23.2
S‐Conjugates
23.2.1 Introduction
Varietal thiols are potent impact compounds contributing pleasant tropical and citrus aromas to several varietal wines, most notably Sauvignon Blanc (Chapter 10), but these compounds are largely absent from grape juice. The majority of these thiols appear to be released by yeast enzyme activity cleaving the C–S bonds of non‐volatile sulfur‐containing precursors. Early studies identified conjugates of the sulfur‐containing amino acid cysteine (Cys) and the tripeptide glutathione (GSH) as precursors capable of releasing key thiols 3‐mercaptohexan‐1‐ol (3‐MH) and 4‐mercapto‐4‐methylpentan‐2‐one (4‐MMP)1 during fermentation (Figure 23.2.1) [1–4]. These thiol precursors2 are found in grape skin and pulp at tens to hundreds of µg/L, with grape botrytis infection and ripeness, grape pressing, skin contact, and other processing operations greatly affecting their concentrations in juice (see Table 23.2.1 for some examples). Identical or related S‐conjugates have been found in other natural matrices such as passion fruit, onion, bell pepper, and even human sweat, where they can serve as precursors to potent odorous thiols [5]. Research surrounding varietal thiols and their precursors is still very active, and complete explanations are not yet available. As a result this chapter includes more speculation based on available data than most other chapters.
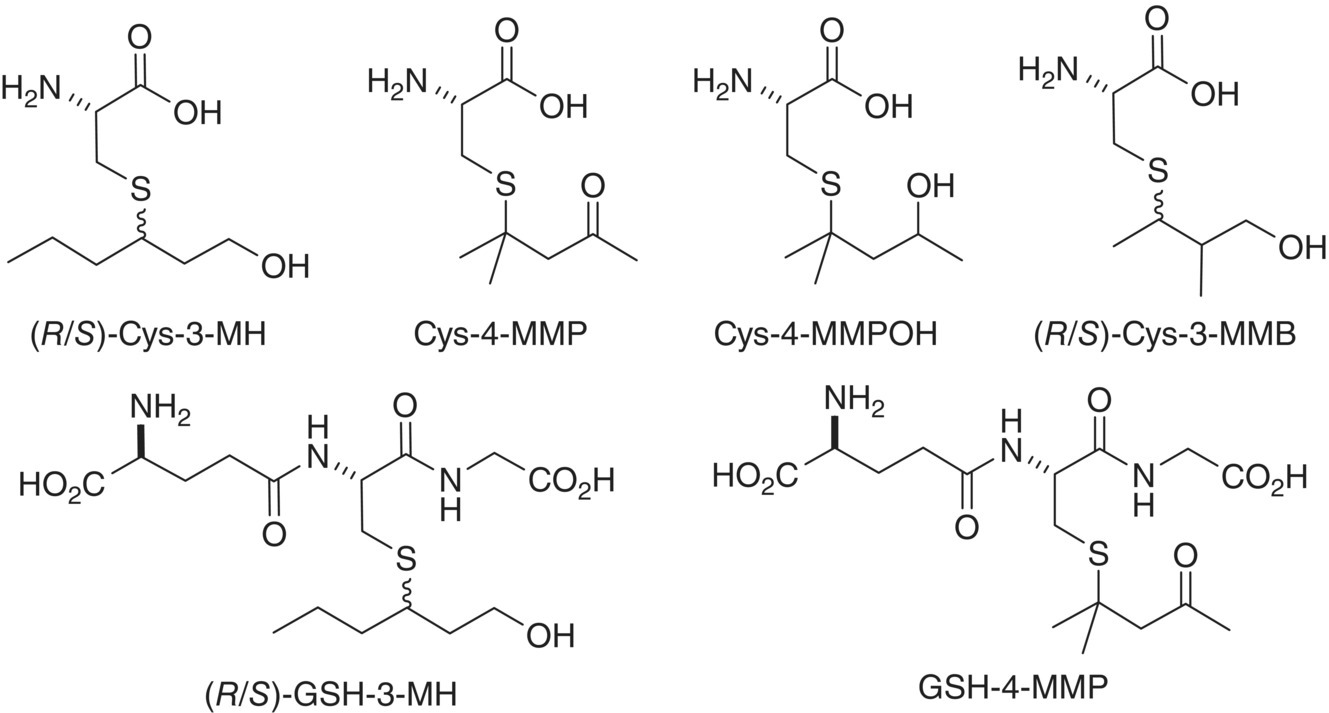
Figure 23.2.1 Varietal thiol‐related cysteine and glutathione conjugates identified in grape juice or wine. MH = mercaptohexan‐1‐ol, MMP = mercapto‐4‐methylpentan‐2‐one, MMPOH = mercapto‐4‐methylpentan‐2‐ol, and MMB = mercapto‐2‐methylbutan‐1‐ol. (R/S) refers to the alkyl chain stereocenter and designates that these chiral compounds are present as pairs of diastereomers. This designation is often omitted but the presence of diastereomers should still be assumed
Table 23.2.1 Viticultural and pre‐fermentation processing effects on Cys‐3‐MH and GSH‐3‐MH concentrations in Sauvignon Blanc berries and juices
| Harvest method | Treatment | Precursor concentration, sum of diastereomers (µg/L) | Interpretation | Study | |
| Cys‐3MH | GSH‐3MHa | ||||
| Hand | –b | 11.7–11.8 | 73.0–77.8 | Hand harvesting tends to yield lower concentrations of precursors due to minimizing both berry damage and precursor extraction | [6] |
| –c | 7.6–57.0 | 33–224 | [7] | ||
| Separated skind | 30 | 32 | Although variable for different thiol precursors, greater amounts are mostly found in the skins of berries | [8] | |
| Separated pulpd | 8 | 24 | |||
| Botrytis 0% | 100 | NR | Botrytis infection stimulates production of precursors by grape berries, in line with a berry damage and detoxification mechanism | [9] | |
| Botrytis 100%, not desiccated | 2188 | NR | |||
