheses have been proposed to explain their benefits to yeast [8]:
- The equilibrium constant associated with the transamination reaction is roughly unity, and removing excess α‐keto acids favors complete deamination of amino acids and thus is more efficient.
- Yeast growth results in excess NADH:NAD+. To restore the redox balance, yeast will divert glycolysis products to glycerol at the expense of ATP production (Chapter 22.1). By using aldehydes formed through the catabolic pathway as electron acceptors, yeast can restore the redox balance without a loss of ATP production.
- Some higher alcohols (e.g., 2‐phenylethanol, tryptophanol) may be needed by yeast for a hypothetical role in quorum sensing and signaling [8].
Finally, in addition to the Ehrlich pathway, two other catabolic pathways are of importance to wine chemistry:
- Sulfur‐containing amino acids, cysteine, and methionine. In this pathway, the C–S bond is cleaved to yield an α‐keto acid, NH4+, and the malodorous H2S or CH3SH, respectively (Chapter 10).
- Arginine can be metabolized to ornithine (a polynitrogenous α‐amino acid) and urea. Under low nitrogen conditions, the urea will subsequently be used by yeast as a nitrogen source. However, in nitrogen‐rich musts, the urea may accumulate and eventually form the possible human carcinogen, ethyl carbamate (Chapter 5).
22.3.5 Acetate ester formation
The formation of acetate esters is distinct from the formation of ethyl esters, which result from fatty acid metabolism (Chapter 22.2). Acetate esters are formed from higher alcohols that can serve as substrates for enzymatic acetylation by acetyl‐CoA. Analogous acetylation reactions can occur for other alcohols, such as grape‐derived 3‐mercaptohexanol (Chapter 10) and 1‐hexanol (Chapter 6). This reaction is catalyzed by acetyltransferase enzymes, particularly Atf1p (Figure 22.3.4). As discussed earlier (Chapter 7), acetate esters typically have fruity or floral aromas and are important contributors to the fermentation aromas of young wines.
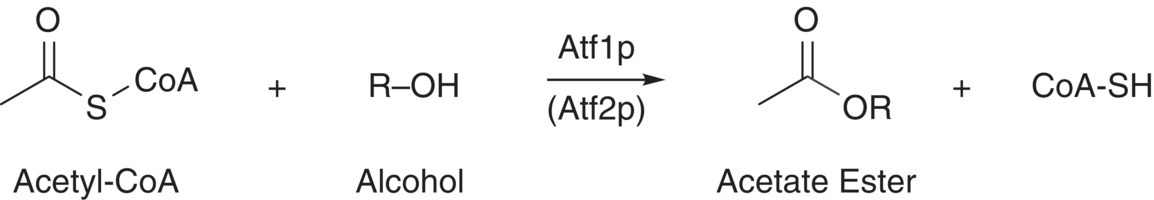
Figure 22.3.4 Reaction depicting formation of acetate esters from acetyl‐CoA and higher alcohols by yeast via acetyltransferase enzymes (Atf1p and Atf2p)
The percentage of alcohol substrate that undergoes acetylation is low, and experiments in which 1‐hexanol, 1‐octanol, and other alcohols were spiked into wines showed that 0.2‐–1.0% were converted to their corresponding acetate esters on a molar basis [17]. Most data indicate that acetate ester production during fermentation is more highly controlled by ATF1 expression than by alcohol substrate availability [18], perhaps because the conversion efficiency is so low. This observation is in contrast to ethyl ester formation, for which fatty acid substrate availability appears to be more important (Chapter 22.2).
Several approaches are available to winemakers to manipulate acetate ester production. Commercial yeast strains differ in acetate ester production, even under identical fermentation conditions, by an order of magnitude [19]. This variation is plausibly due to variation in Atf1p activity, as yeast genetically modified to overexpress ATF1 show comparable order of magnitude changes in acetate ester formation (Figure 22.3.5) [20]. Overexpression of a different acetyltransferase enzyme (Atf2p) or an enzyme associated with ethyl ester formation (Eht1p, Chapter 22.2) resulted in minor increases in acetate ester concentrations. Acetate ester accumulation was decreased in strains expressing high esterase activity, for example, high activity of Iah1p (Figure 22.3.5).