| Botrytis 100%, desiccated | 4486 | NR | |||
| As above, picked 1 week later | 3449 | NR | |||
| Veraisone | 0.1–1.5 | 4.8–13.2 | Precursor concentrations increase as grapes ripen, in line with a berry damage and detoxification mechanism | [10] | |
| Two weeks before harveste | 2.9–4.9 | 18.8–41.8 | |||
| Harveste | 27.4–49.7 | 144.3–225.6 | |||
| Machine | No SO2 | 38.1 | 253.7 | Precursor concentrations increase due to transportation of grapes (as a result of berry damage, enzymatic reactions, and maceration), and decrease when high levels of SO2 are added during harvest (due to an inhibitory effect on enzymatic reactions) | [6] |
| 50 mg/L SO2 | 26.2 | 214.8 | |||
| 500 mg/L SO2 | 9.46 | 114.0 | |||
| Transport, no SO2 additionf | 269.0 | 507.8 | |||
| Transport, 50 mg/L SO2f | 213.2 | 440.3 | |||
| Transport, 500 mg/L SO2f | 92.9 | 252.1 | |||
| Free run juicec | 9.6–39.5 | 33–322 | Precursor concentrations increase with skin contact and pressing at higher pressures, in accord with their localization within berry skins | [11] | |
| Pressed to 1 barc | 16.5–111 | 200–541 | |||
| Free run juiceg | 14–18 | NR | |||
| Skin contact 1 hourg | 18–22 | NR | |||
| Pressed to 1.2–1.4 atmg | 31–74 | NR | |||
a NR, not reported.
b Range for two different locations within a single vineyard.
c Range for five different juices from NZ.
d Mean result for three different regions of France. Units are µg/kg of fresh skin or pulp.
e Five different clones co‐located in one vineyard in the Adelaide Hills, Australia. Units are µg/kg of fresh berries.
f Transported 800 km in 12 h from SA to NSW, Australia.
g Range for three different juices from NZ.
23.2.2 Formation of S‐conjugate precursors in berries and juice
S‐conjugate precursors of both 3‐MH and 4‐MMP are well characterized in grapes. In contrast, O‐acetates such as 3‐mercaptohexyl acetate (3‐MHA) do not have a direct grape‐derived precursor; rather, 3‐MHA arises from acetylation of 3‐MH liberated during fermentation (see Section 23.2.3). Formation of both Cys‐ and GSH‐conjugates in grapes likely results from conjugation of GSH to electrophilic α,β‐unsaturated alkenals (e.g., (E)‐2‐hexenal to yield 3‐MH precursors, Figure 23.2.2) or alkenones (e.g., 4‐methylpent‐3‐en‐2‐one [mesityl oxide], giving 4‐MMP precursors) [5, 12, 13].3 GSH conjugation reactions catalyzed by glutathione S‐transferases (GSTs) are well‐known detoxification mechanisms in the plant kingdom [14, 15], as well as among animals.4 The resulting adducts typically show lower reactivity and greater solubility than their precursors. The same role of GSTs in grapevines appears to at least partially explain the presence of S‐conjugates in grapes [16]. Additional S‐conjugates may be formed post‐harvest during grape processing or fermentation [3].
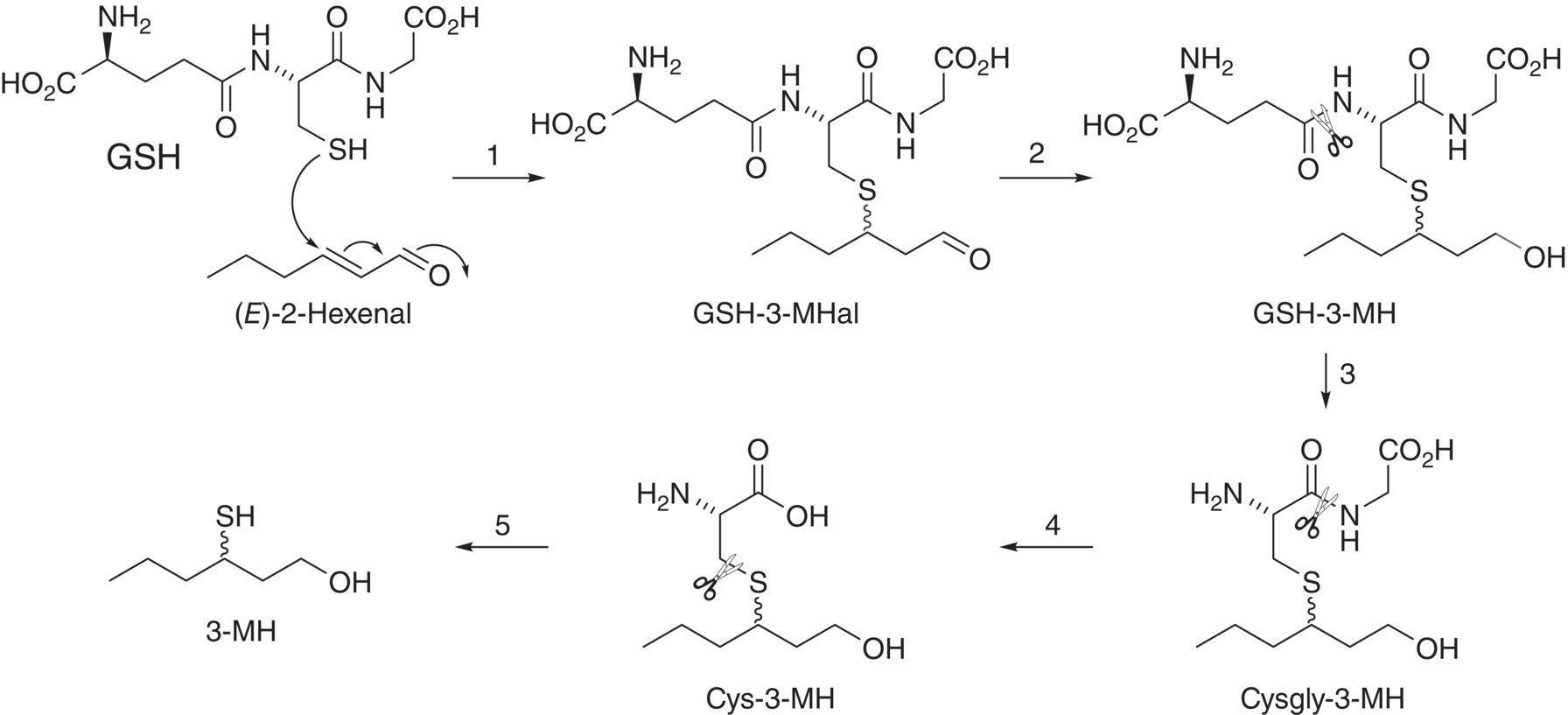
Figure 23.2.2 Formation of thiol precursor GSH‐3‐MH from nucleophilic GSH and electrophilic (E)‐2‐hexenal, and degradation to yield other precursors and varietal aroma compound 3‐MH in wine. Steps: 1 = conjugation by glutathione S‐transferase (possibly non‐enzymatic); 2 = enzymatic carbonyl reduction; 3 = removal of glutamyl residue by γ‐glutamyltranspeptidase; 4 = removal of glycine residue by peptidases; 5 = release of varietal thiol by carbon–sulfur lyase. Note that the precise order of carbonyl reduction relative to cleavage of amino acids is unknown.
Metabolism of GSH‐conjugates is well studied in other plants [17], and it appears that some of these metabolites also form in grape berries. The tripeptide GSH is enzymatically hydrolyzed to first yield the dipeptide conjugate (Cysgly) followed by the Cys conjugate [18]. There is strong evidence that these steps also occur in grapes (or juice), as these conjugates have been detected for 3‐MH [19, 20]. Intermediates necessarily undergo other enzymatic transformations, such as reduction of an aldehyde to yield the corresponding alcohol (Figure 23.2.2).
The concentrations of GSH‐3‐MH and Cys‐3‐MH are strongly affected by both growing and processing conditions (e.g., see Reference [2]), as outlined in Table 23.2.1. In brief:
- Hand harvesting leads to lower precursor concentrations compared to mechanical harvesting.
- Precursor concentrations increase as a result of disease, grape maturity, and transportation of harvested fruit.
- Precursors are found in grape skins and their concentrations increase with skin contact and pressure used during pressing to juice.
23.2.3 Conversion of S‐conjugate precursors during fermentation
Unlike glycosylated precursors, which can liberate key odorants through both enzymatic and non‐enzymatic pathways, S‐conjugates appear to only release volatile thiols through enzymatic mechanisms, primarily during fermentation [2, 21] (Figures 23.2.2 and 23.2.3). This release requires two steps:
- S‐conjugate precursor uptake into the cell.
- Precursor cleavage by carbon–sulfur (C‐S) lyases (i.e. β‐lyase enzymes).