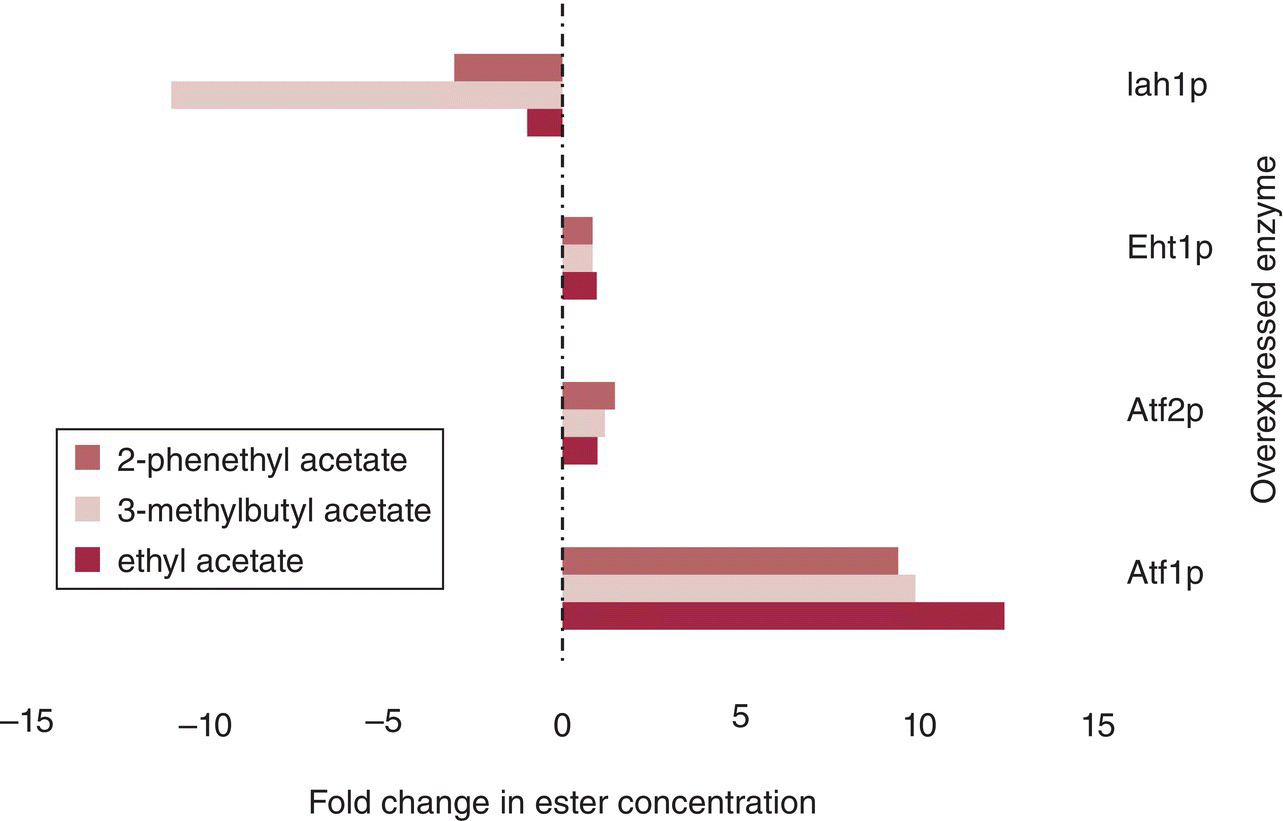
Figure 22.3.5 Effects of overexpression of genes involved in ester metabolism on acetate ester formation by transformed yeast. The largest increases are seen with Atf1p overexpression due to its role in catalyzing the acetylation of corresponding higher alcohols (Figure 22.3.4). Iah1p results in a decrease in acetate esters, likely due to esterase activity.
Data from Lilly et al. [20]
Fermentation conditions may also affect ATF1 expression. Most notably, aeration and unsaturated fatty acid additions are well known to decrease acetate ester formation [14], and these conditions can repress expression by at least four‐fold [21]. High temperatures can also decrease acetate ester formation. Increasing the fermentation temperature from 20 °C to 30 °C reportedly lowers total isoamyl acetate production by 30% [22], and final wine concentrations are decreased by half due to additional losses resulting from increased volatilization at higher temperatures (Chapter 22.1.1). These factors also decrease fatty acid ethyl ester formation (Chapter 22.2), and in part explain the common winemaking practice of clarifying musts, minimizing oxygen exposure, and using cool fermentation temperatures when making fruity white wines.
22.3.6 YAN in the winery – requirements, approaches, and consequences
The effects of lower YAN concentrations on fermentation outcomes are summarized in Table 22.3.1. Typically, lower YAN results in slower fermentation kinetics and higher final sugar concentrations (Figure 22.3.6). While this and many of the other consequences of low YAN (e.g., greater H2S and fusel alcohol production) are undesirable, excessively high YAN in must can result in higher YAN at the end of fermentation (Figure 22.3.6), leading to challenges after alcoholic fermentation is complete (e.g., decreased microbial stability, greater potential for ethyl carbamate or biogenic amine formation). However, because the consequences of insufficient YAN are usually more immediately apparent and problematic to the winemaker (e.g., stuck or sluggish fermentations, off‐aroma formation), it is more common to focus on the minimum necessary amount of YAN. Typical recommendations for YAN are in the order of 150–200 mg/L as N, but targets will vary depending on the criteria used. Concentrations as low as 70–140 mg/L are reportedly necessary to avoid any detectable residual nitrogen, while concentrations of 267 mg/L are recommended to avoid residual sugar at the end of fermentation [1].