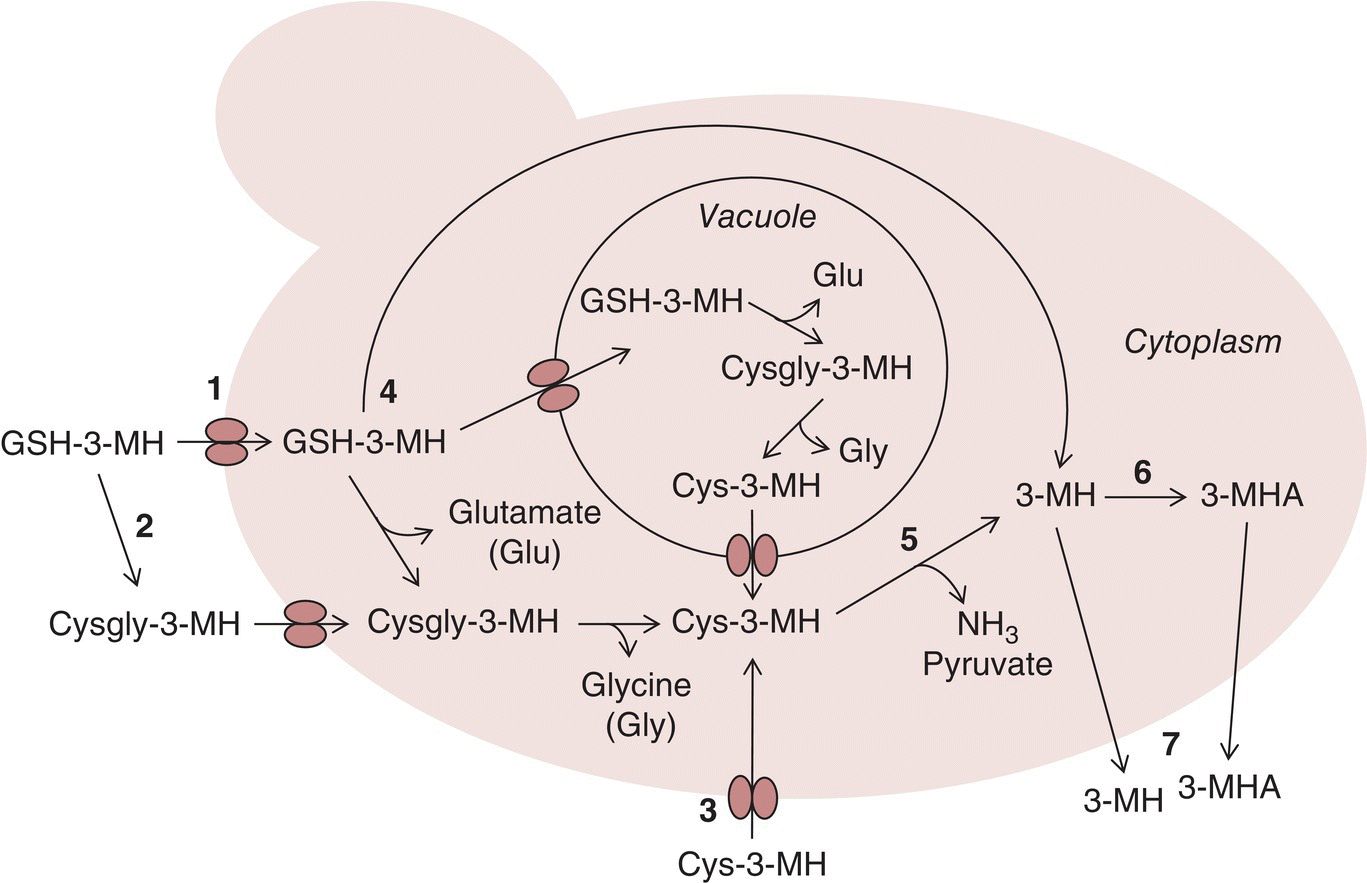
Figure 23.2.3 Representation of potential pathways for 3‐MH liberation from grape‐derived GSH‐3‐MH, Cysgly‐3‐MH, and Cys‐3‐MH present in juice, and conversion to 3‐MHA during fermentation based on information compiled in Reference [24]. Bolded numbers indicate metabolic processes as follows: 1, GSH transporter encoded by OPT1 enables uptake of GSH‐3‐MH; 2, γ‐glutamyltranspeptidase cleavage of glutamate and transport of Cysgly‐3‐MH; 3, general amino acid transporter encoded by GAP1 for uptake of Cys‐3‐MH; 4, metabolism of GSH‐3‐MH to 3‐MH either directly or step‐wise via other precursors; 5, cleavage of 3‐MH from Cys‐3‐MH by carbon–sulfur lyase; 6, acetylation of 3‐MH by alcohol acetyltransferase (AAT) to afford 3‐MHA; 7, unknown mechanism leading to volatile thiol release into wine
Historically, aspects of C‐S lyase activity were determined before those related to uptake of precursors by transporters; however, since uptake by yeast has to occur before thiol release, this phenomenon is discussed first.
23.2.3.1 Precursor uptake
As discussed later, the primary reason for microbial catabolism of S‐conjugates appears to be that they can serve as a source of yeast assimilable nitrogen (YAN). These conjugates are not a preferred nitrogen source, however, and their uptake decreases under conditions that induce nitrogen catabolite repression (NCR), that is, when a preferred nitrogen source is present (see Chapter 22.3 for a full discussion of NCR). For example, fermentations performed in the presence of excess diammonium phosphate (DAP), a more preferred source of YAN, led to lower consumption of Cys‐3‐MH. In contrast, addition of urea, a less preferred nitrogen source, had no effect on Cys‐3‐MH consumption [22]. In comparison, oxygen, vitamins, and sugars had negligible effects on thiol formation.
Yeast transporters and permeases are linked to precursor uptake (Figure 23.2.3). Mutant yeast strains that express the general amino acid permease (GAP1) gene, even under NCR conditions (Chapter 22.3), also produced more 3‐MH from Cys‐3MH than the parent strains in a synthetic medium, indicating that Gap1p is likely critical for Cys‐precursor uptake [22]. Analogous work indicated that the oligopeptide transporter gene OPT1, which encodes for the main GSH transporter Opt1p, is critical for GSH‐3‐MH uptake [23]. Deletion of OPT1 in a strain of S. cerevisiae not only limited GSH uptake but also led to a 2‐fold decrease in 3‐MH (and 3‐MHA) production during fermentation of Sauvignon Blanc juice in comparison to the wild‐type strain. The authors hypothesized that half of the 3‐MH arose from a precursor entering via Opt1p (i.e., the precursor resembled GSH and was likely to be GSH‐3‐MH) or that GSH was possibly an activator of intracellular 3‐MH release from S‐conjugate precursors, so the limited uptake of GSH without Opt1p resulted in a lower 3‐MH concentration [23].
23.2.3.2 Precursor cleavage
Volatile thiols can be liberated from S‐conjugates as a result of C‐S lyase activity. This was first demonstrated with bacterial β‐lyase cleavage of synthetic cysteine conjugates and conjugates isolated from Sauvignon Blanc grape juice; the work also showed thiol release from fermentations with S. cerevisiae [25].5 This spurred further interest in using yeast strain selection to optimize release of varietal thiols during winemaking (Chapter 29).
Cleavage of S‐conjugate precursors by yeast has been investigated through studies of S. cerevisiae mutants in which putative β‐lyase genes were either deleted or overexpressed, and allowed to ferment synthetic media containing S‐conjugate precursors. Several candidate genes have been shown to affect 4‐MMP release from Cys‐4‐MMP in synthetic media [26]. Summarizing the key points regarding these β‐lyases:
- IRC7 (called METC at times) appears to be the major β‐lyase gene responsible for liberation of 4‐MMP (in particular) and 3‐MH from cysteine conjugates during fermentation in synthetic media with VL3 strains, as seen with the decrease of 4‐MMP and 3‐MH of around 95 and 40%, respectively, for a deletion strain [27]. In support of this result, IRC7 overexpression resulted in a 100‐fold increase in 4‐MMP and doubling of 3‐MH (and 3‐MHA) using Sauvignon Blanc juice [28].
- However, substrate preferences will vary among β‐lyases, which provides an explanation for how gene expression differences among yeast strains could result in different thiol profiles. For example, another β‐lyase gene (STR3) with primary activity towards cystathionine was also identified in S. cerevisiae; the corresponding enzyme (Str3p) was purified and shown to have β‐lyase side activity towards Cys‐4‐MMP and Cys‐3‐MH. Str3p showed a preference for Cys‐3‐MH over Cys‐4‐MMP at low concentrations (0.25 mM) although at higher precursor concentrations (2 mM) Cys‐4‐MMP was preferred [29]. Overexpression of STR3 in commercial wine yeast VIN13 and fermentation of Sauvignon Blanc juice revealed increases in 3‐MH in the order of 25%.
- IRC7 expression, and presumably the expression of other β‐lyase genes, is influenced by NCR transcriptional regulation, and shows stereoselectivity in the production of 3‐MH enantiomers from Cys‐3‐MH diastereomers [27].
- Genetic modification of a commercial yeast strain, VIN13, through insertion and overexpression of an E. coli tryptophanase gene (tnaA) has been shown to increase thiol release during model fermentations by up to 25 times compared to the parent strain [30].
These observations also likely explain variation in vendors’ descriptions of yeast strains – several commercial strains are advertised as being ideal for producing Sauvignon Blanc wines in a fruity style, presumably because of either high β‐lyase activity, high uptake efficiency, or a combination of both (although the relative importance of each is unknown). Ultimately, in light of the low conversion efficiency of precursors (see Section 23.2.4) there is great potential for increasing relative or total thiol release through yeast strain selection (Chapter 29).