Table 22.3.1 Effects of lower YAN on fermentations, summarized from Reference [1]
| Outcome | Effect of lower YAN | Cross reference |
| Hydrogen sulfide production | Increase | Chapter 22.4 |
| Release of thiols via β‐lyase activity | Increase | Chapter 23.2 |
| Fermentation rate and biomass production | Decrease | [26] |
| Higher alcohol production | Increase | This chapter |
| Acetic acid production | Decrease | Chapter 22.1 |
| Ester production | Decrease | This chapter and Chapter 22.2 |
| Ethyl carbamate or biogenic amine potential | Decrease | Chapter 5 |
| Risk of microbial spoilage post‐fermentation | Decrease | [27] |
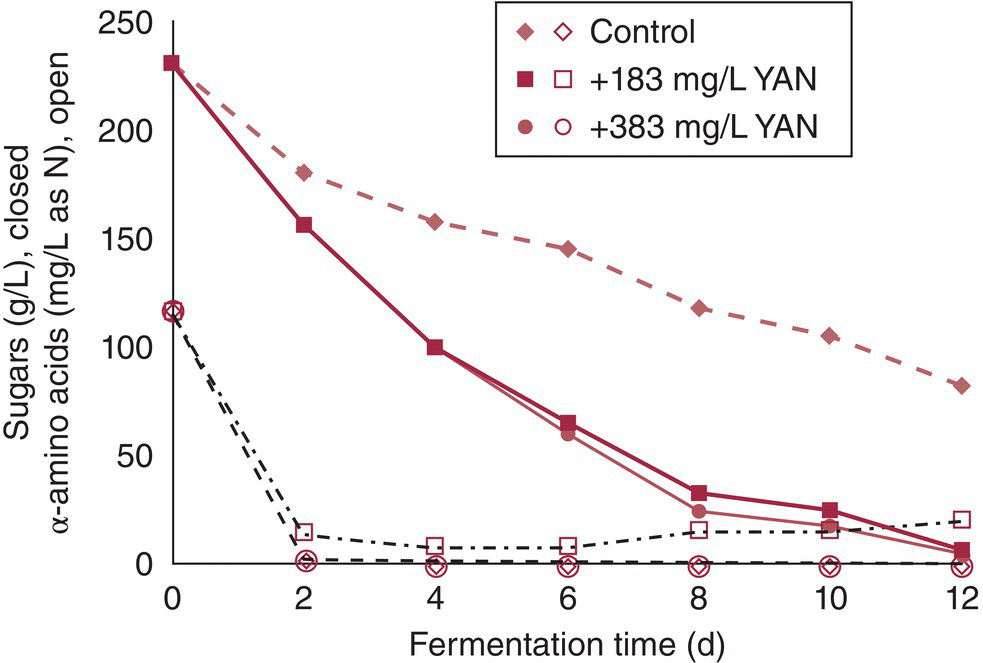
Figure 22.3.6 Effects of DAP supplementation on residual α‐amino acid nitrogen and fermentation kinetics of a synthetic wine‐like media. Fermentations were carried out with AWRI 796 yeast. Closed markers refer to sugar concentrations and open markers refer to α‐amino acid nitrogen. The treatments were: control (diamond, YAN = 117 mg/L N, no DAP addition), + DAP, 183 mg/L as N (circle, total YAN = 300 mg/L), + DAP, 383 mg/L as N (diamond, total YAN = 500 mg/L).
Data from Vilanova et al. [16]
YAN is typically increased by addition of NH4+, generally in the form of diammonium hydrogen phosphate (diammonium phosphate, DAP) [23]. More complex sources containing both amino acids and DAP derived from yeast autolysates are available commercially, although the concentration of amino acids in these preparations is usually less than 10 mg/L and thus likely to be of negligible importance [24]. These additions may be done prior to or after commencement of fermentation, but are recommended to take place in the first half of fermentation since the ability of yeast to take up nitrogen via permeases diminishes in the presence of ethanol [25].
References
- 1. Bell, S.J. and Henschke, P.A. (2005) Implications of nitrogen nutrition for grapes, fermentation and wine. Australian Journal of Grape and Wine Research, 11 (3), 242–295.
- 2. Huang, Z. and Ough, C.S. (1991) Amino‐acid profiles of commercial grape juices and wines. American Journal of Enology and Viticulture, 42 (3), 261–267.
- 3. Theron, L.W. and Divol, B. (2014) Microbial aspartic proteases: current and potential applications in industry. Applied Microbiology and Biotechnology, 98 (21), 8853–8868.