23.2.3.3 Acetylation by yeast
An important outcome of fermentation and varietal thiol release is the conversion of 3‐MH into its O‐acetylated derivative 3‐MHA, which has similar aroma qualities but over a 10‐fold lower sensory threshold (Chapter 10). In general, a small proportion of 3‐MHA may be produced (in the order of 10–20% of 3‐MH concentration), but the impact on overall wine aroma can be substantial, particularly in young wines [31]. As with release of thiol precursors, the extent of 3‐MHA production is dictated by genetic differences among yeast strains. The main enzyme catalyzing this transformation has been identified as alcohol acetyltransferase Atf1p [32], which is also involved in formation of other acetate esters (Chapter 22.3) under the control of S. cerevisiae ATF1 gene [33]. Overexpression of ATF1 in commercial yeast VIN13 led to around a 7‐fold increase in 3‐MHA for model fermentations spiked with 3‐MH (approximately 50% conversion), whereas overexpression of other genes involved in ester metabolism (ATF2, EHT1, IAH1) either produced the same amount of 3‐MH as the parent strain or somewhat less in the case of IAH1, which encodes an esterase.
23.2.4 Mass balance and alternative pathways to volatile thiol formation
Although Cys‐ and GSH‐conjugates can serve as thiol precursors during fermentation, the correlation of these S‐conjugates in must to corresponding volatile thiol concentrations in wine is very poor, even under similar fermentation conditions [34]. Additionally, high precursor concentrations remain in finished wine (and precursors can form during winemaking) [10] and conjecture remains about the dominant contributors to thiols in wine. Put simply, there is currently a mass balance problem, in that the extent of conversion of known precursors does not explain the amounts of thiols formed during fermentation. For example, based on a molar conversion yield of <1% for labeled Cys‐3‐MH, the contribution of naturally present Cys‐precursor (160 nM) to total 3‐MH (42 nM) in Sauvignon Blanc was calculated at just 3% (or 1.26 nM) [23]; a similar result has been reported for GSH‐3‐MH [35]. Other reports have observed similarly low conversion efficiencies, with Cys‐3‐MH yielding roughly twice as much 3‐MH (1% molar conversion of precursor) as GSH‐3‐MH (0.5% conversion) [24].
Accounting for the remaining >90% of 3‐MH precursor is not a simple issue to resolve, and has been a subject of several recent investigations. The transporters discussed above have a role in providing the precursors intracellularly, and differences in uptake efficiency or regulation could explain some variation. Additionally, among other possibilities related to precursor reactivity/stability, GSH‐3‐MH may not be cleaved directly to yield 3‐MH, but rather undergo degradation via several steps to yield Cys‐3‐MH (Figure 23.2.3), akin to GSH metabolism with enzymatic cleavage of glutamate and glycine residues [24]. Essentially this suggests that Cys‐3‐MH is more easily utilized by yeast, and precursor ratios could therefore play a role.
Other studies have investigated alternate pathways leading to volatile thiols or their precursors during fermentation. Potential biogenetic pathways involve conjugate addition of a thiol‐containing compound such as H2S, cysteine, or GSH to (E)‐2‐hexenal or mesityl oxide during processing or fermentation [6, 20, 36, 37], yielding 3‐MH (and 3‐MHA) and 4‐MMP more directly. H2S formed through fermentation does appear to yield 3‐MH, although the contribution is very minor. Formation of additional cysteine and GSH conjugates once fermentation commences is not as well studied, but would presumably augment the initial precursor pool (see Section 23.2.2). Additionally, under certain conditions, yeast may convert (E)‐2‐hexen‐1‐ol (a C6 alcohol, Chapter 6) into (E)‐2‐hexenal, yielding additional 3‐MH and 3‐MHA in wine [38], which may occur after the (E)‐2‐hexenal so produced combines with H2S arising early during fermentation (Chapter 22.4). However, the contribution of H2S and C6 reaction products to the volatile thiol pool seems to be low. This is not an unexpected scenario, since the varietal thiol concentrations show stronger grape variety dependence (Chapter 10) than either C6 compounds or yeast‐derived H2S.
Alternatively, other grape‐derived conjugates may exist that are yet to be identified, and precursor intermediates such as GSH‐3‐MHal and Cysgly‐3‐MH (Figure 23.2.2) and similar conjugates could conceivably contribute to wine varietal thiol concentrations [6, 19]. It is also theoretically possible that other sources of (E)‐2‐hexenal could ultimately lead to 3‐MH, such as the (E)‐2‐hexenal sulfonate adduct (Chapter 17) [39] or other unknown adducts. In the end, additional studies will be necessary to fully account for the presence of varietal thiols, particularly 3‐MH and 3‐MHA.
References
- 1. Roland, A., Schneider, R., Razungles, A., Cavelier, F. (2011) Varietal thiols in wine: discovery, analysis and applications. Chemical Reviews, 111 (11), 7355–7376.
- 2. Coetzee, C. and du Toit, W.J. (2012) A comprehensive review on Sauvignon blanc aroma with a focus on certain positive volatile thiols. Food Research International, 45 (1), 287–298.
- 3. Capone, D.L., Sefton, M.A., Jeffery, D.W. (2012) Analytical investigations of wine odorant 3‐mercaptohexan‐1‐ol and its precursors, in Flavor chemistry of wine and other alcoholic beverages, American Chemical Society, pp. 15–35.
- 4. Peña‐Gallego, A., Hernández‐Orte, P., Cacho, J., Ferreira, V. (2012) S‐Cysteinylated and S‐glutathionylated thiol precursors in grapes. A review. Food Chemistry, 131 (1), 1–13.
- 5. Starkenmann, C., Troccaz, M., Howell, K. (2008) The role of cysteine and cysteine–S conjugates as odour precursors in the flavour and fragrance industry. Flavour and Fragrance Journal, 23 (6), 369–381.
- 6. Capone, D.L. and Jeffery, D.W. (2011) Effects of transporting and processing Sauvignon blanc grapes on 3‐mercaptohexan‐1‐ol precursor concentrations. Journal of Agricultural and Food Chemistry, 59 (9), 4659–4667.
- 7. Allen, T., Herbst‐Johnstone, M., Girault, M., et al. (2011) Influence of grape‐harvesting steps on varietal thiol aromas in Sauvignon blanc wines. Journal of Agricultural and Food Chemistry, 59 (19), 10641–10650.
- 8. Roland, A., Schneider, R., Charrier, F., et al. (2011) Distribution of varietal thiol precursors in the skin and the pulp of Melon B. and Sauvignon Blanc grapes. Food Chemistry, 125 (1), 139–144.
- 9. Thibon, C., Shinkaruk, S., Jourdes, M., et al. (2010) Aromatic potential of botrytized white wine grapes: identification and quantification of new cysteine‐S‐conjugate flavor precursors. Analytica Chimica Acta, 660 (1–2), 190–196.
- 10. Capone, D.L., Sefton, M.A., Jeffery, D.W. (2011) Application of a modified method for 3‐mercaptohexan‐1‐ol determination to investigate the relationship between free thiol and related conjugates in grape juice and wine. Journal of Agricultural and Food Chemistry, 59 (9), 4649–4658.
- 11. Maggu, M., Winz, R., Kilmartin, P.A., et al. (2007) Effect of skin contact and pressure on the composition of Sauvignon Blanc must. Journal of Agricultural and Food Chemistry, 55 (25), 10281–10288.
- 12. Peyrot des Gachons, C., Tominaga, T., Dubourdieu, D. (2002) Sulfur aroma precursor present in S‐glutathione conjugate form: identification of S‐3‐(hexan‐1‐ol)‐glutathione in must from Vitis vinifera L. cv. Sauvignon Blanc. Journal of Agricultural and Food Chemistry, 50 (14), 4076–4079.
- 13. Thibon, C., Cluzet, S., Merillon, J.M., et al. (2011) 3‐Sulfanylhexanol precursor biogenesis in grapevine cells: the stimulating effect of Botrytis cinerea. Journal of Agricultural and Food Chemistry, 59 (4), 1344–1351.
- 14. Marrs, K.A. (1996) The functions and regulation of glutathione S‐transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 47, 127–158.
- 15. Dixon, D.P., Skipsey, M., Edwards, R. (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry, 71 (4), 338–350.
- 16. Kobayashi, H., Takase, H., Suzuki, Y., et al. (2011) Environmental stress enhances biosynthesis of flavor precursors, S‐3‐(hexan‐1‐ol)‐glutathione and S‐3‐(hexan‐1‐ol)‐L‐cysteine, in grapevine through glutathione S‐transferase activation. Journal of Experimental Botany, 62 (3), 1325–1336.
- 17. Schroder, P. (1997) Fate of glutathione S‐conjugates in plants. Degradation of the glutathione moiety, Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 18. Rennenberg, H. (1982) Glutathione metabolism and possible biological roles in higher plants. Phytochemistry, 21 (12), 2771–2781.
- 19. Capone, D.L., Pardon, K.H., Cordente, A.G., Jeffery, D.W. (2011) Identification and quantitation of 3‐S‐cysteinylglycinehexan‐1‐ol (Cysgly‐3‐MH) in Sauvignon blanc grape juice by HPLC‐MS/MS. Journal of Agricultural and Food Chemistry, 59 (20), 11204–11210.
- 20. Capone, D.L., Black, C.A., Jeffery, D.W. (2012) Effects on 3‐mercaptohexan‐1‐ol precursor concentrations from prolonged storage of Sauvignon Blanc grapes prior to crushing and pressing. Journal of Agricultural and Food Chemistry, 60 (13), 3515–3523.
- 21. Dufour, M., Zimmer, A., Thibon, C., Marullo, P. (2013) Enhancement of volatile thiol release of Saccharomyces cerevisiae strains using molecular breeding. Applied Microbiology and Biotechnology, 97 (13), 5893–5905.