- 4. Beltran, G., Novo, M., Rozes, N., et al. (2004) Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentations. FEMS Yeast Research, 4 (6), 625–632.
- 5. Feldmann, H. (2010) Yeast: molecular and cell biology, Wiley‐VCH, Weinheim.
- 6. Magasanik, B. and Kaiser, C.A. (2002) Nitrogen regulation in Saccharomyces cerevisiae. Gene, 290 (1–2), 1–18.
- 7. Cooper, T.C. (1982) Nitrogen metabolism in Saccharomyces cerevisiae, in The molecular biology of the yeast Saccharomyces: metabolism and gene expression (eds Strathern, J.N., Jones, E.W., Broach, J.R.), Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 39–99.
- 8. Hazelwood, L.A., Daran, J.‐M., van Maris, A.J.A., et al. (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Applied and Environmental Microbiology, 74 (8), 2259–2266.
- 9. Ehrlich, F. (1907) Über die Bedingungen der Fuselölbildung und über ihren Zusammenhang mit dem Eiweissaufbau der Hefe. Ber. Dtsch Chem. Ges., 40, 1027–1047.
- 10. Vuralhan, Z., Morais, M.A., Tai, S.‐L., et al. (2003) Identification and characterization of phenylpyruvate decarboxylase genes in Saccharomyces cerevisiae. Applied and Environmental Microbiology, 69 (8), 4534–4541.
- 11. Hernández‐Orte, P., Ibarz, M.J., Cacho, J., Ferreira, V. (2005) Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chemistry, 89 (2), 163–174.
- 12. Dickinson, J.R., Harrison, S.J., Hewlins, M.J.E. (1998) An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. Journal of Biological Chemistry, 273 (40), 25751–25756.
- 13. Hernández‐Orte, P., Cacho, J.F., Ferreira, V. (2002) Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. Journal of Agricultural and Food Chemistry, 50 (10), 2891–2899.
- 14. Malcorps, P., Cheval, J., Jamil, S., Dufour, J. (1991) A new model for the regulation of ester synthesis by alcohol acetyltransferase in Saccharomyces cerevisiae during fermentation. Journal of the American Society of Brewing Chemists, 49 (2), 47–53.
- 15. Nisbet, M.A., Tobias, H.J., Brenna, J.T., et al. (2014) Quantifying the contribution of grape hexoses to wine volatiles by high‐precision [U13C]‐glucose tracer studies. Journal of Agricultural and Food Chemistry, 62 (28), 6820–6827.
- 16. Vilanova, M., Ugliano, M., Varela, C., et al. (2007) Assimilable nitrogen utilisation and production of volatile and non‐volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Applied Microbiology and Biotechnology, 77 (1), 145‐157.
- 17. Dennis, E.G., Keyzers, R.A., Kalua, C.M., et al. (2012) Grape contribution to wine aroma: production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. Journal of Agricultural and Food Chemistry, 60 (10), 2638–2646.
- 18. Saerens, S.M.G., Delvaux, F.R., Verstrepen, K.J., Thevelein, J.M. (2010) Production and biological function of volatile esters in Saccharomyces cerevisiae. Microbial Biotechnology, 3 (2), 165–177.
- 19. Steensels, J., Meersman, E., Snoek, T., et al. (2014) Large‐scale selection and breeding to generate industrial yeasts with superior aroma production. Applied and Environmental Microbiology, 80 (22), 6965–6975.
- 20. Lilly, M., Bauer, F.F., Lambrechts, M.G., et al. (2006) The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast, 23 (9), 641–659.
- 21. Fujii, T., Kobayashi, O., Yoshimoto, H., et al. (1997) Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Applied and Environmental Microbiology, 63 (3), 910–915.
- 22. Morakul, S., Mouret, J.‐R., Nicolle, P., et al. (2013) A dynamic analysis of higher alcohol and ester release during winemaking fermentations. Food and Bioprocess Technology, 6 (3), 818–827.
- 23. Boulton, R.B., Singleton, V.L., Bisson, L.F., Kunkee, R.E. (1999) Principles and practices of winemaking, Springer, New York.
- 24. Stewart, A.C. and Butzke, C.E. (2011) Assessment of yeast nutrient supplements and residual nitrogen in wine. American Journal of Enology and Viticulture, 62 (3), 390A–391A.
- 25. Sablayrolles, J.‐M., Dubois, C., Manginot, C., et al. (1996) Effectiveness of combined ammoniacal nitrogen and oxygen additions for completion of sluggish and stuck wine fermentations. Journal of Fermentation and Bioengineering, 82 (4), 377–381.
- 26. Bisson, L.F. (1999) Stuck and sluggish fermentations. American Journal of Enology and Viticulture, 50 (1), 107–119.
- 27. Fugelsang, K.C. and Edwards, C.G. (2007) Wine microbiology – practical applications and procedures, Springer, New York.