- 22. Subileau, M., Schneider, R., Salmon, J.‐M., Degryse, E. (2008) Nitrogen catabolite repression modulates the production of aromatic thiols characteristic of Sauvignon Blanc at the level of precursor transport. FEMS Yeast Research, 8 (5), 771–780.
- 23. Subileau, M., Schneider, R., Salmon, J.‐M., Degryse, E. (2008) New insights on 3‐mercaptohexanol (3MH) biogenesis in Sauvignon Blanc wines: Cys‐3MH and (E)‐hexen‐2‐al are not the major precursors. Journal of Agricultural and Food Chemistry, 56 (19), 9230–9235.
- 24. Winter, G., Van Der Westhuizen, T., Higgins, V.J., et al. (2011) Contribution of cysteine and glutathione conjugates to the formation of the volatile thiols 3‐mercaptohexan‐1‐ol (3MH) and 3‐mercaptohexyl acetate (3MHA) during fermentation by Saccharomyces cerevisiae. Australian Journal of Grape and Wine Research, 17 (2), 285–290.
- 25. Tominaga, T., Peyrot des Gachons, C., Dubourdieu, D. (1998) A new type of flavor precursors in Vitis vinifera L. cv. Sauvignon Blanc: S‐cysteine conjugates. Journal of Agricultural and Food Chemistry, 46 (12), 5215–5219.
- 26. Howell, K.S., Klein, M., Swiegers, J.H., et al. (2005) Genetic determinants of volatile‐thiol release by Saccharomyces cerevisiae during wine fermentation. Applied and Environmental Microbiology, 71 (9), 5420–5426.
- 27. Thibon, C., Marullo, P., Claisse, O., et al. (2008) Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation. FEMS Yeast Research, 8 (7), 1076–1086.
- 28. Roncoroni, M., Santiago, M., Hooks, D.O., et al. (2011) The yeast IRC7 gene encodes a β‐lyase responsible for production of the varietal thiol 4‐mercapto‐4‐methylpentan‐2‐one in wine. Food Microbiology, 28 (5), 926–935.
- 29. Holt, S., Cordente, A.G., Williams, S.J., et al. (2011) Engineering Saccharomyces cerevisiae to release 3‐mercaptohexan‐1‐ol during fermentation through overexpression of an S. cerevisiae gene, STR3, for improvement of wine aroma. Applied and Environmental Microbiology, 77 (11), 3626–3632.
- 30. Swiegers, J.H., Capone, D.L., Pardon, K.H., et al. (2007) Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast, 24 (7), 561–574.
- 31. Dubourdieu, D., Tominaga, T., Masneuf, I., et al. (2006) The role of yeasts in grape flavor development during fermentation: the example of Sauvignon blanc. American Journal of Enology and Viticulture, 57 (1), 81–88.
- 32. Swiegers, J.H., Willmott, R., Hill‐Ling, A., et al. (2006) Modulation of volatile thiol and ester aromas by modified wine yeast, in Developments in food science (eds Bredie, W.L.P. and Petersen, M.A.), Elsevier, Amsterdam, The Netherlands, pp. 113–116.
- 33. Mason, A.B. and Dufour, J.‐P. (2000) Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast, 16 (14), 1287–1298.
- 34. Pinu, F.R., Jouanneau, S., Nicolau, L., et al. (2012) Concentrations of the volatile thiol 3‐mercaptohexanol in Sauvignon blanc wines: no correlation with juice precursors. American Journal of Enology and Viticulture, 63 (3), 407–412.
- 35. Roland, A., Schneider, R., Guernevé, C.L., et al. (2010) Identification and quantification by LC‐MS/MS of a new precursor of 3‐mercaptohexan‐1‐ol (3MH) using stable isotope dilution assay: elements for understanding the 3MH production in wine. Food Chemistry, 121 (3), 847–855.
- 36. Schneider, R., Charrier, F., Razungles, A., Baumes, R. (2006) Evidence for an alternative biogenetic pathway leading to 3‐mercaptohexanol and 4‐mercapto‐4‐methylpentan‐2‐one in wines. Analytica Chimica Acta, 563 (1–2), 58–64.
- 37. Roland, A., Vialaret, J., Razungles, A., et al. (2010) Evolution of S‐cysteinylated and S‐glutathionylated thiol precursors during oxidation of Melon B. and Sauvignon blanc musts. Journal of Agricultural and Food Chemistry, 58 (7), 4406–4413.
- 38. Harsch, M.J., Benkwitz, F., Frost, A., et al. (2013) A new precursor of 3‐mercaptohexan‐1‐ol in grape juice: thiol‐forming potential and kinetics during early stages of must fermentation. Journal of Agricultural and Food Chemistry, 61 (15), 3703–3713.
- 39. Duhamel, N., Piano, F., Davidson, S.J., et al. (2015) Synthesis of alkyl sulfonic acid aldehydes and alcohols, putative precursors to important wine aroma thiols. Tetrahedron Letters, 56 (13), 1728–1731.